User login
Atopic Dermatitis Triggered by Omalizumab and Treated With Dupilumab
To the Editor:
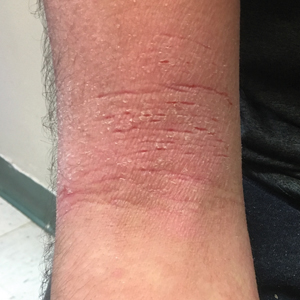
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

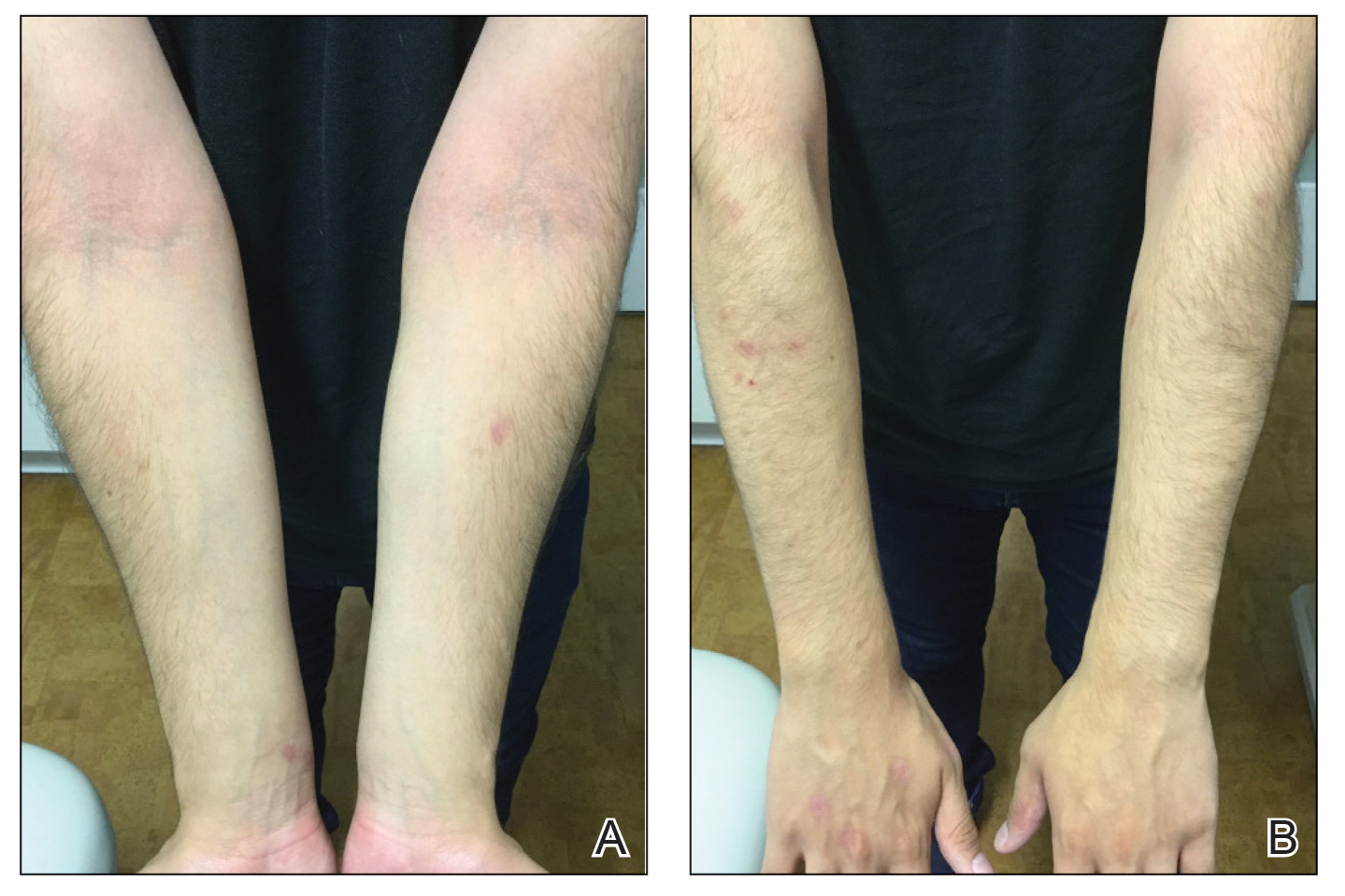
Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
Practice Points
- Monoclonal antibodies are promising therapies for atopic conditions, although its efficacy for atopic dermatitis (AD) is debated and the side-effect profile is not entirely known.
