User login
FDA grants new indication to lumateperone (Caplyta) for bipolar depression
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has expanded approval of lumateperone (Caplyta) to include treatment of adults with depressive episodes associated with bipolar I and II disorder, as monotherapy or adjunctive therapy with lithium or valproate.
This makes lumateperone the only FDA-approved drug for this indication.
“The efficacy, and favorable safety and tolerability profile, make Caplyta an important treatment option for the millions of patients living with bipolar I or II depression and represents a major development for these patients,” Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said in a company news release.
Lumateperone was first approved by the FDA in 2019 for the treatment of adults with schizophrenia.
‘Positioned to launch immediately’
that showed treatment with lumateperone, alone or with lithium or valproate, significantly improved depressive symptoms for patients with major depressive episodes associated with bipolar I and bipolar II disorders.
In these studies, treatment with a 42-mg once-daily dose was associated with significantly greater improvement from baseline in Montgomery-Åsberg Depression Rating Scale score versus placebo.
Lumateperone also showed a statistically significant improvement in the key secondary endpoint relating to clinical global impression of bipolar disorder.
Somnolence/sedation, dizziness, nausea, and dry mouth were the most commonly reported adverse events associated with the medication. Minimal changes were observed in weight and vital signs and in results of metabolic or endocrine assessments. Incidence of extrapyramidal symptom–related events was low and was similar to those with placebo.
Sharon Mates, PhD, chairman and CEO of Intra-Cellular Therapies, noted in the same press release that the company is “positioned to launch immediately and are excited to offer Caplyta to the millions of patients living with bipolar depression.”
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
More evidence ties some antipsychotics to increased breast cancer risk
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
New research provides more evidence that antipsychotics that raise prolactin levels are tied to a significantly increased risk for breast cancer.
The relative risk for breast cancer was 62% higher in women who took category 1 antipsychotic medications associated with high prolactin levels. These include haloperidol (Haldol), paliperidone (Invega), and risperidone (Risperdal). Additionally, the risk was 54% higher in those taking category 2 antipsychotics that have mid-range effects on prolactin. These include iloperidone (Fanapt), lurasidone (Latuda), and olanzapine (Zyprexa).
In contrast, category 3 antipsychotics which have a lesser effect on prolactin levels were not associated with any increase in breast cancer risk. These drugs include aripiprazole (Abilify), asenapine (Saphris), brexpiprazole (Rexulti), cariprazine (Vraylar), clozapine (multiple brands), quetiapine (Seroquel), and ziprasidone (Geodon).
While the “absolute” breast cancer risk for these drugs is unclear, “we can make the case that high circulating prolactin levels are associated with breast cancer risk. This follows what is already known about prolactin from prior studies, notably the nurses’ health studies,” Tahir Rahman, MD, associate professor of psychiatry, Washington University School of Medicine, St. Louis, told this news organization.
“We don’t want to alarm patients taking antipsychotic drugs for life-threatening mental health problems, but we also think it is time for doctors to track prolactin levels and vigilantly monitor their patients who are being treated with antipsychotics,” Dr. Rahman added in a news release.
The study was published online Dec. 3 in the Journal of Clinical Psychopharmacology.
Test prolactin levels
Using administrative claims data, the researchers evaluated breast cancer risk in women aged 18-64 exposed to antipsychotic medications compared with anticonvulsants and/or lithium.
They identified 914 cases of invasive breast cancer among 540,737 women.
Roughly 52% of the study population filled at least one prescription for a category 3 antipsychotic agent, whereas 15% filled at least one prescription for a category 1 agent; 49% of women filled at least one prescription for an anticonvulsant medication during the study period.
Exposure to all antipsychotics was independently associated with a 35% increased risk for breast cancer (adjusted hazard ratio, 1.35; 95% CI, 1.14-1.61), the study team found.
Compared with anticonvulsants or lithium, the risk for breast cancer was significantly increased for high prolactin (category 1) antipsychotics (adjusted hazard ratio, 1.62; 95% CI, 1.30-2.03) and for mid-prolactin (category 2) drugs (aHR 1.54; 95% CI, 1.19-1.99), with no increased risk for category 3 antipsychotics.
“Our research is obviously of interest for preventing breast cancer in antipsychotic-treated patients. Checking a blood prolactin level is cheap and easy [and a high level is] fairly simple to mitigate,” said Dr. Rahman.
A matter of debate
Reached for comment, Christoph Correll, MD, professor of psychiatry and molecular medicine, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, said, “The potential elevation of breast cancer risk depending on the dose and time of treatment with antipsychotic medications with varying degrees of prolactin-raising properties has been a topic of research and matter of debate.”
This new study “adds another data point indicating that antipsychotics that are associated on average with a higher prolactin-raising effect than other antipsychotics may increase the risk of breast cancer in women to some degree,” said Dr. Correll, who was not involved with the study.
However, he cautioned that “naturalistic data are always vulnerable to residual confounding, for example, unmeasured effects that could also at least partially explain the results, and the follow-up time of only 4 years (maximum 6 years) in this study was relatively short.
“Nevertheless, given availability of many different antipsychotics with varying degrees of prolactin-raising potential, in women requiring antipsychotic treatment, less prolactin-raising antipsychotics may be preferable,” Dr. Correll said.
“In women receiving prolactin-raising antipsychotics for medium- and longer-term maintenance therapy, prolactin levels should be monitored,” he added.
When an elevated prolactin level is detected, this should be addressed “either via dose reduction, a switch to an alternative antipsychotic that does not raise prolactin levels significantly, or the addition of a partial or full D2 agonist when the prolactin-raising antipsychotic should be continued based on individualized risk assessment,” Dr. Correll advised.
This work was supported by an award from the Alvin J. Siteman Cancer Center; the National Cancer Institute and the National Center for Advancing Translational Sciences of the National Institutes of Health; the Taylor Family Institute for Innovative Psychiatric Research; and the Center for Brain Research in Mood Disorders. The authors have disclosed no relevant financial relationships. Dr. Correll has received royalties from UpToDate and is a stock option holder of LB Pharma.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHOPHARMACOLOGY
Case report: ECT for delirious mania
Delirious mania is a diagnostic term used in variety of settings, including the emergency department and inpatient psychiatry, but it does not have formal criteria established in the DSM-5. Delirious mania was first described in the 1800s and was referred to as “Bell’s Mania.”
As the late Max Fink, MD, wrote in the journal Bipolar Disorders (2002 Feb 23. doi: 10.1034/j.1399-561.1999.10112.x), delirious mania is considered to be a syndrome of the acute onset of the excitement, grandiosity, emotional lability, delusions, and insomnia characteristic of mania, and the disorientation and altered consciousness characteristic of delirium.
Such patients can be considered as having a component of bipolar I disorder, comprising mania with psychotic features. Delirious mania is associated with higher rates of morbidity and mortality, and demonstrates limited response to conventional treatment guidelines. Therefore, early detection and decisive treatment are imperative. The concurrence of delirium and mania is not unusual, yet currently there are no universal accepted treatment guidelines for delirious mania (BMC Psychiatry. 2012 Jun 21. doi: 10.1186/1471-244X-12-65). The purpose of this case report is to inspire and support community psychiatric clinicians in managing such complex cases and to improve behavioral health care outcomes. To protect our patient’s identity, we changed several key identifiers.
The treatment plan emerges
This case is of a middle-aged man with an established diagnosis of bipolar disorder. He was referred to the ED because of worsening manic symptoms marked by mood lability, pressured speech, grandiose delusions, tangential thought processes, poor insight, and impaired sleep.
Laboratory studies in the ED revealed hyponatremia and serum sodium of 126meq/l (ref. range: 135-146). The patient’s toxicology screen was positive for benzodiazepines. He was stabilized on the medical floor and then transitioned to inpatient psychiatry.
Before his admission to psychiatry, the patient’s medications were alprazolam 1 mg at bed time, bupropion 100 mg twice daily, loxapine 25 mg morning and 50 mg at bed time, olanzapine 20 mg at bedtime and 5 mg twice daily, risperidone 2 mg twice daily and oxcarbazepine 900 mg twice daily.
The bupropion was discontinued because of manic behavior, and the patient’s dose of oxcarbazepine was lowered from 900 mg twice daily to 450 mg twice daily because of hyponatremia. Our team continued to administer risperidone, olanzapine, loxapine, and alprazolam to the patient. However, he was agitated and disorganized on the psychiatry floor. In addition, we noticed that the patient exhibited confusion, disorientation, an inability to connect with reality, and periods of profound agitation.
The patient was frequently restrained physically, and medications were administered to him for safety and containment. The use of benzodiazepines and anticholinergics was minimized. However, we noticed that the patient acted paranoid, disinhibited, and combative, and he became difficult to restrain. He seemed to have a high pain tolerance, responded to internal stimuli, and began hallucinating and displaying aggressive behavior toward staff persons.
It became apparent that the patient’s circadian rhythm had been altered. He slept for only a couple of hours during the day. During the course of treatment, in one incidence, the patient became agitated and charged at a nurse. Subsequently, the patient hit his head on a wall and fell – suffering a head strike and lacerations.
The team conducted investigations, including labs and neuroimaging, to make sure that the patient was OK. His CT head scan proved unremarkable. Liver function tests revealed mild transaminitis. His TSH, folate, B12, and B1 levels were normal.
We then placed the patient in a single room with continuous behavior monitoring. His recovery seemed to take a long time with trials of different antipsychotic medications, including olanzapine, loxapine, risperidone, and paliperidone. Because of his poor response to medications, the team considered using electroconvulsive therapy (ECT).
However, the patient was unable to give informed consent for ECT because of his impaired mental status. At this point, our team submitted a substitute treatment plan that included ECT to the court for approval, and the court approved our plan.
After receiving approximately four bilateral ECT procedures three times a week, the patient’s condition started to improve gradually. He received total of 11 procedures.
Our patient became alert to time, place, and person, and his circadian rhythm normalized. Soon, his delirium cleared, and he demonstrated marked improvement in both insight into his illness and behavioral control. His grandiose delusions were still present, but he was easily redirectable. In addition, our patient demonstrated improved reality testing. He was able to be discharged home following medication adjustments and with community supports within a few short weeks of receiving ECT.
