User login
Schizophrenia, bipolar disorder associated with increased risk of secondary TD
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
Psychiatric inpatients, particularly those with schizophrenia or bipolar disorder, have both a greater risk of having a secondary diagnosis of tardive dyskinesia and having worse illness when tardive dyskinesia is also present, according to results of a case-control study of more than 77,000 inpatients.
For the study, the investigators conducted an analysis of 77,022 adults from the Nationwide Inpatient Sample who had been admitted between January 2010 and December 2014 for mood disorders and schizophrenia; 38,382 patients in this group also had a secondary diagnosis of tardive dyskinesia (TD), reported Rikinkumar S. Patel, MD, of the department of psychiatry at Griffin Memorial Hospital in Norman, Okla., and associates. The study was published in Heliyon.
They investigators found that patients with schizophrenia and bipolar disorder were four to five times more likely to also have TD, and patients with TD were six times more likely to have severe morbidity because of a major loss of function. Compared with non-TD controls, patients with TD had a longer hospital length of stay by 6.36 days and higher cost by $20,415.
More than 60% of TD patients came from below the 50th percentile in median household income, compared with less than 40% of the non-TD group. Dr. Patel and associates also found that almost half of the patients with TD were aged 40-60 years and that the prevalence of TD in the study population increased with age.
“Our findings support the previous evidence that advanced age is a risk factor for the development of TD,” they wrote, citing research by Criscely L. Go, MD, and associates (Parkinsonism Relat Disord. 2019. 15[9]:655-9).
Dr. Patel and associates concluded that more systematic research is needed to prevent TD and “optimize inpatient outcomes in psychiatric patients with TD.”
The study authors reported having no conflicts of interest.
SOURCE: Patel RS et al. Heliyon. 2019. doi: 10.1016/j.heliyon.2019.e01745.
FROM HELIYON
Abnormal gaze processing found in patients with bipolar disorder
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
Patients with bipolar disorder show altered gaze processing on EEG recordings taken during working memory exercises, new study results suggest.
The study, led by Cristina Berchio of the department of basic neurosciences at the University of Geneva, recruited 19 euthymic patients with bipolar I or II from the Mood Disorders Unit at the University Hospital of Geneva and 19 controls matched for age, gender, education level, and handedness. While undergoing high-density EEG recording, participants performed a two-back working memory exercise that involved neutral faces with either direct or averted gazes. The study was published in NeuroImage: Clinical.
; both of those functions are thought to be impaired in patients with bipolar disorder. They suggested that this might reflect early-life dysfunctional parental-infant gaze experiences that could affect how those patients with bipolar disorder learned emotion-regulation strategies. “In this sense, our early gaze experiences might also be considered an environmental risk factor, that might remain as a vulnerability trait in [bipolar patients],” they wrote.
Limitations of the study include the working memory exercise’s design, which could have led to misleading anticipatory effects. The small sample size is another limitation that affected the ability to perform certain analyses. The surface nature of EEG also limited evaluation of deeper brain structures that might have proved salient to this exercise.
The study was supported by several entities, including the Swiss National Center of Competence in Research. The authors declared no conflicts of interest.
SOURCE: Berchio C et al. NeuroImage Clin. 2019. doi: 10.1016/j.nicl.2017.09.006.
FROM NEUROIMAGE: CLINICAL
High recurrence, shortening cycle length in bipolar disorder associated with several biomarkers
Several potential biomarkers may indicate whether patients with bipolar disorder may have high-recurrence disease with a cycle length that progressively shortens, according to Erik Smedler, MD, PhD, of the department of psychiatry and neurochemistry at Gothenburg (Sweden) University.
For the analysis, published in European Neuropsychopharmacology, A total of 745 patients had serum samples available; assays for 203 different protein biomarkers were performed on those samples.
The investigators clustered patients according to frequency – with low-frequency recurrence defined as a maximum of one inpatient episode per year – and by cycle length – with sensitized patients having progressively shorter periods between inpatient episodes. No difference in biomarkers or clinical features were seen between high- and low-frequency recurrence patients, but sensitized patients were significantly more ill and were more likely to be treated with antidepressants.
In addition, in a specific cohort of patients who were both sensitized and had a high recurrence rate (at least five inpatient episodes), four proteins were expressed at a significantly lower level than that of nonsensitized patients: tumor necrosis factor receptor-2, tumor necrosis factor receptor superfamily member 4, placenta growth factor, and adrenomedullin. Sensitization also was associated with a single nucleotide polymorphism near the calcium channel gene CACNA2D3.
“These results suggest the potential for translational research aimed at preventive actions,” the investigators wrote.
The authors reported that they had no conflicts of interest.
SOURCE: Smedler E et al. Eur Neuropsychopharmacol. 2019 Aug 1. doi: 10.1016/j.euroneuro.2019.07.132.
Several potential biomarkers may indicate whether patients with bipolar disorder may have high-recurrence disease with a cycle length that progressively shortens, according to Erik Smedler, MD, PhD, of the department of psychiatry and neurochemistry at Gothenburg (Sweden) University.
For the analysis, published in European Neuropsychopharmacology, A total of 745 patients had serum samples available; assays for 203 different protein biomarkers were performed on those samples.
The investigators clustered patients according to frequency – with low-frequency recurrence defined as a maximum of one inpatient episode per year – and by cycle length – with sensitized patients having progressively shorter periods between inpatient episodes. No difference in biomarkers or clinical features were seen between high- and low-frequency recurrence patients, but sensitized patients were significantly more ill and were more likely to be treated with antidepressants.
In addition, in a specific cohort of patients who were both sensitized and had a high recurrence rate (at least five inpatient episodes), four proteins were expressed at a significantly lower level than that of nonsensitized patients: tumor necrosis factor receptor-2, tumor necrosis factor receptor superfamily member 4, placenta growth factor, and adrenomedullin. Sensitization also was associated with a single nucleotide polymorphism near the calcium channel gene CACNA2D3.
“These results suggest the potential for translational research aimed at preventive actions,” the investigators wrote.
The authors reported that they had no conflicts of interest.
SOURCE: Smedler E et al. Eur Neuropsychopharmacol. 2019 Aug 1. doi: 10.1016/j.euroneuro.2019.07.132.
