User login
Caution urged for antidepressant use in bipolar depression
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Although patients with bipolar disorder commonly experience depressive symptoms, clinicians should be very cautious about treating them with antidepressants, especially as monotherapy, experts asserted in a recent debate on the topic as part of the European Psychiatric Association (EPA) 2020 Congress.
At the Congress, which was virtual this year because of the COVID-19 pandemic, psychiatric experts said that clinicians should also screen patients for mixed symptoms that are better treated with mood stabilizers. These same experts also raised concerns over long-term antidepressant use, recommending continued use only in patients who relapse after stopping antidepressants.
Isabella Pacchiarotti, MD, PhD, Centro de Investigación Biomédica en Red de Salud Mental, Barcelona, Spain, argued against the use of antidepressants in treating bipolar disorder; Guy Goodwin, PhD, however, took the “pro” stance.
Goodwin, a professor of psychiatry at the University of Oxford in the UK, admitted that there is a “paucity of data” on the role of antidepressants in bipolar disorder.
Nevertheless, there are “circumstances that one really has to treat with antidepressants simply because other things have been tried and have not worked,” he told conference attendees.
Challenging, Controversial Topic
The debate was chaired by Eduard Vieta, MD, PhD, chair of the Department of Psychiatry and Psychology at the University of Barcelona Hospital Clinic, Spain.
Vieta said the question over whether antidepressants should be used in the depressive phase of bipolar illness is “perhaps the most challenging ... especially in the area of bipolar disorder.”
At the beginning of the presentation, Vieta asked the audience for their opinion in order to have a “baseline” for the debate: among 164 respondents, 73% were in favor of using antidepressants in bipolar depression.
“Clearly there is a majority, so Isabella [Dr Pacchiarotti] is going to have a hard time improving these numbers,” Vieta noted.
Up first, Pacchiarotti began by noting that this topic remains “an area of big controversy.” However, the real question “should not be the pros and cons of antidepressants but more when and how to use them.»
Of the three phases of bipolar disorder, acute depression «poses the greatest difficulties,» she added.
This is because of the relative paucity of studies in the area, the often heated debates on the specific role of antidepressants, the discrepancy in conclusions between meta-analyses, and the currently approved therapeutic options being associated with “not very high response rates,” Pacchiarotti said.
The diagnostic criteria for unipolar and bipolar depression are “basically the same,” she noted. However, it’s important to be able to distinguish between the two conditions, as up to one fifth of patients with unipolar depression suffer from undiagnosed bipolar disorder, she explained.
Moreover, several studies have identified key symptoms in bipolar depression, such as hyperphagia and hypersomnia, increased anxiety, and psychotic and psychomotor symptoms.
As previously reported by Medscape Medical News, a task force report was released in 2013 by the International Society for Bipolar Disorder (ISBD) on antidepressant use in bipolar disorders. Pacchiarotti and Goodwin were among the report’s authors, which concluded that available evidence on this issue is methodologically weak.
This is largely because of a lack of placebo-controlled studies in this patient population (bipolar depression, alongside suicidal ideation, is often an exclusion criteria in clinical antidepressant trials).
Many guidelines consequently do not consider antidepressants to be a first-line option as monotherapy in bipolar depression, although some name the drugs as second- or third-line options.
In 2013, the ISBD recommended that antidepressant monotherapy should be “avoided” in bipolar I disorder; and in bipolar I and II depression, the treatment should be accompanied by at least two concomitant core manic symptoms.
“What Has Changed?”
Antidepressants should be used “only if there is a history of a positive response,” whereas maintenance therapy should be considered if a patient relapses into a depressive episode after stopping the drugs, the report notes.
Pacchiarotti noted that since the recommendations were published nothing has changed, noting that antidepressant efficacy in bipolar depression “remains unproven.”
The issue is not whether antidepressants are effective in bipolar depression but rather are there subpopulations where these medications are helpful or harmful, she added.
The key to understanding the heterogeneity of responses to antidepressants, she said, is the concept of a bipolar spectrum and a dimensional approach to distinguishing between bipolar disorder and unipolar depression.
In addition, the definition of a mixed episode in the DSM-IV-TR differs from that of an episode with mixed characteristics in the DSM-5, which Pacchiarotti said offers a better understanding of the phenomenon while seemingly disposing with the idea of mixed depression.
Based on previous research, there is some suggestion that a depressive state exists between major depressive disorder and bipolar I disorder with mixed features, and hypomania state between bipolar II and I disorder, also with mixed features.
Pacchiarotti said the role of antidepressants in the treatment of bipolar depression remains “controversial” and there is a need for both short- and long-term studies of their use in both bipolar I and bipolar II disorder with real-world inclusion criteria.
The concept of a bipolar spectrum needs to be considered a more “dimensional approach” to depression, with mixed features seen as a “transversal” contraindication for antidepressant use, she concluded.
In Favor — With Caveats
Taking the opposite position and arguing in favor of antidepressant use, albeit cautiously, Goodwin said previous work has shown that stable patients with bipolar disorder experience depression of variable severity about 50% of the time.
The truth is that patients do not have a depressive episode for extended periods but instead have depressive symptoms, he said. “So how we manage and treat depression really matters.”
In an analysis, Goodwin and his colleagues estimated that the cost of bipolar disorder is approximately £12,600 ($16,000) per patient per year, of which only 30.6% is attributable to healthcare costs and 68.1% to indirect costs. This means the impact on the patient is also felt by society.
He agreed with Pacchiarotti’s assertion of a bipolar spectrum and the need for a dimensional approach.
“All the patients along the spectrum have the symptoms of depression and they differ in the extent to which they show symptoms of mania, which will include irritability,” he added.
Goodwin argued that there is no evidence to suggest that the depression experienced at one end of the scale is any different from that at the other. However, safety issues around antidepressant use “really relate to the additional symptoms you see with increasing evidence of bipolarity.”
In addition, the whole discussion is confounded by comorbidity, “with symptoms that sometimes coalesce into our concept of borderline personality disorder” or attention deficit hyperactivity disorder, he said.
Goodwin said there is “very little doubt” that antidepressants have an effect vs placebo. “The argument is over whether the effect is large and whether we should regard it as clinically significant.”
He noted that previous studies have shown a range of effect sizes with antidepressants, but the “massive” confidence intervals mean that “one is free to believe pretty much what one likes.”
The only antidepressant medication that is statistically significantly different from placebo is fluoxetine combined with olanzapine. However, that conclusion is based on “little data,” said Goodwin.
In terms of long-term management, there is “extremely little” randomized data for maintenance treatment with antidepressants in bipolar disorder. “So this does not support” long-term use, he added.
Still, although choice of antidepressant remains a guess, there is “just about support” for using them, Goodwin noted.
He urged clinicians not to dismiss antidepressant use, but to use them only where there is a clinical need and for as little time as possible. Patients with bipolar disorder should continue to take antidepressants if they relapse after they come off these medications.
However, all of that sits “in contrast” to how they’re currently used in clinical practice, Goodwin said.
Caution Urged
After the debate, the audience was asked to vote again. This time, The remaining 12% voted against the practice.
Summarizing the discussion, Vieta said that “we should be cautious” when using antidepressants in bipolar depression. However, “we should be able to use them when necessary,” he added.
Although their use as monotherapy is not best practice, especially in bipolar I disorder, there may be a subset of bipolar II patients in whom monotherapy “might still be acceptable; but I don’t think it’s a good idea,” Vieta said.
He added that clinicians should very carefully screen for mixed symptoms, which call for the prescription of other drugs, such as olanzapine and fluoxetine.
“The other important message is that we have to be even more cautious in the long term with the use of antidepressants, and we should be able to use them when there is a comorbidity” that calls for their use, Vieta concluded.
Pacchiarotti reported having received speaker fees and educational grants from Adamed, AstraZeneca, Janssen-Cilag, and Lundbeck. Goodwin reported having received honoraria from Angellini, Medscape, Pfizer, Servier, Shire, and Sun; having shares in P1vital Products; past employment as medical director of P1vital Products; and advisory board membership for Compass Pathways, Minerva, MSD, Novartis, Lundbeck, Sage, Servier, and Shire. Vieta has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
New long-term data for antipsychotic in pediatric bipolar depression
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.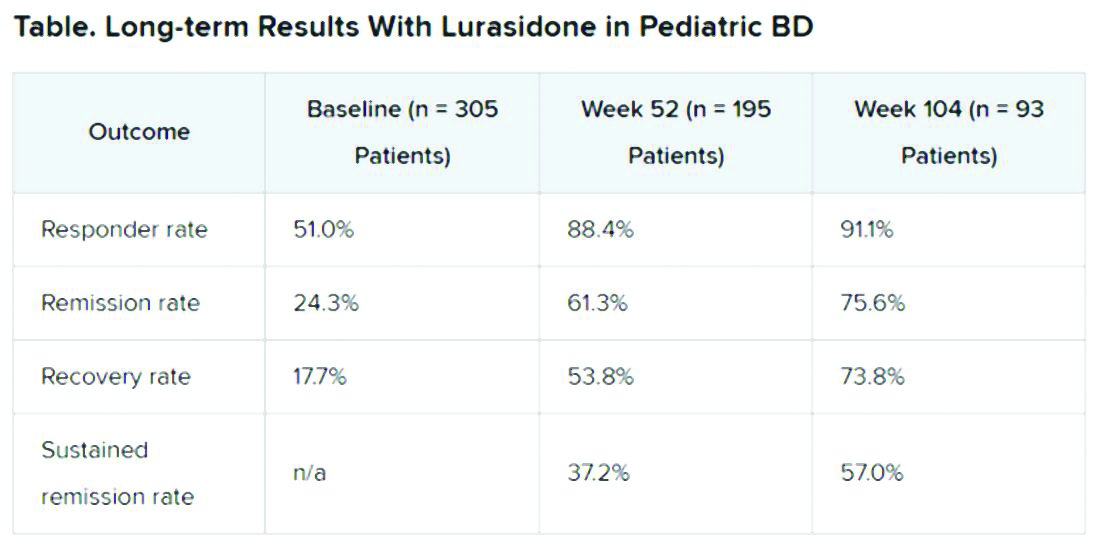
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.
Potential Limitations
Commenting on the study, Christoph U. Correll, MD, professor of child and adolescent psychiatry, Charite Universitatsmedizin, Berlin, Germany, noted that BD is difficult to treat, especially for patients who are going through “a developmentally vulnerable phase of their lives.”
