User login
Plantar Hyperpigmentation
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
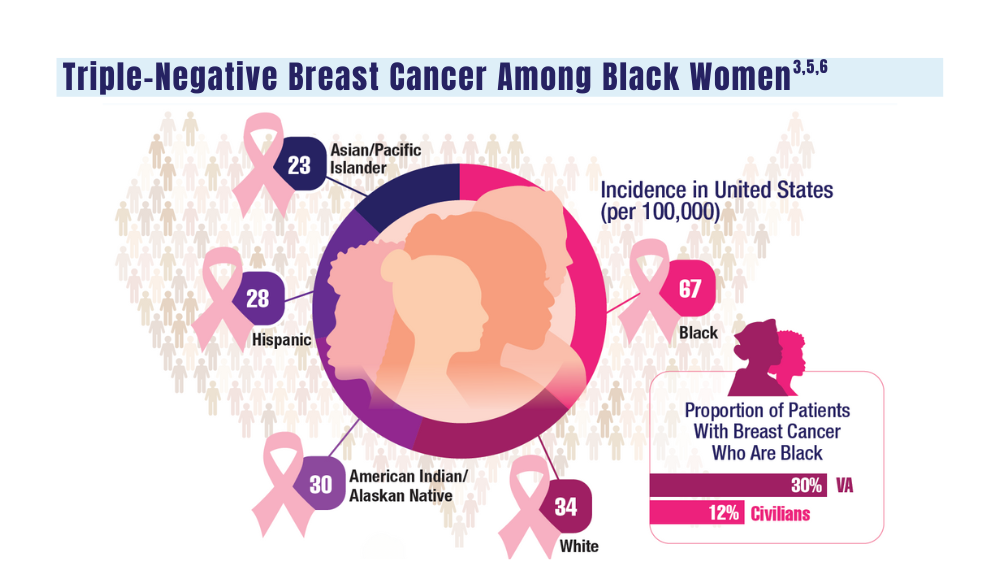
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
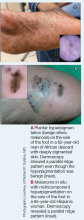
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
Plantar hyperpigmentation (also known as plantar melanosis [increased melanin], volar pigmented macules, benign racial melanosis, acral pigmentation, acral ethnic melanosis, or mottled hyperpigmentation of the plantar surface) is a benign finding in many individuals and is especially prevalent in those with darker skin tones. Acral refers to manifestation on the hands and feet, volar on the palms and soles, and plantar on the soles only. Here, we focus on plantar hyper-pigmentation. We use the terms ethnic and racial interchangeably.
It is critically important to differentiate benign hyperpigmentation, which is common in patients with skin of color, from melanoma. Although rare, Black patients in the United States experience high morbidity and mortality from acral melanoma, which often is diagnosed late in the disease course.1
There are many causes of hyperpigmentation on the plantar surfaces, including benign ethnic melanosis, nevi, melanoma, infections such as syphilis and tinea nigra, conditions such as Peutz-Jeghers syndrome and Laugier-Hunziker syndrome, and postinflammatory hyperpigmentation secondary to atopic dermatitis and psoriasis. We focus on the most common causes, ethnic melanosis and nevi, as well as melanoma, which is the deadliest cause.
Epidemiology
In a 1980 study (N=251), Black Americans had a high incidence of plantar hyperpigmentation, with 52% of affected patients having dark brown skin and 31% having light brown skin.2
The epidemiology of melanoma varies by race/ethnicity. Melanoma in Black individuals is relatively rare, with an annual incidence of approximately 1 in 100,000 individuals.3 However, when individuals with skin of color develop melanoma, they are more likely than their White counterparts to have acral melanoma (acral lentiginous melanoma), one of the deadliest types.1 In a case series of Black patients with melanoma (N=48) from 2 tertiary care centers in Texas, 30 of 40 primary cutaneous melanomas (75%) were located on acral skin.4 Overall, 13 patients developed stage IV disease and 12 died due to disease progression. All patients who developed distant metastases or died of melanoma had acral melanoma.4 Individuals of Asian descent also have a high incidence of acral melanoma, as shown in research from Japan.5-9
Key Clinical Features in Individuals With Darker Skin Tones
Dermoscopy is an evidence-based clinical examination method for earlier diagnosis of cutaneous melanoma, including on acral skin.10,11 Benign nevi on the volar skin as well as the palms and soles tend to have one of these 3 dermoscopic patterns: parallel furrow, lattice, or irregular fibrillar. The pattern that is most predictive of volar melanoma is the parallel ridge pattern (PRP) (Figures A and B [insets]), which showed a high specificity (99.0%) and very high negative predictive value (97.7%) for malignant melanoma in a Japanese population.7 The PRP data from this study cannot be applied reliably to Black individuals, especially because benign ethnic melanosis and other benign conditions can demonstrate PRP.12 Reliance on the PRP as a diagnostic clue could result in unneccessary biopsies in as many as 50% of Black patients with benign plantar hyperpigmentation.2 Furthermore, biopsies of the plantar surface can be painful and cause pain while walking.
It has been suggested that PRP seen on dermoscopy in benign hyperpigmentation such as ethnic melanosis and nevi may preserve the acrosyringia (eccrine gland openings on the ridge), whereas PRP in melanoma may obliterate the acrosyringia.13 This observation is based on case reports only and needs further study. However, if validated, it could be a useful diagnostic clue.
Worth noting
In a retrospective cohort study of skin cancer in Black individuals (n=165) at a New York City–based cancer center from 2000 to 2020, 68% of patients were diagnosed with melanomas—80% were the acral subtype and 75% displayed a PRP. However, the surrounding uninvolved background skin, which was visible in most cases, also demonstrated a PRP.14 Because of the high morbidity and mortality rates of acral melanoma, clinicians should biopsy or immediately refer patients with concerning plantar hyperpigmentation to a dermatologist.
Health disparity highlight
The mortality rate for acral melanoma in Black patients is disproportionately high for the following reasons15,16:
• Patients and health care providers do not expect to see melanoma in Black patients (it truly is rare!), so screening and education on sun protection are limited.
• Benign ethnic melanosis makes it more difficult to distinguish between early acral melanoma and benign skin changes.
• Black patients and other US patient populations with skin of color may be less likely to have health insurance, which contributes to inequities in access to health care. As of 2022, the uninsured rates for nonelderly American Indian and Alaska Native, Hispanic, Native Hawaiian and Other Pacific Islander, Black, and White individuals were 19.1%, 18.0%, 12.7%, 10.0%, and 6.6%, respectively.17
Multi-institutional registries could improve understanding of acral melanoma in Black patients.4 More studies are needed to help differentiate between the dermoscopic finding of PRP in benign ethnic melanosis vs malignant melanoma.
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
1. Huang K, Fan J, Misra S. Acral lentiginous melanoma: incidence and survival in the United States, 2006-2015: an analysis of the SEER registry. J Surg Res. 2020;251:329-339. doi:10.1016/j.jss.2020.02.010
2. Coleman WP, Gately LE, Krementz AB, et al. Nevi, lentigines, and melanomas in blacks. Arch Dermatol. 1980;116:548-551.
