User login
Psoriasis Symptoms With the Greatest Impact on Patients
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Flaking/scaling and itching, followed by dry cracked skin that may bleed, pain or soreness, and burning/stinging were noted by psoriasis patients as the symptoms with the most significant impact on daily life in a public meeting hosted by the US Food and Drug Administration (FDA) to hear patient perspectives on psoriasis. Approximately 70 psoriasis patients or patient representatives attended the meeting in person and others attended through a live webcast.
More than two-thirds of respondents identified flaking/scaling as one of their most significant symptoms of psoriasis, either localized to psoriasis-prone areas such as the elbows and knees or more widespread. Patients reported that this symptom is constant, leaving them to absentmindedly rub certain areas of the skin.
A similar number of respondents indicated that itching was their most significant symptom. One patient called it “an intense subcutaneous itch… deep down in the skin,” a description that resonated with other patients in the room.
Nearly 40% identified dry cracked skin that may bleed as a significant symptom, noting that areas where skin is thinner are affected more, such as the folds of the body. Patients described this symptom as interrelated with other symptoms such as itching. “The thicker the scales get on my skin, the more they itch, and the more they itch, the more I am likely to scratch them, and the more I scratch them, the more they start to crack, and then more come back and it keeps going and going,” one patient said.
More than one-quarter of respondents indicated that pain, soreness, or burning/stinging were the most significant symptoms. Patients indicated that the stinging/burning was more episodic, while the pain was more constant, with the pain being under the skin.
Triggers of these symptoms included stress (primary trigger), changes in weather, hormonal changes, diet, lotions, prolonged exposure to sunlight, sweat, aging, and other medical conditions.
Dermatologists may use these patient insights to prescribe therapies that target these symptoms.
The psoriasis public meeting in March 2016 was the FDA’s 18th patient-focused drug development meeting. The FDA sought this information to have a greater understanding of the burden of psoriasis on patients and the treatments currently used to treat psoriasis and its symptoms. This information will help guide the FDA as they consider future drug approvals.
Novel Solution for Massive Glenoid Defects in Shoulder Arthroplasty: A Patient-Specific Glenoid Vault Reconstruction System
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
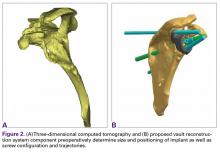
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
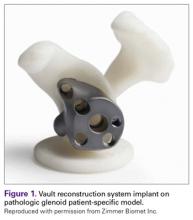
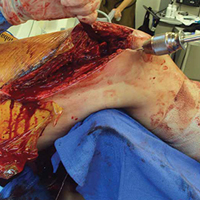
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
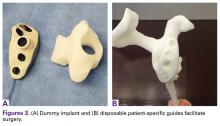
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
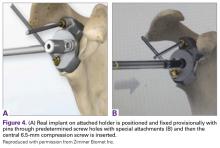
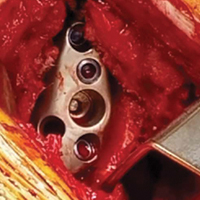
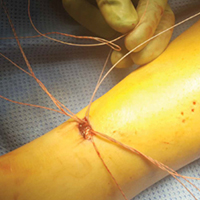
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
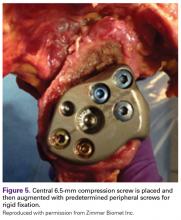
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
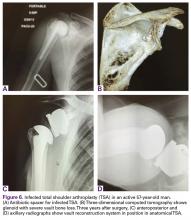
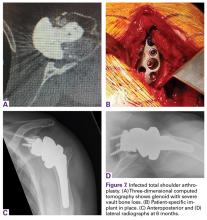
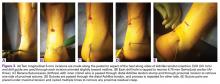
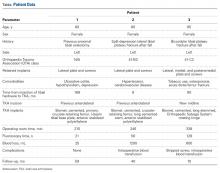
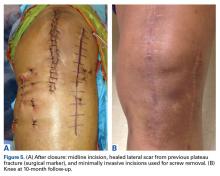
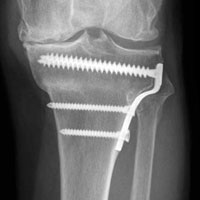
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- With more shoulder arthroplasties being performed on younger patients, we can expect more revisions in the future.
- Many of these revision cases will have profound glenoid bone loss.
- Bone grafting the glenoid defects in shoulder arthroplasty has been less successful especially with significant vault defects.
- Based on the CAD-CAM success in total hip and knee replacement surgery, a patient-specific glenoid vault reconstruction system has been developed by Zimmer Biomet to deal with profound glenoid bone loss and cuff insufficiency.
- Early results of this vault reconstruction system have been promising in these most difficult clinical situations.
Early results of this vault reconstruction system have been promising in these most difficult clinical situations. Complex glenoid deformities present the most difficult challenges in shoulder arthroplasty (SA). These deformities may be caused by severe degenerative or congenital deformity, posttraumatic anatomy, tumor, or, in most cases, bone loss after glenoid failure in anatomical total SA.
Walch and colleagues1 described the pathologic glenoid lesions seen in progressive degenerative arthritis and some congenital defects. The most severe were initially characterized as Walch B2 and Walch C deformities. These lesions have been further classified to include Walch B3 posteroinferior glenoid deformities.2,3 Each of these deformities can result in severe glenoid vault deficiency.
In some revision cases and in severe rheumatoid cases, these deformities can present as cavitary lesions with or without failure of the glenoid rim or wall resulting in significant compromise of glenoid vault lesions.4,5 In these cases, the degree of “medialization” of the native glenohumeral joint line and the amount of peripheral bone loss can have profound effects on the amount of bone available for fixation and on the ability to allow component positioning for best surgical and biomechanical outcomes.
Other bone loss deformities, which have been described by Antuna and colleagues6 and Seebauer and colleagues,7 often accompany disease processes with severe cuff deficiency. These deformities historically have been treated with intercalary-type bone grafts in 1- or 2-stage revision of reverse SA or in salvage to hemiarthroplasty. Treatment of these pathologies with the technique described produced only fair results in short-term to midterm follow-up. The most commonly reported complications have been component loosening, bone graft failure, infection, and instability.8-11Borrowing from hip and knee arthroplasty surgeons’ experience in using CAD/CAM (computer-aided design/computer-aided manufacturing) patient-specific implants to fill significant bony defects, Dr. D. M. Dines and Dr. Craig developed a patient-specific glenoid vault reconstruction system (VRS) in conjunction with the Comprehensive Shoulder Arthroplasty System (Zimmer Biomet). For a number of years, the Food and Drug Administration allowed this patient-specific glenoid VRS component to be made available only as a custom implant. Recently, however, full 510K clearance was granted to use the VRS in reverse SA patients with severe soft-tissue deficiency and significant glenoid bone loss.
In this article, we describe the implant and its indications, technical aspects of production, and surgical technique.
Vault Reconstruction System
Severe glenoid bone loss often requires an implant that specifically matches the patient’s anatomy. The patient-specific glenoid VRS (Figure 1) is made from a 3-dimensional reconstruction of a 2-dimensional computed tomography image.
In some cases in which the bone is sufficient to enhance fixation in the deficient glenoid vault, a custom boss may be added to the implant, as well as a custom guide matching the implant.
Glenoid Exposure
In most cases of severe glenoid bone loss, the associated soft-tissue deficiency allows for easier glenoid exposure. In this implant system, however, maximal peripheral en face exposure of the glenoid is required. In addition, it is mandatory to avoid disturbing the remaining glenoid bone surfaces, which often are thin or fragile, because the patient-specific implant is referenced to this anatomy. Bone that is not maintained changes the orientation of the patient-specific guide and ultimately the fixation of the component. Using the correct retractors and meticulously excising soft-tissue scar tissue are crucial for success.
Implant Positioning
With the glenoid surface properly exposed, the removable inserter handle and the built-in lip on the implant are used to position the patient-specific guide. Next, a central guide pin is placed through the inserter for temporary fixation and further instrumentation. If enough bone is present, a boss reamer can be used over the guide pin to prepare and increase the fixation surface.
The central 6.5-mm nonlocking compression screw is placed to provide strong initial compressive fixation in best bone.
With the patient-specific glenoid VRS implant now rigidly fixed in the glenoid, the sized and offset glenosphere is properly positioned, and the reverse SA is completed in routine fashion.
Case Examples
A 49-year-old man underwent hemiarthroplasty for osteoarthritis. The procedure failed and, 3 years later, was revised to conventional total SA. Unfortunately, the cemented all-polyethylene glenoid loosened secondary to active Propionibacterium acnes infection, which required excisional arthroplasty with antibiotic spacer. Significant cavitary bone loss was found with anterior glenoid wall bone loss compromising the glenoid vault. Given the history of bone loss and infection, patient-specific glenoid vault reconstruction was performed after infection eradication. Within 4 years after this surgery, the patient had resumed all activities. At age 57 years, he had restricted active forward elevation and abduction to 120° but was satisfied with the outcome.
A 71-year-old man underwent reverse SA for rotator cuff-deficient osteoarthritis. After implant excision and spacer placement, he was left with severe soft-tissue deficiency and glenoid bone loss, which caused substantial disability. After treatment for infection, a work-up was performed for glenoid bone deficiency and insertion of a patient-specific glenoid VRS implant.
Discussion
Glenoid bone deformity and deficiency are among the most difficult challenges in SA—a particularly compelling fact given the increasing number of SAs being performed in younger, more active patients. SA surgeons can now expect to be performing even more revisions with concomitant bone defects, which may be severe in some cases.
In addition to these causes of extreme bone loss, recent awareness of the importance of recognizing and treating bone deficits in osteoarthritis, rheumatoid arthritis, trauma, and instability has led to the development of patient-specific guides, instrumentation, and implants. Concepts from the use of CAD/CAM acetabular implants in total hip arthroplasty for severe acetabular bony defects were applied to the use of patient-specific glenoid reconstruction implants without bone graft augmentation.12 In different form, this idea was reported by Chammaa and colleagues13 in 30 cases, and clinical and durable results were very promising.
We have described use of this technique in 2 extreme cases of glenoid vault deficiency. In each case, short-term results were quite satisfactory. However, both patients were relatively young, and long-term clinical and radiographic follow-up is needed.
Many of the severe cases of glenoid bone loss require an implant that specifically matches the patient’s anatomy. The glenoid VRS implant described here may be of great benefit in these difficult reconstructions and is a valuable addition to the armamentarium of treatments for distorted glenoid anatomy. Eventually, the idea may become useful in treating other, less significant defects by re-creating more-normal biomechanics in SA without bone graft.
Am J Orthop. 2017;46(2):104-108. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
1. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
2. Chan K, Knowles NK, Chaoui J, et al. Characterization of the Walch B3 glenoid in primary osteoarthritis [published online January 11, 2017]. J Shoulder Elbow Surg. doi:10.1016/j.jse.2016.10.003.
3. Bercik MJ, Kruse K 2nd, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25(10):1601-1606.
4. Sears BW, Johnston PS, Ramsay ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20(9):604-613.
5. Kocsis G, Thyagarajan DS, Fairbairn KJ, Wallace WA. A new classification of glenoid bone loss to help plan the implantation of a glenoid component before revision arthroplasty of the shoulder. Bone Joint J. 2016;98(3):374-380.
6. Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10(3):217-224.
7. Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy [in English, German]. Oper Orthop Traumatol. 2005;17(1):1-24.
8. Iannotti JP, Frangiamore SJ. Fate of large structural allograft for treatment of severe uncontained glenoid bone deficiency. J Shoulder Elbow Surg. 2012:21(6):765-771.
9. Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83(6):877-883.
10. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
11. Hsu JE, Ricchetti ET, Huffman GR, Iannotti JP, Glaser DL. Addressing glenoid bone deficiency and asymptomatic posterior erosion in shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(9):1298-1308.
12. Gunther SB, Lynch TL. Total shoulder replacement surgery with custom glenoid implants for severe bone deficiency. J Shoulder Elbow Surg. 2012;21(5):675-684.
13. Chammaa R, Uri O, Lambert S. Primary shoulder arthroplasty using a custom-made hip-inspired implant for the treatment of advanced glenohumeral arthritis in the presence of severe glenoid bone loss. J Shoulder Elbow Surg. 2017;26(1):101-107.
