User login
Older-age bipolar disorder: A case series
Although the peak age of onset of bipolar disorder (BD) is between 20 and 40 years,1 some patients develop BD later in life. The International Society for Bipolar Disorders Task Force has classified the illness into 3 categories:
- early-onset bipolar disorder (EOBD), in which the first manic episode occurs before age 40
- late-onset bipolar disorder (LOBD), in which the initial manic/hypomanic episode occurs after age 50
- older-age bipolar disorder (OABD), in which the first manic/hypomanic episode occurs after age 60.2
OABD represents 25% of the population with BD.3 OABD differs from EOBD in its clinical presentation, biological factors, and psychiatric and somatic comorbidities.4 Studies suggest OABD warrants a more extensive workup to rule out organic causes because symptoms are often attributable to a variety of organic etiologies.
This article describes 3 cases of OABD, including treatments and outcomes. We discuss general treatment recommendations for patients with OABD as cited in the literature. Further research is needed to expand our ability to better care for this unique population.
CASE 1
Mr. D was a 66-year-old African American male with no psychiatric history. His medical history was significant for hypertension, poorly controlled diabetes mellitus, and chronic kidney disease. One year ago, he was diagnosed with cholangiocarcinoma, and underwent uncomplicated right trisegmentectomy, resection of extrahepatic biliary tree, and complete portal lymphadenectomy, with Roux-en-Y hepaticojejunostomy to 2 intrahepatic ducts. He presented to the emergency department (ED) with disorganized behavior for 3 weeks. During that time, Mr. D reported increased distractibility, irritability, hyper-religiosity, racing thoughts, decreased appetite, and decreased need for sleep. There was no pertinent family history.
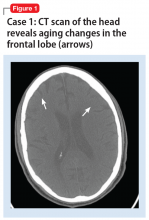
On mental status examination, Mr. D was agitated, noncooperative, and guarded. His speech was loud and pressured. Mr. D was distractible, tangential, and goal-directed. His Young Mania Rating Scale (YMRS) score was 31, which is highly indicative of mania.5 Computed tomography (CT) scan of the head (Figure 1)
CASE 2
Mr. M was a 63-year-old African American male with no psychiatric history and a medical history significant for hypertension and hypercholesterolemia. He presented to the ED with behavioral changes for 2 weeks. During this time, he experienced decreased need for sleep, agitation, excessive spending, self-conversing, hypersexuality, and paranoia. His family history was significant for schizoaffective disorder, bipolar type.
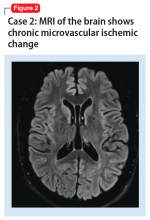
A mental status examination revealed pressured speech, grandiose delusions, hyper-religiosity, flight of ideas, looseness of association, auditory hallucinations, and tangential thought processes. Mr. M’s initial YMRS score was 56. A CT scan of the head revealed no acute abnormality, but MRI of the brain (Figure 2) showed chronic microvascular ischemic change. Mr. M was diagnosed with bipolar I disorder and admitted. He was started on quetiapine extended release, which was titrated to 600 mg nightly. Divalproex sodium extended release was titrated to 1,500 mg nightly, with subsequent improvement. At discharge, his YMRS score was 15.
Continue to: CASE 3
CASE 3
Ms. F was a 69-year-old White female with no psychiatric history. Her medical history was significant for hypertension, osteoarthritis, and stage III-C ovarian adenocarcinoma with a debulking surgical procedure 5 years earlier. After that, she received adjuvant therapy with paclitaxel and carboplatin, which resulted in a 10-month disease-free interval. Subsequent progression led to cycles of doxorubicin liposomal and gemcitabine. She was in remission until 1 week earlier, when a CT scan of the abdomen/pelvis showed recurrence. She presented to the hospital after disrobing in the street due to hyper-religiosity and divine instruction. She endorsed elevated mood and increased energy despite sleeping only 2 hours daily. Her family psychiatric history was significant for her daughter’s suicide attempt.
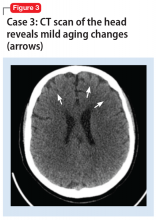
A mental status examination revealed disorganized behavior and agitation. Her speech was loud and pressured. She described a “great” mood with congruent affect. Her thought process was circumstantial and illogical. She displayed flight of ideas, grandiose delusions, and paranoia. Ms. F’s initial YMRS score was 38. Vital signs were significant for an elevated blood pressure of 153/113 mm Hg. A CT scan of the head (Figure 3) showed age-related change with no acute findings. Ms. F was admitted with a diagnosis of bipolar I disorder and prescribed olanzapine, 2.5 mg nightly. Due to continued manic symptoms, olanzapine was discontinued, and Ms. F was started on quetiapine, 300 mg nightly, with subsequent improvement. At discharge, her YMRS score was 10.
Differences between EOBD and OABD
BD has always been considered a multi-system illness; however, comorbidity is much more common in OABD than in EOBD. Comorbid conditions are 3 to 4 times more common in patients with OABD.2 Common comorbidities include metabolic syndrome, allergic rhinitis, arthritis, asthma, and cardiovascular disease.
Compared with younger individuals, older patients with BD score lower on the YMRS in the areas of increased activity-energy, language-thought disorder, and sexual interest.6 Psychotic symptoms are less common or less severe in OABD. Although symptom severity is lower, the prevalence of rapid cycling illness is 20% higher in patients with OABD.6 OABD is less commonly associated with a family history.7 This may suggest a difference from the popular genetic component typically found in patients with EOBD.
Cognitive impairment is more commonly found in OABD. Patients with OABD suffer from neuropsychological deficits even during euthymic phases.8 While these deficits may also be found in patients with EOBD, compared with younger patients, older adults are more susceptible to accelerated decline in cognition. OABD can first present within the context of cardiovascular or neuropsychological impairment. It has also been linked to a greater prevalence of white matter hyperintensities compared with EOBD.9,10
Continue to: Treatment is not specific to OABD
Treatment is not specific to OABD
No established treatment guidelines specifically address OABD. It has been treated similarly to EOBD, with antipsychotics, mood stabilizers, antidepressants, and electroconvulsive therapy (ECT). Although lithium is effective, special precautions should be taken when prescribing it to older adults because these patients may be more sensitive to adverse events.11 Drug–drug interactions may also be more likely due to concomitant use of medications for common medical issues such as hypertension.
Treatment with antipsychotics in older patients carries risks. Use of antipsychotics may result in higher rates of morbidity and mortality related to cardiovascular, metabolic, and infectious etiologies. Some literature recommends the use of antipsychotics for OABD; however, the potential benefits must outweigh the risks.6 Monotherapy followed by combination therapy has demonstrated effectiveness in OABD.11 Because symptoms of OABD are often less severe, it may be best to avoid maintenance antipsychotic therapy when possible. With a higher prevalence of depressed mood following manic episodes, use of antidepressant therapy is common in OABD.6 ECT should be considered for patients with treatment-refractory BD.11
Lessons from our case series
Our case series included 3 patients with OABD. These patients’ comorbid conditions included hypertension, hypercholesteremia, and diabetes mellitus. Two patients had a history of cancer, but there was no metastasis to the brain in either case. However, we considered the possibility of structural changes in the brain or cognitive impairment secondary to cancer or its treatment. A literature review confirmed that adult patients treated for noncentral nervous system cancer experienced cancer-related cognitive impairment (CRCI).12 New research suggests that CRCI could be related to altered neuronal integrity along with a disturbance of brain structure networks that process and integrate information.13
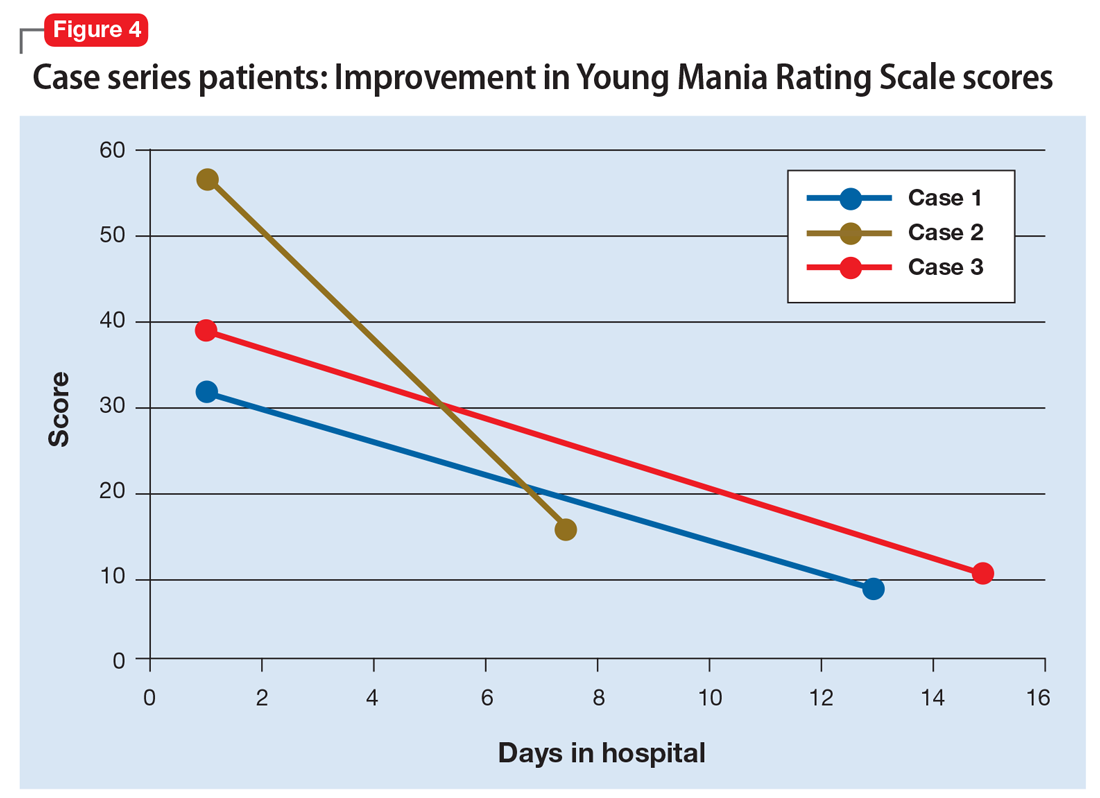
We used the YMRS to compare symptom severity and treatment response (Figure 4). Two patients were treated with atypical antipsychotics with a mood stabilizer, and the third patient was prescribed an antipsychotic only. We avoided lithium and carbamazepine as mood stabilizers due to their adverse effect profiles and potential for drug–drug interactions. Each patient responded well to treatment without adverse events.
Future studies are needed to clearly define the safest and most effective treatment guidelines in patients with OABD. We believe that OABD may require the development of a unique treatment algorithm due to the high likelihood of medical comorbidity and age-related variations in treatment response.
Continue to: Etiology of OABD may be different
Etiology of OABD may be different
OABD may be associated with manic presentations and vascular risk factors. MRI imaging that found more white matter hyperintensities and cerebrovascular lesions in patients with OABD compared with younger patients provides evidence of possible differing etiologies.14 Cassidy and Carroll15 found a higher incidence of smoking, hypertension, diabetes mellitus, coronary heart disease, and atrial fibrillation in patients in the older onset group. Bellivier et al16 proposed 3 subgroups of bipolar I disorder; the late-onset subgroup’s etiology was multifactorial. EOBD and OABD subgroups have similar gender ratios,17 first-episode descriptions, and alcohol use rates; however, OABD subgroups have more neurological comorbidity, lesser severe psychosis, and less genetic predisposition.
Although 25% of BD cases are late onset,3 there is still little consensus regarding subgroups and etiological causes. Therefore, additional research specifically focusing on vascular risks may provide much-needed information. Controlling and mitigating vascular risks in OABD may affect its development and course. Despite debated etiologies, the treatment of BD remains consistent, with anticonvulsants preferred over lithium in older individuals.
The Table summarizes clinical pearls about the features and treatment of OABD.
Bottom Line
Compared with younger patients with bipolar disorder (BD), those who develop BD later in life may be more likely to have rapid cycling, medical comorbidities, and cognitive impairment. Older patients with BD also may be more likely to experience adverse effects of the medications commonly used to treat BD, including antipsychotics, lithium, and carbamazepine.
Related Resources
- Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
- Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Carboplatin • Paraplatin
Divalproex sodium • Depakote
Doxorubicin liposome injection • Doxil
Gemcitabine injection • Gemzar
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Paclitaxel injection • Abraxane
Quetiapine • Seroquel
1. Prabhakar D, Balon R. Late-onset bipolar disorder: a case for careful appraisal. Psychiatry (Edgmont). 2010;7(1):34-37.
2. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry. 2006;163(2):198-203.
4. Chou P-H, Tseng W-J, Chen L-M, et al. Late onset bipolar disorder: a case report and review of the literature. Journal of Clinical Gerontology and Geriatrics. 2015;6(1):27-29.
5. Lukasiewicz M, Gerard S, Besnard A, et al; Emblem Study Group. Young Mania Rating Scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22(1):46-58.
6. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
7. Depp CA, Jeste D V. Bipolar disorder in older adults: A critical review. Bipolar Disord. 2004;6(5):343-367.8.
8. Gildengers AG, Butters MA, et al. Cognitive functioning in late-life bipolar disorder. Am J Psychiatry. 2004. doi:10.1176/appi.ajp.161.4.736
9. Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biological Psychiatry. 1998;43(10):705-712.
10. Tamashiro JH, Zung S, Zanetti MV, et al. Increased rates of white matter hyperintensities in late-onset bipolar disorder. Bipolar Disord. 2008;10(7):765-775.
11. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
12. Wefel JS, Kesler SR, Noll KR, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123-138.
13. Amidi A, Hosseini SMH, Leemans A, et al. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109(12). doi: 10.1093/jnci/djx085.
14. Torrence C, Jackson J. New onset mania in late life: case report and literature review. J Mississippi Acad Sci. 2016;61(1):159.
15. Cassidy F, Carroll BJ. Vascular risk factors in late onset mania. Psychol Med. 2002;32(2):359-362.
16. Bellivier F, Golmard JL, Rietschel M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999-1001.
17. Almeida OP, Fenner S. Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr. 2002;14(3):311-322.
18. Schürhoff F, Bellivier F, Jouvent R, et al. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000;58(3):215-21.
Although the peak age of onset of bipolar disorder (BD) is between 20 and 40 years,1 some patients develop BD later in life. The International Society for Bipolar Disorders Task Force has classified the illness into 3 categories:
- early-onset bipolar disorder (EOBD), in which the first manic episode occurs before age 40
- late-onset bipolar disorder (LOBD), in which the initial manic/hypomanic episode occurs after age 50
- older-age bipolar disorder (OABD), in which the first manic/hypomanic episode occurs after age 60.2
OABD represents 25% of the population with BD.3 OABD differs from EOBD in its clinical presentation, biological factors, and psychiatric and somatic comorbidities.4 Studies suggest OABD warrants a more extensive workup to rule out organic causes because symptoms are often attributable to a variety of organic etiologies.
This article describes 3 cases of OABD, including treatments and outcomes. We discuss general treatment recommendations for patients with OABD as cited in the literature. Further research is needed to expand our ability to better care for this unique population.
CASE 1
Mr. D was a 66-year-old African American male with no psychiatric history. His medical history was significant for hypertension, poorly controlled diabetes mellitus, and chronic kidney disease. One year ago, he was diagnosed with cholangiocarcinoma, and underwent uncomplicated right trisegmentectomy, resection of extrahepatic biliary tree, and complete portal lymphadenectomy, with Roux-en-Y hepaticojejunostomy to 2 intrahepatic ducts. He presented to the emergency department (ED) with disorganized behavior for 3 weeks. During that time, Mr. D reported increased distractibility, irritability, hyper-religiosity, racing thoughts, decreased appetite, and decreased need for sleep. There was no pertinent family history.
