User login
Some physicians still lack access to COVID-19 vaccines
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
It would be overused and trite to say that the pandemic has drastically altered all of our lives and will cause lasting impact on how we function in society and medicine for years to come. While it seems that the current trend of the latest Omicron variant is on the downslope, the path to get to this point has been fraught with challenges that have struck at the very core of our society. As a primary care physician on the front lines seeing COVID patients, I have had to deal with not only the disease but the politics around it. I practice in Florida, and I still cannot give COVID vaccines in my office.
I am a firm believer in the ability for physicians to be able to give all the necessary adult vaccines and provide them for their patients. The COVID vaccine exacerbated a majorly flawed system that further increased the health care disparities in the country. The current vaccine system for the majority of adult vaccines involves the physician’s being able to directly purchase supplies from the vaccine manufacturer, administer them to the patients, and be reimbursed.
Third parties can purchase vaccines at lower rates than those for physicians
The Affordable Care Act mandates that all vaccines approved by the Advisory Committee on Immunization Practices (ACIP) at the Centers for Disease Control and Prevention must be covered. This allows for better access to care as physicians will be able to purchase, store, and deliver vaccines to their patients. The fallacy in this system is that third parties get involved and rebates or incentives are given to these groups to purchase vaccines at a rate lower than those for physicians.
In addition, many organizations can get access to vaccines before physicians and at a lower cost. That system was flawed to begin with and created a deterrent for access to care and physician involvement in the vaccination process. This was worsened by different states being given the ability to decide how vaccines would be distributed for COVID.
Many pharmacies were able to give out COVID vaccines while many physician offices still have not received access to any of the vaccines. One of the major safety issues with this is that no physicians were involved in the administration of the vaccine, and it is unclear what training was given to the individuals injecting that vaccine. Finally, different places were interpreting the recommendations from ACIP on their own and not necessarily following the appropriate guidelines. All of these factors have further widened the health care disparity gap and made it difficult to provide the COVID vaccines in doctors’ offices.
Recommended next steps, solutions to problem
The question is what to do about this. The most important thing is to get the vaccines in arms so they can save lives. In addition, doctors need to be able to get the vaccines in their offices.
Many patients trust their physicians to advise them on what to do regarding health care. The majority of patients want to know if they should get the vaccine and ask for counseling. Physicians answering patients’ questions about vaccines is an important step in overcoming vaccine hesitancy.
Also, doctors need to be informed and supportive of the vaccine process.
The next step is the governmental aspect with those in power making sure that vaccines are accessible to all. Even if the vaccine cannot be given in the office, doctors should still be recommending that patients receive them. Plus, doctors should take every opportunity to ask about what vaccines their patients have received and encourage their patients to get vaccinated.
The COVID-19 vaccines are safe and effective and have been monitored for safety more than any other vaccine. There are multiple systems in place to look for any signals that could indicate an issue was caused by a COVID-19 vaccine. These vaccines can be administered with other vaccines, and there is a great opportunity for physicians to encourage patients to receive these life-saving vaccines.
While it may seem that the COVID-19 case counts are on the downslope, the importance of continuing to vaccinate is predicated on the very real concern that the disease is still circulating and the unvaccinated are still at risk for severe infection.
Dr. Goldman is immediate past governor of the Florida chapter of the American College of Physicians, a regent for the American College of Physicians, vice-president of the Florida Medical Association, and president of the Florida Medical Association Political Action Committee. You can reach Dr. Goldman at [email protected].
Columbia names interim chair of psychiatry after Twitter controversy
Helen Blair Simpson, MD, PhD, will take over for Jeffrey Lieberman, MD, who was suspended over a tweet he sent that was widely condemned as both racist and sexist.
She will also serve as interim director of the New York State Psychiatric Institute and interim psychiatrist-in-chief at New York–Presbyterian/Columbia University Irving Medical Center, the email stated.
All appointments were effective on Feb. 28.
Latest response
Dr. Simpson, who joined the faculty at Columbia in 1999, previously served as a professor and vice chair of research for the psychiatry department, director of Columbia’s Center for Obsessive-Compulsive and Related Disorders, and director of psychiatry research at the New York State Psychiatric Institute. Dr. Simpson is associate editor of JAMA Psychiatry and is president-elect of the Anxiety and Depression Association of America.
Her research has been continuously funded by the National Institute of Mental Health since 1999, and she has advised both the World Health Organization and the American Psychiatric Association on the diagnosis and treatment of OCD.
Dr. Simpson has a bachelor’s degree in biology from Yale University and completed an MD-PhD program at The Rockefeller University and Weill Cornell Medicine, New York. She did her residency in psychiatry at New York–Presbyterian.
The announcement is Columbia’s latest response to the furor that erupted on social media following Dr. Lieberman’s tweet about Sudanese model Nyakim Gatwech, in which he wrote, “Whether a work of art or a freak of nature she’s a beautiful sight to behold.”
Twitter reacted immediately and negatively to the tweet, which even Dr. Lieberman later acknowledged was “racist and sexist” in an email apology he sent Feb. 22 to faculty and staff in the department of psychiatry.
As reported by this news organization, Columbia suspended Dr. Lieberman from his chair position on Feb. 23 and permanently removed him from the post of psychiatrist-in-chief at New York–Presbyterian Hospital/Columbia University Irving Medical Center. Lieberman also resigned as executive director of the New York State Psychiatric Institute.
The email announcing Simpson’s appointment was signed by Katrina Armstrong, MD, incoming CEO, Columbia University Irving Medical Center and dean of the Faculties of Health Sciences and the Vagelos College of Physicians and Surgeons; Anil K. Rustgi, MD, interim executive vice president and dean of the Faculties of Health Sciences and Medicine; Steven J. Corwin, MD; president and CEO, New York–Presbyterian; and Ann Marie Sullivan, MD, commissioner of the New York State Office of Mental Health.
This is a developing story.
A version of this article first appeared on Medscape.com.
