User login
Bright light therapy for bipolar depression
Bright light therapy (BLT) refers to the use of bright light to treat symptoms of depression. BLT was initially prescribed as a treatment for patients with seasonal affective disorder.1 It was later found helpful for nonseasonal depression,2 premenstrual dysphoric disorder, postpartum depression, and phase shift circadian disorders, including for patients with dementia whose cognitive function improved after treatment with BLT.3 More recent studies suggest year-round benefit for nonseasonal depression.2 The American Psychiatric Association practice guidelines for the treatment of depression list BLT as an alternative and/or addition to pharmacologic and psychological treatment.4 BLT also may be beneficial for patients who are in the depressive phase of bipolar illness.
This article describes the evidence, rationale for use, mechanism of action, benefits, and safety profile of BLT for treating patients with bipolar depression.
Circadian rhythm disruption in bipolar disorder
Clinical manifestation. Patients with bipolar disorder (BD) spend more time in depression than in mania.5 Sleep disturbance is a core symptom of BD; patients typically have little need for sleep during a manic episode, and excess sleepiness during a depressive episode. Sleep complaints can be both precipitating factors and consequences of mood disorders. Patients with seasonal depression have excess sleepiness and weight gain in the winter followed by hypomanic-like symptoms in the spring, including decreased need for sleep and weight loss with psychomotor activation. In a recent review of sleep problems in patients with BD, Steinan et al6 reported that 20% of patients with euthymic mood in bipolar disorder experience a sleep disorder. Circadian disruption and “eveningness” (being more active during the evening) have been associated with mood episodes, functional impairment, poor quality of life, and treatment resistance.7-10
Pathophysiology. Existing hypotheses for the biological mechanism underlying dysregulation of circadian rhythm in BD include changes in melatonin levels, expression of melatonin receptors in the CNS, and daily cortisol profiles.11 Genetic evidence also links circadian rhythm dysregulation with BD. Two polymorphisms on the circadian locomotor output cycles kaput (CLOCK) gene that control circadian rhythm—aryl hydrocarbon receptor nuclear translocator-like (ARNTL) and timeless circadian clock (TIMELESS)—have been linked to lithium responsiveness in BD.12 In addition, Per2, Cry1, and Rev-Erbα expression—all components of the circadian clock—have been found to increase individual susceptibility to the therapeutic effects of lithium in mice.13 In addition, circadian rhythm dysregulation is associated with metabolic problems encountered by patients with BD, including weight gain, diabetes mellitus, and cardiovascular disease.14
Rationale for use
Regulation of a patient’s circadian rhythm disruption is a potential treatment for BD. Hashimoto et al15 demonstrated that midday bright light exposure can phase advance and increase the amplitude of nocturnal melatonin production in healthy individuals. Morning light therapy has been shown to increase blood serotonin throughout the day in both healthy individuals and in patients with nonseasonal depression; the effect was apparent with light intensities as low as 50 lux.16 Lithium may exert its therapeutic effect through its influence on the retino-hypothalamic-pineal tract and thus its effect on melatonin secretion.17
BLT is a logical choice to treat the depression phase of BD when exposure to sunlight is not feasible due to geographical location, season, or other factor. For patients who live in areas that receive frequent sunshine, an outside stroll for half an hour will likely achieve similar benefit to BLT.
The precise mechanism of action of BLT for bipolar depression has not yet been determined. It may be attributed to a phase-resetting effect via melanopsin and the suprachiasmatic nucleus (Box18-24).
Box
Bright light therapy: How it works
The mechanism of action of bright light therapy is yet to be elucidated. The suprachiasmatic nucleus (SCN) in the hypothalamus is the center of circadian rhythm regulation and receives direct input from the retina through the retinohypothalamic tract.18 Melanopsin, a short-wavelength, light-sensitive G-protein–coupled receptor located in human retinal ganglion cells, is known to transduce short-wavelength light signals into neural signals.19 Since melanopsin is primarily responsible for resetting the timing of the SCN, suppressing pineal gland melatonin secretion and improving alertness and electroencephalogram-derived correlates of arousal,20 short-wavelength light with a low light intensity might be a better stimulator for melanopsin-containing retinal ganglion cells and the behaviors mediated via this photoreceptor system.21,22 Whether the antidepressant effect of light is also related to its alerting property is unclear.23 However, the acute alerting and performance-enhancing effects of light are increasingly taken into account for the design of indoor light standards in office environments.24 Response to light therapy is thus attributed to its phase-resetting effect.
Continue to: BLT for BD...
BLT for BD: What’s the evidence?
Several studies and case reports have evaluated the use of BLT for bipolar depression. The number of participants in these studies is small, and there is no uniformity of methodology or patient selection.
Dauphinais et al (2012)25 randomly assigned 44 patients with bipolar depression to BLT or a high-density or low-density negative ion generator for 8 weeks. They reported no difference in outcome between the various groups (50% vs 55.6%, remission and response rate). Only one patient in each group showed a switch to hypomania.
Carmadese et al (2015)26 reported an open-label study of adjunctive BLT in 31 difficult-to-treat patients with depression (16 unipolar and 15 bipolar). Significant improvement was noted within 3 weeks and was sustained in 1 patient with bipolar depression 5 weeks after cessation of BLT.
Papatheodorou and Kutcher (1995)27 treated 7 adolescents with bipolar depression with adjunctive BLT (10,000 lux twice per day). Three patients showed a marked response (>70% decrease from baseline Beck Depression Inventory and Symptom Check List scores). Two patients had a moderate response (40% to 47% decrease) and 2 patients obtained mild to no response. There were no reported adverse effects.
Benedetti et al (2014)28 studied 141 patients with treatment-resistant bipolar depression. Approximately one-quarter (23%) had a history of attempted suicide, and 83% had a history of drug resistance. The authors found a combination of total sleep deprivation, BLT, and lithium rapidly decreased suicidality and improved patients’ depressive symptoms.
Liebenluft et al (1995)29 administered 13 trials of BLT to 9 patients with rapid-cycling BD during a 3-month period. Five patients received the treatment in the morning, 5 around midday, and 3 in the evening. Patients who received BLT at midday had the best outcome, while 3 of the 5 patients who received morning BLT developed unstable mood. The authors recommended titrating the duration of light exposure so that patients could skip a treatment if their mood was trending toward hypomania.
Sit et al (2007)30 evaluated BLT in a case series of 9 women with bipolar I or II disorder in the depression phase. Patients were exposed to 50 lux of red light for 2 weeks, and then they received 7,000 lux BLT for 15, 30, and 45 minutes daily for 2 weeks (4 patients received morning light and 5 received midday light). Mood was assessed using the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement and the Mania Rating Scale. Of the 4 patients receiving morning BLT, one patient had a full response and the other 3 developed hypomania. Of the 5 patients who received midday BLT, 2 achieved full response, 2 showed early improvement but required a dose increase, and one remained depressed but had a full response when she was switched to morning BLT.
Tseng et al (2016)31 reported a meta-analysis of BLT for bipolar depression that included a total of 567 patients from 11 studies. They reported significant improvement with BLT alone or in combination with antidepressants or total sleep deprivation. They also reported significant improvement with BLT in 130 patients who were not receiving other treatments. There was no difference in the frequency of mood shifts between patients on BLT alone or in combination with other modalities. The authors reported no mood shift in any of the patients receiving concurrent mood stabilizers. They also reported no difference with the color of light, gender, or duration of illness.
Yorguner et al (2017)32 conducted a 2-week randomized, single-blind study of BLT as an add-on treatment for 32 patients with bipolar depression. Patients were randomly assigned to BLT or dim light, which they were administered each morning for 30 mins for 2 weeks. Sixteen patients who received BLT showed a significantly greater reduction in Hamilton Depression Rating Scale scores (mean score of 24 at baseline down to 12) compared with 16 patients who received dim light (mean score of 24 at baseline down to 18). The authors also reported remission in 4 out of 4 patients who had seasonal depression, compared with 3 out of 12 who did not have seasonal depression (the other 9 showed response but not remission).
Zhou et al (2018)33 conducted a multi-center, randomized, single-blind clinical trial of 63 patients with bipolar depression. Thirty-three patients received morning BLT, and 30 received dim red light therapy (control group). The authors reported a significantly higher response rate in the BLT group (78%) compared with the control group (43%).
Sit et al (2018)34 conducted a 6-week randomized, double-blind, placebo-controlled trial of BLT vs dim red light in patients with bipolar I or II depression. Twenty-three patients were administered 7,000 lux bright white light, and 23 patients received 50 lux dim red light, at midday 5 days a week. The light dose was increased by 15 minutes every week up to 60 minutes by Week 4, unless the patient achieved remission. Patients were maintained on their usual medications, which included mood stabilizers and/or antidepressants. At Week 6, the group randomized to BLT had a significantly higher remission rate (68%) compared with patients who received dim red light (22%). Improvement was noted by Week 4. Patients receiving BLT also had significantly fewer depressive symptoms, and no mood polarity switch was noted.
Prescribing bright light therapy
Light box selection criteria. When selecting a light box or related BLT treatment apparatus, the Center for Environmental Therapeutics recommends consideration of the following factors35:
- clinical efficacy
- ocular and dermatologic safety
- visual comfort.
Selecting a dose. The dose received is determined by the intensity emitted from the light source, distance from the light box, and duration of exposure.36 Begin with midday light therapy between 12 noon and 2
Monitor for adverse effects. Generally, BLT is well tolerated.37 Adverse effects are rare; the most common ones include headache, eyestrain, nausea, and agitation.38 One study found no adverse ocular effects from light therapy after 5 years of treatment.39 Adverse effects tend to remit spontaneously or after dose reduction.35 Evening administration of BLT may increase the incidence of sleep disturbances.40 Like other biologic treatments for bipolar depression, BLT can precipitate manic/hypomanic and mixed states in susceptible patients, although the light dose can be titrated against emergent symptoms of hypomania.41
Bottom Line
Evidence suggests that bright light therapy is an effective, well tolerated, and affordable adjunct treatment for bipolar depression. Exposure to 5,000 to 7,000 lux around noon for 15 to 60 minutes will enhance the remission rate.
Related Resource
Mostert M, Dubovsky S. When bipolar treatment fails: what’s your next step? Current Psychiatry. 2008;7(1):39-46.
Drug Brand Name
Lithium • Eskalith, Lithobid
1. Pjrek E, Winkler D, Stastny J, et al. Bright light therapy in seasonal affective disorder--does it suffice? Eur Neuropsychopharmacol. 2004.14(4):347-351.
2. Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64-71.
3. Sekiguchi H, Iritani S, Fujita K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer’s type dementia: a case series. Psychogeriatrics, 2017;17(5):275-281.
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder, third edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf American Psychiatric Association. 2010. Accessed August, 10, 2017.
5. Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531-535.
6. Steinan MK, Krane-Gartiser K, Morken G, et al. Sleep problems in euthymic bipolar disorders: a review of clinical studies. Current Psychiatry Reviews. 2015;11:235-243.
7. Cudney LE, Frey BN, Streiner D, et al. Biological rhythms are independently associated with quality of life in bipolar disorder. Int J Bipolar Disord. 2016;4(1):9.
8. Duarte FA, Cardoso TA, Campos MT, et al. Biological rhythms in bipolar and depressive disorders: a community study with drug-naive young adults. J Affect Disord, 2015;186:145-148.
9. Pinho M, Sehmbi M, Cudney LE, et al. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Acta Psychiatr Scand. 2015:133(2);102-108.
10. Ng TH, Chung KF, Lee CT, et al. Eveningness and its associated impairments in remitted bipolar disorder. Behav Sleep Med. 2016:14(6):650-664.
11. Wu YH, Ursinus J, Zahn JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord, 2013:148(2-3):357-367.
12. Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, et al. Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord. 2014;16(2):151-158.
13. Schnell A, Sandrelli F, Ranc V, et al. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol Int. 2015;32(8):1075-1089.
14. Kim Y, Santos R, Gage FH, et al. Molecular mechanisms of bipolar disorder: progress made and future challenges. Front Cell Neurosci. 2017;11:30.
15. Hashimoto S, Kohsaka M, Nakamura K. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 1997;221(2-3):
89-92.
16. Rao ML, Müller-Oerlinghausen B, Mackert A, et al. The influence of phototherapy on serotonin and melatonin in non-seasonal depression. Pharmacopsychiatry.1990;23(3):155-158.
17. Moreira J, Geoffroy PA. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol Int. 2016;33(4):351-373.
18. Oldham MA, Ciraulo DA. Bright light therapy for depression: a review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int. 2014;31(3):305-319.
19. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
20. Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49(3):331-339.
21. Anderson JL, Glod CA, Dai J, et al. Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr Scand. 2009;120(3):203-212.
22. Wirz-Justice A, Graw P, Kräuchi K, et al. Effect of light on unmasked circadian rhythms in winter depression. In: Wetterberg L, ed. Light and biological rhythms in man. Oxford, United Kingdom:Pergamon Press;1993:385-393.
23. Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453-464.
24. Aries MBC. Human lighting demands: healthy lighting in an office environment. Eindhoven, Eindhoven University Press. 2005;158. doi:10.6100/IR594257.
25. Dauphinais DR, Rosenthal JZ, Terman M, et al. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012;196(1):57-61.
26. Camardese G, Leone B, Serrani R, et al. Augmentation of light therapy in difficult-to-treat depressed patients: an open-label trial in both unipolar and bipolar patients. Neuropsychiatr Dis Treat. 2015;11:2331-2338.
27. Papatheodorou G, Kutcher S. The effect of adjunctive light therapy on ameliorating breakthrough depressive symptoms in adolescent-onset bipolar disorder.
J Psychiatry Neurosci. 1995;20(3):226-232.
28. Benedetti F, Riccaboni R, Locatelli C, et al. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133-140.
29. Liebenluft E, Turner EH, Felman-Naim S, et al. Light therapy in patients with rapid cycling bipolar disorder: preliminary results. Psychopharmacol Bull. 1995;31(4):
705-710.
30. Sit DK, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9(8):918-927.
31. Tseng PT, Chen YW, Tu KY, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):
1037-1047.
32. Yorguner KN, Bulut NS, Carkaxhiu BG, et al. Efficacy of bright light therapy in bipolar depression. Psychiatry Res. 2017;260:432-438.
33. Zhou TH, Dang WM, Ma YT, et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord. 2018;227:90-96.
34. Sit DK, McGowan J, Wiltrout C, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175(2):
131-139.
