User login
‘Obesity paradox’ in AFib challenged as mortality climbs with BMI
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.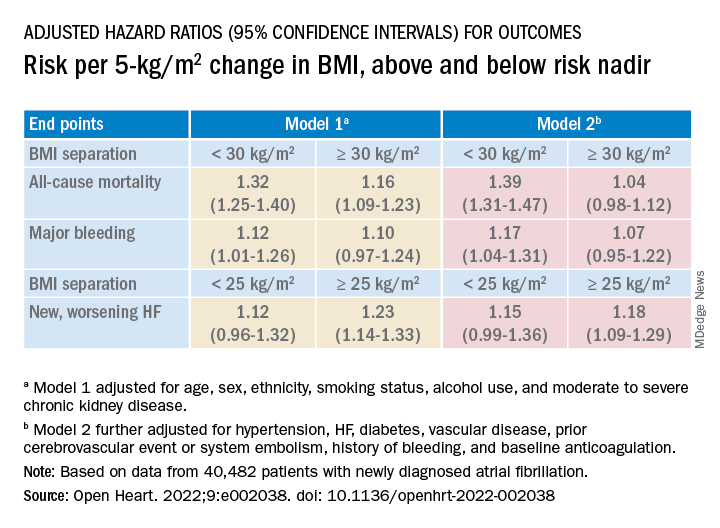
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
The relationship between body mass index (BMI) and all-cause mortality in patients with atrial fibrillation (AFib) is U-shaped, with the risk highest in those who are underweight or severely obese and lowest in patients defined simply as obese, a registry analysis suggests. It also showed a similar relationship between BMI and risk for new or worsening heart failure (HF).
Mortality bottomed out at a BMI of about 30-35 kg/m2, which suggests that mild obesity was protective, compared even with “normal-weight” or “overweight” BMI. Still, mortality went up sharply from there with rising BMI.
But higher BMI, a surrogate for obesity, apparently didn’t worsen outcomes by itself. The risk for death from any cause at higher obesity levels was found to depend a lot on related risk factors and comorbidities when the analysis controlled for conditions such as diabetes and hypertension.
The findings suggest an inverse relationship between BMI and all-cause mortality in AFib only for patients with BMI less than about 30. They therefore argue against any “obesity paradox” in AFib that posits consistently better survival with increasing levels of obesity, say researchers, based on their analysis of patients with new-onset AFib in the GARFIELD-AF registry.
“It’s common practice now for clinicians to discuss weight within a clinic setting when they’re talking to their AFib patients,” observed Christian Fielder Camm, BM, BCh, University of Oxford (England), and Royal Berkshire NHS Foundation Trust, Reading, England. So studies suggesting an inverse association between BMI and AFib-related risk can be a concern.
Such studies “seem to suggest that once you’ve got AFib, maintaining a high or very high BMI may in some way be protective – which is contrary to what would seem to make sense and certainly contrary to what our results have shown,” Dr. Camm told this news organization.
“I think that having further evidence now to suggest, actually, that greater BMI is associated with a greater risk of all-cause mortality and heart failure helps reframe that discussion at the physician-patient interaction level more clearly, and ensures that we’re able to talk to our patients appropriately about risks associated with BMI and atrial fibrillation,” said Dr. Camm, who is lead author on the analysis published in Open Heart.
“Obesity is a cause of most cardiovascular diseases, but [these] data would support that being overweight or having mild obesity does not increase the risk,” observed Carl J. Lavie, MD, of the John Ochsner Heart and Vascular Institute, New Orleans, La., and the Ochsner Clinical School at the University of Queensland, Brisbane, Australia.
“At a BMI of 40, it’s very important for them to lose weight for their long-term prognosis,” Dr. Lavie noted, but “at a BMI of 30, the important thing would be to prevent further weight gain. And if they could keep their BMI of 30, they should have a good prognosis. Their prognosis would be particularly good if they didn’t gain weight and put themselves in a more extreme obesity class that is associated with worse risk.”
The current analysis, Dr. Lavie said, “is way better than the AFFIRM study,” which yielded an obesity-paradox report on its patients with AFib about a dozen years ago. “It’s got more data, more numbers, more statistical power,” and breaks BMI into more categories.
That previous analysis based on the influential AFFIRM randomized trial separated its 4,060 patients with AFib into normal (BMI, 18.5-25), overweight (BMI, 25-30), and obese (BMI, > 30) categories, per the convention at the time. It concluded that “obese patients with atrial fibrillation appear to have better long-term outcomes than nonobese patients.”
Bleeding risk on oral anticoagulants
Also noteworthy in the current analysis, variation in BMI didn’t seem to affect mortality or risk for major bleeding or nonhemorrhagic stroke according to choice of oral anticoagulant – whether a new oral anticoagulant (NOAC) or a vitamin K antagonist (VKA).
“We saw that even in the obese and extremely obese group, all-cause mortality was lower in the group taking NOACs, compared with taking warfarin,” Dr. Camm observed, “which goes against the idea that we would need any kind of dose adjustments for increased BMI.”
