User login
Developing a Cancer Rehabilitation Program—Improving Access to Ancillary Services to Mitigate the Impact of Cancer and its Treatments for Veterans Diagnosed With Cancer
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Background
Approximately 56,000 Veterans are diagnosed with cancer every year in the VA system. Up to 90% of survivors have at least one impairment that decreases their quality of life, but only 2-9% are receiving cancer rehabilitation. Current research in cancer care demonstrates the importance of prospective surveillance, rehabilitation, and a multidisciplinary (MultiD) approach to cancer survivorship. Multi-D treatments help mitigate the effects of cancer and its treatments as the veterans proceed through care, improve outcomes, and streamline the process to meet all rehabilitation needs for those affected by cancer. Prior to the development of this program all services except navigation were available. Those diagnosed with cancer were not receiving prehabilitation and consults to ancillary services did not occur until after active cancer treatment was completed. CCRP united existing Multi-D programs to better serve the needs of veterans with cancer. Development of the CCRP CPRS Consult menu has allowed for improved access for both providers and veterans.
Methods
Identified the need for ancillary services during cancer survivorship, regardless of Veterans treatment location within or outside the VA system. Initiated tracking via CCR consults, developed a CCRP guidebook to identify all services available and how to access them as well as the CCCRP consult menu to create easier access for providers and veterans. Tracking via Multi-D departments that allow for tracking in CPRS via CCRP Consult.
Results
Prior to FY23 no cancer rehab consults existed. Consults received since program implementation: Navigation: 144, Physical Therapy: 102, Occupational Therapy: 7, Speech: 15. All other Multi-D did not track CCRP-specific consults. Other tools for data analysis are utilized in other departments in which gaps in coordination of care have been caught/resolved, and advocacy has increased.
Conclusions
Comprehensive cancer care from diagnosis throughout survivorship improves quality of life. A Multi-D comprehensive Cancer rehabilitation provides an opportunity to streamline care via a CPRS Menu. Other VA medical centers can develop a Multi-D cancer rehabilitation program to coordinate treatments from diagnosis through survivorship. This is an opportunity to make the VA the forefront of oncology care – by providing all services within one system.
Whole Health Oncology—Just Do It: Making Whole Person Cancer Care Routine and Regular at the Dayton VA Medical Center (DVAMC)
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
A Time to Heal for Veterans With Cancer
Background
Cancer diagnosis and treatment can be devastating! After treatment, a person often feels tired, weak, and worried while trying to put their life back together. This transition period is known to be difficult (www.cancer.gov/about-cancer/coping/survivorship/new-normal). A Time to Heal for Veterans and their Caregivers (“wellness rehabilitation”) was created to provide support, information, and skills to help with this transition.
Methods
This 9-week program is based on a successful, well documented, evidence-based book and protocol developed in 2005, that has been updated and adapted for specific populations. The VA program has a customized participant book and is facilitated by a VA social worker and a VA oncology nurse. It includes weekly protocols of research-based educational presentations on the following topics: Building Resilience, Physical Side Effects, Calming Worries and Fears, Nutrition and Exercise for Cancer Survivors, Relationships After Cancer, Nurturing Inner Strength, Planning for the Future, and Happiness Going Forward. It also includes facilitated discussions to share experiences, demonstration/ practices of simple strategies for relaxation or health, and journaling/affirmation writing. The program is held in person at the VA for locals and via Zoom for non-local participants (hybrid format).
Results
A Time to Heal program for Veterans has been offered since 2016. In 2020 it was shortened from 12 weeks to 9 weeks. Since then, 24 veterans and 8 caregivers have completed the program and 13 have completed the evaluation/survey. On a scale of 1 (below expectations) to 5 (exceeded expectations), the program and book have consistently received rating averages of 4.5/5.0. Testimonials include: “Awesome program!” “Was hesitant at first, but so glad I decided to participate. I was able to open up my feelings and express them. I am grateful for the VA to have these resources.”
Conclusions
Recruitment for the program has relied on fliers and education from oncology staff. The feedback received from veterans with cancer, caregivers, and providers indicates a positive impact of this program. More study is needed to evaluate specific aspects of the program, guide participant recruitment, and determine best delivery methods for participants.
Background
Cancer diagnosis and treatment can be devastating! After treatment, a person often feels tired, weak, and worried while trying to put their life back together. This transition period is known to be difficult (www.cancer.gov/about-cancer/coping/survivorship/new-normal). A Time to Heal for Veterans and their Caregivers (“wellness rehabilitation”) was created to provide support, information, and skills to help with this transition.
Methods
This 9-week program is based on a successful, well documented, evidence-based book and protocol developed in 2005, that has been updated and adapted for specific populations. The VA program has a customized participant book and is facilitated by a VA social worker and a VA oncology nurse. It includes weekly protocols of research-based educational presentations on the following topics: Building Resilience, Physical Side Effects, Calming Worries and Fears, Nutrition and Exercise for Cancer Survivors, Relationships After Cancer, Nurturing Inner Strength, Planning for the Future, and Happiness Going Forward. It also includes facilitated discussions to share experiences, demonstration/ practices of simple strategies for relaxation or health, and journaling/affirmation writing. The program is held in person at the VA for locals and via Zoom for non-local participants (hybrid format).
Results
A Time to Heal program for Veterans has been offered since 2016. In 2020 it was shortened from 12 weeks to 9 weeks. Since then, 24 veterans and 8 caregivers have completed the program and 13 have completed the evaluation/survey. On a scale of 1 (below expectations) to 5 (exceeded expectations), the program and book have consistently received rating averages of 4.5/5.0. Testimonials include: “Awesome program!” “Was hesitant at first, but so glad I decided to participate. I was able to open up my feelings and express them. I am grateful for the VA to have these resources.”
Conclusions
Recruitment for the program has relied on fliers and education from oncology staff. The feedback received from veterans with cancer, caregivers, and providers indicates a positive impact of this program. More study is needed to evaluate specific aspects of the program, guide participant recruitment, and determine best delivery methods for participants.
Background
Cancer diagnosis and treatment can be devastating! After treatment, a person often feels tired, weak, and worried while trying to put their life back together. This transition period is known to be difficult (www.cancer.gov/about-cancer/coping/survivorship/new-normal). A Time to Heal for Veterans and their Caregivers (“wellness rehabilitation”) was created to provide support, information, and skills to help with this transition.
Methods
This 9-week program is based on a successful, well documented, evidence-based book and protocol developed in 2005, that has been updated and adapted for specific populations. The VA program has a customized participant book and is facilitated by a VA social worker and a VA oncology nurse. It includes weekly protocols of research-based educational presentations on the following topics: Building Resilience, Physical Side Effects, Calming Worries and Fears, Nutrition and Exercise for Cancer Survivors, Relationships After Cancer, Nurturing Inner Strength, Planning for the Future, and Happiness Going Forward. It also includes facilitated discussions to share experiences, demonstration/ practices of simple strategies for relaxation or health, and journaling/affirmation writing. The program is held in person at the VA for locals and via Zoom for non-local participants (hybrid format).
Results
A Time to Heal program for Veterans has been offered since 2016. In 2020 it was shortened from 12 weeks to 9 weeks. Since then, 24 veterans and 8 caregivers have completed the program and 13 have completed the evaluation/survey. On a scale of 1 (below expectations) to 5 (exceeded expectations), the program and book have consistently received rating averages of 4.5/5.0. Testimonials include: “Awesome program!” “Was hesitant at first, but so glad I decided to participate. I was able to open up my feelings and express them. I am grateful for the VA to have these resources.”
Conclusions
Recruitment for the program has relied on fliers and education from oncology staff. The feedback received from veterans with cancer, caregivers, and providers indicates a positive impact of this program. More study is needed to evaluate specific aspects of the program, guide participant recruitment, and determine best delivery methods for participants.
“It Takes a Village”: Benefits and Challenges of Navigating Cancer Care with the Pacific Community and the Veterans Health Administration
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.
Background
The Palliative Care in Hawaii/Pacific Island Communities for Veterans (PaCiHPIC Veterans) study is a VA-funded research study that explores social determinants of health, cultural values, and cancer disparities impacting Native Hawaiian/Pacific Islander/US-affiliated Pacific Island resident (NHPI/USAPI) Veterans.Cancer prevalence and mortality are increasing among NHPI/ USAPI Veterans which can be partly attributed to nuclear fallout from U.S. military activities in the region. This population faces geographic, financial, and logistical barriers to cancer care. There is an imminent need to understand and address access to cancer care and palliative care to reduce disparities within this population.
Methods
We interviewed 15 clinicians including physicians, nurses, nurse practitioners, social workers, and clinical psychologists specializing in primary care, palliative care, and oncology, self-identifying as White, Asian American, NHPI, and Multiracial. Interviews were transcribed verbatim and de-identified. Using inductive and deductive strategies, we iteratively collapsed content into codes formulating a codebook. Thematic analyses were performed using dual-coder review in Atlas.ti v23. Themes were mapped to the socioecological model.
Results
Clinicians described how NHPI/USAPI Veterans receive healthcare and instrumental support at individual, community, and systems levels, including from family caregivers, “high-talking chiefs,” traditional healers (“suruhanu”), community health clinics, and the VHA. Clinicians identified challenges and opportunities for care coordination: (1) financial and logistical barriers to involve family and decision-makers; (2) clinician understanding of cultural values and influence on medical decision-making; (3) care fragmentation resulting from transitions between community care and VHA; and (4) collaboration with key individuals in Pacific social hierarchies.
