User login
Parabens: The 2019 Nonallergen of the Year
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
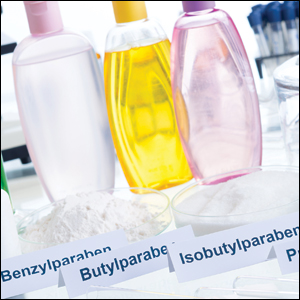
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
Each year, the American Contact Dermatitis Society (ACDS) names an allergen of the year with the purpose of promoting greater awareness of a key allergen and its impact on patients. Often, the allergen of the year is an emerging allergen that may represent an underrecognized or novel cause of allergic contact dermatitis (ACD). In 2019, the ACDS chose parabens as the “nonallergen” of the year to draw attention to their low rate of associated ACD despite high public interest in limiting exposure to parabens.1
What types of products contain parabens?
Parabens are preservatives commonly found in many different categories of personal care products. Preservatives inhibit microbial growth and are necessary ingredients in water-based products. The 4 most common parabens used in personal care products are methylparaben, ethylparaben, propylparaben, and butylparaben.1 Parabens are metabolized to 4-hydroxybenzoic acid and are excreted in urine. When parabens are applied topically, there is minimal penetration through intact human skin.2 In the United States, parabens are allowed as preservatives in cosmetics at concentrations up to 0.4% when used alone or up to 0.8% when used in combination with other parabens.3
Consumers are exposed to parabens in a wide variety of personal care products. The Contact Allergen Management Program (CAMP) is a system owned and managed by the ACDS that typically is used to generate lists of safe personal care products for patients and also can be queried for the presence of individual chemicals in products. According to a 2018 query of the CAMP, parabens were found in 19% of all products.1 A more recent query of CAMP (http://www.contactderm.org/resources/acds-camp) in March 2019 showed parabens were present in 39.3% of makeup products, especially in eye products, foundations, and concealers; parabens also were found in 34% of moisturizers, 11.5% of soaps, and 19% of sunscreens. Notably, 14.8% of prescription topical steroids listed in the CAMP contained a paraben. Another method for evaluating chemical contents of personal care products is a review of the Voluntary Cosmetic Registration Program, a US Food and Drug Administration–based registry for cosmetic products. Survey data from the Voluntary Cosmetic Registration Program in 2018 documented methylparaben in 11,626 formulations.4 Other parabens included propylparaben (8885 products), butylparaben (3915 products), and ethylparaben (3860 products). Parabens were reported more frequently in leave-on rather than rinse-off products.4
In medications, parabens are recommended at concentrations of no more than 0.1%.1 Fransway et al1 compiled a list of medications that contain parabens, including commonly prescribed dermatologic topical medications such as corticosteroids, several acne preparations, eflornithine, fluorouracil, hydroquinone, imiquimod, urea, and sertaconazole. Oral and parenteral medications including local anesthetics and corticosteroids also may contain parabens.
Consumers also may be exposed to parabens through foodstuffs. Methylparaben and propylparaben have been classified as generally recognized as safe in foods by the US Food and Drug Administration.5 The acceptable daily intake of parabens in food is 0 to 10 mg/kg of body weight,1 and the estimated dietary intake for a typical adult is 307 mg/kg of body weight daily.6 Several studies on paraben content in foodstuffs have confirmed their presence in both natural and processed foods.1,6 Systemic contact dermatitis caused by ingestion of parabens is rare. In general, individuals with positive patch test reactions to parabens should not routinely avoid them in foods or oral medications,1 but they should, of course, be avoided in topical medications.
What is the rate of ACD with parabens?
One of the main reasons that parabens were designated as the ACDS nonallergen of the year is the very low rate of ACD associated with parabens. The North American Contact Dermatitis Group, a research group with members in the United States and Canada, reported a 0.6% positive reaction rate when patch testing with paraben mix 12%,7 which closely compares with a 0.8% positive reaction rate when patch testing with paraben mix 16% using the Mayo Clinic standard series.8 From the standpoint of ACD, this very low patch test reaction rate makes parabens one of the safest preservative options for use in cosmetic products.
Are there health risks associated with parabens?
The paraben controversy in the scientific literature and in the lay press centers around potential health risks and endocrine disruption. We will focus on the conversation regarding parabens and the risk for endocrine disruption and association with breast cancer.
Parabens have been reported to have estrogenic effects; however, the bulk of the data is limited to in vitro and animal studies, with less evidence of endocrine disruption in humans.2 In vitro studies have demonstrated that the estrogenic potency of parabens is much less than that of estrogen. In one study, parabens were shown to be 10,000-fold less potent than 17β-estradiol9; in a separate study, they had a maximum potency of only 1/4000 that of estrogen.10 Additionally, an in vitro study showed varying ability for parabens to bind estrogen receptors, with a greater ability to bind with longer alkyl side chains.11 The result is decreased or increased estrogen activity, dependent on side chain length and type of receptor.2 Finally, some studies add conflicting results that parabens may actually create an antiestrogenic effect in human breast cancer cells.12 From the standpoint of estrogen mimicry, there are no known studies in humans confirming harmful effects associated with paraben exposure.
The reported association between parabens and breast cancer is closely related to their theoretical estrogenic effects. The conversation regarding parabens and breast cancer has been fueled by the identification of parabens in human breast tumors and their presence in concentrations similar to what is needed to stimulate in vitro breast cancer cells.2 The existing data do not confirm causation. An association with parabens in topical axillary personal care products has been theorized but not confirmed; for example, it was shown that paraben levels were highest in the axillary region of breast cancer tissue, including women who had never used deodorant. It was concluded that the presence of axillary parabens was due to sources other than topical axillary personal care products.13 Another study confirmed there was not an increased risk for breast cancer in patients who applied personal care products to the axillary area within an hour of shaving.14 The existing data do not support topical paraben exposure as a risk for breast cancer.
Final Thoughts
Parabens are preservatives frequently found in personal care products and exhibit a very low rate of associated ACD. Consumers may be exposed to parabens through foods, cosmetics, and medications. Although there have been consumer concerns regarding endocrine disruption or carcinogenicity associated with parabens, definite evidence of their harm is lacking in the scientific literature, and many studies confirm their safety.2 With their high prevalence in personal care products and low rates of associated contact allergy, parabens remain ideal preservative agents.
Ultimately, contact dermatitis is a common yet often underrecognized dermatologic condition. To address this knowledge gap in clinical practice, we are proud to launch Final Interpretation, a new column in Cutis covering emerging trends in contact dermatitis. We will address pearls, pitfalls, and updates in contact dermatitis. Although our primary focus will be ACD, other important causes of contact dermatitis will be highlighted. Look for the inaugural column in the June 2019 issue of Cutis.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
- Fransway AF, Fransway PJ, Belsito DV, et al. Parabens: contact (non)allergen of the year. Dermatitis. 2019;30:3-31.
- Fransway AF, Fransway PJ, Belsito DV, et al. Paraben toxicology. Dermatitis. 2019;30:32-45.
- Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
- Cosmetic Ingredient Review. Amended safety assessment of parabens as used in cosmetics. https://www.cir-safety.org/sites/default/files/Parabens.pdf. Published August 29, 2018. Accessed March 12, 2019.
- Methylparaben. Fed Regist. 2018;21(3):1490. To be codified at 21 CFR §184.
- Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918-3925.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015-2016. Dermatitis. 2018;29:297-309.
- Veverka KK, Hall MR, Yiannias JA, et al. Trends in patch testing with the Mayo Clinic standard series, 2011-2015. Dermatitis. 2018;29:310-315.
- Routledge EJ, Parker J, Odum J, et al. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12-19.
- Miller D, Brian B, Wheals BB, et al. Estrogenic activity of phenolic additives determined by an in vitro yeast bioassay. Environ Health Perspect. 2001;109:133-138.
- Blair RM, Fang H, Branham WS. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000;54:138-153.
- van Meeuwen JA, van Son O, Piersma AH, et al. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol Appl Pharmacol. 2008;230:372-382.
- Barr L, Metaxas G, Harbach CA, et al. Measurement of paraben concentrations in human breast tissue at serial locations across the breast from axilla to sternum. J Appl Toxicol. 2012;32:219-232.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002;94:1578-1580
.