On mental status examination, Mr. D was agitated, noncooperative, and guarded. His speech was loud and pressured. Mr. D was distractible, tangential, and goal-directed. His Young Mania Rating Scale (YMRS) score was 31, which is highly indicative of mania.5 Computed tomography (CT) scan of the head (Figure 1)
CASE 2
Mr. M was a 63-year-old African American male with no psychiatric history and a medical history significant for hypertension and hypercholesterolemia. He presented to the ED with behavioral changes for 2 weeks. During this time, he experienced decreased need for sleep, agitation, excessive spending, self-conversing, hypersexuality, and paranoia. His family history was significant for schizoaffective disorder, bipolar type.
A mental status examination revealed pressured speech, grandiose delusions, hyper-religiosity, flight of ideas, looseness of association, auditory hallucinations, and tangential thought processes. Mr. M’s initial YMRS score was 56. A CT scan of the head revealed no acute abnormality, but MRI of the brain (Figure 2) showed chronic microvascular ischemic change. Mr. M was diagnosed with bipolar I disorder and admitted. He was started on quetiapine extended release, which was titrated to 600 mg nightly. Divalproex sodium extended release was titrated to 1,500 mg nightly, with subsequent improvement. At discharge, his YMRS score was 15.
Continue to: CASE 3
CASE 3
Ms. F was a 69-year-old White female with no psychiatric history. Her medical history was significant for hypertension, osteoarthritis, and stage III-C ovarian adenocarcinoma with a debulking surgical procedure 5 years earlier. After that, she received adjuvant therapy with paclitaxel and carboplatin, which resulted in a 10-month disease-free interval. Subsequent progression led to cycles of doxorubicin liposomal and gemcitabine. She was in remission until 1 week earlier, when a CT scan of the abdomen/pelvis showed recurrence. She presented to the hospital after disrobing in the street due to hyper-religiosity and divine instruction. She endorsed elevated mood and increased energy despite sleeping only 2 hours daily. Her family psychiatric history was significant for her daughter’s suicide attempt.
A mental status examination revealed disorganized behavior and agitation. Her speech was loud and pressured. She described a “great” mood with congruent affect. Her thought process was circumstantial and illogical. She displayed flight of ideas, grandiose delusions, and paranoia. Ms. F’s initial YMRS score was 38. Vital signs were significant for an elevated blood pressure of 153/113 mm Hg. A CT scan of the head (Figure 3) showed age-related change with no acute findings. Ms. F was admitted with a diagnosis of bipolar I disorder and prescribed olanzapine, 2.5 mg nightly. Due to continued manic symptoms, olanzapine was discontinued, and Ms. F was started on quetiapine, 300 mg nightly, with subsequent improvement. At discharge, her YMRS score was 10.
Differences between EOBD and OABD
BD has always been considered a multi-system illness; however, comorbidity is much more common in OABD than in EOBD. Comorbid conditions are 3 to 4 times more common in patients with OABD.2 Common comorbidities include metabolic syndrome, allergic rhinitis, arthritis, asthma, and cardiovascular disease.
Compared with younger individuals, older patients with BD score lower on the YMRS in the areas of increased activity-energy, language-thought disorder, and sexual interest.6 Psychotic symptoms are less common or less severe in OABD. Although symptom severity is lower, the prevalence of rapid cycling illness is 20% higher in patients with OABD.6 OABD is less commonly associated with a family history.7 This may suggest a difference from the popular genetic component typically found in patients with EOBD.
Cognitive impairment is more commonly found in OABD. Patients with OABD suffer from neuropsychological deficits even during euthymic phases.8 While these deficits may also be found in patients with EOBD, compared with younger patients, older adults are more susceptible to accelerated decline in cognition. OABD can first present within the context of cardiovascular or neuropsychological impairment. It has also been linked to a greater prevalence of white matter hyperintensities compared with EOBD.9,10
Continue to: Treatment is not specific to OABD
Treatment is not specific to OABD
No established treatment guidelines specifically address OABD. It has been treated similarly to EOBD, with antipsychotics, mood stabilizers, antidepressants, and electroconvulsive therapy (ECT). Although lithium is effective, special precautions should be taken when prescribing it to older adults because these patients may be more sensitive to adverse events.11 Drug–drug interactions may also be more likely due to concomitant use of medications for common medical issues such as hypertension.
Treatment with antipsychotics in older patients carries risks. Use of antipsychotics may result in higher rates of morbidity and mortality related to cardiovascular, metabolic, and infectious etiologies. Some literature recommends the use of antipsychotics for OABD; however, the potential benefits must outweigh the risks.6 Monotherapy followed by combination therapy has demonstrated effectiveness in OABD.11 Because symptoms of OABD are often less severe, it may be best to avoid maintenance antipsychotic therapy when possible. With a higher prevalence of depressed mood following manic episodes, use of antidepressant therapy is common in OABD.6 ECT should be considered for patients with treatment-refractory BD.11
Lessons from our case series
Our case series included 3 patients with OABD. These patients’ comorbid conditions included hypertension, hypercholesteremia, and diabetes mellitus. Two patients had a history of cancer, but there was no metastasis to the brain in either case. However, we considered the possibility of structural changes in the brain or cognitive impairment secondary to cancer or its treatment. A literature review confirmed that adult patients treated for noncentral nervous system cancer experienced cancer-related cognitive impairment (CRCI).12 New research suggests that CRCI could be related to altered neuronal integrity along with a disturbance of brain structure networks that process and integrate information.13
We used the YMRS to compare symptom severity and treatment response (Figure 4). Two patients were treated with atypical antipsychotics with a mood stabilizer, and the third patient was prescribed an antipsychotic only. We avoided lithium and carbamazepine as mood stabilizers due to their adverse effect profiles and potential for drug–drug interactions. Each patient responded well to treatment without adverse events.

Future studies are needed to clearly define the safest and most effective treatment guidelines in patients with OABD. We believe that OABD may require the development of a unique treatment algorithm due to the high likelihood of medical comorbidity and age-related variations in treatment response.
Continue to: Etiology of OABD may be different
Etiology of OABD may be different
OABD may be associated with manic presentations and vascular risk factors. MRI imaging that found more white matter hyperintensities and cerebrovascular lesions in patients with OABD compared with younger patients provides evidence of possible differing etiologies.14 Cassidy and Carroll15 found a higher incidence of smoking, hypertension, diabetes mellitus, coronary heart disease, and atrial fibrillation in patients in the older onset group. Bellivier et al16 proposed 3 subgroups of bipolar I disorder; the late-onset subgroup’s etiology was multifactorial. EOBD and OABD subgroups have similar gender ratios,17 first-episode descriptions, and alcohol use rates; however, OABD subgroups have more neurological comorbidity, lesser severe psychosis, and less genetic predisposition.
Although 25% of BD cases are late onset,3 there is still little consensus regarding subgroups and etiological causes. Therefore, additional research specifically focusing on vascular risks may provide much-needed information. Controlling and mitigating vascular risks in OABD may affect its development and course. Despite debated etiologies, the treatment of BD remains consistent, with anticonvulsants preferred over lithium in older individuals.
The Table summarizes clinical pearls about the features and treatment of OABD.
Bottom Line
Compared with younger patients with bipolar disorder (BD), those who develop BD later in life may be more likely to have rapid cycling, medical comorbidities, and cognitive impairment. Older patients with BD also may be more likely to experience adverse effects of the medications commonly used to treat BD, including antipsychotics, lithium, and carbamazepine.
Related Resources
- Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
- Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Carboplatin • Paraplatin
Divalproex sodium • Depakote
Doxorubicin liposome injection • Doxil
Gemcitabine injection • Gemzar
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Paclitaxel injection • Abraxane
Quetiapine • Seroquel
Although the peak age of onset of bipolar disorder (BD) is between 20 and 40 years,1 some patients develop BD later in life. The International Society for Bipolar Disorders Task Force has classified the illness into 3 categories:
- early-onset bipolar disorder (EOBD), in which the first manic episode occurs before age 40
- late-onset bipolar disorder (LOBD), in which the initial manic/hypomanic episode occurs after age 50
- older-age bipolar disorder (OABD), in which the first manic/hypomanic episode occurs after age 60.2
OABD represents 25% of the population with BD.3 OABD differs from EOBD in its clinical presentation, biological factors, and psychiatric and somatic comorbidities.4 Studies suggest OABD warrants a more extensive workup to rule out organic causes because symptoms are often attributable to a variety of organic etiologies.
This article describes 3 cases of OABD, including treatments and outcomes. We discuss general treatment recommendations for patients with OABD as cited in the literature. Further research is needed to expand our ability to better care for this unique population.
CASE 1
Mr. D was a 66-year-old African American male with no psychiatric history. His medical history was significant for hypertension, poorly controlled diabetes mellitus, and chronic kidney disease. One year ago, he was diagnosed with cholangiocarcinoma, and underwent uncomplicated right trisegmentectomy, resection of extrahepatic biliary tree, and complete portal lymphadenectomy, with Roux-en-Y hepaticojejunostomy to 2 intrahepatic ducts. He presented to the emergency department (ED) with disorganized behavior for 3 weeks. During that time, Mr. D reported increased distractibility, irritability, hyper-religiosity, racing thoughts, decreased appetite, and decreased need for sleep. There was no pertinent family history.
On mental status examination, Mr. D was agitated, noncooperative, and guarded. His speech was loud and pressured. Mr. D was distractible, tangential, and goal-directed. His Young Mania Rating Scale (YMRS) score was 31, which is highly indicative of mania.5 Computed tomography (CT) scan of the head (Figure 1)
CASE 2
Mr. M was a 63-year-old African American male with no psychiatric history and a medical history significant for hypertension and hypercholesterolemia. He presented to the ED with behavioral changes for 2 weeks. During this time, he experienced decreased need for sleep, agitation, excessive spending, self-conversing, hypersexuality, and paranoia. His family history was significant for schizoaffective disorder, bipolar type.
A mental status examination revealed pressured speech, grandiose delusions, hyper-religiosity, flight of ideas, looseness of association, auditory hallucinations, and tangential thought processes. Mr. M’s initial YMRS score was 56. A CT scan of the head revealed no acute abnormality, but MRI of the brain (Figure 2) showed chronic microvascular ischemic change. Mr. M was diagnosed with bipolar I disorder and admitted. He was started on quetiapine extended release, which was titrated to 600 mg nightly. Divalproex sodium extended release was titrated to 1,500 mg nightly, with subsequent improvement. At discharge, his YMRS score was 15.
Continue to: CASE 3
CASE 3
Ms. F was a 69-year-old White female with no psychiatric history. Her medical history was significant for hypertension, osteoarthritis, and stage III-C ovarian adenocarcinoma with a debulking surgical procedure 5 years earlier. After that, she received adjuvant therapy with paclitaxel and carboplatin, which resulted in a 10-month disease-free interval. Subsequent progression led to cycles of doxorubicin liposomal and gemcitabine. She was in remission until 1 week earlier, when a CT scan of the abdomen/pelvis showed recurrence. She presented to the hospital after disrobing in the street due to hyper-religiosity and divine instruction. She endorsed elevated mood and increased energy despite sleeping only 2 hours daily. Her family psychiatric history was significant for her daughter’s suicide attempt.
A mental status examination revealed disorganized behavior and agitation. Her speech was loud and pressured. She described a “great” mood with congruent affect. Her thought process was circumstantial and illogical. She displayed flight of ideas, grandiose delusions, and paranoia. Ms. F’s initial YMRS score was 38. Vital signs were significant for an elevated blood pressure of 153/113 mm Hg. A CT scan of the head (Figure 3) showed age-related change with no acute findings. Ms. F was admitted with a diagnosis of bipolar I disorder and prescribed olanzapine, 2.5 mg nightly. Due to continued manic symptoms, olanzapine was discontinued, and Ms. F was started on quetiapine, 300 mg nightly, with subsequent improvement. At discharge, her YMRS score was 10.
Differences between EOBD and OABD
BD has always been considered a multi-system illness; however, comorbidity is much more common in OABD than in EOBD. Comorbid conditions are 3 to 4 times more common in patients with OABD.2 Common comorbidities include metabolic syndrome, allergic rhinitis, arthritis, asthma, and cardiovascular disease.
Compared with younger individuals, older patients with BD score lower on the YMRS in the areas of increased activity-energy, language-thought disorder, and sexual interest.6 Psychotic symptoms are less common or less severe in OABD. Although symptom severity is lower, the prevalence of rapid cycling illness is 20% higher in patients with OABD.6 OABD is less commonly associated with a family history.7 This may suggest a difference from the popular genetic component typically found in patients with EOBD.
Cognitive impairment is more commonly found in OABD. Patients with OABD suffer from neuropsychological deficits even during euthymic phases.8 While these deficits may also be found in patients with EOBD, compared with younger patients, older adults are more susceptible to accelerated decline in cognition. OABD can first present within the context of cardiovascular or neuropsychological impairment. It has also been linked to a greater prevalence of white matter hyperintensities compared with EOBD.9,10
Continue to: Treatment is not specific to OABD
Treatment is not specific to OABD
No established treatment guidelines specifically address OABD. It has been treated similarly to EOBD, with antipsychotics, mood stabilizers, antidepressants, and electroconvulsive therapy (ECT). Although lithium is effective, special precautions should be taken when prescribing it to older adults because these patients may be more sensitive to adverse events.11 Drug–drug interactions may also be more likely due to concomitant use of medications for common medical issues such as hypertension.
Treatment with antipsychotics in older patients carries risks. Use of antipsychotics may result in higher rates of morbidity and mortality related to cardiovascular, metabolic, and infectious etiologies. Some literature recommends the use of antipsychotics for OABD; however, the potential benefits must outweigh the risks.6 Monotherapy followed by combination therapy has demonstrated effectiveness in OABD.11 Because symptoms of OABD are often less severe, it may be best to avoid maintenance antipsychotic therapy when possible. With a higher prevalence of depressed mood following manic episodes, use of antidepressant therapy is common in OABD.6 ECT should be considered for patients with treatment-refractory BD.11
Lessons from our case series
Our case series included 3 patients with OABD. These patients’ comorbid conditions included hypertension, hypercholesteremia, and diabetes mellitus. Two patients had a history of cancer, but there was no metastasis to the brain in either case. However, we considered the possibility of structural changes in the brain or cognitive impairment secondary to cancer or its treatment. A literature review confirmed that adult patients treated for noncentral nervous system cancer experienced cancer-related cognitive impairment (CRCI).12 New research suggests that CRCI could be related to altered neuronal integrity along with a disturbance of brain structure networks that process and integrate information.13
We used the YMRS to compare symptom severity and treatment response (Figure 4). Two patients were treated with atypical antipsychotics with a mood stabilizer, and the third patient was prescribed an antipsychotic only. We avoided lithium and carbamazepine as mood stabilizers due to their adverse effect profiles and potential for drug–drug interactions. Each patient responded well to treatment without adverse events.

Future studies are needed to clearly define the safest and most effective treatment guidelines in patients with OABD. We believe that OABD may require the development of a unique treatment algorithm due to the high likelihood of medical comorbidity and age-related variations in treatment response.
Continue to: Etiology of OABD may be different
Etiology of OABD may be different
OABD may be associated with manic presentations and vascular risk factors. MRI imaging that found more white matter hyperintensities and cerebrovascular lesions in patients with OABD compared with younger patients provides evidence of possible differing etiologies.14 Cassidy and Carroll15 found a higher incidence of smoking, hypertension, diabetes mellitus, coronary heart disease, and atrial fibrillation in patients in the older onset group. Bellivier et al16 proposed 3 subgroups of bipolar I disorder; the late-onset subgroup’s etiology was multifactorial. EOBD and OABD subgroups have similar gender ratios,17 first-episode descriptions, and alcohol use rates; however, OABD subgroups have more neurological comorbidity, lesser severe psychosis, and less genetic predisposition.
Although 25% of BD cases are late onset,3 there is still little consensus regarding subgroups and etiological causes. Therefore, additional research specifically focusing on vascular risks may provide much-needed information. Controlling and mitigating vascular risks in OABD may affect its development and course. Despite debated etiologies, the treatment of BD remains consistent, with anticonvulsants preferred over lithium in older individuals.
The Table summarizes clinical pearls about the features and treatment of OABD.