Conclusions
Cancer navigation and care coordination gaps create challenges for clinicians and NHPI/USAPI Veterans managing cancer in the Pacific Islands. Better understanding of these systems of care and associated gaps can inform the development of an intervention to improve cancer care delivery to this population. NHPI/ USAPI Veterans may experience care fragmentation due to care transitions between community care and the VHA. At the same time, these sources also create multiple layers of support for Veterans. Interventions to address these challenges can leverage the strengths of Pacific communities, while striving to better integrate care between community healthcare providers and VHA.
Treatment Patterns and Outcomes of Older (Age ≥ 80) Veterans With Newly Diagnosed Diffuse Large B-Cell Lymphoma (DLBCL)
Background
Over one-third of newly diagnosed Diffuse Large B-Cell Lymphoma (DLBCL) cases are in people age ≥75. Although a potentially curable malignancy, older adults have a comparatively lower survival rate. This may be due to multiple factors including suboptimal management. In one study, up to 23% of patients age ≥80 did not receive any therapy for DLBCL. This age-related survival disparity is potentially magnified in patients who reside in rural areas. As there is no standard of care for this population, we speculate that there is wide variation in treatment practices which may influence outcomes. The purpose of this study is to describe treatment patterns and outcomes in in veterans age ≥80 with DLBCL by area of residence.
Methods
We conducted a retrospective study of veterans age ≥80 newly diagnosed with Stage II-IV DLBCL between 2006-2023 using the Veterans Affairs (VA) Cancer Registry System (VACRS). Patient, disease, and treatment variables were extracted from the VA Corporate Data Warehouse (CDW) and via chart review. Variables were compared amongst Veterans residing at urban vs. rural addresses.
Results
We evaluated a total of 181 Veterans. Most veterans resided in an urban area (60.2%). At least 18.8% of veterans failed to start lymphoma-directed therapy, but only 6.6% of veterans were not explicitly offered treatment per documentation. In total, 68.5% of veterans were offered a curative treatment regimen by their provider; curative treatment was more likely to be offered to urban patients (68.8% vs 61.5%, p=0.86). Pre-phase steroids and geriatric assessments prior to treatment were severely underutilized (2.8% and 0.6%). More urban veterans started treatment (75.2% vs 65.4%, p=0.38) and 40.9% started an anthracyclinecontaining regimen. Only 27.6% of veterans completed 6 total cycles of treatment. Only 37.6% of veterans achieved a complete response at end of treatment, although response was not reported in 46.4% of patients.
Conclusions
Most elderly veterans with DLBCL are being offered and started on a curative treatment regimen; however, most do not complete a full course of treatment. Although not statistically significant, more urban veterans were offered a curative regimen and received treatment. Wider adoption of pre-phase steroids and geriatric assessments could improve response outcomes.
Background
Over one-third of newly diagnosed Diffuse Large B-Cell Lymphoma (DLBCL) cases are in people age ≥75. Although a potentially curable malignancy, older adults have a comparatively lower survival rate. This may be due to multiple factors including suboptimal management. In one study, up to 23% of patients age ≥80 did not receive any therapy for DLBCL. This age-related survival disparity is potentially magnified in patients who reside in rural areas. As there is no standard of care for this population, we speculate that there is wide variation in treatment practices which may influence outcomes. The purpose of this study is to describe treatment patterns and outcomes in in veterans age ≥80 with DLBCL by area of residence.
Methods
We conducted a retrospective study of veterans age ≥80 newly diagnosed with Stage II-IV DLBCL between 2006-2023 using the Veterans Affairs (VA) Cancer Registry System (VACRS). Patient, disease, and treatment variables were extracted from the VA Corporate Data Warehouse (CDW) and via chart review. Variables were compared amongst Veterans residing at urban vs. rural addresses.
Results
We evaluated a total of 181 Veterans. Most veterans resided in an urban area (60.2%). At least 18.8% of veterans failed to start lymphoma-directed therapy, but only 6.6% of veterans were not explicitly offered treatment per documentation. In total, 68.5% of veterans were offered a curative treatment regimen by their provider; curative treatment was more likely to be offered to urban patients (68.8% vs 61.5%, p=0.86). Pre-phase steroids and geriatric assessments prior to treatment were severely underutilized (2.8% and 0.6%). More urban veterans started treatment (75.2% vs 65.4%, p=0.38) and 40.9% started an anthracyclinecontaining regimen. Only 27.6% of veterans completed 6 total cycles of treatment. Only 37.6% of veterans achieved a complete response at end of treatment, although response was not reported in 46.4% of patients.
Conclusions
Most elderly veterans with DLBCL are being offered and started on a curative treatment regimen; however, most do not complete a full course of treatment. Although not statistically significant, more urban veterans were offered a curative regimen and received treatment. Wider adoption of pre-phase steroids and geriatric assessments could improve response outcomes.
Background
Over one-third of newly diagnosed Diffuse Large B-Cell Lymphoma (DLBCL) cases are in people age ≥75. Although a potentially curable malignancy, older adults have a comparatively lower survival rate. This may be due to multiple factors including suboptimal management. In one study, up to 23% of patients age ≥80 did not receive any therapy for DLBCL. This age-related survival disparity is potentially magnified in patients who reside in rural areas. As there is no standard of care for this population, we speculate that there is wide variation in treatment practices which may influence outcomes. The purpose of this study is to describe treatment patterns and outcomes in in veterans age ≥80 with DLBCL by area of residence.
Methods
We conducted a retrospective study of veterans age ≥80 newly diagnosed with Stage II-IV DLBCL between 2006-2023 using the Veterans Affairs (VA) Cancer Registry System (VACRS). Patient, disease, and treatment variables were extracted from the VA Corporate Data Warehouse (CDW) and via chart review. Variables were compared amongst Veterans residing at urban vs. rural addresses.
Results
We evaluated a total of 181 Veterans. Most veterans resided in an urban area (60.2%). At least 18.8% of veterans failed to start lymphoma-directed therapy, but only 6.6% of veterans were not explicitly offered treatment per documentation. In total, 68.5% of veterans were offered a curative treatment regimen by their provider; curative treatment was more likely to be offered to urban patients (68.8% vs 61.5%, p=0.86). Pre-phase steroids and geriatric assessments prior to treatment were severely underutilized (2.8% and 0.6%). More urban veterans started treatment (75.2% vs 65.4%, p=0.38) and 40.9% started an anthracyclinecontaining regimen. Only 27.6% of veterans completed 6 total cycles of treatment. Only 37.6% of veterans achieved a complete response at end of treatment, although response was not reported in 46.4% of patients.
Conclusions
Most elderly veterans with DLBCL are being offered and started on a curative treatment regimen; however, most do not complete a full course of treatment. Although not statistically significant, more urban veterans were offered a curative regimen and received treatment. Wider adoption of pre-phase steroids and geriatric assessments could improve response outcomes.
Recent Incidence and Survival Trends in Pancreatic Cancer at Young Age (<50 Years)
Background
Pancreatic cancer stands as a prominent contributor to cancer-related mortality in the United States. In this abstract, we reviewed the SEER database to uncover the latest trends in pancreatic cancer among individuals diagnosed under the age of 50.
Methods
Information was obtained from the SEER database November 2023 which covers 22 national cancer registries. Only patients with age < 50 years were included. Age adjusted incidence and 5-year relative survival were compared between different ethnic groups.
Results
We identified 124691 patients with pancreatic cancer diagnosed between 2017-2021, among them 6477 were with age less than 50 years at the time of diagnosis. 3074 were male and 3403 were male. Age adjusted incidence rate was 1.2/100,000 in females and 1.4/100,000 in males. Overall, Average Annual Percent Change (AAPC) of 2.6% (95% CI: 1.9 – 4.3) was noticed between 2017-2021 when compared to previously reported rates. AAPC among different ethnic groups were Hispanics, any race: 5.3% (CI: 4-7.5), Non-Hispanic American Indian/Alaska Native: 1.1 (CI: -2.7-5.1), Non-Hispanic Asian/Pacific Islander: 1.9 (CI: 1.1-2.9), Non-Hispanic Black: 1.0 (CI: 0.3-1.7), and Non-Hispanic White: 1.6 (CI: 1.1-2.1). Stage 4 was the most common stage. Overall, the 5-year relative survival from 2014- 2020 was 37.4% (CI: 36.1-38.7). 5-year relative survival among ethnic groups from 2014-2020 were: Hispanics, any race: 40.3% (CI: 37.6-43.0), Non-Hispanic American Indian/Alaska Native: 21.4 (CI: 8.5-38.2), Non-Hispanic Asian/Pacific Islander: 40.2 (CI: 35.7-44.7), Non-Hispanic Black: 33.1 (CI: 29.9-36.3), and Non-Hispanic White: 36.6 (CI: 34.8-38.4).
Conclusions
Our analysis reveals a rise in the ageadjusted incidence of pancreatic cancer among younger demographics. Particularly noteworthy is the sharp increase observed over the past five years among Hispanics when compared to other ethnic populations. This rise is observed in both males and females. Further studies need to be done to study the risk factors associated with this increase in trend of pancreatic cancer at young age specifically in Hispanic population.
Background
Pancreatic cancer stands as a prominent contributor to cancer-related mortality in the United States. In this abstract, we reviewed the SEER database to uncover the latest trends in pancreatic cancer among individuals diagnosed under the age of 50.