3. Centers for Disease Control and Prevention. Melanoma Incidence and Mortality, United States: 2012-2016. USCS Data Brief, no. 9. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no9-melanoma-incidence-mortality-UnitedStates-2012-2016.htm
4. Wix SN, Brown AB, Heberton M, et al. Clinical features and outcomes of black patients with melanoma. JAMA Dermatol. 2024;160:328-333. doi:10.1001/jamadermatol.2023.5789
5. Saida T, Koga H. Dermoscopic patterns of acral melanocytic nevi: their variations, changes, and significance. Arch Dermatol. 2007;143:1423-1426. doi:10.1001/archderm.143.11.1423
6. Saida T, Koga H, Uhara H. Key points in dermoscopic differentiation between early acral melanoma and acral nevus. J Dermatol. 2011;38:25-34. doi:10.1111/j.1346-8138.2010.01174.x
7. Saida T, Miyazaki A, Oguchi S. Significance of dermoscopic patterns in detecting malignant melanoma on acral volar skin: results of a multicenter study in Japan. Arch Dermatol. 2004;140:1233-1238. doi:10.1001/archderm.140.10.1233
8. Saida T, Koga H, Uhara H. Dermoscopy for acral melanocytic lesions: revision of the 3-step algorithm and refined definition of the regular and irregular fibrillar pattern. Dermatol Pract Concept. 2022;12:e2022123. doi:10.5826/dpc.1203a123
9. Heath CR, Usatine RP. Melanoma. Cutis. 2022;109:284-285. doi:10.12788/cutis.0513.
10. Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Visual inspection and dermoscopy, alone or in combination, for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018; 12:CD011901. doi:10.1002/14651858.CD011901.pub2
11. Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked-eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676. doi:10.1111/j.1365-2133.2008.08713.x
12. Phan A, Dalle S, Marcilly MC, et al. Benign dermoscopic parallel ridge pattern variants. Arch Dermatol. 2011;147:634. doi:10.1001/archdermatol.2011.47
13. Fracaroli TS, Lavorato FG, Maceira JP, et al. Parallel ridge pattern on dermoscopy: observation in non-melanoma cases. An Bras Dermatol. 2013;88:646-648. doi:10.1590/abd1806-4841.20132058
14. Manci RN, Dauscher M, Marchetti MA, et al. Features of skin cancer in black individuals: a single-institution retrospective cohort study. Dermatol Pract Concept. 2022;12:e2022075. doi:10.5826/dpc.1202a75
15. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dematol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
16. Ingrassia JP, Stein JA, Levine A, et al. Diagnosis and management of acral pigmented lesions. Dermatol Surg Off Publ Am Soc Dermatol Surg Al. 2023;49:926-931. doi:10.1097/DSS.0000000000003891
17. Hill L, Artiga S, Damico A. Health coverage by race and ethnicity, 2010-2022. Kaiser Family Foundation. Published January 11, 2024. Accessed May 9, 2024. https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity
In HPV-Positive Head and Neck Cancer, Treatment Is a Quandary
The topic of head and neck cancer is especially timely since the disease is evolving. A hematologist/oncologist with the Association of VA Hematology/Oncology (AVAHO) told colleagues that specialists are grappling with how to de-escalate treatment.
Molly Tokaz, MD, of Veterans Affairs Puget Sound Health Care and the University of Washington said tobacco is fading as a cause as fewer people smoke, and that human papillomavirus (HPV) is triggering more cases. HPV-positive patients have better prognoses, raising the prospect that their treatment could be adjusted.
“Instead of increasing the amount of therapy we're giving, we’re trying to peel it back,” she said. “If they’re going to respond no matter what we do, why are we going in with these huge weapons of mass destruction if we can get the same results with something more like a light infantry?”
Tokaz spoke about deescalating therapy at a May 2024 regional AVAHO meeting in Seattle that was focused on head and neck cancer. She elaborated on her presentation in an interview with Federal Practitioner. according to Tokaz, 90% of head and neck cancers are mucosal squamous cell carcinomas (SCC). HPV is associated specifically with nasopharyngeal cancer, which is distinct from SCC, and oropharyngeal cancer, which has been linked to better prognoses.
HPV-positive head and neck cancer is a unique entity with its own epidemiology, clinical prognosis, and treatment. “Patients tend to be younger without the same number of comorbid conditions,” Tokaz said. “Some of them are never smokers or light smokers. So, it's a different demographic than we’ve seen traditionally.”
The bad news is that HPV-associated head and neck cancer numbers are on the rise. Fortunately, outcomes tend to be better for the HPV-positive forms.
As for therapy for head and neck cancer, immunotherapy and targeted therapy play smaller roles than in some other cancers because the form tends to be diagnosed in early stages before metastases appear. Surgery, chemotherapy, and radiation remain the major treatments. According to Tokaz’s presentation, surgery, or radiation—often with minimal adjuvant chemotherapy—can be appropriate for the earliest stage I and II cases of head and neck SCC. (She noted that HPV-positive oropharyngeal squamous cell carcinoma has its own staging system.)
Stage I and II cases make up 15% of new diagnoses and have a 5-year survival rate of > 70%. “In the earliest days, our main role was to make radiation work better and reduce it while adding a minimum amount of toxicity mutations,” she said. “Chemotherapy can help, but it’s only demonstrated improvement in overall survival in patients with positive surgical margins and extracapsular extension.”
In Stage III, IVA, and IVB cases, which make up 70% of new diagnoses, chemotherapy plus radiation is recommended. Five-year survival drops to 30% to 50%. Finally, 10% of new diagnoses are Stage IVC, which is incurable and median survival is < 1 year.
Since HPV-positive patients generally have better prognoses, oncologists are considering how to adjust their treatment. However, Tokaz notes that clinical trials have not shown a benefit from less intensive treatment in these patients. “At this point, we still treat them the same way as HPV-negative patients. But it's an ongoing area of research.”
Researchers are also exploring how to optimize regimens in patients ineligible for treatment with the chemotherapy agent cisplatin. “These folks have been traditionally excluded from clinical trials because they’re sicker,” Tokaz explained. “Researchers normally want the fittest and the best patients [in trials]. If you give a drug to someone with a lot of other comorbid conditions, they might not do as well with it, and it makes your drug look bad.”
Figuring out how to treat these patients is an especially urgent task in head and neck cancer because so many patients are frail and have comorbidities. More globally, Tokaz said the rise of HPV-related head and neck cancer highlights the importance of HPV vaccination, which is crucial for preventing cervical and anal cancer in addition to head and neck cancer. “HPV vaccination for children and young adults is crucial.”
Molly Tokaz, MD, reported no relevant financial relationships.
The topic of head and neck cancer is especially timely since the disease is evolving. A hematologist/oncologist with the Association of VA Hematology/Oncology (AVAHO) told colleagues that specialists are grappling with how to de-escalate treatment.
Molly Tokaz, MD, of Veterans Affairs Puget Sound Health Care and the University of Washington said tobacco is fading as a cause as fewer people smoke, and that human papillomavirus (HPV) is triggering more cases. HPV-positive patients have better prognoses, raising the prospect that their treatment could be adjusted.
“Instead of increasing the amount of therapy we're giving, we’re trying to peel it back,” she said. “If they’re going to respond no matter what we do, why are we going in with these huge weapons of mass destruction if we can get the same results with something more like a light infantry?”
Tokaz spoke about deescalating therapy at a May 2024 regional AVAHO meeting in Seattle that was focused on head and neck cancer. She elaborated on her presentation in an interview with Federal Practitioner. according to Tokaz, 90% of head and neck cancers are mucosal squamous cell carcinomas (SCC). HPV is associated specifically with nasopharyngeal cancer, which is distinct from SCC, and oropharyngeal cancer, which has been linked to better prognoses.
HPV-positive head and neck cancer is a unique entity with its own epidemiology, clinical prognosis, and treatment. “Patients tend to be younger without the same number of comorbid conditions,” Tokaz said. “Some of them are never smokers or light smokers. So, it's a different demographic than we’ve seen traditionally.”
The bad news is that HPV-associated head and neck cancer numbers are on the rise. Fortunately, outcomes tend to be better for the HPV-positive forms.
As for therapy for head and neck cancer, immunotherapy and targeted therapy play smaller roles than in some other cancers because the form tends to be diagnosed in early stages before metastases appear. Surgery, chemotherapy, and radiation remain the major treatments. According to Tokaz’s presentation, surgery, or radiation—often with minimal adjuvant chemotherapy—can be appropriate for the earliest stage I and II cases of head and neck SCC. (She noted that HPV-positive oropharyngeal squamous cell carcinoma has its own staging system.)