Bottom Line
Compared with younger patients with bipolar disorder (BD), those who develop BD later in life may be more likely to have rapid cycling, medical comorbidities, and cognitive impairment. Older patients with BD also may be more likely to experience adverse effects of the medications commonly used to treat BD, including antipsychotics, lithium, and carbamazepine.
Related Resources
- Carlino AR, Stinnett JL, Kim DR. New onset of bipolar disorder in late life. Psychosomatics. 2013;54(1):94-97.
- Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
Drug Brand Names
Carbamazepine • Carbatrol, Tegretol
Carboplatin • Paraplatin
Divalproex sodium • Depakote
Doxorubicin liposome injection • Doxil
Gemcitabine injection • Gemzar
Lithium • Eskalith, Lithobid
Olanzapine • Zyprexa
Paclitaxel injection • Abraxane
Quetiapine • Seroquel
1. Prabhakar D, Balon R. Late-onset bipolar disorder: a case for careful appraisal. Psychiatry (Edgmont). 2010;7(1):34-37.
2. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry. 2006;163(2):198-203.
4. Chou P-H, Tseng W-J, Chen L-M, et al. Late onset bipolar disorder: a case report and review of the literature. Journal of Clinical Gerontology and Geriatrics. 2015;6(1):27-29.
5. Lukasiewicz M, Gerard S, Besnard A, et al; Emblem Study Group. Young Mania Rating Scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22(1):46-58.
6. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
7. Depp CA, Jeste D V. Bipolar disorder in older adults: A critical review. Bipolar Disord. 2004;6(5):343-367.8.
8. Gildengers AG, Butters MA, et al. Cognitive functioning in late-life bipolar disorder. Am J Psychiatry. 2004. doi:10.1176/appi.ajp.161.4.736
9. Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biological Psychiatry. 1998;43(10):705-712.
10. Tamashiro JH, Zung S, Zanetti MV, et al. Increased rates of white matter hyperintensities in late-onset bipolar disorder. Bipolar Disord. 2008;10(7):765-775.
11. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
12. Wefel JS, Kesler SR, Noll KR, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123-138.
13. Amidi A, Hosseini SMH, Leemans A, et al. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109(12). doi: 10.1093/jnci/djx085.
14. Torrence C, Jackson J. New onset mania in late life: case report and literature review. J Mississippi Acad Sci. 2016;61(1):159.
15. Cassidy F, Carroll BJ. Vascular risk factors in late onset mania. Psychol Med. 2002;32(2):359-362.
16. Bellivier F, Golmard JL, Rietschel M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999-1001.
17. Almeida OP, Fenner S. Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr. 2002;14(3):311-322.
18. Schürhoff F, Bellivier F, Jouvent R, et al. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000;58(3):215-21.
1. Prabhakar D, Balon R. Late-onset bipolar disorder: a case for careful appraisal. Psychiatry (Edgmont). 2010;7(1):34-37.
2. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry. 2006;163(2):198-203.
4. Chou P-H, Tseng W-J, Chen L-M, et al. Late onset bipolar disorder: a case report and review of the literature. Journal of Clinical Gerontology and Geriatrics. 2015;6(1):27-29.
5. Lukasiewicz M, Gerard S, Besnard A, et al; Emblem Study Group. Young Mania Rating Scale: how to interpret the numbers? Determination of a severity threshold and of the minimal clinically significant difference in the EMBLEM cohort. Int J Methods Psychiatr Res. 2013;22(1):46-58.
6. Oostervink F, Boomsma MM, Nolen WA; EMBLEM Advisory Board. Bipolar disorder in the elderly; different effects of age and of age of onset. J Affect Disord. 2009;116(3):176-183.
7. Depp CA, Jeste D V. Bipolar disorder in older adults: A critical review. Bipolar Disord. 2004;6(5):343-367.8.
8. Gildengers AG, Butters MA, et al. Cognitive functioning in late-life bipolar disorder. Am J Psychiatry. 2004. doi:10.1176/appi.ajp.161.4.736
9. Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biological Psychiatry. 1998;43(10):705-712.
10. Tamashiro JH, Zung S, Zanetti MV, et al. Increased rates of white matter hyperintensities in late-onset bipolar disorder. Bipolar Disord. 2008;10(7):765-775.
11. Aziz R, Lorberg B, Tampi RR. Treatments for late-life bipolar disorder. Am J Geriatr Pharmacother. 2006;4(4):347-364.
12. Wefel JS, Kesler SR, Noll KR, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123-138.
13. Amidi A, Hosseini SMH, Leemans A, et al. Changes in brain structural networks and cognitive functions in testicular cancer patients receiving cisplatin-based chemotherapy. J Natl Cancer Inst. 2017;109(12). doi: 10.1093/jnci/djx085.
14. Torrence C, Jackson J. New onset mania in late life: case report and literature review. J Mississippi Acad Sci. 2016;61(1):159.
15. Cassidy F, Carroll BJ. Vascular risk factors in late onset mania. Psychol Med. 2002;32(2):359-362.
16. Bellivier F, Golmard JL, Rietschel M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999-1001.
17. Almeida OP, Fenner S. Bipolar disorder: similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr. 2002;14(3):311-322.
18. Schürhoff F, Bellivier F, Jouvent R, et al. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000;58(3):215-21.
Bipolar patients’ ability to consent can be measured with MacCAT-CR
, according to Christina C. Klein and her associates.
A total of 50 patients who were enrolled in a clinical trial of approved, standard treatments for bipolar disease were included in the consent study. The MacCAT-CR was administered after patients had given consent to be included in the trial, but before the trial had started. Four patients lacked the ability to provide consent for the trial after receiving the MacCAT-CR. After these patients were reeducated and went through the consent process a second time, three were enrolled and one declined enrollment.
Patients with higher Schedule for Assessment of Positive Symptoms scores were more likely to have worse MacCAT-CR Understanding and Appreciation subscale scores; lower Hamilton Depression Rating Scale and higher Clinical Global Impression–Severity scores were associated with worse Reasoning and Understanding subscale scores.
Comorbid ADHD, sex, IQ scores, and age at onset of bipolar disorder were not correlated with any subscale scores. In addition, a history of substance use disorder was associated with higher Appreciation and Reasoning subscale scores.
“The current study provides important information for clinicians and researchers to consider when obtaining informed consent from an individual with bipolar disorder. The MacCAT-CR may serve to identify patients, specifically those with higher psychotic symptoms or global illness severity, as needing additional education regarding informed consent,” the investigators concluded.
Three study coauthors reported conflicts of interest with numerous pharmaceutical companies.
SOURCE: Klein CC et al. J Affect Disord. 2018 Aug 13. doi: 10.1016/j.jad.2018.08.049.
, according to Christina C. Klein and her associates.
A total of 50 patients who were enrolled in a clinical trial of approved, standard treatments for bipolar disease were included in the consent study. The MacCAT-CR was administered after patients had given consent to be included in the trial, but before the trial had started. Four patients lacked the ability to provide consent for the trial after receiving the MacCAT-CR. After these patients were reeducated and went through the consent process a second time, three were enrolled and one declined enrollment.
Patients with higher Schedule for Assessment of Positive Symptoms scores were more likely to have worse MacCAT-CR Understanding and Appreciation subscale scores; lower Hamilton Depression Rating Scale and higher Clinical Global Impression–Severity scores were associated with worse Reasoning and Understanding subscale scores.
Comorbid ADHD, sex, IQ scores, and age at onset of bipolar disorder were not correlated with any subscale scores. In addition, a history of substance use disorder was associated with higher Appreciation and Reasoning subscale scores.
“The current study provides important information for clinicians and researchers to consider when obtaining informed consent from an individual with bipolar disorder. The MacCAT-CR may serve to identify patients, specifically those with higher psychotic symptoms or global illness severity, as needing additional education regarding informed consent,” the investigators concluded.
Three study coauthors reported conflicts of interest with numerous pharmaceutical companies.
SOURCE: Klein CC et al. J Affect Disord. 2018 Aug 13. doi: 10.1016/j.jad.2018.08.049.
, according to Christina C. Klein and her associates.
A total of 50 patients who were enrolled in a clinical trial of approved, standard treatments for bipolar disease were included in the consent study. The MacCAT-CR was administered after patients had given consent to be included in the trial, but before the trial had started. Four patients lacked the ability to provide consent for the trial after receiving the MacCAT-CR. After these patients were reeducated and went through the consent process a second time, three were enrolled and one declined enrollment.
Patients with higher Schedule for Assessment of Positive Symptoms scores were more likely to have worse MacCAT-CR Understanding and Appreciation subscale scores; lower Hamilton Depression Rating Scale and higher Clinical Global Impression–Severity scores were associated with worse Reasoning and Understanding subscale scores.
Comorbid ADHD, sex, IQ scores, and age at onset of bipolar disorder were not correlated with any subscale scores. In addition, a history of substance use disorder was associated with higher Appreciation and Reasoning subscale scores.
“The current study provides important information for clinicians and researchers to consider when obtaining informed consent from an individual with bipolar disorder. The MacCAT-CR may serve to identify patients, specifically those with higher psychotic symptoms or global illness severity, as needing additional education regarding informed consent,” the investigators concluded.
Three study coauthors reported conflicts of interest with numerous pharmaceutical companies.
SOURCE: Klein CC et al. J Affect Disord. 2018 Aug 13. doi: 10.1016/j.jad.2018.08.049.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Early maladaptive schemas increase suicide risk, ideation in bipolar
The entitlement, social isolation, and defectiveness early maladaptive schemas (EMSs) were associated with increased suicide risk and ideation in patients with bipolar disorder, according to Vahid Khosravani of Shahid Beheshti University of Medical Sciences, Tehran, Iran, and his associates.
“These findings were in line with previous studies (J Nerv Ment Dis. 2016 Mar. 204[3]:236-9) showing higher scores of social isolation and entitlement in [bipolar disorder] patients with suicide attempts than those without such attempts,” Mr. Khosravani and his associates wrote in Psychiatry Research.
For the study, 100 inpatients with bipolar disorder completed the Young Schema Questionnaire–Short Form (YSQ-SF), the Bipolar Depression Rating Scale (BDRS), the Young Mania Rating Scale (YMRS), and the Beck Scale for Suicidal Ideation (BSSI). Of that group, 59% had attempted suicide and 59% had a BSSI score of 6 or higher, indicating high suicidal risk, reported Mr. Khosravani and his associates.
; they also had higher levels of depressive and hypomanic/manic symptoms. Current suicide ideation was associated with higher entitlement and defectiveness EMS scores, as well as with increased hypomanic/manic symptoms.
“The findings suggest that manic symptoms as well as specific EMSs including social isolation, entitlement, and defectiveness emerge as potentially implicated in suicidality in BD patients,” the investigators noted. “Therefore, providing social support in the economic, social, political, cultural, and educational spheres may be a factor in preventing suicide.”
Mr. Khosravani and his associates said their study received no funding from public, commercial, or nonprofit agencies. The investigators declared no conflicts of interest.
SOURCE: Khosravani V et al. Psychiatry Res. 2019 Jan. (271):351-9.
The entitlement, social isolation, and defectiveness early maladaptive schemas (EMSs) were associated with increased suicide risk and ideation in patients with bipolar disorder, according to Vahid Khosravani of Shahid Beheshti University of Medical Sciences, Tehran, Iran, and his associates.
“These findings were in line with previous studies (J Nerv Ment Dis. 2016 Mar. 204[3]:236-9) showing higher scores of social isolation and entitlement in [bipolar disorder] patients with suicide attempts than those without such attempts,” Mr. Khosravani and his associates wrote in Psychiatry Research.
For the study, 100 inpatients with bipolar disorder completed the Young Schema Questionnaire–Short Form (YSQ-SF), the Bipolar Depression Rating Scale (BDRS), the Young Mania Rating Scale (YMRS), and the Beck Scale for Suicidal Ideation (BSSI). Of that group, 59% had attempted suicide and 59% had a BSSI score of 6 or higher, indicating high suicidal risk, reported Mr. Khosravani and his associates.
; they also had higher levels of depressive and hypomanic/manic symptoms. Current suicide ideation was associated with higher entitlement and defectiveness EMS scores, as well as with increased hypomanic/manic symptoms.
“The findings suggest that manic symptoms as well as specific EMSs including social isolation, entitlement, and defectiveness emerge as potentially implicated in suicidality in BD patients,” the investigators noted. “Therefore, providing social support in the economic, social, political, cultural, and educational spheres may be a factor in preventing suicide.”
Mr. Khosravani and his associates said their study received no funding from public, commercial, or nonprofit agencies. The investigators declared no conflicts of interest.
SOURCE: Khosravani V et al. Psychiatry Res. 2019 Jan. (271):351-9.
The entitlement, social isolation, and defectiveness early maladaptive schemas (EMSs) were associated with increased suicide risk and ideation in patients with bipolar disorder, according to Vahid Khosravani of Shahid Beheshti University of Medical Sciences, Tehran, Iran, and his associates.
“These findings were in line with previous studies (J Nerv Ment Dis. 2016 Mar. 204[3]:236-9) showing higher scores of social isolation and entitlement in [bipolar disorder] patients with suicide attempts than those without such attempts,” Mr. Khosravani and his associates wrote in Psychiatry Research.
For the study, 100 inpatients with bipolar disorder completed the Young Schema Questionnaire–Short Form (YSQ-SF), the Bipolar Depression Rating Scale (BDRS), the Young Mania Rating Scale (YMRS), and the Beck Scale for Suicidal Ideation (BSSI). Of that group, 59% had attempted suicide and 59% had a BSSI score of 6 or higher, indicating high suicidal risk, reported Mr. Khosravani and his associates.
; they also had higher levels of depressive and hypomanic/manic symptoms. Current suicide ideation was associated with higher entitlement and defectiveness EMS scores, as well as with increased hypomanic/manic symptoms.
“The findings suggest that manic symptoms as well as specific EMSs including social isolation, entitlement, and defectiveness emerge as potentially implicated in suicidality in BD patients,” the investigators noted. “Therefore, providing social support in the economic, social, political, cultural, and educational spheres may be a factor in preventing suicide.”
Mr. Khosravani and his associates said their study received no funding from public, commercial, or nonprofit agencies. The investigators declared no conflicts of interest.
SOURCE: Khosravani V et al. Psychiatry Res. 2019 Jan. (271):351-9.
FROM PSYCHIATRY RESEARCH
ADHD more likely, causes worse outcomes in patients with BD
ADHD is significantly more common and is associated with worse outcomes in patients with bipolar disorder, according to Ross J. Baldessarini, MD, of McLean Hospital and Harvard Medical School, Boston, and his associates.
In a study of 703 patients diagnosed with bipolar disorder (BD) type I or II who were evaluated, treated, and followed at the Lucio Bini Mood Disorder Centers in Rome and Cagliari, Italy, 173 patients had co-occurring lifetime ADHD. Co-occurring conditions were more likely in men and in those with BD-I. The lifetime ADHD prevalence rate of 24.6% in patients with bipolar disorder is significantly higher than the incidence in the general population, the investigators wrote in the Journal of Affective Disorders.
Patients with co-occurring ADHD and BD were more likely to have performed worse in school, have higher Adult ADHD Self-Report Scale scores, be unemployed, have lower socioeconomic status, be married, have separated, have substance abuse, have attempted suicide, and have hypomania, compared with patients with only BD. However, they were less likely to have an anxiety disorder or a family history of mood disorders.
“The association of ADHD with a less successful and stable educational history, more unemployment, lack of or failed marriages, and greater risk of suicide attempts and substance abuse indicates unfavorable effects of having ADHD with BD. Such effects may arise by the impact of ADHD early during development,” the investigators concluded.
The study was partly supported by a research award from the Aretaeus Association of Rome and grants from the Bruce J. Anderson Foundation and the McLean Private Donors Research Fund. No conflicts of interest were reported.
SOURCE: Baldessarini RJ et al. J Affect Disord. 2018 Sep 17. doi: 10.1016/j.jad.2018.09.038.