- Omalizumab may cause a paradoxical exacerbation of AD in select patients analogous to tumor necrosis factor α inhibitor–induced psoriasis.
Topical botanical drug coacillium curbs childhood alopecia
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Patch testing finds higher prevalence of ACD among children with AD
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
, a finding that investigators say underscores the value of considering ACD in patients with AD and referring more children for testing.
ACD is underdetected in children with AD. In some cases, it may be misconstrued to be AD, and patch testing, the gold standard for diagnosing ACD, is often not performed, said senior author JiaDe Yu, MD, MS, a pediatric dermatologist and director of contact and occupational dermatology at Massachusetts General Hospital, Boston, and his co-authors, in the study published in the Journal of the American Academy of Dermatology.
Dr. Yu and his colleagues utilized a database in which dermatologists and some allergists, all of whom had substantive experience in patch testing and in diagnosing and managing ACD in children, entered information about children who were referred to them for testing.
Of 912 children referred for patch testing between 2018 and 2022 from 14 geographically diverse centers in the United States (615 with AD and 297 without AD), those with AD were more likely to have more than one positive reaction (odds radio, 1.57; 95% confidence interval, 1.14-2.14; P = .005) and had a greater number of positive results overall (2.3 vs. 1.9; P = .012).
AD and ACD both present with red, itchy, eczema-like patches and plaques and can be “really hard to differentiate,” Dr. Yu said in an interview.
“Not everybody with AD needs patch testing,” he said, “but I do think some [patients] who have rashes in unusual locations or rashes that don’t seem to improve within an appropriate amount of time to topical medications ... are the children who probably should have patch testing.”
Candidates for patch testing include children with AD who present with isolated head or neck, hand or foot, or anal or genital dermatitis, Dr. Yu and his colleagues write in the study. In addition, Dr. Yu said in the interview, “if you have a child who has AD that involves the elbow and back of the knees but then they get new-onset facial dermatitis, say, or new-onset eyelid dermatitis ... there’s [significant] value in patch testing.”
Children with AD in the study had a more generalized distribution of dermatitis and were significantly less likely to have dermatitis affecting the anal or genital region, the authors note in the study.
Asked to comment on the results, Jennifer Perryman, MD, a dermatologist at UCHealth, Greeley, Colo., who performs patch testing in children and adults, said that ACD is indeed “often underdiagnosed” in children with AD, and the study “solidifies” the importance of considering ACD in this population.
“Clinicians should think about testing children when AD is [not well controlled or] is getting worse, is in an atypical distribution, or if they are considering systemic treatment,” she said in an e-mail.
“I tell my patients, ‘I know you have AD, but you could also have comorbid ACD, and if we can find and control that, we can make you better without adding more to your routine, medications, etc.’ ” said Dr. Perryman, who was not involved in the research.
Top allergens
The top 10 allergens between children with and without AD were largely similar, the authors of the study report. Nickel was the most common allergen identified in both groups, and cobalt was in the top five for both groups. Fragrances (including hydroperoxides of linalool), preservatives (including methylisothiazolinone [MI]), and neomycin ranked in the top 10 in both groups, though prevalence differed.
MI, a preservative frequently used in personal care products and in other products like school glue and paint, was the second most common allergen identified in children with AD. Allergy to MI has “recently become an epidemic in the United States, with rapidly increasing prevalence and importance as a source of ACD among both children and adults,” the authors note.