35. Center for Environmental Therapeutics. https://www.cet.org/. Center for Environmental Therapeutics. Accessed November 15, 2017.
36. Lam RW, Levitt AJ. Canadian consensus guidelines for the treatment of seasonal affective disorder. https://mdsc.ca/documents/Consumer%20and%20Family%20Support/CCG_on_Seasonal_Affective_Disorder.pdf. 1999. Accessed August 2, 2017.
37. Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999; 60(11):799-808;quiz 809.
38. Labbate LA, et al. Side effects induced by bright light treatment for seasonal affective disorder. J Clin Psychiatry. 1994; 55(5):189-191.
39. Gallin PF, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119(2):202-210.
40. Chan PK, Lam RW, Perry KF. Mania precipitated by light therapy for patients with SAD. J Clin Psychiatry. 1994;55(10):454.
41. Kripke DF. Timing of phototherapy and occurrence of mania. Biol Psychiatry. 1991; 29(11):1156-1157.
Bright light therapy (BLT) refers to the use of bright light to treat symptoms of depression. BLT was initially prescribed as a treatment for patients with seasonal affective disorder.1 It was later found helpful for nonseasonal depression,2 premenstrual dysphoric disorder, postpartum depression, and phase shift circadian disorders, including for patients with dementia whose cognitive function improved after treatment with BLT.3 More recent studies suggest year-round benefit for nonseasonal depression.2 The American Psychiatric Association practice guidelines for the treatment of depression list BLT as an alternative and/or addition to pharmacologic and psychological treatment.4 BLT also may be beneficial for patients who are in the depressive phase of bipolar illness.
This article describes the evidence, rationale for use, mechanism of action, benefits, and safety profile of BLT for treating patients with bipolar depression.
Circadian rhythm disruption in bipolar disorder
Clinical manifestation. Patients with bipolar disorder (BD) spend more time in depression than in mania.5 Sleep disturbance is a core symptom of BD; patients typically have little need for sleep during a manic episode, and excess sleepiness during a depressive episode. Sleep complaints can be both precipitating factors and consequences of mood disorders. Patients with seasonal depression have excess sleepiness and weight gain in the winter followed by hypomanic-like symptoms in the spring, including decreased need for sleep and weight loss with psychomotor activation. In a recent review of sleep problems in patients with BD, Steinan et al6 reported that 20% of patients with euthymic mood in bipolar disorder experience a sleep disorder. Circadian disruption and “eveningness” (being more active during the evening) have been associated with mood episodes, functional impairment, poor quality of life, and treatment resistance.7-10
Pathophysiology. Existing hypotheses for the biological mechanism underlying dysregulation of circadian rhythm in BD include changes in melatonin levels, expression of melatonin receptors in the CNS, and daily cortisol profiles.11 Genetic evidence also links circadian rhythm dysregulation with BD. Two polymorphisms on the circadian locomotor output cycles kaput (CLOCK) gene that control circadian rhythm—aryl hydrocarbon receptor nuclear translocator-like (ARNTL) and timeless circadian clock (TIMELESS)—have been linked to lithium responsiveness in BD.12 In addition, Per2, Cry1, and Rev-Erbα expression—all components of the circadian clock—have been found to increase individual susceptibility to the therapeutic effects of lithium in mice.13 In addition, circadian rhythm dysregulation is associated with metabolic problems encountered by patients with BD, including weight gain, diabetes mellitus, and cardiovascular disease.14
Rationale for use
Regulation of a patient’s circadian rhythm disruption is a potential treatment for BD. Hashimoto et al15 demonstrated that midday bright light exposure can phase advance and increase the amplitude of nocturnal melatonin production in healthy individuals. Morning light therapy has been shown to increase blood serotonin throughout the day in both healthy individuals and in patients with nonseasonal depression; the effect was apparent with light intensities as low as 50 lux.16 Lithium may exert its therapeutic effect through its influence on the retino-hypothalamic-pineal tract and thus its effect on melatonin secretion.17
BLT is a logical choice to treat the depression phase of BD when exposure to sunlight is not feasible due to geographical location, season, or other factor. For patients who live in areas that receive frequent sunshine, an outside stroll for half an hour will likely achieve similar benefit to BLT.
The precise mechanism of action of BLT for bipolar depression has not yet been determined. It may be attributed to a phase-resetting effect via melanopsin and the suprachiasmatic nucleus (Box18-24).
Box
Bright light therapy: How it works
The mechanism of action of bright light therapy is yet to be elucidated. The suprachiasmatic nucleus (SCN) in the hypothalamus is the center of circadian rhythm regulation and receives direct input from the retina through the retinohypothalamic tract.18 Melanopsin, a short-wavelength, light-sensitive G-protein–coupled receptor located in human retinal ganglion cells, is known to transduce short-wavelength light signals into neural signals.19 Since melanopsin is primarily responsible for resetting the timing of the SCN, suppressing pineal gland melatonin secretion and improving alertness and electroencephalogram-derived correlates of arousal,20 short-wavelength light with a low light intensity might be a better stimulator for melanopsin-containing retinal ganglion cells and the behaviors mediated via this photoreceptor system.21,22 Whether the antidepressant effect of light is also related to its alerting property is unclear.23 However, the acute alerting and performance-enhancing effects of light are increasingly taken into account for the design of indoor light standards in office environments.24 Response to light therapy is thus attributed to its phase-resetting effect.
Continue to: BLT for BD...
BLT for BD: What’s the evidence?
Several studies and case reports have evaluated the use of BLT for bipolar depression. The number of participants in these studies is small, and there is no uniformity of methodology or patient selection.
Dauphinais et al (2012)25 randomly assigned 44 patients with bipolar depression to BLT or a high-density or low-density negative ion generator for 8 weeks. They reported no difference in outcome between the various groups (50% vs 55.6%, remission and response rate). Only one patient in each group showed a switch to hypomania.
Carmadese et al (2015)26 reported an open-label study of adjunctive BLT in 31 difficult-to-treat patients with depression (16 unipolar and 15 bipolar). Significant improvement was noted within 3 weeks and was sustained in 1 patient with bipolar depression 5 weeks after cessation of BLT.
Papatheodorou and Kutcher (1995)27 treated 7 adolescents with bipolar depression with adjunctive BLT (10,000 lux twice per day). Three patients showed a marked response (>70% decrease from baseline Beck Depression Inventory and Symptom Check List scores). Two patients had a moderate response (40% to 47% decrease) and 2 patients obtained mild to no response. There were no reported adverse effects.
Benedetti et al (2014)28 studied 141 patients with treatment-resistant bipolar depression. Approximately one-quarter (23%) had a history of attempted suicide, and 83% had a history of drug resistance. The authors found a combination of total sleep deprivation, BLT, and lithium rapidly decreased suicidality and improved patients’ depressive symptoms.
Liebenluft et al (1995)29 administered 13 trials of BLT to 9 patients with rapid-cycling BD during a 3-month period. Five patients received the treatment in the morning, 5 around midday, and 3 in the evening. Patients who received BLT at midday had the best outcome, while 3 of the 5 patients who received morning BLT developed unstable mood. The authors recommended titrating the duration of light exposure so that patients could skip a treatment if their mood was trending toward hypomania.
Sit et al (2007)30 evaluated BLT in a case series of 9 women with bipolar I or II disorder in the depression phase. Patients were exposed to 50 lux of red light for 2 weeks, and then they received 7,000 lux BLT for 15, 30, and 45 minutes daily for 2 weeks (4 patients received morning light and 5 received midday light). Mood was assessed using the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement and the Mania Rating Scale. Of the 4 patients receiving morning BLT, one patient had a full response and the other 3 developed hypomania. Of the 5 patients who received midday BLT, 2 achieved full response, 2 showed early improvement but required a dose increase, and one remained depressed but had a full response when she was switched to morning BLT.
Tseng et al (2016)31 reported a meta-analysis of BLT for bipolar depression that included a total of 567 patients from 11 studies. They reported significant improvement with BLT alone or in combination with antidepressants or total sleep deprivation. They also reported significant improvement with BLT in 130 patients who were not receiving other treatments. There was no difference in the frequency of mood shifts between patients on BLT alone or in combination with other modalities. The authors reported no mood shift in any of the patients receiving concurrent mood stabilizers. They also reported no difference with the color of light, gender, or duration of illness.
Yorguner et al (2017)32 conducted a 2-week randomized, single-blind study of BLT as an add-on treatment for 32 patients with bipolar depression. Patients were randomly assigned to BLT or dim light, which they were administered each morning for 30 mins for 2 weeks. Sixteen patients who received BLT showed a significantly greater reduction in Hamilton Depression Rating Scale scores (mean score of 24 at baseline down to 12) compared with 16 patients who received dim light (mean score of 24 at baseline down to 18). The authors also reported remission in 4 out of 4 patients who had seasonal depression, compared with 3 out of 12 who did not have seasonal depression (the other 9 showed response but not remission).
Zhou et al (2018)33 conducted a multi-center, randomized, single-blind clinical trial of 63 patients with bipolar depression. Thirty-three patients received morning BLT, and 30 received dim red light therapy (control group). The authors reported a significantly higher response rate in the BLT group (78%) compared with the control group (43%).
Sit et al (2018)34 conducted a 6-week randomized, double-blind, placebo-controlled trial of BLT vs dim red light in patients with bipolar I or II depression. Twenty-three patients were administered 7,000 lux bright white light, and 23 patients received 50 lux dim red light, at midday 5 days a week. The light dose was increased by 15 minutes every week up to 60 minutes by Week 4, unless the patient achieved remission. Patients were maintained on their usual medications, which included mood stabilizers and/or antidepressants. At Week 6, the group randomized to BLT had a significantly higher remission rate (68%) compared with patients who received dim red light (22%). Improvement was noted by Week 4. Patients receiving BLT also had significantly fewer depressive symptoms, and no mood polarity switch was noted.
Prescribing bright light therapy
Light box selection criteria. When selecting a light box or related BLT treatment apparatus, the Center for Environmental Therapeutics recommends consideration of the following factors35:
- clinical efficacy
- ocular and dermatologic safety
- visual comfort.
Selecting a dose. The dose received is determined by the intensity emitted from the light source, distance from the light box, and duration of exposure.36 Begin with midday light therapy between 12 noon and 2
Monitor for adverse effects. Generally, BLT is well tolerated.37 Adverse effects are rare; the most common ones include headache, eyestrain, nausea, and agitation.38 One study found no adverse ocular effects from light therapy after 5 years of treatment.39 Adverse effects tend to remit spontaneously or after dose reduction.35 Evening administration of BLT may increase the incidence of sleep disturbances.40 Like other biologic treatments for bipolar depression, BLT can precipitate manic/hypomanic and mixed states in susceptible patients, although the light dose can be titrated against emergent symptoms of hypomania.41
Bottom Line
Evidence suggests that bright light therapy is an effective, well tolerated, and affordable adjunct treatment for bipolar depression. Exposure to 5,000 to 7,000 lux around noon for 15 to 60 minutes will enhance the remission rate.
Related Resource
Mostert M, Dubovsky S. When bipolar treatment fails: what’s your next step? Current Psychiatry. 2008;7(1):39-46.
Drug Brand Name
Lithium • Eskalith, Lithobid
Bright light therapy (BLT) refers to the use of bright light to treat symptoms of depression. BLT was initially prescribed as a treatment for patients with seasonal affective disorder.1 It was later found helpful for nonseasonal depression,2 premenstrual dysphoric disorder, postpartum depression, and phase shift circadian disorders, including for patients with dementia whose cognitive function improved after treatment with BLT.3 More recent studies suggest year-round benefit for nonseasonal depression.2 The American Psychiatric Association practice guidelines for the treatment of depression list BLT as an alternative and/or addition to pharmacologic and psychological treatment.4 BLT also may be beneficial for patients who are in the depressive phase of bipolar illness.
This article describes the evidence, rationale for use, mechanism of action, benefits, and safety profile of BLT for treating patients with bipolar depression.
Circadian rhythm disruption in bipolar disorder
Clinical manifestation. Patients with bipolar disorder (BD) spend more time in depression than in mania.5 Sleep disturbance is a core symptom of BD; patients typically have little need for sleep during a manic episode, and excess sleepiness during a depressive episode. Sleep complaints can be both precipitating factors and consequences of mood disorders. Patients with seasonal depression have excess sleepiness and weight gain in the winter followed by hypomanic-like symptoms in the spring, including decreased need for sleep and weight loss with psychomotor activation. In a recent review of sleep problems in patients with BD, Steinan et al6 reported that 20% of patients with euthymic mood in bipolar disorder experience a sleep disorder. Circadian disruption and “eveningness” (being more active during the evening) have been associated with mood episodes, functional impairment, poor quality of life, and treatment resistance.7-10
Pathophysiology. Existing hypotheses for the biological mechanism underlying dysregulation of circadian rhythm in BD include changes in melatonin levels, expression of melatonin receptors in the CNS, and daily cortisol profiles.11 Genetic evidence also links circadian rhythm dysregulation with BD. Two polymorphisms on the circadian locomotor output cycles kaput (CLOCK) gene that control circadian rhythm—aryl hydrocarbon receptor nuclear translocator-like (ARNTL) and timeless circadian clock (TIMELESS)—have been linked to lithium responsiveness in BD.12 In addition, Per2, Cry1, and Rev-Erbα expression—all components of the circadian clock—have been found to increase individual susceptibility to the therapeutic effects of lithium in mice.13 In addition, circadian rhythm dysregulation is associated with metabolic problems encountered by patients with BD, including weight gain, diabetes mellitus, and cardiovascular disease.14
Rationale for use
Regulation of a patient’s circadian rhythm disruption is a potential treatment for BD. Hashimoto et al15 demonstrated that midday bright light exposure can phase advance and increase the amplitude of nocturnal melatonin production in healthy individuals. Morning light therapy has been shown to increase blood serotonin throughout the day in both healthy individuals and in patients with nonseasonal depression; the effect was apparent with light intensities as low as 50 lux.16 Lithium may exert its therapeutic effect through its influence on the retino-hypothalamic-pineal tract and thus its effect on melatonin secretion.17
BLT is a logical choice to treat the depression phase of BD when exposure to sunlight is not feasible due to geographical location, season, or other factor. For patients who live in areas that receive frequent sunshine, an outside stroll for half an hour will likely achieve similar benefit to BLT.
The precise mechanism of action of BLT for bipolar depression has not yet been determined. It may be attributed to a phase-resetting effect via melanopsin and the suprachiasmatic nucleus (Box18-24).
