User login
Cuffless blood pressure monitors: Still a numbers game
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Medscape’s Editor-in-Chief Eric Topol, MD, referred to continual noninvasive, cuffless, accurate blood pressure devices as “a holy grail in sensor technology.”
He personally tested a cuff-calibrated, over-the-counter device available in Europe that claims to monitor daily blood pressure changes and produce data that can help physicians titrate medications.
Dr. Topol does not believe that it is ready for prime time. Yes, cuffless devices are easy to use, and generate lots of data. But are those data accurate?
Many experts say not yet, even as the market continues to grow and more devices are introduced and highlighted at high-profile consumer events.
Burned before
Limitations of cuffed devices are well known, including errors related to cuff size, patient positioning, patient habits or behaviors (for example, caffeine/nicotine use, acute meal digestion, full bladder, very recent physical activity) and clinicians’ failure to take accurate measurements.
Like many clinicians, Timothy B. Plante, MD, MHS, assistant professor at the University of Vermont Medical Center thrombosis & hemostasis program in Burlington, is very excited about cuffless technology. However, “we’ve been burned by it before,” he said in an interview.
Dr. Plante’s 2016 validation study of an instant blood pressure smartphone app found that its measurements were “highly inaccurate,” with such low sensitivity that more than three-quarters of individuals with hypertensive blood levels would be falsely reassured that their blood pressure was in the normal range.
His team’s 2023 review of the current landscape, which includes more sophisticated devices, concluded that accuracy remains an issue: “Unfortunately, the pace of regulation of these devices has failed to match the speed of innovation and direct availability to patient consumers. There is an urgent need to develop a consensus on standards by which cuffless BP devices can be tested for accuracy.”
Devices, indications differ
Cuffless devices estimate blood pressure indirectly. Most operate based on pulse wave analysis and pulse arrival time (PWA-PAT), explained Ramakrishna Mukkamala, PhD, in a commentary. Dr. Mukkamala is a professor in the departments of bioengineering and anesthesiology and perioperative medicine at the University of Pittsburgh.
PWA involves measuring a peripheral arterial waveform using an optical sensor such as the green lights on the back of a wrist-worn device, or a ‘force sensor’ such as a finger cuff or pressing on a smartphone. Certain features are extracted from the waveform using machine learning and calibrated to blood pressure values.
PAT techniques work together with PWA; they record the ECG and extract features from that signal as well as the arterial waveform for calibration to blood pressure values.
The algorithm used to generate the BP numbers comprises a proprietary baseline model that may include demographics and other patient characteristics. A cuff measurement is often part of the baseline model because most cuffless devices require periodic (typically weekly or monthly) calibration using a cuffed device.
Cuffless devices that require cuff calibration compare the estimate they get to the cuff-calibrated number. In this scenario, the cuffless device may come up with the same blood pressure numbers simply because the baseline model – which is made up of thousands of data points relevant to the patient – has not changed.
This has led some experts to question whether PWA-PAT cuffless device readings actually add anything to the baseline model.
They don’t, according to Microsoft Research in what Dr. Mukkamala and coauthors referred to (in a review published in Hypertension) as “a complex article describing perhaps the most important and highest resource project to date (Aurora Project) on assessing the accuracy of PWA and PWA devices.”
The Microsoft article was written for bioengineers. The review in Hypertension explains the project for clinicians, and concludes that, “Cuffless BP devices based on PWA and PWA-PAT, which are similar to some regulatory-cleared devices, were of no additional value in measuring auscultatory or 24-hour ambulatory cuff BP when compared with a baseline model in which BP was predicted without an actual measurement.”
IEEE and FDA validation
Despite these concerns, several cuffless devices using PWA and PAT have been cleared by the Food and Drug Administration.
Validating cuffless devices is no simple matter. The Institute of Electrical and Electronics Engineers published a validation protocol for cuffless blood pressure devices in 2014 that was amended in 2019 to include a requirement to evaluate performance in different positions and in the presence of motion with varying degrees of noise artifact.
However, Daichi Shimbo, MD, codirector of the Columbia Hypertension Center in New York and vice chair of the American Heart Association Statement on blood pressure monitoring, and colleagues point out limitations, even in the updated standard. These include not requiring evaluation for drift over time; lack of specific dynamic testing protocols for stressors such as exercise or environmental temperatures; and an unsuitable reference standard (oscillometric cuff-based devices) during movement.
Dr. Shimbo said in an interview that, although he is excited about them, “these cuffless devices are not aligned with regulatory bodies. If a device gives someone a wrong blood pressure, they might be diagnosed with hypertension when they don’t have it or might miss the fact that they’re hypertensive because they get a normal blood pressure reading. If there’s no yardstick by which you say these devices are good, what are we really doing – helping, or causing a problem?”
“The specifics of how a device estimates blood pressure can determine what testing is needed to ensure that it is providing accurate performance in the intended conditions of use,” Jeremy Kahn, an FDA press officer, said in an interview. “For example, for cuffless devices that are calibrated initially with a cuff-based blood pressure device, the cuffless device needs to specify the period over which it can provide accurate readings and have testing to demonstrate that it provides accurate results over that period of use.”
The FDA said its testing is different from what the Microsoft Aurora Project used in their study.
“The intent of that testing, as the agency understands it, is to evaluate whether the device is providing useful input based on the current physiology of the patient rather than relying on predetermined values based on calibration or patient attributes. We evaluate this clinically in two separate tests: an induced change in blood pressure test and tracking of natural blood pressure changes with longer term device use,” Mr. Kahn explained.
Analyzing a device’s performance on individuals who have had natural changes in blood pressure as compared to a calibration value or initial reading “can also help discern if the device is using physiological data from the patient to determine their blood pressure accurately,” he said.
Experts interviewed for this article who remain skeptical about cuffless BP monitoring question whether the numbers that appear during the induced blood pressure change, and with the natural blood pressure changes that may occur over time, accurately reflect a patient’s blood pressure.
“The FDA doesn’t approve these devices; they clear them,” Dr. Shimbo pointed out. “Clearing them means they can be sold to the general public in the U.S. It’s not a strong statement that they’re accurate.”
Moving toward validation, standards
Ultimately, cuffless BP monitors may require more than one validation protocol and standard, depending on their technology, how and where they will be used, and by whom.
And as Dr. Plante and colleagues write, “Importantly, validation should be performed in diverse and special populations, including pregnant women and individuals across a range of heart rates, skin tones, wrist sizes, common arrhythmias, and beta-blocker use.”
Organizations that might be expected to help move validation and standards forward have mostly remained silent. The American Medical Association’s US Blood Pressure Validated Device Listing website includes only cuffed devices, as does the website of the international scientific nonprofit STRIDE BP.
The European Society of Hypertension 2022 consensus statement on cuffless devices concluded that, until there is an internationally accepted accuracy standard and the devices have been tested in healthy people and those with suspected or diagnosed hypertension, “cuffless BP devices should not be used for the evaluation or management of hypertension in clinical practice.”
This month, ESH published recommendations for “specific, clinically meaningful, and pragmatic validation procedures for different types of intermittent cuffless devices” that will be presented at their upcoming annual meeting June 26.
Updated protocols from IEEE “are coming out soon,” according to Dr. Shimbo. The FDA says currently cleared devices won’t need to revalidate according to new standards unless the sponsor makes significant modifications in software algorithms, device hardware, or targeted patient populations.
Device makers take the initiative
In the face of conflicting reports on accuracy and lack of a robust standard, some device makers are publishing their own tests or encouraging validation by potential customers.
For example, institutions that are considering using the Biobeat cuffless blood pressure monitor watch “usually start with small pilots with our devices to do internal validation,” Lior Ben Shettrit, the company’s vice president of business development, said in an interview. “Only after they complete the internal validation are they willing to move forward to full implementation.”
Cardiologist Dean Nachman, MD, is leading validation studies of the Biobeat device at the Hadassah Ein Kerem Medical Center in Jerusalem. For the first validation, the team recruited 1,057 volunteers who did a single blood pressure measurement with the cuffless device and with a cuffed device.
“We found 96.3% agreement in identifying hypertension and an interclass correlation coefficient of 0.99 and 0.97 for systolic and diastolic measurements, respectively,” he said. “Then we took it to the next level and compared the device to ambulatory 24-hour blood pressure monitoring and found comparable measurements.”
The investigators are not done yet. “We need data from thousands of patients, with subgroups, to not have any concerns,” he says. “Right now, we are using the device as a general monitor – as an EKG plus heart rate plus oxygen saturation level monitor – and as a blood pressure monitor for 24-hour blood pressure monitoring.”
The developers of the Aktiia device, which is the one Dr. Topol tested, take a different perspective. “When somebody introduces a new technology that is disrupting something that has been in place for over 100 years, there will always be some grumblings, ruffling of feathers, people saying it’s not ready, it’s not ready, it’s not ready,” Aktiia’s chief medical officer Jay Shah, MD, noted.
“But a lot of those comments are coming from the isolation of an ivory tower,” he said.
Aktiia cofounder and chief technology officer Josep Solà said that “no device is probably as accurate as if you have an invasive catheter,” adding that “we engage patients to look at their blood pressure day by day. … If each individual measurement of each of those patient is slightly less accurate than a cuff, who cares? We have 40 measurements per day on each patient. The accuracy and precision of each of those is good.”
Researchers from the George Institute for Global Health recently compared the Aktiia device to conventional ambulatory monitoring in 41 patients and found that “it did not accurately track night-time BP decline and results suggested it was unable to track medication-induced BP changes.”
“In the context of 24/7 monitoring of hypertensive patients,” Mr. Solà said, “whatever you do, if it’s better than a sham device or a baseline model and you track the blood pressure changes, it’s a hundred times much better than doing nothing.”
Dr. Nachman and Dr. Plante reported no relevant financial relationships. Dr. Shimbo reported that he received funding from NIH and has consulted for Abbott Vascular, Edward Lifesciences, Medtronic, and Tryton Medical.
A version of this article first appeared on Medscape.com.
Early hysterectomy linked to higher CVD, stroke risk
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Is there benefit to adding ezetimibe to a statin for the secondary prevention of CVD?
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.
