User login
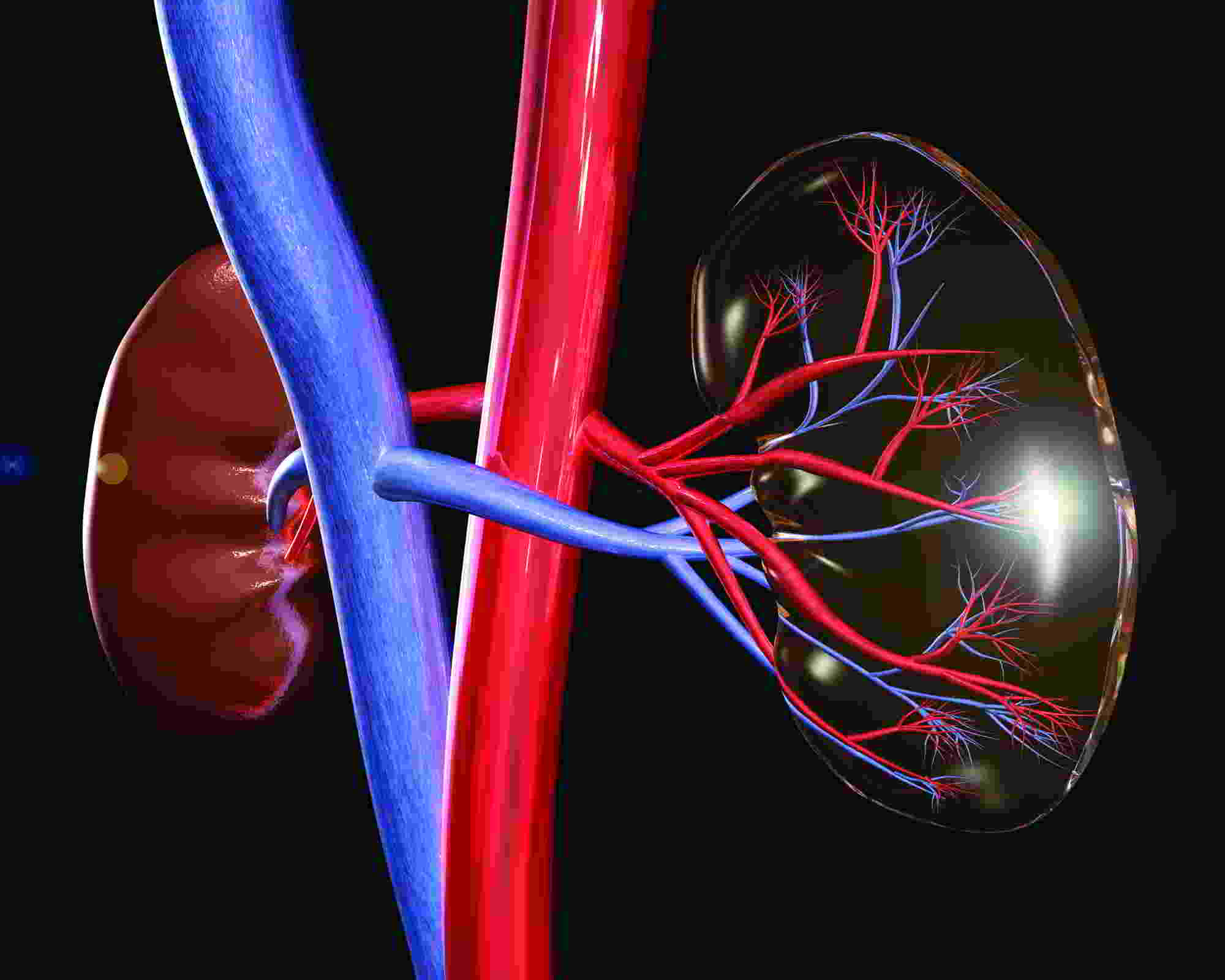
In angiography, intracoronary contrast damaged kidneys more than IV contrast
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
SAN DIEGO – Contrast agents administered through the coronary vessels for invasive angiography led to significantly more kidney damage than contrast agents administered intravenously for coronary computed tomography angiography, according to a randomized study.
In the Coronary Artery Disease-Management (CAD-Man) study, contrast-induced kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration, explained study investigators Dr. Eva Schönenberger and Dr. Marc Dewey of Charité Medical University, Berlin.
Contrast agents used to detect and treat blockages in coronary arteries are known to damage the kidneys in 2%-20% of patients. In the United States, about 4 million doses of contrast are administered directly into the coronary vessels during invasive catheterization, and 40 million into superficial veins, said Dr. Dewey, Heisenberg Professor of Radiology at the German Research Foundation and vice chair of the department of radiology at Charité.
That makes contrast administration a significant clinical decision for physicians, he added, not just because of potential for harm, but also the potential for added costs.
CAD-Man included 326 patients with suspected coronary disease. Researchers randomized 161 patients to intracoronary contrast agent (ICA) for invasive coronary angiography and 165 patients to IV contrast agent for coronary computed tomography angiography (CTA). All patients received the same contrast agent.
Blood samples were taken at baseline before the procedure, and at two time points after: between 18 and 24 hours, and between 46 and 50 hours. Baseline creatinine levels were similar in the two groups. The researchers defined contrast-associated nephrotoxicity as an increase in creatinine of at least 0.5 mg/dL, or 25%.
At follow-up, 21 of 158 ICA patients (13%) and 9 of 160 CTA patients (6%) had contrast-associated nephropathy, a significant difference (P less than .05). In patients without coronary disease, 13% of ICA patients and 4% of CTA patients developed contrast-associated nephropathy, also a significant difference (P less than .05).
Catheter administration concentrates more contrast in the heart and above the kidneys than intravenous administration, Dr. Schönenberger explained at the meeting sponsored by the American Society of Nephrology. Thus, the increased kidney damage in invasive-angiography patients may be due to higher dosages of contrast in their kidneys.
Physicians “have to keep in mind that putting contrast agents directly into the coronaries might produce more of an increase of creatinine, and more acute kidney injury, than just giving it through an IV,” explained Dr. Schönenberger, a nephrologist in the department of anesthesiology and operative intensive care medicine at Charité.
Physicians should take this information into consideration when deciding how to administer contrast for patients suspected of having coronary artery disease, Dr. Dewey noted. “In addition to being noninvasive, cardiac CT may thus also have the advantage of reducing kidney risk.”
Cost should be a big concern as well. Dr. Dewey referred to published literature indicating that contrast-induced kidney injury can lead to “longer hospital and intensive care unit stays, [increased] dialysis, cost of adverse events, and higher mortality rates. The in-hospital cost was $10,000 per contrast-induced acute kidney injury, and the 1-year cost of treatment was more than $11,000.”
Because CAD-Man’s last patient was enrolled in mid-September, the data are still being analyzed, Dr. Schönenberger noted. Therefore, some confounders may be discovered that influenced the results.
For example, cardiologists may select their sicker patients for invasive procedures in order to be ready to insert stents, so there may not be as much flexibility in which approach to use.
Also unclear is the amount of contrast used for each patient in each arm of this study. Some physicians may have used more contrast for patients suspected of having disease that was harder to detect, although that part of the analysis remains under review, Dr. Dewey and Dr. Schönenberger said.
It remains unclear whether the nephrotoxicity found in the invasive angiography group was all due to the contrast, Dr. Schönenberger noted, or whether some of it might have been caused by small particles of hardened cholesterol spreading to blood vessels in the kidneys – a process known as atheroembolic renal disease. That, too, is under review.
The contrast agent used in the study, low-osmolar nonionic Xenetix 350, is used in 96 countries but is not approved by the U.S. Food and Drug Administration, Dr. Schönenberger said. However, it is very similar to those agents that are in use in the United States, she added.
The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
AT KIDNEY WEEK 2015
Key clinical point: Patients undergoing angiography with intracoronary contrast agent instead of IV contrast agent may be at greater risk of kidney injury.
Major finding: Kidney injury was two to three times more likely after intracoronary than after intravenous contrast administration in patients undergoing angiography for suspected heart disease.
Data source: A randomized study of 326 patients with atypical angina pectoris who were scheduled for angiography.
Disclosures: The study was funded by the German Research Foundation through the Heisenberg Professorship Program. The researchers reported no financial disclosures.
AHA: SPRINT’s results upend hypertension targets
ORLANDO – Results from the SPRINT hypertension trial had been highly anticipated ever since the study stopped early in August and the sponsoring National Heart, Lung, and Blood Institute released the top-line positive result in September that treating systolic blood pressure to a target of less than 120 mm Hg led to statistically significant drops in a composite of cardiovascular endpoints as well as in all-cause death, compared with the standard target of less than 140 mm Hg.
When the much fuller report on the results finally came out in a special session at the American Heart Association scientific sessions as well as in a simultaneous publication (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939), the data left attendees buzzing and debating what the results will mean for revised hypertension guidelines and for clinical practice.
The most prominent reactions were accolades for the trial, starting with the independent discussants that the AHA invited to comment at the session, an outpouring of praise reminiscent of that showered on a hit movie:
“A major coup. Thank you, NHLBI,” declared Dr. Marc A. Pfeffer, professor of medicine at Harvard and a cardiologist at Brigham and Women’s Hospital in Boston.
“Thank you for this groundbreaking study,” said Dr. Clive Rosendorff, professor and cardiologist at Mount Sinai Hospital in New York.
“A remarkable trial. The most important blood pressure study in the last 40 years,” gushed Dr. Daniel W. Jones, professor of medicine at the University of Mississippi, Oxford, and director of clinical and population sciences at the Mississippi Center for Obesity Research, Jackson.
Following the huzzahs came a more substantive discussion among meeting attendees of what results from the 9,361-patient Systolic Blood Pressure Intervention Trial will mean for revised blood pressure goals in U.S. guidelines, what it might mean for defining who has hypertension, and how it might influence practice. Perhaps the most pressing issue for the AHA and American College of Cardiology panel that began work on a new revision of hypertension treatment guidelines earlier this year is how to reconcile the SPRINT results with finding from prior studies, especially the 2010 report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N Engl J Med. 2010;362[17]:1575-85.).
ACCORD, at half the size of SPRINT with 4,733 patients, had a very similar design as SPRINT but included only patients with diabetes while SPRINT excluded patients with diabetes. ACCORD failed to show a significant difference in its primary composite outcome after an average of 4.7 years between patients randomized to a hypertension treatment target of less than 140 mm Hg or less than 120 mm Hg, the same goals as in SPRINT. ACCORD did show a statistically significant 41% relative risk reduction for stroke, also in contrast to SPRINT, which showed a much less robust and nonsignificant 11% relative risk reduction in stroke.
In his commentary on SPRINT, Dr. Jones offered several possible explanations for the divergent results, including a possible inherent difference in vascular physiology between patients with diabetes and those with normal glycemic control; the younger patients enrolled in ACCORD (patients averaged 62 years old in ACCORD and 68 years old in SPRINT, and 28% of patients in SPRINT were at least 75 years old); the use of hydrochlorothiazide as the predominant diuretic in ACCORD versus predominant use of chlorthalidone in SPRINT; and the multiple interventions simultaneously tested in ACCORD, which also randomized patients into two arms with respect to glycemic control and into two arms of different lipid-controlling treatment.
SPRINT’s results “need to be assessed in the context of ACCORD,” commented Dr. Salim Yusuf in an interview. “I think the real result is somewhere in between the results of SPRINT and ACCORD” in terms of the appropriate systolic blood pressure target. What we need is a balanced perspective that takes all the trials. SPRINT was a very good trial, but like all studies it should be interpreted in the context of all the other related studies, not in isolation,” said Dr. Yusuf, professor and director of the Population Health Research Institute of McMaster University in Hamilton, Ont.