As Bo-Shyan Lee, MD, and associates reported (BMC Psychiatry. 2012 Jun 21;12:65. doi: 10.1186/1471-244X-12-65), delirious mania is closely related to catatonia. Although there are different definitions for delirium and catatonia, even the most lethal form of catatonia meets the criteria for delirium. ECT is a well established first-line treatment for catatonia. This tool has been shown to be highly effective in the treatment of delirious mania. Delirious mania can be life-threatening and should be managed aggressively. The most common causes of death are heart failure from arrhythmias, cardiac arrest, and respiratory failure. ECT is a safe treatment, and, as Dr. Fink argued, the mortality rate is even less than that associated with normal pregnancies (World J Biol Psychiatry. 2001 Jan;2[1]:1-8). In light of the safety and effectiveness of ECT, we think the tool should be considered not only in university hospital settings but as an early intervention in community settings. This case warrants further research in exploring hyperactive delirium and delirious mania.
Dr. Lamba is BR-2 unit medical director at BayRidge Hospital in Lynn, Mass. Ms. Kennedy is an attending clinician at BayRidge. Dr. Vu is medical director at BayRidge. He also serves as associate chief of psychiatry at Beverly (Mass.) Hospital and at Addison Gilbert Hospital in Gloucester, Mass. Dr. Lamba, Ms. Kennedy, and Dr. Vu have no disclosures.
Delirious mania is a diagnostic term used in variety of settings, including the emergency department and inpatient psychiatry, but it does not have formal criteria established in the DSM-5. Delirious mania was first described in the 1800s and was referred to as “Bell’s Mania.”
As the late Max Fink, MD, wrote in the journal Bipolar Disorders (2002 Feb 23. doi: 10.1034/j.1399-561.1999.10112.x), delirious mania is considered to be a syndrome of the acute onset of the excitement, grandiosity, emotional lability, delusions, and insomnia characteristic of mania, and the disorientation and altered consciousness characteristic of delirium.
Such patients can be considered as having a component of bipolar I disorder, comprising mania with psychotic features. Delirious mania is associated with higher rates of morbidity and mortality, and demonstrates limited response to conventional treatment guidelines. Therefore, early detection and decisive treatment are imperative. The concurrence of delirium and mania is not unusual, yet currently there are no universal accepted treatment guidelines for delirious mania (BMC Psychiatry. 2012 Jun 21. doi: 10.1186/1471-244X-12-65). The purpose of this case report is to inspire and support community psychiatric clinicians in managing such complex cases and to improve behavioral health care outcomes. To protect our patient’s identity, we changed several key identifiers.
The treatment plan emerges
This case is of a middle-aged man with an established diagnosis of bipolar disorder. He was referred to the ED because of worsening manic symptoms marked by mood lability, pressured speech, grandiose delusions, tangential thought processes, poor insight, and impaired sleep.
Laboratory studies in the ED revealed hyponatremia and serum sodium of 126meq/l (ref. range: 135-146). The patient’s toxicology screen was positive for benzodiazepines. He was stabilized on the medical floor and then transitioned to inpatient psychiatry.
Before his admission to psychiatry, the patient’s medications were alprazolam 1 mg at bed time, bupropion 100 mg twice daily, loxapine 25 mg morning and 50 mg at bed time, olanzapine 20 mg at bedtime and 5 mg twice daily, risperidone 2 mg twice daily and oxcarbazepine 900 mg twice daily.
The bupropion was discontinued because of manic behavior, and the patient’s dose of oxcarbazepine was lowered from 900 mg twice daily to 450 mg twice daily because of hyponatremia. Our team continued to administer risperidone, olanzapine, loxapine, and alprazolam to the patient. However, he was agitated and disorganized on the psychiatry floor. In addition, we noticed that the patient exhibited confusion, disorientation, an inability to connect with reality, and periods of profound agitation.
The patient was frequently restrained physically, and medications were administered to him for safety and containment. The use of benzodiazepines and anticholinergics was minimized. However, we noticed that the patient acted paranoid, disinhibited, and combative, and he became difficult to restrain. He seemed to have a high pain tolerance, responded to internal stimuli, and began hallucinating and displaying aggressive behavior toward staff persons.
It became apparent that the patient’s circadian rhythm had been altered. He slept for only a couple of hours during the day. During the course of treatment, in one incidence, the patient became agitated and charged at a nurse. Subsequently, the patient hit his head on a wall and fell – suffering a head strike and lacerations.
The team conducted investigations, including labs and neuroimaging, to make sure that the patient was OK. His CT head scan proved unremarkable. Liver function tests revealed mild transaminitis. His TSH, folate, B12, and B1 levels were normal.
We then placed the patient in a single room with continuous behavior monitoring. His recovery seemed to take a long time with trials of different antipsychotic medications, including olanzapine, loxapine, risperidone, and paliperidone. Because of his poor response to medications, the team considered using electroconvulsive therapy (ECT).
However, the patient was unable to give informed consent for ECT because of his impaired mental status. At this point, our team submitted a substitute treatment plan that included ECT to the court for approval, and the court approved our plan.
After receiving approximately four bilateral ECT procedures three times a week, the patient’s condition started to improve gradually. He received total of 11 procedures.
Our patient became alert to time, place, and person, and his circadian rhythm normalized. Soon, his delirium cleared, and he demonstrated marked improvement in both insight into his illness and behavioral control. His grandiose delusions were still present, but he was easily redirectable. In addition, our patient demonstrated improved reality testing. He was able to be discharged home following medication adjustments and with community supports within a few short weeks of receiving ECT.
As Bo-Shyan Lee, MD, and associates reported (BMC Psychiatry. 2012 Jun 21;12:65. doi: 10.1186/1471-244X-12-65), delirious mania is closely related to catatonia. Although there are different definitions for delirium and catatonia, even the most lethal form of catatonia meets the criteria for delirium. ECT is a well established first-line treatment for catatonia. This tool has been shown to be highly effective in the treatment of delirious mania. Delirious mania can be life-threatening and should be managed aggressively. The most common causes of death are heart failure from arrhythmias, cardiac arrest, and respiratory failure. ECT is a safe treatment, and, as Dr. Fink argued, the mortality rate is even less than that associated with normal pregnancies (World J Biol Psychiatry. 2001 Jan;2[1]:1-8). In light of the safety and effectiveness of ECT, we think the tool should be considered not only in university hospital settings but as an early intervention in community settings. This case warrants further research in exploring hyperactive delirium and delirious mania.
Dr. Lamba is BR-2 unit medical director at BayRidge Hospital in Lynn, Mass. Ms. Kennedy is an attending clinician at BayRidge. Dr. Vu is medical director at BayRidge. He also serves as associate chief of psychiatry at Beverly (Mass.) Hospital and at Addison Gilbert Hospital in Gloucester, Mass. Dr. Lamba, Ms. Kennedy, and Dr. Vu have no disclosures.
Delirious mania is a diagnostic term used in variety of settings, including the emergency department and inpatient psychiatry, but it does not have formal criteria established in the DSM-5. Delirious mania was first described in the 1800s and was referred to as “Bell’s Mania.”
As the late Max Fink, MD, wrote in the journal Bipolar Disorders (2002 Feb 23. doi: 10.1034/j.1399-561.1999.10112.x), delirious mania is considered to be a syndrome of the acute onset of the excitement, grandiosity, emotional lability, delusions, and insomnia characteristic of mania, and the disorientation and altered consciousness characteristic of delirium.
Such patients can be considered as having a component of bipolar I disorder, comprising mania with psychotic features. Delirious mania is associated with higher rates of morbidity and mortality, and demonstrates limited response to conventional treatment guidelines. Therefore, early detection and decisive treatment are imperative. The concurrence of delirium and mania is not unusual, yet currently there are no universal accepted treatment guidelines for delirious mania (BMC Psychiatry. 2012 Jun 21. doi: 10.1186/1471-244X-12-65). The purpose of this case report is to inspire and support community psychiatric clinicians in managing such complex cases and to improve behavioral health care outcomes. To protect our patient’s identity, we changed several key identifiers.
The treatment plan emerges
This case is of a middle-aged man with an established diagnosis of bipolar disorder. He was referred to the ED because of worsening manic symptoms marked by mood lability, pressured speech, grandiose delusions, tangential thought processes, poor insight, and impaired sleep.
Laboratory studies in the ED revealed hyponatremia and serum sodium of 126meq/l (ref. range: 135-146). The patient’s toxicology screen was positive for benzodiazepines. He was stabilized on the medical floor and then transitioned to inpatient psychiatry.
Before his admission to psychiatry, the patient’s medications were alprazolam 1 mg at bed time, bupropion 100 mg twice daily, loxapine 25 mg morning and 50 mg at bed time, olanzapine 20 mg at bedtime and 5 mg twice daily, risperidone 2 mg twice daily and oxcarbazepine 900 mg twice daily.
The bupropion was discontinued because of manic behavior, and the patient’s dose of oxcarbazepine was lowered from 900 mg twice daily to 450 mg twice daily because of hyponatremia. Our team continued to administer risperidone, olanzapine, loxapine, and alprazolam to the patient. However, he was agitated and disorganized on the psychiatry floor. In addition, we noticed that the patient exhibited confusion, disorientation, an inability to connect with reality, and periods of profound agitation.
The patient was frequently restrained physically, and medications were administered to him for safety and containment. The use of benzodiazepines and anticholinergics was minimized. However, we noticed that the patient acted paranoid, disinhibited, and combative, and he became difficult to restrain. He seemed to have a high pain tolerance, responded to internal stimuli, and began hallucinating and displaying aggressive behavior toward staff persons.
It became apparent that the patient’s circadian rhythm had been altered. He slept for only a couple of hours during the day. During the course of treatment, in one incidence, the patient became agitated and charged at a nurse. Subsequently, the patient hit his head on a wall and fell – suffering a head strike and lacerations.
The team conducted investigations, including labs and neuroimaging, to make sure that the patient was OK. His CT head scan proved unremarkable. Liver function tests revealed mild transaminitis. His TSH, folate, B12, and B1 levels were normal.
We then placed the patient in a single room with continuous behavior monitoring. His recovery seemed to take a long time with trials of different antipsychotic medications, including olanzapine, loxapine, risperidone, and paliperidone. Because of his poor response to medications, the team considered using electroconvulsive therapy (ECT).