“Lurasidone is the only monotherapy approved for bipolar depression in youth and is fairly well tolerated,” said Dr. Correll, who was not part of the research. He added that the long-term effectiveness data on response and remission “add relevant information” to the field.
However, he noted that it is not clear whether the high and increasing rates of response and remission were based on the reporting of observed cases or on last-observation-carried-forward analyses. “Given the naturally high dropout rate in such a long-term study and the potential for a survival bias, this is a relevant methodological question that affects the interpretation of the data,” he said.
“Nevertheless, the very favorable results for cumulative response, remission, and sustained remission add to the evidence that lurasidone is an effective treatment for youth with bipolar depression. Since efficacy cannot be interpreted in isolation, data describing the tolerability, including long-term cardiometabolic effects, will be important complementary data to consider,” Dr. Correll said.
The study was funded by Sunovion Pharmaceuticals. Dr. Singh is on the advisory board for Sunovion, is a consultant for Google X and Limbix, and receives royalties from American Psychiatric Association Publishing. She has also received research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, the National Institute of Mental Health, the National Institute on Aging, Johnson and Johnson, Allergan, PCORI, and the Brain and Behavior Research Foundation. Dr. Correll has been a consultant or adviser to and has received honoraria from Sunovion, as well as Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Supernus, Takeda, and Teva.
A version of this article originally appeared on Medscape.com.
FROM ASCP 2020
Atopic dermatitis in adults, children linked to neuropsychiatric disorders
according to a study presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
“The risk increase ranges from as low as 5% up to 59%, depending on the outcome, with generally greater effects observed among the adults,” Joy Wan, MD, a postdoctoral dermatology fellow at the University of Pennsylvania, Philadelphia, said in her presentation. The risk was independent of other atopic disease, gender, age, and socioeconomic status.
Dr. Wan and colleagues conducted a cohort study of patients with AD in the United Kingdom using data from the Health Improvement Network (THIN) electronic records database, matching AD patients in THIN with up to five patients without AD, similar in age and also registered to general practices. The researchers validated AD disease status using an algorithm that identified patients with a diagnostic code and two therapy codes related to AD. Outcomes of interest included anxiety, depression, bipolar disorder, obsessive-compulsive disorder, ADHD, schizophrenia, and autism. Patients entered into the cohort when they were diagnosed with AD, registered by a practice, or when data from a practice was reported to THIN. The researchers stopped following patients when they developed a neuropsychiatric outcome of interest, left a practice, died, or when the study ended.
“Previous studies have found associations between atopic dermatitis and anxiety, depression, and attention-deficit/hyperactivity disorder. However, many previous studies had been cross-sectional and they were unable to evaluate the directionality of association between atopic dermatitis and neuropsychiatric outcomes, while other previous studies have relied on the self-report of atopic dermatitis and outcomes as well,” Dr. Wan said. “Thus, longitudinal studies, using validated measures of atopic dermatitis, and those that include the entire age span, are really needed.”
Overall, 434,859 children and adolescents under aged 18 with AD in the THIN database were matched to 1,983,589 controls, and 644,802 adults with AD were matched to almost 2,900,000 adults without AD. In the pediatric group, demographics were mostly balanced between children with and without AD: the average age ranged between about 5 and almost 6 years. In pediatric patients with AD, there was a higher rate of allergic rhinitis (6.2% vs. 4%) and asthma (13.5% vs. 9.3%) than in the control group.
For adults, the average age was about 48 years in both groups. Compared with patients who did not have AD, adults with AD also had higher rates of allergic rhinitis (15.2% vs. 9.6%) and asthma (19.9% vs. 12.6%).
After adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis, Dr. Wan and colleagues found greater rates of bipolar disorder (hazard ratio, 1.34; 95% confidence interval, 1.09-1.65), obsessive-compulsive disorder (HR, 1.30; 95% CI, 1.21-1.41), anxiety (HR, 1.09; 95% CI, 1.07-1.11), and depression (HR, 1.06; 95% CI, 1.04-1.08) among children and adolescents with AD, compared with controls.
In the adult cohort, a diagnosis of AD was associated with an increased risk of autism (HR, 1.53; 95% CI, 1.30-1.80), obsessive-compulsive disorder (HR, 1.49; 95% CI, 1.40-1.59), ADHD (HR, 1.31; 95% CI, 1.13-1.53), anxiety (HR, 1.17; 95% CI, 1.15-1.18), depression (HR, 1.15; 95% CI, 1.14-1.16), and bipolar disorder (HR, 1.12; 95% CI, 1.04-1.21), after adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis.
One reason for the increased associations among the adults, even for ADHD and autism, which are more characteristically diagnosed in childhood, Dr. Wan said, is that, since they looked at incident outcomes, “many children may already have had these prevalent comorbidities at the time of the entry in the cohort.”
She noted that the study may have observation bias or unknown confounders, but she hopes these results raise awareness of the association between AD and neuropsychiatric disorders, although more research is needed to determine how AD severity affects neuropsychiatric outcomes. “Additional work is needed to really understand the mechanisms that drive these associations, whether it’s mediated through symptoms of atopic dermatitis such as itch and poor sleep, or potentially the stigma of having a chronic skin disease, or perhaps shared pathophysiology between atopic dermatitis and these neuropsychiatric diseases,” she said.
The study was funded by a grant from Pfizer. Dr. Wan reports receiving research funding from Pfizer paid to the University of Pennsylvania.
according to a study presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
“The risk increase ranges from as low as 5% up to 59%, depending on the outcome, with generally greater effects observed among the adults,” Joy Wan, MD, a postdoctoral dermatology fellow at the University of Pennsylvania, Philadelphia, said in her presentation. The risk was independent of other atopic disease, gender, age, and socioeconomic status.
Dr. Wan and colleagues conducted a cohort study of patients with AD in the United Kingdom using data from the Health Improvement Network (THIN) electronic records database, matching AD patients in THIN with up to five patients without AD, similar in age and also registered to general practices. The researchers validated AD disease status using an algorithm that identified patients with a diagnostic code and two therapy codes related to AD. Outcomes of interest included anxiety, depression, bipolar disorder, obsessive-compulsive disorder, ADHD, schizophrenia, and autism. Patients entered into the cohort when they were diagnosed with AD, registered by a practice, or when data from a practice was reported to THIN. The researchers stopped following patients when they developed a neuropsychiatric outcome of interest, left a practice, died, or when the study ended.
“Previous studies have found associations between atopic dermatitis and anxiety, depression, and attention-deficit/hyperactivity disorder. However, many previous studies had been cross-sectional and they were unable to evaluate the directionality of association between atopic dermatitis and neuropsychiatric outcomes, while other previous studies have relied on the self-report of atopic dermatitis and outcomes as well,” Dr. Wan said. “Thus, longitudinal studies, using validated measures of atopic dermatitis, and those that include the entire age span, are really needed.”
Overall, 434,859 children and adolescents under aged 18 with AD in the THIN database were matched to 1,983,589 controls, and 644,802 adults with AD were matched to almost 2,900,000 adults without AD. In the pediatric group, demographics were mostly balanced between children with and without AD: the average age ranged between about 5 and almost 6 years. In pediatric patients with AD, there was a higher rate of allergic rhinitis (6.2% vs. 4%) and asthma (13.5% vs. 9.3%) than in the control group.
For adults, the average age was about 48 years in both groups. Compared with patients who did not have AD, adults with AD also had higher rates of allergic rhinitis (15.2% vs. 9.6%) and asthma (19.9% vs. 12.6%).
After adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis, Dr. Wan and colleagues found greater rates of bipolar disorder (hazard ratio, 1.34; 95% confidence interval, 1.09-1.65), obsessive-compulsive disorder (HR, 1.30; 95% CI, 1.21-1.41), anxiety (HR, 1.09; 95% CI, 1.07-1.11), and depression (HR, 1.06; 95% CI, 1.04-1.08) among children and adolescents with AD, compared with controls.
In the adult cohort, a diagnosis of AD was associated with an increased risk of autism (HR, 1.53; 95% CI, 1.30-1.80), obsessive-compulsive disorder (HR, 1.49; 95% CI, 1.40-1.59), ADHD (HR, 1.31; 95% CI, 1.13-1.53), anxiety (HR, 1.17; 95% CI, 1.15-1.18), depression (HR, 1.15; 95% CI, 1.14-1.16), and bipolar disorder (HR, 1.12; 95% CI, 1.04-1.21), after adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis.
One reason for the increased associations among the adults, even for ADHD and autism, which are more characteristically diagnosed in childhood, Dr. Wan said, is that, since they looked at incident outcomes, “many children may already have had these prevalent comorbidities at the time of the entry in the cohort.”
She noted that the study may have observation bias or unknown confounders, but she hopes these results raise awareness of the association between AD and neuropsychiatric disorders, although more research is needed to determine how AD severity affects neuropsychiatric outcomes. “Additional work is needed to really understand the mechanisms that drive these associations, whether it’s mediated through symptoms of atopic dermatitis such as itch and poor sleep, or potentially the stigma of having a chronic skin disease, or perhaps shared pathophysiology between atopic dermatitis and these neuropsychiatric diseases,” she said.
The study was funded by a grant from Pfizer. Dr. Wan reports receiving research funding from Pfizer paid to the University of Pennsylvania.
according to a study presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
“The risk increase ranges from as low as 5% up to 59%, depending on the outcome, with generally greater effects observed among the adults,” Joy Wan, MD, a postdoctoral dermatology fellow at the University of Pennsylvania, Philadelphia, said in her presentation. The risk was independent of other atopic disease, gender, age, and socioeconomic status.
Dr. Wan and colleagues conducted a cohort study of patients with AD in the United Kingdom using data from the Health Improvement Network (THIN) electronic records database, matching AD patients in THIN with up to five patients without AD, similar in age and also registered to general practices. The researchers validated AD disease status using an algorithm that identified patients with a diagnostic code and two therapy codes related to AD. Outcomes of interest included anxiety, depression, bipolar disorder, obsessive-compulsive disorder, ADHD, schizophrenia, and autism. Patients entered into the cohort when they were diagnosed with AD, registered by a practice, or when data from a practice was reported to THIN. The researchers stopped following patients when they developed a neuropsychiatric outcome of interest, left a practice, died, or when the study ended.
