User login
VTE, sepsis risk increased among COVID-19 patients with cancer
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
, according to data from a registry study.
Researchers analyzed data on 5,556 patients with COVID-19 who had an inpatient or emergency encounter at Mount Sinai Health System (MSHS) in New York between March 1 and May 27, 2020. Patients were included in an anonymous MSHS COVID-19 registry.
There were 421 patients who had cancer: 96 with a hematologic malignancy and 325 with solid tumors.
After adjustment for age, gender, and number of comorbidities, the odds ratios for acute VTE and sepsis for patients with cancer (versus those without cancer) were 1.77 and 1.34, respectively. The adjusted odds ratio for mortality in cancer patients was 1.02.
The results remained “relatively consistent” after stratification by solid and nonsolid cancer types, with no significant difference in outcomes between those two groups, and results remained consistent in a propensity-matched model, according to Naomi Alpert, a biostatistician at Icahn School of Medicine at Mount Sinai, New York.
Ms. Alpert reported these findings at the AACR virtual meeting: COVID-19 and Cancer.
She noted that the cancer patients were older than the noncancer patients (mean age, 69.2 years vs. 63.8 years), and cancer patients were more likely to have two or more comorbid conditions (48.2% vs. 30.4%). Cancer patients also had significantly lower hemoglobin levels and red blood cell, platelet, and white blood cell counts (P < .01 for all).
“Low white blood cell count may be one of the reasons for higher risk of sepsis in cancer patients, as it may lead to a higher risk of infection,” Ms. Alpert said. “However, it’s not clear what role cancer therapies play in the risks of COVID-19 morbidity and mortality, so there is still quite a bit to learn.”
In fact, the findings are limited by a lack of information about cancer treatment, as the registry was not designed for that purpose, she noted.
Another study limitation is the short follow-up of a month or less in most patients, due, in part, to the novelty of COVID-19, but also to the lack of information on patients after they left the hospital.
“However, we had a very large sample size, with more than 400 cancer patients included, and, to our knowledge, this is the largest analysis of its kind to be done so far,” Ms. Alpert said. “In the future, it’s going to be very important to assess the effect of cancer therapies on COVID-19 complications and to see if prior therapies had any effect on outcomes.”
Longer follow-up would also be helpful for assessing the chronic effects of COVID-19 on cancer patients over time, she said. “It would be important to see whether some of these elevated risks of venous thromboembolism and sepsis are associated with longer-term mortality risks than what we were able to measure here,” she added.
Asked about the discrepancy between mortality in this study and those of larger registries, such as the COVID-19 and Cancer Consortium (CCC19) and TERAVOLT, Ms. Alpert noted that the current study included only patients who required hospitalization or emergency care.
“Our mortality rate was actually a bit higher than what was reported in some of the other studies,” she said. “We had about a 30% mortality rate in the cancer patients and about 25% for the noncancer patients, so ... we’re sort of looking at a subset of patients who we know are the sickest of the sick, which may explain some of the higher mortality that we’re seeing.”
Ms. Alpert reported having no disclosures.
SOURCE: Alpert N et al. AACR COVID-19 and Cancer, Abstract S12-02.
FROM AACR: COVID-19 AND CANCER
First guideline on NGS testing in cancer, from ESMO
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Recommendations on the use of next-generation sequencing (NGS) tests for patients with metastatic cancer have been issued by the European Society for Medical Oncology, the first recommendations of their kind to be published by any medical society.
“Until now, there were no recommendations from scientific societies on how to use this technique in daily clinical practice to profile metastatic cancers,” Fernanda Mosele, MD, medical oncologist, Gustave Roussy, Villejuif, France, said in a statement.
NGS testing is already used extensively in oncology, particularly in metastatic cancer, she noted. The technology is used to assess the sequence of DNA in genes from a tumor tissue sample. Numerous genes can be quickly sequenced at the same time at relatively low cost. The results provide information on mutations that are present, which, in turn, helps with deciding which treatments to use, including drugs targeting the identified mutations.
“Our intent is that they [the guidelines] will unify decision-making about how NGS should be used for patients with metastatic cancer,” Dr. Mosele said.
The recommendations were published online August 25 in Annals of Oncology.
Overall, ESMO recommends the use of tumor multigene NGS for non–small cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma.
For other cancers, the authors said that NGS is not recommended in clinical practice but could be used for research purposes.
However, patients should be informed that it is unlikely that test results would benefit them much personally.
Physicians and patients may decide together to subject the tumor to mutational testing using a large panel of genes, provided testing doesn’t burden the health care system with additional costs.
“This recommendation acknowledges that a small number of patients could benefit from a drug because they have a rare mutation,” Joaquin Mateo, MD, chair of the ESMO working group, said in a statement.
“So beyond the cancers in which everyone should receive NGS, there is room for physicians and patients to discuss the pros and cons of ordering these tests,” he added.
ESMO also does not recommend the use of off-label drugs matched to any genomic alteration detected by NGS unless an access program and a decisional procedure have been developed, either regionally or nationally.
No need for NGS testing of other cancers
In contrast to NSCLC, “there is currently no need to perform tumor multigene NGS for patients with mBC [metastatic breast cancer] in the context of daily practice,” ESMO stated.
This is largely because somatic sequencing cannot fully substitute for germline testing for BRCA status, and other mutations, such as HER2, can be detected using immunohistochemistry (IHC).
The same can be said for patients with metastatic gastric cancer, inasmuch as detection of alterations can and should be done using cheaper testing methods, ESMO pointed out.