Children with AD were significantly more likely, however, to have ACD to bacitracin (OR, 3.23; P = .030) and to cocamidopropyl betaine (OR, 3.69; P = .0007), the latter of which is a popular surfactant used in “baby” and “gentle” skincare products. This is unsurprising, given that children with AD are “more often exposed to a myriad of topical treatments,” Dr. Yu and his colleagues write.
Although not a top 10 allergen for either group, ACD to “carba mix,” a combination of three chemicals used to make medical adhesives and other rubber products (such as pacifiers, toys, school supplies, and rubber gloves) was significantly more common in children with AD than in those without (OR, 3.36; P = .025).
Among other findings from the study: Children with AD were more likely to have a longer history of dermatitis (4.1 vs. 1.6 years, P < .0001) prior to patch testing. Testing occurred at a mean age of 11 and 12.3 years for children with and without AD, respectively.
The number of allergens tested and the patch testing series chosen per patient were “not statistically different” between the children with and without AD, the researchers report.
Patch testing availability
Clinicians may be hesitant to subject a child to patch testing, but the process is well tolerated in most children, Dr. Perryman said. She uses a modified panel for children that omits less relevant allergens and usually limits patch testing to age 2 years or older due to a young child’s smaller surface area.
Dr. Yu, who developed an interest in patch testing during his residency at the Medical College of Wisconsin, Milwaukee, where he worked with a patch-testing expert, will test children as young as 3-4 months with a “small selection of patches.”
The challenge with a call for more patch testing is a shortage of trained physicians. “In all of Boston, where we have hundreds of dermatologists, there are only about four of us who really do patch testing. My wait time is about 6 months,” said Dr. Yu, who is also an assistant professor at Harvard Medical School, Boston.
Allergists at Massachusetts General Hospital do “some patch testing ... but they refer a lot of the most complicated cases to me,” he said, noting that patch testing and management of ACD involves detailed counseling for patients about avoidance of allergens. “Overall dermatologists represent the largest group of doctors who have proficiency in patch testing, and there just aren’t many of us.”
Dr. Perryman also said that patch testing is often performed by dermatologists who specialize in treating ACD and AD, though there seems to be “regional variance” in the level of involvement of dermatologists and allergists in patch testing.
Not all residency programs have hands-on patch testing opportunities, Dr. Yu said. A study published in Dermatitis, which he co-authored, showed that in 2020, 47.5% of dermatology residency programs had formal patch testing rotations. This represented improvement but is still not enough, he said.
The American Contact Dermatitis Society offers patch-testing mentorship programs, and the American Academy of Dermatology has recently begun offered a patch testing workshop at its annual meetings, said Dr. Yu, who received 4 weeks of training in the Society’s mentorship program and is now involved in the American Academy of Dermatology’s workshops and as a trainer/lecturer at the Contact Dermatitis Institute.
The study was supported by the Dermatology Foundation. Dr. Yu and his co-investigators reported no conflicts of interest. Dr. Perryman had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
What’s Eating You? Noble False Widow Spider (Steatoda nobilis)
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
PRACTICE POINTS
- With evidence of a recent population boom of noble false widow spiders in Europe and spread to California, dermatologists should be aware of these spiders and their bites.
- Symptoms of Steatoda bites (steatodism) include immediate pain followed by intense pruritus, swelling, erythema, and possibly systemic symptoms such as fever. Secondary infections such as cellulitis and septicemia are risks.
- The envenomation site should be kept clean to prevent secondary infection, and medical care should be sought when there is evidence of ulceration or cellulitis.
FDA issues letter regarding lebrikizumab review for atopic dermatitis
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
Study finds inflammatory bowel disease risk higher in children, adults with atopic dermatitis
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
The published recently in JAMA Dermatology.
The study also found an increased risk for Crohn’s disease (CD) in adults and children with AD, as well as an increased risk for ulcerative colitis (UC) in adults with AD and in children with severe AD, researchers reported.