Dermatologists name isobornyl acrylate contact allergen of the year
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
AT ACDS 2019
Chronic Lymphocytic Leukemia and Infiltrates Seen During Excision of Nonmelanoma Skin Cancer
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
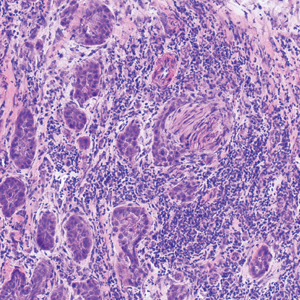
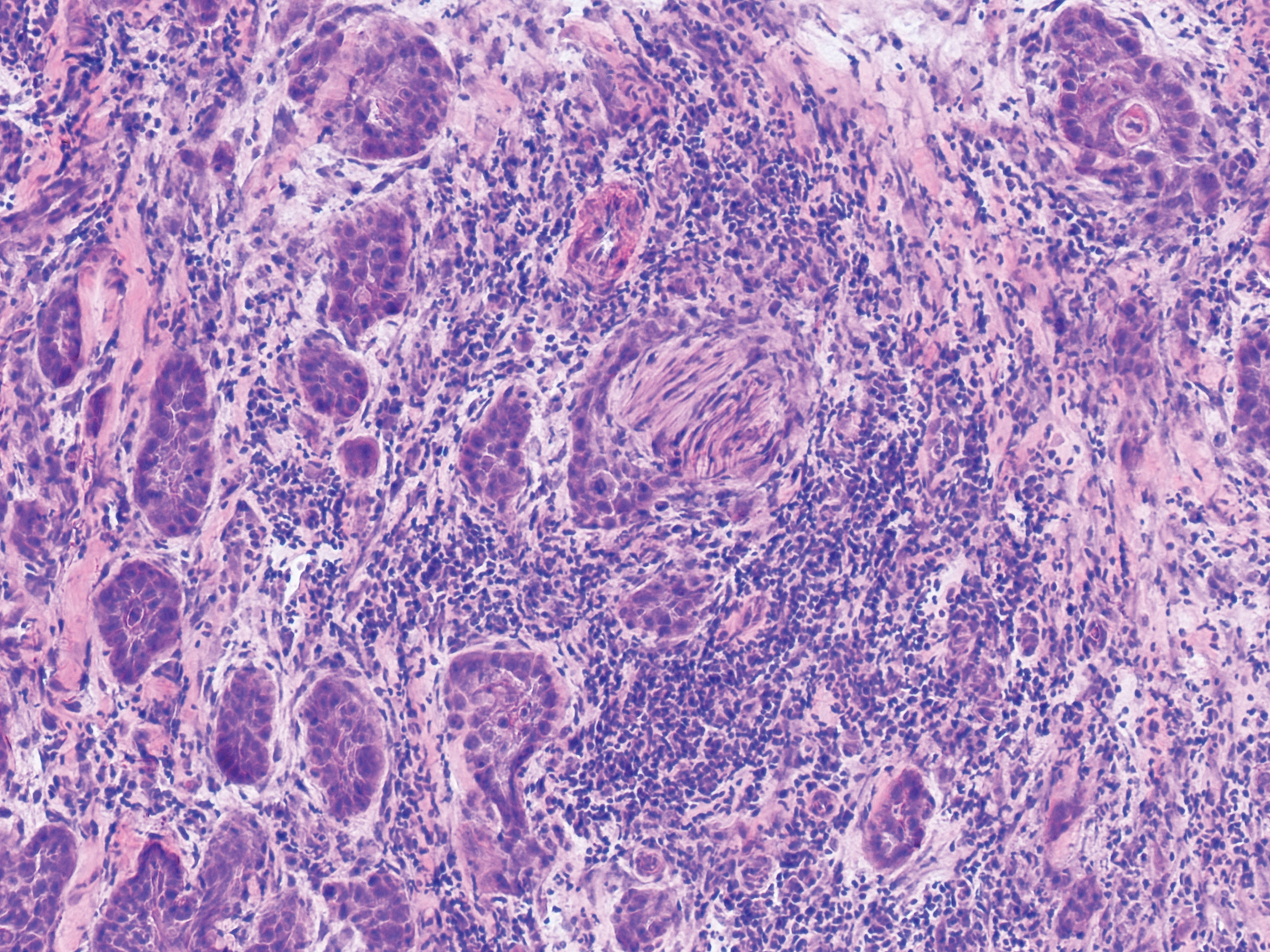
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.
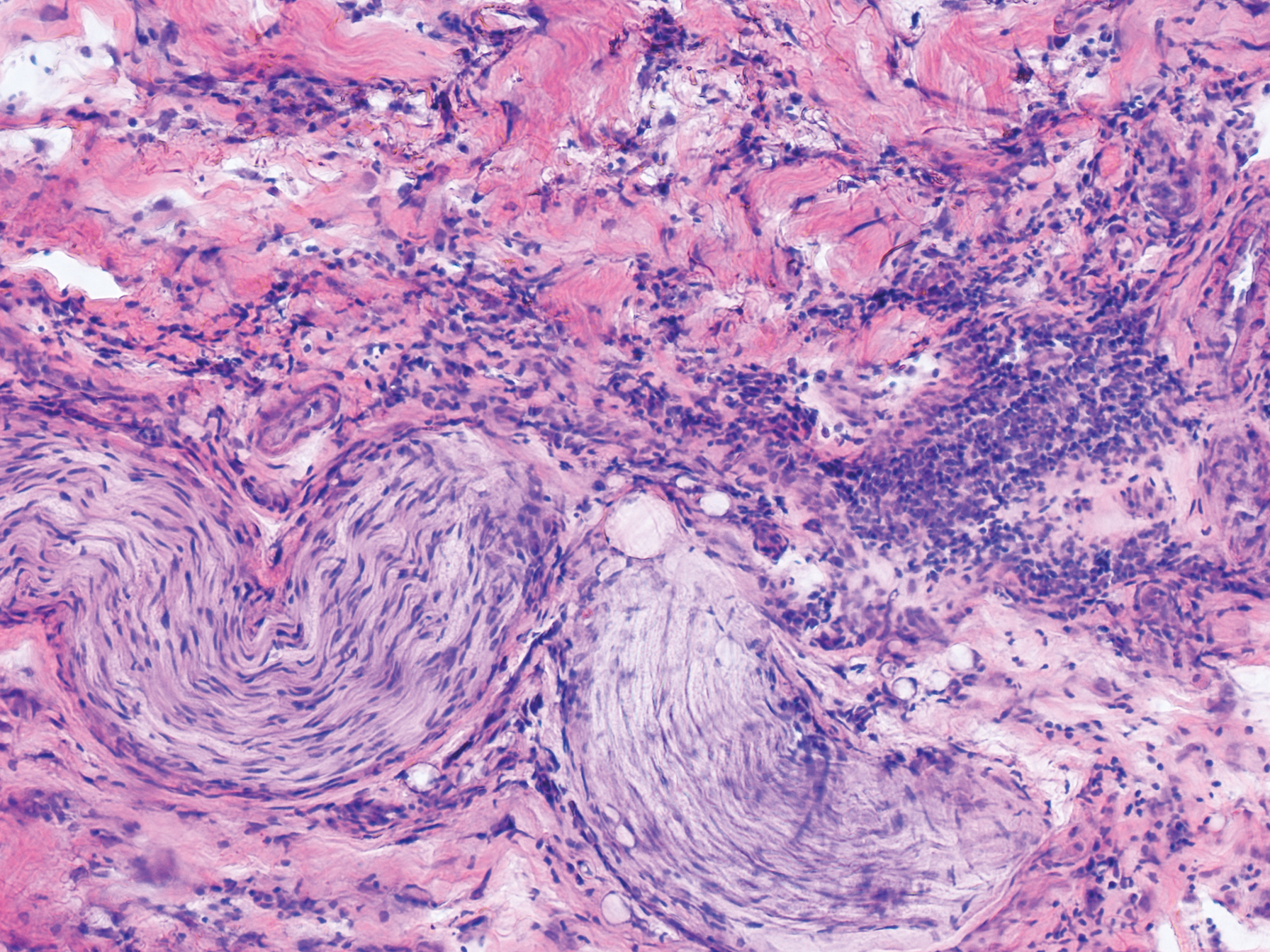
An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
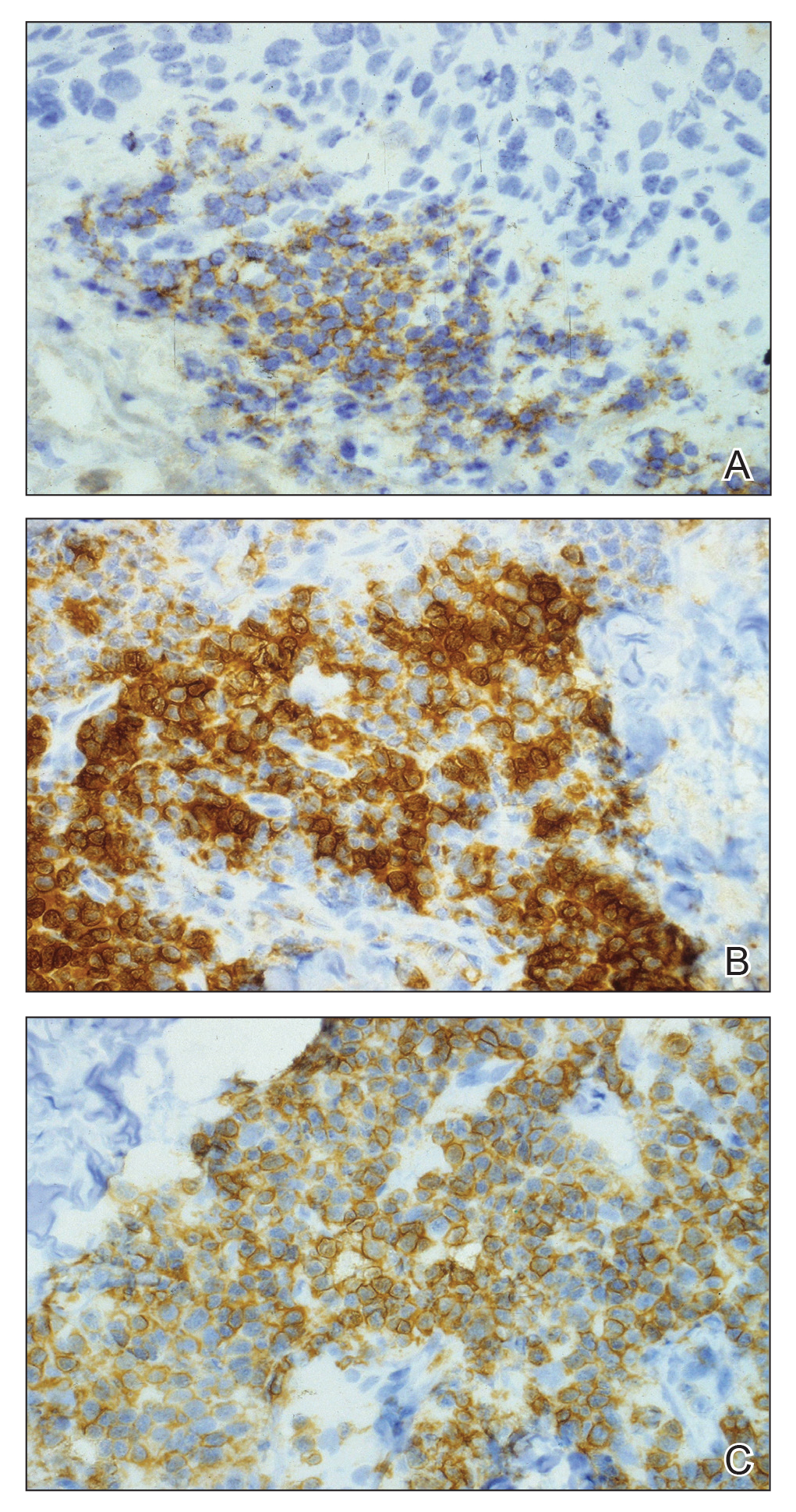
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7

The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
To the Editor:
Specific characteristics of a lymphocytic infiltrate noted on frozen section histologic examination during Mohs micrographic surgery (MMS) tumor excision should raise suspicion of an underlying chronic lymphocytic leukemia (CLL). This infiltrate may be the presenting sign of the underlying leukemia and has variable presentation that may mimic aggressive features. The following 3 cases highlight this phenomenon.
