User login
Lumateperone for major depressive episodes in bipolar I or bipolar II disorder
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8
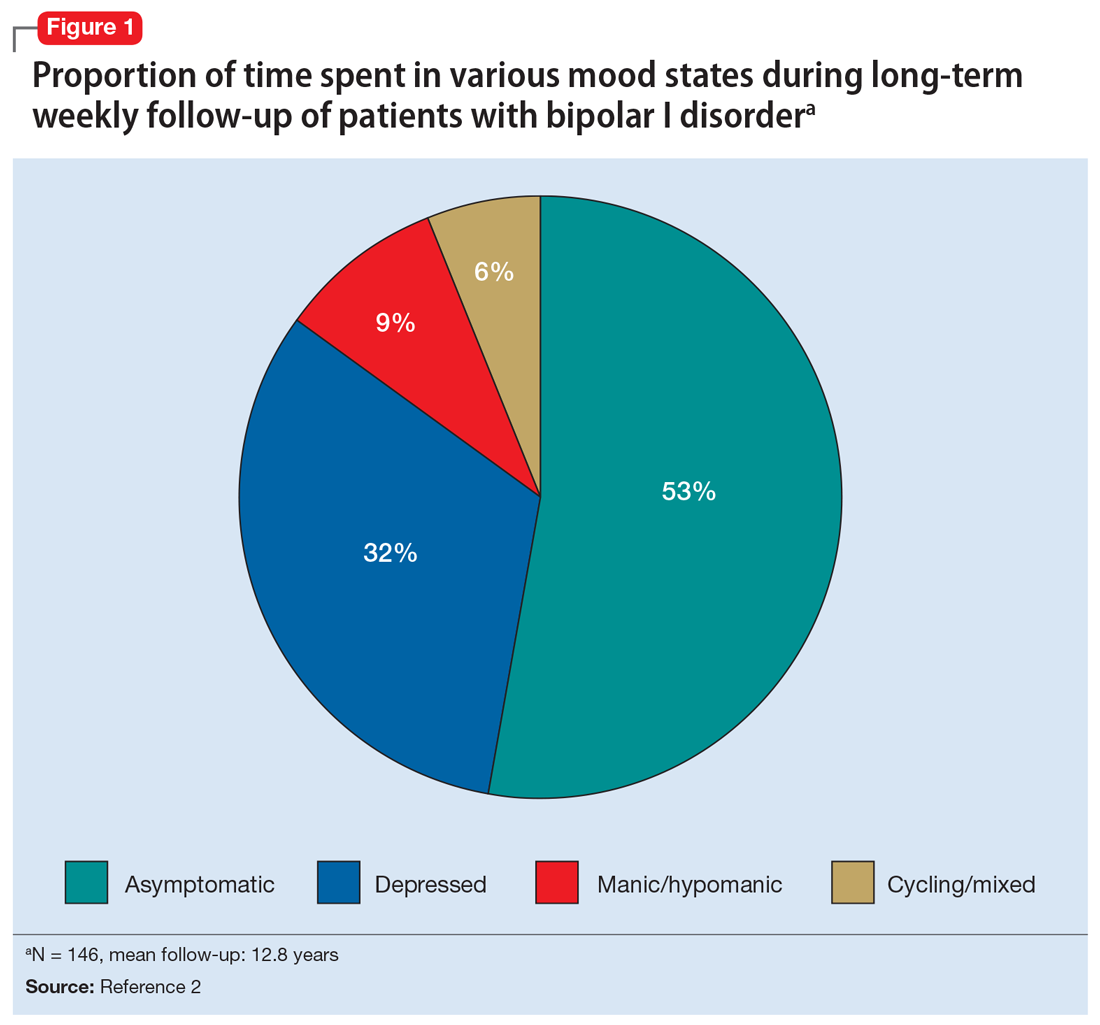
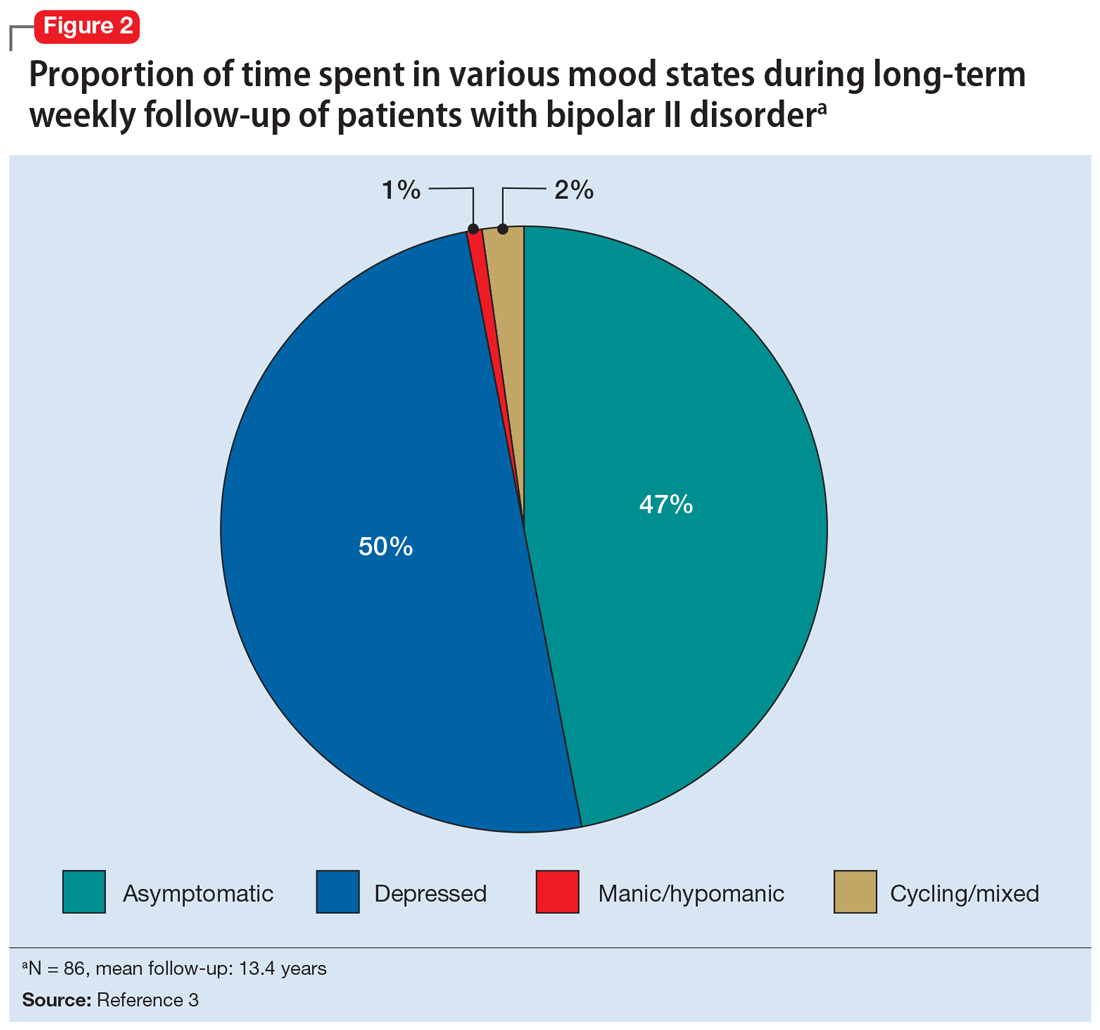
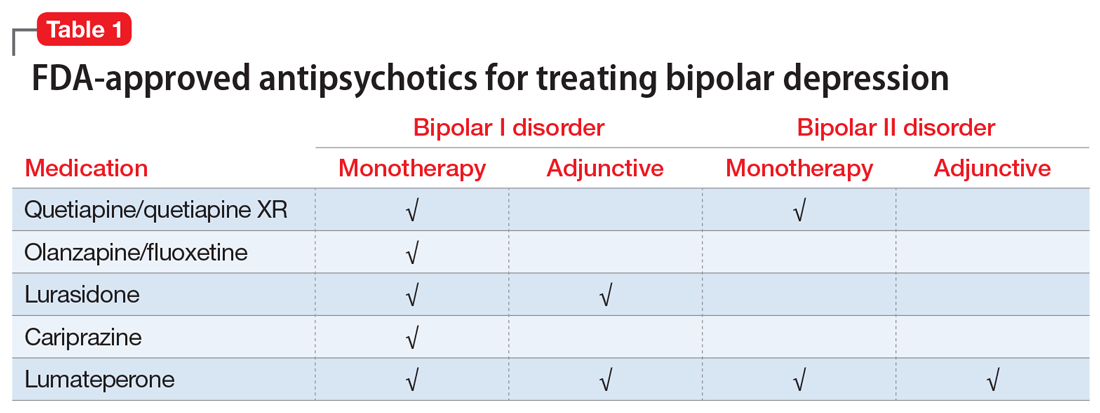
Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...
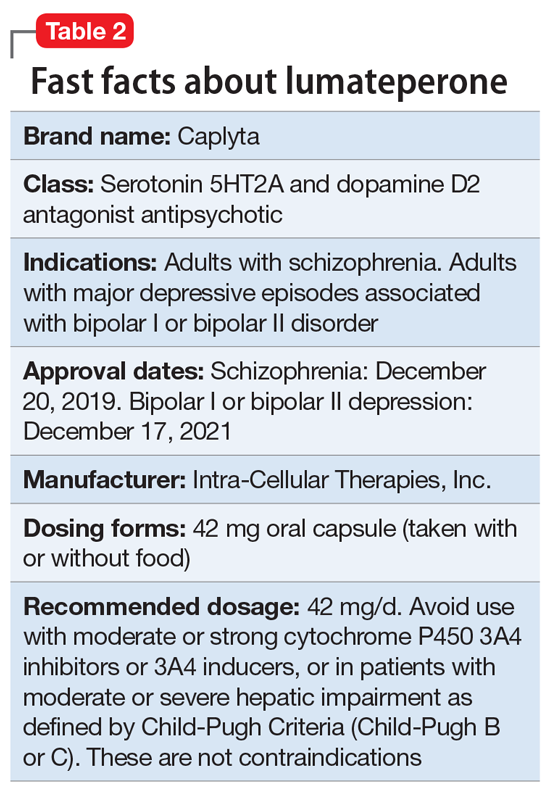
Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13
In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
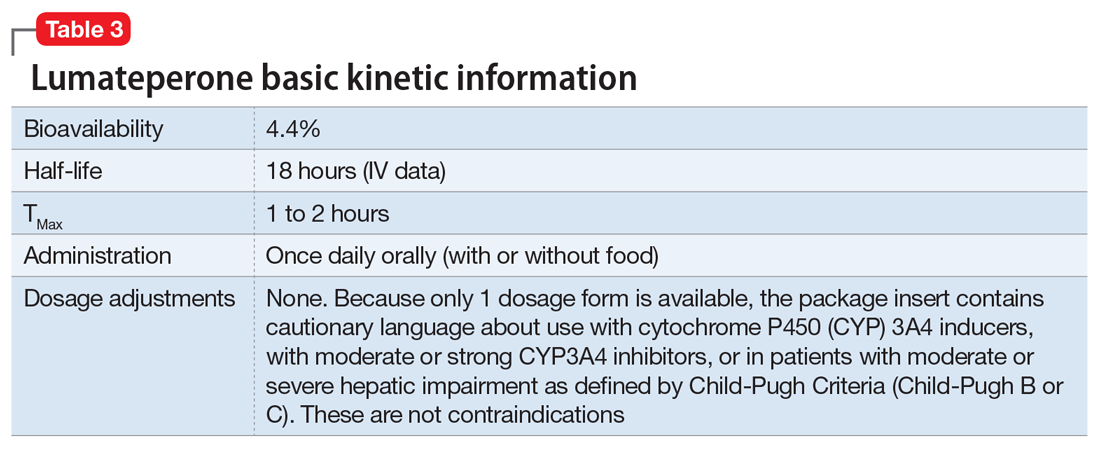
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.
Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
Among patients with bipolar I or II disorder (BD I or II), major depressive episodes represent the predominant mood state when not euthymic, and are disproportionately associated with the functional disability of BD and its suicide risk.1 Long-term naturalistic studies of weekly mood states in patients with BD I or II found that the proportion of time spent depressed greatly exceeded that spent in a mixed, hypomanic, or manic state during >12 years of follow-up (Figure 12and Figure 23). In the 20th century, traditional antidepressants represented the sole option for management of bipolar depression despite concerns of manic switching or lack of efficacy.4,5 Efficacy concerns were subsequently confirmed by placebo-controlled studies, such as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial, which found limited effectiveness of adjunctive antidepressants for bipolar depression.6 Comprehensive reviews of randomized controlled trials and observational studies documented the risk of mood cycling and manic switching, especially in patients with BD I, even if antidepressants were used in the presence of mood-stabilizing medications.7,8


Several newer antipsychotics have been FDA-approved for treating depressive episodes associated with BD (Table 1). Approval of olanzapine/fluoxetine combination (OFC) in December 2003 for depressive episodes associated with BD I established that mechanisms exist which can effectively treat acute depressive episodes in patients with BD without an inordinate risk of mood instability. Subsequent approval of quetiapine in October 2006 for depression associated with BD I or II, lurasidone in June 2013, and cariprazine in May 2019 for major depression in BD I greatly expanded the options for management of acute bipolar depression. However, despite the array of molecules available, for certain patients these agents presented tolerability issues such as sedation, weight gain, akathisia, or parkinsonism that could hamper effective treatment.9 Safety and efficacy data in bipolar depression for adjunctive use with lithium or divalproex/valproate (VPA) also are lacking for quetiapine, OFC, and cariprazine.10,11 Moreover, despite the fact that BD II is as prevalent as BD I, and that patients with BD II have comparable rates of comorbidity, chronicity, disability, and suicidality,12 only quetiapine was approved for acute treatment of depression in patients with BD II. This omission is particularly problematic because the depressive episodes of BD II predominate over the time spent in hypomanic and cycling/mixed states (50.3% for depression vs 3.6% for hypomania/cycling/mixed combined), much more than is seen with BD I (31.9% for depression vs 14.8% for hypomania/cycling/mixed combined).2,3 The paucity of data for the use of newer antipsychotics in BD II depression presents a problem when patients cannot tolerate or refuse to consider quetiapine. This prevents clinicians from engaging in evidence-based efficacy discussions of other options, even if it is assumed that the tolerability profile for BD II depression patients may be similar to that seen when these agents are used for BD I depression.
Continue to: Table 1...

Lumateperone (Caplyta) is a novel oral antipsychotic initially approved in 2019 for the treatment of adult patients with schizophrenia. It was approved in December 2021 for the management of depression associated with BD I or II in adults as monotherapy or when used adjunctively with the mood stabilizers lithium or VPA (Table 2).13 Lumateperone possesses certain binding affinities not unlike those in other newer antipsychotics, including high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), low affinity for dopamine D2 receptors (Ki 32 nM), and low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).13,14 However, there are some distinguishing features: the ratio of 5HT2A receptor affinity to D2 affinity is 60, greater than that for other second-generation antipsychotics (SGAs) such as risperidone (12), olanzapine (12.4) or aripiprazole (0.18).15 At steady state, D2 receptor occupancy remains <40%, and the corresponding rates of extrapyramidal side effects (EPS)/akathisia differed by only 0.4% for lumateperone vs placebo in short-term adult clinical schizophrenia trials,13,16 by 0.2% for lumateperone vs placebo in the monotherapy BD depression study, and by 1.7% in the adjunctive BD depression study.13,17,18 Lumateperone also exhibited no clinically significant impact on metabolic measures or serum prolactin during the 4-week schizophrenia trials, with mean weight gain ≤1 kg for the 42 mg dose across all studies.19 This favorable tolerability profile for endocrine and metabolic adverse effects was also seen in the BD depression studies. Across the 2 BD depression monotherapy trials and the single adjunctive study, the only adverse reactions occurring in ≥5% of lumateperone-treated patients and more than twice the rate of placebo were somnolence/sedation, dizziness, nausea, and dry mouth.13 There was also no single adverse reaction leading to discontinuation in the BD depression studies that occurred at a rate >2% in patients treated with lumateperone.13

In addition to the low risk of adverse events of all types, lumateperone has several pharmacologic features that distinguish it from other agents in its class. One unique aspect of lumateperone’s pharmacology is differential actions at presynaptic and postsynaptic dopamine D2 receptors noted in preclinical assays, a property that may explain its ability to act as an antipsychotic despite low D2 receptor occupancy.16 Preclinical assays also predicted that lumateperone was likely to have antidepressant effects.15,19,20 Unlike every SGA except ziprasidone, lumateperone also possesses moderate binding affinity for serotonin transporters (SERT) (Ki 33 nM), with SERT occupancy of approximately 30% at 42 mg.21 Lumateperone facilitates dopamine D1-mediated neurotransmission, and this is associated with increased glutamate signaling in the prefrontal cortex and antidepressant actions.14,22 While the extent of SERT occupancy is significantly below the ≥80% SERT occupancy seen with selective serotonin reuptake inhibitors, it is hypothesized that near saturation of the 5HT2A receptor might act synergistically with modest 5HT reuptake inhibition and D1-mediated effects to promote the downstream glutamatergic effects that correlate with antidepressant activity (eg, changes in markers such as phosphorylation of glutamate N-methyl-D-aspartate receptor subunits, potentiation of AMPA receptor-mediated transmission).15,22
Continue to: Clinical implications...
