User login
Tips and tools for safe opioid prescribing
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
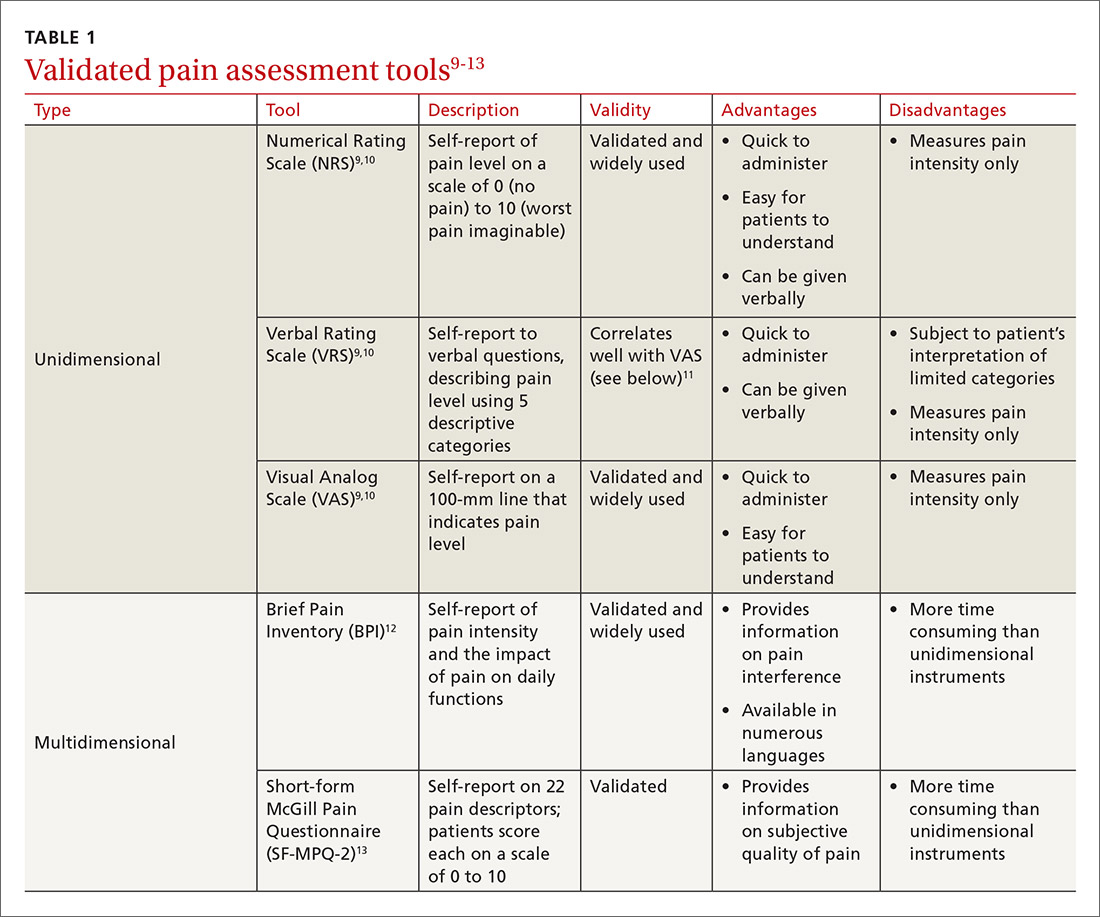
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
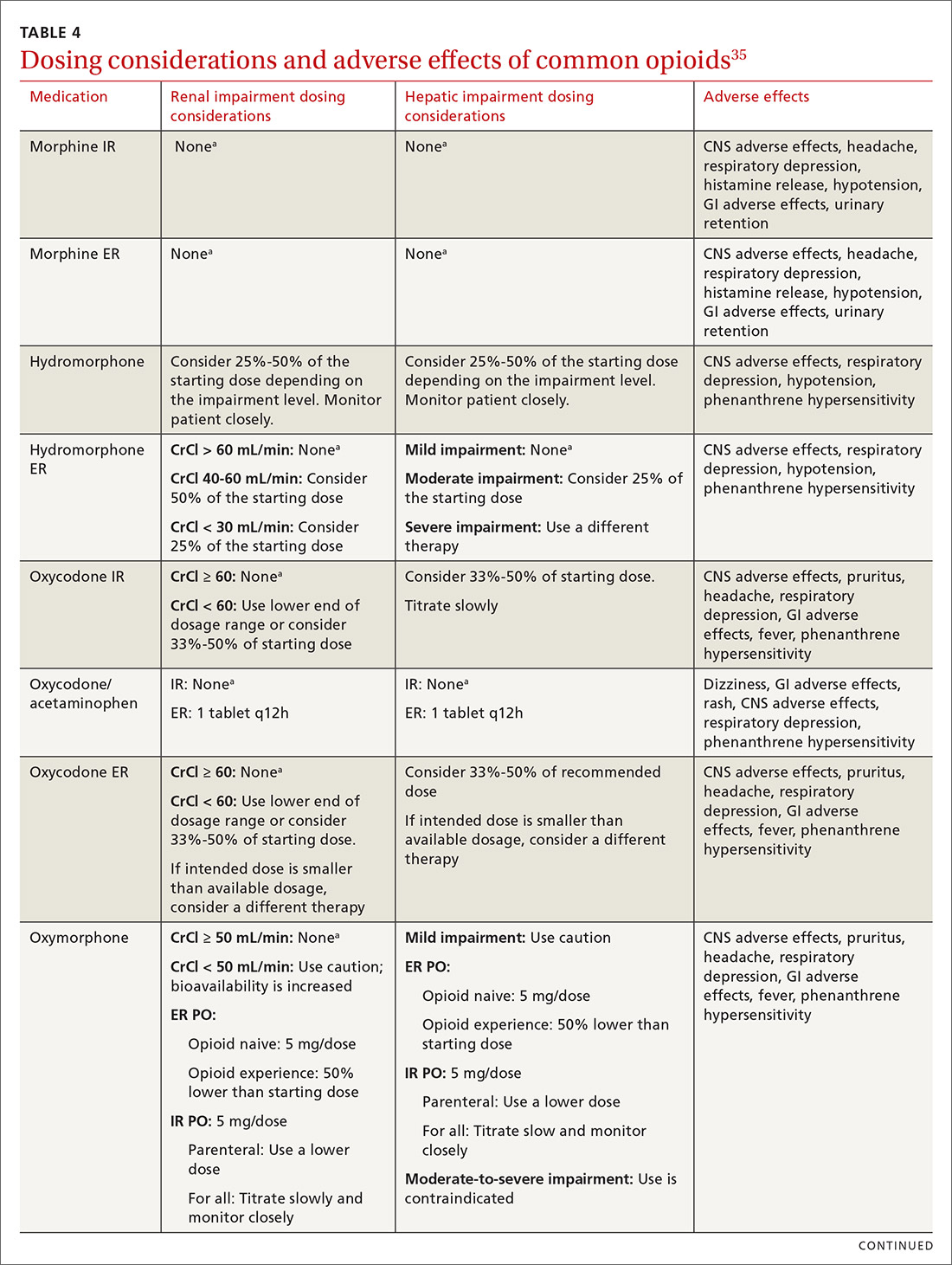
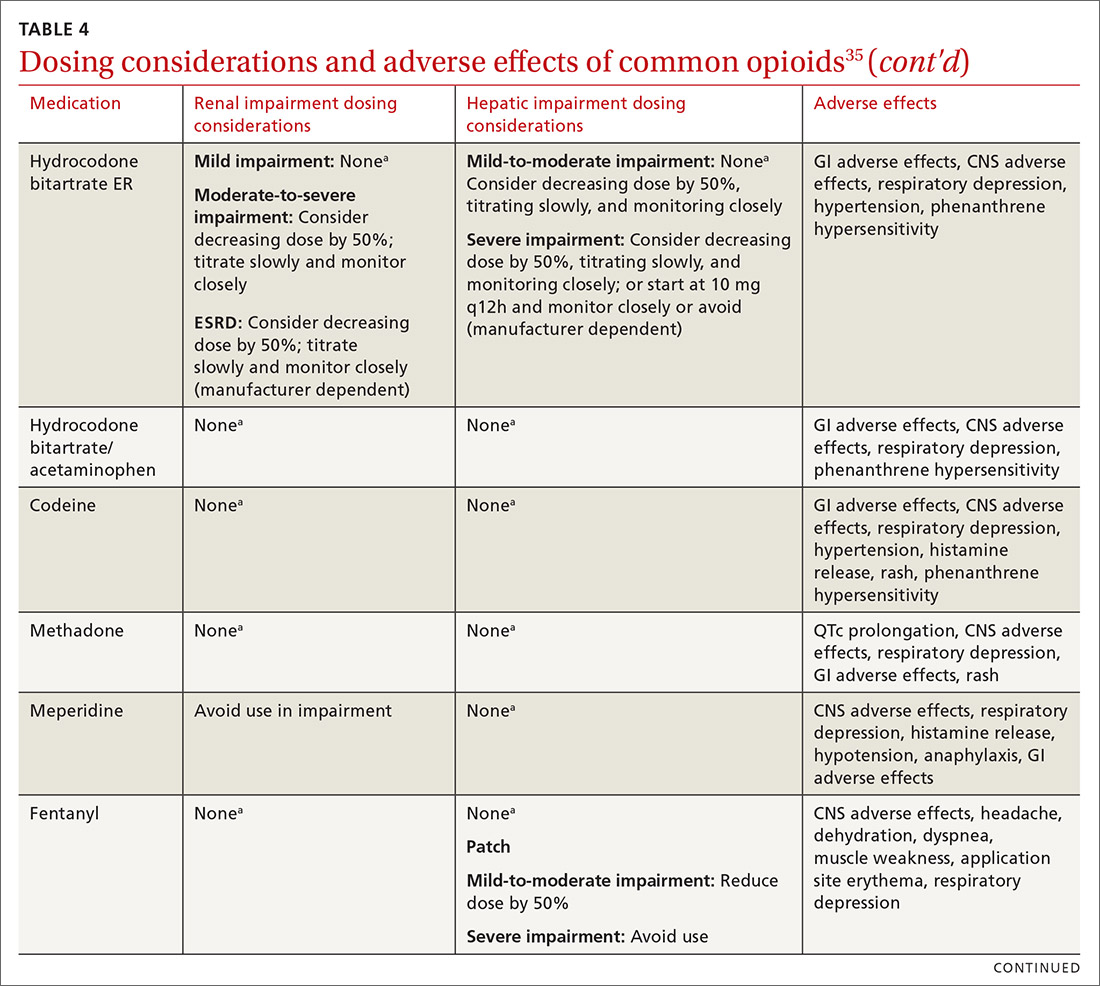
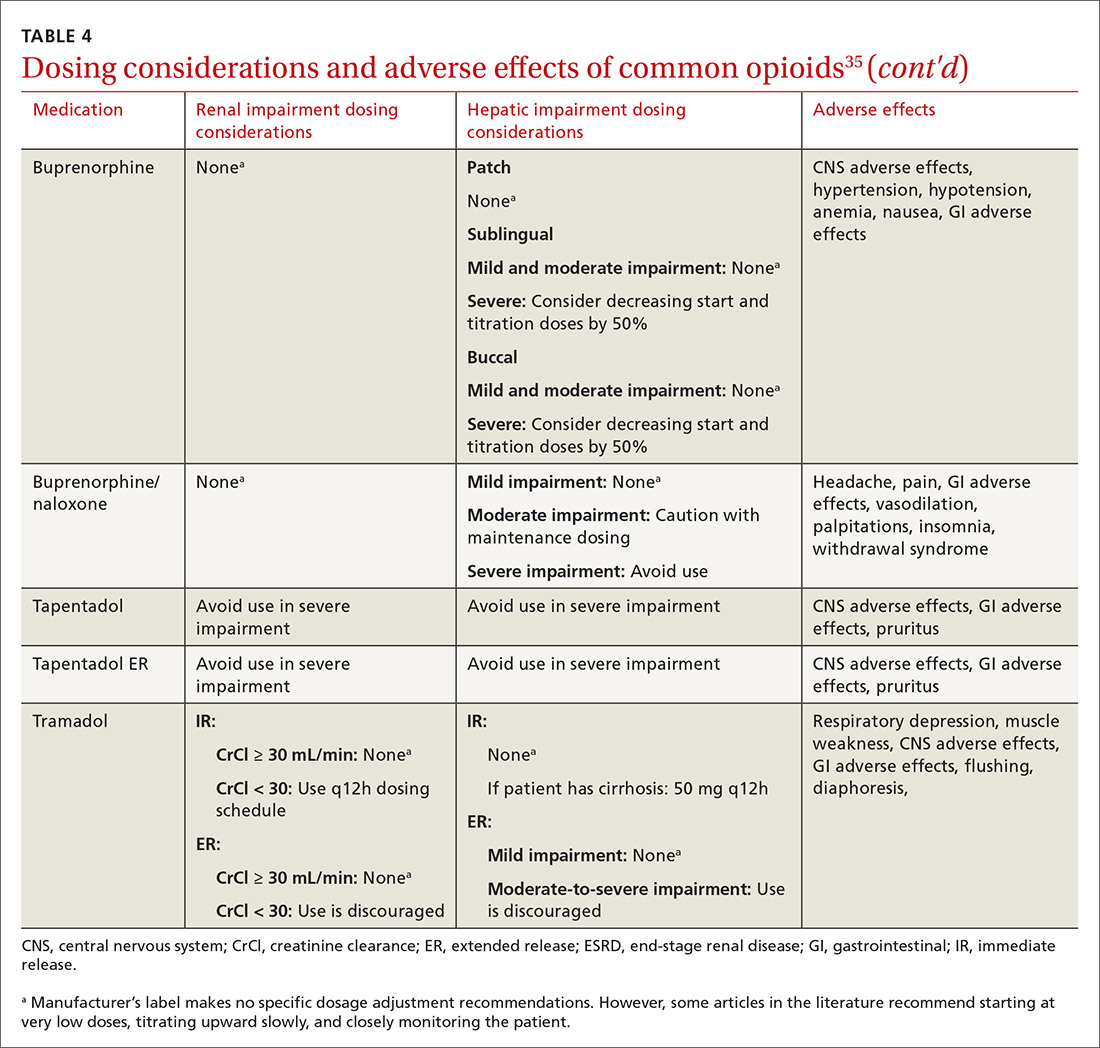
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).