Debunking Psoriasis Myths: Do Systemic Steroids Used in Psoriasis Patients Cause Pustular Psoriasis?
Myth: Systemic steroids cause pustular psoriasis
The advent of biologic therapy for psoriasis has changed the landscape of treatments offered to patients. Nevertheless, systemic therapies still play an important role, according to the American Academy of Dermatology psoriasis treatment guidelines, due to their oral route of administration and low cost compared to biologics. They are options for patients with moderate to severe psoriasis that is unresponsive to topical therapies or phototherapy. However, many dermatologists feel that it is inappropriate to prescribe oral steroids to psoriasis patients due to the risk for steroid-induced conversion to pustular psoriasis, the long-term side effects of steroids, and deterioration of psoriasis after withdrawal of steroids.
Pustular psoriasis appears clinically as white pustules (blisters of noninfectious pus) surrounded by red skin. The pus consists of white blood cells. There are a number of triggers in addition to systemic steroids, such as internal medications, irritating topical agents, overexposure to UV light, and pregnancy. Stopping an oral steroid abruptly can cause serious disease flares, fatigue, and joint pain.
Westphal et al described the case of a 70-year-old woman with palmoplantar psoriasis who was diagnosed with acute generalized exanthematous pustulosis that was treated with corticotherapy by injection and then oral prednisone. She experienced improvement, but her symptoms worsened when she was in the process of reducing the prednisone dose. The dose was increased again, and the same worsening of symptoms was experienced when the dose was reduced. After completely abandoning oral steroid therapy, she developed a severe case of generalized pustular psoriasis that was treated with acitretin. This case illustrates the dangerous consequences of abruptly discontinuing oral steroids.
However, dermatologists may be using oral steroids for psoriasis more often than treatment guidelines suggest. In 2014, Al-Dabagh et al evaluated how frequently systemic corticosteroids are prescribed for psoriasis in the United States. The researchers reported, "Despite the absence or discouragement of systemic corticosteroids in psoriasis management guidelines, systemic corticosteroids are among the most common systemic treatments used for psoriasis." They found that systemic corticosteroids were prescribed at 650,000 of 21,020,000 psoriasis visits, of which 93% were visits to dermatologists. Prednisone was the most commonly prescribed systemic corticosteroid, followed by methylprednisolone and dexamethasone. To prevent rebound flares, systemic corticosteroids were prescribed with a topical corticosteroid in 45% of the visits in patients with psoriasis as the sole diagnosis. They concluded, "The striking contrast between the guidelines for psoriasis management and actual practice suggests that there is an acute need to better understand the use of systemic corticosteroids for psoriasis."
The benefits of systemic corticosteroids versus the frequency of adverse reactions should be weighed by dermatologists and patients to make evidence-based decisions about treatment. Patients should take oral steroids exactly as prescribed by physicians.
References
Al-Dabagh A, Al-Dabagh R, Davis SA, et al. Systemic corticosteroids are frequently prescribed for psoriasis. J Cutan Med Surg. 2014;18:195-199.
Delzell E. What you need to know about steroids. National Psoriasis Foundation website. https://www.psoriasis.org/advance/what-you-need-to-know-about-steroids. Published September 2, 2015. Accessed January 13, 2017.
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
Pustular psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/types/pustular. Accessed January 13, 2017.
Westphal DC, Schettini APM, de Souza PP, et al. Generalized pustular psoriasis induced by systemic steroid dose reduction. An Bras Dermatol. 2016;91:664-666.
Expert Commentaries on next page
Expert Commentaries
When I was a resident, I was trained not to use systemic steroids in psoriasis patients for the reasons noted above, and I have faithfully followed these instructions 9 years into practice. However, I see many patients with severe psoriasis who are given systemic steroids by other physicians (ie, rheumatologists for psoriatic arthritis, pulmonologists for asthma). I often tell patients afterwards of the dangers of systemic steroids and to have them tell their other doctors to be cautious when giving another course of systemic steroids. However, I have yet to see a generalized pustular psoriasis outbreak or flare in psoriasis vulgaris after a course of systemic steroids. While I do not recommend systemic steroids for psoriasis patients since we have so many other systemic agents, I wonder if the risks that we were all trained about are really that high.
—Jashin J. Wu, MD (Los Angeles, California)
How bad is it to give patients with psoriasis systemic steroids? Are psoriasis patients treated with systemic steroids likely to get a pustular flare? Are patients with psoriasis who suddenly stop their corticosteroids more likely to get a pustular flare than psoriasis patients who suddenly stop other systemic psoriasis treatments? I don't have the answers to these questions. My sense is that we have a lot of dogma and strong opinions but very little hard evidence to answer these questions.
I don't typically prescribe systemic steroids to psoriasis patients, but systemic steroids are widely used. Sometimes there are problems. I have seen patients who received systemic steroids for psoriasis and who went on to have a pustular flare, but it's possible the systemic steroid was given because those patients were headed toward the pustular flare already.
I once had a psoriasis patient who came to see me with a suddenly inflamed tender joint. Not knowing what to do, I called a rheumatologist to see the patient. The rheumatologist, too busy to work the patient in, told me to give the patient a 2-week prednisone taper. I did, and nothing untoward happened with the psoriasis. This one anecdote doesn't give me much confidence that systemic steroids are safe for psoriasis patients.
Clearly, long-term steroids cause a host of problems (eg, osteoporosis, diabetes). But I'm not sure that the dogma that systemic steroids should be avoided in patients with psoriasis is well supported. Systemic steroids are being widely used, and I don't see an epidemic of pustular flares.
Is it a mistake to give systemic steroids to psoriasis patients? I just don't know.
—Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina)
Myth: Systemic steroids cause pustular psoriasis
The advent of biologic therapy for psoriasis has changed the landscape of treatments offered to patients. Nevertheless, systemic therapies still play an important role, according to the American Academy of Dermatology psoriasis treatment guidelines, due to their oral route of administration and low cost compared to biologics. They are options for patients with moderate to severe psoriasis that is unresponsive to topical therapies or phototherapy. However, many dermatologists feel that it is inappropriate to prescribe oral steroids to psoriasis patients due to the risk for steroid-induced conversion to pustular psoriasis, the long-term side effects of steroids, and deterioration of psoriasis after withdrawal of steroids.
Pustular psoriasis appears clinically as white pustules (blisters of noninfectious pus) surrounded by red skin. The pus consists of white blood cells. There are a number of triggers in addition to systemic steroids, such as internal medications, irritating topical agents, overexposure to UV light, and pregnancy. Stopping an oral steroid abruptly can cause serious disease flares, fatigue, and joint pain.
Westphal et al described the case of a 70-year-old woman with palmoplantar psoriasis who was diagnosed with acute generalized exanthematous pustulosis that was treated with corticotherapy by injection and then oral prednisone. She experienced improvement, but her symptoms worsened when she was in the process of reducing the prednisone dose. The dose was increased again, and the same worsening of symptoms was experienced when the dose was reduced. After completely abandoning oral steroid therapy, she developed a severe case of generalized pustular psoriasis that was treated with acitretin. This case illustrates the dangerous consequences of abruptly discontinuing oral steroids.
However, dermatologists may be using oral steroids for psoriasis more often than treatment guidelines suggest. In 2014, Al-Dabagh et al evaluated how frequently systemic corticosteroids are prescribed for psoriasis in the United States. The researchers reported, "Despite the absence or discouragement of systemic corticosteroids in psoriasis management guidelines, systemic corticosteroids are among the most common systemic treatments used for psoriasis." They found that systemic corticosteroids were prescribed at 650,000 of 21,020,000 psoriasis visits, of which 93% were visits to dermatologists. Prednisone was the most commonly prescribed systemic corticosteroid, followed by methylprednisolone and dexamethasone. To prevent rebound flares, systemic corticosteroids were prescribed with a topical corticosteroid in 45% of the visits in patients with psoriasis as the sole diagnosis. They concluded, "The striking contrast between the guidelines for psoriasis management and actual practice suggests that there is an acute need to better understand the use of systemic corticosteroids for psoriasis."
The benefits of systemic corticosteroids versus the frequency of adverse reactions should be weighed by dermatologists and patients to make evidence-based decisions about treatment. Patients should take oral steroids exactly as prescribed by physicians.
References
Al-Dabagh A, Al-Dabagh R, Davis SA, et al. Systemic corticosteroids are frequently prescribed for psoriasis. J Cutan Med Surg. 2014;18:195-199.
Delzell E. What you need to know about steroids. National Psoriasis Foundation website. https://www.psoriasis.org/advance/what-you-need-to-know-about-steroids. Published September 2, 2015. Accessed January 13, 2017.
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
Pustular psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/types/pustular. Accessed January 13, 2017.
Westphal DC, Schettini APM, de Souza PP, et al. Generalized pustular psoriasis induced by systemic steroid dose reduction. An Bras Dermatol. 2016;91:664-666.
Expert Commentaries on next page
Expert Commentaries
When I was a resident, I was trained not to use systemic steroids in psoriasis patients for the reasons noted above, and I have faithfully followed these instructions 9 years into practice. However, I see many patients with severe psoriasis who are given systemic steroids by other physicians (ie, rheumatologists for psoriatic arthritis, pulmonologists for asthma). I often tell patients afterwards of the dangers of systemic steroids and to have them tell their other doctors to be cautious when giving another course of systemic steroids. However, I have yet to see a generalized pustular psoriasis outbreak or flare in psoriasis vulgaris after a course of systemic steroids. While I do not recommend systemic steroids for psoriasis patients since we have so many other systemic agents, I wonder if the risks that we were all trained about are really that high.
—Jashin J. Wu, MD (Los Angeles, California)
How bad is it to give patients with psoriasis systemic steroids? Are psoriasis patients treated with systemic steroids likely to get a pustular flare? Are patients with psoriasis who suddenly stop their corticosteroids more likely to get a pustular flare than psoriasis patients who suddenly stop other systemic psoriasis treatments? I don't have the answers to these questions. My sense is that we have a lot of dogma and strong opinions but very little hard evidence to answer these questions.
I don't typically prescribe systemic steroids to psoriasis patients, but systemic steroids are widely used. Sometimes there are problems. I have seen patients who received systemic steroids for psoriasis and who went on to have a pustular flare, but it's possible the systemic steroid was given because those patients were headed toward the pustular flare already.
I once had a psoriasis patient who came to see me with a suddenly inflamed tender joint. Not knowing what to do, I called a rheumatologist to see the patient. The rheumatologist, too busy to work the patient in, told me to give the patient a 2-week prednisone taper. I did, and nothing untoward happened with the psoriasis. This one anecdote doesn't give me much confidence that systemic steroids are safe for psoriasis patients.
Clearly, long-term steroids cause a host of problems (eg, osteoporosis, diabetes). But I'm not sure that the dogma that systemic steroids should be avoided in patients with psoriasis is well supported. Systemic steroids are being widely used, and I don't see an epidemic of pustular flares.
Is it a mistake to give systemic steroids to psoriasis patients? I just don't know.
—Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina)
Myth: Systemic steroids cause pustular psoriasis
The advent of biologic therapy for psoriasis has changed the landscape of treatments offered to patients. Nevertheless, systemic therapies still play an important role, according to the American Academy of Dermatology psoriasis treatment guidelines, due to their oral route of administration and low cost compared to biologics. They are options for patients with moderate to severe psoriasis that is unresponsive to topical therapies or phototherapy. However, many dermatologists feel that it is inappropriate to prescribe oral steroids to psoriasis patients due to the risk for steroid-induced conversion to pustular psoriasis, the long-term side effects of steroids, and deterioration of psoriasis after withdrawal of steroids.
Pustular psoriasis appears clinically as white pustules (blisters of noninfectious pus) surrounded by red skin. The pus consists of white blood cells. There are a number of triggers in addition to systemic steroids, such as internal medications, irritating topical agents, overexposure to UV light, and pregnancy. Stopping an oral steroid abruptly can cause serious disease flares, fatigue, and joint pain.
Westphal et al described the case of a 70-year-old woman with palmoplantar psoriasis who was diagnosed with acute generalized exanthematous pustulosis that was treated with corticotherapy by injection and then oral prednisone. She experienced improvement, but her symptoms worsened when she was in the process of reducing the prednisone dose. The dose was increased again, and the same worsening of symptoms was experienced when the dose was reduced. After completely abandoning oral steroid therapy, she developed a severe case of generalized pustular psoriasis that was treated with acitretin. This case illustrates the dangerous consequences of abruptly discontinuing oral steroids.
