User login
Very high HDL-C: Too much of a good thing?
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.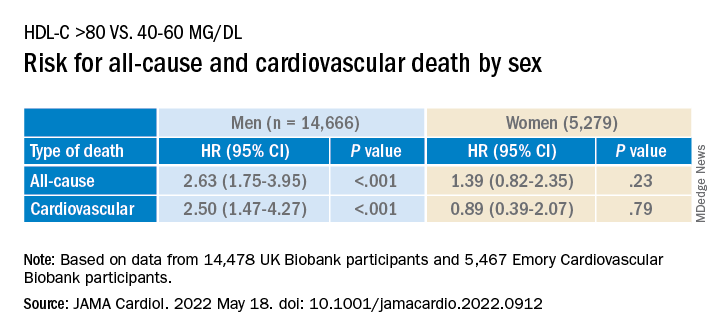
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
A new study suggests that .
Investigators studied close to 10,000 patients with CAD in two separate cohorts. After adjusting for an array of covariates, they found that individuals with HDL-C levels greater than 80 mg/dL had a 96% higher risk for all-cause mortality and a 71% higher risk for cardiovascular mortality than those with HDL-C levels between 40 and 60 mg/dL.
A U-shaped association was found, with higher risk for all-cause and cardiovascular mortality in patients with both very low and very high, compared with midrange, HDL-C values.
“Very high HDL levels are associated with increased risk of adverse outcomes, not lower risk, as previously thought. This is true not only in the general population, but also in people with known coronary artery disease,” senior author Arshed A. Quyyumi, MD, professor of medicine, division of cardiology, Emory University, Atlanta, told this news organization.
“Physicians have to be cognizant of the fact that, at levels of HDL-C above 80 mg/dL, they [should be] more aggressive with risk reduction and not believe that the patient is at ‘low risk’ because of high levels of ‘good’ cholesterol,” said Dr. Quyyumi, director of the Emory Clinical Cardiovascular Research Institute.
The study was published online in JAMA Cardiology.
Inverse association?
HDL-C levels have “historically been inversely associated with increased cardiovascular disease (CVD) risk; however, recent studies have questioned the efficacy of therapies designed to increase HDL-C levels,” the authors wrote. Moreover, genetic variants associated with HDL-C have not been found to be linked to CVD risk.
Whether “very high HDL-C levels in patients with coronary artery disease (CAD) are associated with mortality risk remains unknown,” they wrote. In this study, the researchers investigated not only the potential risk of elevated HDL-C levels in these patients, but also the association of known HDL-C genetic variants with this risk.
To do so, they analyzed data from a subset of patients with CAD in two independent study groups: the UK Biobank (UKB; n = 14,478; mean [standard deviation] age, 61.2 [5.8] years; 76.2% male; 93.8% White) and the Emory Cardiovascular Biobank (EmCAB; n = 5,467; mean age, 63.8 [12.3] years; 66.4% male; 73.2% White). Participants were followed prospectively for a median of 8.9 (interquartile range, 8.0-9.7) years and 6.7 (IQR, 4.0-10.8) years, respectively.
Additional data collected included medical and medication history and demographic characteristics, which were used as covariates, as well as genomic information.
Of the UKB cohort, 12.4% and 7.9% sustained all-cause or cardiovascular death, respectively, during the follow-up period, and 1.8% of participants had an HDL-C level above 80 mg/dL.
Among these participants with very high HDL-C levels, 16.9% and 8.6% had all-cause or cardiovascular death, respectively. Compared with the reference category (HDL-C level of 40-60 mg/dL), those with low HDL-C levels (≤ 30 mg/dL) had an expected higher risk for both all-cause and cardiovascular mortality, even after adjustment for covariates (hazard ratio, 1.33; 95% confidence interval, 1.07-1.64 and HR, 1.42; 95% CI, 1.09-1.85, respectively; P = .009).
“Importantly,” the authors stated, “compared with the reference category, individuals with very high HDL-C levels (>80 mg/dL) also had a higher risk of all-cause death (HR, 1.58 [1.16-2.14], P = .004).”
Although cardiovascular death rates were not significantly greater in unadjusted analyses, after adjustment, the highest HDL-C group had an increased risk for all-cause and cardiovascular death (HR, 1.96; 95% CI, 1.42-2.71; P < .001 and HR, 1.71; 95% CI, 1.09-2.68, respectively; P = .02).
Compared with females, males with HDL-C levels above 80 mg/dL had a higher risk for all-cause and cardiovascular death.
Similar findings were obtained in the EmCAB patients, 1.6% of whom had HDL-C levels above 80 mg/dL. During the follow-up period, 26.9% and 13.8% of participants sustained all-cause and cardiovascular death, respectively. Of those with HDL-C levels above 80 mg/dL, 30.0% and 16.7% experienced all-cause and cardiovascular death, respectively.
Compared with those with HDL-C levels of 40-60 mg/dL, those in the lowest (≤30 mg/dL) and highest (>80 mg/dL) groups had a “significant or near-significant greater risk for all-cause death in both unadjusted and fully adjusted models.
“Using adjusted HR curves, a U-shaped association between HDL-C and adverse events was evident with higher mortality at both very high and low HDL-C levels,” the authors noted.
Compared with patients without diabetes, those with diabetes and an HDL-C level above 80 mg/dL had a higher risk for all-cause and cardiovascular death, and patients younger than 65 years had a higher risk for cardiovascular death than patients 65 years and older.
The researchers found a “positive linear association” between the HDL-C genetic risk score (GRS) and HDL levels, wherein a 1-SD higher HDL-C GRS was associated with a 3.03 mg/dL higher HDL-C level (2.83-3.22; P < .001; R 2 = 0.06).
The HDL-C GRS was not associated with the risk for all-cause or cardiovascular death in unadjusted models, and after the HDL-C GRS was added to the fully adjusted models, the association with HDL-C level above 80 mg/dL was not attenuated, “indicating that HDL-C genetic variations in the GRS do not contribute substantially to the risk.”
“Potential mechanisms through which very high HDL-C might cause adverse cardiovascular outcomes in patients with CAD need to be studied,” Dr. Quyyumi said. “Whether the functional capacity of the HDL particle is altered when the level is very high remains unknown. Whether it is more able to oxidize and thus shift from being protective to harmful also needs to be investigated.”
Red flag
Commenting for this news organization, Sadiya Sana Khan, MD, MSc, assistant professor of medicine (cardiology) and preventive medicine (epidemiology), Northwestern University, Chicago, said: “I think the most important point [of the study] is to identify people with very high HDL-C. This can serve as a reminder to discuss heart-healthy lifestyles and discussion of statin therapy if needed, based on LDL-C.”
In an accompanying editorial coauthored with Gregg Fonarow, MD, Ahmanson-UCLA Cardiomyopathy Center, University of California, Los Angeles, the pair wrote: “Although the present findings may be related to residual confounding, high HDL-C levels should not automatically be assumed to be protective.”
They advised clinicians to “use HDL-C levels as a surrogate marker, with very low and very high levels as a red flag to target for more intensive primary and secondary prevention, as the maxim for HDL-C as ‘good’ cholesterol only holds for HDL-C levels of 80 mg/dL or less.”
This study was supported in part by grants from the National Institutes of Health, the American Heart Association, and the Abraham J. & Phyllis Katz Foundation. Dr. Quyyumi and coauthors report no relevant financial relationships. Dr. Khan reports receiving grants from the American Heart Association and the National Institutes of Health outside the submitted work. Dr. Fonarow reports receiving personal fees from Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis outside the submitted work. No other disclosures were reported.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
SGLT2 inhibitors as first-line therapy in type 2 diabetes?
