User login
Not just a bad dream: Nightmares may predict dementia
Results from a large cohort study showed that healthy middle-aged adults who had bad dreams at least once a week were four times more likely to experience cognitive decline over the following decade, and older adults were twice as likely to be diagnosed with dementia, compared with peers who never had bad dreams.
Frequent nightmares may “identify people who are at high risk of developing dementia in the future, several years or decades before the characteristic memory and thinking problems emerge,” study investigator Abidemi Otaiku, BMBS, University of Birmingham, England, said in an interview.
“This would be the optimum time for doctors to intervene to try and slow down or prevent dementia from developing,” Dr. Otaiku said.
The findings were published online in The Lancet journal eClinicalMedicine).
Distressing dreams
Distressing dreams have been previously associated with faster cognitive decline and increased dementia risk in patients with Parkinson’s disease (PD), but whether the same holds for individuals from the general population without PD is unknown.
To investigate, Dr. Otaiku examined data from three community-based cohorts in the United States. This included 605 middle-aged adults (aged 35-64 years) who were followed for up to 13 years and 2,600 adults aged 79 and older who were followed for up to 7 years. All were considered cognitively normal at baseline.
The prevalence of frequent distressing dreams, defined as occurring “once a week or more,” was higher in the older cohort compared with the middle-aged cohort (6.9% vs. 6.0%, respectively).
This is in line with other research that showed distressing dreams remain relatively stable throughout early adulthood and then progressively increase in prevalence from middle to older adulthood.
After adjustment for all covariates, a higher frequency of distressing dreams was linearly and statistically significantly associated with a higher risk for cognitive decline in middle-aged adults (P = .016) and a higher risk for dementia in older adults (P = .001).
In the fully adjusted model, compared with middle-aged adults who never had bad dreams, those who reported having one or more bad dreams weekly had a fourfold risk for cognitive decline (adjusted odds ratio [aOR], 3.99; 95% confidence interval [CI], 1.07-14.85).
Older adults who had one or more bad dreams weekly had a greater than twofold increased risk for developing dementia (aOR, 2.21; 95% CI, 1.35-3.62).
Early days
In sex-stratified analyses, distressing dreams were strongly and statistically significantly associated with cognitive decline and dementia in men, but were only weakly and nonsignificantly associated with cognitive decline and dementia in women.
Dr. Otaiku said he suspects some individuals in the preclinical phase of dementia have “subtle neurodegeneration occurring over time in the right frontal lobe: the area of the brain that helps to downregulate negative emotions whilst we are awake, and also whilst we are dreaming.”
This could result in “depression and anxiety in the day, and nightmares and bad dreams during the night,” he said.
It is possible that treatment for frequent nightmares may help to slow cognitive decline and delay or prevent dementia, Dr. Otaiku added.
He noted that prazosin is used to treat nightmares and has been shown to prevent memory decline and reduce amyloid B generation in preclinical studies of Alzheimer’s disease.
“This is an exciting prospect [but] it is still early days and we will need research to see whether treating nightmares might help to reduce dementia risk down the line,” Dr. Otaiku said.
Credible research
In an interview regarding these findings, Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said: “This is credible research consistent with the idea that sleep disturbances may be a risk factor or warning sign of cognitive decline.”
She added that “what’s novel here” is the researchers examined distressing dreams – not more physical sleep disturbances and disorders such as insomnia or apnea.
“However, nightmares can disturb sleep in the same way these disorders do by waking people up in the middle of the night,” said Dr. Carrillo, who was not involved with the study.
“Previous research has pointed to nightmares being indicative of potential changes in the brain that can precede other dementias like Parkinson’s disease. More research is needed to tease out what exactly is happening in the brain during nightmares that may be contributing to this increased risk,” she said.
Dr. Carrillo noted that “getting good sleep” is important for overall health, which includes brain health.
“The good news is there are treatments – both drug and nondrug – that can help address sleep disturbances,” she added.
This study received no external funding. Dr. Otaiku and Dr. Carrillo have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a large cohort study showed that healthy middle-aged adults who had bad dreams at least once a week were four times more likely to experience cognitive decline over the following decade, and older adults were twice as likely to be diagnosed with dementia, compared with peers who never had bad dreams.
Frequent nightmares may “identify people who are at high risk of developing dementia in the future, several years or decades before the characteristic memory and thinking problems emerge,” study investigator Abidemi Otaiku, BMBS, University of Birmingham, England, said in an interview.
“This would be the optimum time for doctors to intervene to try and slow down or prevent dementia from developing,” Dr. Otaiku said.
The findings were published online in The Lancet journal eClinicalMedicine).
Distressing dreams
Distressing dreams have been previously associated with faster cognitive decline and increased dementia risk in patients with Parkinson’s disease (PD), but whether the same holds for individuals from the general population without PD is unknown.
To investigate, Dr. Otaiku examined data from three community-based cohorts in the United States. This included 605 middle-aged adults (aged 35-64 years) who were followed for up to 13 years and 2,600 adults aged 79 and older who were followed for up to 7 years. All were considered cognitively normal at baseline.
The prevalence of frequent distressing dreams, defined as occurring “once a week or more,” was higher in the older cohort compared with the middle-aged cohort (6.9% vs. 6.0%, respectively).
This is in line with other research that showed distressing dreams remain relatively stable throughout early adulthood and then progressively increase in prevalence from middle to older adulthood.
After adjustment for all covariates, a higher frequency of distressing dreams was linearly and statistically significantly associated with a higher risk for cognitive decline in middle-aged adults (P = .016) and a higher risk for dementia in older adults (P = .001).
In the fully adjusted model, compared with middle-aged adults who never had bad dreams, those who reported having one or more bad dreams weekly had a fourfold risk for cognitive decline (adjusted odds ratio [aOR], 3.99; 95% confidence interval [CI], 1.07-14.85).
Older adults who had one or more bad dreams weekly had a greater than twofold increased risk for developing dementia (aOR, 2.21; 95% CI, 1.35-3.62).
Early days
In sex-stratified analyses, distressing dreams were strongly and statistically significantly associated with cognitive decline and dementia in men, but were only weakly and nonsignificantly associated with cognitive decline and dementia in women.
Dr. Otaiku said he suspects some individuals in the preclinical phase of dementia have “subtle neurodegeneration occurring over time in the right frontal lobe: the area of the brain that helps to downregulate negative emotions whilst we are awake, and also whilst we are dreaming.”
This could result in “depression and anxiety in the day, and nightmares and bad dreams during the night,” he said.