Methods
Information was obtained from the SEER database November 2023 which covers 22 national cancer registries. Only patients with age < 50 years were included. Age adjusted incidence and 5-year relative survival were compared between different ethnic groups.
Results
We identified 124691 patients with pancreatic cancer diagnosed between 2017-2021, among them 6477 were with age less than 50 years at the time of diagnosis. 3074 were male and 3403 were male. Age adjusted incidence rate was 1.2/100,000 in females and 1.4/100,000 in males. Overall, Average Annual Percent Change (AAPC) of 2.6% (95% CI: 1.9 – 4.3) was noticed between 2017-2021 when compared to previously reported rates. AAPC among different ethnic groups were Hispanics, any race: 5.3% (CI: 4-7.5), Non-Hispanic American Indian/Alaska Native: 1.1 (CI: -2.7-5.1), Non-Hispanic Asian/Pacific Islander: 1.9 (CI: 1.1-2.9), Non-Hispanic Black: 1.0 (CI: 0.3-1.7), and Non-Hispanic White: 1.6 (CI: 1.1-2.1). Stage 4 was the most common stage. Overall, the 5-year relative survival from 2014- 2020 was 37.4% (CI: 36.1-38.7). 5-year relative survival among ethnic groups from 2014-2020 were: Hispanics, any race: 40.3% (CI: 37.6-43.0), Non-Hispanic American Indian/Alaska Native: 21.4 (CI: 8.5-38.2), Non-Hispanic Asian/Pacific Islander: 40.2 (CI: 35.7-44.7), Non-Hispanic Black: 33.1 (CI: 29.9-36.3), and Non-Hispanic White: 36.6 (CI: 34.8-38.4).
Conclusions
Our analysis reveals a rise in the ageadjusted incidence of pancreatic cancer among younger demographics. Particularly noteworthy is the sharp increase observed over the past five years among Hispanics when compared to other ethnic populations. This rise is observed in both males and females. Further studies need to be done to study the risk factors associated with this increase in trend of pancreatic cancer at young age specifically in Hispanic population.
Background
Pancreatic cancer stands as a prominent contributor to cancer-related mortality in the United States. In this abstract, we reviewed the SEER database to uncover the latest trends in pancreatic cancer among individuals diagnosed under the age of 50.
Methods
Information was obtained from the SEER database November 2023 which covers 22 national cancer registries. Only patients with age < 50 years were included. Age adjusted incidence and 5-year relative survival were compared between different ethnic groups.
Results
We identified 124691 patients with pancreatic cancer diagnosed between 2017-2021, among them 6477 were with age less than 50 years at the time of diagnosis. 3074 were male and 3403 were male. Age adjusted incidence rate was 1.2/100,000 in females and 1.4/100,000 in males. Overall, Average Annual Percent Change (AAPC) of 2.6% (95% CI: 1.9 – 4.3) was noticed between 2017-2021 when compared to previously reported rates. AAPC among different ethnic groups were Hispanics, any race: 5.3% (CI: 4-7.5), Non-Hispanic American Indian/Alaska Native: 1.1 (CI: -2.7-5.1), Non-Hispanic Asian/Pacific Islander: 1.9 (CI: 1.1-2.9), Non-Hispanic Black: 1.0 (CI: 0.3-1.7), and Non-Hispanic White: 1.6 (CI: 1.1-2.1). Stage 4 was the most common stage. Overall, the 5-year relative survival from 2014- 2020 was 37.4% (CI: 36.1-38.7). 5-year relative survival among ethnic groups from 2014-2020 were: Hispanics, any race: 40.3% (CI: 37.6-43.0), Non-Hispanic American Indian/Alaska Native: 21.4 (CI: 8.5-38.2), Non-Hispanic Asian/Pacific Islander: 40.2 (CI: 35.7-44.7), Non-Hispanic Black: 33.1 (CI: 29.9-36.3), and Non-Hispanic White: 36.6 (CI: 34.8-38.4).
Conclusions
Our analysis reveals a rise in the ageadjusted incidence of pancreatic cancer among younger demographics. Particularly noteworthy is the sharp increase observed over the past five years among Hispanics when compared to other ethnic populations. This rise is observed in both males and females. Further studies need to be done to study the risk factors associated with this increase in trend of pancreatic cancer at young age specifically in Hispanic population.
Laterality in Renal Cancer: Effect on Survival in Veteran Population
Background
Kidney and renal pelvis cancers (KC) represent 4% of new cancer cases in the US. Although it is a common cancer, there is no data to compare the effect of laterality on survival in veteran population. In this abstract, we attempt to bridge this gap and compare the effect of laterality on survival.
Methods
We obtained data from Albany VA (VAMC) for patients diagnosed with KC between 2010-2020. Data were analyzed for age, stage at diagnosis, histopathological type, laterality of tumor, and 6,12 and 60-months survival after the diagnosis and performed a comparison of overall survival of left versus rightsided cancer by calculating odds ratio using logistic regression, significance level was established at p< 0.05.
Results
We reviewed 130 patients diagnosed with KC at VAMC. 62 had right-sided, 62 had left-sided, and 6 had bilateral cancer. Clear cell (40.8%) was predominant type. Other less common histopathological types include Papillary RCC, mixed, papillary urothelial and transitional types. 58 patients had stage 1 (28 right versus 30 left), 8 had stage 2 (5 versus 3), 29 had stage 3 (13 versus 16), 16 with stage 4 (12 versus 4), and 14 had stage 0 (papillary-urothelial). 59.2% patients underwent surgical treatment after diagnosis (R=35, L=39). At 6-months, 60 patients (96.8%) with left-sided and 53 (85.5%) with right-sided cancer survived. The odds of surviving 6-months were 12% higher (95% CI: 1.014, 1.236; p=0.03) in left versus right-sided cancer. For 1-year survival, the results were similar. 111 patients completed a 5-year follow-up and there was no evidence to support a difference in survival between cohorts at 5-years: OR (95% CI: 0.88, 1.47; p=0.32).
Conclusions
In this study, we discovered that leftsided cancer showed better survival at 6-months and 1-year compared to right-sided cancer, but 5-year survival rates appeared similar irrespective of laterality of cancer. Both subgroups had similar distribution for baseline characteristics with majority of patients being males, older than 60 years, with stage 1 disease. Further studies in larger populations with wider distribution of baseline characteristics are needed to establish clear role of laterality as a prognostic factor.
Background
Kidney and renal pelvis cancers (KC) represent 4% of new cancer cases in the US. Although it is a common cancer, there is no data to compare the effect of laterality on survival in veteran population. In this abstract, we attempt to bridge this gap and compare the effect of laterality on survival.
Methods
We obtained data from Albany VA (VAMC) for patients diagnosed with KC between 2010-2020. Data were analyzed for age, stage at diagnosis, histopathological type, laterality of tumor, and 6,12 and 60-months survival after the diagnosis and performed a comparison of overall survival of left versus rightsided cancer by calculating odds ratio using logistic regression, significance level was established at p< 0.05.
Results
We reviewed 130 patients diagnosed with KC at VAMC. 62 had right-sided, 62 had left-sided, and 6 had bilateral cancer. Clear cell (40.8%) was predominant type. Other less common histopathological types include Papillary RCC, mixed, papillary urothelial and transitional types. 58 patients had stage 1 (28 right versus 30 left), 8 had stage 2 (5 versus 3), 29 had stage 3 (13 versus 16), 16 with stage 4 (12 versus 4), and 14 had stage 0 (papillary-urothelial). 59.2% patients underwent surgical treatment after diagnosis (R=35, L=39). At 6-months, 60 patients (96.8%) with left-sided and 53 (85.5%) with right-sided cancer survived. The odds of surviving 6-months were 12% higher (95% CI: 1.014, 1.236; p=0.03) in left versus right-sided cancer. For 1-year survival, the results were similar. 111 patients completed a 5-year follow-up and there was no evidence to support a difference in survival between cohorts at 5-years: OR (95% CI: 0.88, 1.47; p=0.32).
Conclusions
In this study, we discovered that leftsided cancer showed better survival at 6-months and 1-year compared to right-sided cancer, but 5-year survival rates appeared similar irrespective of laterality of cancer. Both subgroups had similar distribution for baseline characteristics with majority of patients being males, older than 60 years, with stage 1 disease. Further studies in larger populations with wider distribution of baseline characteristics are needed to establish clear role of laterality as a prognostic factor.
Background
Kidney and renal pelvis cancers (KC) represent 4% of new cancer cases in the US. Although it is a common cancer, there is no data to compare the effect of laterality on survival in veteran population. In this abstract, we attempt to bridge this gap and compare the effect of laterality on survival.
Methods
We obtained data from Albany VA (VAMC) for patients diagnosed with KC between 2010-2020. Data were analyzed for age, stage at diagnosis, histopathological type, laterality of tumor, and 6,12 and 60-months survival after the diagnosis and performed a comparison of overall survival of left versus rightsided cancer by calculating odds ratio using logistic regression, significance level was established at p< 0.05.
