User login
Chemo-Free Olaparib with or Without Durvalumab Maintains Platinum Therapy’s Benefits in TNBC
Key clinical point: In patients with platinum-pretreated advanced triple-negative breast cancer (TNBC), a chemotherapy-free maintenance regimen containing olaparib with or without durvalumab vs continued platinum-based chemotherapy led to a greater improvement in progression-free survival (PFS) with no new safety signals.
Major finding: The median PFS was 4.0 months with olaparib and 6.1 months with olaparib + durvalumab, with both treatments providing greater PFS benefit than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). No treatment-related deaths or new safety signals were reported.
Study details: Findings are from the phase 2 DORA trial which included 45 patients with advanced TNBC who had stable disease or complete or partial response after receiving platinum-based chemotherapy and were randomly assigned to receive olaparib or olaparib + durvalumab.
Disclosures: This study was supported by AstraZeneca Pharmaceuticals LP. Some authors declared receiving honoraria, research funding, or fees; owning stocks; or having other ties with AstraZeneca and various other sources.
Source: Tan TJ, Sammons S, Im YH, et al. Phase II DORA study of olaparib with or without durvalumab as a chemotherapy-free maintenance strategy in platinum-pretreated advanced triple-negative breast cancer. Clin Cancer Res. 2024 (Jan 18). doi: 10.1158/1078-0432.CCR-23-2513 Source
Key clinical point: In patients with platinum-pretreated advanced triple-negative breast cancer (TNBC), a chemotherapy-free maintenance regimen containing olaparib with or without durvalumab vs continued platinum-based chemotherapy led to a greater improvement in progression-free survival (PFS) with no new safety signals.
Major finding: The median PFS was 4.0 months with olaparib and 6.1 months with olaparib + durvalumab, with both treatments providing greater PFS benefit than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). No treatment-related deaths or new safety signals were reported.
Study details: Findings are from the phase 2 DORA trial which included 45 patients with advanced TNBC who had stable disease or complete or partial response after receiving platinum-based chemotherapy and were randomly assigned to receive olaparib or olaparib + durvalumab.
Disclosures: This study was supported by AstraZeneca Pharmaceuticals LP. Some authors declared receiving honoraria, research funding, or fees; owning stocks; or having other ties with AstraZeneca and various other sources.
Source: Tan TJ, Sammons S, Im YH, et al. Phase II DORA study of olaparib with or without durvalumab as a chemotherapy-free maintenance strategy in platinum-pretreated advanced triple-negative breast cancer. Clin Cancer Res. 2024 (Jan 18). doi: 10.1158/1078-0432.CCR-23-2513 Source
Key clinical point: In patients with platinum-pretreated advanced triple-negative breast cancer (TNBC), a chemotherapy-free maintenance regimen containing olaparib with or without durvalumab vs continued platinum-based chemotherapy led to a greater improvement in progression-free survival (PFS) with no new safety signals.
Major finding: The median PFS was 4.0 months with olaparib and 6.1 months with olaparib + durvalumab, with both treatments providing greater PFS benefit than the historical control of continued platinum-based therapy (P = .0023 and P < .0001, respectively). No treatment-related deaths or new safety signals were reported.
Study details: Findings are from the phase 2 DORA trial which included 45 patients with advanced TNBC who had stable disease or complete or partial response after receiving platinum-based chemotherapy and were randomly assigned to receive olaparib or olaparib + durvalumab.
Disclosures: This study was supported by AstraZeneca Pharmaceuticals LP. Some authors declared receiving honoraria, research funding, or fees; owning stocks; or having other ties with AstraZeneca and various other sources.
Source: Tan TJ, Sammons S, Im YH, et al. Phase II DORA study of olaparib with or without durvalumab as a chemotherapy-free maintenance strategy in platinum-pretreated advanced triple-negative breast cancer. Clin Cancer Res. 2024 (Jan 18). doi: 10.1158/1078-0432.CCR-23-2513 Source
Fulvestrant-Containing Therapy Not Superior to Anastrozole in ER-Rich/ERBB2− BC
Key clinical point: Neoadjuvant endocrine therapy with fulvestrant or anastrozole + fulvestrant led to an endocrine-sensitive disease rate (ESDR) comparable to anastrozole alone in postmenopausal women with estrogen receptor (ER)-rich, human epidermal growth factor receptor 2-negative (ERBB2−) breast cancer (BC).
Major finding: Both fulvestrant and anastrozole + fulvestrant vs anastrozole alone demonstrated no significant improvement in the ESDR at 6 months (22.8% and 20.5% vs 18.7%, respectively; Wald test, P ≥ .98) and median percentage change in Ki67 at 4 weeks (−79.1% and −83.5% vs −80.6%, respectively; P ≥ .15).
Study details: Findings are from the phase 3 ALTERNATE trial, which included 1298 treatment-naive postmenopausal women with ER-rich/ERBB2− BC who were randomly assigned to receive anastrozole, fulvestrant, or anastrozole + fulvestrant for 6 months in a neoadjuvant setting.
Disclosures: This study was supported by the US National Institutes of Health (NIH)/National Cancer Institute (NCI) and other sources. Several authors declared receiving grants, personal fees, or research support or having other ties with various sources, including NIH/NCI.
Source: Ma CX, Suman VJ, Sanati S, et al. Endocrine-sensitive disease rate in postmenopausal patients with estrogen receptor-rich/ERBB2-negative breast cancer receiving neoadjuvant anastrozole, fulvestrant, or their combination: A phase 3 randomized clinical trial. JAMA Oncol. 2024 (Jan 18). doi: 10.1001/jamaoncol.2023.6038 Source
Key clinical point: Neoadjuvant endocrine therapy with fulvestrant or anastrozole + fulvestrant led to an endocrine-sensitive disease rate (ESDR) comparable to anastrozole alone in postmenopausal women with estrogen receptor (ER)-rich, human epidermal growth factor receptor 2-negative (ERBB2−) breast cancer (BC).
Major finding: Both fulvestrant and anastrozole + fulvestrant vs anastrozole alone demonstrated no significant improvement in the ESDR at 6 months (22.8% and 20.5% vs 18.7%, respectively; Wald test, P ≥ .98) and median percentage change in Ki67 at 4 weeks (−79.1% and −83.5% vs −80.6%, respectively; P ≥ .15).
Study details: Findings are from the phase 3 ALTERNATE trial, which included 1298 treatment-naive postmenopausal women with ER-rich/ERBB2− BC who were randomly assigned to receive anastrozole, fulvestrant, or anastrozole + fulvestrant for 6 months in a neoadjuvant setting.
Disclosures: This study was supported by the US National Institutes of Health (NIH)/National Cancer Institute (NCI) and other sources. Several authors declared receiving grants, personal fees, or research support or having other ties with various sources, including NIH/NCI.
Source: Ma CX, Suman VJ, Sanati S, et al. Endocrine-sensitive disease rate in postmenopausal patients with estrogen receptor-rich/ERBB2-negative breast cancer receiving neoadjuvant anastrozole, fulvestrant, or their combination: A phase 3 randomized clinical trial. JAMA Oncol. 2024 (Jan 18). doi: 10.1001/jamaoncol.2023.6038 Source
Key clinical point: Neoadjuvant endocrine therapy with fulvestrant or anastrozole + fulvestrant led to an endocrine-sensitive disease rate (ESDR) comparable to anastrozole alone in postmenopausal women with estrogen receptor (ER)-rich, human epidermal growth factor receptor 2-negative (ERBB2−) breast cancer (BC).
Major finding: Both fulvestrant and anastrozole + fulvestrant vs anastrozole alone demonstrated no significant improvement in the ESDR at 6 months (22.8% and 20.5% vs 18.7%, respectively; Wald test, P ≥ .98) and median percentage change in Ki67 at 4 weeks (−79.1% and −83.5% vs −80.6%, respectively; P ≥ .15).
Study details: Findings are from the phase 3 ALTERNATE trial, which included 1298 treatment-naive postmenopausal women with ER-rich/ERBB2− BC who were randomly assigned to receive anastrozole, fulvestrant, or anastrozole + fulvestrant for 6 months in a neoadjuvant setting.
Disclosures: This study was supported by the US National Institutes of Health (NIH)/National Cancer Institute (NCI) and other sources. Several authors declared receiving grants, personal fees, or research support or having other ties with various sources, including NIH/NCI.
Source: Ma CX, Suman VJ, Sanati S, et al. Endocrine-sensitive disease rate in postmenopausal patients with estrogen receptor-rich/ERBB2-negative breast cancer receiving neoadjuvant anastrozole, fulvestrant, or their combination: A phase 3 randomized clinical trial. JAMA Oncol. 2024 (Jan 18). doi: 10.1001/jamaoncol.2023.6038 Source
Body Fat Tied to Skeletal Fragility in Postmenopausal Women with AI-Treated Breast Cancer
Key clinical point: High initial fat body mass was associated with a risk for vertebral fracture (VF) progression in postmenopausal women with hormone receptor-positive (HR+) early breast cancer (BC) who received aromatase inhibitors (AI) plus denosumab.
Major finding: After 18 months of adjuvant therapy with AI and denosumab, 4.4% of patients had VF progression, with percentage of fat body mass (odds ratio [OR] 5.41; P = .01) and Fracture Risk Assessment Tool score (OR 3.95; P = .04) being independently associated with VF progression risk.
Study details: Findings are from a prospective cohort study including 237 postmenopausal women with HR+ early BC who received adjuvant therapy with AI and denosumab.
