User login
The federal government paid private doctors twice by mistake for veterans’ care
The U.S. federal government wrote duplicate checks to private doctors who treated veterans, costing taxpayers up to $128 million in extra payments over 5 years, a new report by a federal watchdog revealed in April.
Private doctors were paid twice in nearly 300,000 cases from 2017 to 2021 involving veterans who were eligible for Veterans Health Administration and Medicare benefits, according to the report by the Health & Human Services Office of Inspector General.
The doctors were paid by Medicare for medical services that the VHA had authorized and already paid for, the OIG reported after it conducted a 5-year audit.
Duplicate Medicare payments have doubled from $22 million in 2019 when the Veterans Community Care Program was implemented to $45 million in 2021, according to the OIG report. The program allows veterans to seek care from private doctors when the VHA can’t provide the care they need.
Roughly 1.9 million veterans every year receive government-paid health care from private doctors.
The OIG said it decided to audit Medicare’s claims because “duplicate payments were a long-standing issue.”
The problem dates back to a 1979 General Accounting Office (now the Government Accountability Office) report that found Medicare and the Department of Veterans Affairs VHA made duplicate payments of more than $72,000 for certain medical services provided to veterans, the OIG reported.
The HHS OIG’s audit examined $19.2 billion in Medicare payments for 36 million claims for individuals who enrolled in Medicare and were eligible for VA services. About 90% of those claims were for doctor evaluations and visits, according to the OIG report.
The OIG found “these duplicate payments occurred because CMS did not implement controls to address duplicate payments for services provided to individuals with Medicare and VHA benefits.”
Specifically, the OIG found that the CMS and the VHA were not sharing enrollment, claims, and payment data with each other, as required by federal law.
If CMS had access to that information, the agency could have compared the VHA claims data with existing Medicare claims data to identify duplicate claims, the OIG claimed.
The OIG recommended that CMS take the following four steps to fix the problem, which CMS has agreed to do, according to the report:
- Integrate VHA enrollment, claims, and payment data into the CMS centralized claims data system so it can identify potential fraud, waste, and abuse under the Medicare program.
- Issue guidance to medical professionals on not billing Medicare for a medical service that was authorized by the VHA.
- Establish a comprehensive data-sharing agreement with the VHA.
- Establish an internal process (such as system edits) to address duplicate payments.
“CMS previously informed [the OIG] that establishing a long-term solution to address duplicate payments will take time,” the OIG reported.
A version of this article first appeared on Medscape.com.
The U.S. federal government wrote duplicate checks to private doctors who treated veterans, costing taxpayers up to $128 million in extra payments over 5 years, a new report by a federal watchdog revealed in April.
Private doctors were paid twice in nearly 300,000 cases from 2017 to 2021 involving veterans who were eligible for Veterans Health Administration and Medicare benefits, according to the report by the Health & Human Services Office of Inspector General.
The doctors were paid by Medicare for medical services that the VHA had authorized and already paid for, the OIG reported after it conducted a 5-year audit.
Duplicate Medicare payments have doubled from $22 million in 2019 when the Veterans Community Care Program was implemented to $45 million in 2021, according to the OIG report. The program allows veterans to seek care from private doctors when the VHA can’t provide the care they need.
Roughly 1.9 million veterans every year receive government-paid health care from private doctors.
The OIG said it decided to audit Medicare’s claims because “duplicate payments were a long-standing issue.”
The problem dates back to a 1979 General Accounting Office (now the Government Accountability Office) report that found Medicare and the Department of Veterans Affairs VHA made duplicate payments of more than $72,000 for certain medical services provided to veterans, the OIG reported.
The HHS OIG’s audit examined $19.2 billion in Medicare payments for 36 million claims for individuals who enrolled in Medicare and were eligible for VA services. About 90% of those claims were for doctor evaluations and visits, according to the OIG report.
The OIG found “these duplicate payments occurred because CMS did not implement controls to address duplicate payments for services provided to individuals with Medicare and VHA benefits.”
Specifically, the OIG found that the CMS and the VHA were not sharing enrollment, claims, and payment data with each other, as required by federal law.
If CMS had access to that information, the agency could have compared the VHA claims data with existing Medicare claims data to identify duplicate claims, the OIG claimed.
The OIG recommended that CMS take the following four steps to fix the problem, which CMS has agreed to do, according to the report:
- Integrate VHA enrollment, claims, and payment data into the CMS centralized claims data system so it can identify potential fraud, waste, and abuse under the Medicare program.
- Issue guidance to medical professionals on not billing Medicare for a medical service that was authorized by the VHA.
- Establish a comprehensive data-sharing agreement with the VHA.
- Establish an internal process (such as system edits) to address duplicate payments.
“CMS previously informed [the OIG] that establishing a long-term solution to address duplicate payments will take time,” the OIG reported.
A version of this article first appeared on Medscape.com.
The U.S. federal government wrote duplicate checks to private doctors who treated veterans, costing taxpayers up to $128 million in extra payments over 5 years, a new report by a federal watchdog revealed in April.
Private doctors were paid twice in nearly 300,000 cases from 2017 to 2021 involving veterans who were eligible for Veterans Health Administration and Medicare benefits, according to the report by the Health & Human Services Office of Inspector General.
The doctors were paid by Medicare for medical services that the VHA had authorized and already paid for, the OIG reported after it conducted a 5-year audit.
Duplicate Medicare payments have doubled from $22 million in 2019 when the Veterans Community Care Program was implemented to $45 million in 2021, according to the OIG report. The program allows veterans to seek care from private doctors when the VHA can’t provide the care they need.
Roughly 1.9 million veterans every year receive government-paid health care from private doctors.
The OIG said it decided to audit Medicare’s claims because “duplicate payments were a long-standing issue.”
The problem dates back to a 1979 General Accounting Office (now the Government Accountability Office) report that found Medicare and the Department of Veterans Affairs VHA made duplicate payments of more than $72,000 for certain medical services provided to veterans, the OIG reported.
The HHS OIG’s audit examined $19.2 billion in Medicare payments for 36 million claims for individuals who enrolled in Medicare and were eligible for VA services. About 90% of those claims were for doctor evaluations and visits, according to the OIG report.
The OIG found “these duplicate payments occurred because CMS did not implement controls to address duplicate payments for services provided to individuals with Medicare and VHA benefits.”
Specifically, the OIG found that the CMS and the VHA were not sharing enrollment, claims, and payment data with each other, as required by federal law.
