User login
APA, others lobby to make COVID-19 telehealth waivers permanent
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The American Psychiatric Association (APA) is calling on Congress to permanently lift restrictions that have allowed unfettered delivery of telehealth services during the COVID-19 pandemic, which experts say has been a boon to patients and physicians alike.
“We ask Congress to extend the telehealth waiver authority under COVID-19 beyond the emergency and to study its impact while doing so,” said APA President Jeffrey Geller, MD, in a May 27 video briefing with congressional staff and reporters.
The APA is also seeking to make permanent certain waivers granted by the Centers for Medicare & Medicaid Services on April 30, including elimination of geographic restrictions on behavioral health and allowing patients be seen at home, said Dr. Geller.
The APA also is asking for the elimination of the rule that requires clinicians to have an initial face-to-face meeting with patients before they can prescribe controlled substances, Dr. Geller said. The Drug Enforcement Administration waived that requirement, known as the Ryan Haight Act, on March 17 for the duration of the national emergency.
Telemedicine has supporters on both sides of the aisle in Congress, including Rep. Paul Tonko (D-N.Y.) who said at the APA briefing he would fight to make the waivers permanent.
“The expanded use of telehealth has enormous potential during normal times as well, especially in behavioral health,” said Mr. Tonko. “I am pushing fiercely for these current flexibilities to be extended for a reasonable time after the public health emergency so that we can have time to evaluate which should be made permanent,” he said.
Dr. Geller, other clinicians, and advocates in the briefing praised CMS for facilitating telepsychiatry for Medicare. That follows in the footsteps of most private insurers, who have also relaxed requirements into the summer, according to the Medical Group Management Association.
Game changer
The Medicare waivers “have dramatically changed the entire scene for someone like myself as a clinician to allow me to see my patients in a much easier way,” said Peter Yellowlees, MBBS, MD, chief wellness officer, University of California Davis Health. Within 2 weeks in March, the health system converted almost all of its regular outpatient visits to telemedicine, he said.
Dr. Yellowlees added government still needs to address, what he called, outdated HIPAA regulations that ban certain technologies.
“It makes no sense that I can talk to someone on an iPhone, but the moment I talk to them on FaceTime, it’s illegal,” said Dr. Yellowlees, a former president of the American Telemedicine Association.
Dr. Geller said that “psychiatric care provided by telehealth is as effective as in-person psychiatric services,” adding that “some patients prefer telepsychiatry because of its convenience and as a means of reducing stigma associated with seeking help for mental health.”
Shabana Khan, MD, a child psychiatrist and director of telepsychiatry at New York University Langone Health, said audio and video conferencing are helping address a shortage and maldistribution of child and adolescent psychiatrists.
Americans’ mental health is suffering during the pandemic. The U.S. Census Bureau recently released data showing that half of those surveyed reported depressed mood and that one-third are reporting anxiety, depression, or both, as reported by the Washington Post.
“At this very time that anxiety, depression, substance use, and other mental health problems are rising, our nation’s already strained mental health system is really being pushed to the brink,” said Jodi Kwarciany, manager for mental health policy for the National Alliance on Mental Illness, during the briefing.
Telemedicine can help “by connecting people to providers at the time and the place and using the technology that works best for them,” she said, adding that NAMI would press policymakers to address barriers to access.
The clinicians on the briefing said they’ve observed that some patients are more comfortable with video or audio interactions than with in-person visits.
Increased access to care
Telepsychiatry seems to be convincing some to reconsider therapy, since they can do it at home, said Dr. Yellowlees. he said.
For instance, he said, he has been able to consult by phone and video with several patients who receive care through the Indian Health Service who had not be able to get into the physical clinic.
Dr. Yellowlees said video sessions also may encourage patients to be more, not less, talkative. “Video is actually counterintuitively a very intimate experience,” he said, in part because of the perceived distance and people’s tendency to be less inhibited on technology platforms.“It’s less embarrassing,” he said. “If you’ve got really dramatic, difficult, traumatic things to talk about, it’s slightly easier to talk to someone who’s slightly further apart from you on video,” said Dr. Yellowlees.
“Individuals who have a significant amount of anxiety may actually feel more comfortable with the distance that this technology affords,” agreed Dr. Khan. She said telemedicine had made sessions more comfortable for some of her patients with autism spectrum disorder.
Dr. Geller said audio and video have been important to his practice during the pandemic. One of his patients never leaves the house and does not use computers. “He spends his time sequestered at home listening to records on his record player,” said Dr. Geller. But he’s been amenable to phone sessions. “What I’ve found with him, and I’ve found with several other patients, is that they actually talk more easily when they’re not face to face,” he said.
Far fewer no-shows
Another plus for his New England–based practice during the last few months: patients have not been anxious about missing sessions because of the weather. The clinicians all noted that telepsychiatry seemed to reduce missed visits.
Dr. Yellowlees said that no-show rates had decreased by half at UC Davis. “That means no significant loss of income,” during the pandemic, he said.
“The no-show rate is incredibly low, particularly because when you call the patients and they don’t remember they had an appointment, you have the appointment anyway, most of the time,” said Dr. Geller.
For Dr. Khan, being able to conduct audio and video sessions during the pandemic has meant keeping up continuity of care.
As a result of the pandemic, many college students in New York City had to go home – often to another state. The waivers granted by New York’s Medicaid program and other insurers have allowed Dr. Khan to continue care for these patients.
The NYU clinic also operates day programs in rural areas 5 hours from the city. Dr. Khan recently evaluated a 12-year-old girl with significant anxiety and low mood, both of which had worsened.