A 74-year-old man (patient 1) with a medical history of multiple nonmelanoma skin cancers (NMSCs) presented for definitive treatment of a biopsy-proven infiltrative basal cell carcinoma involving the right infra-auricular region. Mohs section histologic evaluation revealed patches of lymphocytic infiltrates so dense they obscured the tumor margins. The lymphocytic infiltrates persisted even after 3 MMS stages, though they were moderately less dense compared to the initial MMS stage. Clinical interpretation determined no relationship between the lymphocytic infiltrates and residual tumor. Due to concerns that this lymphocytic infiltrate may indicate an underlying leukemic process, preoperative laboratory tests were ordered prior to closure of the surgical wound, which demonstrated an elevated white blood cell count of 65,000/µL (reference range, 4500–11,000/µL) with 93% lymphocytes. A follow-up complete blood cell count (CBC) and blood smear confirmed the diagnosis of CLL. The patient was referred to a hematologist/oncologist.
An 84-year old man (patient 2) with a medical history of numerous precancerous lesions and 1 squamous cell carcinoma (SCC) presented for a biopsy, which determined moderately differentiated SCC. Mohs micrographic surgery was performed. The initial stage of MMS histologic examination demonstrated basosquamous carcinoma in the specimen margins, including perineural growth, with an extensive lymphoid infiltrate surrounding the tumor (Figure 1). A second stage of MMS was performed, and although margins appeared to be clear of the basosquamous histology, complete assessment was difficult due to areas of dense inflammatory infiltrate (Figure 2), including perineural infiltration that remained and appeared to extend deeper into the tissues. Pathology was consulted and it was determined that the perineural infiltration was unlikely related to tumor spread but rather secondary to an unknown cause. Further investigation of the patient’s medical history revealed previously diagnosed CLL, which had been omitted by the patient, as he had forgotten this diagnosis and denied a history of cancer, lymphoma, and even leukemia. A query to the patient’s primary care physician found the most recent CBC demonstrated an elevated white blood cell count of 37,600/µL with 78% lymphocytes.


An 84-year-old man (patient 3) with a known history of CLL was referred for MMS excision of a 3.5×4.0-cm SCC on the right anterior temple extending onto the lateral upper and lower eyelids. Mohs frozen section histologic examination of excised tissue revealed patches of heavy lymphocytic infiltrates not found exclusively around the residual tumor but additionally around superficial and deep neurovascular bundles. The second stage of MMS appeared to be clear of tumor cells, but lymphocytic infiltrates remained. Because this patient had a clear history of CLL, the decision was made in conjunction with a dermatopathologist to conclude the surgery at this point. However, secondary to the aggressive, deeply invasive growth of this SCC, the patient was referred for adjunctive radiation therapy to the surgical site after wound reconstruction.
Chronic lymphocytic leukemia is the most common leukemia in the Western world1 and is estimated to account for 27% of all new cases of leukemia. An individual’s lifetime risk is 0.5%. Chronic lymphocytic leukemia is predominantly a disease of the elderly, with an average age at diagnosis of 71 years. It is more common among males, North American and European populations, and those with a positive family history. Although epidemiologic factors including farming, prolonged pesticide exposure, and contact with Agent Orange have tentative links to CLL, the relationships are poorly established.2
Symptoms associated with acute leukemia only rarely manifest in patients with CLL.3 If present, symptoms are vague and include weakness, fatigue, weight loss, fever, night sweats, and a feeling of abdominal fullness.2,3 On clinical examination, patients also may have lymphadenopathy, splenomegaly, or hepatomegaly. Increasing severity of symptoms at time of presentation directly correlates with the severity and staging at the time of diagnosis.4 Not only do patients with CLL have a greater incidence of NMSCs with more notable subclinical tumor extension than the average person, but these individuals also have a greatly increased risk for skin cancer recurrence posttreatment.5,6
Although tissue pathology is not routinely part of the diagnosis of patients with CLL, findings can add to clinical suspicion. Smudge cells, which are cell debris, are characteristic morphologic features found in CLL. Most CLL cells are characteristically small mature lymphocytes with a dense nucleus.3 The presence of aggregates of these cells may obscure tumor margins during resection of NMSCs.7 This infiltrate is present in more than one-third of patients with CLL, as described in one retrospective cohort. This study simultaneously demonstrated the relationship between CLL and a 2-fold increase in postoperative defect size, which was attributed to either subclinical tumor spread or extra tissue removal to ensure clearance due to the leukemic infiltrates themselves.8 The presence of perineural tumor growth, which can occur with aggressive SCC and basal cell carcinoma, may be mimicked by perineural involvement of CLL cells rather than the reactive inflammation associated with continued tumor margins.7
When evaluating a patient with suspected CLL, laboratory tests should include a CBC with differential and examination of the peripheral smear. If abnormal, immunophenotyping of lymphocytes by flow cytometry will rule out other lymphoproliferative diseases and verify CLL as the diagnosis.3 Diagnosis of CLL requires the presence of monoclonal B lymphocytes (≥5×109/L) in the peripheral blood as confirmed by flow cytometry.3 Clonality of circulating B lymphocytes must be confirmed, and immunophenotyping will establish a diagnosis with leukemic cells having positive expression of CD20 (Figure 3A) and CD23 (Figure 3B)(characteristic of B-cell lineage) with coexpression of CD43 and CD5 (Figure 3C)(characteristic of T-cell lineage).7,9 This pattern of immunohistochemical markers can be differentiated from the normal immune response to cutaneous malignancies, which have the pattern of being CD3+, CD5+, and CD43+ with absence of B-cell markers (ie, CD20, CD23)(Table).7


The pathogenesis of this peritumoral infiltrate is unknown, though multiple theories exist. One theory is that the neoplastic lymphocytes are responding as a dysfunctional arm of the immune system to tumor-specific antigens. In patients with CLL, leukemic lymphocytes comprise a large portion of the circulating leukocyte population and this peritumoral infiltrate may simply be a reflection of the circulating leukocytic population. Another theory contends that neoplastic lymphocytes are simply nonspecific aggregations secondary to tumor neovascularization and increased vascular permeability.10
This neoplastic infiltrate seen incidentally during MMS excision of NMSCs not only provides a unique opportunity to diagnose and intervene in those with unknown CLL but also to be aware of complicating features that can spare the patient from unnecessary tissue removal, thereby maximizing the benefit of MMS. This infiltrate can obscure tumor margins; is unusually dense and patchy, with or without infiltrating perineural or perivascular components; and persists beyond what would seem to be an adequate margin to clear a tumor. These cases show these findings, which exemplify the peritumoral infiltrate of CLL and should prompt further workup.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
- Rozman C, Monserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052-1057.
- What are the risk factors for chronic lymphocytic leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-lymphocytic-leukemia/causes-risks-prevention/risk-factors.html. Revised May 10, 2018. Accessed February 11, 2019.
- Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446-5456.
- Rai KR, Wasil T, Iqbal U, et al. Clinical staging and prognostic markers in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2004;18:795-805, vii.
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs’ surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38-42.
- Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester epidemiology project population-based study in Minnesota. J Am Acad Dermatol. 2015;72:302-309.
- Wilson ML, Elston DM, Tyler WB, et al. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16:4.
- Mehrany K, Byrd DR, Roenigk RK, et al. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29:129-134.
- Khandelwal A, Seilstad KH, Magro CM. Subclinical chronic lymphocytic leukaemia associated with a 13q deletion presenting initially in the skin: apropos of a case. J Cutan Pathol. 2006;33:256-259.
- Padgett JK, Parlette HL, English JC. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29:769-771.
Practice Points
- Chronic lymphocytic leukemia (CLL) may be seen during histologic examination of specimens during Mohs micrographic surgery as a monomorphic infiltrate of small mature lymphocytes with dense nuclei. Patients may be unaware of their diagnosis, which can be the presenting feature.
- An infiltrate of CLL may mimic aggressive behavior of nonmelanoma skin cancers including perineural invasion. A leukemic infiltrate may appear more dense and monomorphic. If needed, immunohistochemical staining of leukemic cells will show CD5 and CD23 positivity.
- Anecdotally, patients with CLL may not remember this pertinent medical history. Whether due to its asymptomatic nature or lack of treatment in early stages, direct questioning about CLL may be warranted if this characteristic infiltrate is encountered.