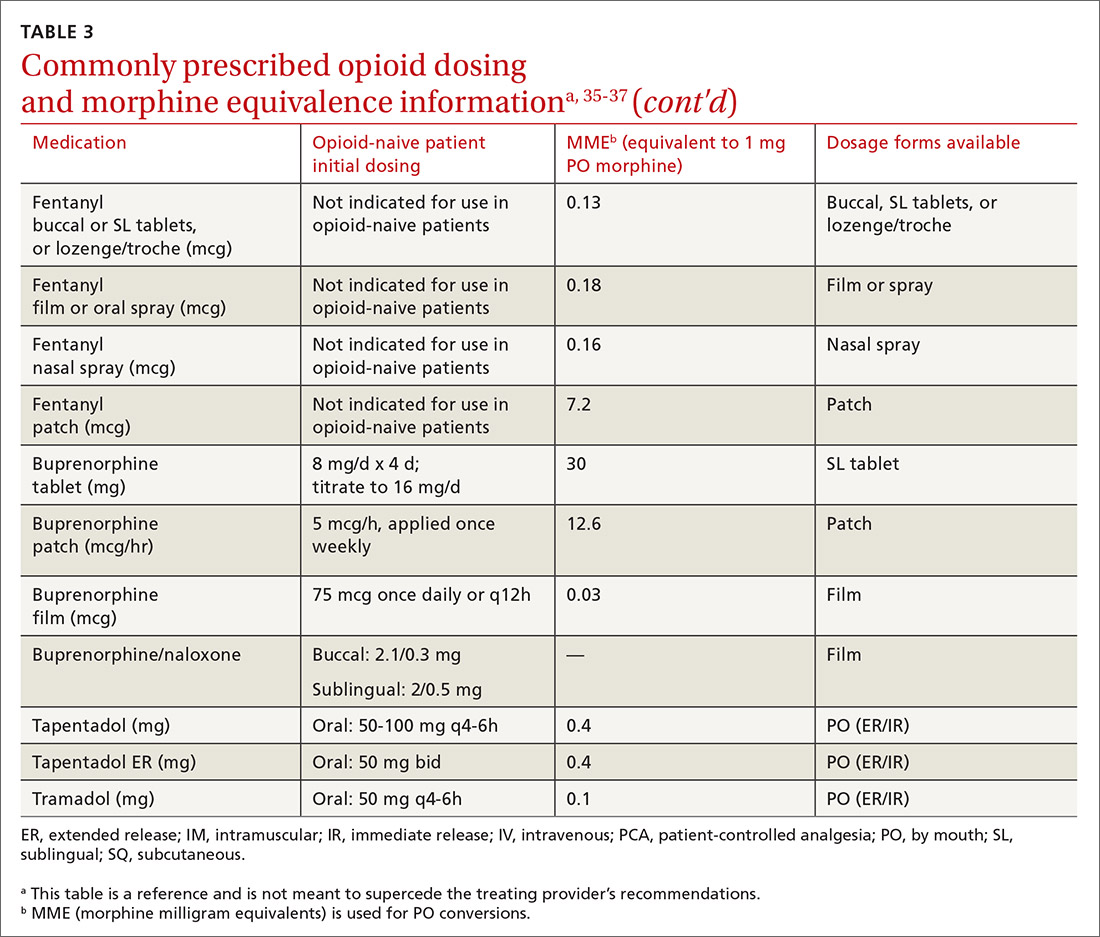
Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
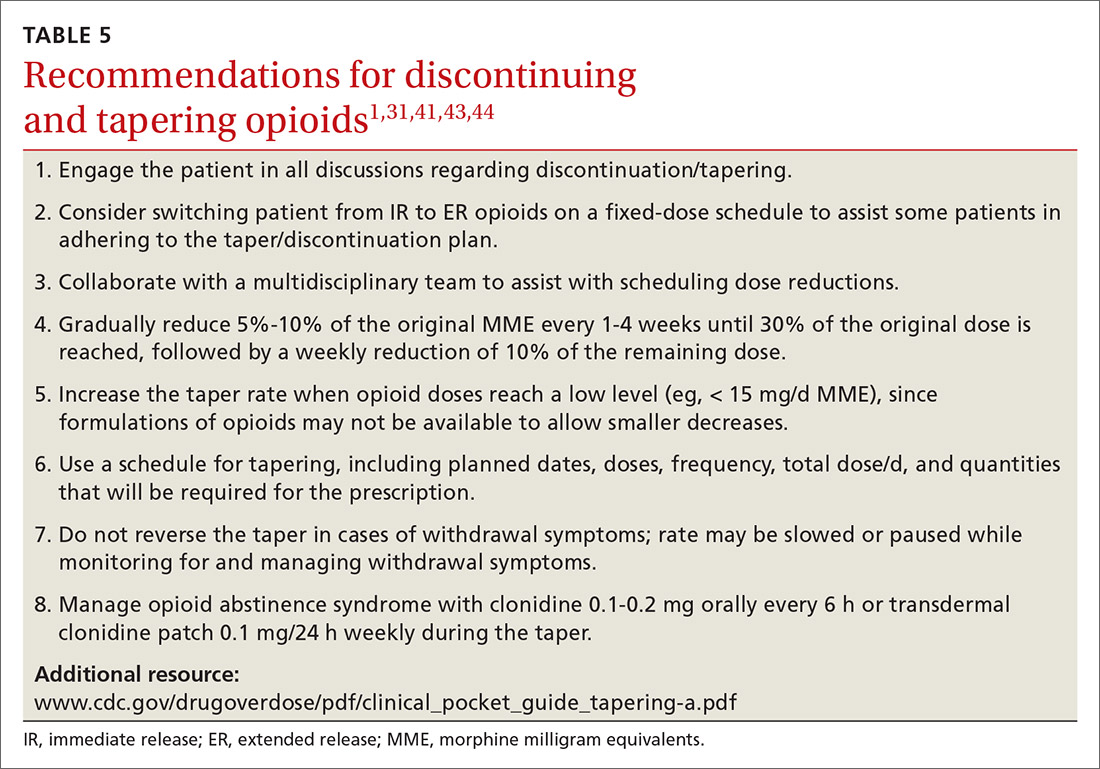
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
CASE
Marcelo G* is a 46-year-old man who presented to our family medicine clinic with a complex medical history including end-stage renal disease (ESRD) and hemodialysis, chronic anemia, peripheral vascular disease, venous thromboembolism and anticoagulation, major depressive disorder, osteoarthritis, and lumbosacral radiculopathy. His current medications included vitamin B complex, cholecalciferol, atorvastatin, warfarin, acetaminophen, diclofenac gel, and capsaicin cream. Mr. G reported bothersome bilateral knee and back pain despite physical therapy and consistent use of his current medications in addition to occasional intra-articular glucocorticoid injections. He mentioned that he had benefited in the past from intermittent opioid use.
How would you manage this patient’s care?
*The patient’s name has been changed to protect his identity.
In 2013, an estimated 191 million prescriptions for opioids were written by health care providers, which is the equivalent of all adults living in the United States having their own opioid prescription.1 This large expansion in opioid prescribing and use has also led to a rise in opioid overdose deaths, whether from prescribed or illicit use.1 The Centers for Disease Control and Prevention (CDC) points out that each day, approximately 128 Americans die from an opioid overdose.1 Deaths that occur from opioid overdose often involve the prescribed opioids methadone, oxycodone, and hydrocodone, the illicit opioid heroin, and, of particular concern, prescription and illicit fentanyl.1
The extent of this problem has sparked the development of health safety initiatives and research efforts. Through production quotas, the US Drug Enforcement Administration (DEA) reduced the number of opioids produced across all schedule I and schedule II lists in 2017 by as much as 25%.2 The DEA again reduced the amounts produced in 2018.3 For 2020, the DEA has determined that the production quotas and assessment of annual needs are sufficient.4
The CDC has also promoted access to naloxone and prevention initiatives; pharmacies in some states have standing orders for naloxone, and medical personnel and law enforcement now carry it.1,5 Finally, new research has identified risk factors that influence one’s potential for addiction, such as mental illness, history of substance and alcohol abuse, and a low income.6 Interestingly, while numerous initiatives and strategies have been implemented across health systems, there is little evidence that demonstrates how implementation of safe prescribing strategies has affected overall patient safety and avoidance of opioid-related harms.
Nevertheless, concerns related to opioids are especially important for primary care providers, who manage many patients with acute and chronic diseases and disorders that require pain control.7 Family physicians write more opioid prescriptions than any other specialty,8 and they are therefore uniquely positioned to protect patients, improve the quality of their care, and ultimately produce a meaningful public health impact. This article provides a guide to safe opioid prescribing.
Continue to: Use the patient interview to ensure that Tx aligns with patient goals
Use the patient interview to ensure that Tx aligns with patient goals
For patients presenting with chronic pain, conduct a complete general history and physical examination that includes a review of available records; a medical, surgical, social, family, medication, and allergy history; a review of systems; and documentation of any psychiatric comorbidities (ie, depression, anxiety, psychiatric disorders, personality traits). Inquiries about social history and current medications should explore the possibility of previous and current substance use and misuse.
While causes of pain can be assessed through physical examination and diagnostic tests, the patient interview is an invaluable source of information. No single means of assessment has consistently demonstrated superiority over another in measuring pain, and numerous standard assessment tools are available (TABLE 19-13).14 Unidimensional tools are often easy and quick ways to assess pain intensity. Multidimensional tools, although more time intensive, are designed to gather more subjective information about the patient’s pain. Finally, use an instrument such as the 9-item Patient Health Questionnaire (PHQ-9) to screen patients for psychological distress.15,16
Provide an environment for patients to openly discuss their experiences, expectations, preferences, fears, and coping efforts, as well as the impact that pain has had on their lives.17,18 Without this foundational understanding, medical treatment may work against the patient’s goals. An empathic approach allows for effective communication, shared decision making, and ultimately, an avenue for individualized therapy.
Balancing treatment with risk mitigation
The challenge of managing chronic pain is to balance treating the patient with the basic principle of nonmaleficence (primum non nocere: “first, do no harm”). The literature has shown that risk factors such as a family history of substance abuse or sexual abuse, younger age, and psychological disease may be linked to greater risk for opioid misuse.19,20 However, despite the many risk-screening tools available, no single instrument has reliably and accurately predicted those at higher propensity for prescription addiction. In fact, risk-screening tools as a whole remain unregulated by the US Food and Drug Administration (FDA) and other authorities.21 Still, screening tools provide useful information as one component of the risk-mitigation process.
Screening tools. The tools most commonly used clinically to stratify risk prior to prescribing opioids are the 5-item Opioid Risk Tool (ORT),22 the revised 24-item Screener and Opioid Assessment for Patients with Pain (SOAPP-R),23 which are patient self-administered assessments, and the 7-item clinician-administered DIRE (Diagnosis, Intractability, Risk, Efficacy).24 Given the subtle differences in criteria and the time required for each of these risk assessments, we recommend choosing one based on site-specific resources and overall clinician comfort.25 Risk stratification helps to determine the optimal frequency and intensity of monitoring, not necessarily to deny care to “high-risk” patients.
Continue to: In fact, just as the "universal precautions"...
In fact, just as the “universal precautions” approach has been applied to infection control, many have suggested using a similar approach to pain management. Risk screening should never be misunderstood as an attempt to diminish or undermine the patient’s burden of pain. By routinely conducting thorough and respectful inquiries of risk factors for all patients, clinicians can reduce stigma, improve care, and contain overall risk.26,27
Monitoring programs and patient agreements. In addition to risk-screening tools, the CDC recommends using state prescription drug monitoring programs (PDMP) and urine drug testing (UDT) data to confirm the use of prescribed and illicit substances.28 All 50 states have implemented PDMPs.29 Consider incorporating these components into controlled-substance agreements, which ultimately aim to promote safety and trust between patients and providers. Of course, such agreements do not eliminate all risks associated with opioid prescribing, nor do they guarantee the absence of adverse outcomes. However, when used correctly, they can provide safeguards to reduce misuse and abuse. They also have the potential to preserve the patient-provider relationship, as opposed to providers cursorily refusing to prescribe opioids altogether. The term “controlled-substance agreement” is preferable to “pain contract” or “narcotic contract” as the latter 2 terms may feel stigmatizing and threatening.30
Risk evaluation and mitigation strategy (REMS). In an effort to ensure that benefits of opioid analgesics continue to outweigh the risks, the FDA approved the extended-release (ER)/long-acting (LA) opioid analgesics shared system REMS. Under this REMS, a consortium of ER/LA opioid manufacturers is mandated to provide prescriber education in the form of accredited continuing education and patient educational materials, available at https://opioidanalgesicrems.com/RpcUI/home.u.
CASE
After reviewing Mr. G’s chart and conducting a history, we learned that his bilateral knee osteoarthritis was atraumatic and likely due to overuse—although possibly affected by major trauma in a motor vehicle accident 5 years earlier. Imaging also revealed multilevel disc degeneration contributing to his radicular back pain, which seemed to be worse on days after working as a caterer. Poor lifting form at work may have contributed to his pain. Nevertheless, he had been consistent with medical follow-up and denied current or past use of illicit substances. Per the numeric rating scale (NRS), he reported 8 out of 10 pain in his knees and 6 out of 10 in his back. In addition to obtaining a PHQ-9 score of 4, we conducted a DIRE assessment and obtained a score of 19 out of a possible 21, indicating that he may be a good candidate for long-term opioid analgesia.
Criteria for prescribing opioids and for guiding treatment goals
Prescribing an opioid requires establishing a medical necessity based on 3 criteria:31
- pain of moderate-to-severe degree
- a physical diagnosis or suspected organic problem
- documented treatment failure of a noncontrolled substance, adjuvant agents, physician-ordered physical therapy, structured exercise program, and interventional techniques.
Continue to: Treatment goals should be established...
Treatment goals should be established and understood by the prescriber and patient prior to initiation of opioids.28 Overarching treatment goals for all opioids prescribed are pain relief (but not necessarily a focus on pain scores), improvement in functional activity, and minimization of adverse effects, with the latter 2 goals taking precedence.31 To assess outcomes, formally measure progress toward goals from baseline evaluations. This can be achieved through repeated use of validated tools such as those mentioned earlier, or may be more broadly considered as progress toward employment status or increasing participation in activities.31 All pain management plans involving opioids should include continued efforts with nonpharmacologic therapy (eg, exercise therapy, weight loss, behavioral training) and nonopioid pharmacologic therapy (eg, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, anticonvulsants).28
Have an “exit strategy.” As part of goal setting, also consider how therapy will be discontinued if benefits do not outweigh the risks of harm.28 Weigh functional status gains against adverse opioid consequences using the PEG scale (pain, enjoyment of life, and general activity) (TABLE 232).33 Improvements of 30% from baseline have been deemed clinically meaningful by some,32 but not all benefits will be easy to quantify. At the start of treatment dialogue, use the term “therapeutic trial” instead of ”treatment plan” to more effectively convey that opioids will be continued only if safe and effective, and will be prescribed at the lowest effective dose as one component of the multimodal approach to pain.30
Initiation of treatment: Opioid selection and dosing
When initiating opioid therapy, prescribe an immediate-release, short-acting agent instead of an ER/LA formulation.28
For moderate pain, first consider tramadol, codeine, tapentadol, or hydrocodone.31 Second-line agents for moderate pain are hydrocodone or oxycodone.31
For severe pain, first-line agents include hydrocodone, oxycodone, hydromorphone, or morphine.31 Second-line agents for severe pain are fentanyl and, with careful supervision or referral to a pain specialist, methadone or buprenorphine.31
Continue to: Of special note...
Of special note,
At the start, prescribe the lowest effective dosage (referring to the product labeling for guidance) and calculate total daily dose in terms of morphine milligram equivalents (MME) (TABLE 335-37).28 Exercise caution when considering opioids for patients with respiratory sleep disorders and for patients ≥ 65 years due to altered pharmacokinetics in the elderly population.38 Also make dose adjustments for renal and hepatic insufficiency (TABLE 435).

Doses between 20 to 50 MME/d are considered relatively low dosages.28 Be cautious when prescribing an opioid at any dosage, and reassess evidence of individual benefits and risks before increasing the dosage to ≥ 50 MME/d.28 Regard a dosage of 90 MME/d as maximal.28 While there is no analgesic ceiling, doses greater than 90 MME/d are associated with risk for overdose and should prompt referral to a pain specialist.31 Veterans Administration guidelines cite strong evidence that risk for overdose and death significantly increases at a range of 20 to 50 MME/d.33 Daily doses exceeding 90 MME/d should be documented with rational justification.28
CASE
Noncontrolled medications are preferred in the treatment of chronic pain. However, the utility of adjuvant options such as NSAIDs, duloxetine, or gabapentin were limited in Mr. G’s case due to his ESRD. Calcium channel α2-δ ligands may have been effective in reducing symptoms of neuropathic pain but would have had limited efficacy against osteoarthritis. Based on his low risk for opioid misuse, we decided to start Mr. G on oxycodone 2.5 mg PO, every 6 hours as needed for moderate-to-severe pain, and to follow up in 1 month. We also explained proper lifting form to him and encouraged him to continue with physical therapy.
Deciding to continue therapy with opioids
There is a lack of convincing evidence that opioid use beyond 6 months improves quality of life; patients do not report a significant reduction in pain beyond this time.28 Thus, a repeat evaluation of continued medical necessity is essential before deciding in favor of ongoing, long-term treatment with opioids. Continue prescribing opioids only if there is meaningful pain relief and improved function that outweighs the harms that may be expected for a given patient.31 With all patients, consider prescribing naloxone to accompany dispensed opioid prescriptions.28 This is particularly important for those at risk for misuse (history of overdose, history of substance use disorder, dosages ≥ 50 MME/d, or concurrent benzodiazepine use). Resources for prescribing naloxone in primary care settings can be found through Prescribe to Prevent at http://prescribetoprevent.org. Due to the established risk of overdose, avoid, if possible, concomitant prescriptions of benzodiazepines and opioids.31
Continue to: Follow-up and monitoring
Follow-up and monitoring
Responsiveness to opioids varies greatly among individuals.38,39 An opioid that leads to a therapeutic analgesic effect in one patient may cause adverse events or toxicity in another. Periodically reassess the appropriateness of chronic opioid therapy and modify treatment based on its ability to meet therapeutic goals. While practice behaviors and clinic policies vary across institutions, risk stratification can provide guidance on the frequency and intensity of follow-up and monitoring. Kaye et al21 describe a triage system in which low-risk patients may be managed by a primary care provider with routine follow-up and reassessment every 3 months.21 Moderate-risk patients may warrant additional management by specialists and a monthly follow-up. High-risk patients may need referrals to interdisciplinary pain centers or addiction specialists.21
Along these lines, the CDC recommends conducting a PDMP review and UDT before initiating therapy, followed by a periodic PDMP (every 1-3 months) and a UDT at least annually. Keep in mind, providers should follow their state-specific regulations, as monitoring requirements may vary. In addition, clinicians should always be alert to adverse reactions (TABLE 435) and sudden behavior changes such as respiratory depression, nausea, constipation, pruritus, cognitive impairment, falls, motor vehicle accidents, and aberrant behaviors. Under these circumstances, consider a dose reduction and, in certain cases, discontinuation.