However, the patient was unable to give informed consent for ECT because of his impaired mental status. At this point, our team submitted a substitute treatment plan that included ECT to the court for approval, and the court approved our plan.
After receiving approximately four bilateral ECT procedures three times a week, the patient’s condition started to improve gradually. He received total of 11 procedures.
Our patient became alert to time, place, and person, and his circadian rhythm normalized. Soon, his delirium cleared, and he demonstrated marked improvement in both insight into his illness and behavioral control. His grandiose delusions were still present, but he was easily redirectable. In addition, our patient demonstrated improved reality testing. He was able to be discharged home following medication adjustments and with community supports within a few short weeks of receiving ECT.
As Bo-Shyan Lee, MD, and associates reported (BMC Psychiatry. 2012 Jun 21;12:65. doi: 10.1186/1471-244X-12-65), delirious mania is closely related to catatonia. Although there are different definitions for delirium and catatonia, even the most lethal form of catatonia meets the criteria for delirium. ECT is a well established first-line treatment for catatonia. This tool has been shown to be highly effective in the treatment of delirious mania. Delirious mania can be life-threatening and should be managed aggressively. The most common causes of death are heart failure from arrhythmias, cardiac arrest, and respiratory failure. ECT is a safe treatment, and, as Dr. Fink argued, the mortality rate is even less than that associated with normal pregnancies (World J Biol Psychiatry. 2001 Jan;2[1]:1-8). In light of the safety and effectiveness of ECT, we think the tool should be considered not only in university hospital settings but as an early intervention in community settings. This case warrants further research in exploring hyperactive delirium and delirious mania.
Dr. Lamba is BR-2 unit medical director at BayRidge Hospital in Lynn, Mass. Ms. Kennedy is an attending clinician at BayRidge. Dr. Vu is medical director at BayRidge. He also serves as associate chief of psychiatry at Beverly (Mass.) Hospital and at Addison Gilbert Hospital in Gloucester, Mass. Dr. Lamba, Ms. Kennedy, and Dr. Vu have no disclosures.
Is it bipolar disorder, or a complex form of PTSD?
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
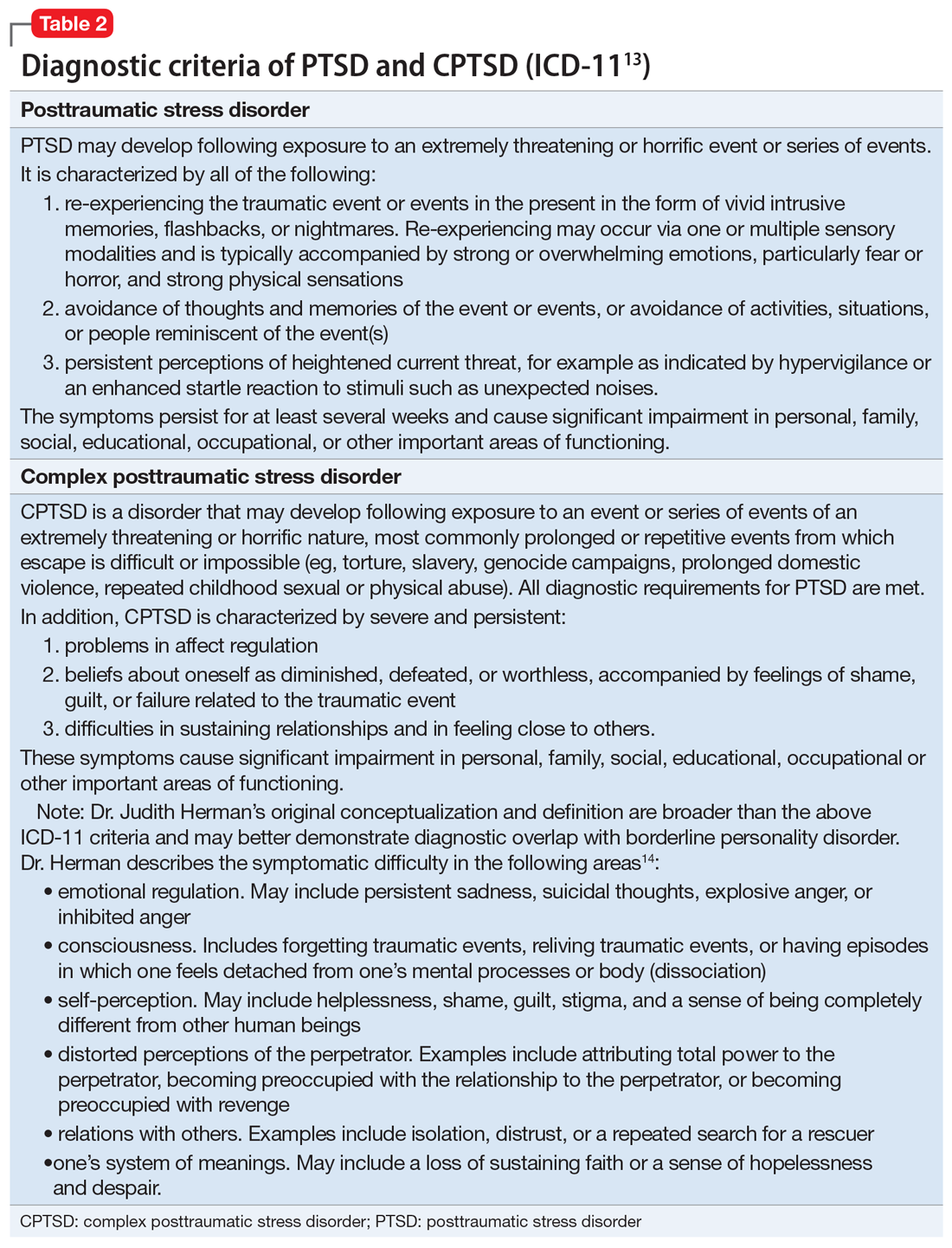
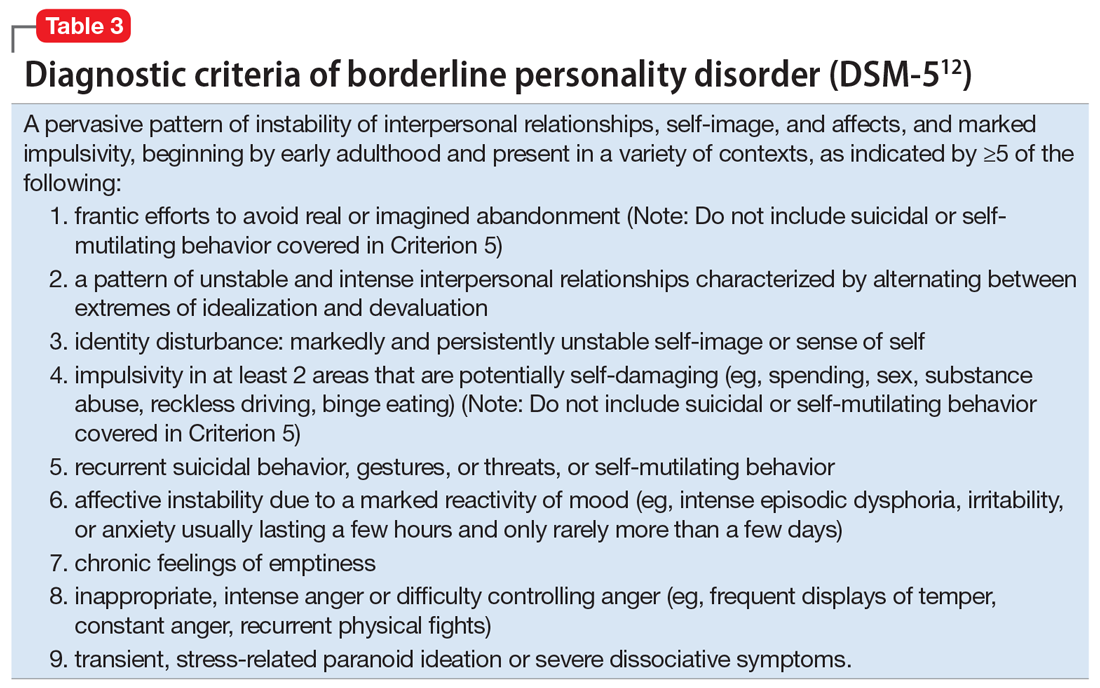
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.

Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
CASE A long history of suicidality
Mr. X, age 26, who has a history of bipolar II disorder and multiple inpatient admissions, presents to a state hospital after a suicide attempt by gunshot. He reports that throughout his lifetime, he has had >20 suicide attempts, often by overdose.
Mr. X is admitted to the hospital under a temporary detention order. He is initially adherent and cooperative with his psychiatric evaluations.
HISTORY Chronic physical and emotional pain
Mr. X is single, unemployed, and lives with his mother and nephew. He was diagnosed with bipolar II disorder during adolescence and receives sertraline, 50 mg twice a day, and lamotrigine, 100 mg twice a day, to which he reports adherence. He also was taking clonazepam and zolpidem, dosages unknown.
His medical history is significant for severe childhood liver disease and inflammatory bowel disease. He dropped out of school during high school due to his multiple medical conditions, which resulted in a significantly diminished overall childhood experience, interrupted developmental trajectory, and chronic physical and emotional pain. He has never been employed and receives financial support through disability benefits. He spends his days on the internet or watching television. He reports daily cigarette and marijuana use and occasional alcohol use, but no other substance use. His mother helps manage his medical conditions and is his main support. His biological father was abusive towards his mother and absent for most of Mr. X’s life. Beyond his mother and therapist, Mr. X has minimal other interpersonal interactions, and reports feeling isolated, lonely, and frustrated.
EVALUATION Agitated and aggressive while hospitalized
Upon learning that he is being involuntarily committed, Mr. X becomes physically aggressive, makes verbal threats, and throws objects across his room. He is given diphenhydramine, 50 mg, haloperidol, 5 mg, and lorazepam, 2 mg, all of which are ordered on an as-needed basis. Mr. X is placed in an emergency restraint chair and put in seclusion. The episode resolves within an hour with reassurance and attention from the treatment team; the rapid escalation from and return to a calmer state is indicative of situational, stress-induced mood lability and impulsivity. Mr. X is counseled on maintaining safety and appropriate behavior, and is advised to ask for medication if he feels agitated or unable to control his behaviors. To maintain safe and appropriate behavior, he requires daily counseling and expectation management regarding his treatment timeline. No further aggressive incidents are noted throughout his hospitalization, and he requires only minimal use of the as-needed medications.
[polldaddy:10983392]
The authors’ observations
The least appropriate therapy for Mr. X would be exposure and response prevention, which allows patients to face their fears without the need to soothe or relieve related feelings with a compulsive act. It is designed to improve specific behavioral deficits most often associated with obsessive-compulsive disorder, a diagnosis inconsistent with Mr. X’s history and presentation. Trauma-focused CBT could facilitate healing from Mr. X’s childhood trauma/adverse childhood experiences, and DBT might help with his anger, maladaptive coping strategies, and chronic suicidality. Motivational interviewing might help with his substance use and his apparent lack of motivation for other forms of social engagement, including seeking employment.
Based on Mr. X’s history of trauma and chronic physical and emotional pain, the treatment team reevaluated him and reconsidered his original diagnosis.
Continue to: EVALUATION A closer look at the diagnosis...
EVALUATION A closer look at the diagnosis
After meeting with Mr. X, the treatment team begins to piece together a more robust picture of him. They review his childhood trauma involving his biological father, his chronic and limiting medical illnesses, and his restricted and somewhat regressive level of functioning. Further, they consider his >20 suicide attempts, numerous psychiatric hospitalizations, and mood and behavioral lability and reactivity. Based on its review, the treatment team concludes that a diagnosis of bipolar disorder II or major depressive disorder is not fully adequate to describe Mr. X’s clinical picture.
At no point during his hospitalization does Mr. X meet full criteria for a major depressive episode or display mania or hypomania. The treatment team considers posttraumatic stress disorder (PTSD) in the setting of chronic, repetitive trauma given Mr. X’s nightmares, dissociative behavior, anger, negative cognitions, and intrusive symptoms. However, not all his symptoms fall within the diagnostic criteria of PTSD. There are also elements of borderline personality disorder in Mr. X’s history, most notably his multiple suicide attempts, emotional lability, and disrupted interpersonal attachments. In this context, a diagnosis of complex PTSD (CPTSD) seems most appropriate in capturing the array of trauma-related symptoms with which he presents.
Complex PTSD
Since at least the early to mid-1990s, there has been recognition of a qualitatively distinct clinical picture that can emerge when an individual’s exposure to trauma or adversity is chronic or repetitive, causing not only familiar PTSD symptomatology but also alterations in self-perception, interpersonal functioning, and affective instability. Complex PTSD was first described by Judith Herman, MD, in 1992 as a distinct entity from PTSD.1 She theorized that PTSD derives primarily from singular traumatic events, while a distinct clinical syndrome might arise after prolonged, repeated trauma.1 A diagnosis of CPTSD might arise in situations with more chronicity than a classic single circumscribed traumatic event, such as being held in captivity, under the control of perpetrators for extended periods of time, imprisoned, or subject to prolonged sexual abuse. Herman’s description of CPTSD identifies 3 areas of psychopathology that extend beyond PTSD1:
- symptomatic refers to the complex, diffuse, and tenacious symptom presentation
- characterological focuses on the personality changes in terms of dissociation, ego-fragmentation, and identity complications
- vulnerability describes characteristic repeated harm with respect to self-mutilation or other self-injurious behaviors, and suicidality.
Taxometrics, official recognition, and controversy
Complex PTSD was proposed for inclusion in DSM-IV as “Disorders of Extreme Stress Not Otherwise Specified,” or DESNOS. Reportedly, it was interpreted as a severe presentation of PTSD, and therefore not included in the manual as a separate diagnosis.2 In contrast, ICD-10 included a CPTSD-like entity of “Enduring Personality Change After Catastrophic Event” (EPCACE). Although the existence of CPTSD as a categorically distinct diagnosis in the psychiatric mainstream has been debated and discussed for years, with many arguably unaware of its existence, clinicians and researchers specializing in trauma are well-versed in its clinical utility. As such, CPTSD was again discussed during the development of DSM-5. In an apparent attempt to balance this clinical utility with ongoing concerns about its validity as a diagnostically distinct syndrome, DSM-5 did not officially recognize CPTSD, but added several criteria to PTSD referencing changes in self-perception, affective instability, and dysphoria, as well as a dissociative subtype, effectively expanding the scope of a PTSD diagnosis to also include CPTSD symptoms when applicable. ICD-11 has taken a different direction, and officially recognizes CPTSD as a distinct diagnosis.
ICD-11 presents CPTSD as a “sibling” disorder, which it distinguishes from PTSD with high levels of dissociation, depression, and borderline personality disorder traits.3 Within this framework, the diagnosis of CPTSD requires that the PTSD criteria be met in addition to symptoms that fall into a “disturbances of self-organization” category. When parsing the symptoms of the “disturbances of self-organization” category, the overlap with borderline personality disorder symptoms is apparent.4 This overlap has given rise to yet another controversy regarding CPTSD’s categorical validity; in addition to its distinctness from PTSD, its distinctness from borderline personality disorder has also been debated. In a study examining the similarity between CPTSD and borderline personality disorder, Jowett et al5 concluded that CPTSD was associated with greater exposure to multiple traumas earlier in life and resulted in higher functional impairment than borderline personality disorder, ultimately supporting CPTSD as a separate entity with features that overlap borderline personality disorder.5 According to Ford and Courtois6 “the evidence ... suggests that a sub-group of BPD patients—who often but not always have comorbid PTSD—may be best understood and treated if CPTSD is explicitly addressed as well—and in some cases, in lieu of—BPD.”
PTSD and CPTSD may therefore both be understood to fall within a spectrum of trauma diagnoses; this paradigm postulates that there exists a wide variety of posttraumatic patient presentations, perhaps on a continuum. On the less severe side of the trauma spectrum, the symptoms traditionally seen and characterized as PTSD (such as hypervigilance, nightmares, and flashbacks) may be found, while, with increasingly severe or prolonged trauma, there may be a tendency to see more complex elements (such as dissociation, personality changes mimicking borderline personality disorder, depression, anxiety, self-injurious behavior, and suicidality).7 Nevertheless, controversy about discriminant validity still exists. A review article by Resnick et al8 argued that the existing evidence is not strong enough to support CPTSD as a standalone entity. However, Resnick et al8 agreed that a singular PTSD diagnosis has limitations, and that there is a need for more research in the field of trauma psychiatry.
Continue to: Utility of the diagnostic conceptualization...
Utility of the diagnostic conceptualization
Although the controversy surrounding the distinction of CPTSD demands categorical clarity with respect to PTSD and borderline personality disorder as a means of resolution, the diagnosis has practical applications that should not limit its use in clinical formulation or treatment planning. Comorbid diagnoses do not prevent clinicians from diagnosing and treating patients who present with complicated manifestations of trauma.9 In fact, having overlapping diagnoses would highlight the array of patient presentations that can be seen in the posttraumatic condition. Furthermore, in the pursuit of individualized care approaches, the addition of CPTSD as a diagnostic conception would allow for more integrated treatment options using a multi-modular approach.10
The addition of CPTSD as a diagnosis is helpful in determining the etiology of a patient’s presentation and therefore formulating the most appropriate treatment plan. While the 2-pronged approach of psychopharmacology and therapy is the central dogma of psychiatric care, there are many specific options to consider for each. By viewing such patients through the lens of trauma as opposed to depression and anxiety, there is a clear shift in treatment that has the potential to make more lasting impacts and progress.11
CPTSD may coexist with PTSD, but it extends beyond it to include a pleomorphic symptom picture encompassing personality changes and a high risk for repeated harm. Failure to correctly classify a patient’s presentation as a response to repetitive, prolonged trauma may result in discrimination and inappropriate or ineffective treatment recommendations.
For a comparison of the diagnostic criteria of PTSD, CPTSD, and borderline personality disorder, see Table 112, Table 2,13,14, and Table 312.



Patients with CPTSD
One of the authors (NR) has cared for several similar individuals presenting for treatment with vague diagnoses of “chronic depression and anxiety” for years, sometimes with a speculative bipolar disorder diagnosis due to situational mood swings or reactivity, and a generally poor response to both medications and psychotherapy. These patients were frustrated because none of the diagnoses seemed to fully “fit” with their pattern of symptoms or subjective experience, and treatment seemed minimally helpful. Very often, their social history revealed a variety of adversities or traumatic events, such as childhood sexual or physical abuse, a home environment plagued by domestic violence, or being raised by one or both parents with their own history of trauma, or perhaps a personality or substance use disorder. Although many of these patients’ symptom profiles aligned only partially with “typical” PTSD, they were often better captured by CPTSD, with a focus on negative self-perception and impact on close relationships. Helping the patient “connect the dots” to create a more continuous narrative, and consequently reconceptualizing the diagnosis as a complex trauma disorder, has proven effective in a number of these cases, allowing the patient to make sense of their symptoms in the context of their personal history, reducing stigma, and allowing for different avenues with medication, therapy, and self-understanding. It can also help to validate the impact of a patient’s adverse experiences and encourage a patient to view their symptoms as an understandable or even once-adaptive response to traumatic stress, rather than a sign of personal weakness or defectiveness.
TREATMENT A trauma-focused approach
Once the treatment team considersMr. X’s significant childhood trauma and reconceptualizes his behaviors through this lens, treatment is adjusted accordingly. His significant reactivity, dissociative symptoms, social impairment, and repeated suicide attempts are better understood and have more significance through a trauma lens, which provides a better explanation than a primary mood disorder.
Therapeutic interventions in the hospital are tailored according to the treatment team’s new insight. Specific DBT skills are practiced, insight-oriented therapy and motivational interviewing are used, and Mr. X and his therapist begin to explore his trauma, both from his biological father and from his intense stressors experienced because of his medical issues.
Mr. X’s mother, who is very involved in his care, is provided with education on this conceptualization and given instruction on trauma-focused therapies in the outpatient setting. While Mr. X’s medication regimen is not changed significantly, for some patients, the reformulation from a primary mood or anxiety disorder to a trauma disorder might require a change in the pharmacotherapy regimen to address behavioral symptoms such as mood reactivity or issues with sleep.