It is possible that treatment for frequent nightmares may help to slow cognitive decline and delay or prevent dementia, Dr. Otaiku added.
He noted that prazosin is used to treat nightmares and has been shown to prevent memory decline and reduce amyloid B generation in preclinical studies of Alzheimer’s disease.
“This is an exciting prospect [but] it is still early days and we will need research to see whether treating nightmares might help to reduce dementia risk down the line,” Dr. Otaiku said.
Credible research
In an interview regarding these findings, Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said: “This is credible research consistent with the idea that sleep disturbances may be a risk factor or warning sign of cognitive decline.”
She added that “what’s novel here” is the researchers examined distressing dreams – not more physical sleep disturbances and disorders such as insomnia or apnea.
“However, nightmares can disturb sleep in the same way these disorders do by waking people up in the middle of the night,” said Dr. Carrillo, who was not involved with the study.
“Previous research has pointed to nightmares being indicative of potential changes in the brain that can precede other dementias like Parkinson’s disease. More research is needed to tease out what exactly is happening in the brain during nightmares that may be contributing to this increased risk,” she said.
Dr. Carrillo noted that “getting good sleep” is important for overall health, which includes brain health.
“The good news is there are treatments – both drug and nondrug – that can help address sleep disturbances,” she added.
This study received no external funding. Dr. Otaiku and Dr. Carrillo have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from a large cohort study showed that healthy middle-aged adults who had bad dreams at least once a week were four times more likely to experience cognitive decline over the following decade, and older adults were twice as likely to be diagnosed with dementia, compared with peers who never had bad dreams.
Frequent nightmares may “identify people who are at high risk of developing dementia in the future, several years or decades before the characteristic memory and thinking problems emerge,” study investigator Abidemi Otaiku, BMBS, University of Birmingham, England, said in an interview.
“This would be the optimum time for doctors to intervene to try and slow down or prevent dementia from developing,” Dr. Otaiku said.
The findings were published online in The Lancet journal eClinicalMedicine).
Distressing dreams
Distressing dreams have been previously associated with faster cognitive decline and increased dementia risk in patients with Parkinson’s disease (PD), but whether the same holds for individuals from the general population without PD is unknown.
To investigate, Dr. Otaiku examined data from three community-based cohorts in the United States. This included 605 middle-aged adults (aged 35-64 years) who were followed for up to 13 years and 2,600 adults aged 79 and older who were followed for up to 7 years. All were considered cognitively normal at baseline.
The prevalence of frequent distressing dreams, defined as occurring “once a week or more,” was higher in the older cohort compared with the middle-aged cohort (6.9% vs. 6.0%, respectively).
This is in line with other research that showed distressing dreams remain relatively stable throughout early adulthood and then progressively increase in prevalence from middle to older adulthood.
After adjustment for all covariates, a higher frequency of distressing dreams was linearly and statistically significantly associated with a higher risk for cognitive decline in middle-aged adults (P = .016) and a higher risk for dementia in older adults (P = .001).
In the fully adjusted model, compared with middle-aged adults who never had bad dreams, those who reported having one or more bad dreams weekly had a fourfold risk for cognitive decline (adjusted odds ratio [aOR], 3.99; 95% confidence interval [CI], 1.07-14.85).
Older adults who had one or more bad dreams weekly had a greater than twofold increased risk for developing dementia (aOR, 2.21; 95% CI, 1.35-3.62).
Early days
In sex-stratified analyses, distressing dreams were strongly and statistically significantly associated with cognitive decline and dementia in men, but were only weakly and nonsignificantly associated with cognitive decline and dementia in women.
Dr. Otaiku said he suspects some individuals in the preclinical phase of dementia have “subtle neurodegeneration occurring over time in the right frontal lobe: the area of the brain that helps to downregulate negative emotions whilst we are awake, and also whilst we are dreaming.”
This could result in “depression and anxiety in the day, and nightmares and bad dreams during the night,” he said.
It is possible that treatment for frequent nightmares may help to slow cognitive decline and delay or prevent dementia, Dr. Otaiku added.
He noted that prazosin is used to treat nightmares and has been shown to prevent memory decline and reduce amyloid B generation in preclinical studies of Alzheimer’s disease.
“This is an exciting prospect [but] it is still early days and we will need research to see whether treating nightmares might help to reduce dementia risk down the line,” Dr. Otaiku said.
Credible research
In an interview regarding these findings, Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said: “This is credible research consistent with the idea that sleep disturbances may be a risk factor or warning sign of cognitive decline.”
She added that “what’s novel here” is the researchers examined distressing dreams – not more physical sleep disturbances and disorders such as insomnia or apnea.
“However, nightmares can disturb sleep in the same way these disorders do by waking people up in the middle of the night,” said Dr. Carrillo, who was not involved with the study.
“Previous research has pointed to nightmares being indicative of potential changes in the brain that can precede other dementias like Parkinson’s disease. More research is needed to tease out what exactly is happening in the brain during nightmares that may be contributing to this increased risk,” she said.
Dr. Carrillo noted that “getting good sleep” is important for overall health, which includes brain health.
“The good news is there are treatments – both drug and nondrug – that can help address sleep disturbances,” she added.
This study received no external funding. Dr. Otaiku and Dr. Carrillo have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
COVID-19 linked to increased Alzheimer’s risk
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
The study of more than 6 million people aged 65 years or older found a 50%-80% increased risk for AD in the year after COVID-19; the risk was especially high for women older than 85 years.
However, the investigators were quick to point out that the observational retrospective study offers no evidence that COVID-19 causes AD. There could be a viral etiology at play, or the connection could be related to inflammation in neural tissue from the SARS-CoV-2 infection. Or it could simply be that exposure to the health care system for COVID-19 increased the odds of detection of existing undiagnosed AD cases.
Whatever the case, these findings point to a potential spike in AD cases, which is a cause for concern, study investigator Pamela Davis, MD, PhD, a professor in the Center for Community Health Integration at Case Western Reserve University, Cleveland, said in an interview.
“COVID may be giving us a legacy of ongoing medical difficulties,” Dr. Davis said. “We were already concerned about having a very large care burden and cost burden from Alzheimer’s disease. If this is another burden that’s increased by COVID, this is something we’re really going to have to prepare for.”
The findings were published online in Journal of Alzheimer’s Disease.
Increased risk
Earlier research points to a potential link between COVID-19 and increased risk for AD and Parkinson’s disease.
For the current study, researchers analyzed anonymous electronic health records of 6.2 million adults aged 65 years or older who received medical treatment between February 2020 and May 2021 and had no prior diagnosis of AD. The database includes information on almost 30% of the entire U.S. population.