However, dermatologists may be using oral steroids for psoriasis more often than treatment guidelines suggest. In 2014, Al-Dabagh et al evaluated how frequently systemic corticosteroids are prescribed for psoriasis in the United States. The researchers reported, "Despite the absence or discouragement of systemic corticosteroids in psoriasis management guidelines, systemic corticosteroids are among the most common systemic treatments used for psoriasis." They found that systemic corticosteroids were prescribed at 650,000 of 21,020,000 psoriasis visits, of which 93% were visits to dermatologists. Prednisone was the most commonly prescribed systemic corticosteroid, followed by methylprednisolone and dexamethasone. To prevent rebound flares, systemic corticosteroids were prescribed with a topical corticosteroid in 45% of the visits in patients with psoriasis as the sole diagnosis. They concluded, "The striking contrast between the guidelines for psoriasis management and actual practice suggests that there is an acute need to better understand the use of systemic corticosteroids for psoriasis."
The benefits of systemic corticosteroids versus the frequency of adverse reactions should be weighed by dermatologists and patients to make evidence-based decisions about treatment. Patients should take oral steroids exactly as prescribed by physicians.
References
Al-Dabagh A, Al-Dabagh R, Davis SA, et al. Systemic corticosteroids are frequently prescribed for psoriasis. J Cutan Med Surg. 2014;18:195-199.
Delzell E. What you need to know about steroids. National Psoriasis Foundation website. https://www.psoriasis.org/advance/what-you-need-to-know-about-steroids. Published September 2, 2015. Accessed January 13, 2017.
Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
Pustular psoriasis. National Psoriasis Foundation website. https://www.psoriasis.org/about-psoriasis/types/pustular. Accessed January 13, 2017.
Westphal DC, Schettini APM, de Souza PP, et al. Generalized pustular psoriasis induced by systemic steroid dose reduction. An Bras Dermatol. 2016;91:664-666.
Expert Commentaries on next page
Expert Commentaries
When I was a resident, I was trained not to use systemic steroids in psoriasis patients for the reasons noted above, and I have faithfully followed these instructions 9 years into practice. However, I see many patients with severe psoriasis who are given systemic steroids by other physicians (ie, rheumatologists for psoriatic arthritis, pulmonologists for asthma). I often tell patients afterwards of the dangers of systemic steroids and to have them tell their other doctors to be cautious when giving another course of systemic steroids. However, I have yet to see a generalized pustular psoriasis outbreak or flare in psoriasis vulgaris after a course of systemic steroids. While I do not recommend systemic steroids for psoriasis patients since we have so many other systemic agents, I wonder if the risks that we were all trained about are really that high.
—Jashin J. Wu, MD (Los Angeles, California)
How bad is it to give patients with psoriasis systemic steroids? Are psoriasis patients treated with systemic steroids likely to get a pustular flare? Are patients with psoriasis who suddenly stop their corticosteroids more likely to get a pustular flare than psoriasis patients who suddenly stop other systemic psoriasis treatments? I don't have the answers to these questions. My sense is that we have a lot of dogma and strong opinions but very little hard evidence to answer these questions.
I don't typically prescribe systemic steroids to psoriasis patients, but systemic steroids are widely used. Sometimes there are problems. I have seen patients who received systemic steroids for psoriasis and who went on to have a pustular flare, but it's possible the systemic steroid was given because those patients were headed toward the pustular flare already.
I once had a psoriasis patient who came to see me with a suddenly inflamed tender joint. Not knowing what to do, I called a rheumatologist to see the patient. The rheumatologist, too busy to work the patient in, told me to give the patient a 2-week prednisone taper. I did, and nothing untoward happened with the psoriasis. This one anecdote doesn't give me much confidence that systemic steroids are safe for psoriasis patients.
Clearly, long-term steroids cause a host of problems (eg, osteoporosis, diabetes). But I'm not sure that the dogma that systemic steroids should be avoided in patients with psoriasis is well supported. Systemic steroids are being widely used, and I don't see an epidemic of pustular flares.
Is it a mistake to give systemic steroids to psoriasis patients? I just don't know.
—Steven R. Feldman, MD, PhD (Winston-Salem, North Carolina)
Using a Modified Ball-Tip Guide Rod to Equalize Leg Length and Restore Femoral Offset
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
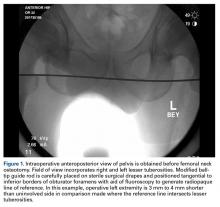
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
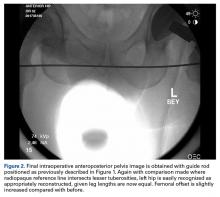
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Current Techniques in Treating Femoroacetabular Impingement: Capsular Repair and Plication
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Hip capsule provides static stabilization for the hip joint.
- Capsular management must weigh visualization to address underlying osseous deformity but also repair/plication of the capsule to maintain biomechanical characteristics.
- T-capsulotomy provides optimal visualization with a small interportal incision with a vertical incision along the femoral neck.
- Extensile interportal capsulotomy is the most widely used capsulotomy and size may vary depending on capsular and patient characteristics.
- Orthopedic surgeons should be equipped to employ either technique depending on the patients individual hip pathomorphology.
Hip arthroscopy has emerged as a common surgical treatment for a number of hip pathologies. Surgical treatment strategies, including management of the hip capsule, have evolved. Whereas earlier hip arthroscopies often involved capsulectomy or capsulotomy without repair, more recently capsular closure has been considered an important step in restoring the anatomy of the hip joint and preventing microinstability or gross macroinstability.
The anatomy of the hip joint includes both static and dynamic stabilizers designed to maintain a functioning articulation. The osseous articulation of the femoral head and acetabulum is the first static stabilizer, with variations in offset, version, and inclination of the acetabulum and the proximal femur. The joint capsule consists of 3 ligaments—iliofemoral, pubofemoral, and ischiofemoral—that converge to form the zona orbicularis. Other soft-tissue structures, such as the articular cartilage, the labrum, the transverse acetabular ligament, the pulvinar, and the ligamentum teres, also provide static constraint.1 The surrounding musculature provides the hip joint with dynamic stability, which contributes to overall maintenance of proper joint kinematics.
Management of the hip capsule has evolved as our understanding of hip pathology and biomechanics has matured. Initial articles on using hip arthroscopy to treat labral tears described improvement in clinical outcomes,2 but the cases involved limited focal capsulotomy. Not until the idea of femoroacetabular impingement (FAI) was introduced were extensive capsulotomies and capsulectomies performed to address the underlying osseous deformities and emulate open techniques. Soon after our ability to access osseous pathomorphology improved with enhanced visualization and comprehensive resection, cases of hip instability after hip arthroscopy surfaced.3-5 Although frank dislocation after hip arthroscopy is rare and largely underreported, it is a catastrophic complication. In addition, focal capsular defects were also described in cases of failed hip arthroscopy and thought to lead to microinstability of the hip.6 Iatrogenic microinstability is thought to be more common, but it is also underrecognized as a cause of failure of hip arthroscopy.7Microinstability is a pathologic condition that can affect hip function. In cases of recurrent pain and unimproved functional status after surgery, microinstability should be considered. In an imaging study of capsule integrity, McCormick and colleagues6 found that 78% of patients who underwent revision arthroscopic surgery after hip arthroscopic surgery for FAI showed evidence of capsular and iliofemoral defects on magnetic resonance angiography. Frank and colleagues8 reported that, though all patients showed preoperative-to-postoperative improvement on outcome measures, those who underwent complete repair of their T-capsulotomy (vs repair of only its longitudinal portion) had superior outcomes, particularly increased sport-specific activity.
For patients undergoing hip arthroscopy, several predisposing factors can increase the risk of postoperative instability. Patient-related hip instability factors include generalized ligamentous laxity, supraphysiologic athletics (eg, dance), and borderline or true hip dysplasia. Surgeon-related factors include overaggressive acetabular rim resection, excessive labral débridement, and lack of capsular repair.5,9 Although there are multiple techniques for accessing the hip joint and addressing capsular closure at the end of surgery,9-14 we think capsular closure is an important aspect of the case.
Surgical Technique
For a demonstration of this technique, click here to see the video that accompanies this article. The patient is moved to a traction table and placed in the supine position. Induction of general anesthesia with muscle relaxation allows for atraumatic axial traction. The anesthetized patient is assessed for passive motion and ligamentous laxity. Well-padded boots are applied, and a well-padded perineal post is used for positioning. Gentle traction is applied to the contralateral limb, and axial traction is applied through the surgical limb with the hip abducted and minimally flexed. The leg is then adducted and neutrally extended, inducing a transverse vector cantilever moment to the proximal femur. The foot is internally rotated to optimize femoral neck length on an anteroposterior radiograph. The circulating nursing staff notes the onset of hip distraction in order to ensure safe traction duration.
Bony landmarks are marked with a sterile marking pen. Under fluoroscopic guidance, an anterolateral (AL) portal is established 1 cm proximal and 1 cm anterior to the AL tip of the greater trochanter. Standard cannulation allows for intra-articular visualization with a 70° arthroscope. A needle is used to localize placement of a modified anterior portal. After cannulation, the arthroscope is placed in the modified anterior portal to confirm safe entry of the portal without labral violation. An arthroscopic scalpel (Samurai Blade; Stryker Sports Medicine) is used to make a transverse interportal capsulotomy 8 mm to 10 mm from the labrum and extending from 12 to 2 o’clock; length is 2 cm to 4 cm, depending on the extent of the intra-articular injury (Figure 1A).
The acetabular rim is trimmed with a 5.0-mm arthroscopic burr. Distal AL accessory (DALA) portal placement (4-6 cm distal to and in line with the AL portal) allows for suture anchor–based labral refixation. Generally, 2 to 4 anchors (1.4-mm NanoTack Anatomic Labrum Restoration System; Stryker Sports Medicine) are placed as near the articular cartilage as possible without penetration (Figure 1B). On completion of labral refixation, traction is released, and the hip is flexed to 20° to 30°.
T-Capsulotomy
Pericapsular fatty tissue is débrided with an arthroscopic shaver to visualize the interval between the iliocapsularis and gluteus minimus muscles. An arthroscopic scalpel is used, through a 5.0-mm cannula in the DALA portal, to extend the capsulotomy longitudinally and perpendicular to the interportal capsulotomy (Figure 1C). The T-capsulotomy is performed along the length of the femoral neck distally to the capsular reflection at the intertrochanteric line. The arthroscopic burr is used to perform a femoral osteochondroplasty between the lateral synovial folds (12 o’clock) and the medial synovial folds (6 o’clock). Dynamic examination and fluoroscopic imaging confirm that the entire cam deformity has been excised and that there is no evidence of impingement.
Although various suture-shuttling or tissue-penetrating/retrieving devices may be used, we recommend whichever device is appropriate for closing the capsule in its entirety. With the arthroscope in the modified anterior portal, an 8.25-mm × 90-mm cannula is placed in the AL portal, and an 8.25-mm × 110-mm cannula in the DALA portal. These portals will facilitate suture passage.
The vertical limb of the T-capsulotomy is closed with 2 to 4 side-to-side sutures, and the interportal capsulotomy limb with 2 or 3 sutures. Capsular closure begins with the distal portion of the longitudinal limb at the base of the iliofemoral ligament (IFL). A crescent tissue penetrating device (Slingshot; Stryker Sports Medicine) is loaded with high-strength No. 2 suture (Zipline; Stryker Sports Medicine) and placed through the AL portal to sharply pierce the lateral leaflet of the IFL (Figure 1D). The No. 2 suture is shuttled into the intra-articular side of the capsule (Figure 1E). Through the DALA portal, the penetrating device is used to pierce the medial leaflet to retrieve the free suture (Figure 1F). Next, the looped suture retriever is used to pull the suture from the AL portal to the DALA portal so the suture can be tied. We prefer to tie each suture individually after it is passed, but all of the sutures can be passed first, and then tied. As successive suture placement and knot tying inherently tighten the capsule, successive visualization requires more precision. Each subsequent suture is similarly passed, about 1 cm proximal to the previous stitch.