Bedbugs in the Workplace



What’s Eating You? Bedbugs
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
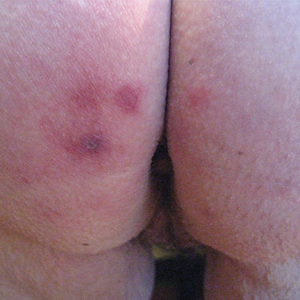
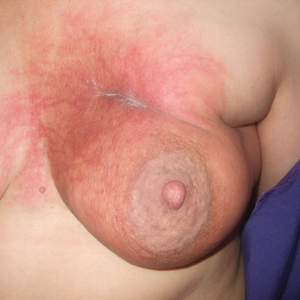
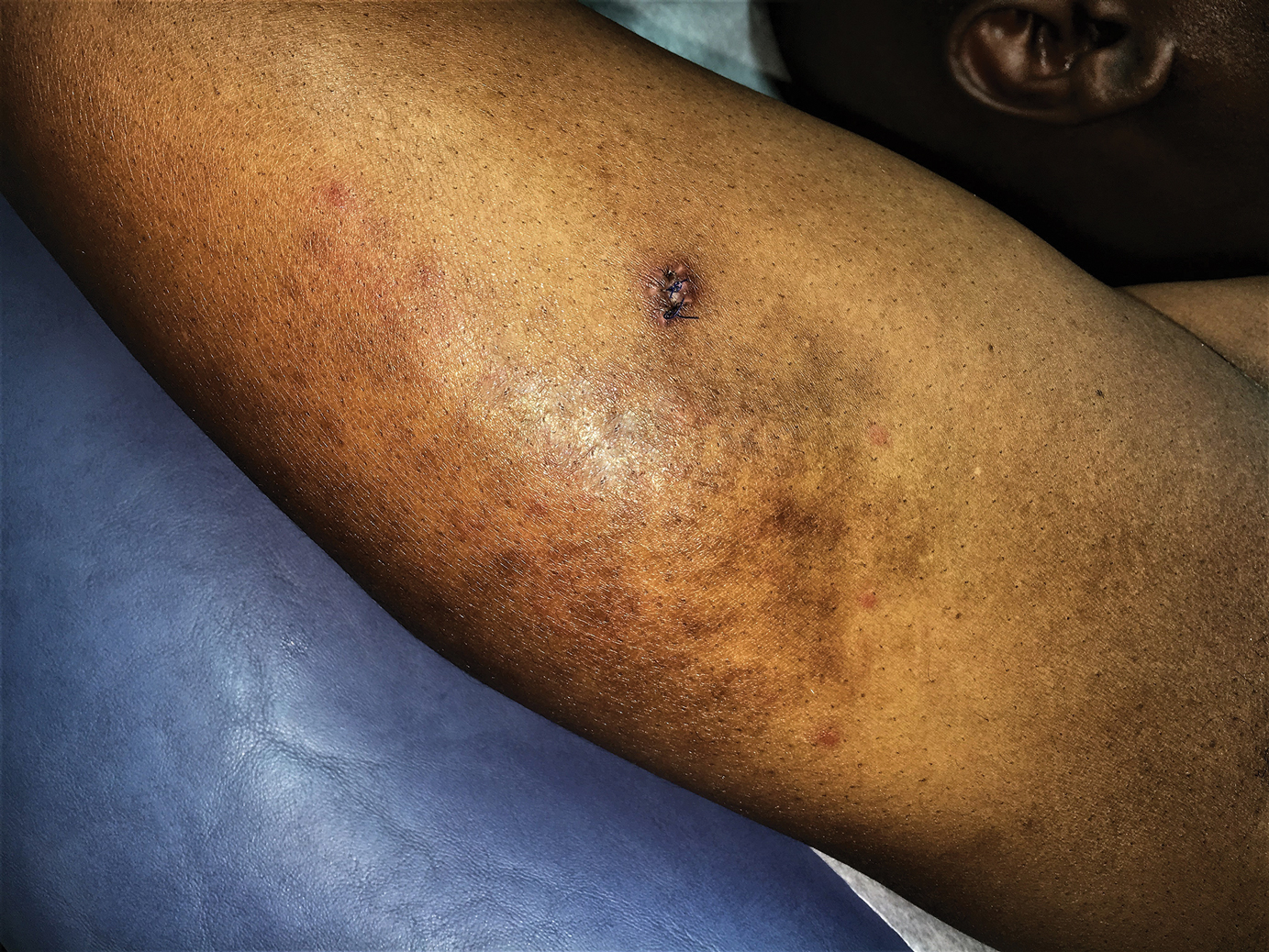
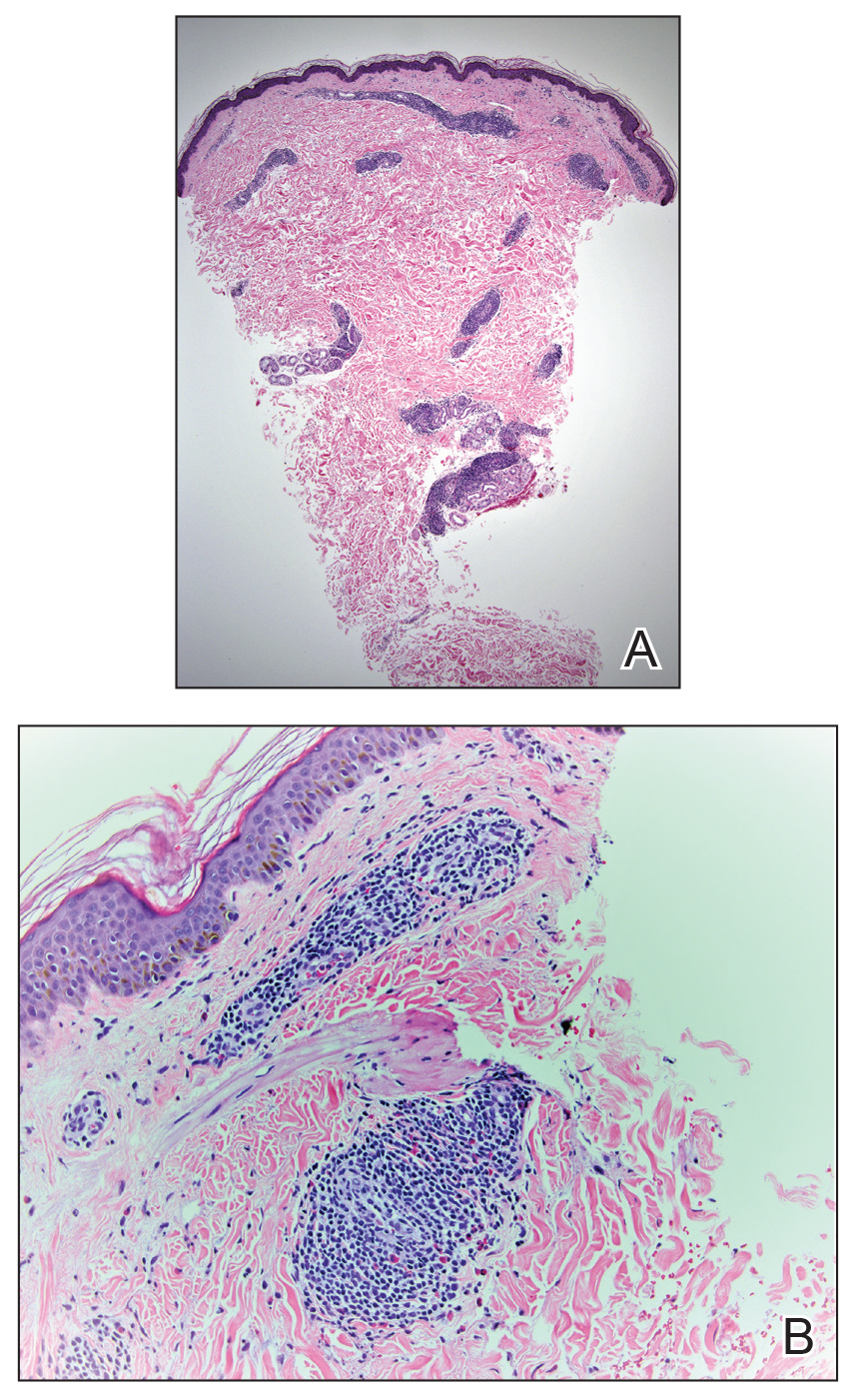
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.
At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Practice Points
- Bedbug exposures in the workplace are on the rise.
- High clinical suspicion is required when atypical dermatoses are not responding to therapy and histology suggests arthropod exposure.
- Once detected, partnership with occupational health and pest management experts is critical to eradicate bedbugs.
Blanchable Erythematous Patches on the Fingers
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
The Diagnosis: Irritant Contact Dermatitis
The diagnosis of irritant contact dermatitis secondary to skateboarding is similar to pool palms, a benign, self-limiting irritant contact dermatitis.1 We propose that contact with concrete surfaces during skateboarding can lead to a presentation similar to pool palms. In our case, it was likely that the finger pulpitis noted in the physical examination was due to daily skateboarding rather than once-weekly swimming. Furthermore, the fingertip contact with concrete in pool palms is similar to the rough surface exposure on the skateboard.
Pool palms is more commonly reported in children due to their participation in sports and other activities with recent exposure to rough surfaces, most commonly the floor of swimming pools.2 The condition resolves after eliminating exposures.3 The frequency and duration of exposure to rough surfaces in swimming pools leading to development of this condition is unknown.
There have been mixed reports on the pathogenesis of pool palms. Some literature supports the idea that it is a wet dermatitis, a combination of prolonged water contact, friction, chemicals, and microbes leading to a chronic dermatitis. This theory states that the primary factor influencing the development of erythematous patches on the fingers, palms, and soles is the hyperhydration of the corneal layer at these sites.4 A different theory attributes pool palms to a mechanical origin, such as repeated microtrauma from contact with the rough concrete surfaces of swimming pools.5 This theory further states that the chemicals in pool water, such as chlorine and sodium hypochlorite, rarely produce irritant, allergic, or urticarial reactions.3
Based on these theories, we hypothesized that fingertip pulpitis can result from activities other than swimming (eg, skateboarding). Our case supports the latter theory on fingertip pulpitis in pool palms being a result of frictional dermatitis rather than wet dermatitis because we attributed our patient’s findings to contact with rough surfaces during skateboarding. Although the patient did swim, he only did so once weekly in the summer months, and the lesions had been persistent for 2 years consistently. His skateboarding hobby was more frequent, and he endorsed contact of the pads of the bilateral second to fifth fingers to the rough surfaces of the road and skateboard. The patient did not have lesions on the toes, further supporting the hypothesis that skateboarding led to the current presentation.