Box
Bright light therapy: How it works
The mechanism of action of bright light therapy is yet to be elucidated. The suprachiasmatic nucleus (SCN) in the hypothalamus is the center of circadian rhythm regulation and receives direct input from the retina through the retinohypothalamic tract.18 Melanopsin, a short-wavelength, light-sensitive G-protein–coupled receptor located in human retinal ganglion cells, is known to transduce short-wavelength light signals into neural signals.19 Since melanopsin is primarily responsible for resetting the timing of the SCN, suppressing pineal gland melatonin secretion and improving alertness and electroencephalogram-derived correlates of arousal,20 short-wavelength light with a low light intensity might be a better stimulator for melanopsin-containing retinal ganglion cells and the behaviors mediated via this photoreceptor system.21,22 Whether the antidepressant effect of light is also related to its alerting property is unclear.23 However, the acute alerting and performance-enhancing effects of light are increasingly taken into account for the design of indoor light standards in office environments.24 Response to light therapy is thus attributed to its phase-resetting effect.
Continue to: BLT for BD...
BLT for BD: What’s the evidence?
Several studies and case reports have evaluated the use of BLT for bipolar depression. The number of participants in these studies is small, and there is no uniformity of methodology or patient selection.
Dauphinais et al (2012)25 randomly assigned 44 patients with bipolar depression to BLT or a high-density or low-density negative ion generator for 8 weeks. They reported no difference in outcome between the various groups (50% vs 55.6%, remission and response rate). Only one patient in each group showed a switch to hypomania.
Carmadese et al (2015)26 reported an open-label study of adjunctive BLT in 31 difficult-to-treat patients with depression (16 unipolar and 15 bipolar). Significant improvement was noted within 3 weeks and was sustained in 1 patient with bipolar depression 5 weeks after cessation of BLT.
Papatheodorou and Kutcher (1995)27 treated 7 adolescents with bipolar depression with adjunctive BLT (10,000 lux twice per day). Three patients showed a marked response (>70% decrease from baseline Beck Depression Inventory and Symptom Check List scores). Two patients had a moderate response (40% to 47% decrease) and 2 patients obtained mild to no response. There were no reported adverse effects.
Benedetti et al (2014)28 studied 141 patients with treatment-resistant bipolar depression. Approximately one-quarter (23%) had a history of attempted suicide, and 83% had a history of drug resistance. The authors found a combination of total sleep deprivation, BLT, and lithium rapidly decreased suicidality and improved patients’ depressive symptoms.
Liebenluft et al (1995)29 administered 13 trials of BLT to 9 patients with rapid-cycling BD during a 3-month period. Five patients received the treatment in the morning, 5 around midday, and 3 in the evening. Patients who received BLT at midday had the best outcome, while 3 of the 5 patients who received morning BLT developed unstable mood. The authors recommended titrating the duration of light exposure so that patients could skip a treatment if their mood was trending toward hypomania.
Sit et al (2007)30 evaluated BLT in a case series of 9 women with bipolar I or II disorder in the depression phase. Patients were exposed to 50 lux of red light for 2 weeks, and then they received 7,000 lux BLT for 15, 30, and 45 minutes daily for 2 weeks (4 patients received morning light and 5 received midday light). Mood was assessed using the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement and the Mania Rating Scale. Of the 4 patients receiving morning BLT, one patient had a full response and the other 3 developed hypomania. Of the 5 patients who received midday BLT, 2 achieved full response, 2 showed early improvement but required a dose increase, and one remained depressed but had a full response when she was switched to morning BLT.
Tseng et al (2016)31 reported a meta-analysis of BLT for bipolar depression that included a total of 567 patients from 11 studies. They reported significant improvement with BLT alone or in combination with antidepressants or total sleep deprivation. They also reported significant improvement with BLT in 130 patients who were not receiving other treatments. There was no difference in the frequency of mood shifts between patients on BLT alone or in combination with other modalities. The authors reported no mood shift in any of the patients receiving concurrent mood stabilizers. They also reported no difference with the color of light, gender, or duration of illness.
Yorguner et al (2017)32 conducted a 2-week randomized, single-blind study of BLT as an add-on treatment for 32 patients with bipolar depression. Patients were randomly assigned to BLT or dim light, which they were administered each morning for 30 mins for 2 weeks. Sixteen patients who received BLT showed a significantly greater reduction in Hamilton Depression Rating Scale scores (mean score of 24 at baseline down to 12) compared with 16 patients who received dim light (mean score of 24 at baseline down to 18). The authors also reported remission in 4 out of 4 patients who had seasonal depression, compared with 3 out of 12 who did not have seasonal depression (the other 9 showed response but not remission).
Zhou et al (2018)33 conducted a multi-center, randomized, single-blind clinical trial of 63 patients with bipolar depression. Thirty-three patients received morning BLT, and 30 received dim red light therapy (control group). The authors reported a significantly higher response rate in the BLT group (78%) compared with the control group (43%).
Sit et al (2018)34 conducted a 6-week randomized, double-blind, placebo-controlled trial of BLT vs dim red light in patients with bipolar I or II depression. Twenty-three patients were administered 7,000 lux bright white light, and 23 patients received 50 lux dim red light, at midday 5 days a week. The light dose was increased by 15 minutes every week up to 60 minutes by Week 4, unless the patient achieved remission. Patients were maintained on their usual medications, which included mood stabilizers and/or antidepressants. At Week 6, the group randomized to BLT had a significantly higher remission rate (68%) compared with patients who received dim red light (22%). Improvement was noted by Week 4. Patients receiving BLT also had significantly fewer depressive symptoms, and no mood polarity switch was noted.
Prescribing bright light therapy
Light box selection criteria. When selecting a light box or related BLT treatment apparatus, the Center for Environmental Therapeutics recommends consideration of the following factors35:
- clinical efficacy
- ocular and dermatologic safety
- visual comfort.
Selecting a dose. The dose received is determined by the intensity emitted from the light source, distance from the light box, and duration of exposure.36 Begin with midday light therapy between 12 noon and 2
Monitor for adverse effects. Generally, BLT is well tolerated.37 Adverse effects are rare; the most common ones include headache, eyestrain, nausea, and agitation.38 One study found no adverse ocular effects from light therapy after 5 years of treatment.39 Adverse effects tend to remit spontaneously or after dose reduction.35 Evening administration of BLT may increase the incidence of sleep disturbances.40 Like other biologic treatments for bipolar depression, BLT can precipitate manic/hypomanic and mixed states in susceptible patients, although the light dose can be titrated against emergent symptoms of hypomania.41
Bottom Line
Evidence suggests that bright light therapy is an effective, well tolerated, and affordable adjunct treatment for bipolar depression. Exposure to 5,000 to 7,000 lux around noon for 15 to 60 minutes will enhance the remission rate.
Related Resource
Mostert M, Dubovsky S. When bipolar treatment fails: what’s your next step? Current Psychiatry. 2008;7(1):39-46.
Drug Brand Name
Lithium • Eskalith, Lithobid
1. Pjrek E, Winkler D, Stastny J, et al. Bright light therapy in seasonal affective disorder--does it suffice? Eur Neuropsychopharmacol. 2004.14(4):347-351.
2. Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64-71.
3. Sekiguchi H, Iritani S, Fujita K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer’s type dementia: a case series. Psychogeriatrics, 2017;17(5):275-281.
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder, third edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf American Psychiatric Association. 2010. Accessed August, 10, 2017.
5. Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531-535.
6. Steinan MK, Krane-Gartiser K, Morken G, et al. Sleep problems in euthymic bipolar disorders: a review of clinical studies. Current Psychiatry Reviews. 2015;11:235-243.
7. Cudney LE, Frey BN, Streiner D, et al. Biological rhythms are independently associated with quality of life in bipolar disorder. Int J Bipolar Disord. 2016;4(1):9.
8. Duarte FA, Cardoso TA, Campos MT, et al. Biological rhythms in bipolar and depressive disorders: a community study with drug-naive young adults. J Affect Disord, 2015;186:145-148.
9. Pinho M, Sehmbi M, Cudney LE, et al. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Acta Psychiatr Scand. 2015:133(2);102-108.
10. Ng TH, Chung KF, Lee CT, et al. Eveningness and its associated impairments in remitted bipolar disorder. Behav Sleep Med. 2016:14(6):650-664.
11. Wu YH, Ursinus J, Zahn JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord, 2013:148(2-3):357-367.
12. Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, et al. Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord. 2014;16(2):151-158.
13. Schnell A, Sandrelli F, Ranc V, et al. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol Int. 2015;32(8):1075-1089.
14. Kim Y, Santos R, Gage FH, et al. Molecular mechanisms of bipolar disorder: progress made and future challenges. Front Cell Neurosci. 2017;11:30.
15. Hashimoto S, Kohsaka M, Nakamura K. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 1997;221(2-3):
89-92.
16. Rao ML, Müller-Oerlinghausen B, Mackert A, et al. The influence of phototherapy on serotonin and melatonin in non-seasonal depression. Pharmacopsychiatry.1990;23(3):155-158.
17. Moreira J, Geoffroy PA. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol Int. 2016;33(4):351-373.
18. Oldham MA, Ciraulo DA. Bright light therapy for depression: a review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int. 2014;31(3):305-319.
19. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
20. Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49(3):331-339.
21. Anderson JL, Glod CA, Dai J, et al. Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr Scand. 2009;120(3):203-212.
22. Wirz-Justice A, Graw P, Kräuchi K, et al. Effect of light on unmasked circadian rhythms in winter depression. In: Wetterberg L, ed. Light and biological rhythms in man. Oxford, United Kingdom:Pergamon Press;1993:385-393.
23. Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453-464.
24. Aries MBC. Human lighting demands: healthy lighting in an office environment. Eindhoven, Eindhoven University Press. 2005;158. doi:10.6100/IR594257.
25. Dauphinais DR, Rosenthal JZ, Terman M, et al. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012;196(1):57-61.
26. Camardese G, Leone B, Serrani R, et al. Augmentation of light therapy in difficult-to-treat depressed patients: an open-label trial in both unipolar and bipolar patients. Neuropsychiatr Dis Treat. 2015;11:2331-2338.
27. Papatheodorou G, Kutcher S. The effect of adjunctive light therapy on ameliorating breakthrough depressive symptoms in adolescent-onset bipolar disorder.
J Psychiatry Neurosci. 1995;20(3):226-232.
28. Benedetti F, Riccaboni R, Locatelli C, et al. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133-140.
29. Liebenluft E, Turner EH, Felman-Naim S, et al. Light therapy in patients with rapid cycling bipolar disorder: preliminary results. Psychopharmacol Bull. 1995;31(4):
705-710.
30. Sit DK, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9(8):918-927.
31. Tseng PT, Chen YW, Tu KY, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):
1037-1047.
32. Yorguner KN, Bulut NS, Carkaxhiu BG, et al. Efficacy of bright light therapy in bipolar depression. Psychiatry Res. 2017;260:432-438.
33. Zhou TH, Dang WM, Ma YT, et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord. 2018;227:90-96.
34. Sit DK, McGowan J, Wiltrout C, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175(2):
131-139.
35. Center for Environmental Therapeutics. https://www.cet.org/. Center for Environmental Therapeutics. Accessed November 15, 2017.
36. Lam RW, Levitt AJ. Canadian consensus guidelines for the treatment of seasonal affective disorder. https://mdsc.ca/documents/Consumer%20and%20Family%20Support/CCG_on_Seasonal_Affective_Disorder.pdf. 1999. Accessed August 2, 2017.
37. Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999; 60(11):799-808;quiz 809.
38. Labbate LA, et al. Side effects induced by bright light treatment for seasonal affective disorder. J Clin Psychiatry. 1994; 55(5):189-191.
39. Gallin PF, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119(2):202-210.
40. Chan PK, Lam RW, Perry KF. Mania precipitated by light therapy for patients with SAD. J Clin Psychiatry. 1994;55(10):454.
41. Kripke DF. Timing of phototherapy and occurrence of mania. Biol Psychiatry. 1991; 29(11):1156-1157.
1. Pjrek E, Winkler D, Stastny J, et al. Bright light therapy in seasonal affective disorder--does it suffice? Eur Neuropsychopharmacol. 2004.14(4):347-351.
2. Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: meta-analysis of clinical trials. J Affect Disord. 2016;198:64-71.
3. Sekiguchi H, Iritani S, Fujita K. Bright light therapy for sleep disturbance in dementia is most effective for mild to moderate Alzheimer’s type dementia: a case series. Psychogeriatrics, 2017;17(5):275-281.
4. Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder, third edition. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf American Psychiatric Association. 2010. Accessed August, 10, 2017.
5. Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007;9(5):531-535.
6. Steinan MK, Krane-Gartiser K, Morken G, et al. Sleep problems in euthymic bipolar disorders: a review of clinical studies. Current Psychiatry Reviews. 2015;11:235-243.
7. Cudney LE, Frey BN, Streiner D, et al. Biological rhythms are independently associated with quality of life in bipolar disorder. Int J Bipolar Disord. 2016;4(1):9.
8. Duarte FA, Cardoso TA, Campos MT, et al. Biological rhythms in bipolar and depressive disorders: a community study with drug-naive young adults. J Affect Disord, 2015;186:145-148.
9. Pinho M, Sehmbi M, Cudney LE, et al. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Acta Psychiatr Scand. 2015:133(2);102-108.
10. Ng TH, Chung KF, Lee CT, et al. Eveningness and its associated impairments in remitted bipolar disorder. Behav Sleep Med. 2016:14(6):650-664.
11. Wu YH, Ursinus J, Zahn JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord, 2013:148(2-3):357-367.
12. Rybakowski JK, Dmitrzak-Weglar M, Kliwicki S, et al. Polymorphism of circadian clock genes and prophylactic lithium response. Bipolar Disord. 2014;16(2):151-158.
13. Schnell A, Sandrelli F, Ranc V, et al. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol Int. 2015;32(8):1075-1089.
14. Kim Y, Santos R, Gage FH, et al. Molecular mechanisms of bipolar disorder: progress made and future challenges. Front Cell Neurosci. 2017;11:30.
15. Hashimoto S, Kohsaka M, Nakamura K. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 1997;221(2-3):
89-92.
16. Rao ML, Müller-Oerlinghausen B, Mackert A, et al. The influence of phototherapy on serotonin and melatonin in non-seasonal depression. Pharmacopsychiatry.1990;23(3):155-158.