Results
We reviewed 130 patients diagnosed with KC at VAMC. 62 had right-sided, 62 had left-sided, and 6 had bilateral cancer. Clear cell (40.8%) was predominant type. Other less common histopathological types include Papillary RCC, mixed, papillary urothelial and transitional types. 58 patients had stage 1 (28 right versus 30 left), 8 had stage 2 (5 versus 3), 29 had stage 3 (13 versus 16), 16 with stage 4 (12 versus 4), and 14 had stage 0 (papillary-urothelial). 59.2% patients underwent surgical treatment after diagnosis (R=35, L=39). At 6-months, 60 patients (96.8%) with left-sided and 53 (85.5%) with right-sided cancer survived. The odds of surviving 6-months were 12% higher (95% CI: 1.014, 1.236; p=0.03) in left versus right-sided cancer. For 1-year survival, the results were similar. 111 patients completed a 5-year follow-up and there was no evidence to support a difference in survival between cohorts at 5-years: OR (95% CI: 0.88, 1.47; p=0.32).
Conclusions
In this study, we discovered that leftsided cancer showed better survival at 6-months and 1-year compared to right-sided cancer, but 5-year survival rates appeared similar irrespective of laterality of cancer. Both subgroups had similar distribution for baseline characteristics with majority of patients being males, older than 60 years, with stage 1 disease. Further studies in larger populations with wider distribution of baseline characteristics are needed to establish clear role of laterality as a prognostic factor.
Paclitaxel Drug-Drug Interactions in the Military Health System
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
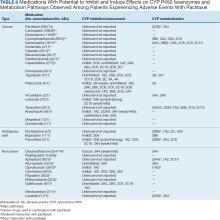
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
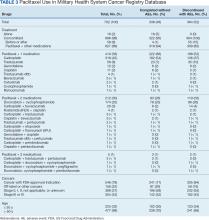
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
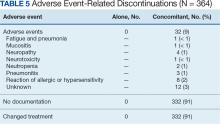
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
Background
Paclitaxel was first derived from the bark of the yew tree (Taxus brevifolia). It was discovered as part of a National Cancer Institute program screen of plants and natural products with putative anticancer activity during the 1960s.1-9 Paclitaxel works by suppressing spindle microtube dynamics, which results in the blockage of the metaphase-anaphase transitions, inhibition of mitosis, and induction of apoptosis in a broad spectrum of cancer cells. Paclitaxel also displayed additional anticancer activities, including the suppression of cell proliferation and antiangiogenic effects. However, since the growth of normal body cells may also be affected, other adverse effects (AEs) will also occur.8-18
Two different chemotherapy drugs contain paclitaxel—paclitaxel and nab-paclitaxel—and the US Food and Drug Administration (FDA) recognizes them as separate entities.19-21 Taxol (paclitaxel) was approved by the FDA in 1992 for treating advanced ovarian cancer.20 It has since been approved for the treatment of metastatic breast cancer, AIDS-related Kaposi sarcoma (as an orphan drug), non-small cell lung cancer (NSCLC), and cervical cancers (in combination withbevacizumab) in 1994, 1997, 1999, and 2014, respectively.21 Since 2002, a generic version of Taxol, known as paclitaxel injectable, has been FDA-approved from different manufacturers. According to the National Cancer Institute, a combination of carboplatin and Taxol is approved to treat carcinoma of unknown primary, cervical, endometrial, NSCLC, ovarian, and thymoma cancers.19 Abraxane (nab-paclitaxel) was FDA-approved to treat metastatic breast cancer in 2005. It was later approved for first-line treatment of advanced NSCLC and late-stage pancreatic cancer in 2012 and 2013, respectively. In 2018 and 2020, both Taxol and Abraxane were approved for first-line treatment of metastatic squamous cell NSCLC in combination with carboplatin and pembrolizumab and metastatic triple-negative breast cancer in combination with pembrolizumab, respectively.22-26 In 2019, Abraxane was approved with atezolizumab to treat metastatic triple-negative breast cancer, but this approval was withdrawn in 2021. In 2022, a generic version of Abraxane, known as paclitaxel protein-bound, was released in the United States. Furthermore, paclitaxel-containing formulations also are being studied in the treatment of other types of cancer.19-32
One of the main limitations of paclitaxel is its low solubility in water, which complicates its drug supply. To distribute this hydrophobic anticancer drug efficiently, paclitaxel is formulated and administered to patients via polyethoxylated castor oil or albumin-bound (nab-paclitaxel). However, polyethoxylated castor oil induces complement activation and is the cause of common hypersensitivity reactions related to paclitaxel use.2,17,33-38 Therefore, many alternatives to polyethoxylated castor oil have been researched.
Since 2000, new paclitaxel formulations have emerged using nanomedicine techniques. The difference between these formulations is the drug vehicle. Different paclitaxel-based nanotechnological vehicles have been developed and approved, such as albumin-based nanoparticles, polymeric lipidic nanoparticles, polymeric micelles, and liposomes, with many others in clinical trial phases.3,37 Albumin-based nanoparticles have a high response rate (33%), whereas the response rate for polyethoxylated castor oil is 25% in patients with metastatic breast cancer.33,39-52 The use of paclitaxel dimer nanoparticles also has been proposed as a method for increasing drug solubility.33,53
Paclitaxel is metabolized by cytochrome P450 (CYP) isoenzymes 2C8 and 3A4. When administering paclitaxel with known inhibitors, inducers, or substrates of CYP2C8 or CYP3A4, caution is required.19-22 Regulations for CYP research were not issued until 2008, so potential interactions between paclitaxel and other drugs have not been extensively evaluated in clinical trials. A study of 12 kinase inhibitors showed strong inhibition of CYP2C8 and/or CYP3A4 pathways by these inhibitors, which could alter the ratio of paclitaxel metabolites in vivo, leading to clinically relevant changes.54 Differential metabolism has been linked to paclitaxel-induced neurotoxicity in patients with cancer.55 Nonetheless, variants in the CYP2C8, CYP3A4, CYP3A5, and ABCB1 genes do not account for significant interindividual variability in paclitaxel pharmacokinetics.56 In liver microsomes, losartan inhibited paclitaxel metabolism when used at concentrations > 50 µmol/L.57 Many drug-drug interaction (DDI) studies of CYP2C8 and CYP3A4 have shown similar results for paclitaxel.58-64
The goals of this study are to investigate prescribed drugs used with paclitaxel and determine patient outcomes through several Military Health System (MHS) databases. The investigation focused on (1) the functions of paclitaxel; (2) identifying AEs that patients experienced; (3) evaluating differences when paclitaxel is used alone vs concomitantly and between the completed vs discontinued treatment groups; (4) identifying all drugs used during paclitaxel treatment; and (5) evaluating DDIs with antidepressants (that have an FDA boxed warning and are known to have DDIs confirmed in previous publications) and other drugs.65-67
The Walter Reed National Military Medical Center in Bethesda, Maryland, institutionalreview board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and MHS data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and the Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
METHODS
The DoD Cancer Registry Program was established in 1986 and currently contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in the CAPER record represents an ambulatory encounter at a military treatment facility (MTF). CAPER includes data from 2003 to 2024.
Each observation in the PDTS record represents a prescription filled for an MHS beneficiary at an MTF through the TRICARE mail-order program or a US retail pharmacy. Missing from this record are prescriptions filled at international civilian pharmacies and inpatient pharmacy prescriptions. The MHS Data Repository PDTS record is available from 2002 to 2024. The legacy Composite Health Care System is being replaced by GENESIS at MTFs.
Data Extraction Design
The study design involved a cross-sectional analysis. We requested data extraction for paclitaxel from 1998 to 2022. Data from the DoD Cancer Registry Program were used to identify patients who received cancer treatment. Once patients were identified, the CAPER database was searched for diagnoses to identify other health conditions, whereas the PDTS database was used to populate a list of prescription medications filled during chemotherapy treatment.
Data collected from the JPC included cancer treatment, cancer information, demographics, and physicians’ comments on AEs. Collected data from the MHS include diagnosis and filled prescription history from initiation to completion of the therapy period (or 2 years after the diagnosis date). For the analysis of the DoD Cancer Registry Program and CAPER databases, we used all collected data without excluding any. When analyzing PDTS data, we excluded patients with PDTS data but without a record of paclitaxel being filled, or medications filled outside the chemotherapy period (by evaluating the dispensed date and day of supply).
Data Extraction Analysis
The Surveillance, Epidemiology, and End Results Program Coding and Staging Manual 2016 and the International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.68,69 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the paclitaxel groups divided by the total number of patients or data variables. The subgroup percentage was calculated by using the number of patients or data available within the subgroup divided by the total number of patients in that subgroup.
In alone vs concomitant and completed vs discontinued treatment groups, a 2-tailed, 2-sample z test was used to statistical significance (P < .05) using a statistics website.70 Concomitant was defined as paclitaxel taken with other antineoplastic agent(s) before, after, or at the same time as cancer therapy. For the retrospective data analysis, physicians’ notes with a period, comma, forward slash, semicolon, or space between medication names were interpreted as concurrent, whereas plus (+), minus/plus (-/+), or “and” between drug names that were dispensed on the same day were interpreted as combined with known common combinations: 2 drugs (DM886 paclitaxel and carboplatin and DM881-TC-1 paclitaxel and cisplatin) or 3 drugs (DM887-ACT doxorubicin, cyclophosphamide, and paclitaxel). Completed treatment was defined as paclitaxel as the last medication the patient took without recorded AEs; switching or experiencing AEs was defined as discontinued treatment.