OUTCOME Decreased intensity of suicidal thoughts
By the time of discharge, Mr. X has maintained safety, with no further outbursts, and subjectively reports feeling more understood and validated. Although chronic suicidal ideation can take months or years of treatment to resolve, at the time of discharge Mr. X reports a decreased intensity of these thoughts, and no acute suicidal ideation, plan, or intent. His discharge planning emphasizes ongoing work specifically related to coping with symptoms of traumatic stress, and the involvement of his main social support in facilitating this work.
The authors’ observations
As a caveat, it may be in some cases that chronic negative affect, dysphoria, and self-perception are better understood as a comorbid depressive disorder rather than subsumed into a PTSD/ CPTSD diagnosis. Also, because situational mood instability and impulsivity are often interpreted as bipolar disorder, a history of hypomania and mania should be ruled out. In Mr. X’s case, the diagnostic reformulation did not significantly impact pharmacotherapy because the target symptoms of mood instability, irritability, anxiety, and depression remained, despite the change in diagnosis.
Although the DSM-5 PTSD criteria effectively incorporate many CPTSD elements, we argue that this inclusivity comes at the expense of appreciating CPTSD as a qualitatively distinct condition, and we prefer ICD-11’s recognition of CPTSD as a separate diagnosis that incorporates PTSD criteria but extends the definition to include negative self-concept, affect dysregulation, and interpersonal difficulties.
Related Resources
- US Department of Veterans Affairs. PTSD: National Center for PTSD. Published January 1, 2007. https://www.ptsd.va.gov/ professional/treat/essentials/complex_ptsd.asp
- Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality disorders: theory, research, and treatment. 2020;11(1):36.
Drug Brand Names
Clonazepam • Klonopin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lorazepam • Ativan
Sertraline • Zoloft
Zolpidem • Ambien
Bottom Line
Consider a diagnosis of complex posttraumatic stress disorder (CPTSD) when providing care for patients with chronic depression and suicidality with a history of trauma or childhood adversity. This reformulation can allow clinicians to understand the contributing factors more holistically; align with the patient more effectively; appreciate past and present interpersonal, psychological, and psychosocial factors that may precipitate and perpetuate symptoms; and allow for treatment recommendations beyond those of mood and anxiety disorders.
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
1. Herman JL. Complex PTSD: a syndrome in survivors of prolonged and repeated trauma. J Trauma Stress. 1992;5(3):377-391.
2. Friedman MJ. Finalizing PTSD in DSM-5: getting here from there and where to go next. J Trauma Stress. 2013;26(5):548-556. doi: 10.1002/jts.21840 3. Hyland P, Shevlin M, Fyvie C, et al. Posttraumatic stress disorder and complex posttraumatic stress disorder in DSM-5 and ICD-11: clinical and behavioral correlates. J Trauma Stress. 2018; 31(12):174-180.
4. Brand B, Loewenstein R. Dissociative disorders: an overview of assessment, phenomenology and treatment. Psychiatric Times. Published 2010. Accessed October 4, 2021. https://www.researchgate.net/profile/Bethany-Brand/publication/231337464_Dissociative_Disorders_An_Overview_of_Assessment_Phenomonology_and_Treatment/links/09e415068c721ef9b5000000/Dissociative-Disorders-An-Overview-of-Assessment-Phenomonology-and-Treatment.pdf
5. Jowett S, Karatzias T, Shevlin M, et al. Differentiating symptom profiles of ICD-11 PTSD, complex PTSD, and borderline personality disorder: a latent class analysis in a multiply traumatized sample. Personality Disorders: theory, research, and treatment. 2020;11(1):36.
6. Ford JD, Courtois CA. Complex PTSD, affect dysregulation, and borderline personality disorder. Bord Personal Disord Emot Dysregul. 2014;1:9. doi.org/10.1186/2051-6673-1-9
7. van der Kolk BA. The trauma spectrum: the interaction of biological and social events in the genesis of the trauma response. J Trauma Stress. 1998;1(3):273-290.
8. Resnick PA, Bovin MJ, Calloway AL, et al. A critical evaluation of the complex PTSD literature: implications for DSM-5. J Trauma Stress. 2012;25(3);241-251.
9. Herman J. CPTSD is a distinct entity: comment on Resick et al. J Trauma Stress. 2012;25(3): 256-257.
10. Karatzias T, Cloitre M. Treating adults with complex posttraumatic stress disorder using a modular approach to treatment: rationale, evidence, and directions for future research. J Trauma Stress. 2019;32(6):870-876.
11. Perry S, Cooper AM, Michels R. The psychodynamic formulation: its purpose, structure, and clinical application. Am J Psych. 1987;144(5):543-550.
12. Diagnostic and statistical manual of mental disorders, 5th ed. American Psychiatric Association; 2013.
13. International Classification of Diseases, 11th revision. 2019; World Health Organization.
14. US Department of Veterans Affairs. PTSD: National Center for PTSD. Complex PTSD. Published January 1, 2007. Accessed October 4, 2021. https://www.ptsd.va.gov/professional/treat/essentials/complex_ptsd.asp
COVID-19 mortality risk factors: An unexpected finding
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Lithium’s antisuicidal effects questioned
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
Adding lithium to usual care does not decrease the risk of suicide-related events in those with major depressive disorder (MDD) or bipolar disorder (BD) who have survived a recent suicidal event, new research shows.
The results of a randomized, double-blind, placebo-controlled trial in veterans showed no apparent advantage of the drug in preventing self-injury, suicide attempts, or urgent hospitalization to prevent suicide.
“Lithium is an important therapy for bipolar disorders and depression subsets. Our study indicates that, in patients who are actively followed and treated in a system of care that the VA provides, simply adding lithium to their existing management, including medications, is unlikely to be effective for preventing a broad range of suicide-related events,” study investigator Ryan Ferguson, MPH, ScD, Boston Cooperative Studies Coordinating Center, VA Boston Healthcare System, told this news organization.
The study was published online JAMA Psychiatry.
Surprising findings
The results were somewhat surprising, Dr. Ferguson added. “Lithium showed little or no effect in our study, compared to observational data and results from previous trials. Many clinicians and practice guidelines had assumed that lithium was an effective agent in preventing suicide,” he said.
However, the authors of an accompanying editorial urge caution in concluding that lithium has no antisuicidal effects.
This “rigorously designed and conducted trial has much to teach but cannot be taken as evidence that lithium treatment is ineffective regarding suicidal risk,” write Ross Baldessarini, MD, and Leonardo Tondo, MD, department of psychiatry, Harvard Medical School, Boston.
Study participants were veterans with MDD or BD receiving care at one of 29 Veterans Administration medical centers who survived a recent suicide-related event. In addition to usual care, they were randomly assigned to receive oral extended-release lithium carbonate starting at 600 mg/day or matching placebo for 52 weeks.
The primary outcome was time to the first repeated suicide-related event, including suicide attempts, interrupted attempts, hospitalizations specifically to prevent suicide, and deaths from suicide.
The trial was stopped for futility after 519 veterans (mean age, 42.8 years; 84% male) were randomly assigned to receive lithium (n = 255) or placebo (n = 264). At 3 months, mean lithium concentrations were 0.54 mEq/L for patients with BD and 0.46 mEq/L for those with MDD.
There was no significant difference in the primary outcome (hazard ratio, 1.10; 95% confidence interval, 0.77-1.55; P = .61).
One death occurred in the lithium group and three in the placebo group. There were no unanticipated drug-related safety concerns.
Caveats, cautionary notes
The researchers note that the study did not reach its original recruitment goal. “One of the barriers to recruitment was the perception of many of the clinicians caring for potential participants that the effectiveness of lithium was already established; in fact, this perception was supported by the VA/U.S. Department of Defense Clinical Practice Guideline,” they point out.
They also note that most veterans in the study had depression rather than BD, which is the most common indication for lithium use. Most also had substance use disorders, posttraumatic stress disorder, or both, which could influence outcomes.
As a result of small numbers, it wasn’t possible to evaluate outcomes for patients with BD, test whether outcomes differed among patients with BD and MDD, or assess whether comorbidities attenuated the effects of lithium.
The study’s protocol increased participants’ contacts with the VA, which also may have affected outcomes, the researchers note.
In addition, high rates of attrition and low rates of substantial adherence to lithium meant only about half (48.1%) of the study population achieved target serum lithium concentrations.
Editorial writers Dr. Baldessarini and Dr. Tondo note that the low circulating concentrations of lithium and the fact that adherence to assigned treatment was considered adequate in only 17% of participants are key limitations of the study.
“In general, controlled treatment trials aimed at detecting suicide preventive effects are difficult to design, perform, and interpret,” they point out.
Evidence supporting an antisuicidal effect of lithium treatment includes nearly three dozen observational trials that have shown fewer suicides or attempts with lithium treatment, as well as “marked, temporary” increases in suicidal behavior soon after stopping lithium treatment.
Dr. Baldessarini and Dr. Tondo note the current findings “cannot be taken as evidence that lithium lacks antisuicidal effects. An ironic final note is that recruiting participants to such trials may be made difficult by an evidently prevalent belief that the question of antisuicidal effects of lithium is already settled, which it certainly is not,” they write.
Dr. Ferguson “agrees that more work needs to be done to understand the antisuicidal effect of lithium.
The study received financial and material support from a grant from the Cooperative Studies Program, Office of Research and Development, U.S. Department of Veterans Affairs. Dr. Ferguson has disclosed no relevant financial relationships. A complete list of author disclosures is available with the original article.
Dr. Baldessarini and Dr. Tondo have disclosed no relevant financial relationships. Their editorial was supported by grants from the Bruce J. Anderson Foundation, the McLean Private Donors Fund for Psychiatric Research, and the Aretaeus Foundation of Rome.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Britney Spears – Reflections on conservatorship
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
If you are a psychiatrist who has done a public lecture in the past year, you likely encountered the question, “What about Britney’s conservatorship?” Many psychiatrists are far removed from conservatorship evaluations, doing the different yet still important work of alleviating mental suffering without paddling in the controversial waters of involuntary treatment. Others judiciously hide behind the veil of the prudent Goldwater Rule in avoiding such discussions altogether. Regardless of whether psychiatry attempts to stay out of such affairs publicly, our field remains intimately involved in the process itself. This can lead to negative views of psychiatry among the public – that of a medical specialty with ulterior motives operating at the behest of the state.
