User login
Off-label medications for addictive disorders
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
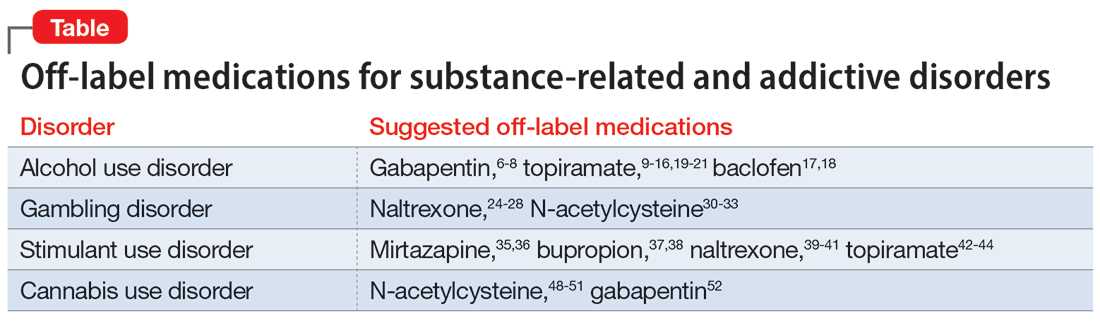
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52
The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Burnout among surgeons: Lessons for psychiatrists
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.
Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.
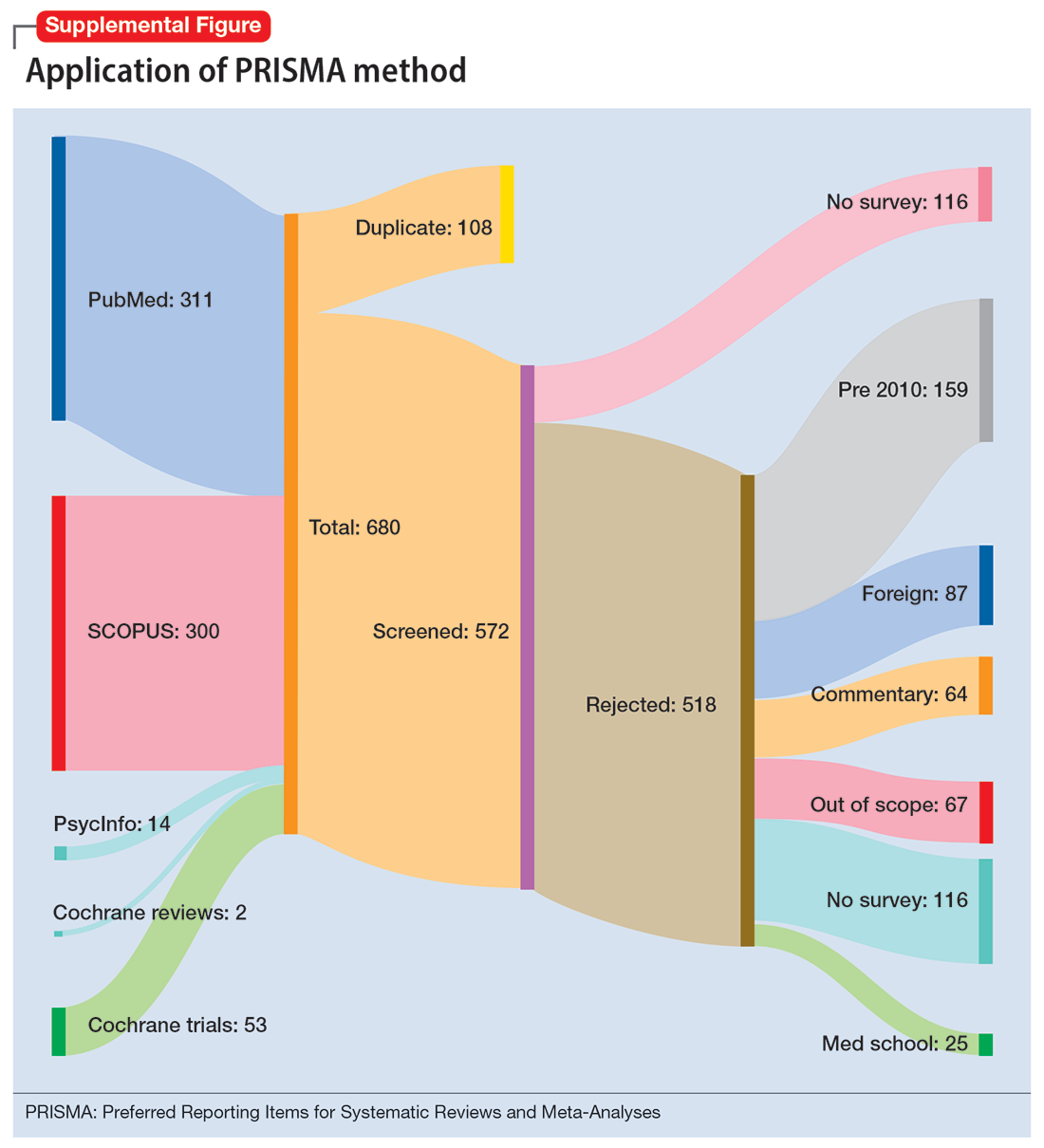
Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.
Work/life balance factors
Fifteen studies
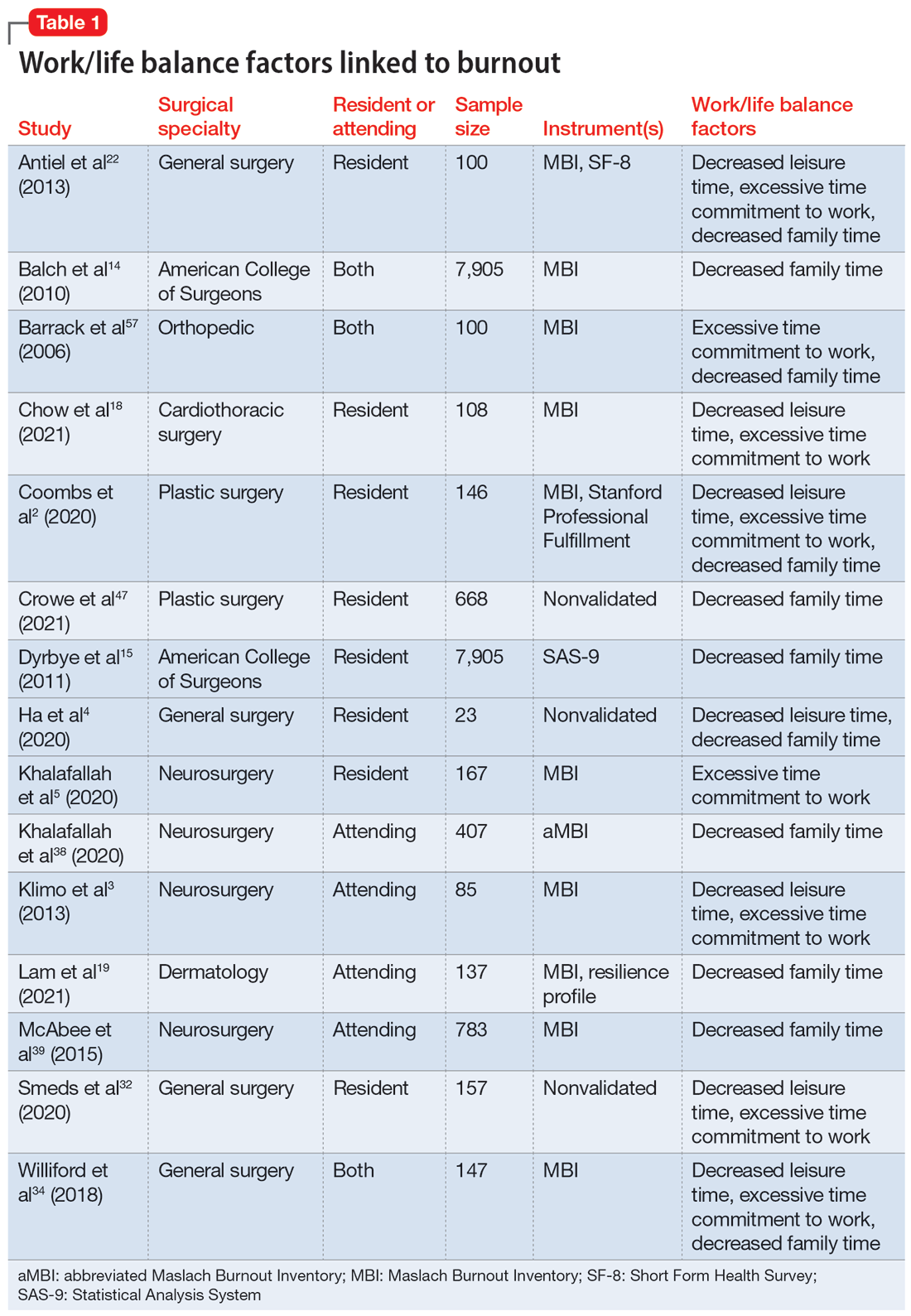
Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies
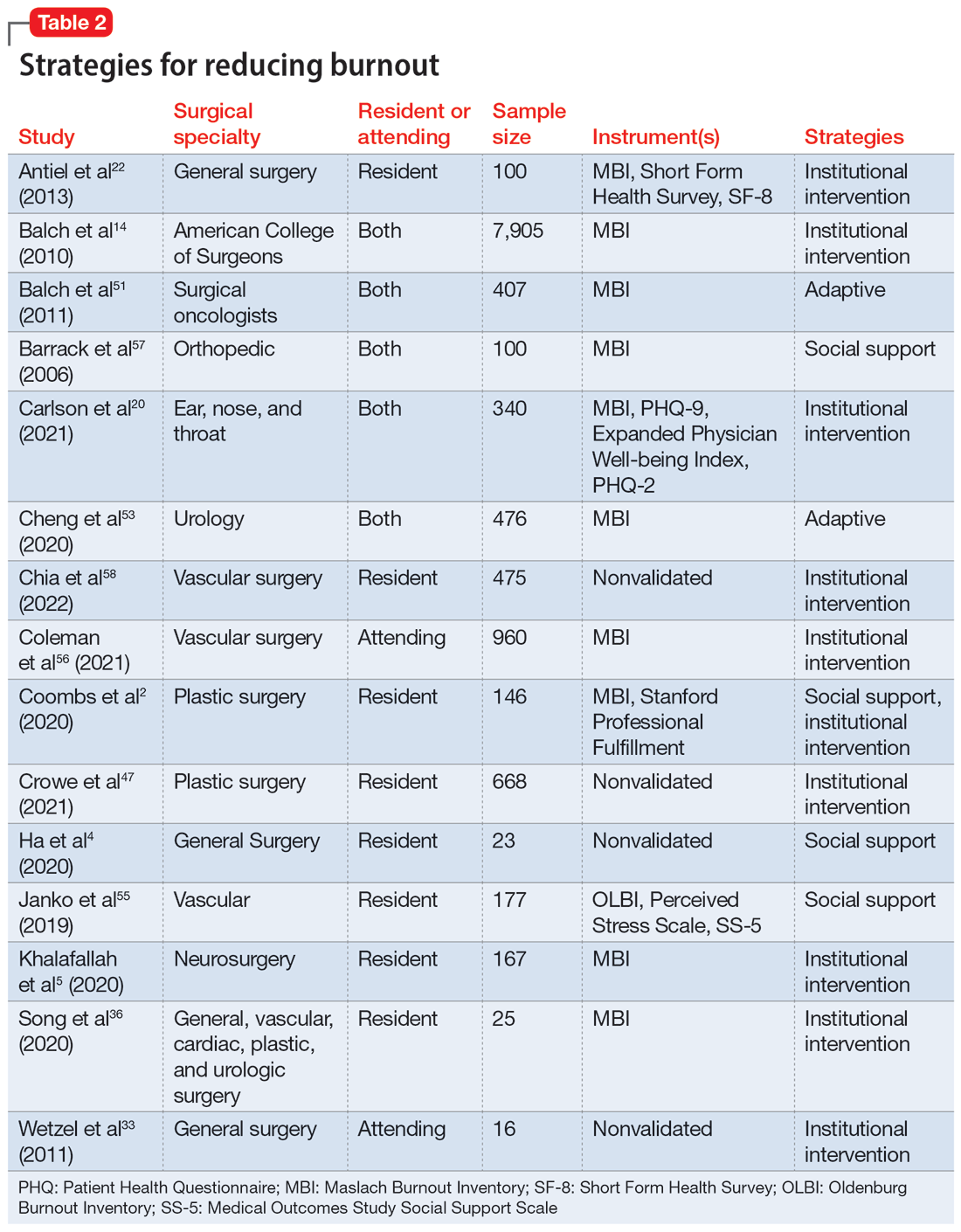
Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Risk Evaluation and Mitigation Strategy programs: How they can be improved
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).
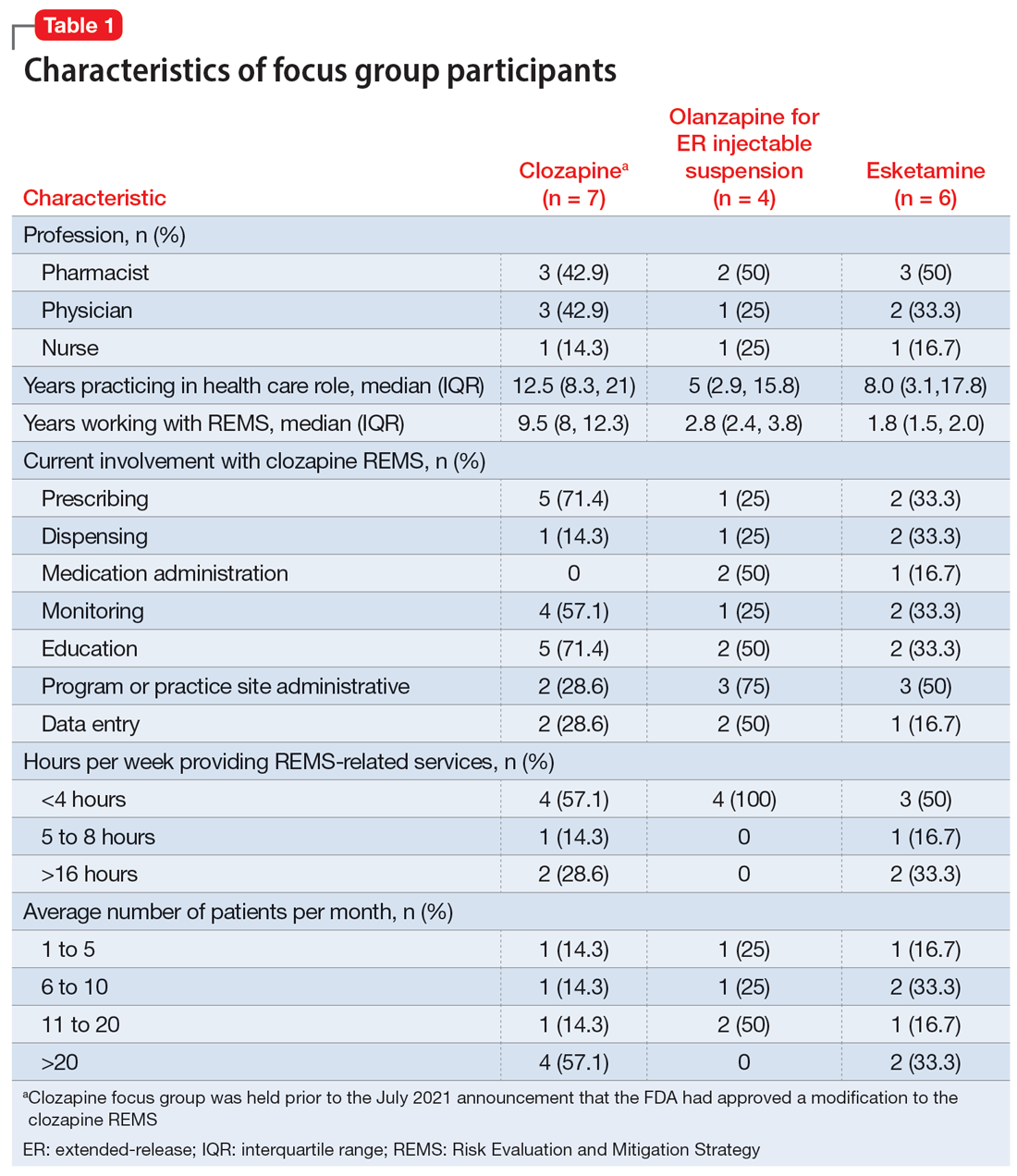
Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.
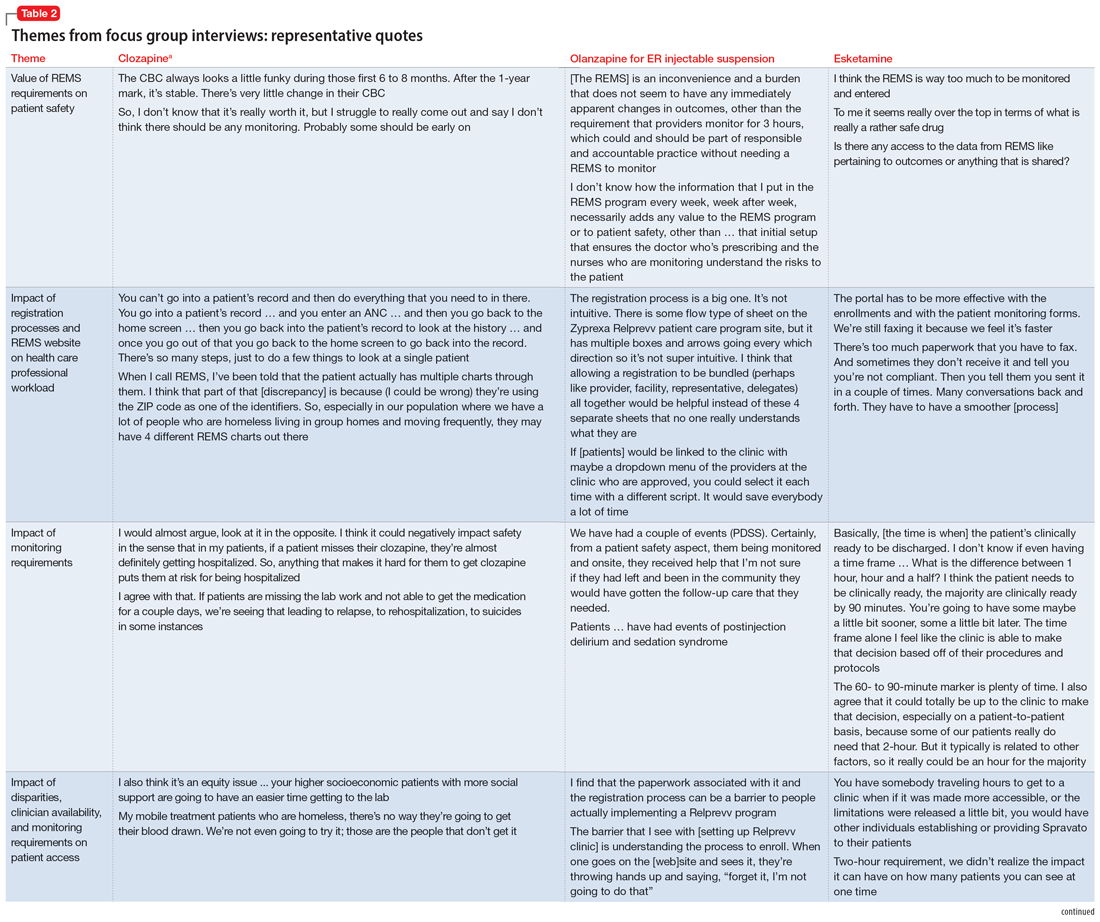

Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.
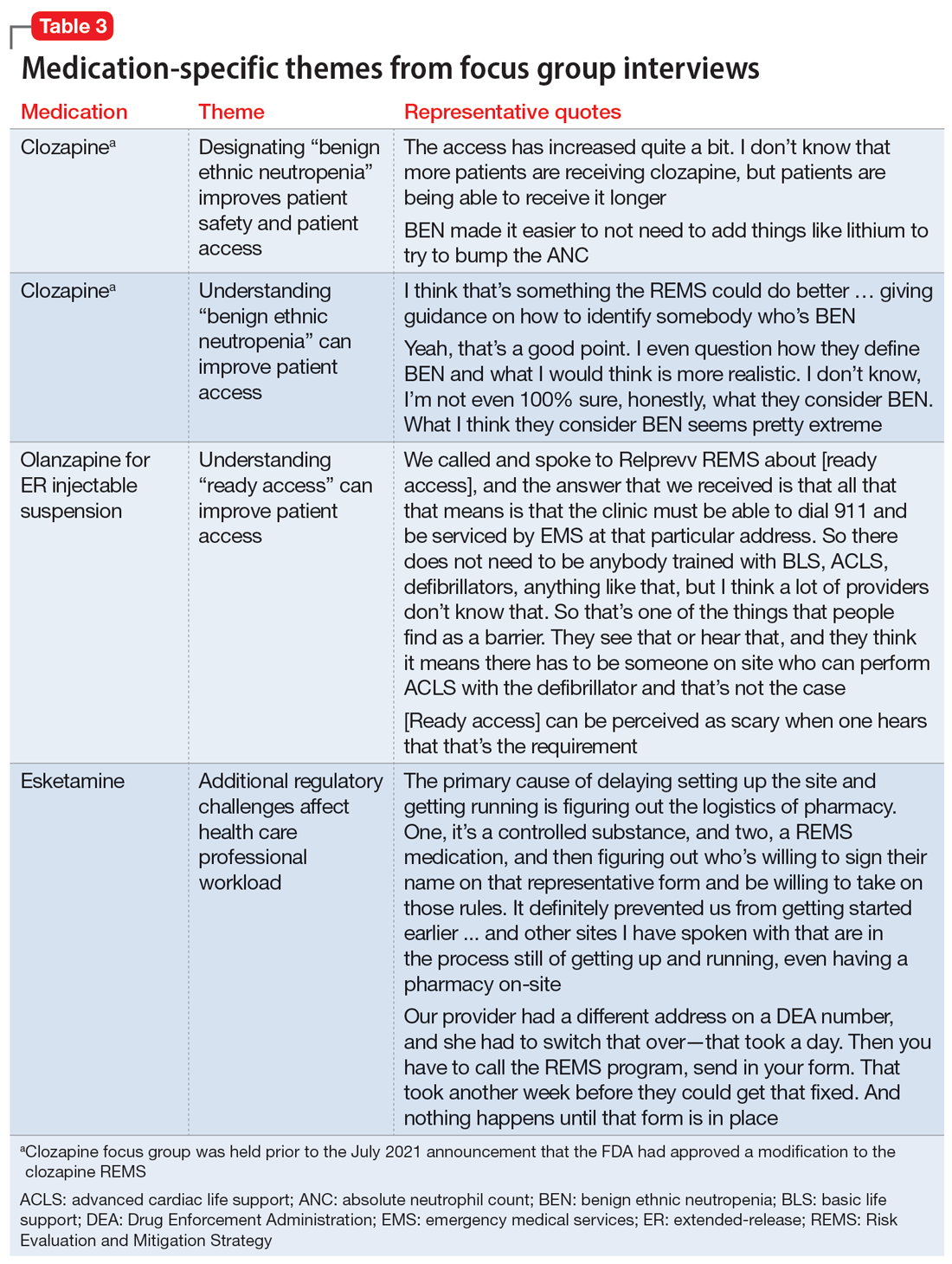
Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.
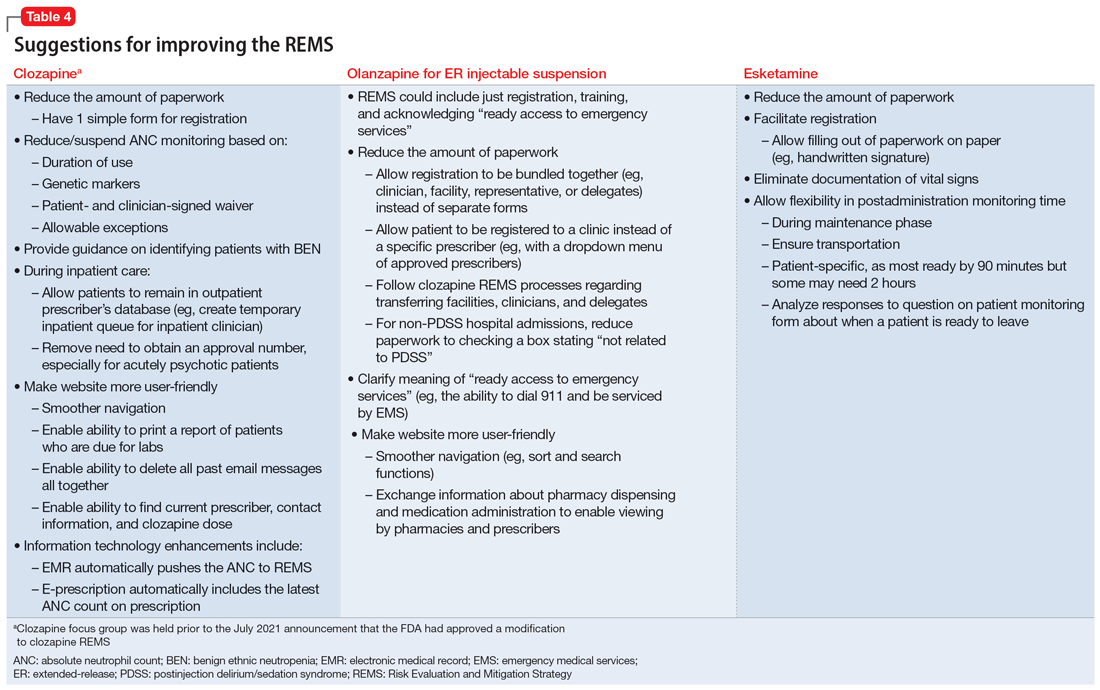
Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
Interventional psychiatry (Part 2)
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.

Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
Optimizing benzodiazepine treatment of anxiety disorders
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).
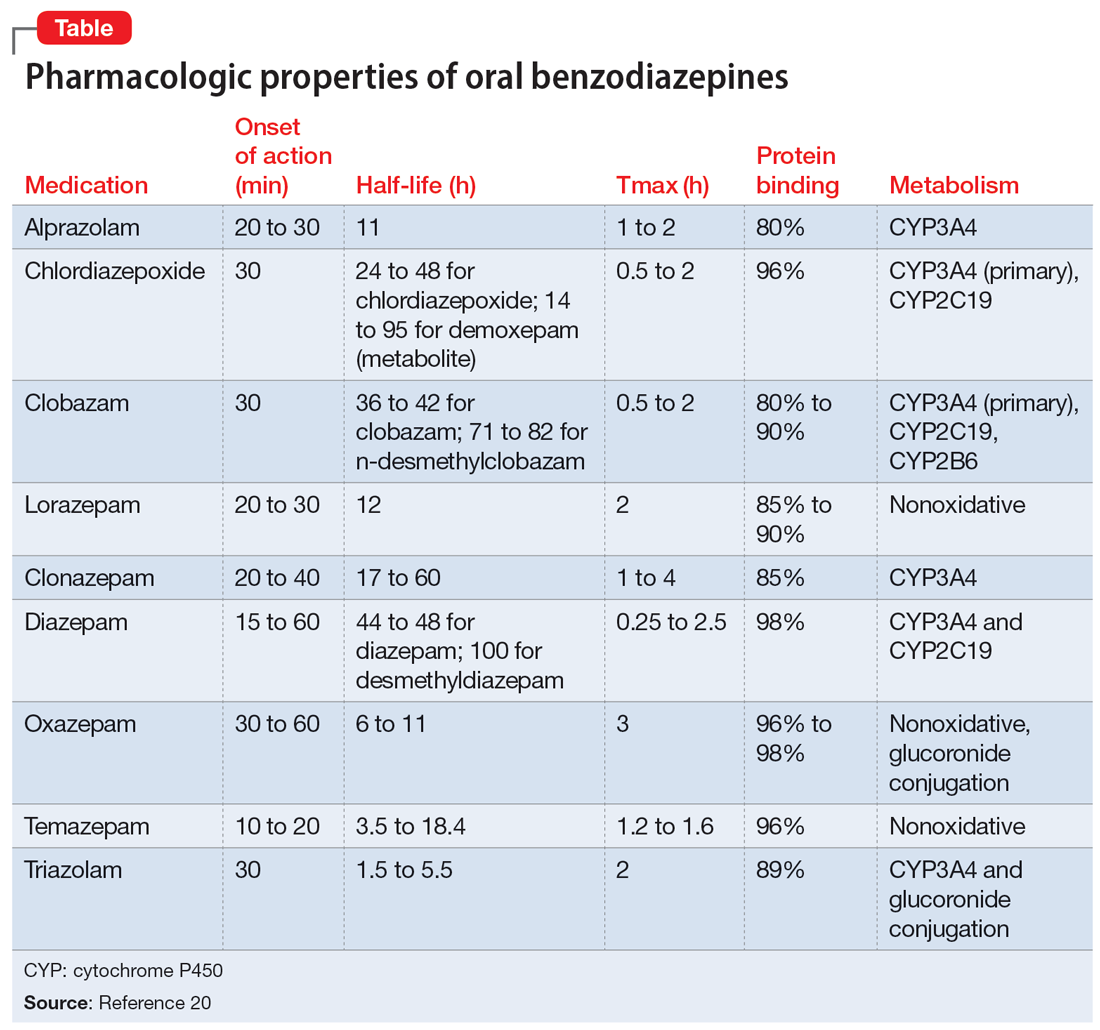
Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.
CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51
Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.
What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).
Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).

Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.

CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51

Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.

What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).

Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
Though once the main treatment for anxiety disorders—often as monotherapy1—benzodiazepines are now primarily used as adjunctive agents.2-4 Their ability to produce rapid anxiolysis represents a significant therapeutic advantage, but in recent decades their tolerability, class-specific risks, and lack of antidepressant properties contributed to benzodiazepines being largely replaced by selective serotonin reuptake inhibitors (SSRIs) for the pharmacologic treatment of anxiety. This shift within the pharmacologic armamentarium has decreased many clinicians’ familiarity with benzodiazepines.
While benzodiazepines continue to have an important role in managing anxiety disorders, particularly treatment-resistant anxiety,4 clinicians must consider the limitations of these agents. Benzodiazepines can be associated with abuse and dependence, and overdose risk when combined with opiates.5,6 They may cause memory impairment7,8 and conflicting data suggest they may contribute to the risk of developing cognitive disorders.9-11 Benzodiazepines also have been associated with falls and fractures,12 and worse outcomes in patients with posttraumatic stress disorder.13 Some studies of patients with chronic obstructive pulmonary disease (COPD) found benzodiazepines may increase the risk of COPD exacerbations and accidental overdose,14 though others found that was not always the case.15 Benzodiazepines may be associated with an increased risk of spontaneous abortion when used early in pregnancy.16 Prospective research in women who were breastfeeding found benzodiazepines may cause sedation in up to 2% of infants.17
Despite the potential for adverse effects, benzodiazepine use remains common.18 These medications have a rapid onset of action, are useful for breakthrough symptoms, may enhance treatment adherence, and alleviate activating symptoms of SSRIs. Like other commonly used medications, benzodiazepines have the potential for both harm and benefit.19 Similar to other medications with tolerability concerns but established efficacy, particularly in treatment-resistant anxiety disorders, it is important to balance “overprescribing … to patients at risk and underusing these effective medications when indicated.”19 Though the use of benzodiazepines has been discouraged and perceptions have shifted, knowledge of benzodiazepines and benzodiazepine pharmacology also has been degraded contemporaneously.
This article provides a synthesis of the clinically relevant pharmacology of benzodiazepines, with a focus on orally administered benzodiazepines, which are more common in outpatient clinical practice. Specifically, this review describes the pharmacology of benzodiazepines, benzodiazepine medication interactions, the relationship between pharmacologic characteristics and treatment response/tolerability, and selection and dosing of oral benzodiazepines (Table20).

Benzodiazepine pharmacodynamics
Benzodiazepines act at the gamma-aminobutyric acid (GABA)-A receptor complex and bind allosterically.21-23 Comprised of 5 glycoprotein subunits (2 alpha subunits, 2 beta subunits, and 1 gamma subunit), the receptor has 2 distinct sites at which the endogenous inhibitory transmitter GABA binds and 1 benzodiazepine binding site. Benzodiazepines bind within a socket created by the alpha and gamma subunits22 and after binding induce a conformational change in the receptor, which enhances GABA binding. There are 2 types of benzodiazepine receptors: BZ1 and BZ2. The subunits play a critical role in driving the pharmacologic characteristics of the receptor.24 BZ1 and BZ2 receptors bind benzodiazepines, although they are differentially distributed within the brain. Binding at BZ1 receptors—which are distributed in cortical, thalamic, and cerebellar regions—contributes to sedation and deleterious effects of benzodiazepines on memory (eg, anterograde amnesia). BZ2 receptors (which contain gamma-2 subunits) are responsible for anxiolytic and muscle-relaxing effects. They are distributed throughout limbic regions and motor tracts, including motor neurons and neurons in the dorsal horn of the spinal cord.24
Benzodiazepines—positive GABA-A receptor allosteric modulators—produce phasic inhibition, largely through the alpha and gamma subunits discussed above. In contrast, newer positive allosteric modulators (eg, zuranolone) bind at the alpha/beta subunits.25 Mechanistically, endogenous neuroactive steroids and nonbenzodiazepine GABA-A–positive allosteric modulators such as zuranolone and ganaxolone also differ in their regulation of GABA-A (downregulated with benzodiazepines and hypothetically upregulated with zuranolone)26 and their synaptic effects (benzodiazepines synaptically vs endogenous neurosteroids and nonbenzodiazpine positive allosteric modulators extrasynaptically).27
From a developmental perspective, benzodiazepines may have less efficacy for anxiolysis and worse tolerability in some pediatric patients,28 although they generally appear effective for immediate use to treat anxiety in acute settings.29 The differences in efficacy and tolerability may be related to pharmacodynamic differences between pediatric populations and adults. GABA receptor expression and function do not reach adult levels until age 14 to 17½ for subcortical regions and age 18 to 22 for cortical regions, although girls reach adult expression of GABA receptors slightly earlier than boys.30 D
Continue to: Pharmacology and clinical effects
Pharmacology and clinical effects
Benzodiazepine pharmacokinetics are intimately linked with the onset of action and duration of clinical effect and vary based on the route of administration, absorption, and distribution/redistribution.31 In this review, we focus on oral administration as opposed to IV, IM, sublingual, or intranasal administration.
Absorption
Benzodiazepines are rapidly absorbed after oral administration and quickly enter the systemic circulation. However, absorption rates vary depending on specific aspects of the gastrointestinal milieu and intrinsic properties of the benzodiazepine. For example, alprazolam is more rapidly absorbed than most other benzodiazepines, with a Tmax of 1.8 hours compared to lorazepam, which has a Tmax of approximately 2 hours. These pharmacokinetic effects instantiate differences in tolerability and efficacy. Thus, following single doses of alprazolam and diazepam, self-rated sedating effects and impairment on a task of working memory suggest that effects have a more rapid onset for alprazolam relative to lorazepam.32 Food and concomitant medications can significantly affect benzodiazepine absorption. A single-dose, 3-way crossover study demonstrated that taking diazepam concomitantly with an antacid (eg, aluminum hydroxide) decreased peak concentrations and prolonged absorption by approximately 30 minutes. However, total absorption of the medication was unaffected.33 Additionally, administration of diazepam with food significantly slows absorption from 1 hour 15 minutes to approximately 2 hours 30 minutes and increases benzodiazepine absorption by 25% (Figure 134); the fat content of the meal appears important in moderating this effect.35 The impact of food on alprazolam varies by formulation. For example, when administered in an extended-release (XR) formulation with a high-fat meal, alprazolam absorption increases by one-third, while absorption for administration of the orally disintegrating tablet with a high-fat meal increases from 1 hour 30 minutes to 2 hours. Similarly, for lorazepam, administration with a meal delays absorption by approximately 2 hours; however, this effect does not appear present with the XR formulation. Administering benzodiazepines with food can be clinically leveraged to either accelerate the onset of action or decrease peak-associated adverse effects. Thus, when a highly lipophilic benzodiazepine is needed to treat acute anxiety or prior to an expected anxiogenic stimuli, administering the medication without food may produce a faster onset of action.

CNS penetration
Benzodiazepines enter the CNS by passive diffusion. Because of this, lipophilicity at physiologic pH influences the rate at which a benzodiazepine crosses the blood-brain barrier. The rate at which benzodiazepines enter the CNS influences their clinical effects and the speed at which both efficacy (ie, anxiolysis) and adverse effects (ie, sedation, slowed cognition) are observed. In general, more lipophilic medications initiate their anxiolytic effect more quickly. However, by quickly leaving the CNS (through the same mechanism that allowed them to enter the CNS at such speed), their effects rapidly cease as they redistribute into fat. Thus, highly lipophilic benzodiazepines produce more intense effects compared to less lipophilic benzodiazepines. For these reasons, lipophilicity is more important than half-life for determining the duration of effect in most patients.
Lipophilicity and duration of effect
Benzodiazepines and their metabolites tend to be highly protein-bound and distributed in fat- and lipid-enriched areas such as the CNS. As a result, the more lipophilic agents generally have the highest rates of absorption and the fastest onset of clinical effects. The duration of action for many benzodiazepines is determined by the rate and extent of distribution (a function of lipophilicity) rather than by the rate of elimination. For example, diazepam has a longer half-life than lorazepam, but its duration of action following a single dose is shorter. This is because diazepam is more lipophilic and therefore more extensively distributed (particularly to adipose tissue). This results in it leaving the brain and blood and distributing to other tissues. In turn, its CNS effect (ie, anxiolytic effects) are more quickly terminated.
By contrast, less lipophilic benzodiazepines maintain their CNS concentrations longer; they have a longer duration of action because of their slower redistribution, which culminates in a shorter half-life, and are less extensively distributed to peripheral tissues. In essence, this means that (other things being equal) a less lipophilic benzodiazepine produces a more sustained anxiolytic effect compared to a highly lipophilic benzodiazepine.36 Lipophilicity is also important in predicting some cognitive adverse effects, including amnesia. Benzodiazepines with high lipophilicity have greater absorption and faster onset of action as well as more rapid amnestic effects.37,38 These effects may relate to overall efficacy differences for oral benzodiazepines. A recent meta-analysis by Stimpfl et al36 found that less lipophilic benzodiazepines produced a greater response compared to more lipophilic benzodiazepines.
Continue to: Metabolism
Metabolism
Regarding cytochrome P450 (CYP) metabolism, polymorphic CYP2C19 and CYP3A4/5 are involved in the metabolism of several benzodiazepines39 and CYP2B6 has been recognized as a contributor to diazepam metabolism. CYP3A5 gene polymorphisms may produce variation in alprazolam metabolism; however, the predominant cytochrome involved in the metabolism of oxidatively metabolized benzodiazepines (ie, benzodiazepines other than lorazepam, oxazepam, and temazepam) is primarily CYP3A4, and most effects on CYP3A4 activity are related to concomitant medications and other nongenetic factors.
Drug-drug interactions
Apart from lorazepam,40,41 oxazepam,42,43 and temazepam, most benzodiazepines are metabolized through oxidative mechanisms that involve CYP3A4 (Figure 220).39 As such, their metabolism is influenced by medications that impact CYP3A4, including antifungals (eg, ketoconazole), calcium channel blockers (eg, verapamil, diltiazem), nefazodone, some protease inhibitors, and macrolide antibiotics. Research has examined the impact of low-dose estrogen oral contraceptives (OCPs) on exposure (eg, plasma concentrations) of several benzodiazepines. The mechanism for this interaction is likely complex and putatively involves multiple pathways, including inhibition of CYP3A4 by OCPs. The effects of OCPs on benzodiazepine pharmacokinetics vary based on the metabolism of the benzodiazepine. In general, medications oxidized and nitroreduced (eg, chlordiazepoxide, alprazolam, diazepam, and nitrazepam) have decreased clearance in patients treated with OCPs. Regarding nonoxidatively metabolized benzodiazepines, data are mixed. Research found no OCP-related effects on the pharmacokinetics of nonoxidatively metabolized benzodiazepines44; another study suggested that clearance of these medications—through increased glucuronidation—may be increased.31 The effect of smoking on benzodiazepine concentration has been well documented. Smoking increases the clearance of orally administered diazepam,45 but not IV diazepam, midazolam, or lorazepam, suggesting that this represents a first-pass effect.46 For alprazolam, plasma concentrations are reduced by 15% to 30% in smokers and total body clearance is 24% greater compared to nonsmokers, which results in an approximately 50% increase in half-life in nonsmokers compared to smokers.47 The most notable interaction between benzodiazepines and SSRIs is seen with fluvoxamine. Because fluvoxamine moderately inhibits CYP2C19 and CYP3A4 and potently inhibits CYP1A2,48 the clearance of oxidatively metabolized benzodiazepines is reduced.49 Additionally, the effects of grapefruit juice—a potent inhibitor of CYP3A4—has been evaluated for several benzodiazepines. Yasui et al50 found grapefruit juice did not alter alprazolam plasma concentrations. However, in separate research, grapefruit juice tripled diazepam exposure, increased peak concentrations 1.5-fold, and prolonged absorption.51

Hepatic disease
Exposure to benzodiazepines—other than lorazepam, oxazepam, and temazepam—is influenced by intrinsic hepatic disease and requires dose adjustment in individuals with significant hepatic impairment. The impact of hepatic disease on the clinical pharmacology of benzodiazepines may relate to 2 factors: protein binding and metabolism. In a study of individuals with cirrhosis, lorazepam binding was decreased, although its metabolism and clearance were largely unaffected.40
Aging and benzodiazepine metabolism/clearance
Aging is associated with myriad physiologic changes (eg, decrease in renal clearance after age 40, changes in body fat distribution, changes in activity of cytochromes) that are relevant to benzodiazepine pharmacology. They may underlie differences in the tolerability of benzodiazepines and other clinically relevant characteristics (eg, duration of action, accumulation).
Several studies have evaluated the impact of aging on the clearance and disposition of selected benzodiazepines. The respective half-lives of chlordiazepoxide and diazepam increase from 4- to 6-fold from age 20 to 80. Further, with chronic dosing, highly lipophilic benzodiazepines may require additional attention in geriatric patients. In a study that included individuals up to age 78, steady-state plasma concentrations of diazepam and its metabolite, desmethyldiazepam (DMDZ), were 30% to 35% higher in older patients compared to younger individuals.52 In this study, the half-lives for the young and older patients were 31 hours and 86 hours, respectively, for diazepam, and 40 hours and 80 hours, respectively, for the active metabolite. The half-life of diazepam is increased by “1 hour for each year of age beginning with a half-life of 20 hours at 20 years of age, as the volume of distribution is increased, and clearance is decreased.”52 Clinically, this implies that in older adults, clinicians should expect lower peak concentrations (Cmax), higher trough concentrations (Cmin), and that diazepam will take longer to reach steady-state concentrations. Taken together, these findings raised concern that “slow accumulation and delayed washout of diazepam and DMDZ is probable.”52 These findings—which may have more clinical relevance than those of single-dose studies—suggest that the effects related to diazepam would also take longer to resolve in older patients. Finally, lorazepam clearance or distribution does not appear to be affected by aging, at least in patients age 15 to 73.40 Alprazolam is more slowly cleared in geriatric patients and its effects may be potentiated by reduced protein binding.
Continue to: Obesity
Obesity
The distribution of medications, including benzodiazepines, is altered in patients who are obese because of increased adipose tissue.53,54 This increase in the volume of distribution can attenuate the onset of action, increase medication accumulation in fat, and potentiate the duration of action.55,56
Obesity may also affect hepatic metabolism by induction of CYP1A2, CYP2C9, and CYP2C19, and inhibition of CYP3A4.57 Triazolam, which is metabolized by CYP3A4, is associated with a greater exposure (ie, plasma concentrations) in individuals who are obese.58 However, when considering differences in benzodiazepine pharmacokinetics in patients who are obese, clinicians must remember that elimination half-life depends on both volume of distribution and clearance. In
How quickly do benzodiazepines work?
Benzodiazepines act quickly. Meta-analyses36 suggest that improvement in anxiety symptoms compared to placebo is greatest initially and then the rate of improvement slows over successive weeks. Research on benzodiazepines reveals statistically significant differences between benzodiazepines and placebo within the first week of treatment, with >80% of the expected improvement by Week 8 of treatment emerging by Week 4 (Figure 336). The rapid reduction in anxiety symptoms seen with benzodiazepines has important treatment implications, given that traditional psychotherapeutic and antidepressant treatments are slow to produce improvements. Consistent data suggesting that benzodiazepines work faster than other treatments support that they may have a role during the initiation of other treatments.

What is the ‘best’ dose?
As seen with other classes of psychotropic medications,4 the relationship between benzodiazepine dose and response is complex. In a recent meta-analysis of 65 placebo-controlled trials of benzodiazepines in adults with anxiety disorders, there was a superior response over time for low-dose benzodiazepines (<3 mg/d in lorazepam equivalents) compared to a medium dose (3 to 6 mg/d; P = .042); high-dose benzodiazepines (>6 mg/d) yielded less improvement compared to medium doses (P = .001).36 A study of adults with panic disorder similarly found the greatest responses with alprazolam plasma concentrations of 20 to 40 ng/mL, with no additional benefit at <20 ng/mL or >40 ng/mL.49 As plasma concentrations increase, adverse effects such as sedation also increase, which may confound the observed loss of a dose-response relationship at higher doses and plasma concentrations.62 This may, in part, account for the observation that higher doses of benzodiazepines are associated with greater depressive symptoms and disrupted sleep.63 As such, low doses may represent a delicate equipoise between efficacy and tolerability, yielding the most optimal clinical response.
Which benzodiazepine should I prescribe?
Comparing benzodiazepines is difficult, given the differences in dosing and disorders studied and differences in how each individual clinical trial was conducted. A meta-analysis by Stimpfl et al36 that used Bayesian hierarchical modeling, which allowed some of this heterogeneity to be addressed, found that relative to the reference benzodiazepine (lorazepam), clonazepam had the greatest trajectory/magnitude of response (other specific benzodiazepines did not statistically differ from lorazepam) (Figure 436).