Disclosures: This study did not declare any specific funding. Three authors declared receiving personal fees or grants or having other ties with various sources.
Source: Cosentini D, Pedersini R, Mauro PD, et al. Fat body mass and vertebral fracture progression in women with breast cancer. JAMA Netw Open. 2024;7:e2350950. doi:10.1001/jamanetworkopen.2023.50950 Source
Key clinical point: High initial fat body mass was associated with a risk for vertebral fracture (VF) progression in postmenopausal women with hormone receptor-positive (HR+) early breast cancer (BC) who received aromatase inhibitors (AI) plus denosumab.
Major finding: After 18 months of adjuvant therapy with AI and denosumab, 4.4% of patients had VF progression, with percentage of fat body mass (odds ratio [OR] 5.41; P = .01) and Fracture Risk Assessment Tool score (OR 3.95; P = .04) being independently associated with VF progression risk.
Study details: Findings are from a prospective cohort study including 237 postmenopausal women with HR+ early BC who received adjuvant therapy with AI and denosumab.
Disclosures: This study did not declare any specific funding. Three authors declared receiving personal fees or grants or having other ties with various sources.
Source: Cosentini D, Pedersini R, Mauro PD, et al. Fat body mass and vertebral fracture progression in women with breast cancer. JAMA Netw Open. 2024;7:e2350950. doi:10.1001/jamanetworkopen.2023.50950 Source
Key clinical point: High initial fat body mass was associated with a risk for vertebral fracture (VF) progression in postmenopausal women with hormone receptor-positive (HR+) early breast cancer (BC) who received aromatase inhibitors (AI) plus denosumab.
Major finding: After 18 months of adjuvant therapy with AI and denosumab, 4.4% of patients had VF progression, with percentage of fat body mass (odds ratio [OR] 5.41; P = .01) and Fracture Risk Assessment Tool score (OR 3.95; P = .04) being independently associated with VF progression risk.
Study details: Findings are from a prospective cohort study including 237 postmenopausal women with HR+ early BC who received adjuvant therapy with AI and denosumab.
Disclosures: This study did not declare any specific funding. Three authors declared receiving personal fees or grants or having other ties with various sources.
Source: Cosentini D, Pedersini R, Mauro PD, et al. Fat body mass and vertebral fracture progression in women with breast cancer. JAMA Netw Open. 2024;7:e2350950. doi:10.1001/jamanetworkopen.2023.50950 Source
Hyperbaric Oxygen: Effective Against Cancer Radiation Harm?
Although a new study of hyperbaric oxygen therapy in JAMA Oncology has been “ anxiously awaited” by breast radiation oncologists, the trial does not provide the smoking gun evidence that would justify its routine use, according to experts.
Here’s a snapshot of the current state of affairs regarding hyperbaric oxygen therapy in breast radiation oncology.
What Is Hyperbaric Oxygen Therapy?
Hyperbaric oxygen therapy is a medical procedure aimed at reducing the late toxic effects of breast irradiation, including pain, fibrosis, and edema. Patients breathe pure oxygen at greater than atmospheric pressure in a specialized chamber or room. The process leads to increased partial pressures of oxygen in blood and tissues, which can help form new blood vessels and repair damaged irradiated tissue.
What Is the Current State of Play?
In 2021, the US Food and Drug Administration (FDA) cleared the therapy for a variety of disorders, including radiation injuries. Some health insurers may cover the procedure as well.
Still, the FDA has cautioned clinicians “to be wary of unproven claims of effect,” University of Toronto radiation oncologist Ezra Hahn, MD, and colleagues Aron Popovtzer, MD, and Benjamin W. Corn, MD, said in a JAMA Oncology editorial.
While the procedure is “seldom used by many in practice,” there is growing industry for the procedure. More than 1000 facilities in the United States offer hyperbaric oxygen therapy, but only about 15% are accredited by the Undersea and Hyperbaric Medical Society, which may signal misuse of the procedure.
Does the Latest Study Clarify Whether This Therapy Works?
The most recent evidence on hyperbaric oxygen therapy comes from a single-institution, randomized trial from the Netherlands, dubbed HONEY. In the trial, 189 women who experienced late toxic effects following adjuvant breast radiation were randomized 2:1 to hyperbaric oxygen therapy or a control arm. Of the 125 women offered hyperbaric oxygen therapy, only 25% (31 patients) accepted and completed treatment; those who declined received usual follow-up care.
Among women who completed hyperbaric oxygen therapy, 32% (10 of 31) reported moderate or severe pain at follow-up vs 75% of controls — a 66% reduction. Similarly, 17% of women who completed hyperbaric oxygen therapy reported moderate or severe fibrosis at follow-up vs 86% among the hypothetical treatment-completing controls — an 86% reduction. However, the authors did not observe a significant effect of hyperbaric oxygen therapy on breast edema, movement restriction, or overall quality of life.
The authors also included an intention-to-treat analysis, which included patients who declined hyperbaric oxygen therapy as part of the intervention group. This analysis estimated clinical outcomes among patients who had the intervention available to them, with some taking advantage and others not.
Overall, hyperbaric oxygen therapy “seems effective for reducing pain and fibrosis in women with late local toxic effects after breast irradiation,” concluded investigators led by Dieuwke R. Mink van der Molen, PhD, a researcher at the University Medical Centre Utrecht, the Netherlands. However, most patients offered the therapy declined the invitation, largely because of the “high treatment intensity” burden.
What Are the Limitations of the Current Study?
The investigators and editorialists highlighted a handful of limitations.
For one, the trial had no sham hyperbaric oxygen therapy procedure in the control group. In fact, control patients were selected from a larger cohort of ongoing studies in the Netherlands who were not aware the trial was being conducted.
Because radiation toxicity fluctuates over time and can improve on its own, “a high-quality control arm” would be needed in such a trial, especially to account for subjective and patient-reported outcomes, the editorialists said.
Another key issue: Only a quarter of women offered hyperbaric oxygen therapy agreed to and completed treatment. The treatment burden was the most common reason for declining the procedure. Study participants underwent 30-40 2-hour sessions over 6-8 weeks.
Will the Latest Evidence Usher This Therapy Into More Standard Use?
Probably not, the editorialists concluded.
The HONEY trial “reminds us that convenience has become a factor weighted heavily by patients during the process of decision-making,” Dr. Hahn and colleagues wrote. “Despite experiencing relatively severe symptoms, many declined hyperbaric therapy after being counseled by HONEY investigators about the time commitment.”
Despite its limitations, the trial does offer “modest evidence to justify the use of [hyperbaric oxygen therapy] in treating the chronic morbidities associated with breast irradiation,” the editorialists said. But an “adequately powered randomized, sham-controlled, double-blind trials will be necessary to truly determine the benefit.”
HONEY was partially funded by The Da Vinci Clinic, the Netherlands. The investigators didn’t have any disclosures. One of Dr. Hahn’s coauthors reported personal fees from Lutris Pharma as Chief Medical Officer.
A version of this article appeared on Medscape.com.
Although a new study of hyperbaric oxygen therapy in JAMA Oncology has been “ anxiously awaited” by breast radiation oncologists, the trial does not provide the smoking gun evidence that would justify its routine use, according to experts.
Here’s a snapshot of the current state of affairs regarding hyperbaric oxygen therapy in breast radiation oncology.
What Is Hyperbaric Oxygen Therapy?
Hyperbaric oxygen therapy is a medical procedure aimed at reducing the late toxic effects of breast irradiation, including pain, fibrosis, and edema. Patients breathe pure oxygen at greater than atmospheric pressure in a specialized chamber or room. The process leads to increased partial pressures of oxygen in blood and tissues, which can help form new blood vessels and repair damaged irradiated tissue.
What Is the Current State of Play?
In 2021, the US Food and Drug Administration (FDA) cleared the therapy for a variety of disorders, including radiation injuries. Some health insurers may cover the procedure as well.
Still, the FDA has cautioned clinicians “to be wary of unproven claims of effect,” University of Toronto radiation oncologist Ezra Hahn, MD, and colleagues Aron Popovtzer, MD, and Benjamin W. Corn, MD, said in a JAMA Oncology editorial.
While the procedure is “seldom used by many in practice,” there is growing industry for the procedure. More than 1000 facilities in the United States offer hyperbaric oxygen therapy, but only about 15% are accredited by the Undersea and Hyperbaric Medical Society, which may signal misuse of the procedure.
Does the Latest Study Clarify Whether This Therapy Works?
The most recent evidence on hyperbaric oxygen therapy comes from a single-institution, randomized trial from the Netherlands, dubbed HONEY. In the trial, 189 women who experienced late toxic effects following adjuvant breast radiation were randomized 2:1 to hyperbaric oxygen therapy or a control arm. Of the 125 women offered hyperbaric oxygen therapy, only 25% (31 patients) accepted and completed treatment; those who declined received usual follow-up care.
Among women who completed hyperbaric oxygen therapy, 32% (10 of 31) reported moderate or severe pain at follow-up vs 75% of controls — a 66% reduction. Similarly, 17% of women who completed hyperbaric oxygen therapy reported moderate or severe fibrosis at follow-up vs 86% among the hypothetical treatment-completing controls — an 86% reduction. However, the authors did not observe a significant effect of hyperbaric oxygen therapy on breast edema, movement restriction, or overall quality of life.
The authors also included an intention-to-treat analysis, which included patients who declined hyperbaric oxygen therapy as part of the intervention group. This analysis estimated clinical outcomes among patients who had the intervention available to them, with some taking advantage and others not.