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Use of sodium–glucose cotransporter-2 (SGLT-2) inhibitors rather than metformin as first-line treatment for type 2 diabetes appears to cut the risk for heart failure hospitalization but not myocardial infarction, stroke, or all-cause mortality, a new analysis of real-world data suggests.
Safety findings were similar, except for the fact that genital infections were more common with SGLT-2 inhibitors.
The study was conducted using claims data from two large U.S. insurance databases and Medicare. Propensity score matching was used to account for baseline differences.
The study was conducted by HoJin Shin, BPharm, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, and colleagues. The findings were published online in Annals of Internal Medicine.
“Those who start SGLT-2 inhibitors as first line show similar risks, compared with metformin in MI, stroke, and all-cause mortality outcomes. Strikingly and consistently, SGLT-2 inhibitors show lower risk for hospitalization for heart failure, which is consistent with the findings from cardiovascular outcomes trials,” Dr. Shin said in an interview.
Just a beginning step, although trial probably wasn’t long enough
However, she added, “I don’t want to overstate anything. ... We aren’t powered enough to investigate who would benefit the most. ... As a pharmacoepidemiologist, I think it’s my duty to provide high-quality evidence so we can actually help physicians and patients make better decisions on their medication. Our current research is just a beginning step.”
Asked to comment, Simeon I. Taylor, MD, PhD, professor of medicine at the University of Maryland, Baltimore, told this news organization, “This study generally confirmed conclusions from published RCTs [randomized clinical trials]. No real surprises, albeit the conclusions may not fully support some of the most enthusiastic claims for SGLT-2 inhibitors with respect to MI, stroke, and cardiovascular death.”
Indeed, Dr. Taylor noted that only two SGLT-2 inhibitors, canagliflozin and empagliflozin, were shown to have a statistically significant association with decreased major adverse cardiovascular events.
In contrast, neither dapagliflozin nor ertugliflozin showed significant benefit regarding those outcomes.
He also pointed out that those four major SLGT-2 inhibitor cardiovascular outcomes trials were placebo-controlled rather than head-to-head trials in which they were compared to an active comparator such as metformin.
“Viewed in this light, it’s probably not surprising that the present study did not demonstrate a robust benefit for SGLT-2 inhibitors to decrease [major adverse CV events].”
The duration of follow-up in the current study is also a limitation, he added.
“The majority of patients were followed for a year or less. This is probably sufficient to assess the impact of some pharmacological mechanisms, for example, the beneficial impact to decrease risk of heart failure by promoting urinary sodium excretion. However, it’s probably insufficient time to observe a beneficial impact on atherosclerosis. For example, there is typically a lag of several years before statins demonstrate efficacy with respect to adverse cardiovascular events.”
Nevertheless, he said, “it provides strong support for benefit with respect to decreasing risk of hospitalization for heart failure.”
He noted that while metformin is currently significantly cheaper than any SGLT-2 inhibitors, once the latter become available as generics, they will be cheaper, and this will likely have a bearing on prescribing decisions.
“Availability of generic SGLT-2 inhibitors offers potential to transform prescribing patterns for type 2 diabetes,” he noted.
First-line SGLT2 inhibitors versus metformin: Most outcomes similar
The study data came from two commercial U.S. health insurance databases, Optum Clinfomatics Data Mart and IBM Marketscan, and from Medicare fee-for-service enrollees.
From April 2013 through March 2020, a total of 9,334 patients began treatment with first-line SGLT-2 inhibitors; 819,973 patients began taking metformin. After 1:2 propensity score matching for confounders, there were 8,613 participants in the SGLT-2 inhibitor group and 17,226 in the group that began treatment with metformin.
The mean follow-up times were 10.7 months for patients taking SGLT-2 inhibitors and 12.2 months for patients taking metformin.
Incidence rates per 1,000 person-years for the composite of hospitalization for MI, hospitalization for ischemic or hemorrhagic stroke, or all-cause mortality (MI/stroke/mortality) were 15.0 versus 16.2 for SLGT-2 inhibitors versus metformin, not a significant difference (hazard ratio, 0.96).
However, for the composite of heart failure hospitalization or all-cause mortality, the rates were 18.3 versus 23.5, a significant difference, with an HR of 0.80. The benefit was seen beginning at about 6 months.
Compared with metformin, SGLT-2 inhibitors showed a significantly lower risk for heart failure hospitalization (HR, 0.78), a numerically (but not significantly) lower risk for MI (HR, 0.70), and similar risks for stroke, mortality, and MI/stroke/HHF/mortality.
Genital infections were significantly more common with SGLT-2 inhibitors (54.1 vs. 23.7 per 1,000 person-years; HR, 2.19). Other safety measures were similar, including acute kidney injury, bone fractures, severe hypoglycemia, diabetic ketoacidosis, and lower-limb amputations.
How does cost factor in?
A sensitivity analysis aimed at examining the possible effect of unmeasured socioeconomic status showed no difference in cardiovascular benefit for first-line SGLT-2 inhibitors and metformin, compared with first-line dipeptidyl peptidase–4 (DPP-4) inhibitors, which cost more than metformin; it is not known what effect DPP-4 inhibitors have on the cardiovascular outcomes of interest.
Cost and insurance coverage factor into the benefit/risk calculation. Metformin is far less costly than any of the SGLT-2 inhibitors – roughly $10 to $20 per month, compared with more than $500 a month.
However, “for some fortunate patients with the most generous pharmacy benefit insurance coverage, the out-of-pocket cost of brand name drugs like SGLT-2 inhibitors is substantially lower,” Dr. Taylor noted.
He said that the current study “raises questions about whether the clinical benefits of SGLT-2 inhibitors as initial monotherapy justify the higher price relative to metformin. The data in this paper suggest that the value case for SGLT-2 inhibitors is strongest for patients with the greatest risk to be hospitalized for heart failure.”
Indeed, Dr. Shin said, “Once we get more information, it may just help in extending the coverage from insurance companies and Medicare/Medicaid, to lower the barrier to access.”
Dr. Taylor reiterated that patents on some of the early SGLT-2 inhibitors are expected to expire in the next few years, which would make it possible for generic versions to be approved. “At that point, prices would likely fall, possibly to levels similar to metformin.”
The study was funded by grant support from the Division of Pharmacoepidemiology and Pharmacoeconomics, department of medicine, Brigham and Women’s Hospital, and Harvard Medical School, the National Institute on Aging, and the Patient-Centered Outcomes Research Institute. Dr. Shin has disclosed no relevant financial relationships. Dr. Taylor is a consultant for Ionis Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
SAFE-PAD shows long-term safety of paclitaxel devices
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
Patients who have paclitaxel-coated stents and balloons have survival and outcomes comparable to those who have a bare-metal stent or percutaneous transluminal angioplasty, according to updated results from a large study of almost 170,000 Medicare beneficiaries.
The SAFE-PAD study analyzed Medicare claims data of 168,533 patients, including 70,584 who were treated with drug-coated devices (DCD), from April 2015 through 2018.
Notably, Eric A. Secemsky, MD, MSc, said in an interview, that included more than 32,000 patients with more than 5 years of follow-up. He presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“What we’re seeing now with this study is that paclitaxel-coated devices [PCDs] have the same long-term survival compared to those treated with non–drug-coated devices (NDCDs),” said Dr. Secemsky, director of vascular intervention at Beth Israel Deaconess Medical Center in Boston. “I think this is another important piece and some of the longest-term data in this size population to demonstrate the long-term safety of PCD, and hopefully it will help us get back to normal practice that has been halted now for over 3 years.”
