User login
TAVR feasible, comparable with surgery in rheumatic heart disease
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
FDA okays transcatheter pulmonary valve for congenital heart disease
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved Medtronic’s Harmony Transcatheter Pulmonary Valve (TPV) System to treat severe pulmonary regurgitation in pediatric and adult patients who have a native or surgically repaired right ventricular outflow tract (RVOT).
The Harmony TPV is the first nonsurgical heart valve to treat severe pulmonary valve regurgitation, which is common in patients with congenital heart disease, the agency said in a news release. Its use can delay the time before a patient needs open-heart surgery and potentially reduce the number of these surgeries required over a lifetime.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease,” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said in the statement.
“It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease,” he said.
The Harmony valve, which was granted breakthrough device designation, is a 22-mm or 25-mm porcine pericardium valve, sewn to a nitinol frame. It is implanted with a 25-French delivery system using a coil-loading catheter.
The FDA approval was based on the 70-patient prospective, nonrandomized, multicenter Harmony TPV Clinical study, in which 100% of patients achieved the primary safety endpoint of no procedure or device-related deaths 30 days after implantation.
Among 65 patients with evaluable echocardiographic data, 89.2% met the primary effectiveness endpoint of no additional surgical or interventional device-related procedures and acceptable heart blood flow at 6 months.
Adverse events included irregular or abnormal heart rhythms in 23.9% of patients, including 14.1% ventricular tachycardia; leakage around the valve in 8.5%, including 1.4% major leakage; minor bleeding in 7.0%, narrowing of the pulmonary valve in 4.2%, and movement of the implant in 4.2%.
Follow-up was scheduled annually through 5 years and has been extended to 10 years as part of the postapproval study, the FDA noted.
The Harmony TPV device is contraindicated for patients with an infection in the heart or elsewhere, for patients who cannot tolerate blood-thinning medicines, and for those with a sensitivity to nitinol (titanium or nickel).
A version of this article first appeared on Medscape.com.
BASILICA technique prevents TAVR-related coronary obstruction in registry study
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
For patients undergoing transcatheter aortic valve replacement (TAVR), the intentional laceration technique of diseased valve leaflets called BASILICA is effective and reasonably safe for preventing coronary artery obstruction, according to a late-breaking study presented at CRT 2021 sponsored by MedStar Heart & Vascular Institute.
In a series of 214 patients entered into a registry over a recent 30-month period, leaflets posing risk were effectively traversed with the technique in 95% of cases, and complication rates were reasonably low with 30-day stroke and death rate of 3.4%, reported Jaffar M. Khan, BMBCH, PhD, cardiovascular branch of the National Heart, Lung, and Blood Institute.
The rate of complications is acceptable given the large potential risk, according to Dr. Khan. If coronary obstruction occurs, reported mortality rates have been as high as 50%. The 1-year survival rate in the registry following BASILICA was 84%.
Results should ‘push people toward BASILICA’
The acronym BASILICA stands for bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction. In the procedure, performed immediately before TAVR, guidewires are introduced to first traverse and then lacerate aortic leaflets threatening obstruction of a coronary artery.
In cases where diseased valve leaflets pose a risk of coronary obstruction, most interventionalists “are comfortable with surgery when patients are at low or intermediate risk, but the choices for high-risk patients are a snorkel stent or BASILICA. Given the limits of snorkel stenting, these data should be reassuring and push people toward BASILICA,” Dr. Khan said.
The 214 patients were entered into the registry from June 2015 to December 2020. The mean age was 74.9 years. Of valves treated, 73% were failed bioprosthetic devices. The remaining were native aortic valves. Solo BASILICA was performed in most patients, but 21.5% underwent a doppio procedure, meaning the laceration of two leaflets.
Despite BASILICA, 10 patients (4.7%) had some degree of coronary obstruction, including 5 with partial obstruction of the main coronary artery and 1 with partial obstruction of the right coronary artery. All of these partial obstructions were successfully treated with orthotopic stents.
An obstruction of the right coronary artery was successfully treated with balloon angioplasty. Another patient with significant left main coronary artery obstruction required cardiopulmonary bypass but was successfully treated with snorkel stenting. Of two patients with complete obstruction of the left main coronary artery caused by the skirt of the TAVR device, one died in hospital despite several maneuvers to restore perfusion.
Procedural complications included a mitral chord laceration, which subsequently led to valve replacement, and three guidewire transversals into surrounding tissue that did not result in serious sequelae. Hypotension requiring pressors occurred in 8.5%.
There was a “slight trend” for worse outcomes in those undergoing doppio rather than solo BASILICA, but the difference did not reach statistical significance. Cerebral embolic protection was offered to a minority of patients in this series. The trend for a lower risk of stroke in this group did not reach significance, Dr. Khan reported.
Best for high-volume centers, for now
Although these data support the conclusion that BASILICA “is feasible in a real-world setting,” Dr. Khan acknowledged that BASILICA might not be appropriate at low-volume centers. Dr. Khan cited data that indicates obstruction of a coronary artery by a diseased leaflet occurs in less than 1% of TAVR cases.
“Not every site doing a handful of TAVRs is going to want to tackle these cases, but those working in a high-volume center will from time to time encounter patients with coronary obstruction or who are at increased risk,” Dr. Khan said.
In North America, there has been a proctoring program to disseminate the skills required to perform BASILICA, according to Dr. Khan, who explained that proctors typically participate in two or three cases before these are performed without supervision.
So far, the uptake of BASILICA has been limited.
“BASILICA has not been catching on in EUROPE,” said Didier F. Loulmet, MD, chief of cardiac surgery at Tisch Hospital, New York University Langone Health. There might be several reasons, but Dr. Loulmet said that lack of a comparable proctoring program is one factor.”
“This is a relatively complex procedure performed in a small number of patients, so building up expertise is quite a challenge, particularly in small centers,” he added. He encouraged proctoring as “the way that it has to be propagated.”
The results presented by Dr. Khan on March 6 at CRT 2021 were simultaneously published in JACC: Cardiovascular Interventions.
Dr. Khan has patents on several devices, including catheters to lacerate valve leaflet. Dr. Loulmet reported no potential conflicts of interest.
FROM CRT 2021
DOACs offered after heart valve surgery despite absence of data
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
Direct oral anticoagulants (DOACs) are used in about 1% of patients undergoing surgical mechanical aortic and mitral valve replacement, but in up to 6% of surgical bioprosthetic valve replacements, according to registry data presented at CRT 2021.
In an analysis of the Society of Thoracic Surgery (STS) registry during 2014-2017, DOAC use increased steadily among those undergoing surgical bioprosthetic valve replacement, reaching a number that is potentially clinically significant, according to Ankur Kalra, MD, an interventional cardiologist at Akron General Hospital who has an academic appointment at the Cleveland Clinic.
There was no increase in the use of DOACs observed among patients undergoing mechanical valve replacement, “but even if the number is 1%, they should probably not be used at all until we accrue more data,” Dr. Kalra said.
DOACs discouraged in patients with mechanical or bioprosthetic valves
In Food and Drug Administration labeling, DOACs are contraindicated or not recommended. This can be traced to the randomized RE-ALIGN trial, which was stopped prematurely due to evidence of harm from a DOAC, according to Dr. Kalra.
In RE-ALIGN, which enrolled patients undergoing mechanical aortic or mitral valve replacement, dabigatran was associated not only with more bleeding events than warfarin, but also more thromboembolic events.
There are no randomized data comparing the factor Xa inhibitors rivaroxaban or apixaban to warfarin in heart valve surgery, but Dr. Kalra noted cautionary language is found in the labeling of both, “perhaps due to the RE-ALIGN data.”