“It is imperative for clinicians to understand atopic dermatitis and the trajectory of our patients with it in order to provide the best standard of care,” senior author Joel M. Gelfand, MD, MSCE, professor in clinical investigation with the department of dermatology at the University of Pennsylvania, Philadelphia, said in a news release.
“There are new and better treatments for AD today, and there will likely continue to be more,” continued Dr. Gelfand. “But providers have to understand how those treatments could impact other autoimmune diseases. For patients with AD and another autoimmune disease, some currently available medications can exacerbate symptoms of their other disease or can help treat two immune diseases at the same time.”
The study results support the idea that AD and IBD may have some common underlying causes, said Sheilagh Maguiness, MD, pediatric dermatologist and associate professor in the department of dermatology at the University of Minnesota, Minneapolis, who was asked to comment on the findings.
“As the pathogenesis of AD is becoming better understood, we are recognizing that, rather than simply a cutaneous disease, the underlying inflammation and immune dysregulation that leads to AD best categorizes it as a systemic inflammatory disease with significant comorbidities,” she told this news organization. “I will be more likely to ask patients and families about GI symptoms, and if positive, may plan to refer to GI more readily than in the past,” added Dr. Maguiness, who was not involved in the study.
UK general practice cohort
AD has been associated with an increasing number of comorbidities, including IBD, but studies linking AD with IBD, including UC, have had mixed results, the authors wrote. And few studies have separately examined how AD or AD severity may be linked with UC or CD risk.
To examine the risk for new-onset IBD, UC, and CD in children and adults with atopic dermatitis, the researchers conducted a population-based cohort study using the THIN (The Health Improvement Network) electronic medical record database of patients registered with United Kingdom general practices. They used 21 years of data collected from January 1994 to February 2015.
The researchers matched each patient who had AD with up to five controls based on age, practice, and index date. Because THIN does not capture AD severity, they used treatment exposure assessed by dermatologic referrals and treatments patients received as proxy for severity. The authors used logistic regression to examine the risks for IBD, UC, and CD in children (aged 1-10) with AD, and in adults (aged 30-68) with AD, and they compared their outcomes with the outcomes for controls.
In the pediatric cohort, the team compared 409,431 children who had AD with 1.8 million children without AD. Slightly more than half were boys. In the adult cohort, they compared 625,083 people who had AD with 2.68 million controls, and slightly more than half were women. Data on race or ethnicity were not available, the authors wrote, but the THIN database is considered to be representative of the UK population.
AD severity linked with IBD risk
The risk for new-onset inflammatory bowel disease appears to be higher in children and adults with AD, and the risk varies based on age, AD severity, and subtype of inflammatory bowel disease, the authors reported.
Overall, AD in children was associated with a 44% increased risk for IBD (adjusted hazard ratio (HR), 1.44; 95% confidence interval [CI], 1.31-1.58) compared with controls, the authors reported. They found a 74% increased risk for CD in children with AD compared with controls (HR, 1.74; 95% CI, 1.54-1.97). More severe AD was linked with increased risk for both IBD and CD.
AD did not appear to increase risk for UC in children, except those with severe AD (HR, 1.65; 95% CI, 1.02-2.67).
Overall, adults with AD had a 34% (HR, 1.34; 95% CI, 1.27-1.40) increased risk for IBD, a 36% (HR, 1.36; 95% CI, 1.26-1.47) increased risk for CD, and a 32% (HR, 1.32; 95% CI, 1.24-1.41) increased risk for UC, with risk increasing with increased AD severity.
Robust data with cautionary note
“This study provides the most robust data to date on the association between IBD and AD. It provides clear evidence for an association that most dermatologists or primary care providers are not typically taught in training,” Kelly Scarberry, MD, assistant professor of dermatology at Case Western Reserve University in Cleveland, told this news organization. “I will be much more likely to pursue diagnostic workup in my AD patients who have GI complaints.”