However, ESMO members still emphasized that it’s important to include patients with metastatic breast cancer in molecular screening programs as well as in clinical trials testing targeted agents.
Similarly, there is no need to test metastatic colorectal cancer (mCRC) using multigene NGS in daily practice, inasmuch as most level 1 alterations in mCRC can be determined by IHC or PCR.
However, NGS can be considered as an alternative to PCR-based tests in mCRC, provided NGS is not associated with additional cost.
ESMO again recommended that research centers include mCRC patients in molecular screening programs in order for them to have access to innovative clinical trial agents.
As for advanced prostate cancer, ESMO does recommend that clinicians perform NGS on tissue samples to assess the tumor’s mutational status, at least for the presence of BRCA1 and BRCA2 mutations, when patients have access to the poly (ADP-ribose) polymerase inhibitors for treatment.
The authors cautioned, however, that this strategy is unlikely to be cost-effective, so larger panels should be used only when there are specific agreements with payers.
Multigene NGS is also not recommended for patients with advanced pancreatic ductal adenocarcinoma (PDAC), although ESMO points out that it is the role of research centers to propose multigene sequencing for these patients in the context of molecular screening programs.
This is again to facilitate access to innovative drugs for these patients.
Similar to recommendations for patients with advanced PDAC, patients with advanced hepatocellular carcinoma (HCC) do not need to have tumor multigene NGS either.
Considering the high unmet needs of HCC patients, ESMO feels that research centers should propose multigene sequencing to patients with advanced HCC in the context of molecular screening programs.
In contrast, ESMO recommended that tumor multigene NGS be used to detect actionable alterations in patients with advanced cholangiocarcinoma.
Again, they predict that this strategy is unlikely to be cost-effective, so larger panels should only be used if a specific agreement is in place with payers.
ESMO also assessed the frequency of level 1 alterations in less frequent tumor types, including ovarian cancers. Because BRCA1 and BRCA2 somatic mutations in ovarian tumors have been associated with increased response to the PARP inhibitors, the use of multigene NGS is justified with this malignancy, ESMO states.
The authors also recommend that tumor mutational burden be determined in cervical cancer, moderately differentiated neuroendocrine tumors, salivary cancers, vulvar cancer, and thyroid cancers.
Dr. Mosele has disclosed no relevant financial relationships. Many coauthors have relationships with the pharmaceutical industry, as listed in the article.
This article first appeared on Medscape.com.
Immunotherapy should not be withheld because of sex, age, or PS
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
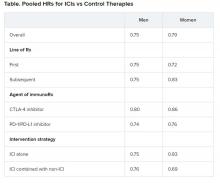
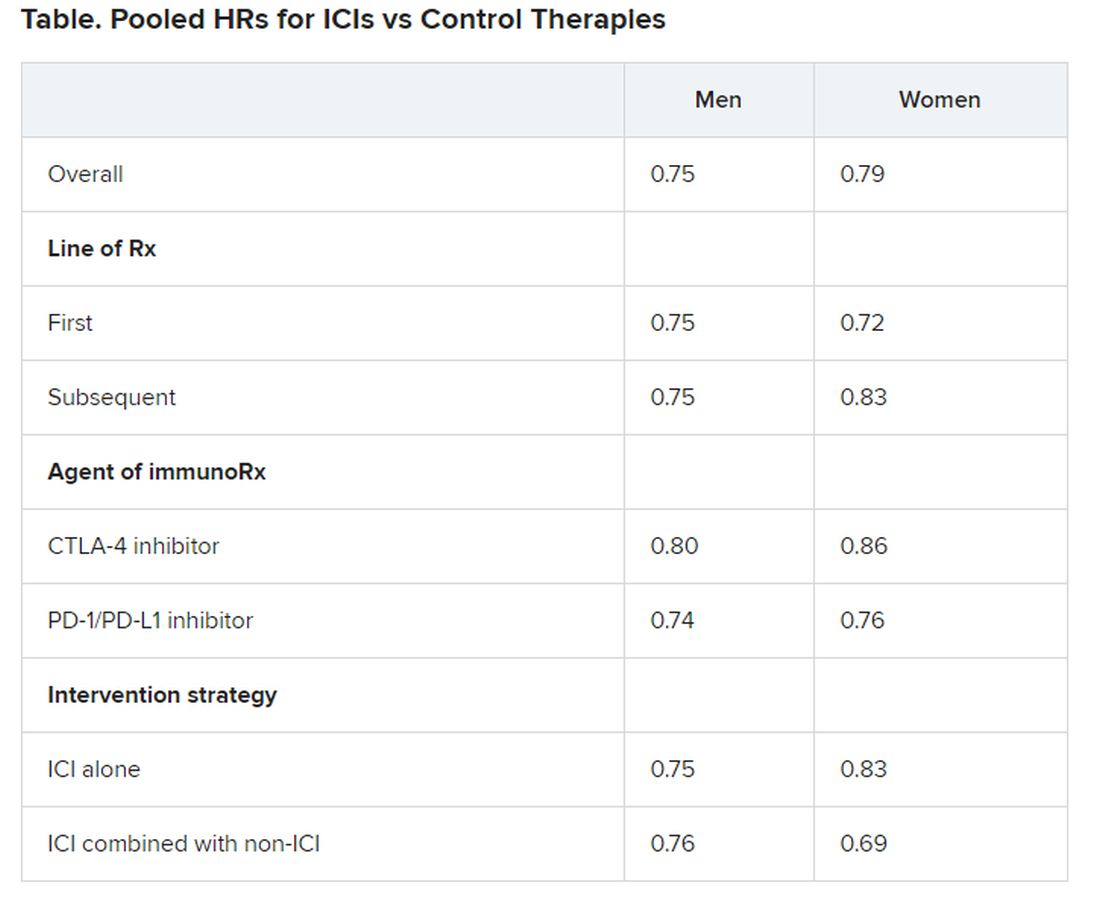
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
The improvement in survival in many cancer types that is seen with immune checkpoint inhibitors (ICIs), when compared to control therapies, is not affected by the patient’s sex, age, or Eastern Cooperative Oncology Group (ECOG) performance status (PS), according to a new meta-analysis.