Indeed, the report notes, use of NOACs, compared with VKA, was associated with a 23% drop in risk for death among patients who were either normal weight or overweight and also in those who were obese or extremely obese.
Those findings “are basically saying that the NOACs look better than warfarin regardless of weight,” agreed Dr. Lavie. “The problem is that the study is not very powered.”
Whereas the benefits of NOACs, compared to VKA, seem similar for patients with a BMI of 30 or 34, compared with a BMI of 23, for example, “none of the studies has many people with 50 BMI.” Many clinicians “feel uncomfortable giving the same dose of NOAC to somebody who has a 60 BMI,” he said. At least with warfarin, “you can check the INR [international normalized ratio].”
The current analysis included 40,482 patients with recently diagnosed AFib and at least one other stroke risk factor from among the registry’s more than 50,000 patients from 35 countries, enrolled from 2010 to 2016. They were followed for 2 years.
The 703 patients with BMI under 18.5 at AFib diagnosis were classified per World Health Organization definitions as underweight; the 13,095 with BMI 18.5-25 as normal weight; the 15,043 with BMI 25-30 as overweight; the 7,560 with BMI 30-35 as obese; and the 4,081 with BMI above 35 as extremely obese. Their ages averaged 71 years, and 55.6% were men.
BMI effects on different outcomes
Relationships between BMI and all-cause mortality and between BMI and new or worsening HF emerged as U-shaped, the risk climbing with both increasing and decreasing BMI. The nadir BMI for risk was about 30 in the case of mortality and about 25 for new or worsening HF.
The all-cause mortality risk rose by 32% for every 5 BMI points lower than a BMI of 30, and by 16% for every 5 BMI points higher than 30, in a partially adjusted analysis. The risk for new or worsening HF rose significantly with increasing but not decreasing BMI, and the reverse was observed for the endpoint of major bleeding.
The effect of BMI on all-cause mortality was “substantially attenuated” when the analysis was further adjusted with “likely mediators of any association between BMI and outcomes,” including hypertension, diabetes, HF, cerebrovascular events, and history of bleeding, Dr. Camm said.
That blunted BMI-mortality relationship, he said, “suggests that a lot of the effect is mediated through relatively traditional risk factors like hypertension and diabetes.”
The 2010 AFFIRM analysis by BMI, Dr. Lavie noted, “didn’t even look at the underweight; they actually threw them out.” Yet, such patients with AFib, who tend to be extremely frail or have chronic diseases or conditions other than the arrhythmia, are common. A take-home of the current study is that “the underweight with atrial fibrillation have a really bad prognosis.”
That message isn’t heard as much, he observed, “but is as important as saying that BMI 30 has the best prognosis. The worst prognosis is with the underweight or the really extreme obese.”
Dr. Camm discloses research funding from the British Heart Foundation. Disclosures for the other authors are in the report. Dr. Lavie has previously disclosed serving as a speaker and consultant for PAI Health and DSM Nutritional Products and is the author of “The Obesity Paradox: When Thinner Means Sicker and Heavier Means Healthier” (Avery, 2014).
A version of this article first appeared on Medscape.com.
FROM OPEN HEART
Using wearable devices to detect AFib ‘cost effective’
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening for atrial fibrillation with wearable devices is cost effective, when compared with either no screening or screening using traditional methods, a new study concludes.
“Undiagnosed atrial fibrillation (AFib) is an important cause of stroke. Screening for AFib using wrist-worn wearable devices may prevent strokes, but their cost effectiveness is unknown,” write Wanyi Chen, PhD, from Massachusetts General Hospital, Boston, and colleagues, in JAMA Health Forum.
The investigators used a microsimulation decision-analytic model to evaluate the cost effectiveness of these devices to screen for undiagnosed AFib.
The model comprised 30 million simulated individuals with an age, sex, and comorbidity profile matching the United States population aged 65 years or older.
The model looked at eight AFib screening strategies: six using wrist-worn wearable devices (either watch or band photoplethysmography with or without watch or band electrocardiography) and two using traditional modalities (that is, pulse palpation and 12-lead electrocardiogram) versus no screening.
The primary outcome was the incremental cost effectiveness ratio, defined as U.S. dollars per quality-adjusted life-year (QALY). Secondary outcomes included rates of stroke and major bleeding.
In the model, the mean age was 72.5 years and 50% were women.
All 6 screening strategies using wrist-worn wearable devices were estimated to be more cost effective than no screening. The model showed that the range of QALYs gained, compared with no screening, was 226 to 957 per 100,000 individuals.
The wrist-worn devices were also associated with greater relative benefit than screening using traditional modalities, as the range of QALYs gained, compared with no screening, was –116 to 93 per 100,000 individuals.
Compared with no screening, screening with wrist-worn wearable devices was associated with a reduction in stroke incidence by 20 to 23 per 100,000 person-years but an increase in major bleeding by 20 to 44 per 100,000 person years.
Overall, the preferred strategy for screening was wearable photoplethysmography, followed by wearable electrocardiography with patch monitor confirmation. This strategy had an incremental cost effectiveness ratio of $57,894 per QALY, “meeting the acceptability threshold of $100,000 per QALY,” the authors write.
