User login
The Ketogenic Diet and Dermatology: A Primer on Current Literature
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
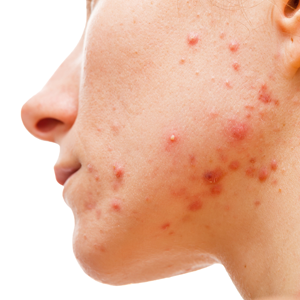
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
The ketogenic diet has been therapeutically employed by physicians since the times of Hippocrates, primarily for its effect on the nervous system.1 The neurologic literature is inundated with the uses of this medicinal diet for applications in the treatment of epilepsy, neurodegenerative disease, malignancy, and enzyme deficiencies, among others.2 In recent years, physicians and scientists have moved to study the application of a ketogenic diet in the realms of cardiovascular disease,3 autoimmune disease,4 management of diabetes mellitus (DM) and obesity,3,5 and enhancement of sports and combat performance,6 all with promising results. Increased interest in alternative therapies among the lay population and the efficacy purported by many adherents has spurred intrigue by health care professionals. Over the last decade, there has seen a boom in so-called holistic approaches to health; included are the Paleo Diet, Primal Blueprint Diet, Bulletproof Diet, and the ketogenic/low-carbohydrate, high-fat diet. The benefits of ketones in these diets—through intermittent fasting or cyclical ketosis—–for cognitive enhancement, overall well-being, amelioration of chronic disease states, and increased health span have been promulgated to the lay population. But to date, there is a large gap in the literature on the applications of ketones as well as the ketogenic diet in dermatology and skin health and disease.
The aim of this article is not to summarize the uses of ketones and the ketogenic diet in dermatologic applications (because, unfortunately, those studies have not been undertaken) but to provide evidence from all available literature to support the need for targeted research and to encourage dermatologists to investigate ketones and their role in treating skin disease, primarily in an adjunctive manner. In doing so, a clearly medicinal diet may gain a foothold in the disease-treatment repertoire and among health-promoting agents of the dermatologist. Given the amount of capital being spent on health care, there is an ever-increasing need for low-cost, safe, and tolerable treatments that can be used for multiple disease processes and to promote health. We believe the ketogenic diet is such an adjunctive therapeutic option, as it has clearly been proven to be tolerable, safe, and efficacious for many people over the last millennia.
We conducted a PubMed search of articles indexed for MEDLINE using varying combinations of the terms ketones, ketogenic, skin, inflammation, metabolic, oxidation, dermatology, and dermatologic and found 12 articles. Herein, we summarize the relevant articles and the works cited by those articles.
Adverse Effects of the Ketogenic Diet
As with all medical therapies, the ketogenic diet is not without risk of adverse effects, which should be communicated at the outset of this article and with patients in the clinic. The only known absolute contraindications to a ketogenic diet are porphyria and pyruvate carboxylase deficiency secondary to underlying metabolic derangements.7 Certain metabolic cytopathies and carnitine deficiency are relative contraindications, and patients with these conditions should be cautiously placed on this diet and closely monitored. Dehydration, acidosis, lethargy, hypoglycemia, dyslipidemia, electrolyte imbalances, prurigo pigmentosa, and gastrointestinal distress may be an acute issue, but these effects are transient and can be managed. Chronic adverse effects are nephrolithiasis (there are recommended screening procedures for those at risk and prophylactic therapies, which is beyond the scope of this article) and weight loss.7
NLRP3 Inflammasome Suppression
Youm et al8 reported their findings in Nature Medicine that β-hydroxybutyrate, a ketone body that naturally circulates in the human body, specifically suppresses activity of the NLRP3 inflammasome. The NLRP3 inflammasome serves as the activating platform for IL-1β.8 Aberrant and elevated IL-1β levels cause or are associated with a number of dermatologic diseases—namely, the autoinflammatory syndromes (familial cold autoinflammatory syndrome, Muckle-Wells syndrome, neonatal-onset multisystemic disease/chronic infantile neurological cutaneous articular syndrome), hyperimmunoglobulinemia D with periodic fever syndrome, tumor necrosis factor–receptor associated periodic syndrome, juvenile idiopathic arthritis, relapsing polychondritis, Schnitzler syndrome, Sweet syndrome, Behçet disease, gout, sunburn and contact hypersensitivity, hidradenitis suppurativa, and metastatic melanoma.7 Clearly, the ketogenic diet may be employed in a therapeutic manner (though to what degree, we need further study) for these dermatologic conditions based on the interaction with the NRLP3 inflammasome and IL-1β.
Acne
A link between acne and diet has long been suspected, but a lack of well-controlled studies has caused only speculation to remain. Recent literature suggests that the effects of insulin may be a notable driver of acne through effects on sex hormones and subsequent effects on sebum production and inflammation. Cordain et al9 discuss the mechanism by which insulin can worsen acne in a valuable article, which Paoli et al10 later corroborated. Essentially, insulin propagates acne by 2 known mechanisms. First, an increase in serum insulin causes a rise in insulinlike growth factor 1 levels and a decrease in insulinlike growth factor binding protein 3 levels, which directly influences keratinocyte proliferation and reduces retinoic acid receptor/retinoid X receptor activity in the skin, causing hyperkeratinization and concomitant abnormal desquamation of the follicular epithelium.9,10 Second, this increase in insulinlike growth factor 1 and insulin causes a decrease in sex hormone–binding globulin and leads to increased androgen production and circulation in the skin, which causes an increase in sebum production. These factors combined with skin that is colonized with Cutibacterium acnes lead to an inflammatory response and the disease known as acne vulgaris.9,10 A ketogenic diet could help ameliorate acne because it results in very little insulin secretion, unlike the typical Western diet, which causes frequent large spikes in insulin levels. Furthermore, the anti-inflammatory effects of ketones would benefit the inflammatory nature of this disease.