ADHD is significantly more common and is associated with worse outcomes in patients with bipolar disorder, according to Ross J. Baldessarini, MD, of McLean Hospital and Harvard Medical School, Boston, and his associates.
In a study of 703 patients diagnosed with bipolar disorder (BD) type I or II who were evaluated, treated, and followed at the Lucio Bini Mood Disorder Centers in Rome and Cagliari, Italy, 173 patients had co-occurring lifetime ADHD. Co-occurring conditions were more likely in men and in those with BD-I. The lifetime ADHD prevalence rate of 24.6% in patients with bipolar disorder is significantly higher than the incidence in the general population, the investigators wrote in the Journal of Affective Disorders.
Patients with co-occurring ADHD and BD were more likely to have performed worse in school, have higher Adult ADHD Self-Report Scale scores, be unemployed, have lower socioeconomic status, be married, have separated, have substance abuse, have attempted suicide, and have hypomania, compared with patients with only BD. However, they were less likely to have an anxiety disorder or a family history of mood disorders.
“The association of ADHD with a less successful and stable educational history, more unemployment, lack of or failed marriages, and greater risk of suicide attempts and substance abuse indicates unfavorable effects of having ADHD with BD. Such effects may arise by the impact of ADHD early during development,” the investigators concluded.
The study was partly supported by a research award from the Aretaeus Association of Rome and grants from the Bruce J. Anderson Foundation and the McLean Private Donors Research Fund. No conflicts of interest were reported.
SOURCE: Baldessarini RJ et al. J Affect Disord. 2018 Sep 17. doi: 10.1016/j.jad.2018.09.038.
ADHD is significantly more common and is associated with worse outcomes in patients with bipolar disorder, according to Ross J. Baldessarini, MD, of McLean Hospital and Harvard Medical School, Boston, and his associates.
In a study of 703 patients diagnosed with bipolar disorder (BD) type I or II who were evaluated, treated, and followed at the Lucio Bini Mood Disorder Centers in Rome and Cagliari, Italy, 173 patients had co-occurring lifetime ADHD. Co-occurring conditions were more likely in men and in those with BD-I. The lifetime ADHD prevalence rate of 24.6% in patients with bipolar disorder is significantly higher than the incidence in the general population, the investigators wrote in the Journal of Affective Disorders.
Patients with co-occurring ADHD and BD were more likely to have performed worse in school, have higher Adult ADHD Self-Report Scale scores, be unemployed, have lower socioeconomic status, be married, have separated, have substance abuse, have attempted suicide, and have hypomania, compared with patients with only BD. However, they were less likely to have an anxiety disorder or a family history of mood disorders.
“The association of ADHD with a less successful and stable educational history, more unemployment, lack of or failed marriages, and greater risk of suicide attempts and substance abuse indicates unfavorable effects of having ADHD with BD. Such effects may arise by the impact of ADHD early during development,” the investigators concluded.
The study was partly supported by a research award from the Aretaeus Association of Rome and grants from the Bruce J. Anderson Foundation and the McLean Private Donors Research Fund. No conflicts of interest were reported.
SOURCE: Baldessarini RJ et al. J Affect Disord. 2018 Sep 17. doi: 10.1016/j.jad.2018.09.038.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Best of Psychopharmacology: Stimulants, ketamine, benzodiazapines
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
In this episode we go back to the summer for two master classes on ketamine and stimulants, respectively and we drop in on two conversations between Lorenzo Norris, MD on anxiety and comorbid ADHD as well as a conversation on benzodiazapines. The Psychcast will be back with new content in 2019.
Amazon
Apple
Google
Spotify
CPN welcomes Andrea Murru, MD, PhD, to CPN board
Clinical Psychiatry News is pleased to announce that Andrea Murru, MD, PhD, has joined the Editorial Advisory Board.
Dr. Murru is senior clinician in the bipolar disorder and sleep disorder units of the Hospital Clinic of Barcelona in Spain, and is a postdoctoral researcher of the Spanish Network of Research in Mental Health, which is led by Eduard Vieta, MD, PhD.
In addition, His research focuses on using, long-term treatments, and implementing clinical guidelines in daily practice. He also researches tolerability in patients with bipolar disorder, schizoaffective disorder, and sleep-related disorders and biomarkers.
He earned his medical degree from the University of Cagliari (Italy).
Clinical Psychiatry News is pleased to announce that Andrea Murru, MD, PhD, has joined the Editorial Advisory Board.
Dr. Murru is senior clinician in the bipolar disorder and sleep disorder units of the Hospital Clinic of Barcelona in Spain, and is a postdoctoral researcher of the Spanish Network of Research in Mental Health, which is led by Eduard Vieta, MD, PhD.
In addition, His research focuses on using, long-term treatments, and implementing clinical guidelines in daily practice. He also researches tolerability in patients with bipolar disorder, schizoaffective disorder, and sleep-related disorders and biomarkers.
He earned his medical degree from the University of Cagliari (Italy).
Clinical Psychiatry News is pleased to announce that Andrea Murru, MD, PhD, has joined the Editorial Advisory Board.
Dr. Murru is senior clinician in the bipolar disorder and sleep disorder units of the Hospital Clinic of Barcelona in Spain, and is a postdoctoral researcher of the Spanish Network of Research in Mental Health, which is led by Eduard Vieta, MD, PhD.
In addition, His research focuses on using, long-term treatments, and implementing clinical guidelines in daily practice. He also researches tolerability in patients with bipolar disorder, schizoaffective disorder, and sleep-related disorders and biomarkers.
He earned his medical degree from the University of Cagliari (Italy).
A mood disorder complicated by multiple sclerosis
CASE Depression, or something else?
Ms. A, age 56, presents to the emergency department (ED) with depressed mood, poor sleep, anhedonia, irritability, agitation, and recent self-injurious behavior; she had superficially cut her wrists. She also has a longstanding history of multiple sclerosis (MS), depression, and anxiety. She is admitted voluntarily to an inpatient psychiatric unit.
According to medical records, at age 32, Ms. A was diagnosed with relapsing-remitting MS, which initially presented with facial numbness, and later with optic neuritis with transient loss of vision. As her disease progressed to the secondary progressive type, she experienced spasticity and vertigo. In the past few years, she also had experienced cognitive difficulties, particularly with memory and focus.
Ms. A has a history of recurrent depressive symptoms that began at an unspecified time after being diagnosed with MS. In the past few years, she had greatly increased her alcohol use in response to multiple psychosocial stressors and as an attempt to self-medicate MS-related pain. Several years ago, Ms. A had been admitted to a rehabilitation facility to address her alcohol use.
In the past, Ms. A’s depressive symptoms had been treated with various antidepressants, including fluoxetine (unspecified dose), which for a time was effective. The most recently prescribed antidepressant was duloxetine, 60 mg/d, which was discontinued because Ms. A felt it activated her mood lability. A few years before this current hospitalization, Ms. A had been started on a trial of dextromethorphan/quinidine (20 mg/10 mg, twice daily), which was discontinued due to concomitant use of an unspecified serotonin-norepinephrine reuptake inhibitor (SNRI) and subsequent precipitation of serotonin syndrome.
At the time of this current admission to the psychiatric unit, Ms. A is being treated for MS with rituximab (10 mg/mL IV, every 6 months). Additionally, just before her admission, she was taking alprazolam (.25 mg, 3 times per day) for anxiety. She denies experiencing any spasticity or vision impairment.
[polldaddy:10175070]
The authors’ observations
We initially considered a diagnosis of MDD due to Ms. A’s past history of depressive episodes, her recent increase in tearfulness and anhedonia, and her self-injurious behaviors. However, diagnosis of a mood disorder was complicated by her complex history of longstanding MS and other psychosocial factors.
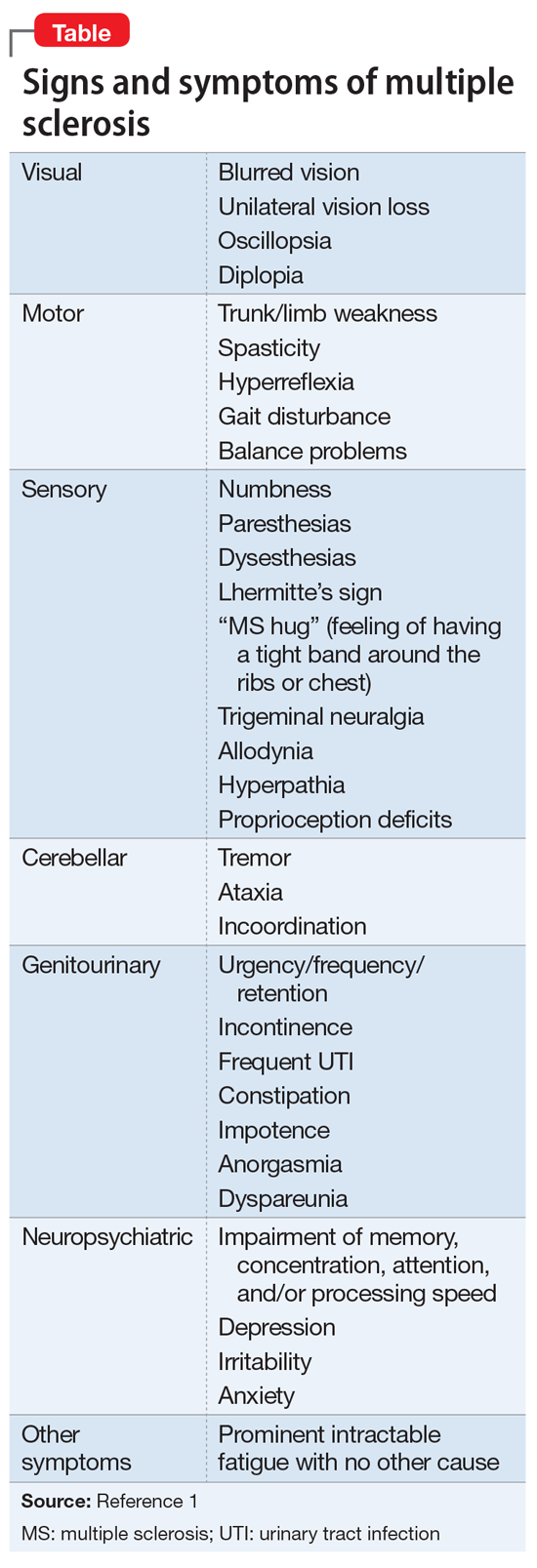
Continue to: Several factors contribute to the neuropsychiatric course of patients with MS...
Several factors contribute to the neuropsychiatric course of patients with MS, including the impact of the patient accepting a chronic and incurable diagnosis, the toll of progressive neurologic/physical disability and subsequent decline in functioning, and the availability of a support system.2 As opposed to disorders such as Parkinson’s disease, where disease progression is relatively more predictable, the culture of MS involves the obscurity of symptom fluctuation, both from the patient’s and/or clinician’s viewpoint. Psychiatric and neurologic symptoms may be difficult to predict, leading to speculation and projection as to the progression of the disease. The diagnosis of psychiatric conditions, such as depression, can be complicated by the fact that MS and psychiatric disorders share presenting symptoms; for example, disturbances in sleep and concentration may be seen in both conditions.
While studies have examined the neurobiology of MS lesions and their effects on mood symptoms, there has been no clear consensus of specific lesion distributions, although lesions in the superior frontal lobe and right temporal lobe regions have been identified in depressed MS patients.8 Lesions in the left frontal lobe may also have some contribution; studies have shown hyperintense lesion load in this area, which was found to be an independent predictor of MDD in MS.9 This, in turn, coincides with the association of left frontal cortex involvement in modulating affective depression, evidenced by studies that have associated depression severity with left frontal lobe damage in post-stroke patients10 as well as the use of transcranial magnetic stimulation of the left prefrontal cortex for treatment-resistant MDD.11 Lesions along the orbitofrontal prefrontal cortex have similarly been connected to mood lability and impulsivity, which are characteristics of bipolar disorder.8 Within the general population, bipolar disorder is associated with areas of hyperintensity on MRI, particularly in the frontal and parietal white matter, which may provide clues as to the role of MS demyelinating lesions in similar locations, although research concerning the relationship between MS and bipolar disorder remains limited.12
EVALUATION No exacerbation of MS
Upon admission, Ms. A’s lability of affect is apparent as she quickly switches from being tearful to bright depending on the topic of discussion. She smiles when talking about the hobbies she enjoys and becomes tearful when speaking of personal problems within her family. She denies suicidal ideation/intent, shows no evidence of psychosis, and denies any history of bipolar disorder or recollection of hypomanic/manic symptoms. Overall, she exhibits low energy and difficulty sleeping, and reiterates her various psychosocial stressors, including her family history of depression and ongoing marital conflicts. Ms. A denies experiencing any acute exacerbations of clinical neurologic features of MS immediately before or during her admission. Laboratory values are normal, except for an elevated thyroid stimulating hormone (TSH) value of 11.136 uIU/mL, which is expected given her history of hypothyroidism. Results of the most recent brain MRI scans for Ms. A are pending.
The authors’ observations
Although we considered a diagnosis of bipolar disorder–mixed subtype, this was less likely to be the diagnosis considering her lack of any frank manic/hypomanic symptoms or history of such symptoms. Additionally, while we also considered a diagnosis of pseudobulbar affect due to her current mood swings and past trial of dextromethorphan/quinidine, this diagnosis was also less likely because Ms. A’s affect was not characterized by uncontrollable outbursts of emotion but was congruent with her experiences and surroundings. For example, Ms. A smiled when talking about her hobbies and became tearful when speaking of conflicts within her family.
Given Ms. A’s mood dysregulation and lability and her history of depressive episodes that began to manifest after her diagnosis of MS was established, and after ruling out other etiologic psychiatric disorders, a diagnosis of mood disorder secondary to MS was made.
[polldaddy:10175136]
Continue to: TREATMENT Mood stabilization
TREATMENT Mood stabilization
We start Ms. A on divalproex sodium, 250 mg 2 times a day, which is eventually titrated to 250 mg every morning with an additional daily 750 mg (total daily dose of 1,000 mg) for mood stabilization. Additionally, quetiapine, 50 mg nightly, is added and eventually titrated to 300 mg to augment mood stabilization and to aid sleep. Before being admitted, Ms. A had been prescribed
The authors’ observations
Definitive treatments for psychiatric conditions in patients with MS have been lacking, and current recommendations are based on regimens used to treat general psychiatric populations. For example, selective serotonin reuptake inhibitors are frequently considered for treatment of MDD in patients with MS, whereas SNRIs are considered for patients with concomitant neuropathic pain.13 Similarly,
OUTCOME Improved mood, energy
After 2 weeks of inpatient treatment, Ms. A shows improvement in mood lability and energy levels, and she is able to tolerate titration of divalproex sodium and quetiapine to therapeutic levels. She is referred to an outpatient psychiatrist after discharge, as well as a follow-up appointment with her neurologist. On discharge, Ms. A expresses a commitment to treatment and hope for the future.
1. National Multiple Sclerosis Society. Signs and symptoms consistent with demyelinating disease (for professionals). https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Diagnosing-MS/Signs-and-Symptoms-Consistent-with-Demyelinating-D. Accessed October 29, 2018.
2. Politte LC, Huffman JC, Stern TA. Neuropsychiatric manifestations of multiple sclerosis. Prim Care Companion J Clin Psychiatry. 2008;10(4):318-324.
3. Siegert RJ, Abernethy D. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
4. Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology. 2013;81(2):184-192.
5. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
6. Duncan A, Malcolm-Smith S, Ameen O, et al. The incidence of euphoria in multiple sclerosis: artefact of measure. Mult Scler Int. 2016;2016:1-8.
7. Paparrigopoulos T, Ferentinos P, Kouzoupis A, et al. The neuropsychiatry of multiple sclerosis: focus on disorders of mood, affect and behaviour. Int Rev Psychiatry. 2010;22(1):14-21.
8. Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport. 2000;11(6):1153-1158.
9. Feinstein A, Roy P, Lobaugh N, et al. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62(4):586-590.
10. Hama S, Yamashita H, Shigenobu M, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):149-152.
11. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
12. Beyer JL, Young R, Kuchibhatla M, et al. Hyperintense MRI lesions in bipolar disorder: a meta-analysis and review. Int Rev Psychiatry. 2009;21(4):394-409.