“She would not have been able to access care otherwise,” said Dr. Khan. And for rural patients who do not have access to broadband or smartphones, audio visits “have been immensely helpful,” she said.
Dr. Khan, Dr. Geller, and Dr. Yellowlees have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Operation Quack Hack: FDA moves to stop fraudulent COVID-19 products
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.
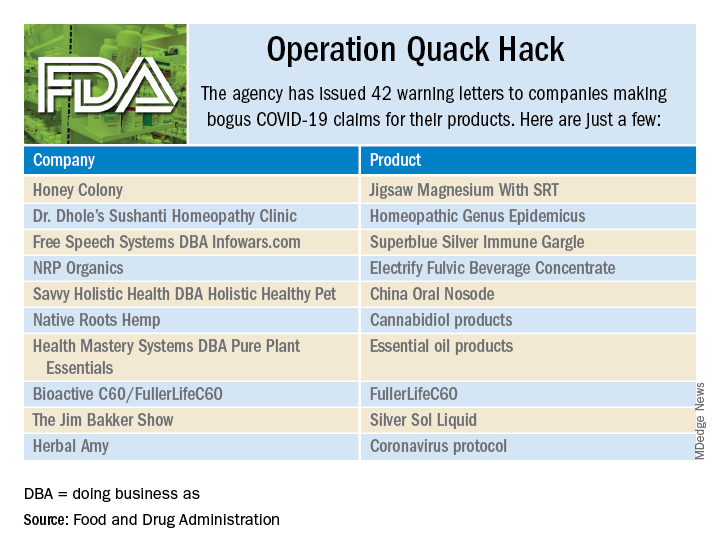
As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
No form of human misery can be allowed to go unexploited, and the pandemic, it seems, is no exception.

As part of Operation Quack Hack, the Food and Drug Administration has stepped up its investigation and enforcement efforts against companies and individuals that are “taking advantage of widespread fear among consumers during the COVID-19 pandemic” by selling fake products and treatments for coronavirus.
As of May 7, 2020, the agency had issued 42 warning letters to companies that were “selling unapproved products that fraudulently claim to mitigate, prevent, treat, diagnose or cure COVID-19,” the FDA announced in a written statement. Of those 42 products, 29 are no longer being sold with any sort of COVID-19 claim.
Since the beginning of the pandemic, Operation Quack Hack has uncovered hundreds of such products – drugs, testing kits, and personal protective equipment – being sold online, and complaints were sent to domain-name registrars and Internet marketplaces that have, in most cases, removed the postings, the FDA said.
“We will continue to monitor the online ecosystem for fraudulent products peddled by bad actors seeking to profit from this global pandemic. We encourage anyone aware of suspected fraudulent medical products for COVID-19 to report them to the FDA,” the statement said.
FDA tightens requirements for COVID-19 antibody tests
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
The U.S. Food and Drug Administration is tightening requirements for companies that develop COVID-19 antibody tests in an effort to combat fraud and better regulate the frenzy of tests coming to market.
The updated policy, announced May 4, requires commercial antibody test developers to apply for Emergency Use Authorization (EUA) from the FDA under a tight time frame and also provides specific performance threshold recommendations for test specificity and sensitivity. The revised requirements follow a March 16 policy that allowed developers to validate their own tests and bring them to market without an agency review. More than 100 coronavirus antibody tests have since entered the market, fueling a congressional investigation into the accuracy of tests.
When the March policy was issued, FDA Commissioner Stephen M. Hahn, MD, said it was critical for the FDA to provide regulatory flexibility for serology test developers, given the nature of the COVID-19 public health emergency and an understanding that the tests were not meant to be used as the sole basis for COVID-19 diagnosis.
“As FDA has authorized more antibody tests and validation data has become available, including through the capability at [the National Cancer Institute] the careful balancing of risks and benefits has shifted to the approach we have outlined today and our policy update,” Dr. Hahn said during a May 4 press conference.
The new approach requires all commercial manufacturers to submit EUA requests with their validation data within 10 business days from the date they notified the FDA of their validation testing or from the date of the May 4 policy, whichever is later. Additionally, the FDA has provided specific performance threshold recommendations for specificity and sensitivity for all serology test developers.
In a statement released May 4, FDA leaders acknowledged the widespread fraud that is occurring in connection to antibody tests entering the market.
“We unfortunately see unscrupulous actors marketing fraudulent test kits and using the pandemic as an opportunity to take advantage of Americans’ anxiety,” wrote Anand Shah, MD, FDA deputy commissioner for medical and scientific affairs in a joint statement with Jeff E. Shuren, MD, director for the FDA’s Center for Devices and Radiological Health. “Some test developers have falsely claimed their serological tests are FDA approved or authorized. Others have falsely claimed that their tests can diagnose COVID-19 or that they are for at-home testing, which would fall outside of the policies outlined in our March 16 guidance, as well as the updated guidance.”
At the same time, FDA officials said they are aware of a “concerning number” of commercial serology tests that are being inappropriately marketed, including for diagnostic use, or that are performing poorly based on an independent evaluation by the National Institutes of Health, according to the May 4 statement.
In addition to tightening its requirements for test developers, the FDA also is introducing a more streamlined process to support EUA submissions and review. Two voluntary EUA templates for antibody tests are now available – one for commercial manufacturers and one for Clinical Laboratory Improvement Amendments-certified high-complexity labs seeking FDA authorization. The templates will facilitate the preparation and submission of EUA requests and can be used by any interested developer, according to the FDA.
