User login
BNP testing improves outcomes in evaluation of dyspnea
Data on treating bronchiolitis severity limited
How Motivational Interviewing Helps Patients with Diabetes
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
Monoclonal Antibodies in MS
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
A 19-year-old man was diagnosed with relapsing multiple sclerosis (MS) at age 7 and is currently being treated with an infusible monoclonal antibody (mAb) therapy. Early in the day, he receives an infusion at an outpatient clinic. That night, he begins to experience numbness and tingling in his right upper extremity, which prompts a visit to an urgent care clinic. There, the clinician administers IV fluids to the patient. After his symptoms improve, the patient is discharged home.
The next morning, he has a new onset of left-side shoulder and neck pain with a pulsating headache. The patient reports his symptoms to his primary care provider (PCP), who instructs him to visit the emergency department (ED) for evaluation and treatment of a possible infection.
EXAMINATION
The patient arrives at the ED with a 102.4°F fever, vomiting, cough, mild congestion, diaphoresis, generalized myalgias, and chills. He also reports depression and anxiety, saying that for the past 7 days, “I haven’t felt like my normal self.”
Medical history includes moderate persistent asthma that is not well controlled, status asthmaticus, and use of an electronic vaporizing device for inhaling nicotine and marijuana/tetrahydrocannabinol (THC). Besides mAb infusions, his medications include hydrocodone/acetaminophen, prochlorperazine, gabapentin, hydroxyzine, trazodone, albuterol, and montelukast.
Examination reveals vital signs within normal limits. Lab work confirms elevated white blood cell count and absolute neutrophil count. Chest x-ray shows diffuse bilateral interstitial and patchy airspace opacities. He is diagnosed with bilateral pneumonia and is admitted and started on an IV antibiotic.
Within 24 hours, a new chest x-ray shows worsening symptoms. CT of the chest with contrast reveals diffuse bilateral ground-glass and airspace opacities suggestive of acute respiratory distress syndrome; bilateral thickening of the pulmonary interstitium; trace bilateral pleural effusions; increased caliber of the main pulmonary artery; and mediastinal and right hilar lymphadenopathy.
Subsequently, the patient developed sepsis and went into acute hypoxemic respiratory failure. He is transferred to the ICU, and pulmonology is consulted. A bronchoscopy with bronchoalveolar lavage (BAL) reveals neutrophil predominance; fungal, bacterial, and viral cultures are negative. The patient is started on broad-spectrum IV antibiotics and high-dose IV steroids. After 4 days, he begins to improve and is transferred out of the ICU. He is discharged with oral steroids and antibiotics.
Continue to: DISCUSSION
DISCUSSION
Fortunately, the PCP and the ED provider identified risk factors that contributed to the patient’s pneumonia and its subsequent worsening to sepsis and acute hypoxemic respiratory failure. The immunosuppressive/immunomodulatory effect of mAb therapy increased the patient’s risk for infection and the severity of infection, which is why vigilant safety monitoring and surveillance is essential with mAb treatment.1 Bloodwork should be performed at least every 6 months and include a complete blood count, complete metabolic panel with differential, and JC virus antibody test. Additionally, urinalysis should be performed prior to every mAb infusion. All testing recommended in the package insert for the patient’s prescribed therapy should be performed.
The patient’s history of asthma and his chronic vaping predisposed him to respiratory infections. In mice studies, exposure to e-cigarette vapor has been shown to be cytotoxic to airway cells and to decrease macrophage and neutrophil antimicrobial function.2 Exposure also alters immunomodulating cytokines in the airway, increases inflammatory markers seen in BAL and serum samples, and increases the virulence of Staphylococcus aureus
TREATMENT AND PATIENT EDUCATION
The PCP’s treatment plan included patient education about the importance of infection control measures when receiving a mAb; this includes practicing good hand and environmental hygiene, maintaining vaccinations, avoiding or reducing exposure to individuals who have infections or colds, avoiding large crowds (especially during flu season), and following recommendations for nutrition and hydration. The PCP also discussed how to recognize the early signs and symptoms of an infection—and the need for vigilant safety monitoring. The PCP described available options for smoking cessation, including nicotine replacement products, prescription non-nicotine medications, behavioral therapy, and/or counseling (individual, group or telephone) and discussed the risks associated with consuming nicotine and/or marijuana/THC and using electronic vaporizing devices.
The PCP emphasized the importance of completing the entire course of the oral antibiotics prescribed at discharge. The patient and the PCP agreed to the following plan of care: appointments with a pulmonologist and a neurologist within the next 2 weeks, and follow-up visits with the
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
1. Celius EG. Infections in patients with multiple sclerosis: implications for disease-modifying therapy. Acta Neurol Scand. 2017;136(suppl 201):34-36.
2. Hwang JH, Lyes M, Sladewski K, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl). 2016;94(6):667-679.
Proteinuria and Albuminuria: What’s the Difference?
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.
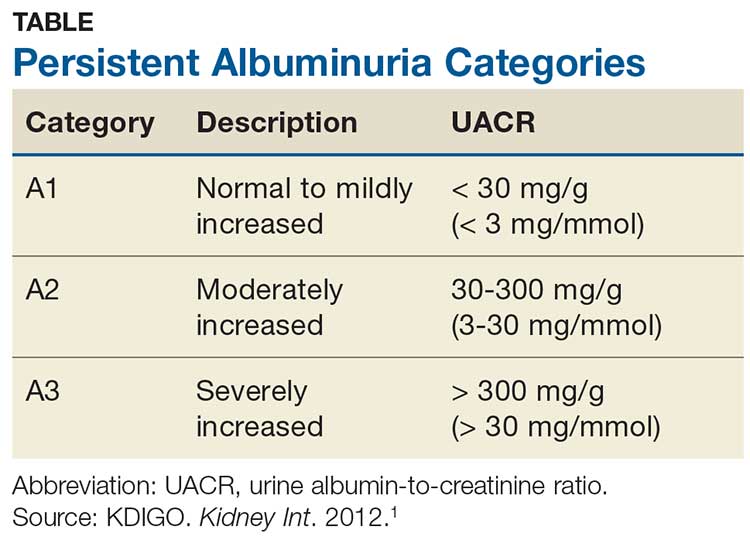
While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.

While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
Q)What exactly is the difference between the protein-to-creatinine ratio and the microalbumin in the lab report? How do they compare?
For the non-nephrology provider, the options for evaluating urine protein or albumin can seem confusing. The first thing to understand is the importance of assessing for proteinuria, an established marker for chronic kidney disease (CKD). Higher protein levels are associated with more rapid progression of CKD to end-stage renal disease and increased risk for cardiovascular events and mortality in both the nondiabetic and diabetic populations. Monitoring proteinuria levels can also aid in evaluating response to treatment.1
Proteinuria and albuminuria are not the same thing. Proteinuria indicates an elevated presence of protein in the urine (normal excretion should be < 150 mg/d), while al
Albuminuria, without or with a reduction in estimated GFR (eGFR), lasting > 3 months is considered a marker of kidney damage. There are 3 categories of persistent albuminuria (see Table).1 Staging of CKD depends on both the eGFR and the albuminuria category; the results affect treatment considerations.

While there are several ways to assess for proteinuria, their accuracy varies. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline on the evaluation and management of CKD recommends the spot urine albumin-to-creatinine ratio (UACR) as the preferred test for both initial and follow-up testing. While the UACR is typically reported as mg/g, it can also be reported in mg/mmol.1 Other options include the spot urine protein-to-creatinine ratio (UPCR) and a manual reading of a reagent strip (urine dipstick test) for total protein. Only if the first 2 choices are unavailable should a provider consider using a dipstick.
