User login
Reducing COVID-19 opioid deaths
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.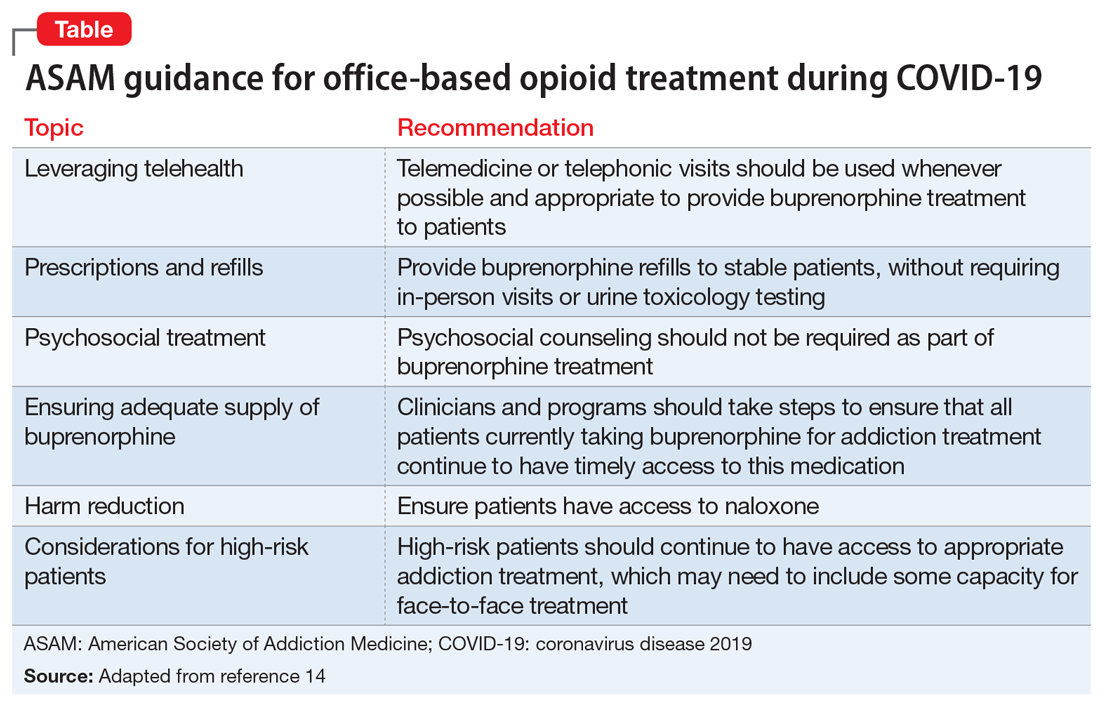
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
Editor's Note: Due to updated statistics from the CDC, the online version of this article has been modified from the version that appears in the printed edition of the January 2021 issue of Current Psychiatry.
Individuals with mental health and substance use disorders (SUDs) are particularly susceptible to negative effects of the coronavirus disease 2019 (COVID-19) pandemic. The collision of the COVID-19 pandemic and the drug overdose epidemic has highlighted the urgent need for physicians, policymakers, and health care professionals to optimize care for individuals with SUDs because they may be particularly vulnerable to the effects of the virus due to compromised respiratory and immune function, and poor social support.1 In this commentary, we highlight the challenges of the drug overdose epidemic, and recommend strategies to mitigate the impact of the COVID-19 pandemic among patients with SUDs.
A crisis exacerbated by COVID-19
The current drug overdose epidemic has become an American public health nightmare. According to preliminary data released by the CDC on December 17, 2020, there were more than 81,000 drug overdose deaths in the United States in the 12 months ending May 2020.2,3 This is the highest number of overdose deaths ever recorded in a 12-month period. The CDC also noted that while overdose deaths were already increasing in the months preceding the COVID-19 pandemic, the latest numbers suggest an acceleration of overdose deaths during the pandemic.
What is causing this significant loss of life? Prescription opioids and illegal opioids such as heroin and illicitly manufactured fentanyl are the main agents associated with overdose deaths. These opioids were responsible for 61% (28,647) of drug overdose deaths in the United States in 2014.4 In 2015, the opioid overdose death rate increased by 15.6%.5
The increase in the number of opioid overdose deaths in part coincides with a sharp increase in the availability and use of heroin. Heroin overdose deaths have more than tripled since 2010, but heroin is not the only opiate involved. Fentanyl, a synthetic, short-acting opioid that is approved for managing pain in patients with advanced cancers, is 50 times more potent than heroin. The abuse of prescribed fentanyl has been accelerating over the past decade, as is the use of illicitly produced fentanyl. Evidence from US Drug Enforcement Administration (DEA) seizure records shows heroin is being adulterated with illicit fentanyl to enhance the potency of the heroin.6,7 Mixing illicit fentanyl with heroin may be contributing to the recent increase in heroin overdose fatalities. According to the CDC, overdose deaths related to synthetic opioids increased 38.4% from the 12-month period leading up to June 2019 compared with the 12-month period leading up to May 2020.2,3 Postmortem studies of individuals who died from a heroin overdose have frequently found the presence of fentanyl along with heroin.8 Overdose deaths involving heroin may be occurring because individuals may be unknowingly using heroin adulterated with fentanyl.9 In addition, carfentanil, a powerful new synthetic fentanyl, has been recently identified in heroin mixtures. Carfentanil is 10,000 times stronger than morphine. Even in miniscule amounts, carfentanil can suppress breathing to the degree that multiple doses of naloxone are needed to restore respirations.
Initial studies indicate that the COVID-19 pandemic has been exacerbating this situation. Wainwright et al10 conducted an analysis of urine drug test results of patients with SUDs from 4 months before and 4 months after COVID-19 was declared a national emergency on March 13, 2020. Compared with before COVID-19, the proportion of specimens testing positive since COVID-19 increased from 3.80% to 7.32% for fentanyl and from 1.29% to 2.09% for heroin.10
A similar drug testing study found that during the pandemic, the proportion of positive results (positivity) increased by 35% for non-prescribed fentanyl and 44% for heroin.11 Positivity for non-prescribed fentanyl increased significantly among patients who tested positive for other drugs, including by 89% for amphetamines; 48% for benzodiazepines; 34% for cocaine; and 39% for opiates (P < .1 for all).11
In a review of electronic medical records, Ochalek et al12 found that the number of nonfatal opioid overdoses in an emergency department in Virginia increased from 102 in March-June 2019 to 227 in March-June 2020. In an issue brief published on October 31, 2020, the American Medical Association reported increase in opioid and other drug-related overdoses in more than 40 states during the COVID-19 pandemic.13
Continue to: Strategies for intervention...
Strategies for intervention
A multi-dimensional approach is needed to protect the public from this growing opioid overdose epidemic. To address this challenging task, we recommend several strategies:
Enhance access to virtual treatment
Even when in-person treatment cannot take place due to COVID-19-related restrictions, it is vital that services are accessible to patients with SUDs during this pandemic. Examples of virtual treatment include:
- Telehealth for medication-assisted treatment (MAT) using buprenorphine (recently updated guidance from the US DEA and Substance Abuse and Mental Health Services Administration [SAMHSA] allows this method of prescribing)
- Teletherapy to prevent relapse
- Remote drug screens by sending saliva or urine kits to patients' homes, visiting patients to collect fluid samples, or asking patients to come to a "drive-through" facility to provide samples
- Virtual (online) Alcoholics Anonymous, Narcotics Anonymous, SMART Recovery, and similar meetings to provide support in the absence of in-person meetings.
The American Society of Addiction Medicine (ASAM) offers guidance to treatment programs to focus on infection control and mitigation. The Table14 summarizes the ASAM recommendations for office-based opioid treatment during COVID-19.
Expand access to treatment
This includes access to MAT (such as buprenorphine/naloxone, methadone, naltrexone, and depot naltrexone) and, equally important, to psychosocial treatment, counseling, and/or recovery services. Recent legislative changes have increased the number of patients that a qualified physician can treat with buprenorphine/naloxone from 100 to 275, and allowed physician extenders to prescribe buprenorphine/naloxone in office-based settings. A recent population-based, retrospective Canadian study showed that opioid agonist treatment decreased the risk of mortality among opioid users, and the protective effects of this treatment increased as fentanyl and other synthetic opioids became common in the illicit drug supply.15 However, because of the shortage of psychiatrists and addiction medicine specialists in several regions of the United States, access to treatment is extremely limited and often inadequate. This constitutes a major public health crisis and contributes to our inability to intervene effectively in the opioid epidemic. Telepsychiatry programs can bring needed services to underserved areas, but they need additional support and development. Further, involving other specialties is paramount for treating this epidemic. Integrating MAT in primary care settings can improve access to treatment. Harm-reduction approaches, such as syringe exchange programs, can play an important role in reducing the adverse consequences associated with heroin use and establish health care relationships with at-risk individuals. Syringe exchange programs can also reduce the rate of infections associated with IV drug use, such as human immunodeficiency virus and hepatitis C virus.
Continue to: Increase education on naloxone...
Increase education on naloxone
Naloxone is a safe and effective opioid antagonist used to treat opioid overdoses. Timely access to naloxone is of the essence when treating opioid-related overdoses. Many states have enacted laws allowing health care professionals, law enforcement officers, and patients and relatives to obtain naloxone without a physician's prescription. It appears this approach may be yielding results. For example, the North Carolina Harm Reduction Coalition distributed >101,000 free overdose rescue kits that included naloxone and recorded 13,392 confirmed cases of overdose rescue with naloxone from 2013 to 2019.16
Divert patients with SUDs from the criminal justice system to treatment
We need to develop programs to divert patients with SUDs from the criminal justice system, which is focused on punishment, to interventions that focus on treatment. Data indicates high recidivism rates for incarcerated individuals with SUDs who do not have access to treatment after they are released. Recognizing this, communities are developing programs that divert low-level offenders from the criminal justice system into treatment. For instance, in Seattle, the Law Enforcement Assisted Diversion is a pilot program developed to divert low-level drug and prostitution offenders into community-based treatment and support services. This helps provide housing, health care, job training, treatment, and mental health support. Innovative programs are needed to provide SUD treatment in the rehabilitation programs of correctional facilities and ensure case managers and discharge planners can transition participants to community treatment programs upon their release.
Develop early identification and prevention programs
These programs should focus on individuals at high risk, such as patients with comorbid SUDs and psychiatric disorders, those with chronic pain, and at-risk children whose parents abuse opiates. Traditional addiction treatment programs typically do not address patients with complex conditions or special populations, such as adolescents or pregnant women with substance use issues. Evidence-based approaches such as Screening, Brief Intervention, and Referral to Treatment (SBIRT), Integrated Dual Diagnosis Treatment (IDDT), and prevention approaches that target students in middle schools and high schools need to be more widely available.
Improve education on opioid prescribing
Responsible opioid prescribing for clinicians should include education about the regular use of prescription drug monitoring programs, urine drug screening, avoiding co-prescription of opioids with sedative-hypnotic medications, and better linkage with addiction treatment.
Treat comorbid psychiatric conditions
It is critical to both identify and effectively treat underlying affective, anxiety, and psychotic disorders in patients with SUDs. Anxiety, depression, and emotional dysregulation often contribute to worsening substance abuse, abuse of prescription drugs, diversion of prescribed drugs, and an increased risk of overdoses and suicides. Effective treatment of comorbid psychiatric conditions also may reduce relapses.
Increase research on causes and treatments
Through research, we must expand our knowledge to better understand the factors that contribute to this epidemic and develop better treatments. These efforts may allow for the development of prevention mechanisms. For example, a recent study found that the continued use of opioid medications after an overdose was associated with a high risk of a repeated overdosecall out material?.17 At the end of a 2-year observation, 17% (confidence interval [CI]: 14% to 20%) of patients receiving a high daily dosage of a prescribed opioid had a repeat overdose compared with 15% (CI: 10% to 21%) of those receiving a moderate dosage, 9% (CI: 6% to 14%) of those receiving a low dosage, and 8% (CI: 6% to 11%) of those receiving no opioids.17 Of the patients who overdosed on prescribed opiates, 30% switched to a new prescriber after their overdose, many of whom may not have been aware of the previous overdose. From a public health perspective, it would make sense for prescribers to know of prior opioid and/or benzodiazepine overdoses. This could be reported by emergency department clinicians, law enforcement, and hospitals into a prescription drug monitoring program, which is readily available to prescribers in most states.