To date, 12 antibody tests have been authorized under an individual EUA, and more than 200 antibody tests are currently the subject of a pre-EUA or EUA review, according to the FDA.
Many unknowns remain about antibody tests and how they might help researchers and clinicians understand and/or potentially treat COVID-19. Antibody tests may be able to provide information on disease prevalence and frequency of asymptomatic infection, as well as identify potential donors of “convalescent plasma,” an approach in which blood plasma containing antibodies from a recovered individual serves as a therapy for an infected patient with severe disease, Dr. Shah wrote in the May 4 statement.
“There are a lot of unanswered questions about this particular issue,” Dr. Hahn said during the press conference. “We need the data because we need to understand this particular aspect of the disease and put it as part of the puzzle around COVID-19.”
CMS hikes telephone visit payments during pandemic
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.
Physicians who are conducting telephone visits during the COVID-19 pandemic will be paid at a higher rate, more closely aligning the rates with payments for face-to-face visits.
On April 30, officials at the Centers for Medicare & Medicaid Services announced the temporary telephone visit rate change and expanded the scope of services that are eligible telephone visits to include many behavioral health and patient education services.
Rates for telephone visits will jump from $14-$41 per visit to about $46-$110. The pay increase is retroactive to March 1, 2020.
The move was welcomed by the American College of Physicians, but the organization said more needs to be done in order help maintain the financial stability of physician practices.
“ACP has repeatedly requested this change from CMS as the country has been dealing with the COVID-19 national emergency, and we are heartened that they have heard our concerns,” ACP President Jacqueline Fincher, MD, said in a statement. “More still needs to be done to ensure that physician practices are able to remain operational and care for their patients, but this change in payment policy addresses one of the biggest issues facing physicians as they struggle to make up for lost revenue and provide appropriate care to patients.”
CMS also is expanding payment availability for audio-only telemedicine services by waiving the video requirement for certain evaluation and management services. The move is aimed at reaching Medicare beneficiaries who may not have access to video technology or choose not to use it.
“This is a major victory for medicine that will enable physicians to care for their patients, especially their elderly patients with chronic conditions who may not have access to audio-visual technology or high-speed Internet,” Patrice Harris, MD, president of the American Medical Association, said in a statement. “This change will help patients address their health challenges that existed before COVID-19.”
Shawn Martin, senior vice president at the American Academy of Family Physicians, said his group is pleased to see CMS roll out this change and noted that it is especially important for patients with underlying health conditions. “This is the only connectivity they may have with a health care system for their ongoing health care needs.”
Samuel Jones, MD, chair of the Health Affairs Committee at the American College of Cardiology, highlighted the expansion and coverage of audio-only telemedicine appointments as a huge plus for patient access.*
“There was a huge hunger to say, ‘Can we just have improvement in the reimbursement for telephone, which is providing a good service, our patients our asking for it,’ and we were able to get that,” Dr. Jones said in an interview. “It really was, I think, a good thing for patient care.”
Dr. Jones also suggested that the temporary policy be extended after the COVID-19 crisis is over.
“Telemedicine is here to stay,” he said. “But if all of these relaxations suddenly go away with a snap of the finger, or if the reimbursement [is lowered], if all that changes as soon as this emergency declaration is over, we are going to have a hard time.”
The pay increase for telephone services was part of a broader package of increased regulatory flexibility CMS rolled out, including expanding the types of providers who can order a COVID-19 test.
*Correction, 5/5/2020: An earlier version of this story misstated Dr. Samuel Jones' affiliation. He is the chair of the Health Affairs Committee at the American College of Cardiology.
Will COVID-19 finally trigger action on health disparities?
Because of stark racial disparities in COVID-19 infection and mortality, the pandemic is being called a “sentinel” and “bellwether” event that should push the United States to finally come to grips with disparities in health care.
When it comes to COVID-19, the pattern is “irrefutable”: Blacks in the United States are being infected with SARS-CoV-2 and are dying of COVID-19 at higher rates than whites, Clyde W. Yancy, MD, Northwestern University, Chicago, wrote in a viewpoint article published online April 15 in JAMA.
According to one recent survey, he noted, the infection rate is threefold higher and the death rate is sixfold higher in predominantly black counties in the United States relative to predominantly white counties.
A sixfold increase in the rate of death for blacks due to a now ubiquitous virus should be deemed “unconscionable” and a moment of “ethical reckoning,” Dr. Yancy wrote.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
The COVID-19 pandemic may be the “bellwether” event that the United States has needed to fully address disparities in health care, Dr. Yancy said.
“Public health is complicated and social reengineering is complex, but change of this magnitude does not happen without a new resolve,” he concluded. “The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event. Certainly, within the broad and powerful economic and legislative engines of the U.S., there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain.”
The question is, he asks, will the nation finally “think differently, and, as has been done in response to other major diseases, declare that a civil society will no longer accept disproportionate suffering?”
Keith C. Ferdinand, MD, Tulane University, New Orleans, doesn’t think so.
In a related editorial published online April 17 in the Journal of the American College of Cardiology, he points out that the 1985 Heckler Report, from the Department of Health and Human Services, documented higher racial/ethnic mortality rates and the need to correct them. This was followed in 2002 by a report from the Institute of Medicine called Unequal Treatment that also underscored health disparities.
Despite some progress, the goal of reducing and eventually eliminating racial/ethnic disparities has not been realized, Dr. Ferdinand said. “I think baked into the consciousness of the American psyche is that there are some people who have and some who have not,” he said in an interview.
“To some extent, some societies at some point become immune. We would not like to think that America, with its sense of egalitarianism, would get to that point, but maybe we have,” said Dr. Ferdinand.