Several potential biomarkers may indicate whether patients with bipolar disorder may have high-recurrence disease with a cycle length that progressively shortens, according to Erik Smedler, MD, PhD, of the department of psychiatry and neurochemistry at Gothenburg (Sweden) University.
For the analysis, published in European Neuropsychopharmacology, A total of 745 patients had serum samples available; assays for 203 different protein biomarkers were performed on those samples.
The investigators clustered patients according to frequency – with low-frequency recurrence defined as a maximum of one inpatient episode per year – and by cycle length – with sensitized patients having progressively shorter periods between inpatient episodes. No difference in biomarkers or clinical features were seen between high- and low-frequency recurrence patients, but sensitized patients were significantly more ill and were more likely to be treated with antidepressants.
In addition, in a specific cohort of patients who were both sensitized and had a high recurrence rate (at least five inpatient episodes), four proteins were expressed at a significantly lower level than that of nonsensitized patients: tumor necrosis factor receptor-2, tumor necrosis factor receptor superfamily member 4, placenta growth factor, and adrenomedullin. Sensitization also was associated with a single nucleotide polymorphism near the calcium channel gene CACNA2D3.
“These results suggest the potential for translational research aimed at preventive actions,” the investigators wrote.
The authors reported that they had no conflicts of interest.
SOURCE: Smedler E et al. Eur Neuropsychopharmacol. 2019 Aug 1. doi: 10.1016/j.euroneuro.2019.07.132.
FROM EUROPEAN NEUROPSYCHOPHARMACOLOGY
Higher risk of bipolar disorder, depression, anxiety found with autism
Individuals with autism spectrum disorder might be at significantly higher risk of bipolar disorder, anxiety, and depression, a new study suggests.
and associates. The report was published in JAMA Pediatrics.
Dr. Kirsch and associates reported the outcomes of a population-based cohort study involving 1,014 individuals with autism spectrum disorder and 2,028 age-and sex-matched controls without autism spectrum disorder. They found that individuals with autism spectrum disorder were more than nine times more likely to be diagnosed with bipolar disorder, 2.81 times more likely to be diagnosed with depression, and 3.45 times more likely to be diagnosed with anxiety, compared with controls.
“Significant psychosocial sequelae associated with having ASD, including difficulties developing and maintaining relationships, challenges succeeding academically and vocationally, and behaviors that can be problematic to manage, particularly increase risk for mood and anxiety symptoms in individuals with ASD,” wrote Dr. Kirsch of the department of psychiatry and psychology at the Mayo Clinic, Rochester, Minn., and associates. “Individuals with ASD also experience greater rates of other mental health challenges, including attention-deficit/hyperactivity disorder and substance abuse.”
Individuals with autism spectrum disorder who received a diagnosis of depression, anxiety, or bipolar disorder also were more likely to be diagnosed at a younger age than were those without autism. In the case of depression, the median age of diagnosis was 15.7 years, compared with 18.1 years among controls. For anxiety, the median age of diagnosis among individuals with autism spectrum disorder was 15.2 years, compared with 20.3 years for controls. For bipolar disorder, it was 20.3 years, compared with 27 years although the small number of individuals meant this was not statistically significant.
The authors suggested that the earlier age at diagnosis might reflect that individuals with autism spectrum disorder generally are monitored more closely, and are more likely to be connected to screening and diagnostic resources because of their original diagnosis.
The researchers also found that the increased risk of depression and anxiety was even higher among men with autism spectrum disorder, even though the cumulative incidence of these conditions was greater in women both with and without autism. In addition, the researchers noted that individuals with autism spectrum disorder were more likely to be diagnosed with multiple psychiatric conditions than were those without autism.
Dr. Kirsch and associates cited several limitations. One is that the population studied came from Olmsted County, Minn., which is wealthier and less diverse than the general population. Nevertheless, the results could help guide treatments for patients with ASD.
“Given the high rates of comorbidity, researchers and practitioners should develop tools that are specific to the unique needs of this population and effective medications and treatments for mood and anxiety concerns, which remain limited in this population,” they wrote.
The study was funded by grants from the National Institutes of Health and the U.S. Public Health Service. No conflicts of interest were disclosed.
SOURCE: Kirsch A et al. JAMA Pediatr. 2019 Dec 2. doi: 10.1001/jamapediatrics.2019.4368.
Individuals with autism spectrum disorder might be at significantly higher risk of bipolar disorder, anxiety, and depression, a new study suggests.
and associates. The report was published in JAMA Pediatrics.
Dr. Kirsch and associates reported the outcomes of a population-based cohort study involving 1,014 individuals with autism spectrum disorder and 2,028 age-and sex-matched controls without autism spectrum disorder. They found that individuals with autism spectrum disorder were more than nine times more likely to be diagnosed with bipolar disorder, 2.81 times more likely to be diagnosed with depression, and 3.45 times more likely to be diagnosed with anxiety, compared with controls.
“Significant psychosocial sequelae associated with having ASD, including difficulties developing and maintaining relationships, challenges succeeding academically and vocationally, and behaviors that can be problematic to manage, particularly increase risk for mood and anxiety symptoms in individuals with ASD,” wrote Dr. Kirsch of the department of psychiatry and psychology at the Mayo Clinic, Rochester, Minn., and associates. “Individuals with ASD also experience greater rates of other mental health challenges, including attention-deficit/hyperactivity disorder and substance abuse.”
Individuals with autism spectrum disorder who received a diagnosis of depression, anxiety, or bipolar disorder also were more likely to be diagnosed at a younger age than were those without autism. In the case of depression, the median age of diagnosis was 15.7 years, compared with 18.1 years among controls. For anxiety, the median age of diagnosis among individuals with autism spectrum disorder was 15.2 years, compared with 20.3 years for controls. For bipolar disorder, it was 20.3 years, compared with 27 years although the small number of individuals meant this was not statistically significant.
The authors suggested that the earlier age at diagnosis might reflect that individuals with autism spectrum disorder generally are monitored more closely, and are more likely to be connected to screening and diagnostic resources because of their original diagnosis.
The researchers also found that the increased risk of depression and anxiety was even higher among men with autism spectrum disorder, even though the cumulative incidence of these conditions was greater in women both with and without autism. In addition, the researchers noted that individuals with autism spectrum disorder were more likely to be diagnosed with multiple psychiatric conditions than were those without autism.