17. Moreira J, Geoffroy PA. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol Int. 2016;33(4):351-373.
18. Oldham MA, Ciraulo DA. Bright light therapy for depression: a review of its effects on chronobiology and the autonomic nervous system. Chronobiol Int. 2014;31(3):305-319.
19. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073.
20. Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49(3):331-339.
21. Anderson JL, Glod CA, Dai J, et al. Lux vs. wavelength in light treatment of seasonal affective disorder. Acta Psychiatr Scand. 2009;120(3):203-212.
22. Wirz-Justice A, Graw P, Kräuchi K, et al. Effect of light on unmasked circadian rhythms in winter depression. In: Wetterberg L, ed. Light and biological rhythms in man. Oxford, United Kingdom:Pergamon Press;1993:385-393.
23. Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11(6):453-464.
24. Aries MBC. Human lighting demands: healthy lighting in an office environment. Eindhoven, Eindhoven University Press. 2005;158. doi:10.6100/IR594257.
25. Dauphinais DR, Rosenthal JZ, Terman M, et al. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012;196(1):57-61.
26. Camardese G, Leone B, Serrani R, et al. Augmentation of light therapy in difficult-to-treat depressed patients: an open-label trial in both unipolar and bipolar patients. Neuropsychiatr Dis Treat. 2015;11:2331-2338.
27. Papatheodorou G, Kutcher S. The effect of adjunctive light therapy on ameliorating breakthrough depressive symptoms in adolescent-onset bipolar disorder.
J Psychiatry Neurosci. 1995;20(3):226-232.
28. Benedetti F, Riccaboni R, Locatelli C, et al. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133-140.
29. Liebenluft E, Turner EH, Felman-Naim S, et al. Light therapy in patients with rapid cycling bipolar disorder: preliminary results. Psychopharmacol Bull. 1995;31(4):
705-710.
30. Sit DK, Wisner KL, Hanusa BH, et al. Light therapy for bipolar disorder: a case series in women. Bipolar Disord. 2007;9(8):918-927.
31. Tseng PT, Chen YW, Tu KY, et al. Light therapy in the treatment of patients with bipolar depression: a meta-analytic study. Eur Neuropsychopharmacol. 2016;26(6):
1037-1047.
32. Yorguner KN, Bulut NS, Carkaxhiu BG, et al. Efficacy of bright light therapy in bipolar depression. Psychiatry Res. 2017;260:432-438.
33. Zhou TH, Dang WM, Ma YT, et al. Clinical efficacy, onset time and safety of bright light therapy in acute bipolar depression as an adjunctive therapy: a randomized controlled trial. J Affect Disord. 2018;227:90-96.
34. Sit DK, McGowan J, Wiltrout C, et al. Adjunctive bright light therapy for bipolar depression: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2018;175(2):
131-139.
35. Center for Environmental Therapeutics. https://www.cet.org/. Center for Environmental Therapeutics. Accessed November 15, 2017.
36. Lam RW, Levitt AJ. Canadian consensus guidelines for the treatment of seasonal affective disorder. https://mdsc.ca/documents/Consumer%20and%20Family%20Support/CCG_on_Seasonal_Affective_Disorder.pdf. 1999. Accessed August 2, 2017.
37. Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psychiatry. 1999; 60(11):799-808;quiz 809.
38. Labbate LA, et al. Side effects induced by bright light treatment for seasonal affective disorder. J Clin Psychiatry. 1994; 55(5):189-191.
39. Gallin PF, et al. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119(2):202-210.
40. Chan PK, Lam RW, Perry KF. Mania precipitated by light therapy for patients with SAD. J Clin Psychiatry. 1994;55(10):454.
41. Kripke DF. Timing of phototherapy and occurrence of mania. Biol Psychiatry. 1991; 29(11):1156-1157.
Bipolar patients’ relatives face increased cardiovascular risk
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
BARCELONA – Young patients recently diagnosed with bipolar disorder are at double the 30-year risk of cardiovascular disease, compared with the general population, and their unaffected first-degree relatives are nearly as high risk, Klara Coello, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The clinical implication of this finding is that unaffected first-degree relatives of patients with bipolar disorder – an affective disorder typically diagnosed at age 15-24 – should be targeted for intensified primary cardiovascular prevention, with a focus on smoking and dyslipidemia, both of which were more prevalent in these patients and their unaffected relatives than in the general population in her study, noted Dr. Coello, a doctoral candidate with the Copenhagen Affective Disorders Research Center at the University of Copenhagen.
She and her coinvestigators presented a cross-sectional study in which they calculated the 30-year Framingham Risk Scores for 221 patients recently diagnosed bipolar disorder – 95% of whom had been diagnosed within the past 2 years – along with 50 unaffected first-degree relatives and 119 age- and sex-matched controls. The investigators used the Framingham Risk Score because the widely used American Heart Association/American College of Cardiology Atherosclerotic Cardiovascular Disease Risk Estimator applies only to individuals aged 40 and up.
The key findings: The 30-year risk of cardiovascular disease for patients with bipolar was 98.5% greater than that of controls, and the calculated risk of the unaffected first-degree relatives was increased by 85.4%, compared with that of controls.
The Framingham Risk Score is determined on the basis of old-school cardiovascular risk factors, including age, gender, lipids, systolic blood pressure, diabetes, and smoking. 45% of the bipolar patients were smokers, as were 20% of their first-degree relatives and 13% of controls.
The Danish finding of increased cardiovascular risk in young adults with bipolar disorder recapitulates an American Heart Association Scientific Statement, which was published in Circulation (2015 Sep 8;132[10]:965-86). The statement was intended to alert clinicians that these affective disorders constitute “moderate-risk” conditions for arterial dysfunction prior to age 30 and for premature cardiovascular disease (CVD). The statement declared that this risk is likely mediated not only by the classic cardiovascular risk factors but also by disease-related inflammation, oxidative stress, sleep disruption, and the adverse metabolic effects of many psychotropic medications.
“The magnitude of increased risk for CVD in adulthood is substantial,” according to the AHA expert panel’s scientific statement.
Dr. Coello’s study only took into account levels of the traditional cardiovascular risk factors. Where the study broke new ground that hadn’t been explored in the AHA scientific statement, however, was in identifying unaffected first-degree relatives as an additional at-risk group.
She reported having no financial conflicts regarding her study, which constitutes her PhD thesis.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: The first-degree relatives of patients with bipolar disorder should be targeted for intensified primary cardiovascular prevention.
Major finding: Thirty-year cardiovascular risk was increased by 98.5% in recently diagnosed bipolar patients and by 85.4% in their unaffected first-degree relatives, compared with the general population.
Study details: This cross-sectional study involved calculation of 30-year Framingham Risk Scores for 221 patients recently diagnosed with bipolar disorder, 50 unaffected first-degree relatives, and 119 age- and sex-matched controls.
Disclosures: The study presenter reported having no financial conflicts of interest.
This year’s top papers on mood disorders
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
BARCELONA – Among the handful of top publications on mood disorders during the first three-quarters of 2018 was a landmark comparison of the efficacy and acceptability of 21 antidepressants for acute treatment of major depressive disorder, Íria Grande, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Dr. Grande, a psychiatrist at the bipolar disorders clinic of the University of Barcelona, shared her personal top picks.
‘Antidepressants work’
This epic systematic review and network meta-analysis (Lancet. 2018 Apr 7;391[10128]:1357-66) encompassed 522 randomized double-blind trials with 116,477 participants with major depressive disorder assigned to 21 antidepressants or placebo, in some instances with an additional active comparator antidepressant arm. The report is a major extension of previous work by the same multinational group of investigators (Lancet. 2009 Feb 28;373[9665]:746-58), who initially scrutinized 12 older antidepressants in a total population only one-quarter the size of the updated analysis.
Based upon this vast randomized trial evidence, some of which came from unpublished studies tracked down by the investigators, the 21 antidepressants were rank-ordered in terms of effectiveness and acceptability. But in Dr. Grande’s view, the most important study finding wasn’t which antidepressant donned the crown of most effective or patient acceptable, it was the fact that all 21 drugs proved significantly more effective than placebo, with odds ratios ranging from 2.13 at the top end to 1.37 for reboxetine.
“The results showed antidepressants work. All of the antipsychiatry system is trying to show us that antidepressants do not work in major depression. Well, in this study, it has been proven that all antidepressants are more effective than placebo in major depressive disorder. I think social media should be made aware of that. (Lead investigator) Dr. Andrea Cipriani talked on the BBC about this article, and it had a high impact,” according to Dr. Grande.
All but three of the 21 antidepressants were deemed to be as acceptable as placebo, based upon study dropout rates. The exceptions were agomelatine and fluoxetine, which were 12%-14% more acceptable than placebo. “That’s strange, I think, but that’s what the clinical trial results showed,” she noted. The findings on clomipramine, which was 30% less acceptable than placebo, make sense, Dr. Grande said, “due to its muscarinic effects.”
She took issue with some of the specific study findings. For example, the two top-rated antidepressants in terms of efficacy were amitriptyline and mirtazapine, with odds ratios of 2.13 and 1.89, respectively.
“As a clinician, I don’t consider mirtazapine to be one of the best antidepressants, especially in major depression,” she said. “But these are the results, and as always, we have to adapt the evidence-based medicine and consider it from our clinical point of view.”
The investigators conducted a subanalysis restricted to placebo-controlled head-to-head studies with a comparator antidepressant which Dr. Grande found more interesting and informative than the overall analysis. In the head-to-head analysis, vortioxetine emerged as the top-rated antidepressant, both in efficacy, with an odds ratio of 2.0, as well as in acceptability.
Lithium vs. quetiapine
Finnish investigators used prospective national databases to examine the rates of psychiatric and all-cause hospitalization during a mean 7.2 years of follow-up in all 18,018 Finns hospitalized for bipolar disorder. The purpose was to assess the impact of various mood stabilizers on overall health outcomes in a real-world setting.
The big winner was lithium. In an analysis adjusted for concomitant psychotropic medications, duration of bipolar illness, and intervals of drug exposure and nonexposure, lithium was associated with the lowest risks of psychiatric rehospitalization and all-cause hospitalization, with relative risk reductions of 33% and 29%, respectively. In contrast, quetiapine, the most widely used antipsychotic agent, paled by comparison, achieving only an 8% reduction in the risk of psychiatric rehospitalization and a 7% decrease in all-cause hospitalization (JAMA Psychiatry. 2018 Apr 1;75[4]:347-55).
In addition, long-acting injectable antipsychotics were significantly more effective for prevention of hospitalization than oral antipsychotics.
“That is kind of shocking, because in some countries, long-acting injectables are not authorized and cannot be used. But I think after this article some regulatory changes are going to take place as a result,” Dr. Grande predicted.
“Another issue I thought was interesting, although it was not the main aim of the study, involved benzodiazepines. They increased the risk of hospitalizations, both for psychiatric illness and all other causes. So apart from giving lithium and long-acting injectable antipsychotics to our bipolar patients, we should also be really careful about the use of benzodiazepines,” she commented.
Intranasal esketamine for suicidality?
Esketamine nasal spray, a fast-acting N-methyl-D-aspartate antagonist whose application for marketing approval in combination with a standard oral antidepressant in treatment-resistant depression is now under Food and Drug Administration review, also is being developed for another indication: reduction of suicidality in patients at imminent suicide risk. In a proof-of-concept study, intranasal esketamine resulted in a significant reduction in suicidal thoughts 4 hours after administration, compared with usual care – but not at 24 hours (Am J Psychiatry. 2018 Jul 1;175[7]:620-30).
New and effective medications for this indication are sorely needed. The only drug approved for the indication of suicide prevention is clozapine.
‘Latest thinking’ on bipolar disorders
Dr. Grande coauthored a comprehensive review article on bipolar disorders that she recommended as worthwhile reading (Nat Rev Dis Primers. 2018 Mar 8;4:18008. doi: 10.1038/nrdp.2018.8).
“It covers all the latest thinking. It focuses on the early stages of the disorder, how epigenetic factors are essential, and many other topics, including the bipolarity index being developed at the University of Barcelona to classify drugs in terms of their capacity to prevent episodes of mania or depression in terms of number needed to treat and number needed to harm. It emphasizes the importance of intervening early and focusing on cognitive dysfunction,” Dr Grande said.
Psychedelics making a comeback
German and Swiss investigators used a facial expression discrimination task to demonstrate that psilocybin, a 5-hydroxytryptamine2A–receptor agonist, decreases connectivity between the amygdala and regions of the brain important in emotion processing, including the striatum and frontal pole. The investigators theorized that this might be the mechanism for the psychedelic’s apparent antidepressant effects (Eur Neuropsychopharmacol. 2018 Jun;28[6]:691-700).
Dr. Grande included this study in her top publications list because it reflects the rapidly growing rebirth of interest in psychedelics research among European psychiatrists.
Indeed, elsewhere at the ECNP congress David J. Nutt, DM, declared, “We now have the beginnings of some swinging of the pendulum back in a modern direction. Over the last 10 years there have been a small number of open studies, all done with psilocybin, which is somewhat easier to use than LSD. There are studies in OCD [obsessive-compulsive disorder], tobacco dependence, alcoholism, resistant depression, end-of-life mood changes with cancer and other terminal diseases, and at least two ongoing randomized trials in resistant depression.”
Dr. Nutt, professor of neuropsychopharmacology at Imperial College London, was senior author of the first proof-of-concept study of psilocybin accompanied by psychologic support as a novel therapy for moderate to severe treatment-resistant major depression (Lancet Psychiatry. 2016 Jul;3[7]:619-27).
Methylphenidate ineffective for treatment of acute mania
The MEMAP study was a randomized, double-blind, placebo-controlled multicenter clinical trial testing what has been called the vigilance regulation model of mania. This model hypothesized that unstable regulation of wakefulness figures prominently in the pathogenesis of both mania and attention-deficit/hyperactivity disorder. If true, investigators reasoned, then 2.5 days of methylphenidate at 20-40 mg/day should have a rapid antimanic effect similar to the drug’s benefits in ADHD. Dr. Grande had been a skeptic, and indeed, the trial was halted early for futility (Eur Neuropsychopharmacol. 2018 Jan;28[1]:185-94).
She reported serving as a paid speaker for Lunbeck, Ferrer, GlaxoSmithKline, and Janssen. Her own research is funded by the Spanish Ministry of Economy and Competitiveness.
REPORTING FROM THE ECNP CONGRESS
Lithium/cancer link debunked
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
BARCELONA – A large Swedish national registry study has found no hint of increased cancer risk in bipolar patients on long-term lithium therapy.
