User login
Multiple mental health woes? Blame it on genetics
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Investigators conducted a genetic analysis of 11 major psychiatric disorders, including schizophrenia and bipolar disorder.
“Our findings confirm that high comorbidity across some disorders in part reflects overlapping pathways of genetic risk,” lead author Andrew Grotzinger, PhD, department of psychology and neuroscience, University of Colorado at Boulder, said in a press release.
The results could lead to the development of treatments that address multiple psychiatric disorders at once and help reshape the way diagnoses are established, the researchers note.
The findings were published online in Nature Genetics.
Common genetic patterns
Using the massive UK Biobank and the Psychiatric Genomics Consortium, the researchers applied novel statistical genetic methods to identify common patterns across 11 major psychiatric disorders: schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, anorexia nervosa, obsessive-compulsive disorder (OCD), Tourette syndrome, post traumatic stress disorder, problematic alcohol use, attention deficit hyperactive disorder, and autism.
The average total sample size per disorder was 156,771 participants, with a range of 9,725 to 802,939 participants.
In all, the investigators identified 152 genetic variants shared across multiple disorders, including those already known to influence certain types of brain cells.
For example, they found that 70% of the genetic signal associated with schizophrenia was also associated with bipolar disorder.
Results also showed that anorexia nervosa and OCD have a strong, shared genetic architecture and that individuals with a genetic predisposition to low body mass index also tend to have a genetic predisposition to these two disorders.
Not surprisingly, the researchers note, there was a large genetic overlap between anxiety disorder and major depressive disorder.
They also observed that psychiatric disorders that tend to cluster together also tend to share genes that influence how and when individuals are physically active during the day.
For example, patients with internalizing disorders such as anxiety and depression tend to have a genetic architecture associated with low movement throughout the day. On the other hand, those with OCD and anorexia tend to have genes associated with higher movement throughout the day.
“When you think about it, it makes sense,” said Dr. Grotzinger. Depressed individuals often experience fatigue or low energy while those with compulsive disorders may have a tough time sitting still, he noted.
One treatment for multiple disorders?
“Collectively, these results offer key insights into the shared and disorder-specific mechanisms of genetic risk for psychiatric disease,” the investigators write.
Their research is also a first step toward developing therapies that can address multiple disorders with one treatment, they add.
“People are more likely today to be prescribed multiple medications intended to treat multiple diagnoses, and in some instances those medicines can have side effects,” Dr. Grotzinger said.
“By identifying what is shared across these issues, we can hopefully come up with ways to target them in a different way that doesn’t require four separate pills or four separate psychotherapy interventions,” he added.
Dr. Grotzinger noted that, for now, the knowledge that genetics are underlying their disorders may provide comfort to some patients.
“It’s important for people to know that they didn’t just get a terrible roll of the dice in life – that they are not facing multiple different issues but rather one set of risk factors bleeding into them all,” he said.
This research had no commercial funding. Dr. Grotzinger reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE GENETICS
Anxiety in America: COVID ‘takes a backseat’ to global events
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
FROM APA 2022
The anxious patient needs psychosomatic primary care
A balance between fear and relaxation is normal. However, mental dispositions and the continuous influence of environmental stimuli can disrupt this balance. A failure in therapy can often conceal unvoiced fears.
This article is based on the lecture “State of the Art: Treating Anxiety Disorders” by Christian Albus, MD, director of the Clinic and Polyclinic for Psychosomatics and Psychotherapy, University Hospital Cologne (Germany), at the 128th conference of the German Society of Internal Medicine.
Hidden fears
Poor compliance often has a simple cause: The patients are scared. They are afraid of bad news, for example through further investigations. Taking medication regularly reminds them, over and over, of their threatening problem. Those affected rarely speak about these delicate issues of their own volition, said Dr. Albus. But latent fears are no trivial issue.
Cardiac prognosis
A third of those affected by acute coronary syndrome (ACS) subsequently suffer from long-term anxiety disorders. The fear that they will relive their experiences overshadows their zest for life. As a result, signs of clinical depression can be detected in 50% of patients with ACS. Posttraumatic stress disorders have even been observed in up to 30% of patients. Fear also exacerbates the prognosis. Patients suffering from heart attack and subsequent cardiac failure demonstrate a significant correlation between stress and increased mortality.
Self-diagnosis
The fact that we are living in an age of fear is influenced by technological advances. “Dr. Google” is the first source to be consulted for almost half of adults who need their symptoms explained. Well-informed patients improve patient-doctor communication. But unfortunately, many people are becoming addicted to searching for diagnoses and symptoms online. Primarily harmless symptoms are associated with catastrophic diagnoses. Regrettably, Google’s search algorithm also increases this tendency. If someone starts to look for serious diseases, Google will show you these sorts of potential catastrophes on an ever more frequent basis. Google ultimately orients itself around the interests of its users. The result is a spiral of fear that can cause illness.
Cyberchondria
Compulsive searching on the internet for more and more new dangers to health has now developed into its own medical condition, termed cyberchondria. The therapy is strict internet abstinence. The gross exaggeration of health problems by the media also contributes to this. This is because it’s not just sex that sells, but also fear. The current example is long COVID. In the much-cited Gutenberg study, over half of coronavirus patients subsequently exhibited the typical symptoms: fatigue, concentration disorders, and breathing issues. Most media ignore the crucial detail that the same problems were also registered in 40% of the coronavirus-free control group. Dr. Albus pointed out that it’s no wonder that so much fear is being spread by long COVID.
The first step
Responsible medicine must counteract these developments. The first step is actively to address the fear problem. Patients who seem tense benefit enormously from the simple question: “How are you otherwise?” This question may open doors. Suddenly, patients begin to talk about their anxieties and fears. Of course, this approach to patients is time consuming. Still, this time must be taken, said Dr. Albus. In a survey of oncology patients, the majority reported that none of their physicians are ever interested in their emotional state. This is a sign of inadequate care, since psychosomatic primary care should be a standard nowadays in every specialty.