Dr. Kirsch and associates cited several limitations. One is that the population studied came from Olmsted County, Minn., which is wealthier and less diverse than the general population. Nevertheless, the results could help guide treatments for patients with ASD.
“Given the high rates of comorbidity, researchers and practitioners should develop tools that are specific to the unique needs of this population and effective medications and treatments for mood and anxiety concerns, which remain limited in this population,” they wrote.
The study was funded by grants from the National Institutes of Health and the U.S. Public Health Service. No conflicts of interest were disclosed.
SOURCE: Kirsch A et al. JAMA Pediatr. 2019 Dec 2. doi: 10.1001/jamapediatrics.2019.4368.
Individuals with autism spectrum disorder might be at significantly higher risk of bipolar disorder, anxiety, and depression, a new study suggests.
and associates. The report was published in JAMA Pediatrics.
Dr. Kirsch and associates reported the outcomes of a population-based cohort study involving 1,014 individuals with autism spectrum disorder and 2,028 age-and sex-matched controls without autism spectrum disorder. They found that individuals with autism spectrum disorder were more than nine times more likely to be diagnosed with bipolar disorder, 2.81 times more likely to be diagnosed with depression, and 3.45 times more likely to be diagnosed with anxiety, compared with controls.
“Significant psychosocial sequelae associated with having ASD, including difficulties developing and maintaining relationships, challenges succeeding academically and vocationally, and behaviors that can be problematic to manage, particularly increase risk for mood and anxiety symptoms in individuals with ASD,” wrote Dr. Kirsch of the department of psychiatry and psychology at the Mayo Clinic, Rochester, Minn., and associates. “Individuals with ASD also experience greater rates of other mental health challenges, including attention-deficit/hyperactivity disorder and substance abuse.”
Individuals with autism spectrum disorder who received a diagnosis of depression, anxiety, or bipolar disorder also were more likely to be diagnosed at a younger age than were those without autism. In the case of depression, the median age of diagnosis was 15.7 years, compared with 18.1 years among controls. For anxiety, the median age of diagnosis among individuals with autism spectrum disorder was 15.2 years, compared with 20.3 years for controls. For bipolar disorder, it was 20.3 years, compared with 27 years although the small number of individuals meant this was not statistically significant.
The authors suggested that the earlier age at diagnosis might reflect that individuals with autism spectrum disorder generally are monitored more closely, and are more likely to be connected to screening and diagnostic resources because of their original diagnosis.
The researchers also found that the increased risk of depression and anxiety was even higher among men with autism spectrum disorder, even though the cumulative incidence of these conditions was greater in women both with and without autism. In addition, the researchers noted that individuals with autism spectrum disorder were more likely to be diagnosed with multiple psychiatric conditions than were those without autism.
Dr. Kirsch and associates cited several limitations. One is that the population studied came from Olmsted County, Minn., which is wealthier and less diverse than the general population. Nevertheless, the results could help guide treatments for patients with ASD.
“Given the high rates of comorbidity, researchers and practitioners should develop tools that are specific to the unique needs of this population and effective medications and treatments for mood and anxiety concerns, which remain limited in this population,” they wrote.
The study was funded by grants from the National Institutes of Health and the U.S. Public Health Service. No conflicts of interest were disclosed.
SOURCE: Kirsch A et al. JAMA Pediatr. 2019 Dec 2. doi: 10.1001/jamapediatrics.2019.4368.
FROM JAMA PEDIATRICS
Alkermes submits NDA for new schizophrenia, bipolar I treatment
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Alkermes has announced that it has submitted a New Drug Application to the Food and Drug Administration for the approval of ALKS 3831 (olanzapine/samidorphan) for the treatment of schizophrenia and bipolar I disorder.
Included in the application for the investigational, novel, once-daily, oral atypical antipsychotic drug candidate is data from the ENLIGHTEN-1 study, which evaluated the antipsychotic efficacy of ALKS 3831, compared with a placebo, over a 4-week period, as well as data from ENLIGHTEN-2, which compared weight gain with ALKS 3831 and olanzapine alone over a 6-month period.
“Antipsychotic medications are an important part of the treatment paradigm for both schizophrenia and bipolar I disorder, yet there remains a persistent unmet need for new treatments,” Craig Hopkinson, MD, chief medical officer and senior vice president of medicines development and medical affairs at Alkermes, said in a press release.
As a combination of olanzapine and samidorphan, Samidorphan, an opioid receptor antagonist, is structurally related to naltrexone.
Alkermes is seeking an indication for the treatment of schizophrenia and an indication for the treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy or as an adjunct to lithium or valproate, as well as for maintenance treatment of bipolar I. Dosage strength would be 10 mg of samidorphan with 5, 10, 15, or 20 mg of olanzapine.
Find the full press release on the Alkermes website.
Blunted ventral striatal responses found in remitted bipolar I
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
People with remitted bipolar I disorder appear to show lower rather than higher activity in the ventral striatum in anticipation of monetary gains, compared with controls, results of a small study suggest. In addition, associations were found between positive urgency measure (PUM) and blunted activity in the nucleus accumbens in individuals with bipolar I.
“Together, these findings suggest that individuals with remitted bipolar disorder show blunted ventral striatal responses during anticipation of conventional rewards (e.g., money),” wrote lead author Sheri L. Johnson, PhD, and associates. The study was published in Neuroimage: Clinical.
Dr. Johnson’s team recruited the participants through advertisements online and in the community. Their study included 24 people with bipolar I (14 men; mean age, 36.22 years) and 24 controls without bipolar (13 men; mean age, 33.92 years), reported Dr. Johnson, of the University of California, Berkeley, and associates. The investigators used a version of the Monetary Incentive Delay Task to elicit neural and behavioral responses and their outcomes during fMRI.
Whole brain analyses found that, compared with the control group, those in the bipolar group “showed less activity for the gain anticipation contrast specifically in the ventral striatum (including the right [nucleus accumbens] and right globus pallidus), as well as less activity for the gain outcome contrast in the left occipital gyrus,” the investigators wrote.
PUM, a dimensional trait, was designed to assess tendencies to act “regrettably” or “impulsively” during states of positive emotion. PUM scores have been correlated with outcomes such as lower functioning in behavior, decreased quality of life, and worse outcomes in bipolar disorder. The investigators hoped that, by including PUM to help evaluate group differences, they could account for inconsistent findings regarding associations of neural imaging and reward responses.