Reagent strip urinalysis may assess for protein or more specifically for albumin; tests for the latter are becoming more common. These tests are often used in a clinic setting, with results typically reported in the protein testing section. It is important to know which reagent strip urinalysis you are using (protein vs albumin) and how this is being reported. Additionally, test results depend on the urine concentration: Concentrated samples are more likely to indicate higher levels than may actually be present, while dilute samples may test negative (or positive for a trace amount) when in reality higher levels are present.
If a reagent strip urinalysis tests positive for protein, confirmatory testing is recommended using the UACR or the UPCR (if the former is not an option). A 24-hour urine screen for total protein excretion or an albumin excretion rate can be obtained if there are concerns about the accuracy of the previously discussed tests. A urine albumin excretion rate ≥ 30 mg/24 h corresponds to a UACR of ≥ 30 mg/g (≥ 3 mg/mmol).1 Although 24-hour urine has been considered the gold standard, it is used less frequently today due to potential for improper sample collection, which can negatively affect accuracy, and inconvenience to patients.2
As a final note, if there is suspicion for nonalbumin proteinuria (eg, when increased plasma levels of low-molecular-weight proteins or immunoglobulin light chains are present), testing for specific urine proteins is recommended. This can include assessment with urine protein electrophoresis.1 —CS
Cynthia A. Smith, DNP, CNN-NP, FNP-BC, APRN, FNKF
Renal Consultants, PLLC, South Charleston, West Virginia
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(suppl):1-150.
2. Ying T, Clayton P, Naresh C, Chadban S. Predictive value of spot versus 24-hour measures of proteinuria for death, end-stage kidney disease or chronic kidney disease progression. BMC Nephrology. 2018;19:55.
Acute Kidney Injury: Treatment Depends on the Cause
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1
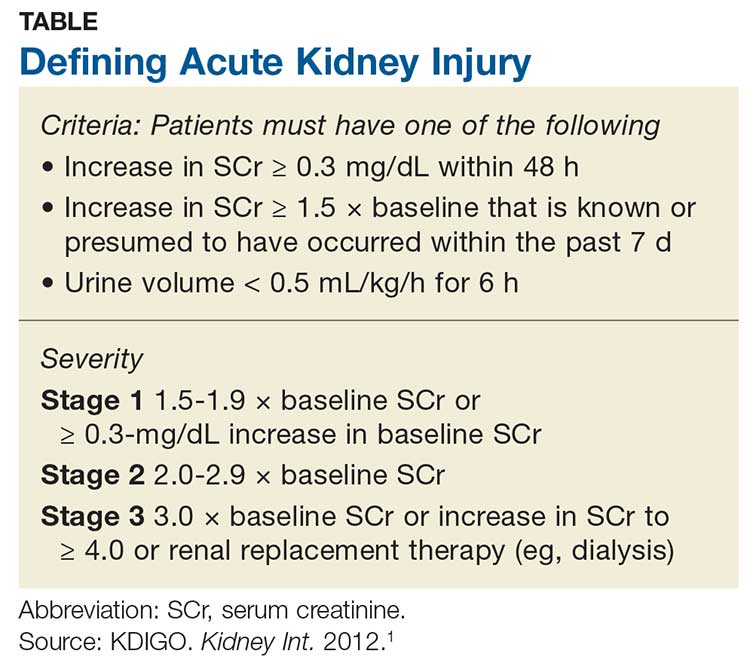
AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1

AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
Q)I have a patient with a discharge diagnosis of community-acquired acute kidney injury. What does this mean? What do I do now?
Acute kidney injury (AKI) refers to an abrupt decrease in kidney function that is possibly reversible or in which harm to the kidney can be modified.1,2 AKI encompasses a broad spectrum of conditions affecting the kidney—including acute renal failure, since even “failure” can sometimes be reversed.1 Criteria for AKI and its severity can be found in the Table.1

AKI can be either community-acquired (CA-AKI) or hospital-acquired (HA-AKI).1,2 In the United States, CA-AKI occurs less frequently than HA-AKI, although cases are likely underreported.1 Evaluation and management are similar for both.
The etiology of the AKI must be determined before treatment of the cause or precipitating factor can be attempted. Causes of AKI can be classified as prerenal (up to 70% of cases), intrinsic, or postrenal.1
Most AKI cases have a prerenal origin.3 Prerenal AKI occurs when there is inadequate blood flow to the kidneys, leading to a rise in blood urea nitrogen (BUN) and serum creatinine (SCr) levels. Reduced blood flow can be caused by
- Diuretic dosing
- Polypharmacy (diuretics, angiotensin-converting enzyme inhibitors [ACEIs]/ angiotensin receptor blockers [ARBs], and/or NSAIDs are common culprits)
- Congestive heart failure exacerbation
- Volume depletion through vomiting or diarrhea
- Massive blood loss (trauma).3
Postrenal causes of AKI include any type of obstructive uropathy. Intrinsic causes involve any condition within the kidney, including interstitial nephritis or acute tubular necrosis. Use of antibiotics (eg, high-dose penicillin or vancomycin) is included in this category.
Obtaining an accurate medical history and examining the patient’s fluid status are critical. Although numerous novel biomarkers have been investigated for detection of AKI, none are yet in wide use. The primary assessment measures remain a serum panel to evaluate SCr and BUN levels; an electrolyte panel to assess for abnormalities; a complete blood count to assess for anemia caused by a less likely source; urinalysis; and imaging to assess for abnormalities or structural changes.
Urinalysis. Urine often holds the key to diagnosis of AKI. Notably in a prerenal injury, its specific gravity will be elevated, but the rest of the urine will likely be bland.3
Continue to: Urinalysis is helpful for...
Urinalysis is helpful for ruling out intrinsic causes of AKI. Patients with intrarenal AKI will have abnormal urine sediment; for example, red blood cell casts are found in glomerulonephritis; granular casts in cases of acute tubular necrosis; and white blood cell casts and eosinophils in acute interstitial nephritis.4
Imaging. The most commonly used imaging for AKI is retroperitoneal ultrasonography of the kidneys, ureters, and bladder, which provides information on the size and shape of the kidneys and can detect stones or masses. It also detects the presence or absence of hydronephrosis, which can occur in postrenal injuries.
Currently, no definitive therapy or pharmacologic agent is approved for AKI; treatment focuses on reversing the cause of the injury. In the immediate aftermath of AKI, it is important to avoid potentially nephrotoxic medications, including NSAIDs. Minimize the use of diuretics and avoid ACEIs and ARB therapy; these can be reintroduced after lab results confirm that the AKI has resolved with a stabilized SCr.
Practice guidelines recommend prompt follow-up at 3 months in most cases of AKI.1 Providers should obtain a metabolic panel and perform a urinalysis to evaluate for chronic kidney disease (CKD), because almost one-third of patients with an AKI episode are newly classified with CKD in the following year.5 Earlier follow-up (< 3 months) is warranted if the patient has a significant comorbidity, such as congestive heart failure.1,2—CS
Christopher Sjoberg, CNN-NP
Idaho Nephrology Associates, Boise
Adjunct Faculty, Boise State University
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
1. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(suppl):1-138.
2. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672.
3. Bellomo R, Ronco C, Kellum JA. Acute kidney injury. Lancet. 2012; 380(9843):756-766.
4. Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Disease. 7thed. Philadelphia, PA: Elsevier; 2017.
5. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
A Veteran Presenting With Leg Swelling, Dyspnea, and Proteinuria
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
*This article has been corrected to include a missing author.