Acknowledgment
The authors thank Scott Proescholdbell, MPH, Injury and Violence Prevention Branch, Chronic Disease and Injury Section, Division of Public Health, North Carolina Department of Health and Human Services, for his assistance.
Bottom Line
The collision of the coronavirus disease 2019 pandemic and the drug overdose epidemic has highlighted the urgent need for health care professionals to optimize care for individuals with substance use disorders. Suggested interventions include enhancing access to medication-assisted treatment and virtual treatment, improving education about naloxone and safe opioid prescribing practices, and diverting at-risk patients from the criminal justice system to interventions that focus on treatment.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
1. Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-62.
2.Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID-19. Accessed December 23, 2020. https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html
3.Centers for Disease Control and Prevention. National Center for Health Statistics Vital Statistics Rapid Release. Provisional drug overdose death counts. Accessed December 30, 2020. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
4.Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths -- United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382.
5.Rudd RA, Seth P, David F, et al. Increases in drug and opioid-involved overdose deaths -- United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
6.US Drug Enforcement Administration. DEA issues nationwide alert on fentanyl as threat to health and public safety. Published March 19, 2015. Accessed October 28, 2020. http://www.dea.gov/divisions/hq/2015/hq031815.shtml
7.Gladden RM, Martinez P, Seth P. Fentanyl law enforcement submissions and increases in synthetic opioid-involved overdose deaths - 27 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2016;65(33):837-843.
8.Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005-May 2006). J Med Toxicol. 2013;9(1):106-115.
9.Centers for Disease Control and Prevention. Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities. HAN Health Advisory. Published October 26, 2015. Accessed October 28, 2020. http://emergency.cdc.gov/han/han00384.asp
10.Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the us declaration of a national emergency concerning the COVID-19 outbreak. JAMA. 2020;324(16):1674-1677.
11.Niles JK, Gudin J, Radliff J, et al. The opioid epidemic within the COVID-19 pandemic: drug testing in 2020 [published online October 8, 2020]. Population Health Management. doi: 10.1089/pop.2020.0230
12.Ochalek TA, Cumpston KL, Wills BK, et al. Nonfatal opioid overdoses at an urban emergency department during the COVID-19 pandemic. JAMA. 2020;324(16):1673-1674.
13.American Medical Association. Issue brief: reports of increases in opioid- and other drug-related overdose and other concerns during COVID pandemic. Published October 31, 2020. Accessed November 9, 2020. https://www.ama-assn.org/system/files/2020-11/issue-brief-increases-in-opioid-related-overdose.pdf
14.American Society of Addiction Medicine. Caring for patients during the COVID-19 pandemic: ASAM COVID-19 Task Force recommendations. Accessed October 30, 2020. https://www.asam.org/docs/default-source/covid-19/medication-formulation-and-dosage-guidance-(1).pdf
15.Pearce LA, Min JE, Piske M, et al. Opioid agonist treatment and risk of mortality during opioid overdose public health emergency: population based retrospective cohort study. BMJ. 2020;368:m772. doi: 10.1136/bmj.m772
16.North Carolina Harm Reduction Coalition. NCHRC'S community-based overdose prevention project. Accessed March 29, 2020. http://www.nchrc.org/programs-and-services
17.Larochelle MR, Liebschutz JM, Zhang F, et al. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1-9.
Pharmacotherapy for alcohol use disorder in patients with hepatic impairment
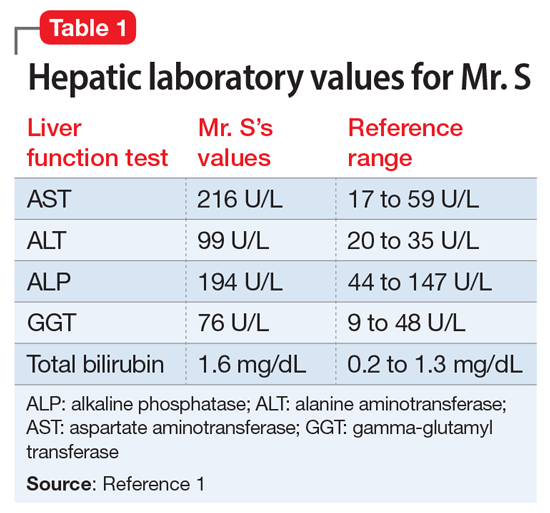
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.
A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
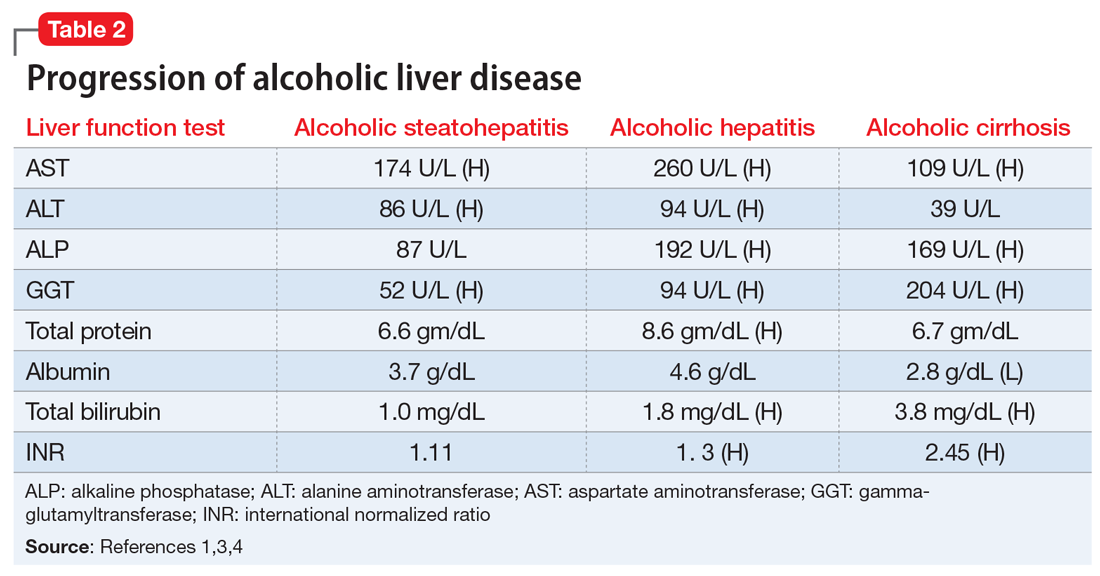
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4
Continue to: FDA-approved medications
FDA-approved medications
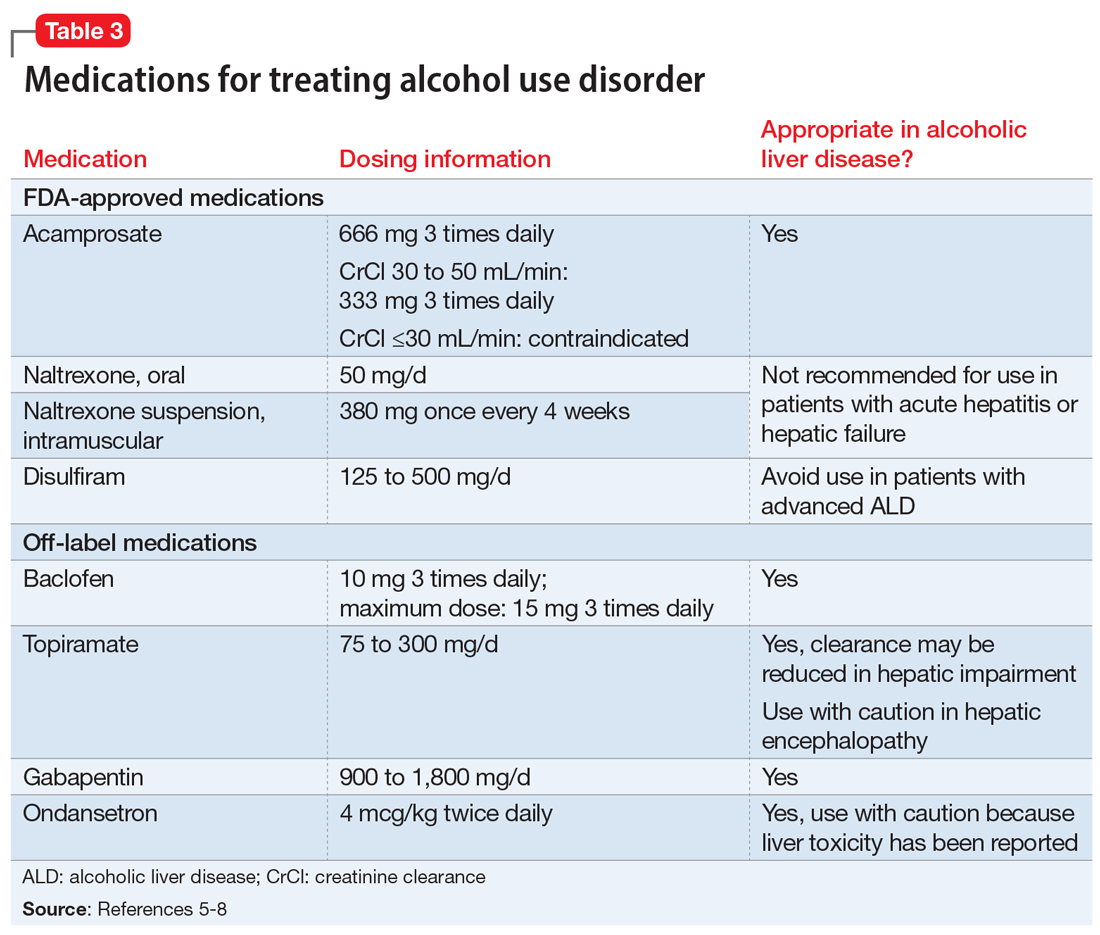
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8
Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Mr. S, age 64, presents for an outpatient follow-up after a recent hospital discharge for alcohol detoxification. He reports a long history of alcohol use, which has resulted in numerous hospital admissions. He has recently been receiving care from a gastroenterologist because the results of laboratory testing suggested hepatic impairment (Table 1). Mr. S says that a friend of his was able to stop drinking by taking a medication, and he wonders if he can be prescribed a medication to help him as well.

A chart review shows that Mr. S recently underwent paracentesis, during which 6 liters of fluid were removed. Additionally, an abdominal ultrasound confirmed hepatic cirrhosis.
According to the World Health Organization, alcohol consumption contributes to 3 million deaths annually.2 The highest proportion of these deaths (21.3%) is due to alcohol-associated gastrointestinal complications, including alcoholic and infectious hepatitis, pancreatitis, and cirrhosis. Because the liver is the primary site of ethanol metabolism, it sustains the greatest degree of tissue injury with heavy alcohol consumption. Additionally, the association of harmful use of alcohol with risky sexual behavior may partially explain the higher prevalence of viral hepatitis among persons with alcohol use disorder (AUD) compared with the general population. Alcoholic liver disease (ALD) progresses through several stages, beginning with hepatic steatosis and progressing through alcohol-related hepatitis, fibrosis, cirrhosis, and potentially hepatocellular carcinoma.3
Liver markers of alcohol use
Although biological markers can be used in clinical practice to screen and monitor for alcohol abuse, making a diagnosis of ALD can be challenging. Typically, a history of heavy alcohol consumption in addition to certain physical signs and laboratory tests for liver disease are the best indicators of ALD. However, the clinical assessment can be confounded by patients who deny or minimize how much alcohol they have consumed. Furthermore, physical and laboratory findings may not be specific to ALD.
Liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT), have historically been used as the basis of diagnosing ALD. In addition to elevated bilirubin and evidence of macrocytic anemia, elevations in these enzymes may suggest heavy alcohol use, but these values alone are inadequate to establish ALD. Gamma-glutamyltransferase is found in cell membranes of several body tissues, including the liver and spleen, and therefore is not specific to liver damage. However, elevated GGT is the best indicator of excessive alcohol consumption because it has greater sensitivity than AST and ALT.1,3,4
Although these biomarkers are helpful in diagnosing ALD, they lose some of their utility in patients with advanced liver disease. Patients with severe liver dysfunction may not have elevated serum aminotransferase levels because the degree of liver enzyme elevation does not correlate well with the severity of ALD. For example, patients with advanced cirrhosis may have liver enzyme levels that appear normal. However, the pattern of elevation in transaminases can be helpful in making a diagnosis of liver dysfunction; using the ratio of AST to ALT may aid in diagnosing ALD, because AST is elevated more than twice that of ALT in >80% of patients with ALD.1,3,4
Table 21,3,4 shows the progression of ALD from steatohepatitis to alcoholic hepatitis to cirrhosis. In steatohepatitis, transaminitis is present but all other biomarkers normal. In alcoholic hepatitis, transaminitis is present along with elevated alkaline phosphatase, elevated bilirubin, and elevated international normalized ratio (INR). In alcoholic cirrhosis, the AST-to-ALT ratio is >2, and hypoalbuminemia, hyperbilirubinemia, and coagulopathy (evidenced by elevated INR) are present, consistent with long-term liver damage.1,3,4

Continue to: FDA-approved medications
FDA-approved medications
Three medications—acamprosate, naltrexone, and disulfiram—currently are FDA-approved for treating AUD.5,6 Additionally, several other medications have shown varying levels of efficacy in treating patients with AUD but are not FDA-approved for this indication (Table 3).5-8

Acamprosate is thought to create a balance of inhibitor and excitatory neurotransmitters by functioning as a glutamate antagonist and gamma-aminobutyric acid (GABA) agonist. This is speculated to aid in abstinence from alcohol. Data suggests that acamprosate may be more effective for maintaining abstinence than for inducing remission in individuals who have not yet detoxified from alcohol. Because of its renal excretion, acamprosate is the only FDA-approved medication for AUD that is not associated with liver toxicity. The most commonly reported adverse effect with acamprosate use is diarrhea.
Naltrexone, a mu-opioid receptor antagonist, is available in both tablet and long-acting IM injection formulations. Naltrexone blocks the binding of endorphins created by alcohol consumption to opioid receptors. This results in diminished dopamine release and is speculated to decrease reward and positive reinforcement with alcohol consumption, leading to fewer heavy drinking days. Due to hepatic metabolism, naltrexone use carries a risk of liver injury. Cases of hepatitis and clinically significant liver dysfunction as well as transient, asymptomatic, hepatic transaminase elevations have been observed in patients who receive naltrexone. Because of the absence of first-pass metabolism, long-acting IM naltrexone may produce less hepatotoxicity than the oral formulation. When the FDA approved both formulations of naltrexone, a “black-box” warning was issued concerning the risk of liver damage; however, these warnings have since been removed from their respective prescribing information.
Disulfiram inhibits acetaldehyde dehydrogenase, resulting in elevated acetaldehyde concentrations after consuming alcohol. In theory, this medication reduces a person’s desire to drink due to the negative physiological and physical effects associated with increased acetaldehyde, including hypotension, flushing, nausea, and vomiting. Although most of these reactions are short-lived, disulfiram can induce hepatotoxicity and liver failure that may prove fatal. Disulfiram should be avoided in patients with advanced ALD.
Off-label medications for AUD
Additional pharmacotherapeutic agents have been evaluated in patients with AUD. Baclofen, topiramate, gabapentin, and ondansetron have shown varying levels of efficacy and pose minimal concern in patients with ALD.
Continue to: Baclofen
Baclofen. Although findings are conflicting, baclofen is the only agent that has been specifically studied for treating AUD in patients with ALD. A GABA B receptor antagonist, baclofen is currently FDA-approved for treating spasticity. In a series of open-label and double-blind studies, baclofen has been shown to effectively reduce alcohol intake, promote abstinence, and prevent relapse.5,6 Further studies identified a possible dose-related response, noting that 20 mg taken 3 times daily may confer additional response over 10 mg taken 3 times daily.5,6 Conversely, the ALPADIR study failed to demonstrate superiority of baclofen vs placebo in the maintenance of abstinence from alcohol despite dosing at 180 mg/d.9 This study did, however, find a significant reduction in alcohol craving in favor of baclofen.9 Further, in a randomized controlled trial (RCT) conducted in veterans with chronic hepatitis C, baclofen 30 mg/d failed to show superiority over placebo with regard to increasing abstinence or reducing alcohol use
Topiramate. A recent meta-analysis found that topiramate use may result in fewer drinking days, heavy drinking days, and number of drinks per drinking day.7 Additionally, topiramate has demonstrated a statistically significant reduction in alcohol craving as well as the ability to decrease all liver function test values.5 This agent should be used with caution in patients with hepatic encephalopathy because the adverse cognitive effects associated with topiramate may confound the clinical course and treatment of such.
Gabapentin. The use of gabapentin to treat patients with AUD is supported by multiple RCTs. In studies that evaluated dose-related response, higher doses of gabapentin (up to 1,800 mg/d) showed greater efficacy than lower doses (ie, 900 mg/d).8 Because gabapentin does not undergo hepatic metabolism, its use in patients with ALD is considered safe. Although the abuse potential of gabapentin is less defined in patients with AUD, there have been reports of abuse in other high-risk populations (ie, those with opioid use disorder, incarcerated persons, and those who misuse prescriptions recreationally).8
Ondansetron is speculated to decrease the reward from alcohol via the down-regulation of dopaminergic neurons. Studies examining ondansetron for patients with AUD have found that it decreases alcohol cravings in those with early-onset alcoholism (initial onset at age ≤25), but not in late-onset alcoholism (initial onset at age >25).5 However, the ondansetron doses used in these trials were very low (4 mcg/kg), and those doses are not available commercially.5
CASE CONTINUED
Following a discussion of available pharmacotherapeutic options for AUD, Mr. S is started on baclofen, 10 mg 3 times daily, with plans for dose titration. At a 2-week follow-up appointment, Mr. S reports that he had not been taking baclofen as often as instructed; however, he denies further alcohol consumption and re-commits to baclofen treatment. Unfortunately, Mr. S is soon admitted to hospice care due to continued decompensation and is unable to attend any additional outpatient follow-up appointments. Three months after his initial outpatient contact, Mr. S dies due to alcoholic cirrhosis.
Related Resources
• Crabb DW, Im GY, Szabo G, et al. Diagnosis and treatment of alcohol-related liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306-333.
• Murail AR, Carey WD. Disease management. Liver test interpretation - approach to the patient with liver disease: a guide to commonly used liver tests. Cleveland Clinic Center for Continuing Education. Updated August 2017. www.clevelandclinicmeded. com/medicalpubs/diseasemanagement/hepatology/ guide-to-common-liver-tests/
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • Revia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
1. Agrawal S, Dhiman RK, Limdi JK. Evaluation of abnormal liver function tests. Postgrad Med J. 2016;92(1086):223-234.
2. World Health Organization. Global status report on alcohol and health 2018. Published 2018. Accessed November 5, 2020. https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/
3. Osna NA, Donohue TM, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 2017;38(2):147-161.
4. Leggio L, Lee MR. Treatment of alcohol use disorder in patients with alcoholic liver disease. Am J Med. 2017;130(2):124-134.
5. Addolorato G, Mirijello A, Leggio L, et al. Management of alcohol dependence in patients with liver disease. CNS Drugs. 2013;27(4):287-299.
6. Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
7. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings. JAMA. 2014;311(18):1889-1900.
8. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
9. Reynaud M, Aubin HJ, Trinquet F, et al. A randomized, placebo-controlled study of high-dose baclofen in alcohol-dependent patients-the ALPADIR study. Alcohol Alcohol. 2017;52(4):439-446.
10. Hauser P, Fuller B, Ho S, et al. The safety and efficacy of baclofen to reduce alcohol use in veterans with chronic hepatitis C: a randomized controlled trial. Addiction. 2017;112(7):1173-1183.
Intense intervention may boost addiction program retention
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
An intense and assertive “won’t take no for an answer” approach is effective for engaging in treatment young adults with substance abuse who have been in and out of various recovery programs for years, new research suggests.
The Youth Opioid Recovery Support (YORS) program is a team effort that includes home delivery of the prescribed medication, family engagement, assertive outreach, and contingency management.
In a new study of 42 patients in recovery for substance use disorder (SUD), those who were treated with extended-release naltrexone or extended-release buprenorphine plus YORS received more outpatient doses of their medication, and rates of opioid relapse at 12 and 24 weeks were lower compared with their peers who received only treatment as usual.
“ coinvestigator Marc Fishman, MD, director of the Maryland Treatment Centers, Johns Hopkins University, Baltimore, said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
Treatment barriers
Young adults with SUD are difficult to reach, which leads to decreased addiction program retention, decreased medication adherence, early drop out, waxing and waning motivation, and worse outcomes, compared with older adults with SUD, Dr. Fishman said.
In July, positive results from a pilot trial conducted by the investigators of YORS were published online in Addiction.
In that study, 41 young adults aged 18-26 years who intended to undergo treatment for SUD with extended-release naltrexone were randomly assigned to also undergo YORS or treatment as usual, which consisted of a standard referral to outpatient care following an inpatient stay.
The primary outcomes were number of medication doses received over 24 weeks and relapse to opioid use, which was defined as 10 or more days of use within 28 days at 24 weeks.
Participants in the YORS group received more doses of extended-release naltrexone (mean, 4.28; standard deviation, 2.3) than participants in the treatment-as-usual group (mean, 0.70; SD, 1.2; P < .01).
In the YORS group, rates of relapse at both 12 and 24 weeks were lower, and there were fewer overall days of opioid use.
For the current study, the investigators wanted to test whether there was a possible effect when patients were given a choice of medication. In the earlier trial, patients did not have a choice – they had to take extended-release naltrexone. In this study, they could opt for it or extended-release buprenorphine.
The researchers recruited 22 young adults (aged 18-26 years) from their inpatient clinic to participate. Half the patients chose to take extended-release naltrexone, and the other half chose extended-release buprenorphine.
The groups were then compared to a historical group of 20 patients who received treatment as usual and served as the control group.
Positive outcomes
As in the first study, outcomes in the new study were better with YORS.
All participants who underwent YORS received more outpatient medication doses at 12 weeks and 24 weeks than those who received treatment as usual (1.91 vs. 0.40 and 3.76 vs. 0.70, respectively; P < .001).
For the YORS group, rates of opioid relapse were lower at 12 weeks (27.3% vs. 75.0%) and at 24 weeks (52.9% vs. 95.0%; P < .01.)
All components of YORS work together to improve retention, Dr. Fishman noted. Patients do much better if a relative such as a mother, father, or grandmother is closely involved, he added.
Also important is drug delivery.
“In some ways, this is similar to the assertive community treatment, or ACT, for schizophrenia. Like substance use disorder, schizophrenia requires long-acting injectable antipsychotics. When that is delivered to the patient through an organized delivery service like YORS, it improves outcomes,” said Dr. Fishman.
SUD is a chronic, relapsing illness in which an individual’s judgment is impaired, he added.
“ACT has become a relatively standard feature of treatment in most communities in this country and internationally and is sustainable under public sector funding, so it’s not an impossible leap to say it could be done. But it will not be cheap,” Dr. Fishman said.
Removing barriers
In a comment, Serra Akyar, MD, a psychiatry resident at Northwell Health’s Staten Island University Hospital, New York, said that the YORS program may appear to be labor intensive.