Therefore, treatment with these immunotherapies should not be withheld on the basis of these factors, the authors concluded.
Asked whether there have been such instances of withholding ICIs, lead author Yucai Wang, MD, PhD, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News: “We did this study solely based on scientific questions we had and not because we were seeing any bias at the moment in the use of ICIs.
“And we saw that the survival benefits were very similar across all of the categories [we analyzed], with a survival benefit of about 20% from immunotherapy across the board, which is clinically meaningful,” he added.
The study was published online August 7 in JAMA Network Open.
“The comparable survival advantage between patients of different sex, age, and ECOG PS may encourage more patients to receive ICI treatment regardless of cancer types, lines of therapy, agents of immunotherapy, and intervention therapies,” the authors commented.
Wang noted that there have been conflicting reports in the literature suggesting that male patients may benefit more from immunotherapy than female patients and that older patients may benefit more from the same treatment than younger patients.
However, there are also suggestions in the literature that women experience a stronger immune response than men and that, with aging, the immune system generally undergoes immunosenescence.
In addition, the PS of oncology patients has been implicated in how well patients respond to immunotherapy.
Wang noted that the findings of past studies have contradicted each other.
Findings of the Meta-Analysis
The meta-analysis included 37 randomized clinical trials that involved a total of 23,760 patients with a variety of advanced cancers. “Most of the trials were phase 3 (n = 34) and conduced for subsequent lines of therapy (n = 22),” the authors explained.
The most common cancers treated with an ICI were non–small cell lung cancer and melanoma.
Pooled overall survival (OS) hazard ratios (HRs) were calculated on the basis of sex, age (younger than 65 years and 65 years and older), and an ECOG PS of 0 and 1 or higher.
Responses were stratified on the basis of cancer type, line of therapy, the ICI used, and the immunotherapy strategy used in the ICI arm.
Most of the drugs evaluated were PD-1 and PD-L1 inhibitors. The specific drugs assessed included ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, and avelumab.
A total of 32 trials that involved more than 20,000 patients reported HRs for death according to the patients’ sex. Thirty-four trials that involved more than 21,000 patients reported HRs for death according to patients’ age, and 30 trials that involved more than 19,000 patients reported HRs for death according to patients’ ECOG PS.
No significant differences in OS benefit were seen by cancer type, line of therapy, agent of immunotherapy, or intervention strategy, the investigators pointed out.
There were also no differences in survival benefit associated with immunotherapy vs control therapies for patients with an ECOG PS of 0 and an ECOG PS of 1 or greater. The OS benefit was 0.81 for those with an ECOG PS of 0 and 0.79 for those with an ECOG PS of 1 or greater.
Wang has disclosed no relevant financial relationships.
This article first appeared on Medscape.com .
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
One-off blast of RT, rather than weeks, for early breast cancer
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Long-term outcomes now being reported confirm earlier reports from the same trial showing efficacy for the use of targeted intraoperative radiotherapy (TARGIT) in patients with early breast cancer.
This novel approach, which delivers a one-off blast of radiation directed at the tumor bed and is given during lumpectomy, has similar efficacy and lowers non–breast cancer mortality when compared with whole-breast external beam radiotherapy (EBRT), which is delivered in fractions over 3-6 weeks after surgery.
Giving the boost of radiation during surgery has numerous benefits, say the authors: it is more convenient for patients and saves on healthcare costs.
However, the controversy over local recurrence rates, sparked by the earlier results, still remains. The difference in the 5-year local recurrence rate between TARGIT and EBRT was within the 2.5% margin for non-inferiority: the rate was 2.11% in 1140 TARGIT recipients, compared with 0.95% in 1158 EBRT recipients, for a difference of 1.16% (13 recurrences).
The new longer-term results from the TARGIT-A trial were published August 20 in the BMJ, and confirm earlier results from this trial published in 2014. Meanwhile, other approaches to intraoperative radiotherapy (IORT) have also been reported.
Nevertheless, whole-breast radiotherapy remains the standard of care today, note the authors.
“The biggest battle the TARGIT investigator family has faced is our challenge to the conventional dogma that radiotherapy has to be given in multiple daily doses, and moreover that whole-breast radiotherapy is always essential,” said lead author Jayant Vaidya, MD, professor of surgery and oncology at University College London, UK. He was one of the team of investigators that together developed the TARGIT approach in the 1990s, as he recalls in a related BMJ blog post.
It is unclear whether the TARGIT-A long-term outcomes will change practice, Rachel Jimenez, MD, associate program director of the Harvard Radiation Oncology Residency Program and assistant professor of radiation oncology at Massachusetts General Hospital, Boston, told Medscape Medical News.
She noted there was controversy and debate over the earlier reports from TARGIT-A, and those findings “did little to change practice patterns over the past 6 years,” she said.
“With the publication of longer-term follow-up, my expectation would be that perceptions of IORT will remain unchanged in the radiation oncology community, and that those previously supportive of the TARGIT-A approach will continue to embrace it while those initially skeptical will be unlikely to change practice despite the longer-term results,” she said.