RESULTS
The JPC provided 702 entries for 687 patients with a mean age of 56 years (range, 2 months to 88 years) who were treated with paclitaxel from March 1996 to October 2021. Fifteen patients had duplicate entries because they had multiple cancer sites or occurrences. There were 623 patients (89%) who received paclitaxel for FDA-approved indications. The most common types of cancer identified were 344 patients with breast cancer (49%), 91 patients with lung cancer (13%), 79 patients with ovarian cancer (11%), and 75 patients with endometrial cancer (11%) (Table 1). Seventy-nine patients (11%) received paclitaxel for cancers that were not for FDA-approved indications, including 19 for cancers of the fallopian tube (3%) and 17 for esophageal cancer (2%) (Table 2).
There were 477 patients (68%) aged > 50 years. A total of 304 patients (43%) had a stage III or IV cancer diagnosis and 398 (57%) had stage II or lower (combination of data for stages 0, I, and II; not applicable; and unknown) cancer diagnosis. For systemic treatment, 16 patients (2%) were treated with paclitaxel alone and 686 patients (98%) received paclitaxel concomitantly with additional chemotherapy: 59 patients (9%) in the before or after group, 410 patients (58%) had a 2-drug combination, 212 patients (30%) had a 3-drug combination, and 5 patients (1%) had a 4-drug combination. In addition, for doublet therapies, paclitaxel combined with carboplatin, trastuzumab, gemcitabine, or cisplatin had more patients (318, 58, 12, and 11, respectively) than other combinations (≤ 4 patients). For triplet therapies, paclitaxel combined withdoxorubicin plus cyclophosphamide or carboplatin plus bevacizumab had more patients (174 and 20, respectively) than other combinations, including quadruplet therapies (≤ 4 patients) (Table 3).
Patients were more likely to discontinue paclitaxel if they received concomitant treatment. None of the 16 patients receiving paclitaxel monotherapy experienced AEs, whereas 364 of 686 patients (53%) treated concomitantly discontinued (P < .001). Comparisons of 1 drug vs combination (2 to 4 drugs) and use for treating cancers that were FDA-approved indications vs off-label use were significant (P < .001), whereas comparisons of stage II or lower vs stage III and IV cancer and of those aged ≤ 50 years vs aged > 50 years were not significant (P = .50 andP = .30, respectively) (Table 4).
Among the 364 patients who had concomitant treatment and had discontinued their treatment, 332 (91%) switched treatments with no AEs documented and 32 (9%) experienced fatigue with pneumonia, mucositis, neuropathy, neurotoxicity, neutropenia, pneumonitis, allergic or hypersensitivity reaction, or an unknown AE. Patients who discontinued treatment because of unknown AEs had a physician’s note that detailed progressive disease, a significant decline in performance status, and another unknown adverse effect due to a previous sinus tract infection and infectious colitis (Table 5).
Management Analysis and Reporting Tool Database
MHS data analysts provided data on diagnoses for 639 patients among 687 submitteddiagnoses, with 294 patients completing and 345 discontinuing paclitaxel treatment. Patients in the completed treatment group had 3 to 258 unique health conditions documented, while patients in the discontinued treatment group had 4 to 181 unique health conditions documented. The MHS reported 3808 unique diagnosis conditions for the completed group and 3714 for the discontinued group (P = .02).
The mean (SD) number of diagnoses was 51 (31) for the completed and 55 (28) for the discontinued treatment groups (Figure). Among 639 patients who received paclitaxel, the top 5 diagnoses were administrative, including encounters for other administrative examinations; antineoplastic chemotherapy; administrative examination for unspecified; other specified counseling; and adjustment and management of vascular access device. The database does not differentiate between administrative and clinically significant diagnoses.
MHS data analysts provided data for 336 of 687 submitted patients who were prescribed paclitaxel; 46 patients had no PDTS data, and 305 patients had PDTS data without paclitaxel, Taxol, or Abraxane dispensed. Medications that were filled outside the chemotherapy period were removed by evaluating the dispensed date and day of supply. Among these 336 patients, 151 completed the treatment and 185 discontinued, with 14 patients experiencing documented AEs. Patients in the completed treatment group filled 9 to 56 prescriptions while patients in the discontinued treatment group filled 6 to 70 prescriptions.Patients in the discontinued group filled more prescriptions than those who completed treatment: 793 vs 591, respectively (P = .34).
The mean (SD) number of filled prescription drugs was 24 (9) for the completed and 34 (12) for the discontinued treatment group. The 5 most filled prescriptions with paclitaxel from 336 patients with PDTS data were dexamethasone (324 prescriptions with 14 recorded AEs), diphenhydramine (296 prescriptions with 12 recorded AEs), ondansetron (277 prescriptions with 11 recorded AEs), prochlorperazine (265 prescriptions with 12 recorded AEs), and sodium chloride (232 prescriptions with 11 recorded AEs).
DISCUSSION
As a retrospective review, this study is more limited in the strength of its conclusions when compared to randomized control trials. The DoD Cancer Registry Program only contains information about cancer types, stages, treatment regimens, and physicians’ notes. Therefore, noncancer drugs are based solely on the PDTS database. In most cases, physicians' notes on AEs were not detailed. There was no distinction between initial vs later lines of therapy and dosage reductions. The change in status or appearance of a new medical condition did not indicate whether paclitaxel caused the changes to develop or directly worsen a pre-existing condition. The PDTS records prescriptions filled, but that may not reflect patients taking prescriptions.
Paclitaxel
Paclitaxel has a long list of both approved and off-label uses in malignancies as a primary agent and in conjunction with other drugs. The FDA prescribing information for Taxol and Abraxane was last updated in April 2011 and September 2020, respectively.20,21 The National Institutes of Health National Library of Medicine has the current update for paclitaxel on July 2023.19,22 Thus, the prescribed information for paclitaxel referenced in the database may not always be up to date. The combinations of paclitaxel with bevacizumab, carboplatin, or carboplatin and pembrolizumab were not in the Taxol prescribing information. Likewise, a combination of nab-paclitaxel with atezolizumab or carboplatin and pembrolizumab is missing in the Abraxane prescribing information.22-27
The generic name is not the same as a generic drug, which may have slight differences from the brand name product.71 The generic drug versions of Taxol and Abraxane have been approved by the FDA as paclitaxel injectable and paclitaxel-protein bound, respectively. There was a global shortage of nab-paclitaxel from October 2021 to June 2022 because of a manufacturing problem.72 During this shortage, data showed similar comments from physician documents that treatment switched to Taxol due to the Abraxane shortage.
Of 336 patients in the PDTS database with dispensed paclitaxel prescriptions, 276 received paclitaxel (year dispensed, 2013-2022), 27 received Abraxane (year dispensed, 2013-2022), 47 received Taxol (year dispensed, 2004-2015), 8 received both Abraxane and paclitaxel, and 6 received both Taxol and paclitaxel. Based on this information, it appears that the distinction between the drugs was not made in the PDTS until after 2015, 10 years after Abraxane received FDA approval. Abraxane was prescribed in the MHS in 2013, 8 years after FDA approval. There were a few comparison studies of Abraxane and Taxol.73-76
Safety and effectiveness in pediatric patients have not been established for paclitaxel. According to the DoD Cancer Registry Program, the youngest patient was aged 2 months. In 2021, this patient was diagnosed with corpus uteri and treated with carboplatin and Taxol in course 1; in course 2, the patient reacted to Taxol; in course 3, Taxol was replaced with Abraxane; in courses 4 to 7, the patient was treated with carboplatin only.
Discontinued Treatment
Ten patients had prescribed Taxol that was changed due to AEs: 1 was switched to Abraxane and atezolizumab, 3 switched to Abraxane, 2 switched to docetaxel, 1 switched to doxorubicin, and 3 switched to pembrolizumab (based on physician’s comments). Of the 10 patients, 7 had Taxol reaction, 2 experienced disease progression, and 1 experienced high programmed death–ligand 1 expression (this patient with breast cancer was switched to Abraxane and atezolizumab during the accelerated FDA approval phase for atezolizumab, which was later revoked). Five patients were treated with carboplatin and Taxol for cancer of the anal canal (changed to pembrolizumab after disease progression), lung not otherwise specified (changed to carboplatin and pembrolizumab due to Taxol reaction), lower inner quadrant of the breast (changed to doxorubicin due to hypersensitivity reaction), corpus uteri (changed to Abraxane due to Taxol reaction), and ovary (changed to docetaxel due to Taxol reaction). Three patients were treated with doxorubicin, cyclophosphamide, and Taxol for breast cancer; 2 patients with breast cancer not otherwise specified switched to Abraxane due to cardiopulmonary hypersensitivity and Taxol reaction and 1 patient with cancer of the upper outer quadrant of the breast changed to docetaxel due to allergic reaction. One patient, who was treated with paclitaxel, ifosfamide, and cisplatin for metastasis of the lower lobe of the lung and kidney cancer, experienced complications due to infectious colitis (treated with ciprofloxacin) and then switched to pembrolizumab after the disease progressed. These AEs are known in paclitaxel medical literature on paclitaxel AEs.19-24,77-81
Combining 2 or more treatments to target cancer-inducing or cell-sustaining pathways is a cornerstone of chemotherapy.82-84 Most combinations are given on the same day, but some are not. For 3- or 4-drug combinations, doxorubicin and cyclophosphamide were given first, followed by paclitaxel with or withouttrastuzumab, carboplatin, or pembrolizumab. Only 16 patients (2%) were treated with paclitaxel alone; therefore, the completed and discontinued treatment groups are mostly concomitant treatment. As a result, the comparisons of the completed and discontinued treatment groups were almost the same for the diagnosis. The PDTS data have a better result because 2 exclusion criteria were applied before narrowing the analysis down to paclitaxel treatment specifically.