Helen Blair Simpson, MD, PhD, will take over for Jeffrey Lieberman, MD, who was suspended over a tweet he sent that was widely condemned as both racist and sexist.
She will also serve as interim director of the New York State Psychiatric Institute and interim psychiatrist-in-chief at New York–Presbyterian/Columbia University Irving Medical Center, the email stated.
All appointments were effective on Feb. 28.
Latest response
Dr. Simpson, who joined the faculty at Columbia in 1999, previously served as a professor and vice chair of research for the psychiatry department, director of Columbia’s Center for Obsessive-Compulsive and Related Disorders, and director of psychiatry research at the New York State Psychiatric Institute. Dr. Simpson is associate editor of JAMA Psychiatry and is president-elect of the Anxiety and Depression Association of America.
Her research has been continuously funded by the National Institute of Mental Health since 1999, and she has advised both the World Health Organization and the American Psychiatric Association on the diagnosis and treatment of OCD.
Dr. Simpson has a bachelor’s degree in biology from Yale University and completed an MD-PhD program at The Rockefeller University and Weill Cornell Medicine, New York. She did her residency in psychiatry at New York–Presbyterian.
The announcement is Columbia’s latest response to the furor that erupted on social media following Dr. Lieberman’s tweet about Sudanese model Nyakim Gatwech, in which he wrote, “Whether a work of art or a freak of nature she’s a beautiful sight to behold.”
Twitter reacted immediately and negatively to the tweet, which even Dr. Lieberman later acknowledged was “racist and sexist” in an email apology he sent Feb. 22 to faculty and staff in the department of psychiatry.
As reported by this news organization, Columbia suspended Dr. Lieberman from his chair position on Feb. 23 and permanently removed him from the post of psychiatrist-in-chief at New York–Presbyterian Hospital/Columbia University Irving Medical Center. Lieberman also resigned as executive director of the New York State Psychiatric Institute.
The email announcing Simpson’s appointment was signed by Katrina Armstrong, MD, incoming CEO, Columbia University Irving Medical Center and dean of the Faculties of Health Sciences and the Vagelos College of Physicians and Surgeons; Anil K. Rustgi, MD, interim executive vice president and dean of the Faculties of Health Sciences and Medicine; Steven J. Corwin, MD; president and CEO, New York–Presbyterian; and Ann Marie Sullivan, MD, commissioner of the New York State Office of Mental Health.
This is a developing story.
A version of this article first appeared on Medscape.com.
Helen Blair Simpson, MD, PhD, will take over for Jeffrey Lieberman, MD, who was suspended over a tweet he sent that was widely condemned as both racist and sexist.
She will also serve as interim director of the New York State Psychiatric Institute and interim psychiatrist-in-chief at New York–Presbyterian/Columbia University Irving Medical Center, the email stated.
All appointments were effective on Feb. 28.
Latest response
Dr. Simpson, who joined the faculty at Columbia in 1999, previously served as a professor and vice chair of research for the psychiatry department, director of Columbia’s Center for Obsessive-Compulsive and Related Disorders, and director of psychiatry research at the New York State Psychiatric Institute. Dr. Simpson is associate editor of JAMA Psychiatry and is president-elect of the Anxiety and Depression Association of America.
Her research has been continuously funded by the National Institute of Mental Health since 1999, and she has advised both the World Health Organization and the American Psychiatric Association on the diagnosis and treatment of OCD.
Dr. Simpson has a bachelor’s degree in biology from Yale University and completed an MD-PhD program at The Rockefeller University and Weill Cornell Medicine, New York. She did her residency in psychiatry at New York–Presbyterian.
The announcement is Columbia’s latest response to the furor that erupted on social media following Dr. Lieberman’s tweet about Sudanese model Nyakim Gatwech, in which he wrote, “Whether a work of art or a freak of nature she’s a beautiful sight to behold.”
Twitter reacted immediately and negatively to the tweet, which even Dr. Lieberman later acknowledged was “racist and sexist” in an email apology he sent Feb. 22 to faculty and staff in the department of psychiatry.
As reported by this news organization, Columbia suspended Dr. Lieberman from his chair position on Feb. 23 and permanently removed him from the post of psychiatrist-in-chief at New York–Presbyterian Hospital/Columbia University Irving Medical Center. Lieberman also resigned as executive director of the New York State Psychiatric Institute.
The email announcing Simpson’s appointment was signed by Katrina Armstrong, MD, incoming CEO, Columbia University Irving Medical Center and dean of the Faculties of Health Sciences and the Vagelos College of Physicians and Surgeons; Anil K. Rustgi, MD, interim executive vice president and dean of the Faculties of Health Sciences and Medicine; Steven J. Corwin, MD; president and CEO, New York–Presbyterian; and Ann Marie Sullivan, MD, commissioner of the New York State Office of Mental Health.
This is a developing story.
A version of this article first appeared on Medscape.com.
Health care on holidays
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My office was open on Presidents Day this year. Granted, I’ve never closed for it.
We’re also open on Veteran’s Day, Columbus Day, and Martin Luther King Jr. Day.
Occasionally (usually MLK or Veteran’s days) we get a call from someone unhappy we’re open that day. Banks, government offices, and schools are closed, and they feel that, by not following suit, I’m insulting the memory of veterans and those who fought for civil rights.
Nothing could be farther from the truth. In fact, I don’t know any doctors’ offices that AREN’T open on those days.
Part of this is patient centered. When people need to see a doctor, they don’t want to wait too long. The emergency room isn’t where the majority of things should be handled. Besides, they’re already swamped with nonemergent cases.
Most practices work 8-5 on weekdays, and are booked out. Every additional weekday you’re closed only adds to the wait. So I try to be there enough days to care for people, but not enough so that I lose my sanity or family.
In my area, a fair number of my patients are schoolteachers, who work the same hours I do. So many of them come in on those days, and appreciate that I’m open when they’re off.