Continue to: Another aspect of the superiority...
Another aspect of the superiority of clonazepam in some research relates to its pharmacokinetic properties, particularly when compared with benzodiazepines that have very short half-lives. Short half-life benzodiazepines have been associated with rebound anxiety, which is defined as “the relative worsening of symptoms on discontinuation of treatment as compared to baseline symptoms” and is distinct from withdrawal.64 While it is difficult to assess this in clinical trials, Herman et al65 provided insight into the contribution of rebound anxiety in a study of patients with panic disorder treated with alprazolam who experienced “interdose anxiety symptoms.” Of the 48 patients in this study, 41 switched to clonazepam, and most who switched (82%) experienced improvement. The improvement was attributed to the decreased frequency of clonazepam (vs alprazolam) administration and lack of interdose anxiety. When selecting an oral benzodiazepine, consider the duration, onset of action, and differences in metabolism that produce varying levels of effectiveness for individual patients. In situations where rapid onset is desired, a short-acting benzodiazepine may be preferable, while a longer-acting benzodiazepine would be preferable in situations where the patient needs sustained effects.
Regarding lipophilicity, differences among benzodiazepines could contribute to differences in psychological dependence and differential utility in some situations. For example, alprazolam rapidly enters the CNS, producing an immediate anxiolytic effect. However, its egress from the CNS is equally rapid, and its anxiolytic effects disappear quickly. This may be desirable for addressing acute, predictable anxiety, but could have unintended consequences in treating chronic anxiety, where it could facilitate psychological dependence.
Practical considerations
When prescribing benzodiazepines, consider a myriad of patient- and medication-specific factors, as these have clinically relevant implications on treatment response. This information, taken together, supports the importance of an individualized approach to benzodiazepine use. Before selecting a benzodiazepine and during treatment, important elements of the patient’s history must be considered, including age, body weight, concomitant medication use (eg, antacids, CYP3A4 inhibitors, OCPs), smoking status, and history of hepatic or renal disease.
Patients age <18 are unlikely to have full expression of GABA receptors in the brain30 and therefore benzodiazepines may not be as efficacious for anxiolysis in this population. Moreover, compared to younger patients, older patients may experience higher steady-state concentrations of benzodiazepines, especially lipophilic agents, due to an increased volume of distribution and decreased clearance. In patients treated with OCPs, some benzodiazepines may take longer to reach steady-state, and dose adjustments may need to be considered. In patients who smoke, clearance of some oral benzodiazepines is also accelerated, potentially decreasing half-life by up to 50%.
When dosing and titrating benzodiazepines, consider the patient’s body weight, particularly if they are obese. The effects of obesity on benzodiazepine pharmacokinetics are complex. For glucuronidated benzodiazepines, clearance is increased in patients who are obese; however, the volume of distribution is also increased in such patients, meaning it will take longer for benzodiazepines to achieve steady-state in these individuals compared to patients who are not obese. These effects suggest it may take longer to achieve a response at a given dose in patients who are obese compared to individuals who are not obese.
Continue to: The properties of individual benzodiazepines...
The properties of individual benzodiazepines should also be considered when selecting a benzodiazepine treatment. If circumstances necessitate rapid symptom relief, a lipophilic benzodiazepine, such as diazepam, may be preferred for quick onset and offset of action. Onset of action may also be hastened by taking the benzodiazepine without food; conversely, if peak adverse effects are problematic, concurrent consumption of a high-fat meal may help decrease peak concentration and prolonging absorption. In other circumstances, such as if sustained anxiolysis is desired, a clinician may opt for a less lipophilic benzodiazepine, such as clonazepam. Finally, in terms of general treatment response, benzodiazepines separate from placebo in the first week of treatment, which supports the idea they may be useful during the introduction of other medications (eg, SSRIs) that take a longer time to achieve clinical effect.
Bottom Line
The pharmacokinetics of benzodiazepines are intimately linked with the onset of action and duration of clinical effect and vary based on individual absorption and distribution/redistribution. Benzodiazepines’ clinical profile derives from their pharmacokinetic differences and is influenced by many factors, including age, body weight, concomitant medication use, smoking status, and hepatic or renal disease. Consider these factors to individualize the approach to using benzodiazepines and optimize tolerability and efficacy.
Related Resources
- Weber SR, Duchemin AM. Benzodiazepines: sensible prescribing in light of the risks. Current Psychiatry. 2018;17(2):22-27.
- Balon R. Benzodiazepines for anxious depression. Current Psychiatry. 2018;17(8):9-12.
Drug Brand Names
Alprazolam • Xanax
Chlordiazepoxide • Librium
Clobazam • Onfi
Clonazepam • Klonopin
Clorazepate • Gen-Xene
Diazepam • Valium
Diltiazem • Cardizem
Fluvoxamine • Luvox
Ganaxolone • Ztalmy
Ketoconazole • Nizoral
Lorazepam • Ativan
Midazolam • Versed
Temazepam • Restoril
Triazolam • Halcion
Verapamil • Calan
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
1. Rickels K, Moeller HJ. Benzodiazepines in anxiety disorders: reassessment of usefulness and safety. World J Biol Psychiatry. 2019;20(7):514-518. doi:10.1080/15622975.2018.1500031
2. Stevens JC, Pollack MH. Benzodiazepines in clinical practice: consideration of their long-term use and alternative agents. J Clin Psychiatry. 2005;66(Suppl 2):21-27.
3. Pollack MH, van Ameringen M, Simon NM, et al. A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry. 2014;171(1):44-53. doi:10.1176/appi.ajp.2013.12101353
4. Strawn JR, Geracioti L, Rajdev N, et al. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19(10):1057-1070. doi:10.1080/14656566.2018.1491966
5. Karaca-Mandic P, Meara E, Morden NE. The growing problem of co-treatment with opioids and benzodiazepines. BMJ. 2017;356:j1224. doi:10.1136/bmj.j1224
6. Bachhuber MA, Hennessy S, Cunningham CO, et al. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996-2013. Am J Public Health. 2016;106(4):686-688. doi:10.2105/AJPH.2016.303061
7. Bentué-Ferrer D, Akwa Y. Benzodiazepines: Effects on memory functioning. In: Pandi-Perumal SR, Verster J, Monti J, et al, eds. Sleep Disorders: Diagnosis and Therapeutics. CRC Press; 2008:104-114. doi:10.3109/9780203091715-15
8. Pomara N, Facelle TM, Roth AE, et al. Dose-dependent retrograde facilitation of verbal memory in healthy elderly after acute oral lorazepam administration.Psychopharmacology (Berl). 2006;185(4):487-494. doi:10.1007/s00213-006-0336-0
9. Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi:10.1136/bmj.i90
10. Biétry FA, Pfeil AM, Reich O, et al. Benzodiazepine use and risk of developing Alzheimer’s disease: a case-control study based on Swiss claims data. CNS Drugs. 2017;31(3):245-251. doi:10.1007/s40263-016-0404-x
11. de Gage SB, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349g5205. doi:10.1136/bmj.g5205
12. Shah R, Raji MA, Westra J, et al. Association of co-prescribing of opioid and benzodiazepine substitutes with incident falls and fractures among older adults: a cohort study. BMJ Open. 2021;11(12):e052057. doi:10.1136/bmjopen-2021-052057
13. Guina J, Rossetter SR, DeRhodes BJ, et al. Benzodiazepines for PTSD: a systematic review and meta-analysis. J Psychiatr Pract. 2015;21(4):281-303.
14. Ekström MP, Bornefalk-Hermansson A, Abernethy AP, et al. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi:10.1136/bmj.g445
15. Donovan LM, Malte CA, Spece LJ, et al. Center predictors of long-term benzodiazepine use in chronic obstructive pulmonary disease and post-traumatic stress disorder. Ann Am Thorac Soc. 2019;16(9):1151-1157. doi:10.1513/AnnalsATS.201901-048OC
16. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. doi:10.1001/jamapsychiatry.2019.0963
17. Kelly LE, Poon S, Madadi P, et al. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161(3):448-451. doi:10.1016/j.jpeds.2012.03.003
18. Agarwal SD, Landon BE. Patterns in outpatient benzodiazepine prescribing in the United States. JAMA Netw Open. 2019;2(1):e187399. doi:10.1001/jamanetworkopen.2018.7399
19. Hirschtritt ME, Olfson M, Kroenke K. Balancing the risks and benefits of benzodiazepines. JAMA. 2021;325(4):347-348. doi:10.1001/jama.2020.22106
20. Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics. McGraw-Hill Education; 2018.
21. Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. British J Psychiatry. 2001;179:390-396. doi:10.1192/bjp.179.5.390
22. Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2(8):833-839. doi:10.2174/1568026023393444
23. Savic
24. Smith TA. Type A gamma-aminobutyric acid (GABAA) receptor subunits and benzodiazepine binding: significance to clinical syndromes and their treatment. Br J Biomed Sci. 2001;58(2):111-121.
25. Althaus AL, Ackley MA, Belfort GM, et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology. 2020;181:108333. doi:10.1016/j.neuropharm.2020.108333
26. Jacob TC, Michels G, Silayeva L, et al. Benzodiazepine treatment induces subtype-specific changes in GABA(A) receptor trafficking and decreases synaptic inhibition. Proc Natl Acad Sci U S A. 2012;109(45):18595-18600. doi:10.1073/pnas.1204994109
27. Nicholson MW, Sweeney A, Pekle E, et al. Diazepam-induced loss of inhibitory synapses mediated by PLCδ/ Ca2+/calcineurin signalling downstream of GABAA receptors. Mol Psychiatry. 2018;23(9):1851-1867. doi:10.1038/s41380-018-0100-y
28. Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. doi:10.4088/JCP.17r12064
29. Kuang H, Johnson JA, Mulqueen JM, et al. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. 2017;34(10):888-896. doi:10.1002/da.22643
30. Chugani DC, Muzik O, Juhász C, et al. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49(5):618-626. doi:10.1002/ana.1003
31. Jochemsen R, Breimer DD. Pharmacokinetics of benzodiazepines: metabolic pathways and plasma level profiles. Curr Med Res Opin. 1984;8(Suppl 4):60-79. doi:10.1185/03007998409109545
32. Greenblatt DJ, Harmatz JS, Dorsey C, et al. Comparative single-dose kinetics and dynamics of lorazepam, alprazolam, prazepam, and placebo. Clin Pharmacol Ther. 1988;44(3)326-334. doi:10.1038/clpt.1988.158
33. Shader RI, Georgotas A, Greenblatt DJ, et al. Impaired absorption of desmethydiazepam from clorazepate by magnesium aluminum hydroxide. Clin Pharmacol Ther. 1978;24(3):308-315. doi:10.1002/cpt1978243308
34. Greenblatt DJ, Allen MD, MacLaughlin DS, et al. Diazepam absorption: effect of antacids and food. Clin Pharmacol Ther. 1978;24(5):600-609. doi:10.1002/cpt1978245600
35. Yamazaki A, Kumagai Y, Fujita T, et al. Different effects of light food on pharmacokinetics and pharmacodynamics of three benzodiazepines, quazepam, nitrazepam and diazepam. J Clin Pharm Ther. 2007;32(1):31-39. doi:10.1111/j.1365-2710.2007.00795.x
36. Stimpfl J, Mills JA, Strawn JR. Pharmacologic predictors of benzodiazepine response trajectory in anxiety disorders: a Bayesian hierarchical modeling meta-analysis. CNS Spectr. 2023;28(1):53-60. doi:10.1017/S1092852921000870
37. Griffin CE 3rd, Kaye AM, Bueno FR, et al. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214-223.
38. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2005;8(1):45-58. doi:10.2174/1381612023396654
39. Fukasawa T, Suzuki A, Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J Clin Pharm Ther. 2007;32(4):333-341. doi:10.1111/j.1365-2710.2007.00829.x
40. Kraus JW, Desmond PV, Marshall JP, et al. Effects of aging and liver disease on disposition of lorazepam. Clin Pharmacol Ther. 1978;24(4):411-419. doi:10.1002/cpt1978244411
41. Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981;6(2):89-105. doi:10.2165/00003088-198106020-00001
42. Walkenstein SS, Wiser R, Gudmundsen CH, et al. Absorption, metabolism, and excretion of oxazepam and its succinate half‐ester. J Pharm Sci. 1964;53(10):1181-1186. doi:10.1002/jps.2600531010
43. Shull HJ, Wilkinson GR, Johnson R, et al. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern Med. 1976;84(4):420-425. doi:10.7326/0003-4819-84-4-420
44. Abernethy DR, Greenblatt DJ, Ochs HR, et al. Lorazepam and oxazepam kinetics in women on low-dose oral contraceptives. Clin Pharmacol Ther. 1983;33(5):628-632. doi:10.1038/clpt.1983.85
45. Greenblatt DJ, Allen MD, Harmatz JS, et al. Diazepam disposition determinants. Clin Pharmacol Ther. 1980;27(3):301-312. doi:10.1038/clpt.1980.40
46. Ochs HR, Greenblatt DJ, Knüchel M. Kinetics of diazepam, midazolam, and lorazepam, in cigarette smokers. Chest. 1985;87(2):223-226. doi:10.1378/chest.87.2.223
47. Smith RB, Gwilt PR, Wright CE 3rd. Single- and multiple-dose pharmacokinetics of oral alprazolam in healthy smoking and nonsmoking men. Clin Pharm. 1983;2(2):139-143.
48. Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. Drugs. 2000;60(4):925-954. doi:10.2165/00003495-200060040-00006
49. Greenblatt DJ, Wright CE. Clinical pharmacokinetics of alprazolam. Therapeutic implications. Clin Pharmacokinet. 1993;24(6):453-471. doi:10.2165/00003088-199324060-00003
50. Yasui N, Kondo T, Furukori H, et al. Effects of repeated ingestion of grapefruit juice on the single and multiple oral-dose pharmacokinetics and pharmacodynamics of alprazolam. Psychopharmacology (Berl). 2000;150(2):185-190. doi:10.1007/s002130000438
51. Özdemir M, Aktan Y, Boydagˇ BS, et al. Interaction between grapefruit juice and diazepam in humans. Eur J Drug Metab Pharmacokinet. 1998;23(1):55-59. doi:10.1007/BF03189827
52. Greenblatt DJ, Harmatz JS, Zhang Q, et al. Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol. 2021;61(2):193-203. doi:10.1002/jcph.1726
53. Abernethy DR, Greenblatt DJ. Drug disposition in obese humans: an update. Clin Pharmacokinet. 1986;11(3):199-213. doi:10.2165/00003088-198611030-00002
54. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71-87. doi:10.2165/11318100-000000000-00000
55. Bauer LA. Drug Dosing in special populations: renal and hepatic disease, dialysis, heart failure, obesity, and drug interactions. In: Weitz M, Thomas, CM, eds. Applied Clinical Pharmacokinetics. 3rd ed. McGraw-Hill Education; 2014. https://accesspharmacy.mhmedical.com/book.aspx?bookid=1374
56. Kendrick JG, Carr RR, Ensom MHH. Pharmacokinetics and drug dosing in obese children. J Pediatr Pharmacol Ther. 2010;15(2):94-109. doi:10.5863/1551-6776-15.2.94
57. Brill MJE, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277-304. doi:10.2165/11599410-000000000-00000
58. Derry CL, Kroboth PD, Pittenger AL, et al. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197-205. doi:10.1097/00004714-199506000-00008
59. Abernethy DR, Greenblatt DJ, Divoll M, et al. The influence of obesity on the pharmacokinetics of oral alprazolam and triazolam. Clin Pharmacokinet. 1984;9(2):177-183. doi:10.2165/00003088-198409020-00005
60. Abernethy DR, Greenblatt DJ, Divoll M, et al. Prolonged accumulation of diazepam in obesity. J Clin Pharmacol. 1983;23(8-9):369-376. doi:10.1002/j.1552-4604.1983.tb02750.x
61. Abernethy DR, Greenblatt DJ, Divoll M, et al. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873-880.
62. Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Alprazolam pharmacokinetics, metabolism, and plasma levels: clinical implications. J Clin Psychiatry. 1993;54 Suppl:4-11.
63. Chen YT, Liu CY, Chang CM, et al. Perceptions, clinical characteristics, and other factors associated with prolonged and high daily dose of benzodiazepine use among patients with anxiety or depressive disorders. J Affect Disord. 2020;271:215-223. doi:10.1016/j.jad.2020.03.077
64. Herman JB, Brotman AW, Rosenbaum JF. Rebound anxiety in panic disorder patients treated with shorter-acting benzodiazepines. J Clin Psychiatry. 1987;48(Suppl):22-28.
65. Herman JB, Rosenbaum JF, Brotman AW. The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol. 1987;7(3):175-178.
Child murder by parents: Toward prevention
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21
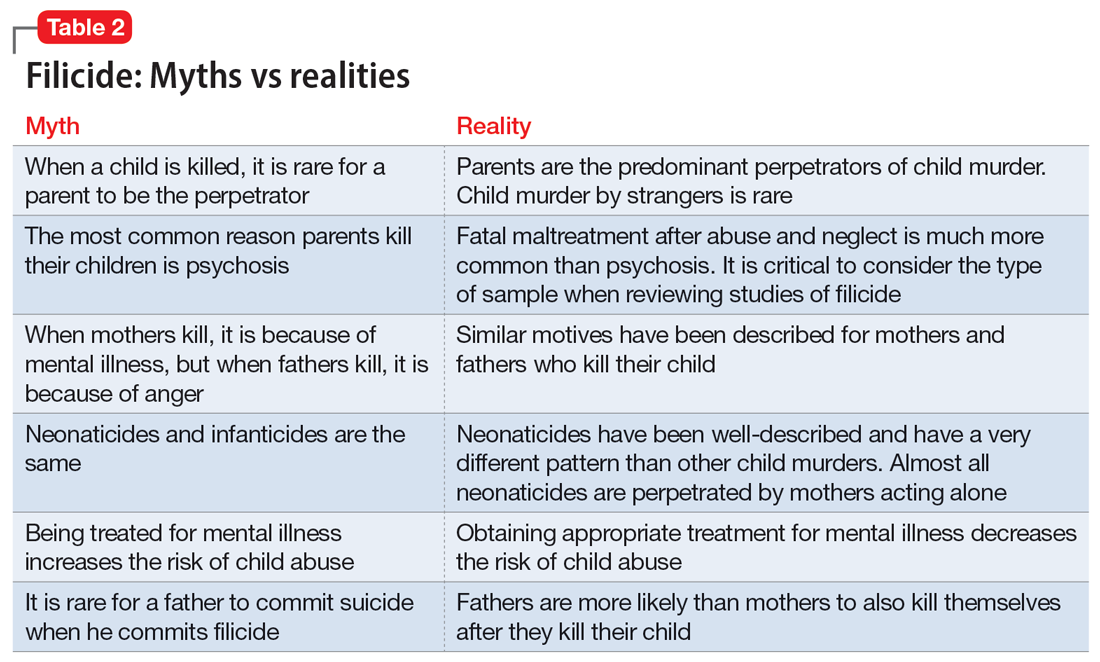
Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.
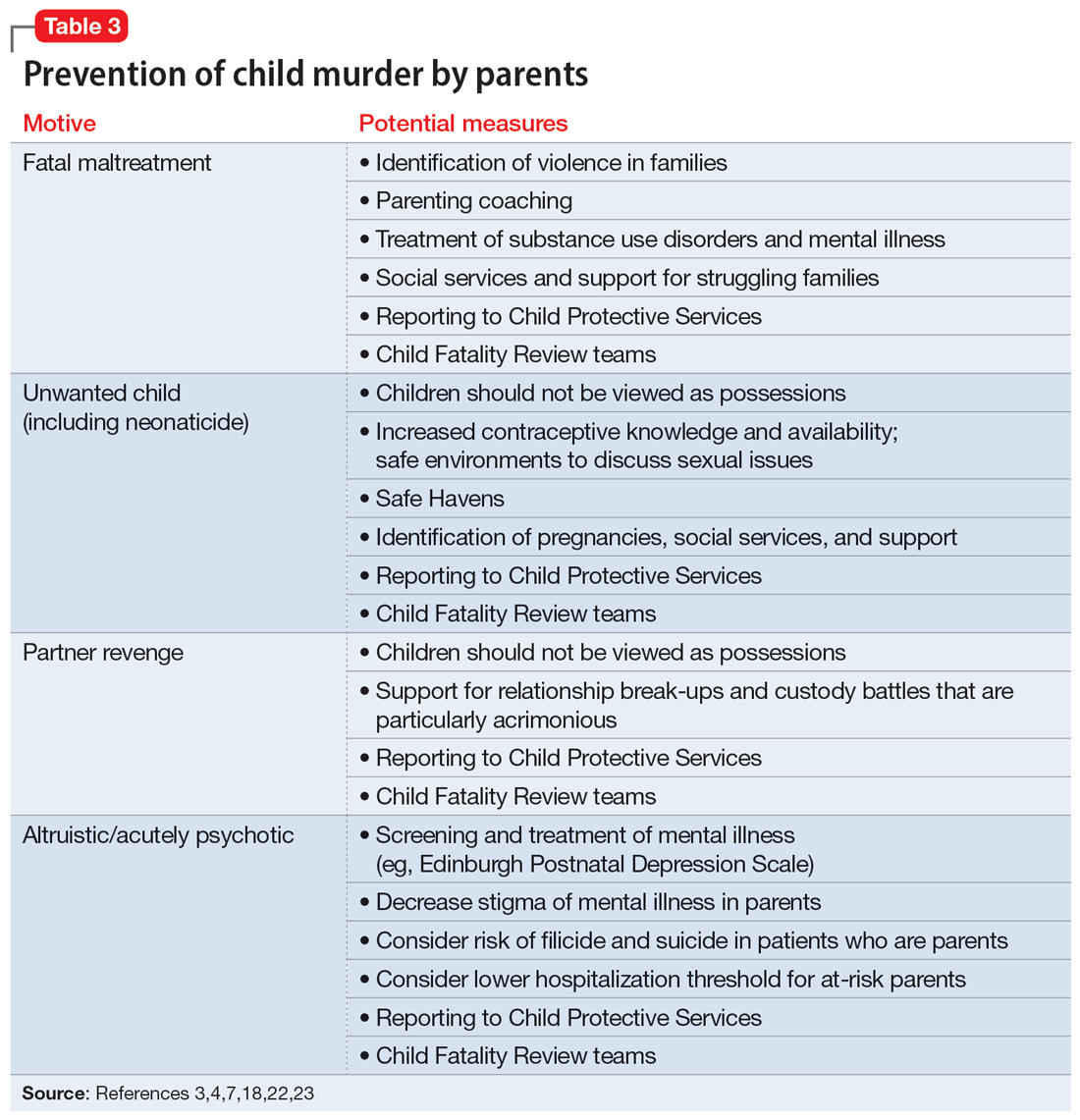
Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
Deaths of children who are killed by their parents often make the news. Cases of maternal infanticide may be particularly shocking, since women are expected to be selfless nurturers. Yet when a child is murdered, the most common perpetrator is their parent, and mothers and fathers kill at similar rates.1
As psychiatrists, we may see these cases in the news and worry about the risks of our own patients killing their children. In approximately 500 cases annually, an American parent is arrested for the homicide of their child.2 This is not even the entire story, since a large percentage of such cases end in suicide—and no arrest. This article reviews the reasons parents kill their children, and considers common characteristics of these parents, dispelling some myths, before discussing the importance of prevention efforts.
Types of child murder by parents
Child murder by parents is termed filicide. Infanticide has various meanings but often refers to the murder of a child younger than age 1. Approximately 2 dozen nations (but not the United States) have Infanticide Acts that decrease the penalty for mothers who kill their young child.3 Neonaticide refers to murder of the infant at birth or in the first day of life.4
Epidemiology and common characteristics
Approximately 15%—or 1 in 7 murders with an arrest—is a filicide.2 The younger the child, the greater the risk, but older children are killed as well.2 Internationally, fathers and mothers are found to kill at similar rates. For other types of homicide, offenders are overwhelmingly male. This makes child murder by parents the singular type of murder in which women and men perpetrate in equal numbers. Fathers are more likely than mothers to also commit suicide after they kill their children.5 The “Cinderella effect” refers to the elevated risk of a stepchild being killed compared to the risk for a biological child.6
In the general international population, mothers who commit filicide tend to have multiple stressors and limited resources. They may be socially isolated and may be victims themselves as well as potentially experiencing substance abuse.1 Some mothers view the child they killed as abnormal.
Less research has been conducted about fathers who kill. Fathers are more likely to also commit partner homicide.5,7 They are more likely to complete filicide-suicide and use firearms or other violent means.5,7-9 Fathers may have a history of violence, substance abuse, and/or mental illness.7
Neonaticide
Mothers are the most common perpetrator of neonaticide.4 It is unusual for a father to be involved in a neonaticide, or for the father and mother to perpetrate the act together. Rates of neonaticide are considered underestimates because of the number of hidden pregnancies, hidden corpses, and the difficulty that forensic pathologists may have in determining whether a baby was born alive or dead.
Continue to: Perpetrators of neonaticide...
Perpetrators of neonaticide tend to be single, relatively young women acting alone. They often live with their parents and are fearful of the repercussions of being pregnant. Pregnancies are often hidden, with no prenatal care. This includes both denial and concealment of pregnancy.4 Perpetrators of neonaticide commonly lack a premorbid serious mental illness, though after the homicide they may develop anxiety, depression, posttraumatic stress disorder (PTSD), or adjustment disorder.4 (Individuals who unwittingly find a murdered baby’s corpse may also be at risk of PTSD.)
Hidden pregnancies may be due to concealment or denial of pregnancy.10,11 Concealment of pregnancy involves a woman knowing she is pregnant, but purposely hiding from others. Concealment may occur after a period of denial of pregnancy. Denial of pregnancy has several subtypes: pervasive denial, affective denial, and psychotic denial. In cases of pervasive denial, the existence of the pregnancy and the pregnancy’s emotional significance is outside the woman’s awareness. Alternatively, in affective denial, she is intellectually aware that she is pregnant but makes little emotional or physical preparation. In the rarest form, psychotic denial, a woman with a psychotic disorder such as schizophrenia may intermittently deny her pregnancy. This may be correlated with a history of custody loss.10,11 Unlike denial of other medical conditions, in cases of denial of pregnancy, there will exist a very specific point in time (delivery) when the reality of the baby confronts the woman. Risks in cases of hidden pregnancies include those from lack of prenatal care and an assisted delivery as well as neonaticide. An FBI study12 of law enforcement files found most neonaticide offenders were single young women with no criminal or psychological history. A caveat is that in the rare cases in which a woman with psychotic illness commits neonaticide, she may have different characteristics from those generally reported.13
Motives
Fathers and mothers have a similar set of motives for killing their child (Table 113-15). Motives are critical to understand not only within forensics, but also for prevention. In performing assessments after a filicide, forensic psychiatrists must be mindful of gender bias.7,16 Resnick15 initially described 5 motives based on his 1969 review of the world literature. Our work5,17 has subsequently further explored these motives.