Antidepressants and Other Drugs
Drug response can vary from person to person and can lead to treatment failure related to AEs. One major factor in drug metabolism is CYP.85 CYP2C8 is the major pathway for paclitaxel and CYP3A4 is the minor pathway. When evaluating the noncancer drugs, there were no reports of CYP2C8 inhibition or induction. Over the years, many DDI warnings have been issued for paclitaxel with different drugs in various electronic resources.
Oncologists follow guidelines to prevent DDIs, as paclitaxel is known to have severe, moderate, and minor interactions with other drugs. Among 687 patients, 261 (38%) were prescribed any of 14 antidepressants. Eight of these antidepressants (amitriptyline, citalopram, desipramine, doxepin, venlafaxine, escitalopram, nortriptyline, and trazodone) are metabolized, 3 (mirtazapine, sertraline, and fluoxetine) are metabolized and inhibited, 2 (bupropion and duloxetine) are neither metabolized nor inhibited, and 1 (paroxetine) is inhibited by CYP3A4. Duloxetine, venlafaxine, and trazodone were more commonly dispensed (84, 78, and 42 patients, respectively) than others (≤ 33 patients).
Of 32 patients with documented AEs,14 (44%) had 168 dispensed drugs in the PDTS database. Six patients (19%) were treated with doxorubicin and cyclophosphamide followed by paclitaxel for breast cancer; 6 (19%) were treated with carboplatin and paclitaxel for cancer of the lung (n = 3), corpus uteri (n = 2), and ovary (n = 1); 1 patient (3%) was treated with carboplatin and paclitaxel, then switched to carboplatin, bevacizumab, and paclitaxel, and then completed treatment with carboplatin and paclitaxel for an unspecified female genital cancer; and 1 patient (3%) was treated with cisplatin, ifosfamide, and paclitaxel for metastasis of the lower lobe lung and kidney cancer.
The 14 patients with PDTS data had 18 cancer drugs dispensed. Eleven had moderate interaction reports and 7 had no interaction reports. A total of 165 noncancer drugs were dispensed, of which 3 were antidepressants and had no interactions reported, 8 had moderate interactions reported, and 2 had minor interactions with Taxol and Abraxane, respectively (Table 6).86-129
Of 3 patients who were dispensed bupropion, nortriptyline, or paroxetine, 1 patient with breast cancer was treated with doxorubicin andcyclophosphamide, followed by paclitaxel with bupropion, nortriptyline, pegfilgrastim,dexamethasone, and 17 other noncancer drugs that had no interaction report dispensed during paclitaxel treatment. Of 2 patients with lung cancer, 1 patient was treated with carboplatin and paclitaxel with nortriptyline, dexamethasone, and 13 additional medications, and the second patient was treated with paroxetine, cimetidine, dexamethasone, and 12 other medications. Patients were dispensed up to6 noncancer medications on the same day as paclitaxel administration to control the AEs, not including the prodrugs filled before the treatments. Paroxetine and cimetidine have weak inhibition, and dexamethasone has weak induction of CYP3A4. Therefore, while 1:1 DDIs might have little or no effect with weak inhibit/induce CYP3A4 drugs, 1:1:1 or more combinations could have a different outcome (confirmed in previous publications).65-67
Dispensed on the same day may not mean taken at the same time. One patient experienced an AE with dispensed 50 mg losartan, carboplatin plus paclitaxel, dexamethasone, and 6 other noncancer drugs. Losartan inhibits paclitaxel, which can lead to negative AEs.57,66,67 However, there were no blood or plasma samples taken to confirm the losartan was taken at the same time as the paclitaxel given this was not a clinical trial.
Conclusions
This retrospective study discusses the use of paclitaxel in the MHS and the potential DDIs associated with it. The study population consisted mostly of active-duty personnel, who are required to be healthy or have controlled or nonactive medical diagnoses and be physically fit. This group is mixed with dependents and retirees that are more reflective of the average US population. As a result, this patient population is healthier than the general population, with a lower prevalence of common illnesses such as diabetes and obesity. The study aimed to identify drugs used alongside paclitaxel treatment. While further research is needed to identify potential DDIs among patients who experienced AEs, in vitro testing will need to be conducted before confirming causality. The low number of AEs experienced by only 32 of 702 patients (5%), with no deaths during paclitaxel treatment, indicates that the drug is generally well tolerated. Although this study cannot conclude that concomitant use with noncancer drugs led to the discontinuation of paclitaxel, we can conclude that there seems to be no significant DDIsidentified between paclitaxel and antidepressants. This comprehensive overview provides clinicians with a complete picture of paclitaxel use for 27 years (1996-2022), enabling them to make informed decisions about paclitaxel treatment.
Acknowledgments
The Department of Research Program funds at Walter Reed National Military Medical Center supported this protocol. We sincerely appreciate the contribution of data extraction from the Joint Pathology Center teams (Francisco J. Rentas, John D. McGeeney, Beatriz A. Hallo, and Johnny P. Beason) and the MHS database personnel (Maj Ryan Costantino, Brandon E. Jenkins, and Alexander G. Rittel). We gratefully thank you for the protocol support from the Department of Research programs: CDR Martin L. Boese, CDR Wesley R. Campbell, Maj. Abhimanyu Chandel, CDR Ling Ye, Chelsea N. Powers, Yaling Zhou, Elizabeth Schafer, Micah Stretch, Diane Beaner, and Adrienne Woodard.
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
1. American Chemical Society. Discovery of camptothecin and taxol. acs.org. Accessed June 4, 2024. https://www.acs.org/education/whatischemistry/landmarks/camptothecintaxol.html
2. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16(3):481-492. doi:10.1007/s10456-013-9334-0.
3. Meštrovic T. Paclitaxel history. News Medical Life Sciences. Updated March 11, 2023. Accessed June 4, 2024. https://www.news-medical.net/health/Paclitaxel-History.aspx
4. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004-1014. doi:10.1056/NEJM199504133321507
5. Walsh V, Goodman J. The billion dollar molecule: Taxol in historical and theoretical perspective. Clio Med. 2002;66:245-267. doi:10.1163/9789004333499_013
6. Perdue RE, Jr, Hartwell JL. The search for plant sources of anticancer drugs. Morris Arboretum Bull. 1969;20:35-53.
7. Wall ME, Wani MC. Camptothecin and taxol: discovery to clinic—thirteenth Bruce F. Cain Memorial Award lecture. Cancer Res. 1995;55:753-760.
8. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc. 1971;93(9):2325-2327. doi:10.1021/ja00738a045
9. Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677-2681. doi:10.1091/mbc.E14-04-0916
10. Chen JG, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and-destabilizing drugs. Cancer Res. 2002;62(7):1935-1938.
11. Singh S, Dash AK. Paclitaxel in cancer treatment: perspectives and prospects of its delivery challenges. Crit Rev Ther Drug Carrier Syst. 2009;26(4):333-372. doi:10.1615/critrevtherdrugcarriersyst.v26.i4.10
12. Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277(5698):665-667. doi:10.1038/277665a0
13. Fuchs DA, Johnson RK. Cytologic evidence that taxol, an antineoplastic agent from taxus brevifolia, acts as a mitotic spindle poison. Cancer Treat Rep. 1978;62(8):1219-1222.
14. Walsh V, Goodman J. From taxol to taxol: the changing identities and ownership of an anti-cancer drug. Med Anthropol. 2002;21(3-4):307-336. doi:10.1080/01459740214074
15. Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug taxol. Soc Sci Med. 1999;49(9):1215-1225. doi:10.1016/s0277-9536(99)00161-6
16. Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56(4):816-825.
17. Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol. 2015;49(2):177-191. doi:10.1007/s12016-014-8416-0
18. Zasadil LM, Andersen KA, Yeum D, et al. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med. 2014;6:229ra243. doi:10.1126/scitranslmed.3007965
19. National Cancer Institute. Carboplatin-Taxol. Published May 30, 2012. Updated March 22, 2023. Accessed June 4, 2024. https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin-taxol
20. Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb; 2011. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
21. Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2021. Accessed June 4, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
22. Awosika AO, Farrar MC, Jacobs TF. Paclitaxel. StatPearls. Updated November 18, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK536917/
23. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated September 1, 2022. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
24. American Cancer Society. Chemotherapy for endometrial cancer. Updated March 27, 2019. Accessed June 4, 2024. https://www.cancer.org/cancer/types/endometrial-cancer/treating/chemotherapy.html
25. US Food and Drug Administration. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. October 30, 2018. Updated December 14, 2018. Accessed June 4, 2024. https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc
26. US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. November 13, 2020. Accessed June 4, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple
27. US Food and Drug Administration. FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast. March 8, 2019. Updated March 18, 2019. Accessed June 5, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
28. US Food and Drug Administration. FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. September 8, 2020. Accessed June 5, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-issues-alert-about-efficacy-and-potential-safety-concerns-atezolizumab-combination-paclitaxel
29. Tan AR. Chemoimmunotherapy: still the standard of care for metastatic triple-negative breast cancer. ASCO Daily News. February 23, 2022. Accessed June 5, 2024. https://dailynews.ascopubs.org/do/chemoimmunotherapy-still-standard-care-metastatic-triple-negative-breast-cancer
30. McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111(4):273-279. doi:10.7326/0003-4819-111-4-273
31. Milas L, Hunter NR, Kurdoglu B, et al. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother Pharmacol. 1995;35(4):297-303. doi:10.1007/BF00689448
32. Searle J, Collins DJ, Harmon B, Kerr JF. The spontaneous occurrence of apoptosis in squamous carcinomas of the uterine cervix. Pathology. 1973;5(2):163-169. doi:10.3109/00313027309060831
33. Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente T. A compressive review about taxol®: history and future challenges. Molecules. 2020;25(24):5986. doi:10.3390/molecules25245986
34. Bernabeu E, Cagel M, Lagomarsino E, Moretton M, Chiappetta DA. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm. 2017;526(1-2):474-495. doi:10.1016/j.ijpharm.2017.05.016
35. Nehate C, Jain S, Saneja A, et al. Paclitaxel formulations: challenges and novel delivery options. Curr Drug Deliv. 2014;11(6):666-686. doi:10.2174/1567201811666140609154949
36. Gelderblom H, Verweij J, Nooter K, Sparreboom A, Cremophor EL. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37(13):1590-1598. doi:10.1016/S0959-8049(01)00171-x
37. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. In vivo biocompatibility, pharmacokinetics, antitumor efficacy, and hypersensitivity evaluation of ionic liquid-mediated paclitaxel formulations. Int J Pharm. 2019;565:219-226. doi:10.1016/j.ijpharm.2019.05.020
38. Borgå O, Henriksson R, Bjermo H, Lilienberg E, Heldring N, Loman N. Maximum tolerated dose and pharmacokinetics of paclitaxel micellar in patients with recurrent malignant solid tumours: a dose-escalation study. Adv Ther. 2019;36(5):1150-1163. doi:10.1007/s12325-019-00909-6
39. Rouzier R, Rajan R, Wagner P, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci USA. 2005;102(23):8315-8320. doi:10.1073/pnas.0408974102
40. Choudhury H, Gorain B, Tekade RK, Pandey M, Karmakar S, Pal TK. Safety against nephrotoxicity in paclitaxel treatment: oral nanocarrier as an effective tool in preclinical evaluation with marked in vivo antitumor activity. Regul Toxicol Pharmacol. 2017;91:179-189. doi:10.1016/j.yrtph.2017.10.023
41. Barkat MA, Beg S, Pottoo FH, Ahmad FJ. Nanopaclitaxel therapy: an evidence based review on the battle for next-generation formulation challenges. Nanomedicine (Lond). 2019;14(10):1323-1341. doi:10.2217/nnm-2018-0313
42. Sofias AM, Dunne M, Storm G, Allen C. The battle of “nano” paclitaxel. Adv Drug Deliv Rev. 2017;122:20-30. doi:10.1016/j.addr.2017.02.003
43. Yang N, Wang C, Wang J, et al. Aurora inase a stabilizes FOXM1 to enhance paclitaxel resistance in triple-negative breast cancer. J Cell Mol Med. 2019;23(9):6442-6453. doi:10.1111/jcmm.14538
44. Chowdhury MR, Moshikur RM, Wakabayashi R, et al. Ionic-liquid-based paclitaxel preparation: a new potential formulation for cancer treatment. Mol Pharm. 2018;15(16):2484-2488. doi:10.1021/acs.molpharmaceut.8b00305
45. Chung HJ, Kim HJ, Hong ST. Tumor-specific delivery of a paclitaxel-loading HSA-haemin nanoparticle for cancer treatment. Nanomedicine. 2020;23:102089. doi:10.1016/j.nano.2019.102089
46. Ye L, He J, Hu Z, et al. Antitumor effect and toxicity of lipusu in rat ovarian cancer xenografts. Food Chem Toxicol. 2013;52:200-206. doi:10.1016/j.fct.2012.11.004
47. Ma WW, Lam ET, Dy GK, et al. A pharmacokinetic and dose-escalating study of paclitaxel injection concentrate for nano-dispersion (PICN) alone and with arboplatin in patients with advanced solid tumors. J Clin Oncol. 2013;31:2557. doi:10.1200/jco.2013.31.15_suppl.2557
48. Micha JP, Goldstein BH, Birk CL, Rettenmaier MA, Brown JV. Abraxane in the treatment of ovarian cancer: the absence of hypersensitivity reactions. Gynecol Oncol. 2006;100(2):437-438. doi:10.1016/j.ygyno.2005.09.012
49. Ingle SG, Pai RV, Monpara JD, Vavia PR. Liposils: an effective strategy for stabilizing paclitaxel loaded liposomes by surface coating with silica. Eur J Pharm Sci. 2018;122:51-63. doi:10.1016/j.ejps.2018.06.025
50. Abriata JP, Turatti RC, Luiz MT, et al. Development, characterization and biological in vitro assays of paclitaxel-loaded PCL polymeric nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;96:347-355. doi:10.1016/j.msec.2018.11.035
51. Hu J, Fu S, Peng Q, et al. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: in vitro and in vivo evaluation. Int J Pharm. 2017;516(1-2):313-322. doi:10.1016/j.ijpharm.2016.11.047
52. Dranitsaris G, Yu B, Wang L, et al. Abraxane® vs Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
53. Pei Q, Hu X, Liu S, Li Y, Xie Z, Jing X. Paclitaxel dimers assembling nanomedicines for treatment of cervix carcinoma. J Control Release. 2017;254:23-33. doi:10.1016/j.jconrel.2017.03.391
54. Wang Y, Wang M, Qi H, et al. Pathway-dependent inhibition of paclitaxel hydroxylation by kinase inhibitors and assessment of drug-drug interaction potentials. Drug Metab Dispos. 2014;42(4):782-795. doi:10.1124/dmd.113.053793
55. Shen F, Jiang G, Philips S, et al. Cytochrome P450 oxidoreductase (POR) associated with severe paclitaxel-induced peripheral neuropathy in patients of european ancestry from ECOG-ACRIN E5103. Clin Cancer Res. 2023;29(13):2494-2500. doi:10.1158/1078-0432.CCR-22-2431
56. Henningsson A, Marsh S, Loos WJ, et al. Association of CYP2C8, CYP3A4, CYP3A5, and ABCB1 polymorphisms with the pharmacokinetics of paclitaxel. Clin Cancer Res. 2005;11(22):8097-8104. doi:10.1158/1078-0432.CCR-05-1152
57. Mukai Y, Senda A, Toda T, et al. Drug-drug interaction between losartan and paclitaxel in human liver microsomes with different CYP2C8 genotypes. Basic Clin Pharmacol Toxicol. 2015;116(6):493-498. doi:10.1111/bcpt.12355
58. Kawahara B, Faull KF, Janzen C, Mascharak PK. Carbon monoxide inhibits cytochrome P450 enzymes CYP3A4/2C8 in human breast cancer cells, increasing sensitivity to paclitaxel. J Med Chem. 2021;64(12):8437-8446. doi:10.1021/acs.jmedchem.1c00404
59. Cresteil T, Monsarrat B, Dubois J, Sonnier M, Alvinerie P, Gueritte F. Regioselective metabolism of taxoids by human CYP3A4 and 2C8: structure-activity relationship. Drug Metab Dispos. 2002;30(4):438-445. doi:10.1124/dmd.30.4.438
60. Taniguchi R, Kumai T, Matsumoto N, et al. Utilization of human liver microsomes to explain individual differences in paclitaxel metabolism by CYP2C8 and CYP3A4. J Pharmacol Sci. 2005;97(1):83-90. doi:10.1254/jphs.fp0040603
61. Nakayama A, Tsuchiya K, Xu L, Matsumoto T, Makino T. Drug-interaction between paclitaxel and goshajinkigan extract and its constituents. J Nat Med. 2022;76(1):59-67. doi:10.1007/s11418-021-01552-8
62. Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos. 1998;26(3):229-233.
63. Walle T. Assays of CYP2C8- and CYP3A4-mediated metabolism of taxol in vivo and in vitro. Methods Enzymol. 1996;272:145-151. doi:10.1016/s0076-6879(96)72018-9
64. Hanioka N, Matsumoto K, Saito Y, Narimatsu S. Functional characterization of CYP2C8.13 and CYP2C8.14: catalytic activities toward paclitaxel. Basic Clin Pharmacol Toxicol. 2010;107(1):565-569. doi:10.1111/j.1742-7843.2010.00543.x
65. Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
66. Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with Cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217-224. doi:10.1016/j.crtox.2021.05.006
67. Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the military health system. Fed Pract. 2023;40(suppl 3):S24-S34. doi:10.12788/fp.0401
68. Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute. Accessed June 5, 2024. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
69. World Health Organization. International classification of diseases for oncology (ICD-O) 3rd ed, 1st revision. World Health Organization; 2013. Accessed June 5, 2024. https://apps.who.int/iris/handle/10665/96612
70. Z score calculator for 2 population proportions. Social science statistics. Accessed June 5, 2024. https://www.socscistatistics.com/tests/ztest/default2.aspx
71. US Food and Drug Administration. Generic drugs: question & answers. FDA.gov. Accessed June 5, 2024. https://www.fda.gov/drugs/frequently-asked-questions-popular-topics/generic-drugs-questions-answers
72. Oura M, Saito H, Nishikawa Y. Shortage of nab-paclitaxel in Japan and around the world: issues in global information sharing. JMA J. 2023;6(2):192-195. doi:10.31662/jmaj.2022-0179
73. Yuan H, Guo H, Luan X, et al. Albumin nanoparticle of paclitaxel (abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol Pharm. 2020;17(7):2275-2286. doi:10.1021/acs.molpharmaceut.9b01221
74. Dranitsaris G, Yu B, Wang L, et al. Abraxane® versus Taxol® for patients with advanced breast cancer: a prospective time and motion analysis from a Chinese health care perspective. J Oncol Pharm Pract. 2016;22(2):205-211. doi:10.1177/1078155214556008
75. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794-7803. doi:10.1200/JCO.2005.04.