Registry shows trends in prescribing
In the STS registry data, 193 (1.1%) of the 18,142 patients undergoing mechanical aortic valve surgery, 139 (1.0%) of the 13,942 patients undergoing mechanical mitral valve surgery, 5,625 (4.7%) of the 116,203 patients undergoing aortic bioprosthetic aortic valve surgery, and 2,180 (5.9%) of the 39,243 patients undergoing bioprosthetic mitral valve surgery were on a DOAC at discharge.
Among those receiving a mechanical value and placed on a DOAC, about two-thirds were on a factor Xa inhibitor rather than dabigatran. For those receiving a bioprosthetic value, the proportion was greater than 80%. Dr. Kalra speculated that the RE-ALIGN trial might be the reason factor Xa inhibitors were favored.
In both types of valves, whether mechanical or bioprosthetic, more comorbidities predicted a greater likelihood of receiving a DOAC rather than warfarin. For those receiving mechanical values, the comorbidities with a significant association with greater DOAC use included hypertension (P = .003), dyslipidemia (P = .02), arrhythmia (P < .001), and peripheral arterial disease (P < 0.001).
The same factors were significant for predicting increased likelihood of a DOAC following bioprosthetic valve replacement, but there were additional factors, including atrial fibrillation independent of other types of arrhythmias (P < .001), a factor not significant for mechanical valves, as well as diabetes (P < .001), cerebrovascular disease (P < .001), dialysis (P < .001), and endocarditis (P < .001).
“This is probably intuitive, but patients who were on a factor Xa inhibitor before their valve replacement were also more likely to be discharged on a factor Xa inhibitor,” Dr. Kalra said at the virtual meeting, sponsored by MedStar Heart & Vascular Institute.
The year-to-year increase in DOAC use among those undergoing bioprosthetic valve replacement over the study period, which was a significant trend, was not observed among those undergoing mechanical valve replacement. Rather, the 1% proportion remained stable over the study period.
“We wanted to look at outcomes, but we found that the STS database, which only includes data out to 30 days, is not structured for this type of analysis,” Dr. Kalra said. He was also concerned about the limitations of a comparison in which 1% of the sample was being compared to 99%.
Expert: One percent is ‘very small number’
David J. Cohen, MD, commented on the 1% figure, which was so low that a moderator questioned whether it could be due mostly to coding errors.
“This is a very, very small number so at some level it is reassuring that it is so low in the mechanical valves,” Dr. Cohen said. However, he was more circumspect about the larger number in bioprosthetic valves.
“I have always thought it was a bit strange there was a warning against using them in bioprosthetic valves, especially in the aortic position,” he said.
“The trials that established the benefits of DOACs were all in nonvalvular atrial fibrillation, but this did not mean non–aortic stenosis; it meant non–mitral valvular. There have been articles written about how that has been misinterpreted,” said Dr. Cohen, director of clinical and outcomes research at the Cardiovascular Research Foundation and director of academic affairs at St. Francis Hospital, Roslyn, N.Y.
For his part, Dr. Kalra reported that he does not consider DOACs in patients who have undergone a surgical mechanical valve replacement. For bioprosthetic valves, he “prefers” warfarin over DOACs.
Overall, the evidence from the registry led Dr. Kalra to suggest that physicians should continue to “exercise caution” in using DOACs instead of warfarin after any surgical valve replacement “until randomized clinical trials provide sufficient evidence” to make a judgment about relative efficacy and safety.
Results of the study were published online as a research letter in Jama Network Open after Dr. Kalra’s presentation. Dr. Kalra and Dr. Cohen report no potential conflicts of interest.
FROM CRT 2021
Is the tide turning on the ‘grubby’ affair of EXCEL and the European guidelines?
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
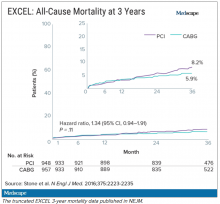
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
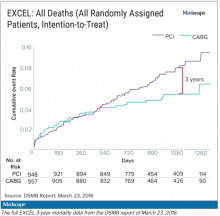
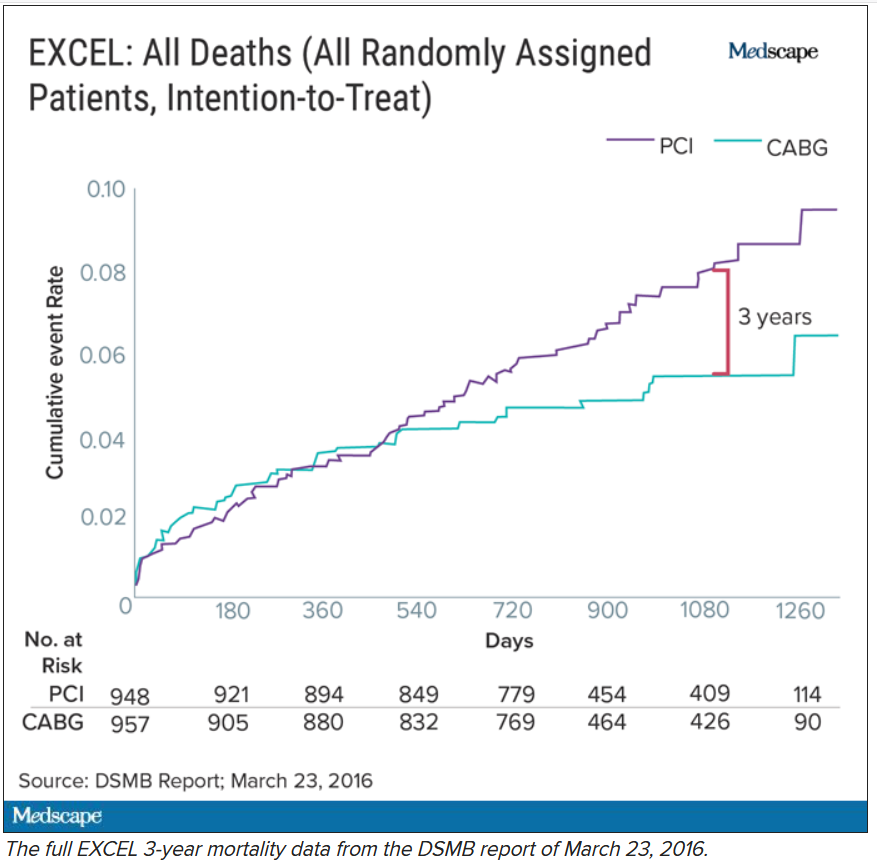
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
“I disapprove of what you say, but I will defend to the death your right to say it.” The choice of the secretary general of the European Association for Cardio-Thoracic Surgery to open with this quote was the first hint that the next presentation at the 2019 annual meeting would be anything but dull. The session chair followed with a reminder to keep the discussion polite and civil.
Presenter David Taggart, MD, PhD, did not disappoint. The professor of cardiovascular surgery at the University of Oxford (England) began with the announcement that he had withdrawn his name from a recent paper in the New England Journal of Medicine. He then proceeded to accuse his coinvestigators of misrepresenting the findings of a major clinical trial.
Dr. Taggart was chair of the surgical committee for the Abbott-sponsored EXCEL trial, which compared two procedures for patients who had blockages in their left main coronary artery: percutaneous coronary intervention (PCI) using coronary stents, and coronary artery bypass graft surgery (CABG). The investigators designed the trial to compare outcomes for the two treatments using a composite endpoint of death, stroke, and MI. The 3-year follow-up data had been published in NEJM without controversy – or, at least, without public controversy.
But when it came time to publish the 5-year follow-up, there was a significantly higher rate of death in the stent group, and both Dr. Taggart and the journal editors were concerned that this finding was being downplayed in the manuscript.
In their comments to the authors, the journal editors had recommended including the mortality difference (unless clearly trivial) ‘”in the concluding statement in the final paragraph.” Yet, the concluding statement of the published paper read that there “was no significant difference between PCI and CABG.”