“This is a very important null result. There is no increased risk for cancer for bipolar patients on lithium. It’s a bad rumor. It’s important to tell patients we’re very confident this is true. We studied every single type of cancer. We would have seen something here if there was something to see,” Lina Martinsson, MD, PhD, said at the annual congress of the European College of Neuropsychopharmacology.
Using comprehensive registry data on nearly 2.6 million Swedes aged 50-84 years with 4 years of follow-up, including 2,393 patients with bipolar disorder on long-term lithium and 3,049 patients not on lithium, the overall cancer incidence rate was 5.9% in the group on lithium and 6.0% in those not taking the drug. Those rates were not different from the general Swedish population, reported Dr. Martinsson, a senior psychiatrist at the Karolinska Institute in Stockholm.
Such patients had a 72% greater risk of lung cancer and other cancers of the respiratory system than the general population, a 47% increased risk of GI cancers, and a 150% greater risk of endocrine organ cancers.
“The increase in respiratory and digestive organ cancers might depend upon bipolar patients’ tendency for smoking and other types of hard living. We can’t explain the increase in endocrine cancers,” she said.
In contrast, the rates of these types of cancer were no different from the general population in bipolar patients taking lithium, hinting at a possible protective effect of the drug, although this remains speculative, the psychiatrist added.
The question of whether lithium is associated with increased cancer risk has been controversial. In particular, several groups have reported a possible increased risk of renal cancer on the basis of what Dr. Martinsson considers weak evidence. She felt a responsibility to undertake this definitive Swedish national study examining the issue because the cancer speculation arose following her earlier study demonstrating that bipolar patients on lithium had much longer telomeres than those not on the drug, and that the ones who responded well to lithium had longer telomeres than those who did not (Transl Psychiatry. 2013 May 21. doi: 10.1038/tp.2013.37).
“If longer telomere length gives longer life to the wrong cells, it might enhance the risk of cancer,” she noted.
But this theoretical concern did not hold up under close Swedish scrutiny. “Warnings for cancer in patients with long-term lithium treatment are unnecessary and ought to be omitted from the current policies,” Dr. Martinsson said.
She reported no financial conflicts regarding her study, which was funded by the Swedish Research Council.
REPORTING FROM THE ECNP CONGRESS
Key clinical point: Long-term lithium therapy does not increase cancer risk.
Major finding: The overall incidence of cancer during 4 years of follow-up was 5.9% in bipolar patients on long-term lithium and 6.0% in those who were not.
Study details: This Swedish national registry study compared cancer incidence rates in more than 5,400 patients with bipolar disorder and nearly 2.6 million controls.
Disclosures: The presenter reported no financial conflicts regarding the study, which was supported by the Swedish Research Council.
Mood disorders worsen multiple sclerosis disability
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
BERLIN – Depression and bipolar disorder are major risk factors for worsening disability in people with multiple sclerosis, according to the results of a large Swedish registry-based study.
The presence of depression increased the risk of having a sustained Expanded Disability Status Scale (EDSS) score of 3.0 by 54% and 4.0 by 87%, and it doubled the risk of an EDSS of 6.0.
Selective serotonin reuptake inhibitor treatment also upped the risk of greater disability, with patients exposed to SSRIs having a 40% increased risk of a sustained EDSS of 3.0, a 97% chance of having a sustained EDSS of 4.0, and 2.2-fold increased risk of a sustained EDSS of 6.0.
“We know that mood disorders are highly prevalent in people with multiple sclerosis,” Stefanie Binzer, MD, said at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. She gave her presentation at the meeting on Oct. 10, which was World Mental Health Day.
The presence of mood disorders is associated with reduced quality of life, said Dr. Binzer of the department of clinical neuroscience at the Karolinska Institute in Stockholm. Furthermore, depression is the major risk factor for suicidality in patients with MS. However, before this study the effect of having a comorbid mood disorder on MS patients’ disability levels had not been established.
The investigators analyzed data from 5,875 patients in the Swedish MS registry between 2001 and 2014. By matching these patients to records in the Swedish National Patient Registry and the Swedish National Prescribed Drug Registry, they found that 8.5% (n = 502) had an International Classification of Diseases, 10th revision (ICD-10), code for depression. Of these, 261 had received a diagnosis of depression before their diagnosis of MS.
Of 3,817 patients with MS onset between 2005 and 2014, 27.4% (n = 1,048) had collected at least one prescription for an SSRI.
“What we found was that MS patients with either an ICD code for depression or having been exposed to SSRIs had a significantly increased risk of reaching EDSS 3.0,” Dr. Binzer reported. The age at which patients reached these milestones were younger in both groups when compared with MS patients without depression, she observed.
“The difference between the groups [MS with and MS without depression] seemed to increased with EDSS,” Dr. Binzer said.
Although not statistically significant, there was a trend for patients with depression to be more likely to convert to secondary progressive MS, with a hazard ratio of 1.38 (95% confidence interval, 0.91-2.1).
“For a sensitivity analysis, we found that those who had depression prior to their first MS symptom, the median age when they reached EDSS 3.0 and 4.0 was reduced by 3 and 7 years, respectively,” Dr. Binzer said, adding that, unfortunately, there wasn’t enough power to look at the other endpoints.
In regard to bipolar disorder, 1.5% (n = 200) of 13,125 MS patients diagnosed between 1973 and 2014 were identified with this mood disorder. Its presence significantly increased the risk of MS patients reaching an EDSS score of 4.0 by 58% (95% CI, 1.1-2.28), but not EDSS 3.0 (HR = 1.34; 95% CI, 0.94-1.92) or 6.0 (HR = 1.16; 95% CI, 0.79-1.69). The latter could be due to smaller sample size, Dr. Binzer suggested.
The investigators’ analysis of the results stratified by sex, conducted because men tend to fare worse than women with MS and progress faster, showed that for both depression and bipolar disorder, men were at significantly higher risk of reaching sustained disability milestones. Indeed, compared with women, men with depression had a 61% increased risk and those with bipolar disorder a 31% increased risk of reaching an EDSS score of 6.0. They also had 51% and 32% increased risks of conversion to secondary progressive MS.
“We don’t know the mechanisms that underlie these associations,” Dr. Binzer noted. “Irrespective of the underlying mechanisms, [the study] clearly shows that it’s imperative that we recognize, early, mood disorders in MS patients, and manage them effectively in order to provide better care and hopefully reduce MS disability worsening.”
The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
SOURCE: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
REPORTING FROM ECTRIMS 2018
Key clinical point:
Major finding: Depression and bipolar disorder increased the risk of reaching Expanded Disability Status Scale scores of 3.0, 4.0, and 6.0, particularly in men with MS.
Study details: Swedish registry study of nearly 6,000 individuals with confirmed MS, 8.5% of whom had depression and 1.5% of whom had bipolar disorder.
Disclosures: The research was funded by the Swedish Research Council and the Swedish Brain Foundation. Dr. Binzer has received speaker fees and travel grants from Biogen.
Source: Binzer S et al. Mult Scler. 2018;24(Suppl 2):41. Abstract 99.
Protein binding changes and drug interactions: What do we know?
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.
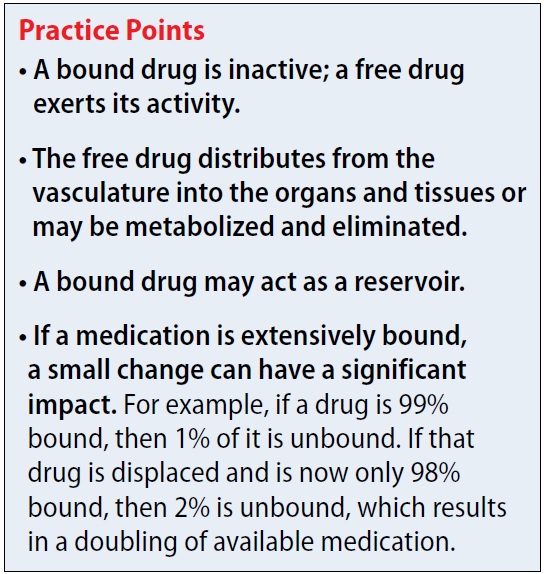
A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.
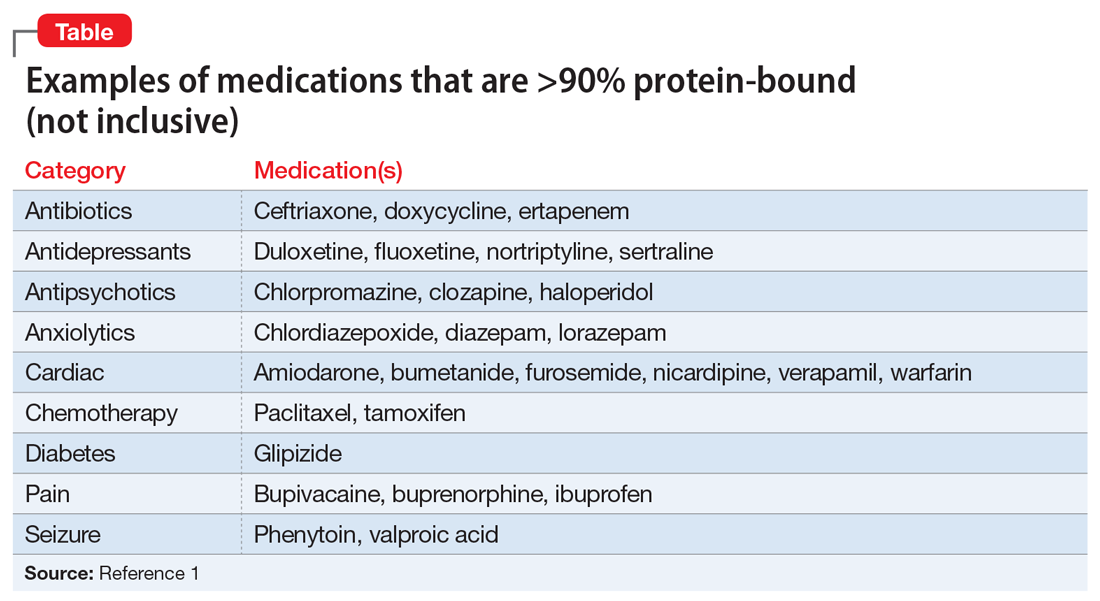
For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
Mr. S, age 47, weighs 209 lb and has a history of seizure disorder, bipolar disorder not otherwise specified, hypertension, and type 2 diabetes mellitus. He presents to the emergency department after not taking his medications for 2 days while on vacation. He has increased energy, decreased sleep, and pressured speech, and insists on walking for up to 10 hours per day “in preparation for a marathon,” even though he has a 4-cm foot ulcer. His family reports that he had been compliant with his medications until the present incident.
Mr. S has no known drug allergies. His medications include oral divalproex sodium delayed release (valproic acid [VPA]), 1,000 mg twice a day, oral lisinopril, 20 mg every morning, and insulin glargine, 22 units subcutaneously every evening.
A complete blood count, basic metabolic panel, creatine kinase level, VPA level, and urine drug screen are ordered. Relevant results include a serum creatinine level of 1.4 mg/dL (normal range: 0.6 to 1.2 mg/dL), a glucose serum level of 188 mg/dL (normal range: 70 to 100 mg/dL), and a VPA level of 23 mcg/mL (therapeutic range: 50 to 125 mcg/mL). A liver function panel is within normal limits: albumin level of 3.9 g/dL, aspartate aminotransferase level of 18 IU/L, and alanine aminotransferase level of 14 IU/L. In light of Mr. S’s seizure history, neurology is consulted and the decision is made to continue treating him with VPA because he has been seizure-free for 4.5 years and this medication has also helped with his bipolar disorder.
Mr. S is admitted to the hospital and his home medications are resumed at the current doses. On hospital Day 3, Mr. S’s VPA level is 62 mcg/mL, his obsession with a marathon has remitted, and his sleep pattern has normalized. Infectious disease and podiatry services are consulted for his diabetic foot infection, which has ulcerated down to the bone. IV ertapenem, 1,000 mg/d, is initiated with plans for debridement the following week. Two days later, Mr. S has a witnessed seizure; his VPA level is 9 mcg/mL.

A common question asked of pharmacists is, “Will protein binding changes affect drug dosages?” In this article, I describe how protein binding changes may occur, and the complexity of the dynamic. Being highly bound to a protein typically does not mean all medications will interact, but some interactions can be important. This article does not cover medications that bind to hormones.
Why is protein binding important? When a medication is bound to plasma protein, it is not free to act. There can be a delay in therapeutic effect (because no drug is available to react), delayed elimination, or possibly displacement of another protein-bound medication. Additionally, medications tend not to cross the blood-brain barrier or be eliminated when bound. For example, if a drug is 99% bound (leaving 1% free) and displacement now leaves 2% of the drug free, this event has doubled the amount of free drug. As the unbound medication is eliminated, the drug that is bound to the protein can act as a reservoir. A dynamic relationship exists between bound drug, unbound drug, and rate of elimination.
Which proteins do drugs commonly bind to? The proteins often associated with binding include albumin, alpha-1-acid glycoprotein (AAG), and lipoproteins. Albumin comprises 60% of total plasma protein in the plasma. Lipoproteins include very high-density lipoprotein (VHDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and low-density lipoprotein (LDL).1 Medications that bind to lipoproteins include cyclosporine, tacrolimus, and propofol.2
Continued to: What common disease states can cause hypoalbuminemia?
What common disease states can cause hypoalbuminemia? Many disease states can result in low albumin levels. The most common ones are malnutrition, malignancies, stress, injury, burns, pregnancy, and diabetes.3 When there is less albumin to bind to, free drug levels may be increased.
Can AAG levels change with disease states as well? Because AAG accounts for a lower percentage of total plasma protein than albumin, there may be less clinical concern regarding AAG. AAG levels usually do not drop, but instead can become elevated during times of trauma, inflammation, and acute myocardial infarction. This could result in increased binding of the free drug.4Which medications bind to red blood cells (RBCs)? There are several locations for drugs to bind to RBCs, including to hemoglobin and the plasma membrane. Medications that commonly bind to RBCs include barbiturates, chlorpromazine, imipramine, and phenytoin.5
What are common highly-bound medications? The Table1 provides examples of medications that are >90% protein-bound. However, this information may be misleading because many medications are highly bound. Zhang et al1 compiled binding data for 222 drugs, half of which bind 90% to 100%. However, the literature does not indicate that they all have clinically significant interactions. Benet and Hoener6 discuss how factors other than protein binding affect potential drug interactions, and the complexity of the body’s ability to compensate for increased free drug. Medication characteristics that may contribute to producing a significant interaction include, but are not limited to:
- free vs protein-bound drug in the plasma or tissue
- volume of distribution
- organs affected
- hepatic bioavailability
- drug clearance.