“The current findings imply that, in individuals with bipolar disorder, individual differences in PUM may also help account for blunted [nucleus accumbens] during reward anticipation.”
The study’s sample size limited the power to detect small effects or interactions. The researchers felt, though, that the sample was well matched and adequately powered for the primary variables of interest.
The authors had no conflicts of interest to disclose. The study was funded by a grant from the National Institute of Mental Health.
SOURCE: Johnson SL et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2019.102018.
FROM NEUROIMAGE: CLINICAL
Disentangling sleep problems and bipolar disorder
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
COPENHAGEN – Sleep spindle density is diminished in euthymic patients with bipolar disorder, suggesting that this sleep architecture abnormality might offer potential for early differentiation of bipolar from unipolar depression, Philipp S. Ritter, MD, said at the annual congress of the European College of Neuropsychopharmacology.
“Hopefully in the future our finding, if replicated, might have clinical utility. It might be a kind of soft biomarker that could be used in early detection, or, in people having their first depressive episode, you could perhaps use this to risk-stratify. And if you see there’s a great reduction in spindle density then a patient might have a higher likelihood of a bipolar disorder, so you might not want to treat with antidepressants that have a high switch rate,” explained Dr. Ritter, a psychiatrist at Technical University of Dresden (Germany).
Sleep spindles are a specific sleep architecture formation evident on the sleep EEG. They are sudden high-amplitude bursts occurring in stage N2 sleep. They are thought to be associated with sensory gating and memory processes. Other investigators have repeatedly demonstrated that patients with schizophrenia, as well as their asymptomatic first-degree relatives, have a reduced density of fast spindles greater than 13 Hz, compared with the general population. In contrast, patients with unipolar depression do not display this polysomnographic abnormality.
These findings prompted Dr. Ritter and his coinvestigators to conduct an all-night polysomnographic study in 24 euthymic patients with bipolar disorder and 25 healthy controls. The bipolar patients demonstrated a reduced density and mean frequency of fast sleep spindles, but not slow spindles (Acta Psychiatr Scand. 2018 Aug;138[2]:163-72).
These sleep spindle findings implicate thalamic dysfunction as a potential neurobiologic mechanism in bipolar disorder, since spindles are generated in the thalamus and spun off in thalamocortical feedback loops, Dr. Ritter observed.
Which came first: the chicken (bipolar disorder) or the egg (sleep disturbance)?
Sleep problems are a prominent issue in patients with bipolar disorder, even when they are euthymic.
“Anybody who deals with bipolar patients knows that sleep is a constant issue. You are always talking to your patients about their sleep. They’re sleeping too much, or not enough, or they’re sleeping just about right but it’s unsatisfactory. They do not sleep well. And if there’s something that disrupts their sleep, it can precipitate episodes,” Dr. Ritter said.
He wondered whether sleep problems are an intrinsic part of the bipolar illness, or a byproduct of the stress of having a severe mental disorder, perhaps a medication side effect, or whether the disordered sleep actually precedes the clinical expression of the mood disorder. So he and his coinvestigators turned to a Munich-based cohort sample of 3,021 adolescents and young adults assessed via the standardized Composite International Diagnostic Interview four times during 10 years of prospective follow-up.
Among 1,943 participants in the epidemiologic study who were free of major mental disorders at entry, the presence of sleep disturbance at baseline as quantified using the Symptom Checklist-90-Revised doubled the risk of developing bipolar disorder within the next 10 years. After the researchers controlled for potential confounders, including parental mood disorder, gender, age, and a history of alcohol or cannabis dependence, poor sleep quality at baseline remained independently associated with a 1.75-fold increased chance of subsequently developing bipolar disorder (J Psychiatr Res. 2015 Sep;68:76-82).
“This is a little bit higher, actually, than the odds ratio usually found for depressive disorders,” said Dr. Ritter.
he added.
Dr. Ritter reported having no financial conflicts regarding these studies.
REPORTING FROM ECNP 2019
Blood-brain barrier imaging could predict disease progression in bipolar
Blood-brain barrier imaging can serve as a biomarker for progression of disease in adults with bipolar disorder, results from a small study suggest.
“While the pathophysiology of bipolar disorder remains poorly understood, converging evidence points to the presence of neuroinflammation in bipolar patients,” wrote Lyna Kamintsky, a PhD candidate at Dalhousie University, Halifax, N.S., and colleagues.
The researchers examined MRI data from 36 patients with bipolar disorder and compared them with 14 matched controls. The average age of the patients was 49 years and the average duration of illness was 28 years. The study was published in NeuroImage: Clinical (2019 Oct 22. doi: 10.1016/j.nicl.2019.102049).
“Leakage rates were considered pathological when exceeding 0.02, the 95th percentile of all values in a cohort of control subjects,” the researchers said. Overall, 10 subjects (all patients with bipolar disorder) met criteria for “extensive blood-brain barrier leakage.” The researchers found that those patients also had higher rates of chronic illness, more frequent and/or severe manic episodes, and more severe anxiety, depression, and social/occupational dysfunction, compared with those without blood-brain barrier leakage.
The patients with extensive blood-brain barrier leakage also had higher body mass indexes, greater risk of cardiovascular disease, and advanced heart age. In addition, all patients in this group had comorbid insulin resistance.
The study findings were limited by the small sample size, but , the researchers said.
The study was supported by the European Union’s Seventh Framework Program, the Nova Scotia Health Research Foundation, Brain Canada, and the Brain & Behavior Research Foundation. The researchers disclosed having no financial conflicts.
SOURCE: Kamintsky L et al. NeuroImage: Clinical. 2019 Oct 22. doi: 10.1016/j.nicl.2019.102049.
Blood-brain barrier imaging can serve as a biomarker for progression of disease in adults with bipolar disorder, results from a small study suggest.
“While the pathophysiology of bipolar disorder remains poorly understood, converging evidence points to the presence of neuroinflammation in bipolar patients,” wrote Lyna Kamintsky, a PhD candidate at Dalhousie University, Halifax, N.S., and colleagues.
The researchers examined MRI data from 36 patients with bipolar disorder and compared them with 14 matched controls. The average age of the patients was 49 years and the average duration of illness was 28 years. The study was published in NeuroImage: Clinical (2019 Oct 22. doi: 10.1016/j.nicl.2019.102049).