However, AD severity was measured by proxy, added Dr. Scarberry, who was not involved in the study, and the study lacked important racial and ethnic data.
Lindsay C. Strowd, MD, associate professor of dermatology at Wake Forest University, Winston-Salem, N.C., also not involved in the study, said in an interview that she found the size of the cohort and the longitudinal data to be strengths of the study.
But, she added, the “lack of family IBD history, race and ethnicity, and comorbidities, are limitations, as is treatment exposure used as a proxy for disease severity, given that physician treatment practices differ.”
For Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest, “the most important conclusion, and it is a definitive finding, [is] that IBD is uncommon, even in patients with AD.
“The findings could be misinterpreted,” cautioned Dr. Feldman, who was not involved in the study. “While there is an increased relative risk, the absolute risk is small.” The study found that “the highest relative risk group is children with severe AD, who have a roughly fivefold increased risk for CD.” However, he added, the incidence rates of CD were 0.68 per 1,000 person-years in children with severe AD and 0.08 per 1,000 person-years in controls.
“Basically, because Crohn’s disease and IBD don’t happen very often, the modest increase in relative risk the investigators found doesn’t amount to much we’d have to worry about,” he said. “The findings do not show any need to screen patients with atopic dermatitis for IBD any more than we’d need to screen patients without atopic dermatitis.”