Overall, there were 410,748 cases of COVID-19 during the study period.
The overall risk for new diagnosis of AD in the COVID-19 cohort was close to double that of those who did not have COVID-19 (0.68% vs. 0.35%, respectively).
After propensity-score matching, those who have had COVID-19 had a significantly higher risk for an AD diagnosis compared with those who were not infected (hazard ratio [HR], 1.69; 95% confidence interval [CI],1.53-1.72).
Risk for AD was elevated in all age groups, regardless of gender or ethnicity. Researchers did not collect data on COVID-19 severity, and the medical codes for long COVID were not published until after the study had ended.
Those with the highest risk were individuals older than 85 years (HR, 1.89; 95% CI, 1.73-2.07) and women (HR, 1.82; 95% CI, 1.69-1.97).
“We expected to see some impact, but I was surprised that it was as potent as it was,” Dr. Davis said.
Association, not causation
Heather Snyder, PhD, Alzheimer’s Association vice president of medical and scientific relations, who commented on the findings for this article, called the study interesting but emphasized caution in interpreting the results.
“Because this study only showed an association through medical records, we cannot know what the underlying mechanisms driving this association are without more research,” Dr. Snyder said. “If you have had COVID-19, it doesn’t mean you’re going to get dementia. But if you have had COVID-19 and are experiencing long-term symptoms including cognitive difficulties, talk to your doctor.”
Dr. Davis agreed, noting that this type of study offers information on association, but not causation. “I do think that this makes it imperative that we continue to follow the population for what’s going on in various neurodegenerative diseases,” Dr. Davis said.
The study was funded by the National Institute of Aging, National Institute on Alcohol Abuse and Alcoholism, the Clinical and Translational Science Collaborative of Cleveland, and the National Cancer Institute. Dr. Synder reports no relevant financial conflicts.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Vitamins or cocoa: Which preserves cognition?
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Sacubitril/valsartan shows cognitive safety in heart failure: PERSPECTIVE
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
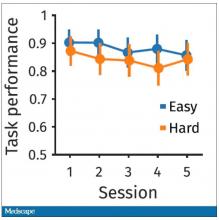
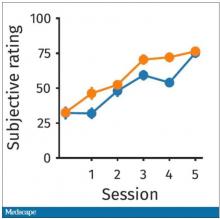
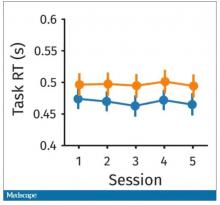
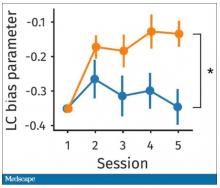
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
AT ESC CONGRESS 2022
Watching TV, using computer have opposite ties to dementia risk
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
The relationship to dementia with these activities remained strong no matter how much physical activity a person did, the authors wrote in Proceedings of the National Academy of Sciences.
Both watching TV and using a computer have been linked to increased risk of chronic disease and mortality, while exercise and physical activity (PA) have shown benefit in reducing cognitive decline, structural brain atrophy, and dementia risk in older adults, the authors wrote.
The authors said they wanted to try to understand the effects of watching TV and using computers on dementia risk, because people in the United States and Europe have been engaging in both of these activities more often.
They concluded that it’s not the sitting part of sedentary behavior (SB) that potentially has the effect on dementia but what people are doing while sitting.
Some of the results were surprising, lead author David Raichlen, PhD, professor of Human and Evolutionary Biology at University of Southern California, Los Angeles, said in an interview.
Previous literature on sedentary behaviors have documented their negative effects on a wide range of health outcomes, rather than finding positive associations, he explained.
More than 140,000 included in study
The researchers conducted their prospective cohort study using data from the United Kingdom Biobank. After excluding people younger than 60, those with prevalent dementia at the start of follow-up, and those without complete data, 146,651 participants were included.
The participants were followed from their baseline visit until they received a dementia diagnosis, died, were lost to follow-up, or were last admitted to the hospital.
TV-watching time was linked with an increased risk of incident dementia (HR [95% confidence interval] = 1.31 [1.23-1.40]), and computer use was linked with a reduced risk of incident dementia HR [95% CI] = 0.80 [0.76-0.85]).
TV’s link with higher dementia risk increased in those who had the highest use, compared with those who had the lowest use (HR [95% CI] = 1.28 [1.18-1.39].
Similarly, the link with risk reduction for dementia with computer use increased with more use.
Both medium and high computer time were associated with reduced risk of incident dementia (HR [95% CI] = 0.70 [0.64-0.76] and HR [95% CI] = 0.76 [0.70-0.83] respectively).
Dr. Raichlen pointed out that the high use of TV in this study was 4 or more hours a day and computer use – which included leisure use, not work use – had benefits on dementia risk after just half an hour.
These results remained significant after researchers adjusted for demographic, health, and lifestyle variables, including time spent on physical activity, sleeping, obesity, alcohol consumption, smoking status, diet scores, education level, body mass index, and employment type.
Physical is still better than sedentary activity
One potential reason for the different effects on dementia risk in the two activities studied, the authors write, is that sitting down to watch TV is associated with “uniquely low levels of muscle activity and energy expenditure, compared with sitting to use a computer.”
Andrew Budson, MD, chief of Cognitive & Behavioral Neurology and Associate Chief of Staff for Education for the VA Boston Healthcare System, Mass., who was not part of the study, said he thinks a more likely explanation for the study findings lies in the active versus passive tasks required in the two kinds of viewing that the authors reference.
“When we’re doing cognitive activity involving using the computer, we’re using large parts of our cortex to carry out that activity, whereas when we’re watching TV, there are probably relatively small amounts of our brain that are actually active,” Dr. Budson, author of Seven Steps to Managing Your Memory, explained in an interview.
“This is one of the first times I’ve been convinced that even when the computer activity isn’t completely new and novel, it may be beneficial,” Dr. Budson said.
It would be much better to do physical activity, but if the choice is sedentary activity, active cognitive activities, such as computer use, are better than TV watching, he continued.
The results of the current study are consistent with previous work showing that the type of sedentary behavior matters, according to the authors.
“Several studies have shown that TV time is associated with mortality and poor cardiometabolic biomarkers, whereas computer time is not,” they wrote.
A limitation of the study is that sedentary behaviors were self-reported via questionnaires, and there may be errors in recall.
“The use of objective methods for measuring both SB and PA are needed in future studies,” they write.