In child homicides from “fatal maltreatment,” the child has often been a chronic victim of abuse or neglect. National American data indicate that approximately 2 per 100,000 children are killed from child maltreatment annually. Of note in conceptualizing prevention, out of the same population of 100,000, there will be 471 referrals to Child Protective Services and 91 substantiated cases.18 However, only a minority of children who die from maltreatment had previous Child Protective Services involvement. While a child may be killed by fatal maltreatment at any age, one-half are younger than age 1, and three-quarters are younger than age 3.18 In rare cases, a parent who engages in medical child abuse (including factitious disorder imposed upon another) kills the child. Depending on the location and whether or not the death appeared to be intended, parents who kill because of fatal maltreatment might face charges of various levels of murder or manslaughter.
“Unwanted child” homicides occur when the parent has determined that they do not want to have the child, especially in comparison to another need or want. Unwanted child motive is the most common in neonaticide cases, occurring after a hidden pregnancy.4
Continue to: In "partner revenge" cases...
In “partner revenge” cases, parenting disputes, a custody battle, infidelity, or a difficult relationship breakup is often present. The parent wants to make the other parent suffer, and does so by killing their child. A parent may make statements such as “If I can’t have [the child], no one can,” and the child is used as a pawn.
In the final 2 motives—“altruistic” and “acutely psychotic”—mental illness is common. These are the populations we tend to find in samples of filicide-suicide cases where the parent has killed themselves and their child, and those found not guilty by reason of insanity.5,17 Altruistic filicide has been described as “murder out of love.” How can a parent kill their child out of love? Our research has shown several ways. First, the parent may be severely depressed and suicidal. They may be planning their own suicide, and as a parent who loves their child, they plan to take their child with them in death and not leave them alone in the “cruel world” that they themselves are departing. Or the parent may believe they are killing the child out of love to prevent or relieve the child’s suffering. The psychotic parent may believe that a terrible fate will befall their child, and they are killing them “gently.” For example, the parent may believe the child will be tortured or sex trafficked. Some parents may believe that their child has a devastating disease and think they would be better off dead. (Similar thinking of misguided altruism is seen in some cases of intimate partner homicide among older adults.19)
Alternatively, in rare cases of acutely psychotic filicide, parents with psychosis kill their child with no comprehensible motive. For example, they may be in a postictal state or may hear a command hallucination from God in the context of their psychosis.15
Myths vs realities of filicide
Common myths vs the realities of filicide are noted in Table 2. There are issues with believing these myths. For example, if we believe that most parents who kill their child have mental illness, this conflates mental illness and child homicide in our minds as well as the mind of the public. This can lead to further stigmatization of mental illness, and a lack of help-seeking behaviors because parents experiencing psychiatric symptoms may be afraid that if they report their symptoms, their child will be removed by Child Protective Services. However, treated mental illness decreases the risks of child abuse, similar to how treating mental illness decreases risks of other types of violence.20,21

Focusing on prevention
On a local level, we need to understand these tragedies to better understand prevention. To this end, across the United States, counties have Child Fatality Review teams.22 These teams are a partnership across sectors and disciplines, including professionals from health services, law enforcement, and social services—among others—working together to understand cases and consider preventive strategies and additional services needed within our communities.
Continue to: When conceptualizing prevention...
When conceptualizing prevention of child murder by parents, we can think of primary, secondary, and tertiary prevention. This means we want to encourage healthy families and healthy relationships within the family, as well as screening for risk and targeting interventions for families that have experienced difficulties, as well as for parents who have mental illness or substance use disorders.
Understanding the motive behind an individual committing filicide is also critical so that we do not conflate filicide and mental illness. Conflating these concepts leads to increased stigmatization and less help-seeking behavior.
Table 33,4,7,18,22,23 describes the importance of understanding the motives for child murder by a parent in order to conceptualize appropriate prevention. Prevention efforts for 1 type of child murder will not necessarily help prevent murders that occur due to the other motives. Regarding prevention for fatal maltreatment cases, poor parenting skills, including inappropriate expressions of discipline, anger, and frustration, are common. In some cases, substance abuse is involved or the parent was acutely mentally unwell. Reporting to Child Protective Services can be helpful, but as previously noted, it is difficult to ascertain which cases will lead to a homicide. Recommendations from Child Fatality Review teams also are valuable.

Though many parents have frustrations with their children or thoughts of child harm, the act of filicide is rare, and individual cases may be difficult to predict. Regarding prediction, some mothers who committed filicide saw their psychiatrist within days to weeks before the murders.17 A small New Zealand study found that psychotic mothers reported no plans for killing their children in advance, whereas depressed mothers had contemplated the killing for days to weeks.24
Several studies have asked mothers about thoughts of harming their child. Among mothers with colicky infants, 70% reported “explicit aggressive thoughts and fantasies” while 26% had “infanticidal thoughts” during a colic episode.25 Another study26 found that among depressed mothers of infants and toddlers, 41% revealed thoughts of harming their child. Women with postpartum depression preferred not to share infanticidal thoughts with their doctor but were more likely to disclose that they were having suicidal thoughts in order to get needed help.27 Psychiatrists need to feel comfortable asking mothers about their coping skills, their suicidal thoughts, and their filicidal thoughts.14,23,28 Screening and treatment of mental illness is critical. Postpartum psychosis is well-known to pose an elevated risk of filicide and suicide.23 Obsessive-compulsive disorder may cause a parent to ruminate over ego-dystonic child harm but should be treated and the risk conceptualized very differently than in postpartum psychosis.23,29 Screening for postpartum depression and appropriate treatment of depression during pregnancy and the postpartum period decrease risk.30
Continue to: Regarding prevention of neonaticide...
Regarding prevention of neonaticide, Safe Haven laws, baby boxes, anonymous birth options, and increased contraceptive information and availability can help decrease the risk of this well-defined type of homicide.4 Safe Haven laws originated from Child Fatality Review teams.24 Though each state has its own variation, in general, parents can drop off an unharmed unwanted infant into Safe Havens in their state, which may include hospitals, police stations, or fire stations. In general, the mother remains anonymous and has immunity from prosecution for (safe) abandonment. There are drawbacks, such as lack of information regarding adoption and paternal rights. Safe Haven laws do not prevent all deaths and all unsafe abandonments. Baby boxes and baby hatches are used in various nations, including in Europe, and in some places have been used for centuries. In anonymous birth options, such as in France, a mother is not identified but is able to give birth at a hospital. This can decrease the risk from unattended delivery, but many women with denial of pregnancy report that they did not realize when they were about to give birth.4
Bottom Line
Knowledge about the intersection of mental illness and filicide can help in prevention. Parents who experience mental health concerns should be encouraged to obtain needed treatment, which aids prevention. However, many other factors elevate the risk of child murder by parents.
Related Resources
- National Center for Fatality Review and Prevention. https://ncfrp.org/
- Child Welfare Information Gateway. https://www.childwelfare.gov/topics/preventing/overview/federal-agencies/
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
1. Friedman SH, Horwitz SM, Resnick PJ. Child murder by mothers: a critical analysis of the current state of knowledge and a research agenda. Am J Psych. 2005;162(9):1578-1587.
2. Mariano TY, Chan HC, Myers WC. Toward a more holistic understanding of filicide: a multidisciplinary analysis of 32 years of US arrest data [published corrections appears in Forensic Sci Int. 2014;245:92-94]. Forensic Sci Int. 2014;236:46-53.
3. Hatters Friedman S, Resnick PJ. Child murder by mothers: patterns and prevention. World Psychiatry. 2007;6(3):137-141.
4. Friedman SH, Resnick PJ. Neonaticide: phenomenology and considerations for prevention. Int J Law Psychiatry. 2009;32(1):43-47.
5. Hatters Friedman S, Hrouda DR, Holden CE, et al. Filicide-suicide: common factors in parents who kill their children and themselves. J Am Acad Psychiatry Law. 2005;33(4):496-504.
6. Daly M, Wilson M. Is the “Cinderella effect” controversial? A case study of evolution-minded research and critiques thereof. In: Crawford C, Krebs D, eds. Foundations of Evolutionary Psychology. Taylor & Francis Group/Lawrence Erlbaum Associates; 2008:383-400.
7. Friedman SH. Fathers and filicide: Mental illness and outcomes. In: Wong G, Parnham G, eds. Infanticide and Filicide: Foundations in Maternal Mental Health Forensics. 1st ed. American Psychiatric Association Publishing; 2020:85-107.
8. West SG, Friedman SH, Resnick PJ. Fathers who kill their children: an analysis of the literature. J Forensic Sci. 2009;54(2):463-468.
9. Putkonen H, Amon S, Eronen M, et al. Gender differences in filicide offense characteristics--a comprehensive register-based study of child murder in two European countries. Child Abuse Neglect. 2011;35(5):319-328.
10. Miller LJ. Denial of pregnancy. In: Spinelli MG, ed. Infanticide: Psychosocial and Legal Perspectives on Mothers Who Kill. American Psychiatric Association Publishing; 2003:81-104.
11. Friedman SH, Heneghan A, Rosenthal M. Characteristics of women who deny or conceal pregnancy. Psychosomatics. 2007;48(2):117-122.
12. Beyer K, Mack SM, Shelton JL. Investigative analysis of neonaticide: an exploratory study. Criminal Justice and Behavior. 2008;35(4):522-535.
13. Putkonen H, Weizmann-Henelius G, Collander J, et al. Neonaticides may be more preventable and heterogeneous than previously thought--neonaticides in Finland 1980-2000. Arch Womens Ment Health. 2007;10(1):15-23.
14. Friedman SH, Resnick PJ. Child murder and mental illness in parents: implications for psychiatrists. J Clin Psychiatry. 2011;72(5):587-588.
15. Resnick PJ. Child murder by parents: a psychiatric review of filicide. Am J Psychiatry. 1969;126(3):325-334.
16. Friedman SH. Searching for the whole truth: considering culture and gender in forensic psychiatric practice. J Am Acad Psychiatry Law. 2023;51(1):23-34.
17. Friedman SH, Hrouda DR, Holden CE, et al. Child murder committed by severely mentally ill mothers: an examination of mothers found not guilty by reason of insanity. J Forensic Sci. 2005;50(6):1466-1471.
18. Ash P. Fatal maltreatment and child abuse turned to murder. In: Friedman SH, ed. Family Murder: Pathologies of Love and Hate. Group for the Advancement Psychiatry; 2018.
19. Friedman SH, Appel JM. Murder in the family: intimate partner homicide in the elderly. Psychiatric News. 2018. Accessed April 8, 2023. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2018.12a21
20. Friedman SH, McEwan MV. Treated mental illness and the risk of child abuse perpetration. Psychiatr Serv. 2018;69(2):211-216.
21. McEwan M, Friedman SH. Violence by parents against their children: reporting of maltreatment suspicions, child protection, and risk in mental illness. Psychiatr Clin North Am. 2016;39(4):691-700.
22. Hatters Friedman S, Beaman JW, Friedman JB. Fatality review and the role of the forensic psychiatrist. J Am Acad Psychiatry Law. 2021;49(3):396-405.
23. Friedman SH, Prakash C, Nagle-Yang S. Postpartum psychosis: protecting mother and infant. Current Psychiatry. 2019;18(4):12-21.
24. Stanton J, Simpson AI, Wouldes T. A qualitative study of filicide by mentally ill mothers. Child Abuse Negl. 2000;24(11):1451-1460.
25. Levitzky S, Cooper R. Infant colic syndrome—maternal fantasies of aggression and infanticide. Clin Pediatr (Phila). 2000;39(7):395-400.
26. Jennings KD, Ross S, Popper S, et al. Thoughts of harming infants in depressed and nondepressed mothers. J Affect Disord. 1999;54(1-2):21-28.
27. Barr JA, Beck CT. Infanticide secrets: qualitative study on postpartum depression. Can Fam Physician. 2008;54(12):1716-1717.e5.
28. Friedman SH, Sorrentino RM, Stankowski JE, et al. Psychiatrists’ knowledge about maternal filicidal thoughts. Compr Psychiatry. 2008;49(1):106-110.
29. Booth BD, Friedman SH, Curry S, et al. Obsessions of child murder: underrecognized manifestations of obsessive-compulsive disorder. J Am Acad Psychiatry Law. 2014;42(1):66-74.
30. Friedman SH, Hall RCW. Avoiding malpractice while treating depression in pregnant women. Current Psychiatry. 2021;20(8):30-36.
Interventional psychiatry (Part 1)
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.
Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
Advances in the understanding of neurobiological and neuropsychiatric pathophysiology have opened new avenues of treatment for psychiatric patients. Historically, with a few exceptions, most psychiatric medications have been administered orally. However, many of the newer treatments require some form of specialized administration because they cannot be taken orally due to their chemical property (such as aducanumab); because there is the need to produce stable blood levels of the medication (such as brexanolone); because oral administration greatly diminished efficacy (such as oral vs IV magnesium or scopolamine), or because the treatment is focused on specific brain structures. This need for specialized administration has created a subspecialty called interventional psychiatry.
Part 1 of this 2-part article provides an overview of 1 type of interventional psychiatry: parenterally administered medications, including those administered via IV. We also describe 3 other interventional approaches to treatment: stellate ganglion blocks, glabellar botulinum toxin (BT) injections, and trigger point injections. In Part 2 we will review interventional approaches that involve neuromodulation.
Parenteral medications in psychiatry
In general, IV and IM medications can be more potent that oral medications due to their overall faster onset of action and higher blood concentrations. These more invasive forms of administration can have significant limitations, such as a risk of infection at the injection site, the need to be administered in a medical setting, additional time, and patient discomfort.