DM and Diabetic Skin Disease
Diabetes mellitus carries with it the risk for skin diseases specific to the diabetic disease process, such as increased risk for bacterial and fungal infections, venous stasis, pruritus (secondary to poor circulation), acanthosis nigricans, diabetic dermopathy, necrobiosis lipoidica diabeticorum, digital sclerosis, and bullosis diabeticorum.11 It is well established that better control of DM results in better disease state outcomes.12 The ketogenic diet has shown itself to be a formidable and successful treatment in the diseases of carbohydrate intolerance (eg, metabolic syndrome, insulin resistance, type 2 DM) because of several known mechanisms, including less glucose entering the body and thus less fat deposition, end-product glycation, and free-radical production (discussed below); enhanced fat loss and metabolic efficiency; increased insulin sensitivity; and decreased inflammation.13 Lowering a patient’s insulin resistance through a ketogenic diet may help prevent or treat diabetic skin disease.
Dermatologic Malignancy
A ketogenic diet has been of interest in oncology research as an adjunctive therapy for several reasons: anti-inflammatory effects, antioxidation effects, possible effects on mammalian target of rapamycin (mTOR) regulation,7 and exploitation of the Warburg effect.14 One article discusses how mTOR, a cell-cycle regulator of particular importance in cancer biology, can be influenced by ketones both directly and indirectly through modulating the inflammatory response.7 It has been shown that suppressing mTOR activity limits and slows tumor growth and spread. Ketones also may prove to be a unique method of metabolically exploiting cancer physiology. The Warburg effect, which earned Otto Warburg the Nobel Prize in Physiology or Medicine in 1931, is the observation that cancerous cells produce adenosine triphosphate solely through aerobic glycolysis followed by lactic acid fermentation.14 This phenomenon is the basis of the positron emission tomography scan. There are several small studies of the effects of ketogenic diets on malignancy, and although none of these studies are of substantial size or control, they show that a ketogenic diet can halt or even reverse tumor growth.15 The hypothesis is that because cancer cells cannot metabolize ketones (but normal cells can), the Warburg effect can be taken advantage of through a ketogenic diet to aid in the treatment of malignant disease.14 If further studies find it a formidable treatment, it most certainly would be helpful for the dermatologist involved in the treatment of cutaneous cancers.
Oxidative Stress
Oxidative stress, a state brought about when reactive oxygen species (ROS) production exceeds the antioxidant capacity of the cell and causes damage, is known to be a central part of certain skin diseases (eg, acne, psoriasis, cutaneous malignancy, varicose ulcers, cutaneous allergic reactions, and drug-induced skin photosensitivity).7 There are 2 proven mechanisms by which a ketogenic diet can augment the body’s innate antioxidation capacity. First, ketones activate a potent antioxidant upregulating protein known as NRF2, which is bound in cytosol and remains inactive until activated by certain stimuli (ie, ketones).16 Migration to the nucleus causes transcriptional changes in DNA to upregulate, via a myriad of pathways, antioxidant production in the cell; most notably, it results in increased glutathione levels.17 NRF2 also targets several genes involved in chronic inflammatory skin diseases that cause an increase in the antioxidant capacity.18 As an aside, several foods encouraged on a ketogenic diet also activate NRF2 independently of ketones (eg, coffee, broccoli).19 Second, a ketogenic diet results in fewer produced ROS and an increase in the nicotinamide adenine dinucleotide ratio produced by the mitochondria; in short, it is a more efficient way of producing cellular energy while enhancing mitochondrial function. When fewer ROS are produced, there is less oxidative stress that needs to be attended to by the cell and less cellular damage. Feichtinger et al19 point out that mitochondrial inefficiency and dysfunction often are overlooked components in several skin diseases, and based on the studies discussed above, these diseases may be aided with a ketogenic diet.
Patient Applications
Clearly, a ketogenic diet is therapeutic, and there are many promising potential roles it may play in the treatment of a wide variety of health and disease states through hormonal normalization, antioxidant effects, anti-inflammatory effects, and improvement of metabolic risk factors. However, there are vast limitations to what is known about the ketogenic diet and how it might be employed, particularly by the dermatologist. First, the ketogenic diet lacks a firm definition. Although processed inflammatory vegetable oils and meats are low in carbohydrates and high in fat by definition, it is impossible to argue that they are healthy options for consumption and disease prevention and treatment. Second, nutrigenomics dictates that there must be an individual role in how the diet is employed (eg, patients who are lactose intolerant will need to stay away from dairy). Third, there are no clear proven clinical results from the ketogenic diet in the realm of dermatology. Fourth, as with everything, there are potential detrimental side effects of the ketogenic diet that must be considered for patients (though there are established screening procedures and prophylactic therapies that are beyond the scope of this article). Further, other diets have shown benefit for many other disease states and health promotion purposes (eg, the Mediterranean diet).20 We do not know yet if the avoidance of certain dietary factors such as processed carbohydrates and fats are more beneficial than adopting a state of ketosis at this time, and therefore we are not claiming superiority of one dietary approach over others that are proven to promote health.