This article was translated from Coliquio.
A balance between fear and relaxation is normal. However, mental dispositions and the continuous influence of environmental stimuli can disrupt this balance. A failure in therapy can often conceal unvoiced fears.
This article is based on the lecture “State of the Art: Treating Anxiety Disorders” by Christian Albus, MD, director of the Clinic and Polyclinic for Psychosomatics and Psychotherapy, University Hospital Cologne (Germany), at the 128th conference of the German Society of Internal Medicine.
Hidden fears
Poor compliance often has a simple cause: The patients are scared. They are afraid of bad news, for example through further investigations. Taking medication regularly reminds them, over and over, of their threatening problem. Those affected rarely speak about these delicate issues of their own volition, said Dr. Albus. But latent fears are no trivial issue.
Cardiac prognosis
A third of those affected by acute coronary syndrome (ACS) subsequently suffer from long-term anxiety disorders. The fear that they will relive their experiences overshadows their zest for life. As a result, signs of clinical depression can be detected in 50% of patients with ACS. Posttraumatic stress disorders have even been observed in up to 30% of patients. Fear also exacerbates the prognosis. Patients suffering from heart attack and subsequent cardiac failure demonstrate a significant correlation between stress and increased mortality.
Self-diagnosis
The fact that we are living in an age of fear is influenced by technological advances. “Dr. Google” is the first source to be consulted for almost half of adults who need their symptoms explained. Well-informed patients improve patient-doctor communication. But unfortunately, many people are becoming addicted to searching for diagnoses and symptoms online. Primarily harmless symptoms are associated with catastrophic diagnoses. Regrettably, Google’s search algorithm also increases this tendency. If someone starts to look for serious diseases, Google will show you these sorts of potential catastrophes on an ever more frequent basis. Google ultimately orients itself around the interests of its users. The result is a spiral of fear that can cause illness.
Cyberchondria
Compulsive searching on the internet for more and more new dangers to health has now developed into its own medical condition, termed cyberchondria. The therapy is strict internet abstinence. The gross exaggeration of health problems by the media also contributes to this. This is because it’s not just sex that sells, but also fear. The current example is long COVID. In the much-cited Gutenberg study, over half of coronavirus patients subsequently exhibited the typical symptoms: fatigue, concentration disorders, and breathing issues. Most media ignore the crucial detail that the same problems were also registered in 40% of the coronavirus-free control group. Dr. Albus pointed out that it’s no wonder that so much fear is being spread by long COVID.
The first step
Responsible medicine must counteract these developments. The first step is actively to address the fear problem. Patients who seem tense benefit enormously from the simple question: “How are you otherwise?” This question may open doors. Suddenly, patients begin to talk about their anxieties and fears. Of course, this approach to patients is time consuming. Still, this time must be taken, said Dr. Albus. In a survey of oncology patients, the majority reported that none of their physicians are ever interested in their emotional state. This is a sign of inadequate care, since psychosomatic primary care should be a standard nowadays in every specialty.
This article was translated from Coliquio.
A balance between fear and relaxation is normal. However, mental dispositions and the continuous influence of environmental stimuli can disrupt this balance. A failure in therapy can often conceal unvoiced fears.
This article is based on the lecture “State of the Art: Treating Anxiety Disorders” by Christian Albus, MD, director of the Clinic and Polyclinic for Psychosomatics and Psychotherapy, University Hospital Cologne (Germany), at the 128th conference of the German Society of Internal Medicine.
Hidden fears
Poor compliance often has a simple cause: The patients are scared. They are afraid of bad news, for example through further investigations. Taking medication regularly reminds them, over and over, of their threatening problem. Those affected rarely speak about these delicate issues of their own volition, said Dr. Albus. But latent fears are no trivial issue.
Cardiac prognosis
A third of those affected by acute coronary syndrome (ACS) subsequently suffer from long-term anxiety disorders. The fear that they will relive their experiences overshadows their zest for life. As a result, signs of clinical depression can be detected in 50% of patients with ACS. Posttraumatic stress disorders have even been observed in up to 30% of patients. Fear also exacerbates the prognosis. Patients suffering from heart attack and subsequent cardiac failure demonstrate a significant correlation between stress and increased mortality.
Self-diagnosis
The fact that we are living in an age of fear is influenced by technological advances. “Dr. Google” is the first source to be consulted for almost half of adults who need their symptoms explained. Well-informed patients improve patient-doctor communication. But unfortunately, many people are becoming addicted to searching for diagnoses and symptoms online. Primarily harmless symptoms are associated with catastrophic diagnoses. Regrettably, Google’s search algorithm also increases this tendency. If someone starts to look for serious diseases, Google will show you these sorts of potential catastrophes on an ever more frequent basis. Google ultimately orients itself around the interests of its users. The result is a spiral of fear that can cause illness.
Cyberchondria
Compulsive searching on the internet for more and more new dangers to health has now developed into its own medical condition, termed cyberchondria. The therapy is strict internet abstinence. The gross exaggeration of health problems by the media also contributes to this. This is because it’s not just sex that sells, but also fear. The current example is long COVID. In the much-cited Gutenberg study, over half of coronavirus patients subsequently exhibited the typical symptoms: fatigue, concentration disorders, and breathing issues. Most media ignore the crucial detail that the same problems were also registered in 40% of the coronavirus-free control group. Dr. Albus pointed out that it’s no wonder that so much fear is being spread by long COVID.
The first step
Responsible medicine must counteract these developments. The first step is actively to address the fear problem. Patients who seem tense benefit enormously from the simple question: “How are you otherwise?” This question may open doors. Suddenly, patients begin to talk about their anxieties and fears. Of course, this approach to patients is time consuming. Still, this time must be taken, said Dr. Albus. In a survey of oncology patients, the majority reported that none of their physicians are ever interested in their emotional state. This is a sign of inadequate care, since psychosomatic primary care should be a standard nowadays in every specialty.