13. Feinstein A. Neuropsychiatric syndromes associated with multiple sclerosis. J Neurol. 2007;254(S2):1173-1176.
14. Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;(1):CD004431. doi: 10.1002/14651858.cd004431.pub2.
CASE Depression, or something else?
Ms. A, age 56, presents to the emergency department (ED) with depressed mood, poor sleep, anhedonia, irritability, agitation, and recent self-injurious behavior; she had superficially cut her wrists. She also has a longstanding history of multiple sclerosis (MS), depression, and anxiety. She is admitted voluntarily to an inpatient psychiatric unit.
According to medical records, at age 32, Ms. A was diagnosed with relapsing-remitting MS, which initially presented with facial numbness, and later with optic neuritis with transient loss of vision. As her disease progressed to the secondary progressive type, she experienced spasticity and vertigo. In the past few years, she also had experienced cognitive difficulties, particularly with memory and focus.
Ms. A has a history of recurrent depressive symptoms that began at an unspecified time after being diagnosed with MS. In the past few years, she had greatly increased her alcohol use in response to multiple psychosocial stressors and as an attempt to self-medicate MS-related pain. Several years ago, Ms. A had been admitted to a rehabilitation facility to address her alcohol use.
In the past, Ms. A’s depressive symptoms had been treated with various antidepressants, including fluoxetine (unspecified dose), which for a time was effective. The most recently prescribed antidepressant was duloxetine, 60 mg/d, which was discontinued because Ms. A felt it activated her mood lability. A few years before this current hospitalization, Ms. A had been started on a trial of dextromethorphan/quinidine (20 mg/10 mg, twice daily), which was discontinued due to concomitant use of an unspecified serotonin-norepinephrine reuptake inhibitor (SNRI) and subsequent precipitation of serotonin syndrome.
At the time of this current admission to the psychiatric unit, Ms. A is being treated for MS with rituximab (10 mg/mL IV, every 6 months). Additionally, just before her admission, she was taking alprazolam (.25 mg, 3 times per day) for anxiety. She denies experiencing any spasticity or vision impairment.
[polldaddy:10175070]
The authors’ observations
We initially considered a diagnosis of MDD due to Ms. A’s past history of depressive episodes, her recent increase in tearfulness and anhedonia, and her self-injurious behaviors. However, diagnosis of a mood disorder was complicated by her complex history of longstanding MS and other psychosocial factors.

Continue to: Several factors contribute to the neuropsychiatric course of patients with MS...
Several factors contribute to the neuropsychiatric course of patients with MS, including the impact of the patient accepting a chronic and incurable diagnosis, the toll of progressive neurologic/physical disability and subsequent decline in functioning, and the availability of a support system.2 As opposed to disorders such as Parkinson’s disease, where disease progression is relatively more predictable, the culture of MS involves the obscurity of symptom fluctuation, both from the patient’s and/or clinician’s viewpoint. Psychiatric and neurologic symptoms may be difficult to predict, leading to speculation and projection as to the progression of the disease. The diagnosis of psychiatric conditions, such as depression, can be complicated by the fact that MS and psychiatric disorders share presenting symptoms; for example, disturbances in sleep and concentration may be seen in both conditions.
While studies have examined the neurobiology of MS lesions and their effects on mood symptoms, there has been no clear consensus of specific lesion distributions, although lesions in the superior frontal lobe and right temporal lobe regions have been identified in depressed MS patients.8 Lesions in the left frontal lobe may also have some contribution; studies have shown hyperintense lesion load in this area, which was found to be an independent predictor of MDD in MS.9 This, in turn, coincides with the association of left frontal cortex involvement in modulating affective depression, evidenced by studies that have associated depression severity with left frontal lobe damage in post-stroke patients10 as well as the use of transcranial magnetic stimulation of the left prefrontal cortex for treatment-resistant MDD.11 Lesions along the orbitofrontal prefrontal cortex have similarly been connected to mood lability and impulsivity, which are characteristics of bipolar disorder.8 Within the general population, bipolar disorder is associated with areas of hyperintensity on MRI, particularly in the frontal and parietal white matter, which may provide clues as to the role of MS demyelinating lesions in similar locations, although research concerning the relationship between MS and bipolar disorder remains limited.12
EVALUATION No exacerbation of MS
Upon admission, Ms. A’s lability of affect is apparent as she quickly switches from being tearful to bright depending on the topic of discussion. She smiles when talking about the hobbies she enjoys and becomes tearful when speaking of personal problems within her family. She denies suicidal ideation/intent, shows no evidence of psychosis, and denies any history of bipolar disorder or recollection of hypomanic/manic symptoms. Overall, she exhibits low energy and difficulty sleeping, and reiterates her various psychosocial stressors, including her family history of depression and ongoing marital conflicts. Ms. A denies experiencing any acute exacerbations of clinical neurologic features of MS immediately before or during her admission. Laboratory values are normal, except for an elevated thyroid stimulating hormone (TSH) value of 11.136 uIU/mL, which is expected given her history of hypothyroidism. Results of the most recent brain MRI scans for Ms. A are pending.
The authors’ observations
Although we considered a diagnosis of bipolar disorder–mixed subtype, this was less likely to be the diagnosis considering her lack of any frank manic/hypomanic symptoms or history of such symptoms. Additionally, while we also considered a diagnosis of pseudobulbar affect due to her current mood swings and past trial of dextromethorphan/quinidine, this diagnosis was also less likely because Ms. A’s affect was not characterized by uncontrollable outbursts of emotion but was congruent with her experiences and surroundings. For example, Ms. A smiled when talking about her hobbies and became tearful when speaking of conflicts within her family.
Given Ms. A’s mood dysregulation and lability and her history of depressive episodes that began to manifest after her diagnosis of MS was established, and after ruling out other etiologic psychiatric disorders, a diagnosis of mood disorder secondary to MS was made.
[polldaddy:10175136]
Continue to: TREATMENT Mood stabilization
TREATMENT Mood stabilization
We start Ms. A on divalproex sodium, 250 mg 2 times a day, which is eventually titrated to 250 mg every morning with an additional daily 750 mg (total daily dose of 1,000 mg) for mood stabilization. Additionally, quetiapine, 50 mg nightly, is added and eventually titrated to 300 mg to augment mood stabilization and to aid sleep. Before being admitted, Ms. A had been prescribed
The authors’ observations
Definitive treatments for psychiatric conditions in patients with MS have been lacking, and current recommendations are based on regimens used to treat general psychiatric populations. For example, selective serotonin reuptake inhibitors are frequently considered for treatment of MDD in patients with MS, whereas SNRIs are considered for patients with concomitant neuropathic pain.13 Similarly,
OUTCOME Improved mood, energy
After 2 weeks of inpatient treatment, Ms. A shows improvement in mood lability and energy levels, and she is able to tolerate titration of divalproex sodium and quetiapine to therapeutic levels. She is referred to an outpatient psychiatrist after discharge, as well as a follow-up appointment with her neurologist. On discharge, Ms. A expresses a commitment to treatment and hope for the future.
CASE Depression, or something else?
Ms. A, age 56, presents to the emergency department (ED) with depressed mood, poor sleep, anhedonia, irritability, agitation, and recent self-injurious behavior; she had superficially cut her wrists. She also has a longstanding history of multiple sclerosis (MS), depression, and anxiety. She is admitted voluntarily to an inpatient psychiatric unit.
According to medical records, at age 32, Ms. A was diagnosed with relapsing-remitting MS, which initially presented with facial numbness, and later with optic neuritis with transient loss of vision. As her disease progressed to the secondary progressive type, she experienced spasticity and vertigo. In the past few years, she also had experienced cognitive difficulties, particularly with memory and focus.
Ms. A has a history of recurrent depressive symptoms that began at an unspecified time after being diagnosed with MS. In the past few years, she had greatly increased her alcohol use in response to multiple psychosocial stressors and as an attempt to self-medicate MS-related pain. Several years ago, Ms. A had been admitted to a rehabilitation facility to address her alcohol use.
In the past, Ms. A’s depressive symptoms had been treated with various antidepressants, including fluoxetine (unspecified dose), which for a time was effective. The most recently prescribed antidepressant was duloxetine, 60 mg/d, which was discontinued because Ms. A felt it activated her mood lability. A few years before this current hospitalization, Ms. A had been started on a trial of dextromethorphan/quinidine (20 mg/10 mg, twice daily), which was discontinued due to concomitant use of an unspecified serotonin-norepinephrine reuptake inhibitor (SNRI) and subsequent precipitation of serotonin syndrome.
At the time of this current admission to the psychiatric unit, Ms. A is being treated for MS with rituximab (10 mg/mL IV, every 6 months). Additionally, just before her admission, she was taking alprazolam (.25 mg, 3 times per day) for anxiety. She denies experiencing any spasticity or vision impairment.
[polldaddy:10175070]
The authors’ observations
We initially considered a diagnosis of MDD due to Ms. A’s past history of depressive episodes, her recent increase in tearfulness and anhedonia, and her self-injurious behaviors. However, diagnosis of a mood disorder was complicated by her complex history of longstanding MS and other psychosocial factors.

Continue to: Several factors contribute to the neuropsychiatric course of patients with MS...
Several factors contribute to the neuropsychiatric course of patients with MS, including the impact of the patient accepting a chronic and incurable diagnosis, the toll of progressive neurologic/physical disability and subsequent decline in functioning, and the availability of a support system.2 As opposed to disorders such as Parkinson’s disease, where disease progression is relatively more predictable, the culture of MS involves the obscurity of symptom fluctuation, both from the patient’s and/or clinician’s viewpoint. Psychiatric and neurologic symptoms may be difficult to predict, leading to speculation and projection as to the progression of the disease. The diagnosis of psychiatric conditions, such as depression, can be complicated by the fact that MS and psychiatric disorders share presenting symptoms; for example, disturbances in sleep and concentration may be seen in both conditions.
While studies have examined the neurobiology of MS lesions and their effects on mood symptoms, there has been no clear consensus of specific lesion distributions, although lesions in the superior frontal lobe and right temporal lobe regions have been identified in depressed MS patients.8 Lesions in the left frontal lobe may also have some contribution; studies have shown hyperintense lesion load in this area, which was found to be an independent predictor of MDD in MS.9 This, in turn, coincides with the association of left frontal cortex involvement in modulating affective depression, evidenced by studies that have associated depression severity with left frontal lobe damage in post-stroke patients10 as well as the use of transcranial magnetic stimulation of the left prefrontal cortex for treatment-resistant MDD.11 Lesions along the orbitofrontal prefrontal cortex have similarly been connected to mood lability and impulsivity, which are characteristics of bipolar disorder.8 Within the general population, bipolar disorder is associated with areas of hyperintensity on MRI, particularly in the frontal and parietal white matter, which may provide clues as to the role of MS demyelinating lesions in similar locations, although research concerning the relationship between MS and bipolar disorder remains limited.12
EVALUATION No exacerbation of MS
Upon admission, Ms. A’s lability of affect is apparent as she quickly switches from being tearful to bright depending on the topic of discussion. She smiles when talking about the hobbies she enjoys and becomes tearful when speaking of personal problems within her family. She denies suicidal ideation/intent, shows no evidence of psychosis, and denies any history of bipolar disorder or recollection of hypomanic/manic symptoms. Overall, she exhibits low energy and difficulty sleeping, and reiterates her various psychosocial stressors, including her family history of depression and ongoing marital conflicts. Ms. A denies experiencing any acute exacerbations of clinical neurologic features of MS immediately before or during her admission. Laboratory values are normal, except for an elevated thyroid stimulating hormone (TSH) value of 11.136 uIU/mL, which is expected given her history of hypothyroidism. Results of the most recent brain MRI scans for Ms. A are pending.
The authors’ observations
Although we considered a diagnosis of bipolar disorder–mixed subtype, this was less likely to be the diagnosis considering her lack of any frank manic/hypomanic symptoms or history of such symptoms. Additionally, while we also considered a diagnosis of pseudobulbar affect due to her current mood swings and past trial of dextromethorphan/quinidine, this diagnosis was also less likely because Ms. A’s affect was not characterized by uncontrollable outbursts of emotion but was congruent with her experiences and surroundings. For example, Ms. A smiled when talking about her hobbies and became tearful when speaking of conflicts within her family.
Given Ms. A’s mood dysregulation and lability and her history of depressive episodes that began to manifest after her diagnosis of MS was established, and after ruling out other etiologic psychiatric disorders, a diagnosis of mood disorder secondary to MS was made.
[polldaddy:10175136]
Continue to: TREATMENT Mood stabilization
TREATMENT Mood stabilization
We start Ms. A on divalproex sodium, 250 mg 2 times a day, which is eventually titrated to 250 mg every morning with an additional daily 750 mg (total daily dose of 1,000 mg) for mood stabilization. Additionally, quetiapine, 50 mg nightly, is added and eventually titrated to 300 mg to augment mood stabilization and to aid sleep. Before being admitted, Ms. A had been prescribed
The authors’ observations
Definitive treatments for psychiatric conditions in patients with MS have been lacking, and current recommendations are based on regimens used to treat general psychiatric populations. For example, selective serotonin reuptake inhibitors are frequently considered for treatment of MDD in patients with MS, whereas SNRIs are considered for patients with concomitant neuropathic pain.13 Similarly,
OUTCOME Improved mood, energy
After 2 weeks of inpatient treatment, Ms. A shows improvement in mood lability and energy levels, and she is able to tolerate titration of divalproex sodium and quetiapine to therapeutic levels. She is referred to an outpatient psychiatrist after discharge, as well as a follow-up appointment with her neurologist. On discharge, Ms. A expresses a commitment to treatment and hope for the future.
1. National Multiple Sclerosis Society. Signs and symptoms consistent with demyelinating disease (for professionals). https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Diagnosing-MS/Signs-and-Symptoms-Consistent-with-Demyelinating-D. Accessed October 29, 2018.
2. Politte LC, Huffman JC, Stern TA. Neuropsychiatric manifestations of multiple sclerosis. Prim Care Companion J Clin Psychiatry. 2008;10(4):318-324.
3. Siegert RJ, Abernethy D. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
4. Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology. 2013;81(2):184-192.
5. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
6. Duncan A, Malcolm-Smith S, Ameen O, et al. The incidence of euphoria in multiple sclerosis: artefact of measure. Mult Scler Int. 2016;2016:1-8.
7. Paparrigopoulos T, Ferentinos P, Kouzoupis A, et al. The neuropsychiatry of multiple sclerosis: focus on disorders of mood, affect and behaviour. Int Rev Psychiatry. 2010;22(1):14-21.
8. Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport. 2000;11(6):1153-1158.
9. Feinstein A, Roy P, Lobaugh N, et al. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62(4):586-590.
10. Hama S, Yamashita H, Shigenobu M, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):149-152.
11. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
12. Beyer JL, Young R, Kuchibhatla M, et al. Hyperintense MRI lesions in bipolar disorder: a meta-analysis and review. Int Rev Psychiatry. 2009;21(4):394-409.
13. Feinstein A. Neuropsychiatric syndromes associated with multiple sclerosis. J Neurol. 2007;254(S2):1173-1176.
14. Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;(1):CD004431. doi: 10.1002/14651858.cd004431.pub2.
1. National Multiple Sclerosis Society. Signs and symptoms consistent with demyelinating disease (for professionals). https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Diagnosing-MS/Signs-and-Symptoms-Consistent-with-Demyelinating-D. Accessed October 29, 2018.
2. Politte LC, Huffman JC, Stern TA. Neuropsychiatric manifestations of multiple sclerosis. Prim Care Companion J Clin Psychiatry. 2008;10(4):318-324.
3. Siegert RJ, Abernethy D. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76(4):469-475.
4. Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology. 2013;81(2):184-192.
5. Ghaffar O, Feinstein A. The neuropsychiatry of multiple sclerosis: a review of recent developments. Curr Opin Psychiatry. 2007;20(3):278-285.