After closure of the vertical limb of the T-capsulotomy, we prefer to close the interportal capsulotomy with the InJector II Capsule Restoration System (Stryker Sports Medicine), a device that allows for closure through a single cannula lateral to medial. This device is passed through the AL cannula in order to bring the suture end through the proximal IFL attached to the acetabulum (Figure 1G). The device is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL (Figure 1H). The stitch is then tensioned and tied. Likewise, closure of the medial IFL involves passing the InJector through the DALA cannula and bringing the first suture end through the proximal IFL attached to the acetabulum. The Injector is removed from the cannula, and the other suture end is placed in the device and passed through the distal IFL. The stitch is then tensioned and tied with the hip in neutral extension. Generally, 2 or 3 stitches are used to close the interportal capsulotomy. Complete capsular closure is confirmed by the inability to visualize the underlying femoral head/neck and by probing the anterior capsule to ensure proper tension (Figure 1I).
Extensile Interportal Capsulotomy
An alternative to T-capsulotomy is interportal capsulotomy. Just as with T-capsulotomy closure, multiple different suture passing devices can be used. Good visualization for accessing the peripheral compartment generally is achieved by making the interportal capsulotomy 4 cm to 6 cm longer than the horizontal limb of the T-capsulotomy (Figures 2A, 2B). Capsular closure usually begins with the medial portion of the interportal capsulotomy. With the arthroscope in the AL portal, the 8.25-mm × 90-mm cannula is placed in the midanterior portal (MAP), and an 8.25-mm × 110-mm cannula is placed in the DALA portal.
Ligamentous laxity determines degree of capsular closure. The capsular leaflets can be closed end to end if there is little concern for laxity and instability. If there is more concern for capsular laxity, a larger bite of the capsular tissue can be taken to allow for a greater degree of plication. Further, the interportal capsule can be tightened by alternately advancing the location where sutures are passed through the capsule. Specifically, the sutures are passed such that larger bites of the distal capsule are taken, increasing the tightness of the capsule in external rotation.9
Rehabilitation
After surgery, hip extension and external rotation are limited to decrease stress on the capsular closure. The patient is placed into a hip orthosis with 0° to 90° of flexion and a night abduction pillow to limit hip external rotation. Crutch-assisted gait with 20 lb of foot-flat weight-bearing is maintained the first 3 weeks. Continuous passive motion and use of a stationary bicycle are recommended for the first 3 weeks, and then the patient slowly progresses to muscle strengthening, including core and proximal motor control. Closed-chain exercises are begun 6 weeks after surgery. Treadmill running may start at 12 weeks, with the goal of returning to sport at 4 to 6 months.
Discussion
Capsular closure during hip arthroscopy restores the normal anatomy of the IFL and therefore restores the biomechanical characteristics of the hip joint. Scientific studies have found that capsular repair or plication after hip arthroscopy restores normal hip translation, rotation, and strain. Clinical studies have also demonstrated a lower revision rate and more rapid return to athletic activity. Capsular closure, however, is technically challenging and increases operative time, but gross instability and microinstability can be avoided with meticulous closure/plication.
Am J Orthop. 2017;46(1):49-54. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
1. Boykin RE, Anz AW, Bushnell BD, Kocher MS, Stubbs AJ, Philippon MJ. Hip instability. J Am Acad Orthop Surg. 2011;19(6):340-349.
2. Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25(4):365-368.
3. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
4. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. McCormick F, Slikker W 3rd, Harris JD, et al. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):902-905.
7. Wylie JD, Beckmann JT, Maak TG, Aoki SK. Arthroscopic capsular repair for symptomatic hip instability after previous hip arthroscopic surgery. Am J Sports Med. 2016;44(1):39-45.
8. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
9. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
10. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
11. Camp CL, Reardon PJ, Levy BA, Krych AJ. A simple technique for capsular repair after hip arthroscopy. Arthrosc Tech. 2015;4(6):e737-e740.
12. Chow RM, Engasser WM, Krych AJ, Levy BA. Arthroscopic capsular repair in the treatment of femoroacetabular impingement. Arthrosc Tech. 2014;3(1):e27-e30.
13. Harris JD, Slikker W 3rd, Gupta AK, McCormick FM, Nho SJ. Routine complete capsular closure during hip arthroscopy. Arthrosc Tech. 2013;2(2):e89-e94.
14. Kuhns BD, Weber AE, Levy DM, et al. Capsular management in hip arthroscopy: an anatomic, biomechanical, and technical review. Front Surg. 2016;3:13.
Ulnar Collateral Ligament Reconstruction: Current Philosophy in 2016
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The ulnar collateral ligament (UCL) is the primary restraint to valgus stress between 20° and 125° of motion.1-5 Overhead athletes, most commonly baseball pitchers, are at risk of developing UCL insufficiency, and dysfunction presents as pain with loss of velocity and control. Some injuries may present acutely while throwing, but many patients, when questioned, report a preceding period of either pain or loss of velocity and control.
Authors have documented a significant rise in elbow injuries in young athletes, especially pitchers.6 Extended seasons, higher pitch counts, year-round pitching, pitching while fatigued, and pitching for multiple teams are risk factors for elbow injuries.7 Pitchers in the southern United States are more likely to undergo UCL reconstruction than those from the northern states.8 Pitchers who also play catcher are at a higher risk due to more total throws than those who pitch and play other positions or pitch only. Throwers with higher velocity are more likely to pitch in showcases, pitch for multiple teams, and pitch with pain and fatigue, and these are all risk factors.6 Also, in one study of youth baseball injuries, individuals in the injured group were found to be taller and heavier than those in the uninjured group.6 Pitch counts, rest from pitching during the off-season, adequate rest, and ensuring pain-free pitching can lessen the risk of injury.6 As expected with the rise in throwing injuries, the rise in medial elbow procedures has risen.9
While throwing, stress across the medial elbow has been measured to be nearly 300 N. A maximum varus force during pitching was measured to be 64 N-m at 95° ± 14°.10 Morrey and An4 determined that the UCL generated 54% of the varus force at 90° of flexion. During active pitching, this value is likely reduced due to simultaneous muscle contraction, but if one assumes the UCL bears 54% of the maximal load, the UCL must be able to withstand 34 N-m. The UCL can withstand a maximum valgus torque between 22.7 and 34 N-m11-13; therefore, during pitching, the UCL is at or above its failure load. After thousands of cycles over many years, one can imagine how the UCL might be injured.
Multiple techniques have been proposed in the surgical treatment of UCL injuries. Jobe14 pioneered UCL reconstruction in 1974 in Tommy John, a Major League Baseball pitcher. John returned to pitch successfully, and both the UCL and the reconstruction are commonly called by his name. Jobe14 reported his technique in 1986, and it has remained, with a few modifications, the primary method for reconstruction of the UCL (Figure 1).
Evaluation
A standard evaluation with physical examination and imaging is completed in all throwers with elbow pain. In our prior study,16 we found that 100% of patients experienced pain during athletic activity and that 96% of throwers complained of pain during late cocking and acceleration phases of the throwing motion. Nearly half reported an acute onset of pain, while 53% were unable to identify a single inciting event. Seventy-five percent of the acute injuries were during competition. Delayed diagnosis was very common, with an average time to diagnosis after onset of symptoms of 6.4 months. Neurologic symptoms were seen in 23% of athletes, most of which were ulnar nerve paresthesias during throwing.16
Physical examination includes inspection for swelling, hand intrinsic atrophy, neurovascular examination, range of motion, shoulder examination, and elbow stress examination. Range of motion at presentation averaged 5° to 135° with 85° of supination and pronation.16 All patients need neurologic evaluation for ulnar nerve dysfunction. Tinel test of the cubital tunnel was positive in 21%.16 Significant ulnar nerve dysfunction, including hand weakness, is much less common but must be well examined and documented. The shoulder must also be evaluated for loss of rotation, which can lead to increased stress on the elbow. An evaluation of mechanics may point out flaws in technique, which may be contributing to elbow stress. The UCL stress examination includes static stress at 30° of flexion, the milking test at 90°, and the moving valgus stress test. The presence of pain directly over the UCL or laxity compared to the uninvolved side is suggestive of UCL injury.
Radiographic evaluation is completed in all patients with concern for UCL injury. Standard x-rays of the elbow, including anteroposterior, medial, and lateral obliques, axial olecranon, and lateral views, are obtained to evaluate bony abnormalities. Fifty-seven percent of our series showed some abnormality, most commonly olecranon osteophyte formation or ectopic calcification within the UCL substance. Stress radiography rarely changed the treatment course and is somewhat difficult to interpret because of the reports documenting normal increased medial elbow opening in the dominant arm of throwing athletes.21 Magnetic resonance imaging (MRI) is obtained very commonly in this patient population, and intra-articular contrast is crucial. Partial, undersurface tears are common, and a contrasted study better demonstrates undersurface tears or avulsions. The T-sign as described by Timmerman and colleagues22 using computed tomography (CT) arthrography shows partial undersurface detachment, which can be difficult to see without intra-articular contrast.22 This finding is very well visualized on MRI arthrogram as well (Figure 3).
Nonoperative Management
Nonoperative treatment is recommended for 3 months prior to performing reconstruction. Patients are given complete rest from throwing, but rehabilitation is initiated immediately. Rehabilitation exercises and nonsteroidal anti-inflammatory medications are prescribed, and activities that place valgus stress across the elbow are avoided. After resolution of symptoms, an interval throwing program is initiated, and the athlete is gradually returned to sport. Unfortunately, due to season-specific schedules and time-sensitive demands in high-level throwers, operative treatment is often chosen without an extended period of conservative treatment.
Platelet-rich plasma (PRP) therapy has recently been shown to improve healing rates and promote healing in partial UCL tears,23 and as orthobiologics are advanced, they will likely play a larger role in the treatment of UCL injuries.
Surgical Technique
At our institution, UCL reconstruction is performed with the modified Jobe technique as described by Azar and colleagues.17 Arthroscopy prior to reconstruction was routinely performed at our institution until we recognized that arthroscopy rarely changed the preoperative plan.16 Currently, the presence of anterior pathology such as loose bodies or osteochondral defect is our only indication for arthroscopy before reconstruction.
Ipsilateral palmaris autograft is our current graft of choice. This must be examined preoperatively because 16% of patients have unilateral absence and 9% have bilateral absence.24 In revision cases or in patients with insufficient or absent palmaris, contralateral palmaris followed by contralateral gracilis tendon is used. The contralateral gracilis is chosen because of ease of setup and position of the surgeon during the harvest. Gracilis tendon is also used in cases with bony involvement of the ligament based on the results from Dugas and colleagues.25 Toe extensors, plantaris, and patellar tendon grafts have also been used. One recent study showed that neither graft choice nor diameter affected resistance to valgus stress, and that all reconstruction types restored strength at 60° to 120° of flexion.26
Ulnar nerve transposition is performed in all cases regardless of the presence of preoperative nerve symptoms. A complete decompression is completed proximally to the Arcade of Struthers and distally to the deep portion of the flexor carpi ulnaris. A single fascial sling of medial intermuscular septum originating from the epicondylar attachment is used to stabilize the nerve without compression. At wound closure, the deep fascia on the posterior skin flap is also sewn into the cubital tunnel to prevent the nerve from subluxating back into the groove. A single suture is placed distally closing the muscle fascia to prevent propagation of the fascial incision, which can lead to herniation. Transposition is necessary because of the ulnar nerve exposure required in the modified Jobe technique to allow elevation of the deep flexor muscle mass for ligament exposure.
The reconstruction is completed as described by Jobe14 but with a few modifications as described by Azar and colleagues17 and slight adaptations implemented since that time. The flexor-pronator mass is retracted laterally instead of detachment or splitting as described by Thompson and colleagues.27 A subcutaneous rather than a submuscular ulnar nerve transposition is used.
The patient is positioned supine using an arm board. If gracilis tendon is chosen, the contralateral leg is prepped and draped simultaneously. A tourniquet is inflated after exsanguination. A medial approach is performed, and the medial antebrachial nerve is located and protected. The ulnar nerve is then located in the cubital tunnel and mobilized. The neurolysis extends to the deep portion of the flexor carpi ulnaris distally and proximally to the Arcade of Struthers, and the nerve is retracted with a vessel loop. The flexor muscle mass is not elevated from the medial epicondyle; rather, it is retracted anteriorly by small Hohmann retractors. The dissection is carried down to the UCL and found at its attachments to the medial epicondyle and sublime tubercle. If no tear is seen on the superficial surface of the ligament, a longitudinal incision is made through the ligament. Undersurface tears, partial tears, and avulsions can then be identified (Figure 4).