The authors receive support from the National Institutes of Health, the State of Arizona, the Arizona Department of Health Services, and the McKnight Brain Research Foundation. Neither the authors nor Dr. Budson declared relevant financial relationships.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Atrial cardiopathy linked to 35% higher dementia risk
“We cautiously suggest that an understanding of this relationship might provide a basis for new interventional strategies to help thwart the development of dementia,” the authors write.
The research, led by Michelle C. Johansen, MD, department of neurology, Johns Hopkins University, Baltimore, was published online in the Journal of the American Heart Association.
Atrial cardiopathy, characterized by abnormal size and function of the left atrium, has been associated with an increased risk of stroke and atrial fibrillation (AFib), and because both stroke and AFib are associated with an increased dementia risk, the authors write, it was important to investigate whether atrial cardiopathy is linked to dementia.
If that’s the case, they reasoned, the next question was whether that link is independent of AFib and stroke, and their new research suggests that it is.
For this analysis, the researchers conducted a prospective cohort analysis of participants in the Atherosclerosis Risk in Communities (ARIC) study who were attending visit 5 (2011-2013). During their fifth, sixth, and seventh clinical visits, the ARIC participants were evaluated for cognitive decline indicating dementia.
They studied a diverse population of 5,078 older adults living in four U.S. communities: Washington County, Md.; Forsyth County, N.C.; the northwestern suburbs of Minneapolis; and Jackson, Miss.
Just more than a third (34%) had atrial cardiopathy (average age, 75 years; 59% female; 21% Black) and 763 participants developed dementia.
Investigators found that atrial cardiopathy was significantly associated with dementia (adjusted hazard ratio, 1.35 [95% confidence interval, 1.16-1.58]).
They considered ARIC participants to have atrial cardiopathy if they had at least one of the following: P-wave terminal force greater than 5,000 mV·ms in ECG lead V1; NTproBNP greater than 250 pg/mL; or left atrial volume index greater than or equal to 34 mL/m2 by transthoracic echocardiography.
The risk of dementia was even stronger when the researchers defined cardiopathy by at least two biomarkers instead of one (aHR, 1.54 [95% CI, 1.25-1.89]).
The authors point out, however, that this study is observational and cannot make a causal link.
Clifford Kavinsky, MD, PhD, head of the Comprehensive Stroke and Cardiology Clinic at Rush University Medical Center, Chicago, told this news organization that much more research would need to be done to show convincingly that atrial cardiopathy causes dementia.
He called the findings “provocative in trying to understand in a general sense how cardiac dysfunction leads to dementia.”
“We all know heart failure leads to dementia, but now we see there may be a relationship with just dysfunction of the upper chambers,” he said.
Unresolved questions
But it still not clear is what is mediating the connection, who is at risk, and how the increased risk can be prevented, he said.
He said he also wonders whether the results eliminated all patients with atrial fibrillation, a point the authors acknowledge as well.
Researchers list in the limitations that “asymptomatic AFib or silent cerebral infarction may have been missed by the ARIC adjudication process.”
There is broad understanding that preventing heart disease is important for a wide array of reasons, Dr. Kavinsky noted, and one of the reasons is cognitive deterioration.
He said this study helps identify that “even dysfunction of the upper chambers of the heart contributes to the evolution of dementia.”
The study amplifies the need to shift to prevention with heart disease in general, and more specifically in atrial dysfunction, Dr. Kavinsky said, noting a lot of atrial dysfunction is mediated by underlying hypertension and coronary disease.
Researchers evaluated cognitive decline in all participants with a comprehensive array of neuropsychological tests and interviewed some of the patients.
“A diagnosis of dementia was generated based on testing results by a computer diagnostic algorithm and then decided upon by an expert based on the Diagnostic and Statistical Manual of Mental Disorders and the criteria outlined by the National Institutes of Health and the National Institutes of Health,” they write.
Dr. Johansen reported funding from National Institute of Neurological Disorders and Stroke. Study coauthor disclosures are listed in the paper. Dr. Kavinsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We cautiously suggest that an understanding of this relationship might provide a basis for new interventional strategies to help thwart the development of dementia,” the authors write.
The research, led by Michelle C. Johansen, MD, department of neurology, Johns Hopkins University, Baltimore, was published online in the Journal of the American Heart Association.
Atrial cardiopathy, characterized by abnormal size and function of the left atrium, has been associated with an increased risk of stroke and atrial fibrillation (AFib), and because both stroke and AFib are associated with an increased dementia risk, the authors write, it was important to investigate whether atrial cardiopathy is linked to dementia.
If that’s the case, they reasoned, the next question was whether that link is independent of AFib and stroke, and their new research suggests that it is.
For this analysis, the researchers conducted a prospective cohort analysis of participants in the Atherosclerosis Risk in Communities (ARIC) study who were attending visit 5 (2011-2013). During their fifth, sixth, and seventh clinical visits, the ARIC participants were evaluated for cognitive decline indicating dementia.
They studied a diverse population of 5,078 older adults living in four U.S. communities: Washington County, Md.; Forsyth County, N.C.; the northwestern suburbs of Minneapolis; and Jackson, Miss.
Just more than a third (34%) had atrial cardiopathy (average age, 75 years; 59% female; 21% Black) and 763 participants developed dementia.
Investigators found that atrial cardiopathy was significantly associated with dementia (adjusted hazard ratio, 1.35 [95% confidence interval, 1.16-1.58]).
They considered ARIC participants to have atrial cardiopathy if they had at least one of the following: P-wave terminal force greater than 5,000 mV·ms in ECG lead V1; NTproBNP greater than 250 pg/mL; or left atrial volume index greater than or equal to 34 mL/m2 by transthoracic echocardiography.
The risk of dementia was even stronger when the researchers defined cardiopathy by at least two biomarkers instead of one (aHR, 1.54 [95% CI, 1.25-1.89]).
The authors point out, however, that this study is observational and cannot make a causal link.
Clifford Kavinsky, MD, PhD, head of the Comprehensive Stroke and Cardiology Clinic at Rush University Medical Center, Chicago, told this news organization that much more research would need to be done to show convincingly that atrial cardiopathy causes dementia.
He called the findings “provocative in trying to understand in a general sense how cardiac dysfunction leads to dementia.”
“We all know heart failure leads to dementia, but now we see there may be a relationship with just dysfunction of the upper chambers,” he said.
Unresolved questions
But it still not clear is what is mediating the connection, who is at risk, and how the increased risk can be prevented, he said.
He said he also wonders whether the results eliminated all patients with atrial fibrillation, a point the authors acknowledge as well.
Researchers list in the limitations that “asymptomatic AFib or silent cerebral infarction may have been missed by the ARIC adjudication process.”