Clinical implications
The approval of lumateperone for both BD I and BD II depression, and for its use as monotherapy and for adjunctive use with lithium or VPA, satisfies several unmet needs for the management of acute major depressive episodes in patients with BD. Clinicians now have both safety and tolerability data to present to their bipolar spectrum patients regardless of subtype, and regardless of whether the patient requires mood stabilizer therapy. The tolerability advantages for lumateperone seen in schizophrenia trials were replicated in a diagnostic group that is very sensitive to D2-related adverse effects, and for whom any signal of clinically significant weight gain or sedation often represents an insuperable barrier to patient acceptance.23
Efficacy in adults with BD I or II depression.
The efficacy of lumateperone for major depressive episodes has been established in 2 pivotal, double-blind, placebo-controlled trials in BD I or II patients: 1 monotherapy study,17 and 1 study when used adjunctively to lithium or VPA.18 The first study was a 6-week, double-blind, placebo-controlled monotherapy trial (study 404) in which 377 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode were randomized in a 1:1 manner to lumateperone 42 mg/d or placebo given once daily in the evening. Symptom entry criteria included a Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥20, and scores ≥4 on the depression and overall BD illness subscales of the Clinical Global Impressions Scale–Bipolar Version Severity scale (CGI-BP-S) at screening and at baseline.17 Study entry also required a score ≤12 on the Young Mania Rating Scale (YMRS) at screening and at baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS. Several secondary efficacy measures were examined, including the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS), or remission (MADRS score ≤12), and differential changes in MADRS scores from baseline for BD I and BD II subgroups.17
The patient population was 58% female and 91% White, with 79.9% diagnosed as BD I and 20.1% as BD II. The least squares mean changes on the MADRS total score from baseline to Day 43 were lumateperone 42 mg/d: -16.7 points; placebo: -12.1 points (P < .0001), and the effect size for this difference was moderate: 0.56. Secondary analyses indicated that 51.1% of those taking lumateperone 42 mg/d and 36.7% taking placebo met response criteria (P < .001), while 39.9% of those taking lumateperone 42 mg/d and 33.5% taking placebo met remission criteria (P = .018). Importantly, depression improvement was observed both in patients with BD I (effect size 0.49, P < .0001) and in those with BD II (effect size 0.81, P < .001).
The second pivotal trial (study 402) was a 6-week, double-blind, placebo-controlled adjunctive trial in which 528 patients age 18 to 75 with BD I or BD II experiencing a major depressive episode despite treatment with lithium or VPA were randomized in a 1:1:1 manner to lumateperone 28 mg/d, lumateperone 42 mg/d, or placebo given once daily in the evening.18 Like the monotherapy trial, symptom entry criteria included a MADRS total score ≥20, and scores ≥4 on the depression and overall illness CGI-BP-S subscales at screening and baseline.18 Study entry also required a score ≤12 on the YMRS at screening and baseline. The duration of the major depressive episode must have been ≥2 weeks but <6 months before screening, with symptoms causing clinically significant distress or functional impairment. The primary outcome measure was change from baseline in MADRS for lumateperone 42 mg/d compared to placebo. Secondary efficacy measures included MADRS changes for lumateperone 28 mg/d and the proportion of patients meeting criteria for treatment response (≥50% decrease in MADRS) or remission (MADRS score ≤12).
The patient population was 58% female and 88% White, with 83.3% diagnosed as BD I, 16.7% diagnosed as BD II, and 28.6% treated with lithium vs 71.4% on VPA. The effect size for the difference in MADRS total score from baseline to Day 43 for lumateperone 42 mg/d was 0.27 (P < .05), while that for the lumateperone 28 mg/d dose did not reach statistical significance. Secondary analyses indicated that response rates for lumateperone 28 mg/d and lumateperone 42 mg/d were significantly higher than for placebo (both P < .05). Response rates were placebo: 39%; lumateperone 28 mg/d: 50%; and lumateperone 42 mg/d: 45%. Remission rates were similar at Day 43 in both lumateperone groups compared with placebo: placebo: 31%, lumateperone 28 mg/d: 31%, and lumateperone 42 mg/d: 28%.18 As of this writing, a secondary analysis by BD subtype has not yet been presented.
A third study examining lumateperone monotherapy failed to establish superiority of lumateperone over placebo (NCT02600494). The data regarding tolerability from that study were incorporated in product labeling describing adverse reactions.
Continue on to: Adverse reactions...
Adverse reactions
In the positive monotherapy trial, there were 376 patients in the modified intent-to-treat efficacy population to receive lumateperone (N = 188) or placebo (N = 188) with nearly identical completion rates in the active treatment and placebo cohorts: lumateperone, 88.8%; placebo, 88.3%.17 The proportion experiencing mania was low in both cohorts (lumateperone, 1.1%; placebo, 2.1%), and there was 1 case of hypomania in each group. One participant in the lumateperone group and 1 in the placebo group discontinued the study due to a serious adverse event of mania. There was no worsening of mania in either group as measured by mean change in the YMRS score. There was also no suicidal behavior in either cohort during the study. Pooling the 2 monotherapy trials, the adverse events that occurred at ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone 42 mg/d: 13%, placebo: 3%), dizziness (lumateperone 42 mg/d: 8%, placebo: 4%), and nausea (lumateperone 42 mg/d: 8%, placebo: 3%).13 Rates of EPS were low for both groups: lumateperone 42 mg/d: 1.3%, placebo: 1.1%.13 Mean weight change at Day 43 was +0.11 kg for lumateperone and +0.03 kg for placebo in the positive monotherapy trial.17 Moreover, compared to placebo, lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant. No patient exhibited a corrected QT interval >500 ms at any time, and increases ≥60 ms from baseline were similar between the lumateperone (n = 1, 0.6%) and placebo (n = 3, 1.8%) cohorts.
Complete safety and tolerability data for the adjunctive trial has not yet been published, but discontinuation rates due to treatment-emergent adverse effects for the 3 arms were: lumateperone 42 mg/d: 5.6%; lumateperone 28 mg/d: 1.7%; and placebo: 1.7%. Overall, 81.4% of patients completed the trial, with only 1 serious adverse event (lithium toxicity) occurring in a patient taking lumateperone 42 mg/d. While this led to study discontinuation, it was not considered related to lumateperone exposure by the investigator. There was no worsening of mania in either lumateperone dosage group or the placebo cohort as measured by mean change in YMRS score: -1.2 for placebo, -1.4 for lumateperone 28 mg/d, and -1.6 for lumateperone 42 mg/d. Suicidal behavior was not observed in any group during treatment. The adverse events that occurred at rates ≥5% in lumateperone-treated patients and at more than twice the rate of the placebo group were somnolence/sedation (lumateperone, 13%; placebo, 3%), dizziness (lumateperone, 11%; placebo, 2%), and nausea (lumateperone, 9%; placebo, 4%).13 Rates of EPS were low for both groups: lumateperone, 4.0%, placebo, 2.3%.13 Mean weight changes at Day 43 were +0.23 kg for placebo, +0.02 kg for lumateperone 28 mg/d, and 0.00 kg for lumateperone 42 mg/d.18 Compared to placebo, both doses of lumateperone exhibited comparable effects on serum prolactin and all metabolic parameters, including fasting insulin, fasting glucose, and fasting lipids, none of which were clinically significant.18
Lastly, the package insert notes that in an uncontrolled, open-label trial of lumateperone for up to 6 months in patients with BD depression, the mean weight change was -0.01 ± 3.1 kg at Day 175.13
Continue on to: Pharmacologic profile...
Pharmacologic profile
Lumateperone’s preclinical discovery program found an impact on markers associated with increased glutamatergic neurotransmission, properties that were predicted to yield antidepressant benefit.14,15,24 This is hypothesized to be based on the complex pharmacology of lumateperone, including dopamine D1 agonism, modest SERT occupancy, and near saturation of the 5HT2A receptor.15,22 Dopamine D2 affinity is modest (32 nM), and the D2 receptor occupancy at the 42 mg dose is low. These properties translate to rates of EPS in clinical studies of schizophrenia and BD that are close to that of placebo. Lumateperone has very high affinity for serotonin 5HT2A receptors (Ki 0.54 nM), which also helps mitigate D2-related adverse effects and may be part of the therapeutic antidepressant mechanism. Underlying the tolerability profile is the low affinity for alpha 1-adrenergic receptors (Ki 73 nM), muscarinic and histaminergic receptors (Ki >100 nM for both).
Clinical considerations
Data from the lumateperone BD depression trials led to it being only the second agent approved for acute major depression in BD II patients, and the only agent which has approvals as monotherapy and adjunctive therapy for both BD subtypes. The monotherapy trial results substantiate that lumateperone was robustly effective regardless of BD subtype, with significant improvement in depressive symptoms experienced by patients with BD I (effect size 0.49, P < .0001) and those with BD II (effect size 0.81, P < .001). Effect sizes in acute BD depression studies are much larger in monotherapy trials than in adjunctive trials, as the latter group represents patients who have already failed pretreatment with a mood stabilizer.25,26 In the lurasidone BD I depression trials, the effect size based on mean change in MADRS score over the course of 6 weeks was 0.51 in the monotherapy study compared to 0.34 when used adjunctively with lithium or VPA.25,26 In the lumateperone adjunctive study, the effect size for the difference in mean MADRS total score from baseline for lumateperone 42 mg/d, was 0.27 (P < .05). Subgroup analyses by BD subtype are not yet available for adjunctive use, but the data presented to FDA were sufficient to permit an indication for adjunctive use across both diagnostic groups.
The absence of clinically significant EPS, the minimal impact on metabolic or endocrine parameters, and the lack of a need for titration are all appealing properties. At the present there is only 1 marketed dose (42 mg capsules), so the package insert includes cautionary language regarding situations when a patient might encounter less drug exposure (concurrent use of cytochrome P450 [CYP] 3A4 inducers), or greater drug exposure due to concurrent use of moderate or strong CYP3A4 inhibitors, as well as in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria (Child-Pugh B or C). These are not contraindications.
Unique properties of lumateperone include efficacy established as monotherapy for BD I and BD II patients, and efficacy for adjunctive use with lithium or VPA. Additionally, the extremely low rates of significant EPS and lack of clinically significant metabolic or endocrine adverse effects are unique properties of lumateperone.13
Why Rx? Reasons to prescribe lumateperone for adult BD depression patients include:
- data support efficacy for BD I and BD II patients, and for monotherapy or adjunctive use with lithium/VPA
- favorable tolerability profile, including no significant signal for EPS, endocrine or metabolic adverse effects, or QT prolongation
- no need for titration.
Dosing. There is only 1 dose available for lumateperone: 42 mg capsules (Table 3). As the dose cannot be modified, the package insert contains cautionary language regarding situations with less drug exposure (use of CYP3A4 inducers), or greater drug exposure (use with moderate or strong CYP3A4 inhibitors or in patients with moderate or severe hepatic impairment as defined by Child-Pugh Criteria [Child-Pugh B or C]). These are not contraindications. Based on newer pharmacokinetic studies, lumateperone does not need to be dosed with food, and there is no clinically significant interaction with UGT1A4 inhibitors such as VPA.

Contraindications. The only contraindication is known hypersensitivity to lumateperone.
Bottom Line
Data support the efficacy of lumateperone for treating depressive episodes in adults with bipolar I or bipolar II disorder, either as monotherapy or adjunctive to lithium or divalproex/valproate. Potential advantages of lumateperone for this indication include a favorable tolerability profile and no need for titration.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
1. Malhi GS, Bell E, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: bipolar disorder summary. Bipolar Disord. 2020;22(8):805-821.
2. Judd LL, Akishal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530-537.
3. Judd LL, Akishal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261-269.
4. Post RM. Treatment of bipolar depression: evolving recommendations. Psychiatr Clin North Am. 2016;39(1):11-33.
5. Pacchiarotti I, Verdolini N. Antidepressants in bipolar II depression: yes and no. Eur Neuropsychopharmacol 2021;47:48-50.
6. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
7. Allain N, Leven C, Falissard B, et al. Manic switches induced by antidepressants: an umbrella review comparing randomized controlled trials and observational studies. Acta Psychiatr Scand. 2017;135(2):106-116.
8. Gitlin MJ. Antidepressants in bipolar depression: an enduring controversy. Int J Bipolar Disord. 2018;6(1):25.
9. Verdolini N, Hidalgo-Mazzei D, Del Matto L, et al. Long-term treatment of bipolar disorder type I: a systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324-340.
10. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180-195.
11. Vraylar [package insert]. Madison, NJ: Allergan USA, Inc.; 2019.
12. Chakrabarty T, Hadijpavlou G, Bond DJ, et al. Bipolar II disorder in context: a review of its epidemiology, disability and economic burden. In: Parker G. Bipolar II Disorder: Modelling, Measuring and Managing. 3rd ed. Cambridge University Press; 2019:49-59.
13. Caplyta [package insert]. New York, NY: Intra-Cellular Therapies, Inc.; 2021.
14. Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614.
15. Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology (Berl). 2015;232:605-621.
16. Vanover KE, Davis RE, Zhou Y, et al. Dopamine D2 receptor occupancy of lumateperone (ITI-007): a positron emission tomography study in patients with schizophrenia. Neuropsychopharmacology. 2019;44(3):598-605.
17. Calabrese JR, Durgam S, Satlin A, et al. Efficacy and safety of lumateperone for major depressive episodes associated with bipolar I or bipolar II disorder: a phase 3 randomized placebo-controlled trial. Am J Psychiatry 2021;178(12):1098-1106.
18. Yatham LN, et al. Adjunctive lumateperone (ITI-007) in the treatment of bipolar depression: results from a randomized clinical trial. Poster presented at: American Psychiatric Association Annual Meeting. May 1-3, 2021; virtual conference.
19. Vanover K, Glass S, Kozauer S, et al. 30 Lumateperone (ITI-007) for the treatment of schizophrenia: overview of placebo-controlled clinical trials and an open-label safety switching study. CNS Spectrums. 2019;24(1):190-191.
20. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today (Barc). 2018;54(12):713-719.
21. Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology (Berl). 2015;232(15):2863-72.
22. Björkholm C, Marcus MM, Konradsson-Geuken Å, et al. The novel antipsychotic drug brexpiprazole, alone and in combination with escitalopram, facilitates prefrontal glutamatergic transmission via a dopamine D1 receptor-dependent mechanism. Eur Neuropsychopharmacol. 2017;27(4):411-417.
23. Bai Y, Yang H, Chen G, et al. Acceptability of acute and maintenance pharmacotherapy of bipolar disorder: a systematic review of randomized, double-blind, placebo-controlled clinical trials. J Clin Psychopharmacol. 2020;40(2):167-179.
24. Vyas P, Hwang BJ, Braši´c JR. An evaluation of lumateperone tosylate for the treatment of schizophrenia. Expert Opin Pharmacother. 2020;21(2):139-145.
25. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160-168.
26. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169-77.
When is your patient a candidate for ECT?
LAS VEGAS – How do you know when a patient is a candidate for electroconvulsive therapy (ECT)?
In the opinion of Mark S. George, MD, it depends on the level of treatment resistance, other treatments the person may be receiving for severe depression or bipolar disorder, and the level of acuity.
“Acute ECT is also useful for catatonia that does not resolve with benzodiazepines, and it also works well for acute suicidality,” Dr. George, distinguished professor of psychiatry, radiology, and neurology at the Medical University of South Carolina, Charleston, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The other reason you would go straight to ECT would be if someone has had good prior ECT response.”
It is lifesaving. Some studies suggests that ECT is effective in Parkinson’s disease and schizophrenia. Antidepressant effects generally take 2-3 weeks, but quicker responses are sometimes seen, especially in patients with bipolar depression.”
In the past 20 years of research studies involving ECT, investigators have discovered that a generalized seizure of adequate duration is necessary for adequate antidepressant effects; reduced therapeutic effects are seen with parietal placement, meaning that proper scalp placement matters; a dose titration over the 12 treatments improves efficacy, and smaller pulse widths are more effective and may result in fewer toxic side effects. “ECT is still relatively spatially crude compared with the other brain stimulation treatments,” said Dr. George, editor-in-chief of Brain Stimulation. “It’s also invasive, requiring repeated anesthesia, and sometimes has possible side effects including impacts on short-term memory.”
An emerging adjunct to ECT is cervical invasive vagus nerve stimulation (VNS) therapy, in which mild electrical pulses applied to the left vagus nerve in the neck send signals to the brain. “Surgeons wrap a wire around the vagus nerve and connect the wire to a generator which is embedded in the chest wall,” Dr. George explained. “The generator sends out a signal through the vagus nerve intermittently. You can program how it does that.”