Table 1 lists short-acting injectable medications used in psychiatry, and Table 2 lists long-acting injectable medications. Parenteral administration of antipsychotics is performed to alleviate acute agitation or for chronic symptom control. These medications generally are not considered interventional treatments, but could be classified as such due to their invasive nature.1 Furthermore, inhalable loxapine—which is indicated for managing acute agitation—requires a Risk Evaluation and Mitigation Strategy program consisting of 2 hours observation and monitoring of respiratory status.2,3 Other indications for parenteral treatments include IM naltrexone extended release4 and subcutaneous injections of buprenorphine extended release5 and risperidone.6
IV administration
Most IV treatments described in this article are not FDA-approved for psychiatric treatment. Despite this, many interventional psychiatric treatments are part of clinical practice. IV infusion of ketamine is the most widely known and most researched of these. Table 3 lists other IV treatments that could be used as psychiatric treatment.
Ketamine
Since the early 1960s, ketamine has been used as a surgical anesthetic for animals. In the United States, it was approved for human surgical anesthesia in 1970. It was widely used during the Vietnam War due to its lack of inhibition of respiratory drive; medical staff first noticed an improvement in depressive symptoms and the resolution of suicidal ideation in patients who received ketamine. This led to further research on ketamine, particularly to determine its application in treatment-resistant depression (TRD) and other conditions.7 IV ketamine administration is most widely researched, but IM injections, intranasal sprays, and lozenges are also available. The dissociative properties of ketamine have led to its recreational use.8
Hypotheses for the mechanism of action of ketamine as an antidepressant include direct synaptic or extrasynaptic (GluN2B-selective), N-methyl-
Continue to: Ketamine is a schedule...
Ketamine is a schedule III medication with addictive properties. Delirium, panic attacks, hallucinations, nightmares, dysphoria, and paranoia may occur during and after use.13 Premedication with benzodiazepines, most notably lorazepam, is occasionally used to minimize ketamine’s adverse effects, but this generally is not recommended because doing so reduces ketamine’s antidepressant effects.14 Driving and operating heavy machinery is contraindicated after IV infusion. The usual protocol involves an IV infusion of ketamine 0.4 mg/kg to 1 mg/kg dosing over 1 hour. Doses between 0.4 mg/kg and 0.6 mg/kg are most common. Ketamine has a therapeutic window; doses >0.5 mg/kg are progressively less effective.15 Unlike the recommendation after esketamine administration, after receiving ketamine, patients remain in the care of their treatment team for <2 hours.
Esketamine, the S enantiomer of ketamine, was FDA-approved for TRD as an intranasal formulation. Esketamine is more commonly used than IV ketamine because it is FDA-approved and typically covered by insurance, but it may not be as effective.16 An economic analysis by Brendle et al17 suggested insurance companies would lower costs if they covered ketamine infusions vs intranasal esketamine.
Aducanumab and lecanemab
The most recent FDA-approved interventional agents are aducanumab and lecanemab, which are indicated for treating Alzheimer disease.18,19 Both are human monoclonal antibodies that bind selectively and with high affinity to amyloid beta plaque aggregates and promote their removal by Fc receptor–mediated phagocytosis.20
FDA approval of aducanumab and lecanemab was controversial. Initially, aducanumab’s safety monitoring board performed a futility analysis that suggested aducanumab was unlikely to separate from placebo, and the research was stopped.21 The manufacturer petitioned the FDA to consider the medication for accelerated approval on the basis of biomarker data showing that amyloid beta plaque aggregates become smaller. Current FDA approval is temporary to allow patients access to this potentially beneficial agent, but the manufacturer must supply clinical evidence that the reduction of amyloid beta plaques is associated with desirable changes in the course of Alzheimer disease, or risk losing the approval.
Lecanemab is also a human monoclonal antibody intended to remove amyloid beta plaques that was FDA-approved under the accelerated approval pathway.22 Unlike aducanumab, lecanemab demonstrated a statistically significant (although clinically imperceptible) reduction in the rate of cognitive decline; it did not show cognitive improvement.23 Lecanemab also significantly reduced amyloid beta plaques.23
Continue to: Aducanumab and lecanemab are generally...
Aducanumab and lecanemab are generally not covered by insurance and typically cost >$26,000 annually. Both are administered by IV infusion once a month. More monoclonal antibody medications for treating early Alzheimer disease are in the late stages of development, most notably donanebab.24 Observations during clinical trials found that in the later stages of Alzheimer disease, forceful removal of plaques by the autoimmune process damages neurons, while in less dense deposits of early dementia such removal is not harmful to the cells and prevents amyloid buildup.
Brexanolone is an aqueous formulation of allopregnanolone, a major metabolite of progesterone and a positive allosteric modulator of GABA-A receptors.25 Its levels are maximal at the end of the third trimester of pregnancy and fall rapidly following delivery. Research showed a 3-day infusion was rapidly and significantly effective for treating postpartum depression26 and brexanolone received FDA approval for this indication in March 2019.27 However, various administrative, economic, insurance, and other hurdles make it difficult for patients to access this treatment. Despite its rapid onset of action (usually 48 hours), brexanolone takes an average of 15 days to go through the prior authorization process.28 In addition to the need for prior authorization, the main impediment to the use of brexanolone is the 3-day infusion schedule, which greatly magnifies the cost but is partially circumvented by the availability of dedicated outpatient centers.
Magnesium
Magnesium is on the World Health Organization’s Model List of Essential Medicines.29 There has been extensive research on the use of magnesium sulfate for psychiatric indications, especially for depression.30 Magnesium functions similarly to calcium channel blockers by competitively blocking intracellular calcium channels, decreasing calcium availability, and inhibiting smooth muscle contractility.31 It also competes with calcium at the motor end plate, reducing excitation by inhibiting the release of acetylcholine.32 This property is used for high-dose IV magnesium treatment of impending preterm labor in obstetrics. Magnesium sulfate is the drug of choice in treating eclamptic seizures and preventing seizures in severe preeclampsia or gestational hypertension with severe features.33 It is also used to treat torsade de pointes, severe asthma exacerbations, constipation, and barium poisoning.34 Beneficial use in asthma treatment35 and the treatment of migraine36 have also been reported.
IV magnesium in myocardial infarction may be harmful,37 though outside of acute cardiac events, magnesium is found to be safe.38
Oral magnesium sulfate is a common over-the-counter anxiety remedy. As a general cell stabilizer (mediated by the reduction of intracellular calcium), magnesium is potentially beneficial outside of its muscle-relaxing properties, although muscle relaxing can benefit anxious patients. It is used to treat anxiety,39 alcohol withdrawal,40 and fear.41 Low magnesium blood levels are found in patients with depression, schizophrenia,42 and attention-deficit/hyperactivity disorder.43 However, it is important to note that the therapeutic effect of magnesium when treating anxiety and headache is independent of preinfusion magnesium blood levels.43
Continue to: The efficacy of oral magnesium...
The efficacy of oral magnesium is not robust. However, IV administration has a pronounced beneficial effect as an abortive and preventative treatment in many patients with anxiety.44
IV administration of magnesium can produce adverse effects, including flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, and cardiac and CNS depression. These complications are very rare and dose-dependent.45 Magnesium is excreted by the kidneys, and dosing must be decreased in patients with kidney failure. The most common adverse effect is local burning along the vein upon infusion; small doses of IV lidocaine can remedy this. Hot flashes are also common.45
Various dosing strategies are available. In patients with anxiety, application dosing is based on the recommended preeclampsia IV dose of 4 g diluted in 250 mL of 5% dextrose. Much higher doses may be used in obstetrics. Unlike in obstetrics, for psychiatric indications, magnesium is administered over 60 to 90 minutes. Heart monitoring is recommended.
Scopolamine
Scopolamine is primarily used to relieve nausea, vomiting, and dizziness associated with motion sickness and recovery from anesthesia. It is also used in ophthalmology and in patients with excessive sweating. In off-label and experimental applications, scopolamine has been used in patients with TRD.46
Scopolamine is an anticholinergic medication. It is a nonselective antagonist at muscarinic receptors.47 Tricyclic antidepressants (TCAs) possess strong anticholinergic function. Newer generations of antidepressants were designed specifically not to have this function because it was believed to be an unwanted and potentially dangerous adverse effect. However, data suggest that anticholinergic action is important in decreasing depressive symptoms. Several hypotheses of anticholinergic effects on depression have been published since the 1970s. They include the cholinergic-adrenergic hypothesis,48 acetylcholine predominance relative to adrenergic action hypothesis,49 and insecticide poisoning observations.50 Centrally acting physostigmine (but not peripherally acting neostigmine) was reported to control mania.48,51 A genetic connection between the M2acetylcholine receptor in patients with major depressive disorder (MDD) and alcohol use disorder is also suggestive.52
Continue to: Multiple animal studies show...
Multiple animal studies show direct improvement in mobility and a decrease in despair upon introducing anticholinergic substances.53-55 The cholinergic theory of depression has been studied in several controlled clinical human studies.56,57 Use of a short-acting anticholinergic glycopyrrolate during electroconvulsive therapy (ECT) may contribute to the procedure’s efficacy.
Human research shows scopolamine has a higher efficacy as an antidepressant and anti-anxiety medication in women than in men,58 possibly because estrogen increases the activity of choline acetyltransferase and release of acetylcholine.59,60 M2receptors mediate estrogen influence on the NMDAR, which may explain the anticholinergic effects of depression treatments in women.61
Another proposed mechanism of action of scopolamine is a potent inhibition of the NMDAR.62 Rapid treatments of depression may be based on this mechanism. Examples of such treatments include IV ketamine and sleep deprivation.63 IV scopolamine shows potency in treating MDD and bipolar depression. This treatment should be reserved for patients who do not respond to or are not candidates for other usual treatment modalities of MDD and for the most severe cases. Scopolamine is 30 times more potent than amitriptyline in anticholinergic effect and reportedly produces sustained improvement in MDD.64
Scopolamine has no black-box warnings. It has not been studied in pregnant women and is not recommended for use during pregnancy. Due to possible negative cardiovascular effects, a normal electrocardiogram is required before the start of treatment. Exercise caution in patients with glaucoma, benign prostatic enlargement, gastroparesis, unstable cardiovascular status, or severe renal impairment.
Treatment with scopolamine is not indicated for patients with myasthenia gravis, psychosis, or seizures. Patients must be off potassium for 3 days before beginning scopolamine treatment. Patients should consult with their cardiologist before having a scopolamine infusion. Adverse reactions may include psychosis, tachycardia, seizures, paralytic ileus, and glaucoma exacerbation. The most common adverse effects of scopolamine infusion treatment include drowsiness, dry mouth, blurred vision, lightheadedness, and dizziness. Due to possible drowsiness, operating motor vehicles or heavy machinery must be avoided on the day of treatment.65 Overall, the adverse effects of scopolamine are preventable and manageable, and its antidepressant efficacy is noteworthy.66
Continue to: Treatment typically consists of 3 consecutive infusions...
Treatment typically consists of 3 consecutive infusions of 4 mcg/kg separated by 3 to 5 days.56 It is possible to have a longer treatment course if the patient experiences only partial improvement. Repeated courses or maintenance treatment (similar to ECT maintenance) are utilized in some patients if indicated. Cardiac monitoring is mandatory.
Clomipramine
Clomipramine, a TCA, acts as a preferential inhibitor of 5-hydroxytryptamine uptake and has proven effective in managing depression, TRD, and obsessive-compulsive disorder (OCD).67 Although this medication has reported treatment benefits for patients with phobia, panic disorder,15 chronic pain,68 Tourette syndrome,69 premature ejaculation, anorexia nervosa,70 cataplexy,49 and enuresis,71 it is FDA-approved only for the treatment of OCD.72 Clomipramine may also be beneficial for pain and headache, possibly because of its anti-inflammatory action.73 The anticholinergic effects of clomipramine may add to its efficacy in depression.
The pathophysiology of MDD is connected to hyperactivity of the HPA axis and elevated cortisol levels. Higher clomipramine plasma levels show a linear correlation with lower cortisol secretion and levels, possibly aiding in the treatment of MDD and anxiety.74 The higher the blood level, the more pronounced clomipramine’s therapeutic effect across multiple domains.75
IV infusion of clomipramine produces the highest concentration in the shortest time, but overall, research does not necessarily support increased efficacy of IV over oral administration. There is evidence suggesting that subgroups of patients with severe, treatment-refractory OCD may benefit from IV agents and research suggests a faster onset of action.76 Faster onset of symptom relief is the basis for IV clomipramine use. In patients with OCD, it can take several months for oral medications to produce therapeutic benefits; not all patients can tolerate this. In such scenarios, IV administration may be considered, though it is not appropriate for routine use until more research is available. Patients with treatment-resistant OCD who have exhausted other means of symptom relief may also be candidates for IV treatment.
The adverse effects of IV clomipramine are no different from oral use, though they may be more pronounced. A pretreatment cardiac exam is desirable because clomipramine, like other TCAs, may be cardiotoxic. The anticholinergic adverse effects of TCAs are well known to clinicians77 and partially explained in the scopolamine section of this article. It is not advisable to combine clomipramine with other TCAs or serotonin reuptake inhibitors. Clomipramine also should not be combined with monoamine oxidase inhibitors, though such a combination was reported in medical literature.78 Combination with antiarrhythmics such as lidocaine or opioids such as fentanyl or and tramadol is highly discouraged (fentanyl and tramadol also have serotonergic effects).79
Continue to: Clomipramine for IV use is not commercially available...
Clomipramine for IV use is not commercially available and must be sterilely compounded. The usual course of treatment is a series of 3 infusions: 150 mg on Day 1, 200 mg on Day 2 or Day 3, and 250 mg on Day 3, Day 4, or Day 5, depending on tolerability. A protocol with a 50 mg/d starting dose and titration up to a maximum dose of 225 mg/d over 5 to 7 days has been suggested for inpatient settings.67 Titration to 250 mg is more common.80
A longer series may be performed, but this increases the likelihood of adverse effects. Booster and maintenance treatments are also completed when required. Cardiac monitoring is mandatory.
Vortioxetine and citalopram
IV treatment of depression with
Injections and blocks
Three interventional approaches to treatment that possess psychotherapeutic potential include stellate ganglion blocks (SGBs), glabellar BT injections, and trigger point injections (TPIs). None of these are FDA-approved for psychiatric treatment.
Stellate ganglion blocks
The sympathetic nervous system is involved in autonomic hyperarousal and is at the core of posttraumatic symptomatology.83 Insomnia, anxiety, irritability, hypervigilance, and other excitatory CNS events are connected to the sympathetic nervous system and amygdala activation is commonly observed in those exposed to extreme stress or traumatic events.84
Continue to: SGBs were first performed 100 years ago...
SGBs were first performed 100 years ago and reported to have beneficial psychiatric effects at the end of the 1940s. In 1998 in Finland, improvement of posttraumatic stress disorder (PTSD) symptoms was observed accidentally via thoracic level spine blocks.85 In 2006, cervical level sympathetic blocks were shown to be effective for PTSD symptom control.86 By the end of 2010, Veterans Administration hospitals adopted SGBs to treat veterans with PTSD.87,88 The first multisite, randomized clinical trial of
Since the stellate ganglion is connected to the amygdala, SGB has also been assessed for treating anxiety and depression.89,90 Outside of PTSD, SGBs are used to treat complex regional pain syndrome,91 phantom limb pain, trigeminal neuralgia,92 intractable angina,93 and postherpetic neuralgia in the head, neck, upper chest, or arms.94 The procedure consists of an injection of a local anesthetic through a 25-gauge needle into the stellate sympathetic ganglion at the C6 or C7 vertebral levels. An injection into C6 is considered safer because of specific cervical spine anatomy. Ideally, fluoroscopic guidance or ultrasound is used to guide needle insertion.95
A severe drop in blood pressure may be associated with SGBs and is mitigated by IV hydration. Other adverse effects include red eyes, drooping of the eyelids, nasal congestion, hoarseness, difficulty swallowing, a sensation of a “lump” in the throat, and a sensation of warmth or tingling in the arm or hand. Bilateral SGB is contraindicated due to the danger of respiratory arrest.96
Glabellar BT injections
OnabotulinumtoxinA (BT) injection was first approved for therapeutic use in 1989 for eye muscle disorders such as strabismus97 and blepharospasm.98 It was later approved for several other indications, including cosmetic use, hyperhidrosis, migraine prevention, neurogenic bladder disorder, overactive bladder, urinary incontinence, and spasticity.99-104 BT is used off-label for achalasia and sialorrhea.105,106 Its mechanism of action is primarily attributed to muscle paralysis by blocking presynaptic acetylcholine release into neuromuscular junctions.107
Facial BT injections are usually administered for cosmetic purposes, but smoothing forehead wrinkles and frown lines (the glabellar region of the face) both have antidepressant effects.108 BT injections into the glabellar region also demonstrate antidepressant effects, particularly in patients with comorbid migraines and MDD.109 Early case observations supported the independent benefit of the toxin on MDD when the toxin was injected into the glabellar region.110,111 The most frequent protocol involves injections in the procerus and corrugator muscles.
Continue to: The facial feedback/emotional proprioception hypothesis...
The facial feedback/emotional proprioception hypothesis has dominated thinking about the mood-improving effects of BT. The theory is that blocking muscular expression of sadness (especially in the face) interrupts the experience of sadness; therefore, depression subsides.112,113 However, BT injections in the muscles involved in the smile and an expression of positive emotions (lateral part of the musculus orbicularis oculi) have been associated with increased MDD scores.114 Thus, the mechanism clearly involves more than the cosmetic effect, since facial muscle injections in rats also have antidepressant effects.115
The use of progressive muscle relaxation is well-established in psychiatric treatment. The investigated conditions of increased muscle tone, especially torticollis and blepharospasm, are associated with MDD, and it may be speculated that proprioceptive feedback from the affected muscles may be causally involved in this association.116-118 Activity of the corrugator muscle has been positively associated with increased amygdala activity.119 This suggests a potential similar mechanism to that hypothesized for SGB.
Alternatively, BT is commonly used to treat chronic conditions that may contribute to depression; its success in relieving the underlying problem may indirectly relieve MDD. Thus, in a postmarketing safety evaluation of BT, MDD was demonstrated 40% to 88% less often by patients treated with BT for 6 of the 8 conditions and injection sites, such as in spasms and spasticity of arms and legs, torticollis and neck pain, and axilla and palm injections for hyperhidrosis. In a parotid and submandibular glands BT injection subcohort, no patients experienced depressive symptoms.120
Medicinal BT is generally considered safe. The most common adverse effects are hypersensitivity, injection site reactions, and other adverse effects specific to the injection site.121 Additionally, the cosmetic effects are transient, given the nature of the medication.
Trigger point injections
TPIs in the neck and shoulders are frequently used to treat tension headaches and various referred pain locations in the face and arms. Tension and depression frequently overlap in clinical practice.122 Relieving muscle tension (with resulting trigger points) improves muscle function and mood.
Continue to: The higher the number of active...
The higher the number of active trigger points (TPs), the greater the physical burden of headache and the higher the anxiety level. Gender differences in TP prevalence and TPI efficacy have been described in the literature. For example, the number of active TPs seems directly associated with anxiety levels in women but not in men.123 Although TPs appear to be more closely associated with anxiety than depression,124 depression associated with muscle tension does improve with TPIs. European studies have demonstrated a decrease in scores on the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale following TPI treatment.125 The effect may be indirect, as when a patient’s pain is relieved, sleep and other psychiatric symptoms improve.126