Case Presentation. A 63-year-old male with well-controlled HIV (CD4 count 757, undetectable viral load), epilepsy, and hypertension presented to the VA Boston Healthcare System (VABHS) emergency department with 1 week of bilateral leg swelling and exertional shortness of breath. He reported having no fever, cough, chest pain, pain with inspiration and orthopnea. There was no personal or family history of pulmonary embolism. He reported weight gain but was unable to quantify how much. He also reported flare up of chronic knee pain, without swelling for which he had taken up to 4 tablets of naproxen daily for several weeks. His physical examination was notable for a heart rate of 105 beats per minute and bilateral pitting edema to his knees. Laboratory testing revealed a creatinine level of 2.5 mg/dL, which was increased from a baseline of 1.0 mg/dL (Table 1), and a urine protein-to-creatinine ratio of 7.8 mg/mg (Table 2). A renal ultrasound showed normal-sized kidneys without hydronephrosis or obstructing renal calculi. The patient was admitted for further workup of his dyspnea and acute kidney injury.
► Jonathan Li, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC). Dr. William, based on the degree of proteinuria and edema, a diagnosis of nephrotic syndrome was made. How is nephrotic syndrome defined, and how is it distinguished from glomerulonephritis?
► Jeffrey William, MD, Nephrologist, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The pathophysiology of nephrotic disease and glomerulonephritis are quite distinct, resulting in symptoms and systemic manifestations that only slightly overlap. Glomerulonephritis is characterized by inflammation of the endothelial cells of the trilayered glomerular capillary, with a resulting active urine sediment with red blood cells, white blood cells, and casts. Nephrotic syndrome mostly affects the visceral epithelial cells of the glomerular capillary, commonly referred to as podocytes, and hence, the urine sediment in nephrotic disease is often inactive. Patients with nephrotic syndrome have nephrotic-range proteinuria (excretion of > 3.5 g per 24 h or a spot urine protein-creatinine ratio > 3.5 g in the steady state) and both hypoalbuminemia (< 3 g/dL) and peripheral edema. Lipiduria and hyperlipidemia are common findings in nephrotic syndrome but are not required for a clinical diagnosis.1 In contrast, glomerulonephritis is defined by a constellation of findings that include renal insufficiency (often indicated by an elevation in blood urea nitrogen and creatinine), hypertension, hematuria, and subnephrotic range proteinuria. In practice, patients may fulfill criteria of both nephrotic and nephritic syndromes, but the preponderance of clinical evidence often points one way or the other. In this case, nephrotic syndrome was diagnosed based on the urine protein-to-creatinine ratio of 7.8 mg/mg, hypoalbuminemia, and edema.
► Dr. Li. What would be your first-line workup for evaluation of the etiology of this patient’s nephrotic syndrome?
► Dr. William. Rather than memorizing a list of etiologies of nephrotic syndrome, it is essential to consider the pathophysiology of heavy proteinuria. Though the glomerular filtration barrier is extremely complex and defects in any component can cause proteinuria, disruption of the podocyte is often involved. Common disease processes that chiefly target the podocyte include minimal change disease, primary focal and segmental glomerulosclerosis (FSGS), and membranous nephropathy, all by differing mechanisms. Minimal change disease and idiopathic/primary FSGS are increasingly thought to be at differing points on a spectrum of the same disease.2 Secondary FSGS, on the other hand, is a progressive disease, commonly resulting from longstanding hypertension, diabetes mellitus, and obesity in adults. Membranous nephropathy can also be either primary or secondary. Primary membranous nephropathy is chiefly caused by a circulating IgG4 antibody to the podocyte membrane antigen PLA2R (M-type phospholipase A2 receptor), whereas secondary membranous nephropathy can be caused by a variety of systemic etiologies, including autoimmune disease (eg, systemic lupus erythematosus), certain malignancies, chronic infections (eg, hepatitis B and C), and many medications, including nonsteroidal anti-inflammatory drugs (NSAIDs).3-5 Paraprotein deposition diseases can also cause glomerular damage leading to nephrotic-range proteinuria.
Given these potential diagnoses, a careful history should be taken to assess exposures and recent medication use. Urine sediment evaluation is essential in the evaluation of nephrotic syndrome to determine if there is an underlying nephritic process. Select serologies may be sent to look for autoimmune disease, such as systemic lupus erythematosus and common viral exposures like hepatitis B or C. Serum and urine protein electrophoreses would be appropriate initial tests of suspected paraprotein-related diseases. Other serologies, such as antineutrophil cytoplasmic antibodies or antiglomerular basement membrane antibodies, would not necessarily be indicated here given the lack of hematuria and presence of nephrotic-range proteinuria.
► Dr. Li. The initial evaluation was notable for an erythrocyte sedimentation rate > 120 (mm/h) and a weakly positive antinuclear antibody (ANA) titer of 1:40. The remainder of his initial workup did not reveal an etiology for his nephrotic syndrome (Table 3).
Dr. William, is there a role for starting urgent empiric steroids in nephrotic syndrome while workup is ongoing? If so, do the severity of proteinuria and/or symptoms play a role or is this determination based on something else?
► Dr. William. Edema is a primary symptom of nephrotic syndrome and can often be managed with diuretics alone. If a clear medication-mediated cause is suspected, discontinuation of this agent may result in spontaneous improvement without steroid treatment. However,in cases where an etiology is unclear and there are serious thrombotic complications requiring anticoagulation, and a renal biopsy is deemed to be too risky, then empiric steroid therapy may be necessary. Children with new-onset nephrotic syndrome are presumed to have minimal change disease, given its prevalence in this patient population, and are often given empiric steroids without obtaining a renal biopsy. However, in the adult population, a renal biopsy can typically be performed quickly and safely, with pathology results interpreted within days. In this patient, since a diagnosis was unclear and there was no contraindication to renal biopsy, a biopsy should be obtained before consideration of steroids.
► Dr. Li. Steroids were deferred in anticipation of renal biopsy, which showed stage I membranous nephropathy, suggestive of membranous lupus nephritis Class V. The deposits were strongly reactive for immunoglobuline G (IgG), IgA, and complement 1q (C1q), showed co-dominant staining for IgG1, IgG2, and IgG3, and were weakly positive for the PLA2 receptor. Focal intimal arteritis in a small interlobular vessel was seen.
Dr. William, the pathology returned suggestive of lupus nephritis. Does the overall clinical picture fit with lupus nephritis?
► Dr. William. Given the history and a rather low ANA, the diagnosis of lupus nephritis seems unlikely. The lack of IgG4 and PLA2R staining in the biopsy suggests that this membranous pattern on the biopsy is likely to be secondary to a systemic etiology, but further investigation should be pursued.
► Dr. Li. The patient was discharged after the biopsy with a planned outpatient nephrology follow-up to discuss results and treatment. He was prescribed an oral diuretic, and his symptoms improved. Several days after discharge, he developed blurry vision and was evaluated in the Ophthalmology clinic. On fundoscopy, he was found to have acute papillitis, a form of optic neuritis. As part of initial evaluation of infectious etiologies of papillitis, ophthalmology recommended testing for syphilis.
Dr. Strymish, when we are considering secondary syphilis, what is the recommended approach to diagnostic testing?
► Judith Strymish, MD, Infectious Diseases, BIDMC, Assistant Professor of Medicine, Harvard Medical School. The diagnosis of syphilis is usually made through serologic testing of blood specimens. Methods that detect the spirochete directly like dark-field smears are not readily available. Serologic tests include treponemal tests (eg, Treponema pallidum particle agglutination assay [TPPA]) and nontreponemal tests (eg, rapid plasma reagin [RPR]). One needs a confirmatory test because either test is associated with false positives. Either test can be done first. Most laboratories, including those at VABHS are now performing treponemal tests first as these have become more cost-effective.6 The TPPA treponemal test was found to have a lower false negative rate in primary syphilis compared with that of nontreponemal tests.7 Nontreponemal tests can be followed for response to therapy. If a patient has a history of treated syphilis, a nontreponemal test should be sent, since the treponemal test will remain positive for life.