Additionally, in cases of pain unresponsive to escalating opioid doses, include opioid-induced hyperalgesia (OIH) in the differential. Dose reductions, opioid rotations, and office-based detoxifications are all options for the treatment of OIH.40 Assessment of pain and function can be accomplished using the PEG scale (TABLE 2).32
CASE
Two weeks into Mr. G’s initial regimen, he called to report no change in pain or functional status. We increased his dose to 5 mg PO every 6 hours as needed. At his 1-month follow-up appointment, he reported his pain as 6/10 and no adverse effects. We again increased his dose to 10 mg PO every 6 hours as needed, with follow-up in another month.
Discontinuation and tapering of opioids
Indications for discontinuing opioids are patient request, resolution of pain, doses ≥ 90 MME/d (in which case a pain specialist should be consulted), inadequate response, untoward adverse effects, and abuse and misuse.1,31,41 However, providers may also face the challenge of working with patients for whom the benefit of opioid therapy is uncertain but who do not have an absolute contraindication. Guidance on this matter may be found in a 2017 systematic review of studies on reducing or discontinuing long-term opioid therapy.42 Although evidence on the whole was low quality, it showed that tapering or discontinuing opioids may actually reduce pain and improve function and quality of life.
Continue to: When working with a patient to taper treatment
When working with a patient to taper treatment, consider using a multidisciplinary approach. Also, assess the patient’s pain level and perception of needs for opioids, make clear the substantial effort that will be asked of the patient, and agree on coping strategies the patient can use to manage the taper.31,43 While the evidence does not appear to support one tapering regimen over another, we can offer some recommendations on ways to individualize a tapering regimen (TABLE 5).1,31,41,43,44
General recommendations. Gradually reduce the original MME dose by 5% to 10% every week to every 4 weeks, with frequent follow-up and adjustments as needed based on the individual’s response.1,31,41,43 In the event that the patient does not tolerate this dose-reduction schedule, tapering can be slowed further.31 Avoid abrupt discontinuation.33 Opioid abstinence syndrome, a myriad of symptoms caused by deprivation of opioids in physiologically dependent individuals, although rare, can occur during tapering and can be managed with clonidine 0.1 to 0.2 mg orally every 6 hours or transdermal clonidine patch 0.1 mg/24 hours weekly during the taper.31
Tapering of long-term opioid treatment is not without risk. Immediate risks include withdrawal syndrome, hyperalgesia, and dropout, while ongoing issues are potential relapse, problems in increasing and maintaining function, and medicolegal implications.43 Withdrawal symptoms begin 2 to 3 half-lives after the last dose of opioid, and resolution varies depending on the duration of use, the most recent dose, and speed of tapering.43 In general, a patient needs 20% to 25% of the previous day’s dose to prevent withdrawal symptoms.31 Increased pain appears to be a brief, time-limited occurance.43 Dropout and relapse tend to be attributed to patient factors such as depressive symptoms and higher pain scores at initiation of the taper.43 Low pain at the end of tapering has been shown to predict long-term abstinence from opioids.43
CASE
Two months into his oxycodone regimen, Mr. G reported improved functional status at his catering job and overall improved quality of life. He had improved his lifting form and was attending biweekly physical therapy sessions. His pain score was 3/10. He expressed a desire to “not get hooked on opioids,” and mentioned he had “tried stopping the medicine last week” but experienced withdrawal symptoms. We discussed and prescribed the following 5-week taper plan: 2.5 mg reduction of oxycodone per dose, every 2 weeks x 2. Then 2.5 mg PO every 6 hours as needed x 1 week before stopping.
Organizing your approach
To optimize the chance for success in opioid treatment and to heighten vigilance and minimize harm to patients, we believe an organized approach is key (TABLE 614,22-24,28,30-32), particularly since this class of medication lacks strong evidence to support its long-term use.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected].
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
1. CDC. Opioid overdose. www.cdc.gov/drugoverdose/opioids/prescribed.html. Accessed June 26, 2020.
2. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2017. www.deadiversion.usdoj.gov/fed_regs/quotas/2016/fr1005.htm. Accessed June 26, 2020.
3. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2018. www.deadiversion.usdoj.gov/fed_regs/quotas/2017/fr1108.htm. Accessed June 26, 2020.
4. DEA, Department of Justice. Established aggregate production quotas for schedule I and II controlled substances and assessment of annual needs for the list I chemicals ephedrine, pseudoephedrine, and phenylpropanolamine for 2020. www.deadiversion.usdoj.gov/fed_regs/quotas/2019/fr1202.htm. Accessed June 26, 2020.
5. US Department of Veterans Affairs. Pharmacy benefits management services: academic detailing service—opioid overdose education & naloxone distribution (OEND). www.pbm.va.gov/AcademicDetailingService/Opioid_Overdose_Education_and_Naloxone_Distribution.asp. Accessed June 26, 2020.
6. McCarberg BH. Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med. 2011;123:119-130.
7. Dean L. Tramadol therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Rubinstein W, et al (eds). Medical Genetics Summaries [Internet]. Bethesda, Md: National Center for Biotechnology Information (US); 2015. www.ncbi.nlm.nih.gov/books/NBK315950/. Accessed June 26, 2020.
8. Chen JH, Humphreys K, Shah NH, et al. Distribution of opioids by different types of Medicare prescribers. JAMA Intern Med. 2016;176:259-261.
9. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. 3rd ed. New York, NY: Guilford Press; 2011;19-41.
10. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798-804.
11. Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379-384.
12. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129-138.
13. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35-42.
14. Dansie EJ, Turk DC. Assessment of patients with chronic pain. Br J Anaesth. 2013;111:19-25.
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
16. Choi Y, Mayer TG, Williams MJ, Gatchel RJ. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014;14:1175-1182.
17. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;122:810-833.
18. Gallagher RM. Empathy: a timeless skill for the pain medicine toolbox. Pain Med. 2006;7:213-214.
19. Koyyalagunta D, Bruera E, Aigner C, et al. Risk stratification of opioid misuse among patients with cancer pain using the SOAPP-SF. Pain Med. 2013;14:667-675.
20. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) guidelines. Pain Physician. 2008;11:S5-S62.
21. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20:S111-S133.
22. Webster LR, Webster RM. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
23. Butler SF, Fernandez K, Benoit C, et al. Validation of the revised screener and opioid assessment for patients with pain (SOAPP-R). J Pain. 2008;9:360-372.
24. Belgrade MJ, Schamber CD, Lindgren BR. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 2006;7:671-681.
25. Fine PG, Finnegan T, Portenoy RK. Protect your patients, protect your practice: practical risk assessment in the structuring of opioid therapy in chronic pain. J Fam Pract. 2010;59(9 suppl 2):S1-16.
26. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6:107-112.
27. Manubay JM, Muchow C, Sullivan MA. Prescription drug abuse: epidemiology, regulatory issues, chronic pain management with narcotic analgesics. Prim Care. 2011;38:71-90.
28. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
29. Prescription Drug Monitoring Program Training and Technical Assistance Center. State PDMP profiles and contacts. www.pdmpassist.org/State. Accessed June 26, 2020.
30. Tobin DG, Andrews R, Becker WC. Prescribing opioids in primary care: Safely starting, monitoring, and stopping. Cleve Clin J Med. 2016;83:207-215.
31. Manchikanti L, Kaye AM, Knezevis NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: American Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3-S92.
32. HHS. Checklist for prescribing opioids for chronic pain. www.cdc.gov/drugoverdose/pdf/PDO_Checklist-a.pdf. Accessed June 26, 2020.
33. VA/DoD. VA/DoD clinical practice guideline for opioid therapy for chronic pain. www.healthquality.va.gov/guidelines/Pain/cot/VADoDOTCPG022717.pdf. Accessed June 26, 2020.
34. Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38-47.
35. Lexi-Comp Online. Hudson (OH): Wolters Kluwer Clinical Drug Information, Inc; 2018. https://online.lexi.com/lco/action/login. Accessed July 9, 2020.
36. CMS. Opioid oral morphine milligram equivalent (MME) conversion factors. www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed June 26, 2020.
37. Cupp M. Equianalgesic dosing of opioids for pain management. Pharmacist’s Letter/Prescriber’s Letter. 2018:340406. Stockton (CA): Therapeutic Research Center, LLC; 2018. www.nhms.org/sites/default/files/Pdfs/Opioid-Comparison-Chart-Prescriber-Letter-2012.pdf. Accessed June 26, 2020.
38. Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11:237-248.
39. Bronstein K, Passik S, Munitz L, et al. Can clinicians accurately predict which patients are misusing their medications? J Pain. 2011;12(suppl):P3.
40. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12:679-684.
41. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic non-cancer pain. CMAJ. 2017;189:E659-E666.
42. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191.
43. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic non-cancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2015;90:828-842.
44. Washington State Agency Medical Director’s Group. Interagency guideline on prescribing opioids for pain. June 2015. www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed June 26, 2020.
PRACTICE RECOMMENDATIONS
› Use a screening instrument such as the Opioid Risk Tool or the DIRE assessment to gauge a patient’s risk of opioid misuse and determine the frequency of monitoring. C
› Give as much priority to improving functional activity and minimizing adverse opioid effects as you do to relieving pain. C
› Prescribe an immediate-release, short-acting agent at first instead of a long-acting formulation; start with the lowest effective dosage and calculate total daily dose in terms of morphine milligram equivalents (MME). C
› Reduce the original MME dose by 5% to 10% every week when discontinuing an opioid. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Revisiting Xanax amid the coronavirus crisis
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
One of the more alarming trends that has emerged during the coronavirus crisis is the concomitant rise in the use of benzodiazepines, such as Xanax. It has been reported that at-risk individuals began seeking prescription anxiolytics as early as mid-February with a consequent peak of 34% the following month, coinciding with the World Health Organization’s declaration of a global pandemic.1
Consistent with the available literature indicating that women are twice as likely to be affected by anxiety disorders, the prescription spikes were almost double when compared with those of their male counterparts.2 The pandemic has instilled a sense of fear in people, leading to social repercussions, such as estrangement, insomnia, and paranoia for at-risk populations.3,4
“Benzos” are commonly prescribed to help people sleep or to assist them in overcoming a host of anxiety disorders. The rapid onset of effects make Xanax a desirable and efficacious benzodiazepine.5 The use of these medications might not be an immediate cause for concern because patients might be taking it as intended. Nevertheless, clinicians are shying away from medical management in favor of counseling or therapy.
Dangerous trends
Numerous factors might contribute to this grim scenario, including patient dependence on benzodiazepines, paranoia about engaging with health care professionals because of fear tied to potential COVID-19 exposure, and/or increased access to illicit counterfeit pills from drug dealers or the dark web markets.
Lessons can be gleaned from the most extensive dark web drug busts in Britain’s history, in which a deluge of “pharmaceutical grade” Xanax pills made it to the hands of drug dealers and consumers between 2015 and 2017.6 A similar phenomenon emerged stateside.7 Virtually indistinguishable from recognized 2-mg Xanax pills, these fake pills posed a serious challenge to forensic scientists.8 The threat of overdose is very real for users targeted by the counterfeit Xanax trade, especially since those at risk often bypass professional health care guidelines.
In broad daylight, the drug dealers ran their operations revolving around two fake Xanax products: a primary knockoff and a limited edition – and vastly more potent “Red Devil” variant that was intentionally dyed for branding purposes. Because the “Red Devil” formulations contained 2.5 times the dose of the 2-mg pill, it had even more pronounced tolerance, dependence, and withdrawal effects (for example, panic attacks, anxiety, and/or hallucinations) – fatal consequences for users involved in consuming other drugs, such as alcohol or opioids. Preexisting drug users tend to gravitate toward benzodiazepines, such as alprazolam (Xanax), perhaps in part, because of its relatively rapid onset of action. Xanax also is known for inducing proeuphoric states at higher doses, hence the appeal of the “Red Devil” pills.
Benzodiazepines, as a class of drugs, facilitate the neurotransmitter gamma-aminobutryric acid’s (GABA) effect on the brain, producing anxiolytic, hypnotic, and/or anticonvulsant states within the user.9 Unbeknownst to numerous users is the fact that drugs such as alcohol and opioids, like Xanax, also serve as respiratory depressants, overriding the brain’s governance of the breathing mechanism. This, in turn, leads to unintended overdose deaths, even among seasoned drug seekers.
Overdose deaths have been steadily climbing over the years because it is common for some users to consume alcohol while being on Xanax therapy – without realizing that both substances are depressants and that taking them together can lead to side effects such as respiratory depression.
Forensic cases also have revealed that preexisting opioid consumers were drawn to Xanax; the drug’s potent mechanism of action would likely appeal to habituated users. A typical behavioral pattern has emerged among users and must be addressed. According to Australian Professor Shane Darke: “So they take their Xanax, they take their painkiller, then they get drunk, that could be enough to kill them.”
Fatalities are more likely when benzodiazepines are combined with other drug classes or if the existing supply is contaminated or laced (for example, with fentanyl).8
As far as deaths by accidental benzodiazepine overdose are concerned, a similar epidemic has been recorded in the United States. In 2013, almost one-third of all prescription overdose deaths can be attributed to the use of benzodiazepines (for example, Xanax, Valium, and Ativan). However, media attention has been considerably muted, especially when compared with that of narcotic abuse. This is even more puzzling when taking into account that three-quarters of benzodiazepine mortalities co-occur within the context of narcotic consumption. Substance Abuse and Mental Health Services Administration data confirm the ubiquitous nature of benzodiazepine (such as alprazolam) coprescriptions, accounting for roughly half of the 176,000 emergency department cases for 2011. The Centers for Disease Control and Prevention noted that there was a 67% increase in benzodiazepine prescriptions between 1996 and 2013, which warranted more stringent regulations for this particular class of drugs.
In 2016, the CDC issued new guidelines for opioid use acknowledging the danger of benzodiazepine coprescriptions. Food and Drug Administration “black box” warnings now grace the prescriptions of both of these drug classes.10 This trend remains on an upward trajectory, even more so during the pandemic, as there are 9.7 million prescriptions of anxiolytics/hypnotics such as Xanax, Ativan, and Klonopin in the United States as of March 2020, which represents a 10% increase over the previous year. , as well as the implementation of urine drug screening monitoring for drug adherence/compliance and diversion in those with suspected benzodiazepine addiction or a history of polysubstance abuse.11,12
Clinical correlates
For patients who present acutely with Xanax toxicity in the emergency room setting, we will need to initially stabilize the vital signs and address the ongoing symptoms. It is advisable to arrange health care accommodations for patients with physical dependence to monitor and treat their withdrawal symptoms. The patient should be enrolled in a comprehensive addiction facility after undergoing formal detoxification; a tapered treatment protocol will need to be implemented because quitting “cold turkey” can lead to convulsions and, in some cases, death. Patient education, talk therapy, and alternatives to benzodiazepines should be discussed with the clinician.13,145
However, to truly address the elephant in the room, we will need to consider institutional reforms to prevent a similar situation from arising in the future. Primary care physician shortages are compounded by changes in insurance policies. Nurses and physician assistants will need to be trained to manage benzodiazepine prescriptions. If there are community shortages in physicians, patients might turn to illegal means to secure their benzodiazepine supply, and it is imperative that we have the necessary fellowship and education programs to educate nonphysician health care clinicians with benzodiazepine management. Because physicians were prescribing benzodiazepines liberally, the Prescription Drug Monitoring Programs (PDMP) was enacted to monitor physician practices. Unfortunately, this ultimately intimidated physicians and effectively curbed reasonable physician prescribing patterns. It might be necessary to revisit existing prescription monitoring programs, encourage drug evaluations and guidelines based on evidence-based medicine and embrace telemedicine in order to facilitate patient-physician communication.