“Understandably, when something like SPRINT comes out there is a lot of enthusiasm. The first reaction is always ‘Wow!’ For patients who meet SPRINT’s enrollment criteria I think we will treat to a target of less than 120 mm Hg. But the guideline writers need to discuss SPRINT and balance it,” he said.
Despite his regard for SPRINT, Dr. Yusuf cited several additional concerns he has about the trial:
• Its early stoppage (SPRINT had originally been designed to run 5-6 years, but it was halted after an average treatment duration of just over 3 years). “When you stop a trial early there is always an upward bias. The apparent treatment effect gets inflated,” he said.
• The increased rate of acute kidney injury among patients randomized to the more aggressive treatment arm, a 4.1% rate, compared with a 2.5% rate in the control patients randomized to treatment to a goal of systolic pressure less than 140 mm Hg, a statistically significant difference.
• The “highly selected, high-risk” patients enrolled into SPRINT. “You can’t extrapolate the results to the average patient,” Dr. Yusuf said.
Some of these concerns and cautions were shared by Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco, although overall he called the SPRINT results “very exciting.”
“Superficially, SPRINT seems to say treat everyone to a blood pressure of less than 120 mm Hg, but that’s not the case. The patients in SPRINT were primarily very well established patients with hypertension. I’d be concerned about an elderly patient with cardiovascular disease and a blood pressure of 130 mm Hg. If you reduce that to less than 120 mm Hg the diastolic pressure may also fall and that’s important for coronary perfusion.” He also cited the absence so far of a subanalysis of what happened to patients with preexisting renal disease and the lack of data on the outcomes of patients whose systolic pressure fell to levels well below 120 mm Hg.
For others, however, the overall, statistically significant 27% reduction in overall mortality was a reassuring indicator of the safety of the aggressive treatment regimen used in SPRINT. “If there was a meaningful worsening of renal function that harmed patients, you would not see a reduction in all-cause mortality,” commented Dr. Gregg C. Fonarow, professor and associate chief of cardiology at the University of California, Los Angeles.
“We have had so many trials that couldn’t dream of producing a reduction in all-cause mortality. Here we have a trial with a robust, clinically meaningful reduction in all-cause mortality that ultimately demonstrates the benefits outweigh the risks,” he said in an interview.
SPRINT “is a phenomenal breakthrough. It’s data we’ve been awaiting for 20-plus years, to now know that a lower blood pressure target is safe and absolutely essential, and where the benefits outweigh the risks,” Dr. Fonarow said. “Now implementation becomes critical. The SPRINT results are truly practice changing.”
SPRINT received no commercial support. The study received antihypertensive drugs from Arbor and Takeda at no charge for a small percentage of enrolled patients. Dr. Pfeffer has been a consultant to more than 20 companies. Dr. Rosendorff has been a consultant to McNeil and received research funding from Eisai. Dr. Yusuf has received honoraria and research grants from Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, Bayer, and Astra Zeneca. Dr. Jones, Dr. Deedwania, and Dr. Fonarow had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Results from the SPRINT hypertension trial had been highly anticipated ever since the study stopped early in August and the sponsoring National Heart, Lung, and Blood Institute released the top-line positive result in September that treating systolic blood pressure to a target of less than 120 mm Hg led to statistically significant drops in a composite of cardiovascular endpoints as well as in all-cause death, compared with the standard target of less than 140 mm Hg.
When the much fuller report on the results finally came out in a special session at the American Heart Association scientific sessions as well as in a simultaneous publication (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939), the data left attendees buzzing and debating what the results will mean for revised hypertension guidelines and for clinical practice.
The most prominent reactions were accolades for the trial, starting with the independent discussants that the AHA invited to comment at the session, an outpouring of praise reminiscent of that showered on a hit movie:
“A major coup. Thank you, NHLBI,” declared Dr. Marc A. Pfeffer, professor of medicine at Harvard and a cardiologist at Brigham and Women’s Hospital in Boston.
“Thank you for this groundbreaking study,” said Dr. Clive Rosendorff, professor and cardiologist at Mount Sinai Hospital in New York.
“A remarkable trial. The most important blood pressure study in the last 40 years,” gushed Dr. Daniel W. Jones, professor of medicine at the University of Mississippi, Oxford, and director of clinical and population sciences at the Mississippi Center for Obesity Research, Jackson.
Following the huzzahs came a more substantive discussion among meeting attendees of what results from the 9,361-patient Systolic Blood Pressure Intervention Trial will mean for revised blood pressure goals in U.S. guidelines, what it might mean for defining who has hypertension, and how it might influence practice. Perhaps the most pressing issue for the AHA and American College of Cardiology panel that began work on a new revision of hypertension treatment guidelines earlier this year is how to reconcile the SPRINT results with finding from prior studies, especially the 2010 report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N Engl J Med. 2010;362[17]:1575-85.).
ACCORD, at half the size of SPRINT with 4,733 patients, had a very similar design as SPRINT but included only patients with diabetes while SPRINT excluded patients with diabetes. ACCORD failed to show a significant difference in its primary composite outcome after an average of 4.7 years between patients randomized to a hypertension treatment target of less than 140 mm Hg or less than 120 mm Hg, the same goals as in SPRINT. ACCORD did show a statistically significant 41% relative risk reduction for stroke, also in contrast to SPRINT, which showed a much less robust and nonsignificant 11% relative risk reduction in stroke.
In his commentary on SPRINT, Dr. Jones offered several possible explanations for the divergent results, including a possible inherent difference in vascular physiology between patients with diabetes and those with normal glycemic control; the younger patients enrolled in ACCORD (patients averaged 62 years old in ACCORD and 68 years old in SPRINT, and 28% of patients in SPRINT were at least 75 years old); the use of hydrochlorothiazide as the predominant diuretic in ACCORD versus predominant use of chlorthalidone in SPRINT; and the multiple interventions simultaneously tested in ACCORD, which also randomized patients into two arms with respect to glycemic control and into two arms of different lipid-controlling treatment.
SPRINT’s results “need to be assessed in the context of ACCORD,” commented Dr. Salim Yusuf in an interview. “I think the real result is somewhere in between the results of SPRINT and ACCORD” in terms of the appropriate systolic blood pressure target. What we need is a balanced perspective that takes all the trials. SPRINT was a very good trial, but like all studies it should be interpreted in the context of all the other related studies, not in isolation,” said Dr. Yusuf, professor and director of the Population Health Research Institute of McMaster University in Hamilton, Ont.
“Understandably, when something like SPRINT comes out there is a lot of enthusiasm. The first reaction is always ‘Wow!’ For patients who meet SPRINT’s enrollment criteria I think we will treat to a target of less than 120 mm Hg. But the guideline writers need to discuss SPRINT and balance it,” he said.
Despite his regard for SPRINT, Dr. Yusuf cited several additional concerns he has about the trial:
• Its early stoppage (SPRINT had originally been designed to run 5-6 years, but it was halted after an average treatment duration of just over 3 years). “When you stop a trial early there is always an upward bias. The apparent treatment effect gets inflated,” he said.
• The increased rate of acute kidney injury among patients randomized to the more aggressive treatment arm, a 4.1% rate, compared with a 2.5% rate in the control patients randomized to treatment to a goal of systolic pressure less than 140 mm Hg, a statistically significant difference.
• The “highly selected, high-risk” patients enrolled into SPRINT. “You can’t extrapolate the results to the average patient,” Dr. Yusuf said.
Some of these concerns and cautions were shared by Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco, although overall he called the SPRINT results “very exciting.”
“Superficially, SPRINT seems to say treat everyone to a blood pressure of less than 120 mm Hg, but that’s not the case. The patients in SPRINT were primarily very well established patients with hypertension. I’d be concerned about an elderly patient with cardiovascular disease and a blood pressure of 130 mm Hg. If you reduce that to less than 120 mm Hg the diastolic pressure may also fall and that’s important for coronary perfusion.” He also cited the absence so far of a subanalysis of what happened to patients with preexisting renal disease and the lack of data on the outcomes of patients whose systolic pressure fell to levels well below 120 mm Hg.
For others, however, the overall, statistically significant 27% reduction in overall mortality was a reassuring indicator of the safety of the aggressive treatment regimen used in SPRINT. “If there was a meaningful worsening of renal function that harmed patients, you would not see a reduction in all-cause mortality,” commented Dr. Gregg C. Fonarow, professor and associate chief of cardiology at the University of California, Los Angeles.
“We have had so many trials that couldn’t dream of producing a reduction in all-cause mortality. Here we have a trial with a robust, clinically meaningful reduction in all-cause mortality that ultimately demonstrates the benefits outweigh the risks,” he said in an interview.
SPRINT “is a phenomenal breakthrough. It’s data we’ve been awaiting for 20-plus years, to now know that a lower blood pressure target is safe and absolutely essential, and where the benefits outweigh the risks,” Dr. Fonarow said. “Now implementation becomes critical. The SPRINT results are truly practice changing.”
SPRINT received no commercial support. The study received antihypertensive drugs from Arbor and Takeda at no charge for a small percentage of enrolled patients. Dr. Pfeffer has been a consultant to more than 20 companies. Dr. Rosendorff has been a consultant to McNeil and received research funding from Eisai. Dr. Yusuf has received honoraria and research grants from Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, Bayer, and Astra Zeneca. Dr. Jones, Dr. Deedwania, and Dr. Fonarow had no disclosures.
On Twitter @mitchelzoler
ORLANDO – Results from the SPRINT hypertension trial had been highly anticipated ever since the study stopped early in August and the sponsoring National Heart, Lung, and Blood Institute released the top-line positive result in September that treating systolic blood pressure to a target of less than 120 mm Hg led to statistically significant drops in a composite of cardiovascular endpoints as well as in all-cause death, compared with the standard target of less than 140 mm Hg.
When the much fuller report on the results finally came out in a special session at the American Heart Association scientific sessions as well as in a simultaneous publication (N Engl J Med. 2015 Nov 9. doi: 10.1056/NEJMoa1511939), the data left attendees buzzing and debating what the results will mean for revised hypertension guidelines and for clinical practice.
The most prominent reactions were accolades for the trial, starting with the independent discussants that the AHA invited to comment at the session, an outpouring of praise reminiscent of that showered on a hit movie:
“A major coup. Thank you, NHLBI,” declared Dr. Marc A. Pfeffer, professor of medicine at Harvard and a cardiologist at Brigham and Women’s Hospital in Boston.
“Thank you for this groundbreaking study,” said Dr. Clive Rosendorff, professor and cardiologist at Mount Sinai Hospital in New York.
“A remarkable trial. The most important blood pressure study in the last 40 years,” gushed Dr. Daniel W. Jones, professor of medicine at the University of Mississippi, Oxford, and director of clinical and population sciences at the Mississippi Center for Obesity Research, Jackson.
Following the huzzahs came a more substantive discussion among meeting attendees of what results from the 9,361-patient Systolic Blood Pressure Intervention Trial will mean for revised blood pressure goals in U.S. guidelines, what it might mean for defining who has hypertension, and how it might influence practice. Perhaps the most pressing issue for the AHA and American College of Cardiology panel that began work on a new revision of hypertension treatment guidelines earlier this year is how to reconcile the SPRINT results with finding from prior studies, especially the 2010 report of results from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial (N Engl J Med. 2010;362[17]:1575-85.).