The autologous graft of choice is then harvested. Our technique for palmaris harvest is performed with three 1-cm transverse incisions. The palmaris is palpated and marked with the first incision made near the distal wrist crease, and the second incision is made 3 to 4 cm proximal to the first. The tendon is found in both distal incisions and cut distally with the wrist flexed to maximize tendon length. The tendon is then pulled through the second incision and tensioned to identify the most proximal location the tendon can be palpated. A third incision is made directly over this point and carried down to cut the tendon. This usually provides a graft length of 15 to 20 cm; 13 cm is the minimum graft length to ensure good graft fixation. Muscle is removed from the tendon and each end is secured with a No. 1 nonabsorbable suture in a locking fashion.
If posterior osteophytes are present, they are removed through a posterior, vertical arthrotomy. Over-resection of the olecranon must be avoided, as this can further destabilize the elbow and place increased stress on the reconstruction. Posterior loose bodies can also be removed through this arthrotomy. The arthrotomy is then closed with absorbable suture.
Tunnel placement is critical to success. A 3.2-mm drill bit is used with palmaris grafts and a 4-mm drill bit is used with gracilis grafts. Two convergent tunnels are drilled in the medial epicondyle in a Y fashion and 2 convergent tunnels are drilled at the sublime tubercle in a U or V fashion. After drilling the first tunnel on each side, a hemostat is placed in the tunnel as an aiming point to ensure a complete tunnel is made. The junction is smoothed with a curette, leaving a 5-mm bone bridge between the articular surface and the tunnels. A bent Hewson suture passer is used to pass one end of the graft through the ulna. The 2 limbs of the tendon graft are then passed through the humeral tunnels, creating a figure-of-eight. A varus stress is applied with the elbow at roughly 30° and the 2 limbs are tied together with a No. 1 nonabsorbable suture. If enough graft remains, one or both limbs are passed back through the tunnels and secured again with No. 1 nonabsorbable suture. The 2 limbs are then tied side-to-side, incorporating the native ligament to further secure and tighten the reconstruction.
The ulnar nerve is then secured using a strip of medial intermuscular septum left intact to its insertion at the medial epicondyle. This is attached to the flexor-pronator muscle fascia with a 3-0 nonabsorbable suture. Enough length should be harvested from the septum to ensure there is no compression on the nerve. The deep posterior fascial tissue is then sewn to the periosteum of the medial epicondyle to further prevent subluxation of the nerve back into the groove. The skin is then closed in layered fashion over a superficial drain. The patient is placed in a well-padded posterior splint for 1 week, then the rehabilitation protocol is initiated as discussed below.
Postoperative Rehabilitation
A standardized postoperative 4-phase rehabilitation program for ulnar collateral reconstruction is followed as described by Wilk and colleagues.28-30 The first phase begins immediately after surgery and continues for 4 weeks. During surgery, the patient’s elbow is placed in a compression dressing with a posterior splint to immobilize the elbow in 90° of flexion with wrist motion for 1 week to allow initial healing. Full range of motion of the elbow joint is restored by the end of the fifth to sixth week after surgery.
During phase II (weeks 4-10), a progressive isotonic strengthening program is initiated. Exercises are focused on scapular, rotator cuff, deltoid, and arm musculature. Shoulder range of motion and stretching exercises are performed during this phase and the Thrower’s Ten exercise program is initiated. Any adaptations or strength deficits are addressed during this phase.
During the advanced strengthening phase (phase III), from weeks 10 to 16, a sport-specific exercise/rehabilitation program is initiated. During this phase, stretching and flexibility exercises are performed to enhance strength, power, and endurance. During this phase the patient is placed on the advanced Thrower’s Ten program. Isotonic strengthening exercises are progressed, and at week 12, the athlete is allowed to begin an isotonic lifting program, including bench press, seated rowing, latissimus dorsi pull downs, triceps push downs, and biceps curls. In addition, the athlete performs specific exercises to emphasize sport-specific movements. At week 12, overhead athletes begin a 2-hand plyometric throwing program, and at 14 weeks, a 1
Discussion
Results after ulnar collateral reconstruction have been good. In our series of 743 patients, 83% returned to the same or higher level at an average of 11.6 months.16 There was a 4% major complication rate and 16% minor complication rate. Major complications included medial epicondyle fracture (0.5%), significant ulnar nerve dysfunction (1 patient), rupture of graft (1%), and graft site infection. Sixteen percent of patients had ulnar nerve dysfunction, and 82% of these resolved within 6 weeks. All but 1 patient’s paresthesias resolved within 1 year.16 The 10-year follow-up of this group of patients included 256 patients and was reported by Osbahr and colleagues31 in 2014. Retirement from baseball was due to reasons other than the elbow in 86%, and 98% were still able to throw on at least a recreational level. The overall longevity was 3.6 years, with 2.9 years at pre-injury level or higher. Statistically, pitchers performed at a higher level after reconstruction.31
A recent review by Erickson and colleagues9 showed an overall 82% excellent and 8% good result when evaluating different techniques, including the American Sports Medicine Institute (ASMI) modification of Jobe’s technique, docking technique, and Jobe’s technique. With an overall complication rate of 10% (75% of which was transient ulnar neuritis), the procedure was deemed overall a safe surgical option. Collegiate athletes had the highest return to sport (95%) compared with high school athletes (89%) and professional athletes (86%). The docking technique had the highest rate of return to play (97%) compared with ASMI technique (93%) and Jobe technique (66%).9 Results after repair have not been as good as reconstruction, as reported in 2 studies.16,32 Savoie and colleagues,15 however, reported 93% good/excellent results after primary UCL repair alone.
Another recent review of outcomes showed an overall return to same or higher level was best with docking or modified docking techniques (90.4% and 91.3%, respectively).19 Overall return with modified Jobe technique was 77%.19 O’Brien and colleagues20 performed a review of 33 patients with either modified Jobe or docking technique that showed 81% return to same or higher level with modified Jobe vs 92% with docking technique. The Kerlan-Jobe Orthopaedic Clinic scores were higher in the modified Jobe group (79 vs 74) and the docking technique group returned to play nearly 1 month sooner (12.4 months vs 11.8 months).20 However, comparing different techniques in a heterogenous patient population over 40 years is difficult. Many of the modified Jobe technique cases were performed in the early evolution of the rehabilitation and return-to-play programs. We believe that the current modified Jobe technique has results equal to any other variation.
Despite good results with reconstructions, the recovery is lengthy and most pitchers cannot fully return to competition level for 12 to 18 months. Extensive research has been performed in exploring alternatives to the traditional reconstruction. Advancements in orthobiologics and development of new surgical options seem to provide an alternative to reconstruction, and may allow faster return to competition with less morbidity.
PRP has been at the forefront of orthopedic research for the last 2 decades, mostly focused in tendon and bone healing. Due to the release of many inflammatory mediators, PRP is theorized to initiate a healing response with growth factors that can direct healing towards normal tissue.33 Two main types of PRP are reported based on the presence or absence of leukocytes. PRP has been studied in many applications, but only one clinical study on the UCL has been published to date. Podesta and colleagues23 injected PRP into the elbow of 34 baseball players with MRI-confirmed partial UCL tear. The athletes then underwent a rehabilitation program, which limited stress across the UCL. Type 1A PRP was used (leukocyte-rich, unactivated, 5x or greater platelet concentration33). Athletes were allowed to return to sport based on symptoms and examination findings. Eighty-eight percent returned to same level of play without complaints at average 70 week follow-up, and average return to play ranged from 10 to 15 weeks.23 No specific data were given on the 16 pitchers in the group, but with such a high rate of return, PRP needs to be further evaluated in the treatment of UCL injuries.
Another recent study from Dugas and colleagues18 presented primary UCL repair using a tape augment (InternalBrace, Arthrex). Nine matched cadaver elbows underwent UCL sectioning and then either modified Jobe reconstruction or primary repair of the UCL with placement of the InternalBrace. The biomechanical data showed the repair with internal brace to have slightly less gap, more stiffness, and higher failure strength, although these findings were not statistically significant.18 This bone-preserving technique with less exposure and healing of the native ligament may be another step towards good results with a quicker return to throwing.
Conclusion
UCL injuries can be disabling in throwers. Reconstruction has afforded throwers a high rate of return to preinjury function or better, and several techniques have been presented that produce acceptable results. Overall complication rates range from 10% to 15%, and the majority of complications are transient ulnar neuropraxias. Orthobiologics and repair with augmentation have more recently offered additional options that may improve success of nonoperative treatment or allow less-invasive surgical treatment. Increased involvement in youth sports and early specialization is driving injury rates in young athletes. The orthopedic community must continue to look for better ways to prevent these injuries and investigate better methods to return athletes to high-level competition.
Am J Orthop. 2016;45(7):E534-E540. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
1. Fuss FK. The ulnar collateral ligament of the human elbow joint. Anatomy, function and biomechanics. J Anat. 1991;175:203-212.
2. Hotchkiss RN, Weiland AJ. Valgus stability of the elbow. J Orthop Res. 1987;5(3):372-377.
3. Morrey BF. Applied anatomy and biomechanics of the elbow joint. Instr Course Lect. 1986;35:59-68.
4. Morrey BF, An KN. Articular and ligamentous contributions to the stability of the elbow joint. Am J Sports Med. 1983;11(5):315-319.
5. Morrey BF, An KN. Functional anatomy of the ligaments of the elbow. Clin Orthop. 1985;(201):84-90.
6. Olsen SJ 2nd, Fleisig GS, Dun S, Loftice J, Andrews JR. Risk factors for shoulder and elbow injuries in adolescent baseball pitchers. Am J Sports Med. 2006;34(6):905-912.
7. Fleisig GS, Andrews JR. Prevention of elbow injuries in youth baseball pitchers. Sports Health. 2012;4(5):419-424.
8. Zaremski JL, Horodyski M, Donlan RM, Brisbane ST, Farmer KW. Does geographic location matter on the prevalence of ulnar collateral ligament reconstruction in collegiate baseball pitchers? Orthop J Sports Med. 2015;3(11):2325967115616582.
9. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
10. Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23(2):233-239.
11. Dillman CJ, Smutz P, Werner S. Valgus extension overload in baseball pitching. Med Sci Sports Exerc. 1991;23(suppl 4):S135.
12. Hechtman KS, Tjin-A-Tsoi EW, Zvijac JE, Uribe JW, Latta LL. Biomechanics of a less invasive procedure for reconstruction of the ulnar collateral ligament of the elbow. Am J Sports Med. 1998;26(5):620-624.
13. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
14. Jobe FW, Stark HE, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Savoie FH 3rd, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
16. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
17. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2016;44(3):735-741.
19. Watson JN, McQueen P, Hutchinson MR. A systematic review of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2014;42(10):2510-2516.
20. O’Brien DF, O’Hagan T, Stewart R, et al. Outcomes for ulnar collateral ligament reconstruction: A retrospective review using the KJOC assessment score with two-year follow-up in an overhead throwing population. J Shoulder Elbow Surg. 2015;24(6):934-940.
21. Ellenbecker TS, Mattalino AJ, Elam EA, Caplinger RA. Medial elbow joint laxity in professional baseball pitchers a bilateral comparison using stress radiography. Am J Sports Med. 1998;26(3):420-424.
22. Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22(1):26-32.
23. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689-1694.
24. Thompson NW, Mockford BJ, Cran GW. Absence of the palmaris longus muscle: a population study. Ulster Med J. 2001;70(1):22-24.
25. Dugas JR, Bilotta J, Watts CD, et al. Ulnar collateral ligament reconstruction with gracilis tendon in athletes with intraligamentous bony excision technique and results. Am J Sports Med. 2012;40(7):1578-1582.
26. Dargel J, Küpper F, Wegmann K, Oppermann J, Eysel P, Müller LP. Graft diameter does not influence primary stability of ulnar collateral ligament reconstruction of the elbow. J Orthop Sci. 2015;20(2):307-313.
27. Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10(2):152-157.
28. Wilk KE, Arrigo CA, Andrews JR. Rehabilitation of the elbow in the throwing athlete. J Orthop Sports Phys Ther. 1993;17(6):305-317.