“However, the combination of medication-assisted treatment and support are essential to the treatment of opioid use disorder, especially for young adults. Developing effective interventions for young adults is particularly important, given the plasticity of their brains,” said Dr. Akyar, who was not involved with the research.
Inability to access medication and a lack of a supportive environment, both in everyday life and in regards to therapy, are barriers to successful treatment, she noted.
“The YORS intervention aims to remove these barriers to further enhance engagement to care through a combination of medication delivery and family engagement and assertive outreach via text messaging, a modality presumed to be well received by youth,” Dr. Akyar said.
Despite having a limited sample size, the study shows how a comprehensive intervention can have a large impact on the maintenance of medication adherence and reduction of relapse in young adults, she added.
“Its early success is encouraging and warrants further study on a larger scale to determine long-term effectiveness, overall costs and feasibility, generalizability, and whether certain independent factors exist that may predict medication adherence and reduction of relapse,” she said.
Wraparound support
The study is also a significant reminder that the opioid crisis has affected the young adult population, who are very vulnerable to OUD, said Jose Vito, MD, child, adolescent, and addiction psychiatrist at New York University.
“The study made me realize the importance of the four components of YORS, which were the outreach, family involvement, home delivery, and monetary incentives,” Dr. Vito said in an interview.
All of these components, in addition to extended-release naltrexone or extended-release buprenorphine, “have contributed to lower rates of opioid relapse, and the relapses are much later in the course of treatment if they do occur,” he said.
Overall, the findings demonstrate the importance of not giving up on these youths, he noted.
“Programs like YORS that provide wraparound support can help alleviate the opioid health care crisis by keeping these young adults in treatment,” Dr. Vito concluded.
The study was funded by the University of Maryland Center for Addiction Research, Education, and Service. Dr. Fishman has a financial relationship with Alkermes.
A version of this article first appeared on Medscape.com.
Shared medical appointments may bridge the opioid treatment gap
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Shared medical appointments (SMAs) are an acceptable way to receive treatment for opioid use disorder (OUD), new research suggests.
In a survey study, participants attending an urban outpatient buprenorphine clinic reported a high degree of satisfaction with SMAs. However, the majority also reported they preferred individual appointments.
Still, SMAs may serve a role in providing comprehensive care for certain subpopulations with OUD who are prone to isolation and may also increase capacity to treat more patients with a substance use disorder (SUD), said coinvestigator Serra Akyar, MD, Northwell Health Staten Island University Hospital, New York.
“By providing education and a forum for sharing, SMAs can lead to changes in behavior and enhance and reinforce coping and problem-solving skills,” Dr. Akyar said in an interview.
The findings were presented at the virtual American Academy of Addiction Psychiatry 31st Annual Meeting.
SMA vs. group therapy
SMA is not a form of group therapy, Dr. Akyar noted. Group therapy has a psychotherapy component and is led by a therapist. SMAs do not have a psychotherapeutic or a behavioral therapy component but provide education and an opportunity for sharing personal experiences of recovery.
“For example, the doctor participating in the group describes what happens in the brain to drive addiction and fellow participants share their personal anecdotes of recovery, including their struggles and successes,” Dr. Akyar said.
“ given the differences in the type of care each group provides,” she added.
Recent research on SMAs for OUD is limited. Although previous studies have shown that the practice is highly acceptable and has comparable or better retention in care rates with buprenorphine versus individual appointments, these studies have been conducted in predominantly White populations and in suburban settings.
For the new study, the investigators wanted to examine how acceptable SMAs for OUD would be in an urban setting involving predominantly racial and ethnic minorities.
They administered a 15-minute survey to patients with OUD who were attending the Comprehensive Addiction Resources and Education Center, an outpatient psychiatry clinic located at New Jersey Medical School, from December 2019 to February 2020.
Of the 42 participants who initially consented, 39 completed the survey. The majority of the responders were Black (64.1%), had an annual income that was less than $20,000 (61.5%), and/or were unemployed or disabled (69.3%).
Most of the participants agreed or strongly agreed with the following statements:
- Scheduling appointments for SMAs is easy.
- I gain valuable information from the responses to other patients’ questions in SMAs.
- There is enough time for questions during SMAs.
- I gain valuable information from the doctor and social worker in SMAs.
- My medical needs are met during SMAs.
- I would recommend an SMA to other patients.
- Since starting SMAs, I find it easier to stick to my treatment plan.
- I have a lot of support outside of SMAs.
- People in SMAs give me the support I need to stick to my treatment plan.
Interestingly, despite the overall high satisfaction with SMAs, just 33% of participants said they preferred them to one-on-one visits, Dr. Akyar noted.
Further analyses showed that total satisfaction scores were positively associated with older age, being on disability, or being in retirement.
Bridging the gap
In a comment, Philip Wong, MD, New Jersey Medical School, Newark, noted that a more widespread use of SMAs could potentially bridge the treatment gap that currently exists in the United States.
“For providers, SMAs help reduce costs, improve productivity, prevent repeating of common advice, and increase outreach. These are all important at a time when the need for OUD treatment is increasing. This is especially true for places like Newark, which is one of the prime epicenters of the opioid epidemic,” said Dr. Wong.
Although he was not involved with this research, he and his colleagues recently conducted a literature review of publications relating to SMAs and found seven peer-reviewed articles. However, none was appropriately designed to compare SMAs with traditional one-on-one recovery treatment.
“We definitely need more clinical studies to further our understanding of SMAs as a tool for the medication-assisted treatment of opioid use disorder,” Dr. Wong said.
“There are currently a very limited number of physicians who can prescribe medication-assisted treatment in the first place. So, if that one provider can reach a larger community by doing these SMAs, then the potential is very great in terms of addressing the opioid epidemic,” he said.
David Kan, MD, chief medical officer of Bright Heart Health, San Ramon, Calif., agreed.
“SMAs are promising because they are efficient and allow more people to access treatment,” Dr. Kan said in an interview.
“Although the mechanism of SMA satisfaction is unclear, other research shows peer support and groups helpful for SUD treatment as a whole. SMA takes the best of many worlds and increases the potential number of patients treated for SUD,” he said.
Also asked to comment, Lewei (Allison) Lin, MD, University of Michigan, Ann Arbor, said SMAs “are one of a number of important interventions that should be considered” in order to increase availability and access to medication providers for OUD.
However, more research is needed “to examine the impact on treatment uptake and patient and provider experiences,” said Dr. Lin.
Dr. Akyar, Dr. Wong, Dr. Kan, and Dr. Lin disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The rebirth of psychedelic psychiatry
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.
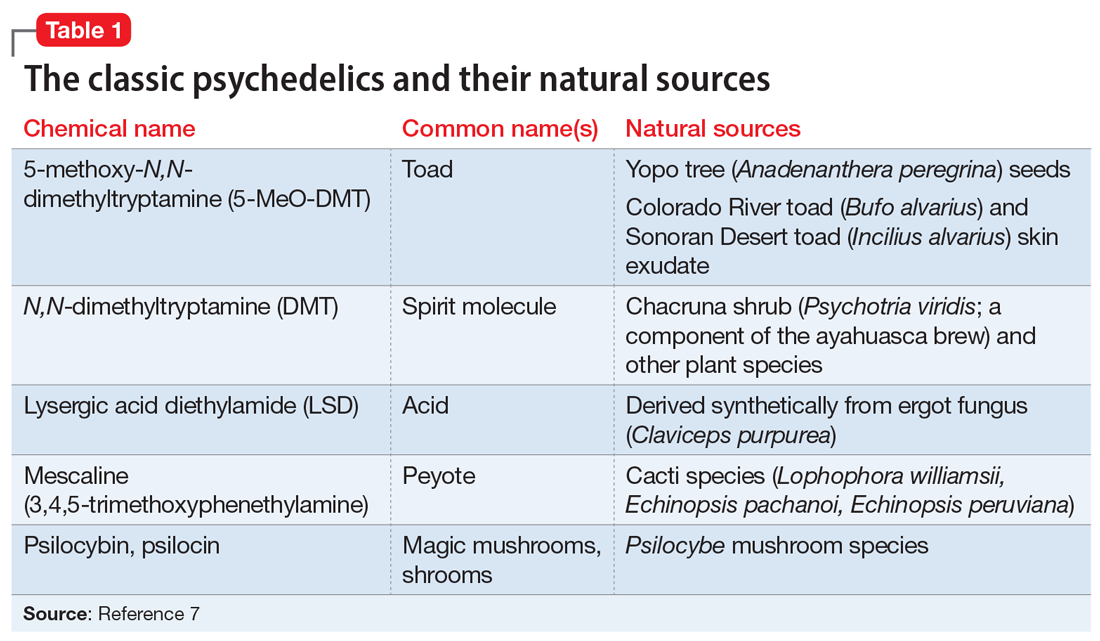
Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.
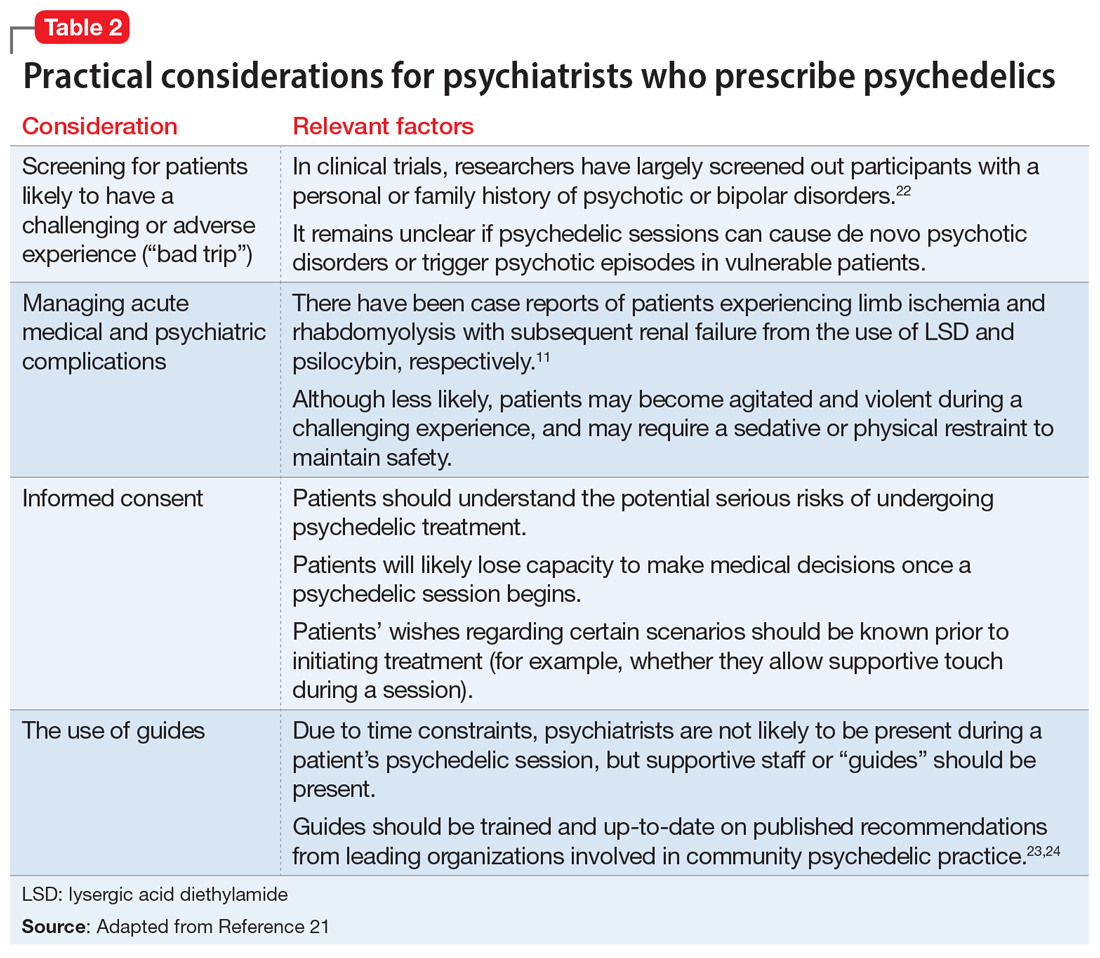
Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24
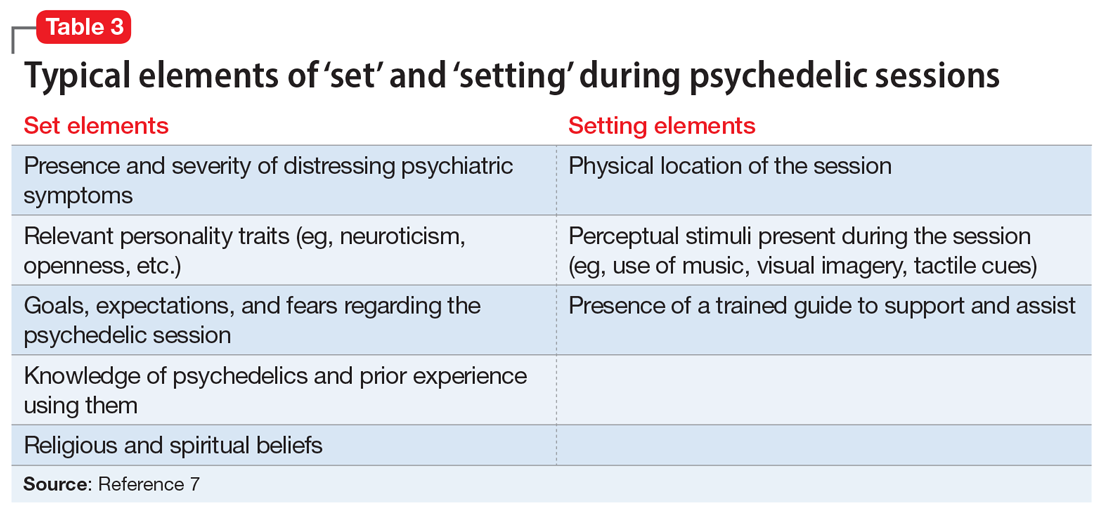
Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.

Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.

Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24

Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
Mr. P, age 65, has a history of major depressive disorder (MDD), generalized anxiety disorder, and social phobia. Mr. P’s personality is high in neuroticism and he has often responded to new situations with feelings of impending doom. For him, fear, anxious rumination, helplessness, and catastrophizing are familiar mental processes.
When he was in his 30s, Mr. P had a severe major depressive episode with suicidal ideation and sought care from a psychiatrist. He began a treatment program of psychotherapy and concomitant psychopharmacotherapy with consecutive trials of fluoxetine, sertraline, and amitriptyline, each of an adequate dose and duration. With each medication, Mr. P experienced new adverse effects, including nausea, constipation, tremors, and headache. His psychiatrist transitioned him to bupropion, which helped Mr. P most. For the next several decades, Mr. P continued to experience low-grade depressive symptoms with intermittent exacerbation to mild-to-moderate major depressive episodes, but he remained adherent to his medication and continued psychotherapy.
Shortly after his 65th birthday, Mr. P experiences progressively worsening nausea and abdominal pain. Initially, he assumes the symptoms are secondary to anxiety. Taking his psychiatrist’s advice, Mr. P visits his primary care physician. A work-up reveals that Mr. P has advanced pancreatic cancer, and an oncologist estimates Mr. P has 6 months of life remaining.
Following his cancer diagnosis, Mr. P quickly develops symptoms of MDD despite continuing to take bupropion. Within a week he becomes withdrawn and hopeless, and thinks about ending his life “before God does.” His psychiatrist urges Mr. P to contact the local academic medical center because it is conducting a trial of a “new” drug, psilocybin, to treat anxiety and depression in patients with terminal illness.
Beginning in the 1940s, a growing body of scientific evidence suggested that psychedelic compounds such as lysergic acid diethylamide (LSD) could benefit individuals with various psychiatric maladies. Research interest in LSD and substances with similar effects persisted until the late 1960s. In response to the growing counterculture movement in the United States and the efforts of Harvard researchers Timothy Leary and Richard Alpert to popularize psychedelic drug use in the general population, in 1970 President Richard M. Nixon signed the Controlled Substances Act (CSA) into law. The CSA categorized LSD as a Schedule I drug, rendering its manufacture and distribution illegal. Research into the potential therapeutic benefits of LSD was effectively halted.1 In recent decades, however, there has been a quiet but growing renaissance of scientific interest in the effects of psychedelics on a variety of conditions, including terminal illness–related anxiety and depression, treatment-resistant depression, and substance use disorders (SUDs). One example is psilocybin, which is currently undergoing Phase 2 and 3 clinical trials in North America and Europe for treatment-resistant depression.
As researchers have once again picked up the torch in the pursuit of psychedelic therapeutics, jurisdictions in the United States are also relaxing their stance on these drugs. In 2019 and early 2020, Denver, Oakland, and Santa Cruz became the first 3 cities in the United States to decriminalize the possession of various psychedelic substances.2-4 With the passage of Measure 109 in November 2020, Oregon became the first state to decriminalize the use of psychedelic mushrooms in therapeutic settings.5 The combined forces of increased research and relaxed political concern related to psychedelics might make it possible for the FDA to approve their use for psychiatric conditions. Therefore, it is critical for psychiatrists to understand the psychopharmacology, range of effects, and potential risks and benefits of these agents. In this article, I describe what psychedelics are and how they work, summarize a few research findings about psilocybin, and offer a framework for psychedelic psychiatric practice in the years to come.
What are psychedelics?
Psychiatrist Humphry Osmond first coined the term “psychedelic” in 1957 at a meeting of the New York Academy of Sciences, where he was discussing his research on the effect of LSD on patients at the Weyburn Mental Hospital in Saskatchewan, Canada.6 Prior to 1957, LSD had been described as a “psychotomimetic” drug because it was believed to induce a state of psychosis similar to that experienced in schizophrenia. But LSD does not generally induce frank auditory hallucinations or clearly defined delusional beliefs. Osmond’s new term—derived from the Greek words psyche, meaning “mind,” and delos, meaning “to show”—referred to the “mind-manifesting” capacities of LSD and related drugs.6 Psychedelic drugs can cause an array of changes to an individual’s conscious experience, from relatively mild changes in visual perception to profound derangements in sense of self and reality.
Continue to: Before describing the effects...
Classic psychedelics vs other compounds
Before describing the effects of psychedelic drugs and how they may relate to their therapeutic potential, it is useful to define which compounds are considered “classic psychedelics.”
The classic psychedelics are substances that operate primarily through activation of the serotonin 5-hydroxytryptamine receptor 2A receptor (5-HT2A) (Table 17). Many psychedelic drugs are derived from natural sources, including plants, fungi, and animals. For example, N, N-dimethyltryptamine (DMT), which is one of the most potent psychedelic compounds, is found in various plant species and can be imbibed in a tea known as ayahuasca, most commonly in the context of spiritual ceremonies.

Other compounds. Some researchers continue to classify other compounds as “psychedelics,” although the mechanisms of action and effects of these compounds may vary greatly from those of the classic psychedelics. These include the dissociative anesthetics ketamine and phencyclidine (PCP), which exert their effects via N-methyl-
The DSM-58 does not differentiate between classic psychedelics and related compounds. In its chapter on Substance-Related and Addictive Disorders, the section Hallucinogen-Related Disorders provides criteria for the diagnoses of phencyclidine use disorder and other hallucinogen use disorder. Researchers generally have abandoned the term “hallucinogen” because psychedelics typically do not induce frank hallucinations. Furthermore, lumping psychedelics and compounds such as MDMA and ketamine into the category of “other hallucinogen” fails to address important distinctions between them, including diagnostically relevant issues. For example, psychedelics do not cause symptoms of physiologic dependence such as craving or a withdrawal syndrome, whereas MDMA can.9 The DSM-5 also contains a diagnosis called hallucinogen persisting perception disorder (HPPD), referring to residual distortions of visual perception that remain following psychedelic intoxication. Although the text notes the estimated prevalence of HPPD in individuals who use psychedelics is 4.2%, the condition is thought to occur infrequently in both therapeutic and recreational users.10
How psychedelics work
Psychedelics can induce a spectrum of effects that are not necessarily dose-dependent. Mild effects of intoxication include altered sensory perception in visual, auditory, proprioceptive, and somatosensory spheres, including synesthesia. Progressively more severe changes include a distorted or eliminated perception or awareness of space, time, body, and self, resulting in derealization and depersonalization. Some of the most extreme alterations of consciousness reported by users include mystical or transcendent experiences of birth, giving birth, death, exchanging bodies with a nonhuman species, and meeting otherworldly beings.11 In terms of neurophysiology, psychedelics cause altered cerebral blood flow and metabolism, increased connectivity between brain regions that do not typically communicate, and a reduction in the activity of a group of cortical structures called the default mode network (DMN).12
Continue to: Researchers hypothesize that...
Researchers hypothesize that the disruption of DMN activity may be a key mechanism accounting for psychedelics’ therapeutic effects in mental illness. The DMN is a group of structures that includes the posterior cingulate cortex, the medial prefrontal cortex, the angular gyrus, and other cortical areas that are active when an individual is not engaged in a particular mental task (for example, during mind wandering). It is thought to underlie introspection and to serve as an “orchestrator” of global brain function.13 Theoretically, then, by temporarily disrupting the neural circuits responsible for maintaining ingrained, negative thought and behavioral patterns, as observed in patients with depression or SUDs, psychedelics can help patients develop greater emotional and cognitive flexibility and identify new ways to view the world and to solve problems.
Evaluating psychedelics as therapeutic agents
The renaissance of research into psychedelics as therapeutic agents during the last 2 decades has produced some promising preliminary findings. In 2020, the American Psychiatric Association’s Work Group on Biomarkers and Novel Treatments published a review of the best evidence on the topic.14 Psilocybin is the most studied drug because compared with LSD, it carries less of a stigma and has a shorter duration of action. Psilocybin has been studied as a potential treatment for several psychiatric disorders, including terminal illness–related depression and anxiety, and SUDs.
Griffiths et al.15 In a double-blind randomized crossover study at Johns Hopkins School of Medicine, Griffiths et al15 administered a high dose (22 or 30 mg/70 kg) and a very low, placebo-like dose (1 or 3 mg/70 kg) of psilocybin at 2 separate sessions to 51 patients with terminal cancer and associated depressive and anxiety disorders. After 5 weeks, the participants assigned to one condition crossed over to the other condition. High-dose psilocybin had a significant effect on depression and anxiety symptoms within 5 weeks that persisted over 6 months of follow-up. At 6 months, 78% of participants experienced a response in depressive symptoms (≥50% decrease in GRID-Hamilton Depression Rating Scale [HAM-D-17] baseline scores) and 65% remitted (GRID-HAM-D-17 score ≤7). At 6 months, 83% of participants had a response in anxiety symptoms (≥50% decrease in Hamilton Rating Scale for Anxiety [HAM-A] baseline scores) and 57% remitted (HAM-A ≤7).
Johnson et al.16,17 In an open-label pilot study16 and ≥12-month follow-up study,17 Johnson et al administered a moderate (20 mg/70 kg) and high (30 mg/70 kg) dose of psilocybin to 15 participants enrolled in a 15-week smoking session program. The psilocybin sessions were scheduled at Weeks 5 and 7, with an optional psilocybin session at Week 13. The sessions included nondirective support from program staff, but not smoking cessation content. Relying on laboratory-verified exhaled carbon monoxide and urine cotinine measures, researchers found an 80% abstinence rate at 6 months, a 67% abstinence rate at 12 months, and a 75% abstinence rate at 2.5 years.16,17
Bogenschutz et al18 conducted a study of 10 patients who met DSM-IV criteria for alcohol dependence and had at least 2 heavy drinking days in the previous 30 days. They found that a 14-session treatment program that included 2 psilocybin-assisted psychotherapy sessions with dosages of 0.4 mg/kg resulted in a significant increase in self-reported alcohol abstinence at 4 weeks that persisted for 36 weeks.18
Although these studies were small, open-label, and had other methodologic flaws, their pilot work has led to larger-scale projects assessing psilocybin’s therapeutic potential. Psilocybin has also been studied for treatment-resistant depression and obsessive-compulsive disorder. Other clinical trials underway are investigating psilocybin for the treatment of cocaine and opioid use disorder, anorexia nervosa, and depression in Alzheimer’s disease.14 Although psilocybin is currently the best-studied psychedelic, there is some research demonstrating that LSD can also induce a persistent reduction in anxiety symptoms associated with terminal illness19 and that ayahuasca causes a rapid reduction in depressive symptoms that persists over 21 days.20
Continue to: The future of psychedelic psychiatry...