ACCORD, at half the size of SPRINT with 4,733 patients, had a very similar design as SPRINT but included only patients with diabetes while SPRINT excluded patients with diabetes. ACCORD failed to show a significant difference in its primary composite outcome after an average of 4.7 years between patients randomized to a hypertension treatment target of less than 140 mm Hg or less than 120 mm Hg, the same goals as in SPRINT. ACCORD did show a statistically significant 41% relative risk reduction for stroke, also in contrast to SPRINT, which showed a much less robust and nonsignificant 11% relative risk reduction in stroke.
In his commentary on SPRINT, Dr. Jones offered several possible explanations for the divergent results, including a possible inherent difference in vascular physiology between patients with diabetes and those with normal glycemic control; the younger patients enrolled in ACCORD (patients averaged 62 years old in ACCORD and 68 years old in SPRINT, and 28% of patients in SPRINT were at least 75 years old); the use of hydrochlorothiazide as the predominant diuretic in ACCORD versus predominant use of chlorthalidone in SPRINT; and the multiple interventions simultaneously tested in ACCORD, which also randomized patients into two arms with respect to glycemic control and into two arms of different lipid-controlling treatment.
SPRINT’s results “need to be assessed in the context of ACCORD,” commented Dr. Salim Yusuf in an interview. “I think the real result is somewhere in between the results of SPRINT and ACCORD” in terms of the appropriate systolic blood pressure target. What we need is a balanced perspective that takes all the trials. SPRINT was a very good trial, but like all studies it should be interpreted in the context of all the other related studies, not in isolation,” said Dr. Yusuf, professor and director of the Population Health Research Institute of McMaster University in Hamilton, Ont.
“Understandably, when something like SPRINT comes out there is a lot of enthusiasm. The first reaction is always ‘Wow!’ For patients who meet SPRINT’s enrollment criteria I think we will treat to a target of less than 120 mm Hg. But the guideline writers need to discuss SPRINT and balance it,” he said.
Despite his regard for SPRINT, Dr. Yusuf cited several additional concerns he has about the trial:
• Its early stoppage (SPRINT had originally been designed to run 5-6 years, but it was halted after an average treatment duration of just over 3 years). “When you stop a trial early there is always an upward bias. The apparent treatment effect gets inflated,” he said.
• The increased rate of acute kidney injury among patients randomized to the more aggressive treatment arm, a 4.1% rate, compared with a 2.5% rate in the control patients randomized to treatment to a goal of systolic pressure less than 140 mm Hg, a statistically significant difference.
• The “highly selected, high-risk” patients enrolled into SPRINT. “You can’t extrapolate the results to the average patient,” Dr. Yusuf said.
Some of these concerns and cautions were shared by Dr. Prakash Deedwania, professor of medicine at the University of California, San Francisco, although overall he called the SPRINT results “very exciting.”
“Superficially, SPRINT seems to say treat everyone to a blood pressure of less than 120 mm Hg, but that’s not the case. The patients in SPRINT were primarily very well established patients with hypertension. I’d be concerned about an elderly patient with cardiovascular disease and a blood pressure of 130 mm Hg. If you reduce that to less than 120 mm Hg the diastolic pressure may also fall and that’s important for coronary perfusion.” He also cited the absence so far of a subanalysis of what happened to patients with preexisting renal disease and the lack of data on the outcomes of patients whose systolic pressure fell to levels well below 120 mm Hg.
For others, however, the overall, statistically significant 27% reduction in overall mortality was a reassuring indicator of the safety of the aggressive treatment regimen used in SPRINT. “If there was a meaningful worsening of renal function that harmed patients, you would not see a reduction in all-cause mortality,” commented Dr. Gregg C. Fonarow, professor and associate chief of cardiology at the University of California, Los Angeles.
“We have had so many trials that couldn’t dream of producing a reduction in all-cause mortality. Here we have a trial with a robust, clinically meaningful reduction in all-cause mortality that ultimately demonstrates the benefits outweigh the risks,” he said in an interview.
SPRINT “is a phenomenal breakthrough. It’s data we’ve been awaiting for 20-plus years, to now know that a lower blood pressure target is safe and absolutely essential, and where the benefits outweigh the risks,” Dr. Fonarow said. “Now implementation becomes critical. The SPRINT results are truly practice changing.”
SPRINT received no commercial support. The study received antihypertensive drugs from Arbor and Takeda at no charge for a small percentage of enrolled patients. Dr. Pfeffer has been a consultant to more than 20 companies. Dr. Rosendorff has been a consultant to McNeil and received research funding from Eisai. Dr. Yusuf has received honoraria and research grants from Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, Bayer, and Astra Zeneca. Dr. Jones, Dr. Deedwania, and Dr. Fonarow had no disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The first full report of results from the SPRINT trial of hypertension treatment targets generated lots of opinions on their implications.
Major finding: Combined cardiovascular events occurred in 5.2% of patients treated to a target systolic blood pressure of less than 120 mm Hg and 6.8% of patients treated to a target of less than 140 mm Hg.
Data source: The multicenter, randomized trial involved 9,361 patients.
Disclosures: SPRINT received no commercial support. The study received antihypertensive drugs from Arbor and Takeda at no charge for a small percentage of enrolled patients. Dr. Pfeffer has been a consultant to more than 20 companies. Dr. Rosendorff has been a consultant to McNeil and received research funding from Eisai. Dr. Yusuf has received honoraria and research grants from Sanofi-Aventis, Bristol-Myers Squibb, Pfizer, Boehringer-Ingelheim, Bayer, and Astra Zeneca. Dr. Jones, Dr. Deedwania, and Dr. Fonarow had no disclosures.
Conservative management for AR safe at 10 years
Whether to operate on patients with severe aortic regurgitation (AR) before or after symptoms appear has been a point of controversy among cardiothoracic surgeons, but a recent study has found that patients who have early surgery may not fare any better for up to 10 years than those who opt for a more conservative “watchful waiting” course of care.
Investigators from Belgium reported results from an analysis of 160 patients in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1100-08). “In asymptomatic severe AR, delaying surgery until the onset of class I/IIa operative triggers is safe, supporting current guidelines,” said Dr. Christophe de Meester and colleagues at the Catholic University of Louvain and St. Luc University Clinic in Brussels.
The goal of the study was to evaluate long-term outcomes and incidence of cardiac complications in patients with severe AR who did not have any signs and symptoms that called for surgery, and who either had surgery early on or entered conservative management and eventually had an operation when signs and symptoms did appear.
The study found that close follow-up and monitoring of patients with severe AR was a cornerstone of successful conservative management. “We found that survival was similar between the two groups,” Dr. De Meester and coauthors said. “Better survival was nonetheless observed in conservatively managed patients with regular as opposed to no or a looser follow-up.”
The most recent European Society of Cardiology (ESC) guidelines and American Heart Association/American College of Cardiology guidelines state that symptomatic severe AR is a class I indication for surgery regardless of left ventricular (LV) systolic function.
However, Dr. De Meester and colleagues said, the timing of that surgery is not so clear-cut. Earlier studies have shown that surgery could be delayed for patients with minimal symptoms, but more recent evidence has suggested the opposite, according to the study. Two factors favor surgery before symptoms arise – poor aortic valve repair outcomes in patients with symptoms of heart failure and long-standing severe AR, which eventually leads to LV dysfunction.
Yet, the latest ESC guidelines have been “reluctant” to make a strong case for early surgery before symptoms of LV dysfunction appear, and the AHA/ACC guidelines call for surgery only when symptoms of LV dysfunction or LV dilatation develop, Dr. de Meester and his coauthors said.
In the past, the risks of aortic valve replacement were too high to consider early surgery, the study authors said. “However, with the advent of aortic valve repair, operative mortality and long-term outcomes have improved to such an extent that early surgery has become a plausible option for patients.”
But the risk of these patients developing symptoms for surgery was nonetheless low over 10 years, the study found: 7.4% for developing severe LV dilatation; 0.6% for becoming symptomatic; and 0.9% for developing LV dysfunction. Overall, the rate of adverse events in the study population was 9.9% at 10 years.
In the study, 69 patients were initially managed conservatively, 49 of whom were in the watchful waiting group that visited a cardiologist at least annually and another 20 considered an “irregular follow-up subgroup.” Among the watchful waiting group, 31 developed symptoms for surgery (only two declined surgery). Watchful waiting patients had five- and 10-year survival of 100% and 95%, respectively, compared with 90% and 79% among those who had irregular follow-up.
Overall, the conservatively managed group had outcomes better than or equal to the early surgery group. Ten-year cardiovascular survival was 96% in both groups, whereas event-free survival was 92% at 10 years in the conservatively managed group vs. 81% in the early surgery group.
The study was supported by the Belgium National Fund for Scientific Research. The authors had no conflicts to disclose.
The design of the Belgium study “challenges” existing treatment guidelines for asymptomatic chronic aortic insufficiency in two ways, Dr. Leora Balsam and Dr. Abe deAndra Jr., both of the New York University-Langone Medical Center, write in their commentary (J Thorac Cardiovasc Surg. 2015;150:1108-10): first, by making aortic valve repair the preferred surgical treatment in the study and, secondly, by offering surgery to both symptomatic and asymptomatic patients.
“In the era of evidence-based medicine,” Dr. Balsam and Dr. deAndra wrote, “there remains a need for research and innovation even in areas where guidelines exist.”
While many authors have described aortic valve repair as an alternative to aortic valve replacement for chronic severe aortic insufficiency, Dr. Balsam and Dr. deAndra explained that the term aortic valve repair “encompasses a wide array of techniques,” among them valve-sparing aortic root replacement, subcommissural annuloplasty and “myriad” leaf resection, plication, and reconstruction techniques. Because of mounting reports of excellent results with aortic valve repair techniques, growing ranks of cardiothoracic surgeons have advocated for repair as an early intervention for aortic valve problems. But the question remains: “Have we identified the optimal triggers for intervention for aortic insufficiency?” they asked. “The answer is probably no, and that newer technology and diagnostic studies will better discriminate between patients that can benefit from intervention and those that will not.”
Dr. Balsam and Dr. deAndra had no disclosures.
The design of the Belgium study “challenges” existing treatment guidelines for asymptomatic chronic aortic insufficiency in two ways, Dr. Leora Balsam and Dr. Abe deAndra Jr., both of the New York University-Langone Medical Center, write in their commentary (J Thorac Cardiovasc Surg. 2015;150:1108-10): first, by making aortic valve repair the preferred surgical treatment in the study and, secondly, by offering surgery to both symptomatic and asymptomatic patients.
“In the era of evidence-based medicine,” Dr. Balsam and Dr. deAndra wrote, “there remains a need for research and innovation even in areas where guidelines exist.”
While many authors have described aortic valve repair as an alternative to aortic valve replacement for chronic severe aortic insufficiency, Dr. Balsam and Dr. deAndra explained that the term aortic valve repair “encompasses a wide array of techniques,” among them valve-sparing aortic root replacement, subcommissural annuloplasty and “myriad” leaf resection, plication, and reconstruction techniques. Because of mounting reports of excellent results with aortic valve repair techniques, growing ranks of cardiothoracic surgeons have advocated for repair as an early intervention for aortic valve problems. But the question remains: “Have we identified the optimal triggers for intervention for aortic insufficiency?” they asked. “The answer is probably no, and that newer technology and diagnostic studies will better discriminate between patients that can benefit from intervention and those that will not.”
Dr. Balsam and Dr. deAndra had no disclosures.