Stage I and II cases make up 15% of new diagnoses and have a 5-year survival rate of > 70%. “In the earliest days, our main role was to make radiation work better and reduce it while adding a minimum amount of toxicity mutations,” she said. “Chemotherapy can help, but it’s only demonstrated improvement in overall survival in patients with positive surgical margins and extracapsular extension.”
In Stage III, IVA, and IVB cases, which make up 70% of new diagnoses, chemotherapy plus radiation is recommended. Five-year survival drops to 30% to 50%. Finally, 10% of new diagnoses are Stage IVC, which is incurable and median survival is < 1 year.
Since HPV-positive patients generally have better prognoses, oncologists are considering how to adjust their treatment. However, Tokaz notes that clinical trials have not shown a benefit from less intensive treatment in these patients. “At this point, we still treat them the same way as HPV-negative patients. But it's an ongoing area of research.”
Researchers are also exploring how to optimize regimens in patients ineligible for treatment with the chemotherapy agent cisplatin. “These folks have been traditionally excluded from clinical trials because they’re sicker,” Tokaz explained. “Researchers normally want the fittest and the best patients [in trials]. If you give a drug to someone with a lot of other comorbid conditions, they might not do as well with it, and it makes your drug look bad.”
Figuring out how to treat these patients is an especially urgent task in head and neck cancer because so many patients are frail and have comorbidities. More globally, Tokaz said the rise of HPV-related head and neck cancer highlights the importance of HPV vaccination, which is crucial for preventing cervical and anal cancer in addition to head and neck cancer. “HPV vaccination for children and young adults is crucial.”
Molly Tokaz, MD, reported no relevant financial relationships.
The topic of head and neck cancer is especially timely since the disease is evolving. A hematologist/oncologist with the Association of VA Hematology/Oncology (AVAHO) told colleagues that specialists are grappling with how to de-escalate treatment.
Molly Tokaz, MD, of Veterans Affairs Puget Sound Health Care and the University of Washington said tobacco is fading as a cause as fewer people smoke, and that human papillomavirus (HPV) is triggering more cases. HPV-positive patients have better prognoses, raising the prospect that their treatment could be adjusted.
“Instead of increasing the amount of therapy we're giving, we’re trying to peel it back,” she said. “If they’re going to respond no matter what we do, why are we going in with these huge weapons of mass destruction if we can get the same results with something more like a light infantry?”
Tokaz spoke about deescalating therapy at a May 2024 regional AVAHO meeting in Seattle that was focused on head and neck cancer. She elaborated on her presentation in an interview with Federal Practitioner. according to Tokaz, 90% of head and neck cancers are mucosal squamous cell carcinomas (SCC). HPV is associated specifically with nasopharyngeal cancer, which is distinct from SCC, and oropharyngeal cancer, which has been linked to better prognoses.
HPV-positive head and neck cancer is a unique entity with its own epidemiology, clinical prognosis, and treatment. “Patients tend to be younger without the same number of comorbid conditions,” Tokaz said. “Some of them are never smokers or light smokers. So, it's a different demographic than we’ve seen traditionally.”
The bad news is that HPV-associated head and neck cancer numbers are on the rise. Fortunately, outcomes tend to be better for the HPV-positive forms.
As for therapy for head and neck cancer, immunotherapy and targeted therapy play smaller roles than in some other cancers because the form tends to be diagnosed in early stages before metastases appear. Surgery, chemotherapy, and radiation remain the major treatments. According to Tokaz’s presentation, surgery, or radiation—often with minimal adjuvant chemotherapy—can be appropriate for the earliest stage I and II cases of head and neck SCC. (She noted that HPV-positive oropharyngeal squamous cell carcinoma has its own staging system.)
Stage I and II cases make up 15% of new diagnoses and have a 5-year survival rate of > 70%. “In the earliest days, our main role was to make radiation work better and reduce it while adding a minimum amount of toxicity mutations,” she said. “Chemotherapy can help, but it’s only demonstrated improvement in overall survival in patients with positive surgical margins and extracapsular extension.”