There is broad understanding that preventing heart disease is important for a wide array of reasons, Dr. Kavinsky noted, and one of the reasons is cognitive deterioration.
He said this study helps identify that “even dysfunction of the upper chambers of the heart contributes to the evolution of dementia.”
The study amplifies the need to shift to prevention with heart disease in general, and more specifically in atrial dysfunction, Dr. Kavinsky said, noting a lot of atrial dysfunction is mediated by underlying hypertension and coronary disease.
Researchers evaluated cognitive decline in all participants with a comprehensive array of neuropsychological tests and interviewed some of the patients.
“A diagnosis of dementia was generated based on testing results by a computer diagnostic algorithm and then decided upon by an expert based on the Diagnostic and Statistical Manual of Mental Disorders and the criteria outlined by the National Institutes of Health and the National Institutes of Health,” they write.
Dr. Johansen reported funding from National Institute of Neurological Disorders and Stroke. Study coauthor disclosures are listed in the paper. Dr. Kavinsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“We cautiously suggest that an understanding of this relationship might provide a basis for new interventional strategies to help thwart the development of dementia,” the authors write.
The research, led by Michelle C. Johansen, MD, department of neurology, Johns Hopkins University, Baltimore, was published online in the Journal of the American Heart Association.
Atrial cardiopathy, characterized by abnormal size and function of the left atrium, has been associated with an increased risk of stroke and atrial fibrillation (AFib), and because both stroke and AFib are associated with an increased dementia risk, the authors write, it was important to investigate whether atrial cardiopathy is linked to dementia.
If that’s the case, they reasoned, the next question was whether that link is independent of AFib and stroke, and their new research suggests that it is.
For this analysis, the researchers conducted a prospective cohort analysis of participants in the Atherosclerosis Risk in Communities (ARIC) study who were attending visit 5 (2011-2013). During their fifth, sixth, and seventh clinical visits, the ARIC participants were evaluated for cognitive decline indicating dementia.
They studied a diverse population of 5,078 older adults living in four U.S. communities: Washington County, Md.; Forsyth County, N.C.; the northwestern suburbs of Minneapolis; and Jackson, Miss.
Just more than a third (34%) had atrial cardiopathy (average age, 75 years; 59% female; 21% Black) and 763 participants developed dementia.
Investigators found that atrial cardiopathy was significantly associated with dementia (adjusted hazard ratio, 1.35 [95% confidence interval, 1.16-1.58]).
They considered ARIC participants to have atrial cardiopathy if they had at least one of the following: P-wave terminal force greater than 5,000 mV·ms in ECG lead V1; NTproBNP greater than 250 pg/mL; or left atrial volume index greater than or equal to 34 mL/m2 by transthoracic echocardiography.
The risk of dementia was even stronger when the researchers defined cardiopathy by at least two biomarkers instead of one (aHR, 1.54 [95% CI, 1.25-1.89]).
The authors point out, however, that this study is observational and cannot make a causal link.
Clifford Kavinsky, MD, PhD, head of the Comprehensive Stroke and Cardiology Clinic at Rush University Medical Center, Chicago, told this news organization that much more research would need to be done to show convincingly that atrial cardiopathy causes dementia.
He called the findings “provocative in trying to understand in a general sense how cardiac dysfunction leads to dementia.”
“We all know heart failure leads to dementia, but now we see there may be a relationship with just dysfunction of the upper chambers,” he said.
Unresolved questions
But it still not clear is what is mediating the connection, who is at risk, and how the increased risk can be prevented, he said.
He said he also wonders whether the results eliminated all patients with atrial fibrillation, a point the authors acknowledge as well.
Researchers list in the limitations that “asymptomatic AFib or silent cerebral infarction may have been missed by the ARIC adjudication process.”
There is broad understanding that preventing heart disease is important for a wide array of reasons, Dr. Kavinsky noted, and one of the reasons is cognitive deterioration.
He said this study helps identify that “even dysfunction of the upper chambers of the heart contributes to the evolution of dementia.”
The study amplifies the need to shift to prevention with heart disease in general, and more specifically in atrial dysfunction, Dr. Kavinsky said, noting a lot of atrial dysfunction is mediated by underlying hypertension and coronary disease.
Researchers evaluated cognitive decline in all participants with a comprehensive array of neuropsychological tests and interviewed some of the patients.
“A diagnosis of dementia was generated based on testing results by a computer diagnostic algorithm and then decided upon by an expert based on the Diagnostic and Statistical Manual of Mental Disorders and the criteria outlined by the National Institutes of Health and the National Institutes of Health,” they write.
Dr. Johansen reported funding from National Institute of Neurological Disorders and Stroke. Study coauthor disclosures are listed in the paper. Dr. Kavinsky has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Early dementia but no specialists: Reinforcements needed?
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
Patients in rural areas are also less likely to see psychologists and undergo neuropsychological testing, according to the study, published in JAMA Network Open.
Patients who forgo such specialist visits and testing may be missing information about their condition that could help them prepare for changes in job responsibilities and future care decisions, said Wendy Yi Xu, PhD, of The Ohio State University, Columbus, who led the research.
“A lot of them are still in the workforce,” Dr. Xu said. Patients in the study were an average age of 56 years, well before the conventional age of retirement.
Location, location, location
To examine rural versus urban differences in the use of diagnostic tests and health care visits for early onset Alzheimer’s disease and related dementias, Dr. Xu and colleagues analyzed commercial claims data from 2012-2018. They identified more than 71,000 patients aged 40-64 years with those conditions and focused on health care use by 7,311 patients in urban areas and 1,119 in rural areas within 90 days of a new dementia diagnosis.
The proportion who received neuropsychological testing was 19% among urban patients and 16% among rural patients. Psychological assessments, which are less specialized and detailed than neuropsychological testing, and brain imaging occurred at similar rates in both groups. Similar proportions of rural and urban patients visited neurologists (17.7% and 17.96%, respectively) and psychiatrists (6.02% and 6.47%).
But more urban patients than rural patients visited a psychologist, at 19% versus 15%, according to the researchers.
Approximately 18% of patients in rural areas saw a primary care provider without visiting other specialists, compared with 13% in urban areas.
The researchers found that rural patients were significantly less likely to undergo neuropsychological testing (odds ratio, 0.83; 95% confidence interval, 0.70-0.98) or see a psychologist (OR, 0.72; 95% CI, 0.60-0.85).
Similarly, rural patients had significantly higher odds of having only primary care providers involved in the diagnosis of dementia and symptom management (OR, 1.40; 95% CI, 1.19-1.66).