Because there are no large-scale studies of the ketogenic diet, there is no verified standardization of initiating and monitoring it, though certain academic centers do have published methods of doing so.21 There are ample anecdotal methods of initiating, maintaining, and monitoring the ketogenic diet.22 In short, drastic restriction of carbohydrate intake and increased fat consumption are the staples of initiating the diet. Medium-chain triglyceride oil supplementation, coffee consumption, intermittent fasting, and low-level aerobic activity also are thought to aid in transition to a ketogenic state. As a result, a dermatologist may recommend that patients interested in this option begin by focusing on fat, fiber, and protein consumption while greatly reducing the amount of carbohydrates in the diet. Morning walks or more intense workouts for fitter patients should be encouraged. Consumption of serum ketone–enhancing foods (eg, coffee, medium-chain triglyceride oil, coconut products) also should be encouraged. A popular beverage known as Bulletproof coffee also may be of interest.23 A blood ketone meter can be used for biofeedback to reinforce these behaviors by aiming for proper β-hydroxybutyrate levels. Numerous companies and websites exist for supporting those patients wishing to pursue a ketogenic state, some hosted by physicians/researchers with others hosted by laypeople with an interest in the topic; discretion should be used as to the clinical and scientific accuracy of these sites. The dermatologist in particular can follow these patients and assess for changes in severity of skin disease, subjective well-being, need for medications and adjunctive therapies, and status of comorbid conditions.
For more information on the ketogenic diet, consider reading the works of the following physicians and researchers who all have been involved with or are currently conducting research in the medical use of ketones and ketogenic diets: David Perlmutter, MD; Thomas Seyfried, PhD; Dominic D’Agostino, PhD; Terry Wahls, MD; Jeff Volek, PhD; and Peter Attia, MD.
Conclusion
Based on the available data, there is potential for use of the ketogenic diet in an adjunctive manner for dermatologic applications, and studies should be undertaken to establish the efficacy or inefficacy of this diet as a preventive measure or treatment of skin disease. With the large push for complementary and alternative therapies over the last decade, particularly for skin disease, the time for research on the ketogenic diet is ripe. Over the coming years, it is our hope that larger clinical, randomized, controlled trials will be conducted for the benefit of dermatology patients worldwide.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
- Wheless JW. History of the ketogenic diet. Epilepsia. 2008;49:3-5.
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59.
- Dashti HM, Mathew TC, Hussein T, et al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol. 2004;9:200-205.
- Storoni M, Plant GT. The therapeutic potential of the ketogenic diet in treating progressive multiple sclerosis. Mult Scler Int. 2015;2015:681289. doi:10.1155/2015/681289.
- Yancy WS, Foy M, Chalecki AM, et al. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2005;2:34.
- Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond). 2004;1:2.
- J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatol Treat. 2017;28:484-487.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of western civilization. Arch Dermatol
- Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- American Diabetes Association. Skin complications. http://www.diabetes.org/diabetes/complications/skin-complications. Accessed December 18, 2019.
- Greenapple R. Review of strategies to enhance outcomes for patients with type 2 diabetes: payers’ perspective. Am Health Drug Benefits. 2011;4:377-386.
- Paoli A, Rubini A, Volek JS, et al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789-796.
- Allen BG, Bhatia SK, Anderson CM, et al. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol. 2014;2:963-970.
- Zhou W, Mukherjee P, Kiebish MA. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond). 2007;4:5.
- Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960-14965.
- Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010:40:238-244.
- Vicente SJ, Ishimoto EY, Torres EA. Coffee modulates transcription factor Nrf2 and highly increases the activity of antioxidant enzymes in rats.J Agric Food Chem. 2014;62:116-122.
- Feichtinger R, Sperl W, Bauer JW, et al. Mitochondrial dysfunction: a neglected component of skin diseases. Exp Dermatol. 2014;23:607-614.
- Brandhorst S, Longo VD. Dietary restrictions and nutrition in the prevention and treatment of cardiovascular disease. Circ Res. 2019;124:952-965.
- Johns Hopkins Medicine. Ketogenic diet therapy for epilepsy. https://www.hopkinsmedicine.org/neurology_neurosurgery/
centers_clinics/epilepsy/pediatric_epilepsy/ketogenic_diet.html. Accessed December 18, 2019. - Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and don’ts. Epilepsy Res. 2012;100:261-266.
- Bulletproof. Bulletproof coffee: everything you want to know. https://blog.bulletproof.com/how-to-make-your-coffee-bulletproof-and-your-morning-too/. Accessed December 18, 2019.
Practice Points
- The ketogenic diet has been employed since antiquity for varying ailments and has a good safety and efficacy profile if administered by a knowledgeable provider.
- New literature is showing promising potential roles for the ketogenic diet as an adjunctive therapy, particularly in the realm of inflammatory disorders, metabolic diseases, and malignancy.
- The dermatologist should be aware of this diet because it is gaining popularity with physicians and patients alike. Dermatologists also should know how it can potentially benefit a number of patients with dermatologic diseases based on small clinical trials, population studies, and basic science research.
Annual Skin Check: Examining the Dermatology Headlines of 2019
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
Resident Pearls
- Chemical sunscreen made headlines in 2019 due to concerns over coral reef toxicity and systemic absorption in humans.
- With a total of 654 cases, New York City’s largest measles outbreak in nearly 30 years ended in September 2019.
- From dupilumab for adolescent atopic dermatitis to apremilast for Behçet disease, the US Food and Drug Administration approved several therapies for pediatric dermatology and rare dermatologic conditions in 2019.
- Two popular skin care products—the Neutrogena Light Therapy Acne Mask and Johnson’s Baby Powder—were involved in recalls in 2019.