In Stage III, IVA, and IVB cases, which make up 70% of new diagnoses, chemotherapy plus radiation is recommended. Five-year survival drops to 30% to 50%. Finally, 10% of new diagnoses are Stage IVC, which is incurable and median survival is < 1 year.
Since HPV-positive patients generally have better prognoses, oncologists are considering how to adjust their treatment. However, Tokaz notes that clinical trials have not shown a benefit from less intensive treatment in these patients. “At this point, we still treat them the same way as HPV-negative patients. But it's an ongoing area of research.”
Researchers are also exploring how to optimize regimens in patients ineligible for treatment with the chemotherapy agent cisplatin. “These folks have been traditionally excluded from clinical trials because they’re sicker,” Tokaz explained. “Researchers normally want the fittest and the best patients [in trials]. If you give a drug to someone with a lot of other comorbid conditions, they might not do as well with it, and it makes your drug look bad.”
Figuring out how to treat these patients is an especially urgent task in head and neck cancer because so many patients are frail and have comorbidities. More globally, Tokaz said the rise of HPV-related head and neck cancer highlights the importance of HPV vaccination, which is crucial for preventing cervical and anal cancer in addition to head and neck cancer. “HPV vaccination for children and young adults is crucial.”
Molly Tokaz, MD, reported no relevant financial relationships.
Potential Impact of USPS Mail Delivery Delays on Colorectal Cancer Screening Programs
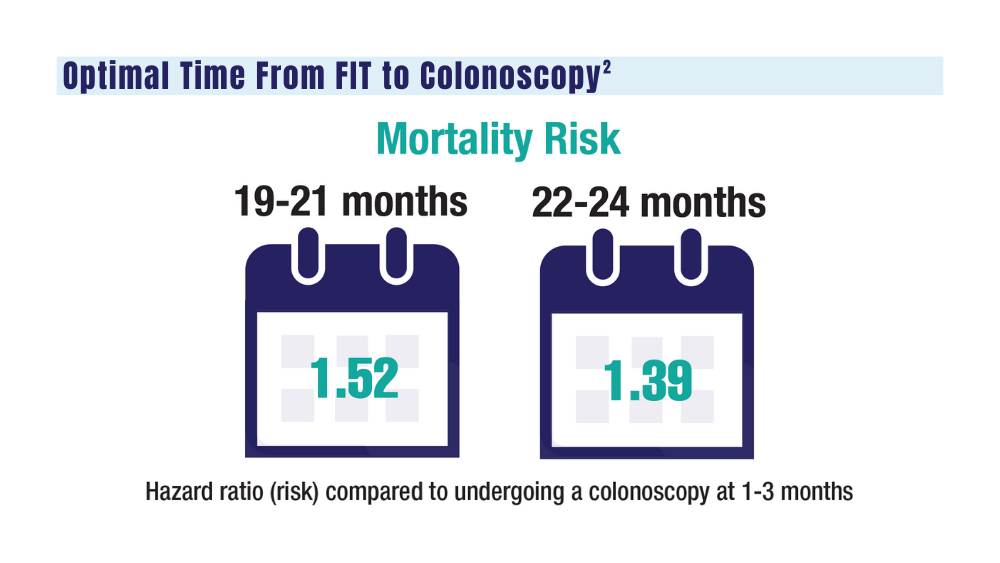
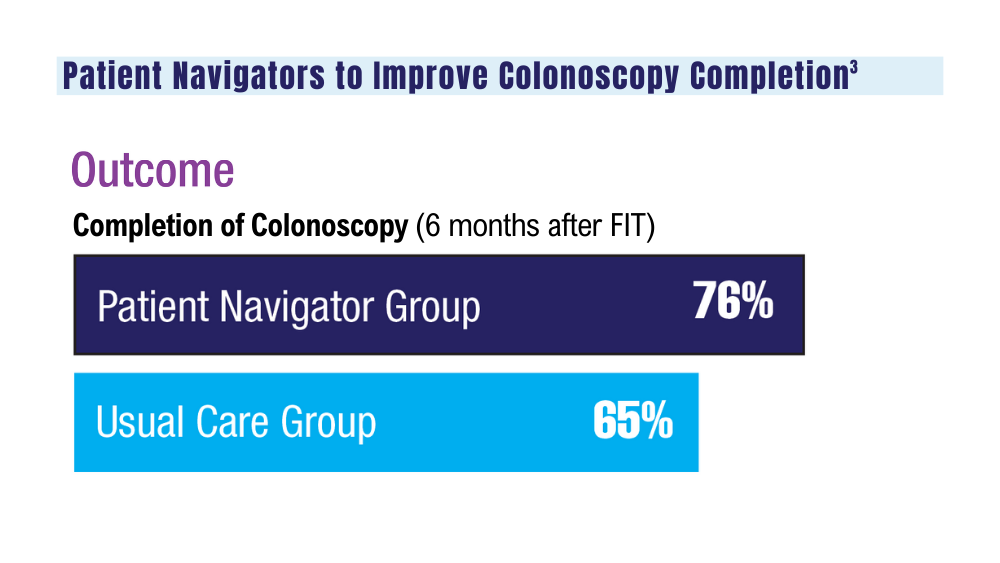
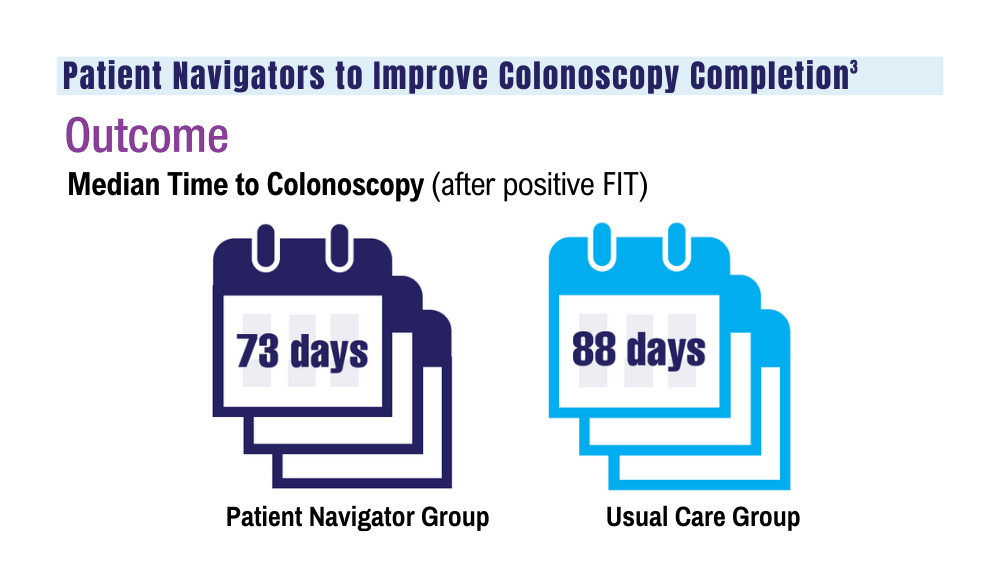
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
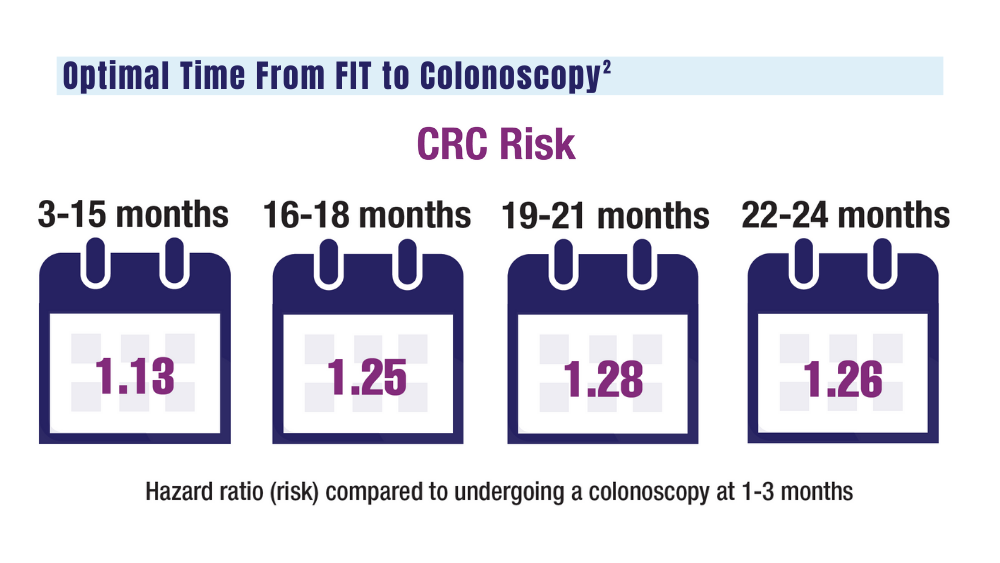
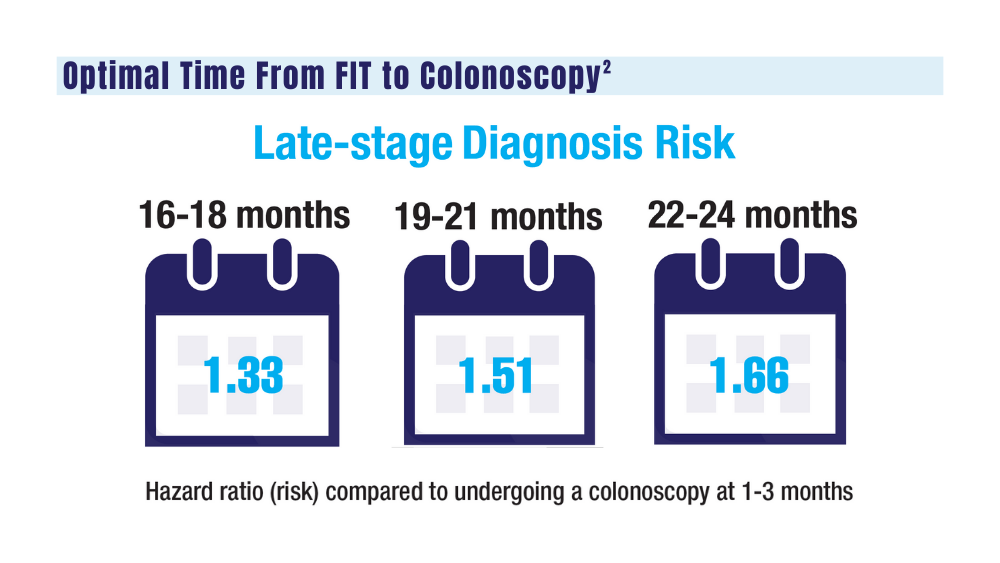
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
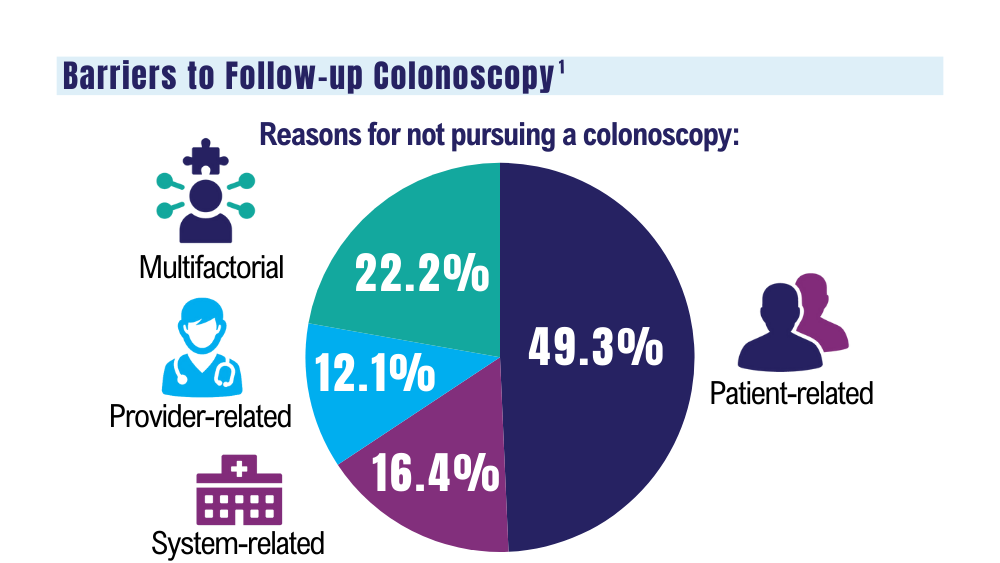
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
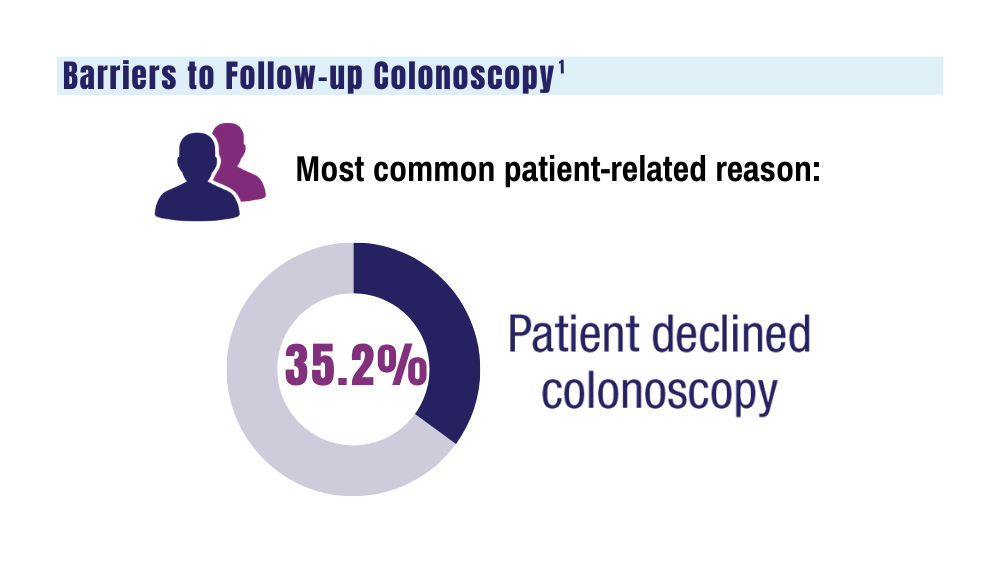
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 In 2022, there were an estimated 151,030 new CRC cases and 52,580 deaths.1 Options for CRC screening of patients at average risk include stool tests (annual fecal immunochemical test [FIT], annual guaiac-based fecal occult blood test, or stool FIT-DNA test every 1 to 3 years), colonoscopies every 10 years, flexible sigmoidoscopies every 5 years (or every 10 years with annual FIT), and computed tomography (CT) colonography every 5 years.2 Many health care systems use annual FIT for patients at average risk. Compared with guaiac-based fecal occult blood testing, FIT does not require dietary or medication modifications and yields greater sensitivity and patient participation.3
The COVID-19 pandemic and staffing issues have caused a scheduling backlog for screening, diagnostic, and surveillance endoscopies at some medical centers. As a result, FIT has become the primary means of CRC screening at these institutions. FIT kits for home use are typically distributed to eligible patients at an office visit or by mail, and patients are then instructed to mail the kits back to the laboratory. For the test to be as sensitive as possible, FIT kit manufacturers advise laboratory analysis within 14 to 15 days of collection, if stored at ambient temperature, and to reject the sample if it does not meet testing criteria for stability. Delayed FIT sample analysis has been associated with higher false-negative rates because of hemoglobin degradation.4 FIT sample exposure to high ambient temperatures also has been linked to decreased sensitivity for detecting CRC.5
US Postal Service (USPS) mail delivery delays have plagued many areas of the country. A variety of factors, including the COVID-19 pandemic, understaffing, changes in USPS policies, closure of post offices, and changes in mail delivery standards, may also be contributory causes. According to the USPS website, delivery standard for first-class mail is 1 to 5 days, but this is not guaranteed.6