The design of the Belgium study “challenges” existing treatment guidelines for asymptomatic chronic aortic insufficiency in two ways, Dr. Leora Balsam and Dr. Abe deAndra Jr., both of the New York University-Langone Medical Center, write in their commentary (J Thorac Cardiovasc Surg. 2015;150:1108-10): first, by making aortic valve repair the preferred surgical treatment in the study and, secondly, by offering surgery to both symptomatic and asymptomatic patients.
“In the era of evidence-based medicine,” Dr. Balsam and Dr. deAndra wrote, “there remains a need for research and innovation even in areas where guidelines exist.”
While many authors have described aortic valve repair as an alternative to aortic valve replacement for chronic severe aortic insufficiency, Dr. Balsam and Dr. deAndra explained that the term aortic valve repair “encompasses a wide array of techniques,” among them valve-sparing aortic root replacement, subcommissural annuloplasty and “myriad” leaf resection, plication, and reconstruction techniques. Because of mounting reports of excellent results with aortic valve repair techniques, growing ranks of cardiothoracic surgeons have advocated for repair as an early intervention for aortic valve problems. But the question remains: “Have we identified the optimal triggers for intervention for aortic insufficiency?” they asked. “The answer is probably no, and that newer technology and diagnostic studies will better discriminate between patients that can benefit from intervention and those that will not.”
Dr. Balsam and Dr. deAndra had no disclosures.
Whether to operate on patients with severe aortic regurgitation (AR) before or after symptoms appear has been a point of controversy among cardiothoracic surgeons, but a recent study has found that patients who have early surgery may not fare any better for up to 10 years than those who opt for a more conservative “watchful waiting” course of care.
Investigators from Belgium reported results from an analysis of 160 patients in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1100-08). “In asymptomatic severe AR, delaying surgery until the onset of class I/IIa operative triggers is safe, supporting current guidelines,” said Dr. Christophe de Meester and colleagues at the Catholic University of Louvain and St. Luc University Clinic in Brussels.
The goal of the study was to evaluate long-term outcomes and incidence of cardiac complications in patients with severe AR who did not have any signs and symptoms that called for surgery, and who either had surgery early on or entered conservative management and eventually had an operation when signs and symptoms did appear.
The study found that close follow-up and monitoring of patients with severe AR was a cornerstone of successful conservative management. “We found that survival was similar between the two groups,” Dr. De Meester and coauthors said. “Better survival was nonetheless observed in conservatively managed patients with regular as opposed to no or a looser follow-up.”
The most recent European Society of Cardiology (ESC) guidelines and American Heart Association/American College of Cardiology guidelines state that symptomatic severe AR is a class I indication for surgery regardless of left ventricular (LV) systolic function.
However, Dr. De Meester and colleagues said, the timing of that surgery is not so clear-cut. Earlier studies have shown that surgery could be delayed for patients with minimal symptoms, but more recent evidence has suggested the opposite, according to the study. Two factors favor surgery before symptoms arise – poor aortic valve repair outcomes in patients with symptoms of heart failure and long-standing severe AR, which eventually leads to LV dysfunction.
Yet, the latest ESC guidelines have been “reluctant” to make a strong case for early surgery before symptoms of LV dysfunction appear, and the AHA/ACC guidelines call for surgery only when symptoms of LV dysfunction or LV dilatation develop, Dr. de Meester and his coauthors said.
In the past, the risks of aortic valve replacement were too high to consider early surgery, the study authors said. “However, with the advent of aortic valve repair, operative mortality and long-term outcomes have improved to such an extent that early surgery has become a plausible option for patients.”
But the risk of these patients developing symptoms for surgery was nonetheless low over 10 years, the study found: 7.4% for developing severe LV dilatation; 0.6% for becoming symptomatic; and 0.9% for developing LV dysfunction. Overall, the rate of adverse events in the study population was 9.9% at 10 years.
In the study, 69 patients were initially managed conservatively, 49 of whom were in the watchful waiting group that visited a cardiologist at least annually and another 20 considered an “irregular follow-up subgroup.” Among the watchful waiting group, 31 developed symptoms for surgery (only two declined surgery). Watchful waiting patients had five- and 10-year survival of 100% and 95%, respectively, compared with 90% and 79% among those who had irregular follow-up.
Overall, the conservatively managed group had outcomes better than or equal to the early surgery group. Ten-year cardiovascular survival was 96% in both groups, whereas event-free survival was 92% at 10 years in the conservatively managed group vs. 81% in the early surgery group.
The study was supported by the Belgium National Fund for Scientific Research. The authors had no conflicts to disclose.
Whether to operate on patients with severe aortic regurgitation (AR) before or after symptoms appear has been a point of controversy among cardiothoracic surgeons, but a recent study has found that patients who have early surgery may not fare any better for up to 10 years than those who opt for a more conservative “watchful waiting” course of care.
Investigators from Belgium reported results from an analysis of 160 patients in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1100-08). “In asymptomatic severe AR, delaying surgery until the onset of class I/IIa operative triggers is safe, supporting current guidelines,” said Dr. Christophe de Meester and colleagues at the Catholic University of Louvain and St. Luc University Clinic in Brussels.
The goal of the study was to evaluate long-term outcomes and incidence of cardiac complications in patients with severe AR who did not have any signs and symptoms that called for surgery, and who either had surgery early on or entered conservative management and eventually had an operation when signs and symptoms did appear.
The study found that close follow-up and monitoring of patients with severe AR was a cornerstone of successful conservative management. “We found that survival was similar between the two groups,” Dr. De Meester and coauthors said. “Better survival was nonetheless observed in conservatively managed patients with regular as opposed to no or a looser follow-up.”
The most recent European Society of Cardiology (ESC) guidelines and American Heart Association/American College of Cardiology guidelines state that symptomatic severe AR is a class I indication for surgery regardless of left ventricular (LV) systolic function.
However, Dr. De Meester and colleagues said, the timing of that surgery is not so clear-cut. Earlier studies have shown that surgery could be delayed for patients with minimal symptoms, but more recent evidence has suggested the opposite, according to the study. Two factors favor surgery before symptoms arise – poor aortic valve repair outcomes in patients with symptoms of heart failure and long-standing severe AR, which eventually leads to LV dysfunction.
Yet, the latest ESC guidelines have been “reluctant” to make a strong case for early surgery before symptoms of LV dysfunction appear, and the AHA/ACC guidelines call for surgery only when symptoms of LV dysfunction or LV dilatation develop, Dr. de Meester and his coauthors said.
In the past, the risks of aortic valve replacement were too high to consider early surgery, the study authors said. “However, with the advent of aortic valve repair, operative mortality and long-term outcomes have improved to such an extent that early surgery has become a plausible option for patients.”
But the risk of these patients developing symptoms for surgery was nonetheless low over 10 years, the study found: 7.4% for developing severe LV dilatation; 0.6% for becoming symptomatic; and 0.9% for developing LV dysfunction. Overall, the rate of adverse events in the study population was 9.9% at 10 years.
In the study, 69 patients were initially managed conservatively, 49 of whom were in the watchful waiting group that visited a cardiologist at least annually and another 20 considered an “irregular follow-up subgroup.” Among the watchful waiting group, 31 developed symptoms for surgery (only two declined surgery). Watchful waiting patients had five- and 10-year survival of 100% and 95%, respectively, compared with 90% and 79% among those who had irregular follow-up.
Overall, the conservatively managed group had outcomes better than or equal to the early surgery group. Ten-year cardiovascular survival was 96% in both groups, whereas event-free survival was 92% at 10 years in the conservatively managed group vs. 81% in the early surgery group.
The study was supported by the Belgium National Fund for Scientific Research. The authors had no conflicts to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Delaying surgery until the onset of symptoms of aortic insufficiency is safe, in support of current clinical guidelines.
Major finding: Ten-year cardiovascular survival was equal among conservatively managed and early-surgery groups, but event free survival was 92% at 10 years in the conservatively managed group vs. 81% in the early surgery group.
Data source: Analysis of 160 consecutive asymptomatic patients with severe aortic regurgitation who were assigned to either conservative management or early surgery and followed up for a median of 7.2 years.
Disclosures: The Belgium National Fund of Scientific Research supported the study. The authors had no disclosures.
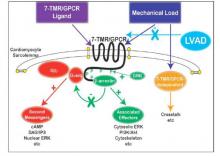
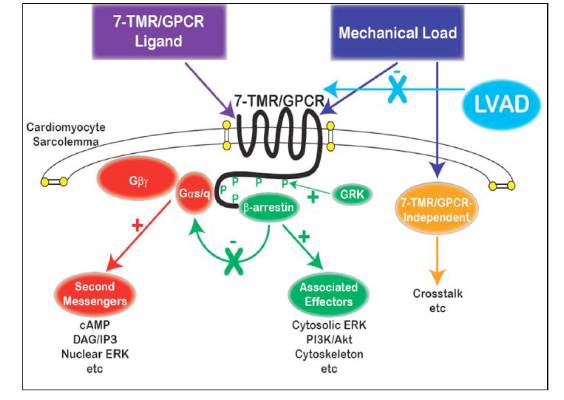
Does LVAD inhibit cardio protection?
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Left ventricular assist device (LVAD) support inhibits pathologic responses to mechanical loading but also can inhibit adaptive responses after myocardial infarction.
Major finding: LVAD support inhibited cardioprotective beta-arrestin–mediated signaling, but net benefits of normalization of load-induced G protein-coupled receptor (GPCR) signaling were observed in the MI-adjacent zone.
Data source: Sheep were induced with myocardial infarction and then placed on LVAD support for 2 weeks and observed for a total of 12 weeks. Then myocardial specimens were harvested and analyzed.
Disclosures: The study authors had no relationships to disclose.
Hybrid revascularization shows promise, but there are concerns
A hybrid coronary revascularization procedure that combines off-pump left internal mammary artery (LIMA) grafting with percutaneous coronary intervention (PCI) showed good results at 1 year after surgery, but nonetheless showed a rate of adverse events that may raise questions about the procedure.
In a study published in the November issue of the Journal of Thoracic and Cardiovascular Surgery, a team of investigators from Aarhus University Hospital in Denmark reported high rates of graft patency and low rates of death and stroke with the procedure 1 year after a series of 100 operations (J Thorac Cardiovasc Surg. 2015;150:1181-6).
“The high left internal mammary artery graft patency rate and low risk of death and stroke at 1 year seem promising for the long-term outcome of this revascularization strategy,” said Dr. Ivy Susanne Modrau and colleagues.
The single-center study evaluated 1-year clinical and angiographic results of 100 consecutive trial patients with multivessel disease who had the hybrid procedure between October 2010 and February 2012. “The rationale of hybrid coronary revascularization is to achieve the survival benefits of the LIMA to LAD (left anterior descending artery) graft with reduced invasiveness to minimize postprocedural discomfort and morbidity, in particular the risk of stroke,” Dr. Modrau and colleagues said.
The study used the LIMA to LAD graft performed off-pump through a reversed J-hemisternotomy “We chose this technique because of its excellent exposure of the heart, technical ease, low risk of complicating chronic pain, and applicability in virtually all patients,” Dr. Modrau said. Eighty-nine patients had surgery prior to PCI and 11 had PCI prior to surgery.
The primary endpoint was rate of major adverse cardiac or cerebrovascular events (MACCE), the composite of all-cause death, stroke, myocardial infarction, and repeat revascularization by PCI or coronary artery bypass grafting at 1 year. Secondary endpoints included individual components and status of stent and graft patency on angiography.
Overall, 20 patients met the 1-year primary endpoint of MACCE. One patient died, one other had a stroke, and three had heart attacks. Sixteen patients had repeat revascularization procedures, eight performed during the index hospitalization. Graft patency was 98% after 1 year.