That was a reference to the 2018 meta-analysis by Konstantinos Katsanos, MD, PhD, of Patras University in Greece, and colleagues, which showed an increased risk of death after PCD placements. That study threw a wet blanket of sorts on PCD use, Dr. Secemsky said.
The median follow-up for SAFE-PAD (formally called the Safety Assessment of Femoropopliteal Endovascular treatment with Paclitaxel-coated Devices) was 3.5 years, with the longest follow-up, 6.3 years. The weighted cumulative incidence of mortality at 6.3 years was 63.6% with NDCDs and 62.5% with DCDs (hazard ratio, 0.98; 95% confidence interval, 0.96-0.99; P < .0001). A subgroup analysis found no link between DCDs and increased death in low-risk patients, low-comorbid patients, inpatient or outpatient treatment, patients without critical limb ischemia, or patients treated with stents or balloon angioplasty alone.
“This report and the length of follow-up is one more piece that has continued to demonstrate safety with PCDs,” Dr. Secemsky said. He added that these results fall in line with smaller studies that failed to show a link between DCDs and long-term mortality, notably the SWEDEPAD randomized study of 2,289 patients evaluated through 4 years, and a subanalysis of 4,000 patients in VOYAGER-PAD through 42 months of follow-up.
“So we’ve really shown through these data sets and others that we can’t replicate any harms that we’ve seen in that Katsanos meta-analysis, and it suggests that there was some bias in that meta-analysis.”
Strengths of the study are its size and the way it followed the patients longitudinally, Sahil A. Parikh, MD, director of endovascular services at Columbia University Vagelos College of Physicians and Surgeons in New York, said in an interview.
With regard to its limitations, Dr. Parikh said, “On the other hand, it’s a claims database which doesn’t have the granularity about the patients’ specific procedural factors,” he said. “There are gaps that might further inform the value of lack thereof of the drug-coated device, but certainly at the topline, which is the hard endpoint of mortality, you can read quite a lot and you can assume that with such large numbers, the signal-to-noise ratio would be sufficiently sensitive that you get a real signal.”
With these updated SAFE-PAD results along with other studies, Dr. Parikh said, “If one weighs the risk benefit of cardiac lesion revascularization regarding requiring a repeat procedure vs. the risk of mortality from paclitaxel, if there is such a thing, I think most physicians have come back and the pendulum has swung back considering it reasonable to use paclitaxel products.”
That’s a message that will resonate with patients reluctant to return to the hospital since the COVID-19 outbreak, he said. “If you can tell them we can avoid a repeat trip to the hospital, they’re all for it,” Dr. Parikh said.
The study results were published simultaneously with Dr. Secemsky’s presentation. Funding for SAFE-PAD came from a multi-industry consortium consisting of BD, Boston Scientific, Cook Medical, Medtronic and Philips, which wasn’t involved in the study design or analysis.
Dr. Secemsky disclosed relationships with Abbott, BD, Bayer, Boston Scientific, Cook Medical, CSI, Endovascular Engineering, Inari, Janssen, Medtronic, Philips, and Venture Med. Dr. Parikh disclosed relationships with TriReme Medical, Boston Scientific, Heartflow, Cordis, Janssen, Terumo, Canon, Shockwave, Abiomed, Abbott, Cardiovascular Systems, Inari and Surmodics.
FROM SCAI 2022
Distal radial snuffbox technique comes up short in DISCO RADIAL
Distal radial access is not superior to conventional radial access with regard to radial artery occlusion (RAO) but is a valid alternative for use in percutaneous procedures, according to results of the DISCO RADIAL trial.
The primary endpoint of forearm RAO at discharge was not met, occurring in 0.31% of patients whose radial artery was accessed distally (DRA) at the anatomical snuffbox and in 0.91% of patients with conventional transradial access (TRA) in the intention-to-treat analysis (P = .29).
The DRA group was also twice as likely to crossover to another access point (7.5% vs. 3.7%; P = .002) and to experience radial artery spasm (5.4% vs. 2.7%; P < .015).
“The message first is that if you do a good job with transradial access you can end up with a lower [occlusion] rate,” said coprincipal investigator Adel Aminian, MD, Hôpital Civil Marie Curie, Charleroi, Belgium. “On the other hand, it’s a trade-off between a more demanding puncture for distal radial access but also a simpler hemostatic process, which I think is one of the main advantages of distal radial access.”
The results were presented during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, and published simultaneously in JACC: Cardiovascular Interventions.
DISCO-RADIAL (Distal Versus Conventional RADIAL Access for Coronary Angiography and Intervention) is the largest trial thus far to compare TRA with the distal radial snuffbox technique, which has shown promise for reducing RAO rates in the recent single-center randomized DAPRAO and ANGIE trials.
The trial was conducted at 15 sites across Europe and Japan in 1,309 patients with an indication for percutaneous coronary procedures using the 6Fr Glidesheath Slender (Terumo). The intention-to-treat population included 657 TRA patients and 650 DRA patients.
The two groups were well matched, with most having a chronic coronary syndrome. Operators had to have performed a minimum of 100 procedures by DRA and follow systematic best practices previously reported by the investigators to prevent RAO, Dr. Aminian said.
The use of DRA did not significantly affect the duration of the coronary procedure (27 minutes vs. 24 minutes with TRA; P = .12) or average radiation dose (1298 mGy vs. 1222 mGy; P = .70).
DRA, however, reduced the need for selective compression devices (88% vs. 99.2%) and shortened the median time to hemostasis from 180 minutes to 153 minutes (P for both < .001).
“These results establish compliance to best practice recommendations for RAO avoidance as a mandatory new reference in transradial practice,” Dr. Aminian concluded. “At the same time, distal radial artery arises as a valid alternative associated with higher crossover rates but with a simpler and shorter hemostasis process.”
A show of hands revealed that about 25% of the audience used distal radial access prior to the presentation but that enthusiasm fell off following the results.
Discussant Hany Eteiba, MD, Glasgow Royal Infirmary, said: “I salute your enthusiasm for presenting a negative trial and you tried to persuade the audience to use the distal radial artery results, but nonetheless.”
Dr. Eteiba said he could see a “potential advantage in the shorter hemostasis time,” and asked whether it might be influencing the rapid turnover for day-case angioplasty.
Dr. Aminian responded that “if you do an angioplasty you have to keep the patient for a certain amount of time, but I think for your nurse work and for the health care resources, having a very short hemostasis time is very interesting. We started with a hemostasis time of 2 hours and now we’ve decreased it to 1 hour and it will decrease even more.”
Session moderator Chaim Lotan, MD, Hadassah-Hebrew University Medical Center, Jerusalem, called DISCO-RADIAL an important study and said, “the question now is what’s the indication in your eyes for using distal radial?”
Dr. Aminian said that one message from the trial is that people who are using transradial access “have to do a better job,” and reminded the audience that RAO rates at many centers are too high, at 10% or upward.
At the same time, Dr. Aminian cautioned that operators wanting to use distal radial access “need to master the technique” or they will “end up with a relatively high failure rate.”
Discussant Eliano Navarese, MD, Nicolaus Copernicus University, Toruń, Poland, said, “I still think that it is a very valid approach, we use it for almost 20 years ... but it is very true, it is very demanding. And the learning curve of 100 cases in the trial maybe needed more cases.”
In an accompanying editorial, Grigorios Tsigkas, MD, PhD, University of Patras, Rio Patras, Greece, and colleagues wrote that the incidence of forearm RAO was “surprisingly low” but could be even lower if the authors administered adequate anticoagulation.
Still, they wrote that distal transradial access “for coronary procedures in combination with the systematic implementation of best practices for RAO prevention may be the final solution against RAO.”