The Jesse Brown Veterans Affairs Medical Center (JBVAMC) laboratory in Chicago has reported receiving FIT kit envelopes in batches by the USPS, with some prepaid first-class business reply envelopes delivered up to 60 days after the time of sample collection. Polymedco, a company that assists US Department of Veterans Affairs (VA) medical centers with logistics of FIT programs for CRC screening, reports that USPS batching of FIT kits leading to delayed delivery has been a periodic problem for medical centers around the country. Polymedco staff remind USPS staff about 4 points when they encounter this issue: Mailers are first-class mail; mailers contain a human biologic specimen that has limited viability; the biological sample used for detecting cancer is time sensitive; and delays in delivery by holding/batching kits could impact morbidity and mortality. Reviewing these key points with local USPS staff usually helps, however, batching and delayed delivery of the FIT kits can sometimes recur with USPS staffing turnover.
Tracking and identifying when a patient receives the FIT kit is difficult. Patients are instructed to write the date of collection on the kit, so the receiving laboratory knows whether the sample can be reliably analyzed. When patients are notified about delayed delivery of their sample, a staff member asks if they postponed dropping the kit in the mail. Most patients report mailing the sample within 1 to 2 days of collection. Tracking and dating each step of FIT kit events is not feasible with a mass mailing campaign. In our experience, most patients write the date of collection on the kit. If a collection date is not provided, the laboratory will call the patient to confirm a date. Cheng and colleagues reviewed the causes for FIT specimen rejection in a laboratory analyzing specimens for VA patients and found that 14% of submitted samples were rejected because the specimen was received > 14 days after collection, and 6% because the patient did not record the collection date. With a series of interventions aimed at reminding patients and improving laboratory procedures, rates of rejection for these 2 causes were reduced to < 4%.7 USPS delays were not identified as a factor or tracked in this study.
It is unclear why the USPS sometimes holds FIT kits at their facilities and then delivers large bins of them at the same time. Because FIT kits should be analyzed within 14 to 15 days of sample collection to assure reliable results, mail delivery delays can result in increased sample rejection. Based on the JBVAMC experience, up to 30% of submitted samples might need to be discarded when batched delivery takes place. In these cases, patients need to be contacted, informed of the problem, and asked to submit new kits. Understandably, patients are reluctant to repeat this type of testing, and we are concerned this could lead to reduced rates of CRC screening in affected communities.
As an alternative to discarding delayed samples, laboratories could report the results of delayed FIT kits with an added comment that “negative test results may be less reliable due to delayed processing,” but this approach would raise quality and medicolegal concerns. Clinicians have reached out to local USPS supervisory personnel with mixed results. Sometimes batching and delayed deliveries stop for a few months, only to resume without warning. Dropping off the sample directly at the laboratory is not a realistic option for most patients. Some patients can be convinced to submit another sample, some elect to switch to other CRC screening strategies, while others, unfortunately, decline further screening efforts.
Laboratory staff can be overwhelmed with having to process hundreds of samples in a short time frame, especially because there is no way of knowing when USPS will make a batched delivery. Laboratory capacities can limit staff at some facilities to performing analysis of only 10 tests at a time. The FIT kits should be delivered on a rolling basis and without delay so that the samples can be reliably analyzed with a predictable workload for the laboratory personnel and without unexpected surges.
When health care facilities identify delayed mail delivery of FIT kits via USPS, laboratories should first ensure that the correct postage rates are used on the prepaid envelopes and that their USPS accounts are properly funded, so that insufficient funds are not contributing to delayed deliveries. Stakeholders should then reach out to local USPS supervisory staff and request that the practice of batching the delivery of FIT kits be stopped. Educating USPS supervisory staff about concerns related to decreased test reliability associated with delayed mail delivery can be a persuasive argument. Adding additional language to the preprinted envelopes, such as “time sensitive,” may also be helpful. Unfortunately, the JBVAMC experience has been that the problem initially gets better after contacting the USPS, only to unexpectedly resurface months later. This cycle has been repeated several times in the past 2 years at JBVAMC.