As of now, it is too early to prescribe Xanax routinely for ongoing anxiety experienced during the coronavirus crisis, and several physicians are cautious about prescribing antianxiety medications for more than a few months.17 Surprisingly, researchers in Barcelona have even explored the role of Xanax as potentially inhibiting Mpro, the primary protease of coronavirus, thereby forestalling the virus’s ability to replicate.16 However, it is worth noting that, given the preliminary nature of the results, any attempts at conclusively integrating Xanax within the context of coronavirus therapy would be premature.
References
1. Luhby T. Anti-anxiety medication prescriptions up 34% since coronavirus. CNN. 2020 Apr 16.
2. Women and Anxiety. Anxiety and Depression Association of America.
3. Shigemura J et al. Psychiatry Clin Neurosci. 2012 Apr 7;74(4):281-2.
4. Petersen A. More people are taking drugs for anxiety and insomnia, and doctors are worried. The Wall Street Journal. 2020 May 25.
5. Downey M. Xanax overdose and related deaths. National Drug & Alcohol Research Centre. UNSW Sydney.
6. Bryant B. Fake Xanax: The UK’s biggest ever dark net drugs bust. BBC. 2018 Mar 10.
7. Reinberg S. Fatal overdoses rising from sedatives like Valium, Xanax. HealthDay. 2016 Feb.
8. Is counterfeit Xanax dangerous? American Addiction Centers. Updated 2018 Nov 14.
9. McLaren E. Xanax history and statistics. Drugabuse.com.
10. Benzodiazepines and opioids. National Institute on Drug Abuse. 2018 Mar 15.
11. Choudhry Z et al. J Psychiatry. 2015;18(5). doi: 10.4172/2378-5756.1000319.
12. Islam FA et al. Current Psychiatry. 2018 Dec 17(12):43-4.
13. Adams M. Xanax death rate on the rise. White Sands Treatment. 2017 Sept.NEED LINK
14. Storrs C. Benzodiazepine overdose deaths soared in recent years, study finds. CNN. 2016 Feb. 18.
15. Hanscom DA. Plan A – Thrive and survive COVID-19. Back in Control. 2020.
16. Smith C. Xanax, a common anxiety medication, might actually block coronavirus. BGR. 2020 May 29.
Dr. Islam is a medical adviser for the International Maternal and Child Health Foundation (IMCHF), Montreal, and is based in New York. He also is a postdoctoral fellow, psychopharmacologist, and a board-certified medical affairs specialist. Dr. Islam disclosed no relevant financial relationships.
Mr. Choudhry is a research assistant at the IMCHF. He has no disclosures.
Dr. Choudhry is the chief scientific officer and head of the department of mental health and clinical research at the IMCHF and is Mr. Choudhry’s father. He has no disclosures.
As a black psychiatrist, she is ‘exhausted’ and ‘furious’
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
I didn’t have any doctors in my family. The only doctor I knew was my pediatrician. At 6 years old – and this gives you a glimpse into my personality – I told my parents I did not think he was a good doctor. I said, “When I grow up to be a doctor, I’m going to be a better doctor than him.” Fast forward to 7th grade, when I saw an orthopedic surgeon for my scoliosis. He was phenomenal. He listened. He explained to me all of the science and medicine and his rationale for decisions. I thought, “That is the kind of doctor I want to be.”
I went to medical school at Penn and didn’t think psychiatry was a medical specialty. I thought it was just Freud and laying on couches. I thought, “Where’s the science, where’s the physiology, where’s the genetics?” I was headed toward surgery.
Then, I rotated with an incredible psychiatrist. I saw behavior was biological, chemical, electrical, and physiological. I realize, looking back, that I had an interest because there is mental illness in my family. And there is so much stigma against psychiatric illnesses and addiction. It’s shocking how badly our patients get treated in the general medicine construct. So, I thought, “This field has science, the human body, activism, and marginalized patients? This is for me!”
I went to Howard University, which was the most freeing time of my life. There was no code-switching, no working hard to be a “presentable” Black person. When I started interviewing for medical schools, I was told by someone I interviewed with at one school that I should straighten my hair if I wanted to get accepted. I marked that school off my list. I decided right then that I would rather not go to medical school than straighten my hair to get into medical school. I went to Penn; they accepted me without my hair straight.
Penn Med was majorly White. There were six of us who were Black in a class of about 150 people. There was this feeling like “we let you in” even though every single one of us who was there was clearly at the top of the game to have been able to get there. I loved Penn Med. My class was amazing. I became the first Black president of medical student government there and I won a lot of awards.
When I was finishing up, my dean at the time, who was a White woman, said, “I’m so proud of you. You came in a piece of coal and look how we shined you up. “What do you say? I have a smart mouth, so I said, “I was already shiny when I got here.” She said, “See, that’s part of your problem, you don’t know how to take a compliment.” That was 2002, and I still remember every word of that conversation.
I was on the psychiatry unit rounding as a medical student and introduced myself to a patient. He said, “What’s your name?” And I thought, here it comes. I said, “Nzinga Ajabu,” my name at the time. He said “Nzinga? You probably have a spear in your closet.” When I tell these stories to White people, they’re always shocked. When I tell these stories to Black people, they say, “Yeah, that sounds about right.”
You can talk to Black medical students, Black interns, Black residents. When patients say something racist to you, nobody speaks up for you, nobody. It should be the attending that professionally approaches the patient and says something, anything. But they just laugh uncomfortably, they let it pass, they pretend they didn’t hear it. Meanwhile, you are fuming, and injured, and have to maintain your professionalism. It happens all the time. When people say, “Oh, you don’t look like a doctor,” I know what that means, but someone else may not even notice it’s an insult. When they do notice an insult, they don’t have the language or the courage to address it. And it’s not always a patient leveling racial insults. It very often is the attending, the fellow, the resident, or another medical student.
These things happen to me less now because I’m in a position of power. I’d say most insults that come my way now are overwhelmingly unintentional. I call people out on it 95% of the time. The other 5% of the time, I’m either exhausted, or I’m in some power structure where I decide it’s too risky. And those are the days – when I decide it’s too risky for me to speak up – when I come home exhausted. Because there will always be a power dynamic, as long as I’m alive, where you can’t speak up because you’re a Black woman, and that just wears me out.
Ultimately, I opted out of academic medicine because I thought it was too constraining, that I wouldn’t be able to raise my voice and do the activism I needed to do. – I’m able to advocate for people who are marginalized by medicine and, in treating addiction, advocate for people who are marginalized by psychiatry, which is marginalized by medicine.
A bias people have is that when you talk about Black people, they think you are talking about poor people. When we talk about police brutality, or being pulled over by the police, or dying in childbirth, our colleagues don’t think that’s happening to us. They think that’s happening to “those” Black people. Regardless of my socioeconomic status, I still have a higher chance of dying in childbirth or dying from COVID.
COVID had already turned my work up to 100 – we had staff losing loved ones and coming down with fevers themselves. And I had just launched my podcast. Then they killed Breonna Taylor, Ahmaud Arbery, Amy Cooper called the cops on Christian Cooper, and they killed George Floyd. This is how it happens. Bam. Bam. Bam.
The series of killings turned up my work at Physicians for Criminal Justice Reform, but it also turned up my work as a mother. My boys are 13 and 14. I personally can’t watch some of the videos because I see my own sons. I was already tired. Now I’m exhausted, I’m furious and I’m desperate to protect my kids. They have this on their backs already. Both of them have already had to deal with overt racism – they’ve had this burden since they were 5 years old, if not younger. I have to teach them to fight this war. Should that be how it is?
Nzinga Harrison, MD, 43, is a psychiatrist and the cofounder and chief medical officer of Eleanor Health, a network of physician clinics that treats people affected by addiction in North Carolina and New Jersey. She is also a cofounder of Physicians for Criminal Justice Reform. and host of the new podcast In Recovery. Harrison was raised in Indianapolis, went to college at Howard University and received her MD from the Perelman School of Medicine at the University of Pennsylvania in 2002. Her mother was an elementary school teacher. Her father, an electrical engineer, was commander of the local Black Panther Militia. Both supported her love of math and science and brought her with them to picket lines and marches.
This article first appeared on Medscape.com.
Medication-assisted treatment in corrections: A life-saving intervention
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Opioid overdose deaths in the United States have more than tripled in recent years, from 6.1 deaths per 100,000 individuals in 1999 to 20.7 per 100,000 individuals in 2018.1 Although the availability of medication-assisted treatment (MAT) has expanded over the past decade, this lifesaving treatment remains largely inaccessible to some of the most vulnerable members of our communities: opioid users facing reentry after incarceration.
Just as abstinence in the community brings a loss of tolerance to opioids, individuals who are incarcerated lose tolerance as well. Clinicians who treat patients with opioid use disorders (OUD) are accustomed to warning patients about the risk of returning to prior levels of use too quickly. Harm reduction strategies include using slowly, using with friends, and having naloxone on hand to prevent unintended overdose.
The risks of opioid use are magnified for those facing reentry; incarceration contributes to a loss of employment, social supports, and connection to care. Those changes can create an exceptionally stressful reentry period – one that places individuals at an acutely high risk of relapse and overdose. Within the first 2 years of release, an individual with a history of incarceration has a risk of death 3.5 times higher than that of someone in the general population. Within the first 2 weeks, those recently incarcerated are 129 times more likely to overdose on opioids and 12.7 times more likely to die than members of the general population.2
Treatment with MAT dramatically reduces deaths during this crucial period. In England, large national studies have shown a similar 75% decrease in all-cause mortality within the first 4 weeks of release among individuals with OUD.4 In California, the counties with the highest overdose death rates are consistently those with fewer opioid treatment programs, which suggests that access to treatment is necessary to prolong the lives of those suffering from OUD.5 In-custody overdose deaths are quite rare, and access to MAT during incarceration has decreased in-custody deaths by 74%.6
Decreased opioid overdose deaths is not the only outcome of MAT. Pharmacotherapy for OUD also has been shown to increase treatment retention,7 reduce reincarceration,8 prevent communicable infections,9 and decrease use of other illicit substances.10 The provision of MAT also has been shown to be cost effective.11
Despite those benefits, as of 2017, only 30 out of 5,100 jails and prisons in the United States provided treatment with methadone or buprenorphine.12 When individuals on maintenance therapy are incarcerated, most correctional facilities force them to taper and discontinue those medications. This practice can cause distressing withdrawal symptoms and actively increase the risk of death for these individuals.
Concerns related to the provision of MAT, and specifically buprenorphine, in the correctional health setting often are related to diversion. Although safe administration of opioid full and partial agonists is a priority, recent literature has suggested that buprenorphine is not a medication frequently used for euphoric properties. In fact, the literature suggests that individuals using illicit buprenorphine primarily do so to treat withdrawal symptoms and that illicit use diminishes with access to formal treatment.13,14
Another concern is that pharmacotherapy for OUD should not be used without adjunctive psychotherapies and social supports. While dual pharmacotherapy and psychotherapy is ideal, the American Society for Addiction Medicine 2020 National Practice Guidelines for the treatment of OUD state: “a patient’s decision to decline psychosocial treatment or the absence of available psychosocial treatment should not preclude or delay pharmacotherapy, with appropriate medication management.”15 Just as some patients wish to engage in mutual help or psychotherapeutic modalities only, some patients wish to engage only in psychopharmacologic interventions. Declaring one modality of treatment better, or worse, or more worthwhile is not borne out by the literature and often places clinicians’ preferences over the preferences of patients.
Individuals who suffer from substance use disorders are at high risk of incarceration, relapse, and overdose death. These patients also suffer from stigmatization from peers and health care workers alike, making the process of engaging in care incredibly burdensome. Because of the disease of addiction, many of our patients cannot envision a healthy future: a future with the potential for intimate relationships, meaningful community engagement, and a rich inner life. The provision of MAT is lifesaving and improves the chances of a successful reentry – an intuitive first step in a long, but worthwhile, journey.
References
1. Hedegaard H et al; National Center for Health Statistics. Drug overdose deaths in the United States, 1999–2018. NCHS Data Brief, 2020 Jan, No. 356.
2. Binswanger IA et al. N Engl J Med. 2007;356:157-65.
3. Green TC et al. JAMA Psychiatry. 2018;75(4):405-7.
4. Marsden J et al. Addiction. 2017;112(8):1408-18.
5. Joshi V and Urada D. State Targeted Response to the Opioid Crisis: California Strategic Plan. 2017 Aug 30.
6. Larney S et al. BMJ Open. 2014. doi: 10.1136/bmjopen-2013-004666.
7. Rich JD et al. Lancet. 2015;386(9991):350-9.
8. Deck D et al. J Addict Dis. 2009. 28(2):89-102.
9. MacArthur GJ et al. BMJ. 2012. doi: 10.1136/bmj.e5945.
10. Tsui J et al. J Subst Abuse Treat. 2019. 109:80-5.
11. Gisev N et al. Addiction. 2015 Dec;110(12):1975-84.
12. National Mental Health and Substance Use Policy Laboratory. “Use of Medication-Assisted Treatment for Opioid Use Disorder in Criminal Justice Settings.” HHS Publication No. PEP19-MATUSECJS. Rockville, Md.: Substance Abuse and Mental Health Services Administration, 2019.
13. Bazazi AR et al. J Addict Med. 2011;5(3):175-80.
14. Schuman-Olivier Z. et al. J Subst Abuse Treat. 2010 Jul;39(1):41-50.
15. Crotty K et al. J Addict Med. 2020;14(2)99-112.
Dr. Barnes is chief resident at San Mateo County Behavioral Health and Recovery Services in California. He disclosed no relevant financial relationships. Dr. Lenane is resident* at San Mateo County Behavioral Health and Recovery Services. He disclosed no relevant financial relationships. The opinions shared in this article represent the viewpoints of the authors and are not necessarily representative of the viewpoints or policies of their academic program or employer.
*This article was updated 7/9/2020.
Could being active reduce cancer death risk from alcohol?
Moderate drinking not a problem
concludes a new observational study involving 50,000-plus British adults.
Being physically active – for example, by walking, house cleaning, or playing a sport – could be promoted as a risk-minimization measure for alcohol-related cancers, say the authors, led by Emmanuel Stamatakis, PhD, professor of Physical Activity, Lifestyle, and Population Health, University of Sydney, Australia.
The researchers found a “strong direct association between alcohol consumption and mortality risk of [10] alcohol-related cancers.”
Specifically, when compared with never drinkers, there was a significantly higher risk of dying from such cancers among drinkers who consumed “hazardous” and “harmful” amounts of alcohol, and also for ex-drinkers.
Notably, occasional drinkers and drinkers within guidelines did not have statistically significantly higher risks for alcohol-related cancer mortality.
But the analysis also found that among the bigger drinkers, the risks were “substantially attenuated” in physically active participants who met at least the lower recommended limit of activity (>7.5 metabolic equivalent task [MET]–hours/week).
That’s not a taxing amount of activity because, for example, general household cleaning results in 3 METs/hour and walking slowly translates into 2 METs/hour. However, nearly a quarter of survey participants reported no physical activity.
The study was published online May 14 in the International Journal of Cancer.
The new results require confirmation because the findings “are limited in their statistical power,” with small numbers of cases in several categories, said Alpa Patel, PhD, an epidemiologist at the American Cancer Society, who was not involved in the study. For example, there were only 55 alcohol-related cancer deaths among the 1540 harmful drinkers.
Patel stressed that, “based on the collective evidence to date, it is best to both avoid alcohol consumption and engage in sufficient amounts of physical activity.” That amount is 150-300 minutes of moderate or 75-150 minutes of vigorous activity per week for cancer prevention.
Her message about abstinence is in-line with new ACS guidelines issued last month, as reported by Medscape Medical News. The ACS’s guidance was criticized by many readers in the comments section, who repeatedly encouraged “moderation.”
However, the ACS also recommended moderation, saying, for those adults who do drink, intake should be no more than 1 drink/day for women or 2 drinks/day for men.
Study author Dr. Stamatakis commented on the alcohol debate.
“Any advice for complete abstinence is bound to alienate many people,” he told Medscape Medical News in an email. “Alcohol drinking has been an integral part of many societies for thousands of years.”
Dr. Stamatakis, who is an occasional beer drinker, also said, “there is no healthy level of alcohol drinking.”
This was also the conclusion of a 2018 study published in the Lancet, which stated that there is “no safe limit,” as even one drink a day increases the risk of cancer. A few years earlier, the 2014 World Cancer Report found a dose-response relationship between alcohol consumption and certain cancers.