“However, despite the controversy surrounding TARGIT-A, it is heartening as a clinician who cares for breast cancer patients to see a trend within the early breast cancer clinical trials space toward the evaluation of increasingly targeted and abbreviated courses of radiation,” Jimenez commented.
Details of new long-term results
TARGIT-A is an open-label, 32-center multinational study conducted in 2298 women aged 45 years or older with early-stage invasive ductal carcinoma who were eligible for breast-conserving surgery. Between March 24, 2000, and June 25, 2012, participants were randomized 1:1 to risk-adapted TARGIT immediately after lumpectomy or to whole-breast EBRT delivered for the standard 3-6 week daily fractionated course.
At a median follow-up of 8.6 years — with some patients followed for nearly 19 years — no significant difference was seen between the treatment groups in local recurrence-free survival (167 vs 147 events; hazard ratio, 1.13); invasive local recurrence-free survival (154 vs 146 events; HR, 1.04); mastectomy-free survival (170 vs 175 events; HR, 0.96); distant disease-free survival (133 vs 148 events; HR, 0.88); overall survival (110 vs 131 events; HR, 0.82); or breast cancer mortality (6 vs 57 events; HR, 1.12).
“Mortality from other causes was significantly lower (45 vs 74 events; HR, 0.59),” the authors note.
Controversy of Earlier Results
Vaidya and colleagues comment that these new results confirm earlier findings from this trial. They were initially presented in 2012 by Vaidya at the San Antonio Breast Cancer Symposium and subsequently published in The Lancet, as previously reported by Medscape Medical News. Those earlier results showed a trend toward lower overall mortality with TARGIT (absolute difference, -1.3%; P = .01) and significantly fewer deaths from causes other than breast cancer (absolute difference, -2.1%; P = .009).
However, TARGIT was associated with slightly more same-breast recurrences at that time (3.3% vs 1.3%; P = 0.42), even though this was still within the 2.5% margin for non-inferiority.
The new longer-term results show a similar pattern.
It was this risk of same-breast recurrences that sparked the heated debate over the findings, as some breast cancer experts argued that this needs to be weighed against various potential benefits of IORT for patients: greater convenience, potentially improved mortality, and lower costs.
The extent of that “vigorous debate” was highlighted in 2015 in the International Journal of Radiation Oncology, Biology, Physics, (the Red Journal), in which editor-in-chief Anthony Zietman, MD, shared numerous letters to the editor, written in response to two editorials, that contained “passionately and articulately expressed” views from “senior investigators and breast cancer physicians from around the globe.”
At the same time, in 2015, another approach to IORT was reported at the American Society for Radiation Oncology (ASTRO) annual meeting and simultaneously published online in The Lancet. This was accelerated partial-breast irradiation (APBI) delivered directly to the tumor bed using multicatheter brachytherapy at the time of lumpectomy in women with early breast cancer. The results showed outcomes that were comparable with whole-breast irradiation, but with fewer side effects.
Those findings prompted a 2016 update to the ASTRO consensus statement on APBI to note that APBI after lumpectomy may be suitable for more women with early-stage breast cancer, including younger patients and those with ductal carcinoma in situ.
In comments to Medscape Medical News, Jimenez noted that several recent studies have shown efficacy for various IORT approaches. There have been two phase 3 non-inferiority studies, namely the NSABP B-39 and the RAPID trial, that evaluate the use of APBI in lieu of whole-breast radiation. There have also been two trials as well as the evaluation of a 5-day ultrahypofractionated whole-breast radiation course per the UK Fast and Fast-Forward trials, compared with several weeks of whole-breast radiation.
“Collectively, these studies lend support for fewer and/or more targeted radiotherapy treatments for our patients and have the potential to reduce patient burden and limit healthcare costs,” Jimenez told Medscape Medical News.
Indeed, the TARGIT-A researchers write that their long-term findings “have shown that risk-adapted single-dose TARGIT-IORT given during lumpectomy can effectively replace the mandatory use of several weeks of daily postoperative whole-breast radiotherapy in patients with breast cancer undergoing breast conservation.”
Given the numerous benefits to patients that this approach provides, the choice should ultimately rest with the patient, the authors conclude.
An extended follow-up of the trial (TARGIT-Ex) is ongoing, as is the TARGIT-B(oost) trial looking at TARGIT-IORT as a tumor bed boost with EBRT boost in younger women and those with higher-risk disease.
The TARGIT-A trial was sponsored by University College London Hospitals (UCLH) Comprehensive Biomedical Research Centre and funded by UCLH Charities, the National Institute for Health Research Health Technology Assessment program, Ninewells Cancer Campaign, the National Health and Medical Research Council, and the German Federal Ministry of Education and Research. The authors reported numerous disclosures, as detailed in the publication.
This article first appeared on Medscape.com.
Tailored messaging needed to get cancer screening back on track
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
Age, smoking among leading cancer risk factors for SLE patients
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
A new study has quantified cancer risk factors in patients with systemic lupus erythematosus, including smoking and the use of certain medications.
“As expected, older age was associated with cancer overall, as well as with the most common cancer subtypes,” wrote Sasha Bernatsky, MD, PhD, of McGill University, Montreal, and coauthors. The study was published in Arthritis Care & Research.