The cost effectiveness of screening was consistent across multiple clinically relevant scenarios, including screening a general population aged 50 years or older with risk factors for stroke, the authors report.
“When deployed within specific AFib screening pathways, wearable devices are likely to be an important component of cost-effective AFib screening,” the investigators conclude.
Study based on modeled data
“This study is the first simulation of various screening strategies for atrial fibrillation using wearable devices and suggests that wearable devices, in particular wrist-worn wearables, in an elderly population, [are] estimated to be cost-effective,” Emma Svennberg, MD, PhD, from the Karolinska University Hospital, Stockholm, told this news organization.
“I find this study interesting, as the adoption of wearables amongst individuals is high and increasing, hence many wearers will screen themselves for arrhythmias (even if health care recommendations are discordant), and the potential costs for society have been unknown,” said Dr. Svennberg, who was not part of this study.
“Of course, no study is without its flaws, and here one must note that the study is based on modeled data alone and not RCTs of the wearable screening strategies ... hence true clinical outcome data is missing,” Dr. Svennberg added.
The large STROKESTOP study, on which she was the lead investigator, “presented data based on true clinical outcomes at ESC 2021 (European Society of Cardiology) and showed cost effectiveness,” Dr. Svennberg said.
The study authors report financial relationships with Bristol Myers Squibb, Fitbit, Medtronic, Pfizer, UpToDate, American Heart Association, IBM, Bayer AG, Novartis, MyoKardia, Boehringer Ingelheim, Heart Rhythm Society, Avania Consulting, Apple, Premier, the National Institutes of Health, Invitae, Blackstone Life Sciences, Flatiron, and Value Analytics Labs. Dr. Svennberg reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.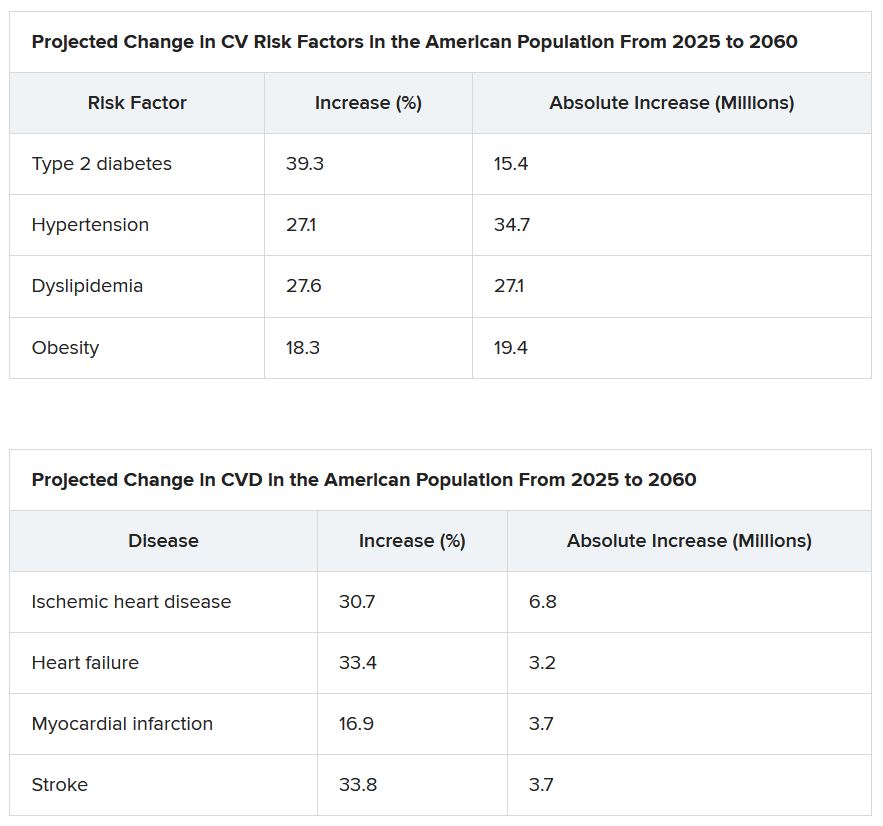
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
Do ICDs still ‘work’ in primary prevention given today’s recommended HF meds?
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
Contemporary guidelines highly recommend patients with heart failure with reduced ejection fraction (HFrEF) be on all four drug classes that together have shown clinical clout, including improved survival, in major randomized trials.
Although many such patients don’t receive all four drug classes, the more that are prescribed to those with primary-prevention implantable defibrillators (ICD), the better their odds of survival, a new analysis suggests.
The cohort study of almost 5,000 patients with HFrEF and such devices saw their all-cause mortality risk improve stepwise with each additional prescription they were given toward the full quadruple drug combo at the core of modern HFrEF guideline-directed medical therapy (GDMT). The four classes are sodium-glucose cotransporter 2 inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and renin-angiotensin system (RAS) inhibitors.