A randomized controlled trial by Castro Sánchez et al127 demonstrated that dry needling therapy in patients with fibromyalgia syndrome (FMS) showed improvements in pain pressure thresholds, body pain, vitality, and social function, as well as total FMS symptoms, quality of sleep, anxiety, hospital anxiety and depression, general pain intensity, and fatigue.
Myofascial pain syndrome, catastrophizing, and muscle tension are common in patients with depression, anxiety, and somatization. Local TPI therapy aimed at inactivating pain generators is supported by moderate quality evidence. All manner of therapies have been described, including injection of saline, corticosteroids, local anesthetic agents, and dry needling. BT injections in chronic TPs are also practiced, though no specific injection therapy has been reliably shown to be more advantageous than another. The benefits of TPIs may be derived from the needle itself rather than from any specific substance injected. Stimulation of a local twitch response with direct needling of the TP appears of importance. There is no established consensus regarding the number of injection points, frequency of administration, and volume or type of injectate.128
Adverse effects of TPIs relate to the nature of the invasive therapy, with the risk of tissue damage and bleeding. Pneumothorax risk is present with needle insertion at the neck and thorax.129 Patients with diabetes may experience variations in blood sugar control if steroids are used.
Bottom Line
Interventional treatment modalities that may have a role in psychiatric treatment include IV administration of ketamine, aducanumab, lecanemab, brexanolone, magnesium, scopolamine, and clomipramine. Other interventional approaches include stellate ganglion blocks, glabellar botulinum toxin injections, and trigger point injections.
Related Resources
- Dokucu ME, Janicak PG. Nontraditional therapies for treatment-resistant depression. Current Psychiatry. 2021; 20(9):38-43,49. doi:10.12788/cp.0166
- Kim J, Khoury R, Grossberg GT. Botulinum toxin: emerging psychiatric indications. Current Psychiatry. 2018;17(12):8-18.
Drug Brand Names
Aducanumab • Aduhelm
Aripiprazole • Abilify
Aripiprazole lauroxil • Aristada
Brexanolone • Zulresso
Buprenorphine • Sublocade
Citalopram • Celexa
Clomipramine • Anafranil
Diazepam • Valium
Droperidol • Inapsine
Esketamine • Spravato
Fentanyl • Actiq
Fluphenazine decanoate • Modecate
Fluphenazine hydrochloride • Prolixin
Haloperidol decanoate • Haldol decanoate
Haloperidol lactate • Haldol
Ketamine • Ketalar
Lecanemab • Leqembi
Lidocaine • Xylocaine
Lorazepam • Ativan
Loxapine inhaled • Adasuve
Naltrexone • Vivitrol
Magnesium sulfate • Sulfamag
Midazolam • Versed
Olanzapine • Zyprexa
OnabotulinumtoxinA injection • Botox
Paliperidone • Invega Hafyera, Invega Sustenna, Invega Trinza
Rapamycin • Rapamune, Sirolimus
Risperidone • Perseris
Risperidone microspheres • Risperdal Consta, Rykindo
Scopolamine • Hyoscine
Tramadol • Conzip
Vortioxetine • Trintellix
Ziprasidone • Geodon
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
1. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574.
2. Allen MH, Feifel D, Lesem MD, et al. Efficacy and safety of loxapine for inhalation in the treatment of agitation in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2011;72(10):1313-1321.
3. Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31-40.
4. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
5. Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2019;393(10173):778-790.
6. Andorn A, Graham J, Csernansky J, et al. Monthly extended-release risperidone (RBP-7000) in the treatment of schizophrenia: results from the phase 3 program. J Clin Psychopharmacol. 2019;39(5):428-433.
7. Dundee TW. Twenty-five years of ketamine. A report of an international meeting. Anaesthesia. 1990;45(2):159. doi:10.1111/j.1365-2044.1990.tb14287.x
8. White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136. doi:10.1097/00000542-198202000-00007
9. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
10. Molero P, Ramos-Quiroga JA, Martin-Santos R, et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32(5):411-420. doi:10.1007/s40263-018-0519-3
11. Williams NR, Heifets BD, Blasey C, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175(12):1205-1215.
12. Witkin JM, Martin AE, Golani LK, et al. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47-96.
13. Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26(9):985-1028. doi:10.1016/j.ajem.2007.12.005
14. Frye MA, Blier P, Tye SJ. Concomitant benzodiazepine use attenuates ketamine response: implications for large scale study design and clinical development. J Clin Psychopharmacol. 2015;35(3):334-336.
15. Fava M, Freeman MP, Flynn M, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25(7):1592-1603.
16. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555. Erratum in: J Affect Disord. 2021;281:1001.
17. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affect Disord. 2022;319:388-396.
18. Dhillon S. Aducanumab: first approval. Drugs. 2021;81(12):1437-1443. Erratum in: Drugs. 2021;81(14):1701.
19. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
20. Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50-56. Update in: Nature. 2017;546(7659):564.
21. Fillit H, Green A. Aducanumab and the FDA – where are we now? Nat Rev Neurol. 2021;17(3):129-130.
22. Reardon S. FDA approves Alzheimer’s drug lecanemab amid safety concerns. Nature. 2023;613(7943):227-228. doi:10.1038/d41586-023-00030-3
23. McDade E, Cummings JL, Dhadda S, et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. doi:10.1186/s13195-022-01124-2
24. Mintun MA, Lo AC, Evans CD, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691-1704.
25. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85(7):2429-2433.
26. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058-1070.
27. Powell JG, Garland S, Preston K, et al. Brexanolone (Zulresso): finally, an FDA-approved treatment for postpartum depression. Ann Pharmacother. 2020;54(2):157-163.
28. Patterson R, Krohn H, Richardson E, et al. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14-22.
29. World Health Organization. WHO model list of essential medicines - 22nd list (2021). World Health Organization. September 30, 2021. Accessed April 7, 2023. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
30. Eby GA, Eby KL, Mruk H. Magnesium and major depression. In: Vink R, Nechifor M, eds. Magnesium in the Central Nervous System. University of Adelaide Press; 2011.
31. Plant TM, Zeleznik AJ. Knobil and Neill’s Physiology of Reproduction. 4th ed. Elsevier Inc.; 2015:2503-2550.
32. Sidebotham D, Le Grice IJ. Physiology and pathophysiology. In: Sidebotham D, McKee A, Gillham M, Levy J. Cardiothoracic Critical Care. Elsevier, Inc.; 2007:3-27.
33. Duley L, Gülmezoglu AM, Henderson-Smart DJ, et al. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;2010(11):CD000025.
34. Emergency supply of medicines. In: British National Formulary. British Medical Association, Royal Pharmaceutical Society; 2015:6. Accessed April 7, 2023. https://www.academia.edu/35076015/british_national_formulary_2015_pdf
35. Kwofie K, Wolfson AB. Intravenous magnesium sulfate for acute asthma exacerbation in children and adults. Am Fam Physician. 2021;103(4):245-246.
36. Patniyot IR, Gelfand AA. Acute treatment therapies for pediatric migraine: a qualitative systematic review. Headache. 2016;56(1):49-70.
37. Wang X, Du X, Yang H, et al. Use of intravenous magnesium sulfate among patients with acute myocardial infarction in China from 2001 to 2015: China PEACE-Retrospective AMI Study. BMJ Open. 2020;10(3):e033269.
38. Karhu E, Atlas SE, Jinrun G, et al. Intravenous infusion of magnesium sulfate is not associated with cardiovascular, liver, kidney, and metabolic toxicity in adults. J Clin Transl Res. 2018;4(1):47-55.
39. Noah L, Pickering G, Mazur A, et al. Impact of magnesium supplementation, in combination with vitamin B6, on stress and magnesium status: secondary data from a randomized controlled trial. Magnes Res. 2020;33(3):45-57.
40. Erstad BL, Cotugno CL. Management of alcohol withdrawal. Am J Health Syst Pharm. 1995;52(7):697-709.
41. Abumaria N, Luo L, Ahn M, et al. Magnesium supplement enhances spatial-context pattern separation and prevents fear overgeneralization. Behav Pharmacol. 2013;24(4):255-263.
42. Kirov GK, Tsachev KN. Magnesium, schizophrenia and manic-depressive disease. Neuropsychobiology. 1990;23(2):79-81.
43. Botturi A, Ciappolino V, Delvecchio G, et al. The role and the effect of magnesium in mental disorders: a systematic review. Nutrients. 2020;12(6):1661.
44. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
45. Magnesium sulfate intravenous side effects by likelihood and severity. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-149570/magnesium-sulfate-intravenous/details/list-sideeffects
46. Scopolamine base transdermal system – uses, side effects, and more. WebMD. Accessed April 9, 2023. https://www.webmd.com/drugs/2/drug-14032/scopolamine-transdermal/details
47. Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576-580.
48. Janowsky DS, el-Yousef MK, Davis JM, et al. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2(7778):632-635.
49. Janowsky DS, Risch SC, Gillin JC. Adrenergic-cholinergic balance and the treatment of affective disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7(2-3):297-307.
50. Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorous insecticides. Lancet. 1972;1(7191):1371-1374.
51. Davis KL, Berger PA, Hollister LE, et al. Physostigmine in mania. Arch Gen Psychiatry. 1978;35(1):119-122.
52. Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13(17):1903-1911.
53. Brown RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58(3):331-334.
54. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266(5604):730-732.
55. Ji CX, Zhang JJ. Effect of scopolamine on depression in mice. Abstract in English. Yao Xue Xue Bao. 2011;46(4):400-405.
56. Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63(10):1121-1129.
57. Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67(5):432-438.
58. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
59. Gibbs RB, Gabor R, Cox T, et al. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29(6):741-748.
60. Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular-signal-regulated kinase activity. Neuroscience. 2004;124(4):809-816.
61. Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949-6956.
62. Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454-464.
63. Voderholzer U. Sleep deprivation and antidepressant treatment. Dialogues Clin Neurosci. 2003;5(4):366-369.
64. Hasselmann H. Scopolamine and depression: a role for muscarinic antagonism? CNS Neurol Disord Drug Targets. 2014;13(4):673-683.
65. Transderm scopolamine [prescribing information]. Warren, NJ: GSK Consumer Healthcare; 2019.
66. Jaffe RJ, Novakovic V, Peselow ED. Scopolamine as an antidepressant: a systematic review. Clin Neuropharmacol. 2013;36(1):24-26.
67. Karameh WK, Khani M. Intravenous clomipramine for treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2015;19(2):pyv084.
68. Andrews ET, Beattie RM, Tighe MP. Functional abdominal pain: what clinicians need to know. Arch Dis Child. 2020;105(10):938-944. doi:10.1136/archdischild-2020-318825
69. Aliane V, Pérez S, Bohren Y, et al. Key role of striatal cholinergic interneurons in processes leading to arrest of motor stereotypies. Brain. 2011;134(Pt 1):110-118. doi:10.1093/brain/awq285
70. Tzavara ET, Bymaster FP, Davis RJ, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18(12):1410-1412. doi:10.1096/fj.04-1575fje
71. Korczyn AD, Kish I. The mechanism of imipramine in enuresis nocturna. Clin Exp Pharmacol Physiol. 1979;6(1):31-35. doi:10.1111/j.1440-1681.1979.tb00004.x
72. Trimble MR. Worldwide use of clomipramine. J Clin Psychiatry. 1990;51(Suppl):51-54; discussion 55-58.
73. Gong W, Zhang S, Zong Y, et al. Involvement of the microglial NLRP3 inflammasome in the anti-inflammatory effect of the antidepressant clomipramine. J Affect Disord. 2019;254:15-25.
74. Piwowarska J, Wrzosek M, Radziwon’-Zaleska M. Serum cortisol concentration in patients with major depression after treatment with clomipramine. Pharmacol Rep. 2009;61(4):604-611.
75. Danish University Antidepressant Group (DUAG). Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clin Pharmacol Ther. 1999;66(2):152-165.
76. Moukaddam NJ, Hirschfeld RMA. Intravenous antidepressants: a review. Depress Anxiety. 2004;19(1):1-9.
77. Gerretsen P, Pollock BG. Rediscovering adverse anticholinergic effects. J Clin Psychiatry. 2011;72(6):869-870. doi:10.4088/JCP.11ac07093
78. Thomas SJ, Shin M, McInnis MG, et al. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35(4):433-449. doi:10.1002/phar.1576
79. Robles LA. Serotonin syndrome induced by fentanyl in a child: case report. Clin Neuropharmacol. 2015;38(5):206-208. doi:10.1097/WNF.0000000000000100
80. Fallon BA, Liebowitz MR, Campeas R, et al. Intravenous clomipramine for obsessive-compulsive disorder refractory to oral clomipramine: a placebo-controlled study. Arch Gen Psychiatry. 1998;55(10):918-924.
81. Vieta E, Florea I, Schmidt SN, et al. Intravenous vortioxetine to accelerate onset of effect in major depressive disorder: a 2-week, randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol. 2019;34(4):153-160.
82. Kasper S, Müller-Spahn F. Intravenous antidepressant treatment: focus on citalopram. Eur Arch Psychiatry Clin Neurosci. 2002;252(3):105-109.
83. Togay B, El-Mallakh RS. Posttraumatic stress disorder: from pathophysiology to pharmacology. Current Psychiatry. 2020;19(5):33-39.
84. Adhikari A, Lerner TN, Finkelstein J, et al. Basomedial amygdala mediates top-down control of anxiety and fear. Nature. 2015;527(7577):179-185. doi:10.1038/nature15698
85. Lipov E. In search of an effective treatment for combat-related post-traumatic stress disorder (PTSD): can the stellate ganglion block be the answer? Pain Pract. 2010;10(4):265-266.
86. Lipov E, Ritchie EC. A review of the use of stellate ganglion block in the treatment of PTSD. Curr Psychiatry Rep. 2015;17(8):599.
87. Olmsted KLR, Bartoszek M, McLean B, et al. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77(2):130-138.
88. Lipov E, Candido K. The successful use of left-sided stellate ganglion block in patients that fail to respond to right-sided stellate ganglion block for the treatment of post-traumatic stress disorder symptoms: a retrospective analysis of 205 patients. Mil Med. 2021;186(11-12):319-320.
89. Li Y, Loshak H. Stellate ganglion block for the treatment of post-traumatic stress disorder, depression, and anxiety. Canadian J Health Technol. 2021;1(3):1-30.
90. Kerzner J, Liu H, Demchenko I, et al. Stellate ganglion block for psychiatric disorders: a systematic review of the clinical research landscape. Chronic Stress (Thousand Oaks). 2021;5:24705470211055176.
91. Wie C, Gupta R, Maloney J, et al. Interventional modalities to treat complex regional pain syndrome. Curr Pain Headache Rep. 2021;25(2):10. doi:10.1007/s11916-020-00904-5
92. Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc. 2001;99(12):698-703.
93. Chester M, Hammond C. Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87(1):103-105. doi:10.1016/S0304-3959(00)00270-0
94. Jeon Y. Therapeutic potential of stellate ganglion block in orofacial pain: a mini review. J Dent Anesth Pain Med. 2016;16(3):159-163. doi:10.17245/jdapm.2016.16.3.159
95. Shan HH, Chen HF, Ni Y, et al. Effects of stellate ganglion block through different approaches under guidance of ultrasound. Front Surg. 2022;8:797793. doi:10.3389/fsurg.2021.797793
96. Goel V, Patwardhan AM, Ibrahim M, et al. Complications associated with stellate ganglion nerve block: a systematic review. Reg Anesth Pain Med. 2019;rapm-2018-100127. doi:10.1136/rapm-2018-100127
97. Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev. 2017;3(3):CD006499.
98. Roggenkämper P, Jost WH, Bihari K, et al. Efficacy and safety of a new botulinum toxin type A free of complexing proteins in the treatment of blepharospasm. J Neural Transm (Vienna). 2006;113(3):303-312.
99. Heckmann M, Ceballos-Baumann AO, Plewig G; Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N Engl J Med. 2001;344(7):488-493.
100. Carruthers JA, Lowe NJ, Menter MA, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002;46(6):840-849.
101. Schurch B, de Sèze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200.
102. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51(9):1358-1373.
103. Dashtipour K, Chen JJ, Walker HW, et al. Systematic literature review of abobotulinumtoxinA in clinical trials for adult upper limb spasticity. Am J Phys Med Rehabil. 2015;94(3):229-238.
104. Nitti VW, Dmochowski R, Herschorn S, et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo-controlled trial. J Urol. 2017;197(2S):S216-S223.
105. Jongerius PH, van den Hoogen FJA, van Limbeek J, et al. Effect of botulinum toxin in the treatment of drooling: a controlled clinical trial. Pediatrics. 2004;114(3):620-627.
106. Zaninotto, G. Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg. 2004;239(3):364-370.
107. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3-9.
108. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
109. Affatato O, Moulin TC, Pisanu C, et al. High efficacy of onabotulinumtoxinA treatment in patients with comorbid migraine and depression: a meta-analysis. J Transl Med. 2021;19(1):133.
110. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
111. Schulze J, Neumann I, Magid M, et al. Botulinum toxin for the management of depression: an updated review of the evidence and meta-analysis. J Psychiatr Res. 2021;135:332-340.
112. Finzi E, Rosenthal NE. Emotional proprioception: treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93-96.
113. Söderkvist S, Ohlén K, Dimberg U. How the experience of emotion is modulated by facial feedback. J Nonverbal Behav. 2018;42(1):129-151.
114. Lewis, MB. The interactions between botulinum-toxin-based facial treatments and embodied emotions. Sci Rep. 2018;8(1):14720.
115. Li Y, Liu J, Liu X, et al. Antidepressant-like action of single facial injection of botulinum neurotoxin A is associated with augmented 5-HT levels and BDNF/ERK/CREB pathways in mouse brain. Neurosci Bull. 2019;35(4):661-672. Erratum in: Neurosci Bull. 2019;35(4):779-780.
116. Gündel H, Wolf A, Xidara V, et al. High psychiatric comorbidity in spasmodic torticollis: a controlled study. J Nerv Ment Dis. 2003;191(7):465-473.
117. Hall TA, McGwin G Jr, Searcey K, et al. Health-related quality of life and psychosocial characteristics of patients with benign essential blepharospasm. Arch Ophthalmol. 2006;124(1):116-119.
118. Ceylan D, Erer S, Zarifog˘lu M, et al. Evaluation of anxiety and depression scales and quality of life in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci. 2019;40(4):725-731.
119. Heller AS, Lapate RC, Mayer KE, et al. The face of negative affect: trial-by-trial corrugator responses to negative pictures are positively associated with amygdala and negatively associated with ventromedial prefrontal cortex activity. J Cogn Neurosci. 2014;26(9):2102-2110.
120. Makunts T, Wollmer MA, Abagyan R. Postmarketing safety surveillance data reveals antidepressant effects of botulinum toxin across various indications and injection sites. Sci Rep. 2020;10(1):12851.
121. Ahsanuddin S, Roy S, Nasser W, et al. Adverse events associated with botox as reported in a Food and Drug Administration database. Aesthetic Plast Surg. 2021;45(3):1201-1209. doi:10.1007/s00266-020-02027-z
122. Kashif M, Tahir S, Ashfaq F, et al. Association of myofascial trigger points in neck and shoulder region with depression, anxiety, and stress among university students. J Pak Med Assoc. 2021;71(9):2139-2142.
123. Cigarán-Méndez M, Jiménez-Antona C, Parás-Bravo P, et al. Active trigger points are associated with anxiety and widespread pressure pain sensitivity in women, but not men, with tension type headache. Pain Pract. 2019;19(5):522-529.
124. Palacios-Ceña M, Castaldo M, Wang K, et al. Relationship of active trigger points with related disability and anxiety in people with tension-type headache. Medicine (Baltimore). 2017;96(13):e6548.
125. Karadas Ö, Inan LE, Ulas Ü, et al. Efficacy of local lidocaine application on anxiety and depression and its curative effect on patients with chronic tension-type headache. Eur Neurol. 2013;70(1-2):95-101.
126. Gerwin RD. Classification, epidemiology and natural history of myofascial pain syndrome. Curr Pain Headache Rep. 2001;5(5):412-420.
127. Castro Sánchez AM, García López H, Fernández Sánchez M, et al. Improvement in clinical outcomes after dry needling versus myofascial release on pain pressure thresholds, quality of life, fatigue, pain intensity, quality of sleep, anxiety, and depression in patients with fibromyalgia syndrome. Disabil Rehabil. 2019;41(19):2235-2246.
128. Healy GM, Finn DP, O’Gorman DA, et al. Pretreatment anxiety and pain acceptance are associated with response to trigger point injection therapy for chronic myofascial pain. Pain Med. 2015;16(10):1955-1966.
129. Morjaria JB, Lakshminarayana UB, Liu-Shiu-Cheong P, et al. Pneumothorax: a tale of pain or spontaneity. Ther Adv Chronic Dis. 2014;5(6):269-273.
Adult ADHD: 6 studies of pharmacologic interventions
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.
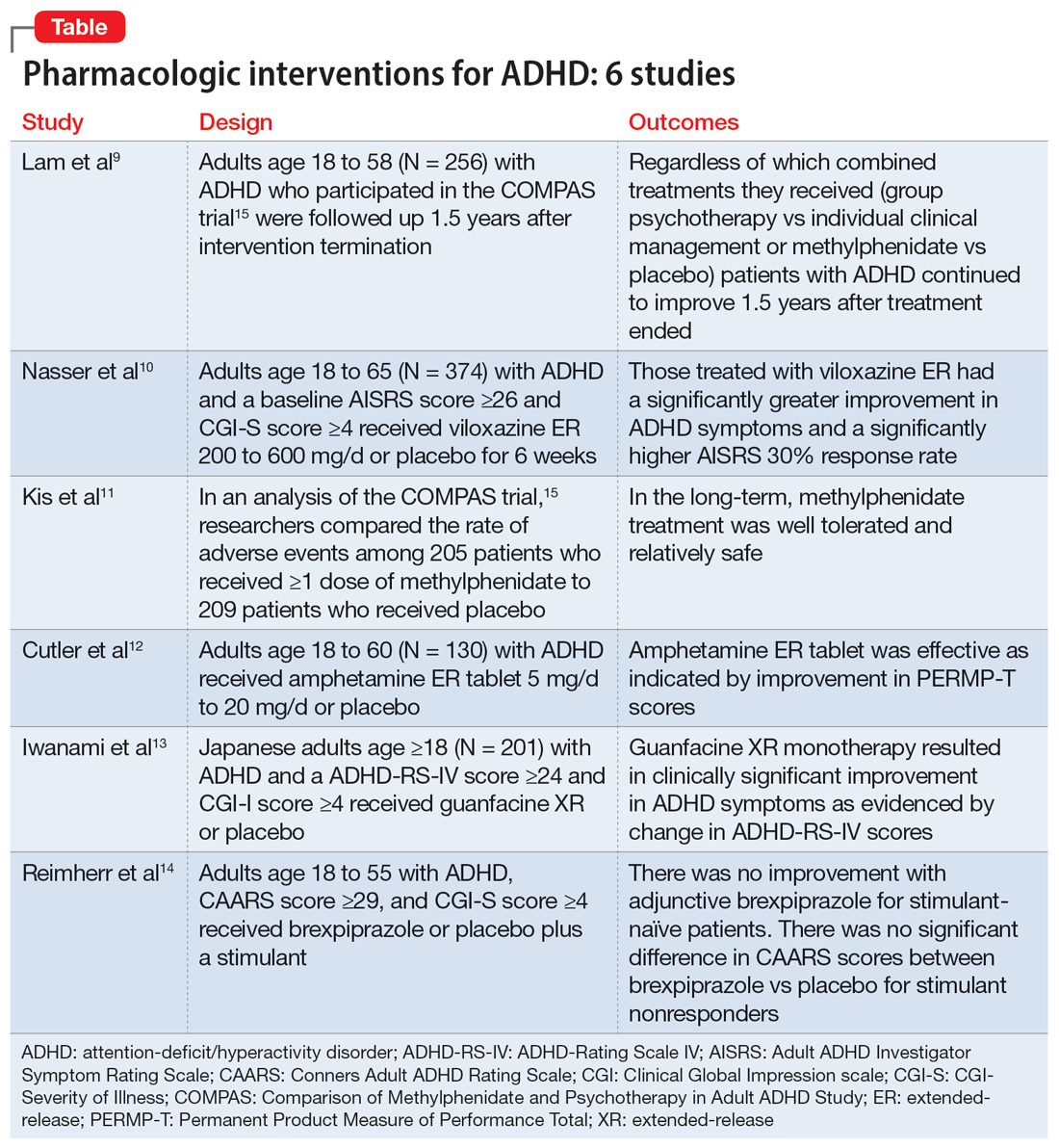
1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
Attention-deficit/hyperactivity disorder (ADHD) is a developmental disorder that begins in childhood and continues into adulthood. The clinical presentation is characterized by a persistent pattern of inattention, impulsivity, and/or hyperactivity that causes functional interference.1 ADHD affects patients’ interpersonal and professional lives as well as their daily functioning.2 Adults with ADHD may suffer from excessive self-criticism, low self-esteem, and sensitivity to criticism.3 The overall prevalence of adult ADHD is 4.4%.4 ADHD in adults is frequently associated with comorbid psychiatric disorders.5 The diagnosis of ADHD in adults requires the presence of ≥5 symptoms of inattention and hyperactivity/impulsivity that persist for ≥6 months. Patients must have first had such symptoms before age 12; symptoms need to be present in ≥2 settings and interfere with functioning.1
Treatment of ADHD includes pharmacologic and nonpharmacologic interventions. For most patients, pharmacotherapy—specifically stimulant medications—is advised as first-line treatment,6 with adequate trials of methylphenidate and amphetamines before using second-line agents such as nonstimulants. However, despite these medications’ efficacy in randomized controlled trials (RCTs), adherence is low.7 This could be due to inadequate response or adverse effects.8 Guidelines also recommend the use of nonpharmacologic interventions for adults who cannot adhere to or tolerate medication or have an inadequate response.6 Potential nonpharmacologic interventions include transcranial direct current stimulation, mindfulness, psychoeducation, cognitive-behavioral therapy, and chronotherapy.
In Part 1 of this 2-part article, we review 6 RCTs of pharmacologic interventions for adult ADHD published within the last 5 years (Table9-14). Part 2 will review nonpharmacologic treatments.

1. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
The Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) was a multicenter prospective, randomized trial of adults age 18 to 58 with ADHD.15 It compared cognitive-behavioral group psychotherapy (GPT) with individual clinical management (CM), and methylphenidate with placebo. When used in conjunction with methylphenidate, psychological treatments produced better results than placebo. However, studies on the long-term effects of multimodal treatment in ADHD are limited. Lam et al9 performed a follow-up analysis of the COMPAS trial.
Study design
- This observer-masked study involved a follow-up of participants in COMPAS 1.5 years after the interventions were terminated. Of the 433 adults with ADHD who participated in COMPAS, 256 participated in this follow-up.
- The inclusion criteria of COMPAS were age 18 to 58; diagnosis of ADHD according to DSM-IV criteria; chronic course of ADHD symptoms from childhood to adulthood; a Wender Utah Rating Scale short version score ≥30; and no pathological abnormality detected on physical examination.
- The exclusion criteria were having an IQ <85; schizophrenia, bipolar disorder (BD), borderline personality disorder, antisocial personality disorder, suicidal or self-injurious behavior, autism, motor tics, or Tourette syndrome; substance abuse/dependence within 6 months prior to screening; positive drug screening; neurologic diseases, seizures, glaucoma, diabetes, hyperlipidemia, uncontrolled arterial hypertension, angina pectoris, tachycardia arrhythmia, or arterial occlusive disease; previous stroke; current bulimia or anorexia; low weight (body mass index [BMI] <20; pregnancy (current or planned) or breastfeeding; treatment with stimulants or ADHD-specific psychotherapy in the past 6 months; methylphenidate intolerance; treatment with antidepressants, norepinephrine reuptake inhibitors, bupropion, antipsychotics, theophylline, amantadine, anticoagulants derived from coumarin, antacids, or alpha-adrenergic agonists in the 2 weeks prior to baseline; and treatment with fluoxetine or monoamine oxidase inhibitors in the 4 weeks prior to baseline.
- The primary outcome was a change from baseline on the ADHD Index of Conners Adult ADHD Rating Scale (CAARS) score. Secondary outcomes were self-ratings on the Beck Depression Inventory (BDI) and observer-masked ratings of the Clinical Global Impression (CGI) scale and other ADHD rating scale scores, such as the Diagnostic Checklist for the diagnosis of ADHD in adults (ADHD-DC) and subscales of the CAARS.
- COMPAS was open regarding patient and therapist assignment to GPT and CM, but double-masked regarding medication. The statistical analysis focused on the 2x2 comparison of GPT vs CM and methylphenidate vs placebo.
Outcomes
- A total of 251 participants had an assessment with the observer-masked CAARS score. The baseline mean (SD) age was 36.3 (10.1), and approximately one-half (49.8%) of participants were male.
- Overall, 9.2% of patients took methylphenidate >31 days from termination of COMPAS before this study but not at the start of this study. Approximately one-third (31.1%) of patients were taking methylphenidate at follow-up. The mean (SD) daily dosage of methylphenidate was 36 (24.77) mg and 0.46 (0.27) mg/kg of body weight.
- The baseline all-group mean ADHD Index of CAARS score was 20.6. At follow-up, it was 14.7 for the CM arm and 14.2 for the GPT arm (difference not significant, P = .48). The mean score decreased to 13.8 for the methylphenidate arm and to 15.2 for the placebo (significant difference, P = .04).
- Overall, methylphenidate was associated with greater improvement in symptoms than placebo. Patients in the GPT arm had fewer severe symptoms as assessed by the self-reported ADHD Symptoms Total Score compared to the CM arm (P = .04).
- There were no significant differences in self-rating CAARS and observer-rated CAARS subscale scores. Compared to CM, GPT significantly decreased pure hyperactive symptoms on the ADHD-DC (P = .08). No significant differences were observed in BDI scores. The difference between GPT and CM remained significant at follow-up in terms of the CGI evaluation of efficacy (P = .04).
Continue to: Conclusions/limitations
Conclusions/limitations
- Regardless of which combined treatments they received, patients with ADHD continued to improve 1.5 years after the 52-week treatment phase ended.
- Patients assigned to methylphenidate performed considerably better on the observer-rated CAARS than patients assigned to placebo.
- Benefits from GPT or CM in addition to methylphenidate therapy lasted 1.5 years. Compared to CM, GPT was not linked to better scores on the CAARS.
- Limitations: Approximately 41% of patients who were recruited did not participate. Daily functioning was measured only by the CGI. There were only marginal differences among the 4 treatments, and the study compared a very regimented approach (GPT) with one that was less focused (CM).
2. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double‐blind, placebo‐controlled trial assessing the efficacy and safety of viloxazine extended‐release capsules in adults with attention‐deficit/hyperactivity disorder. CNS Drugs. 2022;36(8): 897-915. doi:10.1007/s40263-022-00938-w
In 2021, the FDA approved viloxazine extended-release (ER) for treating ADHD in children and adolescents (age 6 to 17). Nasser et al10 reviewed the safety and efficacy of viloxazine ER in adults with ADHD.
Study design
- This phase III, randomized, double-blind, placebo-controlled, multicenter clinical trial included 374 adults with ADHD who received viloxazine ER or placebo.
- Participants were age 18 to 65 and had been given a primary diagnosis of ADHD according to DSM-5 criteria in the last 6 months. Other inclusion criteria were having an Adult ADHD Investigator Symptom Rating Scale (AISRS) total score ≥26 and CGI-Severity of Illness (CGI-S) score ≥4 at baseline, BMI 18 to 35 kg/m2, and being medically healthy.
- Exclusion criteria included having treatment-resistant ADHD, a current diagnosis of any psychiatric disorder other than ADHD, or a history of schizophrenia, schizoaffective disorder, BD, autism, obsessive-compulsive disorder, personality disorder, or posttraumatic stress disorder. Individuals with any significant neurologic disorder, heart condition, arrhythmia, clinically relevant vital sign abnormality, or systemic illness were excluded, as were those with a history (within the past year) or current diagnosis of substance use disorder or a positive drug screen for a drug of abuse. Those with an allergic reaction or intolerance to viloxazine or were breastfeeding, pregnant, or refused to be abstinent or practice birth control were excluded.
- The dosage of viloxazine ER ranged from 200 to 600 mg/d for 6 weeks. This was titrated based on symptom response and adverse effects.
- All individuals received 2 capsules once a day for Week 1 and Week 2. During Week 1 and Week 2, participants in the viloxazine ER group received 200 mg (1 viloxazine ER capsule and 1 placebo capsule) and 400 mg (2 viloxazine ER capsules) of the medication, respectively. Two placebo pills were administered to those in the placebo group. From Week 3 to Week 6, the dose could be titrated or tapered at the investigator’s discretion. Compliance was assessed by comparing the number of pills dispensed vs returned.
- The primary outcome was a change in AISRS score from baselines to Week 6.
- The key secondary outcome was the change in CGI-S score from baseline to Week 6. Scores on the AISRS inattention and hyperactive/impulsivity subscales, Behavioral Regulation Index, Metacognition Index, Behavior Rating Inventory of Executive Function–Adult Version (BRIEF-A), and Generalized Anxiety Disorder-7 item scale (GAD-7) were also evaluated. Also, the rates of 30% and 50% responders on the AISRS (defined as ≥30% or ≥50% reduction from baseline in AISRS total score, respectively), CGI-S scores, and CGI-Improvement (CGI-I) scores were examined.
Outcomes
- Based on change in AISRS total scores, patients who received viloxazine ER had significantly greater improvement in their ADHD symptoms than those taking placebo (P = .0040). Patients in the viloxazine ER group had significantly greater improvement in AISRS hyperactive/impulsive (P = .0380) and inattentive symptoms (P = .0015).
- The decrease in CGI-S score was also significantly greater in the viloxazine ER group than in the placebo group (P = .0023). The viloxazine ER group also had significantly greater improvement in executive function as assessed by the BRIEF-A (P = .0468). The difference in GAD-7 scores between the viloxazine ER group and the placebo group was not significant.
- The viloxazine ER group had a greater AISRS 30% response rate than the placebo group (P = .0395). There were no significant differences between groups in AISRS 50% responder rate or CGI-I responder rate.
- Adverse effects related to viloxazine and occurring in ≥5% of participants included insomnia (14.8%), fatigue (11.6%), nausea, decreased appetite (10.1%), dry mouth (9.0%), and headache (9.0%). The discontinuation rate was 9.0% in the viloxazine ER group vs 4.9% in the placebo group.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, patients treated with viloxazine ER had significantly greater improvements in ADHD symptoms, including both hyperactive/impulsive and inattentive components as well as executive function.
- The viloxazine ER group had a significantly higher AISRS 30% response rate than the placebo group, but there were no significant differences in anxiety symptoms or other measures of response.
- Viloxazine ER was well tolerated and safe.
- Limitations: There was a reduced power to detect differences in treatment due to participants dropping out or discontinuing treatment, a lack of interrater reliability data, and a lack of patient-reported outcome or satisfaction data.
3. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
Kis et al11 analyzed the safety results of COMPAS.15 Details of this trial, including interventions and inclusion/exclusion criteria, are described in the description of Lam et al.9
Study design
- Researchers compared the rate of adverse events (AEs) among 205 patients who received ≥1 dose of methylphenidate with 209 patients who received placebo.
- AEs were documented and analyzed on an “as received” basis during Week 0 to Week 52. Electrocardiogram (ECG) data were recorded at baseline and Week 24. Vital signs were monitored at baseline, every week for the first 12 weeks, then every 4 weeks for the next 52 weeks. Body weight was assessed at Week 6, Week 12, Week 20, Week 28, Week 40, and Week 52. A 12-lead ECG was obtained at baseline and Week 24.
- The sample size was assessed to have 80% power to detect group differences in AEs.
Outcomes
- Overall, 96% of participants in the methylphenidate group and 88% of participants in the placebo group experienced at least 1 AE (difference 8.1%; 95% CI, 2.9% to 13.5%).
- AEs that occurred more frequently with methylphenidate compared to placebo were decreased appetite (22% vs 3.8%); dry mouth (15% vs 4.8%); palpitations (13% vs 3.3%); gastrointestinal (GI) infection (11% vs 4.8%); agitation (11% vs 3.3%); restlessness (10% vs 2.9%); hyperhidrosis, tachycardia, and weight decrease (all 6.3% vs 1.9%); depressive symptoms and influenza (both 4.9% vs 1.0%); and acute tonsillitis (4.4% vs 0.5%). Serious AEs were reported by 7.3% of patients in the methylphenidate group and 4.3% of those in the placebo group, with no difference in frequency (difference 3.0%; 95% CI, 1.6% to 7.9%). The most severe AEs were aggression, depression, somnambulism, and suicidal ideation in the methylphenidate group and car accidents, epicondylitis, and a fall in the placebo group.
- There were no significant differences in AEs between the GPT and CM groups.
- The treatment combinations that included methylphenidate had higher rates of patients experiencing at least 1 AE (CM/methylphenidate 97%, GPT/methylphenidate 96%, CM/placebo 92%, GPT/placebo 84%).
- Overall, 8.8% of patients in the methylphenidate group and 4.8% in the placebo group stopped their medication treatment because of an AE (difference 4.0%; 95% CI, 0.9% to 9.1%). At least 1 dose decrease, increase, or discontinuation was made after an AE in 42% of participants in the placebo group and 69% of those in the methylphenidate group.
- There were no significant differences in clinically pertinent ECG abnormalities between methylphenidate and placebo therapy.
Continue to: Conclusions/limitations
Conclusions/limitations
- AEs were more common in the methylphenidate groups compared to placebo, but there was no significant differences for severe AEs. In the long-term, methylphenidate treatment was well tolerated and relatively safe.
- Limitations: The sample size may have been too small to detect uncommon AEs, all AEs had to be reported and may not have been caused by the treatment, and the original study’s main outcome was efficacy, not safety, which makes this an exploratory analysis of AEs.
4. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
Once-daily dosing of stimulants, which are commonly used to manage adult ADHD,16 can be beneficial because many patients have schedules that limit taking medication multiple times a day. Cutler et al12 looked at the efficacy and safety of amphetamine extended-release tablet (AMPH ER TAB), which is a 3.2:1 mixture of d- and l-amphetamine released by the LiquiXR drug delivery system. This technology allows for a continuous release following an initial quick onset of action.
Study design
- This parallel-study, double-blind study evaluated adults age 18 to 60 who had a diagnosis of ADHD according to DSM-5 criteria and the Adult ADHD Clinical Diagnostic Scale, normal-range IQ, AISRS score ≥26, and baseline CGI-S score ≥4.
- Women were not lactating or pregnant during the study.
- Exclusion criteria included a history of mental illnesses; chronic medical conditions; clinically significant abnormal ECG or cardiac findings on exam; renal or liver disease; family history of sudden death; significant vital sign findings; uncontrolled hypertension or a resting systolic blood pressure (SBP) >140 mmHg or diastolic blood pressure (DBP) >90 mmHg; recent history of or current alcohol or substance use disorder; use of atomoxetine, monoamine oxidase inhibitors, or tricyclic antidepressants within 14 days of study or the use of other stimulant medications within 1 week of screening; use of GI acidifying agents or urinary acidifying agents within 3 days of screening; answering “yes” to questions 4 or 5 of the Suicidal Ideation section of the Columbia Suicide Severity Rating Scale within 2 years prior to the study; taking another investigational medication within 30 days of screening; allergic to amphetamine or components of the study drug, and a lack of prior response to amphetamine.
- Patients were randomized to receive AMPH ER TAB (n = 65) or placebo (n = 65), taken before 10
am . Participants started at 5 mg/d of the drug/placebo and then entered a 5-week titration period in which the medication was increased by 5 mg/d each week until reaching 20 mg/d, and then continued 20 mg/d for 2 weeks. - The primary outcome was the mean Permanent Product Measure of Performance Total (PERMP-T) score averaged across all time points (0.5-, 1-, 2-, 4-, 8-, 10-, 12-, 13-, and 14-hours postdose) at Visit 5.
- Participants underwent AISRS, CGI-S, and safety evaluations at baseline and at the 5 visits at the end of each treatment week.
Outcomes
- Analyses were completed on participants who received ≥1 dose of the medication and who had ≥1 PERMP-T score at Visit 5.
- Predose PERMP-T scores were similar between the AMPH ER TAB group (259.5) and placebo group (260). The mean postdose PERMP-T score in the AMPH ER TAB group (302.8) was significantly higher (P = .0043) than the placebo group (279.6).
- The PERMP-T scores were significantly different at 0.5-, 1-, 2-, 4-, 8-, and 13-hours postdose but not at 10-, 12-, and 14-hours postdose. The first Visit 5 time point at which the difference between groups was statistically different was at 0.5 hours postdose (P = .01), and the last significant time point was 13 hours (P = .006).
- The improvement in CGI-S scores was significantly greater in the AMPH ER TAB group than the placebo group. The improvement in AISRS scores was significantly greater in the AMPH ER TAB group at Visit 3, Visit 4, and Visit 5. More participants in the AMPH ER TAB group had AEs compared to the placebo group (90% vs 60%). The most common AEs (frequency ≥5% and occurring more in the intervention arm) were decreased appetite, insomnia, dry mouth, irritability, headache, anxiety, nausea, dizziness, and tachycardia.
- The AMPH ER TAB group had nonclinically significant increases in SBP (116.8 to 120.7 mmHg), DBP (74.1 to 77.1 mmHg), and heart rate (73.0 to 81.9 bpm) at Visit 5 compared to baseline.
- No serious AEs occurred. Three participants in the AMPH ER TAB group experienced AEs (increased blood pressure, CNS stimulation, and anxiety) that led them to discontinue the study.
Continue to: Conclusions/limitations
Conclusions/limitations
- AMPH ER TAB reduced symptoms in adults with ADHD as assessed by improvement in PERMP-T scores.
- The safety and tolerability profile of AMPH ER TAB were comparable to other stimulants, with expected rises in blood pressure and heart rate.
- Limitations: Patients were required to be titrated to 20 mg/d of AMPH ER TAB, instead of following a flexible titration based on an individual’s response. Some participants may have had greater improvement at a higher or lower dose. This study did not compare AMPH ER TAB to other stimulants. The 5-week duration of this study limited the ability to evaluate long-term efficacy and tolerability. Patients with a wide range of psychiatric or medical comorbidities were excluded.
5. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
Guanfacine extended-release (GXR) is a selective alpha 2A-adrenergic receptor agonist approved for treating ADHD in children and adolescents.17 Iwanami et al13 evaluated the efficacy and safety of GXR for adults.
Study design
- This randomized, double-blinded, placebo-controlled trial enrolled Japanese adults age ≥18 who were diagnosed with ADHD according to DSM-5 criteria and scored ≥24 on the ADHD-Rating Scale IV (ADHD-RS-IV) and ≥4 on CGI- I.
- Exclusion criteria included having anxiety, depression, substance use disorder, tic disorder, BD, personality disorder, schizophrenia, or intellectual disability; a moderate or severe psychiatric disorder requiring treatment other than counseling; seizures; increased risk for suicide; a history of cardiovascular disease, including prolonged QTc/abnormal ECG/abnormal labs, orthostatic hypotension, or continuous bradycardia; or taking medications that affect blood pressure or heart rate.
- Overall, 101 participants were randomized to the GXR group and 100 to the placebo group. Approximately two-thirds of the study population was male. Patients received GXR or placebo once daily at approximately the same time.
- There were 5 phases to the trial. The screening period occurred over 1 to 4 weeks. Part 1 of the treatment period consisted of 5 weeks of medication optimization. Participants were started on GXR 2 mg/d and were required to be receiving a minimum dose of 4 mg/d starting at Week 3. Clinicians were allowed to increase the dose 1 mg/d per week starting at Week 4 based on clinical response to a maximum dosage of 6 mg/d. Part 2 of the treatment period consisted of 5 weeks of maintenance at 4 to 6 mg/d. The tapering period to 2 mg/d occurred over 2 weeks. The follow-up period lasted 1 week.
- Efficacy measurements included the Japanese version of the ADHD-RS-IV and translations of the English-language CAARS, CGI-I, and CGI-S. Participant-reported measures included the Patient Global Impression-Improvement scale (PGI-I), Adult ADHD Quality of Life Questionnaire (AAQoL), and BRIEF-A.
- The primary outcome was the difference in ADHD-RS-IV total score from baseline to the end of the maintenance period (Week 10).
- Safety assessments were completed at Week 5 (end of dose optimization period), Week 10 (end of dose maintenance period), and Week 12 (tapering period).
Outcomes
- The average GXR dose during the maintenance period was 5.07 mg/d.
- Compared to the placebo group, the GXR group had more patients age <30 (47% vs 39%) and fewer patients age ≥40 (17% vs 27%). Baseline ADHD-RS-IV scores in both groups were comparable. At baseline, 51% in the GXR group had a combined inattentive/hyperactive-impulsive presentation and 47% had a predominately inattention presentation, with similar characteristics in the placebo group (49% combined, 49% inattention).
- At Week 10, the least squares mean change from baseline on the ADHD-RS-IV total score was significantly greater in the GXR group than in the placebo group (-11.55 ± 1.10 vs -7.27 ± 1.07; P = .0005), with an effect size of 0.52. There was a greater decrease in the ADHD-RS-IV scores starting at Week 4 and continuing to Week 10 (P < .005).
- There were also significant differences favoring GXR on the ADHD-RS-IV hyperactivity-impulsivity subscale score (P = .0021) and ADHD-RS-IV inattention subscale score (P = .0032).
- There were significant differences in the CAARS total ADHD score (P = .0029) and BRIEF-A scores on the inhibit (P = .0173), initiate (P = .0406), plan/organize (P = .174), and global executive composite index (P = .0404) scales. There was no significant difference in the total AAQoL score (P = .0691), but there was a significant improvement in the AAQoL life productivity subscore (P = .0072).
- At Week 10, there were also significant improvements in the CGI-I scores (P = .0007) and PGI-I scores (P = .0283). The CGI-S scores were similar at all time points.
- Overall, 81.2% of GXR patients reported AEs compared to 62% in the placebo group. There was 1 serious treatment-emergent AE (a suicide attempt) that the authors concluded was unrelated to the study drug. No deaths occurred. The most common AEs (incidence ≥10% in either group) included somnolence, thirst, nasopharyngitis (occurring more in the placebo group), blood pressure decrease, postural dizziness, and constipation. The main AEs leading to discontinuation were somnolence and blood pressure decrease. Overall, 19.8% of patients receiving GXR discontinued treatment due to AEs, compared to 3% in the placebo group.
- Heart rate, blood pressure, and QTc (corrected by the Bazett formula) were decreased in the GXR group at Week 10 while QT and RR intervals increased, and most returned to normal by Week 12.
Continue to: Conclusions/limitations
Conclusions/limitations
- Compared to placebo, GXR monotherapy resulted in clinical improvement in ADHD symptoms, with a moderate effect size.
- The most common AEs were mild to moderate and congruent with known adverse effects of guanfacine. Sedation effects mostly transpired within the first week of medication administration and were transient.
- Limitations: The findings might not be generalizable to non-Japanese patients. The duration of the study was short. Patients with a wide range of psychiatric and medical comorbidities were excluded. Two-thirds of the participants were male, and there was a disparity in participant age in the GXR and placebo groups.
6. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
While stimulants are a mainstay ADHD treatment, some patients have a partial response or do not respond to amphetamines or methylphenidate. Reimherr et el14 assessed the efficacy and safety of adding brexpiprazole (BXP) to a stimulant.
Study design
- This randomized, double-blinded, placebo-controlled trial recruited 559 stimulant-naive patients and 174 patients who had not responded to previous stimulant therapy.
- Participants were adults age 18 to 55 with a primary diagnosis of ADHD according to DSM-IV-TR criteria and the Conners Adult ADHD Diagnostic Interview. Other inclusion criteria were having a CAARS score ≥29 and a CGI-S score ≥4.
- Exclusion criteria included being at risk for suicide; having current substance abuse or positive alcohol/drug screens; a history of good response to prestudy treatment; a clinically significant medical condition; fasting blood glucose >200 mg/dL or hemoglobin A1C >7%; and hospitalization in past 12 months from a diabetic complication, uncontrolled hypertension, ischemic heart disease, or epilepsy. Further exclusion criteria included a history of psychosis, current MDD or BD, current panic disorder, uncontrolled comorbid psychiatric condition, or clinically significant personality disorder. Investigators excluded any patient with severe DSM-IV axis I or II disorders or abnormal/psychopathological behaviors.
- The trial consisted of 3 segments. Part 1 was screening. If the patient was currently receiving a stimulant but not fully responding, the medication was discontinued for at least 5 half-lives.
- Part 2 (5 weeks) involved administering a stimulant plus a single-blind placebo (597 patients completed this phase). The stimulant was chosen by the investigator, who had the option of using 1 of 2 amphetamine derivatives (mixed amphetamine salts capsules or lisdexamfetamine dimesylate capsules) or 1 of 2 methylphenidate derivatives (methylphenidate hydrochloride ER tabs or dexmethylphenidate HCl ER capsules). If a patient did not respond to a particular stimulant prior to the study, they were given a different stimulant from the list. Patients continued the same stimulant throughout the trial. Patients were monitored for a response, defined as a ≥30% decrease in CAARS score or a CAARS score <24, or a CGI-I score of 1 or 2 at Week 5. Patients who did not show this improvement were categorized as open-label nonresponders.
- Part 3 (6 weeks) involved administering a stimulant plus double-blind BXP vs placebo (stimulant-naive n = 167, stimulant nonresponders n = 68). Nonresponders continued the stimulant (at the same dose reached at the end of Part 2) and added either BXP (n = 155) or continued placebo (n = 80). Patients who responded in Part 2 were continued on the stimulant plus placebo and were not randomized. Patients were started on BXP 0.25 mg/d, and the medication could be titrated to 2 mg/d during the following 3 weeks, depending on the benefit vs AE profile. After the third week, the dose could be decreased but not increased.
- The primary outcome was a change in CAARS score. Secondary measurements included the CGI-S, Wender-Reimherr Adult Attention Deficit Disorder Scale (WRAADDS), Montgomery-Åsberg Depression Rating Scale (MADRS), and BDI.
Outcomes
- Stimulant-naive patients were equally divided among the 4 stimulant groups, and previous nonresponders who continued to not respond in Part 2 were more likely to be given methylphenidate HCl or lisdexamfetamine dimesylate.
- Patients with a history of nonresponse had less response to stimulants in Part 2 compared to stimulant-naive patients, as seen by 27% (n = 167) of stimulant-naive patients entering Part 3 compared to 39% of prior nonresponders (n = 68; P = .0249).
- ADHD improvement with BXP appeared to be greater among pretrial nonresponders.
- For stimulant nonresponders before and during the study, at the end of the double-blind endpoint (Part 3; Week 11), WRAADDS total score was significantly improved in the BXP group compared to the placebo group (P = .013; d = 0.74), with most beneficial effects seen in the hyperactivity/restlessness, emotional dysregulation factor, and impulsivity categories.
- For stimulant nonresponders before and during the study, there was no significant difference at the end of Week 11 on the CAARS (P = .64), MADRS (P = .37), or BDI (P = .73). There was a trend toward significance on the CAARS subscale for hyperactive/impulsive (P = .09).
- For prestudy stimulant-naive patients who did not respond to stimulants in Part 2 and were randomized in Part 3, there was not a significant difference between BXP and placebo at Week 11 as assessed on WRAADDS, CAARS, MADRS, or BDI.
- As assessed on WRAADDS, 50% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.334). Under the emotional dysregulation factor category of the WRAADDS, 64% in the BXP group had a response compared to 41% in the placebo group (Fisher exact = 0.064). The attention factor category showed a 40% improvement in the BXP group compared to 32% in the placebo group (Fisher exact = 0.344).
- There were 2 serious AEs in the BXP group (gall bladder inflammation and diarrhea) and 2 in the placebo group (pneumonia and urinary tract infection). There was no statistically significant difference between groups with regards to common AEs (ie, fatigue, heartburn/nausea/stomachache, weight loss), although there was a trend to significant for insomnia in the BXP group (P = .083).
Conclusions/limitations
- Stimulant-naive patients experienced no improvement with adjunctive BXP.
- For prior stimulant nonresponders, there was no significant difference between BXP vs placebo on the primary outcome of the CAARS score, but there was an improvement as observed by assessment with the WRAADDS.
- The largest change in the WRAADDS occurred in the emotional dysregulation factor compared to the attention factor.
- BXP appeared to be well tolerated.
- Limitations: The WRAADDS was administered without the patients’ significant other/collateral. Raters were not trained in the use of the WRAADDS. Patients with a wide range of psychiatric and medical comorbidities were excluded. Fewer patients were recruited in the prior stimulant nonresponder group.
Bottom Line
Recent randomized controlled trials suggest that methylphenidate, amphetamine extended-release, viloxazine extended-release, and guanfacine extended-release improved symptoms of adult attention-deficit/hyperactivity disorder (ADHD). There were no improvements in ADHD symptoms with adjunctive brexpiprazole.
Related Resources
- Parikh AR, Baker SA. Adult ADHD: pharmacologic treatment in the DSM-5 era. Current Psychiatry. 2016;15(10):18-25.
- Akbar HN. Why we should be scrutinizing the rising prevalence of adult ADHD. Current Psychiatry. 2022; 21(7):e1-e2. doi:10.12788/cp.0268
Drug Brand Names
Amantadine • Gocovri
Amphetamine extended-release tablet • Dyanavel XR
Atomoxetine • Strattera
Brexpiprazole • Rexulti
Bupropion • Wellbutrin
Dexmethylphenidate • Focalin
Fluoxetine • Prozac
Guanfacine extended- release • Intuniv
Lisdexamfetamine • Vyvanse
Methylphenidate • Concerta, Methylin
Theophylline • Elixophyllin
Viloxazine • Qelbree
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
2. Harpin V, Mazzone L, Raynaud JP, et al. Long-term outcomes of ADHD: a systematic review of self-esteem and social function. J Atten Disord. 2016;20(4):295-305. doi:10.1177/1087054713486516
3. Beaton DM, Sirois F, Milne E. Experiences of criticism in adults with ADHD: a qualitative study. PLoS One. 2022;17(2):e0263366. doi:10.1371/journal.pone.0263366
4. Attention-deficit/hyperactivity disorder (ADHD). National Institute of Mental Health. Accessed February 9, 2023. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd
5. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
6. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NICE Guideline No. 87. National Institute for Health and Care Excellence (NICE); 2019. Accessed February 9, 2023. http://www.ncbi.nlm.nih.gov/books/NBK493361/
7. Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med. 2010;122(1):184-191. doi:10.3810/pgm.2010.01.2112
8. Cunill R, Castells X, Tobias A, et al. Efficacy, safety and variability in pharmacotherapy for adults with attention deficit hyperactivity disorder: a meta-analysis and meta-regression in over 9000 patients. Psychopharmacology (Berl). 2016;233(2):187-197. doi:10.1007/s00213-015-4099-3
9. Lam AP, Matthies S, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Long-term effects of multimodal treatment on adult attention-deficit/hyperactivity disorder symptoms: follow-up analysis of the COMPAS Trial. JAMA Netw Open. 2019;2(5):e194980. doi:10.1001/jamanetworkopen.2019.4980
10. Nasser A, Hull JT, Chaturvedi SA, et al. A phase III, randomized, double-blind, placebo-controlled trial assessing the efficacy and safety of viloxazine extended-release capsules in adults with attention-deficit/hyperactivity disorder. CNS Drugs. 2022;36(8):897-915. doi:10.1007/s40263-022-00938-w
11. Kis B, Lücke C, Abdel-Hamid M, et al. Safety profile of methylphenidate under long-term treatment in adult ADHD patients - results of the COMPAS study. Pharmacopsychiatry. 2020;53(6):263-271. doi:10.1055/a-1207-9851
12. Cutler AJ, Childress AC, Pardo A, et al. Randomized, double-blind, placebo-controlled, fixed-dose study to evaluate the efficacy and safety of amphetamine extended-release tablets in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2022;83(5):22m14438. doi:10.4088/JCP.22m14438
13. Iwanami A, Saito K, Fujiwara M, et al. Efficacy and safety of guanfacine extended-release in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2020;81(3):19m12979. doi:10.4088/JCP.19m12979
14. Reimherr FW, Gift TE, Steans TA, et al. The use of brexpiprazole combined with a stimulant in adults with treatment-resistant attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2022;42(5):445-453. doi:10.1097/JCP.0000000000001592
15. Philipsen A, Jans T, Graf E, et al; Comparison of Methylphenidate and Psychotherapy in Adult ADHD Study (COMPAS) Consortium. Effects of group psychotherapy, individual counseling, methylphenidate, and placebo in the treatment of adult attention-deficit/hyperactivity disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(12):1199-1210.
16. McGough JJ. Treatment controversies in adult ADHD. Am J Psychiatry. 2016;173(10):960-966. doi:10.1176/appi.ajp.2016.15091207
17. Cruz MP. Guanfacine extended-release tablets (Intuniv), a nonstimulant selective alpha2a-adrenergic receptor agonist for attention-deficit/hyperactivity disorder. P T. 2010;35(8):448-451.
Transient global amnesia: Psychiatric precipitants, features, and comorbidities
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
Ms. A, age 48, is a physician’s assistant with no psychiatric history. She presents to the emergency department (ED) with her partner and daughter due to a 15-minute episode of acute-onset memory loss and concern for stroke. In the ED, Ms. A is confused and repeatedly asks, “Where are we?” “How did we get here?” and “What day is it?” Her partner denies Ms. A has focal neurologic deficits or seizures.
Ms. A had only slept 4 hours the night before she came to the ED because she had just learned that her daughter works in the sex industry. According to her daughter, Ms. A was raped by a soldier many years ago. At that time, her perpetrator told Ms. A that he would kill her entire family if she ever told anyone. As a result, she never pursued any psychological or psychiatric treatment.
During the evaluation, Ms. A shares details regarding her social and medical history; however, she does not recall receiving bad news the night before. She asks the interviewer several times how she got to the hospital, and when a cranial nerve exam is performed, she states, “I am not the patient!”
Ms. A’s vital signs and physical exam are unremarkable. Urinalysis is significant for a ketones level of 20 mmol/L (reference range: negative for ketones), and a urine human chorionic gonadotropin test is negative. A neurologic exam does not identify any focal deficits. No imaging is performed.
Transient global amnesia (TGA) describes an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. On presentation, patients experiencing TGA repeatedly ask, “Where am I?” “What day is it?” and “How did I get here?” However, semantic memory—such as knowledge of the world and autobiographical information—is preserved.1 The first case of TGA was described in 1956, and its diagnostic criteria were most recently modified in 1990 (Table2).
Though TGA is the most common cause of acute-onset amnesia, it is rare, affecting approximately 3 to 10 individuals per 100,000. The average age of onset is 61 to 63, with most cases occurring after age 50. TGA is generally thought to affect males and females equally, though some studies suggest a female predominance.3 In most cases (approximately 90%), there is a precipitating event such as physical or emotional stress, change in temperature, or sexual intercourse.4
In this article, we provide an overview of the classification, presentation, differential diagnosis, workup, and treatment of TGA. While TGA is a neurologic diagnosis, in a subset of patients it can present with psychiatric features resembling conversion disorder. For such patients, we argue that TGA can be considered a neuropsychiatric condition (Box 15-12). This classification may empower emergency psychiatry clinicians and psychotherapists to identify and treat the condition, which is not described by the current psychiatric diagnostic system.
Box 1
Transient global amnesia (TGA) is a neurologic diagnosis. However, in 1956, Bender8 associated the clinical picture of TGA with psychogenic etiology, 2 years before the term was coined. The same year, Courjon et al9 classified TGA as a functional disorder.
As recent literature on TGA has focused on the neuropsychologic mechanism of memory loss, examination of the condition from a psychodynamic standpoint has fallen out of favor. In fact, the earliest discussions of the condition attributed the absence of TGA from literature prior to the 1950s “to erroneous classification of TGA as psychogenic or hysterical amnesia.”10 However, to refer to this condition as purely neurologic—and without any “psychogenic” or functional features— would be reductive.
In a 2019 case report, Espiridion et al6 considered TGA within the same diagnostic realm as—if not actually a form of—dissociative amnesia (DA). They published the case of a 60-year-old woman with a history of posttraumatic stress disorder (PTSD) who experienced an episode of TGA that had manifested as anterograde and retrograde amnesia for 2 days and was precipitated by a psychotherapy session in which she discussed an individual who had assaulted her 5 years earlier. Much like in the case of Ms. A, the report from Espiridion et al6 clearly exemplifies a psychiatric etiology that shares similar context of a stressor unveiling a past memory too unbearable to maintain in consciousness. They concluded that “this case demonstrates anterograde and identity memory impairments likely induced by her PTSD. It is … possible that this presentation may be labeled PTSD-related dissociative amnesia.”6
Considering TGA as a type of DA within a subset of patients represents progression with regards to considering it as a psychiatric disorder. However, a prominent factor distinguishing TGA from DA is that the latter is more commonly associated with loss of personal identity.5 In TGA, memory of autobiographical information typically is preserved.7
Others have argued for a subtype of “emotional arousal–induced TGA”11 or “emotional TGA.”10 We suggest that this “emotional” subtype of TGA, which clearly was affecting Ms. A, shares similarities with functional neurologic symptom disorder, otherwise known as conversion disorder. The psychoanalytic concept that unconscious psychic distress can be “converted” into a neurologic problem is exemplified by Ms. A. Of note, being female and having an emotional stressor are risk factors for conversion disorder. Additionally, migraine— which was not part of Ms. A’s history—is also a risk factor for both TGA and conversion disorder.12 Despite these similarities, however, TGA’s neurophysiological changes on MRI and self-resolving nature still position the disorder as uniquely neuropsychiatric in the term’s purest sense.
Continue to: Differential diagnosis and workup
Differential diagnosis and workup
The differential diagnosis for acute-onset memory loss in the absence of other neurologic or psychiatric features is broad. It includes:
- dissociative amnesia
- ischemic amnesia
- transient epileptic amnesia
- toxic and metabolic amnesia
- posttraumatic amnesia.
Dissociative amnesia (DA), otherwise known as psychogenic amnesia, is “an inability to recall important autobiographical information, usually of a traumatic or stressful nature, that is inconsistent with ordinary forgetting.”13 According to this definition, DA features only retrograde amnesia, as opposed to TGA, which features anterograde amnesia, with possible retrograde amnesia. A subtype of DA—specifically, “continuous amnesia” or “anterograde dissociative amnesia”— is in DSM-5.13 However, the diagnostic criteria are unclear, and no cases have been identified in the literature since 1903, before TGA became a diagnostic entity.5,14 Moreover, patients with DA cannot recall autobiographical information, which is not a feature of TGA. Within DSM-5, TGA is an exclusion criterion for DA.13 Thus, an episode of anterograde amnesia with acute onset best meets criteria for TGA, even if there are substantial psychiatric risk factors.
Ischemic amnesia—including stroke and transient ischemic attack (TIA)—is often the primary concern of patients with TGA and their families upon initial presentation, as was the case with Ms. A.6,15 TIA presenting with isolated, acute-onset amnesia would be highly unusual, because these attacks usually present with focal symptoms including motor deficits, sensory deficits, visual field deficits, and aphasia or dysarthria. A patient with amnesia experiencing a TIA would likely have symptoms lasting from seconds to minutes, which is much shorter than a typical TGA episode.16
Amnesia secondary to stroke may be transient or permanent.7 Amnesia is present in approximately 1% of all strokes and in approximately 19.3% of posterior cerebral artery strokes.7,17 Unlike TIA and TGA, ischemic amnesia would present with MRI findings detectable at symptom onset. TGA does reveal MRI findings, particularly punctate lesions in the CA1 area of the hippocampus; however, these lesions are typically much smaller than those found in stroke, and are not detectable until 12 to 48 hours after episode onset.1,17 MRI findings in ischemic amnesia are typically associated with extrahippocampal lesions.17 Finally, the presence of vascular risk factors such as hyperlipidemia, smoking, diabetes, and hypertension may also favor a diagnosis of stroke or TIA as opposed to TGA, which is not associated with these risk factors.18 Though ischemic amnesia and TGA usually can be differentiated based on history and presentation, MRI with fluid-attenuated inversion recovery and diffusion-weighted imaging may be performed to definitively distinguish stroke from TGA.7
Transient epileptic amnesia (TEA), a focal form of epilepsy within the temporal lobe, should also be considered in patients who present with acute-onset amnesia. Like TGA, TEA may present with simultaneous anterograde and retrograde amnesia accompanied by repetitive questioning.19 Amnesia can be the sole symptom of TEA in up to 24% of cases. However, several key features distinguish TEA from TGA. TEA most often presents with other clinical signs of seizures, such as oral automatisms and/or olfactory hallucinations.20 There is also a significant difference in episode length; TEA episodes last an average of 30 to 60 minutes and tend to occur upon wakening, whereas TGA episodes last an average of 4 to 6 hours and do not preferentially occur at any particular time.1,21 In the interictal period—between seizures—patients with TEA may also experience accelerated long-term forgetting, autobiographical amnesia, and topographical amnesia.19,20 Finally, a diagnosis of TEA also requires recurrent episodes. Recurrence can happen with TGA, but is less frequent.21 Generally, history and presentation can distinguish TEA from TGA. Though there is no formal protocol for TEA workup, Lanzone et al21 recommend 24-hour EEG or EEG sleep monitoring in patients who present with amnesia as well as other clinical manifestations of epileptic phenomenon.
Continue to: Toxic and metabolic
Toxic and metabolic etiologies of amnesia include opioid and cocaine use, general anesthetics,22 and hypoglycemia.7,23 Toxic and metabolic causes of amnesia may mirror TGA in their acute onset as well as anterograde nature. However, these patients will likely present with fluctuating consciousness and/or other neuropsychiatric features, such as pressured speech, delusions, and/or distractability.23 Obtaining a patient’s medical history, including substance use, medication use, and the presence of diabetes,24 is typically sufficient to rule out toxic and metabolic causes.7
Posttraumatic amnesia (PTA) describes transient memory loss that occurs after a traumatic brain injury. Anterograde amnesia is most common, though approximately 20% of patients may also experience retrograde amnesia pertaining to the events near the date of their injury. Unlike TGA, which typically resolves within 24 hours, the recovery time of amnestic symptoms in PTA ranges from minutes to years.7 A distinguishing feature of PTA is the presence of confusion, which often resembles a state of delirium.25 The presentation of PTA can vary immensely with regards to agitation, psychotic symptoms, and the time to resolution of the amnesia. Though TGA can be distinguished from PTA based on a lack of clouding of consciousness, a case of anterograde amnesia warrants inquiry into a potential history of head injuries to rule out a traumatic cause.26
Box 21,3,23,27-33 outlines current theories of the etiology and pathogenesis of TGA.
Box 2
The etiology and pathogenesis of transient global amnesia (TGA) are poorly understood, and TGA remains one of the most enigmatic syndromes in clinical neurology.27 Theories regarding the pathogenesis of TGA are diverse and include vascular, epileptic, migraine, and stress-related etiologies.1,23
Early theories suggested arterial ischemia28 and epileptic phenomena29 as etiologies of TGA. The venous theory posits that TGA stems from jugular venous incompetency, causing venous flow and subsequent venous congestion in the medial temporal lobe, wherein lies the hippocampus. This theory is supported by several studies showing venous valve insufficiency as detected by ultrasonographic evaluation during the Valsalva maneuver in patients with TGA.30 This pathophysiologic mechanism may explain the occurrence of TGA in a specific cluster of cases, including men whose TGA episodes are precipitated by physical stress or the Valsalva maneuver.3 The migraine theory and stress theory share a similar proposed neurophysiologic mechanism.
The migraine theory stems from migraines being a known risk factor for TGA, particularly in middle-aged women.31 The stress theory is based on the known emotional precipitants and psychiatric comorbidities associated with TGA. Notably, both the migraine theory and stress theory implicate the role of excessive glutamate release as well as CNS depression.31,32 Glutamate targets the CA1 region of the hippocampus, which is involved in TGA and is known to have the highest density of N-methyl-D-aspartate receptors among hippocampal regions.33
Given the heterogeneity of the demographics and stressors associated with TGA, multiple mechanisms for the disease process may coexist, leading to a similar clinical picture. In 2006, Quinette et al3 performed a multivariate analysis of variables associated with TGA, including age, sex, medical history, and presentation. They demonstrated 3 “clusters” of TGA pictures: women with anxiety or a personality disorder; men with physical precipitating events; and younger patients (age <56) with a history of migraine. These findings suggest TGA may have unique precipitants corresponding to multiple neurophysiologic mechanisms.
Transient global ischemia: Psychiatric features
Several studies have demonstrated psychiatric precipitants, features, and comorbidities associated with TGA. Of the TGA cases associated with precipitating events, 29% to 50% are associated with an emotional stressor.3,4 Examples of emotional stressors include a quarrel,4 the announcement of a birth or suicide, and a nightmare.15 For Ms. A, learning her daughter worked in the sex industry was an emotional stressor.2
During its acute phase, TGA has been shown to present with mood and anxiety symptoms.34 Moreover, during episodes, patients often demonstrate features of panic attacks, such as dizziness, fainting, choking, palpitations, and paresthesia.3,35
Continue to: Finally, patients with TGA...
Finally, patients with TGA are more likely to have psychiatric comorbidities than those without the condition. In a study of 25 patients who experienced TGA triggered by a precipitant, Inzitari et al4 found a strong association of TGA with phobic personality traits, including agoraphobia and simple phobic attitudes (ie, fear of traveling far from home or the sight of blood). Pantoni et al35 replicated these results in 2005 and found that in comparison to patients with TIA, patients with TGA are more likely to have personal and family histories of psychiatric disease. A 2014 study by Dohring et al36 found that compared to healthy controls, patients with TGA are more likely to have maladaptive coping strategies and stress responses. Patients with TGA tended to exhibit increased feelings of guilt, take more medication, and exhibit more anxiety compared to healthy controls.36
Treatment: Benzodiazepines
There are no published treatment guidelines for TGA. However, in case reports, benzodiazepines (specifically lorazepam37) have been shown to have utility in diagnosing and treating DA. The success of benzodiazepines is attributed to its gamma-aminobutyric acid mechanism, which involves inhibiting activity of the N-methyl-
However, the benzodiazepine midazolam has been identified as a precipitant of TGA. In a case report, Rinehart et al22 identified flumazenil—a benzodiazepine antagonist used primarily to treat retrograde postoperative amnesia—as an antidote. The potentially paradoxical role of benzodiazepines in both the precipitation and treatment of TGA may relate back to the heterogeneity of the etiologies of TGA. Further research comparing the treatment of TGA in patients with stress-induced TGA vs postoperative TGA is needed to better understand the neurochemical basis of TGA and work toward establishing optimal treatment options for different patient demographics.
A generally favorable prognosis
TGA carries a low risk of recurrence. In studies with 3- to 7-year follow-up periods, the recurrence rates ranged from 1.4% to 23.8%.23,35,38
Memory impairments may be present for 5 to 6 months following a TGA episode. The severity of these impairments may range from clinically unnoticeable to the patient meeting the criteria for mild cognitive impairment.23,39 The risk is higher in patients who have had recurrent TGA compared to those patients who have experienced only a single episode.23
Continue to: TGA does not increase...
TGA does not increase the risk of cerebrovascular events. There is controversy regarding a potentially increased risk for dementia as well as epilepsy, though there is insufficient evidence to support these findings.23,40
CASE CONTINUED
Five hours after the onset of Ms. A’s symptoms, the treatment team initiates oral lorazepam 1 mg. One hour after taking lorazepam, Ms. A’s anterograde and retrograde amnesia improve. She cannot recall why she was brought to the hospital but does remember the date and location, which she was not able to do on initial presentation. She feels safe, states a clear plan for self-care, and is discharged in the care of her partner. Though Ms. A’s memory improved soon after she received lorazepam, this improvement also could be attributed to the natural course of time, as TGA tends to resolve on its own within 24 hours.
Bottom Line
Transient global amnesia (TGA) is an episode of anterograde, and possibly retrograde, amnesia that lasts up to 24 hours. It represents an interesting diagnosis at the intersection of psychiatry and neurology. TGA has many established psychiatric risk factors and features—some of which may resemble conversion disorder—but these may only apply to a particular subset of patients, which reflects the heterogeneity of the condition.
Related Resources
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part I: pathophysiology and etiology. J Clin Med. 2022;11(12): 3373. doi:10.3390/jcm1112337
- Sparaco M, Pascarella R, Muccio CF, et al. Forgetting the unforgettable: transient global amnesia part II: a clinical road map. J Clin Med. 2022;11(14):3940. doi:10.3390/ jcm11143940
Drug Brand Names
Flumazenil • Romazicon
Lorazepam • Ativan
Midazolam • Versed
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
1. Miller TD, Butler CR. Acute-onset amnesia: transient global amnesia and other causes. Pract Neurol. 2022;22(3):201-208. doi:10.1136/practneurol-2020-002826
2. Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J Neurol Neurosurg Psychiatry. 1990;53(10):834-843. doi:10.1136/jnnp.53.10.834
3. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129(Pt 7):1640-1658. doi:10.1093/brain/awl105
4. Inzitari D, Pantoni L, Lamassa M, et al. Emotional arousal and phobia in transient global amnesia. Arch Neurol. 1997;54(7):866-873. doi:10.1001/archneur.1997.00550190056015
5. Staniloiu A, Markowitsch HJ. Dissociative amnesia. Lancet Psychiatry. 2014;1(3):226-241. doi:10.1016/S2215-0366(14)70279-2
6. Espiridion ED, Gupta J, Bshara A, et al. Transient global amnesia in a 60-year-old female with post-traumatic stress disorder. Cureus. 2019;11(9):e5792. doi:10.7759/cureus.5792
7. Alessandro L, Ricciardi M, Chaves H, et al. Acute amnestic syndromes. J Neurol Sci. 2020;413:116781. doi:10.1016/j.jns.2020.116781
8. Bender M. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212-215.
9. Courjon J, Guyotat J. Les ictus amnéstiques [Amnesic strokes]. J Med Lyon. 1956;37(882):697-701.
10. Noel A, Quinette P, Hainselin M, et al. The still enigmatic syndrome of transient global amnesia: interactions between neurological and psychopathological factors. Neuropsychol Rev. 2015;25(2):125-133. doi:10.1007/s11065-015-9284-y
11. Merriam AE, Wyszynski B, Betzler T. Emotional arousal-induced transient global amnesia. A clue to the neural transcription of emotion? Psychosomatics. 1992;33(1):109-113. doi:10.1016/S0033-3182(92)72029-5
12. Hallett M, Aybek S, Dworetzky BA, et al. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537-550. doi:10.1016/S1474-4422(21)00422-1
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013.
14. Bourdon B, Dide M. A case of continuous amnesia with tactile asymbolia, complicated by other troubles. Ann Psychol. 1903;10:84-115.
15. Marinella MA. Transient global amnesia and a father’s worst nightmare. N Engl J Med. 2004;350(8):843-844. doi:10.1056/NEJM200402193500821
16. Amarenco P. Transient ischemic attack. N Engl J Med. 2020;382(20):1933-1941. doi:10.1056/NEJMcp1908837
17. Szabo K, Forster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40(6):2042-2045. doi:10.1161/STROKEAHA.108.536144
18. Liampas I, Raptopoulou M, Siokas V, et al. Conventional cardiovascular risk factors in transient global amnesia: systematic review and proposition of a novel hypothesis. Front Neuroendocrinol. 2021;61:100909. doi:10.1016/j.yfrne.2021.100909
19. Zeman A, Butler C. Transient epileptic amnesia. Curr Opin Neurol. 2010;23(6):610-616. doi:10.1097/WCO.0b013e32834027db
20. Baker J, Savage S, Milton F, et al. The syndrome of transient epileptic amnesia: a combined series of 115 cases and literature review. Brain Commun. 2021;3(2):fcab038. doi:10.1093/braincomms/fcab038
21. Lanzone J, Ricci L, Assenza G, et al Transient epileptic and global amnesia: real-life differential diagnosis. Epilepsy Behav. 2018;88:205-211. doi:10.1016/j.yebeh.2018.07.015
22. Rinehart JB, Baker B, Raphael D. Postoperative global amnesia reversed with flumazenil. Neurologist. 2012;18(4):216-218. doi:10.1097/NRL.0b013e31825bbef4
23. Arena JE, Rabinstein AA. Transient global amnesia. Mayo Clin Proc. 2015;90(2):264-272. doi:10.1016/j.mayocp.2014.12.001
24. Holemans X, Dupuis M, Misson N, et al. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18(9):761-763. doi:10.1046/j.1464-5491.2001.00481.x
25. Marshman LAG, Jakabek D, Hennessy M, et al. Post-traumatic amnesia. J Clin Neurosci. 2013;20(11):1475-1481. doi:10.1016/j.jocn.2012.11.022
26. Parker TD, Rees R, Rajagopal S, et al. Post-traumatic amnesia. Pract Neurol. 2022;22(2):129-137. doi:10.1136/practneurol-2021-003056
27. You SH, Kim B, Kim BK. Transient global amnesia: signal alteration in 2D/3D T2-FLAIR sequences. Clin Imaging. 2021;78:154-159. doi:10.1016/j.clinimag.2021.03.029
28. Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5(3):303-311. doi:10.1161/01.str.5.3.303
29. Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(SUPPL 9):1-83.
30. Cejas C, Cisneros LF, Lagos R, et al. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41(1):67-71. doi:10.1161/STROKEAHA.109.566315
31. Liampas I, Siouras AS, Siokas V, et al. Migraine in transient global amnesia: a meta-analysis of observational studies. J Neurol. 2022;269(1):184-196. doi:10.1007/s00415-020-10363-y
32. Ding X, Peng D. Transient global amnesia: an electrophysiological disorder based on cortical spreading depression-transient global amnesia model. Front Hum Neurosci. 2020;14:602496. doi:10.3389/fnhum.2020.602496
33. Bartsch T, Dohring J, Reuter S, et al. Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab. 2015;35(11):1836-1845. doi:10.1038/jcbfm.2015.137
34. Noel A, Quinette P, Guillery-Girard B, et al. Psychopathological factors, memory disorders and transient global amnesia. Br J Psychiatry. 2008;193(2):145-151. doi:10.1192/bjp.bp.107.045716
35. Pantoni L, Bertini E, Lamassa M, et al. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12(5):350-356. doi:10.1111/j.1468-1331.2004.00982.x
36. Dohring J, Schmuck A, Bartsch T. Stress-related factors in the emergence of transient global amnesia with hippocampal lesions. Front Behav Neurosci. 2014;8:287. doi:10.3389/fnbeh.2014.00287
37. Jiang S, Gunther S, Hartney K, et al. An intravenous lorazepam infusion for dissociative amnesia: a case report. Psychosomatics. 2020;61(6):814-818. doi:10.1016/j.psym.2020.01.009
38. He S, Ye Z, Yang Q, et al. Transient global amnesia: risk factors, imaging features, and prognosis. Neuropsychiatr Dis Treat. 2021;17:1611-1619. doi:10.2147/NDT.S299168
39. Borroni B, Agosti C, Brambilla C, et al. Is transient global amnesia a risk factor for amnestic mild cognitive impairment? J Neurol. 2004;251(9):1125-1127. doi:10.1007/s00415-004-0497-x
40. Liampas I, Raptopoulou M, Siokas V, et al. The long-term prognosis of transient global amnesia: a systematic review. Rev Neurosci. 2021;32(5):531-543. doi:10.1515/revneuro-2020-0110
Visual hallucinations: Differentiating psychiatric and neurologic causes
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.
Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.