A ‘sentinel event’
He points out that black people are not genetically or biologically predisposed to COVID-19 but are socially prone to coronavirus exposure and are more likely to have comorbid conditions, such as hypertension, diabetes, obesity, and heart disease, that fuel complications.
The “tragic” higher COVID-19 mortality among African Americans and other racial/ethnic minorities confirms “inadequate” efforts on the part of society to eliminate disparities in cardiovascular disease (CVD) and is a “sentinel event,” Dr. Ferdinand wrote.
A sentinel event, as defined by the Joint Commission, is an unexpected occurrence that leads to death or serious physical or psychological injury or the risk thereof, he explained.
“Conventionally identified sentinel events, such as unintended retention of foreign objects and fall-related events, are used to evaluate quality in hospital care. Similarly, disparate [African American] COVID-19 mortality reflects long-standing, unacceptable U.S. racial/ethnic and socioeconomic CVD inequities and unmasks system failures and unacceptable care to be caught and mitigated,” Dr. Ferdinand concluded.
Dr. Yancy and Dr. Ferdinand have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Because of stark racial disparities in COVID-19 infection and mortality, the pandemic is being called a “sentinel” and “bellwether” event that should push the United States to finally come to grips with disparities in health care.
When it comes to COVID-19, the pattern is “irrefutable”: Blacks in the United States are being infected with SARS-CoV-2 and are dying of COVID-19 at higher rates than whites, Clyde W. Yancy, MD, Northwestern University, Chicago, wrote in a viewpoint article published online April 15 in JAMA.
According to one recent survey, he noted, the infection rate is threefold higher and the death rate is sixfold higher in predominantly black counties in the United States relative to predominantly white counties.
A sixfold increase in the rate of death for blacks due to a now ubiquitous virus should be deemed “unconscionable” and a moment of “ethical reckoning,” Dr. Yancy wrote.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
The COVID-19 pandemic may be the “bellwether” event that the United States has needed to fully address disparities in health care, Dr. Yancy said.
“Public health is complicated and social reengineering is complex, but change of this magnitude does not happen without a new resolve,” he concluded. “The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event. Certainly, within the broad and powerful economic and legislative engines of the U.S., there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain.”
The question is, he asks, will the nation finally “think differently, and, as has been done in response to other major diseases, declare that a civil society will no longer accept disproportionate suffering?”
Keith C. Ferdinand, MD, Tulane University, New Orleans, doesn’t think so.
In a related editorial published online April 17 in the Journal of the American College of Cardiology, he points out that the 1985 Heckler Report, from the Department of Health and Human Services, documented higher racial/ethnic mortality rates and the need to correct them. This was followed in 2002 by a report from the Institute of Medicine called Unequal Treatment that also underscored health disparities.
Despite some progress, the goal of reducing and eventually eliminating racial/ethnic disparities has not been realized, Dr. Ferdinand said. “I think baked into the consciousness of the American psyche is that there are some people who have and some who have not,” he said in an interview.
“To some extent, some societies at some point become immune. We would not like to think that America, with its sense of egalitarianism, would get to that point, but maybe we have,” said Dr. Ferdinand.
A ‘sentinel event’
He points out that black people are not genetically or biologically predisposed to COVID-19 but are socially prone to coronavirus exposure and are more likely to have comorbid conditions, such as hypertension, diabetes, obesity, and heart disease, that fuel complications.
The “tragic” higher COVID-19 mortality among African Americans and other racial/ethnic minorities confirms “inadequate” efforts on the part of society to eliminate disparities in cardiovascular disease (CVD) and is a “sentinel event,” Dr. Ferdinand wrote.
A sentinel event, as defined by the Joint Commission, is an unexpected occurrence that leads to death or serious physical or psychological injury or the risk thereof, he explained.
“Conventionally identified sentinel events, such as unintended retention of foreign objects and fall-related events, are used to evaluate quality in hospital care. Similarly, disparate [African American] COVID-19 mortality reflects long-standing, unacceptable U.S. racial/ethnic and socioeconomic CVD inequities and unmasks system failures and unacceptable care to be caught and mitigated,” Dr. Ferdinand concluded.
Dr. Yancy and Dr. Ferdinand have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Because of stark racial disparities in COVID-19 infection and mortality, the pandemic is being called a “sentinel” and “bellwether” event that should push the United States to finally come to grips with disparities in health care.
When it comes to COVID-19, the pattern is “irrefutable”: Blacks in the United States are being infected with SARS-CoV-2 and are dying of COVID-19 at higher rates than whites, Clyde W. Yancy, MD, Northwestern University, Chicago, wrote in a viewpoint article published online April 15 in JAMA.
According to one recent survey, he noted, the infection rate is threefold higher and the death rate is sixfold higher in predominantly black counties in the United States relative to predominantly white counties.
A sixfold increase in the rate of death for blacks due to a now ubiquitous virus should be deemed “unconscionable” and a moment of “ethical reckoning,” Dr. Yancy wrote.
“Why is this uniquely important to me? I am an academic cardiologist; I study health care disparities; and I am a black man,” he wrote.
The COVID-19 pandemic may be the “bellwether” event that the United States has needed to fully address disparities in health care, Dr. Yancy said.
“Public health is complicated and social reengineering is complex, but change of this magnitude does not happen without a new resolve,” he concluded. “The U.S. has needed a trigger to fully address health care disparities; COVID-19 may be that bellwether event. Certainly, within the broad and powerful economic and legislative engines of the U.S., there is room to definitively address a scourge even worse than COVID-19: health care disparities. It only takes will. It is time to end the refrain.”