If CMS had access to that information, the agency could have compared the VHA claims data with existing Medicare claims data to identify duplicate claims, the OIG claimed.
The OIG recommended that CMS take the following four steps to fix the problem, which CMS has agreed to do, according to the report:
- Integrate VHA enrollment, claims, and payment data into the CMS centralized claims data system so it can identify potential fraud, waste, and abuse under the Medicare program.
- Issue guidance to medical professionals on not billing Medicare for a medical service that was authorized by the VHA.
- Establish a comprehensive data-sharing agreement with the VHA.
- Establish an internal process (such as system edits) to address duplicate payments.
“CMS previously informed [the OIG] that establishing a long-term solution to address duplicate payments will take time,” the OIG reported.
A version of this article first appeared on Medscape.com.
Federal rules don’t require period product ingredients on packaging labels. States are stepping in.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Tens of millions of Americans use menstrual products, and while manufacturers contend they are safe, most disclose little about the chemicals they contain. Now, amid calls for more disclosure and research into the health effects of these products, some states require more transparency.
The manufacture and sale of period and related products is a big business, with revenue expected to top $4.5 billion in the United States this year. On average, a person uses up to 17,000 tampons or pads in their lifetime, and they might also use rubber or silicone cups, or absorbent period underwear.
The FDA regulates and classifies menstrual products as medical devices, meaning they are not subject to the same labeling laws as other consumer items. But companies can voluntarily disclose what’s in their products.
Now, some states are stepping into the breach. In 2021, New York became the first state to enact a menstrual product disclosure law requiring companies to list all intentionally added ingredients on packaging. California’s governor signed a similar law that took effect this year, but it gives manufacturers trade secret protections, so not all ingredients are necessarily disclosed. At least six other states have introduced legislation to address safety and disclosure of ingredients in these products.
Shruthi Mahalingaiah, an assistant professor of environmental, reproductive, and women’s health at Harvard University, Boston, evaluates endocrine disruptors in personal care products and studies menstrual health. She said the health risk depends on the dose, duration, and sensitivity of a person to the ingredients and their mixtures.
Harmful chemicals could come from manufacturing processes, through materials and shipping, from equipment cleaners, from contact with contaminants, or from companies adding them intentionally, said Alexandra Scranton, director of science and research for Women’s Voices for the Earth, a Montana-based nonprofit focused on eliminating toxic chemicals that affect women’s health.
Vaginal and vulvar tissues are capable of absorbing fluids at a higher rate than skin, which can lead to rapid chemical exposure. Ms. Scranton said scarcity of clinical studies and funding for vaginal health research limits understanding about the long-term effects of the ingredients and additives in period products.
“We think manufacturers should do better and be more careful with the ingredients they choose to use,” Ms. Scranton said. “The presence of toxic and hormone-disrupting chemicals in menstrual products is unsettling. We know that chemicals can cause disease, and exposures do add up over time.”
Ms. Scranton’s organization advocates for labels to include the chemical name of the ingredient, the component in which the ingredient is used, and the function of the ingredient.
K. Malaika Walton, operations director for the Center for Baby and Adult Hygiene Products, a trade industry group, said in an email, “BAHP supports accurate and transparent information for users of period products and many of our member companies list ingredients on their packages and websites.”
In a written statement, Procter & Gamble, a major manufacturer of menstrual products, said that ingredients it uses go through rigorous safety evaluations and are continuously tested, and that all fragrance components are added at levels the industry considers safe.
Even though manufacturing of scented tampons for the U.S. market has mostly stopped, companies still use fragrances in other menstrual products. Laws protecting trade secrets keep details about fragrances in pads and tampons confidential so competitors can’t copy the formulas. The Children’s Environmental Health Network lists phthalates, a group of chemicals commonly called plasticizers, that are suspected hormone disruptors, as an ingredient found in fragrances.
Manufacturers follow regulatory guidance issued in 2005 by registering with the Food and Drug Administration and submitting a detailed risk assessment of their products’ components and design, and a safety profile, before being cleared to sell in the United States.
Pads and menstrual cups are considered exempt from regulatory guidance and do not require premarket review, according to FDA spokesperson Carly Kempler. While tampons do require review, the FDA “does not clear or approve individual materials that are used in the fabrication of medical devices.”
“There’s an understanding that the FDA is regulating these products, and they are; it’s just not very adequate,” said Laura Strausfeld, an attorney and a cofounder of Period Law, an organization working to advance state and federal period-equity policies that would stop taxation of products and make them freely available in places like schools and prisons. “The consumer is supposed to trust that when these products are put on shelves they’ve been vetted by the government. But it’s basically a rubber stamp.”
In a 2022 report, a congressional committee directed the FDA to update its guidance for menstrual products to recommend that labels disclose intentionally added ingredients, such as fragrances, and test for contaminants. The FDA is reviewing the directives outlined by the House Appropriations Committee and will update the 2005 guidance as soon as possible, Ms. Kempler said. “We will share additional details when we are able to.”
At least one period product company makes disclosure of its ingredients a selling point. Alex Friedman, cofounder of Lola, said a lack of knowledge is a problem, and more action and awareness are needed to keep people safe.
“The hardest part to swallow is why this is even up for debate. We should all know what’s in these products,” Ms. Friedman said.
New York’s law requires companies to disclose all intentionally added ingredients no matter how much is used, with no trade secret protections for fragrances. Though it applies only to products sold in that state, similar detailed labeling is appearing elsewhere, advocates said.
“We’re also seeing similar or identical disclosure on packaging in other states outside of New York, which is a testament to the power of the law,” said Jamie McConnell, deputy director of Women’s Voices for the Earth.
Manufacturers have 18 months from the passage of the New York law to comply, and some products on shelves in New York still list few ingredients other than “absorbent material,” “surfactant,” “ink,” and “adhesive.”
“We’re like, ‘OK, what is that exactly?’ ” Ms. McConnell said.
Her organization is calling for a federal law at least as strong as New York’s. Previous federal legislation failed to advance, including the most recent, the Menstrual Products Right to Know Act, introduced in 2022.
BAHP, the trade group, supported the federal legislation and the California law. Ms. McConnell said she opposed both bills because they didn’t require companies to list all fragrance ingredients.