For example, VPA is 93% protein-bound and phenytoin is 91% protein-bound.1 However, this interaction is affected by more than just protein binding. VPA not only displaces the protein-bound phenytoin, but also inhibits its metabolism, which together result in increased free phenytoin levels.
Continued to: Another area of concern is a critically ill patient...
Another area of concern is a critically ill patient who has a change in his or her pH. Medications that are highly bound and have high clearance rates may be affected. This is of particular concern when prescribing antibiotics that are time-dependent, such as beta-lactams.3
What happened to Mr. S? Mr. S likely experienced a drug–drug interaction that resulted in a subtherapeutic VPA level and subsequent seizure. Case reports have shown evidence that the carbapenem class of antibiotics, which includes ertapenem, interacts with VPA.7 Proposed mechanisms include a lowering of VPA serum levels due to a redistribution of the VPA onto the RBCs due to carbapenem. Other theories include the possibility that carbapenems may limit oral VPA absorption, decrease VPA enterohepatic recirculation, and increase VPA metabolism.7 Using VPA and ertapenem together is discouraged because seizures have been reported among patients receiving this combination. If it is medically necessary to administer VPA and ertapenem, closely monitor VPA levels. In Mr. S’s case, another broad-spectrum antibiotic, such as piperacillin-tazobactam, could have been used, for his diabetic foot infection.
While many medications may have high protein binding, there are few clinically important known interactions. However, our understanding of the relationship between protein binding and drug interactions may improve with additional research.
CASE CONTINUED
Under neurology’s care, lacosamide is added for treatment of Mr. S’s seizures. No more seizures are noted during the remainder of his hospitalization. Infectious disease services change his antibiotic to piperacillin-tazobactam. Mr. S continues to progress well and is discharged to a rehabilitation center 2 days later.
Related Resource
- DrugBank. www.drugbank.ca. Canadian Institutes of Health Research.
Drug Brand Names
Amiodarone • Cordarone, Pacerone
Bumetanide • Bumex
Bupivacaine • Marcaine, Sensorcaine
Buprenorphine • Belbuca, Subutex
Ceftriaxone • Rocephin
Chlordiazepoxide • Librium
Chlorpromazine • Thorazine
Clozapine • Clozaril
Cyclosporine • Gengraf, Neoral
Diazepam • Valium
Doxycycline • Acticlate, Doryx
Duloxetine • Cymbalta
Ertapenem • Invanz
Fluoxetine • Prozac, Sarafem
Furosemide • Lasix
Glargine (Insulin) • Lantus, Toujeo
Glipizide • Glucotrol
Haloperidol • Haldol
Ibuprofen • Advil, Motrin
Imipramine • Tofranil
Lacosamide • Vimpat
Lisinopril • Prinivil, Zestril
Lorazepam • Ativan
Nicardipine • Cardene
Nortriptyline • Pamelor
Paclitaxel • Abraxane, Taxol
Phenytoin • Dilantin, Phenytek
Piperacillin-tazobactam • Zosyn
Propofol • Diprivan
Sertraline • Zoloft
Tacrolimus • Prograf
Tamoxifen • Soltamox
Valproic acid • Depakene, Depakote
Verapamil • Calan, Verelan
Warfarin • Coumadin, Jantoven
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
1. Zhang F, Xue J, Shao J, et al. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov Today. 2012;17(9-10):475-485.
2. Mehvar R. Role of protein binding in pharmacokinetics. Am J Pharm Edu. 2005;69(5): Article 103;1-8.
3. Roberts JA, Pea F, Lipman J. The clinical relevance of plasma protein binding changes. Clin Pharmacokinet. 2013;52(1):1-8.
4. Schmidt S, Gonzalez D, Derendork H. Significance of protein binding in pharmacokinetics and pharmacodynamics. J Pharm Sci. 2010;99(3):1107-1122.
5. Hinderling P. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49(3):279-295.
6. Benet LZ, Hoener B. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115-121.
7. Park MK, Lim KS, Kim T, et al. Reduced valproic acid serum concentrations due to drug interactions with carbapenem antibiotics: overview of 6 cases. Ther Drug Monit. 2012;34(5):599-603.
Manic after having found a ‘cure’ for Alzheimer’s disease
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.
Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.

Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
CASE Reckless driving, impulse buying
Mr. A, age 73, is admitted to the inpatient psychiatric unit at a community hospital for evaluation of a psychotic episode. His admission to the unit was initiated by his primary care physician, who noted that Mr. A was “not making sense” during a routine visit. Mr. A was speaking rapidly about how he had discovered that high-dose omega-3 fatty acid supplements were a “cure” for Alzheimer’s disease. He also believes that he was recently appointed as CEO of Microsoft and Apple for his discoveries.
Three months earlier, Mr. A had started taking high doses of omega-3 fatty acid supplements (10 to 15 g/d) because he believed they were the cure for memory problems, pain, and depression. At that time, he discontinued taking nortriptyline, 25 mg/d, and citalopram, 40 mg/d, which his outpatient psychiatrist had prescribed for major depressive disorder (MDD). Mr. A also had stopped taking buprenorphine, 2 mg, sublingual, 4 times a day, which he had been prescribed for chronic pain.
Mr. A’s wife reports that during the last 2 months, her husband had become irritable, impulsive, grandiose, and was sleeping very little. She added that although her husband’s ophthalmologist had advised him to not drive due to impaired vision, he had been driving recklessly across the metropolitan area. He had also spent nearly $15,000 buying furniture and other items for their home.
In addition to MDD, Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain. He has been taking vitamin D3, 2,000 U/d, as a nutritional supplement.
[polldaddy:10091672]
The authors’ observations
Mr. A met the DSM-5 criteria for a manic episode (Table 11). His manic and delusional symptoms are new. He has a long-standing diagnosis of MDD, which for many years had been successfully treated with antidepressants without a manic switch. The absence of a manic switch when treated with antidepressants without a mood stabilizer suggested that Mr. A did not have bipolarity in terms of a mood disorder diathesis.2 In addition, it would be unusual for an individual to develop a new-onset or primary bipolar disorder after age 60. Individuals in this age group who present with manic symptoms for the first time are preponderantly found to have a general medical or iatrogenic cause for the emergence of these symptoms.3 Mr. A has a history of chronic kidney disease, Leber’s hereditary optic neuropathy, and chronic pain.

Typically a sedentary man, Mr. A had been exhibiting disinhibited behavior, grandiosity, insomnia, and psychosis. These symptoms began 3 months before he was admitted to the psychiatric unit, when he had started taking high doses of omega-3 fatty acid supplements.
Continue to: EVALUATION Persistent mania
EVALUATION Persistent mania
On initial examination, Mr. A is upset and irritable. He is casually dressed and well-groomed. He lacks insight and says he was brought to the hospital against his will, and it is his wife “who is the one who is crazy.” He is oriented to person, place, and time. At times he is found roaming the hallways, being intrusive, hyperverbal, and tangential with pressured speech. He is very difficult to redirect, and regularly interrupts the interview. His vital signs are stable. He walks well, with slow and steady gait, and displays no tremor or bradykinesia.
[polldaddy:10091674]
The authors’ observations
In order to rule out organic causes, a complete blood count, comprehensive metabolic panel, thyroid profile, urine drug screen, and brain MRI were ordered. No abnormalities were found. DHA and EPA levels were not measured because such testing was not available at the laboratory at the hospital.
Mania emerging after the sixth decade of life is a rare occurrence. Therefore, we made a substantial effort to try to find another cause that might explain Mr. A’s unusual presentation (Table 2).
Omega-3 fatty acid–induced mania. The major types of omega-3 polyunsaturated fatty acids are EPA and DHA and their precursor, alpha-linolenic acid (ALA). EPA and DHA are found primarily in fatty fish, such as salmon, and in fish oil supplements. Omega-3 fatty acids have beneficial anti-inflammatory, antioxidative, and neuroplastic effects.4 Having properties similar to selective serotonin reuptake inhibitors, omega-3 fatty acids are thought to help prevent depression, have few interactions with other medications, and have a lower adverse-effect burden than antidepressants. They have been found to be beneficial as a maintenance treatment and for prevention of depressive episodes in bipolar depression, but no positive association has been found for bipolar mania.5
Continue to: However, very limited evidence suggests...
However, very limited evidence suggests that omega-3 fatty acid supplements, particularly those with flaxseed oil, can induce hypomania or mania. This association was first reported by Rudin6 in 1981, and later reported in other studies.7 How omega-3 fatty acids might induce mania is unclear.
Mr. A was reportedly taking high doses of an omega-3 fatty acid supplement. We hypothesized that the antidepressant effect of this supplement may have precipitated a manic episode. Mr. A had no history of manic episodes in the past and was stable during the treatment with the outpatient psychiatrist. A first episode mania in the seventh decade of life would be highly unusual without an organic etiology. After laboratory tests found no abnormalities that would point to an organic etiology, iatrogenic causes were considered. After a review of the literature, there was anecdotal evidence for the induction of mania in clinical trials studying the effects of omega-3 supplements on affective disorders.
This led us to ask: How much omega-3 fatty acid supplements, if any, can a patient with a depressive or bipolar disorder safely take? Currently, omega-3 fatty acid supplements are not FDA-approved for the treatment of depression or bipolar disorder. However, patients may take 1.5 to 2 g/d for MDD. Further research is needed to determine the optimal dose. It is unclear at this time if omega-3 fatty acid supplementation has any benefit in the acute or maintenance treatment of bipolar disorder.
Alternative nutritional supplements for mood disorders. Traditionally, mood disorders, such as MDD and bipolar disorder, have been treated with psychotropic medications. However, through the years, sporadic studies have examined the efficacy of nutritional interventions as a cost-effective approach to preventing and treating these conditions.5 Proponents of this approach believe such supplements can increase efficacy, as well as decrease the required dose of psychotropic medications, thus potentially minimizing adverse effects. However, their overuse can pose a potential threat of toxicity or unexpected adverse effects, such as precipitation of mania. Table 38 lists over-the-counter nutritional and/or herbal agents that may cause mania.
Continue to: TREATMENT Nonadherence leads to a court order
TREATMENT Nonadherence leads to a court order
On admission, Mr. A receives a dose of
[polldaddy:10091676]
The authors’ observations
During an acute manic episode, the goal of treatment is urgent mood stabilization. Monotherapy can be used; however, in emergent settings, a combination is often used for a rapid response. The most commonly used agents are lithium, anticonvulsants such as valproic acid, and antipsychotics.9 In addition, benzodiazepines can be used for insomnia, agitation, or anxiety. The decision to use lithium, an anticonvulsant, or an antipsychotic depends upon the specific medication’s adverse effects, the patient’s medical history, previous medication trials, drug–drug interactions, patient preference, and cost.
Because Mr. A has a history of chronic kidney disease, lithium was contraindicated.
[polldaddy:10091678]
Continue to: The authors' observations
The authors’ observations
After the acute episode of mania resolves, maintenance pharmacotherapy typically involves continuing the same regimen that achieved mood stabilization. Monotherapy is typically preferred to combination therapy, but it is not always possible after a manic episode.10 A reasonable approach is to slowly taper the antipsychotic after several months of dual therapy if symptoms continue to be well-controlled. Further adjustments may be necessary, depending on the medications’ adverse effects. Moreover, further acute episodes of mania or depression will also determine future treatment.
OUTCOME Resolution of delusions
Mr. A is discharged 30 days after admission. At this point, his acute manic episode has resolved with non-tangential, non-pressured speech, improved sleep, and decreased impulsivity. His grandiose delusions also have resolved. He is prescribed valproic acid, 1,000 mg/d, and risperidone, 6 mg/d at bedtime, under the care of his outpatient psychiatrist.
Bottom Line
Initial presentation of a manic episode in an older patient is rare. It is important to rule out organic causes. Weak evidence suggests omega-3 fatty acid supplements may have the potential to induce mania in certain patients.
Related Resource
- Ramaswamy S, Driscoll D, Rodriguez A, et al. Nutraceuticals for traumatic brain injury: Should you recommend their use? Current Psychiatry. 2017;16(7):34-38,40,41-45.
Drug Brand Names
Buprenorphine • Suboxone, Subutex
Citalopram • Celexa
Hydrocodone/acetaminophen • Vicodin
Lithium • Eskalith, Lithobid
Lorazepam• Ativan
Nortriptyline • Pamelor
Risperidone • Risperdal
Valproic acid • Depakote
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
3. Sami M, Khan H, Nilforooshan R. Late onset mania as an organic syndrome: a review of case reports in the literature. J Affect Disord. 2015:188:226-231.
4. Su KP, Matsuoka Y, Pae CU. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin Psychopharmacol Neurosci. 2015;13(2):129-137.
5. Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81-86.
6. Rudin DO. The major psychoses and neuroses as omega-3 essential fatty acid deficiency syndrome: substrate pellagra. Biol Psychiatry. 1981;16(9):837-850.
7. Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716-717.
8. Joshi K, Faubion M. Mania and psychosis associated with St. John’s wort and ginseng. Psychiatry (Edgmont). 2005;2(9):56-61.
9. Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2009 on the treatment of acute mania. World J Biol Psychiatry. 2009;10(2):85-116.
10. Suppes T, Vieta E, Liu S, et al; Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
Catatonia: How to identify and treat it
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14
Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.