“Leakage rates were considered pathological when exceeding 0.02, the 95th percentile of all values in a cohort of control subjects,” the researchers said. Overall, 10 subjects (all patients with bipolar disorder) met criteria for “extensive blood-brain barrier leakage.” The researchers found that those patients also had higher rates of chronic illness, more frequent and/or severe manic episodes, and more severe anxiety, depression, and social/occupational dysfunction, compared with those without blood-brain barrier leakage.
The patients with extensive blood-brain barrier leakage also had higher body mass indexes, greater risk of cardiovascular disease, and advanced heart age. In addition, all patients in this group had comorbid insulin resistance.
The study findings were limited by the small sample size, but , the researchers said.
The study was supported by the European Union’s Seventh Framework Program, the Nova Scotia Health Research Foundation, Brain Canada, and the Brain & Behavior Research Foundation. The researchers disclosed having no financial conflicts.
SOURCE: Kamintsky L et al. NeuroImage: Clinical. 2019 Oct 22. doi: 10.1016/j.nicl.2019.102049.
Blood-brain barrier imaging can serve as a biomarker for progression of disease in adults with bipolar disorder, results from a small study suggest.
“While the pathophysiology of bipolar disorder remains poorly understood, converging evidence points to the presence of neuroinflammation in bipolar patients,” wrote Lyna Kamintsky, a PhD candidate at Dalhousie University, Halifax, N.S., and colleagues.
The researchers examined MRI data from 36 patients with bipolar disorder and compared them with 14 matched controls. The average age of the patients was 49 years and the average duration of illness was 28 years. The study was published in NeuroImage: Clinical (2019 Oct 22. doi: 10.1016/j.nicl.2019.102049).
“Leakage rates were considered pathological when exceeding 0.02, the 95th percentile of all values in a cohort of control subjects,” the researchers said. Overall, 10 subjects (all patients with bipolar disorder) met criteria for “extensive blood-brain barrier leakage.” The researchers found that those patients also had higher rates of chronic illness, more frequent and/or severe manic episodes, and more severe anxiety, depression, and social/occupational dysfunction, compared with those without blood-brain barrier leakage.
The patients with extensive blood-brain barrier leakage also had higher body mass indexes, greater risk of cardiovascular disease, and advanced heart age. In addition, all patients in this group had comorbid insulin resistance.
The study findings were limited by the small sample size, but , the researchers said.
The study was supported by the European Union’s Seventh Framework Program, the Nova Scotia Health Research Foundation, Brain Canada, and the Brain & Behavior Research Foundation. The researchers disclosed having no financial conflicts.
SOURCE: Kamintsky L et al. NeuroImage: Clinical. 2019 Oct 22. doi: 10.1016/j.nicl.2019.102049.
FROM NEUROIMAGE: CLINICAL
Emotional processing of scenes in bipolar I appears intact
New findings contradict previous studies on processing of faces in bipolar
Differences in self-reported and EEG-measured responses to emotional scenes between patients with bipolar I disorder with and without a history of psychosis, and healthy controls are negligible, results of a cross-sectional study suggest.
“While prior research supports abnormalities in the emotional face response, this study suggests these neural and behavior differences do not fully generalize to scenes, indicating that nonsocial emotional responding may be intact in these patients,” reported Rebekah L. Trotti and colleagues.
The investigators conducted a multisite study among 130 participants with bipolar and a history of psychosis, 75 with bipolar and no history of psychosis, and 181 healthy controls. Although the investigators had hypothesized that, in keeping with findings from face-processing studies, emotional responses would be reduced in patients with bipolar I disorder, The groups were presented with the same 60 scenes that were unpleasant, neutral, or pleasant. The study was published in the Journal of Psychiatric Research.
Participants rated each scene according to the Self-Assessment Manikin after the respective EEG readings were taken. No significant statistical differences were seen on these ratings between groups, reported Ms. Trotti, a graduate student in the behavioral and brain sciences program at the University of Georgia, Athens, and colleagues.
The investigators also assessed whether participants had psychosis and looked at medications they were taking. However, those analyses also showed no statistically significant differences between participants with bipolar I and a history of psychosis, those with bipolar and no history of psychosis, and healthy controls in the processing of emotional scenes. Ms. Trotti and colleagues noted that other ways of differentiating subtypes in this heterogeneous disorder, such as those based on biomarkers and brain structure rather than those laid out by the DSM, might yield the differences in neural activity they had expected.
“Future research on this topic should focus on neurocognitive subtypes of mood and psychotic disorders, as well as other domains of emotional responding and behavior,” Ms. Trotti and colleagues wrote.
SOURCE: Trotti RL et al. J Psychiatr Res. 2019. doi: 10.1016/j.jpsychires.2019.10.005.
New findings contradict previous studies on processing of faces in bipolar
New findings contradict previous studies on processing of faces in bipolar
Differences in self-reported and EEG-measured responses to emotional scenes between patients with bipolar I disorder with and without a history of psychosis, and healthy controls are negligible, results of a cross-sectional study suggest.
“While prior research supports abnormalities in the emotional face response, this study suggests these neural and behavior differences do not fully generalize to scenes, indicating that nonsocial emotional responding may be intact in these patients,” reported Rebekah L. Trotti and colleagues.
The investigators conducted a multisite study among 130 participants with bipolar and a history of psychosis, 75 with bipolar and no history of psychosis, and 181 healthy controls. Although the investigators had hypothesized that, in keeping with findings from face-processing studies, emotional responses would be reduced in patients with bipolar I disorder, The groups were presented with the same 60 scenes that were unpleasant, neutral, or pleasant. The study was published in the Journal of Psychiatric Research.
Participants rated each scene according to the Self-Assessment Manikin after the respective EEG readings were taken. No significant statistical differences were seen on these ratings between groups, reported Ms. Trotti, a graduate student in the behavioral and brain sciences program at the University of Georgia, Athens, and colleagues.
The investigators also assessed whether participants had psychosis and looked at medications they were taking. However, those analyses also showed no statistically significant differences between participants with bipolar I and a history of psychosis, those with bipolar and no history of psychosis, and healthy controls in the processing of emotional scenes. Ms. Trotti and colleagues noted that other ways of differentiating subtypes in this heterogeneous disorder, such as those based on biomarkers and brain structure rather than those laid out by the DSM, might yield the differences in neural activity they had expected.