“I think what it boiled down to at the federal level was the support of corporate interests over public health,” she said.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Medical-level empathy? Yup, ChatGPT can fake that
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Malaria: Not just someone else’s problem
What is the most dangerous animal on Earth? Which one has killed more humans since we first began walking upright?
The mind leaps to the vicious and dangerous – great white sharks. lions. tigers. crocodiles. The fearsome predators of the planet But realistically, more people are killed and injured by large herbivores each year than predators. Just watch news updates from Yellowstone during their busy season.
Anyway, the correct answer is ... none of the above.
It’s the mosquito, and the many microbes it’s a vector for. Malaria, in particular. Even the once-devastating bubonic plague is no longer a major concern.
What do Presidents Washington, Kennedy, Eisenhower, Lincoln, Monroe, Grant, Garfield, Jackson, Teddy Roosevelt, and other historical VIPs like Oliver Cromwell, King Tut, and numerous kings, queens, and popes all have in common? They all had malaria. Cromwell, Tut, and many royal and religious figures died of it.
You can make a solid argument that malaria is the disease that’s affected the course of history more than any other (you could make a good case for the plague, too, but it’s less relevant today). The control of malaria is what allowed the Panama canal to happen.
I’m bringing this up because, mostly overlooked in the news recently as we argued about light beer endorsements, TV pundits, and the NFL draft, is the approval and gradual increase in use of a malaria vaccine.
This is a pretty big deal given the scope of the problem and the fact that the most effective prevention up until recently was a mosquito net.
We tend to see malaria as someone else’s problem, something that affects the tropics, but forget that as recently as the 1940s it was still common in the U.S. During the Civil War as many as 1 million soldiers were infected with it. Given the right conditions it could easily return here.
Which is why we should be more aware of these things. As COVID showed, infectious diseases are never some other country’s, or continent’s, problem. They affect all of us either directly or indirectly. In the interconnected economies of the world illnesses in one area can spread to others. Even if they don’t they can still have significant effects on supply chains, since so much of what we depend on comes from somewhere else.
COVID, by comparison, is small beer. Just think about smallpox, or the plague, or polio, as to what an unchecked disease can do to a society until medicine catches up with it.
There will always be new diseases. Microbes and humans have been in a state of hostilities for a few million years now, and likely always will be. But every victory along the way is a victory for everyone, regardless of who they are or where they live.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What is the most dangerous animal on Earth? Which one has killed more humans since we first began walking upright?
The mind leaps to the vicious and dangerous – great white sharks. lions. tigers. crocodiles. The fearsome predators of the planet But realistically, more people are killed and injured by large herbivores each year than predators. Just watch news updates from Yellowstone during their busy season.
Anyway, the correct answer is ... none of the above.
It’s the mosquito, and the many microbes it’s a vector for. Malaria, in particular. Even the once-devastating bubonic plague is no longer a major concern.
What do Presidents Washington, Kennedy, Eisenhower, Lincoln, Monroe, Grant, Garfield, Jackson, Teddy Roosevelt, and other historical VIPs like Oliver Cromwell, King Tut, and numerous kings, queens, and popes all have in common? They all had malaria. Cromwell, Tut, and many royal and religious figures died of it.
You can make a solid argument that malaria is the disease that’s affected the course of history more than any other (you could make a good case for the plague, too, but it’s less relevant today). The control of malaria is what allowed the Panama canal to happen.
I’m bringing this up because, mostly overlooked in the news recently as we argued about light beer endorsements, TV pundits, and the NFL draft, is the approval and gradual increase in use of a malaria vaccine.
This is a pretty big deal given the scope of the problem and the fact that the most effective prevention up until recently was a mosquito net.
We tend to see malaria as someone else’s problem, something that affects the tropics, but forget that as recently as the 1940s it was still common in the U.S. During the Civil War as many as 1 million soldiers were infected with it. Given the right conditions it could easily return here.
Which is why we should be more aware of these things. As COVID showed, infectious diseases are never some other country’s, or continent’s, problem. They affect all of us either directly or indirectly. In the interconnected economies of the world illnesses in one area can spread to others. Even if they don’t they can still have significant effects on supply chains, since so much of what we depend on comes from somewhere else.
COVID, by comparison, is small beer. Just think about smallpox, or the plague, or polio, as to what an unchecked disease can do to a society until medicine catches up with it.
There will always be new diseases. Microbes and humans have been in a state of hostilities for a few million years now, and likely always will be. But every victory along the way is a victory for everyone, regardless of who they are or where they live.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What is the most dangerous animal on Earth? Which one has killed more humans since we first began walking upright?
The mind leaps to the vicious and dangerous – great white sharks. lions. tigers. crocodiles. The fearsome predators of the planet But realistically, more people are killed and injured by large herbivores each year than predators. Just watch news updates from Yellowstone during their busy season.
Anyway, the correct answer is ... none of the above.
It’s the mosquito, and the many microbes it’s a vector for. Malaria, in particular. Even the once-devastating bubonic plague is no longer a major concern.
What do Presidents Washington, Kennedy, Eisenhower, Lincoln, Monroe, Grant, Garfield, Jackson, Teddy Roosevelt, and other historical VIPs like Oliver Cromwell, King Tut, and numerous kings, queens, and popes all have in common? They all had malaria. Cromwell, Tut, and many royal and religious figures died of it.
You can make a solid argument that malaria is the disease that’s affected the course of history more than any other (you could make a good case for the plague, too, but it’s less relevant today). The control of malaria is what allowed the Panama canal to happen.
I’m bringing this up because, mostly overlooked in the news recently as we argued about light beer endorsements, TV pundits, and the NFL draft, is the approval and gradual increase in use of a malaria vaccine.
This is a pretty big deal given the scope of the problem and the fact that the most effective prevention up until recently was a mosquito net.
We tend to see malaria as someone else’s problem, something that affects the tropics, but forget that as recently as the 1940s it was still common in the U.S. During the Civil War as many as 1 million soldiers were infected with it. Given the right conditions it could easily return here.
Which is why we should be more aware of these things. As COVID showed, infectious diseases are never some other country’s, or continent’s, problem. They affect all of us either directly or indirectly. In the interconnected economies of the world illnesses in one area can spread to others. Even if they don’t they can still have significant effects on supply chains, since so much of what we depend on comes from somewhere else.
COVID, by comparison, is small beer. Just think about smallpox, or the plague, or polio, as to what an unchecked disease can do to a society until medicine catches up with it.