All clinicians involved in CRC screening and treatment at institutions that use FIT kits need to be aware of the impact that local USPS delays can have on the reliability of these results. Health care systems should be prepared to implement mitigation strategies if they encounter significant delays with mail delivery. If delays cannot be reliably resolved by working with the local USPS staff, consider involving national USPS oversight bodies. And if the problems persist despite an attempt to work with the USPS, some institutions might find it feasible to offer drop boxes at their clinics and instruct patients to drop off FIT kits immediately following collection, in lieu of mailing them. Switching to private carriers is not a cost-effective alternative for most health care systems, and some may exclude rural areas. Depending on the local availability and capacity of endoscopists, some clinicians might prioritize referring patients for screening colonoscopies or screening flexible sigmoidoscopies, and might deemphasize FIT kits as a preferred option for CRC screening. CT colonography is an alternative screening method that is not as widely offered, nor as widely accepted at this time.
Conclusions
CRC screening is an essential part of preventive medicine, and the percentage of eligible patients screened is a well-established quality metric in primary care settings. Health care systems, clinicians, and laboratories must be vigilant to ensure that USPS delays in delivering FIT kits do not negatively impact their CRC screening programs. Facilities should actively monitor for delays in the return of FIT kits.
Despite the widespread use of mail-order pharmacies and the use of mail to communicate notifications about test results and follow-up appointments, unreliable or delayed mail delivery traditionally has not been considered a social determinant of health.8 This article highlights the impact delayed mail delivery can have on health outcomes. Disadvantaged communities in inner cities and rural areas have been disproportionately affected by the worsening performance of the USPS over the past few years.9 This represents an underappreciated public health concern in need of a sustainable solution.
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
2. Centers for Disease Control and Prevention. Colorectal cancer screening tests. Updated February 23, 2023. Accessed March 14, 2024. https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm
3. van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135(1):82-90. doi:10.1053/j.gastro.2008.03.040
4. van Rossum LG, van Rijn AF, van Oijen MG, et al. False negative fecal occult blood tests due to delayed sample return in colorectal cancer screening. Int J Cancer. 2009;125(4):746-750. doi:10.1002/ijc.24458
5. Doubeni CA, Jensen CD, Fedewa SA, et al. Fecal immunochemical test (FIT) for colon cancer screening: variable performance with ambient temperature. J Am Board Fam Med. 2016;29(6):672-681. doi:10.3122/jabfm.2016.06.160060
6. United States Postal Service. Shipping and mailing with USPS. Accessed March 14, 2024. https://www.usps.com/ship
7. Cheng C, Ganz DA, Chang ET, Huynh A, De Peralta S. Reducing rejected fecal immunochemical tests received in the laboratory for colorectal cancer screening. J Healthc Qual. 2019;41(2):75-82.doi:10.1097/JHQ.0000000000000181
8. Hussaini SMQ, Alexander GC. The United States Postal Service: an essential public health agency? J Gen Intern Med. 2020;35(12):3699-3701. doi:10.1007/s11606-020-06275-2
9. Hampton DJ. Colorado mountain towns are plagued by post office delays as residents wait weeks for medication and retirement checks. NBC News. February 25, 2023. Accessed March 14, 2024. https://www.nbcnews.com/news/us-news/colo-mountain-towns-are-plagued-post-office-delays-residents-wait-week-rcna72085
VA to Expand Cancer Prevention Services
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
The US Department of Veterans Affairs (VA) announced plans to expand preventive services, health care, and benefits for veterans with cancer.
Urethral cancers are set to be added to the list of > 300 conditions considered presumptive under the Sergeant First Class Heath Robinson Honoring our Promise to Address Comprehensive Toxics (PACT) Act of 2022. Veterans deployed to Iraq, Afghanistan, Somalia, Djibouti, Egypt, Jordan, Lebanon, Syria, Yemen, Uzbekistan, and the entire Southwest Asia theater will not need to prove their service caused their urethral cancer in order to receive treatment for it. Additionally, the VA plans to evaluate whether there is a relationship between urinary bladder and ureteral cancers and toxic exposures for these veterans, and determine whether these conditions are presumptive. The VA has already screened > 5 million veterans for toxic exposures under the PACT Act, as part of an ongoing mission to expand cancer care services.
The VA is also set to expand access to screening programs in 2024 by providing:
- genetic testing to every veteran who may need it;
- lung cancer screening programs to every VA medical center; and
- home tests for colorectal cancer to > 1 million veterans nationwide.
The VA continues to expand the reach of smoking cessation services, with ≥ 6 additional sites added to the Quit VET eReferral program by the end of 2024, and a new pilot program to integrate smoking cessation services into lung cancer screening.
The VA has already taken steps to build on the Biden-Harris Administration Cancer Moonshot program, which has the goals of preventing ≥ 4 million cancer deaths by 2047 and to improve the experience of individuals with cancer. For instance, it has prioritized claims processing for veterans with cancer and expanded cancer risk assessments and mammograms to veterans aged < 40 years, regardless of age, symptoms, family history, or whether they are enrolled in VA health care. In September, the VA and the National Cancer Institute announced a data-sharing collaboration to better understand and treat cancer among veterans.
“VA is planting the seeds for the future of cancer care,” said VHA Under Secretary for Health Shereef Elnahal, MD. “By investing in screenings, expanding access, and embracing cutting-edge technologies, VA is revolutionizing cancer care delivery, providing the best care possible to our nation’s heroes.”
Cancer Data Trends 2024: Breast Cancer
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
1. US Department of Veterans Affairs. Mammogram/breast health. March 28, 2022. Accessed January 10, 2024. https://www.womenshealth.va.gov/topics/mammogram-breast-health.asp
2. First anniversary of Mammography and Medical Options Act (MAMMO Act). VA News. June 6, 2023. Accessed January 10, 2024. https://news.va.gov/120476/anniversary-mammography-and-medicaloptions-act/
3. Moss HA, Rasmussen, KM, Patil, V, et al. Demographic characteristics of veterans diagnosed with breast and gynecologic cancers: a comparative analysis with the general population. Abstract presented at: Annual Meeting of the Association of VA Hematology/ Oncology (AVAHO); September 29–October 1, 2023; Chicago, IL. Abstract 47.
4. US Department of Veterans Affairs. Racial and ethnic minority veterans. Updated July 9, 2020. Accessed January 10, 2024. https:// www.va.gov/HEALTHEQUITY/Race_Ethnicity.asp
5. Stringer-Reasor EM, Elkhanany A, Khoury K, Simon MA, Newman LA. Disparities in breast cancer associated with African American identity. Am Soc Clin Oncol Educ Book. 2021;41:e29-e46. doi:10.1200/EDBK_319929
6. Landry I, Sumbly V, Vest M. Advancements in the treatment of triple- CANCER DATA TRENDS 2024 MARCH 2024 • FEDERAL PRACTITIONER negative breast cancer: a narrative review of the literature. Cureus. 2022;14(2):e21970. doi:10.7759/cureus.21970
7. Schlam I, Tolaney SM, Tarantino P. How I treat HER2-low advanced breast cancer. Breast. 2023;67:116-123. doi:10.1016/j.breast.2023.01.005
8. Modi S, Jacot W, Yamashita T, et al; for the DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9-20. doi:10.1056/NEJMoa2203690
Cancer Data Trends 2024: Colorectal Cancer
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
1. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469-476. doi:10.1016/j.cgh.2018.05.022
2. San Miguel Y, Demb J, Martinez ME, Gupta S, May FP. Time to colonoscopy after abnormal stool-based screening and risk for colorectal cancer incidence and mortality. Gastroenterology. 2021;160(6):1997-2005.e3. doi:10.1053/j.gastro.2021.01.219
3. Coronado GD, Rawlings AM, Petrik AF, et al. Precision patient navigation to improve rates of follow-up colonoscopy, an individual randomized effectiveness trial. Cancer Epidemiol Biomarkers Prev. 2021;30(12):2327-2333. doi:10.1158/1055-9965.EPI-20-1793
4. Barnell EK, Wurtzler EM, La Rocca J, et al. Multitarget stool RNA test for colorectal cancer screening. JAMA. 2023;330(18):1760- 1768. doi:10.1001/jama.2023.22231
5. McNamara D. Two multitarget stool tests show promise for CRC screening: studies. Medscape. Published October 22, 2023. Accessed December 20, 2023. https://www.medscape.com/viewarticle/997609#vp_1
6. Clinical validation of an optimized multi-target stool DNA (Mt-sDNA 2.0) test, for colorectal cancer screening “BLUE-C.” ClinicalTrials. gov identifier: NCT04144738. Updated September 1, 2023. Accessed December 20, 2023. https://www.clinicaltrials.gov/study/ NCT04144738
7. Universal Diagnostics. Universal DX Presents Data from Large, 1,000-patient, Multi-Cohort Study Proving 93% Sensitivity for Colorectal Cancer and 54% Sensitivity for Advanced Adenoma at 92% Specificity. Press Release. Published May 2023. Accessed January 26. 2024.