If there is clinical concern for neurosyphilis, cerebrospinal fluid fluorescent (CSF) treponemal antibody needs to be sampled and sent for the nontreponemal venereal disease research laboratory (VDRL) test. The VDRL is highly specific for neurosyphilis but not as sensitive. Cerebrospinal fluid fluorescent treponemal antibody (CSF FTA) may also be sent; it is very sensitive but not very specific for neurosyphilis.
► Dr. Li. An RPR returned positive at 1:512 (was negative 14 months prior on a routine screening test), with positive reflex TPPA (Table 4). A diagnosis of secondary syphilis was made. Dr. Strymish, at this point, what additional testing and treatment is necessary?
► Dr. Strymish. With papillitis and a very high RPR, we need to assume that he has ophthalmic syphilis. This can occur in any stage of syphilis, but his eye findings and high RPR are consistent with secondary syphilis. Ophthalmic syphilis has been on the upswing, even more than is expected with recent increases in syphilis cases.8 Ophthalmic syphilis is considered a form of neurosyphilis. A lumbar puncture and treatment for neurosyphilis is recommended.9,10
► Dr. Li. A lumbar puncture was performed, and his CSF was VDRL positive. This confirmed a diagnosis of neurosyphilis (Table 4). The patient was treated for neurosyphilis with IV penicillin. The patient shared that he had episodes of unprotected oral sexual activity within the past year and approximately 1 year ago, he came in close contact (but no sexual activity) with a person who had a rash consistent with syphilis.Dr. William, syphilis would be a potential unifying diagnosis of his renal and ophthalmologic manifestations. Is syphilis known to cause membranous nephropathy?
► Dr. William. Though it is uncommon, the nephrotic syndrome is a well-described complication of secondary syphilis.11,12 Syphilis has been shown to cause nephrotic syndrome in a variety of ways. Case reports abound linking syphilis to minimal change disease and other glomerular diseases.13,14 A case report from 1993 shows a membranous pattern of glomerular disease similar to this case.15 As a form of secondary membranous nephropathy, the immunofluorescence pattern can demonstrate staining similar to the “full house” seen in lupus nephritis (IgA, IgM, and C1q, in addition to IgG and C3).16 This explains the initial interpretation of this patient’s biopsy, as lupus nephritis would be a much more common etiology of secondary membranous nephropathy than is acute syphilis with this immunofluorescence pattern. However, the data in this case are highly suggestive of a causal relationship between secondary syphilis and membranous nephropathy.
► Dr. Li. Dr. Strymish, how should this patient be screened for syphilis reinfection, and at what intervals would you recommend?
► Dr. Strymish. He will need follow-up testing to make sure that his syphilis is effectively treated. If CSF pleocytosis was present initially, a CSF examination should be repeated every 6 months until the cell count is normal. He will also need follow-up for normalization of his RPR. Persons with HIV infection and primary or secondary syphilis should be evaluated clinically and serologically for treatment failure at 3, 6, 9, 12, and 24 months after therapy according to US Centers for Disease Control and Prevention guidelines.9
His treponemal test for syphilis will likely stay positive for life. His RPR should decrease significantly with effective treatment. It makes sense to screen with RPR alone as long as he continues to have risk factors for acquiring syphilis. Routine syphilis testing is recommended for pregnant women, sexually active men who have sex with men, sexually active persons with HIV, and persons taking PrEP (pre-exposure prophylaxis) for HIV prevention. He should be screened at least yearly for syphilis.
► Dr. Li. Over the next several months, the patient’s creatinine normalized and his proteinuria resolved. His vision recovered, and he has had no further ophthalmologic complications.
Dr. William, what is his long-term renal prognosis? Do you expect that his acute episode of membranous nephropathy will have permanent effects on his renal function?
► Dr. William. His rapid response to therapy for neurosyphilis provides evidence for this etiology of his renal dysfunction and glomerulonephritis. His long-term prognosis is quite good if the syphilis is the only reason for him to have renal disease. The renal damage is often reversible in these cases. However, given his prior extensive NSAID exposure and history of hypertension, he may be at higher risk for chronic kidney disease than an otherwise healthy patient, especially after an episode of acute kidney injury. Therefore, his renal function should continue to be monitored as an outpatient.
Acknowledgments
The authors thank this veteran for sharing his story and allowing us to learn from this unusual case for the benefit of our future patients.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
1. Rennke H, Denker BM. Renal Pathophysiology: The Essentials. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2014.
2. Maas RJ, Deegens JK, Smeets B, Moeller MJ, Wetzels JF. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat Rev Nephrol. 2016;12(12):768-776.
3. Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11-21.
4. Rennke HG. Secondary membranoproliferative glomerulonephritis. Kidney Int. 1995;47(2):643-656.
5. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 2013;62(5):1012-1017.
6. Pillay A. Centers for Disease Control and Prevention Syphilis Summit—Diagnostics and laboratory issues. Sex Transm Dis. 2018;45(9S)(suppl 1):S13-S16.
7. Levett PN, Fonseca K, Tsang RS, et al. Canadian Public Health Laboratory Network laboratory guidelines for the use of serological tests (excluding point-of-care tests) for the diagnosis of syphilis in Canada. Can J Infect Dis Med Microbiol. 2015;26(suppl A):6A-12A.
8. Oliver SE, Aubin M, Atwell L, et al. Ocular syphilis—eight jurisdictions, United States, 2014-2015. MMWR Morb Mortal Wkly Rep. 2016;65(43):1185-1188.
9. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports 2015;64(RR3):1-137. [Erratum in MMWR Recomm Rep. 2015;64(33):924.]
10. US Centers for Disease Control and Prevention. Clinical advisory: ocular syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Updated March 24, 2016. Accessed August 12, 2019.
11. Braunstein GD, Lewis EJ, Galvanek EG, Hamilton A, Bell WR. The nephrotic syndrome associated with secondary syphilis: an immune deposit disease. Am J Med. 1970;48:643-648.1.
12. Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013:pii:bcr2012008279
13. Krane NK, Espenan P, Walker PD, Bergman SM, Wallin JD. Renal disease and syphilis: a report of nephrotic syndrome with minimal change disease. Am J Kidney Dis. 1987;9(2):176-179.
14. Bhorade MS, Carag HB, Lee HJ, Potter EV, Dunea G. Nephropathy of secondary syphilis: a clinical and pathological spectrum. JAMA. 1971;216(7):1159-1166.
15. Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351-1355.
16. Gamble CN, Reardan JB. Immunopathogenesis of syphilitic glomerulonephritis. Elution of antitreponemal antibody from glomerular immune-complex deposits. N Engl J Med. 1975;292(9):449-454.
10 (Safe) Ways to Reduce Patients’ Insulin Costs
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
Almost a century after its discovery, insulin remains a life-saving yet costly medication: In the past 15 years, prices have risen more than 500%.1 Patients may ask you why the insulin you prescribe is so expensive, and the complex process for determining drug costs makes it difficult to answer. But the bottom line is, patients need their insulin—and they want it without breaking the bank.
Thankfully, there are several strategies for reducing the cost of insulin. First and foremost, patients must be advised that not taking their prescribed insulin, or taking less insulin than prescribed, is not a safe alternative. An individualized cost-benefit analysis between patient and provider can help to determine the best option for each patient. After working in endocrinology for 5 years, I have learned the following 10 ways to help patients whose financial situations limit their access to insulin.