To determine the risk of cancer in people with clinically confirmed incident systemic lupus erythematosus (SLE), the researchers analyzed data from 1,668 newly diagnosed lupus patients with at least one follow-up visit. All patients were enrolled in the Systemic Lupus International Collaborating Clinics inception cohort from across 33 different centers in North America, Europe, and Asia. A total of 89% (n = 1,480) were women, and 49% (n = 824) were white. The average follow-up period was 9 years.
Of the 1,668 SLE patients, 65 developed some type of cancer. The cancers included 15 breast;, 10 nonmelanoma skin; 7 lung; 6 hematologic, 6 prostate; 5 melanoma; 3 cervical; 3 renal; 2 gastric; 2 head and neck; 2 thyroid; and 1 rectal, sarcoma, thymoma, or uterine. No patient had more than one type, and the mean age of the cancer patients at time of SLE diagnosis was 45.6 (standard deviation, 14.5).
Almost half of the 65 cancers occurred in past or current smokers, including all of the lung cancers, while only 33% of patients without cancers smoked prior to baseline. After univariate analysis, characteristics associated with a higher risk of all cancers included older age at SLE diagnosis (adjusted hazard ratio, 1.05; 95% confidence interval, 1.03-1.06), White race/ethnicity (aHR 1.34; 95% CI, 0.76-2.37), and smoking (aHR 1.21; 95% CI, 0.73-2.01).
After multivariate analysis, the two characteristics most associated with increased cancer risk were older age at SLE diagnosis and being male. The analyses also confirmed that older age was a risk factor for breast cancer (aHR 1.06; 95% CI, 1.02-1.10) and nonmelanoma skin cancer (aHR, 1.06; 95% CI, 1.02-1.11), while use of antimalarial drugs was associated with a lower risk of both breast (aHR, 0.28; 95% CI, 0.09-0.90) and nonmelanoma skin (aHR, 0.23; 95% CI, 0.05-0.95) cancers. For lung cancer, the highest risk factor was smoking 15 or more cigarettes a day (aHR, 6.64; 95% CI, 1.43-30.9); for hematologic cancers, it was being in the top quartile of SLE disease activity (aHR, 7.14; 95% CI, 1.13-45.3).
The authors acknowledged their study’s limitations, including the small number of cancers overall and purposefully not comparing cancer risk in SLE patients with risk in the general population. Although their methods – “physicians recording events at annual visits, confirmed by review of charts” – were recognized as very suitable for the current analysis, they noted that a broader comparison would “potentially be problematic due to differential misclassification error” in cancer registry data.
Two of the study’s authors reported potential conflicts of interest, including receiving grants and consulting and personal fees from various pharmaceutical companies. No other potential conflicts were reported.
SOURCE: Bernatsky S et al. Arthritis Care Res. 2020 Aug 19. doi: 10.1002/acr.24425.
FROM ARTHRITIS CARE & RESEARCH
Beyond baseline, DBT no better than mammography for dense breasts
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
In women with extremely dense breasts, digital breast tomosynthesis (DBT) does not outperform digital mammography (DM) after the baseline exam, according to a review of nearly 1.6 million screenings.
At baseline, DBT improved recall and cancer detection rates for all women. On subsequent exams, differences in screening performance between DBT and DM varied by age and density subgroups. However, there were no significant differences in recall or cancer detection rates among women with extremely dense breasts in any age group.
Kathryn Lowry, MD, of the University of Washington in Seattle, and colleagues reported these findings in JAMA Network Open.
“Our findings suggest that density likely should not be used as a criterion to triage use of DBT for routine screening in settings where DBT is not universally available, as has been reported in physician surveys,” the authors wrote. “The largest absolute improvements of DBT screening were achieved on the baseline screening examination, suggesting that women presenting for their first screening examination are particularly important to prioritize for DBT,” regardless of breast density or age.
Study details
Dr. Lowry and colleagues reviewed 1,584,079 screenings in women aged 40-79 years. The exams were done from January 2010 to April 2018 at Breast Cancer Surveillance Consortium facilities across the United States.
Sixty-five percent of the exams were in White, non-Hispanic women, 25.2% were in women younger than 50 years, and 42.4% were in women with heterogeneously dense or extremely dense breasts. Subjects had no history of breast cancer, mastectomy, or breast augmentation.
The investigators compared the performance of 1,273,492 DMs with 310,587 DBTs across the four Breast Imaging Reporting and Database System density types: almost entirely fatty, scattered fibroglandular density, heterogeneously dense, and extremely dense.
Findings were adjusted for race, family breast cancer history, and other potential confounders.
Recall and cancer detection rates
At baseline, recall and cancer detection rates were better with DBT than with DM, regardless of breast density subtype or patient age.
For instance, in women aged 50-59 years, screening recalls per 1,000 exams dropped from 241 with DM to 204 with DBT (relative risk, 0.84; 95% confidence interval, 0.73-0.98). Cancer detection rates per 1,000 exams in this age group increased from 5.9 with DM to 8.8 with DBT (RR, 1.50; 95% CI, 1.10-2.08).
On follow-up exams, recall rates were lower with DBT for women with scattered fibroglandular density and heterogeneously dense breasts in all age groups, as well as in women with almost entirely fatty breasts aged 50-79 years.
“By contrast, there were no significant differences in recall rates in women with extremely dense breasts in any age group,” the authors wrote.
Cancer detection rates on follow-up exams varied by age and breast density.
Cancer detection rates were higher with DBT than with DM in women with heterogeneously dense breasts in all age groups and in women with scattered fibroglandular density at 50-59 years of age and 60-79 years of age. However, cancer detection rates were not significantly different with DBT or DM for women with almost entirely fatty breasts or extremely dense breasts of any age.