That inverse relation between risk and number of GDMT medications held whether patients had solo-ICD or defibrillating cardiac resynchronization therapy (CRT-D) implants, and were independent of device-implantation year and comorbidities, regardless of HFrEF etiology.
“If anybody had doubts about really pushing forward as much of these guideline-directed medical therapies as the patient tolerates, these data confirm that, by doing so, we definitely do better than with two medications or one medication,” Samir Saba, MD, University of Pittsburgh Medical Center, said in an interview.
The analysis begs an old and challenging question: Do primary-prevention ICDs confer clinically important survival gains over those provided by increasingly life-preserving recommended HFrEF medical therapy?
Given the study’s incremental survival bumps with each added GDMT med, “one ought to consider whether ICD therapy can still have an impact on overall survival in this population,” proposes a report published online in JACC Clinical Electrophysiology, with Dr. Saba as senior author.
In the adjusted analysis, the 2-year risk for death from any cause in HFrEF patients with primary-prevention devices fell 36% in those with ICDs and 30% in those with CRT-D devices for each added prescribed GDMT drug, from none up to either three or four such agents (P < .001 in both cases).
Only so much can be made of nonrandomized study results, Saba observed in an interview. But they are enough to justify asking whether primary-prevention ICDs are “still valuable” in HFrEF given current GDMT. One interpretation of the study, the published report noted, is that contemporary GDMT improves HFrEF survival so much that it eclipses any such benefit from a primary-prevention ICD.
Both defibrillators and the four core drug therapies boost survival in such cases, “so the fundamental question is, are they additive. Do we save more lives by having a defibrillator on top of the medications, or is it overlapping?” Dr. Saba asked. “We don’t know the answer.”
For now, at least, the findings could reassure clinicians as they consider whether to recommended a primary-prevention ICD when there might be reasons not to, as long there is full GDMT on board, “especially what we today define as quadruple guideline-directed medical therapy.”
Recently announced North American guidelines defining an HFrEF quadruple regimen prefer – beyond a beta-blocker, MRA, and SGLT2 inhibitor – that the selected RAS inhibitor be sacubitril/valsartan (Entresto, Novartis), with ACE inhibitors or angiotensin-receptor blockers (ARBs) as a substitute, if needed.
Nearly identical European guidelines on HFrEF quad therapy, unveiled in 2021, include but do not necessarily prefer sacubitril/valsartan over ACE inhibitors as the RAS inhibitor of choice.
GDMT a moving target
Primary-prevention defibrillators entered practice at a time when expected background GDMT consisted of beta-blockers and either ACE inhibitors or ARBs, the current report notes. In practice, many patients receive the devices without both drug classes optimally on board. Moreover, many who otherwise meet guidelines for such ICDs won’t tolerate the kind of maximally tolerated GDMT used in the major primary-prevention device trials.
Yet current guidelines give such devices a class I recommendation, based on the highest level of evidence, in HFrEF patients who remain symptomatic despite quad GDMT, observed Gregg C. Fonarow, MD, University of California Los Angeles Medical Center.
The current analysis “further reinforces the importance of providing all four foundational GDMTs” to all eligible HFrEF patients without contraindications who can tolerate them, he said in an interview. Such quad therapy “is associated with incremental 1-year survival advantages” in patients with primary-prevention devices. And in the major trials, “there were reductions in sudden deaths, as well as progressive heart failure deaths.”
But the current study also suggests that in practice “very few patients can actually get to all four drugs on GDMT,” Roderick Tung, MD, University of Arizona, Phoenix, said in an interview. Optimized GDMT in randomized trials probably represents the best-case scenario. “There is a difference between randomized data and real-world data, which is why we need both.”
And it asserts that “the more GDMT you’re on, the better you do,” he said. “But does that obviate the need for an ICD? I think that’s not clear,” in part because of potential confounding in the analysis. For example, patients who can take all four agents tend to be less sick than those who cannot.
“The ones who can get up to four are preselected, because they’re healthier,” Dr. Tung said. “There are real limitations – such as metabolic disturbances, acute kidney injury and cardiorenal syndrome, and hypotension – that actually make it difficult to initiate and titrate these medications.”
Indeed, the major primary-prevention ICD trials usually excluded the sickest patients with the most comorbidities, Dr. Saba observed, which raises issues about their relevance to clinical practice. But his group’s study controlled for many potential confounders by adjusting for, among other things, Elixhauser comorbidity score, ejection fraction, type of cardiomyopathy, and year of device implantation.
“We tried to level the playing field that way, to see if – despite all of this adjustment – the incremental number of heart failure medicines stills make a difference,” Dr. Saba said. “And our results suggest that yes, they still do.”
GDMT coverage in the real world
The analysis of patients with HFrEF involved 3,210 with ICD-only implants and 1,762 with CRT-D devices for primary prevention at a major medical center from 2010 to 2021. Of the total, 5% had not been prescribed any of the four GDMT agents, 20% had been prescribed only one, 52% were prescribed two, and 23% were prescribed three or four. Only 113 patients had been prescribed SGLT2 inhibitors, which have only recently been indicated for HFrEF.