“Future research on this topic should focus on neurocognitive subtypes of mood and psychotic disorders, as well as other domains of emotional responding and behavior,” Ms. Trotti and colleagues wrote.
SOURCE: Trotti RL et al. J Psychiatr Res. 2019. doi: 10.1016/j.jpsychires.2019.10.005.
Differences in self-reported and EEG-measured responses to emotional scenes between patients with bipolar I disorder with and without a history of psychosis, and healthy controls are negligible, results of a cross-sectional study suggest.
“While prior research supports abnormalities in the emotional face response, this study suggests these neural and behavior differences do not fully generalize to scenes, indicating that nonsocial emotional responding may be intact in these patients,” reported Rebekah L. Trotti and colleagues.
The investigators conducted a multisite study among 130 participants with bipolar and a history of psychosis, 75 with bipolar and no history of psychosis, and 181 healthy controls. Although the investigators had hypothesized that, in keeping with findings from face-processing studies, emotional responses would be reduced in patients with bipolar I disorder, The groups were presented with the same 60 scenes that were unpleasant, neutral, or pleasant. The study was published in the Journal of Psychiatric Research.
Participants rated each scene according to the Self-Assessment Manikin after the respective EEG readings were taken. No significant statistical differences were seen on these ratings between groups, reported Ms. Trotti, a graduate student in the behavioral and brain sciences program at the University of Georgia, Athens, and colleagues.
The investigators also assessed whether participants had psychosis and looked at medications they were taking. However, those analyses also showed no statistically significant differences between participants with bipolar I and a history of psychosis, those with bipolar and no history of psychosis, and healthy controls in the processing of emotional scenes. Ms. Trotti and colleagues noted that other ways of differentiating subtypes in this heterogeneous disorder, such as those based on biomarkers and brain structure rather than those laid out by the DSM, might yield the differences in neural activity they had expected.
“Future research on this topic should focus on neurocognitive subtypes of mood and psychotic disorders, as well as other domains of emotional responding and behavior,” Ms. Trotti and colleagues wrote.
SOURCE: Trotti RL et al. J Psychiatr Res. 2019. doi: 10.1016/j.jpsychires.2019.10.005.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
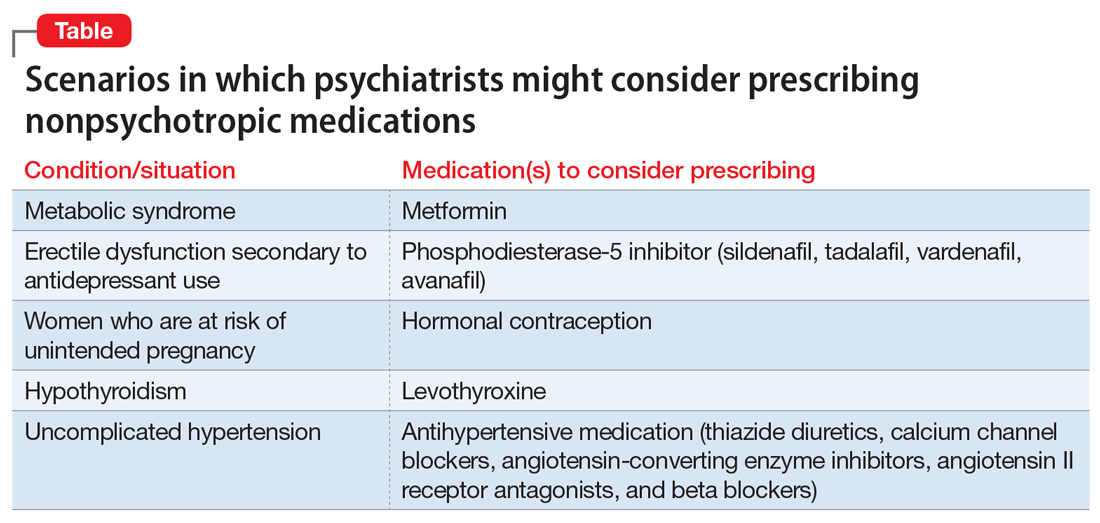
Should psychiatrists prescribe nonpsychotropic medications?
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.
We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
In our experience, most psychiatrists are uncomfortable with prescribing a medication when they feel that doing so would be outside their scope of practice. But there are many situations when prescribing a nonpsychotropic medication would be the correct choice. In this article, we discuss the scope of psychiatric practice, and present 4 case studies that illustrate situations in which psychiatrists should feel comfortable prescribing nonpsychotropic medications.
Defining the scope of practice
What is the scope of a psychiatrist’s practice? Scope of practice usually describes activities that a health care practitioner is allowed to undertake as defined by the terms of his/her license. A license to practice medicine does not include any stipulation restricting practice to a specific medical specialty. However, a local entity may delineate scope of practice within its organization. For instance, local practice standards held by the Detroit Wayne Mental Health Authority (DWMHA) state “Psychiatrists…shall not exceed their scope of practice as per DWMHA credentialing and privileging. For example, a Psychiatrist…who [has] not been appropriately privileged to deliver services to children shall not treat children, excepting crisis situations.”1
Like physicians in other specialties, psychiatrists are not limited to prescribing only a subset of medications commonly associated with their specialty. But for many psychiatrists, prescribing nonpsychotropic medications is complicated by individual and local factors. On one hand, some psychiatrists do not feel it is their role to prescribe nonpsychotropic medications,2 or even some psychotropic medications that may be more complex to prescribe, such as lithium, clozapine, or monoamine oxidase inhibitors.3-5 However, many feel comfortable prescribing complex combinations of psychotropic medications, or prescribing in a way that does not necessarily make sense (eg, prescribing benztropine as prophylaxis for dystonia when starting an antipsychotic).
Reviewing an average day at one urban psychiatric clinic, these questions seem to come up in half of the patient population, especially in patients with chronic mental illness, multiple medical comorbidities, and limited access to health care. When a young patient walks in without an appointment with an acute dystonic reaction secondary to the initiation of antipsychotics a couple of days ago, there is no hesitation to swiftly and appropriately prescribe an IM anticholinergic medication. But why are psychiatrists often hesitant to prescribe nonpsychotropic medications to treat other adverse effects of medications? Lack of knowledge? Lack of training?