“Previous studies have found associations between atopic dermatitis and anxiety, depression, and attention-deficit/hyperactivity disorder. However, many previous studies had been cross-sectional and they were unable to evaluate the directionality of association between atopic dermatitis and neuropsychiatric outcomes, while other previous studies have relied on the self-report of atopic dermatitis and outcomes as well,” Dr. Wan said. “Thus, longitudinal studies, using validated measures of atopic dermatitis, and those that include the entire age span, are really needed.”
Overall, 434,859 children and adolescents under aged 18 with AD in the THIN database were matched to 1,983,589 controls, and 644,802 adults with AD were matched to almost 2,900,000 adults without AD. In the pediatric group, demographics were mostly balanced between children with and without AD: the average age ranged between about 5 and almost 6 years. In pediatric patients with AD, there was a higher rate of allergic rhinitis (6.2% vs. 4%) and asthma (13.5% vs. 9.3%) than in the control group.
For adults, the average age was about 48 years in both groups. Compared with patients who did not have AD, adults with AD also had higher rates of allergic rhinitis (15.2% vs. 9.6%) and asthma (19.9% vs. 12.6%).
After adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis, Dr. Wan and colleagues found greater rates of bipolar disorder (hazard ratio, 1.34; 95% confidence interval, 1.09-1.65), obsessive-compulsive disorder (HR, 1.30; 95% CI, 1.21-1.41), anxiety (HR, 1.09; 95% CI, 1.07-1.11), and depression (HR, 1.06; 95% CI, 1.04-1.08) among children and adolescents with AD, compared with controls.
In the adult cohort, a diagnosis of AD was associated with an increased risk of autism (HR, 1.53; 95% CI, 1.30-1.80), obsessive-compulsive disorder (HR, 1.49; 95% CI, 1.40-1.59), ADHD (HR, 1.31; 95% CI, 1.13-1.53), anxiety (HR, 1.17; 95% CI, 1.15-1.18), depression (HR, 1.15; 95% CI, 1.14-1.16), and bipolar disorder (HR, 1.12; 95% CI, 1.04-1.21), after adjusting for age, gender, socioeconomic status, asthma, and allergic rhinitis.
One reason for the increased associations among the adults, even for ADHD and autism, which are more characteristically diagnosed in childhood, Dr. Wan said, is that, since they looked at incident outcomes, “many children may already have had these prevalent comorbidities at the time of the entry in the cohort.”
She noted that the study may have observation bias or unknown confounders, but she hopes these results raise awareness of the association between AD and neuropsychiatric disorders, although more research is needed to determine how AD severity affects neuropsychiatric outcomes. “Additional work is needed to really understand the mechanisms that drive these associations, whether it’s mediated through symptoms of atopic dermatitis such as itch and poor sleep, or potentially the stigma of having a chronic skin disease, or perhaps shared pathophysiology between atopic dermatitis and these neuropsychiatric diseases,” she said.
The study was funded by a grant from Pfizer. Dr. Wan reports receiving research funding from Pfizer paid to the University of Pennsylvania.
FROM SID 2020
Dietary intervention cuts mood swings, other bipolar symptoms
A nutritional intervention with a focus on fatty acids appears to reduce mood swings in patients with bipolar disorder (BD) when used as an adjunct to pharmacotherapy, early research suggests.
In a single-center study, patients with BD who received a diet consisting of high omega-3 plus low omega-6 fatty acids (H3-L6), in addition to usual care, showed significant reductions in mood variability, irritability, and pain, compared with their counterparts who received a diet with usual levels of omega-3 and omega-6 fatty acids commonly consumed in regular U.S. diets.
“Our findings need replication and validation in other studies,” study coinvestigator Erika Saunders, MD, professor and chair of the department of psychiatry and behavioral health at Penn State Health, Hershey, said in an interview.
“While we got really exciting findings, it’s far from confirmatory or the last word on the subject. The fatty acids do two broad things. They incorporate into the membranes of neurons in the brain and they also create signaling molecules throughout the brain and the body that interact with the immune system and the inflammatory system. And we suspect that it is through those mechanisms that this composition of fatty acids is having an effect on mood stability, but lots more work needs to be done to figure that out,” Dr. Saunders added.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Fewer mood swings
Many patients with bipolar disorder do not achieve complete mood stability with medication, making the need for additional treatments imperative, she added.
“We were interested in looking at treatments that improved mood stability in bipolar disorder that are well tolerated by patients and that can be added to pharmacological treatments. We studied this particular nutritional intervention because biologically it does some of the same things that effective medications for bipolar disorder do and it has been investigated as an effective treatment for conditions like migraine headaches, which has a lot of overlap and comorbidity with bipolar disorder.”
The researchers randomized 41 patients with BD to receive the nutritional intervention of high omega-3 plus low omega-6 (H3-L6) and 41 patients with BD to receive a control diet of usual US levels of omega-3 and omega-6 fatty acids.
The patients were aged 20-75 years (mean age, 43.5 +/– 13.9 years) and 83% were women. They had similar mean levels of mood symptoms and pain.
All patients received group-specific study foods and oils, as well as intensive dietary counseling from a dietitian, access to a website with recipes, and guidance for eating in restaurants. All participants were blinded to the composition of the food that they were eating.
Both the interventional diet and the control diet were tailored for the purposes of the study, noted coinvestigator Sarah Shahriar, a research assistant at Penn State.
“The interventional group had more fatty fish such as salmon and tuna, while the control group had more white fish and fish with less fatty acid content. The interventional group also received a different type of cooking oil, which was a blend of olive and macadamia-nut oil, which was specially formulated by a research nutritional service at the University of North Carolina,” Ms. Shahriar said in an interview.
“They also decreased their red meat consumption and received specially formulated snack foods, which were specifically prepared by [the university’s] research nutritional service. It is important to point out that these diets were for a very specific purpose. We are not saying in any way shape or form that this particular nutritional intervention is good in general,” she added.
After 12 weeks, significant reductions were seen in mood variability, energy, irritability, and pain in the H3-L6 group (P < .001). The only symptom that was significantly lowered in the control group was impulsive thoughts (P = .004).
“The best message for doctors to tell their patients at this point is one of general nutritional health and the importance of nutrition in overall body and brain health, and that [this] can be a very important component of mood,” Dr. Saunders said.
Diet matters
“Highly unsaturated fatty acids are important components of neuronal cell membranes and in cell signaling,” Jessica M. Gannon, MD, University of Pittsburgh, who was not part of the study, said in an interview.
“Omega-6 fatty acids are precursors to proinflammatory compounds. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid are thought to be competitive inhibitors of omega-6 and thought to have anti-inflammatory effects. Supplementation with omega-3 has been explored in cardiovascular disease, diabetes, and in rheumatologic disorders as well as in a host of psychiatric disorders, including bipolar disorders, where a possible treatment effect has been suggested,” Dr. Gannon said.
Dietary interventions targeting not only increasing omega-3 but also decreasing consumption of omega-6 rich foods could be both effective and attractive to patients invested in a healthy lifestyle for promotion of mental health, especially when they are not optimally controlled by prescribed medications, she added.
“This study suggests that such an intervention could prove beneficial, although significant patient support may be necessary to assure adherence to the diet. I would agree that future studies would be worth pursuing,” Dr. Gannon said.
The investigators and Dr. Gannon have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A nutritional intervention with a focus on fatty acids appears to reduce mood swings in patients with bipolar disorder (BD) when used as an adjunct to pharmacotherapy, early research suggests.
In a single-center study, patients with BD who received a diet consisting of high omega-3 plus low omega-6 fatty acids (H3-L6), in addition to usual care, showed significant reductions in mood variability, irritability, and pain, compared with their counterparts who received a diet with usual levels of omega-3 and omega-6 fatty acids commonly consumed in regular U.S. diets.
“Our findings need replication and validation in other studies,” study coinvestigator Erika Saunders, MD, professor and chair of the department of psychiatry and behavioral health at Penn State Health, Hershey, said in an interview.
“While we got really exciting findings, it’s far from confirmatory or the last word on the subject. The fatty acids do two broad things. They incorporate into the membranes of neurons in the brain and they also create signaling molecules throughout the brain and the body that interact with the immune system and the inflammatory system. And we suspect that it is through those mechanisms that this composition of fatty acids is having an effect on mood stability, but lots more work needs to be done to figure that out,” Dr. Saunders added.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Fewer mood swings
Many patients with bipolar disorder do not achieve complete mood stability with medication, making the need for additional treatments imperative, she added.
“We were interested in looking at treatments that improved mood stability in bipolar disorder that are well tolerated by patients and that can be added to pharmacological treatments. We studied this particular nutritional intervention because biologically it does some of the same things that effective medications for bipolar disorder do and it has been investigated as an effective treatment for conditions like migraine headaches, which has a lot of overlap and comorbidity with bipolar disorder.”
The researchers randomized 41 patients with BD to receive the nutritional intervention of high omega-3 plus low omega-6 (H3-L6) and 41 patients with BD to receive a control diet of usual US levels of omega-3 and omega-6 fatty acids.
The patients were aged 20-75 years (mean age, 43.5 +/– 13.9 years) and 83% were women. They had similar mean levels of mood symptoms and pain.
All patients received group-specific study foods and oils, as well as intensive dietary counseling from a dietitian, access to a website with recipes, and guidance for eating in restaurants. All participants were blinded to the composition of the food that they were eating.
Both the interventional diet and the control diet were tailored for the purposes of the study, noted coinvestigator Sarah Shahriar, a research assistant at Penn State.
“The interventional group had more fatty fish such as salmon and tuna, while the control group had more white fish and fish with less fatty acid content. The interventional group also received a different type of cooking oil, which was a blend of olive and macadamia-nut oil, which was specially formulated by a research nutritional service at the University of North Carolina,” Ms. Shahriar said in an interview.
“They also decreased their red meat consumption and received specially formulated snack foods, which were specifically prepared by [the university’s] research nutritional service. It is important to point out that these diets were for a very specific purpose. We are not saying in any way shape or form that this particular nutritional intervention is good in general,” she added.
After 12 weeks, significant reductions were seen in mood variability, energy, irritability, and pain in the H3-L6 group (P < .001). The only symptom that was significantly lowered in the control group was impulsive thoughts (P = .004).
“The best message for doctors to tell their patients at this point is one of general nutritional health and the importance of nutrition in overall body and brain health, and that [this] can be a very important component of mood,” Dr. Saunders said.
Diet matters
“Highly unsaturated fatty acids are important components of neuronal cell membranes and in cell signaling,” Jessica M. Gannon, MD, University of Pittsburgh, who was not part of the study, said in an interview.