This article was translated from Coliquio.
Cluttered consciousness: The mental effects of growing up with a hoarder
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Many of us are reluctant to throw things out.
We buy. We accumulate. We collect. Eventually our attics are packed with dusty heirlooms that we rarely, if ever, look at. Eventually we’re forced to pare down and head to the Goodwill.
But not all of us.
Hoarding – or the prolonged difficulty of discarding unneeded possessions – is pervasive in our culture, affecting nearly 3% of the population. This compulsive collecting, and unwillingness to part with “stuff,” is even the subject of multiple popular television series.
How do you conceptualize hoarding behavior?
The core feature of hoarding is the inability to throw things away. This can be due to many different reasons, whether there’s a strong sentimental attachment or the belief that you will need these items one day. Compulsive buying is often involved, and inevitable clutter.
How was hoarding first conceptualized among psychiatrists and psychologists? And when did the term first enter the lexicon?
It was originally conceptualized as a difficult-to-treat subtype of obsessive-compulsive disorder (OCD). A lot of that work identifying this subgroup was going on in the late 1980s and early 1990s. There was a small but growing group of researchers demonstrating that this is fundamentally different from OCD in several ways.
In terms of the clinical presentation, the comorbidity patterns are different from those for OCD. And the course is a little bit different; we see a progressive development across the lifespan, as opposed to a clear-cut diagnosis earlier in life, as is typically seen with OCD. By the time a lot of people seek treatment, they’re often being brought in by, say, family members when they’re a little bit older. With hoarding, there is also this consistent pattern of poor treatment response across the board, whether to selective serotonin reuptake inhibitors or behavioral therapy.
A lot of this work together led to advocacy for recognizing hoarding as an independent diagnosis in the DSM-5. I think official recognition by our “big book” prompted more attention to this population. Previously these patients probably would have been diagnosed with OCD, and it really isn’t appropriate to think of hoarding as purely an anxiety disorder.
Hoarding exposure and future mental health
You have a new study, published in Annals of Clinical Psychiatry, looking at mental health among adult children of parents with hoarding problems. Can you tell us what inspired you to run this study, and what you found?
There were a couple of factors.
We’d seen a lot of folks with hoarding in OCD specialty clinics, so my clinical experiences with this population certainly drew me to this general area. But then, at the same time, I have this broad training in child mental health. And childhood trauma or adverse childhood experiences, which can include being around hoarding, can be a very difficult thing to live through and deal with. And here I have to give a lot of credit to Suzanne Chabaud, PhD, of the OCD Institute of Greater New Orleans, who’s one of the coauthors on the paper. She’s been beating the drum of thinking about the family and kids of people with hoarding disorders for years. My interests came from some of those experiences, but she had the good idea of really looking at this problem in a detailed way.
Prior to your paper, had there been research on the prevalence of mental illnesses such as anxiety and depression in the children of people with hoarding behaviors?
That particular question was new to our paper. It was the first time anyone, to my knowledge, had looked at a validated assessment of anxiety and depression in this population.
How did you assess their symptoms and what did you find?
We asked study participants to think back on how they felt throughout their teenage years and gauged their responses with the Patient Health Questionnaire (PHQ), a measure of mental health disorders. I should say up front that we didn’t have a control group. But we found that among our 414 study participants, somewhere between 30% and 50% reported clinically significant anxiety or depressive symptoms, far higher than you’d expect in the normal population. So when looking back on how they were feeling as teenagers in that environment, they were struggling, and they often felt rejected by their parents.
We also found that almost 10% of participants were threatened with eviction at some point in their childhood; 15% had to live outside of their home at some point, because of the clutter; and 2% had involvement from child protective services and were removed from the home.
I know you recruited patients from online forums established by the children of hoarding parents. Presumably, these are the people most affected by this phenomenon. How does this play out in people who simply like to, say, collect something? Is this a continuum of behavior, with a breaking point at which it becomes a pathology?
I think it’s safe to conceptualize collecting and hoarding as a continuum, and you’ve got to draw a line somewhere in terms of clinical significance.
Did you assess whether the children of hoarders were more likely to hoard themselves as adults?
This is our follow-up paper; we haven’t looked at it yet.
But in looking at preliminary data, the prevalence seems pretty low, actually, at least in our sample. And as you mentioned, in our study there were folks who were seeking support specifically because they grew up in a really cluttered home.
Management
How do mental health providers typically address and treat hoarding?
To my knowledge, there are no current Food and Drug Administration–approved medications for hoarding, though psychiatrists will prescribe SSRIs and try to treat co-occurring problems such as depression and anxiety symptoms.
I can speak to cognitive-behavioral therapy (CBT) in a bit more detail. A number of randomized controlled trials support CBT for hoarding. I mentioned before that when we as a field treated hoarding akin to OCD and did exposure and response prevention therapy, we didn’t really target the specific features of hoarding. People didn’t do that well.
But now researchers are focusing on CBT interventions focused on discarding tasks that really address hoarding. You can create different categories for different items: Patients can either keep them, throw them out, or donate them. You can explore what thoughts or expectations are associated with these items and try to address them. Clinicians can help patients look at, say, different areas of their house and discuss what they might be willing to part with or at least think about parting with. You find their internal motivations for keeping things.
This sort of therapy generally takes longer than it does for, say, OCD. It can be a little bit slower, particularly if someone has a lot of stuff. And often it can involve doing home visits. In the age of Zoom this is a little bit easier because home visits aren’t always feasible.
What role does family play in managing hoarding? I imagine that including loved ones and friends in the process could be quite helpful.
Yes, absolutely. And social support, more broadly.
A colleague I worked with did a really interesting study where she looked at psychologist-delivered versus peer-delivered CBT for hoarding. They found that the biggest predictor of improved outcomes was having what they called a “clutter buddy,” which follows the Alcoholics Anonymous sponsor model. This would be somebody else struggling with the same problem who’s an accountability partner helping a patient follow through with their goals related to discarding. I think that finding underscores how important that social support is.