Psychiatrists who practice in hospital systems often have immediate access to consultants, and this availability may encourage them to defer to the consultant for treatment of certain adverse effects. We have seen psychiatrists consult Neurology regarding the prescription of donepezil for mild neurocognitive disorder due to Alzheimer’s disease, or Endocrinology regarding prescription of levothyroxine for lithium-induced hypothyroidism.
However, there are numerous scenarios in which psychiatrists should feel comfortable prescribing nonpsychotropic medications or managing medication adverse effects, regardless of whether they consider it to be within or outside their scope of practice. The following case examples illustrate several such situations.
CASE 1
Ms. W, age 30, has been diagnosed with schizophrenia. She requests a refill of quetiapine, 800 mg/d. This medication has been clearly beneficial in alleviating her psychotic symptoms. However, since her last visit 3 months ago, her face appears more round, and she has gained 9 kg. Further evaluation indicates that she has developed metabolic syndrome and pre-diabetes.
Continue to: Metabolic adverse effects
Metabolic adverse effects, such as metabolic syndrome, diabetic ketoacidosis, and cardiovascular disease, are well-known risks of prescribing second-generation antipsychotics.6 In such situations, psychiatrists often advise patients to modify their diet, increase physical activity, and follow up with their primary care physician to determine if other medications are needed. However, getting a patient with a serious mental illness to exercise and modify her/his diet is difficult, and many of these patients do not have a primary care physician.
For patients such as Ms. W, a psychiatrist should consider prescribing metformin. Wu et al7 found that in addition to lifestyle modifications, metformin had the greatest effect on antipsychotic-induced weight gain. In this study, metformin alone had more impact on reversing weight gain and increasing insulin sensitivity than lifestyle modifications alone.7 This is crucial because these patients are especially vulnerable to cardiac disease.8 Metformin is well tolerated and has a low risk of causing hypoglycemia. Concerns regarding lactic acidosis have abated to the extent that the estimated glomerular filtration rate (eGFR) limits for using metformin have been lowered significantly. After reviewing the contraindications, the only knowledge needed to prescribe metformin is the patient’s kidney function and a brief understanding of the titration needed to minimize gastrointestinal adverse effects.9 Thus, prescribing metformin would be a fairly logical and easy first step for managing metabolic syndrome, especially in a patient whose motivation for increasing physical activity and modifying his/her diet is doubtful.
CASE 2
Mr. B, age 45, has major depressive disorder that has been well-controlled on paroxetine, 40 mg/d, for the past 2 years. He has no history of physical illness. On his most recent visit, he appears uncomfortable and nervous. After a long discussion, he discloses that his sex life isn’t what it used to be since starting paroxetine. He is bothered by erectile problems and asks whether he can “get some Viagra.”
Sexual adverse effects, such as erectile dysfunction, are frequently associated with the use of selective serotonin reuptake inhibitors.10 Although managing these adverse effects requires careful evaluation, in most cases, psychiatrists should be able to treat them.10 The logical choice in this case would be to prescribe one of the 4 FDA-approved phosphodiesterase-5 inhibitors (sildenafil [Viagra], tadalafil [Cialis], vardenafil [Levitra], and avanafil [Stendra]. However, Balon et al11 found that few psychiatrists prescribe phosphodiesterase-5 inhibitors, although they believed that they should be prescribing to treat their patients’ sexual dysfunction. Managing these adverse effects is important not only for the patient’s quality of life and relationship with his/her partner, but also for the therapeutic alliance. In a systematic review of 23 trials, Taylor et al12 examined >1,800 patients who were prescribed a medication to address sexual dysfunction secondary to antidepressants. They found that for men, adding a phosphodiesterase-5 inhibitor was appropriate and effective, and for women, adding bupropion at high doses should be considered.12 Like many other adverse effects, sexual adverse effects surely play a role in medication compliance. Dording et al13 found that the addition of sildenafil, 50 to 100 mg as needed, resulted in increased treatment satisfaction and overall contentment in 102 patients who complained of sexual dysfunction in the follow-up phase of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) antidepressant trials. In most cases, with proper psychoeducation, prescription of
CASE 3
Ms. G, age 22, was recently discharged from an inpatient psychiatric unit after an episode of mania. She was prescribed carbamazepine, 600 mg/d, and ziprasidone, 40 mg twice a day, and appears to be doing well on this regimen. When asked about what led to her admission, she recalls having an elevated mood, increased energy, hypersexuality, impulsivity, and poor judgment. She reveals that she had several sexual partners during her manic episode, and worries that if such behavior occurs again, she may get pregnant. Yet Ms. G was not prescribed birth control upon discharge.
Continue to: Contraception
Contraception. We believe that psychiatrists have an obligation to protect patients from consequences of mental illness. Much the same way that psychiatrists hope to prevent suicide in a patient who has depression, patients should be protected from risks encountered in the manic phase of bipolar disorder. Another reason to prescribe contraceptives in such patients is the teratogenic effects of mood stabilizers. Pagano et al14 reviewed 6 studies that examined common forms of hormonal birth control to determine their safety in patients with depression or bipolar disorder. They found that overall, use of hormonal contraception was not associated with a worse clinical course of disease.
Many available forms of birth control are available. When prescribing in an outpatient setting, a daily oral medication or a monthly depot injection are convenient options.
CASE 4
Mr. P, age 65, has bipolar I disorder and is stable on risperidone long-acting injection, 37.7 mg bimonthly, and lithium, 1,200 mg/d. He reports that he is doing well but has noticed a recent decrease in energy and weight gain without any change in mood. Laboratory testing conducted prior to this visit revealed a thyroid-stimulating hormone (TSH) level of 4 mU/L (normal range: 0.4 to 4.0 mU/L). Six months ago, Mr. P’s TSH level was 2.8 mU/L. The resident supervisor suggests discussing the case with an endocrinologist.
Thyroid function. The impact of lithium on the thyroid gland is well established; however, psychiatrists’ response to such changes are not.15 Gitlin16 reviewed the many adverse effects of lithium and presented various management strategies to address findings such as Mr. P’s. Two important points are that lithium should not be discontinued in light of hypothyroidism, and synthetic thyroxine (levothyroxine) can be initiated and titrated to return TSH levels to a normal range.16 Levothyroxine can be started at low doses (eg, 25 to 50 mcg/d) and increased every 6 weeks until a normal TSH level is achieved.17 Managing lithium-induced clinical or subclinical hypothyroidism can prevent further pathology and possible relapse to depression.