The future of psychedelic psychiatry
If psychedelic compounds become approved for the treatment of psychiatric conditions, psychiatrists will likely be responsible for prescribing them and managing patients who receive them.21Table 211,21-24 summarizes practical considerations for psychiatrists who may someday be prescribing psychedelic drugs. Areas of psychedelic treatment in which psychiatric expertise is necessary include:
- screening for patients at increased risk for a challenging or adverse experience or “bad trip”
- conducting a thorough informed consent process in which the risks are discussed and the patient’s wishes regarding potential situations are elicited
- managing acute medical and psychiatric complications, including agitation and violent behavior
- ensuring the use of trained guides during sessions.

Psychiatrists who are interested in providing psychedelic-assisted therapy should understand the concept of “set and setting,” which was defined by Timothy Leary in the 1960s and is thought to play an important role in determining the types of experiences that arise during a psychedelic session.25 “Set” refers to an individual’s mindset going into a session, and “setting” refers to the environment in which the session occurs. Typical elements of each are summarized in Table 3.7 Psychiatrists will play a critical role in assessing and preparing the “set” by screening patients appropriately, assessing patient goals, and providing a thorough informed consent procedure. Psychiatrists should also be mindful of the “setting,” providing a comfortable, safe, familiar environment and access to appropriate music and eyeshades, if desired. Due to time restraints, psychiatrists are not likely to be responsible for guiding patients through sessions, and should educate themselves about ethical practices of psychedelic guides,if they are in the position to hire guides.23,24

Psychiatrists may also play a role in providing psychotherapy to patients receiving treatment with psychedelics. These substances can induce both transcendent and terrifying experiences. Patients therefore require “integration” therapy sessions to assist with processing the content of their psychedelic treatment and incorporating the experiences into day-to-day life. In an online survey of nearly 2,000 individuals who used psilocybin recreationally, 7.6% reported that they had to seek treatment for enduring psychological symptoms that they attributed to their psilocybin use, including persistent anxiety, fear, paranoia, and depression.26 Integrative psychotherapy sessions may help reduce the risk of persistent negative effects from therapeutic psychedelics, as well as enhance their beneficial effects.
CASE CONTINUED
Mr. P is enrolled in the academic medical center study assessing the effect of psilocybin on terminal illness-related anxiety and depression. During a 5-hour, 30-mg psilocybin session, he initially experiences distorted visual cues, with vivid, colorful geometric patterns collapsing into each other. He then loses the concepts and experience of time, space, and his body, as his visual distortions convert to darkness. After what seems like a decade within the darkness, he sees himself lying in a hospital bed with loved ones surrounding him. He watches himself take his last breaths and his family members weep as he dies. As he regains his senses, Mr. P feels that he is being reborn.
In the therapy sessions that follow the psychedelic session, Mr. P reports feeling “finally freed” from the fear, sadness, and anger that he has felt throughout his life. He comes to accept his impending death with gratitude and peace. In his final days, he no longer experiences depression or anxiety. Mr. P’s friends and family members comment that he seems to be the best version of himself in the months that lead up to his death.
Related Resources
• Nutt D. Psychedelic drugs-a new era in psychiatry? Dialogues Clin Neurosci. 2019;21(2):139-147.
• Garcia-Romeu A, Kersgaard B, Addy PH. Clinical applications of hallucinogens: a review. Exp Clin Psychopharmacol. 2016; 24(4):229-268.
Drug Brand Names
Amitriptyline • Amitril, Elavil
Bupropion • Wellbutrin
Fluoxetine • Prozac
Sertraline • Zoloft
Bottom Line
Psychedelics are a class of consciousness-altering agents that have become a potentially promising source of new treatments for psychiatric illness. Although more evidence is needed, compounds such as psilocybin may one day become FDAapproved for conditions such as terminal illness–related depression and anxiety, and substance use disorders. When this occurs, psychiatrists should be responsible for prescribing psychedelics and managing patients who receive treatment.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
1. Smith DE, Raswyck GE, Davidson LD. From Hofmann to the Haight Ashbury, and into the future: the past and potential of lysergic acid diethylamide. J Psychoactive Drugs. 2014;46(1):3-10.
2. Siegel M. Threading Denver’s magic mushrooms needle: promising as medicine, risky as recreation. USA Today. Published May 13, 2019. Accessed December 4, 2020. https://www.usatoday.com/story/opinion/2019/05/13/denver-magic-mushrooms-promising-medicine-reckless-recreation-column/1182543001
3. Epstein, K. Oakland decriminalizes ‘magic mushrooms’ and other natural psychedelics. The Washington Post. Published June 5, 2019. Accessed December 4, 2020. https://www.washingtonpost.com/nation/2019/06/05/oakland-decriminalizes-magic-mushrooms-other-natural-psychedelics
4. York JA. Santa Cruz decriminalizes natural psychedelics. Santa Cruz Sentinel. Published January 30, 2020. Accessed December 4, 2020. https://www.santacruzsentinel.com/2020/01/29/santa-cruz-decriminalizes-natural-psychedelics
5. Acker L. Oregon becomes first state to legalize psychedelic mushrooms. The Oregonian/Oregon Live. Published November 4, 2020. Accessed December 4, 2020. https://www.oregonlive.com/politics/2020/11/oregon-becomes-first-state-to-legalize-psychedelic-mushrooms.html
6. Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50(7):381-388.
7. Holoyda BJ. The psychedelic renaissance and its forensic implications. J Am Acad Psychiatry Law. 2020;48(1):87-97.
8. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
9. Davis AK, Rosenberg H. The prevalence, intensity, and assessment of craving for MDMA/ecstasy in recreational users. J Psychoactive Drugs. 2014;46(2):154-151.
10. Halpern JH, Lerner AG, Passie T. A review of hallucinogen persisting perception disorder (HPPD) and an exploratory study of subjects claiming symptoms of HPPD. Curr Top Behav Neurosci. 2018;36:333-360.
11. Nichols DE. Psychedelics. Pharmacol Rev. 2016;68(2):264-355.
12. Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101(2):131-181.
13. Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci. 2014;8:20.
14. Reiff CM, Richman EE, Nemeroff CB, et al. Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry. 2020;177(5):391-410.
15. Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol. 2016;30(12):1181-1197.
16. Johnson MW, Garcia-Romeu A, Cosimano MP, et al. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol. 2014;28(11):983-992.
17. Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abuse. 2017;43(1):55-60.
18. Bogenschutz MP, Forcehimes AA, Pommy JA, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29(3):1182-1190.
19. Gasser P, Holstein D, Michel Y, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis. 2014;202(7):531-520.
20. Osório F de L, Sanches RF, Macedo LR, et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report. Braz J Psychiatry. 2015;37(1):13-20.
21. Holoyda B. Psychedelic psychiatry: preparing for novel treatments involving altered states of consciousness. Psych Serv. 2020;71(12):1297-1299.
22. Johnson MW, Richards W, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603-620.
23. Council on Spiritual Practices. Code of ethics for spiritual Guides. Published August 10, 2001. Accessed November 25, 2020. https://csp.org/docs/code-of-ethics-for-spiritual-guides
24. Multidisciplinary Association for Psychedelic Studies. Zendo psychedelic harm reduction training manual. Published 2017. Accessed November 25, 2020. https://zendoproject.org/wp-content/uploads/2017/06/Zendo-Manual-2017.pdf
25. Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. Yale University Press; 1984.
26. Carbonaro TM, Bradstreet MP, Barrett FS, et al. Survey study of challenging experiences after ingesting psilocybin mushrooms: acute and enduring positive and negative consequences. J Psychopharmacol. 2016;30(12):1268-1278.
No benefit of cannabis on depression in pregnant women with OUD
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
Cannabis is ineffective at alleviating depression in pregnant women undergoing opioid agonist therapy (OAT), new research shows.
A study of more than 120 pregnant women undergoing treatment of opioid use disorder (OUD) showed that those who used cannabis to alleviate their depressive symptoms while undergoing OAT continued to have high depression scores at the end of opioid treatment.
In addition, depression scores improved for those who abstained from cannabis use after their first positive screen. Interestingly, cannabis use did not affect patient retention in treatment for OUD, the investigators note.
“To our knowledge, this is the first time looking at the impact of cannabis on the specific population of pregnant women with opioid use disorder, who are very vulnerable to depression,” lead author Abigail Richison, MD, University of Arkansas for Medical Sciences, Little Rock, said in an interview.
The findings were presented at the American Academy of Addiction Psychiatry (AAAP) 31st Annual Meeting, which was held online this year because of the COVID-19 pandemic.
A safer alternative?
Data from the National Survey on Drug Use and Health show that perinatal cannabis use increased by 62% between 2002 and 2014. Many women try to ameliorate their depression symptoms by using cannabis in the mistaken belief that it will help their depression, the investigators noted.
In addition, many women consider cannabis safer during pregnancy than prescribed medications for improving mood, said Dr. Richison. She said that cannabis does not alleviate depression and may even worsen it.
Dr. Richison noted that at her center, which has a women’s health program that treats pregnant women with OUDs, she was seeing a lot of patients who reported using cannabis to improve their mood.
“However, it didn’t seem like it was really helping, so I started researching about cannabis and depression,” Dr. Richison said.
“ and can be accused of perinatal substance use. I think it is very important to screen for depression as well as cannabis use in this population,” she added.
To shed some light on the impact of cannabis use by pregnant patients with OUD, the investigators conducted a retrospective chart review of 121 pregnant women with OUD who attended outpatient OAT. All were prescribed buprenorphine.
At each visit, Beck Depression Inventory (BDI) scores were obtained and urine drug screens were administered. The primary outcome was BDI score. Other measures included retention, urinary drug screen results, and antidepressant use.
The women were divided into two groups. The first comprised cannabis users, defined as having more than one urine drug screen that was positive for cannabis (n = 35). The other group comprised nonusers, defined as having urine drug screens that were negative for cannabis (n = 86).
Cannabis users were a little younger (mean age, 27 years) than non–cannabis users (mean age, 29.5 years; P = .006). Most of the participants were White (80.2%). Roughly half were on Medicaid, and most of the other participants had private insurance; a small number of women had no insurance.
Results showed that cannabis users had significantly higher BDI scores than non–cannabis users (mean scores, 16 vs. 9.3; P < .001).
Cannabis use continued to be associated with elevated scores for depression when controlling for opioid misuse and antidepressant use. There were no significant differences in retention or lapse to opioid misuse between the two groups.
More evidence of risk
Commenting on the findings in an interview, Carla Marienfeld, MD, professor of psychiatry at the University of California, San Diego, said there is a growing body of evidence about risks from cannabis use during pregnancy, “a time where we already know the endocannabinoid system is very active in the developing fetus.”
She noted that the current study’s design makes it hard to know whether marijuana use causes worse depression.
However, “it clearly is not associated with helping to improve mood the way people who are using it believe or hope for,” said Dr. Marienfeld, who was not part of the research.
“The risk for harm in terms of worse mood for the pregnant woman or risks for harm to the developing fetus are being better understood with many new studies,” she added.
Yet as more and more states legalize medical marijuana, cannabis use during pregnancy is only going to rise, experts fear.