Adjusted hazard ratios for death from any cause at 2 years for each added GDMT drug (P < .001 in each case), were 0.64 (95% confidence interval, 0.56-0.74) for ICD recipients, 0.70 (95% CI, 0.58-0.86) for those with a CRT-D device, 0.70 (95% CI, 0.60-0.81) for those with ischemic cardiomyopathy, and 0.61 (95% CI, 0.51-0.73) for patients with nonischemic disease.
The results “raise questions rather than answers,” Dr. Saba said. “At some point, someone will need to take patients who are optimized on their heart failure medications and then randomize them to defibrillator versus no defibrillator to see whether there is still an additive impact.”
Current best evidence suggests that primary-prevention ICDs in patients with guideline-based indications confer benefits that far outweigh any risks. But if the major primary-prevention ICD trials were to be repeated in patients on contemporary quad-therapy GDMT, Dr. Tung said, “would the benefit of ICD be attenuated? I think most of us believe it likely would.”
Still, he said, a background of modern GDMT could potentially “optimize” such trials by attenuating mortality from heart failure progression and thereby expanding the proportion of deaths that are arrhythmic, “which the defibrillator can prevent.”
Dr. Saba discloses receiving research support from Boston Scientific and Abbott; and serving on advisory boards for Medtronic and Boston Scientific. The other authors reported no relevant relationships. Dr. Tung has disclosed receiving speaker fees from Abbott and Boston Scientific. Dr. Fonarow has reported receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis.
A version of this article first appeared on Medscape.com.
FROM JACC CLINICAL ELECTROPHYSIOLOGY
‘Stunning variation’ in CV test, procedure costs revealed at top U.S. hospitals
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Heed cardiac risk of BTKis for CLL
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
FROM BLOOD ADVANCES
Interventional imagers take on central role and more radiation
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Interventional echocardiographers have become an increasingly critical part of the structural heart team but may be paying the price in terms of radiation exposure, a new study suggests.
Results showed that interventional echocardiographers receive threefold higher head-level radiation doses than interventional cardiologists during left atrial appendage occlusion (LAAO) closures and 11-fold higher doses during mitral valve transcatheter edge-to-edge repair (TEER).
“Over the last 5-10 years there’s been exponential growth in these two procedures, TEER and LAAO, and while that’s been very exciting, I think there hasn’t been as much research into how to protect these individuals,” lead author David A. McNamara, MD, MPH, Spectrum Health, Grand Rapids, Mich., told this news organization.
The study was published in JAMA Network Open.
Previous studies have focused largely on radiation exposure and mitigation efforts during coronary interventions, but the room set-up for LAAO and TEER and shielding techniques to mitigate radiation exposure are vastly different, he noted.
A 2017 study reported that radiation exposure was significantly higher for imaging specialists than structural heart specialists and varied by procedure type.
For the current study, Dr. McNamara, an echocardiographer by training, and colleagues collected data from 30 consecutive LAAO and 30 consecutive TEER procedures performed at their institution between July 2016 and January 2018.
Interventional imagers, interventional cardiologists, and sonographers all wore a lead skirt, apron, and thyroid collar, as well as a dosimeter to collect radiation data.
Interventional cardiologists stood immediately adjacent to the procedure table and used a ceiling-mounted, upper-body lead shield and a lower-body shield extending from the table to the floor. The echocardiographer stood at the patient’s head and used a mobile accessory shield raised to a height that allowed the imager to extend their arms over the shield to manipulate a transesophageal echocardiogram probe throughout the case.
The median fluoroscopy time was 9.2 minutes for LAAO and 20.9 minutes for TEER. The median air kerma was 164 mGy and 109 mGy, respectively.
Interventional echocardiographers received a median per case radiation dose of 10.6 µSv, compared with 2.1 µSv for interventional cardiologists. The result was similar for TEER (10.5 vs. 0.9 µSv) and LAAO (10.6 vs. 3.5 µSv; P < .001 for all).
The odds of interventional echocardiographers having a radiation dose greater than 20 µSV were 7.5 times greater than for interventional cardiologists (P < .001).
“It’s not the direction of the association, but really the magnitude is what surprised us,” observed Dr. McNamara.
The team was pleasantly surprised, he said, that sonographers, a “vastly understudied group,” received significantly lower median radiation doses than interventional imagers during LAAO (0.2 µSV) and TEER procedures (0.0 µSv; P < .001 for both).
The average distances from the radiation source were 26 cm (10.2 inches) for the echocardiographer, 36 cm (14.2 inches) for the interventional cardiologist, and 250 cm (8.2 feet) for the sonographer.
“These folks [sonographers] were much further away than both the physicians performing these cases, and that is what we hypothesize drove their very low rates, but that should also help inform our mitigation techniques for physicians and for all other cath lab members in the room,” Dr. McNamara said.
He noted that Spectrum Health has been at the forefront in terms of research into radiation exposure and mitigation, has good institutional radiation safety education, and used dose-lowering fluoroscopy systems (AlluraClarity, Philips) with real-time image noise reduction technology and a frame rate of 15 frames per second for the study. “So we’re hopeful that this actually represents a somewhat best-case scenario for what is being done at multiple institutions throughout the nation.”