29. Wilk KE, Arrigo CA, Andrews JR, et al. Rehabilitation following elbow surgery in the throwing athlete. Oper Tech Sports Med. 1996;4:114-132.
30. Wilk KE, Arrigo CA, Andrews JR, et al. Preventative and Rehabilitation Exercises for the Shoulder and Elbow. 4th ed. Birmingham, AL: American Sports Medicine Institute; 1996.
31. Osbahr DC, Cain EL, Raines BT, Fortenbaugh D, Dugas JR, Andrews JR. Long-term outcomes after ulnar collateral ligament reconstruction in competitive baseball players minimum 10-year follow-up. Am J Sports Med. 2014;42(6):1333-1342.
32. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes. Treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
33. Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185-1195.
A New Technique for Obtaining Bone Graft in Cases of Distal Femur Nonunion: Passing a Reamer/Irrigator/Aspirator Retrograde Through the Nonunion Site
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Bone grafting is the main method of treating nonunions.1 The multiple bone graft options available include autogenous bone grafts, allogenic bone grafts, and synthetic bone graft substitutes.2,3 Autogenous bone graft has long been considered the gold standard, as it reduces the risk of infection and eliminates the risk of immune rejection associated with allograft; in addition, autograft has the optimal combination of osteogenic, osteoinductive, and osteoconductive properties.2,4,5 Iliac crest bone graft (ICBG), though the most commonly used autogenous bone graft source, has been associated with infection, hematoma, poor cosmetic outcomes, hernia, neurovascular insults, and chronic persistent pain.6,7 Intramedullary bone graft harvest performed with the Reamer/Irrigator/Aspirator (RIA) system (DePuy Synthes) is a novel technique that allows for simultaneous débridement and collection of bone graft, protects against thermal necrosis and extravasation of marrow contents, and maintains biomechanical strength for weight-bearing.3,4,8,9 Furthermore, RIA aspirate is a rich source of autologous bone graft and provides equal or superior amounts of graft in comparison with ICBG.5-7,10-12
In some cases, RIA is associated with the complication of host bone fracture.4,6,7,11,12 In addition, introducing the reamer may contribute to pain at its entry site and may require violation of local soft-tissue attachments at the hip or knees.4,7,13 In this study, we assessed the possibility of using a new RIA technique to eliminate these adverse effects. We hypothesized that distal femoral nonunions could be successfully treated with the RIA passed retrograde through the nonunion site. This technique may obviate the need for a secondary surgical site (required in traditional intramedullary bone graft harvest), minimize the potential entry-site tissue (eg, hip abductor) damage encountered with the antegrade technique, and yield harvested bone graft in quantities similar to those obtained with the standard technique.
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of all patients with a distal femur nonunion treated with autogenous bone grafting between 2009 and 2013. Identified patients had undergone a novel intramedullary harvest technique that involved passing an RIA retrograde through the nonunion site. Data (patient demographics, volume of graft obtained, perioperative complications, postoperative clinical course) were extracted from the medical records. Before data collection, all patients provided written informed consent for print and electronic publication of their case reports.
Technique
The patient was laid supine on a radiolucent table, and the affected extremity was prepared and draped free. A standard lateral incision previously used for the index procedure was employed. After implant removal, a rongeur, curette, and/or high-speed burr was used to débride the distal femur nonunion of all fibrous tissue. After mobilization and preparation of the distal femoral nonunion, varus angulation was accentuated with delivery of the proximal and distal segments of the nonunion into the wound (Figure A).
Six patients underwent 7 separate procedures for distal femoral nonunion. Of these patients, 5 underwent retrograde RIA through the nonunion site, as described above; the sixth underwent antegrade RIA in the traditional fashion and was therefore excluded. One of the 5 patients underwent another bone grafting procedure after the initial retrograde RIA treatment through the nonunion site. Several outcomes were measured: ability to obtain graft, volume of graft obtained, perioperative complications, and feasibility of the procedure.
Mean age of the 5 patients was 40.4 years (range, 22-66 years). Mean reamer size was 13.4 mm (mode, 14 mm), producing an average bone graft volume of 33 mL. There were no intraoperative or postoperative fractures. In 1 case, the reamer shaft broke during insertion and was retrieved with no retained hardware; passage was made with a new reamer shaft. No patient experienced additional pain or discomfort, as there was no separate entry site for the RIA.
Discussion
Bone grafting for nonunion is one of the most commonly performed procedures in orthopedic trauma surgery. Use of an intramedullary harvest system has become increasingly popular relative to alternative techniques. The RIA system is associated with less donor-site pain and provides relatively more bone graft volume in comparison with ICBG harvest.6,7,10,13 Conversely, intramedullary bone graft harvest may be associated with higher risk of host bone fractures, occurring either during surgery (technical error being the cause) or afterward (a result of patient noncompliance or overaggressive reaming).6,7,11,12 Multiple methods of reducing the risk of iatrogenic fracture caused by technical error of eccentric reaming have been described, including appropriate guide wire placement aided by frequent use of fluoroscopy in 2 planes.4 Despite these potential complications and improved donor-site pain complaints in comparison with ICBG harvest, traditional RIA harvest is still associated with pain at the entry site.4,7,13
In this study, we introduced a novel RIA technique for distal femur nonunion. This technique reduces the complications and adverse effects associated with RIA. It removes the added pain and discomfort associated with a separate entry site. As the reamer is introduced into the medullary canal through the femoral nonunion site, and proximal harvest is limited to the subtrochanteric region, the technique also avoids the complications associated with eccentric reaming of the distal and proximal femur, which may contribute to secondary fracture.6,7,11,12Although the proposed technique is practical, it may present some technical difficulties. First, failed fixation hardware must be removed, and by necessity some stripping of soft tissues is required. These actions are unavoidable, as hardware revision is inherent in the treatment of nonunion. During the procedure, the focus should be on minimizing the insult to bony healing. The nonunion also needs to be completely mobilized to allow adequate angulation, guide wire passage, and sequential reaming. The dual vascular insult of intramedullary reaming combined with the soft-tissue débridement and detachment required for hardware removal and mobilization can be concerning for devascularization of the fracture fragment. However, animal studies have suggested reaming does not affect metaphyseal blood flow; it affects only diaphyseal bone.6,14 The metaphyseal/diaphyseal location of these distal femur nonunions is thought to provide at least partial sparing from the endosteal injury that the RIA may cause. Another difficulty is that the angle of passage of the wire requires a relatively steeper curve to be able to pass beyond the medial distal femoral wall and proceed more proximally. Strong manipulation of the segment is required, which in 1 case caused the reamer shaft to break. This complication had minimal sequelae; the shaft was easily retrieved by withdrawing the ball-tipped guide wire. In addition, strong manipulation of the segment can lead to asymmetric medial reaming or fracture—an outcome easily avoided with a small bend in the distal tip of the guide wire and frequent use of fluoroscopy. In all cases in this series, we achieved proximal passage of the wire and the reamer.
Most RIA bone graft is harvested by reaming the medullary canal at the midshaft of the femur. Passing from the distal femoral nonunion precludes obtaining only a small source of potential distal femoral bone graft, though this metaphyseal bone typically is not used for fear of eccentric reaming and secondary fracture.6,7,11,12 The amount of bone graft obtained from selected patients who undergo retrograde RIA passage through the nonunion site should be similar to the amount obtained with the traditional antegrade method. Our newly proposed technique provided an average bone graft volume of 33 mL, which compares favorably with that reported in the literature for the traditional RIA technique.1,5,6,13,15,16
Conclusion
In distal femoral cases, retrograde passage of the RIA through the nonunion site is technically feasible and has reproducible yields of intramedullary bone graft. Adequate mobilization of the nonunion is a prerequisite for reamer harvest. However, this technique obviates the need for an additional entry point. Furthermore, the technique may limit the perioperative fracture risk previously seen with eccentric reaming of the distal and proximal femur using traditional intramedullary harvest.
Am J Orthop. 2016;45(7):E493-E496. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
1. Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41(1):75-84.
2. Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156-1163.
3. Miller MA, Ivkovic A, Porter R, et al. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2011;35(4):599-605.
4. Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with Reamer-Irrigator-Aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44(10):1263-1269.
5. Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(suppl 4):S28-S34.
6. Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(suppl 2):S3-S15.
7. Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973-2980.
8. Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing Reamer Irrigator Aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5(1):37-42.
9. Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the Reamer-Irrigator-Aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24(1):36-41.
10. Masquelet AC, Benko PE, Mathevon H, Hannouche D, Obert L; French Society of Orthopaedics and Traumatic Surgery (SoFCOT). Harvest of cortico-cancellous intramedullary femoral bone graft using the Reamer-Irrigator-Aspirator (RIA). Orthop Traumatol Surg Res. 2012;98(2):227-232.
11. Quintero AJ, Tarkin IS, Pape HC. Technical tricks when using the Reamer Irrigator Aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):42-45.
12. Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-Irrigator-Aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35(7):951-956.
13. Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer-Irrigator-Aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28(10):584-590.
14. ElMaraghy AW, Humeniuk B, Anderson GI, Schemitsch EH, Richards RR. Femoral bone blood flow after reaming and intramedullary canal preparation: a canine study using laser Doppler flowmetry. J Arthroplasty. 1999;14(2):220-226.
15. Finkemeier CG, Neiman R, Hallare D. RIA: one community’s experience. Orthop Clin North Am. 2010;41(1):99-103.
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227-2236.
Limited-Incision Knotless Achilles Tendon Repair
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The incidence of midsubstance Achilles tendon ruptures is increasing in patients 30 years to 50 years of age, and more than 50% of these injuries occur during recreational basketball.1,2 Achilles ruptures occur more in deconditioned individuals engaged in explosive push-off and jumping activities. Management of these injuries has been controversial over the past decade; there is no consensus on nonoperative treatment, surgical repair, or optimal repair technique.1,3-7 According to American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines, limited-incision approaches have fewer overall complications relative to traditional open repair.3,4
Modern repair techniques, such as the Percutaneous Achilles Repair System (PARS; Arthrex), combine limited soft-tissue dissection with percutaneous suture insertion and knot tying.1,8 This limited-incision technique, employed since 2010, uses a 2-cm transverse incision and nondisposable metal jig with divergent needle passes and locking suture fixation options to secure and fix both tendon ends with minimal dissection of skin, subcutaneous tissue, and paratenon. A review of 270 surgically treated Achilles tendon ruptures (101 PARS, 169 traditional open repair) found that, compared with the open repair group, the PARS group had significantly shorter operative times and more patients returning to baseline physical activities within 5 months after surgery.1 Although the difference was not statistically significant, the overall postoperative complication rate was 5% for the PARS group and 11% for the open repair group. The PARS group had no cases of sural neuritis or deep infection requiring reoperation.
Although the PARS technique has had good outcomes with few complications, care must be taken during surgery to prevent sutures from pulling through the tendon near the rupture site, which can result from overtensioning and from suture knot irritation against superficial soft tissues. Given these potential issues, the PARS procedure was modified (Achilles Midsubstance SpeedBridge; Arthrex) to provide knotless restoration of musculotendinous length in a reliable, reproducible fashion and direct fixation of tendon to bone for early mobilization.9 This new procedure bypasses suture fixation in the compromised tendon ends adjacent to the rupture site, thereby reducing suture slippage and allowing for potential early range of motion and weight-bearing relative to previous techniques. Preliminary results from a cohort of 34 patients treated with this technique are promising: Average return to baseline activities was 18.2 weeks (range, 9-26 weeks), and there were no wound complications, nerve injuries, or reruptures.9Indications are overall health and an acute midsubstance Achilles rupture that presents within 3 weeks after injury (the time limit is used to ensure that both tendon ends can be mobilized and repaired to appropriate length). A relative contraindication is delayed presentation (≥4 weeks), which may require open reconstruction in combination with V-Y lengthening or other adjuvant procedures. Other relative contraindications are insertional rupture, Achilles tendinopathy, and a significant medical comorbidity that prohibits surgical intervention.
Surgical Technique
Operating Room Setup and Approach
The patient is positioned prone with chest rolls and kneepads and with arms at <90° of abduction (Figures 1A-1E).
A “no-touch” technique is used without pickups, and soft tissues are carefully dissected with small scissors down to the paratenon. The sural nerve typically is not visible in the operative field, but, if it is, it can be dissected out and retracted out of the way. A transverse incision is made through the paratenon, and expression of rupture hematoma often follows. Paratenon preservation is key in minimizing disruption of the native vascular supply of the tendon and allowing for repair at the end of the case. A freer can be placed within the wound to confirm that the center of the rupture has been identified.