Dr. Modrau and coauthors noted the MACCE rate of 20% “was higher than expected,” and certainly higher than results in the SYNTAX study (17.8% in the PCI group and 12.4% in the coronary artery bypass grafting [CABG] group) (Euro. Intervention. 2015;10:e1-e6). One possible reason the Danish investigators cited for higher than expected MACCE rates was that they may be attributed to the learning curve involved with LIMA grafting and the use of early angiography possibly revealing “clinically silent LIMA graft dysfunction due to technical errors.”
The number of repeat revascularizations in the study was more in line with the SYNTAX study: 7% in the Aarhus University study and 6% in the SYNTAX CABG group. However, a meta-analysis of six studies with 1,190 patients reported 1-year repeat revascularization rates of 3.8% after a hybrid procedure and 1.4% after CABG (Am Heart J. 2014;167:585-92).
Ultimately, the safety and efficacy of the hybrid revascularization approach will require long-term follow-up data and head-to-head comparison with conventional CABG and PCI in clinical trials. “Meanwhile, LIMA patency, the cornerstone of surgical revascularization, may be used as a surrogate endpoint for long-term survival after HCR,” Dr. Modrau and coauthors said.
They reported having no disclosures.
Hybrid revascularization procedures are “still not ready for prime time,” Dr. Carlos Mestres of Cleveland Clinic Abu Dhabi, United Arab Emirates, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150:1028-9).
The study illuminates two key points of concern, Dr. Mestres said: the “unexpectedly high” 20% rate of major adverse cardiac or cerebrovascular events (MACCE); and the in-hospital revascularization rate that was significantly higher than the 1% after CABG that the authors reported in their own institution. That calls into question the reason the investigators would change their own department strategy away from conventional CABG, where they had optimal results, he said.
Dr. Mestres also said the Danish investigators’ conclusion that the study results seemed promising for long-term outcomes of the hybrid procedure “are to be carefully dissected.”
He commended the investigators for collecting angiographic data at 1 year, but said that 1 year of follow-up “is simply not enough” to credibly compare staged procedures with CABG.
Dr. Mestres had no disclosures.
Hybrid revascularization procedures are “still not ready for prime time,” Dr. Carlos Mestres of Cleveland Clinic Abu Dhabi, United Arab Emirates, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150:1028-9).
The study illuminates two key points of concern, Dr. Mestres said: the “unexpectedly high” 20% rate of major adverse cardiac or cerebrovascular events (MACCE); and the in-hospital revascularization rate that was significantly higher than the 1% after CABG that the authors reported in their own institution. That calls into question the reason the investigators would change their own department strategy away from conventional CABG, where they had optimal results, he said.
Dr. Mestres also said the Danish investigators’ conclusion that the study results seemed promising for long-term outcomes of the hybrid procedure “are to be carefully dissected.”
He commended the investigators for collecting angiographic data at 1 year, but said that 1 year of follow-up “is simply not enough” to credibly compare staged procedures with CABG.
Dr. Mestres had no disclosures.
Hybrid revascularization procedures are “still not ready for prime time,” Dr. Carlos Mestres of Cleveland Clinic Abu Dhabi, United Arab Emirates, said in his invited commentary (J Thorac Cardiovasc Surg. 2015;150:1028-9).
The study illuminates two key points of concern, Dr. Mestres said: the “unexpectedly high” 20% rate of major adverse cardiac or cerebrovascular events (MACCE); and the in-hospital revascularization rate that was significantly higher than the 1% after CABG that the authors reported in their own institution. That calls into question the reason the investigators would change their own department strategy away from conventional CABG, where they had optimal results, he said.
Dr. Mestres also said the Danish investigators’ conclusion that the study results seemed promising for long-term outcomes of the hybrid procedure “are to be carefully dissected.”
He commended the investigators for collecting angiographic data at 1 year, but said that 1 year of follow-up “is simply not enough” to credibly compare staged procedures with CABG.
Dr. Mestres had no disclosures.
A hybrid coronary revascularization procedure that combines off-pump left internal mammary artery (LIMA) grafting with percutaneous coronary intervention (PCI) showed good results at 1 year after surgery, but nonetheless showed a rate of adverse events that may raise questions about the procedure.
In a study published in the November issue of the Journal of Thoracic and Cardiovascular Surgery, a team of investigators from Aarhus University Hospital in Denmark reported high rates of graft patency and low rates of death and stroke with the procedure 1 year after a series of 100 operations (J Thorac Cardiovasc Surg. 2015;150:1181-6).
“The high left internal mammary artery graft patency rate and low risk of death and stroke at 1 year seem promising for the long-term outcome of this revascularization strategy,” said Dr. Ivy Susanne Modrau and colleagues.
The single-center study evaluated 1-year clinical and angiographic results of 100 consecutive trial patients with multivessel disease who had the hybrid procedure between October 2010 and February 2012. “The rationale of hybrid coronary revascularization is to achieve the survival benefits of the LIMA to LAD (left anterior descending artery) graft with reduced invasiveness to minimize postprocedural discomfort and morbidity, in particular the risk of stroke,” Dr. Modrau and colleagues said.
The study used the LIMA to LAD graft performed off-pump through a reversed J-hemisternotomy “We chose this technique because of its excellent exposure of the heart, technical ease, low risk of complicating chronic pain, and applicability in virtually all patients,” Dr. Modrau said. Eighty-nine patients had surgery prior to PCI and 11 had PCI prior to surgery.
The primary endpoint was rate of major adverse cardiac or cerebrovascular events (MACCE), the composite of all-cause death, stroke, myocardial infarction, and repeat revascularization by PCI or coronary artery bypass grafting at 1 year. Secondary endpoints included individual components and status of stent and graft patency on angiography.
Overall, 20 patients met the 1-year primary endpoint of MACCE. One patient died, one other had a stroke, and three had heart attacks. Sixteen patients had repeat revascularization procedures, eight performed during the index hospitalization. Graft patency was 98% after 1 year.
Dr. Modrau and coauthors noted the MACCE rate of 20% “was higher than expected,” and certainly higher than results in the SYNTAX study (17.8% in the PCI group and 12.4% in the coronary artery bypass grafting [CABG] group) (Euro. Intervention. 2015;10:e1-e6). One possible reason the Danish investigators cited for higher than expected MACCE rates was that they may be attributed to the learning curve involved with LIMA grafting and the use of early angiography possibly revealing “clinically silent LIMA graft dysfunction due to technical errors.”
The number of repeat revascularizations in the study was more in line with the SYNTAX study: 7% in the Aarhus University study and 6% in the SYNTAX CABG group. However, a meta-analysis of six studies with 1,190 patients reported 1-year repeat revascularization rates of 3.8% after a hybrid procedure and 1.4% after CABG (Am Heart J. 2014;167:585-92).
Ultimately, the safety and efficacy of the hybrid revascularization approach will require long-term follow-up data and head-to-head comparison with conventional CABG and PCI in clinical trials. “Meanwhile, LIMA patency, the cornerstone of surgical revascularization, may be used as a surrogate endpoint for long-term survival after HCR,” Dr. Modrau and coauthors said.
They reported having no disclosures.
A hybrid coronary revascularization procedure that combines off-pump left internal mammary artery (LIMA) grafting with percutaneous coronary intervention (PCI) showed good results at 1 year after surgery, but nonetheless showed a rate of adverse events that may raise questions about the procedure.
In a study published in the November issue of the Journal of Thoracic and Cardiovascular Surgery, a team of investigators from Aarhus University Hospital in Denmark reported high rates of graft patency and low rates of death and stroke with the procedure 1 year after a series of 100 operations (J Thorac Cardiovasc Surg. 2015;150:1181-6).
“The high left internal mammary artery graft patency rate and low risk of death and stroke at 1 year seem promising for the long-term outcome of this revascularization strategy,” said Dr. Ivy Susanne Modrau and colleagues.
The single-center study evaluated 1-year clinical and angiographic results of 100 consecutive trial patients with multivessel disease who had the hybrid procedure between October 2010 and February 2012. “The rationale of hybrid coronary revascularization is to achieve the survival benefits of the LIMA to LAD (left anterior descending artery) graft with reduced invasiveness to minimize postprocedural discomfort and morbidity, in particular the risk of stroke,” Dr. Modrau and colleagues said.
The study used the LIMA to LAD graft performed off-pump through a reversed J-hemisternotomy “We chose this technique because of its excellent exposure of the heart, technical ease, low risk of complicating chronic pain, and applicability in virtually all patients,” Dr. Modrau said. Eighty-nine patients had surgery prior to PCI and 11 had PCI prior to surgery.
The primary endpoint was rate of major adverse cardiac or cerebrovascular events (MACCE), the composite of all-cause death, stroke, myocardial infarction, and repeat revascularization by PCI or coronary artery bypass grafting at 1 year. Secondary endpoints included individual components and status of stent and graft patency on angiography.
Overall, 20 patients met the 1-year primary endpoint of MACCE. One patient died, one other had a stroke, and three had heart attacks. Sixteen patients had repeat revascularization procedures, eight performed during the index hospitalization. Graft patency was 98% after 1 year.
Dr. Modrau and coauthors noted the MACCE rate of 20% “was higher than expected,” and certainly higher than results in the SYNTAX study (17.8% in the PCI group and 12.4% in the coronary artery bypass grafting [CABG] group) (Euro. Intervention. 2015;10:e1-e6). One possible reason the Danish investigators cited for higher than expected MACCE rates was that they may be attributed to the learning curve involved with LIMA grafting and the use of early angiography possibly revealing “clinically silent LIMA graft dysfunction due to technical errors.”
The number of repeat revascularizations in the study was more in line with the SYNTAX study: 7% in the Aarhus University study and 6% in the SYNTAX CABG group. However, a meta-analysis of six studies with 1,190 patients reported 1-year repeat revascularization rates of 3.8% after a hybrid procedure and 1.4% after CABG (Am Heart J. 2014;167:585-92).
Ultimately, the safety and efficacy of the hybrid revascularization approach will require long-term follow-up data and head-to-head comparison with conventional CABG and PCI in clinical trials. “Meanwhile, LIMA patency, the cornerstone of surgical revascularization, may be used as a surrogate endpoint for long-term survival after HCR,” Dr. Modrau and coauthors said.
They reported having no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:High 1-year left internal mammary artery (LIMA) graft patency and low risk of death and stroke seem promising for long-term outcome after HCR.
Major finding: At 1 year, 98% of patients had patent LIMA grafts but the 20% rate of major adverse cardiac or cerebrovascular events was “higher than expected.”
Data source: Prospective single arm clinical feasibility study including 100 consecutive patients with multivessel disease undergoing staged hybrid coronary revascularization.
Disclosures: The study authors had no disclosures.
New CPR guide sets compression limits, scratches vasopressin
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
New guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) set upper limits on chest compression rate and depth, add naloxone to the care of suspected opioid abusers, and remove vasopressin from the advanced cardiac life support (ACLS) algorithm.
The American Heart Association published its revised guidelines Oct. 15 in Circulation. The AHA released its previous guidelines in 2010.
“Everyone has a role to play in the chain of survival – from bystanders to dispatchers, emergency responders to health care providers,” Dr. Mark Creager said in a statement. “When everyone knows their role, knows CPR, and works together, we can dramatically improve cardiac arrest victims’ chances of survival,” said Dr. Creager, AHA president and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
The 2015 guidelines’ new recommendations include the following:
• Resuscitation pathways. The guidelines note that the resuscitation pathways are very different for patients who experience cardiac arrest present in either a hospital setting (IHCA) or out-of-hospital setting (OHCA). In OHCA, the patient depends on lay rescuers to not only recognize the situation but also call for help, initiate CPR, and, if available, administer defibrillation until emergency medical personnel arrive. However, IHCA involves prevention of cardiac arrest and smooth delivery of care in a multidisciplinary setting.