“Omega-6 fatty acids are precursors to proinflammatory compounds. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid are thought to be competitive inhibitors of omega-6 and thought to have anti-inflammatory effects. Supplementation with omega-3 has been explored in cardiovascular disease, diabetes, and in rheumatologic disorders as well as in a host of psychiatric disorders, including bipolar disorders, where a possible treatment effect has been suggested,” Dr. Gannon said.
Dietary interventions targeting not only increasing omega-3 but also decreasing consumption of omega-6 rich foods could be both effective and attractive to patients invested in a healthy lifestyle for promotion of mental health, especially when they are not optimally controlled by prescribed medications, she added.
“This study suggests that such an intervention could prove beneficial, although significant patient support may be necessary to assure adherence to the diet. I would agree that future studies would be worth pursuing,” Dr. Gannon said.
The investigators and Dr. Gannon have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A nutritional intervention with a focus on fatty acids appears to reduce mood swings in patients with bipolar disorder (BD) when used as an adjunct to pharmacotherapy, early research suggests.
In a single-center study, patients with BD who received a diet consisting of high omega-3 plus low omega-6 fatty acids (H3-L6), in addition to usual care, showed significant reductions in mood variability, irritability, and pain, compared with their counterparts who received a diet with usual levels of omega-3 and omega-6 fatty acids commonly consumed in regular U.S. diets.
“Our findings need replication and validation in other studies,” study coinvestigator Erika Saunders, MD, professor and chair of the department of psychiatry and behavioral health at Penn State Health, Hershey, said in an interview.
“While we got really exciting findings, it’s far from confirmatory or the last word on the subject. The fatty acids do two broad things. They incorporate into the membranes of neurons in the brain and they also create signaling molecules throughout the brain and the body that interact with the immune system and the inflammatory system. And we suspect that it is through those mechanisms that this composition of fatty acids is having an effect on mood stability, but lots more work needs to be done to figure that out,” Dr. Saunders added.
The findings were presented at the American Society of Clinical Psychopharmacology 2020 Virtual Conference.
Fewer mood swings
Many patients with bipolar disorder do not achieve complete mood stability with medication, making the need for additional treatments imperative, she added.
“We were interested in looking at treatments that improved mood stability in bipolar disorder that are well tolerated by patients and that can be added to pharmacological treatments. We studied this particular nutritional intervention because biologically it does some of the same things that effective medications for bipolar disorder do and it has been investigated as an effective treatment for conditions like migraine headaches, which has a lot of overlap and comorbidity with bipolar disorder.”
The researchers randomized 41 patients with BD to receive the nutritional intervention of high omega-3 plus low omega-6 (H3-L6) and 41 patients with BD to receive a control diet of usual US levels of omega-3 and omega-6 fatty acids.
The patients were aged 20-75 years (mean age, 43.5 +/– 13.9 years) and 83% were women. They had similar mean levels of mood symptoms and pain.
All patients received group-specific study foods and oils, as well as intensive dietary counseling from a dietitian, access to a website with recipes, and guidance for eating in restaurants. All participants were blinded to the composition of the food that they were eating.
Both the interventional diet and the control diet were tailored for the purposes of the study, noted coinvestigator Sarah Shahriar, a research assistant at Penn State.
“The interventional group had more fatty fish such as salmon and tuna, while the control group had more white fish and fish with less fatty acid content. The interventional group also received a different type of cooking oil, which was a blend of olive and macadamia-nut oil, which was specially formulated by a research nutritional service at the University of North Carolina,” Ms. Shahriar said in an interview.
“They also decreased their red meat consumption and received specially formulated snack foods, which were specifically prepared by [the university’s] research nutritional service. It is important to point out that these diets were for a very specific purpose. We are not saying in any way shape or form that this particular nutritional intervention is good in general,” she added.
After 12 weeks, significant reductions were seen in mood variability, energy, irritability, and pain in the H3-L6 group (P < .001). The only symptom that was significantly lowered in the control group was impulsive thoughts (P = .004).
“The best message for doctors to tell their patients at this point is one of general nutritional health and the importance of nutrition in overall body and brain health, and that [this] can be a very important component of mood,” Dr. Saunders said.
Diet matters
“Highly unsaturated fatty acids are important components of neuronal cell membranes and in cell signaling,” Jessica M. Gannon, MD, University of Pittsburgh, who was not part of the study, said in an interview.
“Omega-6 fatty acids are precursors to proinflammatory compounds. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid are thought to be competitive inhibitors of omega-6 and thought to have anti-inflammatory effects. Supplementation with omega-3 has been explored in cardiovascular disease, diabetes, and in rheumatologic disorders as well as in a host of psychiatric disorders, including bipolar disorders, where a possible treatment effect has been suggested,” Dr. Gannon said.
Dietary interventions targeting not only increasing omega-3 but also decreasing consumption of omega-6 rich foods could be both effective and attractive to patients invested in a healthy lifestyle for promotion of mental health, especially when they are not optimally controlled by prescribed medications, she added.
“This study suggests that such an intervention could prove beneficial, although significant patient support may be necessary to assure adherence to the diet. I would agree that future studies would be worth pursuing,” Dr. Gannon said.
The investigators and Dr. Gannon have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 exacerbating challenges for Latino patients
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Disproportionate burden of pandemic complicates mental health care
Disproportionate burden of pandemic complicates mental health care
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
Pamela Montano, MD, recalls the recent case of a patient with bipolar II disorder who was improving after treatment with medication and therapy when her life was upended by the COVID-19 pandemic.
The patient, who is Puerto Rican, lost two cousins to the virus, two of her brothers fell ill, and her sister became sick with coronavirus, said Dr. Montano, director of the Latino Bicultural Clinic at Gouverneur Health in New York. The patient was then left to care for her sister’s toddlers along with the patient’s own children, one of whom has special needs.
“After this happened, it increased her anxiety,” Dr. Montano said in an interview. “She’s not sleeping, and she started having panic attacks. My main concern was how to help her cope.”
Across the country, clinicians who treat mental illness and behavioral disorders in Latino patients are facing similar experiences and challenges associated with COVID-19 and the ensuing pandemic response. Current data suggest a disproportionate burden of illness and death from the novel coronavirus among racial and ethnic groups, particularly black and Hispanic patients. The disparities are likely attributable to economic and social conditions more common among such populations, compared with non-Hispanic whites, in addition to isolation from resources, according to the Centers for Disease Control and Prevention.
A recent New York City Department of Health study based on data that were available in late April found that deaths from COVID-19 were substantially higher for black and Hispanic/Latino patients than for white and Asian patients. The death rate per 100,000 population was 209.4 for blacks, 195.3 for Hispanics/Latinos, 107.7 for whites, and 90.8 for Asians.
“The COVID pandemic has highlighted the structural inequities that affect the Latino population [both] immigrant and nonimmigrant,” said Dr. Montano, a board member of the American Society of Hispanic Psychiatry and the officer of infrastructure and advocacy for the Hispanic Caucus of the American Psychiatric Association. “This includes income inequality, poor nutrition, history of trauma and discrimination, employment issues, quality education, access to technology, and overall access to appropriate cultural linguistic health care.”
Navigating challenges
For mental health professionals treating Latino patients, COVID-19 and the pandemic response have generated a range of treatment obstacles.
The transition to telehealth for example, has not been easy for some patients, said Jacqueline Posada, MD, consultation-liaison psychiatry fellow at the Inova Fairfax Hospital–George Washington University program in Falls Church, Va., and an APA Substance Abuse and Mental Health Services Administration minority fellow. Some patients lack Internet services, others forget virtual visits, and some do not have working phones, she said.
“I’ve had to be very flexible,” she said in an interview. “Ideally, I’d love to see everybody via video chat, but a lot of people either don’t have a stable Internet connection or Internet, so I meet the patient where they are. Whatever they have available, that’s what I’m going to use. If they don’t answer on the first call, I will call again at least three to five times in the first 15 minutes to make sure I’m giving them an opportunity to pick up the phone.”
In addition, Dr. Posada has encountered disconnected phones when calling patients for appointments. In such cases, Dr. Posada contacts the patient’s primary care physician to relay medication recommendations in case the patient resurfaces at the clinic.
In other instances, patients are not familiar with video technology, or they must travel to a friend or neighbor’s house to access the technology, said Hector Colón-Rivera, MD, an addiction psychiatrist and medical director of the Asociación Puertorriqueños en Marcha Behavioral Health Program, a nonprofit organization based in the Philadelphia area. Telehealth visits frequently include appearances by children, family members, barking dogs, and other distractions, said Dr. Colón-Rivera, president of the APA Hispanic Caucus.
“We’re seeing things that we didn’t used to see when they came to our office – for good or for bad,” said Dr. Colón-Rivera, an attending telemedicine physician at the University of Pittsburgh Medical Center. “It could be a good chance to meet our patient in a different way. Of course, it creates different stressors. If you have five kids on top of you and you’re the only one at home, it’s hard to do therapy.”
Psychiatrists are also seeing prior health conditions in patients exacerbated by COVID-19 fears and new health problems arising from the current pandemic environment. Dr. Posada recalls a patient whom she successfully treated for premenstrual dysphoric disorder who recently descended into severe clinical depression. The patient, from Colombia, was attending school in the United States on a student visa and supporting herself through child care jobs.
“So much of her depression was based on her social circumstance,” Dr. Posada said. “She had lost her job, her sister had lost her job so they were scraping by on her sister’s husband’s income, and the thing that brought her joy, which was going to school and studying so she could make a different life for herself than what her parents had in Colombia, also seemed like it was out of reach.”
Dr. Colón-Rivera recently received a call from a hospital where one of his patients was admitted after becoming delusional and psychotic. The patient was correctly taking medication prescribed by Dr. Colón-Rivera, but her diabetes had become uncontrolled because she was unable to reach her primary care doctor and couldn’t access the pharmacy. Her blood sugar level became elevated, leading to the delusions.
“A patient that was perfectly stable now is unstable,” he said. “Her diet has not been good enough through the pandemic, exacerbating her diabetes. She was admitted to the hospital for delirium.
Compounding of traumas
For many Latino patients, the adverse impacts of the pandemic comes on top of multiple prior traumas, such as violence exposures, discrimination, and economic issues, said Lisa Fortuna, MD, MPH, MDiv, chief of psychiatry and vice chair at Zuckerberg San Francisco General Hospital. A 2017 analysis found that nearly four in five Latino youth face at least one traumatic childhood experience, like poverty or abuse, and that about 29% of Latino youth experience four or more of these traumas.