However, epidemiological findings are not necessarily “clinically relevant,” commented Jennifer Ligibel, MD, a medical oncologist at the Dana-Farber Cancer Institute, Boston, Massachusetts, in a 2018 interview with Medscape Medical News.
Dr. Ligibel explained that there are 50 years of studies linking alcohol and cancers. “With the huge amount of data we have, even small differences [in consumption] are statistically significant.”
Dr. Ligibel cited an often-repeated statistic: for the average woman, there is a 12% lifetime risk of breast cancer. “If a woman consumes a drink a day, which is considered a low-level intake, that risk may become about 13% – which is statistically significant,” Dr. Ligibel explained.
But that risk increase is not clinically relevant, she added.
Mean 10 years of follow-up
The new study is the first to examine physical activity, drinking, and the 10 cancers that have been linked to alcohol consumption (oral cavity, throat, larynx, esophagus, liver, colorectal, stomach, breast, pancreas, and lung).
The authors used data from 10 British population-based health surveys from 1994-2008 and looked at adults aged 30 years and older. The mean follow-up period was 9.9 years.
Among 54,686 participants, there were 2039 alcohol-related site-specific cancer deaths.
Alcohol consumption categories were based on U.K. guidelines, with 1 unit equal to 8 grams (about 2 ounces) of pure alcohol. The categories were as follows: drinking within guidelines (<14 units/week for women, <21 units/week for men), hazardous level (14-35 units/week for women, 21-49 units/week for men), and harmful level (> 35 units/week for women, >49 units/week for men). The survey also queried participants about being ex-drinkers, occasional drinkers, and never drinkers.
Physical activity was assessed using self-reported accounts of the 4 weeks preceding the health survey and intensity of activity (light, moderate, or vigorous) was queried. Physical activity was categorized using the upper (15 MET-hours/week) and lower (the aforementioned <7 MET-hours/week) recommended limits.
The median age of participants was 51 years; 7.9% were never drinkers and 14.7% exceeded guideline amounts. For physical activity, 23% reported none. The median level of activity was 9 MET-hours/week.
The authors say that the “increased risks [among the harmful, hazardous, and ex-drinker categories] were eliminated” among the individuals who reported physical activity >7.5 MET-hours/week. That meant the hazard ratios for cancer mortality for each category were reduced to the point that they were no longer statistically significant.
For example, for all drinkers in the “hazardous” category, the risk of cancer-related mortality was significantly higher than for nondrinkers (with a hazard ratio of 1.39), but in the subgroup of these participants who were physically active at the lower recommended limit, the hazard ratio dropped to 1.21.
These “broad patterns of effect modification by physical activity persisted when the upper physical activity limit [15 MET-hours/week] was used,” write the authors.
The new study adds to the literature on cancer mortality and alcohol consumption. In another recent study, researchers looked at eight British cohorts and reported overall cancer mortality associated with alcohol consumption was eliminated among those meeting physical activity recommendations (Br J Sports Med. 2017;51:651-7). The new study added two more cohorts to this base of eight and only focused on cancers that have been linked to alcohol consumption. The earlier study included deaths from all types of cancer.
The refinement of focus in the current study is important, say Dr. Stamatakis and colleagues.
“This specificity adds biological plausibility and permits a more immediate translation of our findings into policy and practice,” they write.
Dr. Stamatakis practices what he advocates, but is not a teetotaler.
“I exercise (e.g., dynamic yoga, HIIT cardio workouts, run, cycle, lift weights) for 45-60 minutes a day and I walk 8,000-14,000 steps daily. That would categorize me perhaps in the top 3%-5% for my age/sex group. And I enjoy 1-2 cans of craft beer a couple of times a week,” he said in an email.
Dr. Stamatakis and Dr. Patel have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Moderate drinking not a problem
Moderate drinking not a problem
concludes a new observational study involving 50,000-plus British adults.
Being physically active – for example, by walking, house cleaning, or playing a sport – could be promoted as a risk-minimization measure for alcohol-related cancers, say the authors, led by Emmanuel Stamatakis, PhD, professor of Physical Activity, Lifestyle, and Population Health, University of Sydney, Australia.
The researchers found a “strong direct association between alcohol consumption and mortality risk of [10] alcohol-related cancers.”
Specifically, when compared with never drinkers, there was a significantly higher risk of dying from such cancers among drinkers who consumed “hazardous” and “harmful” amounts of alcohol, and also for ex-drinkers.
Notably, occasional drinkers and drinkers within guidelines did not have statistically significantly higher risks for alcohol-related cancer mortality.
But the analysis also found that among the bigger drinkers, the risks were “substantially attenuated” in physically active participants who met at least the lower recommended limit of activity (>7.5 metabolic equivalent task [MET]–hours/week).
That’s not a taxing amount of activity because, for example, general household cleaning results in 3 METs/hour and walking slowly translates into 2 METs/hour. However, nearly a quarter of survey participants reported no physical activity.
The study was published online May 14 in the International Journal of Cancer.
The new results require confirmation because the findings “are limited in their statistical power,” with small numbers of cases in several categories, said Alpa Patel, PhD, an epidemiologist at the American Cancer Society, who was not involved in the study. For example, there were only 55 alcohol-related cancer deaths among the 1540 harmful drinkers.
Patel stressed that, “based on the collective evidence to date, it is best to both avoid alcohol consumption and engage in sufficient amounts of physical activity.” That amount is 150-300 minutes of moderate or 75-150 minutes of vigorous activity per week for cancer prevention.
Her message about abstinence is in-line with new ACS guidelines issued last month, as reported by Medscape Medical News. The ACS’s guidance was criticized by many readers in the comments section, who repeatedly encouraged “moderation.”
However, the ACS also recommended moderation, saying, for those adults who do drink, intake should be no more than 1 drink/day for women or 2 drinks/day for men.
Study author Dr. Stamatakis commented on the alcohol debate.
“Any advice for complete abstinence is bound to alienate many people,” he told Medscape Medical News in an email. “Alcohol drinking has been an integral part of many societies for thousands of years.”
Dr. Stamatakis, who is an occasional beer drinker, also said, “there is no healthy level of alcohol drinking.”
This was also the conclusion of a 2018 study published in the Lancet, which stated that there is “no safe limit,” as even one drink a day increases the risk of cancer. A few years earlier, the 2014 World Cancer Report found a dose-response relationship between alcohol consumption and certain cancers.
However, epidemiological findings are not necessarily “clinically relevant,” commented Jennifer Ligibel, MD, a medical oncologist at the Dana-Farber Cancer Institute, Boston, Massachusetts, in a 2018 interview with Medscape Medical News.
Dr. Ligibel explained that there are 50 years of studies linking alcohol and cancers. “With the huge amount of data we have, even small differences [in consumption] are statistically significant.”
Dr. Ligibel cited an often-repeated statistic: for the average woman, there is a 12% lifetime risk of breast cancer. “If a woman consumes a drink a day, which is considered a low-level intake, that risk may become about 13% – which is statistically significant,” Dr. Ligibel explained.
But that risk increase is not clinically relevant, she added.
Mean 10 years of follow-up
The new study is the first to examine physical activity, drinking, and the 10 cancers that have been linked to alcohol consumption (oral cavity, throat, larynx, esophagus, liver, colorectal, stomach, breast, pancreas, and lung).
The authors used data from 10 British population-based health surveys from 1994-2008 and looked at adults aged 30 years and older. The mean follow-up period was 9.9 years.
Among 54,686 participants, there were 2039 alcohol-related site-specific cancer deaths.
Alcohol consumption categories were based on U.K. guidelines, with 1 unit equal to 8 grams (about 2 ounces) of pure alcohol. The categories were as follows: drinking within guidelines (<14 units/week for women, <21 units/week for men), hazardous level (14-35 units/week for women, 21-49 units/week for men), and harmful level (> 35 units/week for women, >49 units/week for men). The survey also queried participants about being ex-drinkers, occasional drinkers, and never drinkers.
Physical activity was assessed using self-reported accounts of the 4 weeks preceding the health survey and intensity of activity (light, moderate, or vigorous) was queried. Physical activity was categorized using the upper (15 MET-hours/week) and lower (the aforementioned <7 MET-hours/week) recommended limits.
The median age of participants was 51 years; 7.9% were never drinkers and 14.7% exceeded guideline amounts. For physical activity, 23% reported none. The median level of activity was 9 MET-hours/week.
The authors say that the “increased risks [among the harmful, hazardous, and ex-drinker categories] were eliminated” among the individuals who reported physical activity >7.5 MET-hours/week. That meant the hazard ratios for cancer mortality for each category were reduced to the point that they were no longer statistically significant.
For example, for all drinkers in the “hazardous” category, the risk of cancer-related mortality was significantly higher than for nondrinkers (with a hazard ratio of 1.39), but in the subgroup of these participants who were physically active at the lower recommended limit, the hazard ratio dropped to 1.21.
These “broad patterns of effect modification by physical activity persisted when the upper physical activity limit [15 MET-hours/week] was used,” write the authors.
The new study adds to the literature on cancer mortality and alcohol consumption. In another recent study, researchers looked at eight British cohorts and reported overall cancer mortality associated with alcohol consumption was eliminated among those meeting physical activity recommendations (Br J Sports Med. 2017;51:651-7). The new study added two more cohorts to this base of eight and only focused on cancers that have been linked to alcohol consumption. The earlier study included deaths from all types of cancer.
The refinement of focus in the current study is important, say Dr. Stamatakis and colleagues.
“This specificity adds biological plausibility and permits a more immediate translation of our findings into policy and practice,” they write.
Dr. Stamatakis practices what he advocates, but is not a teetotaler.
“I exercise (e.g., dynamic yoga, HIIT cardio workouts, run, cycle, lift weights) for 45-60 minutes a day and I walk 8,000-14,000 steps daily. That would categorize me perhaps in the top 3%-5% for my age/sex group. And I enjoy 1-2 cans of craft beer a couple of times a week,” he said in an email.
Dr. Stamatakis and Dr. Patel have reported no relevant financial relationships.
This article first appeared on Medscape.com.
concludes a new observational study involving 50,000-plus British adults.
Being physically active – for example, by walking, house cleaning, or playing a sport – could be promoted as a risk-minimization measure for alcohol-related cancers, say the authors, led by Emmanuel Stamatakis, PhD, professor of Physical Activity, Lifestyle, and Population Health, University of Sydney, Australia.
The researchers found a “strong direct association between alcohol consumption and mortality risk of [10] alcohol-related cancers.”
Specifically, when compared with never drinkers, there was a significantly higher risk of dying from such cancers among drinkers who consumed “hazardous” and “harmful” amounts of alcohol, and also for ex-drinkers.
Notably, occasional drinkers and drinkers within guidelines did not have statistically significantly higher risks for alcohol-related cancer mortality.
But the analysis also found that among the bigger drinkers, the risks were “substantially attenuated” in physically active participants who met at least the lower recommended limit of activity (>7.5 metabolic equivalent task [MET]–hours/week).
That’s not a taxing amount of activity because, for example, general household cleaning results in 3 METs/hour and walking slowly translates into 2 METs/hour. However, nearly a quarter of survey participants reported no physical activity.
The study was published online May 14 in the International Journal of Cancer.
The new results require confirmation because the findings “are limited in their statistical power,” with small numbers of cases in several categories, said Alpa Patel, PhD, an epidemiologist at the American Cancer Society, who was not involved in the study. For example, there were only 55 alcohol-related cancer deaths among the 1540 harmful drinkers.
Patel stressed that, “based on the collective evidence to date, it is best to both avoid alcohol consumption and engage in sufficient amounts of physical activity.” That amount is 150-300 minutes of moderate or 75-150 minutes of vigorous activity per week for cancer prevention.
Her message about abstinence is in-line with new ACS guidelines issued last month, as reported by Medscape Medical News. The ACS’s guidance was criticized by many readers in the comments section, who repeatedly encouraged “moderation.”
However, the ACS also recommended moderation, saying, for those adults who do drink, intake should be no more than 1 drink/day for women or 2 drinks/day for men.
Study author Dr. Stamatakis commented on the alcohol debate.
“Any advice for complete abstinence is bound to alienate many people,” he told Medscape Medical News in an email. “Alcohol drinking has been an integral part of many societies for thousands of years.”
Dr. Stamatakis, who is an occasional beer drinker, also said, “there is no healthy level of alcohol drinking.”
This was also the conclusion of a 2018 study published in the Lancet, which stated that there is “no safe limit,” as even one drink a day increases the risk of cancer. A few years earlier, the 2014 World Cancer Report found a dose-response relationship between alcohol consumption and certain cancers.
However, epidemiological findings are not necessarily “clinically relevant,” commented Jennifer Ligibel, MD, a medical oncologist at the Dana-Farber Cancer Institute, Boston, Massachusetts, in a 2018 interview with Medscape Medical News.
Dr. Ligibel explained that there are 50 years of studies linking alcohol and cancers. “With the huge amount of data we have, even small differences [in consumption] are statistically significant.”
Dr. Ligibel cited an often-repeated statistic: for the average woman, there is a 12% lifetime risk of breast cancer. “If a woman consumes a drink a day, which is considered a low-level intake, that risk may become about 13% – which is statistically significant,” Dr. Ligibel explained.
But that risk increase is not clinically relevant, she added.
Mean 10 years of follow-up
The new study is the first to examine physical activity, drinking, and the 10 cancers that have been linked to alcohol consumption (oral cavity, throat, larynx, esophagus, liver, colorectal, stomach, breast, pancreas, and lung).
The authors used data from 10 British population-based health surveys from 1994-2008 and looked at adults aged 30 years and older. The mean follow-up period was 9.9 years.
Among 54,686 participants, there were 2039 alcohol-related site-specific cancer deaths.
Alcohol consumption categories were based on U.K. guidelines, with 1 unit equal to 8 grams (about 2 ounces) of pure alcohol. The categories were as follows: drinking within guidelines (<14 units/week for women, <21 units/week for men), hazardous level (14-35 units/week for women, 21-49 units/week for men), and harmful level (> 35 units/week for women, >49 units/week for men). The survey also queried participants about being ex-drinkers, occasional drinkers, and never drinkers.
Physical activity was assessed using self-reported accounts of the 4 weeks preceding the health survey and intensity of activity (light, moderate, or vigorous) was queried. Physical activity was categorized using the upper (15 MET-hours/week) and lower (the aforementioned <7 MET-hours/week) recommended limits.
The median age of participants was 51 years; 7.9% were never drinkers and 14.7% exceeded guideline amounts. For physical activity, 23% reported none. The median level of activity was 9 MET-hours/week.
The authors say that the “increased risks [among the harmful, hazardous, and ex-drinker categories] were eliminated” among the individuals who reported physical activity >7.5 MET-hours/week. That meant the hazard ratios for cancer mortality for each category were reduced to the point that they were no longer statistically significant.
For example, for all drinkers in the “hazardous” category, the risk of cancer-related mortality was significantly higher than for nondrinkers (with a hazard ratio of 1.39), but in the subgroup of these participants who were physically active at the lower recommended limit, the hazard ratio dropped to 1.21.
These “broad patterns of effect modification by physical activity persisted when the upper physical activity limit [15 MET-hours/week] was used,” write the authors.
The new study adds to the literature on cancer mortality and alcohol consumption. In another recent study, researchers looked at eight British cohorts and reported overall cancer mortality associated with alcohol consumption was eliminated among those meeting physical activity recommendations (Br J Sports Med. 2017;51:651-7). The new study added two more cohorts to this base of eight and only focused on cancers that have been linked to alcohol consumption. The earlier study included deaths from all types of cancer.
The refinement of focus in the current study is important, say Dr. Stamatakis and colleagues.
“This specificity adds biological plausibility and permits a more immediate translation of our findings into policy and practice,” they write.
Dr. Stamatakis practices what he advocates, but is not a teetotaler.
“I exercise (e.g., dynamic yoga, HIIT cardio workouts, run, cycle, lift weights) for 45-60 minutes a day and I walk 8,000-14,000 steps daily. That would categorize me perhaps in the top 3%-5% for my age/sex group. And I enjoy 1-2 cans of craft beer a couple of times a week,” he said in an email.
Dr. Stamatakis and Dr. Patel have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Meditations in an emergency: Talking through pandemic anxiety with a pioneer of mind-body medicine
Andrew N. Wilner, MD: Welcome to Medscape. I’m Dr Andrew Wilner. Today I have a special guest, Dr James Gordon, founder and executive director of the Center for Mind-Body Medicine. Welcome, Dr Gordon.