Addressing workforce deficiencies
More primary care training in dementia care and collaboration with specialist colleagues could help address differences in care, Dr. Xu’s group writes. Such efforts are already underway.
In 2018, the Alzheimer’s Association launched telementoring programs focused on dementia care using the Project ECHO (Extension for Community Healthcare Outcomes) model. Researchers originally developed Project ECHO at the University of New Mexico in 2003 to teach primary care clinicians in remote settings how to treat patients infected with the hepatitis C virus.
With the Alzheimer’s and Dementia Care ECHO Program for Clinicians, primary care clinicians can participate in interactive case-based video conferencing sessions to better understand dementia and how to provide high-quality care in community settings, according to the association.
The program covers guidelines for diagnosis, disclosure, and follow-up; the initiation of care planning; managing disease-related challenges; and resources for patients and caregivers.
Since 2018, nearly 100 primary care practices in the United States have completed training in dementia care using Project ECHO, said Morgan Daven, vice president of health systems for the Alzheimer’s Association. Many cases featured in the program are challenging, he added.
“With primary care being on the front lines, it is really important that primary care physicians are equipped to do what they can to detect or diagnose and know when to refer,” Mr. Daven said.
The association has compiled other resources for clinicians as well.
A 2020 report from the association examined the role that primary care physicians play in dementia care. One survey found that 82% of primary care physicians consider themselves on the front lines of providing care for patients with dementia.
Meanwhile, about half say medical professionals are not prepared to meet rising demands associated with Alzheimer’s disease and dementia care.
Mr. Daven said the geographic disparities Dr. Xu and colleagues found are unsurprising. More than half of primary care physicians who care for people with Alzheimer’s disease say dementia specialists in their communities cannot meet demand. The problem is more urgent in rural areas. Roughly half of nonmetropolitan counties in the United States lack a practicing psychologist, according to a 2018 study published in the American Journal of Preventive Medicine.
“We really need to approach this on both sides – build the capacity in primary care, but we also need to address the dementia care specialty shortages,” Mr. Daven said.
The lack of obvious differences in access to neurologists in the new study “was surprising, given the more than fourfold difference between urban and rural areas in the supply of neurologists,” the researchers note. Health plans may maintain more access to neurologists than psychologists because of relatively higher reimbursement for neurologists, they observed.
One of the study coauthors disclosed ties to Aveanna Healthcare, a company that delivers home health and hospice care.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Understanding the relationship between life satisfaction and cognitive decline
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Every day, we depend on our working memory, spatial cognition, and processing speed abilities to optimize productivity, interpersonal interactions, and psychological wellbeing. These cognitive functioning indices relate closely with academic and work performance, managing emotions, physical fitness, and a sense of fulfillment in personal and work relationships. They are linked intimately to complex cognitive skills (van Dijk et al., 2020). It is thus imperative to identify modifiable predictors of cognitive functioning in the brain to protect against aging-related cognitive decline and maximize the quality of life.
A decline in life satisfaction can worsen cognitive functioning over long periods via lifestyle factors (e.g., suboptimal diet and nutrition, lack of exercise) (Ratigan et al., 2016). Inadequate engagement in these health-enhancing pursuits could build up inflammation in EF-linked brain areas, thus negatively impacting cognitive functioning in adulthood (Grant et al., 2009). Possible pathways include long-term wear and tear of the hypothalamic-pituitary axis and brain regions linked to executive functioning (Zainal and Newman, 2022a). These processes may deteriorate working memory, spatial cognition, and processing speed across time.
Similarly, it is plausible that a reduction in cognitive functioning may lead to a long-term decrease in life satisfaction. Working memory, processing speed, spatial cognition, and related capacities are essential to meaningful activities and feelings of gratification in personal and professional relationships and other spheres of health throughout life (Baumeister et al., 2007). These cognitive functioning markers safeguard against reduced life satisfaction by facilitating effective problem-solving, and choices (Swanson and Fung, 2016). For example, stronger working memory, processing speed, and related domains coincided with better tolerance for stress and trading off immediate rewards for long-term values and life goals (Hofmann et al., 2012). Therefore, reduction in cognitive functioning abilities could precede a future decline in life satisfaction.
Nonetheless, the literature on this topic has several limitations. Most of the studies have been cross-sectional (i.e., across a single time-point) and thus do not permit inferences between cause and effect (e.g., Toh et al., 2020). Also, most studies used statistical methods that did not differentiate between between-person (trait-like individual differences) and within-person (state-like) relations. Distinguishing within- and between-person relations is necessary because they may vary in magnitude and direction. The preceding theories emphasize change-to-future change relations within persons rather than between persons (Wright and Woods, 2020).
Clinical implications
Our recent work (Zainal and Newman, 2022b) added to the literature by using an advanced statistical method to determine the relations between change in life satisfaction and future change in cognitive functioning domains within persons. The choice of an advanced statistical technique minimizes biases due to the passage of time and assessment unreliability. It also adjusts for between-person effects (Klopack and Wickrama, 2020). Improving understanding of the within-person factors leading to the deterioration of cognitive functioning and life satisfaction is crucial given the rising rates of psychiatric and neurocognitive illnesses (Cui et al., 2020). Identifying these changeable risk factors can optimize prevention, early detection, and treatment approaches.
Specifically, we analyzed the publicly available Swedish Adoption/Twin Study of Aging (SATSA) dataset (Petkus et al., 2017). Their dataset comprised 520 middle- to older-aged twin adults without dementia. Participants provided data across 23 years with five time points. Each time lag ranged from 3 to 11 years. The analyses demonstrated that greater decreases in life satisfaction predicted larger future declines in processing speed, verbal working memory, and spatial cognition. Moreover, declines in verbal working memory and processing speed predicted a reduction in life satisfaction. However, change in spatial awareness did not predict change in life satisfaction.
Our study offers multiple theoretical perspectives. Scar theories propose that decreased life satisfaction and related mental health problems can compromise working memory, processing speed, and spatial cognition in the long term. This scarring process occurs through the buildup of allostatic load, such as increased biomarkers of chronic stress (e.g., cortisol) and inflammation (e.g., interleukin-6, C-reactive protein) (Fancourt and Steptoe, 2020; Zainal and Newman, 2021a). Also, findings suggest the importance of executive functioning domains to attain desired milestones and aspirations to enhance a sense of fulfillment (Baddeley, 2013; Toh and Yang, 2020). Reductions in these cognitive functioning capacities could, over time, adversely affect the ability to engage in daily living activities and manage negative moods.