Makeup is contaminated with pathogenic bacteria
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
Recalcitrant acne is a common, unwavering problem in dermatology practices nationwide. However, both gram positive and gram negative infections of the skin can go undiagnosed in patients with acne resistant to the armamentarium of oral and topical therapeutics. Although I often use isotretinoin in patients with cystic or recalcitrant acne, I almost always do a culture prior to initiating therapy, and more often than not, have discovered patients have gram negative and gram positive skin infections resistant to antibiotics commonly used to treat acne.
In a study by Bashir and Lambert published in the Journal of Applied Microbiology, 70%-90% of makeup products tested – including lipstick, lip gloss, beauty blenders, eyeliners, and mascara – were found to be contaminated with bacteria. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the most common culprits, and the product with the highest contamination rates were beauty blenders (the small sponges used to apply makeup), which also had high rates of fungal contamination.
Expiration dates on cosmetic products are used to indicate the length of time a preservative in a product can control bacterial contamination. They are printed on packaging as an open jar symbol with the 3M, 6M, 9M, and 12M label for the number of months the product can be opened and used. Unfortunately and unknowingly, most consumers use products beyond the expiration date, and the most common offender is mascara.
Gram positive and gram negative skin infections should be ruled out in all cases of recalcitrant acne. A reminder to note on all culture requisitions to grow gram negatives because not all labs will grow gram negatives on a skin swab. Counseling should also be given to those patients who wear makeup, which should include techniques to clean and sanitize makeup applicators including brushes, tools, and towels. Blenders are known to be used “wet” and are not dried when washed.
It is my recommendation that blenders be a one-time-use-only tool and disposed of after EVERY application. Instructions provided in my clinic are to wash all devices and brushes once a week with hot soapy water, and blow dry with a hair dryer immediately afterward. Lipsticks, mascara wands, and lip glosses should be sanitized with alcohol once a month. Finally, all products need to be disposed of after their expiry.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Resource
Basher A, Lambert P. J Appl Microbiol. 2019. doi: 10.1111/jam.14479.
iPLEDGE vexes dermatologists treating transgender patients
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
Physicians treating transgender patients – in particular, transgender men who were born female – are faced with a confusing process when prescribing isotretinoin for severe acne.
A research letter published in the Journal of the American Academy of Dermatology reports that established to prevent female patients from starting isotretinoin therapy while pregnant or from becoming pregnant while exposed to the teratogenic medication.
Nearly 90% of respondents favored changing the current gender-specific categories in iPLEDGE to gender-neutral ones, classifying patients only by whether or not they have the ability to become pregnant.
For their research, Courtney Ensslin, MD, of the department of dermatology at Johns Hopkins University, Baltimore, and colleagues, distributed an 18-point questionnaire to 385 members of the Association of Professors of Dermatology that included questions assessing clinicians’ knowledge about the reproductive potential of transgender men and women. The recipients were asked to distribute it to faculty members and residents. The survey also described three clinical scenarios in which the physician needed to decide how to register a patient in iPLEDGE. The clinicians largely opted to class transgender men as women with childbearing potential, even if the category conflicted with the patient’s self-identified and legally recognized male gender.
Of the 136 clinicians who responded, 60% were women, almost half were aged 25-34 years. About 12% of respondents said the complexities of prescribing isotretinoin to a transgender patient led them to choose alternative therapies. And the survey revealed some gaps on providers’ general literacy on transgender patients and their reproductive potential. For example, fewer than a third of respondents answered correctly as to whether testosterone treatment decreases the quality and development of an immature ovum.
The researchers wrote that the survey results, while limited by a small sample of respondents that skewed toward younger women providers, suggest that “continued education on fertility in transgender patients is needed because prescribers must fully understand each patient’s reproductive potential to safely prescribe teratogenic medications.” Additionally, they pointed out, the results support ongoing efforts to reform iPLEDGE, as the current categories “do not offer an inclusive approach to care for transgender patients.”
Earlier this year the American Academy of Dermatology issued a position statement that described a number of ongoing initiatives aimed at improving treatment for patients who are members of gender and sexual minorities. These included the “revision of the AAD position statement on isotretinoin to support a gender-neutral categorization model for [iPLEDGE] … based on child-bearing potential rather than on gender identity,” the statement said.
Dr. Ensslin and colleagues reported conflicts of interest related to their research. The study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health.
SOURCE: Ensslin C et al. J Am Acad Dermatol. 2019 Dec;81(6):1426-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
5 Key Points on Dietary Counseling of Acne Patients



Options for acne treatment continue to advance
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – according to Linda Stein Gold, MD, who reviewed the data on these two therapies, as well as cannabidiol (CBD) and an androgen receptor antagonist, which are currently in clinical trials, at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
When considering antibiotic therapy for patients with moderate to severe acne, sarecycline, approved for that indication in October 2018, has improved anti-inflammatory properties and a narrower spectrum of activity, compared with other tetracycline-class antibiotics used for the condition, according to Dr. Stein Gold, director of dermatology research at Henry Ford Health System in Detroit. In two identically designed, 12-week randomized trials, SC 1401 and SC1402, researchers evaluated the efficacy and safety of an approximate 1.5–mg/kg per day dose of sarecycline in comparison with placebo in patients with moderate to severe facial acne aged 9-45 years (J Drugs Dermatol. 2018 Sept 1;17[9]:987-96). “We started to see separation in the inflammatory lesions as early as week 3, and a nice separation versus placebo over the course of 12 weeks,” said Dr. Stein Gold, one of the study investigators. “In all, 22% of patients got clear or almost clear with monotherapy. That’s fairly good.”
She noted that there was consistency in the reductions of lesion count achieved in both studies. Improvements were seen through to 12 weeks, with statistically significant reduction seen as early as 3 weeks in both studies. Sarecycline was also statistically superior to placebo at every time point studied in both trials.