1 Try older insulins, including mixed insulin 70/30 or 50/50, insulin NPH, or regular insulin. Because the beneficial effects may not be as long lasting with these as with newer insulins on the market, your patient may need to test glucose levels more frequently. Also, insulin NPH and any mixed insulins are suspensions, not solutions, so patients will need to gently roll older insulins prior to use. Those in pen form may also have a shorter shelf life.
2 Switch to a syringe and vial. Although dosing can be less precise, this could be a viable option for patients with good vision and dexterity. This method helps patients save in 3 ways: (1) the insulin is less expensive; (2) syringes generally cost less (about $30 for 100) than pen needle tips (about $50 for 100); and (3) vials of NPH are longer-lasting suspensions that are stable for about 28 days once opened, compared to 14 days for pens.2-4
3 Switch from a 30- to a 90-day supply of refills. This helps to lower copays. For example, a mail-order program (eg, Express Scripts) that ships from a warehouse typically offers lower pricing than a brick-and-mortar pharmacy with greater overhead. Many of these programs provide 2-pharmacist verification for accuracy and free home delivery of medications at a 10% discount, as well as 24-hour pharmacist access.5 The ease of obtaining prescriptions by this method also can help with medication adherence.
4 Patient assistance programs (PAPs) offered by insulin manufacturers can help lower costs for patients who find it difficult to afford their medication. Information on these programs is available on the respective company’s websites, usually in multiple languages (although some are limited to English and Spanish). Patients applying for a PAP must provide a proof of income and adhere to the program’s specific criteria. Renewal is typically required each year.6-8
5 Copay cards are available to many patients with private insurance and may help make insulin more affordable. Patients may be able to receive a $25 monthly supply of insulin for up to 1 year (specific terms vary). Maximum contributions and contributions toward deductibles also vary by program, so patients need to familiarize themselves with what their particular copay card allows. Generally, copay cards are not a sustainable long-term solution; for one thing, they expire, and for another, emphasis should be placed on affordable medications rather than affording expensive medications.
[polldaddy:10400221]
Continue to: 6 External PAPs for patients on Medicare...
6 External PAPs for patients on Medicare can help lower the costs of prescription medications.9 A database of pharmaceutical PAPs is available on the Medicare website.10 Some PAPs may help patients on Medicare pay through the $5,100 coverage gap or “donut hole”—a term referring to a gap in prescription drug coverage once patients have met their prescription limit (all Medicare part D plans have a donut hole).11,12 Patients and providers will need to read the fine print when applying for an external PAP, because some have a monthly or one-time start-up fee for processing the paperwork (and note, there is often paperwork for the relief program in addition to the PAP paperwork through the pharmaceutical company).
7 A Program of All-Inclusive Care for the Elderly (PACE) is available in many states; check medicare.gov to see if your state is eligible. For patients 55 and older on Medicare or Medicaid who do not opt for care at a nursing home facility, PACE may be able to provide care and coverage in the patient’s home or at a PACE facility. Services include primary care, hospital care, laboratory and x-ray services, medical specialty services, and prescription drugs. To be eligible for PACE services, the patient must live in the service area of a PACE organization and have a requirement for a nursing home-level of care (as certified by your state).
8 Shop around for the best deal. Encourage your patients to comparison shop for the best prices rather than accepting the first or only option at their usual pharmacy. Different pharmacies offer drugs at lower prices than competitors. Also, continually compare prices at GoodRx or HealthWarehouse.com. The latter—a fully licensed Internet-based pharmacy—sells FDA-approved medications at affordable prices in all 50 states, without the requirement for insurance coverage.
9 Use of a patch pump may be less expensive for patients with type 2 diabetes who are taking basal-bolus regimens. Patches slowly deliver single short-acting insulin (usually insulin aspart or lispro) that acts as a basal insulin, with an additional reservoir for prandial insulin at mealtime and for snacks. As there is a catheter in the patch, patients would not require the use of needles.13
10 Try removing mealtime insulin for patients with type 2 diabetes who need minimal mealtime insulin. Clinicians can initiate a safe trial of this removal by encouraging the patient to consume a low-carbohydrate diet, increase exercise, and/or use other noninsulin medications that are more affordable.
Continue to: The affordability of insulins...
The affordability of insulins is a potentially uncomfortable but necessary conversation to have with your patient. Providers are one of the best resources for patients who seek relief from financial difficulties. The recommendations discussed here can help providers and patients design a cost-conscious plan for insulin treatment. Although each recommendation is viable, the pros and cons must be weighed on a case-by-case basis. Providers and patients should also pay attention to the Senate Finance Committee’s ongoing discussions and possible resolutions that could result in lower insulin costs. Until legislation that lowers the prices of insulin comes to fruition, however, providers should continue to plan with their patients on how to best get their insulin at the lowest cost.
Test yourself with the poll here.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
1. Grassley, Wyden launch bipartisan investigation into insulin prices. United States Senate Committee on Finance website. www.finance.senate.gov/chairmans-news/grassley-wyden-launch-bipartisan-investigation-into-insulin-prices. Published February 22, 2019. Accessed August 16, 2019.
2. BD Ultra-Fine. Syringe. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=31-gauge-5-16%22-of-1-cc&form=syringe&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
3. BD Ultra-Fine. Pen needle. GoodRx website. www.goodrx.com/bd-ultra-fine?dosage=5-32%22-of-32-gauge&form=pen-needle&label_override=BD+Ultra-Fine&quantity=100. Accessed August 16, 2019.
4. Joffee D. Stability of common insulins in pens and vials. Diabetes in Control website. www.diabetesincontrol.com/wp-content/uploads/PDF/se_insulin_stability_chart.pdf. Published September 2011. Accessed August 16, 2019.
5. Frequently asked questions. Preferred home delivery program for maintenance medications. Express Scripts website. www.express-scripts.com/art/pdf/SST-custom-preferred-faq.pdf. Accessed August 16, 2019.
6. Patient Connection. Sanofi Patient Connection website. www.sanofipatientconnection.com/. Accessed August 16, 2019.
7. The Lilly Cares Foundation Patient Assistance Program. Lilly website. www.lillycares.com/assistanceprograms.aspx. Accessed August 16, 2019.
8. Novo Nordisk Patient Assistance Program. NovoCare website. www.novocare.com/psp/PAP.html. Accessed August 16, 2019.
9. 6 ways to get help with prescription costs. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap/6-ways-to-get-help-with-prescription-costs. Accessed August 16, 2019.
10. Pharmaceutical assistance program. Medicare website. www.medicare.gov/pharmaceutical-assistance-program/Index.aspx. Accessed August 16, 2019.
11. Catastrophic coverage. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/catastrophic-coverage. Accessed August 16, 2019.
12. Costs in the coverage gap. Medicare website. www.medicare.gov/drug-coverage-part-d/costs-for-medicare-drug-coverage/costs-in-the-coverage-gap. Accessed August 16, 2019.
13. V-Go Reimbursement Assistance Program. V-Go website. www.go-vgo.com/coverage-savings/overview/. Accessed August 16, 2019.
Genomic Medicine and Genetic Counseling in the Department of Veterans Affairs and Department of Defense (FULL)
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Vickie Venne, MS. What is the Genomic Medicine Service (GMS) at the US Department of Veterans Affairs (VA)?