In Dr. Taggart’s view, that claim was dangerous for patients, and so he was left with no choice but to remove himself as an author, a first for the academic with over 300 scientific papers to his name.
Earlier publications from the EXCEL trial had influenced European treatment guidelines. But subsequent allegations of misconduct and hidden data spurred the EACTS to repudiate those guidelines out of concern “that some results in the EXCEL trial appear to have been concealed and that some patients may therefore have received the wrong clinical advice.”
The controversy pitted cardiothoracic surgeons against interventional cardiologists, who were seen as increasingly encroaching on the surgeons’ turf. Dr. Taggart was a long-time critic of the subspecialty.
Surgeons demanded an independent analysis of the EXCEL trial data – a demand that the investigators have yet to satisfy. Dr. Taggart was the first to speak publicly, but others had major reservations about the trial reporting and conduct years earlier.
Mortality data held back
One such person was Lars Wallentin, MD, a professor of cardiology at Uppsala (Sweden) University Hospital, who chaired the independent committee that monitored the safety and scientific validity of the EXCEL trial.
The committee, known as the data and safety monitoring board (DSMB), received a report on March 23, 2016, that showed that increasingly more patients who had received stents were dying, compared with the group of patients that had undergone CABG. A graph of the survival curves showed the gap between the two groups widening after 3 years (Figure 1).
By September of that year, Dr. Wallentin and other members of the DSMB were anxious to share the concerning mortality difference with the broader medical community.
They were aware that EACTS and the European Society of Cardiology had started the process of updating their guidelines on myocardial revascularization, and were keen for the guideline writing committee to see all of the data.
Meanwhile, the trial investigators, led by principal investigator Gregg Stone, MD, then at New York–Presbyterian Hospital and Columbia University Medical Center, were preparing to publish a report of the 3-year outcomes. Recruitment for EXCEL started in September 2010, so at the time of the 3-year analysis in 2016, some patients had been followed up for over 5 years. But the data, published in NEJM in October 2016, were capped at 3 years (Figure 2). It didn’t show the widening gap in late mortality that Dr. Wallentin and the rest of the DSMB had seen.
When asked about this, the investigators said they were transparent about their plans to cap the data at 3 years in an amendment to the study protocol. Stone’s coprincipal investigators were interventional cardiologist Patrick Serruys, MD, then of Imperial College London; and two surgeons: Joseph Sabik, MD, then of the Cleveland Clinic Foundation, and A. Pieter Kappetein, MD, PhD, then at Erasmus Medical Center, Rotterdam. The four principal investigators all declared financial payments from stent manufacturers either to themselves or their institutions.
Study sponsor Abbott has distanced itself from the decisions made and has referred all questions about the trial to the EXCEL investigators. Charles Simonton, chief medical officer at Abbott (now at Abiomed) was a coauthor on both the 3- and 5-year papers. Dr. Wallentin believes that the sponsor must have been aware of the DSMB’s concerns.
Continuing DSMB concerns
A year later, the DSMB was still troubled. Dr. Wallentin emailed Dr. Stone in September 2017 asking for an updated analysis of the mortality data without any capping in time.
Dr. Wallentin added that he didn’t think that unblinding the mortality results would be an issue at that stage because these were late deaths in a trial where the interventions were long completed. But, he warned, “it might be very concerning if, in the future, suspicions were raised that already available information on mortality was withheld from the cardiology and thoracic surgery community.”
The investigators took a month to respond. They declined the request, saying that the trial was not statistically powered to measure mortality. In his email to Dr. Wallentin, Dr. Stone stressed that they were committed to complete disclosure of all of the EXCEL data and that the responsible time point to unblind was after 4 years. His coprincipal investigators (Dr. Serruys, Dr. Sabik, and Dr. Kappetein) as well as EXCEL statistical committee chair Stuart Pocock, PhD, and Mr. Simonton were all copied on the email.
Dr. Wallentin deferred to the principal investigators’ arguments.
Missing MI data
Death was not the only outcome of the EXCEL trial to draw scrutiny.
The EXCEL investigators used a unique definition of MI that was almost exclusively based on a rise in the cardiac biomarker CK-MB. This protocol definition of MI was later adapted into the Society for Cardiovascular Angiography and Interventions definition in a paper coauthored by Dr. Stone. The investigators agreed to also measure MIs that met the more commonly used Third Universal Definition as a secondary endpoint. The Third Universal Definition of Myocardial Infarction uses a change in biomarkers – preferably troponin or alternatively CK-MB – coupled with other clinical signs.
It is standard practice to report secondary endpoints in any analysis of the main findings of a study. Yet, the EXCEL investigators did not report the universal definition of MI in either the 3-year or 5-year publications.
This is critical because MI according to one definition may not count according to the other, and the final tally could tip the trial results positive, negative, or neutral for coronary stents.
In Dr. Taggart’s opinion, the protocol definition puts CABG at a disadvantage because it uses the same biomarker threshold for procedural-related MI for both PCI and CABG. Because surgery involves more manipulation of the heart, cardiac enzyme levels will naturally be higher after CABG than PCI. These procedure-related enzyme elevations are not “true clinical MIs,” according to Dr. Taggart and others.
Late last year, a dataset containing the 3-year follow-up of EXCEL, including the information on the universal definition of MI, was leaked to the BBC. Working with biostatisticians, the BBC confirmed that according to this definition, there were more MIs in the stent group.
Originally, the investigators disputed the finding, calling the BBC data “imaginary.” They claimed that they were unable to calculate a rate of MI according to the universal definition because they lacked routine collection of troponins, although the universal definition also allows use of CK-MB. They have since published an analysis of 5-year MI data according to the universal definition, which showed twice the rate of MI in the PCI group.
From the leaked data, the BBC calculated the main composite endpoint of death, stroke, and MI using the universal definition of MI. Now the results swung in favor of CABG.
Impact on guidelines
None of this was known at the time the European cardiology societies convened a committee to write their new guidelines on myocardial revascularization. The writing panel disagreed about whether PCI and CABG were equivalent for patients with left main coronary artery disease (CAD).
Besides EXCEL, another study, the NOBLE trial, compared PCI and CABG in left main CAD and came to opposite conclusions – conclusions that matched the leaked data. In that trial, European investigators chose a slightly different primary endpoint: a composite of death, MI, stroke, and the need for a repeat procedure. They used the universal definition of MI exclusively, and notably, they omitted procedural MI from their clinical event count. The results, published at the same time as the EXCEL 3-year findings, suggested that CABG was better.
Given the discrepant findings of two large trials, the guideline committee considered all of the available data comparing the two methods of revascularization for left main CAD. But even then, things weren’t clear-cut. One draft meta-analysis, supported by the National Institute for Health Research, suggested that results were worse for first- and second-generation drug-eluting coronary stents – including those used in EXCEL – compared with surgery.
Another meta-analysis, later published in The Lancet, drew a different conclusion and found that PCI was just as good as surgery. The main author, Stuart Head, a cardiothoracic surgeon on the ESC/EACTS guideline committee, was a research fellow with EXCEL investigator Dr. Kappetein at Erasmus. EXCEL investigators Dr. Stone, Dr. Kappetein, and Dr. Serruys were coauthors of the Lancet meta-analysis.
There was heated discussion about the committee’s draft recommendations, which gave both CABG and PCI a Class IA recommendation in patients with left main CAD and low anatomical complexity. In October 2017, the ESC commissioned an anonymous external reviewer to weigh in. James Brophy, MD, PhD, a cardiologist and professor of medicine and epidemiology at McGill University, Montreal, confirmed that he was the reviewer after he published an updated version in June 2020.