In children, hand-foot-and-mouth disease classically presents with oval-shaped, erythematous vesicles on the palmar surfaces of the hands and feet and generally is accompanied by fever and sore throat.6 Furthermore, unlike in our case, the viral exanthem usually would be present for up to 3 weeks and would not persist for more than 2 years. Erythema multiforme has an erythematous color and can present on the palms; however, the lesions have a classic targetoid appearance. It would be unique for erythema multiforme to present only on the fingertips rather than more diffusely on the palms or in other areas such as the face.7 Limited cutaneous sclerosis (scleroderma) initially can present with edematous pitted scars on the digital tips; however, with time the fingers will have a taut, white, shiny appearance that can develop into contractures and debilitating ulcerations.8 In our patient, the plaques did not advance to any further disease. Lastly, in contrast to our patient, punctate palmoplantar keratoderma presents as hyperkeratotic, firm, translucent, or opaque papules on the palms and soles. Over time, the papules can appear verrucous or callouslike.9 In our case, the plaques on the fingertips were erythematous rather than translucent or opaque papules.
Our case raises questions on whether prior reports of pool palms can be attributed to other activities involving contact with rough surfaces. More research is needed on the frequency and duration of rough surface exposure resulting in fingertip pulpitis.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
- Lopez-Neyra A, Vano-Galvan S, Alvarez-Twose I, et al. Pool palms [in Spanish]. Dermatol Online J. 2009;15:17.
- Wong LC, Rogers M. Pool palms. Pediatr Dermatol. 2007;24:95.
- Mandojana RM. Pool palms. J Am Acad Dermatol. 1993;28(2 pt 1):280-281.
- Novoa A, Klear S. Pool palms [published online September 30, 2015]. Arch Dis Child. 2016;101:41.
- Martín JM, Martín JM, Ricart JM. Erythematous-violaceous lesions on the palms [in Spanish]. Actas Dermosifiliogr. 2009;100:507-508.
- Marcini AJ, Shani-Adir A. Other viral diseases. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:1345-1366.
- French LE, Prins C. Erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrosis. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:319-334.
- Connoly MK. Systemic sclerosis (scleroderma) and related disorders. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:643-646.
- Krol AL, Siegel D. Keratodermas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Ltd; 2012:871-886.
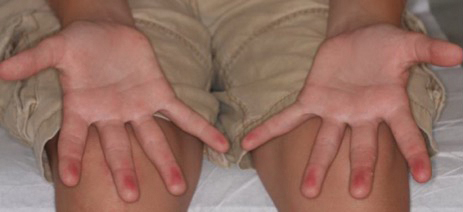
A 12-year-old boy presented with well-defined, blanchable, erythematous patches on the distal bilateral palmar aspects of the second to fifth fingers of 2 years’ duration. The patient stated that he skateboarded daily throughout the year and swam once weekly in the summer months. Furthermore, the patient cited frequent contact with the rough undersurface of the skateboard and concrete road surfaces while skateboarding. He stated that the lesions were always present and worsened in the summer months. The lesions had an occasional burning sensation when they were more prominently erythematous, and the patient denied any pattern of exacerbation, numbness, bleeding, or itching. There was no notable family history or evidence of systemic disease.
Id Reaction Associated With Red Tattoo Ink
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.

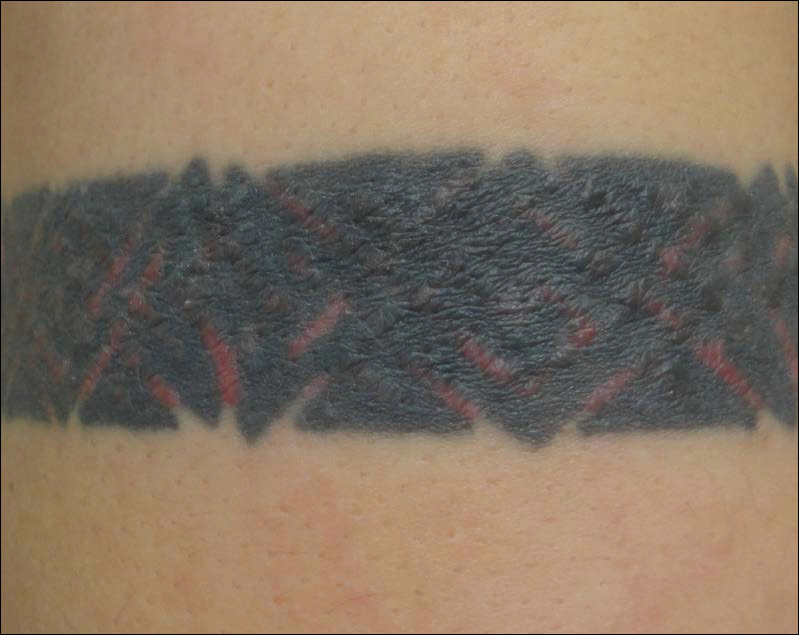
Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.
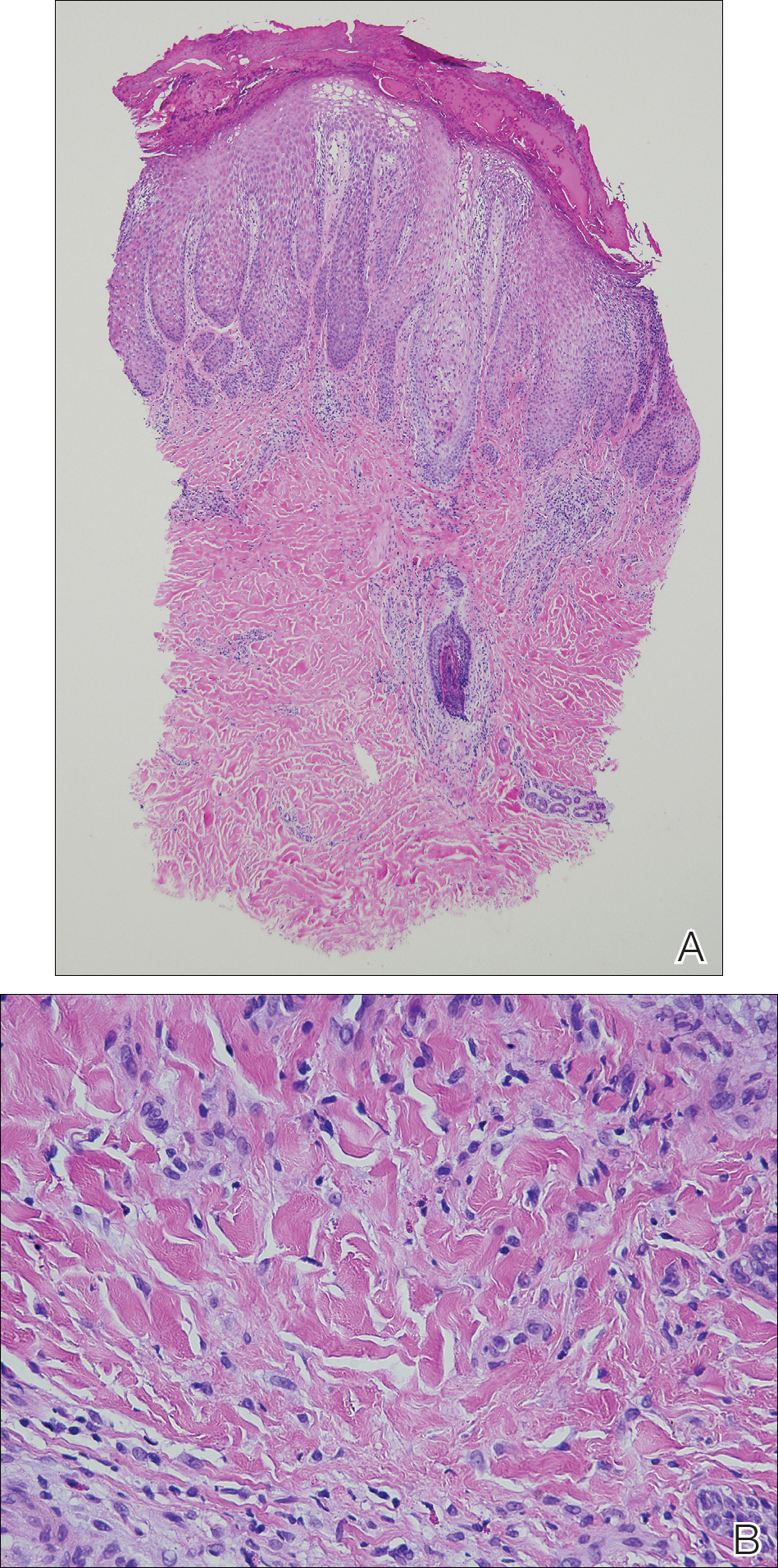
The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.


Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.

The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.


Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.

The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
Practice Points
- Hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos. Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity.
- Id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization.
- Further investigation of color additives in tattoo pigments is warranted to better elucidate the components responsible for cutaneous allergic reactions associated with tattoo ink.
Ice Pack–Induced Perniosis: A Rare and Underrecognized Association
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).