Renee Rider, JD, MS, LCGC. GMS is a telehealth service. We are part of central office and field stationed at the George E. Wahlen VA Medical Center (VAMC) in Salt Lake City, Utah. We provide care to about 90 VAMCs and their associated clinics. Veterans are referred to us by entering an interfacility consult in the VA Computerized Patient Record System (CPRS). We review the consult to determine whether the patient needs to be seen, whether we can answer with an e-consult, or whether we need more information. For the patients who need an appointment, the telehealth department at the veteran’s VA facility will contact the patient to arrange a visit with us. At the time of the appointment, the facility has a staff member available to seat the patient and connect them to us using video equipment.
We provide genetic care for all specialties, including cancer, women’s health, cardiology and neurology. In today’s discussion, we are focusing on cancer care.
Vickie Venne. What do patients do at facilities that don’t get care through GMS?
Renee Rider. There are a handful of facilities that provide their own genetic care in-house. For example, VA Boston Healthcare System in Massachusetts and the Michael E. DeBakey VAMC in Houston, Texas each have their own programs. For veterans who are not at a VA facility that has an agreement with GMS and do not have a different genetics program, their providers need to make referrals to community care.
Vickie Venne. How do patients get referred and what happens at their facility when the patients return to the specialty and primary care providers (PCP)? Ishta, who do you refer to GMS and how do you define them initially?
Ishta Thakar, MD, FACP. Referrals can come at a couple of points during a veteran’s journey at the VA. The VA covers obstetrics care for women veterans. Whenever a PCP or a women’s health provider is doing the initial history and physical on a new patient, if the female veteran has an extensive family history of breast, ovarian, colon, or endometrial cancer, then we take more history and we send a consult to GMS. The second instance would be if she tells us that she has had a personal history of breast, ovarian, or endometrial cancer and she has never had genetic testing. The third instance would be whenever we have a female veteran who is diagnosed with breast, ovarian, endometrial, or colon cancer. We would definitely talk to her about genetic counseling and send a referral to GMS. We would ask for a GMS consult for a patient with advanced maternal age, with exposure to some kind of teratogens, with an abnormal ultrasound, a family history of chromosomal disorders, or if she’s seeing an obstetrician who wants her to be tested. And finally, if a patient has a constellation of multiple cancers in the family and we don’t know what’s going on, we would also refer the patient to GMS.
Vickie Venne. That would be why GMS fields over 150 referrals every week. It is a large list. We also see veterans with personal or family histories of neurologic or cardiologic concerns as well.
Renee, as somebody who fields many of these referrals from unaffected individuals, what is the family history process?
Renee Rider. We don’t expect the referring provider to be a genetic expert. When a provider is seeing a constellation of several different cancers and he or she doesn’t know if there’s anything going on genetically or even if it’s possible, absolutely they should put in a referral to GMS. We have a triage counselor who reviews every consult that comes into our service within 24 hours.
Many cancers are due to exposures that are not concerning for a genetic etiology. We can let you know that it is not concerning, and the PCP can counsel the patient that it is very unlikely to be genetic in nature. We still give feedback even if it’s not someone who is appropriate for genetic counseling and testing. It is important to reach out to GMS even if you don’t know whether a cancer is genetic in nature.
It also is important to take your time when gathering family histories. We get a lot of patients who say, “There’s a lot of cancer in my family. I have no idea who had cancer, but I know a lot of people had cancer.” That’s not the day to put in a referral to GMS. At that point, providers should tell the patient to get as much information as they can about the family history and then reassess. It’s important for us to have accurate information. We’ve had several times where we receive a referral because the veteran says that their sister had ovarian cancer. And then when our staff calls, they later find out it was cervical cancer. That’s not a good use of the veteran’s time, and it’s not a good use of VA resources.
The other important thing about family histories is keeping the questions open-ended. Often a PCP or specialist will ask about a certain type of cancer: “Does anyone in your family have breast cancer, ovarian cancer?” Or if the veteran
is getting a colonoscopy, they ask, “Does anybody have colon cancer?” Where really, we need to be a little bit more open-ended. We prefer questions like, “Has anyone in your family
had cancer?” because that’s the question that prompts a response of, “Yes, 3 people in my family have had thyroid cancer.” That’s very important for us to know, too.
If you do get a positive response, probe a little bit more: what kind of cancer did someone have, how old were they when they had their cancer? And how are they related? Is this an aunt on your mom’s side or on your dad’s side? Those are the types of information that we need to figure out if that person needs a referral.
Vickie Venne. It’s a different story when people already have a cancer diagnosis. Which hematology or oncology patients are good referrals and why?
Lisa Arfons, MD. When patients come in with newly diagnosed cancer, breast for example, it is an emotional diagnosis and psychologicallydistressing. Oftentimes, they want to know why this happened to them. The issues surrounding
genetic testing also becomes very emotional. They want to know whether their children are at risk as well.
Genetic discussions take a long time. I rarely do that on the first visit. I always record for myself in my clinic note if something strikes me regarding the patient’s diagnosis. I quickly run through the National Comprehensive Cancer Network (NCCN) guidelines to remind myself of what I need to go over with the patient at our next meeting. Most patients don’t need to be referred to GMS, and most patients don’t need to be tested once they’re seen.
I often save the referral discussion for after I have established a rapport with a patient, we have a treatment plan, or they already have had their first surgery. Therefore, we are not making decisions about their first surgery based on the genetic medicine results.
If I’m considering a referral, I do a deeper dive with the patient. Is the patient older or younger than 45 years? I pull up NCCN guidelines and we go through the entire checklist.
We have male breast cancer patients at the VA—probably more than the community—so we refer those patients. At the Louis Stokes Cleveland VAMC in Ohio, we have had some in-depth discussions about referring male breast cancer patients for genetic testing and whether it was beneficial to older patients with male breast cancer. Ultimately, we decided that it was important for our male veterans to be tested because it empowered them to have better understanding of their medical conditions that may not just have effect on them but on their offspring, and that that can be a source of psychological and emotional support.
I don’t refer most people to GMS once I go through the checklist. I appreciate the action for an e-consult within the CPRS telemedicine consult itself, as Renee noted. If it is not necessary, GMS makes it an e-consult. I try to communicate that I don’t know whether it is necessary or not so that GMS understands where I’m coming from.
Vickie Venne. In the US Department of Defense (DoD) the process is quite different. Mauricio, can you explain the clinical referral process, who is referred, and how that works from a laboratory perspective?
Maj De Castro, MD, FACMG, USAF. The VA has led the way in demonstrating how to best provide for the medical genetic needs of a large, decentralized population distributed all over the country. Over the last 5 to 10 years, the DoD has made strides in recognizing the role genetics plays in the practice of everyday medicine and redoubling efforts to meet the needs of servicemembers.
The way that it traditionally has worked in the DoD is that military treatment facilities (MTFs) that have dedicated geneticists and genetic counselors: Kessler Medical Center in Mississippi, Walter Reed National Military Medical
Center in Maryland, Tripler Army Medical Center in Hawaii, Madigan Army Medical Center in Washington, Brooke Army Medical Center in Texas, Naval Medical Center San Diego in California, and Naval Medical Center Portsmouth in Virginia. A patient seeking genetic evaluation, counseling, or testing in those larger facilities would be referred to the genetics service by their primary care manager. Wait times vary, but it would usually be weeks, maybe months. However, the great majority of MTFs do not have dedicated genetics support. Most of the time, those patients would have to be referred to the local civilian community—there was no process for them to be seen in in the military healthcare system—with wait times that exceed 6 to 8 months in some cases. This is due to just not a military but a national shortage of genetics professionals (counselors and physicians).
Last year we started the telegenetics initiative, which is small compared to the VA—it is comprised of 2 geneticists and 1 genetic counselor—but with the full intent of growing it over time. Its purpose is to extend the resources we
had to other MTFs. Genetics professionals stationed state-side can provide care to remote facilities with limited access to local genetics support such as Cannon Air Force Base (AFB) or overseas facilities such as Spangdahlem AFB in Germany.