Overall, hyperbaric oxygen therapy “seems effective for reducing pain and fibrosis in women with late local toxic effects after breast irradiation,” concluded investigators led by Dieuwke R. Mink van der Molen, PhD, a researcher at the University Medical Centre Utrecht, the Netherlands. However, most patients offered the therapy declined the invitation, largely because of the “high treatment intensity” burden.
What Are the Limitations of the Current Study?
The investigators and editorialists highlighted a handful of limitations.
For one, the trial had no sham hyperbaric oxygen therapy procedure in the control group. In fact, control patients were selected from a larger cohort of ongoing studies in the Netherlands who were not aware the trial was being conducted.
Because radiation toxicity fluctuates over time and can improve on its own, “a high-quality control arm” would be needed in such a trial, especially to account for subjective and patient-reported outcomes, the editorialists said.
Another key issue: Only a quarter of women offered hyperbaric oxygen therapy agreed to and completed treatment. The treatment burden was the most common reason for declining the procedure. Study participants underwent 30-40 2-hour sessions over 6-8 weeks.
Will the Latest Evidence Usher This Therapy Into More Standard Use?
Probably not, the editorialists concluded.
The HONEY trial “reminds us that convenience has become a factor weighted heavily by patients during the process of decision-making,” Dr. Hahn and colleagues wrote. “Despite experiencing relatively severe symptoms, many declined hyperbaric therapy after being counseled by HONEY investigators about the time commitment.”
Despite its limitations, the trial does offer “modest evidence to justify the use of [hyperbaric oxygen therapy] in treating the chronic morbidities associated with breast irradiation,” the editorialists said. But an “adequately powered randomized, sham-controlled, double-blind trials will be necessary to truly determine the benefit.”
HONEY was partially funded by The Da Vinci Clinic, the Netherlands. The investigators didn’t have any disclosures. One of Dr. Hahn’s coauthors reported personal fees from Lutris Pharma as Chief Medical Officer.
A version of this article appeared on Medscape.com.
Although a new study of hyperbaric oxygen therapy in JAMA Oncology has been “ anxiously awaited” by breast radiation oncologists, the trial does not provide the smoking gun evidence that would justify its routine use, according to experts.
Here’s a snapshot of the current state of affairs regarding hyperbaric oxygen therapy in breast radiation oncology.
What Is Hyperbaric Oxygen Therapy?
Hyperbaric oxygen therapy is a medical procedure aimed at reducing the late toxic effects of breast irradiation, including pain, fibrosis, and edema. Patients breathe pure oxygen at greater than atmospheric pressure in a specialized chamber or room. The process leads to increased partial pressures of oxygen in blood and tissues, which can help form new blood vessels and repair damaged irradiated tissue.
What Is the Current State of Play?
In 2021, the US Food and Drug Administration (FDA) cleared the therapy for a variety of disorders, including radiation injuries. Some health insurers may cover the procedure as well.
Still, the FDA has cautioned clinicians “to be wary of unproven claims of effect,” University of Toronto radiation oncologist Ezra Hahn, MD, and colleagues Aron Popovtzer, MD, and Benjamin W. Corn, MD, said in a JAMA Oncology editorial.
While the procedure is “seldom used by many in practice,” there is growing industry for the procedure. More than 1000 facilities in the United States offer hyperbaric oxygen therapy, but only about 15% are accredited by the Undersea and Hyperbaric Medical Society, which may signal misuse of the procedure.
Does the Latest Study Clarify Whether This Therapy Works?
The most recent evidence on hyperbaric oxygen therapy comes from a single-institution, randomized trial from the Netherlands, dubbed HONEY. In the trial, 189 women who experienced late toxic effects following adjuvant breast radiation were randomized 2:1 to hyperbaric oxygen therapy or a control arm. Of the 125 women offered hyperbaric oxygen therapy, only 25% (31 patients) accepted and completed treatment; those who declined received usual follow-up care.
Among women who completed hyperbaric oxygen therapy, 32% (10 of 31) reported moderate or severe pain at follow-up vs 75% of controls — a 66% reduction. Similarly, 17% of women who completed hyperbaric oxygen therapy reported moderate or severe fibrosis at follow-up vs 86% among the hypothetical treatment-completing controls — an 86% reduction. However, the authors did not observe a significant effect of hyperbaric oxygen therapy on breast edema, movement restriction, or overall quality of life.
The authors also included an intention-to-treat analysis, which included patients who declined hyperbaric oxygen therapy as part of the intervention group. This analysis estimated clinical outcomes among patients who had the intervention available to them, with some taking advantage and others not.
Overall, hyperbaric oxygen therapy “seems effective for reducing pain and fibrosis in women with late local toxic effects after breast irradiation,” concluded investigators led by Dieuwke R. Mink van der Molen, PhD, a researcher at the University Medical Centre Utrecht, the Netherlands. However, most patients offered the therapy declined the invitation, largely because of the “high treatment intensity” burden.
What Are the Limitations of the Current Study?
The investigators and editorialists highlighted a handful of limitations.
For one, the trial had no sham hyperbaric oxygen therapy procedure in the control group. In fact, control patients were selected from a larger cohort of ongoing studies in the Netherlands who were not aware the trial was being conducted.
Because radiation toxicity fluctuates over time and can improve on its own, “a high-quality control arm” would be needed in such a trial, especially to account for subjective and patient-reported outcomes, the editorialists said.
Another key issue: Only a quarter of women offered hyperbaric oxygen therapy agreed to and completed treatment. The treatment burden was the most common reason for declining the procedure. Study participants underwent 30-40 2-hour sessions over 6-8 weeks.
Will the Latest Evidence Usher This Therapy Into More Standard Use?
Probably not, the editorialists concluded.
The HONEY trial “reminds us that convenience has become a factor weighted heavily by patients during the process of decision-making,” Dr. Hahn and colleagues wrote. “Despite experiencing relatively severe symptoms, many declined hyperbaric therapy after being counseled by HONEY investigators about the time commitment.”
Despite its limitations, the trial does offer “modest evidence to justify the use of [hyperbaric oxygen therapy] in treating the chronic morbidities associated with breast irradiation,” the editorialists said. But an “adequately powered randomized, sham-controlled, double-blind trials will be necessary to truly determine the benefit.”
HONEY was partially funded by The Da Vinci Clinic, the Netherlands. The investigators didn’t have any disclosures. One of Dr. Hahn’s coauthors reported personal fees from Lutris Pharma as Chief Medical Officer.
A version of this article appeared on Medscape.com.
Robotic Mastectomy Is Feasible, But Is It Safe?
a type of conservative mastectomy preserving the skin and nipple-areola complex. The new findings potentially expand the application of robotic surgery to a larger patient population but doubts about the safety of this approach linger.
Robotic Mastectomy
The first surgeries involving the Da Vinci robotic surgeon for breast removal date to 2015. Multiport robotic surgery faces significant obstacles in this field, however. Feasibility studies have primarily focused on women with small breasts, corresponding to cup size C or smaller.
In the study that was published in JAMA Surgery, surgeons used the more cost-effective single-port platform for bilateral NSM procedures. Among the 20 patients included in the analysis (age, 29-63 years), 11 underwent prophylactic mastectomy (for a high risk for cancer) and 9 had mastectomy for breast tumors. Breast sizes ranged from A cup to D cup.
The duration of the procedure, from skin incision to suture for both breasts, ranged from 205 to 351 minutes (median, 277 minutes). No immediate operative complications (eg, hematoma) occurred, and there was no need for conversion to open surgery in any case. Over the 36-month follow-up, there were no recurrences. About 95% of patients retained skin sensitivity and 55% retained nipple sensitivity.
Unanswered Questions
In an accompanying article, Monica Morrow, MD, director of surgical breast oncology at the Memorial Sloan-Kettering Cancer Center in New York, acknowledged that the new evidence confirms the surgical approach’s feasibility but deems it insufficient to adopt it lightly. “At this point, the issue is not whether robotic mastectomy can be done but whether there is sufficient information about its oncologic safety that it should be done,” she wrote.
In a 2019 statement that was updated in 2021, the US Food and Drug Administration stated, “The safety and effectiveness of using robotically assisted surgical devices in mastectomy procedures or in the prevention or treatment of breast cancer have not been established.” The significance of this caution is underscored by the experience with laparoscopic and robotic radical hysterectomies. These procedures were widely adopted until a randomized prospective study demonstrated lower disease-free and overall survival for the minimally invasive approach compared with open surgery.
The University of Texas surgeons stated that acceptable safety and oncological outcomes for robotic NSM compared with conventional NSM had been demonstrated. They cited two trials with 238 cases and a median follow-up of less than 3 years. Dr. Morrow wrote, “While these reports provide reassurance that gross residual tumor is not being left behind, they do not address the issue of failure to remove all of the breast tissue due to thick skin flaps, with the potential for development of late recurrence or new cancers.” It is worth noting that even with the traditional surgical approach, the 5-year local recurrence rate after NSM is approximately double when observed with shorter follow-ups.
According to Dr. Morrow, the high rate of sensory preservation observed with robotic surgery, a desirable outcome for patients, is also a cause for concern. “While this may be due to incision placement or minimal skin flap retraction, as suggested by the authors, it is equally plausible that this could be due to thick skin flaps with preservation of the terminal branches of the fourth intercostal nerve.”