Implications and next steps
Dr. Lowry and colleagues noted that use of DBT has increased steadily since it was approved by the Food and Drug Administration in 2011, driven by studies demonstrating, among other things, earlier detection of invasive cancers.
The problem has been that previous investigations “largely dichotomized dense (heterogeneously dense and extremely dense) and nondense (almost entirely fat and scattered fibroglandular densities) categories,” the authors wrote. Therefore, the nuance of benefit across density subtypes hasn’t been clear.
The finding that “screening benefits of DBT differ for women with heterogeneously dense breasts [versus] extremely dense breasts is especially important in the current landscape of density legislation and demand for supplemental screening tests beyond mammography. To date, most state mandates and ... proposed federal legislation have uniformly grouped women with heterogeneously dense breasts and those with extremely dense breasts as a single population,” the authors wrote.
As the new findings suggest, “there are important differences in performance that may not be appreciated by combining density categories,” the authors added.
The results “suggest that women with extremely dense breast tissue may benefit more from additional screening than women with heterogeneously dense breasts who undergo tomosynthesis mammography,” Catherine Tuite, MD, of Fox Chase Cancer Center in Philadelphia, and colleagues wrote in a related editorial.
“Research to determine density and risk-specific outcomes for supplemental screening methods, such as magnetic resonance imaging ... molecular breast imaging, or ultrasonography is necessary to understand which screening method beyond DBT is best for average-risk women with heterogeneous or extremely dense breasts,” the editorialists wrote.
This research was funded by the National Cancer Institute and the Patient-Centered Outcomes Research Institute through the Breast Cancer Surveillance Consortium. Dr. Lowry reported grants from GE Healthcare outside the submitted work. The editorialists didn’t have any disclosures.
SOURCE: Lowry K et al. JAMA Netw Open. 2020 Jul 1;3(7):e2011792.
FROM THE JAMA OPEN NETWORK
How effective is screening mammography for preventing breast cancer mortality?
EXPERT COMMENTARY
Although recommending screening mammograms continues to represent the standard of care, studies from the United States and abroad have questioned their value.1-3
In the June issue of JAMA Network Open, Australian investigators assessed the relative impacts of mammography screening and adjuvant therapy on breast cancer mortality, using data from population-based studies from 1982 through 2013.4 In recent decades, screening has increased substantially among Australian women.
Details of the study
Burton and Stevenson identified 76,630 women included in the Victorian Cancer Registry with invasive breast cancer in the state of Victoria, where women aged 50 to 69 are offered biennial screening.4 During the study’s time period, the use of adjuvant tamoxifen and chemotherapy increased substantially.
In the 31-year period assessed in this study, breast cancer mortality declined considerably. During the same period, however, the incidence of advanced breast cancer doubled.
These findings from Australia parallel those from the United States, Holland, and Norway, where the incidence of advanced breast cancer was stable or increased after screening mammography was introduced.1-3
According to Burton and Stevenson, the increased incidence of advanced cancer clarifies that screening mammography is not responsible for declining breast cancer mortality, but all of the decline in mortality can be attributed to increased uptake of adjuvant therapy.
The authors concluded that since screening mammography does not reduce breast cancer mortality, state-sponsored screens should be discontinued.
Study strengths and limitations
Relevant data for this study were obtained from large population-based surveys for premenopausal and postmenopausal women with breast cancer.
The authors noted, however, that this analysis of observational data examining time trends across the study period can show only associations among breast cancer mortality, mammography screening participation, and adjuvant therapy uptake, and that causality can only be inferred.
The study in perspective
Although some will view the findings and recommendations of these Australian authors with skepticism or even hostility, I view their findings as good news—we have improved the treatment of breast cancer so dramatically that the benefits of finding early tumors with screening mammography have become attenuated.
Although it is challenging given the time constraints of office visits, I try to engage in shared decision making with my patients regarding when to start and how often to have screening mammography. ●
Given our evolving understanding regarding the value of screening mammograms, it is time to stop pressuring patients who are reluctant or unwilling to undergo screening. Likewise, insurance companies and government agencies should stop using screening mammography as a quality metric.
ANDREW M. KAUNITZ, MD
- Bleyer A, Welch GH. Effect of three decades of screening mammography on breast cancer-incidence. N Engl J Med. 2012;367:1998-2005.
- Autier P, Boniol M, Koechlin A, et al. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224.
- Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
EXPERT COMMENTARY
Although recommending screening mammograms continues to represent the standard of care, studies from the United States and abroad have questioned their value.1-3
In the June issue of JAMA Network Open, Australian investigators assessed the relative impacts of mammography screening and adjuvant therapy on breast cancer mortality, using data from population-based studies from 1982 through 2013.4 In recent decades, screening has increased substantially among Australian women.
Details of the study
Burton and Stevenson identified 76,630 women included in the Victorian Cancer Registry with invasive breast cancer in the state of Victoria, where women aged 50 to 69 are offered biennial screening.4 During the study’s time period, the use of adjuvant tamoxifen and chemotherapy increased substantially.
In the 31-year period assessed in this study, breast cancer mortality declined considerably. During the same period, however, the incidence of advanced breast cancer doubled.
These findings from Australia parallel those from the United States, Holland, and Norway, where the incidence of advanced breast cancer was stable or increased after screening mammography was introduced.1-3
According to Burton and Stevenson, the increased incidence of advanced cancer clarifies that screening mammography is not responsible for declining breast cancer mortality, but all of the decline in mortality can be attributed to increased uptake of adjuvant therapy.
The authors concluded that since screening mammography does not reduce breast cancer mortality, state-sponsored screens should be discontinued.