We recognize there are military-specific needs for the DoD regarding the genetic counseling process that have to take into account readiness, genetic discrimination, continued ability to serve and fitness for duty. For this important reason, we are seeking to expand our telegenetics initiative. The goal is to be able to provide 100% of all genetic counseling in-house, so to speak.
Currently, providers at the 4 pilot sites (Cannon AFB, Fort Bragg, Spangdahlem AFB, and Guantanamo Bay) send us referrals. We triage them and assign the patient to see a geneticist or a counselor depending on the indication.
On the laboratory side, it has been a very interesting experience. Because we provide comprehensive germline cancer testing at very little cost to the provider at any MTF, we have had high numbers of test requests over the years.
In addition to saving the DoD millions of dollars in testing, we have learned some interesting lessons in the process. For instance, we have worked closely with several different groups to better understand how to educate providers on the genetic counseling and testing process. This has allowed us to craft a thorough and inclusive consent form that addresses the needs of the DoD. We have also learned valuable lessons about population-based screening vs evidence-based testing, and lessons surrounding narrow-based testing (BRCA1 and BRCA2 only testing) vs ordering a more comprehensive panel that includes other genes supported by strong evidence (such as PALB2, CHEK2, or TP53).
For example, we have found that in a significant proportion of individuals with and without family history, there are clinically relevant variants in genes other than BRCA1 or BRCA2. And so, we have made part of our consent process,
a statement on secondary findings. If the patient consents, we will report pathogenic variants in other genes known to be associated with cancer (with strong evidence) even if the provider ordered a narrow panel such as BRCA1 and BRCA2 testing only. In about 1% to 4% of patients that would otherwise not meet NCCN guidelines, we’ve reported variants that were clinically actionable and changed the medical management of that patient.
We feel strongly that this is a conversation that we need to have in our field, and we realize it’s a complex issue, maybe we need to expand who gets testing. Guideline based testing is missing some patients out there that could benefit from it.
Vickie Venne. There certainly are many sides to the conversation of population-based vs evidence-based genetic testing. Genetic testing policies are changing rapidly. There are teams exploring comprehensive gene sequencing for
newborns and how that potential 1-time test can provide information will be reinterpreted as a person goes from cradle to grave. However, unlike the current DoD process, in the VA there are patients who we don’t see.
Renee Rider. I want to talk about money. When we order a genetic test, that test is paid for by the pathology department at the patient’s VAMC. Most of the pathology departments we work with are clear that they only can provide
genetic testing that is considered medically necessary. Thus, we review each test to make sure it meets established guidelines for testing. We don’t do population genetic screening as there isn’t evidence or guidelines to support offering it. We are strict about who does and does not get genetic testing, partly because we have a responsibility to pathology departments and to the taxpayers.
GMS focuses on conditions that are inherited, that is to say, we deal with germline genetics. Therefore, we discontinue referrals for somatic requests, such as when an OncotypeDX test is requested. It is my understanding that pharmacogenetic referrals may be sent to the new PHASeR initiative, which is a joint collaboration between the VA and Sanford Health and is headed by Deepak Voora, MD.
We generally don’t see patients who still are having diagnostic procedures done. For example, if a veteran has a suspicious breast mass, we recommend that the provider workup the mass before referring to GMS. Regardless of a genetic test result, a suspicious mass needs to be worked up. And, knowing if the mass is cancerous could change how we would proceed with the genetic workup. For example, if the mass were not cancerous, we may recommend that an affected relative have the first genetic evaluation. Furthermore, knowing if the patient has cancer changes how we interpret negative test results.
Another group of patients we don’t see are those who already had genetic testing done by the referring provider. It’s a VA directive that if you order a test, you’re the person who is responsible for giving the results. We agree with
this directive. If you don’t feel comfortable giving back test results, don’t order the test. Often, when a provider sends a patient to us after the test was done, we discover that the patient didn’t have appropriate pretest counseling. A test result, such as a variant of uncertain significance (VUS), should never be a surprise to either the provider or the patient.
Ishta Thakar. For newly diagnosed cancers, the first call is to the patient to inform them that they have cancer. We usually bring up genetic counseling or testing, if applicable, when they are ready to accept the diagnosis and have a conversation about it. All our consults are via telehealth, so none of our patients physically come to GMS in Salt Lake City. All the consults are done virtually.
For newly diagnosed patients, we would send a consult in within a couple of weeks. For patients who had a family history, the referral would not be urgent: They can be seen within about 3 months. The turnaround times for GMS are so much better than what we have available in the community where it’s often at least 6 months, as previously noted.
Vickie Venne. Thank you. We continue to work on that. One of the interesting things that we’ve done, which is the brainchild of Renee, is shared medical appointments.
Renee Rider. We have now created 4 group appointments for people who have concerns surrounding cancer. One group is for people who don’t have cancer but have family members who have cancer who may be the best testing candidate. For example, that might be a 30-year old who tells you that her mother had breast cancer at age 45 years. Her mother is still living, but she’s never had genetic testing. We would put her in a group where we discuss the importance of talking to the family members and encouraging them to go get that first genetic evaluation in the family.
Our second group is for people who don’t have cancer themselves, but have a family history of cancer and those affected relatives have passed away. The family needs a genetic evaluation, and the veteran is the best living testing candidate.
That group is geared towards education about the test and informed consent.
The third group is for people with cancer who qualify for genetic testing. We provide all of the information that they need to make an informed decision on having (or not having) genetic testing.
The final group is for people who have family histories of known genetic mutations in cancer genes. Again, we provide them with all of the information that they need to make an informed decision regarding genetic testing.
With the shared medical appointments, we have been able to greatly increase the number of patients that we can see. Our first 3 groups all meet once a week and can have 10 or 12 veterans. Our last group meets every other week and has a maximum of 6 veterans. Wait times for our groups are generally ≤ 2 weeks. All veterans can choose to have an individual appointment if they prefer. We regularly get unsolicited feedback from veterans that they learn a lot during our groups and appreciate it.
Our group appointments have lowered the wait time for the people in the groups. And, they’ve lowered the wait time for the people who are seen individually. They’ve allowed us to address the backlog of patients waiting to see us in a more timely manner. Our wait time for individual appointment had been approaching 6 months, and it is now about 1.5 months.
We also think that being in a group normalizes the experience. Most people don’t know anyone who has had genetic testing. Now, they are in a group with others going through the same experience. In one of my groups, a male veteran talked about his breast cancer being really rare. Another male in the group volunteer that he had breast cancer, too. They both seemed to appreciate not feeling alone.
Vickie Venne. I want to move to our final piece. What do the referring providers tell the patients about a genetics referral and what should they expect?
Lisa Arfons. First and foremost, I tell the patient that it is a discussion with a genetic counselor. I make it clear that they understand that it is a discussion. They then can agree or not agree to accept genetic testing if it’s recommended.
I talk in general terms about why I think it can be important for them to have the discussion, but that we don’t have great data for decisionmaking. We understand that there are more options for preventive measures but then it ultimately will be a discussion between the PCP, the patient, and their family members about how they proceed about the preventive measures. I want them to start thinking about how the genetic test results, regardless of if they are positive, negative, or a variant that is not yet understood, can impact their offspring.
Probably I am biased, as my mom had breast cancer and she underwent genetic testing. So, I have a bit of an offspring focus as well. I already mentioned that you must discuss about whether or not it’s worth screening or doing any preventive measures on contralateral breast, or screening for things like prostate cancer at age 75 years. And so I focus more on the family members.