James S. Gordon, MD: Thank you very much. It’s good to be with you.
Dr. Wilner: Thanks for joining us. We are recording this in late May 2020, in the midst of the coronavirus pandemic. Millions of people have been infected. Hundreds of thousands have died. Millions have lost their jobs. I think it’s fair to say that people are under a greater degree of stress than they’re normally accustomed to. Would you agree with that?
Dr. Gordon: I think it’s more than fair to say that everybody in the United States, and actually pretty much everyone in the world, is under extreme stress. And that compounds any stresses that they’ve experienced before in their lives. Everyone is affected.
Dr. Wilner: The mind-body medicine concept is one that you’ve pursued for decades. Tell us a little bit about the Center for Mind-Body Medicine and how that’s led to the program that you have to help us deal with the coronavirus.
Dr. Gordon: I started the Center for Mind-Body Medicine about 30 years ago. I’d been a researcher at the National Institute of Mental Health for a number of years, in private practice, and a professor at Georgetown Medical School. But I wanted to really focus on how to change and enrich medicine by making self-care, self-awareness, and group support central to all healthcare.
Western medicine is enormously powerful in certain situations, such as physical trauma, high levels of infection, congenital anomalies. But we’re not so good at working with chronic physical or psychological problems. Those are much more complex.
We’ve been discovering that what is going to make the long-term difference in conditions like type 2 diabetes, pain syndromes, hypertension, depression, and anxiety are those approaches that we can learn to do for ourselves. These are changes we can make in how we deal with stress, eat, exercise, relate to other people, and whether we find meaning and purpose in our lives.
by wars, climate-related disasters, the opioid epidemic, chronic poverty, historical trauma. We do a lot of work with indigenous people here in North America. We’ve worked in a number of communities where school shootings have traumatized everyone.
What we’ve learned over these past 25 years, and what interested me professionally as well as personally over the past 50 years, is what we’re now bringing out on an even larger scale. The kind of approaches that we’ve developed, studied, and published research on are exactly what everyone needs to include and incorporate in their daily life, as well as in their medical and health care, from now on.
Dr. Wilner: Do you have a program that’s specifically for health care providers?
Dr. Gordon: Yes. The Center for Mind-Body Medicine is primarily an educational organization rather than a service organization. Since the beginning, I’ve been focused on training health professionals. My first passion was for training physicians – I’m a physician, so there’s a feeling of fellowship there – but also health care workers and mental health professionals of every kind.
We teach health professionals a whole system, a comprehensive program of techniques of self-awareness and self-care. We teach them so that they can practice on themselves and study the underlying science, so they can then teach what they’ve learned to the patients or clients they work with. They integrate it into what they’re already doing, regardless of their specialty. At times we also offer some of the same kinds of mind-body skills groups that are the fundamental part of our training as a stand-alone intervention. You can’t really teach other people how to take care of themselves unless you’re also doing it yourself. Otherwise, it’s just a theory.
Dr. Wilner: As a neurologist, I’m interested in the mind-body system. You are a psychiatrist and understand that it’s a lot more difficult to objectify certain things. What is stress? What is happiness? What is sadness? It’s very hard to measure. You can have scales, but it requires insight on the part of the individual. So I think it’s certainly an ambitious project.
Dr. Gordon: You’re absolutely right. It requires insight. And one of the shortcomings of our medical education is that it doesn’t encourage us to look inside ourselves enough. There’s so much focus on objectivity and on data, that we’ve lost some of the subjective art of medicine.
My experience with myself, as well as with the thousands of people we’ve trained here in the United States and around the world and the many hundreds of thousands with whom they’ve worked, is that all of us have a greater capacity to understand and help ourselves than we ordinarily think or than most of us learn about in our medical education.
This work is saying to people to take a little bit of time and relax a little in order to allow yourself to come into a meditative state. And I don’t mean anything fancy by that. Meditation is just being relaxed. Moment-to-moment awareness doesn’t have to do with any particular religion or spiritual practice. It’s part of all of them. If you can get into that state, then you can begin to say, “Oh, that’s what’s going on with me. That’s why my pain is worse.”
For example, you often wonder in people with peripheral neuropathy why it becomes worse or better at certain points. I would encourage neurologists and other physicians to ask your patients, “Why do you think it’s worse?” They may say, “I don’t know, doc; that’s why I’m here.” But I would ask them to take a couple of minutes to let me know. They could think it has something to do with the fact that they had a big fight with their wife that morning, they don’t want to go to work, or whatever it is. This is part of the lost art that we need to bring back into medicine for ourselves and especially for our patients.
Dr. Wilner: Can you give me an example of some of the exercises you’d do in a class?
Dr. Gordon: All of the exercises and our entire program that we teach at the Center for Mind-Body Medicine is in this new book of mine, “The Transformation: Discovering Wholeness and Healing After Trauma.” It’s really the distillation of not just the past 25 or 30 years, but really 50 years of work.
The techniques are all pretty simple and, as we say, evidence based. There is evidence that shows how they work on us physiologically, as well as psychologically. And they’re all pretty easy to teach to anyone.
Myself and about 60 or 70 of our faculty at the Center for Mind-Body Medicine are currently leading online groups. Then several hundred of the other people we’ve trained are also leading these groups. We’re still counting it up, but we probably have between 700 and 1,000 groups going around the world, led by our faculty and by people we’ve trained.
We teach a different technique every week in these online groups. Last week, after getting people energized and focused, we did a written dialogue with an emotion. You put down the initial of your name – in my case, “J” for Jim – and create a dialogue with an emotion, such as sadness. I would write it as fast as I can.
I would say, “OK, Sadness. Why are you here? What are you doing? I don’t enjoy having you around.” And Sadness writes back to me, “But you need me.” And J says, “What do you mean I need you?” And Sadness says, “Well, your brother died 7 weeks ago, didn’t he?” And I say, “Yes, he did.” And Sadness says, “Aren’t you sad?” I say, “Yes. I’m terribly sad and grieving all the time. But I wasn’t thinking about him at this moment.” And Sadness says, “But he’s there with you all the time and that sadness is in you.” And I say, “You mean it’s in me even here, now, as I’m talking with Andrew in this interview?” And Sadness says, “Yes. You can talk about your work. But in between the words, as you take a breath, don’t you feel it in your chest?” That’s the way the dialogue goes.
Dr. Wilner: What about specifically with the coronavirus? Fear is certainly an emotion. Nobody wants to get sick and die. Nobody wants to bring this disease home to their family. People are reluctant to even go outside and you can’t shake someone’s hand. Are there precedents for this?
Dr. Gordon: There are precedents, but only relatively small groups were affected before by, for example, severe acute respiratory syndrome or H1N1, at least in the United States. But we haven’t seen a global pandemic like this since 1918. None of us was around then – or I certainly wasn’t around. So for most everyone, not only has it not happened before, but we’ve never been so globally aware of everything that’s going on and how different groups are reacting.
I’ve been reading Daniel Defoe’s book, “A Journal of the Plague Year.” It’s really very interesting. It’s about the bubonic plague in 1665 London, although he wrote it in the 1720s. Some of the same things were going on then: the enormous fear, the isolation; rich people being able to escape, poor people having nowhere to go; conspiracy theories of one kind or another, about where the plague came from or blaming a group of people for it; magical thinking that it’s just going to go away. All of those things that happened several hundred years ago are going on now.
And we’re all simultaneously aware of all those things. There’s not only the fear, which should be universal because it’s a reasonable response to this situation, but also the terrible confusion about what to do. The President is saying one thing, governors something else; Anthony Fauci is saying something else, and Deborah Birx is saying something a little bit different. There’s this tremendous confusion that overlays the fear, and I think everybody is more or less feeling these things.
So yes, a dialogue with fear is a good thing to do because it can be clarifying. What we need here is a sense of, what is it that makes sense for me to do? What precautions should I take? What precautions shouldn’t I take?
I have a 17-year-old son who lives with his mom in California. He and I were on the phone the other day. He’s a basketball player and very serious about it. He said, “I don’t want to put my life on hold.” And my response was, “If you go outside too soon, your life may be on hold for a hell of a lot longer than if you stay inside because, if you get sick, it’s serious. But you also need to start looking at the evidence and asking yourself the right questions because I can’t be there all the time and neither can your mom.”
Everybody really needs to use these kinds of tools to help themselves. The tools we teach are extremely good at bringing us back into a state of psychological and physiological balance — slow, deep breathing being a very basic one. Because it’s only in that state that we’re going to be able to make the most intelligent decisions about what to do. It’s only in that state that we’re going to be able to really look our fear in the face and find out what we should be afraid of and what we shouldn’t be afraid of.
It’s a process that’s very much integrated. We’re talking now about how to deal with the emotions. But the first part of what we do in our groups and our online trainings and webinars is teach people to just take a few deep breaths. Just take a few deep breaths in through the nose, out through the mouth, with your belly soft and relaxed. You can keep breathing this way while talking. That’s the antidote to the fight-or-flight response. We all learn about fight-or-flight in first-year physiology. We need to deal with it. We need to bring ourselves into balance. That’s the way we’re going to make the wisest decisions for ourselves and be best able to help our patients.
Dr. Wilner: As you mentioned, part of modern culture is that we now have access to all of this information worldwide. There’s a continual stream of newsfeeds, people flipping on their phones, receiving constant updates, 24/7. That’s a new phenomenon. Does that steal from us the time we had before for just breathing and synthesizing data as opposed to just acquiring it all the time?
Dr. Gordon: You’re absolutely right. It does and it’s a challenge. It can’t steal from us unless we’re letting our emotional, psychological, and physiological pockets be picked!
What we need to do is to make it our priority to come into balance. I don’t watch news all day long – a little tiny bit in the morning and in the evening, just to get a sense of what’s happening. That’s enough. And I think everybody needs to take a step back, ask if this is really what they want to be doing, and to come into balance.
The other thing that’s really important is physical activity, especially during this time. In addition to using slow, deep breathing to come into balance, physical exercise and movement of any kind is extremely good as an antidote to fight-or-flight and that shut-down, freeze-up response that we get into when we feel completely overwhelmed.
We’ve got to take it into our own hands. The media just want to sell us things. Let’s face it: They’re not here for our good. Our job as physicians and health care professionals is to really reinforce for people not only what we can do for them but what they can do for themselves.
Dr. Wilner: I’m certainly interested in learning more about mind-body medicine. For those who feel the same, where do you recommend they go to learn more?
Dr. Gordon: We have a website, cmbm.org, which features a number of webinars. I do a free webinar there every week. We have mind-body skills groups that meet once a week for 8 weeks. There are six physicians in my group and all kinds of health professionals in other groups. We have a training program that we’re bringing online. We’ve trained well over 6,000 people around the world and would love to train more. You can read about that on the website.
We’re starting to do more and more consulting with health care organizations. We’re working with the largest division of Veterans Affairs, which is in Florida, as well as in south Georgia and the Caribbean. We’re working with a large health system in Indiana and others elsewhere. In addition, we’re working with groups of physicians and mental health professionals, helping them to integrate what we have to offer into what they’re already doing.
That’s our job – to help you do your job.
Dr. Wilner: Dr Gordon, I feel more relaxed just speaking with you. Thank you for talking with me and sharing your experiences with Medscape. I look forward to learning more.
Dr. Gordon: Thank you. My pleasure.
A version of this article originally appeared on Medscape.com.
Andrew N. Wilner, MD: Welcome to Medscape. I’m Dr Andrew Wilner. Today I have a special guest, Dr James Gordon, founder and executive director of the Center for Mind-Body Medicine. Welcome, Dr Gordon.
James S. Gordon, MD: Thank you very much. It’s good to be with you.
Dr. Wilner: Thanks for joining us. We are recording this in late May 2020, in the midst of the coronavirus pandemic. Millions of people have been infected. Hundreds of thousands have died. Millions have lost their jobs. I think it’s fair to say that people are under a greater degree of stress than they’re normally accustomed to. Would you agree with that?
Dr. Gordon: I think it’s more than fair to say that everybody in the United States, and actually pretty much everyone in the world, is under extreme stress. And that compounds any stresses that they’ve experienced before in their lives. Everyone is affected.
Dr. Wilner: The mind-body medicine concept is one that you’ve pursued for decades. Tell us a little bit about the Center for Mind-Body Medicine and how that’s led to the program that you have to help us deal with the coronavirus.
Dr. Gordon: I started the Center for Mind-Body Medicine about 30 years ago. I’d been a researcher at the National Institute of Mental Health for a number of years, in private practice, and a professor at Georgetown Medical School. But I wanted to really focus on how to change and enrich medicine by making self-care, self-awareness, and group support central to all healthcare.
Western medicine is enormously powerful in certain situations, such as physical trauma, high levels of infection, congenital anomalies. But we’re not so good at working with chronic physical or psychological problems. Those are much more complex.
We’ve been discovering that what is going to make the long-term difference in conditions like type 2 diabetes, pain syndromes, hypertension, depression, and anxiety are those approaches that we can learn to do for ourselves. These are changes we can make in how we deal with stress, eat, exercise, relate to other people, and whether we find meaning and purpose in our lives.
by wars, climate-related disasters, the opioid epidemic, chronic poverty, historical trauma. We do a lot of work with indigenous people here in North America. We’ve worked in a number of communities where school shootings have traumatized everyone.
What we’ve learned over these past 25 years, and what interested me professionally as well as personally over the past 50 years, is what we’re now bringing out on an even larger scale. The kind of approaches that we’ve developed, studied, and published research on are exactly what everyone needs to include and incorporate in their daily life, as well as in their medical and health care, from now on.
Dr. Wilner: Do you have a program that’s specifically for health care providers?
Dr. Gordon: Yes. The Center for Mind-Body Medicine is primarily an educational organization rather than a service organization. Since the beginning, I’ve been focused on training health professionals. My first passion was for training physicians – I’m a physician, so there’s a feeling of fellowship there – but also health care workers and mental health professionals of every kind.
We teach health professionals a whole system, a comprehensive program of techniques of self-awareness and self-care. We teach them so that they can practice on themselves and study the underlying science, so they can then teach what they’ve learned to the patients or clients they work with. They integrate it into what they’re already doing, regardless of their specialty. At times we also offer some of the same kinds of mind-body skills groups that are the fundamental part of our training as a stand-alone intervention. You can’t really teach other people how to take care of themselves unless you’re also doing it yourself. Otherwise, it’s just a theory.
Dr. Wilner: As a neurologist, I’m interested in the mind-body system. You are a psychiatrist and understand that it’s a lot more difficult to objectify certain things. What is stress? What is happiness? What is sadness? It’s very hard to measure. You can have scales, but it requires insight on the part of the individual. So I think it’s certainly an ambitious project.
Dr. Gordon: You’re absolutely right. It requires insight. And one of the shortcomings of our medical education is that it doesn’t encourage us to look inside ourselves enough. There’s so much focus on objectivity and on data, that we’ve lost some of the subjective art of medicine.
My experience with myself, as well as with the thousands of people we’ve trained here in the United States and around the world and the many hundreds of thousands with whom they’ve worked, is that all of us have a greater capacity to understand and help ourselves than we ordinarily think or than most of us learn about in our medical education.
This work is saying to people to take a little bit of time and relax a little in order to allow yourself to come into a meditative state. And I don’t mean anything fancy by that. Meditation is just being relaxed. Moment-to-moment awareness doesn’t have to do with any particular religion or spiritual practice. It’s part of all of them. If you can get into that state, then you can begin to say, “Oh, that’s what’s going on with me. That’s why my pain is worse.”
For example, you often wonder in people with peripheral neuropathy why it becomes worse or better at certain points. I would encourage neurologists and other physicians to ask your patients, “Why do you think it’s worse?” They may say, “I don’t know, doc; that’s why I’m here.” But I would ask them to take a couple of minutes to let me know. They could think it has something to do with the fact that they had a big fight with their wife that morning, they don’t want to go to work, or whatever it is. This is part of the lost art that we need to bring back into medicine for ourselves and especially for our patients.
Dr. Wilner: Can you give me an example of some of the exercises you’d do in a class?
Dr. Gordon: All of the exercises and our entire program that we teach at the Center for Mind-Body Medicine is in this new book of mine, “The Transformation: Discovering Wholeness and Healing After Trauma.” It’s really the distillation of not just the past 25 or 30 years, but really 50 years of work.
The techniques are all pretty simple and, as we say, evidence based. There is evidence that shows how they work on us physiologically, as well as psychologically. And they’re all pretty easy to teach to anyone.