Cancer Data Trends 2024: Lung Cancer
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
1. Wolf AMD, Oeffinger KC, Shih TYC, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin. 2023;10.3322/caac.21811. doi:10.3322/caac.21811
2. US Department of Veterans Affairs. VA promotes high-quality, patient-centered lung cancer screening for veterans. Published June 15, 2023. Accessed December 18, 2023. http://www.hsrd.research.va.gov/impacts/lcs.cfm
3. Navuluri N, Morrison S, Green CL, et al. Racial disparities in lung cancer screening among veterans, 2013 to 2021. JAMA Netw Open. 2023;6(6):e2318795. doi:10.1001/jamanetworkopen.2023.18795
4. Bruno DS, Hess LM, Li X, Su EW, Patel M. Disparities in biomarker testing and clinical trial enrollment among patients with lung, breast, or colorectal cancers in the United States. JCO Precis Oncol. 2022;6:e2100427. doi:10.1200/PO.21.00427
5. Jalal SI, Guo A, Ahmed S, Kelley MJ. Analysis of actionable genetic alterations in lung carcinoma from the VA National Precision Oncology Program. Semin Oncol. 2022;S0093-7754(22)00054-9. doi:10.1053/j.seminoncol.2022.06.014
6. Williams CD, Allo MA, Gu L, Vashistha V, Press A, Kelley M. Health outcomes and healthcare resource utilization among veterans with stage IV non-small cell lung cancer treated with second-line chemotherapy versus immunotherapy. PLoS One. 2023;18(2):e0282020. doi:10.1371/journal.pone.0282020
7. US Food and Drug Administration. Oncology (cancer)/hematologic malignancies approval notifications. Updated December 15, 2023. Accessed December 18, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications
8. Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022;7(2):100408. doi:10.1016/j.esmoop.2022.100408
Cancer Data Trends 2024: Multiple Myeloma
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
1. Mahmood S, Gupta P, Ma H. Impact of time period of diagnosis, race, and military exposures on the survival of US military veterans with multiple myeloma and/or plasmacytoma. J Clin Oncol. 2023;41(16 suppl). Abstract e20061. https://doi.org/10.1200/jco.2023.41.16_suppl.e20061
2. National Cancer Institute. Cancer stat facts: myeloma. Accessed January 2, 2024. https://seer.cancer.gov/statfacts/html/mulmy.html
3. Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2022;33(1):117]. Ann Oncol. 2021;32(3):309-322. doi: 10.1016/j.annonc.2020.11.014
4. Su CT, Chen JC, Sussman JB. Virtual care for multiple myeloma in the COVID-19 era: interrupted time series analysis of Veterans Health Administration data. Leuk Lymphoma. 2023;64(5):1035-1039. doi: 10.1080/10428194.2023.2189989
5. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in patients with multiple myeloma. Cancer. 2022;128(10):1996-2004. doi: 10.1002/cncr.34134
6. O’Donnell EK, Shapiro YN, Yee AJ, et al. Quality of life, psychological distress, and prognostic perceptions in caregivers of patients with multiple myeloma. Blood Adv. 2022;6(17):4967-4974. doi: 10.1182/bloodadvances.2022007127
7. Ahmed N, Shahzad M, Shippey E, et al. Socioeconomic and racial disparity in chimeric antigen receptor T cell therapy access. Transplant Cell Ther. 2022;28(7):358-364. doi: 10.1016/j.jtct.2022.04.008
Cancer Data Trends 2024: Hepatocellular Carcinoma
1. Pinheiro PS, Jones PD, Medina H, et al. Incidence of etiology-specific hepatocellular carcinoma: diIverging trends and significant heterogeneity by race and ethnicity. Clin Gastroenterol Hepatol. Published online September 6, 2023. doi:10.1016/j.cgh.2023.08.016
2. Ju MR, Karalis JD, Chansard M, et al. Variation of hepatocellular carcinoma treatment patterns and survival across geographic regions in a veteran population. Ann Surg Oncol. 2022;29(13):8413-8420. doi:10.1245/s10434-022-12390-7
3. Veterans Health Administration. VA collaborative consensus on a pathway for the development of a multidisciplinary team to manage hepatocellular carcinoma. VA Liver Cancer Summit; March 8, 2019; Miami, FL. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/HCC-multidisciplinary-management-best-practices.pdf
4. Emerson L. Hepatocellular carcinoma treatment, survival varies among VA regions. US Medicine. Published June 12, 2023. Accessed December 14, 2023. https://www.usmedicine.com/clinical-topics/cancer/hepatocellular-carcinoma/hepatocellular-carcinoma-treatment-survival-varies-among-va-regions/
5. Agarwal PD, Haftoglou BA, Ziemlewicz TJ, Lucey MR, Said A. Psychosocial barriers and their impact on hepatocellular carcinoma care in US veterans: tumor board model of care. Fed Pract. 2022;39(suppl 2):S32-S36. doi:10.12788/fp.0272
6. Ito T, Nguyen MH. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J Hepatocell Carcinoma. 2023;10:413-428. doi:10.2147/JHC.S347959
7. Garren P, Serper M. Chronic hepatitis B in US veterans. Curr Hepatol Rep. 2019;18(3):310-315. doi:10.1007/s11901-019-00479-9
8. US Department of Veteran Affairs. Hepatitis C: information for veterans. Published November 2022. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/Hepatitis-C-Factsheet-Veterans.pdf
9. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
10. Elderkin J, Al Hallak N, Azmi AS, et al. Hepatocellular carcinoma: surveillance, diagnosis, evaluation and management. Cancers (Basel). 2023;15(21):5118. doi:http://doi.org/10.3390/cancers15215118
11. Wei H, Yang J, Lu R, et al. m6A modification of AC026356.1 facilitates hepatocellular carcinoma progression by regulating the IGF2BP1-IL11 axis. Sci Rep. 2023;13(1):19124. doi:10.1038/s41598-023-45449-w
12. Espinoza M, Muquith M, Lim M, Zhu H, Singal AG, Hsiehchen D. Disease etiology and outcomes after atezolizumab plus bevacizumab in hepatocellular carcinoma: post-hoc analysis of IMbrave150. Gastroenterology. 2023;165(1):286-288.e4. doi:10.1053/j.gastro.2023.02.042
13. Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10(1):3. doi:10.1186/s40364-021-00350-4
14. Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(10):670-681. doi:10.1038/s41575-022-00620-y
15. US Department of Veteran Affairs. VA Cooperative Studies Program (CSP). CSP #2023 PREventing liver cancer Mortality through Imaging with Ultrasound vs MRI (PREMIUM STUDY). Updated July 2022. Accessed December 14, 2023. https://www.vacsp.research.va.gov /CSP_2023/CSP_2023.asp
1. Pinheiro PS, Jones PD, Medina H, et al. Incidence of etiology-specific hepatocellular carcinoma: diIverging trends and significant heterogeneity by race and ethnicity. Clin Gastroenterol Hepatol. Published online September 6, 2023. doi:10.1016/j.cgh.2023.08.016
2. Ju MR, Karalis JD, Chansard M, et al. Variation of hepatocellular carcinoma treatment patterns and survival across geographic regions in a veteran population. Ann Surg Oncol. 2022;29(13):8413-8420. doi:10.1245/s10434-022-12390-7