I try to stay in my lane. I am extremely uncomfortable when I hear about someone in our facility sending off a blood test and then asking someone else to interpret the results and discuss it with the patient. Just because it’s a blood test and it’s easy to order doesn’t mean that it is easy to know what to do with it, and it needs to be respected as such.
Ishta Thakar. Our PCPs let the patients know that GMS will contact the patient to schedule a video appointment and that if they want to bring any family members along with them, they’re welcome to. We also explain that certain cancers are genetically based and that if they have a genetic mutation, it can be passed on to their offspring. I also explain that if they have certain mutations, then we would be more vigilant in screening them for other kinds of cancers. That’s the reason that we refer that they get counseled. After counseling if they’re ready for the testing, then the counselor orders the test and does the posttest discussion with the patient.
Vickie Venne. In the VA, people are invited to attend a genetic counseling session but can certainly decline. Does the the DoD have a different approach?
Maj De Castro. I would say that the great majority of active duty patients have limited knowledge of what to expect out of a genetics appointment. One of the main things we do is educate them on their rights and protections and the potential risks associated with performing genetic testing, in particular when it comes to their continued ability to serve. Genetic testing for clinical purposes is not mandatory in the DoD, patients can certainly decline testing. Because genetic testing has the potential to alter someone’s career, it is critical we have a very thorough and comprehensive pre- and posttest counseling sessions that includes everything from career implications to the Genetic Information Nondiscrimination Act (GINA) and genetic discrimination in the military, in addition to the standard of care medical information.
Scenarios in which a servicemember is negatively impacted by pursuing a genetic diagnosis are very rare. More than 90% of the time, genetic counseling and/or testing has no adverse career effect. When they do, it is out of concern for the safety and wellbeing of a servicemember. For instance, if we diagnosis a patient with a genetic form of some arrhythmogenic disorder, part of the treatment plan can be to limit that person’s level of exertion, because it could potentially lead to death. We don’t want to put someone in a situation that may trigger that.
Vickie Venne. We also have a certain number of veterans who ask us about their service disability pay and the impact of genetic testing on it. One example is veterans with prostate cancer who were exposed to Agent Orange, which has been associated with increased risk for developing prostate cancer. I have had men who have been referred for genetic evaluation ask, “Well, if I have an identifiable mutation, how will that impact my service disability?” So we discuss the carcinogenic process that may include an inherited component as well as the environmental risk factors. I think that’s a unique issue for a population we’re honored to be able to serve.
Renee Rider. When we are talking about how the population of veterans is unique, I think it is also important to acknowledge mental health. I’ve had several patients tell me that they have posttraumatic stress disorder or anxiety and the idea of getting an indeterminant test result, such as VUS, would really weigh on them.
In the community, a lot of providers order the biggest panel they can, but for these patients who are worried about getting those indeterminant test results, I’ve been able to work with them to limit the size of the panel. I order a small panel that only has genes that have implications for that veteran’s clinical management. For example, in a patient with ductal breast cancer, I remove the genes that cause lobular breast cancer. This takes a bit of knowledge and critical thinking that our VA genetic counselors have because they have experience with veterans and their needs.
As our time draws to a close, I have one final thought. This has been a heartwarming conversation today. It is really nice to hear that GMS services are appreciated. We in GMS want to partner with our referring providers. Help us help you! When you enter a referral, please let us know how we can help you. The more we understand why you are sending your veteran to GMS, the more we can help meet your needs. If there are any questions or problems, feel free to send us an email or pick up the phone and call us.
Sexual Dysfunction in MS
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
A 37-year-old woman presents to her primary care clinic with a chief complaint of depression. She was diagnosed with relapsing multiple sclerosis (MS) at age 29 and is currently taking an injectable preventive therapy. Over the past 6 months, she has had increased marital strain secondary to losing her job because “I couldn’t mentally keep up with the work anymore.” This has caused financial difficulties for her family. In addition, she tires easily and has been napping in the afternoon. She and her husband are experiencing intimacy difficulties, and she confirms problems with vaginal dryness and a general loss of her sexual drive.
Sexual dysfunction in MS is common, affecting 40% to 80% of women and 50% to 90% of men with MS. It is an “invisible” symptom, similar to fatigue, cognitive dysfunction, and pain.1-3
There are three ways that MS patients can be affected by sexual dysfunction, and they are categorized as primary, secondary, and tertiary. Primary sexual dysfunction results from demyelination/axonal destruction of the central nervous system, which potentially leads to altered genital sensation or paresthesia. Secondary sexual dysfunction stems from nonsexual MS symptoms, such as fatigue, spasticity, tremor, impairments in concentration/attention, and iatrogenic causes (eg, adverse effects of medication). Tertiary sexual dysfunction involves the psychosocial/cultural aspects of the disease that can impact a patient’s sexual drive.
SYMPTOMS
Like many other symptoms associated with MS, the symptoms of sexual dysfunction are highly variable. In women, the most common complaints are fatigue, decrease in genital sensation (27%-47%), decrease in libido (31%-74%) and vaginal lubrication (36%-48%), and difficulty with orgasm.4 In men with MS, in addition to erectile problems, surveys have identified decreased genital sensation, fatigue (75%), difficulty with ejaculation (18%-50%), decreased interest or arousal (39%), and anorgasmia (37%) as fairly common complaints.2
TREATMENT
Managing sexual dysfunction in a patient with MS is dependent on the underlying problem. Some examples include
- For many patients, their disease causes significant anxiety and worry about current and potentially future disability—which can make intimacy more difficult. Sometimes, referral to a mental health professional may be required to help the patient
with individual and/or couples counseling to further elucidate underlying intimacy issues. - For patients experiencing MS-associated fatigue, suggest planning for sexual activity in the morning, since fatigue is known to worsen throughout the day.
- For those who qualify for antidepressant medications, remember that some (eg, selective serotonin reuptake inhibitors) can further decrease libido and therefore should be avoided if possible.
- For women who have difficulty with lubrication, a nonpetroleum-based lubricant may reduce vaginal dryness, while use of a vibrator may assist with genital stimulation.
- For men who cannot maintain erection, phosphodiesterase inhibitor drugs (eg, sildenafil) can be helpful; other options include alprostadil urethral suppositories and intracavernous injections.
The patient is screened for depression using the Patient Health Questionnaire, which yields a score of 17 (moderately severe). You discuss the need for active treatment with her, and she agrees to start an antidepressant medication. Bupropion is chosen, given its effectiveness and lack of adverse effects (including sexual dysfunction). The patient also is encouraged to use nonpetroleum-based lubricants. Finally, a referral is made for couples counseling, and a 6-week follow-up appointment is scheduled.
CONCLUSION
Sexual dysfunction in MS is quite common in both women and men, and the related symptoms are often multifactorial. Strategies to address sexual dysfunction in MS require a tailored approach. Fortunately, any treatments for sexual dysfunction initiated by the patient’s primary care provider will not have an adverse effect on the patient’s outcome with MS. For more complicated cases of MS-associated sexual dysfunction, urology referral is recommended.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.
1. Foley FW, Sander A. Sexuality, multiple sclerosis and women. Mult Scler Manage. 1997;4:1-9.
2. Calabro RS, De Luca R, Conti-Nibali V, et al. Sexual dysfunction in male patients with multiple sclerosis: a need for counseling! Int J Neurosci. 2014;124(8):547-557.
3. Gava G, Visconti M, Salvi F, et al. Prevalence and psychopathological determinants of sexual dysfunction and related distress in women with and without multiple sclerosis. J Sex Med. 2019;16(6):833-842.
4. Cordeau D, Courtois, F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014; 57(5):337-47.