The increased relative risk “could be a clue to possible genetic connections between diseases,” he added. “But when we’re making clinical decisions, those decisions should be based on the absolute risk that some event may occur.”
Susan Massick, MD, dermatologist and associate professor at The Ohio State University in Columbus, who was not involved with the study, said in an interview, “We are still scratching the surface of the complexity of the immune and inflammatory pathways in AD and IBD.
“It is important to remember that correlation does not mean causation,” Dr. Massick said. “It would be premature to draw direct conclusions based on this study alone.”
The authors recommend future related studies in more diverse populations.
Dr. Gelfand and two coauthors reported ties with Pfizer, which supported the study. Dr. Gelfand and three coauthors reported ties with other pharmaceutical companies. Dr. Maguiness, Dr. Scarberry, Dr. Strowd, and Dr. Massick reported having no relevant disclosures. Dr. Feldman reported ties with Pfizer and other pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
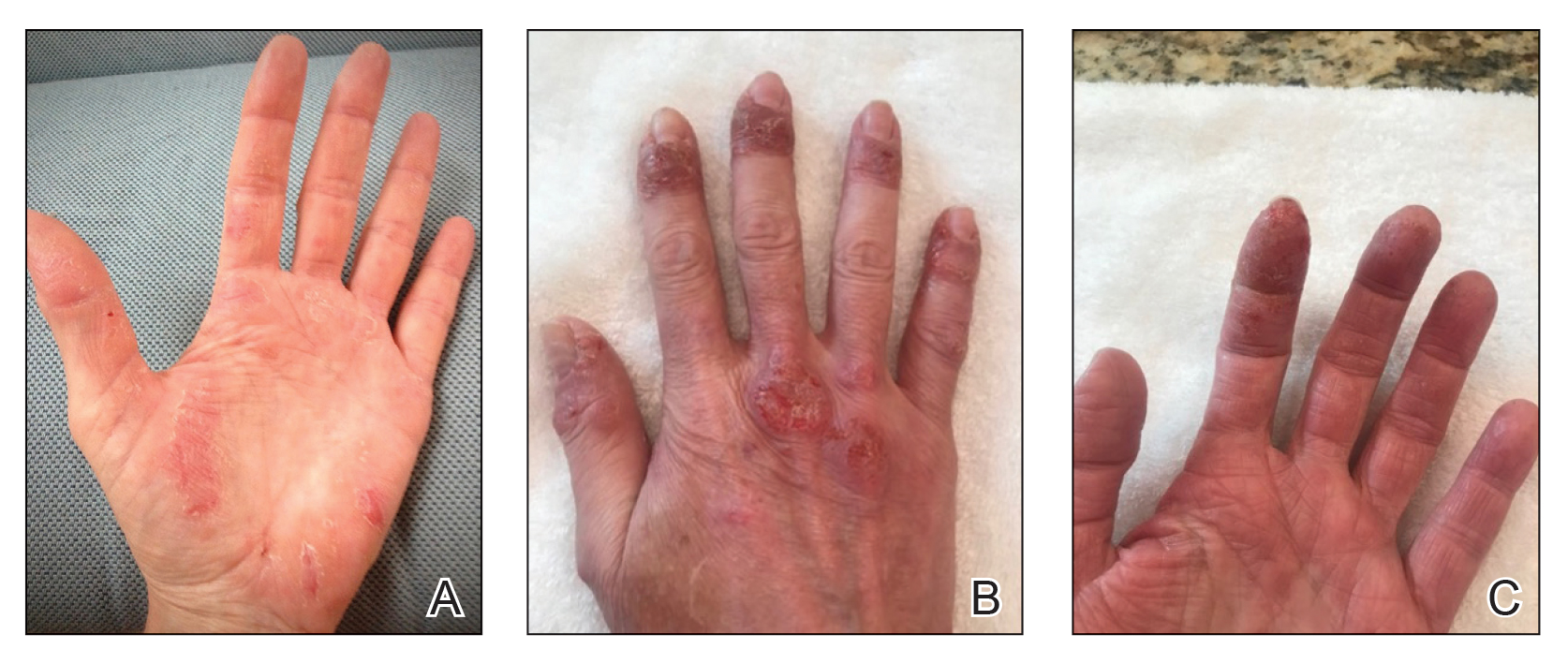
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
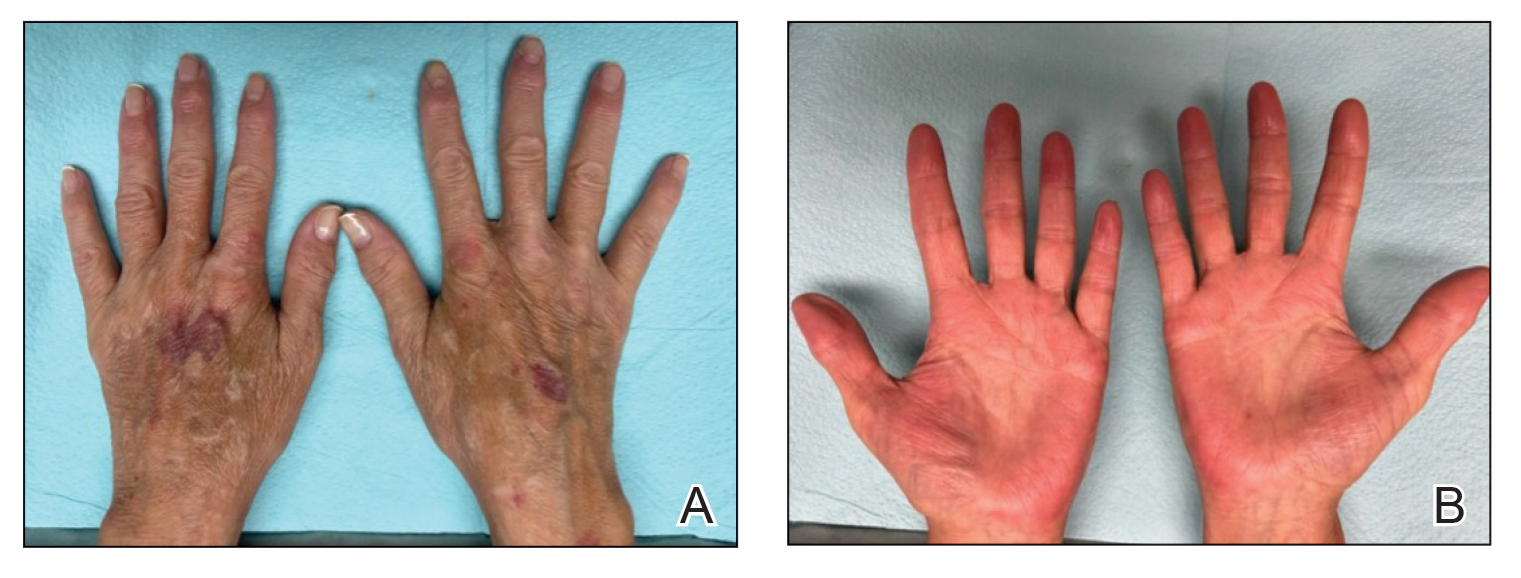
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Commentary: Are "significant" results necessarily clinically meaningful? October 2023
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
CHMP recommends marketing of biologic for atopic dermatitis
The who are candidates for systemic therapy.