A device from LivaNova known as the VNS Pulse Model 102 Generator was granted clearance for depression based on a comparative study, but in the absence of class I evidence. The generator is about the size of a quarter, is embedded under the skin, and its battery lasts for 8-10 years. “Patients are given a static magnet to use to turn the device off if they’re having side effects, as a safety precaution,” said Dr. George, a staff physician at the Ralph H. Johnson VA Medical Center in Charleston. “The side effects are mainly stimulation-based and typically decrease over time. There is a low rate of treatment discontinuation and no signal for treatment-related emergence of suicidal ideation/behavior. Sometimes you can get emergent mania or hypermania, but it’s rare. It’s pretty safe, but the insurance companies have been very slow to pay. You only get about 30% remission, this takes several months to years to achieve, and there’s no way to tell who’s going to respond before you place the device.”
However, results from a 5-year observational study of patients with treatment-resistant depression who were treated at 61 sites with VNS or treatment as usual found that the antidepressant effects built over time compared with treatment as usual (Am J Psychiatry 2017;174[7]:640-8). “There is remarkable durability but it’s not very fast,” he said. “It’s three months before you start seeing any differences.”
According to Dr. George, data from an informal registry of Medicare patients who received VNS treatment “did so much better” than untreated patients. “They didn’t need as much ECT and didn’t require as many hospitalizations,” he said. “They weren’t changing medications nearly as much. They found that VNS was saving money and saving people’s lives.” As a result, in September of 2019 LivaNova launched a prospective, multicenter, randomized, controlled, blinded trial of subjects implanted with VNS therapy, called RECOVER. Active treatment and no stimulation control are randomized at least 2 weeks after implantation and observed for 12 months. The study is ongoing with results expected in 2022 or 2023.
Dr. George disclosed that he is a paid consultant for Neurolief, Microtransponder, and Sooma and that he has been a paid consultant for GSK, Cyberonics, NeuroPace, and Jazz. He is an unpaid consultant to Brainsway, Neuronetics, Neostim, Neosync, and Magnus Medical.
LAS VEGAS – How do you know when a patient is a candidate for electroconvulsive therapy (ECT)?
In the opinion of Mark S. George, MD, it depends on the level of treatment resistance, other treatments the person may be receiving for severe depression or bipolar disorder, and the level of acuity.
“Acute ECT is also useful for catatonia that does not resolve with benzodiazepines, and it also works well for acute suicidality,” Dr. George, distinguished professor of psychiatry, radiology, and neurology at the Medical University of South Carolina, Charleston, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The other reason you would go straight to ECT would be if someone has had good prior ECT response.”
It is lifesaving. Some studies suggests that ECT is effective in Parkinson’s disease and schizophrenia. Antidepressant effects generally take 2-3 weeks, but quicker responses are sometimes seen, especially in patients with bipolar depression.”
In the past 20 years of research studies involving ECT, investigators have discovered that a generalized seizure of adequate duration is necessary for adequate antidepressant effects; reduced therapeutic effects are seen with parietal placement, meaning that proper scalp placement matters; a dose titration over the 12 treatments improves efficacy, and smaller pulse widths are more effective and may result in fewer toxic side effects. “ECT is still relatively spatially crude compared with the other brain stimulation treatments,” said Dr. George, editor-in-chief of Brain Stimulation. “It’s also invasive, requiring repeated anesthesia, and sometimes has possible side effects including impacts on short-term memory.”
An emerging adjunct to ECT is cervical invasive vagus nerve stimulation (VNS) therapy, in which mild electrical pulses applied to the left vagus nerve in the neck send signals to the brain. “Surgeons wrap a wire around the vagus nerve and connect the wire to a generator which is embedded in the chest wall,” Dr. George explained. “The generator sends out a signal through the vagus nerve intermittently. You can program how it does that.”
A device from LivaNova known as the VNS Pulse Model 102 Generator was granted clearance for depression based on a comparative study, but in the absence of class I evidence. The generator is about the size of a quarter, is embedded under the skin, and its battery lasts for 8-10 years. “Patients are given a static magnet to use to turn the device off if they’re having side effects, as a safety precaution,” said Dr. George, a staff physician at the Ralph H. Johnson VA Medical Center in Charleston. “The side effects are mainly stimulation-based and typically decrease over time. There is a low rate of treatment discontinuation and no signal for treatment-related emergence of suicidal ideation/behavior. Sometimes you can get emergent mania or hypermania, but it’s rare. It’s pretty safe, but the insurance companies have been very slow to pay. You only get about 30% remission, this takes several months to years to achieve, and there’s no way to tell who’s going to respond before you place the device.”
However, results from a 5-year observational study of patients with treatment-resistant depression who were treated at 61 sites with VNS or treatment as usual found that the antidepressant effects built over time compared with treatment as usual (Am J Psychiatry 2017;174[7]:640-8). “There is remarkable durability but it’s not very fast,” he said. “It’s three months before you start seeing any differences.”
According to Dr. George, data from an informal registry of Medicare patients who received VNS treatment “did so much better” than untreated patients. “They didn’t need as much ECT and didn’t require as many hospitalizations,” he said. “They weren’t changing medications nearly as much. They found that VNS was saving money and saving people’s lives.” As a result, in September of 2019 LivaNova launched a prospective, multicenter, randomized, controlled, blinded trial of subjects implanted with VNS therapy, called RECOVER. Active treatment and no stimulation control are randomized at least 2 weeks after implantation and observed for 12 months. The study is ongoing with results expected in 2022 or 2023.
Dr. George disclosed that he is a paid consultant for Neurolief, Microtransponder, and Sooma and that he has been a paid consultant for GSK, Cyberonics, NeuroPace, and Jazz. He is an unpaid consultant to Brainsway, Neuronetics, Neostim, Neosync, and Magnus Medical.
LAS VEGAS – How do you know when a patient is a candidate for electroconvulsive therapy (ECT)?
In the opinion of Mark S. George, MD, it depends on the level of treatment resistance, other treatments the person may be receiving for severe depression or bipolar disorder, and the level of acuity.
“Acute ECT is also useful for catatonia that does not resolve with benzodiazepines, and it also works well for acute suicidality,” Dr. George, distinguished professor of psychiatry, radiology, and neurology at the Medical University of South Carolina, Charleston, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “The other reason you would go straight to ECT would be if someone has had good prior ECT response.”
It is lifesaving. Some studies suggests that ECT is effective in Parkinson’s disease and schizophrenia. Antidepressant effects generally take 2-3 weeks, but quicker responses are sometimes seen, especially in patients with bipolar depression.”
In the past 20 years of research studies involving ECT, investigators have discovered that a generalized seizure of adequate duration is necessary for adequate antidepressant effects; reduced therapeutic effects are seen with parietal placement, meaning that proper scalp placement matters; a dose titration over the 12 treatments improves efficacy, and smaller pulse widths are more effective and may result in fewer toxic side effects. “ECT is still relatively spatially crude compared with the other brain stimulation treatments,” said Dr. George, editor-in-chief of Brain Stimulation. “It’s also invasive, requiring repeated anesthesia, and sometimes has possible side effects including impacts on short-term memory.”
An emerging adjunct to ECT is cervical invasive vagus nerve stimulation (VNS) therapy, in which mild electrical pulses applied to the left vagus nerve in the neck send signals to the brain. “Surgeons wrap a wire around the vagus nerve and connect the wire to a generator which is embedded in the chest wall,” Dr. George explained. “The generator sends out a signal through the vagus nerve intermittently. You can program how it does that.”
A device from LivaNova known as the VNS Pulse Model 102 Generator was granted clearance for depression based on a comparative study, but in the absence of class I evidence. The generator is about the size of a quarter, is embedded under the skin, and its battery lasts for 8-10 years. “Patients are given a static magnet to use to turn the device off if they’re having side effects, as a safety precaution,” said Dr. George, a staff physician at the Ralph H. Johnson VA Medical Center in Charleston. “The side effects are mainly stimulation-based and typically decrease over time. There is a low rate of treatment discontinuation and no signal for treatment-related emergence of suicidal ideation/behavior. Sometimes you can get emergent mania or hypermania, but it’s rare. It’s pretty safe, but the insurance companies have been very slow to pay. You only get about 30% remission, this takes several months to years to achieve, and there’s no way to tell who’s going to respond before you place the device.”
However, results from a 5-year observational study of patients with treatment-resistant depression who were treated at 61 sites with VNS or treatment as usual found that the antidepressant effects built over time compared with treatment as usual (Am J Psychiatry 2017;174[7]:640-8). “There is remarkable durability but it’s not very fast,” he said. “It’s three months before you start seeing any differences.”
According to Dr. George, data from an informal registry of Medicare patients who received VNS treatment “did so much better” than untreated patients. “They didn’t need as much ECT and didn’t require as many hospitalizations,” he said. “They weren’t changing medications nearly as much. They found that VNS was saving money and saving people’s lives.” As a result, in September of 2019 LivaNova launched a prospective, multicenter, randomized, controlled, blinded trial of subjects implanted with VNS therapy, called RECOVER. Active treatment and no stimulation control are randomized at least 2 weeks after implantation and observed for 12 months. The study is ongoing with results expected in 2022 or 2023.
Dr. George disclosed that he is a paid consultant for Neurolief, Microtransponder, and Sooma and that he has been a paid consultant for GSK, Cyberonics, NeuroPace, and Jazz. He is an unpaid consultant to Brainsway, Neuronetics, Neostim, Neosync, and Magnus Medical.
FROM NPA 2022
Ketamine fast, effective for suicidal crises
In addition, a strong effect of ketamine was observed in patients with bipolar disorder, “whereas the effect was moderate and did not quite reach significance in those with other psychiatric disorders and unexpectedly was nonsignificant in those with major depressive disorders,” the researchers wrote.
“We assessed for the first time in the same study the effect of ketamine on three a priori–defined groups of nonpsychotic patients: those with a bipolar disorder, those with a depressive disorder, and those with other diagnoses,” study investigator Fabrice Jollant, MD, PhD, professor of psychiatry, University of Paris, said in an interview.
“This allowed us to find that comorbid disorders are important modulators of the clinical effects of ketamine, and that the effect of ketamine is particularly marked among patients with a bipolar disorder,” Dr. Jollant added.
The study was published online Feb. 2, 2022, in the BMJ.
Swift, full remission
The study included 156 adults admitted voluntarily to seven French teaching hospitals with severe suicidal ideation, including 52 with bipolar disorder, 56 with depressive disorder, and 48 with other psychiatric diagnoses.
They were randomly allocated to two 40-minute intravenous infusions of ketamine (0.5 mg/kg) or placebo (saline) administered at baseline and 24 hours, in addition to usual treatment.
The primary outcome was the rate of patients in full suicidal remission at day 3, confirmed by a score of 3 or less on a clinician-rated scale for suicidal ideation based on 19 items scored 0-2 (maximum score, 38).
“We investigated the full remission of suicidal ideas and not only the response, which is usually defined as a reduction of 50% of scores on a given scale. If people remain slightly suicidal, the suicidal risk persists. We want all suicidal ideas to disappear,” said Dr. Jollant.
They found that more patients reached full remission of suicidal ideas at day 3 after two ketamine infusions than after placebo infusions (63% vs. 32%; odds ratio, 3.7; 95% confidence interval, 1.9-7.3; P < .001).
This antisuicidal effect of ketamine was rapid, with 44% remission only 2 hours after the first infusion, the authors reported.
The effect of ketamine on suicidal remission was greatest in patients with bipolar disorder, with 85% achieving full remission at day 3 (OR, 14.1; 95% CI, 3.0-92.2; P < .001), compared with 42% of patients with depressive disorder (OR, 1.3; 95% CI, 0.3-5.2; P = .6) or 62% of those with other disorders (OR, 3.7; 95% CI, 0.9-17.3; P = .07).
At 6 weeks after treatment, remission in the ketamine group remained high, although nonsignificantly versus placebo (69.5% vs. 56.3%; OR, 0.8; 95% CI, 0.3-2.5; P = .7).
The researchers noted the beneficial effect of ketamine on suicidal ideation could be mediated by an effect on psychological pain.
“Although mental pain does not necessarily lead to suicidal ideas, recent studies suggest that individuals with severe suicidal ideas (notably those with a plan) also have high levels of mental pain. Ketamine might therefore exert its effects through analgesic mechanisms that reduce mental pain,” they wrote.
Ketamine’s side effects were “limited” with no manic or psychotic symptoms seen. The main side effects, including sedation, denationalization/derealization, nausea, and dizziness, were of short duration and occurred in about 10% or fewer patients.
The investigators acknowledged that the nonsignificant effect of ketamine in the patients with major depressive disorders in this study is “challenging to interpret.”
They pointed out the study may have lacked power to detect an effect in these patients. In addition, this group might be particularly heterogeneous, with more patients sensitive to a placebo effect and more patients requiring repeated ketamine infusions.
A new perspective on ketamine
In an accompanying editorial, Riccardo De Giorgi, MD, Wellcome Trust doctoral training fellow, department of psychiatry, University of Oxford (England), said the study challenges current thinking about ketamine.
The “unexpected” outcome (no benefit) in the depressive group “perhaps defies the prevailing notion that patients with major depression would benefit most from ketamine,” Dr. De Giorgi wrote.
“In fact, both usual care and ketamine given with usual care led to low, comparable remission rates of 35.7% and 42.3% for suicidal ideation, respectively, in patients with depressive disorder,” Dr. De Giorgi pointed out.
“While this study therefore confirms that many patients with depressive disorder and suicidal ideation remain poorly served by available treatments, it shows that another important group of patients with acute suicidal ideation, those with bipolar disorder, could benefit from ketamine,” Dr. De Giorgi wrote.
“Once again, here is evidence that careful clinical evaluation must precede any consideration of ketamine use, which must be reserved for specific clinical presentations and not given indiscriminately to anyone presenting with suicidal thoughts,” he concluded.
Funding for the study was provided by Programme Hospitalier de Recherche Clinique National. Dr. Jollant and Dr. De Giorgi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, a strong effect of ketamine was observed in patients with bipolar disorder, “whereas the effect was moderate and did not quite reach significance in those with other psychiatric disorders and unexpectedly was nonsignificant in those with major depressive disorders,” the researchers wrote.
“We assessed for the first time in the same study the effect of ketamine on three a priori–defined groups of nonpsychotic patients: those with a bipolar disorder, those with a depressive disorder, and those with other diagnoses,” study investigator Fabrice Jollant, MD, PhD, professor of psychiatry, University of Paris, said in an interview.
“This allowed us to find that comorbid disorders are important modulators of the clinical effects of ketamine, and that the effect of ketamine is particularly marked among patients with a bipolar disorder,” Dr. Jollant added.
The study was published online Feb. 2, 2022, in the BMJ.
Swift, full remission
The study included 156 adults admitted voluntarily to seven French teaching hospitals with severe suicidal ideation, including 52 with bipolar disorder, 56 with depressive disorder, and 48 with other psychiatric diagnoses.
They were randomly allocated to two 40-minute intravenous infusions of ketamine (0.5 mg/kg) or placebo (saline) administered at baseline and 24 hours, in addition to usual treatment.
The primary outcome was the rate of patients in full suicidal remission at day 3, confirmed by a score of 3 or less on a clinician-rated scale for suicidal ideation based on 19 items scored 0-2 (maximum score, 38).
“We investigated the full remission of suicidal ideas and not only the response, which is usually defined as a reduction of 50% of scores on a given scale. If people remain slightly suicidal, the suicidal risk persists. We want all suicidal ideas to disappear,” said Dr. Jollant.
They found that more patients reached full remission of suicidal ideas at day 3 after two ketamine infusions than after placebo infusions (63% vs. 32%; odds ratio, 3.7; 95% confidence interval, 1.9-7.3; P < .001).
This antisuicidal effect of ketamine was rapid, with 44% remission only 2 hours after the first infusion, the authors reported.
The effect of ketamine on suicidal remission was greatest in patients with bipolar disorder, with 85% achieving full remission at day 3 (OR, 14.1; 95% CI, 3.0-92.2; P < .001), compared with 42% of patients with depressive disorder (OR, 1.3; 95% CI, 0.3-5.2; P = .6) or 62% of those with other disorders (OR, 3.7; 95% CI, 0.9-17.3; P = .07).
At 6 weeks after treatment, remission in the ketamine group remained high, although nonsignificantly versus placebo (69.5% vs. 56.3%; OR, 0.8; 95% CI, 0.3-2.5; P = .7).
The researchers noted the beneficial effect of ketamine on suicidal ideation could be mediated by an effect on psychological pain.
“Although mental pain does not necessarily lead to suicidal ideas, recent studies suggest that individuals with severe suicidal ideas (notably those with a plan) also have high levels of mental pain. Ketamine might therefore exert its effects through analgesic mechanisms that reduce mental pain,” they wrote.
Ketamine’s side effects were “limited” with no manic or psychotic symptoms seen. The main side effects, including sedation, denationalization/derealization, nausea, and dizziness, were of short duration and occurred in about 10% or fewer patients.
The investigators acknowledged that the nonsignificant effect of ketamine in the patients with major depressive disorders in this study is “challenging to interpret.”