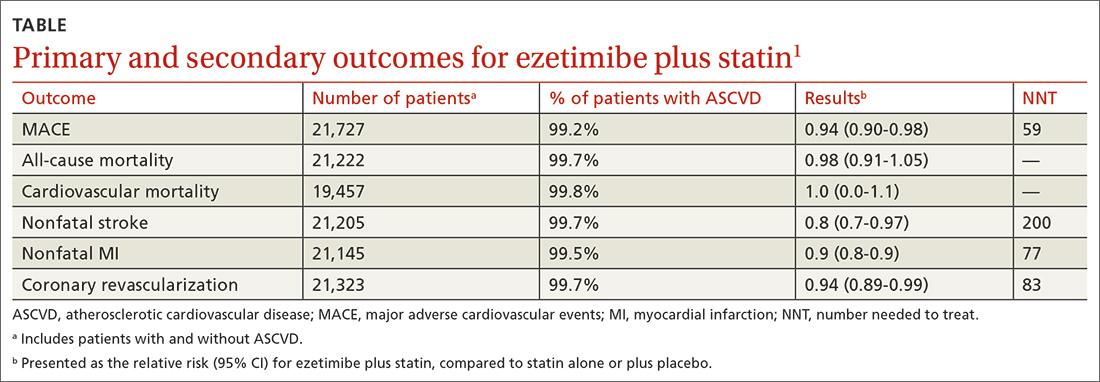
The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
Evidence summary
Adding ezetimibe reduces nonfatal events but does not improve mortality
A 2018 Cochrane meta-analysis included 10 RCTs (N = 21,919 patients) that evaluated the efficacy and safety of ezetimibe plus a statin (dual therapy) vs a statin alone or plus placebo (monotherapy) for the secondary prevention of CVD. Mean age of patients ranged from 55 to 84 years. Almost all of the patients (> 99%) included in the analyses had existing ASCVD. The dose of ezetimibe was 10 mg; statins used included atorvastatin 10 to 80 mg, pitavastatin 2 to 4 mg, rosuvastatin 10 mg, and simvastatin 20 to 80 mg.1
The primary outcomes were MACE and all-cause mortality. MACE is defined as a composite of CVD, nonfatal myocardial infarction (MI), nonfatal stroke, hospitalization for unstable angina, or coronary revascularization procedures. The TABLE1 provides a detailed breakdown of each of the outcomes.

The dual-therapy group compared to the monotherapy group had a lower risk for MACE (26.6% vs 28.3%; 1.7% absolute risk reduction; 6% relative risk reduction; NNT = 59) and little or no difference in the reduction of all-cause mortality. For secondary outcomes, the dual-therapy group had a lower risk for nonfatal MI, nonfatal stroke, and coronary revascularization. There was no difference in cardiovascular mortality or adverse events between the 2 groups. The quality of evidence was high for all-cause mortality and moderate for cardiovascular mortality, MACE, MI, and stroke.1
The 2015 IMPROVE-IT study, the largest included in the Cochrane review, was a double-blind RCT (N = 18,144) conducted at 1147 sites in 39 countries comparing simvastatin 40 mg/d plus ezetimibe 10 mg/d (dual therapy) vs simvastatin 40 mg/d plus placebo (monotherapy). Patients were at least 50 years old (average age, 64 years) and had been hospitalized for acute coronary syndrome (ACS) within the previous 10 days; 76% were male and 84% were White. The average low-density lipoprotein (LDL) concentration at baseline was 94 mg/dL in both groups.2
The primary endpoint was a composite of cardiovascular death, a major coronary event (nonfatal MI, unstable angina requiring hospitalization, coronary revascularization at least 30 days after randomization), or nonfatal stroke, with a median follow-up of 6 years. The simvastatin plus ezetimibe group compared to the simvastatin-only group had a lower risk for the primary end point (HR = 0.94; 95% CI, 0.89-0.99; NNT = 50), but no differences in cardiovascular or all-cause mortality. Since the study only recruited patients with recent ACS, results are only applicable to that specific population.2
The 2022 RACING study was a multicenter, open-label, randomized, noninferiority trial that evaluated the combination of ezetimibe 10 mg and a moderate-intensity statin (rosuvastatin 10 mg) compared to a high-intensity statin alone (rosuvastatin 20 mg) in adults (N = 3780) with ASCVD. Included patients were ages 19 to 80 years (mean, 64 years) and had a baseline LDL concentration of 80 mg/dL (standard deviation, 64-100 mg/dL) with known ASCVD (defined by prior MI, ACS, history of coronary or other arterial revascularization, ischemic stroke, or peripheral artery disease); 75% were male.3
The primary outcome was a composite of cardiovascular death, major cardiovascular events, or nonfatal stroke. At 3 years, an intention-to-treat analysis found no significant difference between the combination and monotherapy groups (9% vs 9.9%; absolute difference, –0.78%; 95% CI, –2.39% to 0.83%). Dose reduction or discontinuation of the study drug(s) due to intolerance was lower in the combination group than in the monotherapy group (4.8% vs 8.2%; P < 0.0001). The study may be limited by the fact that it was nonblinded and all participants were South Korean, which limits generalizability.3
Recommendations from others
A 2022 evidence-based clinical practice guideline published in BMJ recommends adding ezetimibe to a statin to decrease all-cause mortality, cardiovascular mortality, nonfatal stroke, and nonfatal MI in patients with known CVD, regardless of their LDL concentration (weak recommendation based on a systematic review and network meta-analysis).4
In 2019, the American Heart Association and the American College of Cardiology recommended ezetimibe for patients with clinical ASCVD who are on maximally tolerated statin therapy and have an LDL concentration of 70 mg/dL or higher (Class 2b recommendation [meaning it can be considered] based on a meta-analysis of moderate-quality RCTs).5
Editor’s takeaway
The data on this important and well-studied question have inched closer to firm and clear answers. First, adding ezetimibe to a lower-intensity statin when a higher-intensity statin is not tolerated is an effective treatment. Second, adding ezetimibe to a statin improves nonfatal ASCVD outcomes but not fatal ones. What has not yet been made clear, because a noninferiority trial does not answer this question, is whether the highest intensity statin plus ezetimibe is superior to that high-intensity statin alone, regardless of LDL concentration.
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
1. Zhan S, Tang M, Liu F, et al. Ezetimibe for the prevention of cardiovascular disease and all‐cause mortality events. Cochrane Database System Rev. 2018;11:CD012502. doi: 10.1002/14651858.CD012502.pub2
2. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397. doi: 10.1056/NEJMoa1410489 pmid:26039521
3. Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380-390. doi: 10.1016/S0140-6736(22)00916-3
4. Hao Q, Aertgeerts B, Guyatt G, et al. PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations. BMJ. 2022;377:e069066. doi: 10.1136/bmj-2021-069066
5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
EVIDENCE-BASED REVIEW:
YES. In patients with known cardio- vascular disease (CVD), ezetimibe with a statin decreases
In adults with atherosclerotic CVD (ASCVD), the combination of ezetimibe and a moderate-intensity statin (rosuvastatin 10 mg) was noninferior at decreasing cardiovascular death, major cardiovascular events, and nonfatal stroke, but was more tolerable, compared to a high-intensity statin (rosuvastatin 20 mg) alone (SOR, B; 1 RCT).
64-year-old woman • hot flashes, facial flushing, excessive sweating, and palpitations • daily headaches • history of hypertension • Dx?
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.
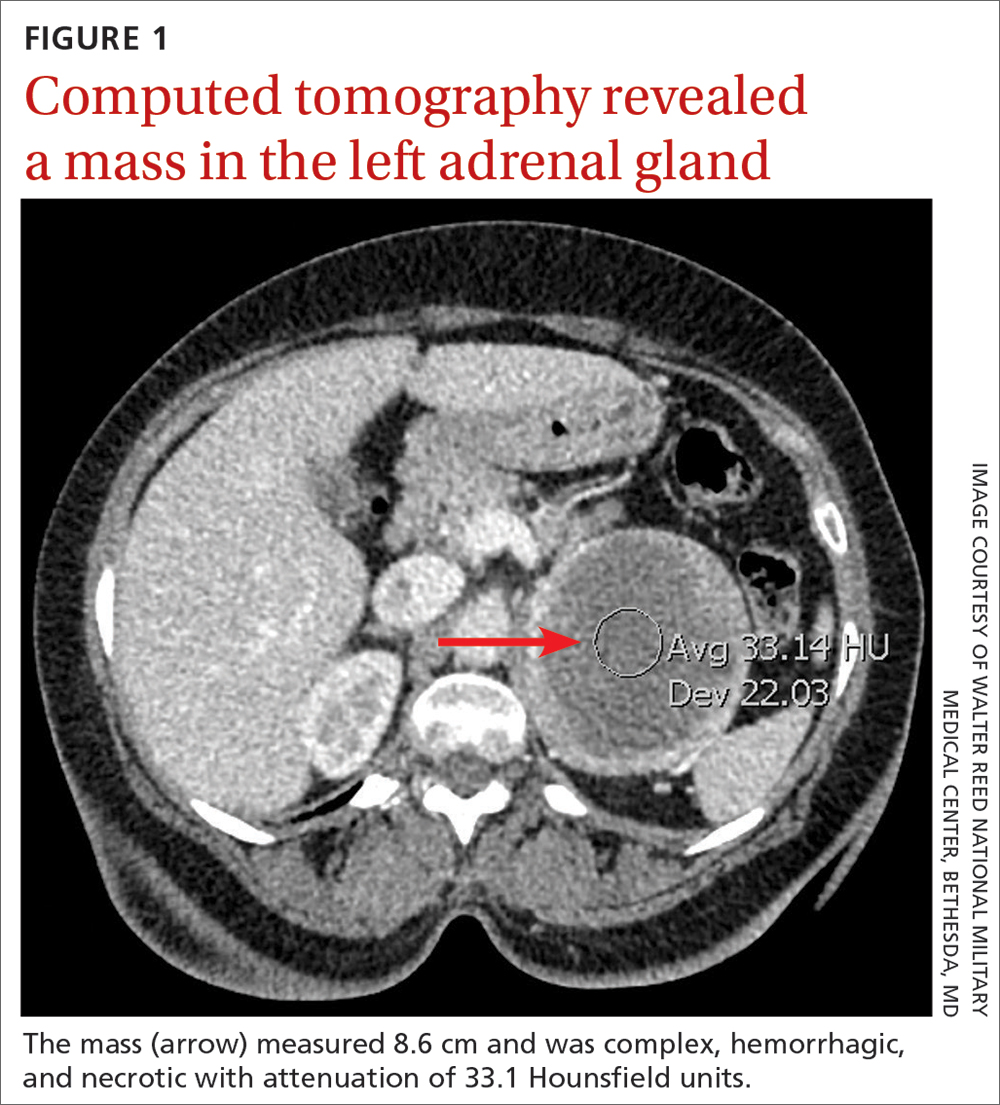
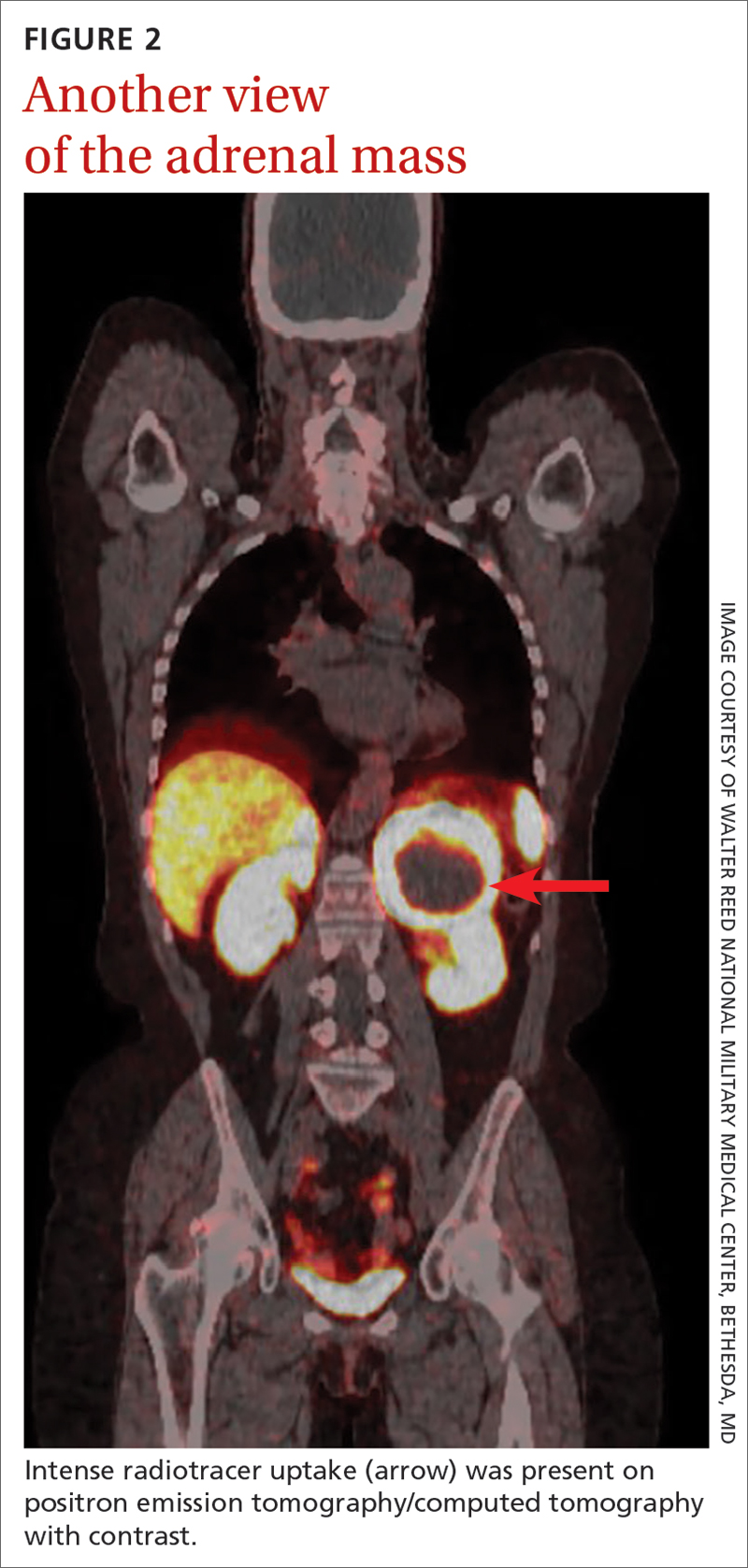
Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).
THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
THE CASE
A 64-year-old woman sought care after having hot flashes, facial flushing, excessive sweating, palpitations, and daily headaches for 1 month. She had a history of hypertension that was well controlled with hydrochlorothiazide 25 mg/d but over the previous month, it had become more difficult to control. Her blood pressure remained elevated to 150/100 mm Hg despite the addition of lisinopril 40 mg/d and amlodipine 10 mg/d, indicating resistant hypertension. She had no family history of hypertension, diabetes, or obesity or any other pertinent medical or surgical history. Physical examination was negative for weight gain, stretch marks, or muscle weakness.

Laboratory tests revealed a normal serum aldosterone-renin ratio, renal function, and thyroid function; however, she had elevated levels of normetanephrine (2429 pg/mL; normal range, 0-145 pg/mL) and metanephrine (143 pg/mL; normal range, 0-62 pg/mL). Computed tomography (CT) revealed an 8.6-cm complex, hemorrhagic, necrotic left adrenal mass with attenuation of 33.1 Hounsfield units (HU) (FIGURE 1). Magnetic resonance imaging (MRI) demonstrated a T2 hyperintense left adrenal mass. An evaluation for Cushing syndrome was negative, and positron emission tomography (PET)/CT with gallium-68 dotatate was ordered. It showed intense radiotracer uptake in the left adrenal gland, with a maximum standardized uptake value of 70.1 (FIGURE 2).

THE DIAGNOSIS
After appropriate preparation with alpha blockade (phenoxybenzamine 20 mg twice daily for 7 days) and fluid resuscitation (normal saline run over 12 hours preoperatively), the patient underwent successful open surgical resection of the adrenal mass, during which her blood pressure was controlled with a nitroprusside infusion and boluses of esmolol and labetalol. Pathology results showed cells in a nested pattern with round to oval nuclei in a vascular background. There was no necrosis, increased mitotic figures, capsular invasion, or increased cellularity. Chromogranin immunohistochemical staining was positive. Given her resistant hypertension, clinical symptoms, and pathology results, the patient was given a diagnosis of pheochromocytoma.
DISCUSSION
Resistant hypertension is defined as blood pressure that is elevated above goal despite the use of 3 maximally titrated antihypertensive agents from different classes or that is well controlled with at least 4 antihypertensive medications.1 The prevalence of resistant hypertension is 12% to 18% in adults being treated for hypertension.1 Patients with resistant hypertension have a higher risk for cardiovascular events and death, are more likely to have a secondary cause of hypertension, and may benefit from special diagnostic testing or treatment approaches to control their blood pressure.1
There are many causes of resistant hypertension; primary aldosteronism is the most common cause (prevalence as high as 20%).2 Given the increased risk for cardiovascular/cerebrovascular disease, all patients with resistant hypertension should be screened for this condition.2 Other causes of resistant hypertension include renal parenchymal disease, renal artery stenosis, coarctation of the aorta, thyroid dysfunction, Cushing syndrome, paraganglioma, and as seen in our case, pheochromocytoma. Although pheochromocytoma is a rare cause of resistant hypertension (0.01%-4%),1 it is associated with high rates of morbidity and mortality if left untreated and may be inherited, making it an essential diagnosis to consider in all patients with resistant hypertension.1,3
Common symptoms of pheochromocytoma are hypertension (paroxysmal or sustained), headaches, palpitations, pallor, and piloerection (or cold sweats).1 Patients with pheochromocytoma typically exhibit metanephrine levels that are more than 4 times the upper limit of normal.4 Therefore, measurement of plasma free metanephrines or urinary fractionated metanephrines is recommended.5 Elevated metanephrine levels also are caused by obesity, obstructive sleep apnea, and certain medications and should be ruled out.5
All pheochromocytomas are potentially malignant. Despite the existence of pathologic scoring systems6,7 and radiographic features that suggest malignancy,8,9 no single risk-stratification tool is recommended in the current literature.10 Ultimately, the only way to confirm malignancy is to see metastases where chromaffin tissue is not normally found on imaging.10
Continue to: Pathologic features to look for...
Pathologic features to look for include capsular/periadrenal adipose invasion, increased cellularity, necrosis, tumor cell spindling, increased/atypical mitotic figures, and nuclear pleomorphism. Radiographic features include larger size (≥ 4-6 cm),11 an irregular shape, necrosis, calcifications, attenuation of 10 HU or higher on noncontrast CT, absolute washout of 60% or lower, and relative washout of 40% or lower.8,12 On MRI, malignant lesions appear hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging.9 Fluorodeoxyglucose avidity on PET scan also is indicative of malignancy.8,9
Treatment for pheochromocytoma is surgical resection. An experienced surgical team and proper preoperative preparation are necessary because the induction of anesthesia, endotracheal intubation, and tumor manipulation can lead to a release of catecholamines, potentially resulting in an intraoperative hypertensive crisis, cardiac arrhythmias, and multiorgan failure.
Proper preoperative preparation includes taking an alpha-adrenergic blocker, such as phenoxybenzamine, prazosin, terazosin, or doxazosin, for at least 7 days to normalize the patient’s blood pressure. Patients should be counseled that they may experience nasal congestion, orthostasis, and fatigue while taking these medications. Volume expansion with intravenous fluids also should be performed and a high-salt diet considered. Beta-adrenergic blockade can be initiated once appropriate alpha-adrenergic blockade is achieved to control the patient’s heart rate; beta-blockers should never be started first because of the risk for severe hypertension. Careful hemodynamic monitoring is vital intraoperatively and postoperatively.5,13 Because metastatic lesions can occur decades after resection, long-term follow-up is critical.5,10
Following tumor resection, our patient’s blood pressure was supported with intravenous fluids and phenylephrine. She was able to discontinue all her antihypertensive medications postoperatively, and her plasma free and urinary fractionated metanephrine levels returned to within normal limits 8 weeks after surgery. Five years after surgery, she continues to have no signs of recurrence, as evidenced by annual negative plasma free metanephrines testing and abdominal/pelvic CT.
THE TAKEAWAY
This case highlights the importance of recognizing resistant hypertension and a potential secondary cause of this disease—pheochromocytoma. Although rare, pheochromocytomas confer increased risk for cardiovascular disease and death. Thus, swift recognition and proper preparation for surgical resection are necessary. Malignant lesions can be diagnosed only upon discovery of metastatic disease and can recur for decades after surgical resection, making diligent long-term follow-up imperative.
CORRESPONDENCE
Nicole O. Vietor, MD, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53-e90. doi: 10.1161/HYP.0000000000000084
2. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285:126-148. doi: 10.1111/joim.12831
3. Young WF Jr, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society Scientific Statement. Endocr Rev. 2017;38:103-122. doi: 10.1210/er.2017-00054
4. Lenders JWM, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427-1434. doi: 10.1001/jama.287.11.1427
5. Lenders JW, Duh Q-Y, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2014;99:1915-1942. doi: 10.1210/jc.2014-1498
6. Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:405-414. doi: 10.1530/ERC-13-0494
7. Thompson LDR. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551-566. doi: 10.1097/00000478-200205000-00002
8. Vaidya A, Hamrahian A, Bancos I, et al. The evaluation of incidentally discovered adrenal masses. Endocr Pract. 2019;25:178-192. doi: 10.4158/DSCR-2018-0565
9. Young WF Jr. Conventional imaging in adrenocortical carcinoma: update and perspectives. Horm Cancer. 2011;2:341-347. doi: 10.1007/s12672-011-0089-z
10. Neumann HPH, Young WF Jr, Eng C. Pheochromocytoma and paraganglioma. N Engl J Med. 2019;381:552-565. doi: 10.1056/NEJMra1806651
11. Iñiguez-Ariza NM, Kohlenberg JD, Delivanis DA, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin Proc Innov Qual Outcomes. 2017;2:30-39. doi: 10.1016/j.mayocpiqo.2017.11.002
12. Marty M, Gaye D, Perez P, et al. Diagnostic accuracy of computed tomography to identify adenomas among adrenal incidentalomas in an endocrinological population. Eur J Endocrinol. 2018;178:439-446. doi: 10.1530/EJE-17-1056
13. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92:4069-4079. doi: 10.1210/jc.2007-1720
► Hot flashes, facial flushing, excessive sweating, and palpitations
► Daily headaches
► History of hypertension
High Lp(a) tied to higher coronary plaque volume, progression
MANNHEIM, GERMANY – , an observational imaging study shows.