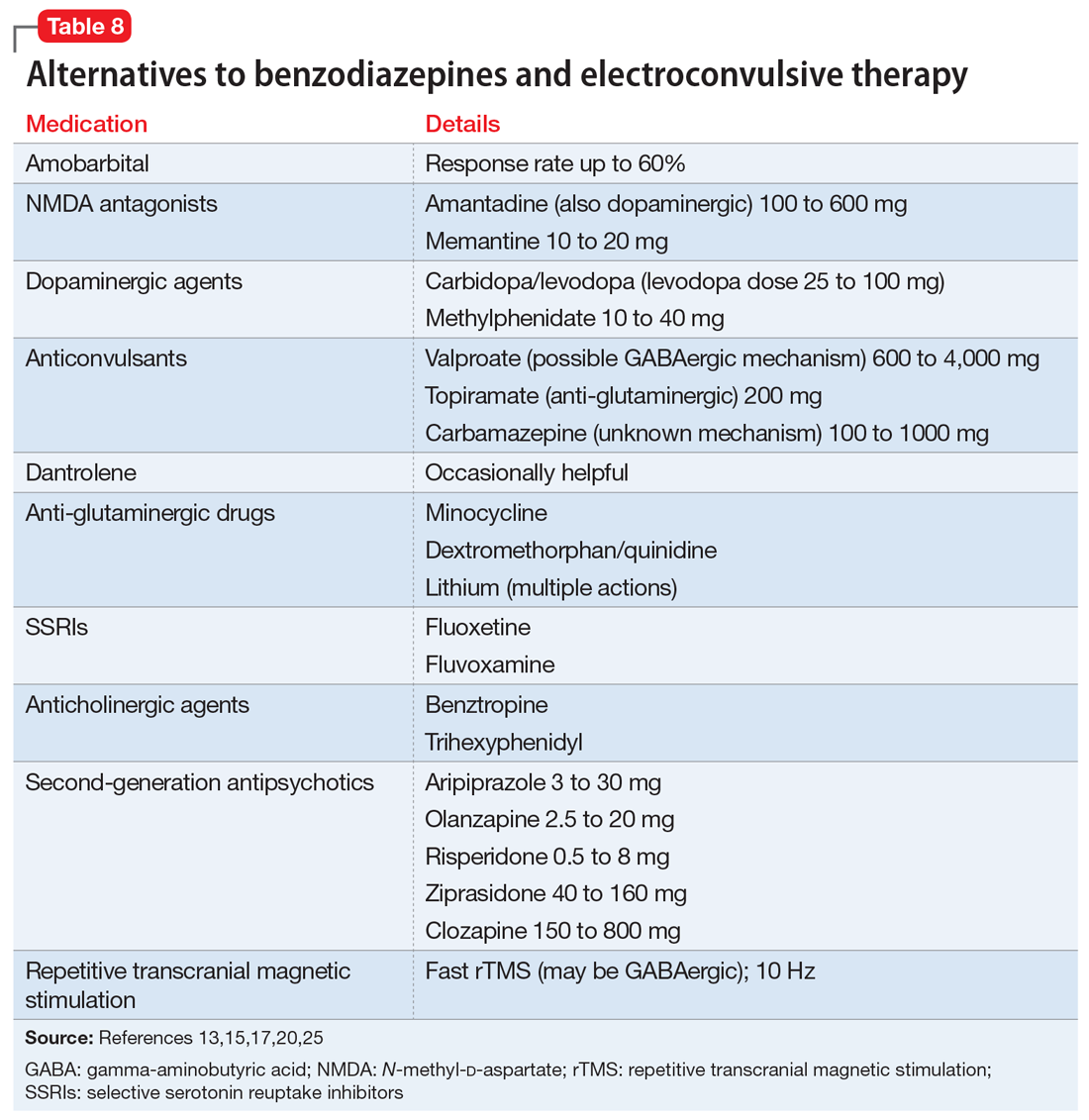
Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
Is catatonia a rare condition that belongs in the history books, or is it more prevalent than we think? If we think we don’t see it often, how will we recognize it? And how do we treat it? This article reviews the evolution of our understanding of the phenomenology and therapy of this interesting and complex condition.
History of the concept
In 1874, Kahlbaum1,2 was the first to propose a syndrome of motor dysfunction characterized by mutism, immobility, staring gaze, negativism, stereotyped behavior, waxy flexibility, and verbal stereotypies that he called catatonia. Kahlbaum conceptualized catatonia as a distinct disorder,3 but Kraepelin reformulated it as a feature of dementia praecox.4 Although Bleuler felt that catatonia could occur in other psychiatric disorders and in normal people,4 he also included catatonia as a marker of schizophrenia, where it remained from DSM-I through DSM-IV.3 As was believed to be true of schizophrenia, Kraepelin considered catatonia to be characterized by poor prognosis, whereas Bleuler eliminated poor prognosis as a criterion for catatonia.3
In DSM-IV, catatonia was still a subtype of schizophrenia, but for the first time it was expanded diagnostically to become both a specifier in mood disorders, and a syndrome resulting from a general medical condition.5,6 In DSM-5, catatonic schizophrenia was deleted, and catatonia became a specifier for 10 disorders, including schizophrenia, mood disorders, and general medical conditions.3,5-9 In ICD-10, however, catatonia is still associated primarily with schizophrenia.10
A wide range of presentations
Catatonia is a cyclical syndrome characterized by alterations in motor, behavioral, and vocal signs occurring in the context of medical, neurologic, and psychiatric disorders.8 The most common features are immobility, waxy flexibility, stupor, mutism, negativism, echolalia, echopraxia, peculiarities of voluntary movement, and rigidity.7,11 Features of catatonia that have been repeatedly described through the years are summarized in Table 1.8,12,13 In general, presentations of catatonia are not specific to any psychiatric or medical etiology.13,14

Catatonia often is described along a continuum from retarded/stuporous to excited,14,15 and from benign to malignant.13 Examples of these ranges of presentation include5,12,13,15-19:
Stuporous/retarded catatonia (Kahlbaum syndrome) is a primarily negative syndrome in which stupor, mutism, negativism, obsessional slowness, and posturing predominate. Akinetic mutism and coma vigil are sometimes considered to be types of stuporous catatonia, as occasionally are locked-in syndrome and abulia caused by anterior cingulate lesions.
Excited catatonia (hyperkinetic variant, Bell’s mania, oneirophrenia, oneroid state/syndrome, catatonia raptus) is characterized by agitation, combativeness, verbigeration, stereotypies, grimacing, and echo phenomena (echopraxia and echolalia).
Continue to: Malignant (lethal) catatonia
Malignant (lethal) catatonia consists of catatonia accompanied by excitement, stupor, altered level of consciousness, catalepsy, hyperthermia, and autonomic instability with tachycardia, tachypnea, hypertension, and labile blood pressure. Autonomic dysregulation, fever, rhabdomyolysis, and acute renal failure can be causes of morbidity and mortality. Neuroleptic malignant syndrome (NMS)—which is associated with dopamine antagonists, especially antipsychotics—is considered a form of malignant catatonia and has a mortality rate of 10% to 20%. Signs of NMS include muscle rigidity, fever, diaphoresis, rigor, altered consciousness, mutism, tachycardia, hypertension, leukocytosis, and laboratory evidence of muscle damage. Serotonin syndrome can be difficult to distinguish from malignant catatonia, but it is usually not associated with waxy flexibility and rigidity.
Several specific subtypes of catatonia that may exist anywhere along dimensions of activity and severity also have been described:
Periodic catatonia. In 1908, Kraepelin described a form of periodic catatonia, with rapid shifts from excitement to stupor.4 Later, Gjessing described periodic catatonia in schizophrenia and reported success treating it with high doses of thyroid hormone.4 Today, periodic catatonia refers to the rapid onset of recurrent, brief hypokinetic or hyperkinetic episodes lasting 4 to 10 days and recurring during the course of weeks to years. Patients often are asymptomatic between episodes except for grimacing, stereotypies, and negativism later in the course.13,15 At least some forms of periodic catatonia are familial,4 with autosomal dominant transmission possibly linked to chromosome 15q15.13
A familial form of catatonia has been described that has a poor response to standard therapies (benzodiazepines and electroconvulsive therapy [ECT]), but in view of the high comorbidity of catatonia and bipolar disorder, it is difficult to determine whether this is a separate condition, or a group of patients with bipolar disorder.5
Late (ie, late-onset) catatonia is well described in the Japanese literature.10 Reported primarily in women without a known medical illness or brain disorder, late catatonia begins with prodromal hypochondriacal or depressive symptoms during a stressful situation, followed by unprovoked anxiety and agitation. Some patients develop hallucinations, delusions, and recurrent excitement, along with anxiety and agitation. The next stage involves typical catatonic features (mainly excitement, retardation, negativism, and autonomic disturbance), progressing to stupor, mutism, verbal stereotypies, and negativism, including refusal of food. Most patients have residual symptoms following improvement. A few cases have been noted to remit with ECT, with relapse when treatment was discontinued. Late catatonia has been thought to be associated with late-onset schizophrenia or bipolar disorder, or to be an independent entity.
Continue to: Untreated catatonia can have...
Untreated catatonia can have serious medical complications, including deep vein thrombosis, pulmonary embolism, aspiration pneumonia, infection, metabolic disorders, decubitus ulcers, malnutrition, dehydration, contractures, thrombosis, urinary retention, rhabdomyolysis, acute renal failure, sepsis, disseminated intravascular coagulation, and cardiac arrest.11,12,16,20,21 Mortality approaches 10%.12 In children and adolescents, catatonia increases the risk of premature death (including by suicide) 60-fold.22
Not as rare as you might think
With the shift from inpatient to outpatient care driven by deinstitutionalization, longitudinal close observation became less common, and clinicians got the impression that the dramatic catatonia that was common in the hospital had become rare.3 The impression that catatonia was unimportant was strengthened by expanding industry promotion of antipsychotic medications while ignoring catatonia, for which the industry had no specific treatment.3 With recent research, however, catatonia has been reported in 7% to 38% of adult psychiatric patients, including 9% to 25% of inpatients, 20% to 25% of patients with mania,3,5 and 20% of patients with major depressive episodes.7 Catatonia has been noted in .6% to 18% of adolescent psychiatric inpatients (especially in communication and social disorders programs),5,8,22 some children,5 and 6% to 18% of adult and juvenile patients with autism spectrum disorder (ASD).23 In the medical setting, catatonia occurs in 12% to 37% of patients with delirium,8,14,17,18,20,24 7% to 45% of medically ill patients, including those with no psychiatric history,12,13 and 4% of ICU patients.12 Several substances have been linked to catatonia; these are discussed later.11 Contrary to earlier impressions, catatonia is more common in mood disorders, particularly mixed bipolar disorder, especially mania,5 than in schizophrenia.7,8,17,25
Pathophysiology/etiology
Conditions associated with catatonia have different features that act through a final common pathway,7 possibly related to the neurobiology of an extreme fear response called tonic immobility that has been conserved through evolution.8 This mechanism may be mediated by decreased dopamine signaling in basal ganglia, orbitofrontal, and limbic systems, including the hypothalamus and basal forebrain.3,17,20 Subcortical reduction of dopaminergic neurotransmission appears to be related to reduced GABAA receptor signaling and dysfunction of N-methyl-
Up to one-quarter of cases of catatonia are secondary to medical (mostly neurologic) factors or substances.15 Table 25,13,15 lists common medical and neurological causes. Medications and substances known to cause catatonia are noted in Table 3.5,8,13,16,26
Catatonia can be a specifier, or a separate condition
DSM-5 criteria for catatonia are summarized in Table 4.28 With these features, catatonia can be a specifier for depressive, bipolar, or psychotic disorders; a complication of a medical disorder; or another separate diagnosis.8 The diagnosis of catatonia in DSM-5 is made when the clinical picture is dominated by ≥3 of the following core features8,15:
- motoric immobility as evidenced by catalepsy (including waxy flexibility) or stupor
- excessive purposeless motor activity that is not influenced by external stimuli
- extreme negativism or mutism
- peculiarities of voluntary movement such as posturing, stereotyped movements, prominent mannerisms, or prominent grimacing
- echolalia or echopraxia.
Continue to: DSM-5 criteria for the diagnosis of catatonia are more...
DSM-5 criteria for the diagnosis of catatonia are more restrictive than DSM-IV criteria. As a result, they exclude a significant number of patients who would be considered catatonic in other systems.29 For example, DSM-5 criteria do not include common features noted in Table 1,8,12,13 such as rigidity and staring.14,29 If the diagnosis is not obvious, it might be suspected in the presence of >1 of posturing, automatic obedience, or waxy flexibility, or >2 of echopraxia/echolalia, gegenhalten, negativism, mitgehen, or stereotypy/vergiberation.12 Clues to catatonia that are not included in formal diagnostic systems and are easily confused with features of psychosis include whispered or robotic speech, uncharacteristic foreign accent, tiptoe walking, hopping, rituals, and odd mannerisms.5
There are several catatonia rating scales containing between 14 and 40 items that are useful in diagnosing and following treatment response in catatonia (Table 58,13,15,29). Of these, the Kanner Scale is primarily applied in neuropsychiatric settings, while the Bush-Francis Catatonia Rating Scale (BFCRS) has had the most widespread use. The BFCRS consists of 23 items, the first 14 of which are used as a screening instrument. It requires 2 of its first 14 items to diagnose catatonia, while DSM-5 requires 3 of 12 signs.29 If the diagnosis remains in doubt, a benzodiazepine agonist test can be instructive.9,12 The presence of catatonia is suggested by significant improvement, ideally assessed prospectively by improvement of BFCRS scores, shortly after administration of a single dose of 1 to 2 mg lorazepam or 5 mg diazepam IV, or 10 mg zolpidem orally. Further evaluation generally consists of a careful medical and psychiatric histories of patient and family, review of all medications, history of substance use with toxicology as indicated, physical examination focusing on autonomic dysregulation, examination for delirium, and laboratory tests as suggested by the history and examination that may include complete blood count, creatine kinase, serum iron, blood urea nitrogen, electrolytes, creatinine, prolactin, anti-NMDA antibodies, thyroid function tests, serology, metabolic panel, human immunodeficiency virus testing, EEG, and neuroimaging.8,15,16
A complex differential diagnosis
Manifestations of numerous psychiatric and neurologic disorders can mimic or be identical to those of catatonia. The differential diagnosis is complicated by the fact that some of these disorders can cause catatonia, which is then masked by the primary disorder; some disorders (eg, NMS) are forms of catatonia. Table 65,8,12,19,26,30 lists conditions to consider.
Some of these conditions warrant discussion. ASD may have catatonia-like features such as echolalia, echopraxia, excitement, combativeness, grimacing, mutism, logorrhea, verbigeration, catalepsy, mannerisms, rigidity, staring and withdrawal.8 Catatonia may also be a stage of deterioration of autism, in which case it is characterized by increases in slowness of movement and speech, reliance on physical or verbal prompting from others, passivity, and lack of motivation.23 At the same time, catatonic features such as mutism, stereotypic speech, repetitive behavior, echolalia, posturing, mannerisms, purposeless agitation, and rigidity in catatonia can be misinterpreted as signs of ASD.8 Catatonia should be suspected as a complication of longstanding ASD in the presence of a consistent, marked change in motor behavior, such as immobility, decreased speech, stupor, excitement, or mixtures or alternations of stupor and excitement.8 Freezing while doing something, difficulty crossing lines, or uncharacteristic persistence of a particular behavior may also herald the presence of catatonia with ASD.8
Catatonia caused by a neurologic or metabolic factor or a substance can be difficult to distinguish from delirium complicated by catatonia. Delirium may be identified in patients with catatonia by the presence of a waxing and waning level of consciousness (vs fluctuating behavior in catatonia) and slowing of the EEG.12,15 Antipsychotic medications can improve delirium but worsen catatonia, while benzodiazepines can improve catatonia but worsen delirium.