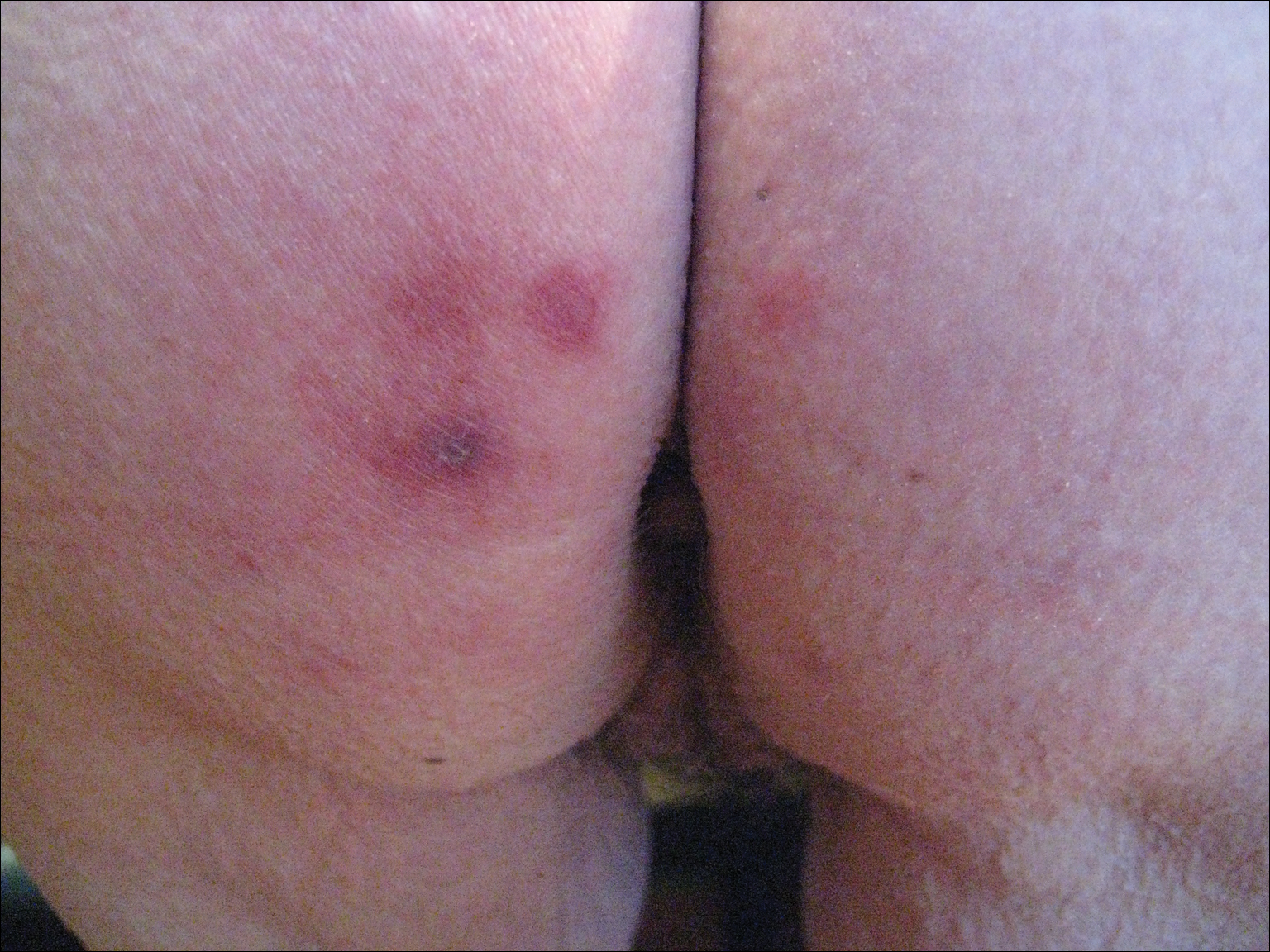
After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.
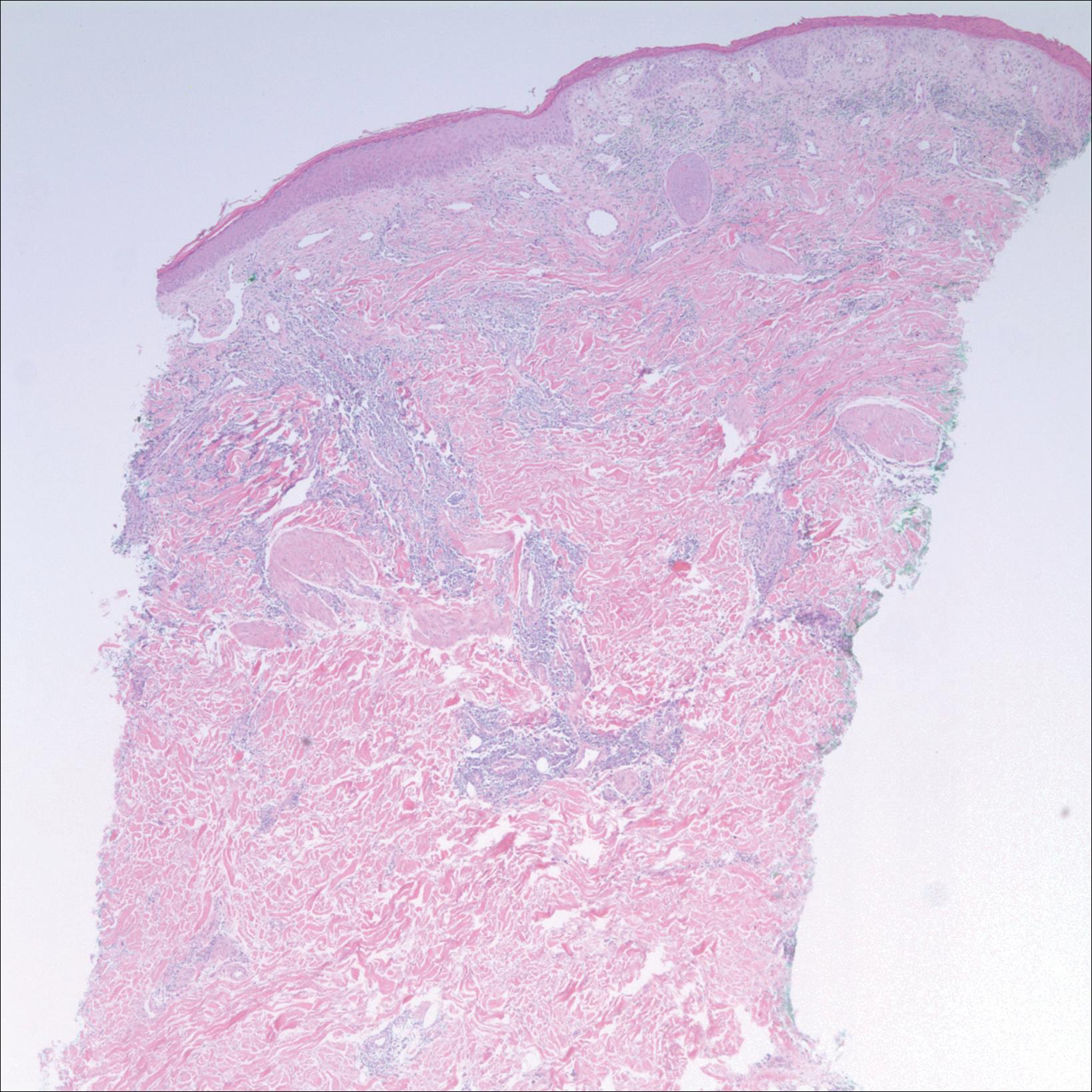
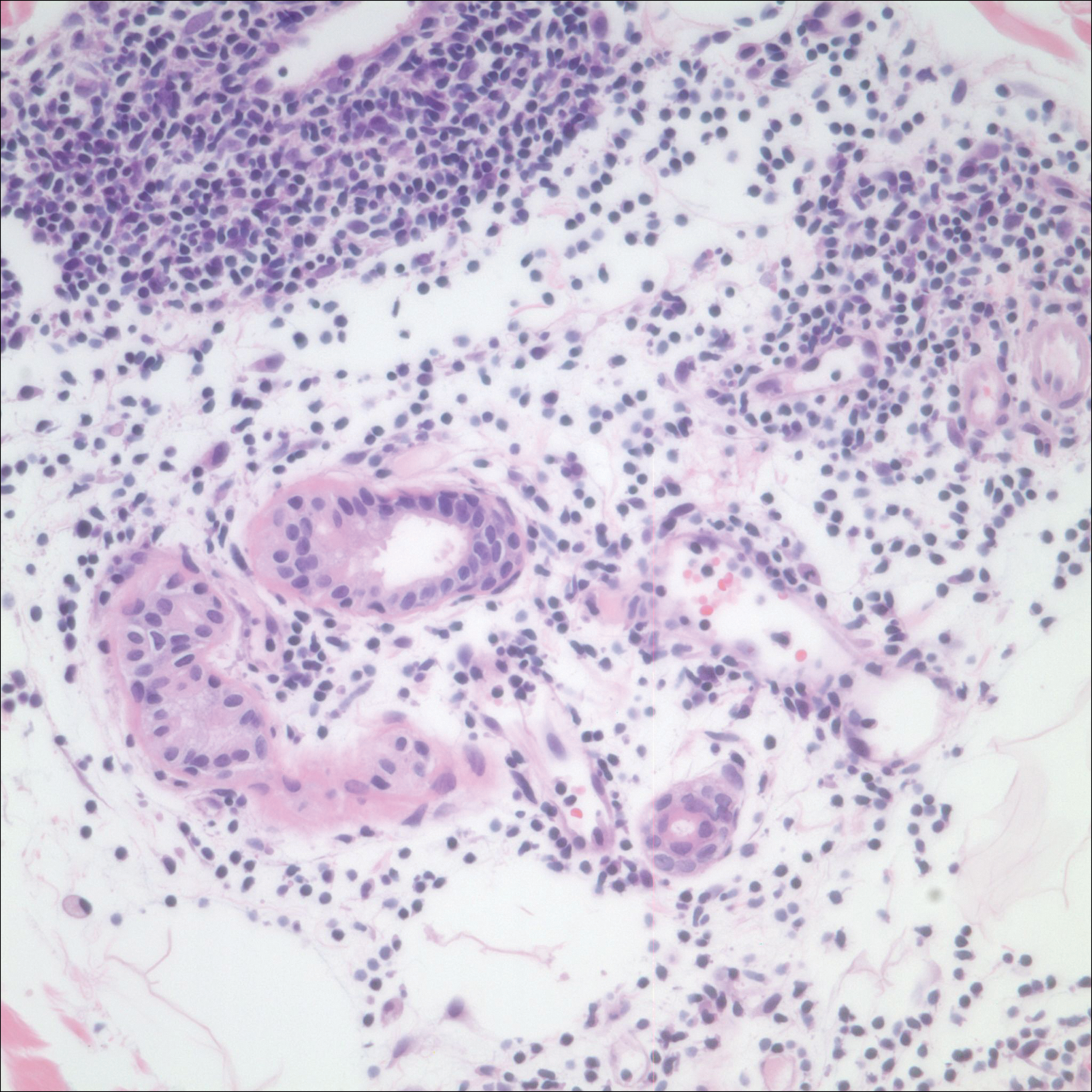
Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Practice Points
- Ice pack-induced perniosis is a rare condition that can occur in patients using long-term ice pack therapy.