• Layperson CPR. Untrained lay rescuers should provide compression-only CPR for OHCA. Trained lay rescuers who are able to provide rescue breaths should begin CPR with compressions followed by breaths at a ratio of 30 compressions to two breaths. Compression-only CPR is easier to perform for untrained lay rescuers, the guidelines note, and survival rates are similar using CPR with or without rescue breaths in adult cardiac arrest with a cardiac etiology.
• Compression rate and depth. The new guidelines set upper limits on chest compression depth and heart rate, recommending a compression rate of 100-120 compressions per minute with a depth of at least 2 inches, not to exceed 2.4 inches in adults.
• Social media dispatching. Despite limited evidence, the guideline authors said that it may be reasonable for communities to use social media technologies to alert lay rescuers with mobile phones about nearby OHCA cases.
• Naloxone and opioid addiction. Also new to the guidelines is the recommended use of naloxone for patients with suspected or known opioid addiction by appropriately trained lay rescuers or basic life support (BLS) providers.
• CPR training. The guidelines highlight several changes to simplify health care provider training in CPR. For example, trained rescuers can simultaneously perform some tasks to reduce the time to initiate chest compressions. Likewise, in a team of trained rescuers, multiple steps such as activating the emergency response system, chest compression, ventilation, and defibrillator retrieval can be accomplished simultaneously.
• High-quality CPR. Finally, the guidelines focus on emphasizing high-quality CPR with adequate compression rate and depth, complete chest recoil, few interruptions to compressions, and appropriate ventilation.
The guidelines offer several changes to advanced cardiac life support (ACLS). The algorithm was simplified by removing vasopressin, because the authors note that “the combined use of vasopressin and epinephrine offers no advantage to using standard-dose epinephrine in cardiac arrest.”
Likewise, the guidelines note conflicting studies to support the use of lidocaine after return of spontaneous circulation (ROSC). “However, the initiation or continuation of lidocaine may be considered immediately after ROSC from VF/pulseless ventricular tachycardia cardiac arrest,” the guideline authors wrote. Finally, the guidelines highlight updates in post–cardiac arrest care, including a wider range of target temperatures, between 32° C and 36° C, to be maintained for at least 24 hours in comatose adults with ROSC after cardiac arrest. In comparison, the 2010 guidelines called for a target temperature range of 32° C to 34° C for 12-24 hours. The guidelines also detail new updates for acute coronary syndrome, pediatric BLS, pediatric ACLS, and neonatal resuscitation.
As the AHA updates its CPR guidelines, it’s also important for lay rescuers and health providers to update their own training, noted Dr. Clifton Callaway, chair of the AHA’s Emergency Cardiovascular Care (ECC) committee.
“Research shows resuscitation skills can decline within a few months after training – far before the 2-year period in which basic and advanced life support skills are currently evaluated,” cautioned Dr. Callaway, professor of emergency medicine at the University of Pittsburgh. “Frequent training with shorter intervals of basic and advanced cardiovascular life support skills may be helpful for providers who are likely to encounter a cardiac arrest to ensure the patient receives high-quality CPR,” he added.
FROM CIRCULATION
ESC: Cancer itself may cause cardiotoxicity
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.
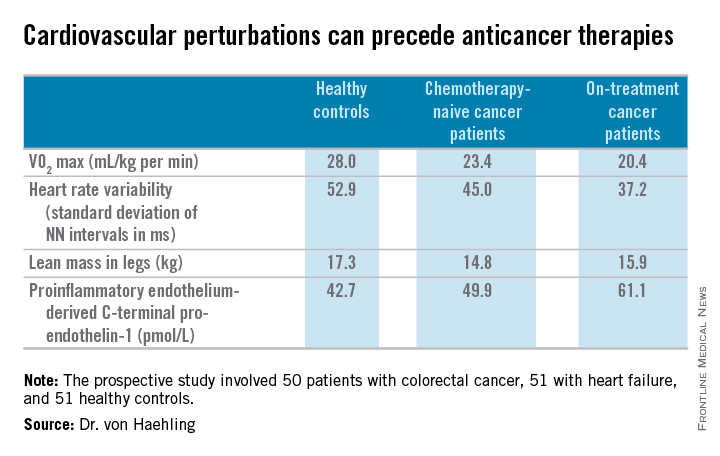
For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
FDA approves first bioabsorbable-polymer stent
The Food and Drug Administration has approved Synergy, first coronary stent with a bioabsorbable polymer on the U.S. market, Boston Scientific announced.
Synergy is a platinum chrome stent that uses the polymer PLGA as the biodegradable carrier to deliver the antirestenosing agent everolimus. Polymers have been identified as potential contributors to coronary artery restenosis after stent deployment, at least partly due to their contribution to a proinflammatory state.
The new stent, which carries the CE mark and has been in use in several European countries since 2013, was studied in the United States in the pivotal EVOLVE II clinical trial, which was a randomized, multicenter trial of 1,684 patients. That trial met its primary endpoints of noninferiority in safety and efficacy when compared to a drug-eluting stent that used a durable polymer (Circ Cardiovasc Interv. 2015 Apr 8. doi: 10.1161/CIRCINTERVENTIONS.114.002372).
EVOLVE II’s primary efficacy endpoint was a combined measure of cardiac death, myocardial infarction with ischemia from a stented artery, or ischemia-driven target vessel revascularization. The Synergy arm saw 6.7% of patients experiencing one of these events, compared with 6.5% of the durable-polymer stent patients. This difference was not statistically significant.
The primary safety outcome of definite or probable stent thrombosis was seen in three patients with the resorbable polymer stent and in five patients with durable polymer stents. This was also a nonsignificant difference.
In EVOLVE II, procedures using Synergy were slightly more likely to be immediately successful than those using the durable polymer stent, with 98.3% vs. 96.9% immediate success rates.
“I’m very excited to have the next generation of the next generation” of stents available as a choice for patients, Dr. Roxana Mehran, professor of medicine and an interventional cardiologist at Mt. Sinai Hospital in New York, said in an interview. She noted that the data are strong, and expects that this stent will prove to be safe and efficacious. But removing the presumed “bad actor” of the proinflammatory durable polymer, she said, may also afford an opportunity to shorten the duration of antiplatelet therapy (DAPT).
She called for a prospective randomized, controlled trial of a shorter duration of DAPT, emphasizing real-world considerations. “Our patients are real people,” she said. “They might need a colonoscopy, spine surgery, knee surgery, dental implants.” The sooner patients can safely stop DAPT, the better, for the mostly elderly population that will be receiving the stents, she said. “It’s very exciting, but I still think we have a ways to go.”
EVOLVE II was sponsored by Boston Scientific, the manufacturer of Synergy. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as several other device and drug companies.
On Twitter @karioakes
The Food and Drug Administration has approved Synergy, first coronary stent with a bioabsorbable polymer on the U.S. market, Boston Scientific announced.
Synergy is a platinum chrome stent that uses the polymer PLGA as the biodegradable carrier to deliver the antirestenosing agent everolimus. Polymers have been identified as potential contributors to coronary artery restenosis after stent deployment, at least partly due to their contribution to a proinflammatory state.
The new stent, which carries the CE mark and has been in use in several European countries since 2013, was studied in the United States in the pivotal EVOLVE II clinical trial, which was a randomized, multicenter trial of 1,684 patients. That trial met its primary endpoints of noninferiority in safety and efficacy when compared to a drug-eluting stent that used a durable polymer (Circ Cardiovasc Interv. 2015 Apr 8. doi: 10.1161/CIRCINTERVENTIONS.114.002372).
EVOLVE II’s primary efficacy endpoint was a combined measure of cardiac death, myocardial infarction with ischemia from a stented artery, or ischemia-driven target vessel revascularization. The Synergy arm saw 6.7% of patients experiencing one of these events, compared with 6.5% of the durable-polymer stent patients. This difference was not statistically significant.
The primary safety outcome of definite or probable stent thrombosis was seen in three patients with the resorbable polymer stent and in five patients with durable polymer stents. This was also a nonsignificant difference.
In EVOLVE II, procedures using Synergy were slightly more likely to be immediately successful than those using the durable polymer stent, with 98.3% vs. 96.9% immediate success rates.
“I’m very excited to have the next generation of the next generation” of stents available as a choice for patients, Dr. Roxana Mehran, professor of medicine and an interventional cardiologist at Mt. Sinai Hospital in New York, said in an interview. She noted that the data are strong, and expects that this stent will prove to be safe and efficacious. But removing the presumed “bad actor” of the proinflammatory durable polymer, she said, may also afford an opportunity to shorten the duration of antiplatelet therapy (DAPT).
She called for a prospective randomized, controlled trial of a shorter duration of DAPT, emphasizing real-world considerations. “Our patients are real people,” she said. “They might need a colonoscopy, spine surgery, knee surgery, dental implants.” The sooner patients can safely stop DAPT, the better, for the mostly elderly population that will be receiving the stents, she said. “It’s very exciting, but I still think we have a ways to go.”
EVOLVE II was sponsored by Boston Scientific, the manufacturer of Synergy. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as several other device and drug companies.
On Twitter @karioakes
The Food and Drug Administration has approved Synergy, first coronary stent with a bioabsorbable polymer on the U.S. market, Boston Scientific announced.
Synergy is a platinum chrome stent that uses the polymer PLGA as the biodegradable carrier to deliver the antirestenosing agent everolimus. Polymers have been identified as potential contributors to coronary artery restenosis after stent deployment, at least partly due to their contribution to a proinflammatory state.
The new stent, which carries the CE mark and has been in use in several European countries since 2013, was studied in the United States in the pivotal EVOLVE II clinical trial, which was a randomized, multicenter trial of 1,684 patients. That trial met its primary endpoints of noninferiority in safety and efficacy when compared to a drug-eluting stent that used a durable polymer (Circ Cardiovasc Interv. 2015 Apr 8. doi: 10.1161/CIRCINTERVENTIONS.114.002372).
EVOLVE II’s primary efficacy endpoint was a combined measure of cardiac death, myocardial infarction with ischemia from a stented artery, or ischemia-driven target vessel revascularization. The Synergy arm saw 6.7% of patients experiencing one of these events, compared with 6.5% of the durable-polymer stent patients. This difference was not statistically significant.
The primary safety outcome of definite or probable stent thrombosis was seen in three patients with the resorbable polymer stent and in five patients with durable polymer stents. This was also a nonsignificant difference.
In EVOLVE II, procedures using Synergy were slightly more likely to be immediately successful than those using the durable polymer stent, with 98.3% vs. 96.9% immediate success rates.
“I’m very excited to have the next generation of the next generation” of stents available as a choice for patients, Dr. Roxana Mehran, professor of medicine and an interventional cardiologist at Mt. Sinai Hospital in New York, said in an interview. She noted that the data are strong, and expects that this stent will prove to be safe and efficacious. But removing the presumed “bad actor” of the proinflammatory durable polymer, she said, may also afford an opportunity to shorten the duration of antiplatelet therapy (DAPT).
She called for a prospective randomized, controlled trial of a shorter duration of DAPT, emphasizing real-world considerations. “Our patients are real people,” she said. “They might need a colonoscopy, spine surgery, knee surgery, dental implants.” The sooner patients can safely stop DAPT, the better, for the mostly elderly population that will be receiving the stents, she said. “It’s very exciting, but I still think we have a ways to go.”