In order to be judged a successful outcome, the subject had to have a 12-week Investigator’s Global Assessment (IGA) score with a 2-point or greater decrease (improvement) from baseline score on the IGA in each location, for patients who have a baseline IGA of 2 or greater, and to be clear (0) or almost clear (1). The same IGA scale was used for the chest and back assessments as was used for facial acne; the researchers observed statistically significant improvements in IGA score for both chest and back acne across both studies at week 12.
Sarecycline also had a favorable safety profile, with no treatment-emergent vertigo or tinnitus adverse events, she noted. Treatment-emergent vestibular and phototoxic adverse events both occurred in fewer than 1% of sarecycline patients. Among females, rates of yeast infections were low. When she recommends a course of this drug for her patients, “I never overpromise,” she said. “I tell my acne patients, ‘We measure your success in weeks and months, not days.’ I always tell them, ‘Take a selfie today and take a selfie every few weeks. When you come back in, we’ll review your progress and see how things went.’ ”
Researchers have also been studying topical minocycline as a treatment option. Minocycline is a large molecule that Dr. Stein Gold characterized as being “very challenging” to deliver topically. “It’s also challenging to keep it stable in a topical formulation.” However, results from two identical phase 3 trials found 4% topical minocycline foam significantly reduced both inflammatory and noninflammatory lesions and improved IGA scores in patients with moderate to severe acne when treated daily for 12 weeks (J Am Acad Dermatol. 2019 Jan;80[1]:168-77).
“This drug has an interesting vehicle,” said Dr. Stein Gold, who was one of the study investigators. “If you take the vehicle itself and you put it next to sebum, it causes sebum to melt at lower temperatures. Why does this matter? If you’re dissolving sebum, maybe you’re creating an easier pathway for the drug to get delivered into the skin and into the hair follicles. We don’t know all the details.” In the two trials, 15%-31% of patients achieved clearance or near clearance of all lesions. “How did it do in terms of decreasing papules and pustules? It did pretty well,” she said. “We want drugs to meet their match in everything that we do.” In terms of tolerability, skin-related adverse events were reported in fewer than 1% of subjects treated with 4% topical minocycline foam. She noted that by delivering minocycline topically, “we get huge concentrations in the skin, but almost negligible amounts in the systemic circulation, which is important. We want to keep [the drug] in the skin; we don’t want it in our system.”
(The Food and Drug Administration approved minocycline foam 4% in October 2019 for treating inflammatory lesions associated with non-nodular moderate to severe acne).
Another potential treatment on the horizon is cortexolone 17a-propionate, a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. “When used around the sebaceous gland, we find that the amount of sebum produced goes down,” she said, noting that phase 3 trials of the agent have been completed.
“We also find that abnormal keratinization subsequently goes down. Just putting this on the skin significantly reduced acne as monotherapy in patients with moderate to severe acne. We were able to get them to clear or almost clear. It worked on comedones, papules, and pustules. Hopefully, it will get FDA approval. This fills the one unmet need we haven’t had topically in terms of decreasing sebum production.”
Clinical trials of CBD are also under way for acne and atopic dermatitis. “It could work for acne because there are some studies showing that might work on sebaceous glands to decrease sebum production,” she said. “CBD has been shown to have positive effects on abnormal keratinization, and it has been shown to have anti-inflammatory effects. Maybe we’ll have another mechanism of action for acne.”
Dr. Gold disclosed that she is on the speakers bureau for Almirall, Galderma, Leo Pharma, Ortho Dermatologics, Pfizer, and Sanofi/Regeneron. She is a consultant for Dermavant, Foamix, Galderma, Leo Pharma, Pfizer, Novartis, Ortho Dermatologics, and holds stock/stock options in AbbVie, Dermavant, Eli Lilly, Foamix, Galderma, Incyte, Leo Pharma, Novartis, Ortho Dermatologics, Pfizer, and Sol-Gel.
SDEF and this news organization are owned by the same parent company.
AT THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
FDA advisory committee supports birth control patch approval
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
Most of the committee members based their decisions on the need for additional contraceptive options for patients. However, most also expressed concerns about its efficacy and offered suggestions for product labeling that called attention to high rates of unintended pregnancies and increased risk of venous thromboembolism (VTE) in obese women.
The agency’s Bone, Reproductive and Urologic Drugs Advisory Committee reviewed safety and efficacy data for AG200-15, a combined hormonal contraceptive patch developed by Agile Therapeutics. The treatment regimen involves application of a patch to the abdomen, buttock, or upper torso, and the patch is changed weekly for 3 weeks, followed by 1 week without a patch.
Elizabeth Garner, MD, consultant and former chief medical officer of Agile, presented study data on safety and effectiveness of the patch. The key study (known as Study 23) considered by the FDA included 1,736 women aged 35 years and younger. The primary efficacy endpoint was the pregnancy rate in the women who used the patch. Women reported sexual activity and back-up contraception use in e-diaries.
A total of 68 pregnancies occurred in the study population after 15,165 evaluable cycles, yielding an overall Pearl Index of 5.83 across all weight and body mass index groups. Historically, a Pearl Index of 5 has been the standard measure for effectiveness in contraceptive products, with lower being better. The index is defined as the number of pregnancies per 100 woman-years of product use. For example, a Pearl Index of 0.1 means that 1 in 1,000 women who use the same contraceptive method for 1 year becomes pregnant.
A subgroup analysis showed reduced efficacy in women with a higher BMI. The Pearl Index for women with a BMI of less than 30 kg/m2 (defined as nonobese) was 4.34, whereas in women with a BMI of 30 kg/m2 and higher (defined as obese), the index was 8.64, nearly double that of nonobese women. No significant differences in the index were noted based on race/ethnicity.