This could explain the greater risk for major adverse cardiovascular events seen in patients with high Lp(a) levels, suggests the research, presented during the annual European Atherosclerosis Society Congress.
The team performed follow-up coronary CT angiography (CCTA) on almost 275 patients who had undergone imaging approximately 10 years earlier, finding that almost one-third had high Lp(a) levels.
At baseline, per cent plaque volumes were 1.8 times greater in high Lp(a) patients versus those with low levels of the protein. After 10 years, plaque volumes were 3.3 times larger in patients with high Lp(a) levels.
Over this period, the rate of increase of plaque volume was 1.9 times greater in patients with high Lp(a) levels.
Study presenter Nick S. Nurmohamed, MD, PhD candidate, department of vascular medicine, Amsterdam University Medical Centers, also showed that high Lp(a) levels were associated with a 2.1-fold increase in rates of MACE.
He said in an interview that this finding could be related to Lp(a) increasing inflammatory signaling in the plaque, “making it more prone to rupture, and we saw that on the CCTA scans,” where high Lp(a) levels were associated with the presence of more high-risk plaques.
He added that in the absence of drugs that target Lp(a) levels directly, the results underline the need to focus on other means of lipid-lowering, as well as “creating awareness that Lp(a) is associated with plaque formation.”
Dr. Nurmohamed said that “for the moment, we have to treat patients with high Lp(a) with other risk-lowering therapies, such as low-density lipoprotein [LDL] cholesterol–lowering drugs, and the management of other risk factors.”
However, he noted that “there are a couple of Lp(a)-lowering medications in trials,” with results expected in the next 2-3 years.
“Then we will have the means to treat those patients, and with CCTA we can identify the patients with the biggest risk,” Dr. Nurmohamed added.
Plaque burden
Philippe Moulin, MD, PhD, head of endocrinology and professor of human nutrition at Faculté Lyon Est, Claude Bernard Lyon (France) 1 University, said that the association between Lp(a) and plaque burden has been seen previously in the literature in a very similar study but with only 1-year follow-up.
Similarly, registry data have suggested that Lp(a) is associated with worsening plaque progression over time.
“Here, with 10-year follow-up, [the study] is much more interesting,” due to its greater statistical power, he said in an interview. It is also “well-documented” and uses an “appropriate” methodology.
But Dr. Moulin underlined that the number of patients with high Lp(a) levels included in the study is relatively small.
Consequently, the researchers were not able to look at the level and rate of progression of atherosclerosis between different quartiles of Lp(a), “so you have no dose-response analysis.”
It also does not “establish causality,” as it remains an observational study, despite being longitudinal, “well done, and so on.”
Dr. Moulin added that the study nevertheless adds “one more stone” to the construct of the idea of high risk around high Lp(a) levels, and “prepares the ground” for the availability of two drugs to decrease Lp(a) levels, expected in 2026 and 2027.
These are expected to substantially reduce Lp(a) levels and achieve a reduction in cardiovascular risk of around 20%-40%, “which would be interesting,” especially as “we have patients who have Lp(a) levels four times above the upper normal value.”
Crucially, they may already have normal LDL cholesterol levels, meaning that, for some patients, “there is clearly a need for such treatment, as long as it is proven that it will decrease cardiovascular risk.”
For the moment, however, the strategy for managing patients with high Lp(a) remains to increase the dose of statin and to have more stringent targets, although Dr. Moulin pointed out that, “when you give statins, you raise slightly Lp(a) levels.”
Dr. Nurmohamed said in an interview that “we know from largely genetic and observational studies that Lp(a) is causally associated with atherosclerotic cardiovascular disease.”
What is less clear is the exact underlying mechanism, he said, noting that there have been several imaging studies in high and low Lp(a) patients that have yielded conflicting results in terms of the relationship with plaque burden.
To investigate the impact of Lp(a) levels on long-term coronary plaque progression, the team invited patients who had taken part in a previous CCTA study to undergo repeat CCTA, regardless of their underlying symptoms.
In all, 299 patients underwent follow-up imaging a median of 10.2 years after their original scan. Plaque volumes were quantified and adjusted for vessel volumes, and the patients were classified as having high (≥ 70 nmol/L) or low (< 70 nmol/L) Lp(a) levels.
After excluding patients who had undergone coronary artery bypass grafting, the team analyzed 274 patients with a mean age at baseline of 57 years. Of these, 159 (58%) were men. High Lp(a) levels were identified in 87 (32%) patients.
The team found that at baseline, patients with high Lp(a) levels had significantly larger percent atheroma volumes than those with low levels, at 3.92% versus 2.17%, or an absolute difference of 1.75% (P = .013).
The difference between the two groups was even greater at the follow-up, when percent atheroma volumes reached 8.75% in patients with high Lp(a) levels versus 3.90% for those with low levels, or an absolute difference of 4.85% (P = .005).
Similar findings were seen when looking separately at percentage of noncalcified and calcified plaque volumes as well as when analyzing for the presence of low-density plaques.
Multivariate analysis taking into account clinical risk factors, statin use, and CT tube voltage found that high Lp(a) levels were associated with a greater percent atheroma volume at baseline, at an odds ratio versus low Lp(a) of 1.83 (95% confidence interval, 0.12-3.54; P = .037).
High Lp(a) levels were also linked to a larger percent atheroma volume on follow-up imaging, at an odds ratio of 3.25 (95% CI, 0.80-5.71; P = .010), and a significantly greater change in atheroma volume from baseline to follow-up imaging, at an odds ratio of 1.86 (95% CI, 0.59-3.14; P = .005)
Finally, the team showed that, after adjusting for clinical risk factors, high baseline Lp(a) levels were associated with an increased risk of MACE during the follow-up period, at a hazard ratio versus low Lp(a) levels of 2.10 (95% CI, 1.01-4.29, P = .048).
No funding was declared. Dr. Nurmohamed is cofounder of Lipid Tools. Other authors declare relationships with Amgen, Novartis, Esperion, Sanofi-Regeneron, Ackee, Cleerly, GW Heart and Vascular Institute, Siemens Healthineers, and HeartFlow.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – , an observational imaging study shows.
This could explain the greater risk for major adverse cardiovascular events seen in patients with high Lp(a) levels, suggests the research, presented during the annual European Atherosclerosis Society Congress.
The team performed follow-up coronary CT angiography (CCTA) on almost 275 patients who had undergone imaging approximately 10 years earlier, finding that almost one-third had high Lp(a) levels.
At baseline, per cent plaque volumes were 1.8 times greater in high Lp(a) patients versus those with low levels of the protein. After 10 years, plaque volumes were 3.3 times larger in patients with high Lp(a) levels.
Over this period, the rate of increase of plaque volume was 1.9 times greater in patients with high Lp(a) levels.
Study presenter Nick S. Nurmohamed, MD, PhD candidate, department of vascular medicine, Amsterdam University Medical Centers, also showed that high Lp(a) levels were associated with a 2.1-fold increase in rates of MACE.
He said in an interview that this finding could be related to Lp(a) increasing inflammatory signaling in the plaque, “making it more prone to rupture, and we saw that on the CCTA scans,” where high Lp(a) levels were associated with the presence of more high-risk plaques.
He added that in the absence of drugs that target Lp(a) levels directly, the results underline the need to focus on other means of lipid-lowering, as well as “creating awareness that Lp(a) is associated with plaque formation.”
Dr. Nurmohamed said that “for the moment, we have to treat patients with high Lp(a) with other risk-lowering therapies, such as low-density lipoprotein [LDL] cholesterol–lowering drugs, and the management of other risk factors.”
However, he noted that “there are a couple of Lp(a)-lowering medications in trials,” with results expected in the next 2-3 years.
“Then we will have the means to treat those patients, and with CCTA we can identify the patients with the biggest risk,” Dr. Nurmohamed added.
Plaque burden
Philippe Moulin, MD, PhD, head of endocrinology and professor of human nutrition at Faculté Lyon Est, Claude Bernard Lyon (France) 1 University, said that the association between Lp(a) and plaque burden has been seen previously in the literature in a very similar study but with only 1-year follow-up.
Similarly, registry data have suggested that Lp(a) is associated with worsening plaque progression over time.
“Here, with 10-year follow-up, [the study] is much more interesting,” due to its greater statistical power, he said in an interview. It is also “well-documented” and uses an “appropriate” methodology.
But Dr. Moulin underlined that the number of patients with high Lp(a) levels included in the study is relatively small.
Consequently, the researchers were not able to look at the level and rate of progression of atherosclerosis between different quartiles of Lp(a), “so you have no dose-response analysis.”
It also does not “establish causality,” as it remains an observational study, despite being longitudinal, “well done, and so on.”
Dr. Moulin added that the study nevertheless adds “one more stone” to the construct of the idea of high risk around high Lp(a) levels, and “prepares the ground” for the availability of two drugs to decrease Lp(a) levels, expected in 2026 and 2027.
These are expected to substantially reduce Lp(a) levels and achieve a reduction in cardiovascular risk of around 20%-40%, “which would be interesting,” especially as “we have patients who have Lp(a) levels four times above the upper normal value.”
Crucially, they may already have normal LDL cholesterol levels, meaning that, for some patients, “there is clearly a need for such treatment, as long as it is proven that it will decrease cardiovascular risk.”
For the moment, however, the strategy for managing patients with high Lp(a) remains to increase the dose of statin and to have more stringent targets, although Dr. Moulin pointed out that, “when you give statins, you raise slightly Lp(a) levels.”
Dr. Nurmohamed said in an interview that “we know from largely genetic and observational studies that Lp(a) is causally associated with atherosclerotic cardiovascular disease.”
What is less clear is the exact underlying mechanism, he said, noting that there have been several imaging studies in high and low Lp(a) patients that have yielded conflicting results in terms of the relationship with plaque burden.
To investigate the impact of Lp(a) levels on long-term coronary plaque progression, the team invited patients who had taken part in a previous CCTA study to undergo repeat CCTA, regardless of their underlying symptoms.
In all, 299 patients underwent follow-up imaging a median of 10.2 years after their original scan. Plaque volumes were quantified and adjusted for vessel volumes, and the patients were classified as having high (≥ 70 nmol/L) or low (< 70 nmol/L) Lp(a) levels.
After excluding patients who had undergone coronary artery bypass grafting, the team analyzed 274 patients with a mean age at baseline of 57 years. Of these, 159 (58%) were men. High Lp(a) levels were identified in 87 (32%) patients.
The team found that at baseline, patients with high Lp(a) levels had significantly larger percent atheroma volumes than those with low levels, at 3.92% versus 2.17%, or an absolute difference of 1.75% (P = .013).
The difference between the two groups was even greater at the follow-up, when percent atheroma volumes reached 8.75% in patients with high Lp(a) levels versus 3.90% for those with low levels, or an absolute difference of 4.85% (P = .005).
Similar findings were seen when looking separately at percentage of noncalcified and calcified plaque volumes as well as when analyzing for the presence of low-density plaques.
Multivariate analysis taking into account clinical risk factors, statin use, and CT tube voltage found that high Lp(a) levels were associated with a greater percent atheroma volume at baseline, at an odds ratio versus low Lp(a) of 1.83 (95% confidence interval, 0.12-3.54; P = .037).