76. Liu M, Liu S, Yang L, Wang S. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):118. doi:10.1186/s12885-021-07831-7
77. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 1993;20(4 Suppl 3):1-15.
78. Banerji A, Lax T, Guyer A, Hurwitz S, Camargo CA Jr, Long AA. Management of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year review. J Allergy Clin Immunol Pract. 2014;2(4):428-433. doi:10.1016/j.jaip.2014.04.010
79. Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S. Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol. 2020;324:113121. doi:10.1016/j.expneurol.2019.113121
80. Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6(5):489-494. doi:10.1093/oxfordjournals.annonc.a059220
81. Liu JM, Chen YM, Chao Y, et al. Paclitaxel-induced severe neuropathy in patients with previous radiotherapy to the head and neck region. J Natl Cancer Inst. 1996;88(14):1000-1002. doi:10.1093/jnci/88.14.1000-a
82. Bayat Mokhtari R, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022-38043. doi:10.18632/oncotarget.16723
83. Blagosklonny MV. Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle. 2004;3(8):1035-1042.
84. Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592-1605. doi:10.1200/JCO.2011.37.6418
85. Gilani B, Cassagnol M. Biochemistry, Cytochrome P450. StatPearls. Updated April 24, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557698/
86. LiverTox: clinical and research information on drug-induced liver injury; 2012. Carboplatin. Updated September 15, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548565/
87. Carboplatin. Prescribing information. Teva Parenteral Medicines; 2012. Accessed June 5, 204. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/077139Orig1s016lbl.pdf
88. Johnson-Arbor K, Dubey R. Doxorubicin. StatPearls. Updated August 8, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459232/
89. Doxorubicin hydrochloride injection. Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/050467s078,050629s030lbl.pdf
90. Gor, PP, Su, HI, Gray, RJ, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: a retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi:10.1186/bcr2570
91. Cyclophosphamide. Prescribing information. Ingenus Pharmaceuticals; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf
92. Gemcitabine. Prescribing information. Hospira; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/200795Orig1s010lbl.pdf
93. Ifex (ifosfamide). Prescribing information. Baxter; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019763s017lbl.pdf
94. Cisplatin. Prescribing information. WG Critical Care; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/018057s089lbl.pdf
95. Gerriets V, Kasi A. Bevacizumab. StatPearls. Updated August 28, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482126/
96. Avastin (bevacizumab). Prescribing information. Genentech; 2022. Accessed June 5, 2024. https://www.accessdata .fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf
 97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
97. Keytruda (pembrolizumab). Prescribing information. Merck; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
98. Dean L, Kane M. Capecitabine therapy and DPYD genotype. National Center for Biotechnology Information (US); 2012. Updated November 2, 2020. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK385155/
99. Xeloda (capecitabine). Prescribing information. Roche; 2000. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf
100. Pemetrexed injection. Prescribing information. Fareva Unterach; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214657s000lbl.pdf
101. Topotecan Injection. Prescribing information. Zydus Hospira Oncology; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200582s001lbl.pdf
102. Ibrance (palbociclib). Prescribing information. Pfizer; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf
103. Navelbine (vinorelbine) injection. Prescribing information. Pierre Fabre Médicament; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020388s037lbl.pdf
104. LiverTox: clinical and research information on drug-induced liver injury; 2012. Letrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548381/
105. Femara (letrozole). Prescribing information. Novartis; 2014. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020726s027lbl.pdf
106. Soltamox (tamoxifen citrate). Prescribing information. Rosemont Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf
107. LiverTox: clinical and research information on drug-induced liver injury; 2012. Anastrozole. Updated July 25, 2017. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK548189/
108. Grimm SW, Dyroff MC. Inhibition of human drug metabolizing cytochromes P450 by anastrozole, a potent and selective inhibitor of aromatase. Drug Metab Dispos. 1997;25(5):598-602.
109. Arimidex (anastrozole). Prescribing information. AstraZeneca; 2010. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020541s026lbl.pdf
110. Megace (megestrol acetate). Prescribing information. Endo Pharmaceuticals; 2018. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021778s024lbl.pdf
111. Imfinzi (durvalumab). Prescribing information. AstraZeneca; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761069s018lbl.pdf
112. Merwar G, Gibbons JR, Hosseini SA, et al. Nortriptyline. StatPearls. Updated June 5, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482214/
113. Pamelor (nortriptyline HCl). Prescribing information. Patheon Inc.; 2012. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
114. Wellbutrin (bupropion hydrochloride). Prescribing information. GlaxoSmithKline; 2017. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018644s052lbl.pdf
115. Paxil (paroxetine). Prescribing information. Apotex Inc.; 2021. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
116. Johnson DB, Lopez MJ, Kelley B. Dexamethasone. StatPearls. Updated May 2, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482130/
117. Hemady (dexamethasone). Prescribing information. Dexcel Pharma; 2019. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211379s000lbl.pdf
118. Parker SD, King N, Jacobs TF. Pegfilgrastim. StatPearls. Updated May 9, 2024. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK532893/
119. Fylnetra (pegfilgrastim-pbbk). Prescribing information. Kashiv BioSciences; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761084s000lbl.pdf
120. Emend (aprepitant). Prescribing information. Merck; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207865lbl.pdf
121. Lipitor (atorvastatin calcium). Prescribing information. Viatris Specialty; 2022. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020702Orig1s079correctedlbl.pdf
122. Cipro (ciprofloxacin hydrochloride). Prescribing information. Bayer HealthCare Pharmaceuticals Inc.; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019537s090,020780s047lbl.pdf
123. Pino MA, Azer SA. Cimetidine. StatPearls. Updated March 6, 2023. Accessed June 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK544255/
124. Tagament (Cimetidine). Prescribing information. Mylan; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020238Orig1s024lbl.pdf
125. Neupogen (filgrastim). Prescribing information. Amgen Inc.; 2015. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/103353s5184lbl.pdf
126. Flagyl (metronidazole). Prescribing information. Pfizer; 2013. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020334s008lbl.pdf
127. Zymaxid (gatifloxacin ophthalmic solution). Prescribing information. Allergan; 2016. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022548s002lbl.pdf
128. Macrobid (nitrofurantoin monohydrate). Prescribing information. Procter and Gamble Pharmaceutical Inc.; 2009. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020064s019lbl.pdf
129. Hyzaar (losartan). Prescribing information. Merck; 2020. Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020387s067lbl.pdf
AVAHO Mtg: Germline Testing Key for Vets With High-Risk PC
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
Is Location a Risk Factor for Early-Onset Cancer?
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”
Early-onset cancer—diagnosed in adults aged ≤ 50 years—is on the rise. Researchers have studied a variety of factors driving the trend, such as type of cancer. However, geographic locality might have as much, if not more, to do with it, according to a study by researchers at Fox Chase Cancer Center, a National Cancer Institute-designated Comprehensive Cancer Center research facility.
Using the US Cancer Statistics Public Use Research Database, the researchers collected data from adults aged 20 to 49 years with invasive cancer (excluding in situ cases) diagnosed from 2015 through 2020. They calculated the incidence for each state using the national rate as the reference. Then, they calculated a second set of rates, comparing each state to the US in terms of overall incidence and advanced-stage incidence for all early-onset cancers.
The resulting maps indicated that early-onset cancer cases are not evenly distributed. States with worse-than-national rates are frequently near each other geographically. For instance, the rate of early-onset female breast cancer was worse than the national rate in 17 states, 16 of which were located in the eastern half of the US (Hawaii was the 17th state). Similarly, most states with worse-than-national rates of digestive cancers were located in the eastern half of the US, with a concentration in the South. Rates of male genital cancers were worse than national rates in 18 states, primarily in the eastern half of the country (plus Montana, Nebraska, and Puerto Rico).
Three states in the Southeast, 7 in the Northeast, and Puerto Rico had the highest incidence of lymphohematopoietic cancers. Incidence rates of endocrine cancers were worse than national rates in 25 states, which the researchers found formed “a horizontal core of the country running from east to west,” plus Puerto Rico. Rates of urinary system cancers were worse than national rates in 17 contiguous states, from New Mexico to Pennsylvania.
Rates of female genital cancers were worse than national rates in 16 states, largely in the Midwest and South, plus California and Puerto Rico. Skin cancer, on the other hand, was a great leveler, with worse-than-national rates in 32 states, mostly in the northern portion of the country.
Kentucky and West Virginia had the highest overall and advanced-stage incidence rates of early-onset cancer for all cancer sites combined. They were followed by Arkansas, Connecticut, Florida, Georgia, Iowa, Louisiana, Maine, Missouri, New Jersey, New York, North Carolina, Ohio, and Pennsylvania.
According to the researchers, this study provides the first analysis of age-adjusted rates of early-onset cancer based on state-level population and case numbers. Geographic patterns in early-onset cancer, they suggest, indicate possible similarities that could relate to demographic, socioeconomic, behavioral, or environmental risks. “Focusing prevention efforts on the highest-incidence states for the most prevalent sites may reduce the rate of early-onset cancer nationally.”