The question is, he asks, will the nation finally “think differently, and, as has been done in response to other major diseases, declare that a civil society will no longer accept disproportionate suffering?”
Keith C. Ferdinand, MD, Tulane University, New Orleans, doesn’t think so.
In a related editorial published online April 17 in the Journal of the American College of Cardiology, he points out that the 1985 Heckler Report, from the Department of Health and Human Services, documented higher racial/ethnic mortality rates and the need to correct them. This was followed in 2002 by a report from the Institute of Medicine called Unequal Treatment that also underscored health disparities.
Despite some progress, the goal of reducing and eventually eliminating racial/ethnic disparities has not been realized, Dr. Ferdinand said. “I think baked into the consciousness of the American psyche is that there are some people who have and some who have not,” he said in an interview.
“To some extent, some societies at some point become immune. We would not like to think that America, with its sense of egalitarianism, would get to that point, but maybe we have,” said Dr. Ferdinand.
A ‘sentinel event’
He points out that black people are not genetically or biologically predisposed to COVID-19 but are socially prone to coronavirus exposure and are more likely to have comorbid conditions, such as hypertension, diabetes, obesity, and heart disease, that fuel complications.
The “tragic” higher COVID-19 mortality among African Americans and other racial/ethnic minorities confirms “inadequate” efforts on the part of society to eliminate disparities in cardiovascular disease (CVD) and is a “sentinel event,” Dr. Ferdinand wrote.
A sentinel event, as defined by the Joint Commission, is an unexpected occurrence that leads to death or serious physical or psychological injury or the risk thereof, he explained.
“Conventionally identified sentinel events, such as unintended retention of foreign objects and fall-related events, are used to evaluate quality in hospital care. Similarly, disparate [African American] COVID-19 mortality reflects long-standing, unacceptable U.S. racial/ethnic and socioeconomic CVD inequities and unmasks system failures and unacceptable care to be caught and mitigated,” Dr. Ferdinand concluded.
Dr. Yancy and Dr. Ferdinand have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CMS suspends advance payment program to clinicians for COVID-19 relief
The Centers for Medicare & Medicaid Services will suspend its Medicare advance payment program for clinicians and is reevaluating how much to pay to hospitals going forward through particular COVID-19 relief initiatives. CMS announced the changes on April 26. Physicians and others who use the accelerated and advance Medicare payments program repay these advances, and they are typically given 1 year or less to repay the funding.
CMS said in a news release it will not accept new applications for the advanced Medicare payment, and it will be reevaluating all pending and new applications “in light of historical direct payments made available through the Department of Health & Human Services’ (HHS) Provider Relief Fund.”
The advance Medicare payment program predates COVID-19, although it previously was used on a much smaller scale. In the past 5 years, CMS approved about 100 total requests for advanced Medicare payment, with most being tied to natural disasters such as hurricanes.
CMS said it has approved, since March, more than 21,000 applications for advanced Medicare payment, totaling $59.6 billion, for hospitals and other organizations that bill its Part A program. In addition, CMS approved almost 24,000 applications for its Part B program, advancing $40.4 billion for physicians, other clinicians, and medical equipment suppliers.
CMS noted that Congress also has provided $175 billion in aid for the medical community that clinicians and medical organizations would not need to repay. The Coronavirus Aid, Relief, and Economic Security (CARES) Act enacted in March included $100 billion, and the Paycheck Protection Program and Health Care Enhancement Act, enacted March 24, includes another $75 billion.
A version of this article was originally published on Medscape.com.
The Centers for Medicare & Medicaid Services will suspend its Medicare advance payment program for clinicians and is reevaluating how much to pay to hospitals going forward through particular COVID-19 relief initiatives. CMS announced the changes on April 26. Physicians and others who use the accelerated and advance Medicare payments program repay these advances, and they are typically given 1 year or less to repay the funding.
CMS said in a news release it will not accept new applications for the advanced Medicare payment, and it will be reevaluating all pending and new applications “in light of historical direct payments made available through the Department of Health & Human Services’ (HHS) Provider Relief Fund.”
The advance Medicare payment program predates COVID-19, although it previously was used on a much smaller scale. In the past 5 years, CMS approved about 100 total requests for advanced Medicare payment, with most being tied to natural disasters such as hurricanes.
CMS said it has approved, since March, more than 21,000 applications for advanced Medicare payment, totaling $59.6 billion, for hospitals and other organizations that bill its Part A program. In addition, CMS approved almost 24,000 applications for its Part B program, advancing $40.4 billion for physicians, other clinicians, and medical equipment suppliers.
CMS noted that Congress also has provided $175 billion in aid for the medical community that clinicians and medical organizations would not need to repay. The Coronavirus Aid, Relief, and Economic Security (CARES) Act enacted in March included $100 billion, and the Paycheck Protection Program and Health Care Enhancement Act, enacted March 24, includes another $75 billion.
A version of this article was originally published on Medscape.com.
The Centers for Medicare & Medicaid Services will suspend its Medicare advance payment program for clinicians and is reevaluating how much to pay to hospitals going forward through particular COVID-19 relief initiatives. CMS announced the changes on April 26. Physicians and others who use the accelerated and advance Medicare payments program repay these advances, and they are typically given 1 year or less to repay the funding.
CMS said in a news release it will not accept new applications for the advanced Medicare payment, and it will be reevaluating all pending and new applications “in light of historical direct payments made available through the Department of Health & Human Services’ (HHS) Provider Relief Fund.”