High Lp(a) levels were also linked to a larger percent atheroma volume on follow-up imaging, at an odds ratio of 3.25 (95% CI, 0.80-5.71; P = .010), and a significantly greater change in atheroma volume from baseline to follow-up imaging, at an odds ratio of 1.86 (95% CI, 0.59-3.14; P = .005)
Finally, the team showed that, after adjusting for clinical risk factors, high baseline Lp(a) levels were associated with an increased risk of MACE during the follow-up period, at a hazard ratio versus low Lp(a) levels of 2.10 (95% CI, 1.01-4.29, P = .048).
No funding was declared. Dr. Nurmohamed is cofounder of Lipid Tools. Other authors declare relationships with Amgen, Novartis, Esperion, Sanofi-Regeneron, Ackee, Cleerly, GW Heart and Vascular Institute, Siemens Healthineers, and HeartFlow.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – , an observational imaging study shows.
This could explain the greater risk for major adverse cardiovascular events seen in patients with high Lp(a) levels, suggests the research, presented during the annual European Atherosclerosis Society Congress.
The team performed follow-up coronary CT angiography (CCTA) on almost 275 patients who had undergone imaging approximately 10 years earlier, finding that almost one-third had high Lp(a) levels.
At baseline, per cent plaque volumes were 1.8 times greater in high Lp(a) patients versus those with low levels of the protein. After 10 years, plaque volumes were 3.3 times larger in patients with high Lp(a) levels.
Over this period, the rate of increase of plaque volume was 1.9 times greater in patients with high Lp(a) levels.
Study presenter Nick S. Nurmohamed, MD, PhD candidate, department of vascular medicine, Amsterdam University Medical Centers, also showed that high Lp(a) levels were associated with a 2.1-fold increase in rates of MACE.
He said in an interview that this finding could be related to Lp(a) increasing inflammatory signaling in the plaque, “making it more prone to rupture, and we saw that on the CCTA scans,” where high Lp(a) levels were associated with the presence of more high-risk plaques.
He added that in the absence of drugs that target Lp(a) levels directly, the results underline the need to focus on other means of lipid-lowering, as well as “creating awareness that Lp(a) is associated with plaque formation.”
Dr. Nurmohamed said that “for the moment, we have to treat patients with high Lp(a) with other risk-lowering therapies, such as low-density lipoprotein [LDL] cholesterol–lowering drugs, and the management of other risk factors.”
However, he noted that “there are a couple of Lp(a)-lowering medications in trials,” with results expected in the next 2-3 years.
“Then we will have the means to treat those patients, and with CCTA we can identify the patients with the biggest risk,” Dr. Nurmohamed added.
Plaque burden
Philippe Moulin, MD, PhD, head of endocrinology and professor of human nutrition at Faculté Lyon Est, Claude Bernard Lyon (France) 1 University, said that the association between Lp(a) and plaque burden has been seen previously in the literature in a very similar study but with only 1-year follow-up.
Similarly, registry data have suggested that Lp(a) is associated with worsening plaque progression over time.
“Here, with 10-year follow-up, [the study] is much more interesting,” due to its greater statistical power, he said in an interview. It is also “well-documented” and uses an “appropriate” methodology.
But Dr. Moulin underlined that the number of patients with high Lp(a) levels included in the study is relatively small.
Consequently, the researchers were not able to look at the level and rate of progression of atherosclerosis between different quartiles of Lp(a), “so you have no dose-response analysis.”
It also does not “establish causality,” as it remains an observational study, despite being longitudinal, “well done, and so on.”
Dr. Moulin added that the study nevertheless adds “one more stone” to the construct of the idea of high risk around high Lp(a) levels, and “prepares the ground” for the availability of two drugs to decrease Lp(a) levels, expected in 2026 and 2027.
These are expected to substantially reduce Lp(a) levels and achieve a reduction in cardiovascular risk of around 20%-40%, “which would be interesting,” especially as “we have patients who have Lp(a) levels four times above the upper normal value.”
Crucially, they may already have normal LDL cholesterol levels, meaning that, for some patients, “there is clearly a need for such treatment, as long as it is proven that it will decrease cardiovascular risk.”
For the moment, however, the strategy for managing patients with high Lp(a) remains to increase the dose of statin and to have more stringent targets, although Dr. Moulin pointed out that, “when you give statins, you raise slightly Lp(a) levels.”
Dr. Nurmohamed said in an interview that “we know from largely genetic and observational studies that Lp(a) is causally associated with atherosclerotic cardiovascular disease.”
What is less clear is the exact underlying mechanism, he said, noting that there have been several imaging studies in high and low Lp(a) patients that have yielded conflicting results in terms of the relationship with plaque burden.
To investigate the impact of Lp(a) levels on long-term coronary plaque progression, the team invited patients who had taken part in a previous CCTA study to undergo repeat CCTA, regardless of their underlying symptoms.
In all, 299 patients underwent follow-up imaging a median of 10.2 years after their original scan. Plaque volumes were quantified and adjusted for vessel volumes, and the patients were classified as having high (≥ 70 nmol/L) or low (< 70 nmol/L) Lp(a) levels.
After excluding patients who had undergone coronary artery bypass grafting, the team analyzed 274 patients with a mean age at baseline of 57 years. Of these, 159 (58%) were men. High Lp(a) levels were identified in 87 (32%) patients.
The team found that at baseline, patients with high Lp(a) levels had significantly larger percent atheroma volumes than those with low levels, at 3.92% versus 2.17%, or an absolute difference of 1.75% (P = .013).
The difference between the two groups was even greater at the follow-up, when percent atheroma volumes reached 8.75% in patients with high Lp(a) levels versus 3.90% for those with low levels, or an absolute difference of 4.85% (P = .005).
Similar findings were seen when looking separately at percentage of noncalcified and calcified plaque volumes as well as when analyzing for the presence of low-density plaques.
Multivariate analysis taking into account clinical risk factors, statin use, and CT tube voltage found that high Lp(a) levels were associated with a greater percent atheroma volume at baseline, at an odds ratio versus low Lp(a) of 1.83 (95% confidence interval, 0.12-3.54; P = .037).
High Lp(a) levels were also linked to a larger percent atheroma volume on follow-up imaging, at an odds ratio of 3.25 (95% CI, 0.80-5.71; P = .010), and a significantly greater change in atheroma volume from baseline to follow-up imaging, at an odds ratio of 1.86 (95% CI, 0.59-3.14; P = .005)
Finally, the team showed that, after adjusting for clinical risk factors, high baseline Lp(a) levels were associated with an increased risk of MACE during the follow-up period, at a hazard ratio versus low Lp(a) levels of 2.10 (95% CI, 1.01-4.29, P = .048).
No funding was declared. Dr. Nurmohamed is cofounder of Lipid Tools. Other authors declare relationships with Amgen, Novartis, Esperion, Sanofi-Regeneron, Ackee, Cleerly, GW Heart and Vascular Institute, Siemens Healthineers, and HeartFlow.
A version of this article first appeared on Medscape.com.
AT EAS 2023
Link between bipolar disorder and CVD mortality explained?
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
in new findings that may explain the “excessive and premature mortality” related to heart disease in this patient population.
The investigators found that higher reactive hyperemia index (RHI) scores, a measure of endothelial function, were tied to mood severity in patients with higher mania, but not depression scores. These findings persisted even after accounting for medications, obesity, and other cardiovascular risk factors (CVRFs).
“From a clinical perspective, these findings highlight the potential value of integrating vascular health in the assessment and management of youth with BD, and from a scientific perspective, these findings call for additional research focused on shared biological mechanisms linking vascular health and mood symptoms of BD,” senior investigator Benjamin Goldstein, MD, PhD, full professor of psychiatry, pharmacology, and psychological clinical science, University of Toronto, said in an interview.
The study was published online in the Journal of Clinical Psychiatry.
‘Excessively present’
BD is associated with “excessive and premature cardiovascular mortality” and CVD is “excessively present” in BD, exceeding what can be explained by traditional cardiovascular risk factors, psychiatric medications, and substance use, the researchers noted.
“In adults, more severe mood symptoms increase the risk of future CVD. Our focus on endothelial function rose due to the fact that CVD is rare in youth, whereas endothelial dysfunction – considered a precursor of CVD – can be assessed in youth,” said Dr. Goldstein, who holds the RBC Investments Chair in children’s mental health and developmental psychopathology at the Centre for Addiction and Mental Health, Toronto, where he is director of the Centre for Youth Bipolar Disorder.
For this reason, he and his colleagues were “interested in researching whether endothelial dysfunction is associated with mood symptoms in youth with BD.” Ultimately, the motivation was to “inspire new therapeutic opportunities that may improve both cardiovascular and mental health simultaneously.”
To investigate the question, the researchers studied 209 youth aged 13-20 years (n = 114 with BD and 94 healthy controls [HCs]).
In the BD group, there were 34 BD-euthymia, 36 BD-depressed, and 44 BD-hypomanic/mixed; and within the groups who had depression or hypomania/mixed features, 72 were experiencing clinically significant depression.
Participants had to be free of chronic inflammatory illness, use of medications that might be addressing traditional CVRFs, recent infectious diseases, or neurologic conditions.
Participants’ bipolar symptoms, psychosocial functioning, and family history were assessed. In addition, they were asked about treatment, physical and/or sexual abuse, smoking status, and socioeconomic status. Height, weight, waist circumference, blood pressure, and blood tests to assess CVRFs, including C-reactive protein (CRP), were also assessed. RHI was measured via pulse amplitude tonometry, with lower values indicating poorer endothelial function.
Positive affect beneficial?
Compared with HCs, there were fewer White participants in the BD group (78% vs. 55%; P < .001). The BD group also had higher Tanner stage development scores (stage 5: 65% vs. 35%; P = .03; V = 0.21), higher body mass index (BMI, 24.4 ± 4.6 vs. 22.0 ± 4.2; P < .001; d = 0.53), and higher CRP (1.94 ± 3.99 vs. 0.76 ± 0.86; P = .009; d = –0.40).
After controlling for age, sex, and BMI (F3,202 = 4.47; P = .005; np2 = 0.06), the researchers found significant between-group differences in RHI.
Post hoc pairwise comparisons showed RHI to be significantly lower in the BD-depressed versus the HC group (P = .04; d = 0.4). Moreover, the BD-hypomanic/mixed group had significantly higher RHI, compared with the other BD groups and the HC group.
RHI was associated with higher mania scores (beta, 0.26; P = .006), but there was no similar significant association with depression mood scores (beta, 0.01; P = .90).
The mood state differences in RHI and the RHI-mania association remained significant in sensitivity analyses examining the effect of current medication use as well as CVRFs, including lipids, CRP, and blood pressure on RHI.
“We found that youth with BD experiencing a depressive episode had lower endothelial function, whereas youth with BD experiencing a hypomanic/mixed episode had higher endothelial function, as compared to healthy youth,” Dr. Goldstein said.