The editorialists suggested that exposure to radiation could be the “main limitation of this novel vascular approach” and that forthcoming trials, such as DOSE, could shed light on this issue.
Increased procedure times in the DISCO RADIAL and ANGIE trials are secondary in stable patients, Dr. Tsigkas said, but could be a limitation in patients presenting with ST-segment elevation myocardial infarction (STEMI). Ongoing research, such as the RESERVE trial from China and a Korean trial, will provide insights into the safety and feasibility of distal transradial access in STEMI.
The study was supported by Terumo Europe. Dr. Aminian reported receiving honoraria or consultation fees from Abbott, Boston Scientific, and Terumo Interventional Systems. Dr. Tsigkas reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Distal radial access is not superior to conventional radial access with regard to radial artery occlusion (RAO) but is a valid alternative for use in percutaneous procedures, according to results of the DISCO RADIAL trial.
The primary endpoint of forearm RAO at discharge was not met, occurring in 0.31% of patients whose radial artery was accessed distally (DRA) at the anatomical snuffbox and in 0.91% of patients with conventional transradial access (TRA) in the intention-to-treat analysis (P = .29).
The DRA group was also twice as likely to crossover to another access point (7.5% vs. 3.7%; P = .002) and to experience radial artery spasm (5.4% vs. 2.7%; P < .015).
“The message first is that if you do a good job with transradial access you can end up with a lower [occlusion] rate,” said coprincipal investigator Adel Aminian, MD, Hôpital Civil Marie Curie, Charleroi, Belgium. “On the other hand, it’s a trade-off between a more demanding puncture for distal radial access but also a simpler hemostatic process, which I think is one of the main advantages of distal radial access.”
The results were presented during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, and published simultaneously in JACC: Cardiovascular Interventions.
DISCO-RADIAL (Distal Versus Conventional RADIAL Access for Coronary Angiography and Intervention) is the largest trial thus far to compare TRA with the distal radial snuffbox technique, which has shown promise for reducing RAO rates in the recent single-center randomized DAPRAO and ANGIE trials.
The trial was conducted at 15 sites across Europe and Japan in 1,309 patients with an indication for percutaneous coronary procedures using the 6Fr Glidesheath Slender (Terumo). The intention-to-treat population included 657 TRA patients and 650 DRA patients.
The two groups were well matched, with most having a chronic coronary syndrome. Operators had to have performed a minimum of 100 procedures by DRA and follow systematic best practices previously reported by the investigators to prevent RAO, Dr. Aminian said.
The use of DRA did not significantly affect the duration of the coronary procedure (27 minutes vs. 24 minutes with TRA; P = .12) or average radiation dose (1298 mGy vs. 1222 mGy; P = .70).
DRA, however, reduced the need for selective compression devices (88% vs. 99.2%) and shortened the median time to hemostasis from 180 minutes to 153 minutes (P for both < .001).
“These results establish compliance to best practice recommendations for RAO avoidance as a mandatory new reference in transradial practice,” Dr. Aminian concluded. “At the same time, distal radial artery arises as a valid alternative associated with higher crossover rates but with a simpler and shorter hemostasis process.”
A show of hands revealed that about 25% of the audience used distal radial access prior to the presentation but that enthusiasm fell off following the results.
Discussant Hany Eteiba, MD, Glasgow Royal Infirmary, said: “I salute your enthusiasm for presenting a negative trial and you tried to persuade the audience to use the distal radial artery results, but nonetheless.”
Dr. Eteiba said he could see a “potential advantage in the shorter hemostasis time,” and asked whether it might be influencing the rapid turnover for day-case angioplasty.
Dr. Aminian responded that “if you do an angioplasty you have to keep the patient for a certain amount of time, but I think for your nurse work and for the health care resources, having a very short hemostasis time is very interesting. We started with a hemostasis time of 2 hours and now we’ve decreased it to 1 hour and it will decrease even more.”
Session moderator Chaim Lotan, MD, Hadassah-Hebrew University Medical Center, Jerusalem, called DISCO-RADIAL an important study and said, “the question now is what’s the indication in your eyes for using distal radial?”
Dr. Aminian said that one message from the trial is that people who are using transradial access “have to do a better job,” and reminded the audience that RAO rates at many centers are too high, at 10% or upward.
At the same time, Dr. Aminian cautioned that operators wanting to use distal radial access “need to master the technique” or they will “end up with a relatively high failure rate.”
Discussant Eliano Navarese, MD, Nicolaus Copernicus University, Toruń, Poland, said, “I still think that it is a very valid approach, we use it for almost 20 years ... but it is very true, it is very demanding. And the learning curve of 100 cases in the trial maybe needed more cases.”
In an accompanying editorial, Grigorios Tsigkas, MD, PhD, University of Patras, Rio Patras, Greece, and colleagues wrote that the incidence of forearm RAO was “surprisingly low” but could be even lower if the authors administered adequate anticoagulation.
Still, they wrote that distal transradial access “for coronary procedures in combination with the systematic implementation of best practices for RAO prevention may be the final solution against RAO.”
The editorialists suggested that exposure to radiation could be the “main limitation of this novel vascular approach” and that forthcoming trials, such as DOSE, could shed light on this issue.
Increased procedure times in the DISCO RADIAL and ANGIE trials are secondary in stable patients, Dr. Tsigkas said, but could be a limitation in patients presenting with ST-segment elevation myocardial infarction (STEMI). Ongoing research, such as the RESERVE trial from China and a Korean trial, will provide insights into the safety and feasibility of distal transradial access in STEMI.
The study was supported by Terumo Europe. Dr. Aminian reported receiving honoraria or consultation fees from Abbott, Boston Scientific, and Terumo Interventional Systems. Dr. Tsigkas reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Distal radial access is not superior to conventional radial access with regard to radial artery occlusion (RAO) but is a valid alternative for use in percutaneous procedures, according to results of the DISCO RADIAL trial.
The primary endpoint of forearm RAO at discharge was not met, occurring in 0.31% of patients whose radial artery was accessed distally (DRA) at the anatomical snuffbox and in 0.91% of patients with conventional transradial access (TRA) in the intention-to-treat analysis (P = .29).
The DRA group was also twice as likely to crossover to another access point (7.5% vs. 3.7%; P = .002) and to experience radial artery spasm (5.4% vs. 2.7%; P < .015).
“The message first is that if you do a good job with transradial access you can end up with a lower [occlusion] rate,” said coprincipal investigator Adel Aminian, MD, Hôpital Civil Marie Curie, Charleroi, Belgium. “On the other hand, it’s a trade-off between a more demanding puncture for distal radial access but also a simpler hemostatic process, which I think is one of the main advantages of distal radial access.”
The results were presented during the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, and published simultaneously in JACC: Cardiovascular Interventions.
DISCO-RADIAL (Distal Versus Conventional RADIAL Access for Coronary Angiography and Intervention) is the largest trial thus far to compare TRA with the distal radial snuffbox technique, which has shown promise for reducing RAO rates in the recent single-center randomized DAPRAO and ANGIE trials.
The trial was conducted at 15 sites across Europe and Japan in 1,309 patients with an indication for percutaneous coronary procedures using the 6Fr Glidesheath Slender (Terumo). The intention-to-treat population included 657 TRA patients and 650 DRA patients.
The two groups were well matched, with most having a chronic coronary syndrome. Operators had to have performed a minimum of 100 procedures by DRA and follow systematic best practices previously reported by the investigators to prevent RAO, Dr. Aminian said.