EVOLVE II was sponsored by Boston Scientific, the manufacturer of Synergy. Dr. Mehran has received honoraria from and has been a consultant to Boston Scientific as well as several other device and drug companies.
On Twitter @karioakes
Reattaching intercostals fails to squelch spinal cord ischemia in TAAA repairs
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
Spinal cord ischemia is a rare but devastating complication of thoracoabdominal aneurysm repair. Crawford and his colleagues documented in 1993 an incidence of spinal cord ischemia (SCI) as high as 30% for extensive thoracoabdominal repairs. Efforts to diminish the risk of SCI were concentrated in identifying and preserving the direct arterial perfusion to the spinal cord from segmental arteries but continued experimental and clinical experience have suggested that multiple factors contribute to SCI.

|
Dr. Luis A. Sanchez |
Some generally accepted principles for minimizing SCI include hypothermia, distal aortic perfusion with atriofemoral bypass or partial cardiopulmonary bypass, cerebrospinal fluid drainage, and avoidance of hemodynamic instability. Reimplantation of intercostal branches has been suggested as an adjunct to these techniques by some investigators with limited data to support its generalized application. More recently, a growing body of evidence supports the concept of a collateral network that can support the perfusion to the spinal cord after interruption of multiple intercostal arteries and the importance of the hypogastric and subclavian arteries as critical branches that perfuse the spinal collateral network.
The retrospective review of the extensive experience at the University of Wisconsin in Madison supports the concept that “physiologic interventions that increase spinal cord tolerance and collateral network perfusion during and after surgery” are more important than the reimplantation of intercostal vessels during this complex procedure, even in patients considered at the highest risk for SCI. Intercostal artery reimplantation failed to achieve significance as an SCI predictor when comparing two large cohorts of patients (540 vs. 265) treated with intercostal ligation vs. reimplantation. Increasingly, available data support the concept of a collateral network that maintains perfusion to the spinal cord after intercostal artery occlusion.
Additional new concepts and techniques including a two-stage approach for extensive thoracoabdominal repair, preliminary occlusion of some segmental arteries, and the use of hybrid and endovascular techniques may further decrease the incidence of SCI by taking advantage of the collateral network and allow some preconditioning of the spinal cord. Fortunately for these challenging patients, significant advances continue to be made to better understand and prevent spinal cord ischemia.
Dr. Luis A. Sanchez is Chief, Section of Vascular Surgery and the Gregorio A. Sicard Distinguished Professor of Surgery and Radiology, Department of Surgery, Washington University in St. Louis.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
CHICAGO – Intercostal artery reimplantation fails to significantly reduce spinal cord injury following thoracoabdominal aortic aneurysm surgery, results of a large retrospective study show.
“Although there was a small decrease in spinal cord ischemia with ICAR, reattaching the intercostals did not produce a statistically significant reduction in spinal cord ischemia, even in the highest risk patients,” Dr. Charles W. Acher of the University of Wisconsin–Madison, said at the annual meeting of the Midwestern Vascular Surgical Society.
Intercostal artery reimplantation (ICAR) is one of several strategies that have been used to prevent spinal cord ischemia (SCI), paraplegia, and paraparesis that occurs from the interruption of the blood supply to intercostal arteries (ICAs) during thoracoabdominal aortic aneurysm (TAAA) repair.
Surgeons at UW–Madison adopted the ICAR strategy in 2005and now reimplant open ICAs located at T7-L2 in all Type I, II, and III TAAAs, using a previously published technique (J Surg Res. 2009;154:99-104).
Using a prospectively maintained database, the current analysis sought to compare outcomes between 540 patients who had TAAA surgery during 1989-2004 when open ICAs were ligated and 265 patients who had surgery during 2005-2013 with ICAR.The surgical technique for both groups was cross clamp without assisted circulation. The anesthetic technique was also uniform during the study period and included moderate systemic hypothermia (32° - 33° C); spinal fluid drainage (spinal fluid pressure less than 5 mm Hg); naloxone 1 mcg/kg per hour; use of mannitol, methylprednisolone, and barbiturate burst suppression; goal-directed therapy for a mean arterial pressure of 90-100 mm Hg and cardiac index of 2.5 L per minute/meter2; and proactive component blood therapy to avoid anemia, hypovolemia, and hypertension.
Aneurysm extent, acuity, mortality, renal failure, and pulmonary failure were the same in both groups.
The incidence of SCI was similar in all TAAAs at 5.25% without ICAR and 3.4% with ICAR (P = .23) and in the subset of patients with Type I, II, and III aneurysms (8.8% vs. 5.1%; P = .152), Dr. Acher reported on behalf of lead author and his colleague, Dr. Martha M. Wynn.
Interestingly, ICAR patients had more dissections than did the open ICA ligation patients (18% vs. 15%; P = .0016), more previous aortic surgery (47% vs. 31%; P = .0004), and longer renal ischemia time (61 minutes vs. 53 minutes; P = .0001), but had a shorter length of stay (14 days vs. 22 days; P = .0001) and were younger (mean age, 66 years vs. 70 years; P = .0001).
In a multivariate model of all TAAAs, significant predictors of spinal cord ischemia/injury were type II TAAA (odds ratio, 7.59; P = .0001), dissection (OR, 4.25; P = .0015), age as a continuous variable (P = .0085), and acute TAAA (OR, 2.1; P = .0525), Dr. Acher said. Time period of surgery, and therefore ICAR, was not significant (OR, 0.78; P = .55).
ICAR also failed to achieve significance as an SCI predictor in a subanalysis restricted to the highest-risk patients, defined as those having Type II TAAA, dissection, and acute surgery (OR, 0.67; P = .3387).
“Interrupting blood supply to the spinal cord causes spinal cord ischemia that can be mitigated almost entirely by physiologic interventions that increase spinal cord ischemic tolerance and collateral network perfusion during and after surgery,” Dr. Acher said. “Although the cause of SCI in TAAA surgery is anatomic, prevention of the injury is largely physiologic.”
During a discussion of the study, Dr. Acher surprised the audience by saying the findings have not changed current practice at the university. He cited several reasons, observing that there were more dissections in the ICAR group, and most of the ischemia in the ICAR group was delayed, suggesting that more patients could be rescued. In addition, there was a slight downward trend in spinal cord injury and immediate paraplegia with ICAR, however, these were not statistically significant.
“Because of those things, I still think it’s valuable, particularly in patients that are at highest risk, which are the dissections, with lots of open intercostals, but the emphasis should still be on physiologic parameters,” he said. “If you want to salvage patients, that’s the most important thing.
“Even if ICAR were ever shown to be statistically significant in a larger patient population, any role it has in reducing spinal cord injury would be extremely small,” he added in an interview.
The authors reported having no conflicts of interest.
AT MIDWESTERN VASCULAR 2015
Key clinical point: Intercostal artery reimplantation (ICAR) did not produce a significant reduction in spinal cord ischemia following thoracoabdominal aortic aneurysm repair, even in the highest risk patients.
Major finding: ICAR was not a significant predictor of spinal cord ischemia (OR, 0.78; P = .55).
Data source: Retrospective analysis of 805 patients undergoing TAAA with or without ICAR.
Disclosures: The authors reported having no conflicts of interest.
‘Minimalist’ TAVR has short learning curve
As a “minimalist” approach to transcatheter aortic valve replacement – known as MA-TAVR – gains in popularity at high-volume centers, questions persist about the surgeon’s learning curve. A small series of MA-TAVR cases at Emory University in Atlanta has shown that the leaning curve may be like the TAVR approach itself: minimal.
Dr. Hanna Jensen and her associates reported on 151 consecutive patients who had MA-TAVR in the October issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.078). They previously reported their findings at the annual meeting of the American Association for Thoracic Surgery in April in Seattle.
This study builds on an Emory study last year that reported the minimalist approach to TAVR cost about $10,000 less per patient than the standard transfemoral approach (JACC Cardiovasc Interv. 2014;7:898-904).
The operation the study authors evaluated is performed in the catheterization laboratory rather than the operating room, as in traditional TAVR. Both approaches use a femoral approach, but where traditional TAVR requires general anesthesia and transesophageal echocardiography (TEE), MA-TAVR uses local anesthesia, minimal conscious sedation, and transthoracic echocardiography (TTE).
The study authors acknowledged concerns that TTE may underestimate the severity of paravalvular leak after the procedure when compared with TEE. Their protocol relies on preoperative TTE and CT scans, or three-dimensional TEE if the case warrants it, to ensure optimal sizing of the transcatheter valve before the operation. “If any concerns arise, our threshold is low to perform intraoperative balloon-sizing,” Dr. Jensen and her coauthors said. They also use TTE, along with a root-angiogram after valve deployment, and invasively measure the aortic regurgitation index before and after deployment.
Most study patients were high-risk surgical candidates with a median Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score of 10%. The overall major stroke rate was 3.3%, while major vascular complications occurred in 3% of patients and the greater-than-mild paravalvular leak rate was 7%.
The study retrospectively evaluated 151 consecutive patients who were divided into three groups at different time points: May 2012 to January 2013, February to August 2013, and September 2013 to July 2014. Complications were similar among all three groups, but the third group had shorter hospital stays and less time in the intensive care unit (ICU).
The first group received only the first-generation SAPIEN valve system; use of the second-generation SAPIEN XT valve increased in latter two groups. The SAPIEN XT valve is available in 23, 26, or 29 mm, but the 29-mm size was not available in the first-generation SAPIEN implant.
A subgroup analysis looked at patients who were discharged within 48 hours of the operation or more than 48 hours afterward. The early-discharge patients had lower STS PROM scores (8.3% vs. 10.3%) and lower rates of diabetes (31% vs. 49%). They also had less need for postoperative pacemakers and less frequent rehospitalization. “This implies that in selected MA-TAVR patients early discharge is feasible and safe, but larger studies are required to identify the optimal profile of patients who can be sent home within the first two postoperative days,” Dr. Jensen and her colleagues said.
Early in the MA-TAVR protocol all patients were sent to the ICU. As the care team gained more experience with the procedure, the protocol changed to send all patients to a regular telemetry floor after surgery unless they had vascular issues or potential need for a pacemaker. The decreasing need for ICU “was the only indication of an institutional learning curve that was discovered, and demonstrated improved resource utilization over time,” the investigators said.
They encouraged other centers to pursue MA-TAVR. “As experience grows, we believe that this procedure can be done with less or no ICU support leading to a shorter hospital stay and improved resource utilization,” Dr. Jensen and her coauthors concluded. They called for further studies to determine the characteristics that make a patient most suitable for a short-admission MA-TAVR procedure.
Study coauthors Dr. Vasilis Babaliaros, Dr. Vinod Thourani, Amy Simone, and Patricia Keegan are research consultants with Edwards Lifesciences. The rest of the authors had no disclosures.
Calling this report an “early milestone in the relentless simplification” of transcatheter aortic valve replacement (TAVR), Dr. Craig Smith of Columbia University Medical Center/New York Presbyterian Hospital, wrote in his invited commentary that it nonetheless leaves a few questions unanswered – and may leave surgeons seeing their role in TAVR marginalized as the procedure moves from the operating room to the catheterization lab (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.082). .

|
Dr. Craig Smith |
One unanswered question revolves around the use of conscious sedation and transthoracic echocardiography (TTE) for the minimalist approach (MA), rather than general anesthesia and transesophageal echocardiography (TEE) of the traditional transfemoral approach. “MA requires reliance on [TTE] for assessment of paravalvular leak, and since TTE can’t be compared to TEE in the same patients and still be MA, the merits of this trade-off cannot be assessed in this population,” he said.