There are several mechanisms potentially underlying the association between endothelial function and hypomania, the investigators noted. For example, positive affect is associated with increased endothelial function in normative samples, so hypomanic symptoms, including elation, may have similar beneficial associations, although those benefits likely do not extend to mania, which has been associated with cardiovascular risk.
They also point to several limitations in the study. The cross-sectional design “precludes making inferences regarding the temporal relationship between RHI and mood.” Moreover, the study focused only on hypomania, so “we cannot draw conclusions about mania.” In addition, the HC group had a “significantly higher proportion” of White participants, and a lower Tanner stage, so it “may not be a representative control sample.”
Nevertheless, the researchers concluded that the study “adds to the existing evidence for the potential value of integrating cardiovascular-related therapeutic approaches in BD,” noting that further research is needed to elucidate the mechanisms of the association.
Observable changes in youth
In a comment, Jess G Fiedorowicz, MD, PhD, head and chief, department of mental health, Ottawa Hospital Research Institute, noted that individuals with BD “have a much higher risk of CVD, which tends to develop earlier and shortens life expectancy by more than a decade.”
This cardiovascular risk “appears to be acquired over the long-term course of illness and proportionate to the persistence and severity of mood symptoms, which implies that mood syndromes, such as depression and mania, themselves may induce changes in the body relevant to CVD,” said Dr. Fiedorowicz, who is also a professor in the department of psychiatry and senior research chair in adult psychiatry at the Brain and Mind Research Institute, University of Ottawa, and was not involved with the study.
The study “adds to a growing body of evidence that mood syndromes may enact physiological changes that may be relevant to risk of CVD. One important aspect of this study is that this can even be observed in young sample,” he said.
This study was funded by the Canadian Institutes of Health Research and a Miner’s Lamp Innovation Fund from the University of Toronto. Dr. Goldstein and coauthors declare no relevant financial relationships. Dr. Fiedorowicz receives an honorarium from Elsevier for his work as editor-in-chief of the Journal of Psychosomatic Research.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Three ‘synergistic’ problems when taking blood pressure
Insufficient blood pressure measurement during medical consultation, use of an inadequate technique for its determination, and lack of validated automatic sphygmomanometers are three problems that convergently complicate the diagnosis and control of arterial hypertension in the Americas, a silent disease that affects 180 million people in the region and is the main risk factor for cardiovascular diseases, said the Pan American Health Organization.
Jarbas Barbosa, MD, MPH, PhD, director of PAHO, said in an interview: “We don’t have specific data for each of these scenarios, but unfortunately, all three doubtless work together to make the situation worse.
“Often, the staff members at our primary care clinics are not prepared to diagnose and treat hypertension, because there aren’t national protocols to raise awareness and prepare them to provide this care to the correct standard. Also, they are often unqualified to take blood pressure readings properly,” he added.
This concern is reflected in the theme the organization chose for World Hypertension Day, which was observed on May 17: Measure your blood pressure accurately, control it, live longer! “We shouldn’t underestimate the importance of taking blood pressure,” warned Silvana Luciani, chief of PAHO’s noncommunicable diseases, violence, and injury prevention unit. But, the experts stressed, it must be done correctly.
Time no problem
It’s important to raise awareness of the value of blood pressure measurement for the general population. However, as multiple studies have shown, one barrier to detecting and controlling hypertension is that doctors and other health care professionals measure blood pressure less frequently in clinic than expected, or they use inappropriate techniques or obsolete or uncalibrated measurement devices.
“The importance of clinic blood pressure measurement has been recognized for many decades, but adherence to guidelines on proper, standardized blood pressure measurement remains uncommon in clinical practice,” concluded a consensus document signed by 25 experts from 13 institutions in the United States, Australia, Germany, the United Kingdom, Canada, Italy, Belgium, and Greece.
The first problem lies in the low quantity of measurements. A recent study in Argentina of nearly 3,000 visits to the doctor’s office at nine health care centers showed that doctors took blood pressure readings in only once in every seven encounters. Even cardiologists, the specialists with the best performance, did so only half of the time.
“Several factors can come into play: lack of awareness, medical inertia, or lack of appropriate equipment. But it is not for lack of time. How long does it take to take blood pressure three times within a 1-minute interval, with the patient seated and their back supported, as indicated? Four minutes. That’s not very much,” said Judith Zilberman, MD, PhD, said in an interview. Dr. Zilberman leads the department of hypertension and the women’s cardiovascular disease area at the Argerich Hospital in Buenos Aires, and is the former chair of the Argentinian Society of Hypertension.
Patricio López-Jaramillo, MD, PhD, said in an interview that the greatest obstacle is the lack of awareness among physicians and other health care staff about the importance of taking proper blood pressure measurements. Dr. López-Jaramillo is president and scientific director of the MASIRA Research Institute at the University of Santander in Bucaramanga, Colombia, and first author of the Manual Práctico de Diagnóstico y Manejo de la Hipertensión Arterial (Practice Guidelines for Diagnosing and Managing Hypertension), published by the Latin American Hypertension Society.
“Medical schools are also responsible for this. They go over this topic very superficially during undergraduate and, even worse, postgraduate training. The lack of time to take correct measurements, or the lack of appropriate instruments, is secondary to this lack of awareness among most health care staff members,” added Dr. López-Jaramillo, who is one of the researchers of the PURE epidemiologic study. Since 2002, it has followed a cohort of 225,000 participants from 27 high-, mid-, and low-income countries.
Dr. Zilberman added that it would be good practice for all primary care physicians to take blood pressure readings regardless of the reason for the visit and whether patients have been diagnosed with hypertension or not. “If a woman goes to her gynecologist because she wants to get pregnant, her blood pressure should also be taken! And any other specialist should interview the patient, ascertain her history, what medications she’s on, and then ask if her blood pressure has been taken recently,” she recommended.
Measure well
The second factor to consider is that a correct technique should be used to take blood pressure readings in the doctor’s office or clinic so as not to produce inaccurate results that could lead to underdiagnosis, overdiagnosis, or a poor assessment of the patient’s response to prescribed treatments. An observational study performed in Uruguay in 2017 showed that only 5% of 302 blood pressure measurements followed appropriate procedures.
A new fact sheet from the PAHO lists the following eight requirements for obtaining an accurate reading: don’t have a conversation, support the arm at heart level, put the cuff on a bare arm, use the correct cuff size, support the feet, keep the legs uncrossed, ensure the patient has an empty bladder, and support the back.
Though most guidelines recommend taking three readings, the “pragmatic” focus proposed in the international consensus accepts at least two readings separated by a minimum of 30 seconds. The two readings should then be averaged out. There is evidence that simplified protocols can be used, at least for population screening.
The authors of the new document also recommend preparing the patient before taking the measurement. The patient should be asked not to smoke, exercise, or consume alcohol or caffeine for at least 30 minutes beforehand. He or she should rest for a period of 3-5 minutes without speaking or being spoken to before the measurement is taken.
Lastly, clinically validated automated measurement devices should be used, as called for by the PAHO HEARTS initiative in the Americas. “The sphygmomanometer or classic aneroid tensiometer for the auscultatory method, which is still used way too often at doctor’s office visits in the region, has many weaknesses – not only the device itself but also the way it’s used (human error). This produces a rounded, approximate reading,” stressed Dr. Zilberman.
Automated devices also minimize interactions with the patient by reducing distractions during the preparation and measurement phases and freeing up time for the health care professional. “To [check for a] fever, we use the appropriate thermometer in the appropriate location. We should do the same for blood pressure,” she added.
The STRIDE-BP database, which is affiliated with the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League, contains an updated list of validated devices for measuring blood pressure.
The signers of the consensus likewise recognized that, beyond taking blood pressure measurements during office visits, the best measurements are those taken at home outside the context of medical care (doctor’s office or clinic) and that the same recommendations are directly applicable. “Few diseases can be detected so easily as with a simple at-home assessment performed by the individual himself or herself. If after three consecutive measurements, readings above 140/90 mm Hg are obtained, the individual should see the doctor to set up a comprehensive treatment program,” said Pablo Rodríguez, MD, secretary of the Argentinian Society of Hypertension. From now through September 14 (Day for Patients With Hypertension), the society is conducting a campaign to take blood pressure measurements at different locations across the country.
Dr. Zilberman and Dr. López-Jiménez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
Insufficient blood pressure measurement during medical consultation, use of an inadequate technique for its determination, and lack of validated automatic sphygmomanometers are three problems that convergently complicate the diagnosis and control of arterial hypertension in the Americas, a silent disease that affects 180 million people in the region and is the main risk factor for cardiovascular diseases, said the Pan American Health Organization.
Jarbas Barbosa, MD, MPH, PhD, director of PAHO, said in an interview: “We don’t have specific data for each of these scenarios, but unfortunately, all three doubtless work together to make the situation worse.
“Often, the staff members at our primary care clinics are not prepared to diagnose and treat hypertension, because there aren’t national protocols to raise awareness and prepare them to provide this care to the correct standard. Also, they are often unqualified to take blood pressure readings properly,” he added.
This concern is reflected in the theme the organization chose for World Hypertension Day, which was observed on May 17: Measure your blood pressure accurately, control it, live longer! “We shouldn’t underestimate the importance of taking blood pressure,” warned Silvana Luciani, chief of PAHO’s noncommunicable diseases, violence, and injury prevention unit. But, the experts stressed, it must be done correctly.
Time no problem
It’s important to raise awareness of the value of blood pressure measurement for the general population. However, as multiple studies have shown, one barrier to detecting and controlling hypertension is that doctors and other health care professionals measure blood pressure less frequently in clinic than expected, or they use inappropriate techniques or obsolete or uncalibrated measurement devices.
“The importance of clinic blood pressure measurement has been recognized for many decades, but adherence to guidelines on proper, standardized blood pressure measurement remains uncommon in clinical practice,” concluded a consensus document signed by 25 experts from 13 institutions in the United States, Australia, Germany, the United Kingdom, Canada, Italy, Belgium, and Greece.
The first problem lies in the low quantity of measurements. A recent study in Argentina of nearly 3,000 visits to the doctor’s office at nine health care centers showed that doctors took blood pressure readings in only once in every seven encounters. Even cardiologists, the specialists with the best performance, did so only half of the time.
“Several factors can come into play: lack of awareness, medical inertia, or lack of appropriate equipment. But it is not for lack of time. How long does it take to take blood pressure three times within a 1-minute interval, with the patient seated and their back supported, as indicated? Four minutes. That’s not very much,” said Judith Zilberman, MD, PhD, said in an interview. Dr. Zilberman leads the department of hypertension and the women’s cardiovascular disease area at the Argerich Hospital in Buenos Aires, and is the former chair of the Argentinian Society of Hypertension.
Patricio López-Jaramillo, MD, PhD, said in an interview that the greatest obstacle is the lack of awareness among physicians and other health care staff about the importance of taking proper blood pressure measurements. Dr. López-Jaramillo is president and scientific director of the MASIRA Research Institute at the University of Santander in Bucaramanga, Colombia, and first author of the Manual Práctico de Diagnóstico y Manejo de la Hipertensión Arterial (Practice Guidelines for Diagnosing and Managing Hypertension), published by the Latin American Hypertension Society.