The use of DRA did not significantly affect the duration of the coronary procedure (27 minutes vs. 24 minutes with TRA; P = .12) or average radiation dose (1298 mGy vs. 1222 mGy; P = .70).
DRA, however, reduced the need for selective compression devices (88% vs. 99.2%) and shortened the median time to hemostasis from 180 minutes to 153 minutes (P for both < .001).
“These results establish compliance to best practice recommendations for RAO avoidance as a mandatory new reference in transradial practice,” Dr. Aminian concluded. “At the same time, distal radial artery arises as a valid alternative associated with higher crossover rates but with a simpler and shorter hemostasis process.”
A show of hands revealed that about 25% of the audience used distal radial access prior to the presentation but that enthusiasm fell off following the results.
Discussant Hany Eteiba, MD, Glasgow Royal Infirmary, said: “I salute your enthusiasm for presenting a negative trial and you tried to persuade the audience to use the distal radial artery results, but nonetheless.”
Dr. Eteiba said he could see a “potential advantage in the shorter hemostasis time,” and asked whether it might be influencing the rapid turnover for day-case angioplasty.
Dr. Aminian responded that “if you do an angioplasty you have to keep the patient for a certain amount of time, but I think for your nurse work and for the health care resources, having a very short hemostasis time is very interesting. We started with a hemostasis time of 2 hours and now we’ve decreased it to 1 hour and it will decrease even more.”
Session moderator Chaim Lotan, MD, Hadassah-Hebrew University Medical Center, Jerusalem, called DISCO-RADIAL an important study and said, “the question now is what’s the indication in your eyes for using distal radial?”
Dr. Aminian said that one message from the trial is that people who are using transradial access “have to do a better job,” and reminded the audience that RAO rates at many centers are too high, at 10% or upward.
At the same time, Dr. Aminian cautioned that operators wanting to use distal radial access “need to master the technique” or they will “end up with a relatively high failure rate.”
Discussant Eliano Navarese, MD, Nicolaus Copernicus University, Toruń, Poland, said, “I still think that it is a very valid approach, we use it for almost 20 years ... but it is very true, it is very demanding. And the learning curve of 100 cases in the trial maybe needed more cases.”
In an accompanying editorial, Grigorios Tsigkas, MD, PhD, University of Patras, Rio Patras, Greece, and colleagues wrote that the incidence of forearm RAO was “surprisingly low” but could be even lower if the authors administered adequate anticoagulation.
Still, they wrote that distal transradial access “for coronary procedures in combination with the systematic implementation of best practices for RAO prevention may be the final solution against RAO.”
The editorialists suggested that exposure to radiation could be the “main limitation of this novel vascular approach” and that forthcoming trials, such as DOSE, could shed light on this issue.
Increased procedure times in the DISCO RADIAL and ANGIE trials are secondary in stable patients, Dr. Tsigkas said, but could be a limitation in patients presenting with ST-segment elevation myocardial infarction (STEMI). Ongoing research, such as the RESERVE trial from China and a Korean trial, will provide insights into the safety and feasibility of distal transradial access in STEMI.
The study was supported by Terumo Europe. Dr. Aminian reported receiving honoraria or consultation fees from Abbott, Boston Scientific, and Terumo Interventional Systems. Dr. Tsigkas reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUROPCR 2022
Updated AHA/ASA guideline changes care for spontaneous intracerebral hemorrhage
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Many strategies widely considered “standard care” for managing spontaneous intracerebral hemorrhage (ICH) are not as effective as previously thought and are no longer recommended in updated guidelines from the American Heart Association/American Stroke Association (ASA).
Compression stockings, antiseizure medication, and steroid treatment are among the treatments with uncertain effectiveness, the writing group says.
The 2022 Guideline for the Management of Patients With Spontaneous ICH was published online in Stroke. The 80-page document contains major changes and refinements to the 2015 guideline on ICH management.
“Advances have been made in an array of fields related to ICH, including the organization of regional health care systems, reversal of the negative effects of blood thinners, minimally invasive surgical procedures, and the underlying disease in small blood vessels,” Steven M. Greenberg, MD, PhD, chair of the guideline writing group with Harvard Medical School and Massachusetts General Hospital, both in Boston, said in a news release.
“We’ve updated sections across the board. There’s probably no area that went untouched with some tweaking and new evidence added that led to some changes in level of evidence or strength of a recommendation,” Dr. Greenberg added in an interview with this news organization.
“Each section comes with knowledge gaps, and it wasn’t hard to come up with knowledge gaps in every section,” Dr. Greenberg acknowledged.
Time-honored treatments no more?
Among the key updates are changes to some “time-honored” treatments that continue to be used with some “regularity” for patients with ICH, yet appear to confer either no benefit or harm, Dr. Greenberg said.
For example, for emergency or critical care treatment of ICH, prophylactic corticosteroids or continuous hyperosmolar therapy is not recommended, because it appears to have no benefit for outcome, while use of platelet transfusions outside the setting of emergency surgery or severe thrombocytopenia appears to worsen outcome, the authors say.
Use of graduated knee- or thigh-high compression stockings alone is not an effective prophylactic therapy for prevention of deep vein thrombosis (DVT). Instead, intermittent pneumatic compression (IPC) starting on the day of diagnosis is now recommended for DVT prophylaxis.
“This is an area where we still have a lot of exploration to do. It is unclear whether even specialized compression devices reduce the risks of deep vein thrombosis or improve the overall health of people with a brain bleed,” Dr. Greenberg said in the release.
The new guidance advises against use of antiseizure or antidepressant medications for ICH patients in whom there is no evidence of seizures or depression.
In clinical trials, antiseizure medication did not contribute to improvements in functionality or long-term seizure control, and the use of antidepressants increased the chance of bone fractures, the authors say.
The guideline also provides updated recommendations for acute reversal of anticoagulation after ICH. It highlights the use of protein complex concentrate for reversal of vitamin K antagonists, such as warfarin; idarucizumab for reversal of the thrombin inhibitor dabigatran; and andexanet alfa for reversal of factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban.
For acute blood pressure lowering after mild to moderate ICH, treatment regimens that limit blood pressure variability and achieve smooth, sustained blood pressure control appear to reduce hematoma expansion and yield better functional outcome, the guideline says.
It also notes that minimally invasive approaches for hematoma evacuation, compared with medical management alone‚ have been shown to reduce mortality.
For patients with cerebellar hemorrhage, indications for immediate surgical evacuation with or without an external ventricular drain to reduce mortality now include larger volume (> 15 mL) in addition to previously recommended indications of neurologic deterioration, brainstem compression, and hydrocephalus, the authors note.
However, a “major knowledge gap is whether we can improve functional outcome with hematoma evacuation,” Dr. Greenberg said.
Multidisciplinary care
For rehabilitation after ICH, the guideline reinforces the importance of having a multidisciplinary team to develop a comprehensive plan for recovery.
Starting rehabilitation activities such as stretching and functional task training may be considered 24 to 48 hours following mild or moderate ICH. However, early aggressive mobilization within the first 24 hours has been linked to an increased risk of death within 14 days after an ICH, the guideline says.
Knowledge gaps include how soon it’s safe to return to work, drive, and participate in other social engagements. Recommendations on sexual activity and exercise levels that are safe after a stroke are also needed.
“People need additional help with these lifestyle changes, whether it’s moving around more, curbing their alcohol use, or eating healthier foods. This all happens after they leave the hospital, and we need to be sure we are empowering families with the information they may need to be properly supportive,” Dr. Greenberg says in the release.
The guideline points to the patient’s home caregiver as a “key and sometimes overlooked” member of the care team. It recommends psychosocial education, practical support, and training for the caregiver to improve the patient’s balance, activity level, and overall quality of life.