Nevertheless, there is a huge amount of variability in radiation exposure, Dr. McNamara observed. “First and foremost, we really just have to identify our problem and highlight that this is something that needs some advocacy from our [professional] groups.”
Sunil Rao, MD, the newly minted president of the Society of Cardiovascular Angiography and Interventions (SCAI), said, “This is a really important study, because it expands the potential occupational hazards outside of what we traditionally think of as the team that does interventional procedures ... we have to recognize that the procedures we’re doing in the cath lab have changed.”
“Showing that our colleagues are getting 3-10 times radiation exposure is a really important piece of information to have out there. I think it’s really sort of a call to action,” Dr. Rao, professor of medicine at Duke University, Durham, N.C., told this news organization.
Nevertheless, he observed that practices have shifted somewhat since the study and that interventional cardiologists working with imaging physicians are more cognizant of radiation exposure issues.
“When I talk with our folks here that are doing structural heart procedures, they’re making sure that they’re not stepping on the fluoro pedal while the echocardiographer is manipulating the TE probe,” Dr. Rao said. “The echocardiographer is oftentimes using a much bigger shield than what was described in the study, and remember there’s an exponential decrease in the radiation exposure by distance, so they’re stepping back during the fluoroscopy time.”
Although the volume of TEER and LAAO procedures, as well as tricuspid interventions, will continue to climb, Dr. Rao said he expects radiation exposure to the imaging cardiologist will fall thanks to greater use of newer-generation imaging systems with dose-reduction features and better shielding strategies.
He noted that several of SCAI’s “best practices” documents call attention to radiation safety and that SCAI is creating a pathway where imaging cardiologists can become fellows of the society, which was traditionally reserved for interventionalists.
Still, imaging and cardiovascular societies have yet to endorse standardized safety procedures for interventional imagers, nor is information routinely collected on radiation exposure in national registries.
“We just don’t have the budgets or the interest nationally to do that kind of thing, so it has to be done locally,” Dr. Rao said. “And the person who I think is responsible for that is really the cath lab director and the cath lab nurse manager, who really should work hand-in-glove to make sure that radiation safety is at the top of the priority list.”
The study was funded by the Frederik Meijer Heart & Vascular Institute, Spectrum Health, and by Corindus. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication. Senior author Ryan Madder, MD, reports receiving research support, speaker honoraria, and grants, and serving on the advisory board of Corindus. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
Cardiologists concerned for patient safety after abortion ruling
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Add AFib to noncardiac surgery risk evaluation: New support
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Practice has gone back and forth on whether atrial fibrillation (AFib) should be considered in the preoperative cardiovascular risk (CV) evaluation of patients slated for noncardiac surgery, and the Revised Cardiac Risk Index (RCRI), currently widely used as an assessment tool, doesn’t include the arrhythmia.
But consideration of preexisting AFib along with the RCRI predicted 30-day mortality more sharply than the RCRI alone in an analysis of data covering several million patients slated for such procedures.
Indeed, AFib emerged as a significant, independent risk factor for a number of bad postoperative outcomes. Mortality within a month of the procedure climbed about 30% for patients with AFib before the noncardiac surgery. Their 30-day risks for stroke and for heart failure hospitalization went up similarly.
The addition of AFib to the RCRI significantly improved its ability to discriminate 30-day postoperative risk levels regardless of age, sex, and type of noncardiac surgery, Amgad Mentias, MD, Cleveland Clinic, told this news organization. And “it was able to correctly up-classify patients to high risk, if AFib was there, and it was able to down-classify some patients to lower risk if it wasn’t there.”
“I think [the findings] are convincing evidence that atrial fib should at least be part of the thought process for the surgical team and the medical team taking care of the patient,” said Dr. Mentias, who is senior author on the study published in the Journal of the American College of Cardiology, with lead author Sameer Prasada, MD, also of the Cleveland Clinic.
The results “call for incorporating AFib as a risk factor in perioperative risk scores for cardiovascular morbidity and mortality,” the published report states.
Supraventricular arrhythmias had been part of the Goldman Risk Index once widely used preoperatively to assess cardiac risk before practice adopted the RCRI in the past decade, observe Anne B. Curtis, MD, and Sai Krishna C. Korada, MD, University at Buffalo, New York, in an accompanying editorial.
The current findings “demonstrate improved prediction of adverse postsurgical outcomes” from supplementing the RCRI with AFib, they write. Given associations between preexisting AFib and serious cardiac events, “it is time to ‘re-revise’ the RCRI and acknowledge the importance of AFib in predicting adverse outcomes” after noncardiac surgery.
The new findings, however, aren’t all straightforward. In one result that remains a bit of a head-scratcher, postoperative risk of myocardial infarction (MI) in patients with preexisting AFib went in the opposite direction of risk for death and other CV outcomes, falling by almost 20%.