“Medical schools are also responsible for this. They go over this topic very superficially during undergraduate and, even worse, postgraduate training. The lack of time to take correct measurements, or the lack of appropriate instruments, is secondary to this lack of awareness among most health care staff members,” added Dr. López-Jaramillo, who is one of the researchers of the PURE epidemiologic study. Since 2002, it has followed a cohort of 225,000 participants from 27 high-, mid-, and low-income countries.
Dr. Zilberman added that it would be good practice for all primary care physicians to take blood pressure readings regardless of the reason for the visit and whether patients have been diagnosed with hypertension or not. “If a woman goes to her gynecologist because she wants to get pregnant, her blood pressure should also be taken! And any other specialist should interview the patient, ascertain her history, what medications she’s on, and then ask if her blood pressure has been taken recently,” she recommended.
Measure well
The second factor to consider is that a correct technique should be used to take blood pressure readings in the doctor’s office or clinic so as not to produce inaccurate results that could lead to underdiagnosis, overdiagnosis, or a poor assessment of the patient’s response to prescribed treatments. An observational study performed in Uruguay in 2017 showed that only 5% of 302 blood pressure measurements followed appropriate procedures.
A new fact sheet from the PAHO lists the following eight requirements for obtaining an accurate reading: don’t have a conversation, support the arm at heart level, put the cuff on a bare arm, use the correct cuff size, support the feet, keep the legs uncrossed, ensure the patient has an empty bladder, and support the back.
Though most guidelines recommend taking three readings, the “pragmatic” focus proposed in the international consensus accepts at least two readings separated by a minimum of 30 seconds. The two readings should then be averaged out. There is evidence that simplified protocols can be used, at least for population screening.
The authors of the new document also recommend preparing the patient before taking the measurement. The patient should be asked not to smoke, exercise, or consume alcohol or caffeine for at least 30 minutes beforehand. He or she should rest for a period of 3-5 minutes without speaking or being spoken to before the measurement is taken.
Lastly, clinically validated automated measurement devices should be used, as called for by the PAHO HEARTS initiative in the Americas. “The sphygmomanometer or classic aneroid tensiometer for the auscultatory method, which is still used way too often at doctor’s office visits in the region, has many weaknesses – not only the device itself but also the way it’s used (human error). This produces a rounded, approximate reading,” stressed Dr. Zilberman.
Automated devices also minimize interactions with the patient by reducing distractions during the preparation and measurement phases and freeing up time for the health care professional. “To [check for a] fever, we use the appropriate thermometer in the appropriate location. We should do the same for blood pressure,” she added.
The STRIDE-BP database, which is affiliated with the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League, contains an updated list of validated devices for measuring blood pressure.
The signers of the consensus likewise recognized that, beyond taking blood pressure measurements during office visits, the best measurements are those taken at home outside the context of medical care (doctor’s office or clinic) and that the same recommendations are directly applicable. “Few diseases can be detected so easily as with a simple at-home assessment performed by the individual himself or herself. If after three consecutive measurements, readings above 140/90 mm Hg are obtained, the individual should see the doctor to set up a comprehensive treatment program,” said Pablo Rodríguez, MD, secretary of the Argentinian Society of Hypertension. From now through September 14 (Day for Patients With Hypertension), the society is conducting a campaign to take blood pressure measurements at different locations across the country.
Dr. Zilberman and Dr. López-Jiménez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
Insufficient blood pressure measurement during medical consultation, use of an inadequate technique for its determination, and lack of validated automatic sphygmomanometers are three problems that convergently complicate the diagnosis and control of arterial hypertension in the Americas, a silent disease that affects 180 million people in the region and is the main risk factor for cardiovascular diseases, said the Pan American Health Organization.
Jarbas Barbosa, MD, MPH, PhD, director of PAHO, said in an interview: “We don’t have specific data for each of these scenarios, but unfortunately, all three doubtless work together to make the situation worse.
“Often, the staff members at our primary care clinics are not prepared to diagnose and treat hypertension, because there aren’t national protocols to raise awareness and prepare them to provide this care to the correct standard. Also, they are often unqualified to take blood pressure readings properly,” he added.
This concern is reflected in the theme the organization chose for World Hypertension Day, which was observed on May 17: Measure your blood pressure accurately, control it, live longer! “We shouldn’t underestimate the importance of taking blood pressure,” warned Silvana Luciani, chief of PAHO’s noncommunicable diseases, violence, and injury prevention unit. But, the experts stressed, it must be done correctly.
Time no problem
It’s important to raise awareness of the value of blood pressure measurement for the general population. However, as multiple studies have shown, one barrier to detecting and controlling hypertension is that doctors and other health care professionals measure blood pressure less frequently in clinic than expected, or they use inappropriate techniques or obsolete or uncalibrated measurement devices.
“The importance of clinic blood pressure measurement has been recognized for many decades, but adherence to guidelines on proper, standardized blood pressure measurement remains uncommon in clinical practice,” concluded a consensus document signed by 25 experts from 13 institutions in the United States, Australia, Germany, the United Kingdom, Canada, Italy, Belgium, and Greece.
The first problem lies in the low quantity of measurements. A recent study in Argentina of nearly 3,000 visits to the doctor’s office at nine health care centers showed that doctors took blood pressure readings in only once in every seven encounters. Even cardiologists, the specialists with the best performance, did so only half of the time.
“Several factors can come into play: lack of awareness, medical inertia, or lack of appropriate equipment. But it is not for lack of time. How long does it take to take blood pressure three times within a 1-minute interval, with the patient seated and their back supported, as indicated? Four minutes. That’s not very much,” said Judith Zilberman, MD, PhD, said in an interview. Dr. Zilberman leads the department of hypertension and the women’s cardiovascular disease area at the Argerich Hospital in Buenos Aires, and is the former chair of the Argentinian Society of Hypertension.
Patricio López-Jaramillo, MD, PhD, said in an interview that the greatest obstacle is the lack of awareness among physicians and other health care staff about the importance of taking proper blood pressure measurements. Dr. López-Jaramillo is president and scientific director of the MASIRA Research Institute at the University of Santander in Bucaramanga, Colombia, and first author of the Manual Práctico de Diagnóstico y Manejo de la Hipertensión Arterial (Practice Guidelines for Diagnosing and Managing Hypertension), published by the Latin American Hypertension Society.
“Medical schools are also responsible for this. They go over this topic very superficially during undergraduate and, even worse, postgraduate training. The lack of time to take correct measurements, or the lack of appropriate instruments, is secondary to this lack of awareness among most health care staff members,” added Dr. López-Jaramillo, who is one of the researchers of the PURE epidemiologic study. Since 2002, it has followed a cohort of 225,000 participants from 27 high-, mid-, and low-income countries.
Dr. Zilberman added that it would be good practice for all primary care physicians to take blood pressure readings regardless of the reason for the visit and whether patients have been diagnosed with hypertension or not. “If a woman goes to her gynecologist because she wants to get pregnant, her blood pressure should also be taken! And any other specialist should interview the patient, ascertain her history, what medications she’s on, and then ask if her blood pressure has been taken recently,” she recommended.
Measure well
The second factor to consider is that a correct technique should be used to take blood pressure readings in the doctor’s office or clinic so as not to produce inaccurate results that could lead to underdiagnosis, overdiagnosis, or a poor assessment of the patient’s response to prescribed treatments. An observational study performed in Uruguay in 2017 showed that only 5% of 302 blood pressure measurements followed appropriate procedures.
A new fact sheet from the PAHO lists the following eight requirements for obtaining an accurate reading: don’t have a conversation, support the arm at heart level, put the cuff on a bare arm, use the correct cuff size, support the feet, keep the legs uncrossed, ensure the patient has an empty bladder, and support the back.
Though most guidelines recommend taking three readings, the “pragmatic” focus proposed in the international consensus accepts at least two readings separated by a minimum of 30 seconds. The two readings should then be averaged out. There is evidence that simplified protocols can be used, at least for population screening.
The authors of the new document also recommend preparing the patient before taking the measurement. The patient should be asked not to smoke, exercise, or consume alcohol or caffeine for at least 30 minutes beforehand. He or she should rest for a period of 3-5 minutes without speaking or being spoken to before the measurement is taken.
Lastly, clinically validated automated measurement devices should be used, as called for by the PAHO HEARTS initiative in the Americas. “The sphygmomanometer or classic aneroid tensiometer for the auscultatory method, which is still used way too often at doctor’s office visits in the region, has many weaknesses – not only the device itself but also the way it’s used (human error). This produces a rounded, approximate reading,” stressed Dr. Zilberman.
Automated devices also minimize interactions with the patient by reducing distractions during the preparation and measurement phases and freeing up time for the health care professional. “To [check for a] fever, we use the appropriate thermometer in the appropriate location. We should do the same for blood pressure,” she added.
The STRIDE-BP database, which is affiliated with the European Society of Hypertension, the International Society of Hypertension, and the World Hypertension League, contains an updated list of validated devices for measuring blood pressure.
The signers of the consensus likewise recognized that, beyond taking blood pressure measurements during office visits, the best measurements are those taken at home outside the context of medical care (doctor’s office or clinic) and that the same recommendations are directly applicable. “Few diseases can be detected so easily as with a simple at-home assessment performed by the individual himself or herself. If after three consecutive measurements, readings above 140/90 mm Hg are obtained, the individual should see the doctor to set up a comprehensive treatment program,” said Pablo Rodríguez, MD, secretary of the Argentinian Society of Hypertension. From now through September 14 (Day for Patients With Hypertension), the society is conducting a campaign to take blood pressure measurements at different locations across the country.
Dr. Zilberman and Dr. López-Jiménez disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
Medicaid patients with heart failure get poor follow-up after hospital discharge
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION
Lomitapide shows promise in pediatric homozygous FH
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.
MANNHEIM, Germany – Lomitapide, which reduces lipoprotein production in the liver, could help manage pediatric homozygous familial hypercholesterolemia (HoFH), suggest results of a trial that showed large reductions in circulating lipids.
The research was presented May 23 at the 91st European Atherosclerosis Society Congress.
Lomitapide inhibits microsomal triglyceride transfer protein, which plays a key role in apolipoprotein B-containing lipoprotein assembly and secretion in the liver and intestines. Crucially, the drug acts independently of the LDL cholesterol receptor.
It was approved in December 2012 by the U.S. Food and Drug Administration for use in adults with HoFH, sold under the name Juxtapid, and by the European Medicines Agency, where the brand name is Lojuxta.
The current trial involved more than 40 children and teenagers with HoFH aged 5-17 years; they were treated with the drug for 24 weeks, resulting in reductions of low density lipoprotein cholesterol of almost 54%, with nearly 42% reaching target levels.
The drug was also associated with marked reductions in other key lipids of at least 50%. However, 67% of patients also experienced gastrointestinal adverse events, and around 25% saw their levels of liver enzymes increase.
Early diagnosis ‘imperative’
The findings show that the “early diagnosis and treatment of HoFH is imperative,” said study presenter Luis Masana, MD, PhD, director of the Vascular Medicine and Metabolism Unit at Sant Joan de Reus University Hospital, Tarragona, Spain.