Opportunity for prevention?
The guideline also suggests there may be an opportunity to prevent ICH in some people through neuroimaging markers.
While neuroimaging is not routinely performed as a part of risk stratification for primary ICH risk, damage to small blood vessels that is associated with ICH may be evident on MRI that could signal future ICH risk, the guideline says.
“We added to the guidelines for the first time a section on mostly imaging markers of risk for having a first-ever hemorrhage,” Dr. Greenberg said in an interview.
“We don’t make any recommendations as to how to act on these markers because there is a knowledge gap. The hope is that we’ll see growth in our ability to predict first-ever hemorrhage and be able to do things to prevent first-ever hemorrhage,” he said.
“We believe the wide range of knowledge set forth in the new guideline will translate into meaningful improvements in ICH care,” Dr. Greenberg adds in the release.
The updated guideline has been endorsed by the American Association of Neurological Surgeons and Congress of Neurological Surgeons, the Society of Vascular and Interventional Neurology, and the Neurocritical Care Society. The American Academy of Neurology has affirmed the value of this statement as an educational tool for neurologists.
This research had no commercial funding. Dr. Greenberg has disclosed no relevant financial relationships. A complete list of disclosures for the guideline group is available with the original article.
A version of this article first appeared on Medscape.com.
Hormones account for 10% of lipid changes after menopause
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
The transition from perimenopause to menopause is accompanied by a proatherogenic shift in lipids and other circulating metabolites that potentially predispose women to cardiovascular disease (CVD). Now, for the first time, a new prospective cohort study quantifies the link between hormonal shifts and these lipid changes.
However, hormone therapy (HT) somewhat mitigates the shift and may help protect menopausal women from some elevated CVD risk, the same study suggests.
“Menopause is not avoidable, but perhaps the negative metabolite shift can be diminished by lifestyle choices such as eating healthily and being physically active,” senior author Eija Laakkonen, MD, University of Jyväskylä, Finland, told this news organization in an email.
“And women should especially pay attention to the quality of dietary fats and amount of exercise [they get] to maintain cardiorespiratory fitness,” she said, adding that women should discuss the option of HT with their health care providers.
Asked to comment, JoAnn Manson, MD, of Harvard Medical School, Boston, and past president of the North American Menopause Society, said there is strong evidence that women undergo negative cardiometabolic changes during the menopausal transition.
Changes include those in body composition (an increase in visceral fat and waist circumference), as well as unfavorable shifts in the lipid profile, as reflected by increases in low-density lipoprotein cholesterol (LDL-C) and triglycerides and a decrease in high-density lipoprotein cholesterol (HDL-C).
It’s also clear from a variety of cohort studies that HT blunts menopausal-related increases in body weight, percentage of body fat, as well as visceral fat, she said.
So the new findings do seem to “parallel” those of other perimenopausal to menopausal transition studies, which include HT having “favorable effects on lipids,” Dr. Manson said. HT “lowers LDL-C and increases HDL-C, and this is especially true when it is given orally,” but even transdermal delivery has shown some benefits, she observed.
Shift in hormones causes 10% of lipid changes after menopause
The new study, by Jari E. Karppinen, also of the University of Jyväskylä, and colleagues, was recently published in the European Journal of Preventive Cardiology. The data are from the Estrogenic Regulation of Muscle Apoptosis (ERMA) prospective cohort study.
In total, 218 women were tracked from perimenopause through to early postmenopause, 35 of whom started HT, mostly oral preparations. The women were followed for a median of 14 months. Their mean age was 51.7 years when their hormone and metabolite profiles were first measured.
Previous studies have shown that menopause is associated with levels of metabolites that promote CVD, but this study is the first to specifically link this shift with changes in female sex hormones, the researchers stress.
“Menopause was associated with a statistically significant change in 85 metabolite measures,” Mr. Karppinen and colleagues report.
Analyses showed that the menopausal hormonal shift directly explained the change in 64 of the 85 metabolites, with effect sizes ranging from 2.1% to 11.2%.
These included increases in LDL-C, triglycerides, and fatty acids. Analyses were adjusted for age at baseline, duration of follow-up, education level, smoking status, alcohol use, physical activity, and diet quality.
More specifically, investigators found that all apoB-containing particle counts as well as particle diameters increased over follow-up, although no change occurred in HDL particles.
They also found cholesterol concentrations in all apoB-containing lipoprotein classes to increase and triglyceride concentrations to increase in very low-density lipoprotein and HDL particles.
“These findings, including HDL triglycerides, can be interpreted as signs of poor metabolic health since, despite higher HDL-C being good for health, high HDL triglyceride levels are associated with a higher risk of coronary heart disease,” Dr. Laakkonen emphasized.
Among the 35 women who initiated HT on study enrollment, investigators did note, on exploratory analysis, increases in HDL-C and reductions in LDL-C.
“The number of women starting HT was small, and the type of HT was not controlled,” Dr. Laakkonen cautioned, however.
“Nevertheless, our observations support clinical guidelines to initiate HT early into menopause, as this timing offers the greatest cardioprotective effect,” she added.
The study was supported by the Academy of Finland. The authors and Dr. Manson have reported no relevant financial relationships. Dr. Manson is a contributor to Medscape.
This article was updated on 5/20/2022.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
ISCHEMIA substudy data don’t add up, cardiac surgeons say
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
A recent ISCHEMIA trial substudy is under scrutiny from surgeons for a data discrepancy, rekindling concerns about reliance on the landmark trial data in the latest coronary revascularization guidelines.
As previously reported, the main ISCHEMIA findings showed no significant benefit for an initial strategy of percutaneous coronary intervention (PCI) or coronary bypass graft surgery (CABG) over medical therapy in patients with stable moderate to severe ischemic heart disease.
The 2021 substudy by Reynolds et al. showed that coronary artery disease (CAD) severity, classified using the modified Duke Prognostic Index score, predicted 4-year mortality and myocardial infarction in the trial, whereas ischemia severity did not.
Cardiac surgeons Joseph F. Sabik III, MD, and Faisal Bakaeen, MD, however, spotted that only 40 patients are in the Duke category 6 group (three-vessel severe stenosis of at least 70% or two-vessel severe stenosis with a proximal left anterior descending lesion) in Supplemental tables 1 and 2, whereas 659 are in the main paper.
In addition, the Supplemental tables list the following:
- 659 patients in Duke group 5, not 894 as in the paper.
- 894 patients in Duke group 4, not 743 as in the paper.
- 743 patients in Duke group 3, not 179 as in the paper.
The surgeons penned a letter to Circulation early in April flagging the discrepancies, but say it was rejected April 15 because it was submitted outside the journal’s 6-week window for letters. They posted a public comment on the Remarq research platform, as advised by Circulation’s editorial office, and reached out directly to the authors and ISCHEMIA leadership.
“They just keep saying it’s a simple formatting error. Well, if it is a simple formatting error, then fix it,” Dr. Sabik, chair of surgery at University Hospitals Cleveland Medical Center, said in an interview. “But here we are now, a month later, and they still haven’t published our letter. Why? We’re the ones who identified the problem.”
Dr. Sabik said the accuracy of the data has important implications because the recent AHA/ACC/SCAI coronary revascularization guidelines used the ISCHEMIA data to downgrade the CABG recommendation for complex multivessel disease from class 1 to class 2B. Patients with a Duke 6 score are also typically the ones referred for CABG by today’s heart teams.
Several surgical societies have contested the guidelines, questioning whether the ISCHEMIA patients are truly reflective of those seen in clinical practice and questioning the decision to treat PCI and surgery as equivalent strategies to decrease ischemic events.