Looking at all of the data available at the time comparing the procedures for left main CAD, Dr. Brophy’s analysis suggested a 73% chance that the excess in death, stroke, or MI represents at least two excess events per 100 patients treated with PCI rather than CABG.
Dr. Brophy thought that most patients would find these differences clinically meaningful and advised against giving both procedures the same class of recommendation. He was also concerned that many readers will skip to the summary recommendation table without reading the entire guideline document.
“I feel this is misleading in its present form,” he wrote in 2017.
Despite Dr. Brophy’s review, the guideline committee stuck with its original recommendations. The final 2018 ESC/EACTS Guidelines on myocardial revascularization gave equal weight to both CABG and PCI in patients with left main CAD and low anatomical complexity. In contrast, US guidelines do not put PCI and CABG on the same footing for any group of patients with left main CAD.
The lead author of the ESC/EACTS guidelines section on left main disease, and around a third of those on the writing task force, all declared financial payments from stent manufacturers either to themselves or their institutions. The EXCEL principal investigator, Dr. Kappetein, was secretary general of EACTS and oversaw the guidelines process for the surgical organization. He left to work for Medtronic midway through the process and was later joined there by his former research fellow, Stuart Head.
Dr. Brophy said in an interview that given the final guideline recommendations, he assumed that the committee had other reviews and went with the majority opinion.
But not everyone involved in the guidelines saw Dr. Brophy’s review. Nick Freemantle, a statistical reviewer appointed by EACTS, expected to see it but didn’t. This omission calls into question the neutrality of the whole process, in his view.
Mr. Freemantle believes that the deck was stacked so that he only saw the pieces of evidence that supported the conclusions that were already decided and that he was not shown “the bits that don’t fit that neatly.”
“And without that narrative, it all feels a bit grubby, to be honest,” he said.
Professor Barbara Casadei, ESC president, disputed this, saying that the guidelines were approved by all surgical members, including the EACTS council.
Missing from Dr. Brophy’s review were the later data from EXCEL. As he had told the DSMB in 2017, Stone presented the 4-year data from EXCEL at the TCT conference in September 2018. At this point, the analysis showed that 10.3% of people had died after PCI and 7.4% after CABG.
But this presentation was not given much prominence at the conference, which Dr. Stone organized, and occurred during a didactic session in a small room rather than on one of the main stages where the 3-year data from EXCEL were announced with much fanfare. The presentation also took place 3 weeks after the European guidelines were published.
Surgeons withdraw support
After the BBC report last year that the universal definition of MI data had been collected but not published in the 3-year follow-up manuscript, and showed more MI in the PCI group than the protocol definition, the EACTS withdrew its support for the guidelines. The ESC continued to uphold the guidelines «until there is robust scientific evidence (as opposed to allegations) indicating we should do otherwise,” said Ms. Casadei.
A spokesperson for NEJM said the journal stood by the EXCEL papers because “there is no credible harm to patients from the publication of the paper and accurate reporting of trial results.” NEJM has since conducted a review and published a series of letters in response. The letters have reinvigorated rather than appeased the dissenters, as reported by Medscape.
A number of cardiologists and researchers started a petition on change.org to revise the EACTS/ESC left main CAD guidelines, and surgical societies across the globe have written to the editor of NEJM asking him to retract or amend the EXCEL papers.
This has not happened. The journal’s editor maintains that the letters containing the analyses are “sufficient information” to allow readers and guideline authors to “evaluate the trial findings.”
Dr. Taggart was dismissive of that response. “There is still no recognition or acknowledgment that failure to publish these data in 2016 ‘misled’ the guideline writers for the ESC/EACTS guidelines, and there is still no formal correction of the 2016 and 2019 NEJM manuscripts.”
Over a year after the BBC received the leaked data, the EXCEL investigators published an analysis of the primary outcome using the universal definition of MI data in the Journal of the American College of Cardiology.
It shows 141 events in the PCI arm, compared with 102 in the CABG arm. The investigators acknowledge that the rates of procedural MI differ depending on the definition used. According to their analysis, the protocol definition was predictive of mortality after both treatments, whereas the universal definition of procedural MI was predictive of mortality only after CABG. Not everyone agrees with this interpretation, and an accompanying editorial questioned these conclusions.
For Dr. Wallentin, it’s a relief that these data are in the public domain so that their interpretation and clinical consequences can be “openly discussed.” He hoped that the whole experience will result in something constructive and useful for the future.
As for the guidelines, the tide may be turning.
In a joint statement with EACTS on Oct. 6, 2020, the ESC agreed to review its guidelines for left main disease in the light of emerging, longer-term outcome data from the trials of CABG versus PCI.
Dr. Taggart has no regrets about speaking out despite this being “an exceedingly painful and bruising experience.”
The saga, he said, “reflects very badly on our specialty, the investigators, industry, and the world’s ‘leading’ medical journal.”
This article first appeared on Medscape.com.
Post-PCI mortality higher in Blacks vs. Whites, regardless of comorbidities
A combined analysis of 10 prospective trials, intended to shed light on racial disparities in percutaneous coronary intervention (PCI) outcomes, saw sharply higher risks of death and myocardial infarction (MI) for Blacks compared with Whites.
The burden of comorbidities, including diabetes, was greater for Hispanics and Blacks, compared with Whites, but only in Blacks were PCI outcomes significantly worse even after controlling for such conditions and other baseline risk factors.
The analysis based on more than 22,000 patients was published July 6 in JACC: Cardiovascular Interventions,with lead author Mordechai Golomb, MD, Cardiovascular Research Foundation, New York.
In the study based on patient-level data from the different trials, the adjusted risk of MI after PCI was increased 45% at 1 year and 55% after 5 years for Blacks, compared with Whites. Their risk of death at 1 year was doubled, and their risk of major adverse cardiac events (MACE) was up by 28% at 5 years.
“Improving health care and outcomes for minorities is essential, and we are hopeful that our work may help direct these efforts, senior author Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“But this won’t happen without active, concerted efforts to promote change and opportunity, a task for government, regulators, payers, hospital administrators, physicians, and all health care providers,” he said. “Understanding patient outcomes according to race and ethnicity is essential to optimize health for all patients,” but “most prior studies in this regard have looked at population-based data.”
In contrast, the current study used hospital source records – which are considered more accurate than administrative databases – and event coding reports, Dr. Stone said, plus angiographic core laboratory analyses for all patients, which allows “an independent assessment of the extent and type of coronary artery disease and procedural outcomes.”
The analysis “demonstrated that even when upfront treatments are presumably similar [across racial groups] in a clinical trial setting, longitudinal outcomes still differ by race,” Michael Nanna, MD, said in an interview.
The “troubling” results “highlight the persistence of racial disparities in health care and the need to renew our focus on closing these gaps [and] is yet another call to action for clinicians, researchers, and the health care system at large,” said Dr. Nanna, of Duke University Medical Center, Durham, N.C., and lead author on an editorial accompanying the published analysis.
Of the 10 randomized controlled trials included in the study, which encompassed 22,638 patients, 9 were stent comparisons and 1 compared antithrombotic regimens in patients with acute coronary syndromes (ACS), the authors noted. The median follow-up was about 1,100 days.
White patients made up 90.9% of the combined cohort, Black patients comprised 4.1%, Hispanics 2.1%, and Asians 1.8% – figures that “confirm the well-known fact that minority groups are underrepresented in clinical trials,” Dr. Stone said.
There were notable demographic and clinical differences at baseline between the four groups.
For example, Black patients tended to be younger than White, Hispanic, and Asian patients. Black and Hispanic patients were also less likely to be male, compared with White patients.
Both Black and Hispanic patients had more comorbidities than Whites did at baseline, the authors observe. For example, Black and Hispanic patients had a greater body mass index, compared with Whites, whereas it was lower for Asians; and they had more diabetes and more hypertension than Whites (P < .0001 for all differences). Hispanics were more likely to have ACS at baseline, compared with Whites, and less likely to have stable coronary artery disease (CAD) (P < .0001 for all differences). Similar proportions of Blacks and of Whites had stable CAD (about 32% of each) and ACS (about 68% in both cases). Rates of hyperlipidemia and stable CAD were greater and rates of ACS was lower in Asians than the other three race groups (P < .0001 for each difference). In adjusted analysis, the risk of MACE at 5 years was significantly increased for Blacks, compared with Whites (hazard ratio, 1.28; 95% CI, 1.05-1.57; P = .01). The same applied to MI (HR, 1.55; 95% CI, 1.15-2.09; P = .004). At 1 year, Blacks showed higher risks for death (HR, 2.06; 95% CI, 1.26-3.36; P = .004) and for MI (HR, 1.45; 95% CI, 1.01-2.10; P = .045), compared with Whites.
No significant increases in risk for outcomes at 1 and 5 years were seen for Hispanics or Asians, compared with Whites.
Covariates in the analyses included age, sex, body mass index, diabetes, current smoking, hypertension, hyperlipidemia, history of MI or coronary revascularization, clinical CAD presentation, category of stent, and race stratified by study.
Even with underlying genotypic differences between Blacks and Whites, much of the difference in risk for outcomes “should have been accounted for when the researchers adjusted for these clinical phenotypes,” the editorial notes.
Some of the difference in risk must have derived from uncontrolled-for variables, and “[b]eyond genetics, it is clear that race is also a surrogate for other socioeconomic factors that influence both medical care and patient outcomes,” the editorialists wrote.
The adjusted analysis, noted Golomb et al, suggests “that for Hispanic patients, the excess risk for adverse clinical outcomes may have been attributable to a higher prevalence of risk factors. In contrast, the excess risk for adverse clinical outcomes for Black patients persisted even after adjustment for baseline risk factors.”
As such, they agreed: “The observed increased risk may be explained by differences that are not fully captured in traditional cardiovascular risk factor assessment, including socioeconomic differences and education, treatment compliance rates, and yet-to-be-elucidated genetic differences and/or other factors.”
Dr. Stone said that such socioeconomic considerations may include reduced access to care and insurance coverage; lack of preventive care, disease awareness, and education; delayed presentation; and varying levels of provided care.
“Possible genetic or environmental-related differences in the development and progression of atherosclerosis and other disease processes” may also be involved.
“Achieving representative proportions of minorities in clinical trials is essential but has proved challenging,” Dr. Stone said. “We must ensure that adequate numbers of hospitals and providers that are serving these patients participate in multicenter trials, and trust has to be developed so that minority populations have confidence to enroll in studies.”
Dr. Stone reported holding equity options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestro Biomed, Aria, Cardiac Success, the MedFocus family of funds, and Valfix and receiving consulting fees from Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore Ablative Solutions, Miracor, Neovasc, W-Wave, Abiomed, and others. Disclosures for the other authors are in the report. Nanna reports no relevant financial relationships; other coauthor disclosures are provided with the editorial.
A version of this article originally appeared on Medscape.com.
A combined analysis of 10 prospective trials, intended to shed light on racial disparities in percutaneous coronary intervention (PCI) outcomes, saw sharply higher risks of death and myocardial infarction (MI) for Blacks compared with Whites.
The burden of comorbidities, including diabetes, was greater for Hispanics and Blacks, compared with Whites, but only in Blacks were PCI outcomes significantly worse even after controlling for such conditions and other baseline risk factors.
The analysis based on more than 22,000 patients was published July 6 in JACC: Cardiovascular Interventions,with lead author Mordechai Golomb, MD, Cardiovascular Research Foundation, New York.
In the study based on patient-level data from the different trials, the adjusted risk of MI after PCI was increased 45% at 1 year and 55% after 5 years for Blacks, compared with Whites. Their risk of death at 1 year was doubled, and their risk of major adverse cardiac events (MACE) was up by 28% at 5 years.
“Improving health care and outcomes for minorities is essential, and we are hopeful that our work may help direct these efforts, senior author Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“But this won’t happen without active, concerted efforts to promote change and opportunity, a task for government, regulators, payers, hospital administrators, physicians, and all health care providers,” he said. “Understanding patient outcomes according to race and ethnicity is essential to optimize health for all patients,” but “most prior studies in this regard have looked at population-based data.”
In contrast, the current study used hospital source records – which are considered more accurate than administrative databases – and event coding reports, Dr. Stone said, plus angiographic core laboratory analyses for all patients, which allows “an independent assessment of the extent and type of coronary artery disease and procedural outcomes.”
The analysis “demonstrated that even when upfront treatments are presumably similar [across racial groups] in a clinical trial setting, longitudinal outcomes still differ by race,” Michael Nanna, MD, said in an interview.
The “troubling” results “highlight the persistence of racial disparities in health care and the need to renew our focus on closing these gaps [and] is yet another call to action for clinicians, researchers, and the health care system at large,” said Dr. Nanna, of Duke University Medical Center, Durham, N.C., and lead author on an editorial accompanying the published analysis.
Of the 10 randomized controlled trials included in the study, which encompassed 22,638 patients, 9 were stent comparisons and 1 compared antithrombotic regimens in patients with acute coronary syndromes (ACS), the authors noted. The median follow-up was about 1,100 days.
White patients made up 90.9% of the combined cohort, Black patients comprised 4.1%, Hispanics 2.1%, and Asians 1.8% – figures that “confirm the well-known fact that minority groups are underrepresented in clinical trials,” Dr. Stone said.
There were notable demographic and clinical differences at baseline between the four groups.
For example, Black patients tended to be younger than White, Hispanic, and Asian patients. Black and Hispanic patients were also less likely to be male, compared with White patients.
Both Black and Hispanic patients had more comorbidities than Whites did at baseline, the authors observe. For example, Black and Hispanic patients had a greater body mass index, compared with Whites, whereas it was lower for Asians; and they had more diabetes and more hypertension than Whites (P < .0001 for all differences). Hispanics were more likely to have ACS at baseline, compared with Whites, and less likely to have stable coronary artery disease (CAD) (P < .0001 for all differences). Similar proportions of Blacks and of Whites had stable CAD (about 32% of each) and ACS (about 68% in both cases). Rates of hyperlipidemia and stable CAD were greater and rates of ACS was lower in Asians than the other three race groups (P < .0001 for each difference). In adjusted analysis, the risk of MACE at 5 years was significantly increased for Blacks, compared with Whites (hazard ratio, 1.28; 95% CI, 1.05-1.57; P = .01). The same applied to MI (HR, 1.55; 95% CI, 1.15-2.09; P = .004). At 1 year, Blacks showed higher risks for death (HR, 2.06; 95% CI, 1.26-3.36; P = .004) and for MI (HR, 1.45; 95% CI, 1.01-2.10; P = .045), compared with Whites.
No significant increases in risk for outcomes at 1 and 5 years were seen for Hispanics or Asians, compared with Whites.
Covariates in the analyses included age, sex, body mass index, diabetes, current smoking, hypertension, hyperlipidemia, history of MI or coronary revascularization, clinical CAD presentation, category of stent, and race stratified by study.
Even with underlying genotypic differences between Blacks and Whites, much of the difference in risk for outcomes “should have been accounted for when the researchers adjusted for these clinical phenotypes,” the editorial notes.
Some of the difference in risk must have derived from uncontrolled-for variables, and “[b]eyond genetics, it is clear that race is also a surrogate for other socioeconomic factors that influence both medical care and patient outcomes,” the editorialists wrote.
The adjusted analysis, noted Golomb et al, suggests “that for Hispanic patients, the excess risk for adverse clinical outcomes may have been attributable to a higher prevalence of risk factors. In contrast, the excess risk for adverse clinical outcomes for Black patients persisted even after adjustment for baseline risk factors.”
As such, they agreed: “The observed increased risk may be explained by differences that are not fully captured in traditional cardiovascular risk factor assessment, including socioeconomic differences and education, treatment compliance rates, and yet-to-be-elucidated genetic differences and/or other factors.”
Dr. Stone said that such socioeconomic considerations may include reduced access to care and insurance coverage; lack of preventive care, disease awareness, and education; delayed presentation; and varying levels of provided care.
“Possible genetic or environmental-related differences in the development and progression of atherosclerosis and other disease processes” may also be involved.
“Achieving representative proportions of minorities in clinical trials is essential but has proved challenging,” Dr. Stone said. “We must ensure that adequate numbers of hospitals and providers that are serving these patients participate in multicenter trials, and trust has to be developed so that minority populations have confidence to enroll in studies.”
Dr. Stone reported holding equity options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestro Biomed, Aria, Cardiac Success, the MedFocus family of funds, and Valfix and receiving consulting fees from Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore Ablative Solutions, Miracor, Neovasc, W-Wave, Abiomed, and others. Disclosures for the other authors are in the report. Nanna reports no relevant financial relationships; other coauthor disclosures are provided with the editorial.
A version of this article originally appeared on Medscape.com.
A combined analysis of 10 prospective trials, intended to shed light on racial disparities in percutaneous coronary intervention (PCI) outcomes, saw sharply higher risks of death and myocardial infarction (MI) for Blacks compared with Whites.
The burden of comorbidities, including diabetes, was greater for Hispanics and Blacks, compared with Whites, but only in Blacks were PCI outcomes significantly worse even after controlling for such conditions and other baseline risk factors.
The analysis based on more than 22,000 patients was published July 6 in JACC: Cardiovascular Interventions,with lead author Mordechai Golomb, MD, Cardiovascular Research Foundation, New York.
In the study based on patient-level data from the different trials, the adjusted risk of MI after PCI was increased 45% at 1 year and 55% after 5 years for Blacks, compared with Whites. Their risk of death at 1 year was doubled, and their risk of major adverse cardiac events (MACE) was up by 28% at 5 years.
“Improving health care and outcomes for minorities is essential, and we are hopeful that our work may help direct these efforts, senior author Gregg W. Stone, MD, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“But this won’t happen without active, concerted efforts to promote change and opportunity, a task for government, regulators, payers, hospital administrators, physicians, and all health care providers,” he said. “Understanding patient outcomes according to race and ethnicity is essential to optimize health for all patients,” but “most prior studies in this regard have looked at population-based data.”
In contrast, the current study used hospital source records – which are considered more accurate than administrative databases – and event coding reports, Dr. Stone said, plus angiographic core laboratory analyses for all patients, which allows “an independent assessment of the extent and type of coronary artery disease and procedural outcomes.”
The analysis “demonstrated that even when upfront treatments are presumably similar [across racial groups] in a clinical trial setting, longitudinal outcomes still differ by race,” Michael Nanna, MD, said in an interview.
The “troubling” results “highlight the persistence of racial disparities in health care and the need to renew our focus on closing these gaps [and] is yet another call to action for clinicians, researchers, and the health care system at large,” said Dr. Nanna, of Duke University Medical Center, Durham, N.C., and lead author on an editorial accompanying the published analysis.
Of the 10 randomized controlled trials included in the study, which encompassed 22,638 patients, 9 were stent comparisons and 1 compared antithrombotic regimens in patients with acute coronary syndromes (ACS), the authors noted. The median follow-up was about 1,100 days.
White patients made up 90.9% of the combined cohort, Black patients comprised 4.1%, Hispanics 2.1%, and Asians 1.8% – figures that “confirm the well-known fact that minority groups are underrepresented in clinical trials,” Dr. Stone said.
There were notable demographic and clinical differences at baseline between the four groups.
For example, Black patients tended to be younger than White, Hispanic, and Asian patients. Black and Hispanic patients were also less likely to be male, compared with White patients.
Both Black and Hispanic patients had more comorbidities than Whites did at baseline, the authors observe. For example, Black and Hispanic patients had a greater body mass index, compared with Whites, whereas it was lower for Asians; and they had more diabetes and more hypertension than Whites (P < .0001 for all differences). Hispanics were more likely to have ACS at baseline, compared with Whites, and less likely to have stable coronary artery disease (CAD) (P < .0001 for all differences). Similar proportions of Blacks and of Whites had stable CAD (about 32% of each) and ACS (about 68% in both cases). Rates of hyperlipidemia and stable CAD were greater and rates of ACS was lower in Asians than the other three race groups (P < .0001 for each difference). In adjusted analysis, the risk of MACE at 5 years was significantly increased for Blacks, compared with Whites (hazard ratio, 1.28; 95% CI, 1.05-1.57; P = .01). The same applied to MI (HR, 1.55; 95% CI, 1.15-2.09; P = .004). At 1 year, Blacks showed higher risks for death (HR, 2.06; 95% CI, 1.26-3.36; P = .004) and for MI (HR, 1.45; 95% CI, 1.01-2.10; P = .045), compared with Whites.
No significant increases in risk for outcomes at 1 and 5 years were seen for Hispanics or Asians, compared with Whites.
Covariates in the analyses included age, sex, body mass index, diabetes, current smoking, hypertension, hyperlipidemia, history of MI or coronary revascularization, clinical CAD presentation, category of stent, and race stratified by study.
Even with underlying genotypic differences between Blacks and Whites, much of the difference in risk for outcomes “should have been accounted for when the researchers adjusted for these clinical phenotypes,” the editorial notes.
Some of the difference in risk must have derived from uncontrolled-for variables, and “[b]eyond genetics, it is clear that race is also a surrogate for other socioeconomic factors that influence both medical care and patient outcomes,” the editorialists wrote.
The adjusted analysis, noted Golomb et al, suggests “that for Hispanic patients, the excess risk for adverse clinical outcomes may have been attributable to a higher prevalence of risk factors. In contrast, the excess risk for adverse clinical outcomes for Black patients persisted even after adjustment for baseline risk factors.”
As such, they agreed: “The observed increased risk may be explained by differences that are not fully captured in traditional cardiovascular risk factor assessment, including socioeconomic differences and education, treatment compliance rates, and yet-to-be-elucidated genetic differences and/or other factors.”
Dr. Stone said that such socioeconomic considerations may include reduced access to care and insurance coverage; lack of preventive care, disease awareness, and education; delayed presentation; and varying levels of provided care.
“Possible genetic or environmental-related differences in the development and progression of atherosclerosis and other disease processes” may also be involved.
“Achieving representative proportions of minorities in clinical trials is essential but has proved challenging,” Dr. Stone said. “We must ensure that adequate numbers of hospitals and providers that are serving these patients participate in multicenter trials, and trust has to be developed so that minority populations have confidence to enroll in studies.”
Dr. Stone reported holding equity options in Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, the Biostar family of funds, SpectraWave, Orchestro Biomed, Aria, Cardiac Success, the MedFocus family of funds, and Valfix and receiving consulting fees from Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore Ablative Solutions, Miracor, Neovasc, W-Wave, Abiomed, and others. Disclosures for the other authors are in the report. Nanna reports no relevant financial relationships; other coauthor disclosures are provided with the editorial.
A version of this article originally appeared on Medscape.com.
Tendyne device shows promise for mitral annular calcification
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
Transcatheter implantation of the Tendyne mitral valve replacement device for treatment of mitral regurgitation in patients at prohibitive surgical risk because of severe mitral annular calcification showed considerable promise in a small feasibility study, Paul Sorajja, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
There is a huge unmet need for safe and effective therapies for severe mitral annular calcification (MAC).
“Severe MAC often precludes surgical treatment, and there’s a poor prognosis in patients with MAC and mitral regurgitation when untreated, with 2-year survival of about 60% in some studies,” noted Dr. Sorajja, a cardiologist at the Minneapolis Heart Institute Foundation.
Attempts at repurposing transcatheter aortic valves for use in the mitral location have been largely unsatisfactory, he added.
The 6-month outcomes in the 11 patients who received the Tendyne device in the multicenter U.S. feasibility study featured low rates of mortality and nonfatal adverse events, elimination of mitral regurgitation, marked improvement on quality of life measures, and a mean gradient of 4.1 mm Hg. The acute procedural outcomes were encouraging as well.
“We had technical success in 11 of 11 patients, no procedural mortality or left ventricular outflow tract obstruction, no valve embolization or malposition, and no conversion to open heart surgery,” he said.
There was one death caused by mesenteric ischemia 16 days post Tendyne implantation. One patient experienced a nondisabling stroke at day 4. Two patients developed new-onset atrial fibrillation, one of whom cardioverted to sinus rhythm. And one patient had a moderate paravalvular leak that resolved with placement of a plug at 3 months. There were no MIs.
At baseline, 9 of 11 patients were New York Heart Association functional class III and the others were class II. At 6 months, six patients were class I, four were class II, and one was class III. The average score on the Kansas City Cardiomyopathy Questionnaire improved from 45.9 at baseline to 65.5 at 1 month, 77.4 at 3 months, and 70.3 at 6 months.
This was a highly selected study population with a Society of Thoracic Surgery Predicted Risk of Mortality score of 9.03%. Part of the screening process for study participation involved preprocedural CT imaging with simulated device overlay in order to identify candidates who were likely to have an optimal device fit.
Discussant Francesco Maisano, MD, was impressed by how well this simulation resembled the actual results as depicted in side-by-side pre- and postprocedural CT images presented by Dr. Sorajja.
“What really surprised me was the correlation between preprocedural simulation data and the actual CT scan after the procedure. This trial shows that the simulation works, and also that Tendyne is a great alternative to aortic valve-in-MAC for these very-high-risk patients,” said Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
Earlier this year the Tendyne device was approved in Europe for patients with mitral regurgitation who aren’t candidates for surgical valve replacement or transcatheter mitral valve repair. The approval does not, however, extend to MAC. The Abbott device remains investigational in the United States, where the pivotal SUMMIT trial is underway. In one arm of the trial, patients with mitral regurgitation are being randomized to the investigational Tendyne device or to Abbott’s MitraClip, which is approved for that indication. In the other arm, patients with severe MAC at prohibitive surgical risk will get the Tendyne device. Results are expected in 2020.
Dr. Sorajja reported receiving research grants from and serving as a consultant to Abbott, the feasibility study sponsor, as well as to several other medical device companies, as did Dr. Maisano.
REPORTING FROM EUROPCR 2020
More fatalities in heart transplant patients with COVID-19
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 infection is associated with a high risk for mortality in heart transplant (HT) recipients, a new case series suggests.
Investigators looked at data on 28 patients with a confirmed diagnosis of COVID-19 who received a HT between March 1, 2020, and April 24, 2020 and found a case-fatality rate of 25%.
“The high case fatality in our case series should alert physicians to the vulnerability of heart transplant recipients during the COVID-19 pandemic,” senior author Nir Uriel, MD, MSc, professor of medicine at Columbia University, New York, said in an interview.
“These patients require extra precautions to prevent the development of infection,” said Dr. Uriel, who is also a cardiologist at New York Presbyterian/Columbia University Irving Medical Center.
The study was published online May 13 in JAMA Cardiology.
Similar presentation
HT recipients can have several comorbidities after the procedure, including hypertension, diabetes, cardiac allograft vasculopathy, and ongoing immunosuppression, all of which can place them at risk for infection and adverse outcomes with COVID-19 infection, the authors wrote.
The researchers therefore embarked on a case series looking at 28 HT recipients with COVID-19 infection (median age, 64.0 years; interquartile range, 53.5-70.5; 79% male) to “describe the outcomes of recipients of HT who are chronically immunosuppressed and develop COVID-19 and raise important questions about the role of the immune system in the process.”
The median time from HT to study period was 8.6 (IQR, 4.2-14.5) years. Most patients had numerous comorbidities.
“The presentation of COVID-19 was similar to nontransplant patients with fever, dyspnea, cough, and GI symptoms,” Dr. Uriel reported.
No protective effect
Twenty-two patients (79%) required admission to the hospital, seven of whom (25%) required admission to the ICU and mechanical ventilation.
Despite the presence of immunosuppressive therapy, all patients had significant elevation of inflammatory biomarkers (median peak high-sensitivity C-reactive protein [hs-CRP], 11.83 mg/dL; IQR, 7.44-19.26; median peak interleukin [IL]-6, 105 pg/mL; IQR, 38-296).
Three-quarters had myocardial injury, with a median high-sensitivity troponin T of 0.055 (0.0205 - 0.1345) ng/mL.
Treatments of COVID-19 included hydroxychloroquine (18 patients; 78%), high-dose corticosteroids (eight patients; 47%), and IL-6 receptor antagonists (six patients; 26%).
Moreover, during hospitalization, mycophenolate mofetil was discontinued in most (70%) patients, and one-quarter had a reduction in their calcineurin inhibitor dose.
“Heart transplant recipients generally require more intense immunosuppressive therapy than most other solid organ transplant recipients, and this high baseline immunosuppression increases their propensity to develop infections and their likelihood of experiencing severe manifestations of infections,” Dr. Uriel commented.
“With COVID-19, in which the body’s inflammatory reaction appears to play a role in disease severity, there has been a question of whether immunosuppression may offer a protective effect,” he continued.
“This case series suggests that this is not the case, although this would need to be confirmed in larger studies,” he said.
Low threshold
Among the 22 patients who were admitted to the hospital, half were discharged home and four (18%) were still hospitalized at the end of the study.
Of the seven patients who died, two died at the study center, and five died in an outside institution.
“In the HT population, social distancing (or isolation), strict use of masks when in public, proper handwashing, and sanitization of surfaces are of paramount importance in the prevention of COVID-19 infection,” Dr. Uriel stated.
“In addition, we have restricted these patients’ contact with the hospital as much as possible during the pandemic,” he said.
However, “there should be a low threshold to hospitalize heart transplant patients who develop infection with COVID-19. Furthermore, in our series, outcomes were better for patients hospitalized at the transplant center; therefore, strong consideration should be given to transferring HT patients when hospitalized at another hospital,” he added.
The authors emphasized that COVID-19 patients “will require ongoing monitoring in the recovery phase, as an immunosuppression regimen is reintroduced and the consequences to the allograft itself become apparent.”
Vulnerable population
Commenting on the study, Mandeep R. Mehra, MD, MSc, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital, Boston, suggested that “in epidemiological terms, [the findings] might not look as bad as the way they are reflected in the paper.”
Given that Columbia is “one of the larger heart transplant centers in the U.S., following probably 1,000 patients, having only 22 out of perhaps thousands whom they transplanted or are actively following would actually represent a low serious infection rate,” said Dr. Mehra, who is also the executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital and a professor of medicine at Harvard Medical School, also in Boston.
“We must not forget to emphasize that, when assessing these case fatality rates, we must look at the entire population at risk, not only the handful that we were able to observe,” explained Dr. Mehra, who was not involved with the study.
Moreover, the patients were “older and had comorbidities, with poor underlying kidney function and other complications, and underlying coronary artery disease in the transplanted heart,” so “it would not surprise me that they had such a high fatality rate, since they had a high degree of vulnerability,” he said.
Dr. Mehra, who is also the editor-in-chief of the Journal of Heart and Lung Transplantation, said that the journal has received manuscripts still in the review process that suggest different fatality rates than those found in the current case series.
However, he acknowledged that, because these are patients with serious vulnerability due to underlying heart disease, “you can’t be lackadaisical and need to do everything to decrease this vulnerability.”
The authors noted that, although their study did not show a protective effect from immunosuppression against COVID-19, further studies are needed to assess each individual immunosuppressive agent and provide a definitive answer.
The study was supported by a grant to one of the investigators from the National Heart, Lung, and Blood Institute. Dr. Uriel reports no relevant financial relationships. The other authors’ disclosures are listed in the publication. Dr. Mehra reports no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FDA okays emergency use for Impella RP in COVID-19 right heart failure
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration issued an emergency use authorization for use of the Impella RP heart pump system in COVID-19 patients with right heart failure or decompensation, Abiomed announced June 1.
“Based on extrapolation of data from the approved indication and reported clinical experience, FDA has concluded that the Impella RP may be effective at providing temporary right ventricular support for the treatment of acute right heart failure or decompensation caused by COVID-19 complications, including PE [pulmonary embolism],” the letter noted.
It cited, for example, use of the temporary heart pump in a 59-year-old woman suffering from COVID-19 who went into right ventricular failure and became hypotensive after an acute PE was removed. After placement of the device, the patient experienced a “dramatic and immediate” improvement in arterial pressure and the device was removed on the fifth day, according to Amir Kaki, MD, and Ted Schreiber, MD, of Ascension St. John Hospital, Detroit, whose review of the case has been posted online.
“Acute pulmonary embolism is clearly being recognized as a life-threatening manifestation of COVID-19. Impella RP is an important tool to help cardiologists save lives during this pandemic,” Dr. Kaki said in the letter. “As we have demonstrated in our series of patients, early recognition of right ventricular dysfunction and early placement of the Impella RP for patients who are hypotensive can be lifesaving.”
Other data cited in support of the Impella RP emergency use authorization (EUA) include a 2019 series of hemodynamically unstable patients with PE in Japan and a 2017 case report of a 47-year-old man with right ventricular failure, profound shock, and a massive PE.
The FDA granted premarket approval of the Impella RP system in 2017 to provide temporary right ventricular support for up to 14 days in patients with a body surface area of at least 1.5 m2 who develop acute right heart failure or decompensation following left ventricular assist device implantation, MI, heart transplant, or open-heart surgery.
The EUA indication for the Impella RP system is to provide temporary right ventricular support for up to 14 days in critical care patients with a body surface area of at least 1.5 m2 for the treatment of acute right heart failure or decompensation caused by complications related to COVID-19, including PE.
The Impella RP is authorized only for emergency use under the EUA and only for the duration of the circumstances justifying use of EUAs, the letter noted.
Last year, concerns were raised about off-indication use after interim results from a postapproval study suggested a higher risk for death than seen in premarket studies treated with the temporary heart pump.
A version of this article originally appeared on Medscape.com.
To fast or not to fast before elective cardiac catheterization
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
No restriction of oral food intake prior to nonemergent cardiac catheterization is as safe as the current traditional NPO [nothing by mouth] strategy, results from a large, single-center, randomized controlled trial showed.
According to lead investigator Abhishek Mishra, MD, NPO after midnight has been a standard practice before major surgery requiring general anesthesia since Mendelson Syndrome was first described in 1946. “The rational for keeping NPO after midnight has been to keep the stomach empty, to reduce gastric contents and acidity – which would reduce emesis – and eventually reduce the risk of aspiration,” Dr. Mishra, a cardiologist at the Heart and Vascular Institute at Vidant Health in Greenville, N.C., said at the at the Society for Cardiovascular Angiography & Interventions virtual annual scientific sessions. “The rationale of NPO in the setting of cardiac catheterization is to reduce the risk of aspiration, and more so, of a patient needing emergent cardiac surgery.” The clinical question was, do we really need to keep our patients NPO prior to elective cardiac catheterization? So far, no large randomized study has been done to answer this question.”
To find out, Dr. Mishra and colleagues carried out CHOW NOW (Can We Safely Have Our Patients Eat With Cardiac Catheterization – Nix or Allow), a single-center, prospective, randomized, single-blinded study that compared the safety of a nonfasting strategy with the current fasting protocol strategies in 599 patients who underwent nonemergent cardiac catheterization at The Guthrie Clinic/Robert Packer Hospital in Sayre, Pa.
Patients in the fasting group were instructed to be NPO after midnight, but could have clear liquids up to 2 hours prior to the procedure, while those in the nonfasting group had no restriction of oral intake, irrespective of time of cardiac catheterization. The primary outcome was a composite of aspiration pneumonia, preprocedural hypertension, preprocedural hypoglycemia or hyperglycemia, incidence of nausea/vomiting, and contrast-induced neuropathy. Secondary outcomes included total cost of the index hospitalization, patient satisfaction via a questionnaire containing seven questions, and in-hospital mortality.
Of the 599 patients, 306 were assigned to the standard fasting group and the remaining 293 to the nonfasting group. Their mean age was 67 years, 45% were on a proton pump inhibitor or H2 blockers, and 33% had diabetes. In addition, 40% had acute coronary syndrome, and 23% underwent percutaneous intervention.
The researchers observed no statistically significant difference in the primary or secondary outcomes between the study groups. In the nonfasting group, 11.3% of patients met the primary endpoint, compared with 9.8% of the patients in the standard fasting group (P = .65). In addition, the nonfasting strategy was found to be noninferior to the standard fasting strategy for the primary outcome at a noninferiority margin threshold of 0.059.
Dr. Mishra and colleagues observed no differences between the standard fasting and nonfasting groups with respect to in-hospital mortality (0.3% vs. 0.7%, respectively; P = .616), patient satisfaction score (a mean of 4.4 vs. a mean of 4.5; P = .257), and mean total cost of hospitalization ($8,446 vs. $6,960; P = .654).
“In this randomized, controlled trial, we found that there was no significant difference in the rate of overall adverse events with an approach of unrestricted oral intake prior to cardiac catheterization compared to strict fasting, and it was associated with better patient satisfaction and lower cost of care, especially for hospitalized patients,” concluded Dr. Mishra, who conducted the research during his fellowship at The Guthrie Clinic.
He acknowledged certain limitations of the trial, including the fact that results are applicable only to cardiac catheterization procedures, including coronary angiographies, percutaneous coronary interventions, and left heart catheterizations. “These results are not applicable to certain high-risk coronary procedures that required the use of a large-bore access or any valve procedures,” he said.
One of the session’s invited panelists, Cindy L. Grines, MD,, said that she and other interventional cardiologists have “gone around and around” on the issue of NPO prior to nonemergent cardiac catheterization. “I actually let my patients get fluids up until the time they’re put on the cath lab table,” said Dr. Grines, chief scientific officer of the Northside Cardiovascular Institute in Atlanta. “I haven’t been giving them solid food like this, though.”
Another panelist, Timothy D. Henry, MD, said that in his clinical experience, “patients don’t like being NPO, and I think we’ve all seen cases where patients are actually volume-depleted in the morning.” Dr. Henry, medical director of The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital in Cincinnati, pointed out that most NPO policy “is not dictated by us as interventional cardiologists; it’s dictated by hospital policies or by anesthesiologists. Will [the results of this study] change what we do?”
The Donald Guthrie Research Foundation funded the study. Daniel P. Sporn, MD, FACC, was the study’s principal investigator. Dr. Mishra reported having no financial disclosures.
SOURCE: Mishra A et al., SCAI 2020, abstract 11758.
REPORTING FROM SCAI 2020