There will always be new diseases. Microbes and humans have been in a state of hostilities for a few million years now, and likely always will be. But every victory along the way is a victory for everyone, regardless of who they are or where they live.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Getting a white-bagging exemption: A win for the patient, employer, and rheumatologist
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Whether it’s filling out a prior authorization form or testifying before Congress, it is an action we perform that ultimately helps our patients achieve that care. We are familiar with many of the obstacles that block the path to the best care and interfere with our patient-doctor relationships. Much work has been done to pass legislation in the states to mitigate some of those obstacles, such as unreasonable step therapy regimens, nonmedical switching, and copay accumulators.
Unfortunately, that state legislation does not cover patients who work for companies that are self-insured. Self-insured employers, which account for about 60% of America’s workers, directly pay for the health benefits offered to employees instead of buying “fully funded” insurance plans. Most of those self-funded plans fall under “ERISA” protections and are regulated by the federal Department of Labor. ERISA stands for Employee Retirement Income Security Act. The law, which was enacted in 1974, also covers employee health plans. These plans must act as a fiduciary, meaning they must look after the well-being of the employees, including their finances and those of the plan itself.
The Coalition of State Rheumatology Organizations (CSRO) has learned of a number of issues involving patients who work for self-funded companies, regulated by ERISA. One such issue is that of mandated “white bagging.” White bagging has been discussed in “Rheum for Action” in the past. There is a long list of white-bagging problems, including dosing issues, lack of “chain of custody” with the medications, delays in treatment, mandatory up-front payments by the patient, and wastage of unused medication. However, there is another issue that is of concern not only to the employees (our patients) but to the employer as well.
Employers’ fiduciary responsibility
As mentioned earlier, the employers who self insure are responsible for the financial well-being of their employee and the plan itself. Therefore, if certain practices are mandated within the health plan that harm our patients or the plan financially, the company could be in violation of their fiduciary duty. Rheumatologists have said that buying and billing the drug to the medical side of the health plan in many cases costs much less than white bagging. Conceivably, that could result in breach of an employer’s fiduciary duty to their employee.
Evidence for violating fiduciary duty
CSRO recently received redacted receipts comparing costs between the two models of drug acquisition for a patient in an ERISA plan. White bagging for the patient occurred in 2021, and in 2022 an exemption was granted for the rheumatologist to buy and bill the administered medication. Unfortunately, the exemption to buy and bill in 2023 was denied and continues to be denied (as of this writing). A comparison of the receipts revealed the company was charged over $40,000 for the white-bagged medication in 2021, and the patient’s cost share for that year was $525. Under the traditional buy-and-bill acquisition model in 2022, the company was charged around $12,000 for the medication and the patient’s cost share was $30. There is a clear difference in cost to the employee and plan between the two acquisition models.
Is this major company unknowingly violating its fiduciary duty by mandating white bagging as per their contract with one of the three big pharmacy benefit managers (PBMs)? If so, how does something like this happen with a large national company that has ERISA attorneys looking over the contracts with the PBMs?
Why is white bagging mandated?
Often, white bagging is mandated because the cost of infusions in a hospital outpatient facility can be very high. Nationally, it has been shown that hospitals charge four to five times the cost they paid for the drug, and the 100 most expensive hospitals charge 10-18 times the cost of their drugs. With these up-charges, white bagging could easily be a lower cost for employee and company. But across-the-board mandating of white bagging ignores that physician office–based infusions may offer a much lower cost to employees and the employer.
Another reason large and small self-funded companies may unknowingly sign contracts that are often more profitable to the PBM than to the employer is that the employer pharmacy benefit consultants are paid handsomely by the big PBMs and have been known to “rig” the contract in favor of the PBM, according to Paul Holmes, an ERISA attorney with a focus in pharmacy health plan contracts. Clearly, the PBM profits more with white-bagged medicines billed through the pharmacy (PBM) side of insurance as opposed to buy-and-bill medications that are billed on the medical side of insurance. So mandated white bagging is often included in these contracts, ignoring the lower cost in an infusion suite at a physician’s office.
Suggestions for employers
Employers and employees should be able to obtain the costs of mandated, white-bagged drugs from their PBMs because the Consolidated Appropriations Act of 2021 (CAA) mandates that group health plans ensure access to cost data. The employer should also have access to their consultant’s compensation from the PBM as Section 202 in the CAA states that employer benefit consultants must “disclose actual and anticipated cash and non-cash compensation they expect to earn in connection with the sale, renewal, and extension of group health insurance.”
It would be wise for all self-insured companies to use this section to see how much their consultants are being influenced by the company that they are recommending. Additionally, the companies should consider hiring ERISA attorneys that understand not only the legalese of the contract with a PBM but also the pharmacy lingo, such as the difference between maximum allowable cost, average wholesale price, average sales price, and average manufacturer’s price.
Suggestion for the rheumatologist
This leads to a suggestion to rheumatologists trying to get an exemption from mandated white bagging. If a patient has already had white-bagged medication, have them obtain a receipt from the PBM for their charges to the plan for the medication. If the patient has not gone through the white bagging yet, the PBM should be able to tell the plan the cost of the white-bagged medication and the cost to the patient. Compare those costs with what would be charged through buy and bill, and if it is less, present that evidence to the employer and remind them of their fiduciary responsibility to their employees.
Granted, this process may take more effort than filling out a prior authorization, but getting the white-bag exemption will help the patient, the employer, and the rheumatologist in the long run. A win-win-win!
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Two Canadian provinces lift licensing barriers for U.S. doctors
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
They’ll no longer have to start with a limited license and take additional exams or be supervised for up to a year to become fully licensed.
Canada is experiencing an acute shortage of licensed physicians that’s expected to intensify over the next decade. The shortfall is estimated to be about 44,000 physicians by 2028, with family doctors accounting for 72% of the deficit.
“Reducing licensing barriers should make Canada a more attractive option for U.S. doctors who may be considering a move north,” said Tom Florence, president of AMN Healthcare’s Physician Solutions division, which recruits American physicians to work in Canada.
“Canada also has a truly expedited work visa process for qualifying physicians who have a job offer and wish to practice there,” said Mr. Florence. It usually takes about 6 months compared with at least 18 months for Canadian physicians who want to work in the United States, he said.
Few U.S.-trained physicians work in Canada, which has a population of nearly 39 million. Just 812 of them practiced in Canada in 2019, the last year data was collected, according to the Canadian Medical Association.