Immigrants in particular, may have faced trauma in their home country and/or immigration trauma, Dr. Fortuna added. A 2013 study on immigrant Latino adolescents for example, found that 29% of foreign-born adolescents and 34% of foreign-born parents experienced trauma during the migration process (Int Migr Rev. 2013 Dec;47(4):10).
“All of these things are cumulative,” Dr. Fortuna said. “Then when you’re hit with a pandemic, all of the disparities that you already have and all the stress that you already have are compounded. This is for the kids, too, who have been exposed to a lot of stressors and now maybe have family members that have been ill or have died. All of these things definitely put people at risk for increased depression [and] the worsening of any preexisting posttraumatic stress disorder. We’ve seen this in previous disasters, and I expect that’s what we’re going to see more of with the COVID-19 pandemic.”
At the same time, a central cultural value of many Latinos is family unity, Dr. Montano said, a foundation that is now being strained by social distancing and severed connections.
“This has separated many families,” she said. “There has been a lot of loneliness and grief.”
Mistrust and fear toward the government, public agencies, and even the health system itself act as further hurdles for some Latinos in the face of COVID-19. In areas with large immigrant populations such as San Francisco, Dr. Fortuna noted, it’s not uncommon for undocumented patients to avoid accessing medical care and social services, or visiting emergency departments for needed care for fear of drawing attention to themselves or possible detainment.
“The fact that so many people showed up at our hospital so ill and ended up in the ICU – that could be a combination of factors. Because the population has high rates of diabetes and hypertension, that might have put people at increased risk for severe illness,” she said. “But some people may have been holding out for care because they wanted to avoid being in places out of fear of immigration scrutiny.”
Overcoming language barriers
Compounding the challenging pandemic landscape for Latino patients is the fact that many state resources about COVID-19 have not been translated to Spanish, Dr. Colón-Rivera said. He was troubled recently when he went to several state websites and found limited to no information in Spanish about the coronavirus. Some data about COVID-19 from the federal government were not translated to Spanish until officials received pushback, he added. Even now, press releases and other information disseminated by the federal government about the virus appear to be translated by an automated service – and lack sense and context.
The state agencies in Pennsylvania have been alerted to the absence of Spanish information, but change has been slow, he noted.
“In Philadelphia, 23% speaks a language other than English,” he said. “So we missed a lot of critical information that could have helped to avoid spreading the illness and access support.”
Dr. Fortuna said that California has done better with providing COVID-19–related information in Spanish, compared with some other states, but misinformation about the virus and lingering myths have still been a problem among the Latino community. The University of California, San Francisco, recently launched a Latino Task Force resource website for the Latino community that includes information in English, Spanish, and Yucatec Maya about COVID-19, health and wellness tips, and resources for various assistance needs.
The concerning lack of COVID-19 information translated to Spanish led Dr. Montano to start a Facebook page in Spanish about mental health tips and guidance for managing COVID-19–related issues. She and her team of clinicians share information, videos, relaxation exercises, and community resources on the page, among other posts. “There is also general info and recommendations about COVID-19 that I think can be useful for the community,” she said. “The idea is that patients, the general community, and providers can have share information, hope messages, and ask questions in Spanish.”
Feeling ‘helpless’
A central part of caring for Latino patients during the COVID-19 crisis has been referring them to outside agencies and social services, psychiatrists say. But finding the right resources amid a pandemic and ensuring that patients connect with the correct aid has been an uphill battle.
“We sometimes feel like our hands are tied,” Dr. Colón-Rivera said. “Sometimes, we need to call a place to bring food. Some of the state agencies and nonprofits don’t have delivery systems, so the patient has to go pick up for food or medication. Some of our patients don’t want to go outside. Some do not have cars.”
As a clinician, it can be easy to feel helpless when trying to navigate new challenges posed by the pandemic in addition to other longstanding barriers, Dr. Posada said.
“Already, mental health disorders are so influenced by social situations like poverty, job insecurity, or family issues, and now it just seems those obstacles are even more insurmountable,” she said. “At the end of the day, I can feel like: ‘Did I make a difference?’ That’s a big struggle.”
Dr. Montano’s team, which includes psychiatrists, psychologists, and social workers, have come to rely on virtual debriefings to vent, express frustrations, and support one another, she said. She also recently joined a virtual mind-body skills group as a participant.
“I recognize the importance of getting additional support and ways to alleviate burnout,” she said. “We need to take care of ourselves or we won’t be able to help others.”
Focusing on resilience during the current crisis can be beneficial for both patients and providers in coping and drawing strength, Dr. Posada said.
“When it comes to fostering resilience during times of hardship, I think it’s most helpful to reflect on what skills or attributes have helped during past crises and apply those now – whether it’s turning to comfort from close relationships, looking to religion and spirituality, practicing self-care like rest or exercise, or really tapping into one’s purpose and reason for practicing psychiatry and being a physician,” she said. “The same advice goes for clinicians: We’ve all been through hard times in the past, it’s part of the human condition and we’ve also witnessed a lot of suffering in our patients, so now is the time to practice those skills that have gotten us through hard times in the past.”
Learning lessons from COVID-19
Despite the challenges with moving to telehealth, Dr. Fortuna said the tool has proved beneficial overall for mental health care. For Dr. Fortuna’s team for example, telehealth by phone has decreased the no-show rate, compared with clinic visits, and improved care access.
“We need to figure out how to maintain that,” she said. “If we can build ways for equity and access to Internet, especially equipment, I think that’s going to help.”
In addition, more data are needed about the ways in which COVID-19 is affecting Latino patients, Dr. Colón-Rivera said. Mortality statistics have been published, but information is needed about the rates of infection and manifestation of illness.
Most importantly, the COVID-19 crisis has emphasized the critical need to address and improve the underlying inequity issues among Latino patients, psychiatrists say.
“We really need to think about how there can be partnerships, in terms of community-based Latino business and leaders, multisector resources, trying to think about how we can improve conditions both work and safety for Latinos,” Dr. Fortuna said. “How can schools get support in integrating mental health and support for families, especially now after COVID-19? And really looking at some of these underlying inequities that are the underpinnings of why people were at risk for the disproportionate effects of the COVID-19 pandemic.”
When mania isn’t what it seems
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).
Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
CASE Aggressive, impulsive, and not sleeping
Mr. S, age 22, is brought by his family to his outpatient psychiatrist because he has begun to
Mr. S has significant language impairment and is unreliable as a narrator. His family reports that Mr. S’s behavior has resulted in declining academic performance, and they have curtailed his social activities due to behavioral issues. Both his family and teachers report that it is increasingly difficult to redirect Mr. S’s behavior. Although not physically aggressive, Mr. S becomes verbally agitated when rituals are incomplete. He has gone from sleeping 8 hours each night to only 3 to 4 hours, but he does not appear tired during the day.
HISTORY Multiple hospitalizations
As a child, Mr. S had been diagnosed with autism and intellectual disability. When he was 13, he began exhibiting marked stereotypy, restlessness, impulsivity, frenzy, agitation, combativeness, and purposeless motor activity. At that time, he was not receiving any medications. Mr. S had not slept for 2 days and had been walking in circles nonstop. He became aggressive whenever anyone attempted to redirect his behavior. The family took Mr. S to the emergency department (ED), where clinicians ruled out organic causes for his behavioral disturbances, including infections, drug intoxication, and use of illicit substances. Mr. S was transferred from the ED to a child and adolescent psychiatry ward at a nearby university hospital for inpatient treatment.
On the inpatient unit, the treatment team diagnosed Mr. S with bipolar disorder and believed that he was experiencing a manic episode. He was prescribed quetiapine, 25 mg by mouth during the day and 75 mg by mouth at night, to stabilize his agitation, and was discharged with a plan to follow up with his outpatient psychiatrist. However, within 1 week, his symptoms returned, with markedly increased aggression and agitation, so he was readmitted, tapered off quetiapine, and prescribed valproic acid, 125 mg by mouth during the day and 375 mg by mouth at bedtime. With this regimen, Mr. S became calmer, but when he was discharged home, he was subdued and withdrawn, overly adherent to rules and routines, constantly irritable, and often unable to focus.
Two years later, Mr. S developed hyperammonemia. Valproic acid was discontinued, and many of his behavioral issues resolved. He flourished both academically and socially. He experienced no exacerbation of symptoms until his current presentation.
[polldaddy:10544547]
EVALUATION Pinpointing the cause
Mr. S’s physical examination reveals that his vital signs are within normal limits. Mr. S is mildly tachycardic (heart rate, 105 bpm), with regular rate and rhythm. No murmurs, gallops, or rubs are auscultated. The remainder of the physical exam, including a detailed neurologic exam, is normal.
On mental status examination, Mr. S makes limited eye contact. He has difficulty sitting in the chair, with increased rocking, finger flicking, and hand flapping from baseline. Some compulsive behaviors are noted, such as tapping his neck. He has increased tics (eye blinking and mouth opening) and increased verbigeration and repetitive verbal statements. He loudly and repeatedly demands to go home, and uses short sentences with incorrect pronouns. His affect is difficult to assess, but he is agitated. His thought process is concrete. There is no evidence of suicidal ideation, homicidal ideation, or psychosis. Mr. S denies auditory hallucinations. His insight and judgment are limited.
Continue to: The psychiatrist rules out...
The psychiatrist rules out a behavioral exacerbation of autism based on an interview with Mr. S’s family and established rapport from treating him for several years. Mr. S’s family reports that many of his behaviors are not new but that the increased drive and intensity is worrisome. Further, his family cannot identify any stressors or precipitants for the behaviors and reports that offering preferred reinforcers did not help. An anxiety disorder is ruled out because according to the family, Mr. S’s drive to constantly move and complete rituals is fueling his anxiety. Schizoaffective disorder is ruled out because Mr. S denies auditory hallucinations and has not been observed responding to internal stimuli.
His Bush-Francis Catatonia Rating Scale (BFCRS) score is 26, which suggests a high likelihood of catatonia. Based on the BFCRS score, Mr. S’s psychiatrist makes the diagnosis of hyperkinetic catatonia.
The authors’ observations
The psychiatrist determined that Mr. S had been misdiagnosed with bipolar disorder at age 13. At that time, he had experienced his first episode of hyperkinetic catatonia and his symptoms decreased after he received lorazepam in the ED. However, the treatment team did not correctly identify this, most likely due to limited knowledge of catatonia among emergency medicine clinicians.