Cornel Stanciu, MD, of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., who was asked for comment, noted that public endorsement for potential benefits of the marijuana plant is at an all-time high.
“To date, 33 states and the District of Columbia have responded by legalizing medical marijuana, with 10 states also having legalized recreational use of marijuana. The current practice is said to be ahead of science, as robust research has been hindered by strict regulations – and most epidemiological studies point toward harmful associations,” Dr. Stanciu said in an interview.
“Given the decreased perception of harm by the general public, women are certainly compelled to seek what they perceive as more natural self-management remedies,” he said.
A harmful habit
Dr. Stanciu cited a recent study conducted in Colorado in which researchers contacted cannabis dispensaries, identified themselves as being pregnant, and asked for guidance in managing pregnancy-related symptoms.
Almost 70% of dispensaries recommended products to treat symptoms, particularly in the vulnerable first trimester; 36% of them also provided reassurance of the safety profile. Very few encouraged a discussion with the physician.
“Consumption of cannabis during pregnancy results in cannabinoid placental crossing and accumulation in the fetal brain, as well as other organs, where it interferes with neurodevelopment and the endocannabinoid system,” he said.
In addition, retrospective studies have shown an association between prenatal cannabis ingestion and anemia in the mothers, low birth weight, greater risk for preterm and stillbirths, and increased need for neonatal ICU admissions.
“Children born to mothers who used cannabis during pregnancy have higher rates of impulsivity, delinquency, learning and memory impairment, as well as executive function deficits. There is also an increased association with proneness to psychosis during middle childhood,” Dr. Stanciu said.
When used during pregnancy, cannabis has been associated with increased anxiety in mothers, as well as increased risk for depressive disorders, incidence of suicidal ideations and behavior, and symptoms of mania and psychosis among those with bipolar and schizophrenia spectrum conditions. Cannabis has also been linked to coingestion of other substances and with alcohol use.
“So cannabis can pose harm, especially when used by those with affective disorders,” Dr. Stanciu said.
The study was funded by the National Institute on Drug Abuse. Dr. Richison, Dr. Marienfeld, and Dr. Stanciu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com
New coalition demands urgent action on COVID-19 mental health crisis
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
Fourteen mental health organizations have formed a coalition to press federal and state officials to tackle the ongoing and growing mental health crisis that is accompanying the COVID-19 pandemic.
The coalition is offering a road map, A Unified Vision for Transforming Mental Health and Substance Abuse Care, which spells out “immediate and long-term changes that will lead to a mental health care system capable of saving our nation,” they said in a statement.
The group includes CEOs from the American Psychiatric Association, the American Psychological Association, the Massachusetts Association for Mental Health, Meadows Mental Health Policy Institute, Mental Health America, the National Association for Behavioral Healthcare, the National Alliance on Mental Illness, the National Council for Behavioral Health, One Mind, Peg’s Foundation, the Steinberg Institute, The Kennedy Forum, the Treatment Advocacy Center, and the Well Being Trust.
They have been meeting in weekly sessions since the beginning of the pandemic. The groups have come together in the spirit of previous efforts to address major health crises, including the 1970s war on cancer and the campaign to curtail the HIV/AIDS epidemic in the 1980s, they report.
The coalition reported that since the pandemic began the prevalence of depression symptoms has jumped threefold, overdose deaths have increased in 40 states, and 25% of young adults have had suicidal ideation.
“It requires immediate action by the new administration, as well as state and local governments in all 50 states, and an acknowledged, consistent commitment to fix what’s broken in our system of care,” Daniel H. Gillison Jr, CEO of the National Alliance on Mental Illness, said in a statement.
SAMHSA chief ‘grateful’
Elinore McCance-Katz, MD, PhD, who is the assistant secretary for mental health and substance use and leads the Substance Abuse and Mental Health Services Administration, U.S. Department of Health & Human Services, applauded the coalition.
“I am very grateful that these organizations are stepping up and putting out a report like this,” Dr. McCance-Katz told this news organization. “I hope that they will continue this kind of advocacy and leadership on these issues going forward,” she said, adding that the need for mental health care and substance use disorders will be much greater going forward because of the pandemic.
Seven policy areas
The group’s 17-page strategic plan emphasizes interventions and methods that have already been tried and tested, focusing on seven policy areas:
- Early identification and prevention, especially for families and young people, by, for instance, bringing telehealth into schools and community centers.
- Rapid deployment of emergency crisis response and prevention, including speeding up the implementation of the new 988 number for the National Suicide Prevention Lifeline.
- Leveling inequities in access to care by addressing social and political constructs and historical systemic injustices such as racism.
- Integrating physical and mental health care and substance use services to ensure “whole-person” well-being.
- Achieving parity in payment by health plans for mental health and substance-use coverage.
- Assuring evidence-based standards of treatments and care.
- Increasing the number and diversity of the mental health care workforce, peer support, and community-based programs.
and will be even more so in the near future as the effects of the pandemic continue to ripple out.
SAMHSA received $425 million in the first COVID-19 relief package signed into law in March – the CARES Act. The money was distributed to states and used for direct care for people with serious mental illness and substance-use disorders who could not otherwise get care because of virus-related restrictions, and for boosting support for mental health support lines, said Dr. McCance-Katz.
A senior SAMHSA spokesperson said the agency is “hopeful that we will see additional resources in the upcoming stimulus for mental health and substance abuse” that Congress is still working on.
“We need bold steps from our government and the business community alike,” former Rep. Patrick J. Kennedy, founder of The Kennedy Forum, said in the statement from the new coalition. “We encourage all state governments to engage with mental health leaders, bring them into pandemic-related responses, and actively facilitate their communication with communities across the country,” said Mr. Kennedy, who is a part of the new coalition.
Mr. Kennedy is also cochair of the Action Alliance’s Mental Health and Suicide Prevention National Response to COVID-19, which unveiled its own six-priority Action Plan earlier in December.
A version of this article first appeared on Medscape.com.
ADHD meds may boost treatment retention in comorbid addiction
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nicotine vaping tapers off among teens
Levels of nicotine and marijuana vaping among adolescents remain elevated but did not increase significantly in the past year, data from the annual Monitoring the Future survey show.
The 2020 survey included responses from 11,821 individuals in 112 schools across the United States from Feb. 11, 2020, to March 14, 2020, at which time data collection ended prematurely because of the COVID-19 pandemic.
A key positive finding in this year’s survey was the relatively stable levels of nicotine vaping from 2019 to 2020, following a trend of notably increased use annually since vaping was added to the survey in 2017.
During the years 2017-2019, the percentage of teens who reported vaping nicotine in the past 12 months increased from 7.5% to 16.5% among 8th graders, from 15.8% to 30.7% among 10th graders, and from 18.8% to 35.3% among 12th graders. However, in 2020, the percentages of teens who reported past-year nicotine vaping were relatively steady at 16.6%, 30.7%, and 34.5%, for 8th-, 10th-, and 12th-grade students, respectively. In addition, reports of daily or near-daily nicotine vaping (defined as 20 occasions in the past 30 days) decreased significantly, from 6.8% to 3.6% among 10th graders and from 11.6% to 5.3% among 12th graders.
“The rapid rise of teen nicotine vaping in recent years has been unprecedented and deeply concerning since we know that nicotine is highly addictive and can be delivered at high doses by vaping devices, which may also contain other toxic chemicals that may be harmful when inhaled,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse in a press release accompanying the release of the findings. “It is encouraging to see a leveling off of this trend though the rates still remain very high.”
Reports of past-year marijuana vaping remained similar to 2019 levels after a twofold increase in the past 2 years, according to the survey. In early 2020, 8.1%, 19.1%, and 22.1% of 8th, 10th, and 12th graders reported past-year use. However, daily marijuana vaping decreased by more than half from 2019, to 1.1% among 10th graders and 1.5% among 12th graders.
Past-year use of the JUUL devices specifically also declined among older teens, from 28.7% in 2019 to 20% in 2020 among 10th graders and from 28.4% in 2019 to 22.7% in 2020 among 12th graders.
Other trends this year included the increased past-year use of amphetamines, inhalants, and cough medicines among 8th graders, and relatively low reported use among 12th graders of LSD (3.9%), synthetic cannabinoids (2.4%), cocaine (2.9%), ecstasy (1.8%), methamphetamine (1.4%), and heroin (0.3%).
The findings were published in JAMA Pediatrics.
Early data show progress
“The MTF survey is the most referenced and reliable longitudinal study reporting current use of tobacco, drugs, and alcohol among young people,” said Mark S. Gold, MD, of Washington University, St. Louis, in an interview.
“The new data, collected before data collection stopped prematurely due to the COVID-19 pandemic, suggests that some progress is being made in slowing the increase in substance use among these, the most vulnerable,” he said.
“The best news was that nicotine vaping decreased significantly after its meteoric increase over the past few years,” Dr. Gold emphasized. “Past-year vaping of marijuana remained steady at alarming levels in 2020, with 8.1% of 8th graders, 19.1% of 10th graders, and 22.1% of 12th graders reporting past-year use, following a two-fold increase over the past 2 years.” The use of all forms of marijuana, including smoking and vaping, did not significantly change in any of the three grades for lifetime use, past 12-month use, past 30-day use, and daily use from 2019 to 2020.
“Teen alcohol use has not significantly changed over the past 5 years,” and cigarette smoking in the last 30 days did not significantly change from 2019 to 2020, said Dr. Gold. However, “as with adults, psychostimulant use is increasing. Past year nonmedical use of amphetamines among 8th graders increased, from 3.5% in 2017 to 5.3% in 2020.”
COVID-era limitations
“The data suggest that pre-COVID pandemic vaping, smoking cigarettes, marijuana, and alcohol use had stabilized,” Dr. Gold said. “However, it is very difficult to predict what the COVID era data will show as many young people are at home, on the streets, and unsupervised; while adult substance misuse, substance use disorders, and overdoses are increasing. Drug supplies and access have increased for alcohol, cannabis, vaping, and tobacco as have supply synthetics like methamphetamine and fentanyl.”
In addition, “access to evaluation, intervention, and treatment have been curtailed during the pandemic,” Dr. Gold said. “The loss of peer role models, daily routine, and teacher or other adult supervision and interventions may interact with increasing despair, social isolation, depression, and anxiety in ways that are unknown. “It will not be clear until the next survey if perceived dangerousness has changed in ways that can protect these 8th, 10th, and 12th graders and increase the numbers of never users or current nonusers.”
The Monitoring the Future survey is conducted each year by the University of Michigan’s Institute for Social Research, Ann Arbor, and supported by NIDA, part of the National Institutes of Health. Dr. Gold had no relevant financial conflicts to disclose.
Levels of nicotine and marijuana vaping among adolescents remain elevated but did not increase significantly in the past year, data from the annual Monitoring the Future survey show.
The 2020 survey included responses from 11,821 individuals in 112 schools across the United States from Feb. 11, 2020, to March 14, 2020, at which time data collection ended prematurely because of the COVID-19 pandemic.
A key positive finding in this year’s survey was the relatively stable levels of nicotine vaping from 2019 to 2020, following a trend of notably increased use annually since vaping was added to the survey in 2017.
During the years 2017-2019, the percentage of teens who reported vaping nicotine in the past 12 months increased from 7.5% to 16.5% among 8th graders, from 15.8% to 30.7% among 10th graders, and from 18.8% to 35.3% among 12th graders. However, in 2020, the percentages of teens who reported past-year nicotine vaping were relatively steady at 16.6%, 30.7%, and 34.5%, for 8th-, 10th-, and 12th-grade students, respectively. In addition, reports of daily or near-daily nicotine vaping (defined as 20 occasions in the past 30 days) decreased significantly, from 6.8% to 3.6% among 10th graders and from 11.6% to 5.3% among 12th graders.
“The rapid rise of teen nicotine vaping in recent years has been unprecedented and deeply concerning since we know that nicotine is highly addictive and can be delivered at high doses by vaping devices, which may also contain other toxic chemicals that may be harmful when inhaled,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse in a press release accompanying the release of the findings. “It is encouraging to see a leveling off of this trend though the rates still remain very high.”