Myself and about 60 or 70 of our faculty at the Center for Mind-Body Medicine are currently leading online groups. Then several hundred of the other people we’ve trained are also leading these groups. We’re still counting it up, but we probably have between 700 and 1,000 groups going around the world, led by our faculty and by people we’ve trained.
We teach a different technique every week in these online groups. Last week, after getting people energized and focused, we did a written dialogue with an emotion. You put down the initial of your name – in my case, “J” for Jim – and create a dialogue with an emotion, such as sadness. I would write it as fast as I can.
I would say, “OK, Sadness. Why are you here? What are you doing? I don’t enjoy having you around.” And Sadness writes back to me, “But you need me.” And J says, “What do you mean I need you?” And Sadness says, “Well, your brother died 7 weeks ago, didn’t he?” And I say, “Yes, he did.” And Sadness says, “Aren’t you sad?” I say, “Yes. I’m terribly sad and grieving all the time. But I wasn’t thinking about him at this moment.” And Sadness says, “But he’s there with you all the time and that sadness is in you.” And I say, “You mean it’s in me even here, now, as I’m talking with Andrew in this interview?” And Sadness says, “Yes. You can talk about your work. But in between the words, as you take a breath, don’t you feel it in your chest?” That’s the way the dialogue goes.
Dr. Wilner: What about specifically with the coronavirus? Fear is certainly an emotion. Nobody wants to get sick and die. Nobody wants to bring this disease home to their family. People are reluctant to even go outside and you can’t shake someone’s hand. Are there precedents for this?
Dr. Gordon: There are precedents, but only relatively small groups were affected before by, for example, severe acute respiratory syndrome or H1N1, at least in the United States. But we haven’t seen a global pandemic like this since 1918. None of us was around then – or I certainly wasn’t around. So for most everyone, not only has it not happened before, but we’ve never been so globally aware of everything that’s going on and how different groups are reacting.
I’ve been reading Daniel Defoe’s book, “A Journal of the Plague Year.” It’s really very interesting. It’s about the bubonic plague in 1665 London, although he wrote it in the 1720s. Some of the same things were going on then: the enormous fear, the isolation; rich people being able to escape, poor people having nowhere to go; conspiracy theories of one kind or another, about where the plague came from or blaming a group of people for it; magical thinking that it’s just going to go away. All of those things that happened several hundred years ago are going on now.
And we’re all simultaneously aware of all those things. There’s not only the fear, which should be universal because it’s a reasonable response to this situation, but also the terrible confusion about what to do. The President is saying one thing, governors something else; Anthony Fauci is saying something else, and Deborah Birx is saying something a little bit different. There’s this tremendous confusion that overlays the fear, and I think everybody is more or less feeling these things.
So yes, a dialogue with fear is a good thing to do because it can be clarifying. What we need here is a sense of, what is it that makes sense for me to do? What precautions should I take? What precautions shouldn’t I take?
I have a 17-year-old son who lives with his mom in California. He and I were on the phone the other day. He’s a basketball player and very serious about it. He said, “I don’t want to put my life on hold.” And my response was, “If you go outside too soon, your life may be on hold for a hell of a lot longer than if you stay inside because, if you get sick, it’s serious. But you also need to start looking at the evidence and asking yourself the right questions because I can’t be there all the time and neither can your mom.”
Everybody really needs to use these kinds of tools to help themselves. The tools we teach are extremely good at bringing us back into a state of psychological and physiological balance — slow, deep breathing being a very basic one. Because it’s only in that state that we’re going to be able to make the most intelligent decisions about what to do. It’s only in that state that we’re going to be able to really look our fear in the face and find out what we should be afraid of and what we shouldn’t be afraid of.
It’s a process that’s very much integrated. We’re talking now about how to deal with the emotions. But the first part of what we do in our groups and our online trainings and webinars is teach people to just take a few deep breaths. Just take a few deep breaths in through the nose, out through the mouth, with your belly soft and relaxed. You can keep breathing this way while talking. That’s the antidote to the fight-or-flight response. We all learn about fight-or-flight in first-year physiology. We need to deal with it. We need to bring ourselves into balance. That’s the way we’re going to make the wisest decisions for ourselves and be best able to help our patients.
Dr. Wilner: As you mentioned, part of modern culture is that we now have access to all of this information worldwide. There’s a continual stream of newsfeeds, people flipping on their phones, receiving constant updates, 24/7. That’s a new phenomenon. Does that steal from us the time we had before for just breathing and synthesizing data as opposed to just acquiring it all the time?
Dr. Gordon: You’re absolutely right. It does and it’s a challenge. It can’t steal from us unless we’re letting our emotional, psychological, and physiological pockets be picked!
What we need to do is to make it our priority to come into balance. I don’t watch news all day long – a little tiny bit in the morning and in the evening, just to get a sense of what’s happening. That’s enough. And I think everybody needs to take a step back, ask if this is really what they want to be doing, and to come into balance.
The other thing that’s really important is physical activity, especially during this time. In addition to using slow, deep breathing to come into balance, physical exercise and movement of any kind is extremely good as an antidote to fight-or-flight and that shut-down, freeze-up response that we get into when we feel completely overwhelmed.
We’ve got to take it into our own hands. The media just want to sell us things. Let’s face it: They’re not here for our good. Our job as physicians and health care professionals is to really reinforce for people not only what we can do for them but what they can do for themselves.
Dr. Wilner: I’m certainly interested in learning more about mind-body medicine. For those who feel the same, where do you recommend they go to learn more?
Dr. Gordon: We have a website, cmbm.org, which features a number of webinars. I do a free webinar there every week. We have mind-body skills groups that meet once a week for 8 weeks. There are six physicians in my group and all kinds of health professionals in other groups. We have a training program that we’re bringing online. We’ve trained well over 6,000 people around the world and would love to train more. You can read about that on the website.
We’re starting to do more and more consulting with health care organizations. We’re working with the largest division of Veterans Affairs, which is in Florida, as well as in south Georgia and the Caribbean. We’re working with a large health system in Indiana and others elsewhere. In addition, we’re working with groups of physicians and mental health professionals, helping them to integrate what we have to offer into what they’re already doing.
That’s our job – to help you do your job.
Dr. Wilner: Dr Gordon, I feel more relaxed just speaking with you. Thank you for talking with me and sharing your experiences with Medscape. I look forward to learning more.
Dr. Gordon: Thank you. My pleasure.
A version of this article originally appeared on Medscape.com.
Andrew N. Wilner, MD: Welcome to Medscape. I’m Dr Andrew Wilner. Today I have a special guest, Dr James Gordon, founder and executive director of the Center for Mind-Body Medicine. Welcome, Dr Gordon.
James S. Gordon, MD: Thank you very much. It’s good to be with you.
Dr. Wilner: Thanks for joining us. We are recording this in late May 2020, in the midst of the coronavirus pandemic. Millions of people have been infected. Hundreds of thousands have died. Millions have lost their jobs. I think it’s fair to say that people are under a greater degree of stress than they’re normally accustomed to. Would you agree with that?
Dr. Gordon: I think it’s more than fair to say that everybody in the United States, and actually pretty much everyone in the world, is under extreme stress. And that compounds any stresses that they’ve experienced before in their lives. Everyone is affected.
Dr. Wilner: The mind-body medicine concept is one that you’ve pursued for decades. Tell us a little bit about the Center for Mind-Body Medicine and how that’s led to the program that you have to help us deal with the coronavirus.
Dr. Gordon: I started the Center for Mind-Body Medicine about 30 years ago. I’d been a researcher at the National Institute of Mental Health for a number of years, in private practice, and a professor at Georgetown Medical School. But I wanted to really focus on how to change and enrich medicine by making self-care, self-awareness, and group support central to all healthcare.
Western medicine is enormously powerful in certain situations, such as physical trauma, high levels of infection, congenital anomalies. But we’re not so good at working with chronic physical or psychological problems. Those are much more complex.
We’ve been discovering that what is going to make the long-term difference in conditions like type 2 diabetes, pain syndromes, hypertension, depression, and anxiety are those approaches that we can learn to do for ourselves. These are changes we can make in how we deal with stress, eat, exercise, relate to other people, and whether we find meaning and purpose in our lives.
by wars, climate-related disasters, the opioid epidemic, chronic poverty, historical trauma. We do a lot of work with indigenous people here in North America. We’ve worked in a number of communities where school shootings have traumatized everyone.
What we’ve learned over these past 25 years, and what interested me professionally as well as personally over the past 50 years, is what we’re now bringing out on an even larger scale. The kind of approaches that we’ve developed, studied, and published research on are exactly what everyone needs to include and incorporate in their daily life, as well as in their medical and health care, from now on.
Dr. Wilner: Do you have a program that’s specifically for health care providers?
Dr. Gordon: Yes. The Center for Mind-Body Medicine is primarily an educational organization rather than a service organization. Since the beginning, I’ve been focused on training health professionals. My first passion was for training physicians – I’m a physician, so there’s a feeling of fellowship there – but also health care workers and mental health professionals of every kind.
We teach health professionals a whole system, a comprehensive program of techniques of self-awareness and self-care. We teach them so that they can practice on themselves and study the underlying science, so they can then teach what they’ve learned to the patients or clients they work with. They integrate it into what they’re already doing, regardless of their specialty. At times we also offer some of the same kinds of mind-body skills groups that are the fundamental part of our training as a stand-alone intervention. You can’t really teach other people how to take care of themselves unless you’re also doing it yourself. Otherwise, it’s just a theory.
Dr. Wilner: As a neurologist, I’m interested in the mind-body system. You are a psychiatrist and understand that it’s a lot more difficult to objectify certain things. What is stress? What is happiness? What is sadness? It’s very hard to measure. You can have scales, but it requires insight on the part of the individual. So I think it’s certainly an ambitious project.
Dr. Gordon: You’re absolutely right. It requires insight. And one of the shortcomings of our medical education is that it doesn’t encourage us to look inside ourselves enough. There’s so much focus on objectivity and on data, that we’ve lost some of the subjective art of medicine.
My experience with myself, as well as with the thousands of people we’ve trained here in the United States and around the world and the many hundreds of thousands with whom they’ve worked, is that all of us have a greater capacity to understand and help ourselves than we ordinarily think or than most of us learn about in our medical education.
This work is saying to people to take a little bit of time and relax a little in order to allow yourself to come into a meditative state. And I don’t mean anything fancy by that. Meditation is just being relaxed. Moment-to-moment awareness doesn’t have to do with any particular religion or spiritual practice. It’s part of all of them. If you can get into that state, then you can begin to say, “Oh, that’s what’s going on with me. That’s why my pain is worse.”
For example, you often wonder in people with peripheral neuropathy why it becomes worse or better at certain points. I would encourage neurologists and other physicians to ask your patients, “Why do you think it’s worse?” They may say, “I don’t know, doc; that’s why I’m here.” But I would ask them to take a couple of minutes to let me know. They could think it has something to do with the fact that they had a big fight with their wife that morning, they don’t want to go to work, or whatever it is. This is part of the lost art that we need to bring back into medicine for ourselves and especially for our patients.
Dr. Wilner: Can you give me an example of some of the exercises you’d do in a class?
Dr. Gordon: All of the exercises and our entire program that we teach at the Center for Mind-Body Medicine is in this new book of mine, “The Transformation: Discovering Wholeness and Healing After Trauma.” It’s really the distillation of not just the past 25 or 30 years, but really 50 years of work.
The techniques are all pretty simple and, as we say, evidence based. There is evidence that shows how they work on us physiologically, as well as psychologically. And they’re all pretty easy to teach to anyone.
Myself and about 60 or 70 of our faculty at the Center for Mind-Body Medicine are currently leading online groups. Then several hundred of the other people we’ve trained are also leading these groups. We’re still counting it up, but we probably have between 700 and 1,000 groups going around the world, led by our faculty and by people we’ve trained.
We teach a different technique every week in these online groups. Last week, after getting people energized and focused, we did a written dialogue with an emotion. You put down the initial of your name – in my case, “J” for Jim – and create a dialogue with an emotion, such as sadness. I would write it as fast as I can.
I would say, “OK, Sadness. Why are you here? What are you doing? I don’t enjoy having you around.” And Sadness writes back to me, “But you need me.” And J says, “What do you mean I need you?” And Sadness says, “Well, your brother died 7 weeks ago, didn’t he?” And I say, “Yes, he did.” And Sadness says, “Aren’t you sad?” I say, “Yes. I’m terribly sad and grieving all the time. But I wasn’t thinking about him at this moment.” And Sadness says, “But he’s there with you all the time and that sadness is in you.” And I say, “You mean it’s in me even here, now, as I’m talking with Andrew in this interview?” And Sadness says, “Yes. You can talk about your work. But in between the words, as you take a breath, don’t you feel it in your chest?” That’s the way the dialogue goes.
Dr. Wilner: What about specifically with the coronavirus? Fear is certainly an emotion. Nobody wants to get sick and die. Nobody wants to bring this disease home to their family. People are reluctant to even go outside and you can’t shake someone’s hand. Are there precedents for this?
Dr. Gordon: There are precedents, but only relatively small groups were affected before by, for example, severe acute respiratory syndrome or H1N1, at least in the United States. But we haven’t seen a global pandemic like this since 1918. None of us was around then – or I certainly wasn’t around. So for most everyone, not only has it not happened before, but we’ve never been so globally aware of everything that’s going on and how different groups are reacting.
I’ve been reading Daniel Defoe’s book, “A Journal of the Plague Year.” It’s really very interesting. It’s about the bubonic plague in 1665 London, although he wrote it in the 1720s. Some of the same things were going on then: the enormous fear, the isolation; rich people being able to escape, poor people having nowhere to go; conspiracy theories of one kind or another, about where the plague came from or blaming a group of people for it; magical thinking that it’s just going to go away. All of those things that happened several hundred years ago are going on now.
And we’re all simultaneously aware of all those things. There’s not only the fear, which should be universal because it’s a reasonable response to this situation, but also the terrible confusion about what to do. The President is saying one thing, governors something else; Anthony Fauci is saying something else, and Deborah Birx is saying something a little bit different. There’s this tremendous confusion that overlays the fear, and I think everybody is more or less feeling these things.
So yes, a dialogue with fear is a good thing to do because it can be clarifying. What we need here is a sense of, what is it that makes sense for me to do? What precautions should I take? What precautions shouldn’t I take?
I have a 17-year-old son who lives with his mom in California. He and I were on the phone the other day. He’s a basketball player and very serious about it. He said, “I don’t want to put my life on hold.” And my response was, “If you go outside too soon, your life may be on hold for a hell of a lot longer than if you stay inside because, if you get sick, it’s serious. But you also need to start looking at the evidence and asking yourself the right questions because I can’t be there all the time and neither can your mom.”
Everybody really needs to use these kinds of tools to help themselves. The tools we teach are extremely good at bringing us back into a state of psychological and physiological balance — slow, deep breathing being a very basic one. Because it’s only in that state that we’re going to be able to make the most intelligent decisions about what to do. It’s only in that state that we’re going to be able to really look our fear in the face and find out what we should be afraid of and what we shouldn’t be afraid of.
It’s a process that’s very much integrated. We’re talking now about how to deal with the emotions. But the first part of what we do in our groups and our online trainings and webinars is teach people to just take a few deep breaths. Just take a few deep breaths in through the nose, out through the mouth, with your belly soft and relaxed. You can keep breathing this way while talking. That’s the antidote to the fight-or-flight response. We all learn about fight-or-flight in first-year physiology. We need to deal with it. We need to bring ourselves into balance. That’s the way we’re going to make the wisest decisions for ourselves and be best able to help our patients.
Dr. Wilner: As you mentioned, part of modern culture is that we now have access to all of this information worldwide. There’s a continual stream of newsfeeds, people flipping on their phones, receiving constant updates, 24/7. That’s a new phenomenon. Does that steal from us the time we had before for just breathing and synthesizing data as opposed to just acquiring it all the time?
Dr. Gordon: You’re absolutely right. It does and it’s a challenge. It can’t steal from us unless we’re letting our emotional, psychological, and physiological pockets be picked!
What we need to do is to make it our priority to come into balance. I don’t watch news all day long – a little tiny bit in the morning and in the evening, just to get a sense of what’s happening. That’s enough. And I think everybody needs to take a step back, ask if this is really what they want to be doing, and to come into balance.