This case exemplifies a cognitive error of premature closure. Rather than considering catatonia as a complication of autism when Mr. S was 13, the clinicians added a second psychiatric diagnosis of bipolar disorder.Although premature closure errors generally occur when the physician assumes the patient is having a common complication of a known illness,1 in Mr. S’s case, the opposite occurred.
Conceptualizing catatonia
One helpful model for conceptualizing catatonia is to think of it as a basal ganglia disorder, with lesions in the basal ganglia thalamocortical tracts and the anterior cingulate/medial orbitofrontal circuit. Disrupting these pathways can result in symptoms such as mutism or repetitive and imitative behaviors. This is likely due to decreased disinhibition by gamma-aminobutyric acid (GABA), resulting in a hypodopaminergic state. This explains why benzodiazepines, which act to increase GABA, are effective for treating catatonia, and antipsychotics that act to decrease dopamine can exacerbate symptoms. Fricchione et al2 developed a model to visually represent the neurobiologic pathophysiology of catatonia (Figure2).

Continue to: Underlying causes of catatonia
Underlying causes of catatonia
Catatonia is most often seen in individuals with an underlying psychiatric condition such as schizophrenia, mood disorders, or autism. However, catatonia also occurs in the context of general neurologic and medical disorders, including (but not limited to) infections, metabolic disorders, endocrinopathies, epilepsy, neurodegenerative diseases, delirium, hypertensive encephalopathy, autoimmune encephalitis, and liver and kidney transplantation.3
Subtypes of catatonia include4:
- hypokinetic catatonia, which presents as stupor, mutism, and negativism
- hyperkinetic catatonia, which presents as hyperactivity, agitation, and stereotypy (as observed in Mr. S)
- malignant catatonia, which is a potentially lethal form of catatonia that occurs when hypo- or hyperkinetic catatonia is accompanied by autonomic instability such as tachycardia, tachypnea, hypertension, fever, and muscle rigidity
- periodic catatonia, which is characterized by brief episodes of stupor or excitatory catatonia lasting 4 to 10 days. These episodes recur over weeks to years, with patients remaining asymptomatic between episodes, or showing mild symptoms, such as facial grimacing or negativisms. Periodic catatonia often is autosomal dominant, involves linkage for the long arm of chromosome 15, and has a better prognosis than the other forms.
Autism and catatonia
Most individuals with autism who experience a catatonic episode first do so between age 10 and 19, and many episodes are precipitated by sudden changes in routine resulting in stress.5 An estimated 12% to 18% of patients with autism are diagnosed with catatonia in their lifetime, but the actual prevalence is likely higher.4
One of the reasons for this might be that although catatonia is well known in the psychiatric community, it is relatively unknown in the general medical community. Children and adolescents with psychiatric illness are likely to have symptoms of catatonia overlooked because catatonia often is not included in the differential diagnosis.6
In Mr. S’s case, it became clear that he did not have a mood disorder, but was prone to episodes of hyperkinetic catatonia due to his autism.
Continue to: Better recognition of catatonia
Better recognition of catatonia
As catatonia becomes better elucidated and more clearly described in the literature, there is increasing awareness that symptoms do not always involve stupor, mutism, and slowed motor activity, but can include increased motor activity, agitation, and stereotypies. The BFCRS is extremely useful for quantifying symptoms of catatonia. The best way to confirm the diagnosis is to use a lorazepam challenge in an inpatient setting, or a trial of lorazepam in an outpatient setting.5
[polldaddy:10544548]
The authors’ observations
Lorazepam is often considered the first-line treatment for catatonia because it is one of the most widely studied medications. Other benzodiazepines, such as oxazepam and clonazepam, and the sedative/hypnotic zolpidem have also been shown to be effective. Antipsychotics with dopamine-blocking mechanisms can exacerbate symptoms of catatonia and should be avoided in these patients. Furthermore, in cases of refractory catatonia, bilateral electroconvulsive therapy is an important and necessary treatment.7
TREATMENT Pharmacologic agents decrease BFCRS score
Mr. S is prescribed a regimen of lorazepam, 2 mg by mouth daily, and the supplement N-acetylcysteine, 600 mg by mouth daily. Within 2 weeks of starting this regimen, Mr. S’s BFCRS score decreases from 26 to 14. After 6 months of treatment with lorazepam, Mr. S shows considerable improvement. The stereotypic behaviors and impulsivity decrease significantly, leading to improved sleep and performance in school. After 6 months Mr. S is successfully tapered off the lorazepam, with a complete return to baseline.
Bottom Line
Hyperkinetic catatonia is easily overlooked, especially in the emergency setting. Catatonia should always be ruled out, particularly in patients with underlying conditions associated with it. Hyperkinetic catatonia is an underrecognized comorbidity in patients with autism.
Related Resources
- Dhossche DM, Wing L, Ohta M, et al. International Review of Neurobiology: Catatonia in autism spectrum disorders, vol 72. New York, NY: Academic Press/Elsevier; 2006.
- Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
Drug Brand Names
Amantadine • Symmetrel
Bromocriptine • Parlodel
Clonazepam • Klonopin
Lorazepam • Ativan
Memantine • Namenda
Oxazepam • Serax
Quetiapine • Seroquel
Valproic acid • Depakene, Depakote
Zolpidem • Ambien
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
1. McGee DL. Cognitive errors in clinical decision making. Merck Manual. https://www.merckmanuals.com/professional/special-subjects/clinical-decision-making/cognitive-errors-in-clinical-decision-making. Published November 2018. Accessed February 10, 2020.
2. Fricchione GL, Gross AF, Stern TA. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Fricchione GL, Huffman JC, Stern TA, Bush G, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 6th ed. Philadelphia, PA: Saunders Elsevier; 2004:513-530.
3. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
4. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2014;86(8):825-832.
5. Dhossche DM, Shah A, Wing L. Blueprints for the assessment, treatment, and future study of catatonia in autism spectrum disorders. Int Rev Neurobiol. 2006:72;267-284.
6. Wing L, Shah A. Catatonia in autistic spectrum disorders. Br J Psychiatry. 2000:176(4):357-362.
7. Seinaert P, Dhossche DM, Vancampfort D, et al. A clinical review of the treatment of catatonia. Front Psychiatry. 2014;5:181.
Family-focused therapy linked to longer remissions in youth at risk for bipolar disorder
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
A 4-month intensive program of family-focused therapy worked better than a less-intensive program in delaying new mood episodes among young people at risk of developing bipolar disorder, new research shows.
“This study extends the results of other randomized clinical trials indicating effects of family psychoeducation and skill training on the long-term trajectory of depressive symptoms in pediatric mood disorders,” wrote David J. Miklowitz, PhD, of the department of psychiatry and biobehavioral sciences at the University of California, Los Angeles, and colleagues. The study was published in JAMA Psychiatry.
For their research, the investigators recruited 127 subjects aged 9-17 years (mean age, 13 years) deemed at high risk for later bipolar I or II disorder for having depression or subthreshold mania along with active mood symptoms and a family history of bipolar disorder. Some 85% of subjects had depression symptoms at enrollment.
Subjects were randomized to 12 sessions over 4 months of family-focused therapy – a psychoeducation, communication, and problem-solving training program incorporating caretakers and also siblings if possible (n = 61) – or to 3 sessions of family-focused therapy and an additional 3 of individual therapy in the same 4-month time frame (n = 66). Medication was allowed for all subjects, and patients were followed for a median 2 years after the intervention. Baseline characteristics, medication use, and dropout rates were similar between the groups.
Both groups saw similarly high rates of new episodes of major depression, mania, or hypomania during follow-up; however, those in the intensive family-focused therapy group saw longer intervals of wellness, with a median 81 weeks (95% confidence interval, 56-123 weeks) from randomization until the first observed mood episode, compared with 63 weeks (95% CI, 44-78 weeks) to an episode for the less-intensive group (P = .03). Dr. Miklowitz and colleagues did not find differences in the severity of mood episodes following either treatment mode or in later conversion to bipolar I or II.
The researchers described as limitations of their study its inability to measure the “temporal relationship between changes in family communication and symptom changes in patients,” which would help answer whether improvements in communication patterns aid symptom regulation, or whether more stable patients are better able to manage difficult family interactions.
Family-focused therapy “may have uniquely enduring effects that extend into the maintenance phase of treatment,” Dr. Miklowitz and colleagues wrote.
The study was funded by the National Institute of Mental Health. Several coauthors, including the lead author, reported receiving research grants from NIMH and other foundations. Two additional coauthors reported receiving pharmaceutical industry funding, including advisory board and consulting fees.
SOURCE: Miklowitz DJ et al. JAMA Psychiatry. 2020 Jan 15. doi: 10.1001/jamapsychiatry.2019.4520.
FROM JAMA PSYCHIATRY
The evolution of manic and hypomanic symptoms
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
Since publication of the first Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1952,1 the diagnosis of manic and hypomanic symptoms has evolved significantly. This evolution has changed my approach to patients who exhibit these symptoms, which include increased goal-directed activity, decreased need for sleep, and racing thoughts. Here I outline these diagnostic changes in each edition of the DSM and discuss their therapeutic importance and the possibility of future changes.
DSM-I (1952) described manic symptoms as having psychotic features.1 The term “manic episode” was not used, but manic symptoms were described as having a “tendency to remission and recurrence.”1
DSM-II (1968) introduced the term “manic episode” as having psychotic features.2 Manic episodes were characterized by symptoms of excessive elation, irritability, talkativeness, flight of ideas, and accelerated speech and motor activity.2
DSM-III (1980) explained that a manic episode could occur without psychotic features.3 The term “hypomanic episode” was introduced. It described manic features that do not meet criteria for a manic episode.3
DSM-IV (1994) reiterated the criteria for a manic episode.4 In addition, it established criteria for a hypomanic episode as lasting at least 4 days and requires ≥3 symptoms.4
DSM-5 (2013) describes hypomanic symptoms that do not meet criteria for a hypomanic episode (Table).5 These symptoms may require treatment with a mood stabilizer or antipsychotic medication.5
Suggested changes for the next DSM
Although DSM-5 does not discuss the duration of different manic or hypomanic symptoms in the same patient, these can vary widely.6 The same patient may have increased activity for 2 days, increased irritability for 2 weeks, and racing thoughts every day. Future versions of the DSM could include the varying durations of different manic or hypomanic symptoms in the same patient.
Continue to: Racing thoughts without...