Therefore, more information on long-term oncological outcomes in a large number of patients will be necessary to confirm the safety of the procedure. In addition, measuring patient-reported outcomes will be useful in demonstrating that the benefits of the procedure outweigh increased operating times and costs.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
a type of conservative mastectomy preserving the skin and nipple-areola complex. The new findings potentially expand the application of robotic surgery to a larger patient population but doubts about the safety of this approach linger.
Robotic Mastectomy
The first surgeries involving the Da Vinci robotic surgeon for breast removal date to 2015. Multiport robotic surgery faces significant obstacles in this field, however. Feasibility studies have primarily focused on women with small breasts, corresponding to cup size C or smaller.
In the study that was published in JAMA Surgery, surgeons used the more cost-effective single-port platform for bilateral NSM procedures. Among the 20 patients included in the analysis (age, 29-63 years), 11 underwent prophylactic mastectomy (for a high risk for cancer) and 9 had mastectomy for breast tumors. Breast sizes ranged from A cup to D cup.
The duration of the procedure, from skin incision to suture for both breasts, ranged from 205 to 351 minutes (median, 277 minutes). No immediate operative complications (eg, hematoma) occurred, and there was no need for conversion to open surgery in any case. Over the 36-month follow-up, there were no recurrences. About 95% of patients retained skin sensitivity and 55% retained nipple sensitivity.
Unanswered Questions
In an accompanying article, Monica Morrow, MD, director of surgical breast oncology at the Memorial Sloan-Kettering Cancer Center in New York, acknowledged that the new evidence confirms the surgical approach’s feasibility but deems it insufficient to adopt it lightly. “At this point, the issue is not whether robotic mastectomy can be done but whether there is sufficient information about its oncologic safety that it should be done,” she wrote.
In a 2019 statement that was updated in 2021, the US Food and Drug Administration stated, “The safety and effectiveness of using robotically assisted surgical devices in mastectomy procedures or in the prevention or treatment of breast cancer have not been established.” The significance of this caution is underscored by the experience with laparoscopic and robotic radical hysterectomies. These procedures were widely adopted until a randomized prospective study demonstrated lower disease-free and overall survival for the minimally invasive approach compared with open surgery.
The University of Texas surgeons stated that acceptable safety and oncological outcomes for robotic NSM compared with conventional NSM had been demonstrated. They cited two trials with 238 cases and a median follow-up of less than 3 years. Dr. Morrow wrote, “While these reports provide reassurance that gross residual tumor is not being left behind, they do not address the issue of failure to remove all of the breast tissue due to thick skin flaps, with the potential for development of late recurrence or new cancers.” It is worth noting that even with the traditional surgical approach, the 5-year local recurrence rate after NSM is approximately double when observed with shorter follow-ups.
According to Dr. Morrow, the high rate of sensory preservation observed with robotic surgery, a desirable outcome for patients, is also a cause for concern. “While this may be due to incision placement or minimal skin flap retraction, as suggested by the authors, it is equally plausible that this could be due to thick skin flaps with preservation of the terminal branches of the fourth intercostal nerve.”
Therefore, more information on long-term oncological outcomes in a large number of patients will be necessary to confirm the safety of the procedure. In addition, measuring patient-reported outcomes will be useful in demonstrating that the benefits of the procedure outweigh increased operating times and costs.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
a type of conservative mastectomy preserving the skin and nipple-areola complex. The new findings potentially expand the application of robotic surgery to a larger patient population but doubts about the safety of this approach linger.
Robotic Mastectomy
The first surgeries involving the Da Vinci robotic surgeon for breast removal date to 2015. Multiport robotic surgery faces significant obstacles in this field, however. Feasibility studies have primarily focused on women with small breasts, corresponding to cup size C or smaller.
In the study that was published in JAMA Surgery, surgeons used the more cost-effective single-port platform for bilateral NSM procedures. Among the 20 patients included in the analysis (age, 29-63 years), 11 underwent prophylactic mastectomy (for a high risk for cancer) and 9 had mastectomy for breast tumors. Breast sizes ranged from A cup to D cup.
The duration of the procedure, from skin incision to suture for both breasts, ranged from 205 to 351 minutes (median, 277 minutes). No immediate operative complications (eg, hematoma) occurred, and there was no need for conversion to open surgery in any case. Over the 36-month follow-up, there were no recurrences. About 95% of patients retained skin sensitivity and 55% retained nipple sensitivity.
Unanswered Questions
In an accompanying article, Monica Morrow, MD, director of surgical breast oncology at the Memorial Sloan-Kettering Cancer Center in New York, acknowledged that the new evidence confirms the surgical approach’s feasibility but deems it insufficient to adopt it lightly. “At this point, the issue is not whether robotic mastectomy can be done but whether there is sufficient information about its oncologic safety that it should be done,” she wrote.
In a 2019 statement that was updated in 2021, the US Food and Drug Administration stated, “The safety and effectiveness of using robotically assisted surgical devices in mastectomy procedures or in the prevention or treatment of breast cancer have not been established.” The significance of this caution is underscored by the experience with laparoscopic and robotic radical hysterectomies. These procedures were widely adopted until a randomized prospective study demonstrated lower disease-free and overall survival for the minimally invasive approach compared with open surgery.
The University of Texas surgeons stated that acceptable safety and oncological outcomes for robotic NSM compared with conventional NSM had been demonstrated. They cited two trials with 238 cases and a median follow-up of less than 3 years. Dr. Morrow wrote, “While these reports provide reassurance that gross residual tumor is not being left behind, they do not address the issue of failure to remove all of the breast tissue due to thick skin flaps, with the potential for development of late recurrence or new cancers.” It is worth noting that even with the traditional surgical approach, the 5-year local recurrence rate after NSM is approximately double when observed with shorter follow-ups.
According to Dr. Morrow, the high rate of sensory preservation observed with robotic surgery, a desirable outcome for patients, is also a cause for concern. “While this may be due to incision placement or minimal skin flap retraction, as suggested by the authors, it is equally plausible that this could be due to thick skin flaps with preservation of the terminal branches of the fourth intercostal nerve.”
Therefore, more information on long-term oncological outcomes in a large number of patients will be necessary to confirm the safety of the procedure. In addition, measuring patient-reported outcomes will be useful in demonstrating that the benefits of the procedure outweigh increased operating times and costs.
This article was translated from Univadis Italy, which is part of the Medscape Professional Network. A version of this article appeared on Medscape.com.
FROM JAMA SURGERY
Commentary: Benign Breast Disease, PD-L1+ TNBC, and Exercise in BC, February 2024
The benefit of immunotherapy in combination with chemotherapy for programmed death–ligand 1–positive (PD-L1+) metastatic triple-negative breast cancer (mTNBC) has been shown in both the IMpassion130 and KEYNOTE-355 trials.[2,3] However, the IMpassion131 trial, which evaluated atezolizumab plus paclitaxel, did not show a progression-free survival (PFS) or overall survival (OS) benefit vs paclitaxel alone in PD-L1+ mTNBC.[4] Various explanations for these divergent results have been proposed, including the inherent properties of the chemotherapy backbone, patient populations, and the heterogenous nature of TNBC, which can affect response to immunotherapy. Of present, the various KEYNOTE-355 regimens (pembrolizumab plus investigator's choice chemotherapy [nab-paclitaxel, paclitaxel, or gemcitabine-carboplatin]) are US Food and Drug Administration approved for PD-L1+ mTNBC in the first-line setting. The phase 2 randomized TBCRC 043 trial investigated the effect of atezolizumab with carboplatin in patients with mTNBC and further looked at clinical and molecular correlates of response (Lehmann et al). A total of 106 patients were randomly assigned to carboplatin or carboplatin plus atezolizumab; the combination improved PFS (median PFS, 4.1 vs 2.2 mo; hazard ratio [HR] 0.66; P = .05) and OS (12.6 vs 8.6 mo; HR 0.60; P = .03). Grade 3/4 serious adverse events were more common with carboplatin-atezolizumab vs carboplatin alone (41% vs 8%). In addition, an association of better responses with PD-L1 immunotherapy was seen in patients with obesity, uncontrolled blood glucose levels, high tumor mutation burden, and increased tumor infiltrating lymphocytes. These data support the role of immunotherapy in mTNBC, highlight tumor heterogeneity within this subtype and encourage correlative studies to better define which patients benefit from immunotherapy.
Various studies have demonstrated the favorable impact of physical activity on breast cancer risk in postmenopausal women.[5] However, data in premenopausal women is less clear. Various mechanisms connecting physical activity to premenopausal breast cancer risk have been proposed including the effect of exercise on sex steroid hormones, fasting insulin levels, and inflammation.[6] A pooled analysis from 19 cohort studies including 547,601 premenopausal women, with 10,231 incident cases of breast cancer, aimed to examine the relationship between leisure-time physical activity (sports, exercise, recreational walking) and breast cancer risk in young women (Timmins et al). Higher (90th percentile) vs lower (10th percentile) levels of leisure-time physical activity were associated with a 10% reduction in breast cancer risk after adjustment for body mass index (BMI; adjusted HR 0.90; 95% CI 0.85-0.95; P < .001). They also found a significant reduction in risk: 32% (HR 0.68; P = .01) and 9% (HR 0.91; P = .005) for women with underweight (BMI < 18.5) and with average weight (BMI 18.5-24.9), respectively. Further, the effect of physical activity was most pronounced in the human epidermal growth factor receptor 2 (HER2)–enriched breast cancer subtype, wherein higher vs lower levels of activity were associated with an estimated 45% reduction in breast cancer risk (adjusted HR 0.55; 95% CI 0.37-0.82). These findings support the beneficial role of aerobic exercise and healthy body weight on breast cancer risk among premenopausal women and highlight the value of incorporating this information into counseling for our patients.