Lebrikizumab is an investigational, monoclonal antibody that binds to cytokine interleukin (IL)-13, which has been implicated in driving the type-2 inflammatory loop in the skin, leading to skin barrier dysfunction, itch, skin thickening, and infection. The biologic is being developed by Almirall and is designed to be administered once per month. Lebrikizumab is not yet available in the United States.
According to a press release from Almirall, the CHMP opinion was based on three pivotal phase 3 studies that showed long-term response in skin clearance and itch control. ADvocate 1 and ADvocate 2 evaluated lebrikizumab as monotherapy, while ADhere assessed lebrikizumab in combination with topical corticosteroids (TCS) in adult and adolescent patients with moderate to severe AD. At week 16, more than 50% of patients with moderate to severe AD experienced at least a 75% reduction in disease severity (EASI-75) when receiving lebrikizumab monotherapy in the ADvocate studies and nearly 70% of patients receiving lebrikizumab combined with standard-of-care TCS achieved EASI-75 in the ADhere trial.
Most adverse events across the studies were mild or moderate. The most common reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
The who are candidates for systemic therapy.
Lebrikizumab is an investigational, monoclonal antibody that binds to cytokine interleukin (IL)-13, which has been implicated in driving the type-2 inflammatory loop in the skin, leading to skin barrier dysfunction, itch, skin thickening, and infection. The biologic is being developed by Almirall and is designed to be administered once per month. Lebrikizumab is not yet available in the United States.
According to a press release from Almirall, the CHMP opinion was based on three pivotal phase 3 studies that showed long-term response in skin clearance and itch control. ADvocate 1 and ADvocate 2 evaluated lebrikizumab as monotherapy, while ADhere assessed lebrikizumab in combination with topical corticosteroids (TCS) in adult and adolescent patients with moderate to severe AD. At week 16, more than 50% of patients with moderate to severe AD experienced at least a 75% reduction in disease severity (EASI-75) when receiving lebrikizumab monotherapy in the ADvocate studies and nearly 70% of patients receiving lebrikizumab combined with standard-of-care TCS achieved EASI-75 in the ADhere trial.
Most adverse events across the studies were mild or moderate. The most common reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
The who are candidates for systemic therapy.
Lebrikizumab is an investigational, monoclonal antibody that binds to cytokine interleukin (IL)-13, which has been implicated in driving the type-2 inflammatory loop in the skin, leading to skin barrier dysfunction, itch, skin thickening, and infection. The biologic is being developed by Almirall and is designed to be administered once per month. Lebrikizumab is not yet available in the United States.
According to a press release from Almirall, the CHMP opinion was based on three pivotal phase 3 studies that showed long-term response in skin clearance and itch control. ADvocate 1 and ADvocate 2 evaluated lebrikizumab as monotherapy, while ADhere assessed lebrikizumab in combination with topical corticosteroids (TCS) in adult and adolescent patients with moderate to severe AD. At week 16, more than 50% of patients with moderate to severe AD experienced at least a 75% reduction in disease severity (EASI-75) when receiving lebrikizumab monotherapy in the ADvocate studies and nearly 70% of patients receiving lebrikizumab combined with standard-of-care TCS achieved EASI-75 in the ADhere trial.