Some psychiatrists simplistically advocate against any form of involuntary treatment.1 In many ways, this may appear noble. However, the reality of mental illness, with its potential harm to self and others, introduces the potential for dire consequences of such a position. If society is unwilling to accept behavior that may lead to harm, but psychiatry is unwilling to intervene, then other avenues of restricting such behavior will emerge. Those avenues traditionally have included conscription of law enforcement and the incarceration of patients with mental illness.
Yet, therein lies the conundrum of Ms. Spears and other celebrities on conservatorship. At face value, they do not appear to require conservatorship. We do not think it violates the Goldwater Rule to render this observation. In fact, it may reassure the public if the American Psychiatric Association, as well as individual psychiatrists, were more open about the goal, intent, and limitations of conservatorships.
The process of establishing conservatorships is not driven solely by mental health professionals. Rather, conservatorship laws permit society to enact, through psychiatrists, its desire to alleviate behaviors considered unacceptable in the context of mental illness.
In California, it has resulted in our famous or infamous “5150,” which asks psychiatrists to comment on the danger to self, danger to others, and grave disability of our patients. It can be helpful to frame these criteria regarding the relationship between society and our patients. The criteria of danger to self represents society’s wish to intervene in cases of patients with imminent intent of self-harm, operating under the presumption that a suicide can be prevented. Danger to others represents the societal angst, at times exaggerated,2 about people with mental illness perpetuating homicides, especially when off their medication. Grave disability represents public shame at the thought of persons so lost to mental illness they are unable to provide for themselves or even accept food, clothing, and shelter.
While an involuntary hold is necessary at times, working against our patients engenders revolting feelings. We often rationalize involuntary holds as illustrative of sincere compassion for our patients’ suffering and an attempt to lift them out of such tragic conditions. Our patients regularly do not feel our compassion when we are making an argument in a hearing for the restriction of their rights. They see our efforts as an attempt to lock them away “for their own good” because of society’s discomfort with homelessness. As such, we wonder whether our role becomes one of doctors for society, prescribing a treatment for the emotional distress of the community, and at times for ourselves, rather than that of the patient.
One may be perplexed as to how a celebrity could be considered gravely disabled. Celebrities generally have enough income to afford food, clothing, and shelter. One could justifiably ask why an individual with no history of violence would be considered a danger to others. Similarly, one may wonder how, in the absence of any reported attempts to engage in self-harm, with no visible marks of self-harm, someone is determined to be a danger to himself or herself. The bafflement on the part of one on the outside of these determinations can be sharply contrasted by the desperation felt by family members whose loved ones with mental illness appear to meet those criteria yet are consistently turned away by mental health programs and hospitals.
Not uncommonly, it is families advocating for involuntary hospitalization – while lamenting our strict criteria – that prevent doctors from intervening until some tragic fate befalls their loved ones. They criticize what they consider to be too-stringent mental health laws and are infuriated by seemingly obtuse insurance policies limiting care to patients. Most of our colleagues working with those who have severe mental illness share the frustration of these families over the scarcity of psychiatry beds. Therefore, it is particularly shocking when the most mediatized story about conservatorship is not about how hard it is to obtain. The story is about a singer who was seemingly safe, caring for herself, and yet still ended up on a conservatorship.
We wonder whether there is a question of magnitude. Are homeless patients more difficult to place on conservatorship because society sees a lesser stake? One could argue that Ms. Spears and other celebrities would have so much to lose in a single episode of mental illness. A week with mania or psychosis could cause irreparable damage to their persona, opportunity for employment, and their fortune. On the contrary, many of our patients on conservatorship have little to their names, and no one keeping up on their reputation. Triers of facts should ask themselves about the nature of their motivations. Envy, a desire to live vicariously through celebrities, or even less ethical motivations – such as a desire to control and exert authority over those individuals – can influence our decisions.
Throughout the past year, when asked about Ms. Spears, we have pointed out the obvious – she seemingly has a life incompatible with meeting criteria for a psychiatric conservatorship. We have outlined the role, history, and limitations of psychiatric conservatorship. We have shared how such cases are often approached, when required for our own patients or when asked by the court to do so. We have discussed the significant oversight of the system, including the public conservator’s office, which frequently refuses petitions outright. There are hearing officers, who, in the early stages of this process, weigh our case against that of the patients, aided by passionately driven patient advocates. There is the public defender’s office, which, at least in San Diego, vigorously defends the rights of those with mental illness. Most importantly, there are judges who adjudicate those cases with diligence and humility.
As the story has continued to be in the news, we have had numerous conversations about Ms. Spears’ conservatorship with colleagues sharing strong opinions on her case. Many of these colleagues do not have forensic practices and we inevitably find ourselves responding along the lines of, “It is easy to say this, but quite a different thing to prove it in court.” It is hard not to imagine testifying in such a high-profile conservatorship case; testifying, in front of jurors, about a celebrity who may have engaged in what some considered to be unusual behavior.
Conservatorship laws are not about the minutia or criteria of a specific mental health disorder. Patients do not meet criteria for conservatorship by having a certain number of delusional thoughts or a specific type of hallucination. Patients meet criteria for conservatorship because of state-enacted laws based on social factors – such as danger and self-care – the population wishes to treat, even if against the will of those treated. Under this light, one must recognize that a conservatorship trial is not just about mental illness but about how society wants to care for human beings. Psychiatric illness itself is not grounds for conservatorship. Oftentimes, severely ill patients win a hearing for grave disability by simply accepting a referral for housing, showing up to court clothed, and eating the meals provided at the hospital.
With understanding that these laws pertain specifically to behaviors resulting from mental illness that society finds unacceptable, the narrative of a celebrity conservatorship can be considered differently. The stories of celebrities being used and abused by deleterious beings and deleterious conditions have become a genre. Paul Prenter’s treatment of Freddie Mercury documented in the 2018 movie “Bohemian Rhapsody” and John Reid’s alleged betrayal of Elton John, who was suffering from a substance use disorder, documented in the 2019 movie “Rocketman,” are recent examples, among many.
Imagine yourself, as a juror, deciding on the fate of a celebrity. Would you require them to have lost all property, including the clothing on their backs, before intervening? Consider the next time you hear of a celebrity swindled from his or her fortune in a time of crisis and whether it would have been righteous to prevent it. We personally have, at times, argued for restraint in psychiatry’s desire to have more power. This concern extends not only to our ability to control people, but also our ability to force them into being subjected to psychotropic medications with well-known side effects.
At the same time, we remain cognizant of the magnified impact of adverse outcomes on public figures. John Hinckley Jr. did not attempt to murder a bystander; he attempted to kill the president of the United States when he shot at President Ronald Reagan in 1981. That incident led to considerable changes in our laws about insanity. More recently, society was particularly affected by Tom Hanks’ COVID-19 diagnosis. Mr. Hanks’ illness led to scientifically measurable changes in the public’s beliefs regarding the pandemic.3
On the other hand, and of equal importance to the desire to protect public figures from adverse events, is the risk that those same laws intended to protect will harm. From unsanitary asylums to disproportionate placements of minorities on psychiatric holds, we are concerned with unbridled control in the hands of those meant to cure and care. Sadly, there is also a cinematic genre of unprincipled and detrimental mental health treatment, from Brian Wilson’s treatment by his psychologist documented in “Love & Mercy,” to the upcoming “The Shrink Next Door,” featuring a psychiatrist swindling his patient.
With this additional understanding and analysis, we now ask our colleagues what it would take for them to intervene. Would a celebrity losing $100,000,000 because of mental illness constitute a form of grave disability despite remaining dressed? Would a celebrity engaging in significant drug use constitute a form of self-harm despite still recording albums? Would a celebrity failing to fulfill a social commitment to others, including children, constitute a form of harm to others? Those are not trivial questions to answer, and we are glad the Goldwater Rule reminds us of the limitations of speculating on people we do not know.
Nonetheless, the question of conservatorship is more complex than simply saying: “They make money; they have clothes on; this is absurd.” While this may be a catchy, compelling, and relevant argument, when confronted with a more complete narrative, triers of facts may feel compelled to intervene because, in the end, conservatorship laws are about what society is willing to accept rather than an enumeration of psychiatric symptoms.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1. Badre N et al. “Coercion and the critical psychiatrist.” In Critical Psychiatry. Springer, Cham, 2019. doi: 10.1007/97-3-030-02732-2_7.
2. Barnes SS and Badre N. Psychiatr Serv. 2016 Jul 1;67(7)784-6.
3. Myrick JG and Willoughby JF. Health Commun. 2021 Jan 14;1-9.
Iatrogenic hyponatremia in a patient with bipolar disorder
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.
The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.
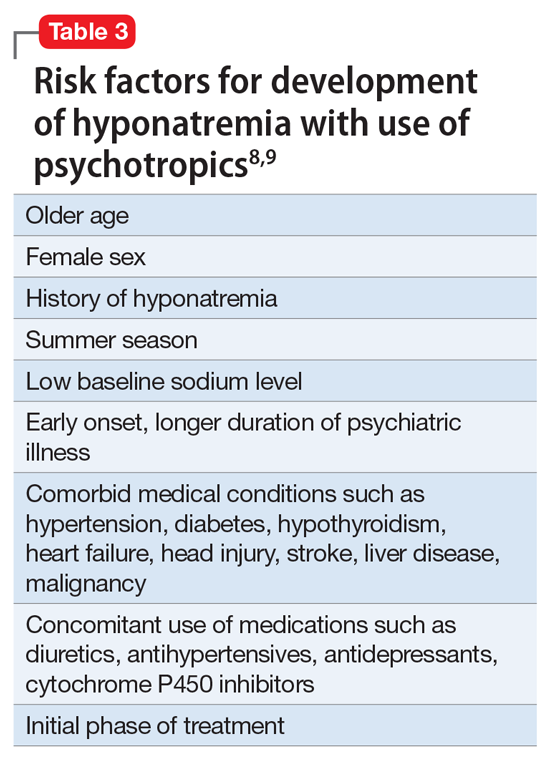
Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Bipolar disorder is a chronic mental disorder, often with onset at a young age. An estimated 4.4% of US adults experience bipolar disorder at some time in their lives.