Another part is practical. In a small practice, cash flow is critical, and there are just so many days in a given year you can be closed without hurting your financial picture. So most practices are closed for the Big 6 (Memorial Day, Independence Day, Labor Day, Thanksgiving, Christmas, and New Years). Usually this also includes Black Friday and Christmas Eve. So a total of 8 days per year (in addition to vacations).
Unlike other businesses (such as stores and restaurants) most medical offices aren’t open on weekends and nights, so our entire revenue stream is dependent on weekdays from 8 to 5. In this day and age, with most practices running on razor-thin margins, every day off adds to the red line. I can’t take care of anyone if I can’t pay my rent and staff.
I mean no disrespect to anyone. Like other doctors I work hard to provide quality care to all. But So I try to be there for them as much as I can, without going overboard and at the same time keeping my small practice afloat.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Survey: Artificial intelligence finds support among dermatologists
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.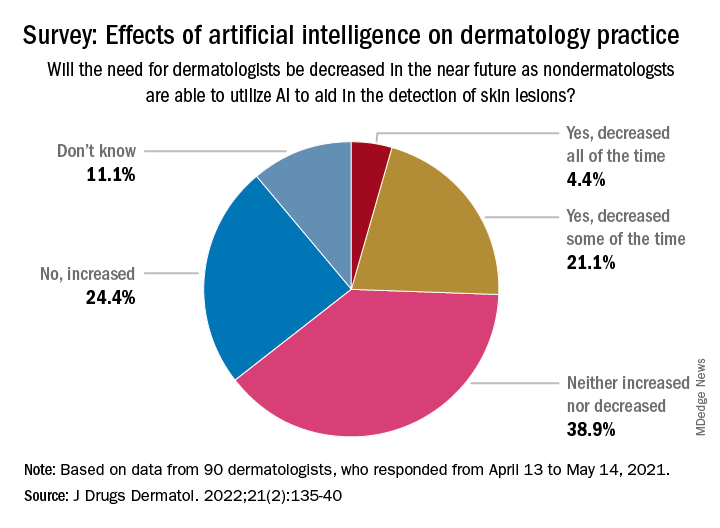
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
according to the results of a small survey.
Just 9% of the 90 respondents acknowledged that they have used AI in their practices, while 81% said they had not, and 10% weren’t sure or didn’t know. Despite that lack of familiarity, however, “many embrace the potential positive benefits, such as reducing misdiagnoses” and a majority (94.5%) “would use it at least in certain scenarios,” Vishal A. Patel, MD, and associates said in the Journal of Drugs in Dermatology.
Dermatologists aged 40 years and under were more likely to have used AI previously: 15% reported previous experience, compared with 4% of those over age 40 – but the difference in “age did not have a significant effect on perception of AI,” the investigators noted, adding that most of the dermatologists over 40 believe “that AI would be most beneficial and used for detection of malignant skin lesions.”
The survey also asked about ways the respondents would use AI to help their patients. Almost two-thirds of respondents (66%) chose analysis and management of electronic health records “for research purposes to improve patient outcomes,” compared with 56% who chose identifying unknown/screening skin lesions “with a list of differential diagnoses,” 32% who chose telemedicine, and 26% who chose primary surveys of skin, said Dr. Patel, director of cutaneous oncology at the George Washington University Cancer Center in Washington, and coauthors.
The respondents were fairly evenly split when asked about the possible impact of nondermatologists using AI in the near future to detect skin lesions, such as melanomas, on the need for dermatologists. Just over a quarter said that the need for dermatologists will be decreased all (about 4.4%) or some (about 21.1%) of the time, and 24.4% said that the need will be increased, with the largest share (39.9%) of respondents choosing the middle ground: neither increased or decreased, the investigators reported.
The survey form was emailed to 850 members of the Orlando Dermatology, Aesthetic & Surgical Conference listserv, with responses accepted from April 13 to May 14, 2021. The investigators noted that the response rate was low enough to be a limiting factor, making selection bias “by those with a particular interest in the topic” a possibility.
No funding sources for the study were disclosed. Dr. Patel disclosed that he is chief medical officer for Lazarus AI, the other authors had no disclosures listed.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Industry payments linked with rheumatologists’ prescribing
Payments to rheumatologists by pharmaceutical companies, whether through food and beverages or consulting fees, are linked with a higher likelihood of prescribing drugs and higher Medicare spending, according to a new study published Feb. 2 in Mayo Clinic Proceedings.
Alí Duarte-García, MD, of the division of rheumatology in the department of medicine at the Mayo Clinic in Rochester, Minn., led the study.
Researchers conducted a cross-sectional analysis of Medicare Part B Public Use File, Medicare Part D Public Use File, and Open Payments data for 2013-2015. They included prescription drugs that accounted for 80% of the total Medicare pharmaceutical expenditures in rheumatology.
They then calculated annual average drug cost per beneficiary per year, the percentage of rheumatologists who received payments, and the average payment per physician per drug per year. Industry payments were categorized as either food/beverage or consulting/compensation.
Multivariable regression models were used to assess links between industry payments, prescribing patterns, and prescription drug spending.
‘Directly associated’ with prescribing probability
The authors concluded that “pharmaceutical company payments to rheumatologists were directly associated with the probability of the physician’s prescribing a drug marketed by that company, the proportion of prescriptions that are for that drug, and the resulting Medicare expenditures.”
The authors noted that food and beverage payments were associated with increased Medicare reimbursement amounts for all drugs except rituximab.
The article includes examples that help quantify the link between payments and prescribing.
The authors wrote that for each $100 in food/beverage payments, Medicare reimbursement increased 6% to 44%. The increases were particularly high for infliximab and rACTH (repository corticotropin injection), “whereby a payment of $100 to a prescriber of these drugs was associated with increases of approximately $72,000 and $30,000 in Medicare reimbursements, respectively. For most of the other drugs, for every $100 in payments, the Medicare reimbursement increased by $8,000 to $13,000.”