The company described the patch as filling a niche and providing an additional alternative for women seeking a noninvasive method of contraception. It proposed a limitation of use (LOU) as part of the product label that would provide detailed information on efficacy based on the Pearl Index for the different categories of BMI and would suggest that the patch may be less effective for women with obesity. Most of the committee members favored use of a LOU statement on the label, but some noted that it might limit prescriptions to nonobese women.
The committee expressed concern over the Pearl data in the study. The FDA has never approved a contraceptive product with a Pearl Index of greater than 5, said Yun Tang, PhD, a statistical reviewer for the agency’s Office of Translational Sciences, who presented the evaluation of the effectiveness of AG200-15.
Key safety concerns raised in discussion included the risk of venous thromboembolism and the risk of unscheduled bleeding. Both of those issues were significantly more common among obese women, said Nneka McNeal-Jackson, MD, clinical reviewer for the FDA, who presented details on the safety profile and risk-benefit considerations for the patch.
Overall, in Study 23, the incidence rate of VTE was 28/10,000 women-years, with cases in five participants. Four of those were deemed related to the patch, and all occurred in obese women.
Virginia C. “Jennie” Leslie, MD, of Oregon Health and Science University, Portland, voted no to recommending approval of the patch mainly because of efficacy concerns. “My goal is to do no harm, and I have concerns regarding efficacy and giving our patients a false sense of hope,” she said.
Even those members who voted yes expressed concerns about the efficacy data and VTE risk in obese women and recommended postmarketing studies and appropriate labeling to help clinicians in shared decision making with their patients.
Esther Eisenberg, MD, of the National Institutes of Health, noted that the patch fills a need, certainly for women with a BMI less than 30 kg/m2, and suggested that use be limited to women in that lower BMI category.
Other committee members suggested that the product not be restricted based on BMI, but rather that the LOU provide clear explanations of how effectiveness decreases as BMI increases.
David J. Margolis, MD, of the University of Pennsylvania, Philadelphia, opted to abstain from voting, in part based on concerns about the study design and a lack of additional data from the company.
FROM THE FDA
Major survey spotlights novel factors influencing acne
MADRID – Do you ask your acne patients if they use cannabis? And if they say yes, do you suggest they consider giving it up? Dermatologist Delphine Kerob, MD, believes you should.
In a late-breaker session at the annual congress of the European Academy of Dermatology and Venereology, she presented . One of the biggest surprises in this first-of-its-kind study was the finding of an association between cannabis use and acne: 21.1% of patients with physician-diagnosed acne were users, compared with 16.6% of controls without acne.
“I think as dermatologists we should ask these kinds of questions when we manage our patients because this may influence the course of their acne,” observed Dr. Kerob, who is the international medical director for Vichy Laboratories in Paris. The survey was sponsored by the company.
This was an Internet-based survey of 2,826 acne patients and 3,853 age- and sex-matched controls without acne. It was conducted in Canada, France, Germany, Italy, Brazil, and Russia.
The survey comprehensively addressed for the first time what lead investigator Brigitte Dreno, MD, PhD, professor and head of dermatology at Nantes (France) University Hospital and EADV Scientific Programming Committee Chair, has previously called the “acne exposome.” The exposome is essentially everything in a patient’s external and internal environment – other than genetics – that influences the occurrence and severity of the disease (J Eur Acad Dermatol Venereol. 2018 May;32[5]:812-9).
The survey probed the six major categories of exposome factors as defined by Dr. Dreno and coauthors: nutrition, air pollution, lifestyle and psychological factors, medications, skin care products, and climate. Here are the highlights:
Lifestyle and psychological factors. While cannabis use emerged as a novel factor linked to increased likelihood of acne, tobacco use was not – a surprising finding because other investigators had previously identified it as an acne trigger.
Feeling burdened by psychological stress was reported by 51% of acne patients and 29% of controls, for an adjusted 1.79-fold increased risk of acne.
Air pollution. Acne patients were significantly more likely to report exposure to solvent vapors, crude oil, tars, frying oil vapors, and living near an airport or close to factories with chimneys. Dr. Kerob noted that these findings are consistent with other investigators’ study of 189 residents of heavily polluted Mexico City or more pristine Cuernavaca, Mexico, with less pollution. The Mexico City cohort demonstrated an increased sebum excretion rate, lower levels of the antioxidants vitamin E and squalene in their sebum, and a less cohesive stratum corneum, along with a higher prevalence of atopic skin and facial seborrheic changes (Int J Cosmet Sci. 2015 Jun;37[3]:329-38).
Nutrition. This is a hot topic that acne patients have many questions about. Myths abound, as detailed by an expert panel including Dr. Dreno in an article entitled, “Acne and Nutrition: Hypotheses, Myths and Facts” (J Eur Acad Dermatol Venereol. 2018 Oct;32[10]:1631-7).
Dr. Kerob reported that the survey showed consumption of dairy products, probiotics, chocolate, cakes and other sweets, soft drinks, fruit juice, and whey protein were each associated with a significantly increased likelihood of acne .
Fifty-seven percent of acne patients indicated they consumed high-alcohol distilled spirits, compared with 43% of controls.
“We know that on our sebaceous glands, as well as on keratinocytes, we have receptors that will be activated by the impact of some nutrients,” she commented.
Among these receptors on sebaceous glands are the insulin growth factor–1 receptor, the leptin receptor, histamine receptors, receptors for substance P, peroxisome proliferator-activated receptors alpha, beta, and gamma, and androgen receptors, she added.