They pointed out the study may have lacked power to detect an effect in these patients. In addition, this group might be particularly heterogeneous, with more patients sensitive to a placebo effect and more patients requiring repeated ketamine infusions.
A new perspective on ketamine
In an accompanying editorial, Riccardo De Giorgi, MD, Wellcome Trust doctoral training fellow, department of psychiatry, University of Oxford (England), said the study challenges current thinking about ketamine.
The “unexpected” outcome (no benefit) in the depressive group “perhaps defies the prevailing notion that patients with major depression would benefit most from ketamine,” Dr. De Giorgi wrote.
“In fact, both usual care and ketamine given with usual care led to low, comparable remission rates of 35.7% and 42.3% for suicidal ideation, respectively, in patients with depressive disorder,” Dr. De Giorgi pointed out.
“While this study therefore confirms that many patients with depressive disorder and suicidal ideation remain poorly served by available treatments, it shows that another important group of patients with acute suicidal ideation, those with bipolar disorder, could benefit from ketamine,” Dr. De Giorgi wrote.
“Once again, here is evidence that careful clinical evaluation must precede any consideration of ketamine use, which must be reserved for specific clinical presentations and not given indiscriminately to anyone presenting with suicidal thoughts,” he concluded.
Funding for the study was provided by Programme Hospitalier de Recherche Clinique National. Dr. Jollant and Dr. De Giorgi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, a strong effect of ketamine was observed in patients with bipolar disorder, “whereas the effect was moderate and did not quite reach significance in those with other psychiatric disorders and unexpectedly was nonsignificant in those with major depressive disorders,” the researchers wrote.
“We assessed for the first time in the same study the effect of ketamine on three a priori–defined groups of nonpsychotic patients: those with a bipolar disorder, those with a depressive disorder, and those with other diagnoses,” study investigator Fabrice Jollant, MD, PhD, professor of psychiatry, University of Paris, said in an interview.
“This allowed us to find that comorbid disorders are important modulators of the clinical effects of ketamine, and that the effect of ketamine is particularly marked among patients with a bipolar disorder,” Dr. Jollant added.
The study was published online Feb. 2, 2022, in the BMJ.
Swift, full remission
The study included 156 adults admitted voluntarily to seven French teaching hospitals with severe suicidal ideation, including 52 with bipolar disorder, 56 with depressive disorder, and 48 with other psychiatric diagnoses.
They were randomly allocated to two 40-minute intravenous infusions of ketamine (0.5 mg/kg) or placebo (saline) administered at baseline and 24 hours, in addition to usual treatment.
The primary outcome was the rate of patients in full suicidal remission at day 3, confirmed by a score of 3 or less on a clinician-rated scale for suicidal ideation based on 19 items scored 0-2 (maximum score, 38).
“We investigated the full remission of suicidal ideas and not only the response, which is usually defined as a reduction of 50% of scores on a given scale. If people remain slightly suicidal, the suicidal risk persists. We want all suicidal ideas to disappear,” said Dr. Jollant.
They found that more patients reached full remission of suicidal ideas at day 3 after two ketamine infusions than after placebo infusions (63% vs. 32%; odds ratio, 3.7; 95% confidence interval, 1.9-7.3; P < .001).
This antisuicidal effect of ketamine was rapid, with 44% remission only 2 hours after the first infusion, the authors reported.
The effect of ketamine on suicidal remission was greatest in patients with bipolar disorder, with 85% achieving full remission at day 3 (OR, 14.1; 95% CI, 3.0-92.2; P < .001), compared with 42% of patients with depressive disorder (OR, 1.3; 95% CI, 0.3-5.2; P = .6) or 62% of those with other disorders (OR, 3.7; 95% CI, 0.9-17.3; P = .07).
At 6 weeks after treatment, remission in the ketamine group remained high, although nonsignificantly versus placebo (69.5% vs. 56.3%; OR, 0.8; 95% CI, 0.3-2.5; P = .7).
The researchers noted the beneficial effect of ketamine on suicidal ideation could be mediated by an effect on psychological pain.
“Although mental pain does not necessarily lead to suicidal ideas, recent studies suggest that individuals with severe suicidal ideas (notably those with a plan) also have high levels of mental pain. Ketamine might therefore exert its effects through analgesic mechanisms that reduce mental pain,” they wrote.
Ketamine’s side effects were “limited” with no manic or psychotic symptoms seen. The main side effects, including sedation, denationalization/derealization, nausea, and dizziness, were of short duration and occurred in about 10% or fewer patients.
The investigators acknowledged that the nonsignificant effect of ketamine in the patients with major depressive disorders in this study is “challenging to interpret.”
They pointed out the study may have lacked power to detect an effect in these patients. In addition, this group might be particularly heterogeneous, with more patients sensitive to a placebo effect and more patients requiring repeated ketamine infusions.
A new perspective on ketamine
In an accompanying editorial, Riccardo De Giorgi, MD, Wellcome Trust doctoral training fellow, department of psychiatry, University of Oxford (England), said the study challenges current thinking about ketamine.
The “unexpected” outcome (no benefit) in the depressive group “perhaps defies the prevailing notion that patients with major depression would benefit most from ketamine,” Dr. De Giorgi wrote.
“In fact, both usual care and ketamine given with usual care led to low, comparable remission rates of 35.7% and 42.3% for suicidal ideation, respectively, in patients with depressive disorder,” Dr. De Giorgi pointed out.
“While this study therefore confirms that many patients with depressive disorder and suicidal ideation remain poorly served by available treatments, it shows that another important group of patients with acute suicidal ideation, those with bipolar disorder, could benefit from ketamine,” Dr. De Giorgi wrote.
“Once again, here is evidence that careful clinical evaluation must precede any consideration of ketamine use, which must be reserved for specific clinical presentations and not given indiscriminately to anyone presenting with suicidal thoughts,” he concluded.
Funding for the study was provided by Programme Hospitalier de Recherche Clinique National. Dr. Jollant and Dr. De Giorgi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Things reproductive psychiatrists might ‘always’ or ‘never’ do in 2022
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The experience of practicing reproductive psychiatry in the context of the pandemic has highlighted unique situations I’ve written about in previous columns that have affected pregnant and postpartum women during the pandemic, such as the management of anxiety and insomnia.
The pandemic has also seen a shift to telemedicine and an opportunity to use virtual platforms to engage with colleagues in our subspecialty across the country. These forums of engagement, which we realize virtually with so many of our colleagues, has prompted me to refine and galvanize what I consider to be some principles that guide frequently encountered clinical scenarios in reproductive psychiatry.
To open 2022, I wanted to revisit the practices I nearly “always” (or conversely, “never”) follow as a reproductive psychiatrist across the numerous clinical situations and variations on the associated clinical themes encountered as we see patients during pregnancy and the postpartum period.
Things we ‘always’ do
1. I continue to make maternal euthymia the North Star of treatment before, during, and after pregnancy.
Before pregnancy, maternal euthymia may be realized through optimization of pharmacologic and nonpharmacologic treatments and waiting to conceive until patients are emotionally well. Sustaining euthymia during pregnancy is a critical issue because of the extent to which euthymia during pregnancy predicts postpartum course. According to many studies, postpartum euthymia is the strongest predictor of long-term neurobehavioral outcome and risk for later child psychopathology. At the end of the day, there are few things I would not do with respect to treatment of maternal psychiatric disorder if the upside afforded maternal euthymia.
2. I almost always treat with consistency of medication across the peripartum period.
Although there have been discussions about the wisdom of changing medications, such as antidepressants, benzodiazepines, and mood stabilizers, that have afforded euthymia during pregnancy as patients approach their delivery date, the evidence base supporting switching medications at that time is exceedingly sparse. The time to adjust or to modify is typically not just prior to delivery unless it is to prevent postpartum psychiatric disorder (see below).
3. I simplify regimens before pregnancy if it’s unclear which medications have afforded patients euthymia.
We have a growing appreciation that polypharmacy is the rule in treatment of affective disorder for both unipolar and bipolar illness. Consultation before pregnancy is the ideal time to take a particularly careful history and think about simplifying regimens where adding medicines hasn’t clearly provided enhanced clinical benefit to the patient.
4. When making a treatment plan for psychiatric disorder during pregnancy, I consider the impact of untreated psychiatric disorder (even if not absolutely quantifiable) on fetal, neonatal, and maternal well-being.
Perhaps now more than even 5-10 years ago, we have better data describing the adverse effects of untreated psychiatric illness on fetal, neonatal, and maternal well-being.
We always try to deliberately consider the effect of a specific treatment on fetal well-being. Less attention (and science) has focused on the effect on pregnancy of deferring treatment; historically, this has not been adequately quantified in the risk-benefit decision. Yet, there is growing evidence of the increased adverse effects of activating the stress axis on everything from intrauterine fetal programming in the brain to effects on obstetrical outcomes such as preterm labor and delivery.
5. I appreciate the value of postpartum prophylaxis for pregnant women with bipolar disorder to mitigate risk of relapse.
We have spoken over the last 20 months of the pandemic, particularly in reproductive psychiatry circles, about the importance of keeping reproductive-age women with bipolar disorder emotionally well as they plan to conceive, during pregnancy, and in the postpartum period. The management of bipolar disorder during this time can be a humbling experience. Clinical roughening can be quick and severe, and so we do everything that we can for these women.
The area in which we have the strongest evidence base for mitigating risk with bipolar women is the value of postpartum prophylaxis during the peripartum period, regardless of what patients have done with their mood-stabilizing medications during pregnancy. Given the risk for postpartum disease, even though there are varying amounts of evidence on prophylactic benefit of specific mood stabilizers (i.e., lithium vs. atypical antipsychotics), the value of prophylaxis against worsening of bipolar disorder postpartum is widely accepted.
The importance of this has been particularly underscored during the pandemic where postpartum support, although available, has been more tenuous given the fluctuations in COVID-19 status around the country. The availability of friends and loved ones as support during the postpartum period has become less reliable in certain circumstances during the pandemic. In some cases, COVID-19 surges have wreaked havoc on travel plans and support persons have contracted the virus, rendering on-site support nonviable given safety concerns. Last-minute shifts of support plans have been responsible for disruption of care plans for new moms and by extension, have affected the ability to protect the sleep of bipolar women, which is critical. Keeping bipolar women well during the postpartum period with plans and backup plans for management remains critical.
Things we ‘never’ do
1. I never taper antidepressants (just prior to delivery), I never check plasma levels of selective serotonin reuptake inhibitors (across pregnancy, or just prior to labor and delivery), and I never use sodium valproate (during pregnancy).
Although there has been some discussion about the potential to mitigate risk for maternal or neonatal toxicity with lowering of agents such as lithium or lamotrigine during pregnancy, I do not routinely check plasma levels or arbitrarily change the dose of antidepressants, lithium, or lamotrigine during pregnancy in the absence of clinical symptoms.
We know full well that plasma levels of medications decline during pregnancy because of hemodilution with lithium and antidepressants and, in the case of lamotrigine, the effects of rising estrogen concentration during pregnancy on the metabolism of lamotrigine. While several studies have shown the decrease of SSRI concentration during pregnancy absent a change in dose of medication, these data have not correlated changes in plasma concentration of SSRI with a frank change in clinical status across pregnancy. Unlike what we see in conditions like epilepsy, where doses are increased to maintain therapeutic plasma levels to mitigate risk for seizure, those therapeutic plasma levels do not clearly exist for the psychiatric medications most widely used to treat psychiatric disorders.
We also almost never use sodium valproate in reproductive-age women despite its efficacy in both the acute and maintenance treatment of bipolar disorder given the risk of both major malformations associated with first-trimester fetal exposure to valproate and the data suggesting longer-term adverse neurobehavioral effects associated with its use during pregnancy.
2. We never suggest patients defer pregnancy based on their underlying psychiatric disorder.
Our role is to provide the best information regarding reproductive safety of psychiatric medications and risks of untreated psychiatric disorder to patients as they and relevant parties weigh the risks of pursuing one treatment or another. Those are private choices, and women and their partners make private decisions applying their own calculus with respect to moving forward with plans to conceive.
3. We never switch antidepressants once a woman has become pregnant.
Although we continue to see patients switched to older SSRIs such as sertraline with documentation of pregnancy, a patient’s road to getting well is sometimes very lengthy. In the absence of indicting reproductive safety data for any particular antidepressant, for patients who have gotten well on an antidepressant, even one for which we have less information, we stay the course and do not switch arbitrarily to an older SSRI for which we may have more reproductive safety data.
If we have the luxury prior to pregnancy to switch a patient to an untried and better studied antidepressant with more data supporting safety, we do so. But this is rarely the case. More often, we see women presenting with a newly documented pregnancy (frequently unplanned, with half of pregnancies across the country still being unplanned across sociodemographic lines) on an antidepressant with varying amounts of reproductive safety information available for the medicine being taken, and frequently after failed previous trials of other antidepressants. In this scenario, we rarely see the time of a newly documented pregnancy as an opportunity to pursue a new trial of an antidepressant without known efficacy for that patient; we stay the course and hope for sustained euthymia on the drug which has afforded euthymia to date.
Final thoughts
Dos and don’ts are relative in reproductive psychiatry. We tend to apply available data and clinical experience as we guide patients on a case-by-case basis, considering the most currently available rigorous reproductive safety data, as well as the individual patient’s clinical status and her personal wishes.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Siblings of people with bipolar disorder have higher cancer risk
, according to new research from Taiwan.
“To our knowledge, our study is the first to report an increased overall cancer risk as well as increased risks of breast and ectodermal cancer among the unaffected siblings aged < 50 years of patients with bipolar disorder,” Ya-Mei Bai, MD, PhD, of National Yang-Ming University, Taipei, Taiwan, and colleagues write in an article published online in the International Journal of Cancer.
Most, but not all, previous studies have shown a link between bipolar disorder and cancer. Whether the elevated risk of malignancy extends to family members without the mental health condition has not been elucidated.
To investigate, the researchers turned to the National Health Insurance Research Database of Taiwan. They identified 25,356 individuals diagnosed with bipolar disorder by a psychiatrist between 1996 and 2010 and the same number of unaffected siblings, as well as more than 100,000 age-, sex-, income-, and residence-matched controls without severe mental illness.
Compared with the control group, people with bipolar disorder (odds ratio, 1.22) and their unaffected siblings (OR, 1.17) both had a higher risk of developing malignant cancer of any kind. The researchers also found that both groups were at higher risk for breast cancer, with odds ratios of 1.98 in individuals with bipolar disorder and 1.73 in their unaffected siblings.
However, the risk of skin cancer was only high in people with bipolar disorder (OR, 2.70) and not in their siblings (OR, 0.62). And conversely, the risk of kidney cancer was significantly increased in unaffected siblings (OR, 2.45) but not in people with bipolar disorder (OR, 0.47).
When stratified by the embryonic developmental layer from which tumors had originated – ectodermal, mesodermal, or endodermal – the authors observed a significantly increased risk for only ectodermal cancers. In addition, only people under age 50 in both groups (OR, 1.90 for those with bipolar disorder; OR, 1.65 for siblings) were more likely to develop an ectodermal cancer, especially of the breast, compared with the control group. The risks remained elevated after excluding breast cancer but were no longer significant.
When stratified by age, the risk of developing any cancer in both groups also only appeared to be greater for those under age 50 (OR, 1.34 in people with bipolar disorder; OR, 1.32 in siblings) compared with those aged 50 and over (OR, 0.97 and 0.99, respectively). The authors highlighted these figures in the supplemental data set but did not discuss it further in the study beyond a brief mention that “younger patients with bipolar disorder and younger unaffected siblings (< 50 years), but not older ones (≥ 50 years), were more likely to develop any malignancy during the follow-up than matched controls.”
“This paper essentially finds what we have found in our previous work – that people with bipolar disorder have a greater risk of cancer,” said Michael Berk, MBBCh, PhD, a professor of psychiatry at the Deakin University School of Medicine in Geelong, Australia, who published a systematic review and meta-analysis last spring on cancer risk and the role of lithium treatment in bipolar disorder.
“The interesting finding in our work,” Dr. Berk told this news organization, “is that this risk is attenuated by use of lithium but not other agents.”
The Taiwanese researchers propose a “biopsychosocial explanation” for their results, noting that both the nervous system and the breast and skin develop from the ectoderm, and that cancer risk factors such as smoking and obesity are more common in people with bipolar disorder and their unaffected siblings.
“The findings,” they write, “imply a genetic overlap in neurodevelopment and malignancy pathogenesis and may encourage clinicians to closely monitor patients with bipolar disorder and their unaffected siblings for cancer warning signs.”
The authors, however, caution that their study needs validation and had several limitations, including lack of adjustment for drug treatment and lifestyle and environmental factors.
“Our findings may persuade clinicians and researchers to reevaluate the cancer risk among the unaffected siblings of patients with schizophrenia and bipolar disorder because these two severe mental disorders may have a common biopsychosocial pathophysiology,” the team writes.
The study was supported by a grant from Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology, Taiwan.
A version of this article first appeared on Medscape.com.
, according to new research from Taiwan.