Reports of past-year marijuana vaping remained similar to 2019 levels after a twofold increase in the past 2 years, according to the survey. In early 2020, 8.1%, 19.1%, and 22.1% of 8th, 10th, and 12th graders reported past-year use. However, daily marijuana vaping decreased by more than half from 2019, to 1.1% among 10th graders and 1.5% among 12th graders.
Past-year use of the JUUL devices specifically also declined among older teens, from 28.7% in 2019 to 20% in 2020 among 10th graders and from 28.4% in 2019 to 22.7% in 2020 among 12th graders.
Other trends this year included the increased past-year use of amphetamines, inhalants, and cough medicines among 8th graders, and relatively low reported use among 12th graders of LSD (3.9%), synthetic cannabinoids (2.4%), cocaine (2.9%), ecstasy (1.8%), methamphetamine (1.4%), and heroin (0.3%).
The findings were published in JAMA Pediatrics.
Early data show progress
“The MTF survey is the most referenced and reliable longitudinal study reporting current use of tobacco, drugs, and alcohol among young people,” said Mark S. Gold, MD, of Washington University, St. Louis, in an interview.
“The new data, collected before data collection stopped prematurely due to the COVID-19 pandemic, suggests that some progress is being made in slowing the increase in substance use among these, the most vulnerable,” he said.
“The best news was that nicotine vaping decreased significantly after its meteoric increase over the past few years,” Dr. Gold emphasized. “Past-year vaping of marijuana remained steady at alarming levels in 2020, with 8.1% of 8th graders, 19.1% of 10th graders, and 22.1% of 12th graders reporting past-year use, following a two-fold increase over the past 2 years.” The use of all forms of marijuana, including smoking and vaping, did not significantly change in any of the three grades for lifetime use, past 12-month use, past 30-day use, and daily use from 2019 to 2020.
“Teen alcohol use has not significantly changed over the past 5 years,” and cigarette smoking in the last 30 days did not significantly change from 2019 to 2020, said Dr. Gold. However, “as with adults, psychostimulant use is increasing. Past year nonmedical use of amphetamines among 8th graders increased, from 3.5% in 2017 to 5.3% in 2020.”
COVID-era limitations
“The data suggest that pre-COVID pandemic vaping, smoking cigarettes, marijuana, and alcohol use had stabilized,” Dr. Gold said. “However, it is very difficult to predict what the COVID era data will show as many young people are at home, on the streets, and unsupervised; while adult substance misuse, substance use disorders, and overdoses are increasing. Drug supplies and access have increased for alcohol, cannabis, vaping, and tobacco as have supply synthetics like methamphetamine and fentanyl.”
In addition, “access to evaluation, intervention, and treatment have been curtailed during the pandemic,” Dr. Gold said. “The loss of peer role models, daily routine, and teacher or other adult supervision and interventions may interact with increasing despair, social isolation, depression, and anxiety in ways that are unknown. “It will not be clear until the next survey if perceived dangerousness has changed in ways that can protect these 8th, 10th, and 12th graders and increase the numbers of never users or current nonusers.”
The Monitoring the Future survey is conducted each year by the University of Michigan’s Institute for Social Research, Ann Arbor, and supported by NIDA, part of the National Institutes of Health. Dr. Gold had no relevant financial conflicts to disclose.
Levels of nicotine and marijuana vaping among adolescents remain elevated but did not increase significantly in the past year, data from the annual Monitoring the Future survey show.
The 2020 survey included responses from 11,821 individuals in 112 schools across the United States from Feb. 11, 2020, to March 14, 2020, at which time data collection ended prematurely because of the COVID-19 pandemic.
A key positive finding in this year’s survey was the relatively stable levels of nicotine vaping from 2019 to 2020, following a trend of notably increased use annually since vaping was added to the survey in 2017.
During the years 2017-2019, the percentage of teens who reported vaping nicotine in the past 12 months increased from 7.5% to 16.5% among 8th graders, from 15.8% to 30.7% among 10th graders, and from 18.8% to 35.3% among 12th graders. However, in 2020, the percentages of teens who reported past-year nicotine vaping were relatively steady at 16.6%, 30.7%, and 34.5%, for 8th-, 10th-, and 12th-grade students, respectively. In addition, reports of daily or near-daily nicotine vaping (defined as 20 occasions in the past 30 days) decreased significantly, from 6.8% to 3.6% among 10th graders and from 11.6% to 5.3% among 12th graders.
“The rapid rise of teen nicotine vaping in recent years has been unprecedented and deeply concerning since we know that nicotine is highly addictive and can be delivered at high doses by vaping devices, which may also contain other toxic chemicals that may be harmful when inhaled,” said Nora D. Volkow, MD, director of the National Institute on Drug Abuse in a press release accompanying the release of the findings. “It is encouraging to see a leveling off of this trend though the rates still remain very high.”
Reports of past-year marijuana vaping remained similar to 2019 levels after a twofold increase in the past 2 years, according to the survey. In early 2020, 8.1%, 19.1%, and 22.1% of 8th, 10th, and 12th graders reported past-year use. However, daily marijuana vaping decreased by more than half from 2019, to 1.1% among 10th graders and 1.5% among 12th graders.
Past-year use of the JUUL devices specifically also declined among older teens, from 28.7% in 2019 to 20% in 2020 among 10th graders and from 28.4% in 2019 to 22.7% in 2020 among 12th graders.
Other trends this year included the increased past-year use of amphetamines, inhalants, and cough medicines among 8th graders, and relatively low reported use among 12th graders of LSD (3.9%), synthetic cannabinoids (2.4%), cocaine (2.9%), ecstasy (1.8%), methamphetamine (1.4%), and heroin (0.3%).
The findings were published in JAMA Pediatrics.
Early data show progress
“The MTF survey is the most referenced and reliable longitudinal study reporting current use of tobacco, drugs, and alcohol among young people,” said Mark S. Gold, MD, of Washington University, St. Louis, in an interview.
“The new data, collected before data collection stopped prematurely due to the COVID-19 pandemic, suggests that some progress is being made in slowing the increase in substance use among these, the most vulnerable,” he said.
“The best news was that nicotine vaping decreased significantly after its meteoric increase over the past few years,” Dr. Gold emphasized. “Past-year vaping of marijuana remained steady at alarming levels in 2020, with 8.1% of 8th graders, 19.1% of 10th graders, and 22.1% of 12th graders reporting past-year use, following a two-fold increase over the past 2 years.” The use of all forms of marijuana, including smoking and vaping, did not significantly change in any of the three grades for lifetime use, past 12-month use, past 30-day use, and daily use from 2019 to 2020.
“Teen alcohol use has not significantly changed over the past 5 years,” and cigarette smoking in the last 30 days did not significantly change from 2019 to 2020, said Dr. Gold. However, “as with adults, psychostimulant use is increasing. Past year nonmedical use of amphetamines among 8th graders increased, from 3.5% in 2017 to 5.3% in 2020.”
COVID-era limitations
“The data suggest that pre-COVID pandemic vaping, smoking cigarettes, marijuana, and alcohol use had stabilized,” Dr. Gold said. “However, it is very difficult to predict what the COVID era data will show as many young people are at home, on the streets, and unsupervised; while adult substance misuse, substance use disorders, and overdoses are increasing. Drug supplies and access have increased for alcohol, cannabis, vaping, and tobacco as have supply synthetics like methamphetamine and fentanyl.”
In addition, “access to evaluation, intervention, and treatment have been curtailed during the pandemic,” Dr. Gold said. “The loss of peer role models, daily routine, and teacher or other adult supervision and interventions may interact with increasing despair, social isolation, depression, and anxiety in ways that are unknown. “It will not be clear until the next survey if perceived dangerousness has changed in ways that can protect these 8th, 10th, and 12th graders and increase the numbers of never users or current nonusers.”
The Monitoring the Future survey is conducted each year by the University of Michigan’s Institute for Social Research, Ann Arbor, and supported by NIDA, part of the National Institutes of Health. Dr. Gold had no relevant financial conflicts to disclose.
E-cigarette use tied to increased COPD, asthma risk
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
Results from a large national prospective cohort study of adults demonstrated that the use of electronic cigarettes is associated with an increased risk of asthma, chronic obstructive pulmonary disease (COPD), emphysema, and chronic bronchitis – independent of cigarette smoking and other combustible tobacco product use.
“Our longitudinal results are consistent with the findings of prior population studies,” researchers led by Wubin Xie, DrPH, MPH, wrote in a study published online in JAMA Network Open. “With a more refined study design assessing multiple respiratory conditions and extensive sensitivity checks to mitigate bias from reverse causation and residual confounding by cigarette smoking and other tobacco product use, our results strengthen the evidence of the potential role of e-cigarette use in pulmonary disease pathogenesis. The findings may be used to inform counseling of patients on the potential risks of e-cigarette use.”
Dr. Xie of Boston University, and colleagues used data from the Population Assessment of Tobacco and Health (PATH) study waves 1-4 to examine the association of e-cigarette use with incident respiratory conditions, including COPD, emphysema, chronic bronchitis, and asthma. An earlier analysis of PATH data found an association between e-cigarette use with a composite respiratory disease outcome, but it did not consider the timing of respiratory events over follow-up and was underpowered to evaluate specific respiratory conditions.
The current analysis included data from 21,618 U.S. adults who were surveyed in four waves of PATH between 2013 and 2018. Of these, 49% were men, 65% were non-Hispanic White, 12% were non-Hispanic Black, 16% were Hispanic, and the remainder were non-Hispanic other. Their mean pack-years was 6.7 at baseline, 26% had self-reported hypertension, and their mean body mass index was 27.8 kg/m2. The analysis was limited to data from the wave 1 cohort of adults and the prospective follow-up at waves 2-4 from public use files. It excluded adults who reported a history of a respiratory condition such as COPD, emphysema, chronic bronchitis, or asthma at wave 1 (baseline).
Two-thirds of respondents (66%) were never e-cigarette users, 12% were former e-cigarette users, and 5% were current e-cigarette users. After the researchers adjusted for cigarette and other combustible tobacco product use, demographic characteristics, and chronic health conditions, they observed an increased risk of respiratory disease among former e-cigarette users (incidence rate ratio, 1.28) and current e-cigarette users (IRR, 1.31). Among respondents with good self-reported health, the IRR for former e-cigarette users was 1.21 and the IRR for current e-cigarette users was 1.43. As for specific respiratory diseases among current e-cigarette users, the IRR was 1.33 for chronic bronchitis, 1.69 for emphysema, 1.57 for COPD, and 1.31 for asthma.
“Our findings on clinical outcome were consistent with studies assessing in vivo biomarkers of e-cigarette exposure in animal subjects, human participants, and population studies,” the authors wrote. “Studies have documented that exclusive e-cigarette use may be associated with higher exposure to harmful and potentially harmful constituents, compared with tobacco nonuse. The potential mechanisms of the association of e-cigarette exposure with pulmonary diseases include pulmonary inflammation, increased oxidative stress, and inhibited immune response. Animal studies have generated substantial evidence on e-cigarette exposure and emphysematous lung destruction, loss of pulmonary capillaries, reduced small airway function, and airway hyperresponsiveness, suggesting the plausibility of e-cigarettes causing chronic lung diseases.”
They acknowledged certain limitations of the study, including its reliance on self-reported measures of e-cigarette and other tobacco product use and its reliance on self-reported diagnoses of respiratory diseases.
The study was supported by grants from the National Heart, Lung, and Blood Institute; the Food and Drug Administration Center for Tobacco Products; and the American Lung Association Public Policy Research Award. Dr. Xie reported having no financial disclosures. His coauthors reported having received research grants and personal fees from a variety of sources.
SOURCE: Xie W et al. JAMA Netw Open. 2020 Nov 12. doi: 10.1001/jamanetworkopen.2020.20816
FROM JAMA NETWORK OPEN