The associations were strongest for food and beverage payments even though those were for lower dollar amounts.
The researchers found that every $100 in food and beverage payments, which also include gifts, entertainment, or educational materials, was linked with more than twofold higher probability of prescribing and higher Medicare reimbursements than for every $1,000 paid in consulting fees and compensation.
That finding and findings from previous studies, they say, “suggest that the current approach to regulating industry influence, which focuses on disclosure of large payments, may be inadequate.”
Aaron P. Mitchell, MD, a medical oncologist and health services researcher with Memorial Sloan Kettering Cancer Center in New York, told this news organization that the findings regarding rACTH show the potential harm of industry payments that are linked with prescribing.
The drug is a naturally produced hormone used for both rheumatologic and neurologic conditions. “Since it is an unpatentable drug – really just a hormone – it’s kind of grandfathered in and never had to get [Food and Drug Administration] approval for its current indications,” Dr. Mitchell said.
The drug, which costs nearly $230,000 per Medicare beneficiary, hasn’t been tested head to head in large randomized trials against much cheaper rheumatology drugs, such as prednisone.
In the case of infliximab, he noted, for every $100 in food/beverage payments, there was an increase of $72,000 in Medicare spending.
Dr. Mitchell studies industry influence in a variety of specialties and said rheumatology follows the universal pattern, although the drugs used in the specialty are more expensive than in many specialties.
He agrees with the authors that focusing on disclosures to address the problem is probably not enough.
“We’ve been in this period of full and open disclosure for going on 10 years, and we haven’t really seen a decline in these payments,” he said.
Results won’t surprise rheumatologists
Karen Onel, MD, a pediatric rheumatologist at the Hospital for Special Surgery, New York, and chair of the American College of Rheumatology’s Ethics and Conflict of Interest Committee, who said she was speaking only for herself and not on behalf of the committee, told this news organization that the study will not be surprising to rheumatologists or to physicians in other specialties who are aware of the plethora of studies that concluded that industry payments influence prescribing.*
Physicians are well aware of the problem, but “they all think it’s not them,” she said.
She said a shortcoming of this study is that it is unclear what the payments were for, because many things are lumped together in the categories. In the case of food and beverage payments, she said that could include payments physicians don’t realize are going on their open payments profile.
Dr. Onel gives a personal example. When she goes to a medical conference, she says, “I don’t eat on pharma’s dime.” But because badge numbers are scanned, it appears she accepted the food provided by the pharmaceutical sponsor when she enters a room where food or coffee is being served.
She said that although there are outliers like rACTH that often get highlighted, “the numbers are very low of the physicians who had [industry] money, and the change in prescribing patterns actually was very low.”
She pointed to an important limitation that the authors list: The study was limited to patients with fee-for-service Medicare coverage and did not include those with private insurance.
“For example, Aetna, Cigna, and UnitedHealthcare have restricted reimbursement for rACTH in recent years, citing its lack of proven efficacy and the availability of more affordable options, and rACTH is not in the Veterans Affairs formulary,” the authors wrote.
Rapid development of specialty drugs
Still, the authors wrote, the associations described in this article are of high interest because even though rheumatologists make up a small proportion of the physician workforce, they have among the highest costs per prescription in Medicare drug claims.
“Rheumatology, second only to oncology, has entered an era of rapid development of specialty drugs, including biologic disease-modifying antirheumatic drugs (DMARDs),” the authors wrote.
The complex drugs are expensive to produce and require expertise in handling. Most are under patent with no generic or approved biosimilar equivalent.
Nearly half of all U.S. rheumatologists received some payment from a pharmaceutical company in the study period, and most payments to rheumatologists were for low dollar amounts.
The highest per-year annual expenditures were attributed to etanercept ($741 million), adalimumab ($620 million), and infliximab ($539 million).
These drugs were expensive per beneficiary ($20,728 for etanercept, $21,492 for adalimumab, and $15,941 for infliximab) and were prescribed by a large number of rheumatologists (4,068, 3,872, and 1,349, respectively).
Addressing the problem
Dr. Mitchell says the leaders of individual societies need to fill the gaps that leave busy clinicians searching for easy ways to get up-to-date information on drugs.
He says in all specialties, “Industry should not be the most easily and readily available source of information.
“We’re in the age of Zoom now,” he said. “There’s no reason you have to be sitting for an hour at lunch with a sales rep and not a brown-bag lunch getting a 1-hour update from a society on a new drug indication.
“We will get higher-quality information if we’re doing the education ourselves, and we’d be removing the sense of obligation to pay back the industry gift givers,” Dr. Mitchell said.
Dr. Onel said among the most critical work is making sure that the leadership of ACR is free of conflicts of interest.
“They are making decisions for the entire organization. We need to feel safe and comfortable that they are acting in the best interest of the rheumatologists and patients versus corporate partners,” she said.
Guidelines must strictly follow the standards from the Institute of Medicine that fewer than 50% of the regular guideline committee members may have commercial conflicts, Dr. Onel said.
She added that training on real or apparent conflicts of interest must start in medical school, residency, and fellowship, before physicians start to think they know their own practice patterns and that the results don’t apply to them.
However, the reality is that the industry and physicians will always need each other, she said.
Dr. Onel describes a tension between rheumatologists needing industry’s help for research and education, especially when federal money for research can be difficult to get, and managing those relationships to avoid real or apparent conflicts.
“We want the public’s trust,” she said.
A study coauthor has served on advisory boards of Boehringer Ingelheim (> $10,000) and Gilead Sciences (> $10,000); has served on speakers bureaus for Simply Speaking (> $10,000) and Boehringer Ingelheim (< $10,000); and has received royalties from UpToDate (> $10,000). Dr. Mitchell and Dr. Onel reported no relevant financial relationships.
* Update, 2/25/22: This article, which originally attributed comments to Dr. Karen Onel personally, has been updated to further emphasize that these comments were not on behalf of the committee that she leads.