But Canada may attract American physicians who find U.S. medicine to be fraught with ethical dilemmas and restrictions from insurance companies and elected officials, said Theresa Rohr-Kirchgraber, MD, an internist and immediate past president of the American Medical Women’s Association.
“Rather than give up practicing medicine, a move to Canada may be a welcome respite for some U.S. physicians,” she said.
Physician recruiters in Ontario and Nova Scotia welcomed the news. About 13% of the population is without a family doctor, according to news reports.
A number of U.S. physicians have started practices in Nova Scotia in recent years, said Katrina Philopoulos, Nova Scotia Health’s director of physician recruitment. “I think this momentum will help us,” she said.
Other Canadian provinces with physician shortages are also considering making similar changes. Alberta recently announced a 5-year pilot project to waive some licensing requirements for family doctors and general practitioners trained in Australia, Ireland, United Kingdom, and the United States.
What are the pros and cons of working in Canada?
“Some U.S. physicians may be attracted by a single-payer system in which all patients have access to coverage, but there are a range of drawbacks and benefits to consider in both systems,” said Mr. Florence.
U.S. physicians generally earn more than their Canadian counterparts, so income is not likely to be a draw, he said.
That appears to be the case for both family medicine physicians and specialists when comparing average net annual salaries. (To obtain Canadian salaries, 2021 gross income data from the Canadian Institute for Health Information were used; 20% was deducted for operation costs; and Canadian dollars were converted into U.S. dollars based on the current exchange rate.)
A family medicine doctor in Canada will earn an annual average salary of $195,853 USD compared with $236,000 in the United States. A cardiologist in Canada will earn $314,051 USD annually compared with $459,000 in the United States. A dermatologist in Canada will earn $270,018 annually compared with $394,000 in the United States.
Everett Fuller, MD, an emergency medicine physician who moved from Texas to Nova Scotia in 2015 for his Canadian wife, recently wrote about the pros and cons of working there compared with the United States. For him, it was a worthwhile move.
“It’s getting back to making medicine and patient care the priority instead of the business of medicine,” Dr. Fuller wrote.
“I have the comfort of knowing that a patient and their family will not go bankrupt trying to pay medical bills if I make a catastrophic diagnosis. There’s no out-of-pocket cost, other than prescriptions (depending on their drug plan).”
Dr. Fuller also doesn’t have to fight insurers for reimbursement or preapprovals, and he pays much less for medical malpractice premiums in a less litigious environment, he said.
But he mentioned a few negatives. Some treatment is rationed, which can lead to long wait times for patients to get appointments. Also, “hospitals aren’t in it for the profit, so you’re not going to get a CT, MRI, and cath lab in every hospital,” he noted.
Mr. Florence doesn’t think either system “offers a panacea for many of the challenges physicians face today. Even with reduced barriers to licensure, we do not anticipate an exodus to U.S. physicians to the north.”
A version of this article first appeared on Medscape.com.
A clash of expectations
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
A few weeks ago I asked what changes would have to occur to return urgent care to its former place under the umbrella of the primary care pediatrician. Several responses that I received and the recent story about screenings in this magazine (April 2023) have prompted me to ask the broader question of what is a pediatrician? More specifically, what is the role of a primary care pediatrician?
I think we can agree that a pediatrician is someone who has dedicated his or her training to learning about and then treating the diseases of children. There are pediatricians whose focus is on newborns. There are others who specialize by organ system or by the intensity of the disease (for example, hospitalists and ED physicians). In Great Britain, and to some extent Canada, “paediatricians” serve primarily as consultants to other health care providers. In this country, however, we tend to think of a pediatrician as a frontline primary care physician with general expertise in children. It is those providers (myself included) to whom I address my questions: “What is our role? What is our primary mission?” Are the expectations that we and others have for us realistic given the realities of 21st-century America? And, is our failure to meet some of those expectations contributing to our burnout?
Are we preventionists? I have always thought that one of the things that sets us apart from other specialties is our focus on prevention. We’ve done a pretty good job with infectious diseases thanks to vaccines and antibiotics. But, when I look at the children who grew to be obese adults under my care I have to say that I and my peers have done an abysmal job of prevention. And that is just one example.
Are we educators responsible for helping parents learn what we consider to be the best child-rearing practices? The Latin root of the word “doctor” means teacher. But, education done well is a very time-consuming process. How many of us have time in the office to really teach? Furthermore, some recent studies on managing vaccine deniers suggests that education doesn’t work with people who have long-held beliefs.
Are we data-entry clerks tasked with documenting our every professional step to validate our value to society and the correctness of our methods? It seems that there are some folks who believe we should be.
Are we screeners? TSA agents with white coats and stethoscopes responsible for screening the entire population for potential threats that weren’t obvious to our thoughtful history taking and careful physical examinations?
And finally, are we healers? If you haven’t already disabused yourself of that myth please take a moment to consider the number of cures you have orchestrated in the last 10 years.
The answer is that we can and maybe should be all of those things but we and those who advise us and support us must have reasonable expectations of how difficult it can be to be all those things to all of our patients in the real world of primary care pediatrics. We aren’t social engineers who can level every inequality nor can we orchestrate changes in a society that leans toward enabling unhealthy lifestyles.
The American Academy of Pediatrics must shoulder some of the blame for this discrepancy between expectations and reality. In the Pediatric News article on screening, Susan Kressly, MD, the chair of the American Academy of Pediatrics’s Section on Administration and Practice shares some common-sense observations on how screening can be applied thoughtfully. However, this isn’t how it is usually portrayed in the top-down rollout as each advocacy group releases its next best screening recommendations.