Racing thoughts without increased energy or activity occur frequently and often go unnoticed.7 They can be mistaken for severe worrying or obsessive ideation. Depending on the severity of the patient’s racing thoughts, treatment might include a mood stabilizer or antipsychotic. All 5 DSM-5 diagnoses listed in the Table5 may include this symptom pattern, but do not specifically mention it. A diagnosis or specifier, such as “racing thoughts without increased energy or activity,” might help clinicians better recognize and treat this symptom pattern.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
1. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 1952:24-25.
2. Diagnostic and statistical manual of mental disorders. 2nd ed. Washington, DC: American Psychiatric Association; 1968:35-37.
3. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association; 1980:208-210,223.
4. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994:332,338.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:139-140,148-149,169,184-185.
6. Wilf TJ. When to treat subthreshold hypomanic episodes. Current Psychiatry. 2012;11(8):55.
7. Benazzi F. Unipolar depression with racing thoughts: a bipolar spectrum disorder? Psychiatry Clin Neurosci. 2005;59(5):570-575.
The paranoid business executive
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
CASE Bipolar-like symptoms
Mr. R, age 48, presents to the psychiatric emergency department (ED) for the third time in 4 days after a change in his behavior over the last 2.5 weeks. He exhibits heightened extroversion, pressured speech, and uncharacteristic irritability. Mr. R’s wife reports that her husband normally is reserved.
Mr. R’s wife first became concerned when she noticed he was not sleeping and spending his nights changing the locks on their home. Mr. R, who is a business executive, occupied his time by taking notes on ways to protect his identity from the senior partners at his company.
Three weeks before his first ED visit, Mr. R had been treated for a neck abscess with incision and drainage. He was sent home with a 10-day course of amoxicillin/clavulanate, 875/125 mg by mouth twice daily. There were no reports of steroid use during or after the procedure. Four days after starting the antibiotic, he stopped taking it because he and his wife felt it was contributing to his mood changes and bizarre behavior.
During his first visit to the ED, Mr. R received a 1-time dose of olanzapine, 5 mg by mouth, which helped temporarily reduce his anxiety; however, he returned the following day with the same anxiety symptoms and was discharged with a 30-day prescription for olanzapine, 5 mg/d, to manage symptoms until he could establish care with an outpatient psychiatrist. Two days later, he returned to the ED yet again convinced people were spying on him and that his coworkers were plotting to have him fired. He was not taking his phone to work due to fears that it would be hacked.
Mr. R’s only home medication is clomiphene citrate, 100 mg/d by mouth, which he’s received for the past 7 months to treat low testosterone. He has no personal or family history of psychiatric illness and no prior signs of mania or hypomania.
At the current ED visit, Mr. R’s testosterone level is checked and is within normal limits. His urine drug screen, head CT, and standard laboratory test results are unremarkable, except for mild transaminitis that does not warrant acute management.
The clinicians in the ED establish a diagnosis of mania, unspecified, and psychotic disorder, unspecified. They recommend that Mr. R be admitted for mood stabilization.
[polldaddy:10485725]
Continue to: The authors' observations
The authors’ observations
Our initial impression was that Mr. R was experiencing a manic episode from undiagnosed bipolar I disorder. The diagnosis was equivocal considering his age, lack of family history, and absence of prior psychiatric symptoms. In most cases, the mean age of onset for mania is late adolescence to early adulthood. It would be less common for a patient to experience a first manic episode at age 48, although mania may emerge at any age. Results from a large British study showed that the incidence of a first manic episode drops from 13.81% in men age 16 to 25 to 2.62% in men age 46 to 55.1 However, some estimates suggest that the prevalence of late-onset mania is much higher than previously expected; medical comorbidities, such as dementia and delirium, may play a significant role in posing as manic-type symptoms in these patients.2
In Mr. R’s case, he remained fully alert and oriented without waxing and waning attentional deficits, which made delirium less likely. His affective symptoms included a reduced need for sleep, anxiety, irritability, rapid speech, and grandiosity lasting at least 2 weeks. He also exhibited psychotic symptoms in the form of paranoia. Altogether, he fit diagnostic criteria for bipolar I disorder well.
At the time of his manic episode, Mr. R was taking clomiphene. Clomiphene-induced mania and psychosis has been reported scarcely in the literature.3 In these cases, behavioral changes occurred within the first month of clomiphene initiation, which is dissimilar from Mr. R’s timeline.4 However, there appeared to be a temporal relationship between Mr. R’s use of amoxicillin/clavulanate and his manic episode.
This led us to consider whether medication-induced bipolar disorder would be a more appropriate diagnosis. There are documented associations between mania and antibiotics5; however, to our knowledge, mania secondary specifically to amoxicillin/clavulanate has not been reported extensively in the American literature. We found 1 case of suspected amoxicillin-induced psychosis,6 as well as a case report from the Netherlands of possible amoxicillin/clavulanate-induced mania.7
EVALUATION Ongoing paranoia
During his psychiatric hospitalization, Mr. R remains cooperative and polite, but exhibits ongoing paranoia, pressured speech, and poor reality testing. He remains convinced that “people are out to get me,” and routinely scans the room for safety during daily evaluations. He reports that he feels safe in the hospital, but does not feel safe to leave. Mr. R does not recall if in the past he had taken any products containing amoxicillin, but he is able to appreciate changes in his mood after being prescribed the antibiotic. He reports that starting the antibiotic made him feel confident in social interactions.
Continue to: During Mr. R's psychiatric hospitalization...
During Mr. R’s psychiatric hospitalization, olanzapine is titrated to 10 mg at bedtime. Clomiphene citrate is discontinued to limit any potential precipitants of mania, and amoxicillin/clavulanate is not restarted.
Mr. R gradually shows improvement in sleep quality and duration and becomes less irritable. His speech returns to a regular rate and rhythm. He eventually begins to question whether his fears were reality-based. After 4 days, Mr. R is ready to be discharged home and return to work.
[polldaddy:10485726]
The authors’ observations
The term “antibiomania” is used to describe manic episodes that coincide with antibiotic usage.8 Clarithromycin and ciprofloxacin are the agents most frequently implicated in antibiomania.9 While numerous reports exist in the literature, antibiomania is still considered a rare or unusual adverse event.
The link between infections and neuropsychiatric symptoms is well documented, which makes it challenging to tease apart the role of the acute infection from the use of antibiotics in precipitating psychiatric symptoms. However, in most reported cases of antibiomania, the onset of manic symptoms typically occurs within the first week of antibiotic initiation and resolves 1 to 3 days after medication discontinuation. The temporal relationship between antibiotic initiation and onset of neuropsychiatric symptoms has been best highlighted in cases where clarithromycin is used to treat a chronic Helicobacter pylori infection.10
While reports of antibiomania date back more than 6 decades, the exact mechanism by which antibiotics cause psychiatric symptoms is mostly unknown, although there are several hypotheses.5 Many hypotheses suggest some antibiotics play a role in reducing gamma-aminobutyric acid (GABA) neurotransmission. Quinolones, for example, have been found to cross the blood–brain barrier and can inhibit GABA from binding to the receptor sites. This can result in hyper-excitability in the CNS. Several quinolones have been implicated in antibiomania (Table 15). Penicillins are also thought to interfere with GABA neurotransmission in a similar fashion; however, amoxicillin-clavulanate has poor CNS penetration in the absence of blood–brain barrier disruption,11 which makes this theory a less plausible explanation for Mr. R’s case.
Continue to: Another possible mechanism...
Another possible mechanism of antibiotic-induced CNS excitability is through the glutamatergic system. Cycloserine, an antitubercular agent, is an N-methyl-D-aspartate receptor (NMDA) partial agonist and has reported neuropsychiatric adverse effects.12 It has been proposed that quinolones may also have NMDA agonist activity.
The prostaglandin hypothesis suggests that a decrease in GABA may increase concentrations of steroid hormones in the rat CNS.13 Steroids have been implicated in the breakdown of prostaglandin E1 (PGE1).13 A disruption in steroid regulation may prevent PGE1 breakdown. Lithium’s antimanic properties are thought to be caused at least in part by limiting prostaglandin production.14 Thus, a shift in PGE1 may lead to mood dysregulation.
Bipolar disorder has been linked with mitochondrial function abnormalities.15 Antibiotics that target ribosomal RNA may disrupt normal mitochondrial function and increase risk for mania precipitation.15 However, amoxicillin exerts its antibiotic effects through binding to penicillin-binding proteins, which leads to inhibition of the cell wall biosynthesis.
Lastly, research into the microbiome has elucidated the gut-brain axis. In animal studies, the microbiome has been found to play a role in immunity, cognitive function, and behavior. Dysbiosis in the microbiome is currently being investigated for its role in schizophrenia and bipolar disorder.16 Both the microbiome and changes in mitochondrial function are thought to develop over time, so while these are plausible explanations, an onset within 4 days of antibiotic initiation is likely too short of an exposure time to produce these changes.
The most likely causes of Mr. R’s manic episode were clomiphene or amoxicillin-clavulanate, and the time course seems to indicate the antibiotic was the most likely culprit. Table 2 lists things to consider if you suspect your patient may be experiencing antibiomania.

Continue to: TREATMENT Stable on olanzapine
TREATMENT Stable on olanzapine
During his first visit to the outpatient clinic 4 weeks after being discharged, Mr. R reports that he has successfully returned to work, and his paranoia has completely resolved. He continues to take olanzapine, 10 mg nightly, and has restarted clomiphene, 100 mg/d.
During this outpatient follow-up visit, Mr. R attributes his manic episode to an adverse reaction to amoxicillin/clavulanate, and requests to be tapered off olanzapine. After he and his psychiatrist discuss the risk of relapse in untreated bipolar disorder, olanzapine is reduced to 7.5 mg at bedtime with a plan to taper to discontinuation.
At his second follow-up visit 1 month later, Mr. R has also stopped clomiphene and is taking a herbal supplement instead, which he reports is helpful for his fatigue.
[polldaddy:10485727]
OUTCOME Lasting euthymic mood
Mr. R agrees to our recommendation of continuing to monitor him every 3 months for at least 1 year. We provide him and his wife with education about early warning signs of mood instability. Eight months after his manic episode, Mr. R no longer receives any psychotropic medications and shows no signs of mood instability. His mood remains euthymic and he is able to function well at work and in his personal life.
Bottom Line
‘Antibiomania’ describes manic episodes that coincide with antibiotic usage. This adverse effect is rare but should be considered in patients who present with unexplained first-episode mania, particularly those with an initial onset of mania after early adulthood.