The advance Medicare payment program predates COVID-19, although it previously was used on a much smaller scale. In the past 5 years, CMS approved about 100 total requests for advanced Medicare payment, with most being tied to natural disasters such as hurricanes.
CMS said it has approved, since March, more than 21,000 applications for advanced Medicare payment, totaling $59.6 billion, for hospitals and other organizations that bill its Part A program. In addition, CMS approved almost 24,000 applications for its Part B program, advancing $40.4 billion for physicians, other clinicians, and medical equipment suppliers.
CMS noted that Congress also has provided $175 billion in aid for the medical community that clinicians and medical organizations would not need to repay. The Coronavirus Aid, Relief, and Economic Security (CARES) Act enacted in March included $100 billion, and the Paycheck Protection Program and Health Care Enhancement Act, enacted March 24, includes another $75 billion.
A version of this article was originally published on Medscape.com.
Visa worries besiege immigrant physicians fighting COVID-19
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Physicians and their sponsoring health care facilities shouldn’t have to worry about visa technicalities as they work on the front lines during the COVID-19 pandemic, said health care leaders and immigration reform advocates.
In a press call hosted by the National Immigration Forum, speakers highlighted the need for fast and flexible solutions to enable health care workers, including physicians, to contribute to efforts to combat the pandemic.
Nationwide, over one in five physicians are immigrants, according to data from the Forum. That figure is over one in three in New York, New Jersey, and California, three states hard-hit by COVID-19 cases.
Many physicians stand willing and able to serve where they’re needed, but visa restrictions often block the ability of immigrant physicians to meet COVID-19 surges across the country, said Amit Vashist, MD, senior vice president and chief clinical officer for Ballad Health, Johnson City, Tenn., and a member of the public policy committee of the Society of Hospital Medicine. Ballad Health is an integrated health care system that serves 29 counties in the rural Southeast.
“This pandemic is a war with an invisible enemy, and immigrant physicians have been absolutely critical to providing quality care, especially on the front lines – but current visa restrictions have limited the ability to deploy these physicians in communities with the greatest need,” said Dr. Vashist during the press conference.
Visa requirements currently tie a non-US citizen resident physician to a particular institution and facility, limiting the ability to meet demand flexibly. “Federal agencies and Congress should provide additional flexibility in visa processing to allow for automatic renewals and expediting processing so immigrant medical workers can focus on treating the sick and not on their visa requirements,” said Dr. Vashist.
Dr. Vashist noted that, when he speaks with the many Ballad Health hospitalists who are waiting on permanent residency or citizenship, many of them also cite worries about the fate of their families should they themselves fall ill. Depending on the physician’s visa status, the family may face deportation without recourse if the physician should die.
“Tens of thousands of our physicians continue to endure years, even decades of waiting to obtain a permanent residency in the United States and at the same time, relentlessly and fearlessly serve their communities including in this COVID-19 pandemic,” said Dr. Vashist. “It’s time we take care of them and their long-term immigration needs, and give them the peace of mind that they so desperately deserve,” he added.
Frank Trinity, chief legal officer for the Association of American Medical Colleges, also participated in the call. “For decades,” he said, the United States “has relied on physicians from other countries, especially in rural and underserved areas.”
One of these physicians, Mihir Patel, MD, FHM, a hospitalist at Ballad Health, came to the United States in 2005, but 15 years later is still waiting for the green card that signifies U.S. permanent residency status. He is the corporate director of Ballad’s telemedicine program and is now also the medical director of the health system’s COVID-10 Strike Team.
“During the COVID crisis, these restrictions can cause significant negative impact for small rural hospitals,” Dr. Patel said. “There are physicians on a visa who cannot legally work outside their primary facilities – even though they are willing to do so.”
Regarding the pandemic, Mr. Trinity expressed concerns about whether the surge of patients would “outstrip our workforce.” He noted that, with an unprecedented number of desperately ill patients needing emergency care all across the country, “now is the time for our government to take every possible action to ensure that these highly qualified and courageous health professionals are available in the fight against the coronavirus.”
Mr. Trinity outlined five governmental actions AAMC is proposing to allow immigrant physicians to participate fully in the battle against COVID-19. The first would be to approve a blanket extension of visa deadlines. The second would be to expedite processing of visa extension applications, including reinstating expedited processing of physicians currently holding H-1B visa status.
The third action proposed by AAMC is to provide flexibility to visa sponsors during the emergency so that an individual whose visa is currently limited to a particular program can provide care at another location or by means of telehealth.
Fourth, AAMC proposes streamlined entry for the 4,200 physicians who are matched into residency programs so that they may begin their residencies on time or early.
Finally, Mr. Trinity said that AAMC is proposing that work authorizations be maintained for the 29,000 physicians who are currently not U.S. citizens and actively participating in the health care workforce.
Jacinta Ma, the Forum’s vice president of policy and advocacy, said immigrants are a critical component of the U.S. health care workforce as a whole.
“With immigrants accounting for 17% of health care workers amid the COVID-19 pandemic, it’s clear that they are vital to our communities,” she said. “Congress and the Trump administration both have an opportunity to advance solutions that protect immigrants, and remove immigration-related barriers for immigrant medical professionals by ensuring that immigrant doctors, nurses, home health care workers, researchers, and others can continue their vital work during this pandemic while being afforded adequate protection from COVID-19.”
Senate Dems call for nationwide COVID-19 testing strategy, more funding
Senate Democrats are calling on the Trump Administration to develop a comprehensive strategy for nationwide COVID-19 testing.