Any final thoughts for our audience of clinicians and researchers on how to approach hoarding?
I think there’s been a stigma – at least in psychology circles – that it’s not really treatable because of that earlier work with OCD. But on the CBT side, there’s now good reason to believe that people can live much happier lives and overcome this problem. CBT does seem to work for a lot of people with hoarding. That’s what I’d like to emphasize.
Dr. Stetka is executive editor for Medscape. A version of this article first appeared on Medscape.com.
Four mental health trajectories in youth: Predicting persistent psychopathology
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
A study that tracked psychopathology in 13,000 children and adolescents found that
Investigators also found a strong correlation between new incidence of high psychopathology and externalizing problems such as hyperactivity. “It is of paramount importance to identify factors that distinguish those with persisting problems and escalating trajectories so that resources can be appropriately directed,” wrote the authors of the study published online in JAMA Network Open.
Recent studies have shown that concurrent and sequential comorbidity of psychiatric disorders are very common in adult populations, lead author Colm Healy, PhD, a postdoctoral researcher for psychiatry with the University of Medicine and Health Sciences, Ireland, said in an interview.
The speculation is that this occurs in early life when psychiatry symptoms experience high fluidity. “This presents a complex scenario to model, where young people’s mental health appears to shift and change across development. Few investigations to date have had the data available to examine these trajectories over the full range of child development,” said Dr. Healy.
He and his colleagues attempted to map the profiles and trajectories of psychopathology in children and adolescents, using latent profile transition analysis (LPTA), a person-centered method, to assess comorbidity and movement in the various phases of childhood development.
“The idea behind person-centered methods such as LTPA is that it identifies unobserved subgroups of participants who respond similarly to specific variables – in this case responses to a broad measure of psychopathology,” explained Dr. Healy.
The study included 7,507 children from the child sample (ages 3, 5, and 9 years) and 6,039 children from the adolescent sample (ages 9, 13, and 17 or 18 years). Data analysis took place from October 2020 to September 2021.
Dr. Healy and colleagues in a supplementary investigation compared cohorts at age 9 years to look for sex and generational differences.
Four developmental profiles
Researchers identified 4 distinct developmental profies for person-centered psychopathological trajectories: no psychopathology (incidence range, 60%-70%), high psychopathology (incidence range, 3%-5%), externalizing problems (incidence range, 15%-25%), and internalizing problems (incidence range, 7%-12%).
Internalizing problems reflect issues with peers and emotional problems whereas externalizing problems more closely associate with hyperactivity and conduct.
Less than 5% of the youth studied experienced persistent symptoms. However, 48.6% in the child cohort and 44.1% in the adolescent cohort moved into one of the 3 psychopathology profiles (high psychopathology, externalizing, internalizing problems) at some point in development.
The spread of trajectories was more diverse in the child cohort, said Dr. Healy. “Children ebbed and flowed between the different profiles over time with a large proportion falling into one of the psychopathology categories and then switching between these profiles.” Switching was also evident in the adolescent cohort but to a lesser extent, he said.
Externalizing problems link to high psychopathology
Rates of remittance were higher among individuals in both cohorts for internalizing problems, compared with externalizing problems.
It’s possible that for some of these young people, internalizing problems are a reaction to environmental stressors such as bullying,” said Dr. Healy. “When that stress is relieved, the internalizing problems may dissipate.”
In a clinically relevant finding, children with externalizing problems (age 5, 129 [61.3%] and age 9, 95 [74.3%]) were more likely to present with new incidents of high psychopathology. This was also true in the adolescent group (age 13, 129 [91.1%] and age 17, 146 [89.9%]).
This suggests that a proportion of youth with externalizing problems have an escalating trajectory of psychopathology. “Thus, it may be possible to distinguish those with an escalating trajectory from a stable or remitting trajectory. The specific distinguishing factors require further investigation, but it has been observed before that some of those reporting externalizing problems in early life continue to have difficulties into later life,” noted Dr. Healy.
A combination of environmental or biological factors may explain this escalation, which could respond to early intervention, he said.
Overall, few children in the study transitioned directly from no psychopathology to high psychopathology.
Differences between boys, girls
In both cohorts, investigators noticed significant differences between the sexes.
Boys in childhood made up a larger proportion of the three psychopathology profiles. But by late adolescence, girls made up a larger proportion of the internalizing profile whereas boys made up a larger proportion of the externalizing profile. “These differences were in line with our expectations,” said Dr. Healy.
Trajectories also differed among boys and girls. In childhood, girls had a higher percentage of de-escalating trajectories relative to boys. “More girls than boys in the psychopathology profiles switched to a non or less severe profile. In adolescence, differences in trajectories were less obvious, with the exception that girls were more likely than boys to transition to internalizing problems from all of the other profiles at age 17,” said Dr. Healy.
Most young people who experience psychopathology will eventually see an improvement in symptoms, noted Dr. Healy. Next steps are to identify markers that distinguish individuals with persistent trajectories from remitting trajectories at the different phases of development, he said.
Study draws mixed reviews
Clinical psychiatrists not involved in the study had varying reactions to the results.
“This study is notable for its data-driven and powerful illustration of how childhood and adolescence are dynamic periods during which psychiatric symptoms can emerge and evolve,” said Sunny X. Tang, MD, a psychiatrist and an assistant professor at the Institute of Behavioral Science and the Feinstein Institutes for Medical Research, Manhasset, New York.
The clinical call for action is for person-centered mental health screening to be a routine part of pediatric and adolescent primary care or school-based services, noted Dr. Tang.
Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore, did not think the study would have a significant impact on clinical practice.
He noted that Dr. Healy and coauthors found that some children stayed true to type, but many fluctuated between the four profile groups. The finding that fluctuation occurred more frequently in younger children is not surprising “and is consistent with what we know about the ‘moving targets’ that make diagnosing children so difficult,” said Dr. Nestadt.