Medications. For Dr. Kerob, another surprise study finding was that 11.9% of acne patients had used an anabolic steroid- or testosterone-based hormonal drug within the previous 12 months, compared with 3.2% of controls without acne.
Cosmetic factors. The use of facial scrubs, harsh cleansers, and dermarollers was significantly more common among the acne patients.
Climate. Acne patients were more likely to live in hot and/or humid locations. For example, 24.6% of the acne group lived in a hot climate, versus 17.1% of controls.
“We think that identifying and reducing the impact of the exposome is very important for an adequate and holistic acne disease management,” the researcher concluded.
However, Eric Simpson, MD, rose from the audience to comment that he finds this plethora of associations to be of little use in advising his acne patients in clinical practice. For example, does cannabis use cause acne, or are acne patients more likely to be cannabis users as a means of coping with the social stigma surrounding their skin disease?
“I’d just caution about confounding association with causation. Let’s look at trials of removing that association to see if it actually improves acne before we make strong recommendations in the clinic,” urged Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
“You’re perfectly right, there,” Dr. Kerob replied. “The methodology of our study can’t separate cause from effect. But as dermatologists, if we have patients with acne that’s resistant to treatment, we need to see if there are other factors that could worsen acne outcome. And we have patients asking us questions all the time about nutrition – now we have some answers that we can provide to those patients.”
The study was sponsored by Vichy Laboratories, and Dr. Kerob is an employee of the company.
MADRID – Do you ask your acne patients if they use cannabis? And if they say yes, do you suggest they consider giving it up? Dermatologist Delphine Kerob, MD, believes you should.
In a late-breaker session at the annual congress of the European Academy of Dermatology and Venereology, she presented . One of the biggest surprises in this first-of-its-kind study was the finding of an association between cannabis use and acne: 21.1% of patients with physician-diagnosed acne were users, compared with 16.6% of controls without acne.
“I think as dermatologists we should ask these kinds of questions when we manage our patients because this may influence the course of their acne,” observed Dr. Kerob, who is the international medical director for Vichy Laboratories in Paris. The survey was sponsored by the company.
This was an Internet-based survey of 2,826 acne patients and 3,853 age- and sex-matched controls without acne. It was conducted in Canada, France, Germany, Italy, Brazil, and Russia.
The survey comprehensively addressed for the first time what lead investigator Brigitte Dreno, MD, PhD, professor and head of dermatology at Nantes (France) University Hospital and EADV Scientific Programming Committee Chair, has previously called the “acne exposome.” The exposome is essentially everything in a patient’s external and internal environment – other than genetics – that influences the occurrence and severity of the disease (J Eur Acad Dermatol Venereol. 2018 May;32[5]:812-9).
The survey probed the six major categories of exposome factors as defined by Dr. Dreno and coauthors: nutrition, air pollution, lifestyle and psychological factors, medications, skin care products, and climate. Here are the highlights:
Lifestyle and psychological factors. While cannabis use emerged as a novel factor linked to increased likelihood of acne, tobacco use was not – a surprising finding because other investigators had previously identified it as an acne trigger.
Feeling burdened by psychological stress was reported by 51% of acne patients and 29% of controls, for an adjusted 1.79-fold increased risk of acne.
Air pollution. Acne patients were significantly more likely to report exposure to solvent vapors, crude oil, tars, frying oil vapors, and living near an airport or close to factories with chimneys. Dr. Kerob noted that these findings are consistent with other investigators’ study of 189 residents of heavily polluted Mexico City or more pristine Cuernavaca, Mexico, with less pollution. The Mexico City cohort demonstrated an increased sebum excretion rate, lower levels of the antioxidants vitamin E and squalene in their sebum, and a less cohesive stratum corneum, along with a higher prevalence of atopic skin and facial seborrheic changes (Int J Cosmet Sci. 2015 Jun;37[3]:329-38).
Nutrition. This is a hot topic that acne patients have many questions about. Myths abound, as detailed by an expert panel including Dr. Dreno in an article entitled, “Acne and Nutrition: Hypotheses, Myths and Facts” (J Eur Acad Dermatol Venereol. 2018 Oct;32[10]:1631-7).
Dr. Kerob reported that the survey showed consumption of dairy products, probiotics, chocolate, cakes and other sweets, soft drinks, fruit juice, and whey protein were each associated with a significantly increased likelihood of acne .
Fifty-seven percent of acne patients indicated they consumed high-alcohol distilled spirits, compared with 43% of controls.
“We know that on our sebaceous glands, as well as on keratinocytes, we have receptors that will be activated by the impact of some nutrients,” she commented.
Among these receptors on sebaceous glands are the insulin growth factor–1 receptor, the leptin receptor, histamine receptors, receptors for substance P, peroxisome proliferator-activated receptors alpha, beta, and gamma, and androgen receptors, she added.
Medications. For Dr. Kerob, another surprise study finding was that 11.9% of acne patients had used an anabolic steroid- or testosterone-based hormonal drug within the previous 12 months, compared with 3.2% of controls without acne.
Cosmetic factors. The use of facial scrubs, harsh cleansers, and dermarollers was significantly more common among the acne patients.
Climate. Acne patients were more likely to live in hot and/or humid locations. For example, 24.6% of the acne group lived in a hot climate, versus 17.1% of controls.
“We think that identifying and reducing the impact of the exposome is very important for an adequate and holistic acne disease management,” the researcher concluded.
However, Eric Simpson, MD, rose from the audience to comment that he finds this plethora of associations to be of little use in advising his acne patients in clinical practice. For example, does cannabis use cause acne, or are acne patients more likely to be cannabis users as a means of coping with the social stigma surrounding their skin disease?