Further, he said that the study data do not conclusively link MA to early discharge because the early discharge patients had lower Society of Thoracic Surgery scores.
Another important unanswered question is whether endocarditis is more frequent in TAVR when it’s performed outside the operating room.
“While I suspect the answer will be ‘yes,’ this question will be left dangling until large numbers have been done in hybrid cath labs, because the frequency will be low, and because the forces propelling a ‘cath lab’ alternative to surgical or transcatheter valve replacement done in an operating room will be too powerful to retard on a hunch,” Dr. Smith wrote. “What will the departure of TAVR from operating rooms mean for the role of the surgeon? That is for surgeons to determine. Stay involved, or say goodbye!”
Calling this report an “early milestone in the relentless simplification” of transcatheter aortic valve replacement (TAVR), Dr. Craig Smith of Columbia University Medical Center/New York Presbyterian Hospital, wrote in his invited commentary that it nonetheless leaves a few questions unanswered – and may leave surgeons seeing their role in TAVR marginalized as the procedure moves from the operating room to the catheterization lab (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.082). .

|
Dr. Craig Smith |
One unanswered question revolves around the use of conscious sedation and transthoracic echocardiography (TTE) for the minimalist approach (MA), rather than general anesthesia and transesophageal echocardiography (TEE) of the traditional transfemoral approach. “MA requires reliance on [TTE] for assessment of paravalvular leak, and since TTE can’t be compared to TEE in the same patients and still be MA, the merits of this trade-off cannot be assessed in this population,” he said.
Further, he said that the study data do not conclusively link MA to early discharge because the early discharge patients had lower Society of Thoracic Surgery scores.
Another important unanswered question is whether endocarditis is more frequent in TAVR when it’s performed outside the operating room.
“While I suspect the answer will be ‘yes,’ this question will be left dangling until large numbers have been done in hybrid cath labs, because the frequency will be low, and because the forces propelling a ‘cath lab’ alternative to surgical or transcatheter valve replacement done in an operating room will be too powerful to retard on a hunch,” Dr. Smith wrote. “What will the departure of TAVR from operating rooms mean for the role of the surgeon? That is for surgeons to determine. Stay involved, or say goodbye!”
Calling this report an “early milestone in the relentless simplification” of transcatheter aortic valve replacement (TAVR), Dr. Craig Smith of Columbia University Medical Center/New York Presbyterian Hospital, wrote in his invited commentary that it nonetheless leaves a few questions unanswered – and may leave surgeons seeing their role in TAVR marginalized as the procedure moves from the operating room to the catheterization lab (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.082). .

|
Dr. Craig Smith |
One unanswered question revolves around the use of conscious sedation and transthoracic echocardiography (TTE) for the minimalist approach (MA), rather than general anesthesia and transesophageal echocardiography (TEE) of the traditional transfemoral approach. “MA requires reliance on [TTE] for assessment of paravalvular leak, and since TTE can’t be compared to TEE in the same patients and still be MA, the merits of this trade-off cannot be assessed in this population,” he said.
Further, he said that the study data do not conclusively link MA to early discharge because the early discharge patients had lower Society of Thoracic Surgery scores.
Another important unanswered question is whether endocarditis is more frequent in TAVR when it’s performed outside the operating room.
“While I suspect the answer will be ‘yes,’ this question will be left dangling until large numbers have been done in hybrid cath labs, because the frequency will be low, and because the forces propelling a ‘cath lab’ alternative to surgical or transcatheter valve replacement done in an operating room will be too powerful to retard on a hunch,” Dr. Smith wrote. “What will the departure of TAVR from operating rooms mean for the role of the surgeon? That is for surgeons to determine. Stay involved, or say goodbye!”
As a “minimalist” approach to transcatheter aortic valve replacement – known as MA-TAVR – gains in popularity at high-volume centers, questions persist about the surgeon’s learning curve. A small series of MA-TAVR cases at Emory University in Atlanta has shown that the leaning curve may be like the TAVR approach itself: minimal.
Dr. Hanna Jensen and her associates reported on 151 consecutive patients who had MA-TAVR in the October issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.078). They previously reported their findings at the annual meeting of the American Association for Thoracic Surgery in April in Seattle.
This study builds on an Emory study last year that reported the minimalist approach to TAVR cost about $10,000 less per patient than the standard transfemoral approach (JACC Cardiovasc Interv. 2014;7:898-904).
The operation the study authors evaluated is performed in the catheterization laboratory rather than the operating room, as in traditional TAVR. Both approaches use a femoral approach, but where traditional TAVR requires general anesthesia and transesophageal echocardiography (TEE), MA-TAVR uses local anesthesia, minimal conscious sedation, and transthoracic echocardiography (TTE).
The study authors acknowledged concerns that TTE may underestimate the severity of paravalvular leak after the procedure when compared with TEE. Their protocol relies on preoperative TTE and CT scans, or three-dimensional TEE if the case warrants it, to ensure optimal sizing of the transcatheter valve before the operation. “If any concerns arise, our threshold is low to perform intraoperative balloon-sizing,” Dr. Jensen and her coauthors said. They also use TTE, along with a root-angiogram after valve deployment, and invasively measure the aortic regurgitation index before and after deployment.
Most study patients were high-risk surgical candidates with a median Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score of 10%. The overall major stroke rate was 3.3%, while major vascular complications occurred in 3% of patients and the greater-than-mild paravalvular leak rate was 7%.
The study retrospectively evaluated 151 consecutive patients who were divided into three groups at different time points: May 2012 to January 2013, February to August 2013, and September 2013 to July 2014. Complications were similar among all three groups, but the third group had shorter hospital stays and less time in the intensive care unit (ICU).
The first group received only the first-generation SAPIEN valve system; use of the second-generation SAPIEN XT valve increased in latter two groups. The SAPIEN XT valve is available in 23, 26, or 29 mm, but the 29-mm size was not available in the first-generation SAPIEN implant.
A subgroup analysis looked at patients who were discharged within 48 hours of the operation or more than 48 hours afterward. The early-discharge patients had lower STS PROM scores (8.3% vs. 10.3%) and lower rates of diabetes (31% vs. 49%). They also had less need for postoperative pacemakers and less frequent rehospitalization. “This implies that in selected MA-TAVR patients early discharge is feasible and safe, but larger studies are required to identify the optimal profile of patients who can be sent home within the first two postoperative days,” Dr. Jensen and her colleagues said.
Early in the MA-TAVR protocol all patients were sent to the ICU. As the care team gained more experience with the procedure, the protocol changed to send all patients to a regular telemetry floor after surgery unless they had vascular issues or potential need for a pacemaker. The decreasing need for ICU “was the only indication of an institutional learning curve that was discovered, and demonstrated improved resource utilization over time,” the investigators said.
They encouraged other centers to pursue MA-TAVR. “As experience grows, we believe that this procedure can be done with less or no ICU support leading to a shorter hospital stay and improved resource utilization,” Dr. Jensen and her coauthors concluded. They called for further studies to determine the characteristics that make a patient most suitable for a short-admission MA-TAVR procedure.
Study coauthors Dr. Vasilis Babaliaros, Dr. Vinod Thourani, Amy Simone, and Patricia Keegan are research consultants with Edwards Lifesciences. The rest of the authors had no disclosures.
As a “minimalist” approach to transcatheter aortic valve replacement – known as MA-TAVR – gains in popularity at high-volume centers, questions persist about the surgeon’s learning curve. A small series of MA-TAVR cases at Emory University in Atlanta has shown that the leaning curve may be like the TAVR approach itself: minimal.
Dr. Hanna Jensen and her associates reported on 151 consecutive patients who had MA-TAVR in the October issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2015. doi: 10.1016/j.jtcvs.2015.07.078). They previously reported their findings at the annual meeting of the American Association for Thoracic Surgery in April in Seattle.
This study builds on an Emory study last year that reported the minimalist approach to TAVR cost about $10,000 less per patient than the standard transfemoral approach (JACC Cardiovasc Interv. 2014;7:898-904).
The operation the study authors evaluated is performed in the catheterization laboratory rather than the operating room, as in traditional TAVR. Both approaches use a femoral approach, but where traditional TAVR requires general anesthesia and transesophageal echocardiography (TEE), MA-TAVR uses local anesthesia, minimal conscious sedation, and transthoracic echocardiography (TTE).
The study authors acknowledged concerns that TTE may underestimate the severity of paravalvular leak after the procedure when compared with TEE. Their protocol relies on preoperative TTE and CT scans, or three-dimensional TEE if the case warrants it, to ensure optimal sizing of the transcatheter valve before the operation. “If any concerns arise, our threshold is low to perform intraoperative balloon-sizing,” Dr. Jensen and her coauthors said. They also use TTE, along with a root-angiogram after valve deployment, and invasively measure the aortic regurgitation index before and after deployment.
Most study patients were high-risk surgical candidates with a median Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) score of 10%. The overall major stroke rate was 3.3%, while major vascular complications occurred in 3% of patients and the greater-than-mild paravalvular leak rate was 7%.
The study retrospectively evaluated 151 consecutive patients who were divided into three groups at different time points: May 2012 to January 2013, February to August 2013, and September 2013 to July 2014. Complications were similar among all three groups, but the third group had shorter hospital stays and less time in the intensive care unit (ICU).
The first group received only the first-generation SAPIEN valve system; use of the second-generation SAPIEN XT valve increased in latter two groups. The SAPIEN XT valve is available in 23, 26, or 29 mm, but the 29-mm size was not available in the first-generation SAPIEN implant.
A subgroup analysis looked at patients who were discharged within 48 hours of the operation or more than 48 hours afterward. The early-discharge patients had lower STS PROM scores (8.3% vs. 10.3%) and lower rates of diabetes (31% vs. 49%). They also had less need for postoperative pacemakers and less frequent rehospitalization. “This implies that in selected MA-TAVR patients early discharge is feasible and safe, but larger studies are required to identify the optimal profile of patients who can be sent home within the first two postoperative days,” Dr. Jensen and her colleagues said.
Early in the MA-TAVR protocol all patients were sent to the ICU. As the care team gained more experience with the procedure, the protocol changed to send all patients to a regular telemetry floor after surgery unless they had vascular issues or potential need for a pacemaker. The decreasing need for ICU “was the only indication of an institutional learning curve that was discovered, and demonstrated improved resource utilization over time,” the investigators said.
They encouraged other centers to pursue MA-TAVR. “As experience grows, we believe that this procedure can be done with less or no ICU support leading to a shorter hospital stay and improved resource utilization,” Dr. Jensen and her coauthors concluded. They called for further studies to determine the characteristics that make a patient most suitable for a short-admission MA-TAVR procedure.
Study coauthors Dr. Vasilis Babaliaros, Dr. Vinod Thourani, Amy Simone, and Patricia Keegan are research consultants with Edwards Lifesciences. The rest of the authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A minimalist approach to transcatheter aortic valve replacement (MA-TAVR) is feasible with acceptable outcomes.
Major finding: Transition to MA-TAVR in a high-volume center had a relatively small learning curve.
Data source: A review of 151 consecutive patients who had MA-TAVR at Emory University between May 2012 and July 2014.
Disclosures: Study coauthors Dr. Vasilis Babaliaros, Dr. Vinod Thourani, Amy Simone, and Patricia Keegan are research consultants with Edwards Lifesciences. The rest of the authors had no disclosures.