Lawmakers released a “roadmap” document with the goal of including its provisions in the next legislative aid package for COVID-19. Sen. Patty Murray (D-Wash.), the ranking member of the Health, Education, Labor & Pensions committee, noted during an April 15 press conference call that testing in the United States is actually slowing because of shortages and glitches.
“At our current pace, getting 100 million tests done would already take far too long,” she said. “We absolutely cannot afford any backsliding.”
The components of the roadmap include requiring the federal government to develop and communicate a detailed strategic plan to rapidly scale and optimize COVID-19 testing, Sen. Murray said. “This is a national crisis. We need a federally coordinated, whole-of-society response, not one that leaves each state to fend for itself.”
The strategic plan called for in the roadmap would need to establish a high-functioning supply chain with a sufficient amount of available testing materials and supplies; assess potential bottlenecks in the supply chain and communicate them to all stakeholders; and develop and validate accurate and reliable tests for COVID-19, with an emphasis on tests that can deliver rapid results.
Legislation would be used to bolster the supply chain enhancements, according to the roadmap, and would include incentives for domestic manufacturing of testing supplies and compel the sharing of intellectual property and guarantees on the purchase of testing materials.
Testing would be available to patients at no cost sharing under this proposal. The plan also calls for strengthening the price gouging policy in the CARES (Coronavirus Aid, Relief, and Economic Security) Act to ensure that health care professionals are fairly reimbursed by insurers.
The roadmap calls for $30 billion in new emergency funding to enable faster scaling of testing and development of different types of test, with an emphasis on rapid response tests. The funding would also be used to address supply chain issues, according to the roadmap document.
Sen. Lamar Alexander (R.-Tenn.), who chairs the Senate Health, Education, Labor & Pensions committee, echoed the need for more testing to be done, but suggested that the funding that has already been approved by Congress should be exhausted before more is allocated.
“In the last month, Congress has given federal agencies up to $38 billion to develop tests, treatments, and vaccines. Nothing is more important than finding a new diagnostic technology that will make it possible to test tens of millions of Americans, something our country has never tried to do before,” he said in a statement issued after the roadmap’s release. “We should start by using the money Congress has already provided, put politics aside, and work together on more tests with quick results.”
Senate Democrats are calling on the Trump Administration to develop a comprehensive strategy for nationwide COVID-19 testing.
Lawmakers released a “roadmap” document with the goal of including its provisions in the next legislative aid package for COVID-19. Sen. Patty Murray (D-Wash.), the ranking member of the Health, Education, Labor & Pensions committee, noted during an April 15 press conference call that testing in the United States is actually slowing because of shortages and glitches.
“At our current pace, getting 100 million tests done would already take far too long,” she said. “We absolutely cannot afford any backsliding.”
The components of the roadmap include requiring the federal government to develop and communicate a detailed strategic plan to rapidly scale and optimize COVID-19 testing, Sen. Murray said. “This is a national crisis. We need a federally coordinated, whole-of-society response, not one that leaves each state to fend for itself.”
The strategic plan called for in the roadmap would need to establish a high-functioning supply chain with a sufficient amount of available testing materials and supplies; assess potential bottlenecks in the supply chain and communicate them to all stakeholders; and develop and validate accurate and reliable tests for COVID-19, with an emphasis on tests that can deliver rapid results.
Legislation would be used to bolster the supply chain enhancements, according to the roadmap, and would include incentives for domestic manufacturing of testing supplies and compel the sharing of intellectual property and guarantees on the purchase of testing materials.
Testing would be available to patients at no cost sharing under this proposal. The plan also calls for strengthening the price gouging policy in the CARES (Coronavirus Aid, Relief, and Economic Security) Act to ensure that health care professionals are fairly reimbursed by insurers.
The roadmap calls for $30 billion in new emergency funding to enable faster scaling of testing and development of different types of test, with an emphasis on rapid response tests. The funding would also be used to address supply chain issues, according to the roadmap document.
Sen. Lamar Alexander (R.-Tenn.), who chairs the Senate Health, Education, Labor & Pensions committee, echoed the need for more testing to be done, but suggested that the funding that has already been approved by Congress should be exhausted before more is allocated.
“In the last month, Congress has given federal agencies up to $38 billion to develop tests, treatments, and vaccines. Nothing is more important than finding a new diagnostic technology that will make it possible to test tens of millions of Americans, something our country has never tried to do before,” he said in a statement issued after the roadmap’s release. “We should start by using the money Congress has already provided, put politics aside, and work together on more tests with quick results.”
Senate Democrats are calling on the Trump Administration to develop a comprehensive strategy for nationwide COVID-19 testing.
Lawmakers released a “roadmap” document with the goal of including its provisions in the next legislative aid package for COVID-19. Sen. Patty Murray (D-Wash.), the ranking member of the Health, Education, Labor & Pensions committee, noted during an April 15 press conference call that testing in the United States is actually slowing because of shortages and glitches.
“At our current pace, getting 100 million tests done would already take far too long,” she said. “We absolutely cannot afford any backsliding.”
The components of the roadmap include requiring the federal government to develop and communicate a detailed strategic plan to rapidly scale and optimize COVID-19 testing, Sen. Murray said. “This is a national crisis. We need a federally coordinated, whole-of-society response, not one that leaves each state to fend for itself.”
The strategic plan called for in the roadmap would need to establish a high-functioning supply chain with a sufficient amount of available testing materials and supplies; assess potential bottlenecks in the supply chain and communicate them to all stakeholders; and develop and validate accurate and reliable tests for COVID-19, with an emphasis on tests that can deliver rapid results.