A variety of medications—including mood stabilizers, lithium, and antipsychotics (Table 1,3,4 and Table 2,4)—and somatic treatments such as electroconvulsive therapy and transcranial magnetic stimulation are used to manage the depressive and manic/mixed episodes of bipolar disorder. Treatment should be individualized based on the patient’s symptom severity, sensitivity, response to treatment, and preferences.


The most common reason for discontinuing a medication is intolerance to adverse effects. Some adverse effects are mild and may lessen over time. Others can be life-threatening. Thus, medications should be chosen carefully and started at low doses, and patients should be closely monitored for adverse effects at regular intervals.
Here I describe the case of a patient with bipolar disorder who developed hyponatremia while being treated with the second-generation antipsychotic lurasidone.
Continue to: CASE REPORT...
CASE REPORT
Mrs. G, age 65, lives with her husband. She has a history of bipolar disorder, chronic kidney disease, diabetes mellitus type 2, obstructive sleep apnea, hypertension associated with hyperaldosteronism, and obesity, for which she has undergone bariatric surgery. Symptoms of bipolar disorder started when she was in her 30s, following the death of her father. Her initial symptoms included depressed mood, anger, irritability, difficulty sleeping, racing thoughts, and impulsive spending. She did not have any suicidal ideation or homicidal ideation. She did not have anxiety, posttraumatic stress disorder, or obsessive-compulsive disorder symptoms. She was diagnosed with bipolar disorder. For some time, she took perphenazine, 16 mg/d, divalproex sodium, 1,500 mg/d, and temazepam, 30 mg/d at bedtime. These doses were reduced as her mood stabilized. Over time, divalproex sodium was tapered and discontinued, and perphenazine was reduced to 4 mg/d at bedtime. Lithium was tried briefly but discontinued because Mrs. G did not tolerate it well. She has never been hospitalized for mental health issues, but did have one emergency department visit a very long time ago. She has no history of suicide attempts, and there is no family history of completed suicide. There is a family history of bipolar disorder in her mother.
Mrs. G was born and raised outside the United States in a stable, two-parent home. She had no maltreatment during childhood. She has a bachelor’s degree and was employed. She is a social drinker, with no history of treatment for alcohol use disorder.
Mrs. G was stable on perphenazine, 4 mg/d, and temazepam, 30 mg/d, until 5 years ago. In 2016, she became concerned about her weight and overall health, and underwent bariatric surgery (gastric sleeve). After this surgery, Mrs. G experienced changes in mood and thought. She felt paranoid and had ideas of reference, social sensitivity, increased irritability, and poor self-esteem. Perphenazine was discontinued, divalproex was reintroduced, and lurasidone was started. Lurasidone was titrated up to 120 mg/d, and divalproex up to 1,500 mg/d. Temazepam, 30 mg/d at bedtime, was continued for her insomnia. She also occasionally took over-the-counter melatonin, 5 to 10 mg, as needed for insomnia.
Mrs. G improved on this combination, and became stable and euthymic in September 2017. Other than a brief hypomanic episode in Spring 2018 that resolved quickly, she remained euthymic. During routine follow-up visits, Mrs. G’s nephrologist noticed that her sodium levels had been fluctuating. Mrs. G said her nephrologist was not sure exactly what was causing these fluctuations, and she continued to take the same medications.
In June 2018, Mrs. G developed tremors, slowing, and lethargy. Lurasidone was gradually reduced to 60 mg/d and divalproex to 750 mg/d. Temazepam, 30 mg/d at bedtime, was continued. In July 2018, divalproex was further reduced to 500 mg/d because Mrs. G’s free valproic acid levels were elevated. In February 2019, lurasidone was further reduced to 40 mg/d due to blunted affect, and in April 2019, escitalopram, 10 mg/d, was added for symptoms of depression (off-label), and anxiety. In June 2019, Mrs. G’s sodium level was 127 mEq/L (reference range: 135 to 145 mEq/L). Because escitalopram can cause hyponatremia, it was discontinued in August 2019, but Mrs. G continued to take lurasidone, 40 mg/d, divalproex, 500 mg/d, and temazepam, 30 mg/d.
In October and November 2020, Mrs. G’s sodium level remained low at 123 and 127 mEq/L. Our treatment team wondered if lurasidone could be causing Mrs. G’s sodium levels to fall. Lurasidone was tapered over 3 days and discontinued. Repeat blood work showed that Mrs. G’s sodium levels soon returned to normal range. In January through March 2021, her sodium levels were 138, 139, and 136 mEq/L, all of which were within normal range. This confirmed our suspicion that lurasidone had caused the hyponatremia, though briefly it may have been made worse by escitalopram. Currently, Mrs. G is stable on perphenazine, 4 mg twice a day, divalproex, 500 mg/d, temazepam, 30 mg/d at bedtime, and melatonin, 5 mg at bedtime.
Continue to: Syndrome of inappropriate antidiuretic hormone secretion...
Syndrome of inappropriate antidiuretic hormone secretion
Syndrome of inappropriate antidiuretic hormone (SIADH) secretion can result in hyponatremia. Classes of medications that can cause SIADH include antidepressants, antipsychotics, anticonvulsants, cytotoxic agents, and pain medications.5 The class of drugs most commonly associated with SIADH is selective serotonin reuptake inhibitors, particularly citalopram.5 Among the antipsychotics, risperidone is most associated with hyponatremia. The proposed mechanism of medication-induced SIADH is an increase in the release of ADH.6 Treatment options include discontinuing the offending medication(s) or switching to a different medication.
Hyponatremia is a rare adverse effect of lurasidone, with a reported incidence <1%.7 Although hyponatremia is potentially life-threatening, there is no recommendation to routinely monitor sodium levels in patients treated with lurasidone or other psychotropics, and patients who are prescribed lurasidone are not routinely monitored for sodium deficiency. Table 38,9 outlines risk factors for developing hyponatremia among patients taking psychotropic medications.

Mrs. G had been taking lurasidone for a few years and experienced fluctuating sodium levels. She had been taking divalproex, which by itself could cause hyponatremia and could have added to the effects of lurasidone in lowering sodium levels. Escitalopram briefly made her hyponatremia worse. Given Mrs. G’s medical illnesses, our focus had been on her underlying medical conditions rather than on a suspected medication-induced adverse effect.
In summary, patients who are prescribed lurasidone may benefit from regular monitoring of sodium levels. Monitoring sodium levels in geriatric patients who have multiple comorbid medical conditions and take multiple medications may reduce the morbidity and mortality associated with SIADH.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
1. National Institute of Mental Health. Bipolar disorder. Accessed October 12, 2021. https://www.nimh.nih.gov/health/statistics/bipolar-disorder
2. Müller JK, Leweke FM. Bipolar disorder: clinical overview. Med Monatsschr Pharm. 2016;39(9):363-369.
3. Bobo WV, Shelton RC. Bipolar major depression in adults: Efficacy and adverse effects of second-generation antipsychotics. UpToDate. Updated September 1, 2020. Accessed October 12, 2021. https://www.uptodate.com/contents/bipolar-major-depression-in-adults-efficacy-and-adverse-effects-of-second-generation-antipsychotics
4. Epocrates. Version 21.9.1. Accessed October 14, 2021. https://www.epocrates.com
5. Shepshelovich D, Schechter A, Calvarysky B, et al. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017;83(8):1801-1807.
6. Guirguis E, Grace Y, Seetaram M. Management of hyponatremia: focus on psychiatric patients. US Pharm. 2013;38(11):HS3-HS6.
7. Drugs.com. Latuda side effects. Accessed October 12, 2021. https://www.drugs.com/sfx/latuda-side-effects.html
8. Ali SN, Bazzano LA. Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J. 2018;18(3):230-235.
9. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
Sleep problems in mental illness highly pervasive
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
An inpatient psychiatric diagnosis at some point over a lifetime is significantly associated with a range of sleep problems, results from the largest study of its kind show.
A prior diagnosis of major depression, schizophrenia, anxiety, or bipolar disorder was associated with a later bedtime, earlier waking time, and significantly poorer sleep quality that included frequent awakenings during the night and shorter sleep bouts.
“We were struck by the pervasiveness of sleep problems across all the diagnoses of mental illness and sleep parameters we looked at,” study investigator Michael Wainberg, PhD, a postdoctoral fellow at the Krembil Centre for Neuroinformatics at the Center for Addiction and Mental Health (CAMH), Toronto, told this news organization. “This suggests there may need to be even more of an emphasis on sleep in these patients than there already is.”
The study, which includes data from nearly 90,000 adults in the United Kingdom, was published online October 12 in PLoS Medicine.
Trove of data
Data for the analysis comes from the UK Biobank, a large-scale biomedical database launched in 2006 that has collected biological and medical data on more than 500,000 individuals who consented to provide blood, urine, and saliva samples and detailed lifestyle information that is matched to their medical records.
Between 2013 and 2015, more than 103,000 of these participants agreed to wear accelerometers on their wrists for 24 hours a day for 7 days, collecting a trove of data for researchers to mine.
“This allows us to get at objectively derived sleep measures and to measure them in greater numbers of people who have experienced mental illness,” said senior author Shreejoy Tripathy, PhD, assistant professor at the University of Toronto and independent scientist for CAMH. “You can study multiple disorders at once and the influence of other variables that might not be possible in the context of other studies.”
The research is the first known large-scale transdiagnostic study of objectively measured sleep and mental health. Insomnia and other sleep disorders are common among people with mental illness, as shown in prior research, including at least one study that used the same dataset the team employed for this project.
The new findings add to that body of work, Dr. Wainberg said, and look beyond just how long a person sleeps to the quality of the sleep they get.
“We found that the metrics of sleep quality seem to be affected more than mere sleep duration,” he said.
Unexpected finding
After excluding participants with faulty accelerometers and those who didn’t wear them for the entire 7-day study period, data from 89,205 participants (aged 43-79, 56% female, 97% self-reported White) was included. Lifetime inpatient psychiatric diagnoses were reported in 2.5% of the entire cohort.
Researchers looked at 10 sleep measures: bedtime, wake-up time, sleep duration, wake after sleep onset, sleep efficiency, number of awakenings, duration of longest sleep bout, number of naps, and variability in bedtime and sleep duration.