“To our knowledge, our study is the first to report an increased overall cancer risk as well as increased risks of breast and ectodermal cancer among the unaffected siblings aged < 50 years of patients with bipolar disorder,” Ya-Mei Bai, MD, PhD, of National Yang-Ming University, Taipei, Taiwan, and colleagues write in an article published online in the International Journal of Cancer.
Most, but not all, previous studies have shown a link between bipolar disorder and cancer. Whether the elevated risk of malignancy extends to family members without the mental health condition has not been elucidated.
To investigate, the researchers turned to the National Health Insurance Research Database of Taiwan. They identified 25,356 individuals diagnosed with bipolar disorder by a psychiatrist between 1996 and 2010 and the same number of unaffected siblings, as well as more than 100,000 age-, sex-, income-, and residence-matched controls without severe mental illness.
Compared with the control group, people with bipolar disorder (odds ratio, 1.22) and their unaffected siblings (OR, 1.17) both had a higher risk of developing malignant cancer of any kind. The researchers also found that both groups were at higher risk for breast cancer, with odds ratios of 1.98 in individuals with bipolar disorder and 1.73 in their unaffected siblings.
However, the risk of skin cancer was only high in people with bipolar disorder (OR, 2.70) and not in their siblings (OR, 0.62). And conversely, the risk of kidney cancer was significantly increased in unaffected siblings (OR, 2.45) but not in people with bipolar disorder (OR, 0.47).
When stratified by the embryonic developmental layer from which tumors had originated – ectodermal, mesodermal, or endodermal – the authors observed a significantly increased risk for only ectodermal cancers. In addition, only people under age 50 in both groups (OR, 1.90 for those with bipolar disorder; OR, 1.65 for siblings) were more likely to develop an ectodermal cancer, especially of the breast, compared with the control group. The risks remained elevated after excluding breast cancer but were no longer significant.
When stratified by age, the risk of developing any cancer in both groups also only appeared to be greater for those under age 50 (OR, 1.34 in people with bipolar disorder; OR, 1.32 in siblings) compared with those aged 50 and over (OR, 0.97 and 0.99, respectively). The authors highlighted these figures in the supplemental data set but did not discuss it further in the study beyond a brief mention that “younger patients with bipolar disorder and younger unaffected siblings (< 50 years), but not older ones (≥ 50 years), were more likely to develop any malignancy during the follow-up than matched controls.”
“This paper essentially finds what we have found in our previous work – that people with bipolar disorder have a greater risk of cancer,” said Michael Berk, MBBCh, PhD, a professor of psychiatry at the Deakin University School of Medicine in Geelong, Australia, who published a systematic review and meta-analysis last spring on cancer risk and the role of lithium treatment in bipolar disorder.
“The interesting finding in our work,” Dr. Berk told this news organization, “is that this risk is attenuated by use of lithium but not other agents.”
The Taiwanese researchers propose a “biopsychosocial explanation” for their results, noting that both the nervous system and the breast and skin develop from the ectoderm, and that cancer risk factors such as smoking and obesity are more common in people with bipolar disorder and their unaffected siblings.
“The findings,” they write, “imply a genetic overlap in neurodevelopment and malignancy pathogenesis and may encourage clinicians to closely monitor patients with bipolar disorder and their unaffected siblings for cancer warning signs.”
The authors, however, caution that their study needs validation and had several limitations, including lack of adjustment for drug treatment and lifestyle and environmental factors.
“Our findings may persuade clinicians and researchers to reevaluate the cancer risk among the unaffected siblings of patients with schizophrenia and bipolar disorder because these two severe mental disorders may have a common biopsychosocial pathophysiology,” the team writes.
The study was supported by a grant from Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology, Taiwan.
A version of this article first appeared on Medscape.com.
, according to new research from Taiwan.
“To our knowledge, our study is the first to report an increased overall cancer risk as well as increased risks of breast and ectodermal cancer among the unaffected siblings aged < 50 years of patients with bipolar disorder,” Ya-Mei Bai, MD, PhD, of National Yang-Ming University, Taipei, Taiwan, and colleagues write in an article published online in the International Journal of Cancer.
Most, but not all, previous studies have shown a link between bipolar disorder and cancer. Whether the elevated risk of malignancy extends to family members without the mental health condition has not been elucidated.
To investigate, the researchers turned to the National Health Insurance Research Database of Taiwan. They identified 25,356 individuals diagnosed with bipolar disorder by a psychiatrist between 1996 and 2010 and the same number of unaffected siblings, as well as more than 100,000 age-, sex-, income-, and residence-matched controls without severe mental illness.
Compared with the control group, people with bipolar disorder (odds ratio, 1.22) and their unaffected siblings (OR, 1.17) both had a higher risk of developing malignant cancer of any kind. The researchers also found that both groups were at higher risk for breast cancer, with odds ratios of 1.98 in individuals with bipolar disorder and 1.73 in their unaffected siblings.
However, the risk of skin cancer was only high in people with bipolar disorder (OR, 2.70) and not in their siblings (OR, 0.62). And conversely, the risk of kidney cancer was significantly increased in unaffected siblings (OR, 2.45) but not in people with bipolar disorder (OR, 0.47).
When stratified by the embryonic developmental layer from which tumors had originated – ectodermal, mesodermal, or endodermal – the authors observed a significantly increased risk for only ectodermal cancers. In addition, only people under age 50 in both groups (OR, 1.90 for those with bipolar disorder; OR, 1.65 for siblings) were more likely to develop an ectodermal cancer, especially of the breast, compared with the control group. The risks remained elevated after excluding breast cancer but were no longer significant.
When stratified by age, the risk of developing any cancer in both groups also only appeared to be greater for those under age 50 (OR, 1.34 in people with bipolar disorder; OR, 1.32 in siblings) compared with those aged 50 and over (OR, 0.97 and 0.99, respectively). The authors highlighted these figures in the supplemental data set but did not discuss it further in the study beyond a brief mention that “younger patients with bipolar disorder and younger unaffected siblings (< 50 years), but not older ones (≥ 50 years), were more likely to develop any malignancy during the follow-up than matched controls.”
“This paper essentially finds what we have found in our previous work – that people with bipolar disorder have a greater risk of cancer,” said Michael Berk, MBBCh, PhD, a professor of psychiatry at the Deakin University School of Medicine in Geelong, Australia, who published a systematic review and meta-analysis last spring on cancer risk and the role of lithium treatment in bipolar disorder.
“The interesting finding in our work,” Dr. Berk told this news organization, “is that this risk is attenuated by use of lithium but not other agents.”
The Taiwanese researchers propose a “biopsychosocial explanation” for their results, noting that both the nervous system and the breast and skin develop from the ectoderm, and that cancer risk factors such as smoking and obesity are more common in people with bipolar disorder and their unaffected siblings.
“The findings,” they write, “imply a genetic overlap in neurodevelopment and malignancy pathogenesis and may encourage clinicians to closely monitor patients with bipolar disorder and their unaffected siblings for cancer warning signs.”
The authors, however, caution that their study needs validation and had several limitations, including lack of adjustment for drug treatment and lifestyle and environmental factors.
“Our findings may persuade clinicians and researchers to reevaluate the cancer risk among the unaffected siblings of patients with schizophrenia and bipolar disorder because these two severe mental disorders may have a common biopsychosocial pathophysiology,” the team writes.
The study was supported by a grant from Taipei Veterans General Hospital, Yen Tjing Ling Medical Foundation, and the Ministry of Science and Technology, Taiwan.
A version of this article first appeared on Medscape.com.
FROM INTERNATIONAL JOURNAL OF CANCER
Family-oriented care in adult psychiatric residency
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
The Group for the Advancement of Psychiatry’s Committee on the Family published an updated curriculum in October 2021 on family-oriented care. The first curriculum, published in 2006, was nominated as the American Association of Directors of Psychiatric Residency Training model curriculum for family-oriented care. The updated curriculum, produced by the GAP family committee and guests, is shorter and more focused.
The following is a summary of the introduction and the highlights.
Introduction
Use of family systems–based techniques in the diagnosis and care of patients is a key evidence-based tool for psychiatric disorders. However, it is not a current Accreditation Council for Graduate Medical Education Training training requirement, and it is possible to complete psychiatry residency without exposure to this key framework.
Here, we highlight the importance of considering patients through a “family systems” lens and the incorporation of multiple individuals from an individual patient’s identified system in their care.
Current medicine curricula emphasize patient autonomy, one of the core pillars of ethics. Autonomy is the cornerstone of the everyday practice within medicine of communicating all risks, benefits, and alternatives of a proposed treatment to a patient making decisions about desired paths forward. This prevents paternalistic care in which the doctor “knows best” and makes decisions for the patient. Unfortunately, the emphasis of this pillar has morphed over time into the idea that the individual patient is the only person to whom this information should be provided or from whom information should be obtained.
Extensive research proves conclusively that family support, education, and psychoeducation improve both patient and family functioning in medical and psychiatric illness. as well as overlook the opportunity to use the structure and support system around a patient to strengthen their care and improve treatment outcomes.
The network and family dynamics around a patient can be critical to providing accurate information on medication adherence and symptoms, supporting recovery, and handling emergencies. Markedly improved patient outcomes occur when family members are seen as allies and offered support, assessment, and psychoeducation. In fact, the American Psychiatric Association’s Practice Guidelines on the treatment of schizophrenia (2020), major depressive disorder (2010), and bipolar disorder (2002) include the expectation that patients’ family members will be involved in the assessment and treatment of patients. Yet, training in how to incorporate these practices is often minimal or nonexistent during residency.
A family systems orientation is distinguished by its view of the family as a transactional system. Stressful events and problems of an individual member affect the whole family as a functional unit, with ripple effects for all members and their relationships. In turn, the family response – how the family handles problems – contributes significantly to positive adaptation or to individual and relational dysfunction. Thus, individual problems are assessed and addressed in the context of the family, with attention to socioeconomic and other environmental stressors.
A family systems approach is distinguished less by who is in the room and more by the clinician’s attention to relationship systems in assessment and treatment planning. We need to consider how family members may contribute to – and be affected by – problem situations. Most importantly, regardless of the source of difficulties, we involve key family members who can contribute to needed changes. Interventions are aimed at modifying dysfunctional patterns, tapping family resources, and strengthening both individual and family functioning.
A family systemic lens is useful for working with all types of families, for example: refugee families, thinking through child adoption processes, working with families with specific social disadvantages, etc. Incorporating issues of race, gender, and sexual orientation is important in this work, as is working with larger systems such as schools, workplaces, and health care settings.
As opposed to previous viewpoints that family therapy is the only “family” skill to be taught during residency, the GAP committee proposes that psychiatric residents should be trained in skills of family inclusion, support, and psychoeducation, and that these skills should be taught throughout the residency. Our goal is to have residents be able to consider any case through a family systems lens, to understand how patients’ illnesses and their family systems have bidirectional effects on each other, to perform a basic assessment of family system functioning, and to use this information in diagnostic and treatment planning.
Training goals
Systems-based thinking will enable trainees to:
1. Ally with family members to work with the patient to comply with goals of care (for example, taking medications, complying with lifestyle changes, and maintaining sobriety).
- Teachers focus on engagement, joining with families.
2. Help patients understand the influences of their families in their own lives, such as intergenerational transmission of trauma and resilience.
- Teachers focus on the creation of a genogram, and the location of the individual within their family system.
3. Understand that mental health includes the creation and maintenance of healthy relationships.
- Teachers focus on assessing a willingness to listen to others’ points of view and the cocreation of a shared reality and belief system: a belief that relationships can change over time and how to create new family narratives.
4. Understand the impact of illness on the family unit and the impact of the family unit on illness.
- Teachers focus on the concept of a family system, clarifying the roles within the family, including caregiving responsibilities.
5. Assess the family for strengths and weaknesses.
- Teachers focus on how families maintain a healthy emotional climate, allocate roles, decide on rules, problem-solving abilities, and so on.
6. Gather information from multiple informants in the same room.
- Teachers focus on using communication techniques to elicit, guide, and redirect information from multiple individuals of a system with varying perspectives in the same room. Teachers help students understand that there are multiple realities in families and learn how to maintain multidirectional partiality.
Knowledge, skills, and attitudes across all treatment settings
Knowledge: Beginning level
- Healthy family functioning at the various phases of the family life cycle. Systems concepts are applicable to families, multidisciplinary teams in clinical settings, and medical/government organizations. However, family systems are distinguished by deep attachment bonds, specific generational hierarchy, goals of emotional safety and, for many families, child rearing.
- Systemic thinking, unlike a linear cause and effect model, examines the feedback loops by which multiple persons or groups arrive at a specific way of functioning.
- Understanding boundaries, subsystems, and feedback loops is critical to understanding interpersonal connections. Understand how the family affects and is affected by psychiatric and medical illnesses. Impact of interpersonal stress on biological systems. The role of expressed emotion (EE) in psychiatric illness. EE describes the level of criticism, hostility, and emotional overinvolvement in families. It has been studied extensively across the health care spectrum, and cultural variance is significant.
- The components of family psychoeducation, and its associated research in improving patient and family outcomes.
Knowledge: Advanced level
- Principles of adaptive and maladaptive relational functioning in family life and family organization, communication, problem solving, and emotional regulation. Role of family strengths, resilience in reducing vulnerability.
- Couple and family development over the life cycle.
- Understanding multigenerational patterns.
- How age, gender, class, culture, and spirituality affect family life.
- The variety of family forms (for example, single parent, stepfamilies, same-sex parents).
- Special issues in couples and families, including loss, divorce and remarriage, immigration, illness, secrets, affairs, violence, alcohol and substance abuse, sexuality, including LGBTQi. Relationship of families to larger systems, for example, schools, work, health care systems, government agencies.
Skills
- Family-interviewing skills, especially managing high levels of emotion and making room for multiple points of view.
- Promoting resilience, hope, and strength.
- Basic psychoeducation techniques, which includes providing a therapeutic space for emotional processing, providing information about the illness, skills such as better communication, problem-solving, and relapse drill and support.
- Collaborative treatment planning with family members and other helping professionals. Treatment planning should include all members of the system: patient, family members, and members of the treatment team. Good planning establishes a role for family members, helps define criteria for managing emergencies, looks for areas of strength and resilience and provides clear and realistic goals for treatment.
- Knowledge of, and referral to, local and national resources, both in the community and online.
Attitudes
- Appreciate the multiple points of view in a family.
- Interest in family members as people with their own needs and history.
- Including family members as a resource in recovery.
- Understand caregiver burden and rewards and that stress extends to all family members.
Training techniques
Most learning takes place at the level of patient, supervisor, and resident. It is critical that the resident sees faculty members dealing with patients in observed or shared family sessions, and /or sees videos made by faculty or professionally made videos. Attitudes are best learned by modeling.
Areas of focus can include time management, addressing the fear that family sessions may get out of control, and the influence of the residents’ own life experiences and background including potential generational or cultural differences on their assessment and interactions with patient family dynamics. In skill development, our goal is efficient interviewing, history taking, and support in controlling sessions.
It is difficult to specify which techniques are most useful in didactic sessions as each presenter will have a different skill set for engaging the class. The techniques that work best are the ones most comfortable to the presenter. Any technique that gets emotions involved, such as role play, sculpting, discussing movie clips, bringing in family members to discuss their experiences, or self-exploration, will generate the most powerful learning. If time permits, exploration of the resident’s own family, including a genogram, is an exceptionally helpful technique, especially if accompanied by asking the residents to interview their own families.
Adult didactic curriculum
The curriculum represents basic concepts. We have vignettes by the authors, if needed, but it is best if the class, including the supervisor, uses vignettes from their own experiences. Material for use in class is in references, but the class is urged to draw on their own experiences as this supports strength-based teaching. The following are key topics and concepts for each of the training years.
Basic concepts for PGY1 and PGY2
1. Where are you in the family and individual life cycles? What are your experiences with psychiatric illness in family/friends? Open discussion about how individual and family life cycles interact. Draw genograms of s/o in the class or with the supervisor.
2. Healthy family functioning and family resilience. Recommend asking residents to talk to their parents/elders about their lives and family life cycle when they were your age. Open discussion about what makes a healthy resilient family.
3. How do I connect with the family rather than just one person? How do you learn to hold multiple perspectives? How do I try not to take sides/multidirectional partiality? How do I see each person in a positive way? How do I focus on family strengths, rather than focusing on someone behaving badly (which is really hard because it is overlearned in individual therapy).
4. What are the common factors used across all therapies, both individual and family? When is it best to use an individual relational approach versus a family systemic approach?
5. How do I decide if a family needs support or education or family therapy?
6. Psychoeducation: Research, current use and cultural adaptations.
7. Attachment styles and couples therapy.
8. What is the evidence base behind our work?
System practice for PGY 3 and 4
These seminars follow the basic seminars. The focus is on clarification of what systems thinking means. Systems thinking or relational thinking is to be differentiated from systems-based practice. These lectures require knowledge of systemic practice. If there are no local experts, residency programs can reach out to national experts at the Association of Family Psychiatrists, for help with virtual/remote or in-person teaching.