Faced with this clash or expectations I have always chosen to think small. I live in a small town in a small state. I look at each patient and each family, one at a time, with its strengths and its vulnerabilities as a given. I try to educate and prevent as their needs and my time allows. I screen when something makes me feel uncomfortable. Long ago I retired my aspirations as a healer and instead have focussed on being a soother.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Obesity drugs overpriced, change needed to tackle issue
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).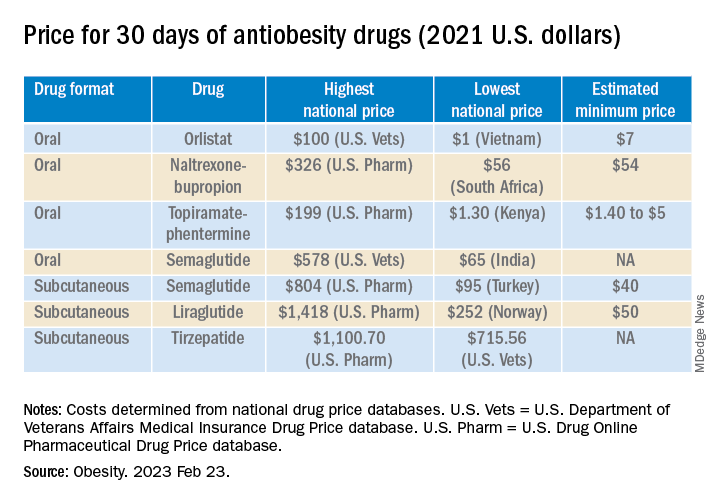
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
The lowest available national prices of drugs to treat obesity are up to 20 times higher than the estimated cost of profitable generic versions of the same agents, according to a new analysis.
The findings by Jacob Levi, MBBS, and colleagues were published in Obesity.
“Our study highlights the inequality in pricing that exists for effective antiobesity medications, which are largely unaffordable in most countries,” Dr. Levi, from Royal Free Hospital NHS Trust, London, said in a press release.
“We show that these drugs can actually be produced and sold profitably for low prices,” he summarized. “A public health approach that prioritizes improving access to medications should be adopted, instead of allowing companies to maximize profits,” Dr. Levi urged.
Dr. Levi and colleagues studied the oral agents orlistat, naltrexone/bupropion, topiramate/phentermine, and semaglutide, and subcutaneous liraglutide, semaglutide, and tirzepatide (all approved by the U.S. Food and Drug Administration to treat obesity, except for oral semaglutide and subcutaneous tirzepatide, which are not yet approved to treat obesity in the absence of type 2 diabetes).
“Worldwide, more people are dying from diabetes and clinical obesity than HIV, tuberculosis, and malaria combined now,” senior author Andrew Hill, MD, department of pharmacology and therapeutics, University of Liverpool, England, pointed out.
We need to repeat the low-cost success story with obesity drugs
“Millions of lives have been saved by treating infectious diseases at low cost in poor countries,” Dr. Hill continued. “Now we need to repeat this medical success story, with mass treatment of diabetes and clinical obesity at low prices.”
However, in an accompanying editorial, Eric A. Finkelstein, MD, and Junxing Chay, PhD, Duke-NUS Medical School, Singapore, maintain that “It would be great if everyone had affordable access to all medicines that might improve their health. Yet that is simply not possible, nor will it ever be.”
“What is truly needed is a better way to ration the health care dollars currently available in efforts to maximize population health. That is the challenge ahead not just for [antiobesity medications] but for all treatments,” they say.
“Greater use of cost-effectiveness analysis and direct negotiations, while maintaining the patent system, represents an appropriate approach for allocating scarce health care resources in the United States and beyond,” they continue.
Lowest current patented drug prices vs. estimated generic drug prices
New medications for obesity were highly effective in recent clinical trials, but high prices limit the ability of patients to get these medications, Dr. Levi and colleagues write.
They analyzed prices for obesity drugs in 16 low-, middle-, and high-income countries: Australia, Bangladesh, China, France, Germany, India, Kenya, Morocco, Norway, Peru, Pakistan, South Africa, Turkey, the United Kingdom, the United States, and Vietnam.
The researchers assessed the price of a 30-day supply of each of the studied branded drugs based on the lowest available price (in 2021 U.S. dollars) from multiple online national price databases.
Then they calculated the estimated minimum price of a 30-day supply of a potential generic version of these drugs, which included the cost of the active medicinal ingredients, the excipients (nonactive ingredients), the prefilled injectable device plus needles (for subcutaneous drugs), transportation, 10% profit, and 27% tax on profit.
The national prices of the branded medications for obesity were significantly higher than the estimated minimum prices of potential generic drugs (see Table).
The highest national price for a branded oral drug for obesity vs. the estimated minimum price for a potential generic version was $100 vs. $7 for orlistat, $199 vs. $5 for phentermine/topiramate, and $326 vs. $54 for naltrexone/bupropion, for a 30-day supply.
There was an even greater difference between highest national branded drug price vs. estimated minimum generic drug price for the newer subcutaneously injectable drugs for obesity.
For example, the price of a 30-day course of subcutaneous semaglutide ranged from $804 (United States) to $95 (Turkey), while the estimated minimum potential generic drug price was $40 (which is 20 times lower).
The study was funded by grants from the Make Medicines Affordable/International Treatment Preparedness Coalition and from the National Heart, Lung, and Blood Institute of the National Institutes of Health. Coauthor Francois Venter has reported receiving support from the Bill and Melinda Gates Foundation, U.S. Agency for International Development, Unitaid, SA Medical Research Council, Foundation for Innovative New Diagnostics, the Children’s Investment Fund Foundation, Gilead, ViiV, Mylan, Merck, Adcock Ingram, Aspen, Abbott, Roche, Johnson & Johnson, Sanofi, Virology Education, SA HIV Clinicians Society, and Dira Sengwe. The other authors and Dr. Chay have reported no relevant financial relationships. Dr. Finkelstein has reported receiving support for serving on the WW scientific advisory board and an educational grant unrelated to the present work from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Branding tattoo removal helps sex trafficking survivor close door on painful past
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
PHOENIX – When Kathy Givens walked onstage during a plenary session at the annual conference of the American Society for Laser Medicine and Surgery to reflect on her 9-month ordeal being sex trafficked in Texas more than 20 years ago, you could hear a pin drop.
“One of the scariest things about the life of sex trafficking is not knowing who’s going to be on the other side,” said Ms. Givens, who now lives in Houston. “There was some violence. There were some horrible things that happened. But you know what was really scary? When I got out. People may ask, ‘How’s that so? You escaped your trafficker. The past is behind you. Why were you afraid?’ I was afraid because I didn’t know that I had community. I didn’t know that community or that society would care about someone like me.”
She said that she found herself immobilized by fear of being shamed in society and labeled a sex trafficking victim, and wondered if she could overcome that fear and if anyone would view her as human again. Once free from her trafficker, she began a “healing journey,” which included getting married, raising four children, and re-enrolling in college with hopes of becoming a social worker. In 2020, she and her husband founded Twelve 11 Partners, an organization committed to supporting human trafficking survivors.