Additional References
- Figueroa JD, Gierach GL, Duggan MA, et al. Risk factors for breast cancer development by tumor characteristics among women with benign breast disease. Breast Cancer Res. 2021;23:34. doi: 10.1186/s13058-021-01410-1 Source
- Schmid P, Adams S, Rugo HS, et al, for the IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121. doi: 10.1056/nejmoa1809615 Source
- Cortes J, Rugo HS, Cescon DW, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217-226. doi: 10.1056/NEJMoa2202809 Source
- Miles D, Gligorov J, André F, et al, on behalf of the IMpassion131 investigators. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994-1004. doi: 10.1016/j.annonc.2021.05.801 Source
- Eliassen AH, Hankinson SE, Rosner B, et al. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758-1764. doi: 10.1001/archinternmed.2010.363 Source
- Swain CTV, Drummond AE, Boing L, et al. Linking physical activity to breast cancer via sex hormones, part 1: The effect of physical activity on sex steroid hormones. Cancer Epidemiol Biomarkers Prev. 2022;31:16-27. doi: 10.1158/1055-9965.EPI-21-0437 Source
The benefit of immunotherapy in combination with chemotherapy for programmed death–ligand 1–positive (PD-L1+) metastatic triple-negative breast cancer (mTNBC) has been shown in both the IMpassion130 and KEYNOTE-355 trials.[2,3] However, the IMpassion131 trial, which evaluated atezolizumab plus paclitaxel, did not show a progression-free survival (PFS) or overall survival (OS) benefit vs paclitaxel alone in PD-L1+ mTNBC.[4] Various explanations for these divergent results have been proposed, including the inherent properties of the chemotherapy backbone, patient populations, and the heterogenous nature of TNBC, which can affect response to immunotherapy. Of present, the various KEYNOTE-355 regimens (pembrolizumab plus investigator's choice chemotherapy [nab-paclitaxel, paclitaxel, or gemcitabine-carboplatin]) are US Food and Drug Administration approved for PD-L1+ mTNBC in the first-line setting. The phase 2 randomized TBCRC 043 trial investigated the effect of atezolizumab with carboplatin in patients with mTNBC and further looked at clinical and molecular correlates of response (Lehmann et al). A total of 106 patients were randomly assigned to carboplatin or carboplatin plus atezolizumab; the combination improved PFS (median PFS, 4.1 vs 2.2 mo; hazard ratio [HR] 0.66; P = .05) and OS (12.6 vs 8.6 mo; HR 0.60; P = .03). Grade 3/4 serious adverse events were more common with carboplatin-atezolizumab vs carboplatin alone (41% vs 8%). In addition, an association of better responses with PD-L1 immunotherapy was seen in patients with obesity, uncontrolled blood glucose levels, high tumor mutation burden, and increased tumor infiltrating lymphocytes. These data support the role of immunotherapy in mTNBC, highlight tumor heterogeneity within this subtype and encourage correlative studies to better define which patients benefit from immunotherapy.
Various studies have demonstrated the favorable impact of physical activity on breast cancer risk in postmenopausal women.[5] However, data in premenopausal women is less clear. Various mechanisms connecting physical activity to premenopausal breast cancer risk have been proposed including the effect of exercise on sex steroid hormones, fasting insulin levels, and inflammation.[6] A pooled analysis from 19 cohort studies including 547,601 premenopausal women, with 10,231 incident cases of breast cancer, aimed to examine the relationship between leisure-time physical activity (sports, exercise, recreational walking) and breast cancer risk in young women (Timmins et al). Higher (90th percentile) vs lower (10th percentile) levels of leisure-time physical activity were associated with a 10% reduction in breast cancer risk after adjustment for body mass index (BMI; adjusted HR 0.90; 95% CI 0.85-0.95; P < .001). They also found a significant reduction in risk: 32% (HR 0.68; P = .01) and 9% (HR 0.91; P = .005) for women with underweight (BMI < 18.5) and with average weight (BMI 18.5-24.9), respectively. Further, the effect of physical activity was most pronounced in the human epidermal growth factor receptor 2 (HER2)–enriched breast cancer subtype, wherein higher vs lower levels of activity were associated with an estimated 45% reduction in breast cancer risk (adjusted HR 0.55; 95% CI 0.37-0.82). These findings support the beneficial role of aerobic exercise and healthy body weight on breast cancer risk among premenopausal women and highlight the value of incorporating this information into counseling for our patients.
Additional References
- Figueroa JD, Gierach GL, Duggan MA, et al. Risk factors for breast cancer development by tumor characteristics among women with benign breast disease. Breast Cancer Res. 2021;23:34. doi: 10.1186/s13058-021-01410-1 Source
- Schmid P, Adams S, Rugo HS, et al, for the IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121. doi: 10.1056/nejmoa1809615 Source
- Cortes J, Rugo HS, Cescon DW, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217-226. doi: 10.1056/NEJMoa2202809 Source
- Miles D, Gligorov J, André F, et al, on behalf of the IMpassion131 investigators. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994-1004. doi: 10.1016/j.annonc.2021.05.801 Source
- Eliassen AH, Hankinson SE, Rosner B, et al. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758-1764. doi: 10.1001/archinternmed.2010.363 Source
- Swain CTV, Drummond AE, Boing L, et al. Linking physical activity to breast cancer via sex hormones, part 1: The effect of physical activity on sex steroid hormones. Cancer Epidemiol Biomarkers Prev. 2022;31:16-27. doi: 10.1158/1055-9965.EPI-21-0437 Source
The benefit of immunotherapy in combination with chemotherapy for programmed death–ligand 1–positive (PD-L1+) metastatic triple-negative breast cancer (mTNBC) has been shown in both the IMpassion130 and KEYNOTE-355 trials.[2,3] However, the IMpassion131 trial, which evaluated atezolizumab plus paclitaxel, did not show a progression-free survival (PFS) or overall survival (OS) benefit vs paclitaxel alone in PD-L1+ mTNBC.[4] Various explanations for these divergent results have been proposed, including the inherent properties of the chemotherapy backbone, patient populations, and the heterogenous nature of TNBC, which can affect response to immunotherapy. Of present, the various KEYNOTE-355 regimens (pembrolizumab plus investigator's choice chemotherapy [nab-paclitaxel, paclitaxel, or gemcitabine-carboplatin]) are US Food and Drug Administration approved for PD-L1+ mTNBC in the first-line setting. The phase 2 randomized TBCRC 043 trial investigated the effect of atezolizumab with carboplatin in patients with mTNBC and further looked at clinical and molecular correlates of response (Lehmann et al). A total of 106 patients were randomly assigned to carboplatin or carboplatin plus atezolizumab; the combination improved PFS (median PFS, 4.1 vs 2.2 mo; hazard ratio [HR] 0.66; P = .05) and OS (12.6 vs 8.6 mo; HR 0.60; P = .03). Grade 3/4 serious adverse events were more common with carboplatin-atezolizumab vs carboplatin alone (41% vs 8%). In addition, an association of better responses with PD-L1 immunotherapy was seen in patients with obesity, uncontrolled blood glucose levels, high tumor mutation burden, and increased tumor infiltrating lymphocytes. These data support the role of immunotherapy in mTNBC, highlight tumor heterogeneity within this subtype and encourage correlative studies to better define which patients benefit from immunotherapy.
Various studies have demonstrated the favorable impact of physical activity on breast cancer risk in postmenopausal women.[5] However, data in premenopausal women is less clear. Various mechanisms connecting physical activity to premenopausal breast cancer risk have been proposed including the effect of exercise on sex steroid hormones, fasting insulin levels, and inflammation.[6] A pooled analysis from 19 cohort studies including 547,601 premenopausal women, with 10,231 incident cases of breast cancer, aimed to examine the relationship between leisure-time physical activity (sports, exercise, recreational walking) and breast cancer risk in young women (Timmins et al). Higher (90th percentile) vs lower (10th percentile) levels of leisure-time physical activity were associated with a 10% reduction in breast cancer risk after adjustment for body mass index (BMI; adjusted HR 0.90; 95% CI 0.85-0.95; P < .001). They also found a significant reduction in risk: 32% (HR 0.68; P = .01) and 9% (HR 0.91; P = .005) for women with underweight (BMI < 18.5) and with average weight (BMI 18.5-24.9), respectively. Further, the effect of physical activity was most pronounced in the human epidermal growth factor receptor 2 (HER2)–enriched breast cancer subtype, wherein higher vs lower levels of activity were associated with an estimated 45% reduction in breast cancer risk (adjusted HR 0.55; 95% CI 0.37-0.82). These findings support the beneficial role of aerobic exercise and healthy body weight on breast cancer risk among premenopausal women and highlight the value of incorporating this information into counseling for our patients.
Additional References
- Figueroa JD, Gierach GL, Duggan MA, et al. Risk factors for breast cancer development by tumor characteristics among women with benign breast disease. Breast Cancer Res. 2021;23:34. doi: 10.1186/s13058-021-01410-1 Source
- Schmid P, Adams S, Rugo HS, et al, for the IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121. doi: 10.1056/nejmoa1809615 Source
- Cortes J, Rugo HS, Cescon DW, et al, for the KEYNOTE-355 Investigators. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med. 2022;387:217-226. doi: 10.1056/NEJMoa2202809 Source
- Miles D, Gligorov J, André F, et al, on behalf of the IMpassion131 investigators. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994-1004. doi: 10.1016/j.annonc.2021.05.801 Source
- Eliassen AH, Hankinson SE, Rosner B, et al. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758-1764. doi: 10.1001/archinternmed.2010.363 Source
- Swain CTV, Drummond AE, Boing L, et al. Linking physical activity to breast cancer via sex hormones, part 1: The effect of physical activity on sex steroid hormones. Cancer Epidemiol Biomarkers Prev. 2022;31:16-27. doi: 10.1158/1055-9965.EPI-21-0437 Source
More Young Women Being Diagnosed With Breast Cancer Than Ever Before
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.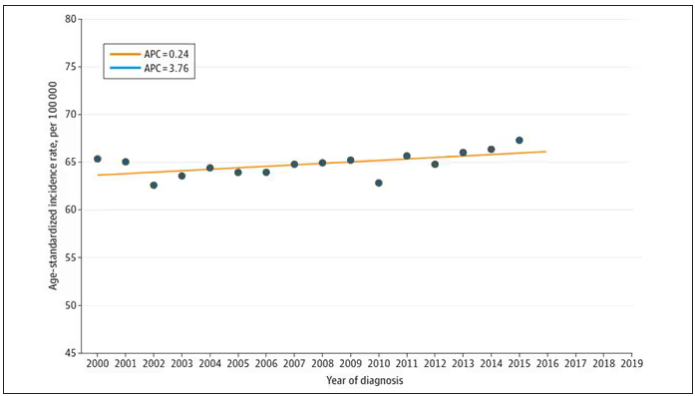
And then this happened: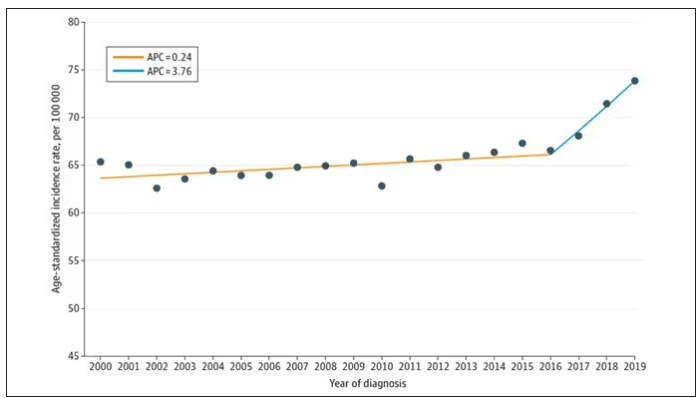
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.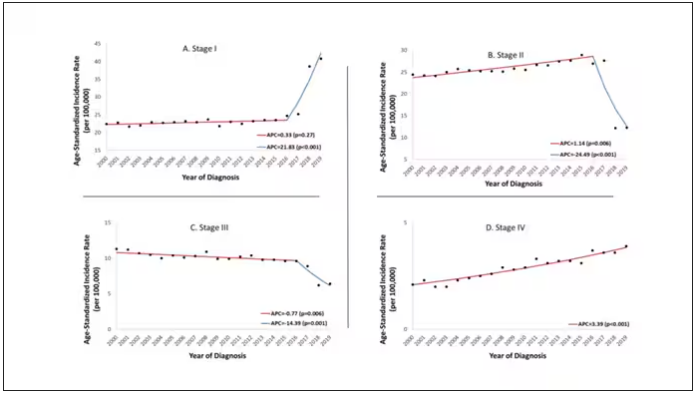
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
From the year 2000 until around 2016, the incidence of breast cancer among young women — those under age 50 — rose steadily, if slowly.
And then this happened:
I look at a lot of graphs in my line of work, and it’s not too often that one actually makes me say “What the hell?” out loud. But this one did. Why are young women all of a sudden more likely to get breast cancer?
The graph comes from this paper, Breast cancer incidence among us women aged 20 to 49 years by race, stage, and hormone receptor status, appearing in JAMA Network Open
Researchers from Washington University in St. Louis utilized SEER registries to conduct their analyses. SEER is a public database from the National Cancer Institute with coverage of 27% of the US population and a long track record of statistical backbone to translate the data from SEER to numbers that are representative of the population at large.
From 2000 to 2019, more than 200,000 women were diagnosed with primary invasive breast cancer in the dataset, and I’ve already given you the top-line results. Of course, when you see a graph like this, the next question really needs to be why?
Fortunately, the SEER dataset contains a lot more information than simply whether someone was diagnosed with cancer. In the case of breast cancer, there is information about the patient’s demographics, the hormone status of the cancer, the stage, and so on. Using those additional data points can help the authors, and us, start to formulate some hypotheses as to what is happening here.
Let’s start with something a bit tricky about this kind of data. We see an uptick in new breast cancer diagnoses among young women in recent years. We need to tease that uptick apart a bit. It could be that it is the year that is the key factor here. In other words, it is simply that more women are getting breast cancer since 2016 and so more young women are getting breast cancer since 2016. These are known as period effects.
Or is there something unique to young women — something about their environmental exposures that put them at higher risk than they would have been had they been born at some other time? These are known as cohort effects.
The researchers teased these two effects apart, as you can see here, and concluded that, well, it’s both.
Stage of cancer at diagnosis can give us some more insight into what is happening. These results are pretty interesting. These higher cancer rates are due primarily to stage I and stage IV cancers, not stage II and stage III cancers.
The rising incidence of stage I cancers could reflect better detection, though many of the women in this cohort would not have been old enough to quality for screening mammograms. That said, increased awareness about genetic risk and family history might be leading younger women to get screened, picking up more early cancers. Additionally, much of the increased incidence was with estrogen receptor–positive tumors, which might reflect the fact that women in this cohort are tending to have fewer children, and children later in life.
So why the rise in stage IV breast cancer? Well, precisely because younger women are not recommended to get screening mammograms; those who detect a lump on their own are likely to be at a more advanced stage. But I’m not sure why that would be changing recently. The authors argue that an increase in overweight and obesity in the country might be to blame here. Prior studies have shown that higher BMI is associated with higher stage at breast cancer diagnosis.
Of course, we can speculate as to multiple other causes as well: environmental toxins, pollution, hormone exposures, and so on. Figuring this out will be the work of multiple other studies. In the meantime, we should remember that the landscape of cancer is continuously changing. And that means we need to adapt to it. If these trends continue, national agencies may need to reconsider their guidelines for when screening mammography should begin — at least in some groups of young women.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Chemo-Free Maintenance Strategies May Boost Survival in TNBC
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- First-line standard therapy for advanced TNBC generally includes taxane- or platinum-based chemotherapy which poses challenging toxicities. Exploring chemotherapy-free maintenance strategies may provide adequate disease control and improve patient quality of life.
- The researchers evaluated 45 patients, at five sites in the Republic of Korea, the United States, and Singapore, with TNBC who had ongoing stable disease or complete/partial response from first- or second-line platinum-based chemotherapy.
- The patients were randomized 1:1 to receive olaparib 300 mg twice daily with or without durvalumab 1500 mg on day 1 every 4 weeks.
- The authors compared PFS with a historical control of continued platinum-based therapy. An improvement to 4 months with maintenance therapy was considered clinically significant.
TAKEAWAY:
- After a follow-up of 9.8 months, patients who received olaparib alone demonstrated median PFS of 4.0 months, and those who received the combination therapy had median PFS of 6.1 months.
- Clinical benefit rates, defined as stable disease for at least 24 weeks or complete/partial response, were reported in 44% of the monotherapy group and 36% of the combination therapy group.
- Sustained clinical benefit was evident irrespective of germline BRCA mutation or programmed death-ligand 1 status, although it tended to be associated with complete or partial response to prior platinum.
- Grade 3-4 adverse events were reported in nine patients (39%) in the olaparib arm and eight patients (36%) in the combination arm. No treatment-related deaths or new safety signals were observed.
IN PRACTICE:
“Maintenance regimens are rarely used in [triple-negative breast cancer] but offer the possibility of more tolerable long-term treatment avoiding some of the chemotherapy-related side effects of more aggressive regimens, as is standard in the first-line treatment of HER2-positive advanced breast cancer,” the researchers concluded.
SOURCE:
This study, led by Tira J. Tan from Duke-NUS Medical School, Singapore, was published online on January 18, 2024, in Clinical Cancer Research.
LIMITATIONS:
The main limitations were the small sample size and lack of a standard control arm. Most patients (76%) were Asian, limiting generalizability. The trial was not designed to compare olaparib monotherapy and olaparib plus durvalumab regimens.
DISCLOSURES:
AstraZeneca Pharmaceuticals LP supported this study. Several authors reported financial support from various sources.
A version of this article appeared on Medscape.com.
Dana-Farber Moves to Retract, Correct Dozens of Cancer Papers Amid Allegations
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
News of the investigation follows a blog post by British molecular biologist Sholto David, MD, who flagged almost 60 papers published between 1997 and 2017 that contained image manipulation and other errors. Some of the papers were published by Dana-Farber’s chief executive officer, Laurie Glimcher, MD, and chief operating officer, William Hahn, MD, on topics including multiple myeloma and immune cells.
Mr. David, who blogs about research integrity, highlighted numerous errors and irregularities, including copying and pasting images across multiple experiments to represent different days within the same experiment, sometimes rotating or stretching images.
In one case, Mr. David equated the manipulation with tactics used by “hapless Chinese papermills” and concluded that “a swathe of research coming out of [Dana-Farber] authored by the most senior researchers and managers appears to be hopelessly corrupt with errors that are obvious from just a cursory reading the papers.”
“Imagine what mistakes might be found in the raw data if anyone was allowed to look!” he wrote.
Barrett Rollins, MD, PhD, Dana-Farber Cancer Institute’s research integrity officer, declined to comment on whether the errors represent scientific misconduct, according to STAT. Rollins told ScienceInsider that the “presence of image discrepancies in a paper is not evidence of an author’s intent to deceive.”
Access to new artificial intelligence tools is making it easier for data sleuths, like Mr. David, to unearth data manipulation and errors.
The current investigation closely follows two other investigations into the published work of Harvard University’s former president, Claudine Gay, and Stanford University’s former president, Marc Tessier-Lavigne, which led both to resign their posts.
A version of this article appeared on Medscape.com.
Advantage of Abemaciclib Plus Endocrine Therapy for Early Breast Cancer Endures at 5 Years
in updated results of a trial.
This was based on data collected over a median follow-up of 54 months. Previously reported data from this phase III study, known as monarchE, showed the same outcomes but over a 2-year treatment period, the researchers said.
Risk of cancer recurrence may be as much as 30% at 5 years in these high-risk patients, who will likely need more intense treatment, wrote Priya Rastogi, MD, of the University of Pittsburgh Medical Center, and colleagues.
In the new study published in the Journal of Clinical Oncology (2023 Jan 9. doi: 10.1200/JCO.23.019), the researchers reported 5-year efficacy results from an interim analysis of overall survival in the monarchE trial.
The intent-to-treat population included 2808 individuals randomized to abemaciclib plus ET and 2814 to ET alone; the median age was 51 years, and approximately 70% of the participants were White.
The addition of abemaciclib significantly reduced the risk of IDFS and DRFS over a median follow-up period of 54 months with hazard ratios of 0.680 and 0.675, respectively. Adjuvant abemaciclib also significantly improved DRFS over ET alone (HR 0.675).
The findings were limited by the lack of statistical significance for overall survival with abemaciclib. However, the increased benefits for IDFS and DRFS with abemaciclib plus ET vs. ET alone were consistent across all subgroups, and the benefit of abemaciclib was consistent regardless of the number of nodes involved, the researchers wrote.
“Prior reports from this trial with shorter follow-up demonstrated benefit of abemaciclib. However, with longer follow-up of a median 54 months, we see that the benefit of the drug is not only sustained (32% reduction in the risk of a disease event), but that there is further separation of the curves with an absolute difference in IDFS and DRFS rates of 7.6% and 6.7, comparing the ET alone vs. ET plus abemaciclib arms,” study coauthor Matthew P. Goetz, MD, said in an interview.
Although statistical significance was not reached for overall survival, fewer deaths occurred in the abemaciclib-plus-ET group compared with the ET-only group, said Dr. Goetz, of the Mayo Clinic, Rochester, Minnesota. However, patients with the worst prognosis (Ki-67–high subgroup) tended to have higher overall survival.
A total of 208 deaths occurred in the combination group vs. 234 in the ET-only group, and no new safety signals were observed. The occurrence of serious adverse events of any cause was similar in the abemaciclib group and the ET-only group (6.5% vs. 7.3%).
“These data are a pleasant surprise, as there were concerns that the benefit of the drug seen with shorter follow-up would wane over time,” Dr. Goetz said. “However, the opposite has occurred; with increasing length of follow-up, the curves continue to separate.”
Based on the new results, “we have high confidence that for patients with ER+/HER2- breast cancer at high risk of recurrence, the addition of 2 years of adjuvant abemaciclib to ET results in clinically significant improvements in IDFS,” he said.
Looking ahead, “we need additional follow-up to determine whether the benefit we now see in terms of IDFS will eventually translate into improvements in overall survival,” Dr. Goetz said. “We need to identify biomarkers that can identify patients at risk for early recurrence despite administration of adjuvant abemaciclib and further, biomarkers that will allow us to select patients that can be safely treated with ET alone.”
Findings Confirm Value of Combined Treatment
“It was reassuring to see the continued benefit at 5 years with adjuvant abemaciclib in combination with endocrine therapy compared to endocrine therapy alone in this high-risk HR+, HER2– EBC [early breast cancer] population,” Manali Ajay Bhave, MD, a medical oncologist at Emory University, Atlanta, said in an interview.
“While the interim overall survival analysis was not significant, further follow-up is necessary to truly discern a survival benefit particularly in this patient population where a survival advantage may not be seen for several years,” she added.
The current study supports the continued use of adjuvant abemaciclib in high-risk HR+, HER2– EBC patients, Dr. Bhave said. “Investigation of novel endocrine agents in the adjuvant setting for patients with high risk, HR+ HER2– EBC is needed to further improve outcomes.”
Urgent Need to Improve Adjuvant Therapy
“The monarchE study is a timely study aimed at improving adjuvant treatments in ER+ breast cancer to reduce risk of late recurrences,” Malinda T. West, MD, of the University of Wisconsin, said in an interview. “Late recurrences occurring decades later is a risk associated with ER+ breast cancer, and the risk of breast cancer recurrence is highest in those with larger tumors and nodal involvement.
“Abemaciclib is one of the three FDA-approved cyclin-dependent kinase 4/6 inhibitors in metastatic ER+ breast cancer based on demonstrated efficacy and safety in the metastatic setting compared to endocrine therapy alone, which was the rationale for expanded use of abemaciclib into the adjuvant setting for those at high risk for recurrence and basis of the monarchE trial,” said Dr. West.
An important criterion for inclusion was the randomization to abemaciclib required within 16 months of definitive breast cancer surgery, which reflected a window of time in which to start adjuvant abemaciclib, Dr. West said. “Exclusion criteria were those with a history of thromboembolic events, as abemaciclib carries a warning for venous thromboembolism,” she added.
In the monarchE follow-up, Dr. West said she was encouraged by the persistent and widening benefit with 2 years of added abemaciclib to endocrine therapy in reducing IDFS and DRFS compared to endocrine therapy alone.
Dr. West advised clinicians to consider initiating the therapy for up to 16 months after definitive breast surgery, because doing so may allow for recovery from surgery, chemotherapy, and radiation.
The findings tell physicians to “use caution with adding abemaciclib in those with a history of thromboembolic events or VTE risk factors as abemaciclib has a known VTE warning and this population was excluded in the monarchE trial,” she noted.
“Continued long-term follow up of those in this study will be important to determine survival benefits and how the predictive biomarker Ki-67 may impact survival outcomes,” she said.
The study was supported by Eli Lilly. Lead author Dr. Rastogi disclosed travel, accommodations, and expenses from Genentech/Roche, Lilly, and AstraZeneca. Several coauthors disclosed stock or ownership interests and/or other relationships with Lilly and other pharmaceutical companies. Dr. Goetz receives research funding from the National Institutes of Health. Dr. Bhave and Dr. West had no financial conflicts to disclose.
in updated results of a trial.
This was based on data collected over a median follow-up of 54 months. Previously reported data from this phase III study, known as monarchE, showed the same outcomes but over a 2-year treatment period, the researchers said.
Risk of cancer recurrence may be as much as 30% at 5 years in these high-risk patients, who will likely need more intense treatment, wrote Priya Rastogi, MD, of the University of Pittsburgh Medical Center, and colleagues.
In the new study published in the Journal of Clinical Oncology (2023 Jan 9. doi: 10.1200/JCO.23.019), the researchers reported 5-year efficacy results from an interim analysis of overall survival in the monarchE trial.
The intent-to-treat population included 2808 individuals randomized to abemaciclib plus ET and 2814 to ET alone; the median age was 51 years, and approximately 70% of the participants were White.
The addition of abemaciclib significantly reduced the risk of IDFS and DRFS over a median follow-up period of 54 months with hazard ratios of 0.680 and 0.675, respectively. Adjuvant abemaciclib also significantly improved DRFS over ET alone (HR 0.675).
The findings were limited by the lack of statistical significance for overall survival with abemaciclib. However, the increased benefits for IDFS and DRFS with abemaciclib plus ET vs. ET alone were consistent across all subgroups, and the benefit of abemaciclib was consistent regardless of the number of nodes involved, the researchers wrote.
“Prior reports from this trial with shorter follow-up demonstrated benefit of abemaciclib. However, with longer follow-up of a median 54 months, we see that the benefit of the drug is not only sustained (32% reduction in the risk of a disease event), but that there is further separation of the curves with an absolute difference in IDFS and DRFS rates of 7.6% and 6.7, comparing the ET alone vs. ET plus abemaciclib arms,” study coauthor Matthew P. Goetz, MD, said in an interview.
Although statistical significance was not reached for overall survival, fewer deaths occurred in the abemaciclib-plus-ET group compared with the ET-only group, said Dr. Goetz, of the Mayo Clinic, Rochester, Minnesota. However, patients with the worst prognosis (Ki-67–high subgroup) tended to have higher overall survival.
A total of 208 deaths occurred in the combination group vs. 234 in the ET-only group, and no new safety signals were observed. The occurrence of serious adverse events of any cause was similar in the abemaciclib group and the ET-only group (6.5% vs. 7.3%).
“These data are a pleasant surprise, as there were concerns that the benefit of the drug seen with shorter follow-up would wane over time,” Dr. Goetz said. “However, the opposite has occurred; with increasing length of follow-up, the curves continue to separate.”