Here is a list of other topics that should be covered:
- Relational formulation, nested subsystems, boundaries, history of these concepts, contributions to the development of family therapy.
- How to define and identify common systems concepts, such as circular patterns, feedback loops, and triangulation. Teach circular questioning. Framing. This concept is the family systems equivalent of insight. How to intervene to effect communication change and behavior change?
- Working at interfaces: community, legal, government, agencies, and so on, and other treaters, consultation. Include systemic and individual racism.
- Understanding the complexity of intimacy.
- Emergency situations. When to report regarding abuse. Dealing with family trauma.
- Varieties of family therapy; assumptions and major concepts.
*The new curriculum was written by The GAP Committee on the Family: Ellen Berman, MD; John Rolland, MD, MPH; John Sargent, MD; and me, and with guests Chayanin Foongsathaporn, MD; Sarah Nguyen, MD, MPH; Neha Sharma, DO; and Jodi Zik, MD. For the full curriculum, which includes residency milestones, site-specific training goals, references, and case studies, please access the Association of Family Psychiatry’s website: www.familypsychiatrists.org.Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
Stabilizing circadian rhythm tied to lower suicide risk in bipolar disorder
Social rhythm therapy (SRT), which uses behavioral strategies to support healthy sleep and other routines, is linked to improved mood and reduced suicide risk in young people with bipolar disorder (BD), early research suggests.
The small study also showed SRT is both feasible and acceptable in this patient population.
Results showed SRT, which was primarily delivered via telehealth sessions, began to show efficacy approximately 6 weeks into the 12-week therapeutic program, the researchers noted.
“Improving the regularity of daily rhythms like sleep, physical activity, and social activities can be really robust in improving mental health and even reducing suicide risk,” study investigator Hilary P. Blumberg, MD, the John and Hope Furth Professor of Psychiatric Neuroscience and director of the mood disorders research program at Yale University, New Haven, Conn., said in an interview.
The findings are published in the American Journal of Psychotherapy.
Trigger for depression, mania
Previous research shows unstable circadian rhythms may trigger depressive and manic symptoms – and are risk factors for suicidal thoughts and behaviors. Although interpersonal and social rhythm therapy has shown promise in patients with mood disorders, there is little research focusing only on the social rhythm aspect of the therapy.
The researchers only examined SRT, modified to create a therapeutic program aimed at adolescents and young adults.
The study included 13 participants (mean age, 20.5 years) with BD and a score of 15 or more on the 29-item Hamilton Depression Rating Scale (HDRS-29) and/or a score of 12 or more on the Young Mania Rating Scale (YMRS).
Participants were enrolled in the National Institute of Mental Health Brain Emotion Circuitry Targeted Self-Monitoring and Regulation Therapy (BE-SMART) program, which requires MRI sessions at three in-person visits to assess brain changes with the therapy. All but one participant was taking mood-stabilizing medications.
“We didn’t ask them to come off medications because we didn’t want to exacerbate things,” said Dr. Blumberg. She added the therapeutic approach “could be adjunctive to further improve symptoms and reduce risk.”
The majority occurred on a secure video platform. Three were conducted in person.
Working with a therapist, participants were taught how to follow a daily routine. Dr. Blumberg noted this is not just a matter of going to sleep and getting up at the same time every day, but thoroughly reviewing details of all daily activities and routines, including who participants eat with and when, their exercise schedule, and social engagements.
Each week, participants completed the five-item version of the Social Rhythm Metric. At the end of the intervention, they also completed the Client Satisfaction Questionnaire (CSQ). Scores on the CSQ range from 8 to 32, with scores of 26-32 indicating “excellent” satisfaction.
In addition, participants and therapists completed the Working Alliance Inventory, which assesses the client-therapist relationship by asking about such things as degree of comfort and respect.
Before and after the intervention, participants reported the regularity of their social rhythms using the Brief Social Rhythm Scale (BSRS) and risk for suicidal behavior using a subscale of the Concise Health Risk Tracking (CHRT) scale.
High retention, ‘excellent satisfaction’
Results showed 10 of the 13 participants (9 females) completed all study procedures, for a retention rate of 77%. Treatment satisfaction was excellent (mean CSQ, 29.4).
Both therapists and participants had high scores on all aspects of the Working Alliance Inventory scale.
“High treatment retention, excellent client satisfaction, and strong working alliance scores support the feasibility and acceptability of this intervention for adolescents and young adults with bipolar disorder,” the investigators wrote.
Participants showed significant improvement in social rhythm regularity and reductions in depression and manic symptoms as well as suicide propensity (P = .016 for BSRS; .024 for HDRS-29; .028 for YMRS; and .028 for CHRT suicide propensity). Effect sizes were in the moderate to high range.
By the midpoint of the therapy, there were significant improvements in social rhythm regularity and suicide propensity and trend-level reductions in depression, suggesting the potential for early benefits.
Dr. Blumberg noted it is difficult to find a therapy that helps with both depressive and mania symptoms. “An antidepressant may reduce depression, but sometimes can worsen manic symptoms.”
Impact on emotional brain circuitry?
The association between improved regularity of social rhythms and reduced suicide propensity persisted even after controlling for mood symptom changes.
“Suicide risk was reduced not just because subjects were less depressed. There’s something about regularizing rhythms that can reduce suicide risk,” said Dr. Blumberg.
The reviewers noted that SRT administered remotely improves accessibility; and this intervention “is well suited to the future of psychotherapy delivery, which will undoubtedly include remote treatment delivery.”
The absence of a comparator condition was cited as a study limitation. The investigators noted the small sample size means the findings should be interpreted cautiously and verified in an adequately powered randomized controlled trial.
The researchers now have early results from the brain scanning component of the study. “Preliminary findings suggest the intervention seems to benefit emotional brain circuitry,” Dr. Blumberg said.
The researchers are about to embark on a new study funded by a grant from the American Foundation of Suicide Prevention. It will investigate SRT in preventing suicide in adolescents and adults to age 29 years with depression or BD.
In addition, the researchers have secured support from the Klingenstein Third Generation Foundation to research prevention in youth at risk for BD – and from Women’s Health Access Matters to examine the therapy in women 50 and older with depression, a population possibly at increased risk for dementia.
‘Promising’ results
Commenting on the findings, Michael Thase, MD, professor of psychiatry, University of Pennsylvania, and research psychiatrist at the Corporal Michael J. Crescenz Veterans Affairs Medical Center, both in Philadelphia, praised the study.
“It’s a very, very promising initial study because even though there’s no control group, it does show that participants liked the program, most finished it, and on average, people got quite a bit better,” said Dr. Thase, who was not involved with the research.
The treatment may be especially beneficial for young patients with bipolar disorder who, just by their very age, experience lifestyle disruptions, Dr. Thase noted. Results from a previous study of the therapeutic approach in adults showed “probably half of the adults didn’t take to it.”
However, not everyone in this new study benefited either, as some dropped out, which Dr. Thase noted is not atypical.
“No form of intervention is suitable for everyone,” he said.
The study was supported by grants from the National Institute of Mental Health, AIM Youth Mental Health Foundation, Klingenstein Third Generation Foundation, American Foundation for Suicide Prevention, International Bipolar Foundation, MQ Brighter Futures Program, For the Love of Travis Foundation, and the John and Hope Furth Endowment. Dr. Blumberg and Dr. Thase reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social rhythm therapy (SRT), which uses behavioral strategies to support healthy sleep and other routines, is linked to improved mood and reduced suicide risk in young people with bipolar disorder (BD), early research suggests.
The small study also showed SRT is both feasible and acceptable in this patient population.
Results showed SRT, which was primarily delivered via telehealth sessions, began to show efficacy approximately 6 weeks into the 12-week therapeutic program, the researchers noted.
“Improving the regularity of daily rhythms like sleep, physical activity, and social activities can be really robust in improving mental health and even reducing suicide risk,” study investigator Hilary P. Blumberg, MD, the John and Hope Furth Professor of Psychiatric Neuroscience and director of the mood disorders research program at Yale University, New Haven, Conn., said in an interview.
The findings are published in the American Journal of Psychotherapy.
Trigger for depression, mania
Previous research shows unstable circadian rhythms may trigger depressive and manic symptoms – and are risk factors for suicidal thoughts and behaviors. Although interpersonal and social rhythm therapy has shown promise in patients with mood disorders, there is little research focusing only on the social rhythm aspect of the therapy.
The researchers only examined SRT, modified to create a therapeutic program aimed at adolescents and young adults.
The study included 13 participants (mean age, 20.5 years) with BD and a score of 15 or more on the 29-item Hamilton Depression Rating Scale (HDRS-29) and/or a score of 12 or more on the Young Mania Rating Scale (YMRS).
Participants were enrolled in the National Institute of Mental Health Brain Emotion Circuitry Targeted Self-Monitoring and Regulation Therapy (BE-SMART) program, which requires MRI sessions at three in-person visits to assess brain changes with the therapy. All but one participant was taking mood-stabilizing medications.
“We didn’t ask them to come off medications because we didn’t want to exacerbate things,” said Dr. Blumberg. She added the therapeutic approach “could be adjunctive to further improve symptoms and reduce risk.”
The majority occurred on a secure video platform. Three were conducted in person.
Working with a therapist, participants were taught how to follow a daily routine. Dr. Blumberg noted this is not just a matter of going to sleep and getting up at the same time every day, but thoroughly reviewing details of all daily activities and routines, including who participants eat with and when, their exercise schedule, and social engagements.
Each week, participants completed the five-item version of the Social Rhythm Metric. At the end of the intervention, they also completed the Client Satisfaction Questionnaire (CSQ). Scores on the CSQ range from 8 to 32, with scores of 26-32 indicating “excellent” satisfaction.
In addition, participants and therapists completed the Working Alliance Inventory, which assesses the client-therapist relationship by asking about such things as degree of comfort and respect.
Before and after the intervention, participants reported the regularity of their social rhythms using the Brief Social Rhythm Scale (BSRS) and risk for suicidal behavior using a subscale of the Concise Health Risk Tracking (CHRT) scale.
High retention, ‘excellent satisfaction’
Results showed 10 of the 13 participants (9 females) completed all study procedures, for a retention rate of 77%. Treatment satisfaction was excellent (mean CSQ, 29.4).
Both therapists and participants had high scores on all aspects of the Working Alliance Inventory scale.
“High treatment retention, excellent client satisfaction, and strong working alliance scores support the feasibility and acceptability of this intervention for adolescents and young adults with bipolar disorder,” the investigators wrote.
Participants showed significant improvement in social rhythm regularity and reductions in depression and manic symptoms as well as suicide propensity (P = .016 for BSRS; .024 for HDRS-29; .028 for YMRS; and .028 for CHRT suicide propensity). Effect sizes were in the moderate to high range.
By the midpoint of the therapy, there were significant improvements in social rhythm regularity and suicide propensity and trend-level reductions in depression, suggesting the potential for early benefits.
Dr. Blumberg noted it is difficult to find a therapy that helps with both depressive and mania symptoms. “An antidepressant may reduce depression, but sometimes can worsen manic symptoms.”
Impact on emotional brain circuitry?
The association between improved regularity of social rhythms and reduced suicide propensity persisted even after controlling for mood symptom changes.
“Suicide risk was reduced not just because subjects were less depressed. There’s something about regularizing rhythms that can reduce suicide risk,” said Dr. Blumberg.
The reviewers noted that SRT administered remotely improves accessibility; and this intervention “is well suited to the future of psychotherapy delivery, which will undoubtedly include remote treatment delivery.”
The absence of a comparator condition was cited as a study limitation. The investigators noted the small sample size means the findings should be interpreted cautiously and verified in an adequately powered randomized controlled trial.
The researchers now have early results from the brain scanning component of the study. “Preliminary findings suggest the intervention seems to benefit emotional brain circuitry,” Dr. Blumberg said.
The researchers are about to embark on a new study funded by a grant from the American Foundation of Suicide Prevention. It will investigate SRT in preventing suicide in adolescents and adults to age 29 years with depression or BD.
In addition, the researchers have secured support from the Klingenstein Third Generation Foundation to research prevention in youth at risk for BD – and from Women’s Health Access Matters to examine the therapy in women 50 and older with depression, a population possibly at increased risk for dementia.
‘Promising’ results
Commenting on the findings, Michael Thase, MD, professor of psychiatry, University of Pennsylvania, and research psychiatrist at the Corporal Michael J. Crescenz Veterans Affairs Medical Center, both in Philadelphia, praised the study.
“It’s a very, very promising initial study because even though there’s no control group, it does show that participants liked the program, most finished it, and on average, people got quite a bit better,” said Dr. Thase, who was not involved with the research.
The treatment may be especially beneficial for young patients with bipolar disorder who, just by their very age, experience lifestyle disruptions, Dr. Thase noted. Results from a previous study of the therapeutic approach in adults showed “probably half of the adults didn’t take to it.”
However, not everyone in this new study benefited either, as some dropped out, which Dr. Thase noted is not atypical.
“No form of intervention is suitable for everyone,” he said.
The study was supported by grants from the National Institute of Mental Health, AIM Youth Mental Health Foundation, Klingenstein Third Generation Foundation, American Foundation for Suicide Prevention, International Bipolar Foundation, MQ Brighter Futures Program, For the Love of Travis Foundation, and the John and Hope Furth Endowment. Dr. Blumberg and Dr. Thase reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Social rhythm therapy (SRT), which uses behavioral strategies to support healthy sleep and other routines, is linked to improved mood and reduced suicide risk in young people with bipolar disorder (BD), early research suggests.
The small study also showed SRT is both feasible and acceptable in this patient population.
Results showed SRT, which was primarily delivered via telehealth sessions, began to show efficacy approximately 6 weeks into the 12-week therapeutic program, the researchers noted.
“Improving the regularity of daily rhythms like sleep, physical activity, and social activities can be really robust in improving mental health and even reducing suicide risk,” study investigator Hilary P. Blumberg, MD, the John and Hope Furth Professor of Psychiatric Neuroscience and director of the mood disorders research program at Yale University, New Haven, Conn., said in an interview.
The findings are published in the American Journal of Psychotherapy.
Trigger for depression, mania
Previous research shows unstable circadian rhythms may trigger depressive and manic symptoms – and are risk factors for suicidal thoughts and behaviors. Although interpersonal and social rhythm therapy has shown promise in patients with mood disorders, there is little research focusing only on the social rhythm aspect of the therapy.
The researchers only examined SRT, modified to create a therapeutic program aimed at adolescents and young adults.
The study included 13 participants (mean age, 20.5 years) with BD and a score of 15 or more on the 29-item Hamilton Depression Rating Scale (HDRS-29) and/or a score of 12 or more on the Young Mania Rating Scale (YMRS).
Participants were enrolled in the National Institute of Mental Health Brain Emotion Circuitry Targeted Self-Monitoring and Regulation Therapy (BE-SMART) program, which requires MRI sessions at three in-person visits to assess brain changes with the therapy. All but one participant was taking mood-stabilizing medications.
“We didn’t ask them to come off medications because we didn’t want to exacerbate things,” said Dr. Blumberg. She added the therapeutic approach “could be adjunctive to further improve symptoms and reduce risk.”
The majority occurred on a secure video platform. Three were conducted in person.
Working with a therapist, participants were taught how to follow a daily routine. Dr. Blumberg noted this is not just a matter of going to sleep and getting up at the same time every day, but thoroughly reviewing details of all daily activities and routines, including who participants eat with and when, their exercise schedule, and social engagements.
Each week, participants completed the five-item version of the Social Rhythm Metric. At the end of the intervention, they also completed the Client Satisfaction Questionnaire (CSQ). Scores on the CSQ range from 8 to 32, with scores of 26-32 indicating “excellent” satisfaction.
In addition, participants and therapists completed the Working Alliance Inventory, which assesses the client-therapist relationship by asking about such things as degree of comfort and respect.
Before and after the intervention, participants reported the regularity of their social rhythms using the Brief Social Rhythm Scale (BSRS) and risk for suicidal behavior using a subscale of the Concise Health Risk Tracking (CHRT) scale.
High retention, ‘excellent satisfaction’
Results showed 10 of the 13 participants (9 females) completed all study procedures, for a retention rate of 77%. Treatment satisfaction was excellent (mean CSQ, 29.4).
Both therapists and participants had high scores on all aspects of the Working Alliance Inventory scale.
“High treatment retention, excellent client satisfaction, and strong working alliance scores support the feasibility and acceptability of this intervention for adolescents and young adults with bipolar disorder,” the investigators wrote.
Participants showed significant improvement in social rhythm regularity and reductions in depression and manic symptoms as well as suicide propensity (P = .016 for BSRS; .024 for HDRS-29; .028 for YMRS; and .028 for CHRT suicide propensity). Effect sizes were in the moderate to high range.
By the midpoint of the therapy, there were significant improvements in social rhythm regularity and suicide propensity and trend-level reductions in depression, suggesting the potential for early benefits.
Dr. Blumberg noted it is difficult to find a therapy that helps with both depressive and mania symptoms. “An antidepressant may reduce depression, but sometimes can worsen manic symptoms.”
Impact on emotional brain circuitry?