Limitations of our study include the lack of a multiple-assessment approach to measuring diverse cognitive functioning domains. Also, the absence of cognitive self-reports is a shortcoming since perceived cognitive difficulties might not align with performance on cognitive tests. Relatedly, future studies should administer cognitive tests that parallel and transfer to everyday tasks. However, our study’s strengths include the robust findings across different intervals between study waves, advanced statistics, and the large sample size.
If future studies replicate a similar pattern of results, the clinical applications of this study merit attention. Mindfulness-based interventions can promote working memory, sustained awareness, and spatial cognition or protect against cognitive decline (Jha et al., 2019; Zainal and Newman, 2021b). Further, clinical science can profit from exploring cognitive-behavioral therapies to improve adults’ cognitive function or life satisfaction (Sok et al., 2021).
Dr. Zainal recently accepted a 2-year postdoctoral research associate position at Harvard Medical School, Boston, starting in summer 2022. She received her Ph.D. from Pennsylvania State University, University Park, and completed a predoctoral clinical fellowship at the HMS-affiliated Massachusetts General Hospital – Cognitive Behavioral Scientist Track. Her research interests focus on how executive functioning, social cognition, and cognitive-behavioral strategies link to the etiology, maintenance, and treatment of anxiety and depressive disorders. Dr. Newman is a professor of psychology and psychiatry, and the director of the Center for the Treatment of Anxiety and Depression, at Pennsylvania State University. She has conducted basic and applied research on anxiety disorders and depression and has published over 200 papers on these topics.
Sources
Baddeley A. Working memory and emotion: Ruminations on a theory of depression. Rev Gen Psychol. 2013;17(1):20-7. doi: 10.1037/a0030029.
Baumeister RF et al. “Self-regulation and the executive function: The self as controlling agent,” in Social Psychology: Handbook of Basic Principles, 2nd ed. (pp. 516-39). The Guilford Press: New York, 2007.
Cui L et al. Prevalence of alzheimer’s disease and parkinson’s disease in China: An updated systematical analysis. Front Aging Neurosci. 2020 Dec 21;12:603854. doi: 10.3389/fnagi.2020.603854.
Fancourt D and Steptoe A. The longitudinal relationship between changes in wellbeing and inflammatory markers: Are associations independent of depression? Brain Behav Immun. 2020 Jan;83:146-52. doi: 10.1016/j.bbi.2019.10.004.
Grant N et al. The relationship between life satisfaction and health behavior: A cross-cultural analysis of young adults. Int J Behav Med. 2009;16(3):259-68. doi: 10.1007/s12529-009-9032-x.
Hofmann W et al. Executive functions and self-regulation. Trends Cogn Sci. 2012 Mar;16(3):174-80. doi: 10.1016/j.tics.2012.01.006.
Jha AP et al. Bolstering cognitive resilience via train-the-trainer delivery of mindfulness training in applied high-demand settings. Mindfulness. 2019;11(3):683-97. doi: 10.1007/s12671-019-01284-7.
Klopack ET and Wickrama K. Modeling latent change score analysis and extensions in Mplus: A practical guide for researchers. Struct Equ Modeling. 2020;27(1):97-110. doi: 10.1080/10705511.2018.1562929.
Petkus AJ et al. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017 May;32(3):278-92. doi: 10.1037/pag0000164.
Ratigan A et al. Sex differences in the association of physical function and cognitive function with life satisfaction in older age: The Rancho Bernardo Study. Maturitas. 2016 Jul;89:29-35. doi: 10.1016/j.maturitas.2016.04.007.
Sok S et al. Effects of cognitive/exercise dual-task program on the cognitive function, health status, depression, and life satisfaction of the elderly living in the community. Int J Environ Res Public Health. 2021 Jul 24;18(15):7848. doi: 10.3390/ijerph18157848.
Swanson HL and Fung W. Working memory components and problem-solving accuracy: Are there multiple pathways? J Educ Psychol. 2016;108(8):1153-77. doi: 10.1037/edu0000116.
Toh WX and Yang H. Executive function moderates the effect of reappraisal on life satisfaction: A latent variable analysis. Emotion. 2020;22(3):554-71. doi: 10.1037/emo0000907.
Toh WX et al. Executive function and subjective wellbeing in middle and late adulthood. J Gerontol B Psychol Sci Soc Sci. 2020 Jun 2;75(6):e69-e77. doi: 10.1093/geronb/gbz006.
van Dijk DM, et al. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS One. 2020 Apr 23;15(4):e0231906. doi: 10.1371/journal.pone.0231906.
Wright AGC and Woods WC. Personalized models of psychopathology. Annu Rev Clin Psychol. 2020 May 7;16:49-74. doi: 10.1146/annurev-clinpsy-102419-125032.
Zainal NH and Newman MG. (2021a). Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021 Mar 3;1-11. doi: 10.1017/S0033291721000398.
Zainal NH and Newman MG. (2021b). Mindfulness enhances cognitive functioning: A meta-analysis of 111 randomized controlled trials. PsyArXiv Preprints. 2021 May 11. doi: 10.31234/osf.io/vzxw7.
Zainal NH and Newman MG. (2022a). Inflammation mediates depression and generalized anxiety symptoms predicting executive function impairment after 18 years. J Affect Disord. 2022 Jan 1;296:465-75. doi: 10.1016/j.jad.2021.08.077.
Zainal NH and Newman MG. (2022b). Life satisfaction prevents decline in working memory, spatial cognition, and processing speed: Latent change score analyses across 23 years. Eur Psychiatry. 2022 Apr 19;65(1):1-55. doi: 10.1192/j.eurpsy.2022.19.
Mechanistic link between herpes virus, Alzheimer’s revealed?
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Why our brains wear out at the end of the day
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
Once again, we’re doing an informal journal club to talk about a really interesting study, “A Neuro-metabolic Account of Why Daylong Cognitive Work Alters the Control of Economic Decisions,” that just came out. It tries to answer the question of why our brains wear out. I’m going to put myself in the corner here. Let’s walk through this study, which appears in Current Biology, by lead author Antonius Wiehler from Paris.
The big question is what’s going on with cognitive fatigue. If you look at chess players who are exerting a lot of cognitive effort, it’s well documented that over hours of play, they get worse and make more mistakes. It takes them longer to make decisions. The question is, why?
Why does your brain get tired?
To date, it’s been a little bit hard to tease that out. Now, there is some suggestion of what is responsible for this. The cognitive control center of the brain is probably somewhere in the left lateral prefrontal cortex (LLPC).