Dr. Bakaeen, from the Cleveland Clinic, told this news organization they don’t want a public battle over the data like the one that befell the EXCEL trial, and that it’s entirely possible the investigators might have inadvertently upgraded all the Duke score assignments by 1.
A systematic error, however, is more plausible than a formatting error, he said, because Supplemental tables 1 and 2 correspond exactly to the Duke 1 to Duke 7 sequence, suggesting the tables are correct and that the error might have occurred downstream, including in the manuscript.
The numbers should be consistent across all the ISCHEMIA manuscripts, Dr. Bakaeen added, but currently “don’t add up,” even after adjustment for different denominators, and especially for participants with left main disease.
They hope that publication of their letter, he said, will convince the authors to publicly share the data for patients in each of the seven modified Duke categories.
Lead author of the ISCHEMIA substudy, Harmony Reynolds, MD, New York (N.Y.) University Langone Health, told this news organization via email that as a result of a “formatting error in the transfer of data from the statistical output file to a Word document, data in Supplemental tables 1 and 2 were incorrect.”
She explained that they planned to present six, not seven, rows for the Duke score in the tables, collapsing the first two categories of nonobstructive disease (Duke 1-2), as they were in all other tables and figures. However, the Supplemental tables had incorrect row headings and because the Word program is designed to fill all available rows, it inserted the data from the output file into a seven-row table shell, duplicating the values for row 1 in the last row for left main disease of at least 50%.
“The data were correctly presented in the main manuscript tables and figures and in the remainder of the supplement, with a total of 659 patients in the subset with modified Duke prognostic index category 6 on coronary CT angiography,” Dr. Reynolds said.
She noted that Circulation will issue a correction. In addition, “we are in the process of preparing the data for public sharing soon. The data will include the Duke prognostic score at all levels.”
Circulation editor-in-chief Joseph A. Hill, MD, PhD, chief of cardiology at UT Southwestern Medical Center, Dallas, declined to be interviewed but confirmed via email that Dr. Bakaeen and Dr. Sabik’s letter and the correction will be published the week of May 16.
As for the delay, he said, “I received their reach-out just over 1 week ago, and per protocol, we conducted an internal evaluation of their allegations, which took a bit of time.”
A version of this article first appeared on Medscape.com.
FDA approves Medtronic’s Onyx Frontier drug-eluting stent
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved the Onyx Frontier drug-eluting stent (DES) to treat patients with coronary artery disease, the device manufacturer, Medtronic, announced today.
The Onyx Frontier shares the same stent platform and clinical indications as the previous-generation Resolute Onyx zotarolimus-eluting stent, including the most recent approval for patients at high risk of bleeding who may benefit from just 1 month dual-antiplatelet therapy.
“Meaningful design changes, including increased catheter flexibility, an innovative dual-layer balloon technology and a lower crossing profile led to a 16% improvement in deliverability with Onyx Frontier vs. the previous generation Resolute Onyx DES,” Medtronic said in a news release.
Onyx Frontier also offers a broad size matrix to treat more patients, and joins the Resolute Onyx as the only 2-mm DES available in the United States, the company noted. The stent is available in 4.5- to 5-mm sizes that can be expanded to 6 mm, specifically designed to support extra-large vessels.
The Onyx Frontier DES is pending CE Mark in Europe.
A version of this article first appeared on Medscape.com.
Reduced exercise capacity predicted mortality in COPD
Reduced exercise capacity and peak ventilation were significant predictors of early mortality in adults with chronic obstructive pulmonary disease, based on data from 126 individuals.
Cardiopulmonary exercise testing (CPET) is a common assessment for cardiorespiratory disease patients, but its role as a predictor of clinically relevant outcomes in chronic obstructive pulmonary disease (COPD) has not been investigated, and data on changes in exercise capacity over time in COPD patients are limited, wrote Cassia da Luz Goulart, MD, of the Federal University of São Carlos, Brazil, and colleagues.
The researchers hypothesized that CPET threshold values could be used as predictors of mortality in COPD.
In a prospective study published in Respiratory Medicine, the researchers identified 126 adults with COPD who were followed for 42 months. At study entry, each patient completed a clinical evaluation, followed by a pulmonary function test and CPET. The average age of the patients was 65 years, and 73% were men. All patients were on optimal medical management for COPD.
The researchers recorded data on peak oxygen consumption (VO2, mL/min), VCO2 (mL/min), minute ventilation (VE, L/min), the oxygen uptake efficiency slope (OUES), and ventilatory efficiency (the VE/VCO2 slope).
The participants performed CPET on a cycle ergometer, with breath-by-breath analysis measured throughout the test using a computer-based system.
A total of 48 patients (38%) died during the 42-month follow-up period. Overall, the significant predictors of mortality were VE/VCO2 slope of 30 or higher, peak VE of 25.7 L/min, and peak VO2 ≤ 13.8 mLO2 kg–1 min–1 were strong predictors of mortality in COPD patients in a Cox regression analysis.
When comparing the 78 survivors to the 48 nonsurvivors, the researchers found that the nonsurvivors were significantly more likely to be women, with worse lung function, inspiratory muscle weakness, and poorer CPET responses (P < .050 for all).
“The VE peak response is directly related to the FEV1 in COPD patients, factors such as dyspnea and increased leg discomfort negatively impact the VE response during exercise,” the researchers wrote in their discussion of the findings. In this context, our results may hold clinical utility in refining the prognostic accuracy when a patient with COPD has a VE peak ≤ 25.7 L/min,” they explained.
The study findings were limited by the inability to assess complete pulmonary function in the COPD patients, and the assessment only of three CPET measures, the researchers noted.
However, the results support the use of CPET as a clinical assessment tool for COPD patients, they said. “Moreover, therapeutic approaches, such as cardiopulmonary rehabilitation, may consider focusing on improving these metabolic and ventilatory markers as an indicator of clinical improvement and prognosis in patients with COPD,” they added.
The study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil, and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil. The researchers had no financial conflicts to disclose.
Reduced exercise capacity and peak ventilation were significant predictors of early mortality in adults with chronic obstructive pulmonary disease, based on data from 126 individuals.
Cardiopulmonary exercise testing (CPET) is a common assessment for cardiorespiratory disease patients, but its role as a predictor of clinically relevant outcomes in chronic obstructive pulmonary disease (COPD) has not been investigated, and data on changes in exercise capacity over time in COPD patients are limited, wrote Cassia da Luz Goulart, MD, of the Federal University of São Carlos, Brazil, and colleagues.
The researchers hypothesized that CPET threshold values could be used as predictors of mortality in COPD.
In a prospective study published in Respiratory Medicine, the researchers identified 126 adults with COPD who were followed for 42 months. At study entry, each patient completed a clinical evaluation, followed by a pulmonary function test and CPET. The average age of the patients was 65 years, and 73% were men. All patients were on optimal medical management for COPD.
The researchers recorded data on peak oxygen consumption (VO2, mL/min), VCO2 (mL/min), minute ventilation (VE, L/min), the oxygen uptake efficiency slope (OUES), and ventilatory efficiency (the VE/VCO2 slope).
The participants performed CPET on a cycle ergometer, with breath-by-breath analysis measured throughout the test using a computer-based system.
A total of 48 patients (38%) died during the 42-month follow-up period. Overall, the significant predictors of mortality were VE/VCO2 slope of 30 or higher, peak VE of 25.7 L/min, and peak VO2 ≤ 13.8 mLO2 kg–1 min–1 were strong predictors of mortality in COPD patients in a Cox regression analysis.