“I was working in the anti-trafficking field helping other survivors ... who have experienced this horrific crime,” she said. “I thought I was on my way.” But one “stain” from her sex trafficking past remained: The name of her trafficker was tattooed on her skin, “a reminder of what I’d gone through.”
Ms. Givens was eventually introduced to Paul M. Friedman, MD, the current ASLMS president and one of the that was formed in 2022. According to a survey that Dr. Friedman and colleagues presented at the 2022 annual ASLMS conference, an estimated 1 in 2 sex trafficking survivors have branding tattoos, and at least 1,000 survivors a year could benefit from removal of those tattoos.
“To date, 87 physicians in the U.S. and one in Canada have stepped forward to volunteer their services to be part of this program,” Dr. Friedman, who directs the Dermatology and Laser Surgery Center in Houston, said at this year’s meeting. “My goal is to double this number by the next annual conference,” he added, noting that trauma-informed training is part of the program, “to support the survivor experience during the treatment process.”
ASLMS is also working on this issue in partnership with the American Academy of Dermatology (AAD) Ad Hoc Task Force on Dermatological Resources for the Intervention and Prevention of Human Trafficking, which is headed by Boston dermatologist Shadi Kourosh, MD.
“Dermatologists are uniquely positioned to aid in efforts to assist those experiences in trafficking with our training to recognize and diagnose relevant signs on the skin and to assist patients with certain aspects of care and recovery including the treatment of the disease of scars and tattoos,” Dr. Friedman said. “Ultimately, we hope to create a database together to improve recognition of branding tattoos to aid in identifying sex trafficking victims.”
Ms. Givens, who sits on the U.S. Advisory Council on Human Trafficking, said that she was able to truly close the door on her sex trafficking past thanks to the tattoo removal Dr. Friedman performed as part of New Beginnings. “It means the world to me to know that I can now be an advocate for other individuals who have experienced human trafficking,” she told meeting attendees.
“Again, one of the scariest things is not knowing that you have community. I was scared of losing hope, but I’m standing here today. I have all the hope that I need. You have the power to change lives.”
AT ASLMS 2023
New ABIM fees to stay listed as ‘board certified’ irk physicians
Abdul Moiz Hafiz, MD, was flabbergasted when he received a phone call from his institution’s credentialing office telling him that he was not certified for interventional cardiology – even though he had passed that exam in 2016.
Dr. Hafiz, who directs the Advanced Structural Heart Disease Program at Southern Illinois University, phoned the American Board of Internal Medicine (ABIM), where he learned that to restore his credentials, he would need to pay $1,225 in maintenance of certification (MOC) fees.
Like Dr. Hafiz,
Even doctors who are participating in mandatory continuing education outside the ABIM’s auspices are finding themselves listed as “not certified.” Some physicians learned of the policy change only after applying for hospital privileges or for jobs that require ABIM certification.
Now that increasing numbers of physicians are employed by hospitals and health care organizations that require ABIM certification, many doctors have no option but to pony up the fees if they want to continue to practice medicine.
“We have no say in the matter,” said Dr. Hafiz, “and there’s no appeal process.”
The change affects nearly 330,000 physicians. Responses to the policy on Twitter included accusations of extortion and denunciations of the ABIM’s “money grab policies.”
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angiography and Interventions (SCAI), has heard from many SCAI members who had experiences similar to Dr. Hafiz’s. While Dr. Rao describes some of the Twitter outrage as “emotional,” he does acknowledge that the ABIM’s moves appear to be financially motivated.
“The issue here was that as soon as they paid the fee, all of a sudden, ABIM flipped the switch and said they were certified,” he said. “It certainly sounds like a purely financial kind of structure.”
Richard Baron, MD, president and CEO of the ABIM, said doctors are misunderstanding the policy change.
“No doctor loses certification solely for failure to pay fees,” Dr. Baron told this news organization. “What caused them to be reported as not certified was that we didn’t have evidence that they had met program requirements. They could say, ‘But I did meet program requirements, you just didn’t know it.’ To which our answer would be, for us to know it, we have to process them. And our policy is that we don’t process them unless you are current on your fees.”
This is not the first time ABIM policies have alienated physicians.
Last year, the ABIM raised its MOC fees from $165 to $220. That also prompted a wave of outrage. Other grievances go further back. At one time, being board certified was a lifetime credential. However, in 1990 the ABIM made periodic recertification mandatory.
The process, which came to be known as “maintenance of certification,” had to be completed every 10 years, and fees were charged for each certification. At that point, said Dr. Baron, the relationship between the ABIM and physicians changed from a one-time interaction to a career-long relationship. He advises doctors to check in periodically on their portal page at the ABIM or download the app so they will always know their status.
Many physicians would prefer not to be bound to a lifetime relationship with the ABIM. There is an alternative licensing board, the National Board of Physicians and Surgeons (NBPAS), but it is accepted by only a limited number of hospitals.
“Until the NBPAS gains wide recognition,” said Dr. Hafiz, “the ABIM is going to continue to have basically a monopoly over the market.”
The value of MOC itself has been called into question. “There are no direct data supporting the value of the MOC process in either improving care, making patient care safer, or making patient care higher quality,” said Dr. Rao. This feeds frustration in a clinical community already dealing with onerous training requirements and expensive board certification exams and adds to the perception that it is a purely financial transaction, he said. (Studies examining whether the MOC system improves patient care have shown mixed results.)
The true value of the ABIM to physicians, Dr. Baron contends, is that the organization is an independent third party that differentiates those doctors from people who don’t have their skills, training, and expertise. “In these days, where anyone can be an ‘expert’ on the Internet, that’s more valuable than ever before,” he said.
A version of this article first appeared on Medscape.com.
Abdul Moiz Hafiz, MD, was flabbergasted when he received a phone call from his institution’s credentialing office telling him that he was not certified for interventional cardiology – even though he had passed that exam in 2016.
Dr. Hafiz, who directs the Advanced Structural Heart Disease Program at Southern Illinois University, phoned the American Board of Internal Medicine (ABIM), where he learned that to restore his credentials, he would need to pay $1,225 in maintenance of certification (MOC) fees.
Like Dr. Hafiz,
Even doctors who are participating in mandatory continuing education outside the ABIM’s auspices are finding themselves listed as “not certified.” Some physicians learned of the policy change only after applying for hospital privileges or for jobs that require ABIM certification.
Now that increasing numbers of physicians are employed by hospitals and health care organizations that require ABIM certification, many doctors have no option but to pony up the fees if they want to continue to practice medicine.