Continue to: Among other neurologic syndromes...
Among other neurologic syndromes that can be confused with catatonia, locked-in syndrome consists of total immobility except for vertical extraocular movements and blinking. In this state, patients attempt to communicate with their eyes, while catatonic patients do not try to communicate. There is no response to a lorazepam challenge test. Stiff man syndrome is associated with painful spasms precipitated by touch, noise, or emotional stimuli. Baclofen can resolve stiff man syndrome, but it can induce catatonia. Paratonia refers to generalized increased motor tone that is idiopathic, or associated with neurodegeneration, encephalopathy, or medications. The only motor sign is increased tone, and other signs of catatonia are absent. Catatonia is usually associated with some motor behaviors and interaction with the environment, even if it is negative, while the coma vigil patient is completely unresponsive. Frontotemporal dementia is progressive, while catatonia usually improves without residual dementia.30
Benzodiazepines, ECT are the usual treatments
Experience dictates that the general principles of treatment noted in Table 712,15,23,31 apply to all patients with catatonia. Since the first reported improvement of catatonia with amobarbital in 1930,6 there have been no controlled studies of specific treatments of catatonia.13 Meaningful treatment trials are either naturalistic, or have been performed only for NMS and malignant catatonia.5 However, multiple case reports and case series suggest that treatments with agents that have anticonvulsant properties (benzodiazepines, barbiturates) and ECT are effective.5
Benzodiazepines and related compounds. Case series have suggested a 60% to 80% remission rate of catatonia with benzodiazepines, the most commonly utilized of which has been lorazepam.7,13,32 Treatment begins with a lorazepam challenge test of 1 to 2 mg in adults and 0.5 to 1 mg in children and geriatric patients,9,15 administered orally (including via nasogastric tube), IM, or IV. Following a response (≥50% improvement), the dose is increased to 2 mg 3 times per day. The dose is further increased to 6 to 16 mg/d, and sometimes up to 30 mg/d.9,11 Oral is less effective than sublingual or IM administration.11 Diazepam can be helpful at doses 5 times the lorazepam dose.9,17 A zo
One alternative benzodiazepine protocol utilizes an initial IV dose of 2 mg lorazepam, repeated 3 to 5 times per day; the dose is increased to 10 to 12 mg/d if the first doses are partially effective.16 A lorazepam/diazepam approach involves a combination of IM lorazepam and IV diazepam.11 The protocol begins with 2 mg of IM lorazepam. If there is no effect within 2 hours, a second 2 mg dose is administered, followed by an IV infusion of 10 mg diazepam in 500 ml of normal saline at 1.25 mg/hour until catatonia remits.
An Indian study of 107 patients (mean age 26) receiving relatively low doses of lorazepam (3 to 6 mg/d for at least 3 days) found that factors suggesting a robust response include a shorter duration of catatonia and waxy flexibility, while passivity, mutism, and auditory hallucinations describing the patient in the third person were associated with a poorer acute response.31 Catatonia with marked retardation and mutism complicating schizophrenia, especially with chronic negative symptoms, may be associated with a lower response rate to benzodiazepines.20,33 Maintenance lorazepam has been effective in reducing relapse and recurrence.11 There are no controlled studies of maintenance treatment with benzodiazepines, but clinical reports suggest that doses in the range of 4 to 10 mg/d are effective.32
Continue to: ECT was used for catatonia in 1934...
ECT was first used for catatonia in 1934, when Laszlo Meduna used chemically induced seizures in catatonic patients who had been on tube feeding for months and no longer needed it after treatment.6,7 As was true for other disorders, this approach was replaced by ECT.7 In various case series, the effectiveness of ECT in catatonia has been 53% to 100%.7,13,15 Right unilateral ECT has been reported to be effective with 1 treatment.21 However, the best-established approach is with bitemporal ECT with a suprathreshold stimulus,9 usually with an acute course of 6 to 20 treatments.20 ECT has been reported to be equally safe and effective in adolescents and adults.34 Continued ECT is usually necessary until the patient has returned to baseline.9
ECT usually is recommended within 24 hours for treatment-resistant malignant catatonia or refusal to eat or drink, and within 2 to 3 days if medications are not sufficiently effective in other forms of catatonia.12,15,20 If ECT is initiated after a benzodiazepine trial, the benzodiazepine antagonist flumazenil is administered first to reverse the anticonvulsant effect.9 Some experts recommend using a muscle relaxant other than succinylcholine in the presence of evidence of muscle damage.7
Alternatives to benzodiazepines and ECT. Based on case reports, the treatments described in Table 813,15,17,20,25 have been used for patients with catatonia who do not tolerate or respond to standard treatments. The largest number of case reports have been with NMDA antagonists, while the presumed involvement of reduced dopamine signaling suggests that dopaminergic medications should be helpful. Dantrolene, which blocks release of calcium from intracellular stores and has been used to treat malignant hyperthermia, is sometimes used for NMS, often with disappointing results.

Whereas first-generation antipsychotics definitely increase the risk of catatonia and second-generation antipsychotics (SGAs) probably do so, SGAs are sometimes necessary to treat persistent psychosis in patients with schizophrenia who develop catatonia. Of these medications, clozapine may be most desirable because of low potency for dopamine receptor blockade and modulation of glutamatergic signaling. Partial dopamine agonism by aripiprazole, and the potential for increased subcortical prefrontal dopamine release resulting from serotonin 5HT2A antagonism and 5HT1A agonism by other SGAs, could also be helpful or at least not harmful in catatonia. Lorazepam is usually administered along with these medications to ameliorate treatment-emergent exacerbation of catatonia.
There are no controlled studies of any of these treatments. Based on case reports, most experts would recommend initiating treatment of catatonia with lorazepam, followed by ECT if necessary or in the presence of life-threatening catatonia. If ECT is not available, ineffective, or not tolerated, the first alternatives to be considered would be an NMDA antagonist or an anticonvulsant.20
Continue to: Course varies by patient, underlying cause
Course varies by patient, underlying cause
The response to benzodiazepines or ECT can vary from episode to episode11 and is similar in adults and younger patients.22 Many patients recover completely after a single episode, while relapse after remission occurs repeatedly in periodic catatonia, which involves chronic alternating stupor and excitement waxing and waning over years.11 Relapses may occur frequently, or every few years.11 Some cases of catatonia initially have an episodic course and become chronic and deteriorating, possibly paralleling the original descriptions of the natural history of untreated catatonia, while malignant catatonia can be complicated by medical morbidity or death.4 The long-term prognosis generally depends on the underlying cause of catatonia.5
Bottom Line
Much more common than many clinicians realize, catatonia can be overlooked because symptoms can mimic or overlap with features of an underlying medical or neurologic disorder. Suspect catatonia when one of these illnesses has an unexpected course or an inadequate treatment response. Be alert to characteristic changes in behavior and speech. A benzodiazepine challenge can be used to diagnose and begin treatment of catatonia. Consider electroconvulsive therapy sooner rather than later, especially for severely ill patients.
Related Resources
- Gibson RC, Walcott G. Benzodiazepines for catatonia in people with schizophrenia and other serious mental illnesses. Cochrane Database Syst Rev. 2008;(4):CD006570.
- Newcastle University. Catatonia. https://youtu.be/_s1lzxHRO4U.
Drug Brand Names
Amantadine • Symmetrel
Amobarbital • Amytal
Aripiprazole • Abilify
Azithromycin • Zithromax
Baclofen • Lioresal
Benztropine • Cogentin
Carbamazepine • Carbatrol, Tegretol
Carbidopa/levodopa • Sinemet
Ciprofloxacin • Cipro
Clozapine • Clozaril
Dantrolene • Dantrium
Dexamethasone • Decadron
Dextromethorphan/quinidine • Neudexta
Diazepam • Valium
Disulfiram • Antabuse
Flumazenil • Romazicon
Fluoxetine • Prozac
Fluvoxamine • Luvox
Levetiracetam • Keppra
Lithium • Eskalith, Lithobid
Lorazepam • Ativan
Memantine • Namenda
Methylphenidate • Ritalin
Minocycline • Minocin
Olanzapine • Zyprexa
Risperidone • Risperdal
Succinylcholine • Anectine
Topiramate • Topamax
Trihexyphenidyl • Artane
Valproate • Depakote
Ziprasidone • Geodon
Zolpidem • Ambien
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
1. Kahlbaum KL. Catatonia. Baltimore, MD: John Hopkins University Press; 1973.
2. Kahlbaum KL. Die Katatonie oder das Spannungsirresein. Berlin: Hirschwald; 1874.
3. Tang VM, Duffin J. Catatonia in the history of psychiatry: construction and deconstruction of a disease concept. Perspect Biol Med. 2014;57(4):524-537.
4. Carroll BT. Kahlbaum’s catatonia revisited. Psychiatry Clin Neurosci. 2001;55(5):431-436.
5. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160(7):1233-1241.
6. Fink M, Fricchione GL, Rummans T, et al. Catatonia is a systemic medical syndrome. Acta Psychiatr Scand. 2016;133(3):250-251.
7. Medda P, Toni C, Luchini F, et al. Catatonia in 26 patients with bipolar disorder: clinical features and response to electroconvulsive therapy. Bipolar Disord. 2015;17(8):892-901.
8. Mazzone L, Postorino V, Valeri G, et al. Catatonia in patients with autism: prevalence and management. CNS Drugs. 2014;28(3):205-215.
9. Fink M, Kellner CH, McCall WV. Optimizing ECT technique in treating catatonia. J ECT. 2016;32(3):149-150.
10. Kocha H, Moriguchi S, Mimura M. Revisiting the concept of late catatonia. Compr Psychiatry. 2014;55(7):1485-1490.
11. Lin CC, Hung YL, Tsai MC, et al. Relapses and recurrences of catatonia: 30-case analysis and literature review. Compr Psychiatry. 2016;66:157-165.
12. Saddawi-Konefka D, Berg SM, Nejad SH, et al. Catatonia in the ICU: An important and underdiagnosed cause of altered mental status. A case series and review of the literature. Crit Care Med. 2013;42(3):e234-e241.
13. Wijemanne S, Jankovic J. Movement disorders in catatonia. J Neurol Neurosurg Psychiatry. 2015;86(8):825-832.
14. Grover S, Chakrabarti S, Ghormode D, et al. Catatonia in inpatients with psychiatric disorders: a comparison of schizophrenia and mood disorders. Psychiatry Res. 2015;229(3):919-925.
15. Oldham MA, Lee HB. Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37(6):554-559.
16. Tuerlings JH, van Waarde JA, Verwey B. A retrospective study of 34 catatonic patients: analysis of clinical ‘care and treatment. Gen Hosp Psychiatry. 2010;32(6):631-635.
17. Ohi K, Kuwata A, Shimada T, et al. Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore). 2017;96(16):e6566. doi: 10.1097/MD.0000000000006566.
18. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
19. Lang FU, Lang S, Becker T, et al. Neuroleptic malignant syndrome or catatonia? Trying to solve the catatonic dilemma. Psychopharmacology (Berl). 2015;232(1):1-5.
20. Beach SR, Gomez-Bernal F, Huffman JC, et al. Alternative treatment strategies for catatonia: a systematic review. Gen Hosp Psychiatry. 2017;48:1-19.
21. Kugler JL, Hauptman AJ, Collier SJ, et al. Treatment of catatonia with ultrabrief right unilateral electroconvulsive therapy: a case series. J ECT. 2015;31(3):192-196.
22. Raffin M, Zugaj-Bensaou L, Bodeau N, et al. Treatment use in a prospective naturalistic cohort of children and adolescents with catatonia. Eur Child Adolesc Psychiatry. 2015;24(4):441-449.
23. DeJong H, Bunton P, Hare DJ. A systematic review of interventions used to treat catatonic symptoms in people with autistic spectrum disorders. J Autism Dev Disord. 2014;44(9):2127-2136.
24. Wachtel L, Commins E, Park MH, et al. Neuroleptic malignant syndrome and delirious mania as malignant catatonia in autism: prompt relief with electroconvulsive therapy. Acta Psychiatr Scand. 2015;132(4):319-320.
25. Fink M, Taylor MA. Catatonia: subtype or syndrome in DSM? Am J Psychiatry. 2006;163(11):1875-1876.
26. Khan M, Pace L, Truong A, et al. Catatonia secondary to synthetic cannabinoid use in two patients with no previous psychosis. Am J Addictions. 2016;25(1):25-27.
27. Komatsu T, Nomura T, Takami H, et al. Catatonic symptoms appearing before autonomic symptoms help distinguish neuroleptic malignant syndrome from malignant catatonia. Intern Med. 2016;55(19):2893-2897.
28. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
29. Wilson JE, Niu K, Nicolson SE, et al. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164(1-3):256-262.
30. Ducharme S, Dickerson BC, Larvie M, et al. Differentiating frontotemporal dementia from catatonia: a complex neuropsychiatric challenge. J Neuropsychiatry Clin Neurosci. 2015;27(2):e174-e176.
31. Narayanaswamy JC, Tibrewal P, Zutshi A, et al. Clinical predictors of response to treatment in catatonia. Gen Hosp Psychiatry. 2012;34(3):312-316.
32. Thamizh JS, Harshini M, Selvakumar N, et al. Maintenance lorazepam for treatment of recurrent catatonic states: a case series and implications. Asian J Psychiatr. 2016;22:147-149
33. Ungvari GS, Chiu HF, Chow LY, et al. Lorazepam for chronic catatonia: a randomized, double-blind, placebo-controlled cross-over study. Psychopharmacology (Berl). 1999;142(4):393-398.
34. Flamarique I, Baeza I, de la Serna E, et al. Long-term effectiveness of electroconvulsive therapy in adolescents with schizophrenia spectrum disorders. Eur Child Adolesc Psychiatry. 2015;24(5):517-524.
Bipolar disorder: How to avoid overdiagnosis
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.