6. Duncan A, Malcolm-Smith S, Ameen O, et al. The incidence of euphoria in multiple sclerosis: artefact of measure. Mult Scler Int. 2016;2016:1-8.
7. Paparrigopoulos T, Ferentinos P, Kouzoupis A, et al. The neuropsychiatry of multiple sclerosis: focus on disorders of mood, affect and behaviour. Int Rev Psychiatry. 2010;22(1):14-21.
8. Bakshi R, Czarnecki D, Shaikh ZA, et al. Brain MRI lesions and atrophy are related to depression in multiple sclerosis. Neuroreport. 2000;11(6):1153-1158.
9. Feinstein A, Roy P, Lobaugh N, et al. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62(4):586-590.
10. Hama S, Yamashita H, Shigenobu M, et al. Post-stroke affective or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. Eur Arch Psychiatry Clin Neurosci. 2007;257(3):149-152.
11. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
12. Beyer JL, Young R, Kuchibhatla M, et al. Hyperintense MRI lesions in bipolar disorder: a meta-analysis and review. Int Rev Psychiatry. 2009;21(4):394-409.
13. Feinstein A. Neuropsychiatric syndromes associated with multiple sclerosis. J Neurol. 2007;254(S2):1173-1176.
14. Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;(1):CD004431. doi: 10.1002/14651858.cd004431.pub2.
Ask depressed patients about hypersomnia to screen for mixicity
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
BARCELONA – Hypersomnia is a novel, previously unappreciated factor useful in tipping the balance in favor of an underlying bipolar predisposition in patients with an acute major depressive episode, Andrea Murru, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
“This may help us in clinical practice. It’s an effective, costless, and highly objective clinical measure. It’s one question, and it takes a second. It’s simply asking the patient: ‘Are you sleeping more hours at night than usual?’ It’s a very simple clinical question that could really change the focus of treatment for a patient,” said Dr. Murru, a clinical psychiatrist in the bipolar disorders program of the University of Barcelona.
He presented a post hoc analysis of 2,514 acutely depressed individuals who participated in the BRIDGE-II-MIX (Bipolar Disorders: Improving Diagnosis, Guidance and Education) study, an international, multicenter, cross-sectional, observational study aimed at better characterizing clinically valid mixed features of depression indicative of concurrent manic symptoms.
“This is one of a whole series of hypothesis-generating studies from BRIDGE-II-MIX that are trying to deal with the struggle of understanding whether all the elements that favor mixicity and an underpinning bipolar diathesis are fairly represented in the diagnostic criteria in DSM-5. And what we are basically finding is the DSM-5 criteria are leaving out important symptoms that really do play a role,” the psychiatrist said in an interview.
One of those missing factors, he continued, is hypersomnia. It was present in 16.8% of the study population, and he and his coinvestigators compared them in terms of clinical variables, current and past psychiatric symptoms, and sociodemographics with the 83.2% of patients with insomnia. That is, patients who got fewer hours of sleep than normal and felt fatigued during the next day were compared with those who felt a reduced need to sleep.
The two groups differed in important ways. Hypersomnia showed a powerful correlation with a physician diagnosis of major depressive episode with atypical features, being present in 32.2% of such patients, while a mere 1.8% had insomnia. Moreover, among patients diagnosed with bipolar disorder I or II, 20.6% reported hypersomnia, a significantly higher proportion than the 16% who had insomnia.
The finding that only 5% of BRIDGE-II-MIX participants with hypersomnia met DSM-5 criteria for a mixed-state specifier, a rate not significantly different from the 8.3% figure in those with insomnia, underscores the drawbacks of the DSM-5 criteria, according to Dr. Murru. He noted that, in contrast to the DSM-5 criteria, 32.9% of the hypersomniac patients with a major depressive episode met Research Diagnostic Criteria (RDC) for a mixed-state specifier, a rate significantly higher than the 27.6% figure in patients with insomnia.
Specifically, the individual RDC mixed-state specifiers that stood out as significantly more frequent among depressed patients with hypersomnia than insomnia were racing thoughts, by a margin of 15.1% to 10.6%; impulsivity, 16.8% versus 13.2%; distractibility, 29.6% versus 23.4%; hypersexuality, which was present in 4% of patients with hypersomnia but only 2.3% with insomnia; irritable mood, 33.1% versus 24.8%; and a history of insufficient response to previous antidepressant therapy, 34.3%, compared with 27.1% in insomniacs.
When Dr. Murru and his coinvestigators performed a stepwise linear regression analysis to identify significant predictors of hypersomnia in patients with a major depressive episode, they found that the sleep abnormality keeps some interesting company. Patients with current bulimia were 4.21-fold more likely to have hypersomnia than those without the eating disorder. Current social phobia was associated with a 1.77-fold increased risk of hypersomnia; mood lability on prior antidepressant therapy carried a 1.37-fold risk, as did current mood lability; prior attempted suicide was associated with a 1.31-fold increased risk; being overweight or obese was associated with a 1.42-fold risk; currently being on a mood stabilizer carried a 1.33-fold increased risk of hypersomnia; and currently being on an atypical antipsychotic agent had a 1.36-fold greater risk.
Dr. Murru concluded that the take-home message of this study – “Of course, conceding it’s highly exploratory nature intrinsic to a post hoc analysis,” he noted – is that hypersomnia should be included among the symptoms that trigger the “with mixed features” specifier in patients with a major depressive episode.
The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. Dr. Murru reported having no financial conflicts of interest regarding the study.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Ask patients with a major depressive episode about hypersomnia.
Major finding: Hypersomnia in patients with an acute major depressive episode clusters with numerous elements of a bipolar diathesis.
Study details: This was a post hoc analysis of 2,514 acutely depressed individuals who participated in an international, multicenter, cross-sectional, observational study.
Disclosures: The BRIDGE-II-MIX study was sponsored by Sanofi-Aventis. The presenter reported having no financial conflicts of interest regarding the study.
Mood stabilizers protect bipolar patients from suicide
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
BARCELONA – Lithium and valproic acid are the treatments of choice for patients with bipolar disorder at increased risk for suicide, according to the findings of a large Finnish national study.
“We found that – surprise, surprise – lithium and valproic acid had the lowest risk of anyone committing suicide on them. And funnily enough, some antidepressants seemed to be correlated with a higher risk of committing suicide,” Markku Lähteenvuo, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
However, he suspects that the correlation between antidepressant therapy and suicide in bipolar patients probably was caused by confounding by indication.
“I wouldn’t have as a take-home message that SSRI [selective serotonin reuptake inhibitor] antidepressants are bad for you; it’s probably rather that these are really bad cases, treatment gets initiated when someone gets worse, and the antidepressants just aren’t quick enough to really give an effect. It takes 3-4 weeks for an SSRI to really kick in, whereas mood stabilizers like lithium and valproic acid usually kick in almost immediately, within a couple of days to a week,” explained Dr. Lähteenvuo, a forensic psychiatrist at Niuvanniemi Hospital and the University of Eastern Finland, in Kuopio.
He presented a study of all suicides among the 18,018 Finnish patients hospitalized for bipolar disorder nationwide during 1996-2012. Using prospective national databases to follow patients for a mean of 7.2 years, he and his coinvestigators were able to determine what medications they were on when they committed suicide and the likelihood they were actually taking their prescribed medications at the time.
In a multivariate proportional hazards analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, intervals of drug exposure and nonexposure, age, sex, and number of hospitalizations for suicidality within the prior 2 years, bipolar patients on lithium had a 67% lower risk of suicide than did those not on the drug. Those on valproic acid were 39% less likely to commit suicide than those not on that mood stabilizer. And bipolar patients on lithium were 42% less likely to die from suicide than those on valproic acid.
At the other extreme, patients on antidepressants were in aggregate 28% more likely to commit suicide than those who were not. This risk varied considerably according to the specific antidepressant: for example, escitalopram and mirtazapine were associated with 71% and 57% greater risks of suicide, respectively, than in patients not on those antidepressants, while patients on paroxetine, venlafaxine, or sertraline were not at increased risk.
Also, the use of sedatives was associated with a 52% greater likelihood of suicide, and benzodiazepines were linked to a 21% increase in risk. Again, as with antidepressants, these associations with increased risk of completed suicide could be attributable in part or in whole to confounding by indication, the psychiatrist noted. He and his colleagues are conducting further analyses examining that possibility.
Pending the outcome of that closer look, however, Dr. Lähteenvuo believes the study’s message to clinicians is clear: “The use of lithium is getting less and less common all the time because of side effects. That’s also true for valproic acid. Many bipolar patients are now prescribed only antidepressants, even though all the guidelines advise against it due to the risk of hypomania. We think that this is a bad trend and that lithium and valproic acid should be prescribed more. Also, as a safety precaution, anyone with bipolar disorder on antidepressants or benzodiazepines or sedatives should be followed especially closely for suicidal signals, because they could carry a really highly increased risk – up to 50% – due to the medication as well.”
He reported having no financial conflicts regarding the study, funded by the Finnish government.
Dr. Lähteenvuo also was first author of a recent study showing that among the various drugs prescribed for treatment of bipolar disorder, lithium was the clear winner for prevention of rehospitalization in the same national Finnish population of more than 18,000 bipolar patients (JAMA Psychiatry. 2018;75[4]:347-55). One expert named this among the top four studies of the year in the field of mood disorders.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Lithium and valproic acid protect patients with bipolar disorder from suicide.
Major finding: The likelihood of suicide was reduced by two-thirds in bipolar patients on lithium, compared with that of patients not on the mood stabilizer.
Study details: This was a retrospective study of more than 18,000 Finnish patients hospitalized at some point for bipolar disorder and the medications they were on when a subset of them committed suicide.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by the Finnish government.
Disruptive mood dysregulation disorder: A better understanding
Disruptive mood dysregulation disorder (DMDD)—a childhood condition of extreme irritability, anger, and frequent, intense temper outbursts—has been a source of controversy among clinicians in the field of pediatric mental health. Before DSM-5 was published, the validity of DMDD had been questioned because DMDD had failed a field trial; agreement between clinicians on the diagnosis of DMDD was poor.1 Axelson2 and Birmaher et al3 examined its validity in their COBY (Course and Outcome of Bipolar Youth) sample. They concluded that only 19% met the criteria for DMDD in 3 times of follow-up. Furthermore, most DMDD criteria overlap with those of other common pediatric psychiatric disorders, including oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and pediatric bipolar disorder (BD). Because diagnosis of pediatric BD increased drastically from 2.9% to 15.1% between 1990 and 2000,4 it was believed that introducing DMDD as a diagnosis might lessen the overdiagnosis of pediatric BD by identifying children with chronic irritability and temper tantrums who previously would have been diagnosed with BD.
It is important to recognize that in pediatric patients, mood disorders present differently than they do in adults.5 In children/adolescents, mood disorders are less likely to present as distinct episodes (narrow band), but more likely to present as chronic, broad symptoms. Also, irritability is a common presentation in many pediatric psychiatric disorders, such as ODD, BD (irritability without elation),6 and depression. Thus, for many clinicians, determining the correct mood disorder diagnosis in pediatric patients can be challenging.
This article describes the diagnosis of DMDD, and how its presentation is similar to—and different from—those of other common pediatric psychiatric disorders.
_
The origin of DMDD
Many researchers have investigated the broadband phenotypical presentation of pediatric mood disorders, which have been mostly diagnosed in the psychiatric community as pediatric BD. Leibenluft7 identified a subtype of mood disorder that they termed “severe mood dysregulation” (SMD). Compared with the narrow-band, clearly episodic BD, SMD has a different trajectory, outcome, and findings on brain imaging. SMD is characterized by chronic irritability with abnormal mood (anger or sadness) at least half of the day on most days, with 3 hyperarousal symptoms, including pressured speech, racing thoughts or flight of ideas, intrusiveness, distractibility, insomnia, and agitation.8 Eventually, SMD became the foundation of the development of DMDD.
DSM-5 diagnostic criteria for DMDD include severe recurrent temper outbursts that are out of proportion to the situation, inconsistent with developmental level, and occurring on average ≥3 times per week, plus persistently irritable or angry mood for most of the day nearly every day.9 Additional criteria include the presence of symptoms for at least 12 months (without a symptom-free period of at least 3 consecutive months) in ≥2 settings (at home, at school, or with peers) with onset before age 10. The course of DMDD typically is chronic with accompanying severe temperament. The estimated 6-month to 1-year prevalence is 2% to 5%; the diagnosis is more common among males and school-age children than it is in females and adolescents.9,10
_
DMDD or bipolar disorder?
A patient cannot be dually diagnosed with both disorders. If a patient exhibits a manic episode for more than 1 day, that would null and void the DMDD diagnosis. However, in a study to evaluate BD in pediatric patients, researchers divided BD symptoms into BD-specific categories (elevated mood, grandiosity, and increased goal-directed activity) and nonspecific symptoms such as irritability and talkativeness, distractibility, and flight of ideas or racing thoughts. They found that in the absence of specific symptoms, a diagnosis of BD is unlikely to be the correct diagnosis.11 Hence, as a nonspecific symptom, chronic irritability should be attributed to the symptom count for DMDD, rather than BD. Most epidemiologic studies have concluded that depression and anxiety, and not irritability, are typically the preceeding presentations prior to the development of BD in young adults.12 Chronic irritability, however, predicts major depressive disorder and anxiety disorders in later adolescence and one’s early twenties.13 Furthermore, BD commonly presents with infrequent and discrete episodes and a later age of onset, while DMDD presents with chronic and frequent, severe temper outbursts. Some differences between DMDD and BD are illustrated in Table 1.11-13
Continue to: CASE 1
CASE 1
Irritable and taking risks
Ms. N, age 16, is brought to the outpatient psychiatry clinic by her parents for evaluation of mood symptoms, including irritability. Her mother claims her daughter was an introverted, anxious, shy child, but by the beginning of middle school, she began to feel irritable and frequently stayed up at night with little sleep. In high school, Ms. N had displayed several episodes of risk-taking behaviors, including taking her father’s vehicle for a drive despite not having a driver’s permit, running away for 2 days, and having unprotected sex.
During her assessment, Ms. N is pleasant and claims she usually has a great mood. She fought with her mother several times this year, which led her to run away. Her parents had divorced when Ms. N was 5 years old and have shared custody. Ms. N is doing well in school despite her parents’ concerns.
Diagnosis. The most likely diagnosis is emerging BD. Notice that Ms. N may have had anxiety symptoms before she developed irritability. She had a relatively late onset of symptoms that were episodic in nature, which further supports a diagnosis of BD.
_
>
DMDD or oppositional defiant disorder?
DMDD and ODD cannot be dually diagnosed. However, if a patient meets the criteria for both DMDD and ODD, only the DMDD diagnosis should be considered. One of many issues of DMDD is its similarity to ODD. In fact, more than 70% of patients with DMDD also meet the diagnostic criteria for ODD.10,14 Some researchers have conceptualized DMDD as a severe form of ODD. However, there are a few differences that clinicians can use to distinguish the 2 disorders.
Compared with patients with ODD, those with DMDD more frequently experience severe irritability.15 Patients with ODD may present with delinquent behaviors and trouble with authority figures. Moreover, comorbidity with ADHD is twice as common in ODD; more than 65% of patients with ADHD have ODD vs 30% who have DMDD.10,16 Finally, in general, children with DMDD have more social impairments compared with those with ODD. Differences between DMDD and BD are illustrated in Table 2.10,14-16
Continue to: CASE 2
CASE 2
Angry and defiant
Mr. R, age 14, is brought to the emergency department (ED) by his parents after becoming very aggressive with them. He punched a wall and vandalized his room after his parents grounded him because of his previous defiant behavior. He had been suspended from school that day for disrespecting his teacher after he was caught fighting another student.
His parents describe Mr. R as a strong-willed, stubborn child. He has difficulty with rules and refuses to follow them. He is grouchy and irritable around adults, including the ED staff. Mr. R enjoys being with his friends and playing video games. He had been diagnosed with ADHD when he was in kindergarten, when his teacher noticed he had a poor attention span and could not sit still. According to his parents, Mr. R has “blown up” a few times before, smashing items in his room and shouting obscenities. Mr. R’s parents noticed that he is more defiant in concurrence with discontinuing his ADHD stimulant medication.