3. Veterans Health Administration. VA collaborative consensus on a pathway for the development of a multidisciplinary team to manage hepatocellular carcinoma. VA Liver Cancer Summit; March 8, 2019; Miami, FL. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/HCC-multidisciplinary-management-best-practices.pdf
4. Emerson L. Hepatocellular carcinoma treatment, survival varies among VA regions. US Medicine. Published June 12, 2023. Accessed December 14, 2023. https://www.usmedicine.com/clinical-topics/cancer/hepatocellular-carcinoma/hepatocellular-carcinoma-treatment-survival-varies-among-va-regions/
5. Agarwal PD, Haftoglou BA, Ziemlewicz TJ, Lucey MR, Said A. Psychosocial barriers and their impact on hepatocellular carcinoma care in US veterans: tumor board model of care. Fed Pract. 2022;39(suppl 2):S32-S36. doi:10.12788/fp.0272
6. Ito T, Nguyen MH. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J Hepatocell Carcinoma. 2023;10:413-428. doi:10.2147/JHC.S347959
7. Garren P, Serper M. Chronic hepatitis B in US veterans. Curr Hepatol Rep. 2019;18(3):310-315. doi:10.1007/s11901-019-00479-9
8. US Department of Veteran Affairs. Hepatitis C: information for veterans. Published November 2022. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/Hepatitis-C-Factsheet-Veterans.pdf
9. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
10. Elderkin J, Al Hallak N, Azmi AS, et al. Hepatocellular carcinoma: surveillance, diagnosis, evaluation and management. Cancers (Basel). 2023;15(21):5118. doi:http://doi.org/10.3390/cancers15215118
11. Wei H, Yang J, Lu R, et al. m6A modification of AC026356.1 facilitates hepatocellular carcinoma progression by regulating the IGF2BP1-IL11 axis. Sci Rep. 2023;13(1):19124. doi:10.1038/s41598-023-45449-w
12. Espinoza M, Muquith M, Lim M, Zhu H, Singal AG, Hsiehchen D. Disease etiology and outcomes after atezolizumab plus bevacizumab in hepatocellular carcinoma: post-hoc analysis of IMbrave150. Gastroenterology. 2023;165(1):286-288.e4. doi:10.1053/j.gastro.2023.02.042
13. Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10(1):3. doi:10.1186/s40364-021-00350-4
14. Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(10):670-681. doi:10.1038/s41575-022-00620-y
15. US Department of Veteran Affairs. VA Cooperative Studies Program (CSP). CSP #2023 PREventing liver cancer Mortality through Imaging with Ultrasound vs MRI (PREMIUM STUDY). Updated July 2022. Accessed December 14, 2023. https://www.vacsp.research.va.gov /CSP_2023/CSP_2023.asp
1. Pinheiro PS, Jones PD, Medina H, et al. Incidence of etiology-specific hepatocellular carcinoma: diIverging trends and significant heterogeneity by race and ethnicity. Clin Gastroenterol Hepatol. Published online September 6, 2023. doi:10.1016/j.cgh.2023.08.016
2. Ju MR, Karalis JD, Chansard M, et al. Variation of hepatocellular carcinoma treatment patterns and survival across geographic regions in a veteran population. Ann Surg Oncol. 2022;29(13):8413-8420. doi:10.1245/s10434-022-12390-7
3. Veterans Health Administration. VA collaborative consensus on a pathway for the development of a multidisciplinary team to manage hepatocellular carcinoma. VA Liver Cancer Summit; March 8, 2019; Miami, FL. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/HCC-multidisciplinary-management-best-practices.pdf
4. Emerson L. Hepatocellular carcinoma treatment, survival varies among VA regions. US Medicine. Published June 12, 2023. Accessed December 14, 2023. https://www.usmedicine.com/clinical-topics/cancer/hepatocellular-carcinoma/hepatocellular-carcinoma-treatment-survival-varies-among-va-regions/
5. Agarwal PD, Haftoglou BA, Ziemlewicz TJ, Lucey MR, Said A. Psychosocial barriers and their impact on hepatocellular carcinoma care in US veterans: tumor board model of care. Fed Pract. 2022;39(suppl 2):S32-S36. doi:10.12788/fp.0272
6. Ito T, Nguyen MH. Perspectives on the underlying etiology of HCC and its effects on treatment outcomes. J Hepatocell Carcinoma. 2023;10:413-428. doi:10.2147/JHC.S347959
7. Garren P, Serper M. Chronic hepatitis B in US veterans. Curr Hepatol Rep. 2019;18(3):310-315. doi:10.1007/s11901-019-00479-9
8. US Department of Veteran Affairs. Hepatitis C: information for veterans. Published November 2022. Accessed December 14, 2023. https://www.hepatitis.va.gov/pdf/Hepatitis-C-Factsheet-Veterans.pdf
9. Janevska D, Chaloska-Ivanova V, Janevski V. Hepatocellular carcinoma: risk factors, diagnosis and treatment. Open Access Maced J Med Sci. 2015;3(4):732-736. doi:10.3889/oamjms.2015.111
10. Elderkin J, Al Hallak N, Azmi AS, et al. Hepatocellular carcinoma: surveillance, diagnosis, evaluation and management. Cancers (Basel). 2023;15(21):5118. doi:http://doi.org/10.3390/cancers15215118
11. Wei H, Yang J, Lu R, et al. m6A modification of AC026356.1 facilitates hepatocellular carcinoma progression by regulating the IGF2BP1-IL11 axis. Sci Rep. 2023;13(1):19124. doi:10.1038/s41598-023-45449-w
12. Espinoza M, Muquith M, Lim M, Zhu H, Singal AG, Hsiehchen D. Disease etiology and outcomes after atezolizumab plus bevacizumab in hepatocellular carcinoma: post-hoc analysis of IMbrave150. Gastroenterology. 2023;165(1):286-288.e4. doi:10.1053/j.gastro.2023.02.042
13. Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10(1):3. doi:10.1186/s40364-021-00350-4
14. Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(10):670-681. doi:10.1038/s41575-022-00620-y
15. US Department of Veteran Affairs. VA Cooperative Studies Program (CSP). CSP #2023 PREventing liver cancer Mortality through Imaging with Ultrasound vs MRI (PREMIUM STUDY). Updated July 2022. Accessed December 14, 2023. https://www.vacsp.research.va.gov /CSP_2023/CSP_2023.asp
Cancer Data Trends 2024
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure
The annual issue of Cancer Data Trends, produced in collaboration with the Association of VA Hematology/Oncology (AVAHO), highlights the latest research in some of the top cancers impacting US veterans.
Click to view the Digital Edition.
In this issue:
Hepatocellular Carcinoma
Special care for veterans, changes in staging, and biomarkers for early diagnosis
Lung Cancer
Guideline updates and racial disparities in veterans
Multiple Myeloma
Improving survival in the VA
Colorectal Cancer
Barriers to follow-up colonoscopies after FIT testing
B-Cell Lymphomas
Findings from the VA's National TeleOncology Program and recent therapy updates
Breast Cancer
A look at the VA's Risk Assessment Pipeline and incidence among veterans vs the general population
Genitourinary Cancers
Molecular testing in prostate cancer, improving survival for metastatic RCC, and links between bladder cancer and Agent Orange exposure