Study strengths and limitations
Relevant data for this study were obtained from large population-based surveys for premenopausal and postmenopausal women with breast cancer.
The authors noted, however, that this analysis of observational data examining time trends across the study period can show only associations among breast cancer mortality, mammography screening participation, and adjuvant therapy uptake, and that causality can only be inferred.
The study in perspective
Although some will view the findings and recommendations of these Australian authors with skepticism or even hostility, I view their findings as good news—we have improved the treatment of breast cancer so dramatically that the benefits of finding early tumors with screening mammography have become attenuated.
Although it is challenging given the time constraints of office visits, I try to engage in shared decision making with my patients regarding when to start and how often to have screening mammography. ●
Given our evolving understanding regarding the value of screening mammograms, it is time to stop pressuring patients who are reluctant or unwilling to undergo screening. Likewise, insurance companies and government agencies should stop using screening mammography as a quality metric.
ANDREW M. KAUNITZ, MD
EXPERT COMMENTARY
Although recommending screening mammograms continues to represent the standard of care, studies from the United States and abroad have questioned their value.1-3
In the June issue of JAMA Network Open, Australian investigators assessed the relative impacts of mammography screening and adjuvant therapy on breast cancer mortality, using data from population-based studies from 1982 through 2013.4 In recent decades, screening has increased substantially among Australian women.
Details of the study
Burton and Stevenson identified 76,630 women included in the Victorian Cancer Registry with invasive breast cancer in the state of Victoria, where women aged 50 to 69 are offered biennial screening.4 During the study’s time period, the use of adjuvant tamoxifen and chemotherapy increased substantially.
In the 31-year period assessed in this study, breast cancer mortality declined considerably. During the same period, however, the incidence of advanced breast cancer doubled.
These findings from Australia parallel those from the United States, Holland, and Norway, where the incidence of advanced breast cancer was stable or increased after screening mammography was introduced.1-3
According to Burton and Stevenson, the increased incidence of advanced cancer clarifies that screening mammography is not responsible for declining breast cancer mortality, but all of the decline in mortality can be attributed to increased uptake of adjuvant therapy.
The authors concluded that since screening mammography does not reduce breast cancer mortality, state-sponsored screens should be discontinued.
Study strengths and limitations
Relevant data for this study were obtained from large population-based surveys for premenopausal and postmenopausal women with breast cancer.
The authors noted, however, that this analysis of observational data examining time trends across the study period can show only associations among breast cancer mortality, mammography screening participation, and adjuvant therapy uptake, and that causality can only be inferred.
The study in perspective
Although some will view the findings and recommendations of these Australian authors with skepticism or even hostility, I view their findings as good news—we have improved the treatment of breast cancer so dramatically that the benefits of finding early tumors with screening mammography have become attenuated.
Although it is challenging given the time constraints of office visits, I try to engage in shared decision making with my patients regarding when to start and how often to have screening mammography. ●
Given our evolving understanding regarding the value of screening mammograms, it is time to stop pressuring patients who are reluctant or unwilling to undergo screening. Likewise, insurance companies and government agencies should stop using screening mammography as a quality metric.
ANDREW M. KAUNITZ, MD
- Bleyer A, Welch GH. Effect of three decades of screening mammography on breast cancer-incidence. N Engl J Med. 2012;367:1998-2005.
- Autier P, Boniol M, Koechlin A, et al. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224.
- Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
- Bleyer A, Welch GH. Effect of three decades of screening mammography on breast cancer-incidence. N Engl J Med. 2012;367:1998-2005.
- Autier P, Boniol M, Koechlin A, et al. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224.
- Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-1210.
- Burton R, Stevenson C. Assessment of breast cancer mortality trends associated with mammographic screening and adjuvant therapy from 1986 to 2013 in the state of Victoria, Australia. JAMA Netw Open. 2020;3:e208249.
Mammography starting at 40 cuts risk of breast cancer death
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.
New data will add fuel to the ongoing debate over the age at which mammography screening for breast cancer should begin. Many guidelines recommend starting at age 50.
But yearly mammography between the ages of 40 and 49 years was associated with a “substantial and significant” 25% reduction in breast cancer mortality during the first 10 years of follow-up, according to new data from the UK Age Trial.
The researchers calculated that 1,150 women needed to undergo screening in the age group of 40-49 years to prevent 1 breast cancer death, or about 1 breast cancer death prevented per 1,000 screened.
However, they also noted that, in the years since the trial first began, there have been improvements in the treatment of breast cancer, so “there might be less scope for screening to reduce mortality in our current era.”
The study was published online August 12 in Lancet Oncology.
“Our results do indicate that screening before age 50 does indeed prevent deaths from breast cancer, with a minimal additional burden of overdiagnosis,” said lead author Stephen W. Duffy, MSc, director of the policy research unit in cancer awareness, screening and early diagnosis, at Queen Mary University, London.
That said, Dr. Duffy explained they do not expect policy makers to extend the age range on the basis of these results alone. “For one thing, they will want to consider costs, both human and financial.” “For another, at this time, the services are concentrating on recovering from the hiatus caused by the COVID-19 crisis, and, at this time, it would be impractical to try to expand the eligibility for screening.”
“I would say our results indicate that lowering the age range, although not necessarily to 40 but to some age below 50, will be at least worth considering when the current crisis is over,” he added.
Guideline recommendations differ
Breast cancer screening guidelines have generated debate, much of which has focused on the age at which to begin screening.
The U.S. Preventive Services Task Force and American College of Physicians recommend screening every other year, on average, for women between the ages of 50 and 74 years.