“We have no say in the matter,” said Dr. Hafiz, “and there’s no appeal process.”
The change affects nearly 330,000 physicians. Responses to the policy on Twitter included accusations of extortion and denunciations of the ABIM’s “money grab policies.”
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angiography and Interventions (SCAI), has heard from many SCAI members who had experiences similar to Dr. Hafiz’s. While Dr. Rao describes some of the Twitter outrage as “emotional,” he does acknowledge that the ABIM’s moves appear to be financially motivated.
“The issue here was that as soon as they paid the fee, all of a sudden, ABIM flipped the switch and said they were certified,” he said. “It certainly sounds like a purely financial kind of structure.”
Richard Baron, MD, president and CEO of the ABIM, said doctors are misunderstanding the policy change.
“No doctor loses certification solely for failure to pay fees,” Dr. Baron told this news organization. “What caused them to be reported as not certified was that we didn’t have evidence that they had met program requirements. They could say, ‘But I did meet program requirements, you just didn’t know it.’ To which our answer would be, for us to know it, we have to process them. And our policy is that we don’t process them unless you are current on your fees.”
This is not the first time ABIM policies have alienated physicians.
Last year, the ABIM raised its MOC fees from $165 to $220. That also prompted a wave of outrage. Other grievances go further back. At one time, being board certified was a lifetime credential. However, in 1990 the ABIM made periodic recertification mandatory.
The process, which came to be known as “maintenance of certification,” had to be completed every 10 years, and fees were charged for each certification. At that point, said Dr. Baron, the relationship between the ABIM and physicians changed from a one-time interaction to a career-long relationship. He advises doctors to check in periodically on their portal page at the ABIM or download the app so they will always know their status.
Many physicians would prefer not to be bound to a lifetime relationship with the ABIM. There is an alternative licensing board, the National Board of Physicians and Surgeons (NBPAS), but it is accepted by only a limited number of hospitals.
“Until the NBPAS gains wide recognition,” said Dr. Hafiz, “the ABIM is going to continue to have basically a monopoly over the market.”
The value of MOC itself has been called into question. “There are no direct data supporting the value of the MOC process in either improving care, making patient care safer, or making patient care higher quality,” said Dr. Rao. This feeds frustration in a clinical community already dealing with onerous training requirements and expensive board certification exams and adds to the perception that it is a purely financial transaction, he said. (Studies examining whether the MOC system improves patient care have shown mixed results.)
The true value of the ABIM to physicians, Dr. Baron contends, is that the organization is an independent third party that differentiates those doctors from people who don’t have their skills, training, and expertise. “In these days, where anyone can be an ‘expert’ on the Internet, that’s more valuable than ever before,” he said.
A version of this article first appeared on Medscape.com.
Abdul Moiz Hafiz, MD, was flabbergasted when he received a phone call from his institution’s credentialing office telling him that he was not certified for interventional cardiology – even though he had passed that exam in 2016.
Dr. Hafiz, who directs the Advanced Structural Heart Disease Program at Southern Illinois University, phoned the American Board of Internal Medicine (ABIM), where he learned that to restore his credentials, he would need to pay $1,225 in maintenance of certification (MOC) fees.
Like Dr. Hafiz,
Even doctors who are participating in mandatory continuing education outside the ABIM’s auspices are finding themselves listed as “not certified.” Some physicians learned of the policy change only after applying for hospital privileges or for jobs that require ABIM certification.
Now that increasing numbers of physicians are employed by hospitals and health care organizations that require ABIM certification, many doctors have no option but to pony up the fees if they want to continue to practice medicine.
“We have no say in the matter,” said Dr. Hafiz, “and there’s no appeal process.”
The change affects nearly 330,000 physicians. Responses to the policy on Twitter included accusations of extortion and denunciations of the ABIM’s “money grab policies.”
Sunil Rao, MD, director of interventional cardiology at NYU Langone Health and president of the Society for Cardiovascular Angiography and Interventions (SCAI), has heard from many SCAI members who had experiences similar to Dr. Hafiz’s. While Dr. Rao describes some of the Twitter outrage as “emotional,” he does acknowledge that the ABIM’s moves appear to be financially motivated.
“The issue here was that as soon as they paid the fee, all of a sudden, ABIM flipped the switch and said they were certified,” he said. “It certainly sounds like a purely financial kind of structure.”
Richard Baron, MD, president and CEO of the ABIM, said doctors are misunderstanding the policy change.
“No doctor loses certification solely for failure to pay fees,” Dr. Baron told this news organization. “What caused them to be reported as not certified was that we didn’t have evidence that they had met program requirements. They could say, ‘But I did meet program requirements, you just didn’t know it.’ To which our answer would be, for us to know it, we have to process them. And our policy is that we don’t process them unless you are current on your fees.”
This is not the first time ABIM policies have alienated physicians.
Last year, the ABIM raised its MOC fees from $165 to $220. That also prompted a wave of outrage. Other grievances go further back. At one time, being board certified was a lifetime credential. However, in 1990 the ABIM made periodic recertification mandatory.
The process, which came to be known as “maintenance of certification,” had to be completed every 10 years, and fees were charged for each certification. At that point, said Dr. Baron, the relationship between the ABIM and physicians changed from a one-time interaction to a career-long relationship. He advises doctors to check in periodically on their portal page at the ABIM or download the app so they will always know their status.
Many physicians would prefer not to be bound to a lifetime relationship with the ABIM. There is an alternative licensing board, the National Board of Physicians and Surgeons (NBPAS), but it is accepted by only a limited number of hospitals.
“Until the NBPAS gains wide recognition,” said Dr. Hafiz, “the ABIM is going to continue to have basically a monopoly over the market.”
The value of MOC itself has been called into question. “There are no direct data supporting the value of the MOC process in either improving care, making patient care safer, or making patient care higher quality,” said Dr. Rao. This feeds frustration in a clinical community already dealing with onerous training requirements and expensive board certification exams and adds to the perception that it is a purely financial transaction, he said. (Studies examining whether the MOC system improves patient care have shown mixed results.)
The true value of the ABIM to physicians, Dr. Baron contends, is that the organization is an independent third party that differentiates those doctors from people who don’t have their skills, training, and expertise. “In these days, where anyone can be an ‘expert’ on the Internet, that’s more valuable than ever before,” he said.
A version of this article first appeared on Medscape.com.