Most adverse events across the studies were mild or moderate. The most common reactions were conjunctivitis, injection site reactions, allergic conjunctivitis, and dry eye.
Upadacitinib an effective treatment option in AD patients with recent discontinuation of dupilumab
Key clinical point: Upadacitinib was safe and effective in patients with atopic dermatitis (AD) who recently discontinued dupilumab therapy due to lack of desired efficacy or an adverse event (AE).
Major finding: By week 16, a ≥75% improvement in the Eczema Area and Severity Index score or an Investigator’s Global Assessment score of 0 or 1 was achieved by 75% and 81.8% of patients receiving 15 mg and 30 mg upadacitinib, respectively. The treatment-related AE rate was 30.7%, and none of the patients discontinued treatment during the 16-week treatment period.
Study details: Findings are from a real-world multicenter retrospective study that included 39 adult patients with AD who were treated with upadacitinib after discontinuing treatment with dupilumab due to inefficacy or an AE.
Disclosures: This study did not receive any funding. Two authors declared serving as advisors, consultants, speakers, or investigators for various organizations. The other authors declared no conflicts of interest.
Source: Georgakopoulos JR et al. Real-world effectiveness and safety of upadacitinib for the treatment of atopic dermatitis in adult patients switched from dupilumab: A multicenter retrospective study. J Am Acad Dermatol. 2023 (Aug 28). doi: 10.1016/j.jaad.2023.08.059
Key clinical point: Upadacitinib was safe and effective in patients with atopic dermatitis (AD) who recently discontinued dupilumab therapy due to lack of desired efficacy or an adverse event (AE).
Major finding: By week 16, a ≥75% improvement in the Eczema Area and Severity Index score or an Investigator’s Global Assessment score of 0 or 1 was achieved by 75% and 81.8% of patients receiving 15 mg and 30 mg upadacitinib, respectively. The treatment-related AE rate was 30.7%, and none of the patients discontinued treatment during the 16-week treatment period.
Study details: Findings are from a real-world multicenter retrospective study that included 39 adult patients with AD who were treated with upadacitinib after discontinuing treatment with dupilumab due to inefficacy or an AE.
Disclosures: This study did not receive any funding. Two authors declared serving as advisors, consultants, speakers, or investigators for various organizations. The other authors declared no conflicts of interest.
Source: Georgakopoulos JR et al. Real-world effectiveness and safety of upadacitinib for the treatment of atopic dermatitis in adult patients switched from dupilumab: A multicenter retrospective study. J Am Acad Dermatol. 2023 (Aug 28). doi: 10.1016/j.jaad.2023.08.059
Key clinical point: Upadacitinib was safe and effective in patients with atopic dermatitis (AD) who recently discontinued dupilumab therapy due to lack of desired efficacy or an adverse event (AE).
Major finding: By week 16, a ≥75% improvement in the Eczema Area and Severity Index score or an Investigator’s Global Assessment score of 0 or 1 was achieved by 75% and 81.8% of patients receiving 15 mg and 30 mg upadacitinib, respectively. The treatment-related AE rate was 30.7%, and none of the patients discontinued treatment during the 16-week treatment period.
Study details: Findings are from a real-world multicenter retrospective study that included 39 adult patients with AD who were treated with upadacitinib after discontinuing treatment with dupilumab due to inefficacy or an AE.
Disclosures: This study did not receive any funding. Two authors declared serving as advisors, consultants, speakers, or investigators for various organizations. The other authors declared no conflicts of interest.
Source: Georgakopoulos JR et al. Real-world effectiveness and safety of upadacitinib for the treatment of atopic dermatitis in adult patients switched from dupilumab: A multicenter retrospective study. J Am Acad Dermatol. 2023 (Aug 28). doi: 10.1016/j.jaad.2023.08.059