Legislation would be used to bolster the supply chain enhancements, according to the roadmap, and would include incentives for domestic manufacturing of testing supplies and compel the sharing of intellectual property and guarantees on the purchase of testing materials.
Testing would be available to patients at no cost sharing under this proposal. The plan also calls for strengthening the price gouging policy in the CARES (Coronavirus Aid, Relief, and Economic Security) Act to ensure that health care professionals are fairly reimbursed by insurers.
The roadmap calls for $30 billion in new emergency funding to enable faster scaling of testing and development of different types of test, with an emphasis on rapid response tests. The funding would also be used to address supply chain issues, according to the roadmap document.
Sen. Lamar Alexander (R.-Tenn.), who chairs the Senate Health, Education, Labor & Pensions committee, echoed the need for more testing to be done, but suggested that the funding that has already been approved by Congress should be exhausted before more is allocated.
“In the last month, Congress has given federal agencies up to $38 billion to develop tests, treatments, and vaccines. Nothing is more important than finding a new diagnostic technology that will make it possible to test tens of millions of Americans, something our country has never tried to do before,” he said in a statement issued after the roadmap’s release. “We should start by using the money Congress has already provided, put politics aside, and work together on more tests with quick results.”
CMS implements temporary regulatory changes to aid COVID-19 response
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” CMS stated in a fact sheet highlighting the regulatory changes. “For example, a health care system can use a hotel to take care of patients needing less intensive care while using inpatient beds for COVID-19 patients.”
With these waivers, hospital systems will not have to rely on the Federal Emergency Management Agency to set up temporary hospitals and can move ahead using available community resources to help deal with the expected surge, Ms. Verma said.
These regulatory changes will be effect for the duration of the public health emergency, according to Ms. Verma.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
Additionally, for Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
CMS also is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet.
Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements, and allowed greater use of verbal orders, to allow clinicians to spend more time on direct patient care.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including emergency department visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” CMS stated in a fact sheet highlighting the regulatory changes. “For example, a health care system can use a hotel to take care of patients needing less intensive care while using inpatient beds for COVID-19 patients.”
With these waivers, hospital systems will not have to rely on the Federal Emergency Management Agency to set up temporary hospitals and can move ahead using available community resources to help deal with the expected surge, Ms. Verma said.
These regulatory changes will be effect for the duration of the public health emergency, according to Ms. Verma.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
Additionally, for Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
CMS also is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet.
Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements, and allowed greater use of verbal orders, to allow clinicians to spend more time on direct patient care.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including emergency department visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
The Centers for Medicare & Medicaid Services has announced a wide range of temporary regulatory moves aimed at helping hospitals and health systems handle the surge of COVID-19 patients.
“We are waiving a wide and unprecedented range of regulatory requirements to equip the American health care system with maximum flexibility to deal with an influx of cases,” CMS Administrator Seema Verma said during a March 30 conference call with reporters. “Many health care systems may not need these waivers and they shouldn’t use them if the situation doesn’t warrant it. But the flexibilities are there if it does. At a time of crisis, no regulatory barriers should stand in the way of patient care.”
Among the changes is an expansion of the venues in which health care systems and hospitals can provide services.
Federal regulations call for hospitals to provide services within their own buildings, raising concerns as to whether there will be enough capacity to handle the anticipated COVID-19 caseload.
“Under CMS’s temporary new rules, hospitals will be able to transfer patients to outside facilities, such as ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories, while still receiving hospital payments under Medicare,” CMS stated in a fact sheet highlighting the regulatory changes. “For example, a health care system can use a hotel to take care of patients needing less intensive care while using inpatient beds for COVID-19 patients.”
With these waivers, hospital systems will not have to rely on the Federal Emergency Management Agency to set up temporary hospitals and can move ahead using available community resources to help deal with the expected surge, Ms. Verma said.
These regulatory changes will be effect for the duration of the public health emergency, according to Ms. Verma.
Ambulatory surgery centers will have the option to contract with local health care systems to provide hospital services or they can enroll and bill as hospitals during the emergency, the fact sheet noted. They will be able to perform hospital services such as cancer procedures, trauma surgeries, and other essential surgeries.
CMS also is waiving the limit on the number of beds a doctor-owned hospital can have.
Additionally, for Medicare patients who may be homebound, CMS will now pay for a laboratory technician to make a home visit to collect a specimen for COVID-19 testing, and hospitals will be able to conduct testing in homes or other community-based settings under certain circumstances.
CMS also is taking actions aimed at expanding the health care workforce.
For instance, the agency is issuing a “blanket waiver” that allows hospitals to provide benefits to medical staff, including multiple daily meals, laundry service for personal clothing, or child care services while the staff is at the hospital providing patient care, according to the fact sheet.
Teaching hospitals will also receive more flexibility in using residents to provide health care services under the virtual direction of a teaching physician, who may be available through audio/video technology.
CMS also is temporarily eliminating paperwork requirements, and allowed greater use of verbal orders, to allow clinicians to spend more time on direct patient care.
On the device/equipment side, Medicare will cover respiratory-related devices and equipment “for any medical reason determined by clinicians,” according to the fact sheet, rather than only under certain circumstances.
And on the telehealth side, CMS is expanding the number of services that it will pay for via telehealth by more than 80, including emergency department visits, initial nursing facility and discharge visits, and home visits, which must be provided by a clinician that is allowed to provide telehealth. CMS will allow the use of commonly available interactive apps with audio and video, as well as audio-only phones.
Wilkie and the VA vs COVID-19: Who’s Winning?
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”