A version of this article first appeared on Medscape.com.
Payments to rheumatologists by pharmaceutical companies, whether through food and beverages or consulting fees, are linked with a higher likelihood of prescribing drugs and higher Medicare spending, according to a new study published Feb. 2 in Mayo Clinic Proceedings.
Alí Duarte-García, MD, of the division of rheumatology in the department of medicine at the Mayo Clinic in Rochester, Minn., led the study.
Researchers conducted a cross-sectional analysis of Medicare Part B Public Use File, Medicare Part D Public Use File, and Open Payments data for 2013-2015. They included prescription drugs that accounted for 80% of the total Medicare pharmaceutical expenditures in rheumatology.
They then calculated annual average drug cost per beneficiary per year, the percentage of rheumatologists who received payments, and the average payment per physician per drug per year. Industry payments were categorized as either food/beverage or consulting/compensation.
Multivariable regression models were used to assess links between industry payments, prescribing patterns, and prescription drug spending.
‘Directly associated’ with prescribing probability
The authors concluded that “pharmaceutical company payments to rheumatologists were directly associated with the probability of the physician’s prescribing a drug marketed by that company, the proportion of prescriptions that are for that drug, and the resulting Medicare expenditures.”
The authors noted that food and beverage payments were associated with increased Medicare reimbursement amounts for all drugs except rituximab.
The article includes examples that help quantify the link between payments and prescribing.
The authors wrote that for each $100 in food/beverage payments, Medicare reimbursement increased 6% to 44%. The increases were particularly high for infliximab and rACTH (repository corticotropin injection), “whereby a payment of $100 to a prescriber of these drugs was associated with increases of approximately $72,000 and $30,000 in Medicare reimbursements, respectively. For most of the other drugs, for every $100 in payments, the Medicare reimbursement increased by $8,000 to $13,000.”
The associations were strongest for food and beverage payments even though those were for lower dollar amounts.
The researchers found that every $100 in food and beverage payments, which also include gifts, entertainment, or educational materials, was linked with more than twofold higher probability of prescribing and higher Medicare reimbursements than for every $1,000 paid in consulting fees and compensation.
That finding and findings from previous studies, they say, “suggest that the current approach to regulating industry influence, which focuses on disclosure of large payments, may be inadequate.”
Aaron P. Mitchell, MD, a medical oncologist and health services researcher with Memorial Sloan Kettering Cancer Center in New York, told this news organization that the findings regarding rACTH show the potential harm of industry payments that are linked with prescribing.
The drug is a naturally produced hormone used for both rheumatologic and neurologic conditions. “Since it is an unpatentable drug – really just a hormone – it’s kind of grandfathered in and never had to get [Food and Drug Administration] approval for its current indications,” Dr. Mitchell said.
The drug, which costs nearly $230,000 per Medicare beneficiary, hasn’t been tested head to head in large randomized trials against much cheaper rheumatology drugs, such as prednisone.
In the case of infliximab, he noted, for every $100 in food/beverage payments, there was an increase of $72,000 in Medicare spending.
Dr. Mitchell studies industry influence in a variety of specialties and said rheumatology follows the universal pattern, although the drugs used in the specialty are more expensive than in many specialties.
He agrees with the authors that focusing on disclosures to address the problem is probably not enough.
“We’ve been in this period of full and open disclosure for going on 10 years, and we haven’t really seen a decline in these payments,” he said.
Results won’t surprise rheumatologists
Karen Onel, MD, a pediatric rheumatologist at the Hospital for Special Surgery, New York, and chair of the American College of Rheumatology’s Ethics and Conflict of Interest Committee, who said she was speaking only for herself and not on behalf of the committee, told this news organization that the study will not be surprising to rheumatologists or to physicians in other specialties who are aware of the plethora of studies that concluded that industry payments influence prescribing.*
Physicians are well aware of the problem, but “they all think it’s not them,” she said.
She said a shortcoming of this study is that it is unclear what the payments were for, because many things are lumped together in the categories. In the case of food and beverage payments, she said that could include payments physicians don’t realize are going on their open payments profile.
Dr. Onel gives a personal example. When she goes to a medical conference, she says, “I don’t eat on pharma’s dime.” But because badge numbers are scanned, it appears she accepted the food provided by the pharmaceutical sponsor when she enters a room where food or coffee is being served.
She said that although there are outliers like rACTH that often get highlighted, “the numbers are very low of the physicians who had [industry] money, and the change in prescribing patterns actually was very low.”
She pointed to an important limitation that the authors list: The study was limited to patients with fee-for-service Medicare coverage and did not include those with private insurance.
“For example, Aetna, Cigna, and UnitedHealthcare have restricted reimbursement for rACTH in recent years, citing its lack of proven efficacy and the availability of more affordable options, and rACTH is not in the Veterans Affairs formulary,” the authors wrote.
Rapid development of specialty drugs
Still, the authors wrote, the associations described in this article are of high interest because even though rheumatologists make up a small proportion of the physician workforce, they have among the highest costs per prescription in Medicare drug claims.
“Rheumatology, second only to oncology, has entered an era of rapid development of specialty drugs, including biologic disease-modifying antirheumatic drugs (DMARDs),” the authors wrote.
The complex drugs are expensive to produce and require expertise in handling. Most are under patent with no generic or approved biosimilar equivalent.
Nearly half of all U.S. rheumatologists received some payment from a pharmaceutical company in the study period, and most payments to rheumatologists were for low dollar amounts.
The highest per-year annual expenditures were attributed to etanercept ($741 million), adalimumab ($620 million), and infliximab ($539 million).
These drugs were expensive per beneficiary ($20,728 for etanercept, $21,492 for adalimumab, and $15,941 for infliximab) and were prescribed by a large number of rheumatologists (4,068, 3,872, and 1,349, respectively).
Addressing the problem
Dr. Mitchell says the leaders of individual societies need to fill the gaps that leave busy clinicians searching for easy ways to get up-to-date information on drugs.