- This entity histopathologically mimics cutaneous lupus erythematosus and can present a diagnostic challenge.
- A thorough clinical history and awareness of this diagnosis is essential for diagnostic accuracy.
Gemcitabine-Induced Pseudocellulitis
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.
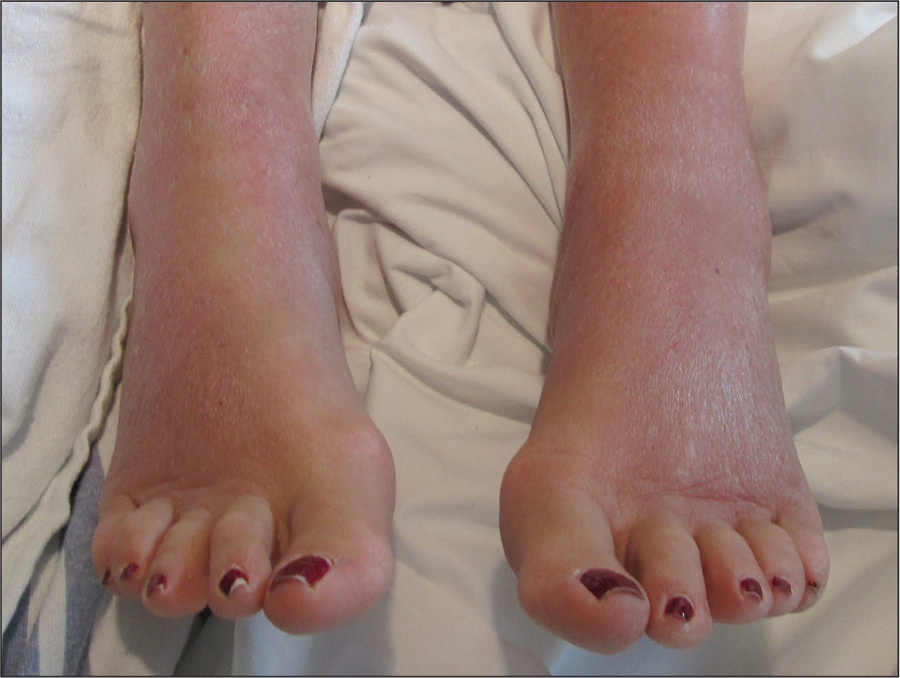
Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.
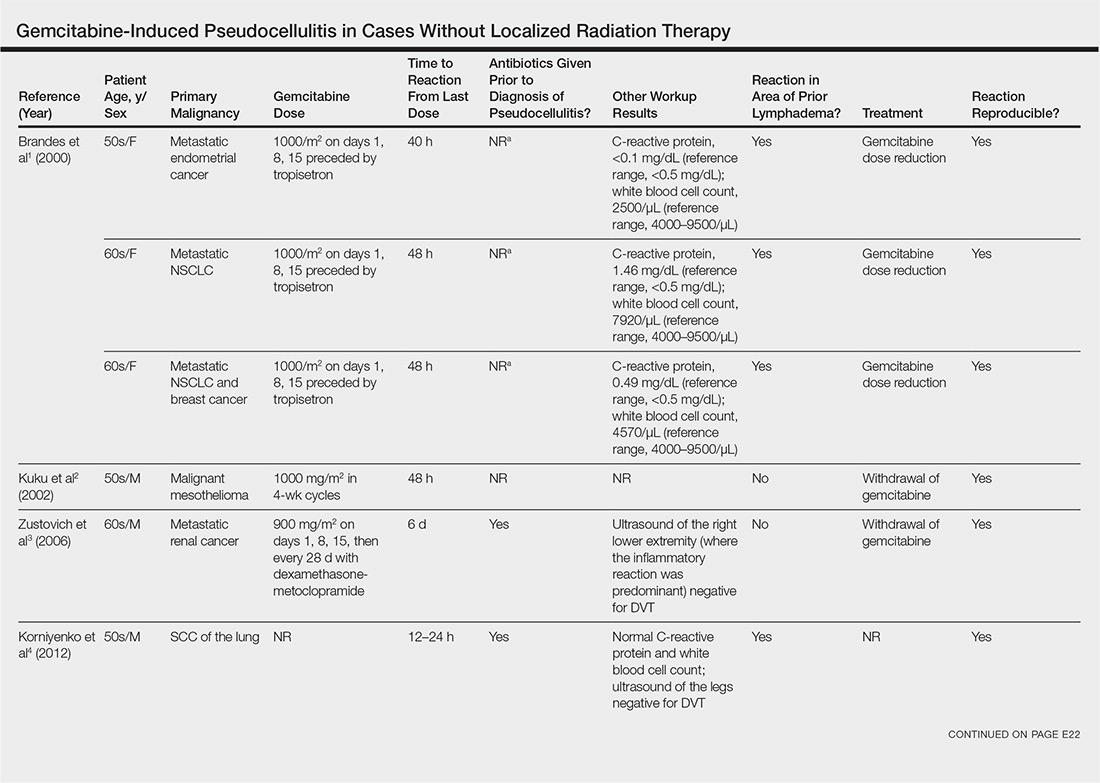
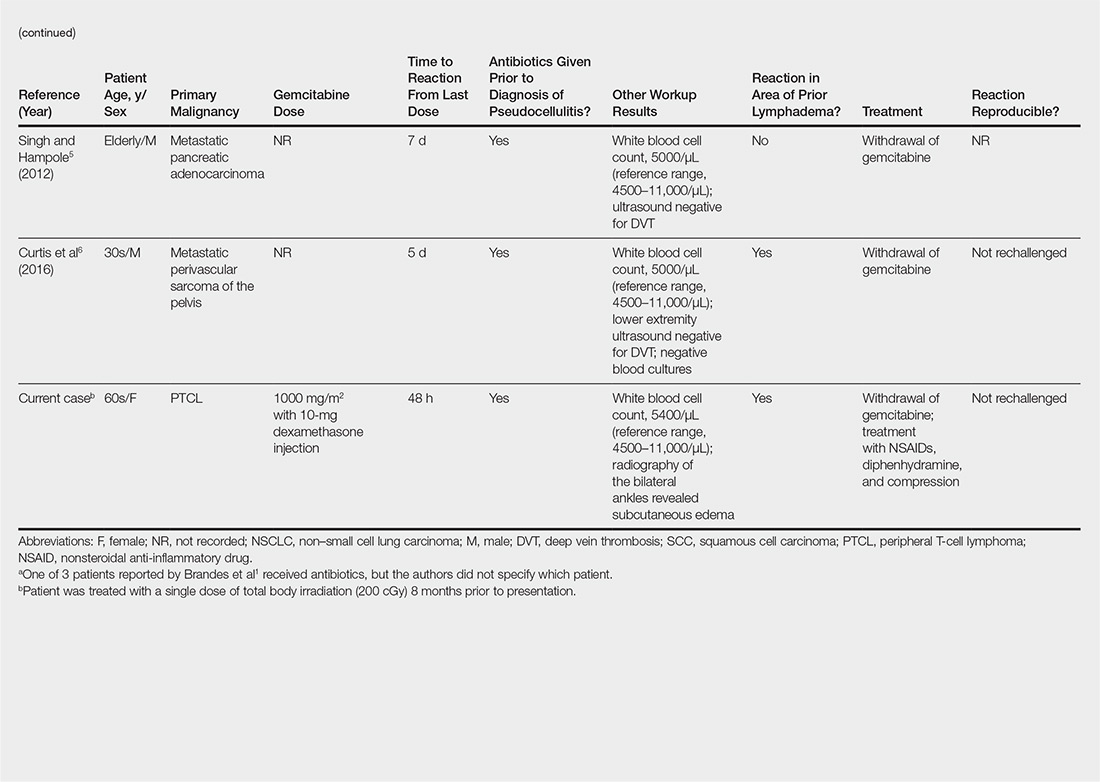
Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.

Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.


Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
To the Editor:
Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies. Cutaneous toxicities include radiation recall dermatitis and erysipelaslike reactions that occur in areas not previously treated with radiation. Often referred to as pseudocellulitis, these reactions generally have been reported in areas of lymphedema in patients with solid malignancies.1-6 Herein, we report a rare case of gemcitabine-induced pseudocellulitis on the legs in a patient with a history of hematologic malignancy and total body irradiation (TBI).