However, other organizations disagree. The American College of Radiology and Society of Breast Imaging both recommend annual mammograms starting at age 40, and continuing “as long as they are in good health.”
In the UK, where the study was conducted, a national breast cancer screening program offers mammography to women aged 50-70 years every 3 years.
Given the uncertainty that continues to exist over the optimal age for average-risk women to begin screening, the UK Age Trial set out to assess if screening should begin at a younger age and if that might lead to overdiagnosis of breast cancer.
Results from the study’s 17-year follow-up, published in 2015, showed a reduction in breast cancer mortality with annual screening, beginning at age 40 years, which was significant in the first 10 years after participants were randomized (Lancet Oncol. 2015;16:1123-32).
In the current study, Dr. Duffy and colleagues report on breast cancer incidence and mortality results in the UK Age trial after 23 years of follow-up.
The cohort included 160,921 women enrolled between Oct. 14, 1990, and Sept. 24, 1997, who were randomized to screening (n = 53,883) or the control group (n = 106,953).
Of those screened during the study period, 7,893 (18.1%) had at least one false-positive result. There were 10,439 deaths, of which 683 (7%) were attributed to breast cancer diagnosed during the study period.
At 10 years of follow-up, death from breast cancer was significantly lower among women in the screening versus control group (83 vs 219 deaths; relative risk, 0.75; P = .029).
However, no significant reduction was observed thereafter, with 126 versus 255 deaths occurring after more than 10 years of follow-up (RR, 0.98; 95% confidence interval, 0.79-1.22; P = .86), the authors note.
“This follow-up indicates that the gain in survival was concentrated in the first 10 years after the women began to be screened,” commented Kevin McConway, PhD, emeritus professor of applied statistics at the Open University, Milton Keynes, England.
“In those first 10 years, out of every 10,000 women invited for screening, on average, about 16 died of breast cancer, while in every 10,000 women in the control group who did not get the screening, on average, 21 died. These numbers indicate that lives were saved,” he said.
“But they also indicate that death from breast cancer was pretty rare in women of that age,” he pointed out.
“Because breast cancer deaths in younger women are not common, the estimates of breast cancer death rates are not very precise, despite the fact that the trial involved 160,000 women,” he said.
“Over the whole follow-up period so far, the difference in numbers of deaths between those who were screened in their 40s and those who were not is 6 deaths for every 10,000 women, but because of the statistical uncertainty, this figure could plausibly be larger, at 13 per 10,000. Or, in fact, the data are also consistent with a very slightly higher death rate [1 death per 10,000 women] in those who had the screening,” Dr. McConway explained.
“But none of those numbers is very large, out of 10,000 women. Allowing for the fact that not every woman invited for screening will actually attend the screening, the researchers estimate that 1,150 women would have to be screened in their 40s to prevent one breast cancer death,” he noted.
U.S. experts support starting screening at 40
“The American Society of Breast Surgeons has continued to recommend screening women at the age of 40,” said Stephanie Bernik, MD, FACS, chief of breast surgery, Mount Sinai West, and associate professor of surgery at the Icahn School of Medicine at Mount Sinai, New York. “There is no question that screening earlier saves more lives, and this study adds to the body of evidence already available.”
She pointed out that the argument against early screening was that there were many false positives, which, in turn, increased cost and anxiety. “Because women in their 40s are in the prime of their lives, often with young children, it seems as though screening would be paramount. Furthermore, it is well known that the sooner you find a cancer, the better, as the treatment needed to cure the cancer is less toxic and less dramatic.”
Catherine Tuite, MD, section chief, breast radiology, Fox Chase Cancer Center, Philadelphia, echoed a similar viewpoint. “There is no real debate on this issue. The USPSTF recommends beginning screening mammography at age 50, and it is no secret that this is a recommendation based on cost, not on saving women’s lives.”
She emphasized that these recommendations were made without the input of expert physicians. “The data, reaffirmed by this publication, have always been clear that the most years of life saved from deaths due to breast cancer are achieved in women who begin screening mammography at age 40. We know that one-sixth of all breast cancers are diagnosed before age 50, and many of these cancers are the most aggressive types of breast cancer.
“The guidelines from every organization representing health care professionals who actually diagnose and care for women with breast cancer recommend that all women of average risk begin annual screening mammography at age 40 and continue as long as the woman is in good health, with life expectancy of 10 years,” she continued.
As for screening intervals, annual mammogram is also recommended for all age groups in the United States. At her institutions, she explained that they are currently enrolling women into the TMIST screening mammogram trial, which is, among other endpoints, evaluating a biannual screening interval for postmenopausal women of lower than average risk, but again, outside of a trial setting, yearly screening for all women is recommended.
Dr. Duffy commented that, in the United Kingdom, the current screening protocol for mammograms is every 3 years, which he said “works well in women over the age of 50 years.” But for younger women, more frequent screening would be need – in this study, screening was done annually.
“The results not only from our study but from others around the world suggest that this [3-year screening interval] would not be very effective in women under 50, due partly to the denser breast tissue of younger women and partly to the faster progression on average of cancers diagnosed in younger women,” he said. “Some counties in Sweden, for example, offer screening to women under 50 at 18-month intervals, which seems more realistic.”
The study was funded by the Health Technology Assessment program of the National Institute for Health Research. Dr. Duffy reported also receiving grants from the NIHR outside this trial. Dr. Bernik, Dr. Tuite, and Dr. Hodgson reported no relevant financial relationships.
This article first appeared on Medscape.com.