Diagnosis. The most likely diagnosis for Mr. R is ODD. Notice the comorbidity of ADHD, which is more commonly associated with ODD. The frequency and severity of his outbursts and irritability symptoms were lower than that typically associated with DMDD.
_
Treatment strategies for DMDD
Management of DMDD should focus on helping children and adolescents improve their emotional dysregulation.
Clinicians should always consider behavioral therapy as a first-line intervention. The behavioral planning team may include (but is not limited to) a behavior specialist, child psychiatrist, psychologist, therapist, skills trainer, teachers, and the caregiver(s). The plan should be implemented across all settings, including home and school. Furthermore, social skills training is necessary for many children with DMDD, who may require intensive behavioral modification planning. Comorbidity with ADHD should be addressed with a combination of behavioral planning and stimulant medications.17 If available, parent training and parent-child interactive therapy can help to improve defiant behavior.
Pharmacotherapy
Currently, no medications are FDA-approved for treating DMDD. Most pharmacologic trials that included patients with DMDD focused on managing chronic irritability and/or stabilizing comorbid disorders (ie, ADHD, depression, and anxiety).
Continue to: Stimulants
Stimulants. Previous trials examined the benefit of CNS stimulant medications, alone or in conjunction with behavioral therapy, in treating DMDD and comorbid ADHD. Methylphenidate results in a significant reduction in aggression18 with a dosing recommendation range from 1 to 1.2 mg/kg/d. CNS stimulants should be considered as first-line pharmacotherapy for DMDD, especially for patients with comorbid ADHD.
Anticonvulsants. Divalproex sodium is superior to placebo in treating aggression in children and adolescents.19 Trials found that divalproex sodium reduces irritability and aggression whether it is prescribed as monotherapy or combined with stimulant medications.19
Lithium is one of the main treatment options for mania in BD. The benefits of lithium for controlling aggression in DMDD are still under investigation. Earlier studies found that lithium significantly improves aggressive behavior in hospitalized pediatric with conduct disorder.20,21 However, a later study that evaluated lithium vs placebo for children with SMD (which arguably is phenotypically related to the DMDD) found there were no significant differences in improvement of irritability symptoms between groups.22 More research is needed to determine if lithium may play a role in treating patients with DMDD.
Antipsychotics. Aripiprazole and risperidone are FDA-approved for treating irritability in autism. A 2017 meta-analysis found both medications were effective in controlling irritability and aggression in other diagnoses as well.23 Other antipsychotic medications did not show sufficient benefits in treating irritability.23 When considering antipsychotics, clinicians should weigh the risks of metabolic adverse effects and follow practice guidelines.
Antidepressants. A systematic review did not find that selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors effectively reduce irritability.24 However, in most of the studies evaluated, irritability was not the primary outcome measure.24
Other medications. Alpha-2 agonists (guanfacine, clonidine), and atomoxetine may help irritability indirectly by improving ADHD symptoms.25
Bottom Line
Disruptive mood dysregulation disorder (DMDD), bipolar disorder, and oppositional defiant disorder have similar presentations and diagnostic criteria. The frequency and severity of irritability can be a distinguishing factor. Behavioral therapy is a first-line treatment. No medications are FDA-approved for treating DMDD, but pharmacotherapy may help reduce irritability and aggression.
Related Resources
- Rao U. DSM-5: disruptive mood dysregulation disorder. Asian J Psychiatr. 2014;11:119-123.
- Roy AK, Lopes V, Klein RG. Disruptive mood dysregulation disorder: a new diagnostic approach to chronic irritability in youth. Am J Psychiatry. 2014;171(9):918-924.
Drug Brand Names
Aripiprazole • Abilify
Atomoxetine • Strattera
Clonidine • Catapres
Divalproex sodium • Depakote, Depakote ER
Guanfacine • Intuniv, Tenex
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta, Ritalin
Risperidone • Risperdal
1. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
2. Axelson D. Taking disruptive mood dysregulation disorder out for a test drive. Am J Psychiatry. 2013;170(2):136-139.
3. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
4. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64(1):89-96.
5. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
6. Hunt J, Birmaher B, Leonard H, et al. Irritability without elation in a large bipolar youth sample: frequency and clinical description. J Am Acad Child Adolesc Psychiatry. 2009;48(7):730-739.
7. Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
8. Rich BA, Carver FW, Holroyd T, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011;45(10):1283-1294.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Copeland WE, Angold A, Costello EJ, et al. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. Am J Psychiatry. 2013;170(2):173-179.
11. Elmaadawi AZ, Jensen PS, Arnold LE, et al. Risk for emerging bipolar disorder, variants, and symptoms in children with attention deficit hyperactivity disorder, now grown up. World J Psychiatry. 2015;5(4):412-424.
12. Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55(8):477-485.
13. Stringaris A, Cohen P, Pine DS, et al. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048-1054.
14. Mayes SD, Waxmonsky JD, Calhoun SL, et al. Disruptive mood dysregulation disorder symptoms and association with oppositional defiant and other disorders in a general population child sample. J Child Adolesc Psychopharmacol. 2016;26(2):101-106.
15. Stringaris A, Vidal-Ribas P, Brotman MA, et al. Practitioner review: definition, recognition, and treatment challenges of irritability in young people. J Child Psychol Psychiatry. 2018;59(7):721-739.
16. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57-87.
17. Fernandez de la Cruz L, Simonoff E, McGough JJ, et al. Treatment of children with attention-deficit/hyperactivity disorder (ADHD) and irritability: results from the multimodal treatment study of children with ADHD (MTA). J Am Acad Child Adolesc Psychiatry. 2015;54(1):62-70.
18. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry. 2000;157(5):818-820.
20. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
21. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
22. Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19(1):61-73.
23. van Schalkwyk GI, Lewis AS, Beyer C, et al. Efficacy of antipsychotics for irritability and aggression in children: a meta-analysis. Expert Rev Neurother. 2017;17(10):1045-1053.
24. Kim S, Boylan K. Effectiveness of antidepressant medications for symptoms of irritability and disruptive behaviors in children and adolescents. J Child Adolesc Psychopharmacol. 2016;26(8):694-704.
25. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067-1074.
Disruptive mood dysregulation disorder (DMDD)—a childhood condition of extreme irritability, anger, and frequent, intense temper outbursts—has been a source of controversy among clinicians in the field of pediatric mental health. Before DSM-5 was published, the validity of DMDD had been questioned because DMDD had failed a field trial; agreement between clinicians on the diagnosis of DMDD was poor.1 Axelson2 and Birmaher et al3 examined its validity in their COBY (Course and Outcome of Bipolar Youth) sample. They concluded that only 19% met the criteria for DMDD in 3 times of follow-up. Furthermore, most DMDD criteria overlap with those of other common pediatric psychiatric disorders, including oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and pediatric bipolar disorder (BD). Because diagnosis of pediatric BD increased drastically from 2.9% to 15.1% between 1990 and 2000,4 it was believed that introducing DMDD as a diagnosis might lessen the overdiagnosis of pediatric BD by identifying children with chronic irritability and temper tantrums who previously would have been diagnosed with BD.
It is important to recognize that in pediatric patients, mood disorders present differently than they do in adults.5 In children/adolescents, mood disorders are less likely to present as distinct episodes (narrow band), but more likely to present as chronic, broad symptoms. Also, irritability is a common presentation in many pediatric psychiatric disorders, such as ODD, BD (irritability without elation),6 and depression. Thus, for many clinicians, determining the correct mood disorder diagnosis in pediatric patients can be challenging.
This article describes the diagnosis of DMDD, and how its presentation is similar to—and different from—those of other common pediatric psychiatric disorders.
_
The origin of DMDD
Many researchers have investigated the broadband phenotypical presentation of pediatric mood disorders, which have been mostly diagnosed in the psychiatric community as pediatric BD. Leibenluft7 identified a subtype of mood disorder that they termed “severe mood dysregulation” (SMD). Compared with the narrow-band, clearly episodic BD, SMD has a different trajectory, outcome, and findings on brain imaging. SMD is characterized by chronic irritability with abnormal mood (anger or sadness) at least half of the day on most days, with 3 hyperarousal symptoms, including pressured speech, racing thoughts or flight of ideas, intrusiveness, distractibility, insomnia, and agitation.8 Eventually, SMD became the foundation of the development of DMDD.
DSM-5 diagnostic criteria for DMDD include severe recurrent temper outbursts that are out of proportion to the situation, inconsistent with developmental level, and occurring on average ≥3 times per week, plus persistently irritable or angry mood for most of the day nearly every day.9 Additional criteria include the presence of symptoms for at least 12 months (without a symptom-free period of at least 3 consecutive months) in ≥2 settings (at home, at school, or with peers) with onset before age 10. The course of DMDD typically is chronic with accompanying severe temperament. The estimated 6-month to 1-year prevalence is 2% to 5%; the diagnosis is more common among males and school-age children than it is in females and adolescents.9,10
_
DMDD or bipolar disorder?
A patient cannot be dually diagnosed with both disorders. If a patient exhibits a manic episode for more than 1 day, that would null and void the DMDD diagnosis. However, in a study to evaluate BD in pediatric patients, researchers divided BD symptoms into BD-specific categories (elevated mood, grandiosity, and increased goal-directed activity) and nonspecific symptoms such as irritability and talkativeness, distractibility, and flight of ideas or racing thoughts. They found that in the absence of specific symptoms, a diagnosis of BD is unlikely to be the correct diagnosis.11 Hence, as a nonspecific symptom, chronic irritability should be attributed to the symptom count for DMDD, rather than BD. Most epidemiologic studies have concluded that depression and anxiety, and not irritability, are typically the preceeding presentations prior to the development of BD in young adults.12 Chronic irritability, however, predicts major depressive disorder and anxiety disorders in later adolescence and one’s early twenties.13 Furthermore, BD commonly presents with infrequent and discrete episodes and a later age of onset, while DMDD presents with chronic and frequent, severe temper outbursts. Some differences between DMDD and BD are illustrated in Table 1.11-13

Continue to: CASE 1
CASE 1
Irritable and taking risks
Ms. N, age 16, is brought to the outpatient psychiatry clinic by her parents for evaluation of mood symptoms, including irritability. Her mother claims her daughter was an introverted, anxious, shy child, but by the beginning of middle school, she began to feel irritable and frequently stayed up at night with little sleep. In high school, Ms. N had displayed several episodes of risk-taking behaviors, including taking her father’s vehicle for a drive despite not having a driver’s permit, running away for 2 days, and having unprotected sex.
During her assessment, Ms. N is pleasant and claims she usually has a great mood. She fought with her mother several times this year, which led her to run away. Her parents had divorced when Ms. N was 5 years old and have shared custody. Ms. N is doing well in school despite her parents’ concerns.
Diagnosis. The most likely diagnosis is emerging BD. Notice that Ms. N may have had anxiety symptoms before she developed irritability. She had a relatively late onset of symptoms that were episodic in nature, which further supports a diagnosis of BD.
_
>
DMDD or oppositional defiant disorder?
DMDD and ODD cannot be dually diagnosed. However, if a patient meets the criteria for both DMDD and ODD, only the DMDD diagnosis should be considered. One of many issues of DMDD is its similarity to ODD. In fact, more than 70% of patients with DMDD also meet the diagnostic criteria for ODD.10,14 Some researchers have conceptualized DMDD as a severe form of ODD. However, there are a few differences that clinicians can use to distinguish the 2 disorders.
Compared with patients with ODD, those with DMDD more frequently experience severe irritability.15 Patients with ODD may present with delinquent behaviors and trouble with authority figures. Moreover, comorbidity with ADHD is twice as common in ODD; more than 65% of patients with ADHD have ODD vs 30% who have DMDD.10,16 Finally, in general, children with DMDD have more social impairments compared with those with ODD. Differences between DMDD and BD are illustrated in Table 2.10,14-16

Continue to: CASE 2
CASE 2
Angry and defiant
Mr. R, age 14, is brought to the emergency department (ED) by his parents after becoming very aggressive with them. He punched a wall and vandalized his room after his parents grounded him because of his previous defiant behavior. He had been suspended from school that day for disrespecting his teacher after he was caught fighting another student.
His parents describe Mr. R as a strong-willed, stubborn child. He has difficulty with rules and refuses to follow them. He is grouchy and irritable around adults, including the ED staff. Mr. R enjoys being with his friends and playing video games. He had been diagnosed with ADHD when he was in kindergarten, when his teacher noticed he had a poor attention span and could not sit still. According to his parents, Mr. R has “blown up” a few times before, smashing items in his room and shouting obscenities. Mr. R’s parents noticed that he is more defiant in concurrence with discontinuing his ADHD stimulant medication.
Diagnosis. The most likely diagnosis for Mr. R is ODD. Notice the comorbidity of ADHD, which is more commonly associated with ODD. The frequency and severity of his outbursts and irritability symptoms were lower than that typically associated with DMDD.
_
Treatment strategies for DMDD
Management of DMDD should focus on helping children and adolescents improve their emotional dysregulation.
Clinicians should always consider behavioral therapy as a first-line intervention. The behavioral planning team may include (but is not limited to) a behavior specialist, child psychiatrist, psychologist, therapist, skills trainer, teachers, and the caregiver(s). The plan should be implemented across all settings, including home and school. Furthermore, social skills training is necessary for many children with DMDD, who may require intensive behavioral modification planning. Comorbidity with ADHD should be addressed with a combination of behavioral planning and stimulant medications.17 If available, parent training and parent-child interactive therapy can help to improve defiant behavior.
Pharmacotherapy
Currently, no medications are FDA-approved for treating DMDD. Most pharmacologic trials that included patients with DMDD focused on managing chronic irritability and/or stabilizing comorbid disorders (ie, ADHD, depression, and anxiety).
Continue to: Stimulants
Stimulants. Previous trials examined the benefit of CNS stimulant medications, alone or in conjunction with behavioral therapy, in treating DMDD and comorbid ADHD. Methylphenidate results in a significant reduction in aggression18 with a dosing recommendation range from 1 to 1.2 mg/kg/d. CNS stimulants should be considered as first-line pharmacotherapy for DMDD, especially for patients with comorbid ADHD.
Anticonvulsants. Divalproex sodium is superior to placebo in treating aggression in children and adolescents.19 Trials found that divalproex sodium reduces irritability and aggression whether it is prescribed as monotherapy or combined with stimulant medications.19
Lithium is one of the main treatment options for mania in BD. The benefits of lithium for controlling aggression in DMDD are still under investigation. Earlier studies found that lithium significantly improves aggressive behavior in hospitalized pediatric with conduct disorder.20,21 However, a later study that evaluated lithium vs placebo for children with SMD (which arguably is phenotypically related to the DMDD) found there were no significant differences in improvement of irritability symptoms between groups.22 More research is needed to determine if lithium may play a role in treating patients with DMDD.
Antipsychotics. Aripiprazole and risperidone are FDA-approved for treating irritability in autism. A 2017 meta-analysis found both medications were effective in controlling irritability and aggression in other diagnoses as well.23 Other antipsychotic medications did not show sufficient benefits in treating irritability.23 When considering antipsychotics, clinicians should weigh the risks of metabolic adverse effects and follow practice guidelines.
Antidepressants. A systematic review did not find that selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors effectively reduce irritability.24 However, in most of the studies evaluated, irritability was not the primary outcome measure.24
Other medications. Alpha-2 agonists (guanfacine, clonidine), and atomoxetine may help irritability indirectly by improving ADHD symptoms.25
Bottom Line
Disruptive mood dysregulation disorder (DMDD), bipolar disorder, and oppositional defiant disorder have similar presentations and diagnostic criteria. The frequency and severity of irritability can be a distinguishing factor. Behavioral therapy is a first-line treatment. No medications are FDA-approved for treating DMDD, but pharmacotherapy may help reduce irritability and aggression.