He says in all specialties, “Industry should not be the most easily and readily available source of information.
“We’re in the age of Zoom now,” he said. “There’s no reason you have to be sitting for an hour at lunch with a sales rep and not a brown-bag lunch getting a 1-hour update from a society on a new drug indication.
“We will get higher-quality information if we’re doing the education ourselves, and we’d be removing the sense of obligation to pay back the industry gift givers,” Dr. Mitchell said.
Dr. Onel said among the most critical work is making sure that the leadership of ACR is free of conflicts of interest.
“They are making decisions for the entire organization. We need to feel safe and comfortable that they are acting in the best interest of the rheumatologists and patients versus corporate partners,” she said.
Guidelines must strictly follow the standards from the Institute of Medicine that fewer than 50% of the regular guideline committee members may have commercial conflicts, Dr. Onel said.
She added that training on real or apparent conflicts of interest must start in medical school, residency, and fellowship, before physicians start to think they know their own practice patterns and that the results don’t apply to them.
However, the reality is that the industry and physicians will always need each other, she said.
Dr. Onel describes a tension between rheumatologists needing industry’s help for research and education, especially when federal money for research can be difficult to get, and managing those relationships to avoid real or apparent conflicts.
“We want the public’s trust,” she said.
A study coauthor has served on advisory boards of Boehringer Ingelheim (> $10,000) and Gilead Sciences (> $10,000); has served on speakers bureaus for Simply Speaking (> $10,000) and Boehringer Ingelheim (< $10,000); and has received royalties from UpToDate (> $10,000). Dr. Mitchell and Dr. Onel reported no relevant financial relationships.
* Update, 2/25/22: This article, which originally attributed comments to Dr. Karen Onel personally, has been updated to further emphasize that these comments were not on behalf of the committee that she leads.
A version of this article first appeared on Medscape.com.
Payments to rheumatologists by pharmaceutical companies, whether through food and beverages or consulting fees, are linked with a higher likelihood of prescribing drugs and higher Medicare spending, according to a new study published Feb. 2 in Mayo Clinic Proceedings.
Alí Duarte-García, MD, of the division of rheumatology in the department of medicine at the Mayo Clinic in Rochester, Minn., led the study.
Researchers conducted a cross-sectional analysis of Medicare Part B Public Use File, Medicare Part D Public Use File, and Open Payments data for 2013-2015. They included prescription drugs that accounted for 80% of the total Medicare pharmaceutical expenditures in rheumatology.
They then calculated annual average drug cost per beneficiary per year, the percentage of rheumatologists who received payments, and the average payment per physician per drug per year. Industry payments were categorized as either food/beverage or consulting/compensation.
Multivariable regression models were used to assess links between industry payments, prescribing patterns, and prescription drug spending.
‘Directly associated’ with prescribing probability
The authors concluded that “pharmaceutical company payments to rheumatologists were directly associated with the probability of the physician’s prescribing a drug marketed by that company, the proportion of prescriptions that are for that drug, and the resulting Medicare expenditures.”
The authors noted that food and beverage payments were associated with increased Medicare reimbursement amounts for all drugs except rituximab.
The article includes examples that help quantify the link between payments and prescribing.
The authors wrote that for each $100 in food/beverage payments, Medicare reimbursement increased 6% to 44%. The increases were particularly high for infliximab and rACTH (repository corticotropin injection), “whereby a payment of $100 to a prescriber of these drugs was associated with increases of approximately $72,000 and $30,000 in Medicare reimbursements, respectively. For most of the other drugs, for every $100 in payments, the Medicare reimbursement increased by $8,000 to $13,000.”
The associations were strongest for food and beverage payments even though those were for lower dollar amounts.
The researchers found that every $100 in food and beverage payments, which also include gifts, entertainment, or educational materials, was linked with more than twofold higher probability of prescribing and higher Medicare reimbursements than for every $1,000 paid in consulting fees and compensation.
That finding and findings from previous studies, they say, “suggest that the current approach to regulating industry influence, which focuses on disclosure of large payments, may be inadequate.”
Aaron P. Mitchell, MD, a medical oncologist and health services researcher with Memorial Sloan Kettering Cancer Center in New York, told this news organization that the findings regarding rACTH show the potential harm of industry payments that are linked with prescribing.
The drug is a naturally produced hormone used for both rheumatologic and neurologic conditions. “Since it is an unpatentable drug – really just a hormone – it’s kind of grandfathered in and never had to get [Food and Drug Administration] approval for its current indications,” Dr. Mitchell said.
The drug, which costs nearly $230,000 per Medicare beneficiary, hasn’t been tested head to head in large randomized trials against much cheaper rheumatology drugs, such as prednisone.
In the case of infliximab, he noted, for every $100 in food/beverage payments, there was an increase of $72,000 in Medicare spending.
Dr. Mitchell studies industry influence in a variety of specialties and said rheumatology follows the universal pattern, although the drugs used in the specialty are more expensive than in many specialties.
He agrees with the authors that focusing on disclosures to address the problem is probably not enough.
“We’ve been in this period of full and open disclosure for going on 10 years, and we haven’t really seen a decline in these payments,” he said.
Results won’t surprise rheumatologists
Karen Onel, MD, a pediatric rheumatologist at the Hospital for Special Surgery, New York, and chair of the American College of Rheumatology’s Ethics and Conflict of Interest Committee, who said she was speaking only for herself and not on behalf of the committee, told this news organization that the study will not be surprising to rheumatologists or to physicians in other specialties who are aware of the plethora of studies that concluded that industry payments influence prescribing.*
Physicians are well aware of the problem, but “they all think it’s not them,” she said.
She said a shortcoming of this study is that it is unclear what the payments were for, because many things are lumped together in the categories. In the case of food and beverage payments, she said that could include payments physicians don’t realize are going on their open payments profile.