“I’d just caution about confounding association with causation. Let’s look at trials of removing that association to see if it actually improves acne before we make strong recommendations in the clinic,” urged Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
“You’re perfectly right, there,” Dr. Kerob replied. “The methodology of our study can’t separate cause from effect. But as dermatologists, if we have patients with acne that’s resistant to treatment, we need to see if there are other factors that could worsen acne outcome. And we have patients asking us questions all the time about nutrition – now we have some answers that we can provide to those patients.”
The study was sponsored by Vichy Laboratories, and Dr. Kerob is an employee of the company.
MADRID – Do you ask your acne patients if they use cannabis? And if they say yes, do you suggest they consider giving it up? Dermatologist Delphine Kerob, MD, believes you should.
In a late-breaker session at the annual congress of the European Academy of Dermatology and Venereology, she presented . One of the biggest surprises in this first-of-its-kind study was the finding of an association between cannabis use and acne: 21.1% of patients with physician-diagnosed acne were users, compared with 16.6% of controls without acne.
“I think as dermatologists we should ask these kinds of questions when we manage our patients because this may influence the course of their acne,” observed Dr. Kerob, who is the international medical director for Vichy Laboratories in Paris. The survey was sponsored by the company.
This was an Internet-based survey of 2,826 acne patients and 3,853 age- and sex-matched controls without acne. It was conducted in Canada, France, Germany, Italy, Brazil, and Russia.
The survey comprehensively addressed for the first time what lead investigator Brigitte Dreno, MD, PhD, professor and head of dermatology at Nantes (France) University Hospital and EADV Scientific Programming Committee Chair, has previously called the “acne exposome.” The exposome is essentially everything in a patient’s external and internal environment – other than genetics – that influences the occurrence and severity of the disease (J Eur Acad Dermatol Venereol. 2018 May;32[5]:812-9).
The survey probed the six major categories of exposome factors as defined by Dr. Dreno and coauthors: nutrition, air pollution, lifestyle and psychological factors, medications, skin care products, and climate. Here are the highlights:
Lifestyle and psychological factors. While cannabis use emerged as a novel factor linked to increased likelihood of acne, tobacco use was not – a surprising finding because other investigators had previously identified it as an acne trigger.
Feeling burdened by psychological stress was reported by 51% of acne patients and 29% of controls, for an adjusted 1.79-fold increased risk of acne.
Air pollution. Acne patients were significantly more likely to report exposure to solvent vapors, crude oil, tars, frying oil vapors, and living near an airport or close to factories with chimneys. Dr. Kerob noted that these findings are consistent with other investigators’ study of 189 residents of heavily polluted Mexico City or more pristine Cuernavaca, Mexico, with less pollution. The Mexico City cohort demonstrated an increased sebum excretion rate, lower levels of the antioxidants vitamin E and squalene in their sebum, and a less cohesive stratum corneum, along with a higher prevalence of atopic skin and facial seborrheic changes (Int J Cosmet Sci. 2015 Jun;37[3]:329-38).
Nutrition. This is a hot topic that acne patients have many questions about. Myths abound, as detailed by an expert panel including Dr. Dreno in an article entitled, “Acne and Nutrition: Hypotheses, Myths and Facts” (J Eur Acad Dermatol Venereol. 2018 Oct;32[10]:1631-7).
Dr. Kerob reported that the survey showed consumption of dairy products, probiotics, chocolate, cakes and other sweets, soft drinks, fruit juice, and whey protein were each associated with a significantly increased likelihood of acne .
Fifty-seven percent of acne patients indicated they consumed high-alcohol distilled spirits, compared with 43% of controls.
“We know that on our sebaceous glands, as well as on keratinocytes, we have receptors that will be activated by the impact of some nutrients,” she commented.
Among these receptors on sebaceous glands are the insulin growth factor–1 receptor, the leptin receptor, histamine receptors, receptors for substance P, peroxisome proliferator-activated receptors alpha, beta, and gamma, and androgen receptors, she added.
Medications. For Dr. Kerob, another surprise study finding was that 11.9% of acne patients had used an anabolic steroid- or testosterone-based hormonal drug within the previous 12 months, compared with 3.2% of controls without acne.
Cosmetic factors. The use of facial scrubs, harsh cleansers, and dermarollers was significantly more common among the acne patients.
Climate. Acne patients were more likely to live in hot and/or humid locations. For example, 24.6% of the acne group lived in a hot climate, versus 17.1% of controls.
“We think that identifying and reducing the impact of the exposome is very important for an adequate and holistic acne disease management,” the researcher concluded.
However, Eric Simpson, MD, rose from the audience to comment that he finds this plethora of associations to be of little use in advising his acne patients in clinical practice. For example, does cannabis use cause acne, or are acne patients more likely to be cannabis users as a means of coping with the social stigma surrounding their skin disease?
“I’d just caution about confounding association with causation. Let’s look at trials of removing that association to see if it actually improves acne before we make strong recommendations in the clinic,” urged Dr. Simpson, professor of dermatology at Oregon Health & Science University, Portland.
“You’re perfectly right, there,” Dr. Kerob replied. “The methodology of our study can’t separate cause from effect. But as dermatologists, if we have patients with acne that’s resistant to treatment, we need to see if there are other factors that could worsen acne outcome. And we have patients asking us questions all the time about nutrition – now we have some answers that we can provide to those patients.”
The study was sponsored by Vichy Laboratories, and Dr. Kerob is an employee of the company.
REPORTING FROM EADV 2019
FDA approves minocycline foam for moderate, severe acne
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.