User login
Survival-Toxicity Trade-off With T-DM1 in HER+ Breast Cancer
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
SAN ANTONIO — in older patients with advanced human epidermal growth factor receptor 2–positive (HER2+) breast cancer, although toxicity is much lower, results from the HERB TEA study show.
Overall, the standard-of-care triple regimen of monoclonal antibodies pertuzumab and trastuzumab plus docetaxel remains the “first-line treatment for HER2-positive advanced breast cancer, regardless of age,” said study author Akihiko Shimomura, MD, PhD, who presented the findings (abstract RF02-04) on December 7 at the San Antonio Breast Cancer Symposium.
However, he noted that the standard-of-care regimen appears to be “intolerable mentally and physically” in those older than 65 years, and “impairs” quality of life.
Therefore a “new standard treatment with less toxicity and noninferior efficacy for older patients is needed,” said Dr. Shimomura, Department of Breast and Medical Oncology, National Center for Global Health and Medicine, Tokyo.
Dr. Shimomura and colleagues recruited patients aged 65 years or older with advanced HER2+ breast cancer who had received no prior chemotherapy for metastatic breast cancer and had a good performance status.
Patients were randomly assigned to either pertuzumab and trastuzumab plus docetaxel or T-DM1 until disease progression. The planned sample size was 250 patients, but the study was terminated after 148 participants were recruited because an interim analysis showed that T-DM1 failed to show noninferiority.
Among 75 patients assigned to the standard-of-care regimen, the mean age was 71 years, with 64% aged 65-74 years. Sixty-five percent had stage IV disease, and 35% had relapsed. These baseline characteristics were similar among the 73 patients given T-DM1.
At the data cutoff of June 15, 2023, the median progression-free survival was comparable between the two groups, at 15.6 months with the triple therapy vs 11.3 months with T-DM1 (hazard ratio [HR], 1.358; P =.1236).
There was also no significant difference in overall survival between the two groups (HR, 1.263; P =.95322).
However, T-DM1 failed to meet its primary endpoint of noninferiority to pertuzumab and trastuzumab plus docetaxel, defined as a hazard ratio for overall survival of 1.35.
Nevertheless, T-DM1 was associated with significantly less toxicity than the standard-of care-regimen, with rates of grade 3 or worse adverse events of 36.1% vs 56.8%, Shimomura reported.
The most common hematologic adverse events with the triple therapy were leukopenia (34.2%) and neutropenia (52.0%), whereas thrombocytopenia was the most common event with T-DM1 (16.7%).
Liver toxicities were also increased with the antibody-drug conjugate, whereas fatigue, diarrhea, and appetite loss were more frequently seen with the standard-of-care regimen.
Although T-DM1 did not achieve noninferiority, given its lower toxicity profile, a “detailed analysis, including geriatric assessment, is needed to identify the patient population for whom T-DM1 may be used as first line treatment,” said Shimomura.
Virginia Kaklamani, MD, codirector of the SABCS and leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center, Texas, said in an interview that the trial shows T-DM1 could be “a good alternative to our first line therapy in HER2+ metastatic breast cancer” for some patients.
“It is, however, unlikely to change the standard of care due to several changes in the field including the results from the KATHERINE trial and the DESTINY-Breast trials,” she said.
The study was funded by the Japanese National Cancer Center. Dr. Shimomura declares relationships with Daiichi Sankyo, Pfizer, AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd, Eli Lilly Japan K.K., MSD Co. Ltd, Eisai Co. Ltd, Gilead Sciences, and Taiho Pharmaceutical Co. Ltd.
A version of this article appeared on Medscape.com.
AT SABCS 2023
Pregnancy safe after BRCA-mutated breast cancer
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
SAN ANTONIO — New research provides some reassuring news for young women hoping to become pregnant after a diagnosis of BRCA-mutated breast cancer.
said Jame Abraham, MD, chair of Hematology & Medical Oncology at the Cleveland Clinic, who was not involved in the research.
The analysis, presented at the San Antonio Breast Cancer Symposium, revealed no issue with women becoming pregnant and carrying a healthy baby to term and reported no sign of worse disease outcomes among BRCA carriers following diagnosis and treatment.
“The final and most important conclusion from our study is that conceiving after proper breast cancer treatment and follow-up should not be contraindicated anymore in young BRCA carriers,” a message of particular importance for oncofertility counseling, lead investigator Matteo Lambertini, MD, a breast cancer oncologist at the University of Genova, Italy, said during his SABCS presentation.
The study was published December 7 in JAMA to coincide with his presentation.
Although pregnancy after breast cancer is generally considered safe, limited data exist for BRCA carriers in particular, Dr. Lambertini said.
The current analysis represents the largest look into the matter to date. The study included 4732 young women from across the globe who had been diagnosed with stage I-III invasive breast cancer. These women, all BRCA carriers, were 40 years or younger (median age at diagnosis, 35 years).
The team compared outcomes between 659 patients who had at least one pregnancy over a median follow-up of almost 8 years with 4073 women who did not become pregnant.
Dr. Lambertini and colleagues reported a median time of 3.5 years from breast cancer diagnosis to conception. Overall, about 1 in 5 young BRCA carriers (22%) conceived within 10 years after their breast cancer diagnosis. Of the 80% of patients with a completed pregnancy, 91% delivered at term and only 4 infants (0.9%) had documented congenital anomalies.
In short, “the rate of pregnancy, fetal, and obstetric complications was low and in line with the expectations in a population of women with similar age and no history of breast cancer,” Dr. Lambertini said. The team cautioned, however, that the data was extracted from oncology medical records, which might have underreported maternal and fetal outcomes.
Disease-free survival was similar among women who became pregnant and those who did not after breast cancer (adjusted HR, 0.99; 95% CI, 0.81-1.20).
When looking at the specific BRCA gene, differences did emerge. BRCA1 carriers had better disease-free survival after pregnancy (aHR, 0.80), while BRCA2 carriers appeared to have worse disease-free survival after pregnancy (aHR, 1.55).
For reasons that remain unclear, the researchers also found that BRCA1 carriers who got pregnant had significantly better breast cancer-specific survival (aHR, 0.59; P < .01) and overall survival (aHR, 0.58; P < .01). These women tended to have HR-negative breast cancer, which the authors also found was associated with improved survival after pregnancy (aHR, 0.76).
It’s possible, the team posited, that hormone receptor status played a role in the observed survival benefit. It’s also possible that these women were healthier overall.
The overall survival advantage, however, did not extend to BRCA2 carriers, who tended to have hormone receptor-positive disease. Hormone receptor-positive status did not appear to have a significant impact on survival (aHR, 1.30; 95% CI, 0.95-1.76).
“While the results appear reassuring for BRCA1 carriers, more caution is needed to counsel BRCA2 carriers, “ the investigators wrote.
The study was funded by the Italian Association for Cancer Research, Gilead, and others. Investigators had numerous ties to industry, including Dr. Lambertini, who is an adviser and speaker for Roche, Pfizer, Novartis, and others. The full list of disclosures can be found with the original article.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Patients with HR-positive breast cancer can safely use ART
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — who pause endocrine therapy to conceive, according to new data from the POSITIVE trial.
“We believe these data are of vital importance for the oncofertility counseling of young breast cancer patients,” Hatem A. Azim Jr., MD, PhD, adjunct professor, School of Medicine and Breast Cancer Center, Monterrey Institute of Technology, Mexico, said in a presentation at the San Antonio Breast Cancer Symposium.
As reported previously by this news organization, the primary results of the POSITIVE trial showed that interrupting endocrine therapy to allow pregnancy does not increase the risk of recurrence at 41 months follow-up.
Yet, there is concern that use of fertility preservation or assisted reproductive technology methods — especially those that entail the use of hormones — could have harmful effects on patients with HR-positive breast cancers, Dr. Azim explained.
To investigate, Dr. Azim and colleagues did a secondary analysis of outcomes from the POSITIVE trial, focusing on resumption of menstruation and use of fertility preservation and assisted reproductive technologies.
Among 516 women evaluated for the menstruation analysis, two thirds were aged 35 and older and a little more than half (53%) reported amenorrhea at enrollment, “which is not surprising,” Dr. Azim said.
“What is encouraging,” he said, is that 85% of women recovered menses within 6 months and 94% within 12 months of pausing endocrine therapy.
Among 497 evaluable participants who paused endocrine therapy to attempt pregnancy, 368 (74%) became pregnant.
Looking at time to pregnancy, there was a clear association between younger age at enrollment and shorter time to pregnancy. The cumulative incidence of pregnancy at 12 months was 64% in women younger than age 35 years, 54% in those aged 35-39, and 38% in those age 40-42. In a multivariable model, age < 35 was the only factor independently associated with a shorter time to pregnancy.
No Harmful Impact on Breast Cancer Outcomes
Turning to fertility preservation and use of assisted reproductive technologies, roughly half of the women (51%) underwent some form of fertility preservation at breast cancer diagnosis and before trial enrollment, most commonly ovarian stimulation for embryo or oocyte cryopreservation.
After enrollment, 43% of women underwent some form of assisted reproductive technology to attempt pregnancy, most commonly ovarian stimulation for in vitro fertilization (IVF) and cryopreserved embryo transfer.
In the multivariable model, cryopreserved embryo transfer was the only assisted reproductive technology significantly associated with a greater chance of becoming pregnant, more than doubling patients’ odds (odds ratio, 2.4).
“This means that at breast cancer diagnosis, we should consider cryopreservation of embryos for future use if desired,” Dr. Azim said.
Again, age mattered. Women younger than 35 undergoing assisted reproductive technologies had a 50% higher chance of becoming pregnant compared with peers aged 35-39, and an 84% higher chance than women aged 40-42.
Importantly, there was no apparent short-term detrimental impact of fertility preservation and/or assisted reproductive technologies on breast cancer outcomes, Dr. Azim reported. At 3 years, the breast cancer-free interval was almost identical between women who underwent ovarian stimulation for cryopreservation and those who did not (9.7% vs 8.7%).
“POSITIVE showed positive results that emphasize the importance of active oncofertility counseling with the patient starting at diagnosis,” said Hee Jeong Kim, MD, PhD, professor, Division of Breast Surgery, Asan Medical Center, Seoul, Republic of Korea, and discussant for the study.
“These data are reassuring for our young patients with a diagnosis of breast cancer and shows that assisted reproductive technology is an option and is probably safe to do with the caveat that it needs longer follow-up,” added SABCS codirector Carlos Arteaga, MD, director, Simmons Comprehensive Cancer Center, UT Southwestern Medical Center, Dallas.
Dr. Azim has no relevant disclosures. Dr. Arteaga is a scientific adviser to Novartis, Lilly, Merck, AstraZeneca, Daiichi Sankyo, OrigiMed, Immunomedics, PUMA Biotechnology, TAIHO Oncology, Sanofi, and the Susan G. Komen Foundation. He has received grant support from Pfizer, Lilly, and Takeda. Dr. Kim reports no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Less is more for axillary surgery in early breast cancer
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
SAN ANTONIO — than do those who have more extensive surgery, according to findings from a large meta-analysis.
Less extensive surgery also reduced patients’ risk for lymphedema, according to research (abstract GS02-05) presented at the San Antonio Breast Cancer Symposium.
These results, which included data from more than 20,000 women, may “reassure” patients and clinicians that more extensive axillary lymph node dissection “does not improve outcomes in many women with early-stage breast cancer,” said Andrea V. Barrio, MD, a breast surgeon at Memorial Sloan Kettering Cancer Center, New York City, who was not involved in the study.
Gurdeep S. Mannu, DPhil, of the University of Oxford, United Kingdom, who presented the findings at SABCS, explained that the optimal surgical management of the axilla remains uncertain in this patient population.
To better understand the long-term risks and benefits of more vs less aggressive axillary surgery in early breast cancer, Dr. Mannu and colleagues performed a meta-analysis of 29 randomized trials conducted over six decades, which included data on 20,285 women. The trials compared more vs less extensive axillary surgery as well as axillary surgery vs axillary radiotherapy.
In trials comparing more vs less extensive axillary surgery, researchers found that 83% of locoregional recurrences occurred in the breast or in multiple sites/unspecified locations, and the remaining 17% occurred in isolated axilla or other local recurrences, such as in the supraclavicular fossa or internal mammary chain.
Those with recurrences in the breast or multiple sites/unspecified locations did not benefit from more extensive surgery, demonstrating similar recurrence rates (RR) (RR for breast, 1.13; 95% CI, 0.92-1.40; RR for other, 0.89; 95% CI, 0.67-1.18).
The group with recurrences in isolated axilla or other local recurrences tended to do better with more extensive surgery (RR, 0.43 and 0.41, respectively).
Overall though, after a median follow-up of 10 years, differences in locoregional recurrence rates at any site did not differ among patients who had more vs less extensive axillary surgery (RR, 0.91; P = .22). This finding held even when restricting the analysis to women with node-positive disease/unknown nodal status (RR, 1.00; P = .98) and for node-negative women (RR, 0.88; P = .15).
Dr. Mannu and colleagues observed similar findings for distant recurrence, breast cancer mortality, and death from any cause.
“But where there was quite a striking difference was in morbidity,” said Dr. Mannu.
To examine rates of lymphedema — the surgical complication that has been “one of the main motivations” for the deescalation trials of the past few decades — the researchers focused on more recent trials, which “are most relevant to women treated today,” Dr. Mannu explained.
These showed that more extensive axillary surgery was associated with almost 2.5-times the rate of lymphedema compared with less extensive treatment (odds ratio [OR], 2.43).
Finally, the team compared axillary dissection with axillary radiotherapy across five trials and found no significant differences in the treatment approaches in terms of locoregional occurrence, distant recurrence, breast cancer mortality, and death from any cause.
However, once again, a notable difference in rates of lymphedema occurred, with axillary dissection associated with higher rates compared with radiotherapy (OR, 1.79).
This is “probably the largest meta-analysis comparing more vs less axillary surgery,” Dr. Barrio said in an interview.
“When we have one or two positive sentinel nodes, anywhere from 30%-50% of women will have additional positive lymph nodes that we’re not removing” with less extensive surgery, she explained. This study shows that, even then, this “doesn’t seem to impact on survival.”
This is “likely related to better medical treatment and radiation techniques that can treat that disease just as well as big surgery, but with less lymphedema,” she added.
Nevertheless, Dr. Barrio believes that there are “situations where we still feel that axillary lymph node dissection is important: in women with advanced cancer, like inflammatory breast cancer, and in women who’ve received chemotherapy upfront, then had surgery, and still have positive nodes after the chemo.”
The study was funded by Cancer Research UK, British Heart Foundation, Medical Research Council.
No relevant financial relationships have been declared.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Few with inflammatory breast cancer get guideline-based care
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
SAN ANTONIO — Yet, a retrospective study of patients with inflammatory breast carcinoma shows that the majority of patients don’t receive it.
The study also showed that overall survival was lowest for Black women who didn’t receive guideline-concordant care, said Brian Diskin, MD, with the Division of Breast Surgery, Memorial Sloan Kettering Cancer Center, New York City, here at the San Antonio Breast Cancer Symposium.
The results highlight the importance of adhering to guidelines in inflammatory breast carcinoma and suggest that improving the rates among Black patients “may help to mitigate racial disparities and survival,” Dr.Diskin told the conference.
Inflammatory breast carcinoma is an aggressive form of breast cancer associated with worse survival outcomes compared with other subtypes of breast cancer. Yet, it’s unclear how often and consistently guideline-concordant care — defined as treatment with neoadjuvant chemotherapy followed by modified radical mastectomy without immediate reconstruction, and postmastectomy radiotherapy — is received and what factors play a role in receiving recommended care.
To investigate, Dr. Diskin and colleagues identified 6945 women from the National Cancer Database with nonmetastatic inflammatory breast cancer treated from 2010-2018. Guideline-concordant care was defined as trimodality treatment administered in the correct sequence, with neoadjuvant chemotherapy started within 60 days of diagnosis.
Most patients (88%) did not start neoadjuvant chemotherapy within 60 days of diagnosis.
Black and Asian patients were less likely than were White patients to start chemotherapy within 60 days (odds ratio [OR] 0.54 and 0.51, respectively; P < .001), while patients with Medicare or private insurance were more likely to receive chemotherapy within 60 days of diagnosis than uninsured patients (OR 1.37 and 1.87, respectively; P < .001).
Roughly half of all patients didn’t receive appropriate surgical treatment (modified radical mastectomy without immediate reconstruction and postmastectomy radiotherapy).
Overall, only about one third of the cohort received guideline-concordant treatment, Dr. Diskin reported.
Patients aged 60-69 were more likely than were patients aged 40-49 to receive guideline-concordant treatment (odds ratio [OR], 1.24; P < .001), as were patients with a higher clinical nodal burden (OR, 1.34 for N1; OR, 1.28 for N2; OR, 1.15 for N3 vs N0; P < .001 for N1 and N2).
Patients treated between 2014 and 2018 were less likely to receive guideline-concordant treatment than patients treated between 2010 and 2013 (OR, 0.63; P <.001).
Receiving guideline-concordant care and being privately insured were both positively associated with improved overall survival (OR, 0.75 and 0.62, respectively; P < .001). Conversely, triple-negative subtype and Black race were associated with worse overall survival (HR, 1.6 and 1.4, respectively; P < .001).
However, timely receipt of guideline-concordant care for Black patients with triple-negative disease did lead to improved overall survival. Among recipients of guideline-based care with triple-negative disease, there was no racial disparity in overall survival.
Study discussant Kathryn Hudson, MD, director of survivorship and medical oncologist at Texas Oncology, Austin, said it’s important to note that Black women have a 4% lower incidence of breast cancer than do White women but a 40% higher breast cancer death rate.
“This study is important because it confirms that those who receive guideline-based care have better outcomes and that Black women have worse survival in [inflammatory breast cancer],” Dr. Hudson said.
The finding that Black and Asian women in the study were less likely to have timely neoadjuvant chemotherapy, “likely reflects worse access to care, and this may play a role in why Black women had worse outcomes,” she added.
Dr. Hudson said she found it “surprising” that only about one third of patients received guideline-concordant care.
In her view, “the take-home message is that improving guideline-concordant will improve outcomes for all patients with inflammatory breast cancer. And it’s really important, as a next step, to examine the barriers to guideline-concordant care in inflammatory breast cancer and continue to understand the reasons for worse [rates of] survival of Black women.”
Dr. Diskin has disclosed no relevant financial relationships. Dr. Hudson has received honoraria from the Menarini Group and Gilead.
A version of this article appeared on Medscape.com.
AT SABCS 2023
‘Baby TAM’ effective, tolerable for breast cancer prevention
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
SAN ANTONIO — The drug can reduce incidence of breast cancer in high-risk individuals, but side effects that mimic menopause have led to low rates of uptake. Lower-dose tamoxifen aims to reduce those side effects, but there remains some uncertainty about the minimum dose required to maintain efficacy.
The TAM-01 study, first published in 2019, demonstrated that a 5-mg dose of tamoxifen led to a reduction in recurrence of invasive breast cancer or ductal carcinoma in situ (DCIS). At the San Antonio Breast Cancer Symposium, two studies were presented that provided insight into dose efficacy and likelihood of medication adherence in women taking baby TAM.
“We all know that women who are at increased risk for breast cancer may benefit from the use of tamoxifen to help lower their risk, although historical uptake to tamoxifen in the prevention setting has been quite low,” said Lauren Cornell, MD, during a presentation. Her team investigated the impact of patient counseling on how well they understood their risk, as well as their likelihood of adherence to the medication.
The study included 41 women, and 31 completed follow-up at 1 year. “We saw that 90% of our patients reported good or complete understanding of their breast cancer risk after the consultation, emphasizing the benefit of that consult, and 73% reported that the availability of baby tamoxifen helped in their decision to consider a preventative medication,” said Dr. Cornell during her presentation. After 1 year of follow-up, 74% said that they had initiated baby tamoxifen, and 78% of those who started taking the drug were still taking it at 1 year.
Participants who continued to take baby TAM at 1 year had a higher estimated breast cancer risk (IBIS 10-year risk, 12.7% vs 7.6%; P = .027) than those who discontinued. “We saw that uptake to baby TAM after informed discussion in patients who qualify is high, especially in those patients with high risk and intraepithelial lesions or DCIS, and adherence and tolerability at 1 year follow up is improved, compared to what we would expect with traditional dosing of tamoxifen. It’s important to note that the NCCN guidelines and the ASCO clinical practice update now include low-dose tamoxifen as an option for select women, and future randomized control trials on de-escalation of tamoxifen and high-risk patients based on their risk model assessment still need to be done. Future study should also focus on markers to identify candidates best suited for low versus standard dose of tamoxifen,” said Dr. Cornell, who is an assistant professor of medicine at Mayo Clinic Florida in Jacksonville.
At another SABCS session, Per Hall, MD, PhD, discussed findings from the previously published KARISMA-2 study, which examined efficacy of various doses of tamoxifen. A total of 1440 participants, 240 in each arm, received tamoxifen doses of 20 mg, 10 mg, 5 mg, 2.5 mg, 1 mg, or placebo. During his talk, Dr. Hall pointed out that measuring outcomes would take a very large number of participants to identify small differences in breast cancer rates. Therefore, the researchers examined breast density changes as a proxy. As a noninferiority outcome, the researchers used the proportion of women in each arm who achieved the median decrease in breast density seen at 20 mg of tamoxifen, which is 10.1%.
The women underwent mammograms at baseline and again at 6 months to determine change in breast density. Among all women in the study, the proportion of patients who had a similar breast density reduction as the 20-mg dose were very similar in the 10 mg (50.0%; P = .002), 5 mg (49.3%; P < .001), and 2.5 mg (52.5%; P < .001) groups. The 1 mg group had a proportion of 39.5% (P = .138), while the placebo group had 38.9% (P = .161). However, the results were driven by premenopausal women, where the values were 63.3%, 70.7%, 74.4%, and 69.7% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, respectively, and 32.9% at 1 mg and 29.7% on placebo. In postmenopausal women, the values were 41.9%, 36.7%, 33.3%, and 41.9% in the 20-mg, 10-mg, 5-mg, and 2.5-mg groups, with values of 44.2% in the 1-mg group and 43.8% in the placebo group.
The median density change was 18.5% in premenopausal women and 4.0% in postmenopausal women.
“We didn’t see anything in the postmenopausal women. The decrease for those on 20 milligrams and those on placebo were exactly the same. Why this is, we still don’t know because we do know that tamoxifen in the adjuvant setting could be used for postmenopausal women. It could be that 6 months is too short of a time [to see a benefit]. We don’t know,” said Dr. Hall, who is a medical epidemiologist and biostatistician at Karolinska Institutet, Stockholm, Sweden.
Severe vasomotor side effects like hot flashes, cold flashes, and night sweats were reduced by about 50% in the lower tamoxifen doses, compared with 20 mg.
Dr. Hall also pointed out that tamoxifen is a prodrug. The CYP2D6 enzyme produces a range of metabolites, with endoxifen having the strongest affinity to the estrogen receptor and being present at the highest plasma concentration. He showed a table of endoxifen plasma levels at various tamoxifen doses in women of various metabolizer status, ranging from poor to ultrafast. Among intermediate, normal, and ultrarapid metabolizers, 5- and 10-mg doses produced plasma endoxifen levels ranging from 2.4 to 6.2 ng/mL, which represents a good therapeutic window. “For intermediate and normal metabolizers, it could be that 5 mg [of tamoxifen] is enough, but I want to underline that we didn’t use breast cancer incidence or recurrence in this study, we used density change, so we should be careful when we use these results,” said Dr. Hall. His group is now conducting the KARISMA Endoxifen trial, which will test endoxifen directly at doses of 1 and 2 mg.
Dr. Cornell has no relevant financial disclosures. Dr. Hall is a member of the scientific advisory board for Atossa Therapeutics.
FROM SABCS 2023
Living in a Food Swamp Tied to High Breast Cancer Mortality
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
SAN ANTONIO — a novel ecological study has found.
“Food deserts and food swamps are both bad, but it’s worse in food swamps,” Malcolm Bevel, PhD, MSPH, with Augusta University in Georgia, said in an interview.
He presented his research at the San Antonio Breast Cancer Symposium.
Breast cancer is the fourth leading cause of cancer death in the United States and is one of 13 obesity-related cancers. Healthy food consumption is a protective factor shown to decrease obesity risk and postmenopausal breast cancer mortality.
However, residing in food deserts or food swamps reduces access to healthy foods and has been severely understudied regarding postmenopausal breast cancer mortality, Dr. Bevel explained.
To investigate, Dr. Bevel and colleagues did a cross-sectional, ecological analysis where they merged 2010 to 2020 postmenopausal breast cancer mortality data from the Centers for Disease Control and Prevention (CDC) with aggregated 2012 to 2020 data from the US Department of Agriculture Food Environment Atlas.
A food swamp score was calculated as the ratio of fast-food and convenience stores to grocery stores and farmer’s markets.
A food desert score was calculated as the proportion of residents living more than 1 mile (urban) or 10 miles (rural) from a grocery store and household income ≤ 200% of the federal poverty threshold.
The researchers categorized food deserts and food swamps as low, moderate, or high, with higher scores denoting counties with fewer resources for healthy food.
Counties with high postmenopausal breast cancer mortality rates had a higher percentage of non-Hispanic Black population (5.8% vs. 2.1%), poverty rates (17.2% vs 14.2%), and adult obesity (32.5% vs 32%) and diabetes rates (11.8% vs 10.5%), compared with counties with low postmenopausal breast cancer mortality rates, Dr. Bevel reported.
The age-adjusted odds of counties having high postmenopausal breast cancer mortality was 53% higher in counties with high food desert scores (adjusted odds ratio [aOR] 1.53; 95% CI, 1.26 - 1.88), and over twofold higher in those with high food swamp scores (aOR, 2.09; 95% CI: 1.69 - 2.58).
In fully adjusted models, the likelihood of counties having moderate postmenopausal breast cancer mortality rates was 32% higher in those with moderate food swamp scores (aOR, 1.32; 95% CI, 1.03 - 1.70).
Growing Epidemic Requires System Change
These findings are in line with another study by Dr. Bevel and his colleagues published earlier this year in JAMA Oncology.
In that study, communities with easy access to fast food were 77% more likely to have high levels of obesity-related cancer mortality, as reported by this news organization.
There is a “growing epidemic” of food deserts and food swamps in the US, which could be due to systemic issues such as gentrification/redlining and lack of investment with chain grocery stores that provide healthy food options, said Dr. Bevel.
Local policymakers and community stakeholders could implement culturally tailored, sustainable interventions for obesity and obesity-related cancer prevention, including postmenopausal breast cancer. These could include creating more walkable neighborhoods and community vegetable gardens, he suggested.
“This is an important study demonstrating how the environment impacts outcomes in postmenopausal women diagnosed with breast cancer,” said Lia Scott, PhD, MPH, discussant for the study.
“Most of the literature is primarily focused on food deserts to characterize the food environment. However, these authors looked at both food deserts and food swamps. And even after adjusting for various factors and age, counties with high food swamp scores at greater odds of having higher postmenopausal breast cancer mortality rates,” said Dr. Scott, who is from Georgia State University School of Public Health in Atlanta.
“There is a clear need for systems change. With ecological studies like this one, we could potentially drive policy by providing actionable data,” she added.
The study had no specific funding. Dr. Bevel and Dr. Scott report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM SABCS 2023
Oncotype Score Helps Avoid Unnecessary Radiation in DCIS
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
SAN ANTONIO — There’s a long-standing concern among oncologists that many women with ductal carcinoma in situ (DCIS), a potential precursor to invasive breast cancer, receive more treatment than they need. The potential for overtreatment largely revolves around the extent of surgery and the use of radiation.
Using the Oncotype DX Breast DCIS Score test, a laboratory test that estimates DCIS recurrence risk, may help identify patients with low-risk DCIS who can safely avoid adjuvant radiation after surgery, according to new research (abstract GS03-01) presented at the San Antonio Breast Cancer Symposium.
Low-risk patients who skipped adjuvant radiotherapy after breast-conserving surgery demonstrated similar 5-year recurrence rates compared with high-risk patients who received adjuvant radiotherapy.
This is the first prospective study to evaluate radiation decisions among patients with DCIS.
Lead author Seema A. Khan, MD, who presented the research, called the findings “reassuring.”
However, “we need larger and better trials” as well as longer follow-up to confirm this less-is-more approach, said Dr. Khan, a breast cancer surgeon and researcher at Northwestern University, Chicago.
Virginia Kaklamani, MD, who moderated the presentation, noted that it is good to finally have prospective data on this topic. And although they are not definitive, “I personally think these results should be used” for counseling, said Dr. Kaklamani, leader of the breast cancer program at UT Health San Antonio.
To reduce the risk for DCIS recurrence or progression to invasive breast cancer, most patients with DCIS undergo breast-conserving surgery followed by adjuvant radiotherapy, Dr. Khan explained. Instead of breast-conserving surgery, about one in four patients opt for mastectomy.
Earlier results from this trial revealed that MRI helped identify patients who can safely receive breast-conserving surgery instead of mastectomy.
The current results assessed whether the Oncotype DX score can guide radiation treatment decisions.
The study included 171 patients with DCIS who had wide local excisions after MRI confirmed that they could forgo more extensive surgery.
Surgical specimens were then sent for testing to determine the DCIS score using the 12-gene Oncotype DX test.
Women who scored < 39 points on the 100-point Oncotype DX scale were considered to be at low risk for recurrence and were advised to skip radiation. Women who scored > 39 were advised to undergo radiation. Overall, 93% of the patients followed the radiation recommendations: 75 of 82 patients (91.4%) deemed as low risk skipped adjuvant radiotherapy and 84 of 89 patients (94.4%) deemed as high risk had radiotherapy.
At a median follow-up of 5 years, 5.1% (4 of 82) of low-risk patients experienced a recurrence vs. 4.5% (4 of 89) of higher-risk patients.
Recurrence rates among patients who followed the radiation recommendations mirrored these overall findings: 5.5% of 75 patients with low-risk DCIS who skipped radiotherapy experienced disease recurrence vs. 4.8% of 84 patients with high-risk DCIS who received radiotherapy.
Age did not appear to affect the outcomes. Among the 33 women younger than 50 years, two experienced a recurrence (4%), both invasive. One occurred in the low-risk group and the other in the higher-risk group. Among the 138 older women, six had recurrences, three in each group, and one recurrence in each was invasive.
In short, “women who skipped radiation based on this score did not experience an excess risk of” ipsilateral recurrence over 5 years, said Dr. Khan.
Overall, the study offers “strong evidence” that the DCIS score might help “prevent excessive treatment for some patients,” she concluded, adding that 10-year outcomes will be reported.
The work was funded by the National Cancer Institute. Dr. Khan has no conflicts of interest. Dr. Kaklamani has extensive industry ties, including being a speaker for Pfizer, Genentech, Novartis, and AstraZeneca.
A version in the article appeared on Medscape.com.
FROM SABCS 2023
Patient counseling for breast cancer screening: Taking changes to USPSTF recommendations into account
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.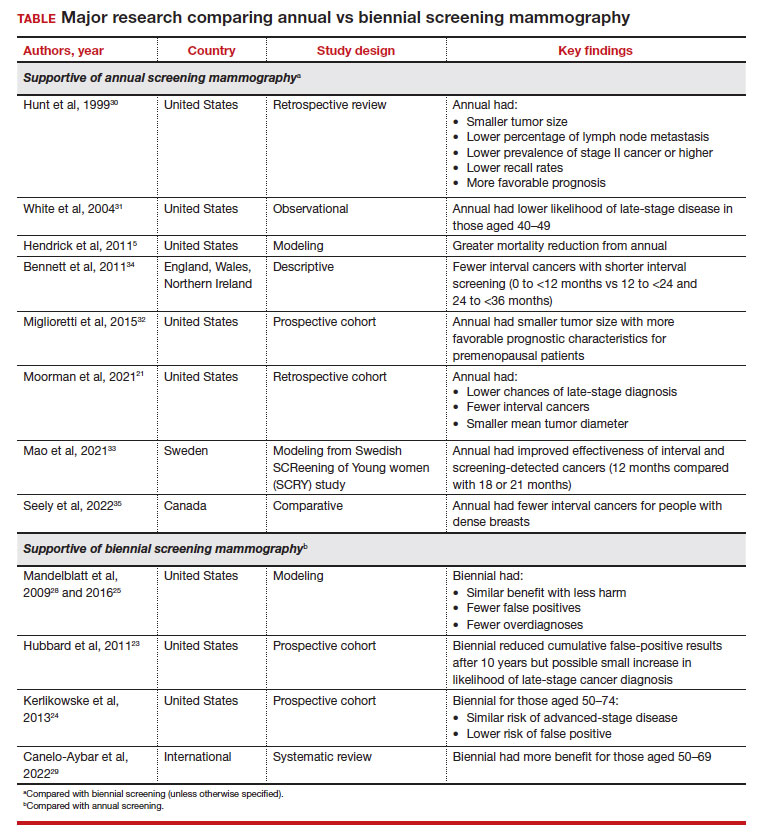
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
Breast cancer represents the most commonly diagnosed cancer in the nation.1 However, unlike other cancers, most breast cancers are identified at stage I and have a 90% survival rate 5-year prognosis.2 These outcomes are attributable to various factors, one of the most significant being screening mammography—a largely accessible, highly sensitive and specific screening tool.3 Data demonstrate that malignant tumors detected on screening mammography have more favorable profiles in tumor size and nodal status compared with symptomatic breast cancers,4 which make it critical for early diagnosis. Most importantly, the research overwhelmingly demonstrates that screening mammography decreases breast cancer–related mortality.5-7
The USPSTF big change: Mammography starting at age 40 for all recommended
Despite the general accessibility and mortality benefits of screening mammography (in light of the high lifetime 12% prevalence of breast cancer in the United States8), recommendations still conflict across medical societies regarding optimal timing and frequency.9-12 Previously, the US Preventive Services Task Force (USPSTF) recommended that screening mammography should occur at age 50 biennially and that screening between ages 40 and 49 should be an individualized decision.13,14 In the draft recommendation statement issued on May 9, 2023, however, the USPSTF now recommends screening every other year starting at age 40 to decrease the risk of dying from breast cancer.15
This change represents a critically important shift. The new guidance:
- acknowledges the increasing incidence of early-onset breast cancer
- reinforces a national consciousness toward screening mammography in decreasing mortality,17 even among a younger age group for whom the perception of risk may be lower.
The USPSTF statement represents a significant change in how patients should be counseled. Practitioners now have more direct guidance that is concordant with what other national medical organizations offer or recommend, including the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), and the National Comprehensive Cancer Network (NCCN).
However, while the USPSTF statement can and should encourage health care practitioners to initiate mammography earlier than prior recommendations, ongoing discussion regarding the optimal screening interval is warranted. The USPSTF recommendations state that mammography should be performed biennially. While the age at initiation represents a step in the right direction, this recommended screening interval should be reevaluated.
Annual vs biennial screening?
The debate between annual and biennial screening mammography is not new. While many randomized trials on screening mammography have evaluated such factors as breast cancer mortality by age or rate of false positives,18 fewer trials have evaluated the optimal screening interval.
One randomized trial from the United Kingdom evaluated 99,389 people aged 50 to 62 from 1989 to 1996 who underwent annual screening (study arm) versus 3 years later (control).19 Findings demonstrated a significantly smaller tumor size in the study arm (P=.05) as well as an increased total cancer detection rate. However, the authors concluded that shortening the screening interval (from 3 years) would not yield a statistically significant decrease in mortality.19
In a randomized trial from Finland, researchers screened those aged older than 50 at biennial intervals and those aged younger than 50 at either annual or triennial intervals.20 Results demonstrated that, among those aged 40 to 49, the frequency of stage I cancers was not significantly different from screen-detected cancers, interval cancers, or cancers detected outside of screening (50%, 42%, and 44%, respectively; P=.73). Furthermore, there was a greater likelihood of interval cancers among those aged 40 to 49 at 1-year (27%) and 3-year (39%) screening intervals compared with those aged older than 50 screened biennially (18%; P=.08 and P=.0009, respectively).20
These randomized trials, however, have been scrutinized because of factors such as discrepancies in screening intervals by country as well as substantial improvements made in screening mammography since the time these trials were conducted.5 Due to the dearth of more contemporary randomized controlled trials accounting for more up-to-date training and technology, most of the more recent data has been largely observational, retrospective, or used modeling.21 The TABLE outlines some of the major studies on this topic.
False-positive results, biopsy rates. The arguments against more frequent screening include the possibility of false positives that require callbacks and biopsies, which may be more frequent among those who undergo annual mammography.22 A systematic review from the Breast Cancer Surveillance Consortium demonstrated a 61.3% annual (confidence interval [CI], 59.4%–63.1%) versus 41.6% biennial (CI, 40.6%–42.5%) false-positive rate, resulting in a 7% (CI, 6.1%–7.8%) versus 4.8% (CI, 4.4–5.2%) rate of biopsy, respectively.23 This false-positive rate, however, also may be increased in younger patients aged 40 to 49 and in those with dense breasts.22,24 These callbacks and biopsies could induce significant patient stress, pain, and anxiety, as well as carry financial implications related to subsequent diagnostic imaging.
Overdiagnosis. There is also the risk of overdiagnosis, in which an indolent breast cancer that otherwise would not grow or progress to become symptomatic is identified. This could lead to overtreatment. While the exact incidence of overdiagnosis is unclear (due to recommendations for universal treatment of ductal carcinoma in situ), some data suggest that overdiagnosis could be decreased with biennial screening.25
While discomfort could also be a barrier, it may not necessarily be prohibitive for some to continue with future screening mammograms.22 Further, increased radiation with annual mammography is a concern. However, modeling studies have shown that the mortality benefit for annual mammography starting at age 40 outweighs (by 60-fold) the mortality risk from a radiation-induced breast cancer.26
Benefit from biennial screening
Some research suggests overall benefit from biennial screening. One study that used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer microsimulation was adapted to measure the incidence, mortality, and life-years gained for Canadian patients.27 This model demonstrated that mortality reduction was linked to greater lifetime screens for breast cancer, but this applied primarily to patients aged 50 and older. Overall, a larger impact was observed by initiating screening at age 40 than by decreasing screening intervals.27
Using modeling, Mandelblatt and colleagues demonstrated that biennial screening could capture most of the benefit of annual screening with less harm.28 In another study in 2016, Mandelblatt and colleagues used updated and revised versions of these simulation models and maintained that biennial screening upheld 79.8% to 81.3% of the benefits of annual screening mammography but with fewer overdiagnoses and false-positive results.25 The authors concluded that while biennial screening is equally effective for average-risk populations, there should be an evaluation of benefits and harms based on the clinical scenario (suggesting that annual screening for those at age 40 who carried elevated risk was similar to biennial screening for average-risk patients starting at age 50).25
Another study that served to inform the European Commission Initiative on Breast Cancer recommendations evaluated randomized controlled trials and observational and modeling studies that assessed breast screening intervals.29 The authors concluded that each screening interval has risks and benefits, with data suggesting more benefit with biennial screening for people aged 50 to 69 years and more possible harm with annual screening in younger people (aged 45–49).29
Continue to: Benefit from annual screening...
Benefit from annual screening
However, these data conflict with other studies that demonstrate the benefit of annual compared with biennial screening mammography. One large retrospective review of prospectively collected data evaluated outcome differences based on mammography frequency.30 For those undergoing annual versus biennial screening, the median tumor size was 11 mm (versus 15 mm), the percentage of lymph node metastasis was 14% (versus 24%), and cancer stage II or higher was 17% (versus 29%). The study overall demonstrated that annual screening resulted in lower recall rates (P<.0001) and detection of smaller tumors that carried a more favorable prognosis (P<.04).30
Another observational study from 2004 that assessed data from 7 different mammography registries nationwide noted that, among those aged 40 to 49, patients who underwent biennial screening had an increased likelihood of late-stage disease compared with those with annual screening (28% vs 21%, respectively; odds ratio [OR], 1.35; 95% CI, 1.01–1.81), although this discrepancy was not observed in people aged 50 or older.31
A study that critiqued the previous 2012 version of the USPSTF guidelines used CISNET modeling, which demonstrated a 39.6% mortality reduction with annual screening for those aged 40 to 84 versus 23.2% for biennial screening for those aged 50 to 74.5
More recent data also reflect these findings. A retrospective cohort study that evaluated patients aged 40 to 84 diagnosed with breast cancer found that those who previously underwent annual versus biennial screening mammography had lower incidences of late-stage diagnoses (24.0% vs 43.8%, respectively; P=.02), fewer interval cancers (10.5% vs 37.5%; P<.001), and smaller mean (SD) tumor diameter (1.4 [1.2] cm vs 1.8 [1.6] cm; P=.04).21 Postmenopausal patients in this cohort also demonstrated similar findings when comparing mammogram frequency. Although not significant, biennial (or greater) frequency of screening mammography also resulted in an increased likelihood of axillary lymph node dissection and chemotherapy.
Similarly, authors of another large prospective cohort study concluded that breast cancers diagnosed in premenopausal patients were more likely to be larger with less favorable prognostic characteristics (tumor size >15 mm, relative risk [RR], 1.21 [95% CI, 1.07–1.37]; P=.002); any less favorable prognostic characteristics (RR, 1.11 [95% CI, 1.00–1.22]; P=.047), and higher stage (stage IIB or higher, RR, 1.28 [95% CI, 1.01–1.63]; P=.04) for those who underwent biennial screening compared with breast cancers diagnosed by annual screening.32 However, this trend was not observed in postmenopausal patients not taking hormone therapy.32
Some international studies also show more favorable outcomes with annual screening mammography. A Swedish study evaluated mammography screening intervals of 21 months compared with 18 or 12 months in patients aged 40 to 49.33 Data showed an improved effectiveness of 1.6% to 9.8% for interval cancers and 2.9% to 17.4% for both interval and screening-detected cancers by reducing the screening frequency to 12 months, with authors suggesting a further reduction in breast cancer–related mortality rates for this age group.33
Results from another descriptive study from Europe also showed increasing interval breast cancer rates with increasing screening intervals.34 After a negative screen, the interval cancer rates and regional ranges for 0 to less than 12 months, 12 to less than 24 months, and 24 to less than 36 months per 1,000 screened were 0.55 (0.43–0.76), 1.13 (0.92–1.47), and 1.22 (0.93–1.57), respectively.34
Finally, a study conducted in Canada evaluated interval breast cancers among people with dense breasts screened between 2008 and 2010.35 Those with screening programs with policies that offered annual screening reported fewer interval cancers (interval cancer rate, 0.89 per 1,000; 95% CI, 0.67–1.11) compared with those who had policies that used biennial screening (interval cancer rate, 1.45 per 1,000 [annualized]; 95% CI, 1.19–1.72), which was 63% higher (P=.002). For those for whom radiologists recommended screening, interval cancer was lower for annual (0.93 per 1,000; 95% CI, 0.71–1.16) versus biennial screening (1.70 per 1,000 [annualized]; 95% CI, 0.70–2.71) (P=.061).35
Continue to: Black patients have a worse breast cancer prognosis...
Black patients have a worse breast cancer prognosis
Additional consideration should be given to populations with worse survival outcomes at baseline for whom screening mammography could play a significant role. In particular, Black people have similar rates of breast cancer compared with White people (127.8 cases per 100,000 vs 133.7 cases per 100,000, respectively) but have a 40% increased breast cancer–related mortality.8 The USPSTF recognizes this disparity and mentions it in their recommendations, encouraging health care clinicians to engage in shared decision making with Black patients and asserting that more research is needed on screening mammography in Black communities.15
While the age modification to the new guidelines better addresses the disparities that impact the Black community (such as increased likelihood of early-onset breast cancer36 and increased rate of breast cancer diagnosis at first mammogram37), the next obvious question is: Can groups with higher breast cancer mortality such as Black communities afford to undergo mammography every 2 years (as opposed to every year)?
Although some data specifically have evaluated the age of initiation and frequency of screening mammography among Black patients,38,39 little data have specifically assessed outcomes for annual versus biennial screening among Black people. Despite these research gaps, risk factors among the Black community should be considered. There is an increased risk of triple-negative breast cancer that can contribute to higher mortality among Black communities.40 Black people also tend to be diagnosed with more aggressive subtypes overall,41,42 are more likely to have dense breasts,43,44 have a higher likelihood of advanced stages at the time of diagnosis compared with White people,8,45 and have a greater chance of diagnosis of a second primary or contralateral breast cancer46-48—all risk factors that support the importance of regular and early-screening mammography.
How I counsel my patients
As Director of the Cancer Genetics and Breast Health Clinic, I am a gynecologist who primarily evaluates patients at increased risk for breast cancer (and other cancers). As an initial step, I strongly encourage all patients (especially Black patients and those of Ashkenazi Jewish ancestry as per the American College of Radiology recommendations9) to undergo risk assessment at age 25 to determine if they may be at increased risk for breast cancer. This first step may include genetic testing if the patient meets NCCN testing criteria based on personal or family history. If results are positive for a germline pathogenic variant, the timing and nature of breast screening would be based on NCCN recommendations for that particular variant (with possible modification of age of initiation based on family history). If testing is negative, lifetime risk assessment would then be performed using risk calculators—such as Tyrer-Cuzick—to determine if the patient meets criteria for intensive surveillance with supplemental breast magnetic resonance imaging. If the patient is subsequently determined to be at average risk after these assessments, I recommend they undergo screening mammography annually starting at age 40. However, it must be recognized that risk may change over time. A patient’s risk can continue to be assessed over a lifetime—with changing family history, personal risk factors, and new discoveries in genetics.
Summary
Ultimately, it is reassuring that the USPSTF guidelines have been updated to be concordant with other national medical society recommendations. They reflect the increasing nationwide trends that clearly demonstrate the high overall prevalence of breast cancer as well as the increasing incidence of early-onset breast cancer.
The updated guidelines, however, do not reflect the entirety of breast cancer trends in this country. With breast cancer being the most commonly diagnosed cancer in the United States, it is imperative to consider the data that demonstrate improved prognostics with annual compared with biennial mammography. Furthermore, the guidelines only begin to explore the disparities that Black patients face regarding breast cancer–related mortality. The risks of younger age at diagnosis, greater likelihood of aggressive subtypes, increased risk of second primary and contralateral breast cancer, and later stage at diagnosis must be seriously evaluated when counseling this patient population.
While the USPSTF recommendations for age at initiation reflect national statistics, recommendations by the ACR and NCCN more appropriately recognize that the benefits of annual screening outweigh the potential risks. Annual screening frequency should be adopted when counseling patients, particularly for the Black community. ●
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
- Cancer stat facts: Common cancer sites. National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Accessed November 7, 2023. https://seer .cancer.gov/statfacts/html/common.html#:~:text=An%20 estimated%20297%2C790%20women%20and,overall%20 with%20288%2C300%20expected%20cases
- Survival rates for breast cancer. American Cancer Society. March 1, 2023. Accessed November 16, 2023. https://www .cancer.org/cancer/breast-cancer/understanding-a-breast -cancer-diagnosis/breast-cancer-survival-rates.html
- Ambinder EB, Lee E, Nguyen DL, et al. Interval breast cancers versus screen detected breast cancers: a retrospective cohort study. Acad Radiol. 2023;30(suppl 2):S154-S160.
- Allgood PC, Duffy SW, Kearins O, et al. Explaining the difference in prognosis between screen-detected and symptomatic breast cancers. Br J Cancer. 2011;104:1680-1685.
- Hendrick RE, Helvie MA. United States Preventive Services Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-W116.
- Oeffinger KC, Fontham ETH, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599-1614.
- Hendrick RE, Baker JA, Helvie MA. Breast cancer deaths averted over 3 decades. Cancer. 2019;125:1482-1488.
- Breast cancer facts & figures 2022-2024. American Cancer Society. 2022. Accessed September 7, 2023. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/breast-cancer-facts-and-figures/2022-2024 -breast-cancer-fact-figures-acs.pdf
- New ACR breast cancer screening guidelines call for earlier and more-intensive screening for high-risk women. American College of Radiology. May 3, 2023. Accessed October 8, 2023. https://www.acr.org/Media-Center/ACR -News-Releases/2023/New-ACR-Breast-Cancer-Screening -Guidelines-call-for-earlier-screening-for-high-risk-women
- American Cancer Society recommendations for the early detection of breast cancer. American Cancer Society. January 14, 2022. Accessed October 30, 2023. https://www.cancer .org/cancer/types/breast-cancer/screening-tests-and-early -detection/american-cancer-society-recommendations-for -the-early-detection-of-breast-cancer.html
- Breast cancer screening and diagnosis. National Comprehensive Cancer Network. Published Version 1.2023. June 19, 2023. Accessed September 21, 2023. https://www .nccn.org/professionals/physician_gls/pdf/breast-screening .pdf
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130:e1-e16.
- Final recommendation statement. Breast cancer: screening. US Preventive Services Task Force. January 11, 2016. Accessed September 1, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/recommendation breast-cancer-screening
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279-296.
- Breast cancer: screening. US Preventive Services Task Force. May 9, 2023. Accessed October 7, 2023. https://www .uspreventiveservicestaskforce.org/uspstf/document/draft -evidence-review/breast-cancer-screening-adults
- Breast cancer in young women. Centers for Disease Control and Prevention. June 21, 2023. Accessed October 30, 2023. https://www.cdc.gov/cancer/breast/young_women/index .htm
- Arleo EK, Hendrick RE, Helvie MA, et al. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123:3673-3680.
- Nelson HD, Tyne K, Naik A, et al; US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727737, W237-W242.
- Breast Screening Frequency Trial Group. The frequency of breast cancer screening: results from the UKCCCR randomised trial. United Kingdom Co-ordinating Committee on Cancer Research. Eur J Cancer. 2002;38:1458-1464.
- Klemi PJ, Toikkanen S, Räsänen O, et al. Mammography screening interval and the frequency of interval cancers in a population-based screening. Br J Cancer. 1997;75:762-766.
- Moorman SEH, Pujara AC, Sakala MD, et al. Annual screening mammography associated with lower stage breast cancer compared with biennial screening. AJR Am J Roentgenol. 2021;217:40-47.
- Nelson HD, Pappas M, Cantor A, et al. Harms of breast cancer screening: systematic review to update the 2009 US Preventive Services Task Force recommendation. Ann Intern Med. 2016;164:256-267.
- Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
- Kerlikowske K, Zhu W, Hubbard RA, et al; Breast Cancer Surveillance Consortium. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173:807-816.
- Mandelblatt JS, Stout NK, Schechter CB, et al. Collaborative modeling of the benefits and harms associated with different US breast cancer screening strategies. Ann Intern Med. 2016;164:215-225.
- Miglioretti DL, Lange J, van den Broek JJ, et al. Radiationinduced breast cancer incidence and mortality from digital mammography screening: a modeling study. Ann Intern Med. 2016;164:205-214.
- Yaffe MJ, Mittmann N, Lee P, et al. Clinical outcomes of modelling mammography screening strategies. Health Rep. 2015;26:9-15.
- Mandelblatt JS, Cronin KA, Bailey S, et al; Breast Cancer Working Group of the Cancer Intervention and Surveillance Modeling Network. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151: 738-747.
- Canelo-Aybar C, Posso M, Montero N, et al. Benefits and harms of annual, biennial, or triennial breast cancer mammography screening for women at average risk of breast cancer: a systematic review for the European Commission Initiative on Breast Cancer (ECIBC). Br J Cancer. 2022;126:673-688.
- Hunt KA, Rosen EL, Sickles EA. Outcome analysis for women undergoing annual versus biennial screening mammography: a review of 24,211 examinations. AJR Am J Roentgenol. 1999;173:285-289.
- White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96:1832-1839.
- Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069-1077.
- Mao Z, Nyström L, Jonsson H. Breast cancer screening with mammography in women aged 40-49 years: impact of length of screening interval on effectiveness of the program. J Med Screen. 2021;28:200-206.
- Bennett RL, Sellars SJ, Moss SM. Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland. Br J Cancer. 2011;104:571-577.
- Seely JM, Peddle SE, Yang H, et al. Breast density and risk of interval cancers: the effect of annual versus biennial screening mammography policies in Canada. Can Assoc Radiol J. 2022;73:90-100.
- Liu Q, Yao S, Zhao H, et al. Early-onset triple-negative breast cancer in multiracial/ethnic populations: distinct trends of prevalence of truncation mutations. Cancer Med. 2019;8:1845-1853.
- Wilkerson AD, Obi M, Ortega C, et al. Young Black women may be more likely to have first mammogram cancers: a new perspective in breast cancer disparities. Ann Surg Oncol. 2023;30:2856-2869.
- Chen T, Kharazmi E, Fallah M. Race and ethnicity-adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open. 2023;6:e238893.
- Chapman CH, Schechter CB, Cadham CJ, et al. Identifying equitable screening mammography strategies for Black women in the United States using simulation modeling. Ann Intern Med. 2021;174:1637-1646.
- Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8-16.
- Stringer-Reasor EM, Elkhanany A, Khoury K, et al. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46.
- Newman LA. Parsing the etiology of breast cancer disparities. J Clin Oncol. 2016;34:1013-1014.
- Moore JX, Han Y, Appleton C, et al. Determinants of mammographic breast density by race among a large screening population. JNCI Cancer Spectr. 2020;4:pkaa010.
- McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst. 2016;108:djw104.
- Chen L, Li CI. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomarkers Prev. 2015;24:1666-1672.
- Terman E, Sheade J, Zhao F, et al. The impact of race and age on response to neoadjuvant therapy and long-term outcomes in Black and White women with early-stage breast cancer. Breast Cancer Res Treat. 2023;200:75-83.
- Watt GP, John EM, Bandera EV, et al. Race, ethnicity and risk of second primary contralateral breast cancer in the United States. Int J Cancer. 2021;148:2748-2758.
- Giannakeas V, Lim DW, Narod SA. The risk of contralateral breast cancer: a SEER-based analysis. Br J Cancer. 2021;125:601-610.
Answering the unknowns of taxanes for breast cancer during pregnancy
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
San Antonio – The findings shed light on a relatively unstudied topic. “Our cohort with 103 patients represents the most extensive study to date, and our main goal was to have homogeneous reporting of adverse events,” Ana Ferrigno Guajardo, MD, said in an interview. She presented the results at the San Antonio Breast Cancer Symposium.
“Breast cancer during pregnancy is a very challenging clinical situation as the expected antineoplastic effects of treatment must be carefully balanced against potential detrimental consequences on the developing fetus,” said Dr. Guajardo. She is a resident physician at Yale University School of Medicine.
Anthracycline-based chemotherapy agents are generally used during pregnancy because there is more safety data available for them, but some studies have shown that taxanes may have better efficacy in some clinical situations. “Cohort studies that have been done in the past [show] that taxane use is mostly deferred to the postpartum period, and we are not really sure of the impact that can have on survival in patients postponing treatment,” said Dr. Guajardo.
There are potential safety concerns with taxanes because neonates lack the cytochrome enzymes to metabolize the drugs, which creates a theoretical risk of adverse effects due to prolonged activity. On the other hand, pregnant women metabolize taxanes faster, and there are placental barriers that can inhibit high molecular weight molecules like taxanes from reaching the fetus, according to Dr. Guajardo.
In addition to pregnancy outcomes, the researchers followed 28 infants, and found that 87% were found to be completely healthy, “so we were relatively reassured. But of course we think that there’s a need for prospective studies that validate our findings regarding the safety taxanes,” said Dr. Guajardo.
Although there is no direct comparison group, the findings correlate well with studies of the general population and other chemotherapy agents. “We have large cohorts with mostly anthracycline-based chemotherapy agents during pregnancy that we can compare our results to, and overall, we were reassured that the prevalence of complications that we found in our cohort was very similar or even lower to those reported in the literature with patients treated with anthracycline-based therapy,” said Dr. Guajardo.
Compared with the general population, the team found higher rates of preterm births, neonatal ICU admissions, and premature membrane rupture, and infants that are small for gestational age. However, with the exception of the latter, all of these risks have been seen in pregnant women treated with other types of chemotherapy. “Perhaps it would be interesting to see if the incidence of small for gestational age neonates might be a bit higher in this population when compared to anthracycline-based therapy agents, but that does require a study that has a comparator group,” said Dr. Guajardo.
The researchers recruited 103 women with an average age of 34 years from 10 centers in 6 countries: United States, France, Spain, Mexico, Italy, and Costa Rica. The great majority were also treated with anthracyclines during gestation, and nearly all (97%) were treated with paclitaxel. The live birth rate was 98%, and 43.4% were preterm, 24% were small for gestational age, 16% were admitted to the neonate ICU, and 12.5% had hyperbilirubinemia.
Obstetric complications included intrauterine growth restriction (9%), preterm premature rupture of membranes (5%), gestational diabetes mellitus (5%), hypertensive disorders (4%), and pregnancy loss (2%).
After the presentation, Virginia Borges, MD, professor of medical oncology at University of Colorado Anschutz Medical Center, served as a discussant.
“Highlights of this study [include] that it is an international cohort from over six countries with over 100 cases of women included specifically focusing on the use of paclitaxel. They demonstrated safe outcomes for the pregnancies and the mothers,” Dr. Borges said during her presentation.
She went on to highlight several key points that physicians should consider when treating pregnancy-related breast cancer. “We want to achieve prepartum treatment wherever feasible to tackle that cancer before delivery of the child to prevent a pregnancy-related breast cancer from potentially turning into a postpartum breast cancer,” she said.
“If the tumor is ER+/HER2-, we now see we can safely give anthracyclines and taxanes from 12 to about 35 weeks of gestation. We don’t want to get too close to the delivery with chemotherapy. If a patient is HER2+, I prefer to give the anthracycline portion while the person is pregnant and then after delivery incorporate the taxane with the HER2 targeted therapies as there’s some older data showing concurrent therapy looks a bit better than sequential. In triple negative breast cancer, again I prefer to give the anthracycline and delay the taxane and carboplatin to overlap with immunotherapy so we are getting the necessary synergy there as well,” Dr. Borges added.
Dr. Guajardo has no relevant financial disclosures. Dr. Borges has consulted for SeaGen, Gilead, and AstraZeneca, and has received research funding from AstraZeneca, Gilead, Olema, and SeaGen.
FROM SABCS 2023