Dr. Onel gives a personal example. When she goes to a medical conference, she says, “I don’t eat on pharma’s dime.” But because badge numbers are scanned, it appears she accepted the food provided by the pharmaceutical sponsor when she enters a room where food or coffee is being served.
She said that although there are outliers like rACTH that often get highlighted, “the numbers are very low of the physicians who had [industry] money, and the change in prescribing patterns actually was very low.”
She pointed to an important limitation that the authors list: The study was limited to patients with fee-for-service Medicare coverage and did not include those with private insurance.
“For example, Aetna, Cigna, and UnitedHealthcare have restricted reimbursement for rACTH in recent years, citing its lack of proven efficacy and the availability of more affordable options, and rACTH is not in the Veterans Affairs formulary,” the authors wrote.
Rapid development of specialty drugs
Still, the authors wrote, the associations described in this article are of high interest because even though rheumatologists make up a small proportion of the physician workforce, they have among the highest costs per prescription in Medicare drug claims.
“Rheumatology, second only to oncology, has entered an era of rapid development of specialty drugs, including biologic disease-modifying antirheumatic drugs (DMARDs),” the authors wrote.
The complex drugs are expensive to produce and require expertise in handling. Most are under patent with no generic or approved biosimilar equivalent.
Nearly half of all U.S. rheumatologists received some payment from a pharmaceutical company in the study period, and most payments to rheumatologists were for low dollar amounts.
The highest per-year annual expenditures were attributed to etanercept ($741 million), adalimumab ($620 million), and infliximab ($539 million).
These drugs were expensive per beneficiary ($20,728 for etanercept, $21,492 for adalimumab, and $15,941 for infliximab) and were prescribed by a large number of rheumatologists (4,068, 3,872, and 1,349, respectively).
Addressing the problem
Dr. Mitchell says the leaders of individual societies need to fill the gaps that leave busy clinicians searching for easy ways to get up-to-date information on drugs.
He says in all specialties, “Industry should not be the most easily and readily available source of information.
“We’re in the age of Zoom now,” he said. “There’s no reason you have to be sitting for an hour at lunch with a sales rep and not a brown-bag lunch getting a 1-hour update from a society on a new drug indication.
“We will get higher-quality information if we’re doing the education ourselves, and we’d be removing the sense of obligation to pay back the industry gift givers,” Dr. Mitchell said.
Dr. Onel said among the most critical work is making sure that the leadership of ACR is free of conflicts of interest.
“They are making decisions for the entire organization. We need to feel safe and comfortable that they are acting in the best interest of the rheumatologists and patients versus corporate partners,” she said.
Guidelines must strictly follow the standards from the Institute of Medicine that fewer than 50% of the regular guideline committee members may have commercial conflicts, Dr. Onel said.
She added that training on real or apparent conflicts of interest must start in medical school, residency, and fellowship, before physicians start to think they know their own practice patterns and that the results don’t apply to them.
However, the reality is that the industry and physicians will always need each other, she said.
Dr. Onel describes a tension between rheumatologists needing industry’s help for research and education, especially when federal money for research can be difficult to get, and managing those relationships to avoid real or apparent conflicts.
“We want the public’s trust,” she said.
A study coauthor has served on advisory boards of Boehringer Ingelheim (> $10,000) and Gilead Sciences (> $10,000); has served on speakers bureaus for Simply Speaking (> $10,000) and Boehringer Ingelheim (< $10,000); and has received royalties from UpToDate (> $10,000). Dr. Mitchell and Dr. Onel reported no relevant financial relationships.
* Update, 2/25/22: This article, which originally attributed comments to Dr. Karen Onel personally, has been updated to further emphasize that these comments were not on behalf of the committee that she leads.
A version of this article first appeared on Medscape.com.
When physicians are the plaintiffs
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Have you experienced malpractice?
No, I’m not asking whether you have experienced litigation. I’m asking whether you, as a physician, have actually experienced substandard care from a colleague. I have heard many such experiences over the years, and mistreatment doesn’t seem to be getting any less frequent.
The first is that, unlike the Pope, who has a dedicated confessor trained to minister to his spiritual needs, no one formally trains physicians to treat physicians. As a result, most of us feel slightly uneasy at treating other physicians. We naturally wish to keep our colleagues well, but at the same time realize that our clinical skills are being very closely scrutinized. What if they are found to be wanting? This discomfiture can make a physician treating a physician overly compulsive, or worse, overtly dismissive.
Second, we physicians are famously poor patients. We pretend we don’t need the advice we give others, to monitor our health and promptly seek care when something feels amiss. And, for the period during which we delay a medical encounter, we often attempt to diagnose and treat ourselves.
Sometimes we are successful, which reinforces this approach. Other times, we fail at being our own caregiver and present to someone else either too late, or with avoidable complications. In the former instance, we congratulate ourselves and learn nothing from the experience. In the latter, we may heap shame upon ourselves for our folly, and we may learn; but it could be a lethal lesson. In the worst scenario, our colleague gives in to frustration (or angst), and heaps even more shame onto their late-presenting physician patient.
Third, when we do submit to being a patient, we often demand VIP treatment. This is probably in response to our anxiety that some of the worst things we have seen happen to patients might happen to us if we are not vigilant to ensure we receive a higher level of care. But of course, such hypervigilance can lead to excessive care and testing, with all the attendant hazards, or alternatively to dilution of care if our caregivers decide we are just too much trouble.
Fourth, as a fifth-generation physician myself, I am convinced that physicians and physician family members are either prone to unusual manifestations of common diseases or unusual diseases, or that rare disease entities and complications are actually more common than literature suggests, and they simply aren’t pursued or diagnosed in nonphysician families.
No matter how we may have arrived in a position to need medical care, how often is such care substandard? And how do we respond when we suspect, or know, this to be the case? Are physicians more, or less, likely to take legal action in the face of it?