“I think that, with these results, we are bringing a new hope for this group of patients,” he continued. “I also think we will increase the quality of life, not just of the patients but also all the families involved in [managing] this problem.”
Session co-chair Andreas Zirlik, MD, PhD, head of the department of cardiology and chairman of the University Heart Center Graz, LKH-University Hospital, and Medical University of Graz (Austria), was more circumspect in his appraisal of the results.
He told this news organization that it is “always very difficult to establish therapy in pediatrics,” and believes that the drug “will give us an additional option” in managing HoFH.
However, Dr. Zirlik warned that he is a “little bit concerned” about lomitapide’s adverse event profile, and “would need to see a little bit deeper into the safety data.”
Highlighting the elevations in liver enzymes of around 25%, he asked: “What does it mean?” And how will it “play out in the long run?”
Beyond lomitapide, Dr. Zirlik pointed out that there are other drugs that have shown potential in managing HoFH and could potentially be used in the pediatric population, such as angiopoietin-like 3 protein (ANGPTL3) inhibitors and small interfering RNA (siRNA) compounds that target upstream production. “So, let’s see how they pan out,” he said.
Life-limiting condition
HoFH is an “ultra-rare, life-limiting condition,” with an estimated prevalence of approximately 3 per 1 million people, and a life expectancy in untreated patients of just 18 years, Dr. Masana said during his presentation.
Case series of lomitapide use in pediatric HoFH patients have shown encouraging results that are consistent with those seen in adults, he noted, with many able to achieve their LDL cholesterol target and stop or reduce apheresis.
To investigate further, a phase 3, single arm, open-label study was conducted. Following screening, 46 children and teenagers with HoFH underwent a 6- to 12-week run-in period, during which they were put on a low-fat diet with nutritional supplements.
“As you can imagine,” Dr. Masana said, “we are reducing the capacity for fat absorption with lomitapide, so the supplements and low-fat diet are necessary.”
Of these, 43 participants then entered a 24-week treatment period in which they were started on one of three doses, before undergoing dose escalation to the maximally tolerated dose. This was followed by an 80-week open-label safety phase, in which they continued on the maximally tolerated dose, then a follow-up period.
For the current presentation, Dr. Masana focused on the efficacy phase, showing that the mean age of participants was 10.7 years and that 55.8% were female. The HoFH diagnosis was confirmed genetically in 88.4% of cases.
Results showed that lomitapide was associated with a significant reduction in LDL cholesterol levels, from 435.8 mg/dL at baseline to 176.5 mg/dL at Week 24, which corresponded to a 53.5% overall reduction (P < .0001).
This meant that 41.9% of patients achieved their EAS LDL cholesterol target of less than 135 mg/dL at some point during the 24-week treatment period.
Stratifying by age, the reduction between baseline and week 24 was 538.5 mg/dL to 207.2 mg/dL, or 56.5%, in the 20 children aged 5-10 years, and 346.5 mg/dL to 149.9 mg/L, or 50.9%, in the 23 patients aged 11-17 years.
Dr. Masana explained that the results were “a little bit better in the younger group because they were receiving less treatment at this stage of the disease” than the older group.
He showed that lomitapide was associated with significant reductions in other lipid markers, including a 53.9% reduction in non–HDL cholesterol (P < .0001), a 50.1% drop in total cholesterol (P < .0001), and a 50.2% fall in very-low-density lipoprotein cholesterol (P < .0001).
Results showed 93% of patients experienced treatment-related adverse events, with 11.6% having serious events and 4.7% having events that led to study discontinuation. There was one (2.3%) major adverse cardiac event but no deaths.
He said that, despite these figures, the adverse events were “mostly mild or moderate.”
The majority (67%) of patients nevertheless had gastrointestinal adverse events, which were, “in general, associated with a lack of adherence to the low-fat diet.”
Aspartate aminotransferase levels were elevated in 23% of patients, while 28% had elevations in alanine aminotransferase, which were described by Dr. Masana as “moderate.”
The study was sponsored by Amryt Pharma. Dr. Masana declares relationships with Amarin, Amryt, Daiichi-Sankyo, Novartis, Sanofi, Servier, Servier, and Viatrix.
A version of this article first appeared on Medscape.com.
Troponin to ID diabetes patients with silent heart disease?
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
– based on data from a representative sample of more than 10,000 U.S. adults.
The finding suggests hs-cTnT maybe a useful marker for adults with diabetes who could benefit from more aggressive CVD risk reduction despite having no clinical indications of CVD.
The results “highlight the substantial burden of subclinical CVD in persons with diabetes and emphasize the importance of early detection and treatment of CVD for this high-risk population,” say the authors of the research, published in the Journal of the American Heart Association.
“This is the first study to examine subclinical CVD, defined by elevated cardiac biomarkers, in a nationally representative population of adults with or without diabetes. It provides novel information on the high burden of subclinical CVD [in American adults with diabetes] and the potential utility of hs-cTnT for monitoring this risk in people with diabetes,” said Elizabeth Selvin, PhD, senior author and a professor of epidemiology at Johns Hopkins University, Baltimore.
“What we are seeing is that many people with type 2 diabetes who have not had a heart attack or a history of cardiovascular disease are at high risk for cardiovascular complications,” added Dr. Selvin in an AHA press release. “When we look at the whole population of people diagnosed with type 2 diabetes, about 27 million adults in the U.S., according to the [Centers for Disease Control and Prevention], some are at low risk and some are at high risk for cardiovascular disease, so the open question is: ‘Who is most at risk?’ These cardiac biomarkers give us a window into cardiovascular risk in people who otherwise might not be recognized as highest risk.”
“Our results provide evidence to support use of cardiac biomarkers for routine risk monitoring in high-risk populations such as people with diabetes,” Dr. Selvin noted in an interview.
Need for aggressive CVD risk reduction
The findings also indicate that people with diabetes and an elevated hs-cTnT “should be targeted for aggressive cardiovascular risk reduction, including lifestyle interventions, weight loss, and treatment with statins, blood pressure medications, and cardioprotective therapies such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors and glucagonlike peptide-1 (GLP-1) receptor agonists,” Dr. Selvin added.
“Cholesterol is often the factor that we target to reduce the risk of cardiovascular disease in people with type 2 diabetes,” she observed. “However, type 2 diabetes may have a direct effect on the heart not related to cholesterol levels. If type 2 diabetes is directly causing damage to the small vessels in the heart unrelated to cholesterol plaque buildup, then cholesterol-lowering medications are not going to prevent cardiac damage,” Dr. Selvin explained. “Our research suggests that additional non–statin-related therapies are needed to lower the cardiovascular disease risk in people with type 2 diabetes.”
However, she noted that a necessary step prior to formally recommending such a strategy is to run clinical trials to assess the efficacy of specific treatments, such as SGLT-2 inhibitors and GLP-1 agonists, in people with diabetes and elevated hs-cTnT.
“Randomized controlled trials would be best to test the relevance of measuring these biomarkers to assess risk in asymptomatic people with diabetes,” as well as prospective study of the value of hs-cTnT to guide treatment, commented Robert H. Eckel, MD, an endocrinologist affiliated with the University of Colorado at Denver, Aurora.
“I doubt measurements [of hs-cTnT] would be reimbursed [by third-party payers] if carried out without such outcome data,” he added.
Dr. Eckel also highlights the need to further validate in additional cohorts the link between elevations in hs-cTnT and CVD events in adults with diabetes, and to confirm that elevated levels of another cardiac biomarker – N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) – do not work as well as troponin as a risk marker for people with diabetes, another finding of the study.
ADA report already recommends testing these biomarkers for HF
However, a consensus report published in 2022 by the American Diabetes Association laid out the case for routinely and regularly measuring levels of both high sensitivity cardiac troponin and natriuretic peptides in people with diabetes for early identification of incident heart failure.
“Among individuals with diabetes, measurement of a natriuretic peptide or high-sensitivity cardiac troponin is recommended on at least a yearly basis to identify the earliest heart failure stages and implement strategies to prevent transition to symptomatic heart failure,” noted the ADA consensus report on heart failure.
The new study run by Dr. Selvin and coauthors used data collected by the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004 from U.S. adults who were at least 20 years old and had no history of CVD: myocardial infarction, stroke, coronary heart disease, or heart failure. This included 9,273 people without diabetes and 1,031 with diabetes, defined as a prior diagnosis or hemoglobin A1c of at least 6.5%.
“Cardiovascular risk varies substantially in adults with type 2 diabetes, highlighting the need for accurate risk stratification,” the authors observed.
All study participants had recorded measures of hs-cTnT and NT-proBNP.
The researchers considered an hs-cTnT level of greater than 14 ng/L and an NT-proBNP level of greater than 125 pg/mL as indicators of subclinical CVD.
The crude prevalence of elevated NT-proBNP was 33.4% among those with diabetes and 16.1% in those without diabetes. Elevated hs-cTnT occurred in 19% of those with diabetes and in 5% of those without diabetes. Elevated levels of both markers existed in 9% of those with diabetes and in 3% of those without diabetes.
“Approximately one in three adults with diabetes had subclinical CVD, with 19% having elevated levels of hs-cTnT, 23% having elevated NT-proBNP, and 9% having elevations in both cardiac biomarkers,” the researchers noted.
Diabetes linked with a doubled prevalence of elevated hs-cTnT
After adjustment for several demographic variables as well as traditional CVD risk factors, people with diabetes had a significant 98% higher rate of elevated hs-cTnT, compared with those without diabetes. But after similar adjustments, the rate of elevated NT-proBNP was significantly lower among people with diabetes, compared with controls, by a relative reduction of 24%.
“Our findings suggest that, in people with diabetes, hs-cTnT may be more useful [than NT-proBNP] for general risk monitoring, as its interpretation is less complicated,” said Dr. Selvin, who explained that “NT-proBNP is affected by overweight and obesity.”
In people with diabetes, the age-adjusted prevalence of elevated hs-cTnT ran higher in those with longer duration diabetes, and in those with less well-controlled diabetes based on a higher level of A1c. Neither of these factors showed any significant relationship with measured levels of NT-proBNP.
Further analysis linked the NHANES findings during 1999-2004 with U.S. national death records through the end of 2019. This showed that elevated levels of both hs-cTnT and NT-proBNP significantly linked with subsequently higher rates of all-cause mortality among people with diabetes. Elevated hs-cTnT linked with a 77% increased mortality and NT-proBNP linked with a 78% increased rate, compared with people with diabetes and no elevations in these markers, after adjustment for demographic variables and CVD risk factors.
However, for the outcome of cardiovascular death, elevated hs-cTnT linked with a nonsignificant 54% relative increase, while elevated NT-proBNP linked with a significant 2.46-fold relative increase.
The study “adds new data on biomarkers that are not routinely measured in asymptomatic people with or without diabetes” and the relationships of these markers to CVD mortality and all-cause mortality, Dr. Eckel concluded.
The study received no commercial funding, but used reagents donated by Abbott Laboratories, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare Diagnostics. Dr. Selvin and Dr. Eckel had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION