User login
Overweight in heterozygous FH tied to even higher CAD risk
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY – – rates that appear to have a substantial impact on these patients’ already increased risk of coronary artery disease, a registry analysis suggests.
Data on almost 36,000 individuals with FH were collated from an international registry, revealing that 55% of adults and 25% of children and adolescents with the homozygous form of FH had overweight or obesity. The figures for heterozygous FH were 52% and 27%, respectively.
Crucially, overweight or obesity was associated with substantially increased rates of coronary artery disease, particularly in persons with heterozygous FH, among whom adults with obesity faced a twofold increased risk, rising to more than sixfold in children and adolescents.
Moreover, “obesity is associated with a worse lipid profile, even from childhood, regardless of whether a patient is on medication,” said study presenter Amany Elshorbagy, DPhil, Cardiovascular Epidemiologist, department of primary care and public health, Imperial College London.
She added that, with the increased risk of coronary artery disease associated with heterozygous FH, the results showed that “together with lipid-lowering medication, weight management is needed.”
The research was presented at the annual meeting of the European Atherosclerosis Society.
Tended to be thin
Alberico L. Catapano, MD, PhD, director of cardiovascular research and of the Lipoproteins and Atherosclerosis Laboratory of IRCCS Multimedica, Milan, and past president of the EAS, said in an interview that, historically, few FH patients were overweight or obese; rather, they tended to be thin.
However, there is now “a trend for people with FH to show more diabetes and obesity,” with the “bottom line” being that, as they are already at increased risk of coronary artery disease, it pushes their risk up even further.
In other words, if a risk factor such as obesity is added “on top of the strongest risk factor, that is LDL cholesterol, it is not one plus one makes two, it is one plus one makes three,” he said.
As such, Dr. Catapano believes that the study is “very interesting,” because it further underlines the importance of weight management for individuals with increased LDL cholesterol, “especially when you have genetic forms, like FH.”
Dr. Catapano’s comments were echoed by session co-chair Ulrike Schatz, MD, leader of the lipidology specialty department at the University Hospital Carl Gustav Carus, Technical University of Dresden (Germany).
Indeed, she told Dr. Elshorbagy before her presentation that she finds “a lot of my FH patients have a tendency towards anorexia.”
In an interview, Dr. Elshorbagy said that that reaction was typical of “most of the clinicians” she had spoken to. Upon seeing her data, especially for homozygous FH patients, they say, “They are on the lean side.”
Consequently, the research team went into the study “with the expectation that they might have a lower prevalence of obesity and overweight than the general population,” but “that’s not what we’re seeing.”
Dr. Elshorbagy noted that it would be helpful to have longitudinal data to determine whether, 50 years ago, patients with HF “were leaner, along with the rest of the population.”
The registry data are cross-sectional, and the team is now reaching out to the respective national lead investigators to submit follow-up data on their patients, with the aim of looking at changes in body weight and the impact on outcomes over time.
Another key question for the researchers is in regard to fat distribution, as body mass index “is not the best predictor of heart disease,” Dr. Elshorbagy said, but is rather central obesity.
Although they have also asked investigators to share waist circumference data, she conceded that it is a measurement that “is a lot harder to standardize across centers and countries; it’s not like putting patients on a scale.”
Overall, Dr. Elshorbagy believes that her findings indicate that clinicians should take a broader, more holistic approach toward their patients – in other words, an approach in which lipid lowering medication is “key but is just one of several things we need to do to make sure the coronary event rate goes down.”
More with than without
Dr. Elshorbagy began her presentation by highlighting that the prevalence of overweight and obesity ranges from 50% to 70% and that it is “the only health condition where you’ve got more people worldwide with the condition than without.”
Crucially, overweight increases the risk of coronary artery disease by approximately 20%. Among patients with obesity, the risk rises to 50%.
Given that FH patients “already have a very high risk of cardiovascular disease from their high cholesterol levels,” the team set out to determine rates of obesity and overweight in this population and their impact on coronary artery disease risk.
They used cross-sectional data from the EAS FH Studies Collaboration Global Registry, which involves 29,262 adults aged greater than or equal to 18 years and 6,275 children and adolescents aged 5 to 17 years with heterozygous FH, and 325 adults and 57 children with homozygous FH.
Dividing the adults into standard BMI categories, they found that 16% of heterozygous and 23% of homozygous FH patients had obesity, while 52% and 55%, respectively, had overweight or obesity.
For children, the team used World Health Organization z score cutoffs, which indicated that 9% of patients with heterozygous FH and 7% of patients with homozygous FH had obesity. Rates of overweight or obesity were 27% and 25%, respectively.
Among patients with heterozygous FH, rates of overweight or obesity among adults were 50% in high-income countries and 63% in other countries; among children, the rates were and 27% and 29%, respectively.
Stratified by region, the team found that the lowest rate of overweight or obesity among adult patients with heterozygous FH was in Eastern Asia, at 27%, while the highest was in Northern Africa/Western Asia (the Middle East), at 82%.
In North America, 56% of adult patients had overweight or obesity. The prevalence of coronary artery disease rose with increasing BMI.
Among adult patients with heterozygous FH, 11.3% of those with normal weight had coronary artery disease; the percentage rose to 22.9% among those with overweight, and 30.9% among those with obesity. Among children, the corresponding figures were 0.1%, 0.2%, and 0.7%.
Putting adults and children with homozygous FH together, the researchers found that 29.0% of patients with normal weight had coronary artery disease, compared with 31.3% of those with overweight and 49.3% of those with obesity.
Moreover, the results showed that levels of LDL and remnant cholesterol were significantly associated with BMI in adults and children with heterozygous FH, even after adjusting for age, sex, and lipid-lowering medication (P < .001 for all).
Multivariate analysis that took into account age, sex, lipid-lowering medication, and LDL cholesterol revealed that having obesity, compared with not having obesity, was associated with a substantial increase in the risk of coronary artery disease among patients with heterozygous FH.
Among adults with the condition, the odds ratio was 2.16 (95% confidence interval, 1.97-2.36), while among children and adolescents, it was 6.87 (95% CI, 1.55-30.46).
The results remained similar after further adjustment for the presence of diabetes and when considering peripheral artery disease and stroke.
No funding for the study was declared. Dr. Elshorbagy has relationships with Amgen, Daiichi Sankyo, and Regeneron.
A version of this article first appeared on Medscape.com.
AT EAS 2023
PTSD, anxiety linked to out-of-hospital cardiac arrest
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.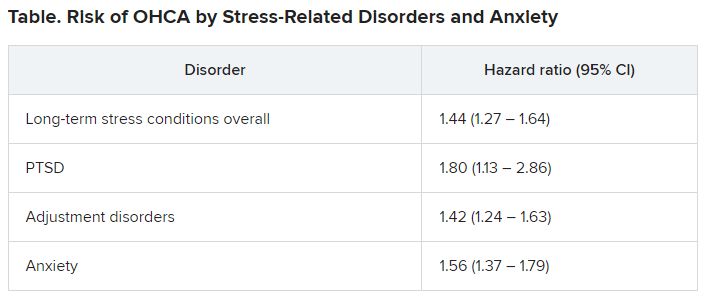
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators compared more than 35,000 OHCA case patients with a similar number of matched control persons and found an almost 1.5 times higher hazard of long-term stress conditions among OHCA case patients, compared with control persons, with a similar hazard for anxiety. Posttraumatic stress disorder was associated with an almost twofold higher risk of OHCA.
The findings applied equally to men and women and were independent of the presence of cardiovascular disease (CVD).
“This study raises awareness of the higher risks of OHCA and early risk monitoring to prevent OHCA in patients with stress-related disorders and anxiety,” write Talip Eroglu, of the department of cardiology, Copenhagen University Hospital, and colleagues.
The study was published online in BMJ Open Heart.
Stress disorders and anxiety overrepresented
OHCA “predominantly arises from lethal cardiac arrhythmias ... that occur most frequently in the setting of coronary heart disease,” the authors write. However, increasing evidence suggests that rates of OHCA may also be increased in association with noncardiac diseases.
Individuals with stress-related disorders and anxiety are “overrepresented” among victims of cardiac arrest as well as those with multiple CVDs. But previous studies of OHCA have been limited by small numbers of cardiac arrests. In addition, those studies involved only data from selected populations or used in-hospital diagnosis to identify cardiac arrest, thereby potentially omitting OHCA patients who died prior to hospital admission.
The researchers therefore turned to data from Danish health registries that include a large, unselected cohort of patients with OHCA to investigate whether long-term stress conditions (that is, PTSD and adjustment disorder) or anxiety disorder were associated with OHCA.
They stratified the cohort according to sex, age, and CVD to identify which risk factor confers the highest risk of OHCA in patients with long-term stress conditions or anxiety, and they conducted sensitivity analyses of potential confounders, such as depression.
The design was a nested-case control model in which records at an individual patient level across registries were cross-linked to data from other national registries and were compared to matched control persons from the general population (35,195 OHCAs and 351,950 matched control persons; median IQR age, 72 [62-81] years; 66.82% men).
The prevalence of comorbidities and use of cardiovascular drugs were higher among OHCA case patients than among non-OHCA control persons.
Keep aware of stress and anxiety as risk factors
Among OHCA and non-OHCA participants, long-term stress conditions were diagnosed in 0.92% and 0.45%, respectively. Anxiety was diagnosed in 0.85% of OHCA case patients and in 0.37% of non-OHCA control persons.
These conditions were associated with a higher rate of OHCA after adjustment for common OHCA risk factors.
There were no significant differences in results when the researchers adjusted for the use of anxiolytics and antidepressants.
When they examined the prevalence of concomitant medication use or comorbidities, they found that depression was more frequent among patients with long-term stress and anxiety, compared with individuals with neither of those diagnoses. Additionally, patients with long-term stress and anxiety more often used anxiolytics, antidepressants, and QT-prolonging drugs.
Stratification of the analyses according to sex revealed that the OHCA rate was increased in both women and men with long-term stress and anxiety. There were no significant differences between the sexes. There were also no significant differences between the association among different age groups, nor between patients with and those without CVD, ischemic heart disease, or heart failure.
Previous research has shown associations of stress-related disorders or anxiety with cardiovascular outcomes, including myocardial infarction, heart failure, and cerebrovascular disease. These disorders might be “biological mediators in the causal pathway of OHCA” and contribute to the increased OHCA rate associated with stress-related disorders and anxiety, the authors suggest.
Nevertheless, they note, stress-related disorders and anxiety remained significantly associated with OHCA after controlling for these variables, “suggesting that it is unlikely that traditional risk factors of OHCA alone explain this relationship.”
They suggest several potential mechanisms. One is that the relationship is likely mediated by the activity of the sympathetic autonomic nervous system, which “leads to an increase in heart rate, release of neurotransmitters into the circulation, and local release of neurotransmitters in the heart.”
Each of these factors “may potentially influence cardiac electrophysiology and facilitate ventricular arrhythmias and OHCA.”
In addition to a biological mechanism, behavioral and psychosocial factors may also contribute to OHCA risk, since stress-related disorders and anxiety “often lead to unhealthy lifestyle, such as smoking and lower physical activity, which in turn may increase the risk of OHCA.” Given the absence of data on these features in the registries the investigators used, they were unable to account for them.
However, “it is unlikely that knowledge of these factors would have altered our conclusions considering that we have adjusted for all the relevant cardiovascular comorbidities.”
Similarly, other psychiatric disorders, such as depression, can contribute to OHCA risk, but they adjusted for depression in their multivariable analyses.
“Awareness of the higher risks of OHCA in patients with stress-related disorders and anxiety is important when treating these patients,” they conclude.
Detrimental to the heart, not just the psyche
Glenn Levine, MD, master clinician and professor of medicine, Baylor College of Medicine, Houston, called it an “important study in that it is a large, nationwide cohort study and thus provides important information to complement much smaller, focused studies.”
Like those other studies, “it finds that negative psychological health, specifically, long-term stress (as well as anxiety), is associated with a significantly increased risk of out-of-hospital cardiac arrest,” continued Dr. Levine, who is the chief of the cardiology section at Michael E. DeBakey VA Medical Center, Houston, and was not involved with the study.
Dr. Levine thinks the study “does a good job, as best one can for such a study, in trying to control for other factors, and zeroing in specifically on stress (and anxiety), trying to assess their independent contributions to the risk of developing cardiac arrest.”
The take-home message for clinicians and patients “is that negative psychological stress factors, such as stress and anxiety, are not only detrimental to one’s psychological health but likely increase one’s risk for adverse cardiac events, such as cardiac arrest,” he stated.
No specific funding for the study was disclosed. Mr. Eroglu has disclosed no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. Levine reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN HEART
Plant-based diet tied to healthier blood lipid levels
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
, in a new meta-analysis of 30 trials.
The findings suggest that “plant-based diets have the potential to lessen the atherosclerotic burden from atherogenic lipoproteins and thereby reduce the risk of cardiovascular disease,” write Caroline Amelie Koch, a medical student at the University of Copenhagen, and colleagues. Their findings were published online in the European Heart Journal (2023 May 24. doi: 10.1093/eurheartj/ehad211).
“Vegetarian and vegan diets were associated with a 14% reduction in all artery-clogging lipoproteins as indicated by apoB,” senior author Ruth Frikke-Schmidt, DMSc, PhD, Rigshospitalet, Copenhagen, and professor, University of Copenhagen, said in a press release from her university.
“This corresponds to a third of the effect of taking cholesterol-lowering medications such as statins,” she added, “and would result in a 7% reduction in the risk of cardiovascular disease in someone who maintained a plant-based diet for 5 years.”
“Importantly, we found similar results, across continents, ages, different ranges of body mass index, and among people in different states of health,” Dr. Frikke-Schmidt stressed.
And combining statins with plant-based diets would likely produce a synergistic effect, she speculated.
“If people start eating vegetarian or vegan diets from an early age,” she said, “the potential for reducing the risk of cardiovascular disease caused by blocked arteries is substantial.”
In addition, the researchers conclude: “Shifting to plant-based diets at a populational level will reduce emissions of greenhouse gases considerably – together making these diets efficient means [moving] towards a more sustainable development, while at the same time reducing the growing burden of atherosclerotic cardiovascular disease.”
More support for vegan, vegetarian diets
These new findings “add to the body of evidence supporting favorable effects of healthy vegan and vegetarian dietary patterns on circulating levels of LDL-C and atherogenic lipoproteins, which would be expected to reduce ASCVD risk,” Kevin C. Maki, PhD, and Carol Kirkpatrick, PhD, MPH, write in an accompanying editorial.
“While it is not necessary to entirely omit foods such as meat, poultry, and fish/seafood to follow a recommended dietary pattern, reducing consumption of such foods is a reasonable option for those who prefer to do so,” note Dr. Maki, of Indiana University School of Public Health, Bloomington, and Kirkpatrick, of Idaho State University, Pocatello.
Plant-based diet needs to be ‘well-planned’
Several experts who were not involved in this meta-analysis shed light on the study and its implications in comments to the U.K. Science Media Center.
“Although a vegetarian and vegan diet can be very healthy and beneficial with respect to cardiovascular risk, it is important that it is well planned so that nutrients it can be low in are included, including iron, iodine, vitamin B12, and vitamin D,” said Duane Mellor, PhD, a registered dietitian and senior lecturer, Aston Medical School, Aston University, Birmingham, England.
Some people “may find it easier to follow a Mediterranean-style diet that features plenty of fruit, vegetables, pulses, wholegrains, fish, eggs and low-fat dairy, with only small amounts of meat,” Tracy Parker, senior dietitian at the British Heart Foundation, London, suggested.
“There is considerable evidence that this type of diet can help lower your risk of developing heart and circulatory diseases by improving cholesterol and blood pressure levels, reducing inflammation, and controlling blood glucose levels,” she added.
And Aedin Cassidy, PhD, chair in nutrition & preventative medicine, Queen’s University Belfast (Ireland), noted that “not all plant-based diets are equal. Healthy plant-based diets, characterized by fruits, vegetables, and whole grains improve health, but other plant diets (for example, those including refined carbohydrates, processed foods high in fat/salt, etc.) do not.”
This new study shows that plant-based diets have the potential to improve health by improving blood lipids, “but this is one of many potential mechanisms, including impact on blood pressure, weight maintenance, and blood sugars,” she added.
“This work represents a well-conducted analysis of 30 clinical trials involving over two thousand participants and highlights the value of a vegetarian diet in reducing the risk of heart attack or stroke through reduction in blood cholesterol levels,” said Robert Storey, BM, DM, professor of cardiology, University of Sheffield, U.K.
However, it also demonstrates that the impact of diet on an individual’s cholesterol level is relatively limited, he added.
“This is because people inherit the tendency for their livers to produce too much cholesterol, meaning that high cholesterol is more strongly influenced by our genes than by our diet,” he explained.
This is “why statins are needed to block cholesterol production in people who are at higher risk of or have already suffered from a heart attack, stroke, or other illness related to cholesterol build-up in blood vessels.”
Beneficial effect on ApoB, LDL-C, and total cholesterol
ApoB is the main apolipoprotein in LDL-C (“bad” cholesterol), the researchers note. Previous studies have shown that LDL-C and apoB-containing particles are associated with increased risk of ASCVD.
They aimed to estimate the effect of vegetarian or vegan diets on blood levels of total cholesterol, LDL-C, triglycerides, and apoB in people randomized to a vegetarian or vegan diet versus an omnivorous diet (that is, including meat and dairy).
They identified 30 studies published between 1982 and 2022 and conducted in the United States (18 studies), Sweden (2), Finland (2), South Korea (2), Australia (1), Brazil (1), Czech Republic (1), Italy (1), Iran (1), and New Zealand (1).
The diet interventions lasted from 10 days to 5 years with a mean of 29 weeks (15 studies ≤ 3 months; 12 studies 3-12 months; and three studies > 1 year). Nine studies used a crossover design, and the rest used a parallel design whereby participants followed only one diet.
The studies had 11 to 291 participants (mean, 79 participants) with a mean BMI of 21.5-35.1 kg/m2 and a mean age of 20-67 years. Thirteen studies included participants treated with lipid-lowering therapy at baseline.
The dietary intervention was vegetarian in 15 trials (three lacto-vegetarian and 12 lacto-ovo-vegetarian) and vegan in the other 15 trials.
On average, compared with people eating an omnivore diet, people eating a plant-based diet had a 7% reduction in total cholesterol from baseline (–0.34 mmol/L), a 10% reduction in LDL-C from baseline (–0.30 mmol/L), and a 14% reduction in apoB from baseline (–12.9 mg/dL) (all P < .01).
The effects were similar across age, continent, study duration, health status, intervention diet, intervention program, and study design subgroups.
There was no significant difference in triglyceride levels in patients in the omnivore versus plant-based diet groups.
Such diets could considerably reduce greenhouse gases
Senior author Dr. Frikke-Schmidt noted: “Recent systematic reviews have shown that if the populations of high-income countries shift to plant-based diets, this can reduce net emissions of greenhouse gases by between 35% to 49%.”
“Plant-based diets are key instruments for changing food production to more environmentally sustainable forms, while at the same time reducing the burden of cardiovascular disease” in an aging population, she said.
“We should be eating a varied, plant-rich diet, not too much, and quenching our thirst with water,” she concluded.
The study was funded by the Lundbeck Foundation, the Danish Heart Foundation, and the Leducq Foundation. The authors, editorialists, Ms. Parker, Dr. Cassidy, and Dr. Storey have reported no relevant financial relationships. Dr. Mellor has disclosed that he is a vegetarian.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
Circulatory support for RV failure caused by pulmonary embolism
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
A new review article highlights approaches for mechanical circulatory support in patients with high-risk acute pulmonary embolism (PE).
Pulmonary embolism with hemodynamic significance is widely underdiagnosed, and the mortality rate can be as high as 30%, but new therapeutic developments offer promise. “Over the past few years, a renewed interest in mechanical circulatory support (MCS; both percutaneous and surgical) for acute RVF has emerged, increasing viable treatment options for high-risk acute PE,” wrote the authors of the review, which was published online in Interventional Cardiology Clinics.
Poor outcomes are often driven by RVF, which is tricky to diagnose and manage, and it stems from a sudden increase in pulmonary vascular resistance (PVR) following PE. “The mechanism for increased PVR in acute PE is multifactorial, including direct blood flow impedance, local hypoxia-induced vasoconstriction, and platelet/thrombin-induced release of vasoactive peptides. The cascade of events that then leads to RVF includes decreased RV stoke volume, increased RV wall tension, and RV dilation,” the authors wrote.
The authors noted that diuretics help to correct changes to RV geometry and can improve left ventricle filling, which improves hemodynamics. Diuretics can be used in patients who are hypotensive and volume overloaded, but vasopressors should be employed to support blood pressure.
When using mechanical ventilation, strategies such as low tidal volumes, minimization of positive end expiratory pressure, and prevention of hypoxemia and acidemia should be employed to prevent an increase of pulmonary vascular resistance, which can worsen RV failure.
Pulmonary vasodilators aren’t recommended for acute PE, but inhaled pulmonary vasodilators may be considered in hemodynamically unstable patients.
Surgically implanted right ventricle assistance device are generally not used for acute RV failure in high-risk PE, unless the patient has not improved after medical management.
Percutaneous devices
Percutaneous mechanical circulatory support devices can be used for patients experiencing refractory shock. The review highlighted three such devices, including the Impella RP, tandem-heart right ventricular assist devices (TH-RVAD) or Protek Duo, and venoarterial extracorporeal membrane oxygenation (VA-ECMO), but they are not without limitations. “Challenges to using these devices in patients with acute PE include clot dislodgement, vascular complications, infections, device migration, and fracture of individual elements,” the authors wrote.
The Impella RP is easy to deploy and bypasses the RV, but it can’t provide blood oxygenation and may cause bleeding or hemolysis. TH-RVAD oxygenates the blood and bypasses the RV, but suffers from a large sheath size. VA-ECMO oxygenates the blood but may cause bleeding.
There are important differences among the mechanical support devices, according to Jonathan Ludmir, MD, who was asked to comment. “In reality, if someone has a large pulmonary embolism burden, to put in the Impella RP or the Protek Duo would be a little bit risky, because you’d be sometimes putting the device right where the clot is. At least what we do in our institution, when someone is in extremis despite using [intravenous] medications like vasopressors or inotropes, VA-ECMO is kind of the go to. This is both the quickest and probably most effective way to support the patient. I say the quickest because this is a procedure you can do at the bedside.”
Benefits of PERT
One message that the review only briefly mentions, but Dr. Ludmir believes is key, is employing a pulmonary embolism response team. “That’s been looked at extensively, and it’s a really key part of any decision-making. If someone presents to the emergency room or someone inside the hospital has an acute pulmonary embolism, you have a team of people that can respond and help assess the next step. Typically, that involves a cardiologist or an interventional cardiologist, a hematologist, vascular surgeon, often a cardiac surgeon, so it’s a whole slew of people. Based on the patient assessment they can quickly decide, can this patient just be okay with a blood thinner like heparin? Does this patient need something more aggressive, like a thrombectomy? Or is this a serious case where you involve the shock team or the ECMO team, and you have to stabilize the patient on mechanical circulatory support, so you can accomplish what you need to do to get rid of the pulmonary embolism,” said Dr. Ludmir, who is an assistant professor of medicine at Corrigan Minehan Heart Center at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“Every case is individualized, hence the importance of having a team of a variety of different backgrounds and thoughts to approach it. And I think that’s kind of like the key takeaway. Yes, you have to be familiar with all the therapies, but at the end of the day, not every patient is going to fit into the algorithm for how you approach pulmonary embolism,” said Dr. Ludmir.
Dr. Ludmir has no relevant conflicts of interest.
FROM INTERVENTIONAL CARDIOLOGY CLINICS
Half of deaths from homozygous FH occur before age 32 years
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
MANNHEIM, GERMANY –
The researchers looked at almost 40 patients from the HoFH International Clinical Collaborators (HICC) registry who had died before data entry, finding that they had a mean age of diagnosis of 12 years.
Even those who received treatment had high LDL cholesterol levels, and 70% developed atherosclerotic cardiovascular disease (ASCVD) at a median age of 28 years.
Worryingly, the results showed that the median age at death was 32 years. Results were presented at the annual congress of the European Atherosclerosis Society.
Patients with HoFH “have severe atherosclerotic cardiovascular disease risk,” said study presenter Janneke Mulder, a PhD candidate at the department of internal medicine, Erasmus University Medical Center, Rotterdam, the Netherlands.
“Therefore, early diagnosis and initiation of treatments, and also a combination of treatments, is really crucial,” she added.
Call to action
Approached for comment, Maciej Banach, MD, PhD, full professor of cardiology, Polish Mother’s Memorial Hospital Research Institute, Lodz, and Secretary of the EAS, described the results as “terrifying.”
He said in an interview that they are a “call to action,” especially given that so few patients in the study received intensive combination lipid-lowering therapy despite having a baseline LDL cholesterol level that was “very, very high.”
Banach underlined that patients who receive triple lipid-lowering therapy with a high-intensity statin, ezetimibe (Nustendi), and a proprotein convertase subtilisin/kexin type 9 inhibitor, could expect, based on current evidence, to see their LDL cholesterol levels reduced by 85% and be on target.
“Obviously, this is kind of academic,” because in the real-world “this 85% is not observed very often,” but it offers a target for steep reductions in cholesterol levels.
“This is something that we should focus on for these patients from the beginning,” said Dr. Banach, either with a stepwise approach “or for experts in pediatric HoFH, “maybe immediately.”
He emphasized that clinicians have everything at hand to “be both effective in the early diagnosis of HoFH, the earlier the better, and obviously to be effective with its treatment.”
“We should do something to prolong the lives of those people,” because the current results are “terrifying,” Dr. Banach added.
Rare genetic condition
Presenting her findings, Ms. Mulder began by highlighting that HoFH is a “rare genetic condition that occurs due to mutations in cholesterol metabolism.”
This, she continued, leads to “severely increased LDL cholesterol levels, and consequently to very premature cardiovascular disease,” with patients potentially experiencing their first cardiovascular event before age 20 years.
Ms. Mulder pointed out that, although there have been case series in the literature on HoFH, they have had “limited numbers” and patients have typically spent decades being treated at the same lipid management clinic.
To broaden the understanding of the clinical characteristics and management of patients dying with HoFH, the team examined data from the HICC registry, which is “the largest contemporary database of homozygous FH patients,” Ms. Mulder said.
It includes 751 patients with HoFH from 88 centers in 38 countries who were alive in 2010 or later. Data entry was between 2016 and 2020. The current analysis focused on 37 patients who had already died by the time they were included on the registry.
Of those, 49% were women, 38% were of White ethnicity, and 43% were from high-income countries.
The median age at diagnosis was 12 years, Ms. Mulder said, explaining that this is similar to that seen in other studies. The majority (86%) underwent genetic testing, and 92% presented with xanthomas.
Ms. Mulder also noted that, at their final clinical evaluation, which was conducted a median age of 18 years after their initial diagnosis, 43% of patients were recorded as current or former smokers.
In terms of their lipid-lowering therapy, 94% were taking a statin, whereas 68% were on ezetimibe, and 23% were undergoing apheresis.
Ms. Mulder said that the median number of lipid-lowering therapies per patient was two, and that “sadly ... 26% of the deceased patients had only one or no treatment.”
Therefore, perhaps unsurprisingly even those patients who were receiving treatment had LDL cholesterol levels that were “too high,” at 9.4 mmol/L versus 15.6 mmol/L among those who were untreated.
There was a high prevalence of ASCVD, at 70% overall, or 41% for aortic stenosis, 30% for myocardial infarction, 30% for angina pectoris, and 22% each for aortic valve replacement and coronary artery bypass grafting. In addition, 19% underwent percutaneous coronary intervention.
The median age of onset for ASCVD was 28 years. Ms. Mulder pointed out, however, that, as data were not available for all patients, “this might be an underestimation.” About 70% of patients experienced recurrent ASCVD.
There was a wide range in the age at which patients with HoFH died, although the median was, “strikingly,” 32 years, Ms. Mulder said. Death was confirmed as stemming from cardiovascular causes in 76% of cases.
During the postpresentation discussion, session chair Antonio J. Vallejo-Vaz, PhD, from the Research Group of Clinical Epidemiology and Vascular Risk, Institute of Biomedicine of Seville (Spain), highlighted that, if 38% of the patients were of White ethnicity, then the remainder must therefore be from other ethnic groups.
“There could be potential issues with accessibility to lipid centers” for these patients, which could affect the findings, noted Dr. Vallejo-Vaz, who is also chief scientist of the EAS Familial Hypercholesterolaemia Studies Collaboration.
Ms. Mulder agreed, replying that their results, though already striking, may be an underestimation because the patients were all from either high or middle-income countries, “so it would be good to have some data on low-income countries.”
She was also asked about two patients who died at a much older age than did the others, at ages 70 years and 86 years, respectively, and whether they had, for example, a protective genetic mutation.
Ms. Mulder said that they do not yet know, but they are planning an extended case series on these and other long-lived patients so that they can be investigated further.
No funding or relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
Coronary artery calcium score bests polygenic risk score in CHD prediction
As a predictor of coronary heart disease (CHD) events, the coronary artery calcium (CAC) score on computed tomography had better risk discrimination than the polygenic risk score, a binational study found. And when added to classic cardiovascular risk factors, the CAC score significantly improved risk classification while the polygenic risk factor score did not.
These findings emerged from two large cohorts of middle-aged and older White adults from the United States and the Netherlands in the first head-to-head comparison of these two approaches. Led by Sadiya S. Kahn, MD, MSc, an assistant professor of medicine (cardiology) and preventive medicine (epidemiology) at Northwestern University, Chicago, the study was published online in JAMA.
There has been much interest in using both genetic factors and CT imaging to better identify individuals at risk for heart disease. “Each approach has advantages and disadvantages, and we wanted to better understand the comparative predictive utility to provide support for what the preferred approach should be,” Dr. Kahn said in an interview. “We focused on middle-aged to older adults for whom current risk prediction equations are relevant in estimating risk with the Pooled Cohort Equation, or PCE.”
The superiority of the CT-imaged coronary artery risk score may be because of its direct visualization of calcification in the arteries and the subclinical disease burden rather than a focus on common genetic variants, Dr. Kahn explained. “In addition, prior studies have demonstrated that genetics, or inherited risk, is not destiny, so this score may not perform as well for risk discrimination as the traditional risk factors themselves along with CT.”
The study
Study participants came from the U.S. Multi-Ethnic Study of Atherosclerosis (MESA, n = 1,991) and the Dutch Rotterdam Study (RS, n = 1,217). Ages ranged from 45 to 79, with the medians in the two cohorts 61 and 68 years, respectively. Slightly more than half of participants in both groups were female.
Traditional risk factors were used to calculate CHD risk with pooled cohort equations, while computed tomography was used to determine the CAC score and genotyped samples for a validated polygenic risk score.
Both scores were significantly associated with 10-year risk of incident CHD.
The median predicted atherosclerotic disease risk based on traditional risk factors was 6.99% in MESA and 5.93% in RS. During the total available follow-up in MESA (median, 16.0 years) and RS (median, 14.2 years), incident CHD occurred in 187 participants (9.4%) and 98 participants (8.1%), respectively.
C (concordance) statistics for the two scores showed the superiority of the CAC. This statistic measures a model’s ability to rank patients from high to low risk, with a value of 1 being perfect risk fit or concordance and 0.70 or more indicating good concordance and risk discrimination. The CAC score had a C statistic of 0.76 (95% confidence interval, 0.71-0.79) vs. 0.69 for the polygenic risk score (95% CI, 0.63-0.71).
When each score was added to PCEs, the C statistics changed as follows: CAC score, 0.09 (95% CI, 0.06-0.13); polygenic risk score, 0.02 (95% CI, 0.00-0.04); and 0.10 (95% CI, 0.07-0.14) for both.
Net reclassification significantly improved with the CAC plus PCEs by the following values: 0.19 (95% CI, 0.06-0.28). The change was not significant, however, with the polygenic risk score plus PCEs: 0.04 (95% CI, –0.05-0.10).
In the clinical setting, Dr. Kahn said, “The use of CT in patients who are at intermediate risk for heart disease can be helpful in refining risk estimation and guiding recommendations for lipid-lowering therapy. Polygenic risk scores are not helpful in middle-aged to older adults above and beyond traditional risk factors for predicting risk of heart disease.”
This study was supported by the National Heart, Lung, and Blood Institute. MESA is supported by the NHLBI. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Dr. Khan reported grants from the NHLBI and the NIH during the study and outside of the submitted work. Several coauthors reported grant support from, variously, the NIH, the NHLBI, and the American Heart Association.
As a predictor of coronary heart disease (CHD) events, the coronary artery calcium (CAC) score on computed tomography had better risk discrimination than the polygenic risk score, a binational study found. And when added to classic cardiovascular risk factors, the CAC score significantly improved risk classification while the polygenic risk factor score did not.
These findings emerged from two large cohorts of middle-aged and older White adults from the United States and the Netherlands in the first head-to-head comparison of these two approaches. Led by Sadiya S. Kahn, MD, MSc, an assistant professor of medicine (cardiology) and preventive medicine (epidemiology) at Northwestern University, Chicago, the study was published online in JAMA.
There has been much interest in using both genetic factors and CT imaging to better identify individuals at risk for heart disease. “Each approach has advantages and disadvantages, and we wanted to better understand the comparative predictive utility to provide support for what the preferred approach should be,” Dr. Kahn said in an interview. “We focused on middle-aged to older adults for whom current risk prediction equations are relevant in estimating risk with the Pooled Cohort Equation, or PCE.”
The superiority of the CT-imaged coronary artery risk score may be because of its direct visualization of calcification in the arteries and the subclinical disease burden rather than a focus on common genetic variants, Dr. Kahn explained. “In addition, prior studies have demonstrated that genetics, or inherited risk, is not destiny, so this score may not perform as well for risk discrimination as the traditional risk factors themselves along with CT.”
The study
Study participants came from the U.S. Multi-Ethnic Study of Atherosclerosis (MESA, n = 1,991) and the Dutch Rotterdam Study (RS, n = 1,217). Ages ranged from 45 to 79, with the medians in the two cohorts 61 and 68 years, respectively. Slightly more than half of participants in both groups were female.
Traditional risk factors were used to calculate CHD risk with pooled cohort equations, while computed tomography was used to determine the CAC score and genotyped samples for a validated polygenic risk score.
Both scores were significantly associated with 10-year risk of incident CHD.
The median predicted atherosclerotic disease risk based on traditional risk factors was 6.99% in MESA and 5.93% in RS. During the total available follow-up in MESA (median, 16.0 years) and RS (median, 14.2 years), incident CHD occurred in 187 participants (9.4%) and 98 participants (8.1%), respectively.
C (concordance) statistics for the two scores showed the superiority of the CAC. This statistic measures a model’s ability to rank patients from high to low risk, with a value of 1 being perfect risk fit or concordance and 0.70 or more indicating good concordance and risk discrimination. The CAC score had a C statistic of 0.76 (95% confidence interval, 0.71-0.79) vs. 0.69 for the polygenic risk score (95% CI, 0.63-0.71).
When each score was added to PCEs, the C statistics changed as follows: CAC score, 0.09 (95% CI, 0.06-0.13); polygenic risk score, 0.02 (95% CI, 0.00-0.04); and 0.10 (95% CI, 0.07-0.14) for both.
Net reclassification significantly improved with the CAC plus PCEs by the following values: 0.19 (95% CI, 0.06-0.28). The change was not significant, however, with the polygenic risk score plus PCEs: 0.04 (95% CI, –0.05-0.10).
In the clinical setting, Dr. Kahn said, “The use of CT in patients who are at intermediate risk for heart disease can be helpful in refining risk estimation and guiding recommendations for lipid-lowering therapy. Polygenic risk scores are not helpful in middle-aged to older adults above and beyond traditional risk factors for predicting risk of heart disease.”
This study was supported by the National Heart, Lung, and Blood Institute. MESA is supported by the NHLBI. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Dr. Khan reported grants from the NHLBI and the NIH during the study and outside of the submitted work. Several coauthors reported grant support from, variously, the NIH, the NHLBI, and the American Heart Association.
As a predictor of coronary heart disease (CHD) events, the coronary artery calcium (CAC) score on computed tomography had better risk discrimination than the polygenic risk score, a binational study found. And when added to classic cardiovascular risk factors, the CAC score significantly improved risk classification while the polygenic risk factor score did not.
These findings emerged from two large cohorts of middle-aged and older White adults from the United States and the Netherlands in the first head-to-head comparison of these two approaches. Led by Sadiya S. Kahn, MD, MSc, an assistant professor of medicine (cardiology) and preventive medicine (epidemiology) at Northwestern University, Chicago, the study was published online in JAMA.
There has been much interest in using both genetic factors and CT imaging to better identify individuals at risk for heart disease. “Each approach has advantages and disadvantages, and we wanted to better understand the comparative predictive utility to provide support for what the preferred approach should be,” Dr. Kahn said in an interview. “We focused on middle-aged to older adults for whom current risk prediction equations are relevant in estimating risk with the Pooled Cohort Equation, or PCE.”
The superiority of the CT-imaged coronary artery risk score may be because of its direct visualization of calcification in the arteries and the subclinical disease burden rather than a focus on common genetic variants, Dr. Kahn explained. “In addition, prior studies have demonstrated that genetics, or inherited risk, is not destiny, so this score may not perform as well for risk discrimination as the traditional risk factors themselves along with CT.”
The study
Study participants came from the U.S. Multi-Ethnic Study of Atherosclerosis (MESA, n = 1,991) and the Dutch Rotterdam Study (RS, n = 1,217). Ages ranged from 45 to 79, with the medians in the two cohorts 61 and 68 years, respectively. Slightly more than half of participants in both groups were female.
Traditional risk factors were used to calculate CHD risk with pooled cohort equations, while computed tomography was used to determine the CAC score and genotyped samples for a validated polygenic risk score.
Both scores were significantly associated with 10-year risk of incident CHD.
The median predicted atherosclerotic disease risk based on traditional risk factors was 6.99% in MESA and 5.93% in RS. During the total available follow-up in MESA (median, 16.0 years) and RS (median, 14.2 years), incident CHD occurred in 187 participants (9.4%) and 98 participants (8.1%), respectively.
C (concordance) statistics for the two scores showed the superiority of the CAC. This statistic measures a model’s ability to rank patients from high to low risk, with a value of 1 being perfect risk fit or concordance and 0.70 or more indicating good concordance and risk discrimination. The CAC score had a C statistic of 0.76 (95% confidence interval, 0.71-0.79) vs. 0.69 for the polygenic risk score (95% CI, 0.63-0.71).
When each score was added to PCEs, the C statistics changed as follows: CAC score, 0.09 (95% CI, 0.06-0.13); polygenic risk score, 0.02 (95% CI, 0.00-0.04); and 0.10 (95% CI, 0.07-0.14) for both.
Net reclassification significantly improved with the CAC plus PCEs by the following values: 0.19 (95% CI, 0.06-0.28). The change was not significant, however, with the polygenic risk score plus PCEs: 0.04 (95% CI, –0.05-0.10).
In the clinical setting, Dr. Kahn said, “The use of CT in patients who are at intermediate risk for heart disease can be helpful in refining risk estimation and guiding recommendations for lipid-lowering therapy. Polygenic risk scores are not helpful in middle-aged to older adults above and beyond traditional risk factors for predicting risk of heart disease.”
This study was supported by the National Heart, Lung, and Blood Institute. MESA is supported by the NHLBI. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development; the Research Institute for Diseases in the Elderly; the Netherlands Genomics Initiative; the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. Dr. Khan reported grants from the NHLBI and the NIH during the study and outside of the submitted work. Several coauthors reported grant support from, variously, the NIH, the NHLBI, and the American Heart Association.
FROM JAMA
Once-daily nifedipine sufficient for hypertension in pregnancy
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
BALTIMORE – , according to research presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.*
The findings suggest that starting patients on a once-daily 60-mg dose is therefore reasonable, Isabelle Band, BA, a medical student at the Icahn School of Medicine at Mount Sinai, New York, told attendees. Ms. Band said in an interview that there does not appear to be a consensus on the standard of care for nifedipine dosing regimen in this population but that previous in vitro studies have shown increased metabolism of nifedipine in a physiologic state that mimics pregnancy.
“I’ve spoken to some colleagues here who say that they frequently have this debate of which dosing regimen to go with,” Ms. Band said. “I was pleasantly surprised that there was no significant difference between the two dosing regimens because once-daily dosing is less burdensome for patients and will likely improve compliance and convenience for patients.” An additional benefit of once-daily dosing relates to payers because anecdotal reports suggest insurance companies do not tend to approve twice-daily dosing as readily as once-daily dosing, Ms. Band added.
Ms. Band and her colleagues conducted a retrospective chart review of all patients with hypertensive disorders of pregnancy who were admitted to the Mount Sinai Health System between Jan. 1, 2015, and April 30, 2021, and were prescribed nifedipine in a once-daily (60-mg) or twice-daily (two 30-mg) dose. They excluded patients with renal disease and those already taking hypertensives prior to admission.
Among 237 patients who met the criteria, 59% received 60 mg in a twice-daily 30-mg dose, and 41% received 60 mg in a once-daily dose. Among patients requiring an up titration, two-thirds (67%) needed an increase in the nifedipine dose – the most common adjustment – and 20.7% needed both an increase in nifedipine and an additional medication.
The researchers observed no statistically significant differences in the proportion of patients who required a dose increase or an additional antihypertensive in the group taking the twice-daily dose (33.8%) or those receiving the once-daily dose (35.7%). This finding remained statistically insignificant after controlling for gestational diabetes, delivery mode, administration of Lasix, and receipt of emergency antihypertensive treatment (P = .71). The time that passed before patients needed a dose increase was also statistically similar between the groups: 24.3 hours in the twice-daily group and 24 hours in the once-daily group (P = .49).
There were no statistically significant differences in the need for a dose increase or an additional hypertensive agent based on race, ethnicity, body mass index, or history of preeclampsia as well. However, 24.5% of those taking the once-daily dosage had a history of preeclampsia, compared with 7.2% of those taking the twice-daily dosage (P < .001). Further, the median number of prior pregnancies was two in the twice-daily group versus three in the once-daily group (P = .002).
The authors found no significant difference between the two dosing groups in the need for emergency hypertensive treatment after reaching the study dose or in readmission for blood pressure control. In the twice-daily group, 21.6% of patients needed emergency antihypertensive treatment, compared with 14.3% in the once-daily group (P = .19). Readmission was necessary for 7.2% of the twice-daily group and 6.1% of the once-daily group (P > .99).
A subgroup analysis compared those who started nifedipine antepartum and those who started it post partum, but again, no significant difference in the dosing regimens existed.
Michael Ruma, MD, a maternal-fetal medicine specialist at Perinatal Associates of New Mexico in Albuquerque, was not involved in the study and said he welcomed the results.
“We have too many choices in medicine, so we need to just simplify the plan of attack,” reducing the number of things that clinicians need to think about, Dr. Ruma said in an interview. “A singular dose is always easiest for the patient, always easier for nursing staff, and usually, if you can optimize the dosing, that’s the best approach.”
Annabeth Brewton, MD, a resident at University of Tennessee, Knoxville, agreed, adding that new parents already have a lot going on immediately post partum.
“They’re going to be breastfeeding, they’re not sleeping, they’re going to forget to take that [second] dose,” Dr. Brewton said.
Ms. Band and Dr. Brewton had no disclosures. Dr. Ruma reported consulting and speaking for Hologic and consulting for Philips Ultrasound.
Correction, 5/24/23: An earlier version of this article misstated the daily doses of nifedipine. The study compared a
AT ACOG 2023
Marijuana linked to higher PAD risk
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
But death, intervention rates same
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
AT SCAI 2023
Differences in 30-Day Readmission Rates in Older Adults With Dementia
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
Study 1 Overview (Park et al)
Objective: To compare rates of adverse events and 30-day readmission among patients with dementia who undergo percutaneous coronary intervention (PCI) with those without dementia.
Design: This cohort study used a national database of hospital readmissions developed by the Agency for Healthcare Research and Quality.
Setting and participants: Data from State Inpatient Databases were used to derive this national readmissions database representing 80% of hospitals from 28 states that contribute data. The study included all individuals aged 18 years and older who were identified to have had a PCI procedure in the years 2017 and 2018. International Classification of Diseases, Tenth Revision (ICD-10) codes were used to identify PCI procedures, including drug-eluting stent placement, bare-metal stent placement, and balloon angioplasty, performed in patients who presented with myocardial infarction and unstable angina and those with stable ischemic heart disease. Patients were stratified into those with or without dementia, also defined using ICD-10 codes. A total of 755,406 index hospitalizations were included; 2.3% of the patients had dementia.
Main outcome measures: The primary study outcome was 30-day all-cause readmission, with the cause classified as cardiovascular or noncardiovascular. Secondary outcome measures examined were delirium, in-hospital mortality, cardiac arrest, blood transfusion, acute kidney injury, fall in hospital, length of hospital stay, and other adverse outcomes. Location at discharge was also examined. Other covariates included in the analysis were age, sex, comorbidities, hospital characteristics, primary payer, and median income. For analysis, a propensity score matching algorithm was applied to match patients with and without dementia. Kaplan-Meier curves were used to examine 30-day readmission rates, and a Cox proportional hazards model was used to calculate hazard ratios (HR) for those with and without dementia. For secondary outcomes, logistic regression models were used to calculate odds ratios (OR) of outcomes between those with and without dementia.
Main results: The average age of those with dementia was 78.8 years vs 64.9 years in those without dementia. Women made up 42.8% of those with dementia and 31.3% of those without dementia. Those with dementia also had higher rates of comorbidities, such as heart failure, renal failure, and depression. After propensity score matching, 17,309 and 17,187 patients with and without dementia, respectively, were included. Covariates were balanced between the 2 groups after matching. For the primary outcome, patients with dementia were more likely to be readmitted at 30 days (HR, 1.11; 95% CI, 1.05-1.18; P < .01) when compared to those without dementia. For other adverse outcomes, delirium was significantly more likely to occur for those with dementia (OR, 4.37; 95% CI, 3.69-5.16; P < .01). Patients with dementia were also more likely to die in hospital (OR, 1.15; 95% CI, 1.01-1.30; P = .03), have cardiac arrest (OR, 1.19; 95% CI, 1.01-1.39; P = .04), receive a blood transfusion (OR, 1.17; 95% CI, 1.00-1.36; P = .05), experience acute kidney injury (OR, 1.30; 95% CI, 1.21-1.39; P < .01), and fall in hospital (OR, 2.51; 95% CI, 2.06-3.07; P < .01). Hospital length of stay was higher for those with dementia, with a mean difference of 1.43 days. For discharge location, patients with dementia were more likely to be sent to a skilled nursing facility (30.1% vs 12.2%) and less likely to be discharged home.
Conclusion: Patients with dementia are more likely to experience adverse events, including delirium, mortality, kidney injury, and falls after PCI, and are more likely to be readmitted to the hospital in 30 days compared to those without dementia.
Study 2 Overview (Gilmore-Bykovskyi et al)
Objective: To examine the association between race and 30-day readmissions in Black and non-Hispanic White Medicare beneficiaries with dementia.
Design: This was a retrospective cohort study that used 100% Medicare fee-for service claims data from all hospitalizations between January 1, 2014, and November 30, 2014, for all enrollees with a dementia diagnosis. The claims data were linked to the patient, hospital stay, and hospital factors. Patients with dementia were identified using a validated algorithm that requires an inpatient, skilled nursing facility, home health, or Part B institutional or noninstitutional claim with a qualifying diagnostic code during a 3-year period. Persons enrolled in a health maintenance organization plan were excluded.
Main outcome measures: The primary outcome examined in this study was 30-day all-cause readmission. Self-reported race and ethnic identity was a baseline covariate. Persons who self-reported Black or non-Hispanic White race were included in the study; other categories of race and ethnicity were excluded because of prior evidence suggesting low accuracy of these categories in Medicare claims data. Other covariates included neighborhood disadvantage, measured using the Area Deprivation Index (ADI), and rurality; hospital-level and hospital stay–level characteristics such as for-profit status and number of annual discharges; and individual demographic characteristics and comorbidities. The ADI is constructed using variables of poverty, education, housing, and employment and is represented as a percentile ranking of level of disadvantage. Unadjusted and adjusted analyses of 30-day hospital readmission were conducted. Models using various levels of adjustment were constructed to examine the contributions of the identified covariates to the estimated association between 30-day readmission and race.
Main results: A total of 1,523,142 index hospital stays among 945,481 beneficiaries were included; 215,815 episodes were among Black beneficiaries and 1,307,327 episodes were among non-Hispanic White beneficiaries. Mean age was 81.5 years, and approximately 61% of beneficiaries were female. Black beneficiaries were younger but had higher rates of dual Medicare/Medicaid eligibility and disability; they were also more likely to reside in disadvantaged neighborhoods. Black beneficiaries had a 30-day readmission rate of 24.1% compared with 18.5% in non-Hispanic White beneficiaries (unadjusted OR, 1.37; 95% CI, 1.35-1.39). The differences in outcomes persisted after adjusting for geographic factors, social factors, hospital characteristics, hospital stay factors, demographics, and comorbidities, suggesting that unmeasured underlying racial disparities not included in this model accounted for the differences. The effects of certain variables, such as neighborhood, differed by race; for example, the protective effect of living in a less disadvantaged neighborhood was observed among White beneficiaries but not Black beneficiaries.
Conclusion: Racial and geographic disparities in 30-day readmission rates were observed among Medicare beneficiaries with dementia. Protective effects associated with neighborhood advantage may confer different levels of benefit for people of different race.
Commentary
Adults living with dementia are at higher risk of adverse outcomes across settings. In the first study, by Park et al, among adults who underwent a cardiac procedure (PCI), those with dementia were more likely to experience adverse events compared to those without dementia. These outcomes include increased rates of 30-day readmissions, delirium, cardiac arrest, and falls. These findings are consistent with other studies that found a similar association among patients who underwent other cardiac procedures, such as transcatheter aortic valve replacement.1 Because dementia is a strong predisposing factor for delirium, it is not surprising that delirium is observed across patients who underwent different procedures or hospitalization episodes.2 Because of the potential hazards for inpatients with dementia, hospitals have developed risk-reduction programs, such as those that promote recognition of dementia, and management strategies that reduce the risk of delirium.3 Delirium prevention may also impact other adverse outcomes, such as falls, discharge to institutional care, and readmissions.
Racial disparities in care outcomes have been documented across settings, including hospital4 and hospice care settings.5 In study 2, by Gilmore-Bykovskyi et al, the findings of higher rates of hospital readmission among Black patients when compared to non-Hispanic White patients were not surprising. The central finding of this study is that even when accounting for various levels of factors, including hospital-level, hospital stay–level, individual (demographics, comorbidities), and neighborhood characteristics (disadvantage), the observed disparity diminished but persisted, suggesting that while these various levels of factors contributed to the observed disparity, other unmeasured factors also contributed. Another key finding is that the effect of the various factors examined in this study may affect different subgroups in different ways, suggesting underlying factors, and thus potential solutions to reduce disparities in care outcomes, could differ among subgroups.
Applications for Clinical Practice and System Implementation
These 2 studies add to the literature on factors that can affect 30-day hospital readmission rates in patients with dementia. These data could allow for more robust discussions of what to anticipate when adults with dementia undergo specific procedures, and also further build the case that improvements in care, such as delirium prevention programs, could offer benefits. The observation about racial and ethnic disparities in care outcomes among patients with dementia highlights the continued need to better understand the drivers of these disparities so that hospital systems and policy makers can consider and test possible solutions. Future studies should further disentangle the relationships among the various levels of factors and observed disparities in outcomes, especially for this vulnerable population of adults living with dementia.
Practice Points
- Clinicians should be aware of the additional risks for poor outcomes that dementia confers.
- Awareness of this increased risk will inform discussions of risks and benefits for older adults considered for procedures.
–William W. Hung, MD, MPH
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
1. Park DY, Sana MK, Shoura S, et al. Readmission and in-hospital outcomes after transcatheter aortic valve replacement in patients with dementia. Cardiovasc Revasc Med. 2023;46:70-77. doi:10.1016/j.carrev.2022.08.016
2. McNicoll L, Pisani MA, Zhang Y, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598. doi:10.1034/j.1600-0579.2003.00201.x
3. Weldingh NM, Mellingsæter MR, Hegna BW, et al. Impact of a dementia-friendly program on detection and management of patients with cognitive impairment and delirium in acute-care hospital units: a controlled clinical trial design. BMC Geriatr. 2022;22(1):266. doi:10.1186/s12877-022-02949-0
4. Hermosura AH, Noonan CJ, Fyfe-Johnson AL, et al. Hospital disparities between native Hawaiian and other pacific islanders and non-Hispanic whites with Alzheimer’s disease and related dementias. J Aging Health. 2020;32(10):1579-1590. doi:10.1177/0898264320945177
5. Zhang Y, Shao H, Zhang M, Li J. Healthcare utilization and mortality after hospice live discharge among Medicare patients with and without Alzheimer’s disease and related dementias. J Gen Intern Med. 2023 Jan 17. doi:10.1007/s11606-023-08031-8
HFpEF: New guidelines are pertinent for primary care
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. The incidence of HFpEF is increasing, yet it’s underrecognized. Now that there are evidence-based treatment approaches that improve outcomes, we’ve started to look for this condition and are diagnosing it more often. HFpEF is commonly encountered in primary care.
We should be thinking about HFpEF when we see adults with shortness of breath and/or fatigue and reduced exercise capacity, particularly in the settings of obesity, hypertension, or diabetes. It may not be simple deconditioning; it could be HFpEF.
I’ll organize this discussion into three topics: when to think about HFpEF, how to diagnosis it. and how to treat it.
When to think about HFpEF. When we see a person with risk factors (e.g., older age, obesity, diabetes, hypertension) experiencing dyspnea or fatigue with physical activity, their symptoms are not always from simple deconditioning. HFpEF should be on our differential as well as chronic obstructive pulmonary disease (COPD).
Making the diagnosis. HFpEF is defined as a clinical diagnosis of HR with left ventricular EF (LVEF) greater than 50%. Remember, in HF with reduced EF (HFrEF), the EF is less than 40%, and the EF in midrange HF is 40%-50%. See this recent HF review for more details on reduced and midrange ejection fractions.
For practical purposes, to diagnose HFpEF, check for an elevated N-terminal pro B-type natriuretic peptide (NT-proBNP) (> 125 pg/mL) and evidence of diastolic dysfunction on echocardiogram. Be aware that patients with obesity and HFpEF have lower BNP concentrations than those without obesity, and one professional society has suggested that a 50% reduction in BNP cutoff values should be used when making the diagnosis in patients with obesity.
Of course, we evaluate for other causes of dyspnea and/or edema including lung (most commonly COPD), liver, or kidney disease. When the diagnosis of HFpEF is made, consider whether further evaluation is warranted for specific underlying causes of HFpEF, such as amyloidosis, sarcoid, hemochromatosis, or hypertrophic cardiomyopathy.
Treatment. The evolution of the management of HFpEF has been intriguing. I recommend that people take a look at the guidelines and read the supporting trials. Finding effective therapies has taken longer than it did for HFrEF, but finally, an effective therapy for HFpEF is available.
To quote the guidelines, diuretics should be used “judiciously as needed” to reduce pulmonary congestion and improve symptoms. But here’s the big deal. The mainstays of treatment for HFpEF are the sodium-glucose cotransporter 2 (SGLT2) inhibitors on the basis of the findings of two trials: DELIVER (dapagliflozin) and EMPEROR-Preserved (empagliflozin), both of which have shown very impressive levels of benefit.
Both trials lasted a little over 2 years and found a statistically significant approximately 30% decline in HF hospitalizations and a numerical reduction of about 10% in cardiovascular death, which was statistically significant in meta-analysis. That’s over 2 years! That’s a large level of effect. They also showed improvements in symptoms and health status. Therefore, SGLT2 inhibitors are first-line treatment for all individuals with HFpEF, currently graded as a Class 2a (moderate) recommendation, but likely soon to be upgraded to Class 1 (strong) recommendation.
After the SGLT2 inhibitors, treatment is based on evidence which is not as strong and the recommendations are graded as Class 2b (weak) recommendations. In men with an LVEF less than 55%-60% and for women with any EF, use of a mineralocorticoid antagonist (MRA), an angiotensin receptor-neprilysin inhibitor, or if an ARN inhibitor is not feasible, an angiotensin receptor blocker (ARB) may be considered.
Nonpharmacologic management is also important. Exercise and weight loss (if the patient is overweight) can improve symptoms and quality of life. A new intervention, an implantable ambulatory pulmonary artery sensor, called CardioMEMS, has been evaluated in two trials, showing a decrease in HF hospitalizations. This may be considered for those who experience hospitalizations for HF and continue to experience New York Heart Association functional Class 3 symptoms despite optimal guideline-directed medical therapy or those who have lability in volume status or other medical problems (such as obesity or COPD) that make it difficult to tell whether their symptoms are from HFpEF or a comorbid condition.
In summary:
- Have a low threshold to evaluate for HFpEF in any patients who have shortness of breath, fatigue with exertion, or fluid overload.
- Initially evaluate with an NT-proBNP level and an echocardiogram.
- First-line treatment is an evidence-based SGLT2 inhibitor along with exercise and perhaps weight loss if needed. A loop diuretic can be used as needed to control volume status. Then you can consider, based on symptoms and details discussed above, an MRA, ARN inhibitor, or ARB.
This is important information for a diagnosis that is common in primary care, HFpEF, and for which we now have impressive, effective treatment.
Dr. Skolnik is a professor in the department of family medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the department of family medicine at Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim; Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.