The prefrontal cortex is responsible for higher-level thinking. It’s what causes you to be inhibited. It gets shut off by alcohol and leads to impulsive behaviors. The LLPC, according to functional MRI studies, has reduced activity as people become more and more cognitively fatigued. The LLPC helps you think through choices. As you become more fatigued, this area of the brain isn’t working as well. But why would it not work as well? What is going on in that particular part of the brain? It doesn’t seem to be something simple, like glucose levels; that’s been investigated and glucose levels are pretty constant throughout the brain, regardless of cognitive task. This paper seeks to tease out what is actually going on in the LLPC when you are becoming cognitively tired.
They did an experiment where they induced cognitive fatigue, and it sounds like a painful experiment. For more than 6 hours, volunteers completed sessions during which they had to perform cognitive switching tasks. Investigators showed participants a letter, in either red or green, and the participant would respond with whether it was a vowel or a consonant or whether it was a capital or lowercase letter, based on the color. If it’s red, say whether it’s a consonant or vowel. If it’s green, say whether it’s upper- or lowercase.
It’s hard, and doing it for 6 hours is likely to induce a lot of cognitive fatigue. They had a control group as well, which is really important here. The control group also did a task like this for 6 hours, but for them, investigators didn’t change the color as often – perhaps only once per session. For the study group, they were switching colors back and forth quite a lot. They also incorporated a memory challenge that worked in a similar way.
So, what are the readouts of this study? They had a group who went through the hard cognitive challenge and a group who went through the easy cognitive challenge. They looked at a variety of metrics. I’ll describe a few.
The first is performance decrement. Did they get it wrong? What percentage of the time did the participant say “consonant” when they should have said “lowercase?”
You can see here that the hard group did a little bit worse overall. It was harder, so they don’t do as well. That makes sense. But both groups kind of waned over time a little bit. It’s not as though the hard group declines much more. The slopes of those lines are pretty similar. So, not very robust findings there.
What about subjective fatigue? They asked the participants how exhausted they were from doing the tasks.
Both groups were worn out. It was a long day. There was a suggestion that the hard group became worn out a little bit sooner, but I don’t think this achieves statistical significance. Everyone was getting tired by hour 6 here.
What about response time? How quickly could the participant say “consonant,” “vowel,” “lowercase,” or “uppercase?”
The hard group took longer to respond because it was a harder task. But over time, the response times were pretty flat.
So far there isn’t a robust readout that would make us say, oh, yeah, that is a good marker of cognitive fatigue. That’s how you measure cognitive fatigue. It’s not what people say. It’s not how quick they are. It’s not even how accurate they are.
But then the investigators got a little bit clever. Participants were asked to play a “would you rather” game, a reward game. Here are two examples.
Would you rather:
- Have a 25% chance of earning $50 OR a 95% chance of earning $17.30?
- Earn $50, but your next task session will be hard or earn $40 and your next task session will be easy?
Participants had to figure out the better odds – what should they be choosing here? They had to tease out whether they preferred lower cost lower-risk choices – when they are cognitively fatigued, which has been shown in prior studies.
This showed a pretty dramatic difference between the groups in terms of the low-cost bias – how much more likely they were to pick the low-cost, easier choice as they became more and more cognitively fatigued. The hard group participants were more likely to pick the easy thing rather than the potentially more lucrative thing, which is really interesting when we think about how our own cognitive fatigue happens at the end of a difficult workday, how you may just be likely to go with the flow and do something easy because you just don’t have that much decision-making power left.
It would be nice to have some objective physiologic measurements for this, and they do. This is pupil dilation.
When you’re paying attention to something, your pupils dilate a little bit. They were able to show that as the hard group became more and more fatigued, pupil dilation sort of went away. In fact, if anything, their pupils constricted a little bit. But basically there was a significant difference here. The easy group’s pupils were still fine; they were still dilating. The hard group’s pupils got more sluggish. This is a physiologic correlate of what’s going on.
But again, these are all downstream of whatever is happening in the LLPC. So the real meat of this study is a functional MRI analysis, and the way they did this is pretty clever. They were looking for metabolites in the various parts of the brain using a labeled hydrogen MRI, which is even fancier than a functional MRI. It’s like MRI spectroscopy, and it can measure the levels of certain chemicals in the brain. They hypothesized that if there is a chemical that builds up when you are tired, it should build up preferentially in the LLPC.
Whereas in the rest of the brain, there shouldn’t be that much difference because we know the action is happening in the LLPC. The control part of the brain is a section called V1. They looked at a variety of metabolites, but the only one that behaved the way they expected was glutamate and glutamic acid (glutamate metabolites). In the hard group, the glutamate is building up over time, so there is a higher concentration of glutamate in the LLPC but not the rest of the brain. There is also a greater diffusion of glutamate from the intracellular to the extracellular space, which suggests that it’s kind of leaking out of the cells.
So the signal here is that the thing that’s impacting that part of the brain is this buildup of glutamate. To tie this together, they showed in the scatterplot the relationship between the increase in glutamate and the low-cost bias from the decision fatigue example.
It’s not the strongest correlation, but it is statistically significant that the more glutamate in your LLPC, the more likely you are to just take the easy decision as opposed to really thinking things through. That is pretty powerful. It’s telling us that your brain making you fatigued, and making you less likely to continue to use your LLPC, may be a self-defense mechanism against a buildup of glutamate, which may be neurotoxic. And that’s a fascinating bit of homeostasis.
Of course, it makes you wonder how we might adjust glutamate levels in the brain, although maybe we should let the brain be tired if the brain wants to be tired. It reminds me of that old Far Side cartoon where the guy is raising his hand and asking: “Can I be excused? My brain is full.” That is essentially what’s happening. This part of your brain is becoming taxed and building up glutamate. There’s some kind of negative feedback loop. The authors don’t know what the receptor pathway is that down-regulates that part of the brain based on the glutamate buildup, but some kind of negative feedback loop is saying, okay, give this part of the brain a rest. Things have gone on too far here.
It’s a fascinating study, although it’s not clear what we can do with this information. It’s not clear whether we can manipulate glutamate levels in this particular part of the brain or not. But it’s nice to see some biologic correlates of a psychological phenomenon that is incredibly well described – the phenomenon of decision fatigue. I think we all feel it at the end of a hard workday. If you’ve been doing a lot of cognitively intensive tasks, you just don’t have it in you anymore. And maybe the act of a good night’s sleep is clearing out some of that glutamate in the LLPC, which lets you start over and make some good decisions again. So I hope you all make some good decisions and keep your glutamate levels low. And I’ll see you next time.
For Medscape, I’m Perry Wilson.
Dr. Wilson is an associate professor of medicine and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.