“It would have been helpful to have identified clinical indicators of likely persistence in psychopathology, but the measure employed here did not allow that. It is also frustrating to not have any information on treatment, such that we cannot know whether the children who shifted to ‘no psychopathology’ did so because of treatment or spontaneously,” he added.
Victor M. Fornari, MD, MS, director of the Division of Child & Adolescent Psychiatry at The Zucker Hillside Hospital and Cohen’s Children’s Medical Center, New York, said the study is an important contribution to understanding the development of psychopathology during childhood.
“Generally, it is felt that nearly one in five youth will meet criteria for at least one psychiatric disorder by the age of 18. It is well known that externalizing disorders like ADHD manifest earlier in childhood and that depression often manifests later in adolescence,” he said.
No disclosures were reported.
FROM JAMA NETWORK OPEN
Neuropsychiatric risks of COVID-19: New data
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.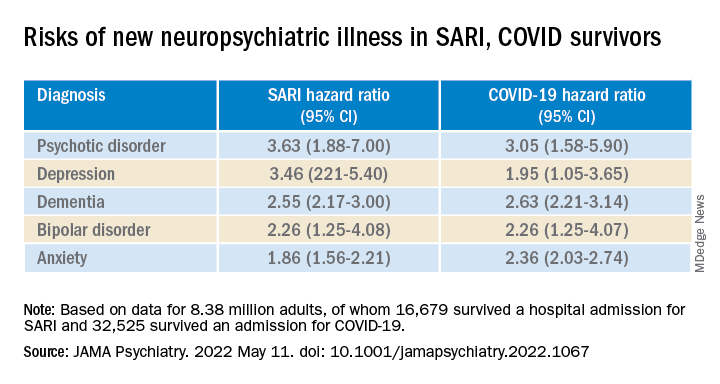
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
The neuropsychiatric ramifications of severe COVID-19 infection appear to be no different than for other severe acute respiratory infections (SARI).
This suggests that disease severity, rather than pathogen, is the most relevant factor in new-onset neuropsychiatric illness, the investigators note.
The risk of new-onset neuropsychological illness after severe COVID-19 infection are “substantial, but similar to those after other severe respiratory infections,” study investigator Peter Watkinson, MD, Nuffield Department of Clinical Neurosciences, University of Oxford, and John Radcliffe Hospital, Oxford, England, told this news organization.
The study was published online in JAMA Psychiatry.
Significant mental health burden
Research has shown a significant burden of neuropsychological illness after severe COVID-19 infection. However, it’s unclear how this risk compares to SARI.
To investigate, Dr. Watkinson and colleagues evaluated electronic health record data on more than 8.3 million adults, including 16,679 (0.02%) who survived a hospital admission for SARI and 32,525 (0.03%) who survived a hospital stay for COVID-19.
Compared with the remaining population, risks of new anxiety disorder, dementia, psychotic disorder, depression, and bipolar disorder diagnoses were significantly and similarly increased in adults surviving hospitalization for either COVID-19 or SARI.
Compared with the wider population, survivors of severe SARI or COVID-19 were also at increased risk of starting treatment with antidepressants, hypnotics/anxiolytics, or antipsychotics.
When comparing survivors of SARI hospitalization to survivors of COVID-19 hospitalization, no significant differences were observed in the postdischarge rates of new-onset anxiety disorder, dementia, depression, or bipolar affective disorder.
The SARI and COVID groups also did not differ in terms of their postdischarge risks of antidepressant or hypnotic/anxiolytic use, but the COVID survivors had a 20% lower risk of starting an antipsychotic.
“In this cohort study, SARI were found to be associated with significant postacute neuropsychiatric morbidity, for which COVID-19 is not distinctly different,” Dr. Watkinson and colleagues write.
“These results may help refine our understanding of the post–severe COVID-19 phenotype and may inform post-discharge support for patients requiring hospital-based and intensive care for SARI regardless of causative pathogen,” they write.
Caveats, cautionary notes
Kevin McConway, PhD, emeritus professor of applied statistics at the Open University in Milton Keynes, England, described the study as “impressive.” However, he pointed out that the study’s observational design is a limitation.
“One can never be absolutely certain about the interpretation of findings of an observational study. What the research can’t tell us is what caused the increased psychiatric risks for people hospitalized with COVID-19 or some other serious respiratory disease,” Dr. McConway said.
“It can’t tell us what might happen in the future, when, we all hope, many fewer are being hospitalized with COVID-19 than was the case in those first two waves, and the current backlog of provision of some health services has decreased,” he added.
“So we can’t just say that, in general, serious COVID-19 has much the same neuropsychiatric consequences as other very serious respiratory illness. Maybe it does, maybe it doesn’t,” Dr. McConway cautioned.
Max Taquet, PhD, with the University of Oxford, noted that the study is limited to hospitalized adult patients, leaving open the question of risk in nonhospitalized individuals – which is the overwhelming majority of patients with COVID-19 – or in children.
Whether the neuropsychiatric risks have remained the same since the emergence of the Omicron variant also remains “an open question since all patients in this study were diagnosed before July 2021,” Dr. Taquet said in statement.
The study was funded by the Wellcome Trust, the John Fell Oxford University Press Research Fund, the Oxford Wellcome Institutional Strategic Support Fund and Cancer Research UK, through the Cancer Research UK Oxford Centre. Dr. Watkinson disclosed grants from the National Institute for Health Research and Sensyne Health outside the submitted work; and serving as chief medical officer for Sensyne Health prior to this work, as well as holding shares in the company. Dr. McConway is a trustee of the UK Science Media Centre and a member of its advisory committee. His comments were provided in his capacity as an independent professional statistician. Dr. Taquet has worked on similar studies trying to identify, quantify, and specify the neurological and psychiatric consequences of COVID-19.
A version of this article first appeared on Medscape.com.
Most COVID-19 survivors return to work within 2 years
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
The burden of persistent COVID-19 symptoms appeared to improve over time, but a higher percentage of former patients reported poor health, compared with the general population. This suggests that some patients need more time to completely recover from COVID-19, wrote the authors of the new study, which was published in The Lancet Respiratory Medicine. Previous research has shown that the health effects of COVID-19 last for up to a year, but data from longer-term studies are limited, said Lixue Huang, MD, of Capital Medical University, Beijing, one of the study authors, and colleagues.
Methods and results
In the new study, the researchers reviewed data from 1,192 adult patients who were discharged from the hospital after surviving COVID-19 between Jan. 7, 2020, and May 29, 2020. The researchers measured the participants’ health outcomes at 6 months, 12 months, and 2 years after their onset of symptoms. A community-based dataset of 3,383 adults with no history of COVID-19 served as controls to measure the recovery of the COVID-19 patients. The median age of the patients at the time of hospital discharge was 57 years, and 46% were women. The median follow-up time after the onset of symptoms was 185 days, 349 days, and 685 days for the 6-month, 12-month, and 2-year visits, respectively. The researchers measured health outcomes using a 6-min walking distance (6MWD) test, laboratory tests, and questionnaires about symptoms, mental health, health-related quality of life, returning to work, and health care use since leaving the hospital.
Overall, the proportion of COVID-19 survivors with at least one symptom decreased from 68% at 6 months to 55% at 2 years (P < .0001). The most frequent symptoms were fatigue and muscle weakness, reported by approximately one-third of the patients (31%); sleep problems also were reported by 31% of the patients.
The proportion of individuals with poor results on the 6MWD decreased continuously over time, not only in COVID-19 survivors overall, but also in three subgroups of varying initial disease severity. Of the 494 survivors who reported working before becoming ill, 438 (89%) had returned to their original jobs 2 years later. The most common reasons for not returning to work were decreased physical function, unwillingness to return, and unemployment, the researchers noted.
However, at 2 years, COVID-19 survivors reported more pain and discomfort, as well as more anxiety and depression, compared with the controls (23% vs. 5% and 12% vs. 5%, respectively).
In addition, significantly more survivors who needed high levels of respiratory support while hospitalized had lung diffusion impairment (65%), reduced residual volume (62%), and total lung capacity (39%), compared with matched controls (36%, 20%, and 6%, respectively) at 2 years.
Long-COVID concerns
Approximately half of the survivors had symptoms of long COVID at 2 years. These individuals were more likely to report pain or discomfort or anxiety or depression, as well as mobility problems, compared to survivors without long COVID. Participants with long-COVID symptoms were more than twice as likely to have an outpatient clinic visit (odds ratio, 2.82), and not quite twice as likely to be rehospitalized (OR, 1.64).
“We found that [health-related quality of life], exercise capacity, and mental health continued to improve throughout the 2 years regardless of initial disease severity, but about half still had symptomatic sequelae at 2 years,” the researchers wrote in their paper.
Findings can inform doctor-patient discussions
“We are increasingly recognizing that the health effects of COVID-19 may persist beyond acute illness, therefore this is a timely study to assess the long-term impact of COVID-19 with a long follow-up period,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
The findings are consistent with the existing literature, said Dr. Pal, who was not involved in the study. The data from the study “can help clinicians have discussions regarding expected recovery and long-term prognosis for patients with COVID-19,” he noted.
What patients should know is that “studies such as this can help COVID-19 survivors understand and monitor persistent symptoms they may experience, and bring them to the attention of their clinicians,” said Dr. Pal.
However, “As a single-center study with high attrition of subjects during the study period, the findings may not be generalizable,” Dr. Pal emphasized. “Larger-scale studies and patient registries distributed over different geographical areas and time periods will help obtain a better understanding of the nature and prevalence of long COVID,” he said.
The study findings were limited by several factors, including the lack of formerly hospitalized controls with respiratory infections other than COVID-19 to determine which outcomes are COVID-19 specific, the researchers noted. Other limitations included the use of data from only patients at a single center, and from the early stages of the pandemic, as well as the use of self-reports for comorbidities and health outcomes, they said.
However, the results represent the longest-known published longitudinal follow-up of patients who recovered from acute COVID-19, the researchers emphasized. Study strengths included the large sample size, longitudinal design, and long-term follow-up with non-COVID controls to determine outcomes. The researchers noted their plans to conduct annual follow-ups in the current study population. They added that more research is needed to explore rehabilitation programs to promote recovery for COVID-19 survivors and to reduce the effects of long COVID.
The study was supported by the Chinese Academy of Medical Sciences, National Natural Science Foundation of China, National Key Research and Development Program of China, National Administration of Traditional Chinese Medicine, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, China Evergrande Group, Jack Ma Foundation, Sino Biopharmaceutical, Ping An Insurance (Group), and New Sunshine Charity Foundation. The researchers and Dr. Pal had no financial conflicts to disclose.
This article was updated on 5/16/2022.
FROM THE LANCET RESPIRATORY MEDICINE
‘Goodie bag’ pill mill doctor sentenced to 2 decades in prison
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
A Pennsylvania-based internist was sentenced to 20 years in prison by a federal judge on May 10 for running a prescription “pill mill” from his medical practice.
Since May 2005, Andrew Berkowitz, MD, 62, of Huntington Valley, Pa., was president and CEO of A+ Pain Management, a clinic in the Philadelphia area, according to his LinkedIn profile.
Prosecutors said patients, no matter their complaint, would leave Dr. Berkowitz’s offices with “goodie bags” filled with a selection of drugs. A typical haul included topical analgesics, such as Relyyt and/or lidocaine; muscle relaxants, including chlorzoxazone and/or cyclobenzaprine; anti-inflammatories, such as celecoxib and/or fenoprofen; and schedule IV substances, including tramadol, eszopiclone, and quazepam.
The practice was registered in Pennsylvania as a nonpharmacy dispensing site, allowing Dr. Berkowitz to bill insurers for the drugs, according to The Pennsylvania Record, a journal covering Pennsylvania’s legal system. Dr. Berkowitz also prescribed oxycodone for “pill seeking” patients, who gave him their tacit approval of submitting claims to their insurance providers, which included Medicare, Aetna, and others, for the items in the goodie bag.
In addition, Dr. Berkowitz fraudulently billed insurers for medically unnecessary physical therapy, acupuncture, and chiropractic adjustments, as well as for treatments that were never provided, according to federal officials.
According to the Department of Justice, Dr. Berkowitz collected more than $4,000 per bag from insurers. From 2015 to 2018, prosecutors estimate that Dr. Berkowitz took in more than $4 million in fraudulent proceeds from his scheme.
The pill mill came to the attention of federal authorities after Blue Cross investigators forwarded to the FBI several complaints it had received about Dr. Berkowitz. In 2017, the FBI sent a cooperating witness to Dr. Berkowitz’s clinic. The undercover patient received a prescription for oxycodone, Motrin, and Flexeril and paid $185, according to The Record.
After being indicted in 2019, Dr. Berkowitz pleaded guilty in January 2020 to 19 counts of health care fraud and to 23 counts of distributing oxycodone outside the course of professional practice and without a legitimate medical purpose.
On May 10, he was sentenced to 20 years in prison, followed by 5 years of supervised release. In addition, he was ordered to pay a $40,000 fine and almost $4 million in restitution. As a result of civil False Claims Act liability for false claims submitted to Medicare, he is also obligated to pay approximately $1.8 million and is subject to a permanent prohibition on prescribing, distributing, or dispensing controlled substances.
Dr. Berkowitz’s actions were deemed especially egregious in light of the opioid epidemic.
“Doctors are supposed to treat illness, not feed it,” said Jacqueline Maguire, special agent in charge of the FBI’s Philadelphia division. “Andrew Berkowitz prescribed patients unnecessary pills and handed out opioids to addicts.” Jennifer Arbittier Williams, acting U.S. Attorney, added upon announcing the sentence, “Doctors who dare engage in health care fraud and drug diversion, two drivers of the opioid epidemic ravaging our communities, should heed this sentence as a warning that they will be held responsible, criminally and financially.”
A version of this article first appeared on Medscape.com.
Can sensitivity to common smells sniff out depression, anxiety?
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sensitivity to specific common odors correlates with symptoms of depression or anxiety, new research shows.
A study of more than 400 participants showed that symptoms of anxiety were associated with heightened awareness of floral scents or kitchen smells, while depression was linked to increased awareness of social odors including “good” and “bad” smells of other people.
“The assessment of meta-cognitive abilities may be a useful tool in assessing depressive, anxiety, and social anxiety symptoms,” study investigator Cinzia Cecchetto, PhD student, postdoctoral researcher, department of general psychology, University of Padua, Italy, told this news organization.
The findings were published online in the Journal of Affective Disorders.
Smell perception
Previous studies have shown a strong relationship between reduced odor detection and symptoms of depression, with less clear evidence of a link between olfactory perception and symptoms of anxiety, Dr. Cecchetto said.
However, few studies have investigated to what extent individuals with symptoms of anxiety or depression are aware of, or pay attention to, odors in their environment, she added.
The study included 429 healthy participants (76.9% women, aged 18-45 years) recruited through social media. The age cut-off was 45 because evidence shows that olfactory perceptions start to decline at that time of life, Dr. Cecchetto noted.
Participants completed psychological questionnaires, including the Beck Depression Inventory-II, the Beck Anxiety Inventory, and the Liebowitz Social Anxiety Scale.
They also completed olfactory questionnaires, including the Odor Awareness Scale for evaluating the degree to which an individual focuses on olfactory stimuli such as that from food; the Affective Impact of Odor scale for assessing how odors affect liking and memory for people, places, and things; the Vividness of Olfactory Imagery Questionnaire, which examines ability to form olfactory images such as fragrances from a garden; and the Social Odor Scale, which assesses awareness of social odors such as sweat in everyday interactions.
Results showed that general anxiety symptoms were a significant predictor of higher levels of awareness of common odors.
The investigators note that this finding is similar to that from previous research in which patients with panic disorder reported higher olfactory sensitivity, reactivity, and awareness of odors compared with a control group. It is also in line with clinical features of anxiety, in which individuals maintain heightened vigilance, hyperarousal, and action readiness to respond to sudden danger.
Individuals with social anxiety symptoms reported being less attentive toward social odors.
This finding is at odds with the tendency of individuals with social anxiety to continuously monitor the environment for signs of potential negative evaluations by others.
In addition, it contradicts findings from previous studies showing that social anxiety is associated with enhanced startle reactivity and faster processing of social odor anxiety signals, compared with healthy controls, the investigators note.
Clinical implications?
“A possible explanation for these conflicting findings could be that in our study we didn’t present real odors to participants, but asked them if they usually pay attention to these odors,” lead author Elisa Dal Bò, PhD student, Padova Neuroscience Center and department of general psychology, University of Padua, told this news organization.
“Indeed, other studies have shown individuals with social anxiety focus their attention more on themselves and avoid paying attention to other people during social interactions,” she added.
Depressive symptoms were a significant predictor of higher social odor awareness and lower affective responses to odors. Some past studies showed that “depression is characterized by an increased attention to social stimuli induced by the fear of social rejection,” Ms. Dal Bò noted.
The current findings showing that depression symptoms were associated with higher social odor awareness while social anxiety symptoms were associated with lower social odor awareness are at odds with what was hypothesized, Dr. Cecchetto said.
“Actually we were expecting the opposite pattern, and that’s why it’s important to investigate more deeply these meta-cognitive abilities,” she said.
Neither depressive nor anxiety symptoms were significant predictors of olfactory imagery.