Continue to: Related Resources
Related Resources
- Rakofsky JJ, Dunlop BW. Nothing to sneeze at: Upper respiratory infections and mood disorders. Current Psychiatry. 2019;18(7):29-34.
- Adiba A, Jackson JC, Torrence CL. Older-age bipolar disorder: A case series. Current Psychiatry. 2019;18(2):24-29
Drug Brand Names
Amoxicillin • Amoxil
Amoxicillin/clavulanate • Augmentin
Ampicillin • Omnipen-N, Polycillin-N
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Cycloserine • Seromycin
Dapsone • Dapsone
Erythromycin • Erythrocin, Pediamycin
Ethambutol • Myambutol
Ethionamide • Trecator-SC
Gentamicin • Garamycin
Isoniazid • Hyzyd, Nydrazid
Lithium • Eskalith, Lithobid
Metronidazole • Flagyl
Minocycline • Dynacin, Solodyn
Norfloxacin • Noroxin
Ofloxacin • Floxin
Olanzapine • Zyprexa
Penicillin G procaine • Duracillin A-S, Pfizerpen
Sulfamethoxazole/trimethoprim • Bactrim, Septra
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
1. Kennedy M, Everitt B, Boydell J, et al. Incidence and distribution of first-episode mania by age: results for a 35-year study. Psychol Med. 2005;35(6):855-863.
2. Dols A, Kupka RW, van Lammeren A, et al. The prevalence of late-life mania: a review. Bipolar Disord. 2014;16:113-118.
3. Siedontopf F, Horstkamp B, Stief G, et al. Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment. Hum Reprod. 1997;12(4):706-707.
4. Oyffe T, Lerner A, Isaacs G, et al. Clomiphene-induced psychosis. Am J Psychiatry. 1997;154(8):1169-1170.
5. Lambrichts S, Van Oudenhove L, Sienaert P. Antibiotics and mania: a systematic review. J Affect Disord. 2017;219:149-156.
6. Beal DM, Hudson B, Zaiac M. Amoxicillin-induced psychosis? Am J Psychiatry. 1986;143(2):255-256.
7. Klain V, Timmerman L. Antibiomania, acute manic psychosis following the use of antibiotics. European Psychiatry. 2013;28(suppl 1):1.
8. Abouesh A, Stone C, Hobbs WR. Antimicrobial-induced mania (antibiomania): a review of spontaneous reports. J Clin Psychopharmacol. 2002;22(1):71-81.
9. Lally L, Mannion L. The potential for antimicrobials to adversely affect mental state. BMJ Case Rep. 2013. pii: bcr2013009659. doi: 10.1136/bcr-2013-009659.
10. Neufeld NH, Mohamed NS, Grujich N, et al. Acute neuropsychiatric symptoms associated with antibiotic treatment of Helicobactor Pylori infections: a review. J Psychiatr Pract. 2017;23(1):25-35.
11. Sutter R, Rüegg S, Tschudin-Sutter S. Seizures as adverse events of antibiotic drugs: a systematic review. Neurology. 2015;85(15):1332-1341.
12. Bakhla A, Gore P, Srivastava S. Cycloserine induced mania. Ind Psychiatry J. 2013;22(1):69-70.
13. Barbaccia ML, Roscetti G, Trabucchi M, et al. Isoniazid-induced inhibition of GABAergic transmission enhances neurosteroid content in the rat brain. Neuropharmacology. 1996;35(9-10):1299-1305.
14. Murphy D, Donnelly C, Moskowitz J. Inhibition by lithium of prostaglandin E1 and norepinephrine effects on cyclic adenosine monophosphate production in human platelets. Clin Pharmacol Ther. 1973;14(5):810-814.
15. Clay H, Sillivan S, Konradi C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int J Dev Neurosci. 2011;29(3):311-324.
16. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun. 2017;62:46-52.
Valproic acid-induced hyperammonemic encephalopathy
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
Mrs. C, age 75, is transferred to our inpatient medical/surgical hospital from a psychiatric hospital after presenting with shortness of breath and altered mental status.
Eight days earlier, Mrs. C had been admitted to the psychiatric hospital for bipolar mania with psychotic features. While there, Mrs. C received quetiapine, 400 mg nightly, and an initial valproic acid (VPA) dosage of 500 mg 2 times daily. While receiving VPA 500 mg 2 times daily, her VPA total level was 62 µg/mL, which is on the lower end of the therapeutic range (50 to 125 µg/mL). This prompted the team at the psychiatric hospital to increase her VPA dosage to 500 mg 3 times daily the day before she was transferred to our hospital.
At our hospital, she is found to be in hypoxic respiratory failure secondary to pneumonia. Upon admission, her laboratory data show evidence of infection and anemia and she also has an
From hospital Day 3 to Day 6, Mrs. C experiences gradual improvement in her respiratory and mental status. However, on hospital Day 7, she has extreme somnolence and altered mental status without respiratory involvement. Our team suspects VPA toxicity and/or VPA-induced hyperammonemic encephalopathy (VHE).
VPA-induced hyperammonemia
Hyperammonemia can occur in individuals receiving VPA and is most often asymptomatic. However, elevations in ammonia may lead to VHE, which is a rare but serious adverse effect. VHE has been reported early in treatment, in acute VPA overdose, and in chronic VPA use despite normal doses and levels.1 It also can occur in the absence of clinical and laboratory evidence of hepatotoxicity. VHE is associated with significant morbidity and CNS damage. Symptoms of VHE include vomiting, lethargy, and confusion. If left untreated, VHE can lead to coma and death.
Mechanism of VHE. The exact mechanism of VHE is unknown.1-3 Ammonia is a toxic base produced by deamination of amino acids. The liver eliminates ammonia via the urea cycle.2 Valproic acid metabolites, propionate and 4-en-VPA, can directly inhibit N-acetyl glutamate, which can disrupt the urea cycle, leading to elevated ammonia levels.3 Long-term or high-dose VPA can lead to carnitine deficiency, primarily by inhibiting its biosynthesis and depleting stores.4 Carnitine deficiency leads to disturbances in mitochondrial function, causing inhibition of the urea cycle and increasing ammonia. CNS toxicity due to hyperammonemia is thought to be due to activation of glutamate receptors.3
Risk factors. Co-administration of other antiepileptic drugs (AEDs) with VPA is a risk factor for VHE.1,5 This happens because enzyme-inducing AEDs such as phenytoin, phenobarbital, and carbamazepine can increase toxic metabolites of VPA, which can lead to hyperammonemia. Topiramate can also inhibit the urea cycle, leading to increased ammonia levels. Additionally, co-administration of VPA with quetiapine, paliperidone, risperidone, or aripiprazole has been reported to increase the risk of VHE.1,5 Intellectual disability, carnitine deficiency, low albumin, and abnormal liver function have also been reported to increase the risk of VHE.1,5
Continue to: Diagnosis and management
Diagnosis and management. If a patient receiving VPA is experiencing nausea, fatigue, or somnolence, it is important to check the patient’s ammonia level (normal range: 11 to 32 µmol/L) and VPA total levels (therapeutic range: 50 to 125 µg/mL). Consider checking a VPA free level, especially in geriatric patients or patients who have low albumin; the therapeutic range of VPA free is 6 to 22 µg/mL.3 If the ammonia level is elevated, discontinue VPA immediately (Table).1-3 Clinicians may also elect to prescribe lactulose until ammonia levels return to normal range. Adding levocarnitine may also help, although evidence is limited to small case series or retrospective studies.3 Currently, there is no known advantage in combining lactulose and levocarnitine to address VHE. Severe cases of VHE (ammonia levels >400 µmol/L) may require hemodialysis.1

Prevention. Strategies to prevent VHE include avoiding polypharmacy, especially concurrent use of enzyme-inducing AEDs and possibly second-generation antipsychotics. Additionally, VPA should not be used in individuals with urea cycle disorders. It is unknown if levocarnitine supplementation is preventive, but this approach has been suggested.3
CASE CONTINUED
Mrs. C has several possible risk factors for VHE, including co-administration of quetiapine and VPA, and a low albumin level. A further laboratory workup for Mrs. C reveals a VPA free level of 19 µg/mL (21.1% free), a VPA total level of 90 µg/mL, and an ammonia level of 79 µmol/L, confirming our suspicions regarding VHE. We determine that Mrs. C’s altered mental status is likely due her elevated ammonia levels, because the infection had been improving in the days leading up to the sudden, extreme somnolence.
VPA is immediately stopped and Mrs. C receives 1 dose of lactulose. The following day, Mrs. C’s mental status improves, and her ammonia levels return to normal. On hospital Day 9, she is transferred back to the psychiatric facility for management of manic and psychotic symptoms.
Related Resources
- Brown LM, Cupples N, Moore TA. Levocarnitine for valproate-induced hyperammonemia in the psychiatric setting: a case series and literature review. Ment Health Clin. 2018;8(3):148-154.
- Aires CCP, van Cruchten A, Ijlat L, et al. New insights on the mechanisms of valproate-induced hyperammonemia: inhibition of hepatic N-acetylglutamate synthase activity by valproyl-CoA. J Hepatol. 2011;55(2):426-434.
Drug Brand Names
Aripiprazole • Abilify
Carbamazepine • Tegretol
Lactulose • Enulose
Levocarnitine • Carnitine, Carnitor
Levofloxacin • Levaquin IV
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Quetiapine • Seroquel
Risperidone • Risperdal
Topiramate • Topamax
Valproic acid • Depakene
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.
1. Chopra A, Kolla BP, Mansukhani MP, et al. Valproate-induced hyperammonemic encephalopathy: an update on risk factors, clinical correlates, and management. Gen Hosp Psychiatry. 2012;34(3):290-298.
2. Kowalski PC, Dowben JS, Keltner NL. Ammonium: the deadly toxin you don’t want to miss when using mood stabilizers. Perspect Psychiatr Care. 2013;49(4):221-225.
3. Baddour E, Tewksbury A, Stauner N. Valproic acid-induced hyper ammonemia: incidence, clinical significance, and treatment management. Ment Health Clin. 2018;8(2):73-77.
4. Raskind JY, El-Chaar GM. The role of carnitine supplementation during valproic acid therapy. Ann Pharmacother. 2000;34(5):630-638. 5. Tseng YL, Huang CR, Lin CH, et al. Risk factors of hyperammonemia in patients with epilepsy. Medicine (Baltimore). 2014;93(11):e66. doi: 10.1097/MD.0000000000000066.