That is “hard to explain with the available data,” the report states, but “the use of anticoagulation, whether oral or parenteral (as a bridge therapy in the perioperative period), is a plausible explanation” given the frequent role of thrombosis in triggering MIs.
Consistent with such a mechanism, the group argues, the MI risk reduction was seen primarily among patients with AFib and a CHA2DS2-VASc score of 2 or higher – that is, those at highest risk for stroke and therefore most likely to be on oral anticoagulation. The MI risk reduction wasn’t seen in such patients with a CHA2DS2-VASc score of 0 or 1.
“I think that’s part of the explanation, that anticoagulation can reduce risk of MI. But it’s not the whole explanation,” Dr. Mentias said in an interview. If it were the sole mechanism, he said, then the same oral anticoagulation that protected against MI should have also cut the postoperative stroke risk. Yet that risk climbed 40% among patients with preexisting AFib.
The analysis started with 8.6 million Medicare patients with planned noncardiac surgery, seen from 2015 to 2019, of whom 16.4% had preexisting AFib. Propensity matching for demographics, urgency and type of surgery, CHA2DS2-VASc score, and RCRI index created two cohorts for comparison: 1.13 million patients with and 1.92 million without preexisting AFib.
Preexisting AFib was associated with a higher 30-day risk for death from any cause, the primary endpoint being 8.3% versus 5.8% for those without such AFib (P < .001), for an odds ratio of 1.31 (95% confidence interval, 1.30-1.32).
Corresponding 30-day ORs for other events, all significant at P < .001, were:
- 1.31 (95% CI, 1.30-1.33) for heart failure
- 1.40 (95% CI, 1.37-1.43) for stroke
- 1.59 (95% CI, 1.43-1.75) for systemic embolism
- 1.14 (95% CI, 1.13-1.16) for major bleeding
- 0.81 (95% CI, 0.79-0.82) for MI
Those with preexisting AFib also had longer hospitalizations at a median 5 days, compared with 4 days for those without such AFib (P < .001).
The study has the limitations of most any retrospective cohort analysis. Other limitations, the report notes, include lack of information on any antiarrhythmic meds given during hospitalization or type of AFib.
For example, AFib that is permanent – compared with paroxysmal or persistent – may be associated with more atrial fibrosis, greater atrial dilatation, “and probably higher pressures inside the heart,” Dr. Mentias observed.
“That’s not always the case, but that’s the notion. So presumably people with persistent or permanent atrial fib would have more advanced heart disease, and that could imply more risk. But we did not have that kind of data.”
Dr. Mentias and Dr. Prasada report no relevant financial relationships; disclosures for the other authors are in the report. Dr. Curtis discloses serving on advisory boards for Abbott, Janssen Pharmaceuticals, Sanofi, and Milestone Pharmaceuticals; receiving honoraria for speaking from Medtronic and Zoll; and serving on a data-monitoring board for Medtronic. Dr. Korada reports he has no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Meta-analysis points to safety of acetylcholine coronary testing
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Provocation testing with intracoronary acetylcholine is safe, particularly among Western patients, suggests a large systematic review that underscores the importance of functional coronary angiography to diagnose epicardial or microvascular spasm.
The results, derived from more than 12,000 patients in 16 studies, showed a 0.5% risk of major complications, defined as death, ventricular tachycardia/ventricular fibrillation, myocardial infarction, and shock requiring resuscitation.
Ventricular tachycardia/fibrillation were the most common events and mainly reported from two Japanese studies. There were no deaths.
Exploratory subgroup analyses revealed significantly fewer major complications in Western populations (0.0%; P for heterogeneity = .938), compared with Asian populations (2.3%; P for heterogeneity < .001).
The pooled positive vasospasm rate was also lower in Western versus Asian studies (37.9% vs. 50.7%; P for between-group heterogeneity = .010), as reported by the Microvascular Network in the Journal of the American College of Cardiology.
“If you look at the data between Asian studies versus others, mainly European or U.S. studies, primarily in Caucasian populations, it’s like zero percent history of major complications. So, it sounds extremely safe to do this testing in Caucasian populations,” Yuhei Kobayashi, MD, NewYork-Presbyterian Brooklyn Methodist Hospital, Weill Cornell Medicine, said.
Safety will need to be assessed in African Americans and other racial/ethnic groups, but “it makes us think we should end up testing more in the United States,” he told this news organization.
Intracoronary acetylcholine testing is daily practice in Japan but is limited in the United States and Europe to a few specialized centers due to safety concerns. Three deaths were reported in 1980 with intravenous ergonovine testing, whereas the safety of acetylcholine protocols has been studied largely in single-center retrospective studies, typically in Asian populations.
Growing recognition of myocardial infarction with nonobstructive coronary arteries (MINOCA) and ischemia with no obstructive coronary arteries (INOCA), however, is changing the landscape. In recent U.S. and European guidelines, intracoronary acetylcholine testing is indicated as a class 2a recommendation in MINOCA/INOCA.
“More and more institutions in Europe and the United States are starting to do acetylcholine testing, because now we know that chest pain isn’t necessarily coming from the blocked arteries,” Dr. Kobayashi said. “There are functional abnormalities, including coronary spasm, and if we diagnose it, we have appropriate medical regimens for this kind of disease.”