An Allis clamp is inserted into the wound, and the proximal tendon stump is secured and then pulled about 1 cm through the wound. A freer is circumferentially run along the sides of the proximal tendon to release any potential adhesions that may limit distal excursion.
PARS Jig Insertion and Suture Passing
The PARS jig is inserted into the wound with the inner prongs in the narrowest position possible. The curved jig is inserted proximally, and the center turn wheel is used to widen the inner prongs so they can slide along the sides of the tendon in the paratenon. Proper jig placement should be smooth and encounter little resistance. The proximal tendon is in a superficial location and can be palpated within the prongs of the jig to double-check that the tendon is centered within the jig. A frequent error is to insert the jig too deep, which subsequently causes needles and sutures to miss the tendon and pull through.
Keeping the jig centralized in neutral rotation minimizes improper suture passing and avoids iatrogenic injury to the medial and lateral neurovascular structures. During suture passing, all needles (1.6 mm) with nitinol loops are first used unloaded without suture. The first 2 needles are inserted into their respective, numbered holes, through the tendon, and then through the opposite side of the jig. Each needle is checked to make sure that it does not pass outside the jig. Having 2 needles within the jig and tendon at all times during suture passing helps stabilize the jig and avoids adjacent suture piercing with the subsequent needle.
A No. 2 FiberWire suture (Arthrex) is then passed through the first hole using the needle suture passer and made even in length on both sides. The specific colors of the suture are not important, but the order of the sutures placed is. An assistant can write down the colors and order of the sutures passed. Before the second suture is passed, the first needle is inserted back through the jig and tendon into the third hole. The third and fourth sutures (green-striped) differ from the other sutures in that one end has a loop and the other has a tail, and they are passed in an oblique, crossing pattern. These sutures later help create a locking suture on either side of the tendon.
After these sutures are passed, the final result should be 1 green-striped loop and 1 green-striped tail on either side of the tendon. The fifth suture is passed straight across the tendon in a trajectory similar to that of the first suture. In large laborers, obese patients, and elite athletes, 2 additional green-striped sutures can be passed through the optional sixth and seventh holes to create an additional locking suture.
PARS Jig Removal and Suture Management
After all sutures are passed, the turn wheel is used to narrow the inner prongs while gentle, controlled tension is applied to the jig to remove it from the wound (Figures 2A-2C).
Pullout of any suture from the tendon indicates that the tendon was not centered in the jig or was not proximal enough along the tendon during suture passing. If a suture pulls out, it is removed, and the previous steps are repeated with close attention paid to tendon positioning within the jig. It is not advised to extend the incision longitudinally on either end of the transverse incision, as doing so can lead to potential wound-healing complications. After proximal fixation is achieved, all sutures on each side of the tendon are neatly spread apart in the following order from proximal to distal: first suture, second suture, looped green-striped (third) suture, tail green-striped (fourth) suture, fifth suture. The second suture on both sides is then looped around the 2 green-striped sutures and back proximally through the looped end of the green-striped suture.
The green-striped suture tail is pulled through the tendon to the opposite side to create a locking suture on both sides of the tendon. In the end, there are 2 nonlocking sutures and 1 locking suture on either side of the tendon. Each pair of sutures is pulled distally to confirm fixation and remove any initial suture creep from the system. A hemostat is placed on each group of 3 sutures to keep them out of the way during distal anchor preparation.
Distal Anchor Preparation and Banana SutureLasso Passing
Two longitudinal 5-mm incisions are made along the posterior aspect of the heel just distal to the area of maximal heel convexity. Incisions are spaced 1.5 cm apart along the sides of the Achilles tendon insertion. A 3.5-mm drill and a drill guide are used through each incision and placed flush against bone (Figures 3A-3E).
A Banana SutureLasso (Arthrex) with inner nitinol wire is passed through the center of the distal Achilles tendon stump and out the proximal incision to retrieve one side of the proximal sutures. SutureLasso passage through tendon can be facilitated with tactile feedback. The surgeon’s nondominant thumb is placed directly against the distal tendon while the dominant hand grasps the SutureLasso with the thumb near the tip. As the SutureLasso is advanced proximally through the tendon, the surgeon can feel its tip meeting mild resistance. Confirm that the tip of the SutureLasso is in the center of the distal tendon by direct visual inspection through the wound.
The inner nitinol wire is advanced 2 cm to 3 cm out of the tip of the SutureLasso, and sutures are passed through the distal Achilles tendon. During suture passing, the nitinol wire is drawn back to the tip of the SutureLasso, and then the entire SutureLasso is removed from the distal incision. Trying to pass the sutures only through the inner nitinol wire can result in suture tangling and increased resistance. The process is then repeated for the sutures on the opposite side. Suture pairs are placed under maximal tension and cycled multiple times (5-10) to remove any residual proximal suture creep.10
Achilles Tensioning and Anchor Insertion
The ankle is plantar flexed to tension the Achilles tendon relative to the contralateral limb and is held in place by an assistant (Figures 4A-4E).
Position of the drill holes can be rechecked with a Kirschner wire before anchor insertion, as their relative position changes with ankle plantar flexion. It is not necessary to premeasure and adjust suture length at the tip of the anchor as in other blind tunnel anchor insertion techniques (eg, InternalBrace; Arthrex). Once the anchor tip is malleted into bone, the free suture ends are released to avoid overtensioning the tendon. Before the anchor insertion handle is completely removed, the tip of a mosquito clamp can be used to feel the bony surface and confirm the anchor is completely seated.
With the ankle still held in the appropriate amount of plantarflexion, the process is repeated and the other SwiveLock anchor inserted. Sutures are cut flush with the anchor, and the surgeon performs wound irrigation and layered closure, with absorbable suture, of the paratenon and subcutaneous tissues. After skin closure with nylon suture, resting ankle plantarflexion is assessed and the Thompson test performed. The patient is placed in a well-padded non-weight-bearing plantar flexion splint for incision and initial tendon healing during the first 2 weeks after surgery.
Discussion
A key aspect of recovery is the balance achieved between skin and tendon healing and early mobilization, as outcomes of surgical repair of Achilles ruptures are improved with early weight-bearing and functional rehabilitation.11-13 Some surgeons recommend weight-bearing immediately after surgery, given the direct tendon-to-bone fixation achieved with repair.9 I prefer 2 weeks of non-weight-bearing, which allows the skin to heal adequately and the initial soft-tissue inflammation to subside. If the incision is healed at 2 weeks, sutures are removed, and the patient is transitioned to a tall, non-weight-bearing CAM (controlled ankle motion) boot, worn for 1 to 2 weeks with initiation of gentle ankle range-of-motion exercises. If there is any concern about wound healing, sutures are maintained for another 1 to 2 weeks.
Between 3 and 8 weeks after surgery, progressive weight-bearing is initiated with a peel-away heel lift (~2 cm thick total, 3 layers). Each lift layer is removed as pain allows, every 2 to 3 days. The goal is full weight-bearing with the foot flat 5 to 6 weeks after surgery. Physical therapy focusing on ankle motion and gentle Achilles stretching and strengthening is started 5 to 6 weeks after surgery, depending on progression and functional needs. Between 8 and 12 weeks after surgery, the patient is transitioned to normal shoe wear with increased activities. Running and jumping are allowed, as pain and swelling allow, starting at 12 weeks.
Although preliminary outcomes and experience with the Achilles Midsubstance SpeedBridge have been favorable, long-term clinical and functional studies are needed to determine the specific advantages and disadvantages of this new technique relative to other repairs. The main benefits observed thus far are reduced subjective knot tying and tensioning, decreased reliance on suture fixation in compromised tissue at the rupture site, reduced risk of FiberWire knot irritation of superficial soft tissues, lower risk of distal suture pullout, and earlier mobilization owing to bony fixation of the tendon. Potential complications include anchor-site heel pain caused by prominent anchors or by the bone edema that occurs when a patient increases physical activity by a significant amount at 12 weeks.9 Heel pain caused by bone edema resolves by 20 weeks without intervention.
Stress shielding of the distal Achilles tendon is a theoretical concern given the tendon–bone construct, but there have been no reports of tendon atrophy or repair failure caused by stress shielding. The original PARS technique was often used to create Achilles tension with the ankle maximally plantar flexed—the idea being that the tendon would gradually stretch over time.1 Overtensioning the Achilles repair is a potential complication with the SpeedBridge, as the distal anchors provide a more rigid point of distal fixation. Surgeons can avoid this complication by cycling the sutures to remove any residual creep and then tensioning the Achilles according to the contralateral limb and/or palpating tendon opposition at the rupture site.
Overall, this new limited-incision knotless Achilles tendon repair technique allows for minimal soft-tissue dissection, restoration of Achilles musculotendinous length, and direct tendon-to-bone fixation. Early results are promising, but long-term clinical outcomes and comparative analysis are needed. In addition, many details of this technique must be clarified—including incidence of short- and long-term complications in larger cohorts, optimal suture material and configuration, and risks and benefits of immediate (<2 weeks) and delayed (2-4 weeks) weight-bearing.
Am J Orthop. 2016;45(7):E487-E492. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
1. Hsu AR, Jones CP, Cohen BE, Davis WH, Ellington JK, Anderson RB. Clinical outcomes and complications of Percutaneous Achilles Repair System versus open technique for acute Achilles tendon ruptures. Foot Ankle Int. 2015;36(11):1279-1286.
2. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int. 2013;34(4):475-480.
3. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. American Academy of Orthopaedic Surgeons clinical practice guideline on treatment of Achilles tendon rupture. J Bone Joint Surg Am. 2010;92(14):2466-2468.
4. Chiodo CP, Glazebrook M, Bluman EM, et al; American Academy of Orthopaedic Surgeons. Diagnosis and treatment of acute Achilles tendon rupture. J Am Acad Orthop Surg. 2010;18(8):503-510.
5. Khan RJ, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute Achilles tendon ruptures. A meta-analysis of randomized, controlled trials. J Bone Joint Surg Am. 2005;87(10):2202-2210.
6. Renninger CH, Kuhn K, Fellars T, Youngblood S, Bellamy J. Operative and nonoperative management of Achilles tendon ruptures in active duty military population. Foot Ankle Int. 2016;37(3):269-273.
7. Khan RJ, Carey Smith RL. Surgical interventions for treating acute Achilles tendon ruptures. Cochrane Database Syst Rev. 2010;(9):CD003674.
8. McCullough KA, Shaw CM, Anderson RB. Mini-open repair of Achilles rupture in the National Football League. J Surg Orthop Adv. 2014;23(4):179-183.
9. McWilliam JR, Mackay G. The internal brace for midsubstance Achilles ruptures. Foot Ankle Int. 2016;37(7):794-800.
10. Clanton TO, Haytmanek CT, Williams BT, et al. A biomechanical comparison of an open repair and 3 minimally invasive percutaneous Achilles tendon repair techniques during a simulated, progressive rehabilitation protocol. Am J Sports Med. 2015;43(8):1957-1964.
11. Aoki M, Ogiwara N, Ohta T, Nabeta Y. Early active motion and weightbearing after cross-stitch Achilles tendon repair. Am J Sports Med. 1998;26(6):794-800.
12. Kangas J, Pajala A, Ohtonen P, Leppilahti J. Achilles tendon elongation after rupture repair: a randomized comparison of 2 postoperative regimens. Am J Sports Med. 2007;35(1):59-64.
13. Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003;54(6):1171-1180.