The association between improved regularity of social rhythms and reduced suicide propensity persisted even after controlling for mood symptom changes.
“Suicide risk was reduced not just because subjects were less depressed. There’s something about regularizing rhythms that can reduce suicide risk,” said Dr. Blumberg.
The reviewers noted that SRT administered remotely improves accessibility; and this intervention “is well suited to the future of psychotherapy delivery, which will undoubtedly include remote treatment delivery.”
The absence of a comparator condition was cited as a study limitation. The investigators noted the small sample size means the findings should be interpreted cautiously and verified in an adequately powered randomized controlled trial.
The researchers now have early results from the brain scanning component of the study. “Preliminary findings suggest the intervention seems to benefit emotional brain circuitry,” Dr. Blumberg said.
The researchers are about to embark on a new study funded by a grant from the American Foundation of Suicide Prevention. It will investigate SRT in preventing suicide in adolescents and adults to age 29 years with depression or BD.
In addition, the researchers have secured support from the Klingenstein Third Generation Foundation to research prevention in youth at risk for BD – and from Women’s Health Access Matters to examine the therapy in women 50 and older with depression, a population possibly at increased risk for dementia.
‘Promising’ results
Commenting on the findings, Michael Thase, MD, professor of psychiatry, University of Pennsylvania, and research psychiatrist at the Corporal Michael J. Crescenz Veterans Affairs Medical Center, both in Philadelphia, praised the study.
“It’s a very, very promising initial study because even though there’s no control group, it does show that participants liked the program, most finished it, and on average, people got quite a bit better,” said Dr. Thase, who was not involved with the research.
The treatment may be especially beneficial for young patients with bipolar disorder who, just by their very age, experience lifestyle disruptions, Dr. Thase noted. Results from a previous study of the therapeutic approach in adults showed “probably half of the adults didn’t take to it.”
However, not everyone in this new study benefited either, as some dropped out, which Dr. Thase noted is not atypical.
“No form of intervention is suitable for everyone,” he said.
The study was supported by grants from the National Institute of Mental Health, AIM Youth Mental Health Foundation, Klingenstein Third Generation Foundation, American Foundation for Suicide Prevention, International Bipolar Foundation, MQ Brighter Futures Program, For the Love of Travis Foundation, and the John and Hope Furth Endowment. Dr. Blumberg and Dr. Thase reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHOTHERAPY
Despite the stigma, ECT remains a gold standard
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
For Clayton Lively, electroconvulsive therapy, or ECT, has been a lifesaver.
“ECT was like a last resort to treat mania and psychosis for bipolar disorder,” said the 31-year-old financial firm associate who lives in Silver Spring, Md. “I had tried lots of different medications.”
The first course of treatments – three times per week for several weeks – was in 2005. They helped tremendously. “I came down from my mania,” Mr. Lively said. “The hallucinations stopped. The psychosis disappeared.”
He reached a point where medications and psychotherapy worked again. And for a decade, his condition was under control.
But in 2017, another episode of hallucinations and mania jolted him off course. Intrusive thoughts returned. For instance, while driving, he would visualize veering off the road. The thoughts were jarring, and yet, he couldn’t stop them from recurring.
“I wasn’t sleeping, and it just kind of wreaked havoc on my life,” Mr. Lively recalled. “I ended up being hospitalized again.”
Once again, ECT came to the rescue – and yet again, in 2018. Now, he’s on an effective maintenance regimen, receiving ECT once every 4 weeks, after tapering down from more frequent sessions.
When a combination of antidepressants and psychotherapy fails to control severe mental illness, there’s hope on the horizon. ECT can be a reliably safe and effective option.
For some patients, using it as maintenance therapy makes sense, said Vaughn McCall, MD, editor-in-chief of The Journal of ECT and professor and chairman of psychiatry at the Medical College of Georgia in Augusta. “I would think of it the same way as you have to treat any chronic illness,” such as blood pressure medicine to keep hypertension in check and dialysis to prevent kidney failure.
Despite a cacophony of contrarian voices – mainly from the Church of Scientology – “the number of psychiatrists who see controversy in ECT is vanishingly small,” Dr. McCall said.
In weighing the pros and cons of ECT, he noted that “when you’re trying to decide if it’s worth doing a treatment, you’re looking at the effectiveness on one hand and the side effects on the other hand.”
The answer to that emerges from several scales measuring patients’ quality of life by posing questions such as: “After receiving ECT, are you more able or less able to take care of yourself, to work, and enjoy the company of other people?”
In the end, Dr. McCall said, “we’ve applied these scales in probably half a dozen studies or more, and they always show that the patients’ qualify of life as a group is improved.”
A recent study published in The Lancet Psychiatry provides a significant degree of reassurance that ECT – also called “electroshock” or colloquially just “shock” therapy – does not increase the risk of serious medical side effects. In fact, the study suggests a potential benefit in reducing suicide risk.
First performed in 1938, the treatment has been well documented in the medical literature. But negative portrayals in books and movies, such as the 1975 film “One Flew Over the Cuckoo’s Nest,” have contributed to casting it in an unfavorable light.
“Unfortunately, over the past decades and years, there’s a lot of stigma and fear around the treatment,” said the study’s lead author, Tyler Kaster, MD, a psychiatrist and clinical fellow in brain stimulation at the University of Toronto.
For the study, Canadian investigators reviewed the admission records of 10,000 patients hospitalized for at least 3 days because of a severe depressive episode. Nearly two-thirds of the patients were women, and the average age for the entire group approached 57 years.
While half of the patients underwent ECT, the others received medication and psychotherapy. Researchers found that the group undergoing ECT did not have a heightened risk of death over the next 30 days and were not any more likely to be hospitalized for a medical problem.
Previous ECT comparative studies were at high risk of bias because of their inability to sufficiently account for confounding variables and differences between those who received the treatment and those who did not. The current study employed “rigorous methods with careful attention to bias and confounding to overcome limitations of previous work,” the authors wrote.
They used propensity score matching, which included more than 75 variables, such as measures of cognitive impairment, depression severity, medication use, other illnesses, and use of psychiatric and various medical services, capacity to consent to treatment, and sociodemographic factors.
“This is really a landmark study in terms of showing the medical safety of ECT,” said Mark S. George, MD, professor of psychiatry and neurology at the Medical University of South Carolina, Charleston, who was not involved in the study.
He added that “ECT is a life-saving treatment” for individuals with severe depression. “It’s good that we have this option for our patients.”
The authors highlight that depression is a major cause of illness and disability worldwide, with many individuals failing to achieve remission from initial therapies. Treatment-resistant depression is often described as being nonresponsive “to two or more medication trials of adequate dose and duration from different classes,” they wrote. In these instances, the authors point out, there is little evidence that psychotherapy would be helpful.
“The reason we consider ECT is someone has very severe depression that hasn’t responded to medications and talk therapy,” Dr. Kaster said. “The advantage of ECT is that it’s very effective in those circumstances.”
Of all therapies for treatment-resistant depression, ECT has the highest success rate, with 60% of patients attaining remission, according to the study, which cites prior research.
Compared with neurosurgery, the procedure is not invasive but requires general anesthesia. While the patient is asleep, Dr. Kaster said, the treating clinician places an electrical stimulus on the patient’s scalp, causing a generalized seizure inside the brain that lasts from 15 seconds to 2 minutes.
A course of ECT usually takes a total of 8-12 treatments, delivered two to three times per week over a month to a month and a half, Dr. George said.
Some patients need a new course of ECT if they relapse after several months. Others are unable to control their depression between courses and require repeated doses for maintenance. The time between these ECT sessions varies for each individual, Dr. George said, but is typically one session every 3-4 weeks.
To improve the odds of staying well, patients typically need to continue taking antidepressants and engaging in psychotherapy.
“It helps improve the efficacy of ECT and also down the road helps prevent relapse,” Dr. Kaster said, noting that “depression is, unfortunately, a chronic illness. We don’t have a cure.”
Murat Altinay, MD, associate professor of psychiatry at the Cleveland Clinic and a mood disorders specialist, said his patients generally need to demonstrate a lack of response to at least three or four antidepressants before he considers recommending ECT.
Confusion, short-term memory impairment, and muscle aches and pains may occur after the procedure, but they are relatively mild. Patients are monitored in a recovery room before discharge from the hospital, Dr. Altinay said.
The first few treatments will affect everyday function. After that initial period, however, people can resume most of their daily activities, he said.
“Maybe they won’t be able to work full-time right away, but anecdotally, we have had patients who were able to go back to the workforce relatively quickly or while they’re getting ECT,” Dr. Altinay said.
More significant adverse events are very rare, he noted, although heart rate and blood pressure can become elevated because of the electrical stimulus.
Dr. Altinay said he is pleased that the large-scale journal article has been published to help dispel myths surrounding ECT. While psychiatrists feel that ECT is generally safe and effective, the public maintains a negative view.
“It is an underutilized treatment,” he said. “In the media, it is almost depicted as a barbaric and archaic treatment in psychiatry.”
Patients are afraid of major side effects such as personality changes. Some fear they will forget someone’s birthday or other important factual information, “but that kind of stuff obviously does not happen,” Dr. Altinay said.
Sometimes it’s not only the patients who are hesitant to try ECT; it’s the family members who express concerns, said Irving Reti, MBBS, professor of psychiatry and neuroscience and director of the brain stimulation program at the Johns Hopkins University, Baltimore.
“It varies from one patient to another how agreeable or reluctant or cautious they are about their treatment if the doctor thinks it’s indicated for them,” Dr. Reti said. “Family members’ concerns may be very legitimate but may also be influenced by stigma and misunderstanding about the treatment. They may also not fully appreciate the severity of their loved one’s depression that warrants the administration of ECT.”
Hospitalized patients who are at risk of suicide have benefited from ECT. “It’s very effective,” he said. “I think it’s still the gold standard for severe treatment-resistant depression and also particularly helpful in people who are acutely suicidal.”
Dr. George cautioned that psychiatrists and the public should beware of questionable online sources that attempt to discredit ECT. “A quick Google search will find plenty of nonmedical doctors, many funded through Scientology, who will speak poorly of ECT. But they do not use evidence-based arguments and commonly do not treat patients,” he said.
“All good practicing psychiatrists that I know are in favor of ECT, as it clearly saves lives,” Dr. George added. “We all hope that the future will provide refinements of ECT, or even disruptive technologies that are more effective and with less hassle and will make ECT as we do it now obsolete. But we are not there yet.”
A version of this article first appeared on Medscape.com.
Olanzapine-samidorphan combination for schizophrenia or bipolar I disorder
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
Approved by the FDA on May 28, 2021, olanzapine-samidorphan combination (OSC) (Lybalvi, manufactured and distributed by Alkermes, Inc. Waltham, MA USA) is intended to help mitigate some of the weight gain that can be anticipated with the use of olanzapine alone (Table).1-3 Olanzapine (Zyprexa, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA) is a second-generation antipsychotic that has been available for a quarter century.4 Although highly efficacious,5,6 olanzapine has been associated with weight gain, at times substantial, as well as disturbances in glucose and lipid metabolism.7 The addition of samidorphan, an opioid antagonist, to olanzapine in a single tablet may act to decrease the amount of long-term weight gain that can be expected for some patients taking olanzapine alone, consequently minimizing the anticipated increase in waist circumference (a proxy for the measurement of burden imposed by metabolically active adipose tissue). Approval of OSC for the treatment of schizophrenia was based on 2 pivotal randomized controlled trials and their extension studies.8-11 Approval of OSC for bipolar I disorder (acute treatment of manic/mixed episodes as a monotherapy or adjunctive to lithium or valproate, and as a monotherapy maintenance treatment) was based on legacy studies conducted with olanzapine, after establishing that samidorphan does not alter the pharmacokinetics of olanzapine, including in combination with lithium or valproate.3,12,13 OSC should be distinguished from a different combination product, olanzapine-fluoxetine combination (Symbyax, originally manufactured and distributed by Eli Lilly and Company/Lilly USA, LLC, Indianapolis, IN USA), approved for acute depressive episodes associated with bipolar I disorder and for treatment-resistant depression.14

OSC offers the potential to consider olanzapine earlier in the treatment of schizophrenia or bipolar I disorder, especially among practitioners who might otherwise be hesitant to prescribe this agent because of concerns over the risk of excessive weight gain.
OSC is available in 4 dosage strengths containing 5 mg, 10 mg, 15 mg, or 20 mg of olanzapine; all tablets contain 10 mg of samidorphan.2 The recommended starting dose for OSC mirrors the language contained in the legacy olanzapine product label.4 For schizophrenia, the recommended initial dose (olanzapine/samidorphan) is 5 mg/10 mg or 10 mg/10 mg once daily. For bipolar I manic or mixed episodes, the recommended starting dose for monotherapy is 10 mg/10 mg or 15 mg/10 mg, and for use with lithium or valproate, 10 mg/10 mg. For all indications, the recommended target dose can be 10 mg/10 mg, 15 mg/10 mg, or 20 mg/10 mg, with 5 mg/10 mg as an additional potential dose for maintenance monotherapy of bipolar I disorder. The maximum dose is 20 mg/10 mg once daily. Because the amount of samidorphan in each tablet is fixed at 10 mg, combining tablets of OSC, or cutting OSC tablets in half, is not advisable.
Continue to: How it works...
How it works
Product labeling notes that olanzapine is an atypical antipsychotic, that its efficacy in schizophrenia or bipolar I disorder could be mediated through a combination of dopamine and serotonin type 2 (5HT2) antagonism, and that the mechanism of action of samidorphan could be mediated through opioid receptor antagonism.2
The pharmacodynamic profile of olanzapine is complex.2 It binds with high affinity to the following receptors: serotonin 5HT2A/2C, 5HT6 (Ki = 4, 11, and 5 nM, respectively), dopamine D1-4 (Ki = 11-31 nM), histamine H1 (Ki = 7 nM), and adrenergic alpha-1 receptors (Ki = 19 nM). Olanzapine is an antagonist with moderate affinity binding for serotonin 5HT3 (Ki = 57 nM) and muscarinic M1-5 (Ki = 73, 96, 132, 32, and 48 nM, respectively). Olanzapine binds with low affinity to gamma aminobutyric acid type A (GABA-A), benzodiazepine, and beta-adrenergic receptors (Ki >10 µM). Olanzapine’s muscarinic receptor affinity can explain why olanzapine can be associated with constipation, dry mouth, and tachycardia, all adverse reactions possibly related to cholinergic antagonism. Thus, OSC should be used with caution in patients with a current diagnosis or prior history of urinary retention, clinically significant prostatic hypertrophy, constipation, or a history of paralytic ileus or related conditions; a potential drug-drug interaction can be anticipated with concomitant use of anticholinergic medications.2 Other pharmacodynamic drug-drug interactions that can occur with the olanzapine component of OSC include the possibility that diazepam, alcohol, or other CNS-acting drugs may potentiate orthostatic hypotension, and there may be a need to reduce the dosage of concomitantly prescribed antihypertensive drugs in patients being treated for hypertension. Moreover, OSC is not recommended in patients receiving levodopa and dopamine agonists.
Samidorphan binds to the mu-, kappa-, and delta-opioid receptors (Ki = .052, .23, and 2.7 nM, respectively).2 Samidorphan is an antagonist at the mu-opioid receptors with partial agonist activity at kappa- and delta-opioid receptors. A major human metabolite of samidorphan (N-dealkylated) binds to the mu-, kappa-, and delta-opioid receptors (Ki = .26, 23, and 56 nM, respectively), and functions as a mu-opioid receptor agonist. The N-oxide major human metabolite binds to mu-, kappa-, and delta-opioid receptors (Ki = 8, 110, and 280 nM, respectively) and functions as a mu-opioid receptor antagonist. This profile differs from that of other opioid antagonists such as naltrexone.15,16
OSC is not a scheduled drug subject to the Controlled Substances Act. Because samidorphan functions as an opioid antagonist, OSC is contraindicated in patients using opioids or undergoing acute opioid withdrawal.2
Regarding cardiac electrophysiology, OSC was not observed to prolong the electrocardiogram QTc interval to any clinically relevant extent when tested at doses up to 30 mg/30 mg (1.5 times and 3 times the maximum recommended daily dosage of olanzapine and samidorphan, respectively).17
Clinical pharmacokinetics
The pharmacokinetics of both olanzapine and samidorphan are linear over the clinical dose range and there is no pharmacokinetic interaction between olanzapine and samidorphan after oral administration of OSC.2 Coadministration of OSC with lithium or valproate does not have a clinically significant effect on systemic exposure of lithium or valproate.13 OSC steady-state concentrations of olanzapine and samidorphan are reached within 7 days, with accumulation at steady state being 2-fold for olanzapine and 1.3-fold for samidorphan (at 5 days). Elimination half-life for olanzapine is 35 to 52 hours, and for samidorphan, 7 to 11 hours. Olanzapine is metabolized primarily via UGT1A4 and CYP1A2, whereas samidorphan is primarily metabolized by CYP3A4. Consequently, concomitant use of OSC with strong CYP3A4 inducers is not recommended. The recommendation regarding CYP1A2 modulators and OSC are similar to those for olanzapine2,4: consider reducing the dosage of the olanzapine component in OSC when used concomitantly with strong CYP1A2 inhibitors, and consider increasing the dosage of the olanzapine component in OSC when used concomitantly with CYP1A2 inducers. Because cigarette smoke contains polycyclic aromatic hydrocarbons that act as CYP1A2 inducers,18 olanzapine clearance is much higher in smokers than in nonsmokers.2 This translates to potentially clinically relevant differences when optimizing the dose. In a study of patients with schizophrenia, olanzapine concentrations were lower in self-reported smokers (16.5, 34.2, and 60.9 ng/mL) than in self-reported nonsmokers (25.6, 43.4, and 113.2 ng/mL) for dosages of 10, 20, and 40 mg/d, respectively.19 In contrast, samidorphan pharmacokinetics are not affected by smoking status.2
No dose adjustment of OSC is needed in patients with hepatic or renal impairment; however, OSC is not recommended for patients with end-stage renal disease because this has not been specifically studied.2
Continue to: Efficacy...