Female respondents were more attentive to, and aware of, odors than men. This finding is “quite common in the literature,” Dr. Cecchetto noted.
Although preliminary, the results could eventually have clinical implications, the investigators note. Olfactory metacognitive abilities, obtained through questionnaires, could be used to assess potential olfactory impairment, which could signal future risk for psychiatric symptoms.
However, the relatively young age of participants in the study and the prevalence of women limits the generalization of the findings, the researchers note. In addition, clinical symptoms were self-reported and were not verified by health care professionals.
‘Not enough evidence’
Commenting for this news organization, Philip R. Muskin, MD, professor of clinical psychiatry, Columbia University Medical Center, New York, said he did not find the results surprising.
For example, that individuals with social anxiety are not particularly aware of others’ smell “makes perfect sense” because “people with social anxiety disorder are concerned with themselves,” he said.
Dr. Muskin, who has an interest in and has written about olfactory function, was not involved with the research.
He noted several study limitations. First, participants just reported on their smell awareness, but “having people actually smell stuff might have been more interesting.”
In addition, the study population was relatively young, mostly women, and women’s olfactory sensitivity changes throughout the menstrual cycle, Dr. Muskin said.
“We don’t know where these women are in their cycles when they’re reporting their awareness of odors,” he said. “It would be good to know if the women were all in the luteal phase or were premenstrual because that might correlate with their anxiety or depressive symptoms.”
Asking a patient about smell awareness may provide some insight when assessing for symptoms of depression, along with obtaining details on such things as sleep, Dr. Muskin noted.
However, he does not think the new findings are enough to include olfactory awareness in the interview process. “It’s not enough evidence to use as a clinical tool for diagnosis, and I don’t see this is clinically useful yet.”
The study was supported by the European Commission Horizon 2020 research and innovation program and the Austrian Science Fund. The investigators and Dr. Muskin have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When coping skills and parenting behavioral interventions ‘don’t work’
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.
You have an appointment with a 14-year-old youth you last saw for an annual camp physical. He had screened positive for depression, and you had referred him to a local therapist. He did not have an appointment until after camp, and you have only met a few times, but since you had spoken with him about his depression, he set up an appointment with you to ask about medications. When you meet him you ask about what he had been doing in therapy and he says, “I’m learning ‘coping skills,’ but they don’t work.”
From breathing exercises and sticker charts to mindfulness and grounding exercise, coping skills can be crucial for learning how to manage distress, regulate emotions, become more effective interpersonally, and function better. Similarly, parenting interventions, which change the way parents and youth interact, are a central family intervention for behavioral problems in youth.
It is very common, however, to hear that they “don’t work” or have a parent say, “We tried that, it doesn’t work.”
When kids and parents reject coping skills and behavioral interventions by saying they do not work, the consequences can be substantial. It can mean the rejection of coping skills and strategies that actually would have helped, given time and support; that kids and families bounce between services with increasing frustration; that they search for a magic bullet (which also won’t work); and, particularly concerning for physicians, a belief that the youth have not received the right medication, resulting in potentially unhelpful concoctions of medication.
One of the biggest challenges in helping youth and parents overcome their difficulties – whether these difficulties are depression and anxiety or being better parents to struggling kids – is helping them understand that despite the fact that coping skills and behavioral interventions do not seem to work, they work.
We just have to do a better job explaining what that “work” is.
There are five points you can make.
- First, the coping skill or behavioral intervention is not supposed to work if that means solving the underlying problem. Coping skills and behavioral interventions do not immediately cure anxiety, mend broken hearts, correct disruptive behaviors, disentangle power struggles, or alleviate depression. That is not what their job is. Coping skills and behavioral interventions are there to help us get better at handling complex situations and feelings. In particular, they are good at helping us manage our thoughts (“I can’t do it,” “He should behave better”) and our affect (anger, frustration, rage, anxiety, sadness), so that over time we get better at solving the problems, and break out of the patterns that perpetuate these problems.
- Second, kids and parents do not give skills credit for when they do work. That time you were spiraling out of control and told your mom you needed a break and watched some YouTube videos and then joined the family for dinner? Your coping skills worked, but nobody noticed because they worked. We need to help our young patients and families identify those times that coping skills and behavioral interventions worked.
- Third, let’s face it: Nothing works all the time. It is no wonder kids and families are disappointed by coping skills and behavioral interventions if they think they magically work once and forever. We need to manage expectations.
- Fourth, we know they are supposed to fail, and we should discuss this openly up front. This may sound surprising, but challenging behaviors often get worse when we begin to work on them. “Extinction bursts” is probably the easiest explanation, but for psychodynamically oriented youth and families we could talk about “resistance.” No matter what, things tend to get worse before they get better. We should let people know this ahead of time.
- Fifth, and this is the one that forces youth and parents to ask how hard they actually tried, these skills need to be practiced. You can’t be in the middle of a panic attack and for the first time start trying to pace your breathing with a technique a therapist told you about 3 weeks ago. This makes about as much sense as not training for a marathon. You need to practice and build up the skills, recognizing that as you become more familiar with them, they will help you manage during stressful situations. Every skill should be practiced, preferably several times or more in sessions, maybe every session, and definitely outside of sessions when not in distress.
We cannot blame children and parents for thinking that coping skills and behavioral interventions do not work. They are struggling, suffering, fighting, frightened, angry, anxious, frustrated, and often desperate for something to make everything better. Helping them recognize this desire for things to be better while managing expectations is an essential complement to supporting the use of coping skills and behavioral interventions, and a fairly easy conversation to have with youth.
So when you are talking about coping skills and parental behavioral interventions, it is important to be prepared for the “it didn’t work” conversation, and even to address these issues up front. After all, these strategies may not solve all the problems in the world, but can be lifelong ways of coping with life’s challenges.
Dr. Henderson is associate professor of clinical psychiatry at New York University and deputy director of child and adolescent psychiatry at Bellevue Hospital, New York.