Total Knee Arthroplasty With Retained Tibial Implants: The Role of Minimally Invasive Hardware Removal
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Technique
The patient is positioned on a radiolucent table, and a mobile fluoroscopy unit is available. A tourniquet is applied to the upper thigh but typically is not inflated during the percutaneous hardware removal portion of the operation. It is crucial to have information on retained implants so the correct screwdrivers for screw removal can be selected. In addition, provisions for stripped screws should be made. In each of the 3 cases we managed, the Synthes Screw Removal Set was available. Presence of an implant system known to have problems with cold welding of screws (eg, Less Invasive Stabilization System; Synthes) may necessitate additional preparations, such as making conical extraction devices available.1
After preoperative administration of antibiotics, the surgeon typically removes only those proximal tibia screws that are preventing insertion of the tibial base plate. Fluoroscopic guidance is used to locate these screws and then remove them with percutaneous stab incisions. (Retained plates are not removed.) The exact method of localizing and removing the screws percutaneously is crucial. A small stab incision is made in the dermal layer. The number of stab incisions to be made depends on the number of screws to be removed. One small incision is needed for each screw hole. Occasionally mobilizing the skin and redirecting the screwdriver in the deep tissues can allow 2 screws to be removed through a single skin wound. The screwdriver head can be inserted through the muscle and fascial layers without the need for deep dissection. The plate is then felt with the screwdriver and the screw head located. It is very important that the screw head be adequately engaged to prevent stripping. The surgeon should not rush this step. The C-arm can be helpful here. Fluoroscopy not only can guide the screwdriver to the screw hole but can confirm the screwdriver is at right angles to the plate, not oblique. Only when the surgeon is completely satisfied that the screw head is well engaged should the attempt to back out the screw be made. If the screw strips, the screwdriver can be removed, and an attempt can be made to insert a percutaneous stripped screw removal device.1 If this fails, then the technique must be abandoned for a more traditional approach.
Plating complex tibial plateau fractures through a separate posteromedial approach is now popular.2 The deep location and screw orientation of posteromedial hardware make percutaneous removal unfeasible. In these cases, a separate posteromedial incision may be needed—usually posterior enough so it minimally compromises the anterior soft tissues. The incision typically uses the old posteromedial surgical scar but may not need to be as large as the original approach, as only selected screws need be removed. The saphenous neurovascular bundle may still be at risk, depending on the location of these incisions. The plate is not removed.
After the necessary screws are removed, the tourniquet can be inflated, if desired. The total knee arthroplasty (TKA) then proceeds in usual fashion through a single incision and a medial parapatellar arthrotomy.
Results
Between January 2009 and February 2014, Dr. Georgiadis converted 3 cases of retained tibial hardware and severe knee arthrosis to a TKA in a single operation. These cases were reviewed after Institutional Review Board approval was obtained. One patient underwent a closing-wedge high tibial osteotomy 14 years earlier, and the other 2 sustained tibial plateau fractures. Clinical details of the 3 cases are presented in the Table.
In 2 of the cases, anterolateral surgical scars were present. New, separate percutaneous stab incisions were used to remove screws, which meant less of the original skin incision could be used for the TKA (Figures 1A, 1B).
In the third case, involving multiple plates, a similar strategy was used, but an additional small posteromedial incision was required (Figures 2-5). The TKA then proceeded through a new midline incision. This case was performed for tibiofemoral arthrosis in the setting of an acute distal femur fracture, but this had no bearing on the technique.
Tibial base plates were inserted in the usual manner. Length and type of tibial stem were left to the discretion of the surgeon. There were no changes from the usual surgical technique. All patients went on to routine, uneventful wound healing. Follow-up ranged from 10 months to 59 months.
Discussion
If the decision is made to proceed with TKA after previous knee surgery, careful preoperative planning is needed.
For young patients with knee arthrosis and angular deformity, it has been recommended that proximal tibial osteotomy be performed to delay the need for joint replacement.3,4 Although a wide variety of osteotomy techniques is available, plates and screws are often used. With long-term follow-up, knee arthrosis can be expected to progress, and some of these cases will be converted to knee arthroplasty.3,4Displaced tibial plateau fractures are intra-articular injuries. Treatment requires surgery.
Blood work for inflammatory markers (erythrocyte sedimentation rate, C-reactive protein level) should be performed before surgery. In the event of an elevated laboratory value or clinical suspicion (joint effusion), the joint should be aspirated before any arthroplasty procedure.
Preoperative planning for hardware removal is essential.22 The correct screwdriver and a metal cutting burr (for stripped screws) should be available. These needs may be anticipated with certain types of locking plates.1
Surgical incision planning is also crucial in preventing wound problems that can lead to deep prosthetic infection.23,24 Blood supply to the skin of the anterior knee is primarily medially derived; incisions that are more medial put lateral skin flaps at risk.25 Use of the most recently healed or previous lateral-based scars has been recommended. In cases of adherent skin or poor soft-tissue envelope, plastic surgery (eg, soft-tissue expansion, gastrocnemius muscle, fasciocutaneous flaps) may be necessary.26-28Surgeons must decide to perform either a single operation or a multiple-stage operation. Naturally, most patients prefer a single procedure. All previous hardware can be removed, or only the hardware that is preventing insertion of the tibial base plate. Removing the least amount of hardware is advantageous in that surgical stripping and soft-tissue damage are reduced.
In this initial series, we successfully converted 3 tibial implants to TKAs (each as a single operation) by removing only screws in percutaneous or minimally invasive fashion—the prosthetic joint approach did not involve additional soft-tissue stripping. We did not specifically record the time needed for implant removal separately from the time needed for TKA. As the Table shows, this technique can lengthen surgery. Operative time and blood loss can be more variable because of numerous factors, including scar tissue and an altered surgical field from previous surgery, in addition to hardware removal difficulties. Therefore, surgeons should budget more operative time for these procedures. Although longer operations theoretically may increase infection rates, we think the risk is mitigated by the percutaneous aspects of the described technique.
We do not think that most orthopedic surgeons addressing retained plate–screw constructs consider minimally invasive screw removal and plate retention. To our knowledge, the literature includes only 1 case report of a similar technique.29This technique has many potential drawbacks, the foremost being use of intraoperative fluoroscopy. For more complex fractures, fluoroscopy time can be significant if the surgeon is committed to a true percutaneous approach (Table). In addition, use of a mobile fluoroscopy unit adds personnel to the operating theater, which potentially increases the infection rate. There may be cases in which tibial hardware interferes with tibial cuts, necessitating plate removal, but we did not encounter this in our series. This technique is potentially time-consuming. Operating room time can be expected to increase relative to wide exposures that allow quick access to existing implants. For this reason, some surgeons may decide to forgo this technique. Most modern proximal tibial fracture plates are contoured to fit well over the bone. However, some may still be prominent, and surgeons may choose to perform an open approach to remove them. Last, the clinical impact of plates retained without screws in the proximal tibia is not known. Theoretically, they may still act as a nidus for occult infection, and may act as a stress riser for peri-implant fracture. Therefore, for each patient, the surgeon must decide if the extra surgical time, fluoroscopy exposure, and plate retention are worthwhile.
In this 3-case series, screws were removed percutaneously over the proximal tibia. There were no neurovascular injuries in these cases, though there is potential for nerve and artery injuries with percutaneous screw removal, as in the anterolateral area of the distal third of the tibia.30,31 Thus, our technique may not be applicable in such cases. Most patients with plates and screws retained after proximal tibial surgery do not need to have the screws removed from the distal tibia. There also is the potential for saphenous nerve injury if a small medial or posteromedial incision is made. No such injury occurred in our small series.
Surgeons must consider many factors when deciding whether to proceed with TKA in the setting of existing tibial hardware. If staged reconstruction is not planned, consideration can be given to percutaneous screw removal without plate removal in an attempt to minimize further soft-tissue stripping. This has the theoretical advantage of decreasing wound complications. We have been pleased with our initial patient experience and continue to use this technique.
Am J Orthop. 2016;45(7):E481-E486. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.
1. Georgiadis GM, Gove NK, Smith AD, Rodway IP. Removal of the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):562-564.
2. Georgiadis GM. Combined anterior and posterior approaches for complex tibial plateau fractures. J Bone Joint Surg Br. 1994;76(2):285-289.
3. Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis. A long-term follow-up study. J Bone Joint Surg Am. 1984;66(7):1040-1048.
4. Sprenger TR, Doerzbacher JF. Tibial osteotomy for the treatment of varus gonarthrosis. Survival and failure analysis to twenty-two years. J Bone Joint Surg Am. 2003;85(3):469-474.
5. Moore TM, Patzakis MJ, Harvey JP. Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction. J Orthop Trauma. 1987;1(2):97-119.
6. Shah SN, Karunakar MA. Early wound complications after operative treatment of high energy tibial plateau fractures through two incisions. Bull NYU Hosp Joint Dis. 2007;65(2):115-119.
7. Yang EC, Weiner L, Strauss E, Sedin E, Kelley M, Raphael J. Metaphyseal dissociation fractures of the proximal tibia. An analysis of treatment and complications. Am J Orthop. 1995;24(9):695-704.
8. Young MJ, Barrack RL. Complications of internal fixation of tibial plateau fractures. Orthop Rev. 1994;23(2):149-154.
9. Luo CF, Sun H, Zhang B, Zeng BF. Three-column fixation for complex tibial plateau fractures. J Orthop Trauma. 2010;24(11):683-692.
10. Barei DP, Nork SE, Mills WJ, Henley MB, Benirschke SK. Complications associated with internal fixation of high-energy bicondylar tibial plateau fractures utilizing a two-incision technique. J Orthop Trauma. 2004;18(10):649-657.
11. Ruffolo MR, Gettys FK, Montijo HE, Seymour RB, Karunakar MA. Complications of high-energy bicondylar tibial plateau fractures treated with dual plating through 2 incisions. J Orthop Trauma. 2015;29(2):85-90.
12. Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273-277.
13. Volpin G, Dowd GS, Stein H, Bentley G. Degenerative arthritis after intra-articular fractures of the knee. Long-term results. J Bone Joint Surg Br. 1990;72(4):634-638.
14. Mehin R, O’Brien P, Broekhuyse H, Blachut P, Guy P. Endstage arthritis following tibia plateau fractures: average 10-year follow-up. Can J Surg. 2012;55(2):87-94.
15. Wasserstein D, Henry P, Paterson JM, Kreder HJ, Jenkinson R. Risk of total knee arthroplasty after operatively treated tibial plateau fracture: a matched-population-based cohort study. J Bone Joint Surg Am. 2014;96(2):144-150.
16. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
17. Parvizi J, Hanssen AD, Spangheli MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
18. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
19. Civinini R, Carulli C, Matassi F, Villano M, Innocenti M. Total knee arthroplasty after complex tibial plateau fractures. Chir Organi Mov. 2009;93(3):143-147.
20. Saleh KJ, Sherman P, Katkin P, et al. Total knee arthroplasty after open reduction and internal fixation of fractures of the tibial plateau: a minimum five-year follow-up study. J Bone Joint Surg Am. 2001;83(8):1144-1148.
21. Weiss NG, Parvizi J, Trousdale RT, Bryce RD, Lewallen DG. Total knee arthroplasty in patients with a prior fracture of the tibial plateau. J Bone Joint Surg Am. 2003;85(2):218-221.
22. Hak DJ, McElvany M. Removal of broken hardware. J Am Acad Orthop Surg. 2008:16(2):113-120.
23. Della Valle CJ, Berger RA, Rosenberg AG. Surgical exposures in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;(446):59-68.
24. Vince KG, Abdeen A. Wound problems in total knee arthroplasty. Clin Orthop Relat Res. 2006;(452):88-90.
25. Colombel M, Mariz Y, Dahhan P, Kénési C. Arterial and lymphatic supply of the knee integuments. Surg Radiol Anat. 1998;20(1):35-40.
26. Namba RS, Diao E. Tissue expansion for staged reimplantation of infected total knee arthroplasty. J Arthroplasty. 1997;12(4):471-474.
27. Markovich GD, Dorr LD, Klein NE, McPherson EJ, Vince KG. Muscle flaps in total knee arthroplasty. Clin Orthop Relat Res. 1995;(321):122-130.
28. Hallock GG. Salvage of total knee arthroplasty with local fasciocutaneous flaps. J Bone Joint Surg Am. 1990;72(8):1236-1239.
29. Roswell M, Gale D. Total knee arthroplasty following internal fixation of a lateral tibial plateau fracture. Injury Extra. 2005;36(8):352-354.
30. Deangelis JP, Deangelis NA, Anderson R. Anatomy of the superficial peroneal nerve in relation to fixation of tibia fractures with the Less Invasive Stabilization System. J Orthop Trauma. 2004;18(8):536-539.
31. Pichler W, Grechenig W, Tesch NP, Weinberg AM, Heidari N, Clement H. The risk of iatrogenic injury to the deep peroneal nerve in minimally invasive osteosynthesis of the tibia with the Less Invasive Stabilisation System: a cadaver study. J Bone Joint Surg Br. 2009;91(3):385-387.