Efficacy
The efficacy of OSC in the treatment of schizophrenia in adults is supported, in part, by the extensive legacy of studies of orally administered olanzapine.2 For OSC specifically, acute efficacy was primarily demonstrated in a randomized, double-blind, phase 3, 4-week study establishing superiority vs placebo in acutely exacerbated patients with schizophrenia.8 Mitigation of weight gain was assessed separately in a randomized, double-blind, phase 3, 24-week study comparing OSC with olanzapine in non-acute outpatients with schizophrenia.10 Both of these 2 trials were accompanied by 52-week open-label extension studies.9,11
The 4-week study evaluated the antipsychotic efficacy of OSC in 401 patients experiencing an acute exacerbation or relapse of schizophrenia who required inpatient treatment.8 Patients were required to have a Positive and Negative Syndrome Scale (PANSS) total score ≥80, with a score ≥4 on at least 3 of selected positive symptoms, and a Clinical Global Impression-Severity (CGI-S) score ≥4 at baseline and screening. Patients were required to be inpatients for the first 2 weeks of the study, and were encouraged to remain as inpatients for all 4 weeks. Patients were randomized to receive OSC, olanzapine, or placebo. Dosing was once-daily and flexible based on clinical response and tolerability for the first 2 weeks of the study, and fixed thereafter. Patients assigned to OSC could receive 10 mg/10 mg or 20 mg/10 mg, and patients randomized to olanzapine could receive 10 mg or 20 mg. The study compared OSC with placebo, with olanzapine serving as an active control. Treatment with OSC resulted in significant improvements in symptoms compared with placebo at Week 4, as measured by changes in PANSS total scores from baseline. Improvement in PANSS scores with OSC relative to placebo was similar to that observed with olanzapine. The antipsychotic efficacy of OSC relative to placebo was also supported by improvements in CGI-S scores. Thus, the inclusion of samidorphan in OSC did not negatively impact the antipsychotic efficacy of olanzapine.
In the 24-week study, 561 patients were randomized to OSC or olanzapine.10 There was no placebo control. Patients were treated with doses of OSC 10 mg/10 mg or 20 mg/10 mg, or with doses of olanzapine 10 mg or 20 mg. Dosing was flexible for the first 4 weeks of the study and fixed thereafter. Eligible patients were age 18 to 55 years (younger than the 4-week study, where the maximum age was 70 years), with a body mass index of 18 to 30 kg/m2 (lower than the upper limit of 40 kg/m2 used in the 4-week study). In contrast to the acutely exacerbated patients in the 4-week study, patients were required to have a PANSS total score of 50 to 90, CGI-S score ≤4, and symptoms suitable for outpatient treatment. The co-primary endpoints were percent change from baseline in body weight and proportion of patients who gained ≥10% body weight at Week 24. Treatment with OSC or olanzapine resulted in similar improvements in PANSS total and CGI-S scores, but treatment with OSC was associated with statistically significantly less weight gain than treatment with olanzapine, and with a smaller proportion of patients who gained ≥10% body weight. The least squares mean percent weight change from baseline to the end of treatment was 4.2% with OSC vs 6.6% with olanzapine. Although patients treated with OSC or olanzapine had similar weight gain for the first 4 weeks of treatment, OSC weight gain stabilized after approximately the 6th week, whereas patients who received olanzapine continued to gain weight throughout the remainder of the treatment period. The risk of gaining ≥10% body weight from baseline was reduced by 50% with OSC compared with olanzapine. Moreover, the odds of gaining ≥7% body weight from baseline at Week 24 were also reduced by 50% for OSC compared with olanzapine. OSC was also associated with smaller increases in waist circumference compared with olanzapine, which was observable as early as Week 1. The risk of experiencing a 5-cm increase in waist circumference was 50% lower for patients treated with OSC vs olanzapine, a relevant threshold in assessing risk of all-cause mortality and cardiovascular disease.20 However, changes in metabolic laboratory parameters in patients treated with OSC or olanzapine were generally small and were similar between groups. In addition, there were little differences between the 2 treatment groups in metabolic parameter changes considered to be of potential clinical significance, based on commonly used thresholds.
Patients on stable, chronic olanzapine therapy were not specifically studied, so the weight effect of switching from olanzapine to OSC is unknown.For bipolar I manic or mixed episodes, the use of OSC as monotherapy or in combination with lithium or valproate, as well as for maintenance monotherapy, was approved based on legacy clinical trials with olanzapine, as described in product labeling,2,4 as well as pharmacokinetic data evidencing that OSC did not have a clinically significant effect on the pharmacokinetics of lithium or valproate.13 A study is in progress to evaluate the effect of OSC compared with olanzapine on body weight in young adults with schizophrenia, schizophreniform, or bipolar I disorder who are early in their illness (ClinicalTrials.gov identifier: NCT03187769).
Overall tolerability and safety
The systemic safety and tolerability profile for OSC would be expected to be similar to that for olanzapine, unless there are adverse events that are specifically related to the samidorphan component. In the 4-week acute study described above,8 adverse events that occurred at least twice the rate of placebo with OSC included increased weight (18.7%, 14.3%, 3.0%, for OSC, olanzapine, and placebo, respectively), somnolence (9.0%, 9.8%, 2.2%), dry mouth (7.5%, 5.3%, 0.7%), and headache (6.0%, 5.3%, 3.0%). In the 24-week study,10 which did not have a placebo control, the most commonly reported adverse events (≥10% of patients) were increased weight (24.8% vs 36.2% for OSC vs olanzapine), somnolence (21.2% vs 18.1%), dry mouth (12.8% vs 8.0%), and increased appetite (10.9% vs 12.3%). In both studies, rates of discontinuation due to adverse events were low and similar between groups (in the 4-week study, 1.5% for OSC, 2.3% for olanzapine, and 5.2% for placebo; in the 24-week study, 12.0% for OSC and 9.8% for olanzapine).
In the 2 open-label, phase 3, 52-week extension studies,9,11 long-term tolerability was evidenced by low rates discontinuation due to adverse events (≤6%). Neither extension study reported any clinically meaningful changes over time in hematology, biochemistry, vital signs, or electrocardiogram parameters.3 In addition to durability of antipsychotic response as evidenced by sustained improvements in PANSS and CGI-S scores over time, waist circumference and weight remained stable, and the observed long-term changes in weight were consistent with weight changes observed with other second-generation antipsychotics.3 Long-term changes in metabolic laboratory parameter values were small and remained stable, and there was little change in glycosylated hemoglobin (hemoglobin A1c) values, which suggests that glycemic control was maintained with long-term OSC treatment.3 Caveats to consider are that the extension studies were open label without comparators, and they may have selected for patients who responded favorably to OSC treatment in the preceding studies.3Warnings and precautions in OSC product labeling are generally similar to those for other second-generation antipsychotics,21 other than warnings and precautions specifically related to samidorphan being an opioid antagonist, and special mention of “Drug Reaction with Eosinophilia and Systemic Symptoms” and “Anticholinergic (Antimuscarinic) Effects” warnings, which also are contained in the olanzapine legacy label.2,4
Summary
Olanzapine has a plethora of evidence supporting its robust efficacy profile5,6; however, its use is stymied by an unfavorable weight and metabolic profile.7 OSC may help mitigate at least some of the weight gain that would be expected with the use of olanzapine alone in the long-term treatment of patients with schizophrenia or bipolar I disorder. The addition of samidorphan does not deleteriously affect the efficacy of olanzapine, but decreases the risk of gaining ≥10% or ≥7% of baseline body weight by approximately 50% compared with olanzapine alone. Increase in waist circumference, a proxy for how much metabolically active fat one has, is lower with OSC than it is with olanzapine. Because samidorphan is an opioid receptor antagonist, OSC is contraindicated in patients using opioids and in those undergoing acute opioid withdrawal. Dosage strengths available for OSC parallel those for olanzapine, and all strengths including the same fixed dose of samidorphan—10 mg—so advise patients not to double up on the tablets, and to not split them.
Related Resource
• Olanzapine and samidorphan (Lybalvi) prescribing information. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
Drug Brand Names
Diazepam • Valium
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Olanzapine-fluoxetine combination • Symbyax
Olanzapine-samidorphan combination • Lybalvi
Valproate • Depakote, Depakene
Bottom Line
Olanzapine-samidorphan combination (OSC) is intended to mitigate some of the weight gain anticipated when using olanzapine alone. For clinicians who have prescribed olanzapine and have seen its therapeutic benefits, OSC will be a welcome addition to the therapeutic armamentarium. For practitioners who may have avoided olanzapine entirely, OSC can provide another means of offering this therapeutic option and counter “olanzapine hesitancy.”
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
1. US Food and Drug Administration. NDA 213378 approval letter. May 28, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000Approv.pdf
2. Alkermes, Inc. LYBALVI™ (olanzapine and samidorphan) tablets, for oral use. Prescribing information. May 2021. Accessed November 24, 2021. https://www.lybalvi.com/lybalvi-prescribing-information.pdf
3. Citrome L, Graham C, Simmons A, et al. An evidence-based review of OLZ/SAM for treatment of adults with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2021;17:2885-2904.
4. Eli Lilly and Company. ZYPREXA (olanzapine) tablet for oral use; ZYPREXA ZYDIS (olanzapine) tablet, orally disintegrating for oral use; ZYPREXA intramuscular (olanzapine) injection, powder, for solution for intramuscular use. Prescribing information. February 2021. Accessed November 24, 2021. https://pi.lilly.com/us/zyprexa-pi.pdf
5. Citrome L, McEvoy JP, Todtenkopf MS, et al. A commentary on the efficacy of olanzapine for the treatment of schizophrenia: the past, present, and future. Neuropsychiatr Dis Treat. 2019;15:2559-2569.
6. Meftah AM, Deckler E, Citrome L, et al. New discoveries for an old drug: a review of recent olanzapine research. Postgrad Med. 2020;132(1):80-90.
7. Citrome L, Holt RI, Walker DJ, et al. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455-482.
8. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m12769.
9. Yagoda S, Graham C, Simmons A, et al. Long-term safety and durability of effect with a combination of olanzapine and samidorphan in patients with schizophrenia: results from a 1-year open-label extension study. CNS Spectr. 2021;26(4):383-392.
10. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168-1178.
11. Kahn RS, Silverman BL, DiPetrillo L, et al. A phase 3, multicenter study to assess the 1-year safety and tolerability of a combination of olanzapine and samidorphan in patients with schizophrenia: results from the ENLIGHTEN-2 long-term extension. Schizophr Res. 2021;232:45-53.
12. US Food and Drug Administration. Drug approval package: Lybalvi. June 26, 2021. Accessed November 24, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213378Orig1Orig2s000TOC.cfm
13. Sun L, Yagoda S, Yao B, et al. Combination of olanzapine and samidorphan has no clinically significant effect on the pharmacokinetics of lithium or valproate. Clin Drug Investig. 2020;40(1):55-64.
14. Eli Lilly and Company. SYMBYAX (olanzapine and fluoxetine) capsules for oral use. Prescribing information. September 2021. Accessed November 24, 2021. https://pi.lilly.com/us/symbyax-pi.pdf
15. Wentland MP, Lu Q, Lou R, et al. Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone. Bioorg Med Chem Lett. 2005;15(8):2107-2110.
16. Lee MW, Fujioka K. Naltrexone for the treatment of obesity: review and update. Expert Opin Pharmacother. 2009;10(11):1841-1845.
17. Sun L, Yagoda S, Xue H, et al. Combination of olanzapine and samidorphan has no clinically relevant effects on ECG parameters, including the QTc interval: results from a phase 1 QT/QTc study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109881.
18. Zhou SF, Yang LP, Zhou ZW, et al. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. AAPS J. 2009;11(3):481-494.
19. Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278-283.
20. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335-345.
21. Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599-1613.
Treating homeless patients: Book offers key insights
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.
As a psychiatrist dedicated to working with people who are experiencing homelessness, I was very impressed with the new book edited by Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, and Maria D. Llorente, MD, about treating and providing services to this vulnerable population.
The book, “Clinical Management of the Homeless Patient: Social, Psychiatric, and Medical Issues” (Cham, Switzerland: Springer Nature Switzerland, 2021), offers an in-depth review and analysis of the biopsychosocial complexities that affect how medical and behavioral health conditions present in those who are unhoused. Notably, the book recommends with great sensitivity best practices to address these conditions with care, understanding, and love.
This text, invaluable in particular for those of us clinicians who work with people experiencing homelessness (PEH), provides a historical context of homelessness in the United States, an evaluation of the current state, and indispensable guidance for medical and behavioral health practitioners, case managers, housing navigators, and policy makers alike. It also serves as an inspiring source for those who are considering work in the public sector while reminding those of us in the field why we continue to do this challenging and rewarding work.
Tips can provide hope to clinicians
The volume is divided into four clear sections that are easy to navigate depending on your area of expertise and interest. Each chapter consolidates an extensive literature review into an intriguing and thought-provoking analysis. Part I, “The Big Picture – Social and Medical Issues,” focuses on conditions that disproportionately affect those who are unhoused. The authors offer a glimpse into the unique challenges of managing routine health conditions. They also detail the practical knowledge that’s needed to best care for our most vulnerable neighbors; for example, promoting a shared decision-making model; simplifying treatment plans; prescribing, when possible, medications that are dosed daily – instead of multiple times per day; allowing for walk-in appointments; and addressing cultural, linguistic, and educational barriers.
Most chapters highlight informative case examples that bring the text to life. It can be heartbreaking to recognize and witness the inhumane conditions in which PEH live, and these practical tips and suggestions for future policies based on best practices can help prevent burnout and provide hope for those who care for this community.
Part II, “Psychiatric Issues and Treatments,” presents a brief yet comprehensive history on homelessness, beginning with the deep shame that PEH experienced in Colonial times as the result of cultural and religious influences. Sadly, that negative judgment continues to this day.
The authors also explain how deinstitutionalization and transinstitutionalization have shaped the current state of homelessness, including why many PEH receive their care in emergency departments while incarcerated. This section highlights the barriers of care that are created not just by the patient, but also by the clinicians and systems of care – and what’s needed practically to overcome those challenges.
I appreciate the chapter on substance use disorders. It reminds us that the most commonly used substance among PEH is tobacco, which has serious health effects and for which we have treatment; nevertheless, . This section also provides examples of the trauma-informed language to use when addressing difficult and sometimes stigmatizing topics, such as survival sex and trauma history.
The evidence-based discussion continues in Part III with a focus on topics that everyone working with PEH should understand, including food insecurity, the criminal justice system, and sex trafficking. Part IV highlights best practices that should be replicated in every community, including Housing First approaches, medical respite care, and multiple Veterans Administration programs.
Throughout the text, major themes reverberate across the chapters, beginning with empathy. All who work with PEH must understand the conditions and challenges PEH face every day that affect their physical and mental health. The authors offer a stark and pointed reminder that being unhoused amounts to a full-time job just to meet basic needs. In addition, the devastating role of trauma and structural racism in creating and promoting the conditions that lead someone to be unhoused cannot be underestimated.
Fortunately, the primary aim of the book is to highlight solutions, and it’s here that the book shines. While some interventions are well-known, such as the importance of working in multidisciplinary teams, building trust and rapport with our patients, and urging clinicians and institutions to examine their own judgments and biases that might interfere with humane treatment, other suggestions will lead some readers into new territory. The authors, for example, maintain that we need more data and evidence-based research that include PEH. They also make a case for more preventive care and enhanced professional education for all health care workers that centers on trauma-informed care, social determinants of health, and the unique needs of especially vulnerable communities, such as the unhoused LBGTQ+ community and policies that promote best practices, such as Housing First. The book is a stirring read. It offers both inspiration and practical guidance for all who are currently working with or interested in caring for people experiencing homelessness.
Dr. Bird is a psychiatrist with Alameda County Health Care for the Homeless and the TRUST Clinic in Oakland, Calif. She also is a cofounder of StreetHealth, a backpack street medicine team that provides psychiatric and substance use disorder treatment to people experiencing homelessness in downtown Oakland.
Dr. Bird has no disclosures.