Based on the new results, “we have high confidence that for patients with ER+/HER2- breast cancer at high risk of recurrence, the addition of 2 years of adjuvant abemaciclib to ET results in clinically significant improvements in IDFS,” he said.
Looking ahead, “we need additional follow-up to determine whether the benefit we now see in terms of IDFS will eventually translate into improvements in overall survival,” Dr. Goetz said. “We need to identify biomarkers that can identify patients at risk for early recurrence despite administration of adjuvant abemaciclib and further, biomarkers that will allow us to select patients that can be safely treated with ET alone.”
Findings Confirm Value of Combined Treatment
“It was reassuring to see the continued benefit at 5 years with adjuvant abemaciclib in combination with endocrine therapy compared to endocrine therapy alone in this high-risk HR+, HER2– EBC [early breast cancer] population,” Manali Ajay Bhave, MD, a medical oncologist at Emory University, Atlanta, said in an interview.
“While the interim overall survival analysis was not significant, further follow-up is necessary to truly discern a survival benefit particularly in this patient population where a survival advantage may not be seen for several years,” she added.
The current study supports the continued use of adjuvant abemaciclib in high-risk HR+, HER2– EBC patients, Dr. Bhave said. “Investigation of novel endocrine agents in the adjuvant setting for patients with high risk, HR+ HER2– EBC is needed to further improve outcomes.”
Urgent Need to Improve Adjuvant Therapy
“The monarchE study is a timely study aimed at improving adjuvant treatments in ER+ breast cancer to reduce risk of late recurrences,” Malinda T. West, MD, of the University of Wisconsin, said in an interview. “Late recurrences occurring decades later is a risk associated with ER+ breast cancer, and the risk of breast cancer recurrence is highest in those with larger tumors and nodal involvement.
“Abemaciclib is one of the three FDA-approved cyclin-dependent kinase 4/6 inhibitors in metastatic ER+ breast cancer based on demonstrated efficacy and safety in the metastatic setting compared to endocrine therapy alone, which was the rationale for expanded use of abemaciclib into the adjuvant setting for those at high risk for recurrence and basis of the monarchE trial,” said Dr. West.
An important criterion for inclusion was the randomization to abemaciclib required within 16 months of definitive breast cancer surgery, which reflected a window of time in which to start adjuvant abemaciclib, Dr. West said. “Exclusion criteria were those with a history of thromboembolic events, as abemaciclib carries a warning for venous thromboembolism,” she added.
In the monarchE follow-up, Dr. West said she was encouraged by the persistent and widening benefit with 2 years of added abemaciclib to endocrine therapy in reducing IDFS and DRFS compared to endocrine therapy alone.
Dr. West advised clinicians to consider initiating the therapy for up to 16 months after definitive breast surgery, because doing so may allow for recovery from surgery, chemotherapy, and radiation.
The findings tell physicians to “use caution with adding abemaciclib in those with a history of thromboembolic events or VTE risk factors as abemaciclib has a known VTE warning and this population was excluded in the monarchE trial,” she noted.
“Continued long-term follow up of those in this study will be important to determine survival benefits and how the predictive biomarker Ki-67 may impact survival outcomes,” she said.
The study was supported by Eli Lilly. Lead author Dr. Rastogi disclosed travel, accommodations, and expenses from Genentech/Roche, Lilly, and AstraZeneca. Several coauthors disclosed stock or ownership interests and/or other relationships with Lilly and other pharmaceutical companies. Dr. Goetz receives research funding from the National Institutes of Health. Dr. Bhave and Dr. West had no financial conflicts to disclose.
in updated results of a trial.
This was based on data collected over a median follow-up of 54 months. Previously reported data from this phase III study, known as monarchE, showed the same outcomes but over a 2-year treatment period, the researchers said.
Risk of cancer recurrence may be as much as 30% at 5 years in these high-risk patients, who will likely need more intense treatment, wrote Priya Rastogi, MD, of the University of Pittsburgh Medical Center, and colleagues.
In the new study published in the Journal of Clinical Oncology (2023 Jan 9. doi: 10.1200/JCO.23.019), the researchers reported 5-year efficacy results from an interim analysis of overall survival in the monarchE trial.
The intent-to-treat population included 2808 individuals randomized to abemaciclib plus ET and 2814 to ET alone; the median age was 51 years, and approximately 70% of the participants were White.
The addition of abemaciclib significantly reduced the risk of IDFS and DRFS over a median follow-up period of 54 months with hazard ratios of 0.680 and 0.675, respectively. Adjuvant abemaciclib also significantly improved DRFS over ET alone (HR 0.675).
The findings were limited by the lack of statistical significance for overall survival with abemaciclib. However, the increased benefits for IDFS and DRFS with abemaciclib plus ET vs. ET alone were consistent across all subgroups, and the benefit of abemaciclib was consistent regardless of the number of nodes involved, the researchers wrote.
“Prior reports from this trial with shorter follow-up demonstrated benefit of abemaciclib. However, with longer follow-up of a median 54 months, we see that the benefit of the drug is not only sustained (32% reduction in the risk of a disease event), but that there is further separation of the curves with an absolute difference in IDFS and DRFS rates of 7.6% and 6.7, comparing the ET alone vs. ET plus abemaciclib arms,” study coauthor Matthew P. Goetz, MD, said in an interview.
Although statistical significance was not reached for overall survival, fewer deaths occurred in the abemaciclib-plus-ET group compared with the ET-only group, said Dr. Goetz, of the Mayo Clinic, Rochester, Minnesota. However, patients with the worst prognosis (Ki-67–high subgroup) tended to have higher overall survival.
A total of 208 deaths occurred in the combination group vs. 234 in the ET-only group, and no new safety signals were observed. The occurrence of serious adverse events of any cause was similar in the abemaciclib group and the ET-only group (6.5% vs. 7.3%).
“These data are a pleasant surprise, as there were concerns that the benefit of the drug seen with shorter follow-up would wane over time,” Dr. Goetz said. “However, the opposite has occurred; with increasing length of follow-up, the curves continue to separate.”
Based on the new results, “we have high confidence that for patients with ER+/HER2- breast cancer at high risk of recurrence, the addition of 2 years of adjuvant abemaciclib to ET results in clinically significant improvements in IDFS,” he said.
Looking ahead, “we need additional follow-up to determine whether the benefit we now see in terms of IDFS will eventually translate into improvements in overall survival,” Dr. Goetz said. “We need to identify biomarkers that can identify patients at risk for early recurrence despite administration of adjuvant abemaciclib and further, biomarkers that will allow us to select patients that can be safely treated with ET alone.”
Findings Confirm Value of Combined Treatment
“It was reassuring to see the continued benefit at 5 years with adjuvant abemaciclib in combination with endocrine therapy compared to endocrine therapy alone in this high-risk HR+, HER2– EBC [early breast cancer] population,” Manali Ajay Bhave, MD, a medical oncologist at Emory University, Atlanta, said in an interview.
“While the interim overall survival analysis was not significant, further follow-up is necessary to truly discern a survival benefit particularly in this patient population where a survival advantage may not be seen for several years,” she added.
The current study supports the continued use of adjuvant abemaciclib in high-risk HR+, HER2– EBC patients, Dr. Bhave said. “Investigation of novel endocrine agents in the adjuvant setting for patients with high risk, HR+ HER2– EBC is needed to further improve outcomes.”
Urgent Need to Improve Adjuvant Therapy
“The monarchE study is a timely study aimed at improving adjuvant treatments in ER+ breast cancer to reduce risk of late recurrences,” Malinda T. West, MD, of the University of Wisconsin, said in an interview. “Late recurrences occurring decades later is a risk associated with ER+ breast cancer, and the risk of breast cancer recurrence is highest in those with larger tumors and nodal involvement.
“Abemaciclib is one of the three FDA-approved cyclin-dependent kinase 4/6 inhibitors in metastatic ER+ breast cancer based on demonstrated efficacy and safety in the metastatic setting compared to endocrine therapy alone, which was the rationale for expanded use of abemaciclib into the adjuvant setting for those at high risk for recurrence and basis of the monarchE trial,” said Dr. West.
An important criterion for inclusion was the randomization to abemaciclib required within 16 months of definitive breast cancer surgery, which reflected a window of time in which to start adjuvant abemaciclib, Dr. West said. “Exclusion criteria were those with a history of thromboembolic events, as abemaciclib carries a warning for venous thromboembolism,” she added.
In the monarchE follow-up, Dr. West said she was encouraged by the persistent and widening benefit with 2 years of added abemaciclib to endocrine therapy in reducing IDFS and DRFS compared to endocrine therapy alone.
Dr. West advised clinicians to consider initiating the therapy for up to 16 months after definitive breast surgery, because doing so may allow for recovery from surgery, chemotherapy, and radiation.
The findings tell physicians to “use caution with adding abemaciclib in those with a history of thromboembolic events or VTE risk factors as abemaciclib has a known VTE warning and this population was excluded in the monarchE trial,” she noted.
“Continued long-term follow up of those in this study will be important to determine survival benefits and how the predictive biomarker Ki-67 may impact survival outcomes,” she said.
The study was supported by Eli Lilly. Lead author Dr. Rastogi disclosed travel, accommodations, and expenses from Genentech/Roche, Lilly, and AstraZeneca. Several coauthors disclosed stock or ownership interests and/or other relationships with Lilly and other pharmaceutical companies. Dr. Goetz receives research funding from the National Institutes of Health. Dr. Bhave and Dr. West had no financial conflicts to disclose.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