Although the effect sizes were small, having any psychiatric diagnosis was associated with significantly lower scores on every sleep measure except sleep duration.
Compared with those with no inpatient psychiatric diagnosis, those with any psychiatric diagnosis were significantly more likely to:
- have a later bedtime (beta = 0.07; 95% confidence interval, 0.06-0.09)
- have later wake-up time (beta = 0.10; 95% CI, 0.09-0.11)
- wake after sleep onset (beta = 0.10; 95% CI, 0.09-0.12)
- have poorer sleep efficiency (beta = –0.12; 95% CI, −0.14 to −0.11)
- have more awakenings (beta = 0.10; 95% CI, 0.09-0.11)
- have shorter duration of their longest sleep bout (beta = –0.09; 95% CI, −0.11 to −0.08)
- take more naps (beta = 0.11; 95% CI, 0.09-0.12)
- have greater variability in their bedtime (beta = 0.08; 95% CI, 0.06-0.09)
- have greater variability in their sleep duration (beta = 0.10; 95% CI, 0.09-0.12)
The only significant differences in sleep duration were found in those with lifetime major depressive disorder, who slept significantly less (beta = −0.02; P = .003), and in those with lifetime schizophrenia, who slept significantly longer (beta = 0.02; P = .0008).
Researchers found similar results when they examined patient-reported sleep measures collected when participants enrolled in the biobank, long before they agreed to wear an accelerometer.
“Everyone with a lifetime mental illness diagnosis trended toward worse sleep quality, regardless of their diagnosis,” Dr. Tripathy said. “We didn’t expect to see that.”
Limitations of the biobank data prohibited analysis by age and past or current use of psychiatric medications. In addition, investigators were unable to determine whether mental illness was active or controlled at the time of the study. Information on these, and other factors, is needed to truly begin to understand the real-world status of sleep patterns in people with mental illness, the researchers note.
However, the biobank data demonstrates how this type of information can be collected, helping Dr. Tripathy and others to design a new study that will launch next year with patients at CAMH. This effort is part of the BrainHealth Databank, a project that aims to develop a patient data bank similar to the one in the UK that was used for this study.
“We’ve shown that you can use wearable devices to measure correlates of sleep and derive insights about the objective measurements of sleep and associate them with mental illness diagnosis,” Dr. Tripathy said.
The study received no outside funding. Dr. Wainberg and Dr. Tripathy report receiving funding from Kavli Foundation, Krembil Foundation, CAMH Discovery Fund, the McLaughlin Foundation, NSERC, and CIHR. Disclosures for other authors are fully listed in the original article.
A version of this article first appeared on Medscape.com.
Substance use or substance use disorder: A question of judgment
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.
Substance use disorders can be a thorny topic in residency because of our role as gatekeepers of mental hospitals during our training. Intoxicated patients often get dismissed as a burden and distraction, malingering their way into a comfortable place to regain sobriety. This is extremely prevalent, often constituting the majority of patients seen during an emergency department call.
A typical interview may elicit any or all symptoms in the DSM yet be best explained by substance use intoxication or withdrawal. Alcohol and other CNS depressants commonly cause feelings of sadness and/or suicidality. Methamphetamine and other CNS stimulants commonly cause symptoms of psychosis or mania, followed by feelings of sadness and/or suicidality.
Different EDs have different degrees of patience for individuals in the process of becoming sober. Some departments will pressure clinicians into quickly discarding those patients and often frown upon any attempt at providing solace by raising the concern of reinforcing maladaptive behavior. A mystery-meat sandwich of admirable blandness may be the extent of help offered. Some more fortunate patients also receive a juice box or even a taxi voucher in an especially generous ED. This is always against our better judgment, of course, as we are told those gestures encourage abuse.
Other EDs will permit patients to remain until sober, allowing for another evaluation without the influence of controlled substances. We are reminded of many conversations with patients with substance use disorders, where topics discussed included: 1. Recommendation to seek substance use services, which are often nonexistent or with wait lists spanning months; 2. Education on the role of mental health hospitals and how patients’ despair in the context of intoxication does not meet some scriptural criteria; 3. Pep talks aided by such previously described sandwiches and juice boxes to encourage a sobering patient to leave the facility of their own will.
Methamphetamine, heroin, and alcohol are rarely one-and-done endeavors. We sparingly see our patients for their very first ED visit while intoxicated or crashing. They know how the system runs and which ED will more readily allow them an overnight stay. The number of times they have been recommended for substance use treatment is beyond counting – they may have been on a wait list a handful of times. They are aware of our reluctance to provide inpatient psychiatric treatment for substance use, but it is worth a shot trying, anyway – sometimes they get lucky. Usually it is the pep talk, relief from hunger pangs, and daylight that get them out the doors – until next time.
It is under this context that many trainees become psychiatrists, a process that solidifies the separation between drug use and mental illness. Many graduate from residency practically equating substance use disorder with malingering or futility. This can take on a surreal quality as many localities have recently adopted particular forms or requirements like the dispensation of naloxone syringes to all patients with substance use disorders. While the desire and effort are noble, it may suggest to a patient presenting for help that society’s main interest is to avoid seeing them die rather than help with available resources for maintaining sobriety.
Therein lies the conundrum, a conundrum that spans psychiatry to society. The conundrum is our ambivalence between punishing the choice of drug use or healing the substance use disorder. Should we discharge the intoxicated patient as soon as they are safe to walk out, or should we make every effort possible to find long-term solutions?
The calculation becomes more complex
A defining moment appears to have been society’s reconsideration of its stance on substance use disorders when affluent White teenagers started dying in the suburbs from pain pills overdoses. Suddenly, those children needed and deserved treatment, not punishment. We find ourselves far away from a time when the loudest societal commentary on substance use entailed mothers advocating for harsher sentences against drunk drivers.
More recently, as psychiatry and large contingents of society have decided to take up the mantle of equity and social justice, we have begun to make progress in decriminalizing substance use in an effort to reverse systemic discrimination toward minority groups. This has taken many shapes, including drug legalization, criminal justice reform, and even the provision of clean substance use paraphernalia for safer use of IV drugs. Police reform has led to reluctance to arrest or press charges for nonviolent crimes and reduced police presence in minority neighborhoods. The “rich White teenager” approach is now recommended in all neighborhoods.
Society’s attempt at decriminalizing drug use has run parallel with psychiatry’s recent attempts at reduced pathologizing of behaviors more prevalent in underprivileged groups and cultures. This runs the gamut, from avoiding the use of the term “agitated” because of its racial connotations, to advocating for reduced rates of schizophrenia diagnoses in Black males.1 A diagnosis of substance use disorder carries with it similar troublesome societal implications. Decriminalization, legalization, provision of substances to the population, normalization, and other societal reforms will likely have an impact on the prevalence of substance use disorder diagnoses, which involve many criteria dependent on societal context.
It would be expected that criteria such as hazardous use, social problems, and attempts to quit will decrease as social acceptance increases. How might this affect access to substance use treatment, an already extremely limited resource?
Now, as forensic psychiatrists, we find ourselves adjudicating on the role of drugs at a time when society is wrestling with its attitude on the breadth of responsibility possessed by people who use drugs. In California, as in many other states, insanity laws exclude those who were insane as a result of drug use, as a testament to or possibly a remnant of how society feels about the role of choice and responsibility in the use of drugs. Yet another defendant who admits to drug use may on the contrary receive a much more lenient plea deal if willing to commit to sobriety. But in a never-ending maze of differing judgments and opinions, a less understanding district attorney may argue that the additional risk posed by the use of drugs and resulting impulsivity may actually warrant a heavier sentence.
In a recent attempt at atonement for our past punitive stance on drug users, we have found a desire to protect those who use drugs by punishing those who sell, at times forgetting that these populations are deeply intertwined. A recent law permits the federal charge of distribution of fentanyl resulting in death, which carries the mandatory minimum of 20 years in prison. Yet, if the user whom we are trying to protect by this law is also the one selling, what are we left with?
Fentanyl has been a particularly tragic development in the history of mankind and drug use. Substance use has rarely been so easily linked to accidental death. While many physicians can easily explain the safety of fentanyl when used as prescribed and in controlled settings, this is certainly not the case in the community. Measuring micrograms of fentanyl is outside the knowledge and capabilities of most drug dealers, who are not equipped with pharmacy-grade scales. Yet, as a result, they sell and customers buy quantities of fentanyl that range from homeopathically low to lethally high because of a mixture of negligence and deliberate indifference.
Another effort at atonement has been attempts at decriminalizing drug use and releasing many nonviolent offenders. This can, however, encourage bystanders to report more acts as crime rather than public intoxication, to ensure a police response when confronted by intoxicated people. Whereas previously an inebriated person who is homeless may have been called for and asked to seek shelter, they now get called on, and subsequently charged for, allegedly mumbling a threat by a frustrated bystander.
The release of offenders has its limits. Many placements on probation require sobriety and result in longer sentences for the use of substances that are otherwise decriminalized. The decriminalization and reexamination of substance use by society should widen the scope from simply considering crime to examining the use of drugs throughout the legal system and even beyond.
The DSM and psychiatry are not intended or equipped to adjudicate disputes on where the lines should be drawn between determinism and free will. We are knowledgeable of patients with substance use disorders, the effect of intoxicating substances, and the capacity of patients with substance use disorders to act in law-abiding ways. Our field can inform without simply advocating whether our patients should be punished. While society is currently struggling with how to apportion blame, psychiatry should resist the urge to impose medical solutions to social problems. Our solutions would almost certainly be grossly limited as we are still struggling to repent for lobotomizing “uppity” young women2 and using electroshock therapy to disrupt perverse impulses in homosexual males.3 Social norms and political zeitgeists change over time while the psychological and physiological principles underlying our understanding of mental illness should, in theory, stay relatively constant. Psychiatry’s answers for societal ills do not usually improve with time but rather have a tendency to be humbling.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
Dr. Compton is a psychiatry resident at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research.
References
1.Medlock MM et al., eds. “Racism and Psychiatry: Contemporary Issues and Interventions” (New York: Springer, 2018).
2. Tone A and Koziol M. CMAJ. 2018:190(20):e624-5.
3. McGuire RJ and Vallance M. BMJ. 1964;1(5376):151-3.