First safety meta-analysis
The present review and meta-analysis included 12,585 participants in 16 studies through November 2021. Of these, 63% were conducted in Western countries, and most were prospective studies published over the past decade in patients with MINOCA or INOCA.
Ten studies used the contemporary diagnostic criteria for epicardial spasm of at least 90% reduction in coronary diameter. Acetylcholine was administered into the left coronary artery at up to 100 mcg and 200 mcg in seven and six studies, respectively, and was used in the other three studies to assess endothelial function with a slower infusion of up to 36.4 mcg.
Major complications were significantly higher in studies following the contemporary diagnostic cutoff than in those using a lower cutoff of at least 75% diameter reduction (1.0% vs. 0.0%; P for between-group heterogeneity < .001).
The incidence of major complications was 0.2% with the slower infusion of up to 36.4 mcg, 0.8% with a maximum dose of 100 mcg, and 0.3% with a maximum dose of 200 mcg. The positive vasospasm rate was similar with the latter two protocols, at 46.3% and 41.4%, respectively.
Minor complications occurred in 3.3% of patients but were not detailed. They can include paroxysmal atrial fibrillation, ventricular ectopic beats, transient hypotension, and bradycardia requiring intervention.
As with major complications, minor complications were lower in studies using noncontemporary versus contemporary diagnostic cutoffs for epicardial spasm (1.8% vs. 4.7%) and in Western versus Asian populations (2.6% vs. 9.4%). Minor complications were similar between protocols with maximum doses of 100 mcg and 200 mcg (3.6% vs. 3.8%).
Dr. Kobayashi suggested that several factors may explain the racial differences, including previously reported smooth muscle hyperresponsiveness to provocation stimuli in Japanese patients and the inclusion of a wide range of patients in Japanese studies, such as those with obstructive coronary disease.
Japanese studies also used sequential acetylcholine injection into both the right and left coronaries, a faster injection speed of 20 seconds, and upfront placement of a temporary pacing catheter in case of acetylcholine-induced bradycardia, particularly with right coronary injection.
Although the protocol is largely settled in Japan, he said, provocation protocols need to be standardized because “depending on the country and depending on the institution, people are doing totally different things.”
A big step forward
Commenting on the study, C. Noel Bairey Merz, MD, from Cedars Sinai, Los Angeles, said it has “widespread relevance” because half of all coronary angiograms done invasively in the United States for suspected ischemia find no obstructive coronary disease. Left untreated, however, MINOCA has a 2.5% annual event rate, and a quarter of that is death.
“This is a big step forward with likely equal opportunity to improve women and men’s ischemic heart disease,” she said.
On the other hand, all studies were conducted at centers of excellence, so safety will need to be carefully watched as testing rolls out to more community care, Dr. Merz said. “And it always needs to be underscored that this is done by an interventional cardiologist because they’re familiar with wires that can dissect arteries, and they’re familiar with minor complications that could turn into major, if someone didn’t act appropriately.”
Dr. Merz also called for unifying protocols and the need to raise awareness within the general cardiology community to ask interventionalists for acetylcholine spasm testing. Randomized controlled data from within the WISE study and the CorMica study showed that diagnostic certainty leads to greater therapeutic certainty. “You do a much better job about who and how to treat,” she said.
There are also three ongoing randomized controlled trials – WARRIOR, MINOCA-BAT, and iCorMica – in the INOCA and MINOCA populations testing different treatment strategies for hard clinical outcomes like death and myocardial infarction.
“So in addition to this publication being guideline-forming for diagnosis, we anticipate in the next several years to have clinical trial evidence about therapeutics, again, for formulation of class 1 guidelines,” Dr. Merz said.
John Beltrame, BMBS, PhD, University of Adelaide, Australia, said the meta-analysis shows that intracoronary acetylcholine spasm testing is safe and should prompt greater adoption of invasive functional angiography.
Interventionalists are quite happy to do fractional flow reserve using intravenous adenosine to assess coronary microvascular dysfunction, he said, and “what we think is that functional angiography should test both – both the spasm as well as the microvasculature – and that will give us a clear direction because the treatments are slightly different when you’re treating the large arteries as compared to the microscopic arteries. It’s an important thing.”
Dr. Beltrame and colleagues further detail the benefits of comprehensive invasive functional angiography over structural angiography in a related editorial.
He also noted that the Coronary Vasomotion Disorders International Study Group published international diagnostic criteria for microvascular angina and that several protocols for acetylcholine spasm testing are in the works, including one from Australia. Australian investigators are also organizing an accreditation program for those performing the test.
“The protocol itself is relatively straightforward, but it’s not merely picking up a manual and following the instructions,” Dr. Beltrame said. “Just the same as when you train someone in angioplasty, you don’t just go out and do it. You need to develop some experience in it and so should be proctored.”
Dr. Kobayashi reported consulting agreements with Abbott Vascular. Coauthor disclosures are listed in the paper. Dr. Beltrame and colleagues have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.