Incorporating integrated care
In all these cases, the prescription of a medication with which some psychiatrists are not comfortable prescribing would have been the logical, easiest, and preferable choice. Of course, when initiating any medication, boxed warnings, contraindications, and drug–drug interactions should be reviewed. Initial dosages and titration schedules can be found in every medication’s FDA-approved prescribing information document (package insert), as well as in numerous reference books and articles.
Continue to: We acknowledge...
We acknowledge that prescribing a nonpsychotropic medication is not always a psychiatrist’s best choice, and that in patients with multiple medical comorbidities and drug–drug interactions that are not clearly defined, referring to or consulting a specialist is appropriate. We in no way support reckless prescribing, but instead present an opportunity to expand the perception of what should be considered within a psychiatrist’s scope of practice, and call for further education of psychiatrists so that they are more comfortable managing these adverse effects and/or prescribing at least some nonpsychotropic medications.

We exhort integrated medical care during this time of a physician shortage; however, we do not practice this way. Interestingly, physicians in primary care, such as those in family medicine or obstetrics and gynecology, frequently attempt to treat patients with psychiatric conditions in an attempt to provide integrated care. Numerous articles have discussed these efforts.18-20 However, this type of integrated care seems less frequent in psychiatry, even though the practice of modern psychiatry in the United States shows substantial overlap with the practice of physicians in primary care specialties.21 There are few articles or practical guidelines for psychiatrists who wish to treat patients’ physical illnesses, particularly patients with severe mental illness (see Related Resources, page 56). If we practice in an integrated manner to treat one of the simple conditions we described above, we can eliminate the need for a patient to visit a second physician, pay another co-pay, pay another bus fare, and take another day off work. This can be particularly helpful for patients who at times have to decide between paying for groceries or for medications. Having one clinician manage a patient’s medications also can decrease the risk of polypharmacy.
In addition to the case scenarios described in this article, there are more clinical situations and nonpsychotropic medications that psychiatrists could manage. Considering them outside the scope of psychiatric practice and being uncomfortable or ambivalent about them is not an excuse. We hope that psychiatrists can increase their expertise in this area, and can start to practice as the primary care physicians they claim they are, and should be.
Bottom Line
Many psychiatrists are uncomfortable prescribing nonpsychotropic medications, but there are numerous clinical scenarios in which the practice would make sense. This could include cases of metabolic syndrome, sexual dysfunction secondary to antidepressant use, or other adverse effects of commonly prescribed psychotropic medications.
Related Resources
- McCarron RM, Xiong GL, Keenan CR, et al. Preventive medical care in psychiatry. A practical guide for clinicians. Arlington, VA: American Psychiatric Association Publishing; 2015.
- McCarron RM, Xiong GL, Keenan CR, et al. Study guide to preventive medical care in psychiatry. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Washington, DC: American Psychiatric Association Publishing; 2019.
Drug Brand Names
Avanafil • Stendra
Benztropine • Cogentin
Bupropion • Wellbutrin, Zyban
Carbamazepine • Carbatrol, Tegretol
Clozapine • Clozaril
Donepezil • Aricept
Levothyroxine • Levoxyl, Synthroid
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone long-acting injection • Risperdal Consta
Sildenafil • Viagra
Tadalafil • Cialis
Vardenafil • Levitra
Ziprasidone • Geodon
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.
1. Detroit Wayne Integrated Health Network. DWMHA psychiatric practice standards. http://dwihn.org/files/2015/6451/9628/Psychiatric_Practice_Standards.pdf. Revised June 2018. Accessed October 8, 2019.
2. Seaman JJ, Cornfield RM, Cummings DM, et al. Exploring psychiatric prescribing practices: the relationship between the role of the provider and the appropriateness of prescribing. Gen Hosp Psychiatry. 1987;9(3):220-224.
3. Zivanovic O. Lithium: a classic drug—frequently discussed, but, sadly, seldom prescribed! Aust N Z J Psychiatry. 2017;51(9):886-896.
4. Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatric Services. 2014;65(2):186-192.
5. Balon R, Mufti R, Arfken C. A survey of prescribing practices for monoamine oxidase inhibitors. Psychiatric Services. 1999;50(7):945-947.
6. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2-3):225-233.
7. Wu RR, Zhao JP, Jin H, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA. 2008;299(2):185-193.
8. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77.
9. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Internal Med. 2002;137(1):25-33.
10. Balon R. SSRI-associated sexual dysfunction. Am J Psychiatry. 2006;163(9):1504-1509.
11. Balon R, Morreale MK, Segraves RT. Prescribing of phosphodiesterase-5 inhibitors among psychiatrists. J Sex Marital Ther. 2014;40(3):165-169.
12. Taylor MJ, Rudkin L, Bullemor-Day P, et al. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;(5):CD003382.
13. Dording CM, LaRocca RA, Hails KA, et al. The effect of sildenafil on quality of life. Ann Clin Psychiatry. 2013;25(1):3-10.
14. Pagano HP, Zapata LB, Berry-Bibee EN, et al. Safety of hormonal contraception and intrauterine devices among women with depressive and bipolar disorders: a systematic review. Contraception. 2016;94(6):641-649.
15. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3.
16. Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27.
17. Devdhar M, Ousman YH, Burman KD. Hypothyroidism. Endocrinol Metab Clin North Am. 2007;36(3):595-615.
18. Hackley B, Sharma C, Kedzior A, et al. Managing mental health conditions in primary care settings. J Midwifery Women’s Health. 2010;55(1):9-19.
19. Fitelson E, McGibbon C. Evaluation and management of behavioral health disorders in women: an overview of major depression, bipolar disorder, anxiety disorders, and sleep in the primary care setting. Obstet Gynecol Clin North Am. 2016;43(2):231-246.
20. Colorafi K, Vanselow J, Nelson T. Treating anxiety and depression in primary care: reducing barriers to access. Fam Pract Manag. 2017;24(4):11-16.
21. McCall WV. Defining the unique scope of psychiatric practice in 2015. J ECT. 2015;31(4):203-204.