When comparing the 78 survivors to the 48 nonsurvivors, the researchers found that the nonsurvivors were significantly more likely to be women, with worse lung function, inspiratory muscle weakness, and poorer CPET responses (P < .050 for all).
“The VE peak response is directly related to the FEV1 in COPD patients, factors such as dyspnea and increased leg discomfort negatively impact the VE response during exercise,” the researchers wrote in their discussion of the findings. In this context, our results may hold clinical utility in refining the prognostic accuracy when a patient with COPD has a VE peak ≤ 25.7 L/min,” they explained.
The study findings were limited by the inability to assess complete pulmonary function in the COPD patients, and the assessment only of three CPET measures, the researchers noted.
However, the results support the use of CPET as a clinical assessment tool for COPD patients, they said. “Moreover, therapeutic approaches, such as cardiopulmonary rehabilitation, may consider focusing on improving these metabolic and ventilatory markers as an indicator of clinical improvement and prognosis in patients with COPD,” they added.
The study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil, and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil. The researchers had no financial conflicts to disclose.
Reduced exercise capacity and peak ventilation were significant predictors of early mortality in adults with chronic obstructive pulmonary disease, based on data from 126 individuals.
Cardiopulmonary exercise testing (CPET) is a common assessment for cardiorespiratory disease patients, but its role as a predictor of clinically relevant outcomes in chronic obstructive pulmonary disease (COPD) has not been investigated, and data on changes in exercise capacity over time in COPD patients are limited, wrote Cassia da Luz Goulart, MD, of the Federal University of São Carlos, Brazil, and colleagues.
The researchers hypothesized that CPET threshold values could be used as predictors of mortality in COPD.
In a prospective study published in Respiratory Medicine, the researchers identified 126 adults with COPD who were followed for 42 months. At study entry, each patient completed a clinical evaluation, followed by a pulmonary function test and CPET. The average age of the patients was 65 years, and 73% were men. All patients were on optimal medical management for COPD.
The researchers recorded data on peak oxygen consumption (VO2, mL/min), VCO2 (mL/min), minute ventilation (VE, L/min), the oxygen uptake efficiency slope (OUES), and ventilatory efficiency (the VE/VCO2 slope).
The participants performed CPET on a cycle ergometer, with breath-by-breath analysis measured throughout the test using a computer-based system.
A total of 48 patients (38%) died during the 42-month follow-up period. Overall, the significant predictors of mortality were VE/VCO2 slope of 30 or higher, peak VE of 25.7 L/min, and peak VO2 ≤ 13.8 mLO2 kg–1 min–1 were strong predictors of mortality in COPD patients in a Cox regression analysis.
When comparing the 78 survivors to the 48 nonsurvivors, the researchers found that the nonsurvivors were significantly more likely to be women, with worse lung function, inspiratory muscle weakness, and poorer CPET responses (P < .050 for all).
“The VE peak response is directly related to the FEV1 in COPD patients, factors such as dyspnea and increased leg discomfort negatively impact the VE response during exercise,” the researchers wrote in their discussion of the findings. In this context, our results may hold clinical utility in refining the prognostic accuracy when a patient with COPD has a VE peak ≤ 25.7 L/min,” they explained.
The study findings were limited by the inability to assess complete pulmonary function in the COPD patients, and the assessment only of three CPET measures, the researchers noted.
However, the results support the use of CPET as a clinical assessment tool for COPD patients, they said. “Moreover, therapeutic approaches, such as cardiopulmonary rehabilitation, may consider focusing on improving these metabolic and ventilatory markers as an indicator of clinical improvement and prognosis in patients with COPD,” they added.
The study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil, and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil. The researchers had no financial conflicts to disclose.
FROM RESPIRATORY MEDICINE
Espresso coffee linked to increased total cholesterol
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Espresso consumption is associated with higher total cholesterol levels, a population-based, cross-sectional study suggests.
Elevations in serum total cholesterol level were significantly linked to espresso consumption, particularly in men, Åsne Lirhus Svatun, of the Arctic University of Norway, Tromsø, and colleagues reported.
Drinking boiled/plunger coffee was associated with significantly higher serum total cholesterol levels in women and men. There was a significant relationship between filtered coffee consumption and total cholesterol, but only among women, the researchers reported.
“Doctors could become mindful of asking about coffee consumption when taking up the history of patients with elevated serum cholesterol,” study author Maja-Lisa Løchen, MD, PhD, of the Arctic University of Norway, said in an interview.
“Guiding patients to change from plunger coffee or other unfiltered coffee types to filtered or instant coffee could be a part of a lifestyle intervention to lower serum cholesterol levels.”
The results were published online in the journal Open Heart.
Previous studies of the relationship between serum cholesterol and espresso have had varying outcomes, the researchers noted.
Given that coffee consumption is high worldwide, even slight health effects can have substantial health consequences, the researchers noted. “Coffee was included for the first time in the 2021 ESC [European Society of Cardiology] guidelines on cardiovascular disease prevention in clinical practice. Increased knowledge on espresso coffee’s association with serum cholesterol will improve the recommendations regarding coffee consumption.”
“I don’t think that the findings in this paper are necessarily enough to change any advice about coffee,” said David Kao, MD, an associate professor medicine at the University of Colorado at Denver, Aurora, in commenting on the findings. “This is partly because the most important thing at the end of the day is whether subsequent events like heart attack or stroke increased or decreased. This analysis was not designed to answer that question.”
“If one has to choose between this study, which would suggest to drink less coffee to maintain low cholesterol, and the others, which would suggest increasing coffee consumption might reduce risk of multiple kinds of CVD, one should choose the latter,” Dr. Kao concluded.
In the current study, the investigators assessed 21,083 participants in the Tromsø Study in Northern Norway. The mean age of the participants was 56.4 years. Using multivariable linear regression, the researchers compared the relationship between each level of coffee consumption with no coffee consumption as the reference point and serum total cholesterol as the dependent variable. They tested for sex differences and adjusted for relevant covariates.
The findings indicate that drinking three to five cups of espresso each day was significantly linked with greater serum total cholesterol by 0.16 mmol/L (95% confidence interval, 0.07-0.24) for men and by 0.09 mmol/L (95% CI, 0.01-0.17) for women in comparison with participants who did not drink espresso daily.
Compared with individuals who did not drink plunger/boiled coffee, consumption of six or more cups of plunger/boiled coffee each day was linked with elevated serum total cholesterol levels by 0.23 mmol/L (95% CI, 0.08-0.38) for men and 0.30 mmol/L (95% CI, 0.13-0.48) for women.
Notably, for women but not men, there was an increase in serum total cholesterol of 0.11 mmol/L (95% CI, 0.03-0.19) in association with drinking six or more cups of filtered coffee per day.
When excluding participants who did not drink instant coffee, drinking instant coffee yielded a significant linear pattern for both men and women, but there was not a dose-dependent association.
These data show that sex differences were significant for every coffee type except plunger/boiled coffee, the authors noted.
Limitations of the study include its cross-sectional design; lack of generalizability of the data, given that the cohort primarily consisted of elderly adults and middle-aged White persons; and the fact that the study did not adjust for all confounding variables, the researchers noted.
Also among the study’s limitations were that some data were self-reported, and the missing indicator approach was implemented to assess data, the authors added.
Future research efforts should focus on following this cohort over many years to determine how consumption of various types of coffee is linked with events such as heart failure, stroke, and myocardial infarction. This insight would be important in offering guidance on whether the style of coffee preparation matters, concluded Dr. Kao.
The study was supported by a number of sources, including the Arctic University of Norway and the Northern Norway Regional Health Authority. The study investigators reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM OPEN HEART