I certainly don’t know any statistics. Physicians are in an excellent position to take such action, because judges and juries will likely believe that a doctor can recognize negligence when we fall victim to it. But we may also be reluctant to publicly admit the way (or ways) in which we may have contributed to substandard care or outcome.
Based on decades of working with physician clients who have been sued, and having been sued myself (thus witnessing and also experiencing the effects of litigation), I am probably more reluctant than normal patients or physicians to consider taking legal action. This, despite the fact that I am also a lawyer and (through organized medicine) know many colleagues in all specialties who might serve as expert witnesses.
I have experienced serial substandard care, which has left me highly conflicted about the efficacy of my chosen profession. As a resident, I had my first odd pain condition and consulted an “elder statesperson” from my institution, whom I assumed to be a “doctor’s doctor” because he was a superb teacher (wrong!)
He completely missed the diagnosis and further belittled (indeed, libeled) me in the medical record. (Some years later, I learned that, during that period, he was increasingly demented and tended to view all female patients as having “wandering uterus” equivalents.) Fortunately, I found a better diagnostician, or at least one more willing to lend credence to my complaints, who successfully removed the first of several “zebra” lesions I have experienced.
As a young faculty member, I had an odd presentation of a recurring gynecologic condition, which was treated surgically, successfully, except that my fertility was cut in half – a possibility about which I had not been informed when giving operative consent. Would I have sued this fellow faculty member for that? Never, because she invariably treated me with respect as a colleague.
Later in my career after leaving academia, the same condition recurred in a new location. My old-school gynecologist desired to do an extensive procedure, to which I demurred unless specific pathology was found intraoperatively. Affronted, he subjected me to laparoscopy, did nothing but look, and then left the hospital leaving me and the PACU nurse to try to decipher his instructions (which said, basically, “I didn’t find anything; don’t bother me again.”). Several years of pain later, a younger gynecologist performed the correct procedure to address my problem, which has never recurred. Would I have sued him? No, because I believe he had a disability.
At age 59, I developed a new mole. My beloved general practitioner, in the waning years of his practice, forgot to consult a colleague to remove it for several months. When I forced the issue, the mole was removed and turned out to be a rare pediatric condition considered a precursor to melanoma. The same general practitioner had told me I needn’t worry about my “mild hypercalcemia.”
Ten years later I diagnosed my own parathyroid adenoma, in the interim losing 10% of my bone density. Would I have sued him? No, for he always showed he cared. (Though maybe, if I had fractured my spine or hip.)
If you have been the victim of physician malpractice, how did you respond?
Do we serve our profession well by how we handle substandard care – upon ourselves (or our loved ones)?
Dr. Andrew is a former assistant professor in the department of emergency medicine, Johns Hopkins University, Baltimore, and founder and principal of MDMentor, Victoria, B.C.
A version of this article first appeared on Medscape.com.
Cardiologist whistleblower lawsuit settled for $3.8 million
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Catholic Medical Center has agreed to pay $3.8 million to settle claims it provided free call coverage to a cardiologist in exchange for patient referrals to the Manchester, N.H., hospital, according to federal officials.
“The cardiologist who received the free call coverage referred millions of dollars in medical procedures and services to CMC over the decade in which the free services were provided,” the Department of Justice said in a news release.
Because the hospital submitted claims for payment to Medicare, Medicaid, and other federal health care programs for the services referred by the cardiologist, the government alleged the claims were the result of unlawful kickbacks.
The settlement resolves allegations brought in a whistleblower lawsuit filed in 2018 by cardiologist David Goldberg, MD, who previously worked at Catholic Medical Center (CMC) and is represented by Douglas, Leonard & Garvey.
The news release did not name the cardiologist involved in the alleged kickback scheme but the recently unsealed lawsuit says CMC paid its cardiologists above market rates ($10,000 per weekend, $3,000 per night) to provide free coverage services for Mary-Claire Paicopolis, MD.
The lawsuit also claims Dr. Paicopolis insisted the hospital implant only Boston Scientific devices in her patients and that her preferred electrophysiologist use only its Rhythmia mapping system during ablation procedures. To keep CMC from objecting, the suit alleges Boston Scientific offered CMC early access to its Watchman left atrial appendage occluder and provided “unprecedented” support to a nonacademic community hospital site.
“It went back several years, and that and the other issues in the suit were strong motivators for Dr. Goldberg to try to rectify the situation and he deserves a lot of credit for having done so,” attorney Charles G. Douglas III told this news organization.
Dr. Goldberg will receive $570,000 of the $3.8 million settlement as well as $145,361 in expenses, attorney fees, and costs.
Although not addressed in the federal news release, the lawsuit also alleges that CMC staff manipulated mortality data by discharging patients from the ICU and then readmitting them to hospice with a new patient number, “thereby avoiding the need to claim a surgical mortality.”
The lawsuit also says CMC “created a practice of covering up medical errors” and detailed 12 patient deaths between 2012 and 2018, alleging that these deaths were the result of substandard care.
CMC spokesperson Lauren Collins-Cline said in an email that the call coverage arrangement is no longer in place and originated almost 15 years ago with the input of legal counsel in order to provide high-quality care for patients.
“While CMC vigorously disagrees with the government’s allegations that this arrangement violated federal law, we have agreed to settle in order to avoid long costly civil litigation,” she said.
As to the other claims in the complaint, Ms. Collins-Cline said they were investigated by the government and dismissed per the settlement agreement. “CMC holds itself to the highest ethical standards in patient care and business conduct. That’s embedded in our mission and will always remain our highest priority.”
Mr. Douglas, however, said the government retains the right to pursue other claims in the lawsuit in the future. “So, [the hospital] is a little more optimistic than the reality of what the government agrees is the situation.”
A version of this article first appeared on Medscape.com.
Why dermatologists should support artificial intelligence efforts
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
FROM ODAC 2022
Doc says sobbing attorney and crying medical expert led to unfair million-dollar verdict
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Twenty-three percent of health care workers likely to leave industry soon: Poll
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.