The other thing that’s really important is physical activity, especially during this time. In addition to using slow, deep breathing to come into balance, physical exercise and movement of any kind is extremely good as an antidote to fight-or-flight and that shut-down, freeze-up response that we get into when we feel completely overwhelmed.
We’ve got to take it into our own hands. The media just want to sell us things. Let’s face it: They’re not here for our good. Our job as physicians and health care professionals is to really reinforce for people not only what we can do for them but what they can do for themselves.
Dr. Wilner: I’m certainly interested in learning more about mind-body medicine. For those who feel the same, where do you recommend they go to learn more?
Dr. Gordon: We have a website, cmbm.org, which features a number of webinars. I do a free webinar there every week. We have mind-body skills groups that meet once a week for 8 weeks. There are six physicians in my group and all kinds of health professionals in other groups. We have a training program that we’re bringing online. We’ve trained well over 6,000 people around the world and would love to train more. You can read about that on the website.
We’re starting to do more and more consulting with health care organizations. We’re working with the largest division of Veterans Affairs, which is in Florida, as well as in south Georgia and the Caribbean. We’re working with a large health system in Indiana and others elsewhere. In addition, we’re working with groups of physicians and mental health professionals, helping them to integrate what we have to offer into what they’re already doing.
That’s our job – to help you do your job.
Dr. Wilner: Dr Gordon, I feel more relaxed just speaking with you. Thank you for talking with me and sharing your experiences with Medscape. I look forward to learning more.
Dr. Gordon: Thank you. My pleasure.
A version of this article originally appeared on Medscape.com.
Amid pandemic, Virginia hospital’s opioid overdoses up nearly 10-fold
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
Opioid overdoses have shot up by almost 10-fold at a Virginia ED since March, a new report finds. The report provides more evidence that the coronavirus pandemic is sparking a severe medical crisis among illicit drug users.
“Health care providers should closely monitor the number of overdoses coming into their hospitals and in the surrounding community during this time,” study lead author and postdoctoral research fellow Taylor Ochalek, PhD, said in an interview. “If they do notice an increasing trend of overdoses, they should spread awareness in the community to the general public, and offer resources and information for those that may be seeking help and/or may be at a high risk of overdosing.”
Dr. Ochalek presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence.
According to the report, opioid overdoses at the VCU Medical Center in Richmond, Va., grew from an average of six a month from February to December 2019 to 50, 57, and 63 in March, April, and May 2020. Of the 171 cases in the later time frame, the average age was 44 years, 72% were male, and 82% were African American.
“The steep increase in overdoses began primarily in March,” said Dr. Ochalek, of Virginia Commonwealth University in Richmond. “This timing coincides with the Virginia governor’s state of emergency declaration, stay-at-home order, and closure of nonessential businesses order.”
The researchers did not provide details about the types of opioids used, the patient outcomes, or whether the patients tested positive for COVID-19. It’s unclear whether the pandemic directly spawned a higher number of overdoses, but there are growing signs of a stark nationwide trend.
“Nationwide, federal and local officials are reporting alarming spikes in drug overdoses – a hidden epidemic within the coronavirus pandemic,” the Washington Post reported on July 1, pointing to increases in Kentucky, Virginia, and the Chicago area.
Meanwhile, the federal Overdose Detection Mapping Application Program, which tracks overdoses nationwide, issued 191% more “spike alerts” in January to April 2020 than in the same time period in 2019. However, the spike alerts began to increase in January, weeks before the pandemic began to take hold.
The findings are consistent with trends in Houston, where overdose calls were up 31% in the first 3 months of 2020, compared with 2019, said psychologist James Bray, PhD, of the University of Texas, San Antonio, in an interview. More recent data suggest that the numbers are rising even higher, said Dr. Bray, who works with Houston first responders and has analyzed data.
Dr. Bray said.
Another potential factor is the disruption in the illicit drug supply chain because of limits on crossings at the southern border, said ED physician Scott Weiner, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston. “As a result, opioids of extremely variable potency have infiltrated markets, and people using drugs may not be used to the new doses, especially if they are high-potency fentanyl analogues.”
Moving forward, Dr. Bray said, “people need continued access to treatment. Telehealth and other virtual services need to be provided so that people can continue to have access to treatment even during the pandemic.”
Dr. Weiner also emphasized the importance of treatment for patients who overdose on opioids. “In my previous work, we discovered that about 1 in 20 patients who are treated in an emergency department and survive would die within 1 year. That number will likely increase drastically during COVID,” he said. “When a patient presents after overdose, we must intervene aggressively with buprenorphine and other harm-reduction techniques to save these lives.”
The study was funded by the National Institutes of Health. Dr. Ochalek, Dr. Weiner, and Dr. Bray reported no relevant disclosures.
FROM CPDD 2020
High percentage of stimulant use found in opioid ED cases
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
Nearly 40% of hundreds of opioid abusers at several emergency departments tested positive for stimulants, and they were more likely to be white than other users, a new study finds. Reflecting national trends, patients in the Midwest and West Coast regions were more likely to show signs of stimulant use.
Stimulant/opioid users were also “younger, with unstable housing, mostly unemployed, and reported high rates of recent incarcerations,” said substance use researcher and study lead author Marek Chawarski, PhD, of Yale University, New Haven, Conn. “They also reported higher rates of injection drug use during 1 month prior to the study admission and had higher rates of HCV infection. And higher proportions of amphetamine-type stimulant (ATS)–positive patients presented in the emergency departments (EDs) for an injury or with drug overdose.”
Dr. Chawarski, who presented the study findings at the virtual annual meeting of the College on Problems of Drug Dependence, said in an interview that the study is the first to analyze stimulant use in ED patients with opioid use disorder.
The researchers analyzed data for the period 2017-2019 from EDs in Baltimore, New York, Cincinnati, and Seattle. Out of 396 patients, 150 (38%) were positive for amphetamine-type stimulants.
Patients in the Midwest and West Coast were more likely to test positive (38%).
In general, stimulant use is higher in the Midwest and West Coast, said epidemiologist Brandon Marshall, PhD, of Brown University, Providence, R.I., in an interview. “This is due to a number of supply-side, historical, and cultural reasons. New England, Appalachia, and large urban centers on the East Coast are the historical hot spots for opioid use, while states west of the Mississippi River have lower rates of opioid overdose, but a much higher prevalence of ATS use and stimulant-related morbidity and mortality.”
Those who showed signs of stimulant use were more likely to be white (69%) vs. the nonusers (46%), and were more likely to have unstable housing (67% vs. 49%).
Those who used stimulants also were more likely to be suffering from an overdose (23% vs. 13%) and to report injecting drugs in the last month (79% vs. 47%). More had unstable housing (67% vs. 49%, P < .05 for all comparisons).
Dr. Chawarski said there are many reasons why users might use more than one kind of drug. For example, they may take one drug to “alleviate problems created by the use of one substance with taking another substance and multiple other reasons,” he said. “Polysubstance use can exacerbate social and medical harms, including overdose risk. It can pose greater treatment challenges, both for the patients and treatment providers, and often is more difficult to overcome.”
Links between opioid and stimulant use are not new. Last year, a study of 2,244 opioid-related overdose deaths in Massachusetts from 2014 to 2015 found that 36% of patients also showed signs of stimulant use. “Persons older than 24 years, nonrural residents, those with comorbid mental illness, non-Hispanic black residents, and persons with recent homelessness were more likely than their counterparts to die with opioids and stimulants than opioids alone,” the researchers reported (Drug Alcohol Depend. 2019 Jul 1;200:59-63).
Dr. Marshall said the study findings are not surprising. However, he said, they do indicate “ongoing, intentional consumption of opioids. The trends and characteristics we are seeing here suggests a large population of persons who are intentionally using both stimulants and opioids, many of whom are also injecting.”
He added that the study sample is probably higher risk than the general population since they’re presenting to the emergency department, so the findings might not reflect the use of stimulants in the general opioid-misusing population.
Dr. Marshall added that “there have been several instances in modern U.S. history during which increases in stimulant use follow a rise in opioid use, so the pattern we are seeing isn’t entirely surprising.”
“What we don’t know,” he added, “is the extent to which overdoses involving both an opioid and a stimulant are due to fentanyl contamination of the methamphetamine supply or intentional concurrent use – e.g., ‘speedballing’ or ‘goof balling’ – or some other pattern of polysubstance use, such as using an opioid to come down off a methamphetamine high.”
The National Institute on Drug Abuse funded the study. The study authors reported no relevant disclosures. Dr. Marshall reported that he has collaborated frequently with two of the study coauthors.
FROM CPDD 2020
Lawmakers question mental health disclosure rules
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
State medical licensing queries criticized
State medical licensing queries criticized
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
Several federal lawmakers on June 30 questioned state policies that require disclosure of mental health treatment as part of medical licensing applications and renewals, citing concerns about creating barriers to psychiatric care for clinicians.
Mental health–related questions on state medical boards’ licensing applications are especially worrisome with many clinicians, including ED staff, immersed in the physical and emotional challenges involved in treating waves of people with COVID-19, lawmakers said during a hearing of the House Energy and Commerce Committee’s health panel.
“We must consider the mental health of the providers on the front lines of the pandemic,” said Rep. Morgan Griffith, a Virginia Republican.
The issue of state medical boards’ disclosure rules was not on the official agenda for the House Energy and Commerce health subcommittee’s hearing. And there was no discussion of any specific state medical board’s regulations. The Energy and Commerce health subcommittee is working on more than 20 bills related to mental health, including measures intended to aid first responders, such as firemen and emergency medical personnel, and students.
This hearing marked an early stage in the process for a planned package of mental health legislation, said Rep. Michael C. Burgess, MD, of Texas, who is the top Republican on the Energy and Commerce health subcommittee. There may be opportunities as this legislation advances to add provisions intended to aid physicians, said Dr. Burgess, who practiced for many years as an ob.gyn. before being elected to Congress.
“We knew that suicide was a problem among our colleagues prior to the onset of this coronavirus epidemic and I know it is more pronounced now,” he said.
Dr. Burgess then solicited specific recommendations from the hearing’s witnesses on steps needed to help clinicians’ mental health.
The first suggestion offered in reply by Jeffrey L. Geller, MD, MPH, appearing in his role as president of the American Psychiatric Association, was that Congress should look for ways to encourage states to alter their licensing procedures.
The hearing comes on the heels of the APA, the American Academy of Family Physicians, and more than 40 other groups having jointly signed a statement calling for changes to disclosure rules about mental health.
“Licensing and credentialing applications by covered entities should only employ narrowly focused questions that address current functional impairment,” the statement said. “Additionally, we strongly support The Joint Commission (TJC) statement on Removing Barriers to Mental Health Care for Clinicians and Health Care Staff. TJC ‘supports the removal of any barriers that inhibit clinicians and health care staff from accessing mental health care services.’ ”
Physicians and other clinicians must be able to safely secure treatment for mental or other health issues, just as any other individual,” the groups wrote. “A provider’s history of mental illness or substance use disorder should not be used as any indication of their current or future ability to practice competently and without impairment.”
Also among the signers to this statement was the Federation of State Medical Boards, which has been leading an effort for years to change licensing.
In 2018, the FSMB recommended state medical boards reconsider whether it is necessary to include probing questions about a physician applicant’s mental health, addiction, or substance use on applications for medical licensure or their renewal. While the intent of these questions may be to protect patients, these queries can discourage physicians from getting needed help, the FSMB said.
Several states have since revised or considered revising their license applications and renewals. In May 2020, The Joint Commission urged broader adoption of recommendations from the FSMB and the American Medical Association to limit queries about clinicians’ mental health to “conditions that currently impair the clinicians’ ability to perform their job.”
“We strongly encourage organizations to not ask about past history of mental health conditions or treatment,” said The Joint Commission, which accredits hospitals, in a statement. “It is critical that we ensure health care workers can feel free to access mental health resources.”
Rep. Susan Brooks, an Indiana Republican who is an attorney, suggested there may need to be a broader look at how state officials pose questions about past mental health treatment to people in many professions, including her own.
“It does build on the stigma on accessing services” to know a state or licensing authority may question a professional about receiving treatment for mental health, she said.
Also at the hearing, Rep. Nanette Diaz Barragán, a California Democrat, spoke of her own reaction to seeing a question about mental health treatment while applying for a White House internship. During her college years, Rep. Barragán had to cope with her father’s terminal illness.
“I remember thinking to myself: ‘Jeez, if I end up seeing a mental health expert maybe one day I couldn’t work in government,’ ” she said.
FROM A HOUSE ENERGY AND COMMERCE’S HEALTH SUBCOMMITTEE HEARING
New data back use of medical cannabis for epilepsy, pain, anxiety
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
Two new studies offer positive news about medical cannabis, suggesting that marijuana products improve physical and cognitive symptoms, boost quality of life, and rarely produce signs of problematic use.
In one study, patients with epilepsy who used medical cannabis were nearly half as likely to have needed an emergency department visit within the last 30 days as was a control group. In the other study, 3 of 54 subjects who used medical cannabis showed signs of possible cannabis use disorder (CUD) over 12 months.
The findings show that “there is improvement in a range of outcome variables, and the adverse effects seem to be minimal, compared to what we might have hypothesized based on the bulk of the literature on the negative effects of cannabis on health outcomes,” cannabis researcher Ziva Cooper, PhD, of the University of California at Los Angeles, said in an interview. Dr. Cooper moderated a session about the studies at the virtual annual meeting of the College on Problems of Drug Dependence.
In one study, cannabis researcher Ryan Vandrey, PhD, of Johns Hopkins University, Baltimore, and colleagues compared medical cannabis users (number, 808; mean age, 38; percentage female, 63%) to a control group of people who were interested in medical cannabis (n, 468; mean age, 35; percentage female, 62%).
In both groups, 79% were White. The groups had similar levels of primary medical conditions, such as neurologic (38% and 36%, respectively, for the medical cannabis group and control group) and chronic pain (25% and 23%, respectively.)
The wide majority of those in the medical cannabis group – 58% – were cannabidiol (CBD) users, relying on a component of cannabis (marijuana) that does not make people high. Fewer than 20% used tetrahydrocannabinol (THC), which does make people high, or a combination of both CBD and THC.
Most of those in the medical cannabis group used the drug as an adjunct (39%) to other treatments or last-resort (29%) treatment instead of first line (11%) or second line (18%).
In patients with epilepsy, about 45% of controls reported a past-month ED visit, compared with about 25% of medical cannabis users. The gap in past-month hospital admissions was even wider, at about 35% for the controls and about 15% for the medical cannabis.
After an initial survey, the researchers followed subjects prospectively; some either started or stopped using medical cannabis. From baseline to follow-up, those in the medical cannabis group improved more, compared with those in the control group on a variety of measures of quality of life, anxiety, and depression.
“Folks who were in the control condition at baseline and then initiated cannabis use started to look more like the baseline cannabis users,” Dr. Vandrey said. “The folks who were cannabis users at baseline and then stopped for whatever reason started to look like the controls. And the controls [who never started using medical cannabis] stayed the same.”
As for adverse effects, two-thirds of medical cannabis users reported no problems; the highest number, 14%, reported high cost.
As for limitations, Dr. Vandrey reported missing data, a reliance on self-reports, and poor follow-up with about a third of participants agreeing to complete follow-up assessments. “We are continuing to collect data on this,” he said, “and we’re hoping we’ll be able to drill down more as we get bigger.”
The study was funded by the Realm of Caring Foundation.
In the other study, led by cannabis researcher Staci Gruber, PhD, of McLean Hospital in Belmont, Mass., and Harvard Medical School in Boston, researchers tracked 54 subjects (mean age, 49; 20 male and 34 female; 48 white) for up to 2 years after they began medical cannabis use. Most had pain (36) or anxiety/PTSD (31), and all had to have abstained from recreational cannabis use for at least 1 year.
At follow-ups, the users reported improved mood and anxiety via various measures, and they saw some improvement in quality of life. “We did not see worsening cognitive performance,” Dr. Gruber said. “In fact,
Research has suggested that as many as 30% of recreational cannabis users develop cannabis use disorder (CUD), Dr. Gruber said. But only 3 of the 54 patients showed signs of possible CUD at 12 months, she said, even though frequency of use jumped substantially vs. baseline.
Information about study funding was not available.
Dr. Cooper disclosed relationships with FSD Pharma, Beckley Canopy Therapeutics, and Insys Therapeutics. Dr. Vandrey disclosed work with Zynerba Pharmaceuticals, Canopy Health Innovations, and FSD Pharma. Dr. Gruber reported no disclosures.
FROM CPDD 2020