Related Resources
- Rao U. DSM-5: disruptive mood dysregulation disorder. Asian J Psychiatr. 2014;11:119-123.
- Roy AK, Lopes V, Klein RG. Disruptive mood dysregulation disorder: a new diagnostic approach to chronic irritability in youth. Am J Psychiatry. 2014;171(9):918-924.
Drug Brand Names
Aripiprazole • Abilify
Atomoxetine • Strattera
Clonidine • Catapres
Divalproex sodium • Depakote, Depakote ER
Guanfacine • Intuniv, Tenex
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta, Ritalin
Risperidone • Risperdal
Disruptive mood dysregulation disorder (DMDD)—a childhood condition of extreme irritability, anger, and frequent, intense temper outbursts—has been a source of controversy among clinicians in the field of pediatric mental health. Before DSM-5 was published, the validity of DMDD had been questioned because DMDD had failed a field trial; agreement between clinicians on the diagnosis of DMDD was poor.1 Axelson2 and Birmaher et al3 examined its validity in their COBY (Course and Outcome of Bipolar Youth) sample. They concluded that only 19% met the criteria for DMDD in 3 times of follow-up. Furthermore, most DMDD criteria overlap with those of other common pediatric psychiatric disorders, including oppositional defiant disorder (ODD), attention-deficit/hyperactivity disorder (ADHD), and pediatric bipolar disorder (BD). Because diagnosis of pediatric BD increased drastically from 2.9% to 15.1% between 1990 and 2000,4 it was believed that introducing DMDD as a diagnosis might lessen the overdiagnosis of pediatric BD by identifying children with chronic irritability and temper tantrums who previously would have been diagnosed with BD.
It is important to recognize that in pediatric patients, mood disorders present differently than they do in adults.5 In children/adolescents, mood disorders are less likely to present as distinct episodes (narrow band), but more likely to present as chronic, broad symptoms. Also, irritability is a common presentation in many pediatric psychiatric disorders, such as ODD, BD (irritability without elation),6 and depression. Thus, for many clinicians, determining the correct mood disorder diagnosis in pediatric patients can be challenging.
This article describes the diagnosis of DMDD, and how its presentation is similar to—and different from—those of other common pediatric psychiatric disorders.
_
The origin of DMDD
Many researchers have investigated the broadband phenotypical presentation of pediatric mood disorders, which have been mostly diagnosed in the psychiatric community as pediatric BD. Leibenluft7 identified a subtype of mood disorder that they termed “severe mood dysregulation” (SMD). Compared with the narrow-band, clearly episodic BD, SMD has a different trajectory, outcome, and findings on brain imaging. SMD is characterized by chronic irritability with abnormal mood (anger or sadness) at least half of the day on most days, with 3 hyperarousal symptoms, including pressured speech, racing thoughts or flight of ideas, intrusiveness, distractibility, insomnia, and agitation.8 Eventually, SMD became the foundation of the development of DMDD.
DSM-5 diagnostic criteria for DMDD include severe recurrent temper outbursts that are out of proportion to the situation, inconsistent with developmental level, and occurring on average ≥3 times per week, plus persistently irritable or angry mood for most of the day nearly every day.9 Additional criteria include the presence of symptoms for at least 12 months (without a symptom-free period of at least 3 consecutive months) in ≥2 settings (at home, at school, or with peers) with onset before age 10. The course of DMDD typically is chronic with accompanying severe temperament. The estimated 6-month to 1-year prevalence is 2% to 5%; the diagnosis is more common among males and school-age children than it is in females and adolescents.9,10
_
DMDD or bipolar disorder?
A patient cannot be dually diagnosed with both disorders. If a patient exhibits a manic episode for more than 1 day, that would null and void the DMDD diagnosis. However, in a study to evaluate BD in pediatric patients, researchers divided BD symptoms into BD-specific categories (elevated mood, grandiosity, and increased goal-directed activity) and nonspecific symptoms such as irritability and talkativeness, distractibility, and flight of ideas or racing thoughts. They found that in the absence of specific symptoms, a diagnosis of BD is unlikely to be the correct diagnosis.11 Hence, as a nonspecific symptom, chronic irritability should be attributed to the symptom count for DMDD, rather than BD. Most epidemiologic studies have concluded that depression and anxiety, and not irritability, are typically the preceeding presentations prior to the development of BD in young adults.12 Chronic irritability, however, predicts major depressive disorder and anxiety disorders in later adolescence and one’s early twenties.13 Furthermore, BD commonly presents with infrequent and discrete episodes and a later age of onset, while DMDD presents with chronic and frequent, severe temper outbursts. Some differences between DMDD and BD are illustrated in Table 1.11-13

Continue to: CASE 1
CASE 1
Irritable and taking risks
Ms. N, age 16, is brought to the outpatient psychiatry clinic by her parents for evaluation of mood symptoms, including irritability. Her mother claims her daughter was an introverted, anxious, shy child, but by the beginning of middle school, she began to feel irritable and frequently stayed up at night with little sleep. In high school, Ms. N had displayed several episodes of risk-taking behaviors, including taking her father’s vehicle for a drive despite not having a driver’s permit, running away for 2 days, and having unprotected sex.
During her assessment, Ms. N is pleasant and claims she usually has a great mood. She fought with her mother several times this year, which led her to run away. Her parents had divorced when Ms. N was 5 years old and have shared custody. Ms. N is doing well in school despite her parents’ concerns.
Diagnosis. The most likely diagnosis is emerging BD. Notice that Ms. N may have had anxiety symptoms before she developed irritability. She had a relatively late onset of symptoms that were episodic in nature, which further supports a diagnosis of BD.
_
>
DMDD or oppositional defiant disorder?
DMDD and ODD cannot be dually diagnosed. However, if a patient meets the criteria for both DMDD and ODD, only the DMDD diagnosis should be considered. One of many issues of DMDD is its similarity to ODD. In fact, more than 70% of patients with DMDD also meet the diagnostic criteria for ODD.10,14 Some researchers have conceptualized DMDD as a severe form of ODD. However, there are a few differences that clinicians can use to distinguish the 2 disorders.
Compared with patients with ODD, those with DMDD more frequently experience severe irritability.15 Patients with ODD may present with delinquent behaviors and trouble with authority figures. Moreover, comorbidity with ADHD is twice as common in ODD; more than 65% of patients with ADHD have ODD vs 30% who have DMDD.10,16 Finally, in general, children with DMDD have more social impairments compared with those with ODD. Differences between DMDD and BD are illustrated in Table 2.10,14-16

Continue to: CASE 2
CASE 2
Angry and defiant
Mr. R, age 14, is brought to the emergency department (ED) by his parents after becoming very aggressive with them. He punched a wall and vandalized his room after his parents grounded him because of his previous defiant behavior. He had been suspended from school that day for disrespecting his teacher after he was caught fighting another student.
His parents describe Mr. R as a strong-willed, stubborn child. He has difficulty with rules and refuses to follow them. He is grouchy and irritable around adults, including the ED staff. Mr. R enjoys being with his friends and playing video games. He had been diagnosed with ADHD when he was in kindergarten, when his teacher noticed he had a poor attention span and could not sit still. According to his parents, Mr. R has “blown up” a few times before, smashing items in his room and shouting obscenities. Mr. R’s parents noticed that he is more defiant in concurrence with discontinuing his ADHD stimulant medication.
Diagnosis. The most likely diagnosis for Mr. R is ODD. Notice the comorbidity of ADHD, which is more commonly associated with ODD. The frequency and severity of his outbursts and irritability symptoms were lower than that typically associated with DMDD.
_
Treatment strategies for DMDD
Management of DMDD should focus on helping children and adolescents improve their emotional dysregulation.
Clinicians should always consider behavioral therapy as a first-line intervention. The behavioral planning team may include (but is not limited to) a behavior specialist, child psychiatrist, psychologist, therapist, skills trainer, teachers, and the caregiver(s). The plan should be implemented across all settings, including home and school. Furthermore, social skills training is necessary for many children with DMDD, who may require intensive behavioral modification planning. Comorbidity with ADHD should be addressed with a combination of behavioral planning and stimulant medications.17 If available, parent training and parent-child interactive therapy can help to improve defiant behavior.
Pharmacotherapy
Currently, no medications are FDA-approved for treating DMDD. Most pharmacologic trials that included patients with DMDD focused on managing chronic irritability and/or stabilizing comorbid disorders (ie, ADHD, depression, and anxiety).
Continue to: Stimulants
Stimulants. Previous trials examined the benefit of CNS stimulant medications, alone or in conjunction with behavioral therapy, in treating DMDD and comorbid ADHD. Methylphenidate results in a significant reduction in aggression18 with a dosing recommendation range from 1 to 1.2 mg/kg/d. CNS stimulants should be considered as first-line pharmacotherapy for DMDD, especially for patients with comorbid ADHD.
Anticonvulsants. Divalproex sodium is superior to placebo in treating aggression in children and adolescents.19 Trials found that divalproex sodium reduces irritability and aggression whether it is prescribed as monotherapy or combined with stimulant medications.19
Lithium is one of the main treatment options for mania in BD. The benefits of lithium for controlling aggression in DMDD are still under investigation. Earlier studies found that lithium significantly improves aggressive behavior in hospitalized pediatric with conduct disorder.20,21 However, a later study that evaluated lithium vs placebo for children with SMD (which arguably is phenotypically related to the DMDD) found there were no significant differences in improvement of irritability symptoms between groups.22 More research is needed to determine if lithium may play a role in treating patients with DMDD.
Antipsychotics. Aripiprazole and risperidone are FDA-approved for treating irritability in autism. A 2017 meta-analysis found both medications were effective in controlling irritability and aggression in other diagnoses as well.23 Other antipsychotic medications did not show sufficient benefits in treating irritability.23 When considering antipsychotics, clinicians should weigh the risks of metabolic adverse effects and follow practice guidelines.
Antidepressants. A systematic review did not find that selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors effectively reduce irritability.24 However, in most of the studies evaluated, irritability was not the primary outcome measure.24
Other medications. Alpha-2 agonists (guanfacine, clonidine), and atomoxetine may help irritability indirectly by improving ADHD symptoms.25
Bottom Line
Disruptive mood dysregulation disorder (DMDD), bipolar disorder, and oppositional defiant disorder have similar presentations and diagnostic criteria. The frequency and severity of irritability can be a distinguishing factor. Behavioral therapy is a first-line treatment. No medications are FDA-approved for treating DMDD, but pharmacotherapy may help reduce irritability and aggression.
Related Resources
- Rao U. DSM-5: disruptive mood dysregulation disorder. Asian J Psychiatr. 2014;11:119-123.
- Roy AK, Lopes V, Klein RG. Disruptive mood dysregulation disorder: a new diagnostic approach to chronic irritability in youth. Am J Psychiatry. 2014;171(9):918-924.
Drug Brand Names
Aripiprazole • Abilify
Atomoxetine • Strattera
Clonidine • Catapres
Divalproex sodium • Depakote, Depakote ER
Guanfacine • Intuniv, Tenex
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta, Ritalin
Risperidone • Risperdal
1. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
2. Axelson D. Taking disruptive mood dysregulation disorder out for a test drive. Am J Psychiatry. 2013;170(2):136-139.
3. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
4. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64(1):89-96.
5. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
6. Hunt J, Birmaher B, Leonard H, et al. Irritability without elation in a large bipolar youth sample: frequency and clinical description. J Am Acad Child Adolesc Psychiatry. 2009;48(7):730-739.
7. Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
8. Rich BA, Carver FW, Holroyd T, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011;45(10):1283-1294.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Copeland WE, Angold A, Costello EJ, et al. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. Am J Psychiatry. 2013;170(2):173-179.
11. Elmaadawi AZ, Jensen PS, Arnold LE, et al. Risk for emerging bipolar disorder, variants, and symptoms in children with attention deficit hyperactivity disorder, now grown up. World J Psychiatry. 2015;5(4):412-424.
12. Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55(8):477-485.
13. Stringaris A, Cohen P, Pine DS, et al. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048-1054.
14. Mayes SD, Waxmonsky JD, Calhoun SL, et al. Disruptive mood dysregulation disorder symptoms and association with oppositional defiant and other disorders in a general population child sample. J Child Adolesc Psychopharmacol. 2016;26(2):101-106.
15. Stringaris A, Vidal-Ribas P, Brotman MA, et al. Practitioner review: definition, recognition, and treatment challenges of irritability in young people. J Child Psychol Psychiatry. 2018;59(7):721-739.
16. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57-87.
17. Fernandez de la Cruz L, Simonoff E, McGough JJ, et al. Treatment of children with attention-deficit/hyperactivity disorder (ADHD) and irritability: results from the multimodal treatment study of children with ADHD (MTA). J Am Acad Child Adolesc Psychiatry. 2015;54(1):62-70.
18. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry. 2000;157(5):818-820.
20. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
21. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
22. Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19(1):61-73.
23. van Schalkwyk GI, Lewis AS, Beyer C, et al. Efficacy of antipsychotics for irritability and aggression in children: a meta-analysis. Expert Rev Neurother. 2017;17(10):1045-1053.
24. Kim S, Boylan K. Effectiveness of antidepressant medications for symptoms of irritability and disruptive behaviors in children and adolescents. J Child Adolesc Psychopharmacol. 2016;26(8):694-704.
25. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067-1074.
1. Regier DA, Narrow WE, Clarke DE, et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry. 2013;170(1):59-70.
2. Axelson D. Taking disruptive mood dysregulation disorder out for a test drive. Am J Psychiatry. 2013;170(2):136-139.
3. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
4. Case BG, Olfson M, Marcus SC, et al. Trends in the inpatient mental health treatment of children and adolescents in US community hospitals between 1990 and 2000. Arch Gen Psychiatry. 2007;64(1):89-96.
5. Pliszka S; AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
6. Hunt J, Birmaher B, Leonard H, et al. Irritability without elation in a large bipolar youth sample: frequency and clinical description. J Am Acad Child Adolesc Psychiatry. 2009;48(7):730-739.
7. Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
8. Rich BA, Carver FW, Holroyd T, et al. Different neural pathways to negative affect in youth with pediatric bipolar disorder and severe mood dysregulation. J Psychiatr Res. 2011;45(10):1283-1294.
9. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
10. Copeland WE, Angold A, Costello EJ, et al. Prevalence, comorbidity, and correlates of DSM-5 proposed disruptive mood dysregulation disorder. Am J Psychiatry. 2013;170(2):173-179.
11. Elmaadawi AZ, Jensen PS, Arnold LE, et al. Risk for emerging bipolar disorder, variants, and symptoms in children with attention deficit hyperactivity disorder, now grown up. World J Psychiatry. 2015;5(4):412-424.
12. Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55(8):477-485.
13. Stringaris A, Cohen P, Pine DS, et al. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048-1054.
14. Mayes SD, Waxmonsky JD, Calhoun SL, et al. Disruptive mood dysregulation disorder symptoms and association with oppositional defiant and other disorders in a general population child sample. J Child Adolesc Psychopharmacol. 2016;26(2):101-106.
15. Stringaris A, Vidal-Ribas P, Brotman MA, et al. Practitioner review: definition, recognition, and treatment challenges of irritability in young people. J Child Psychol Psychiatry. 2018;59(7):721-739.
16. Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57-87.
17. Fernandez de la Cruz L, Simonoff E, McGough JJ, et al. Treatment of children with attention-deficit/hyperactivity disorder (ADHD) and irritability: results from the multimodal treatment study of children with ADHD (MTA). J Am Acad Child Adolesc Psychiatry. 2015;54(1):62-70.
18. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry. 2000;157(5):818-820.
20. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34(4):445-453.
21. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57(7):649-654.
22. Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19(1):61-73.
23. van Schalkwyk GI, Lewis AS, Beyer C, et al. Efficacy of antipsychotics for irritability and aggression in children: a meta-analysis. Expert Rev Neurother. 2017;17(10):1045-1053.
24. Kim S, Boylan K. Effectiveness of antidepressant medications for symptoms of irritability and disruptive behaviors in children and adolescents. J Child Adolesc Psychopharmacol. 2016;26(8):694-704.
25. Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067-1074.