A 61-year-old woman with history of peripheral T-cell lymphoma presented to the emergency department at our institution with acute-onset redness, tenderness, and swelling of the legs that was concerning for cellulitis. The patient’s history was notable for receiving gemcitabine 1000 mg/m2 for treatment of refractory lymphoma (12 and 4 days prior to presentation) as well as lymphedema of the legs. Her complete treatment course included multiple rounds of chemotherapy and matched unrelated donor nonmyeloablative allogeneic stem cell transplantation with a single dose of TBI at 200 cGy at our institution. Her transplant was complicated only by mild cutaneous graft-versus-host disease, which resolved with prednisone and tacrolimus.
On physical examination, the patient was afebrile with symmetric erythema and induration extending from the bilateral knees to the dorsal feet. A complete blood cell count was notable for a white blood cell count of 5400/µL (reference range, 4500–11,000/µL) and a platelet count of 96,000/µL (reference range, 150,000–400,000/µL). Plain film radiographs of the bilateral ankles were remarkable only for moderate subcutaneous edema. She received vancomycin in the emergency department and was admitted to the oncology service. Blood cultures drawn on admission were negative. Dermatology was consulted on admission, and a diagnosis of pseudocellulitis was made in conjunction with oncology (Figure). Antibiotics were held, and the patient was treated symptomatically with ibuprofen and was discharged 1 day after admission. The reaction resolved after 1 week with the use of diphenhydramine, nonsteroidal anti-inflammatory drugs, and compression. The patient was not rechallenged with gemcitabine.

Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy. Reported cases have suggested key characteristics of pseudocellulitis (Table). The reaction is characterized by localized erythema, edema, and tenderness of the skin, with onset generally 48 hours to 1 week after receiving gemcitabine.1-6 Lymphedema appears to be a risk factor.1,3-5 Six cases (including the current case) demonstrated confinement of these findings to areas of prior lymphedema.1,4,6 Infectious workup is negative, and rechallenging with gemcitabine likely will reproduce the reaction. Unlike radiation recall dermatitis, there is no prior localized radiation exposure.


Our patient had a history of hematologic malignancy and a one-time low-dose TBI of 200 cGy, unlike the other reported cases described in the Table. It is difficult to attribute our patient’s localized eruption to radiation recall given the history of TBI. The clinical examination, laboratory findings, and time frame of the reaction were consistent with gemcitabine-induced pseudocellulitis.
It is important to be aware of pseudocellulitis as a possible complication of gemcitabine therapy in patients without history of localized radiation. Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics. Patients’ temperature, white blood cell count, clinical examination, and potentially ancillary studies (eg, vascular studies, inflammatory markers) should be reviewed carefully to determine whether there is an infectious or alternate etiology. In patients with known prior lymphedema, it may be beneficial to educate clinicians and patients alike about this potential adverse effect of gemcitabine and the high likelihood of recurrence on re-exposure.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
- Brandes A, Reichmann U, Plasswilm L, et al. Time- and dose-limiting erysipeloid rash confined to areas of lymphedema following treatment with gemcitabine—a report of three cases. Anticancer Drugs. 2000;11:15-17.
- Kuku I, Kaya E, Sevinc A, et al. Gemcitabine-induced erysipeloid skin lesions in a patient with malignant mesothelioma. J Eur Acad Dermatol Venereol. 2002;16:271-272.
- Zustovich F, Pavei P, Cartei G. Erysipeloid skin toxicity induced by gemcitabine. J Eur Acad Dermatol Venereol. 2006;20:757-758.
- Korniyenko A, Lozada J, Ranade A, et al. Recurrent lower extremity pseudocellulitis. Am J Ther. 2012;19:e141-e142.
- Singh A, Hampole H. Gemcitabine associated pseudocellulitis [published online June 14, 2012]. J Gen Intern Med. 2012;27:1721.
- Curtis S, Hong S, Gucalp R, et al. Gemcitabine-induced pseudocellulitis in a patient with recurrent lymphedema: a case report and review of the current literature. Am J Ther. 2016;23:e321-323.
Practice Points
- Gemcitabine is a nucleoside analogue used to treat a variety of solid and hematologic malignancies.
- Gemcitabine-induced pseudocellulitis is a rare cutaneous side effect of gemcitabine therapy.
- Early recognition of pseudocellulitis may prevent unnecessary exposure to broad-spectrum antibiotics.
Postirradiation Morphea: Unique Presentation on the Breast
To the Editor:
Postirradiation morphea (PIM) is a rare but well-documented phenomenon that primarily occurs in breast cancer patients who have received radiation therapy; however, it also has been reported in patients who have received radiation therapy for lymphoma as well as endocervical, endometrial, and gastric carcinomas.1 Importantly, clinicians must be able to recognize and differentiate this condition from other causes of new-onset induration and erythema of the breast, such as cancer recurrence, a new primary malignancy, or inflammatory etiologies (eg, radiation or contact dermatitis). Typically, PIM presents months to years after radiation therapy as an erythematous patch within the irradiated area that progressively becomes indurated. We report an unusual case of PIM with a reticulated appearance occurring 3 weeks after radiotherapy, chemotherapy, and surgery for an infiltrating ductal carcinoma of the left breast.
A 62-year-old woman presented to the dermatology department with a stage IIA, lymph node–negative, estrogen and progesterone receptor–negative, human epidermal growth factor receptor 2–negative infiltrating ductal carcinoma of the left breast. She was treated with a partial mastectomy of the left breast followed by external beam radiotherapy to the entire left breast in combination with chemotherapy (doxorubicin, cyclophosphamide, paclitaxel). The patient received 15 fractions of 270 cGy (4050 cGy total) with a weekly 600-cGy boost over 21 days without any complications.
Three weeks after finishing radiation therapy, the patient developed redness and swelling of the left breast that did not encompass the entire radiation field. There was no associated pain or pruritus. She was treated by her surgical oncologist with topical calendula and 3 courses of cephalexin for suspected mastitis with only modest improvement, then was referred to dermatology 3 months later.
At the initial dermatology evaluation, the patient reported little improvement after antibiotics and topical calendula. On physical examination, there were erythematous, reticulated, dusky, indurated patches on the entire left breast. The area of most pronounced induration surrounded the surgical scar on the left superior breast. Punch biopsy for hematoxylin and eosin staining and tissue cultures was obtained at this appointment. The patient was started on doxycycline 100 mg twice daily and was instructed to apply triamcinolone ointment 0.1% twice daily to the affected area. After 1 month of therapy, she reported slight improvement in the degree of erythema with this regimen, but the involved area continued to extend outside of the radiation field to the central chest wall and medial right breast (Figure 1). Two additional biopsies—one from the central chest and another from the right breast—were then taken over the course of 4 months, given the consistently inconclusive clinicopathologic nature and failure of the eruption to respond to antibiotics plus topical corticosteroids.
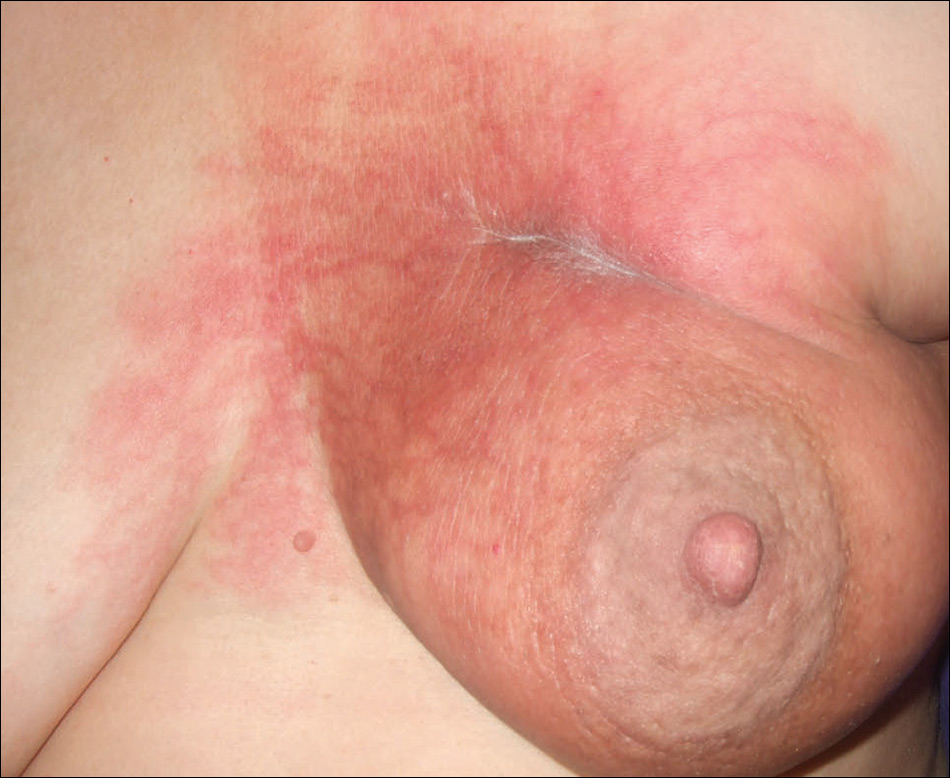
Punch biopsy from the central chest revealed a sparse perivascular infiltrate comprised predominantly of lymphocytes with occasional eosinophils (Figure 2). There were foci suggestive of early dermal sclerosis, an increased number of small blood vessels in the dermis, and scattered enlarged fibroblasts. Metastatic carcinoma was not identified. Although the histologic findings were not entirely specific, the changes were most suggestive of PIM, for which the patient was started on pentoxifylline (400 mg 3 times daily) and oral vitamin E supplementation (400 IU daily). At subsequent follow-up appointments, she showed markedly decreased skin erythema and induration.
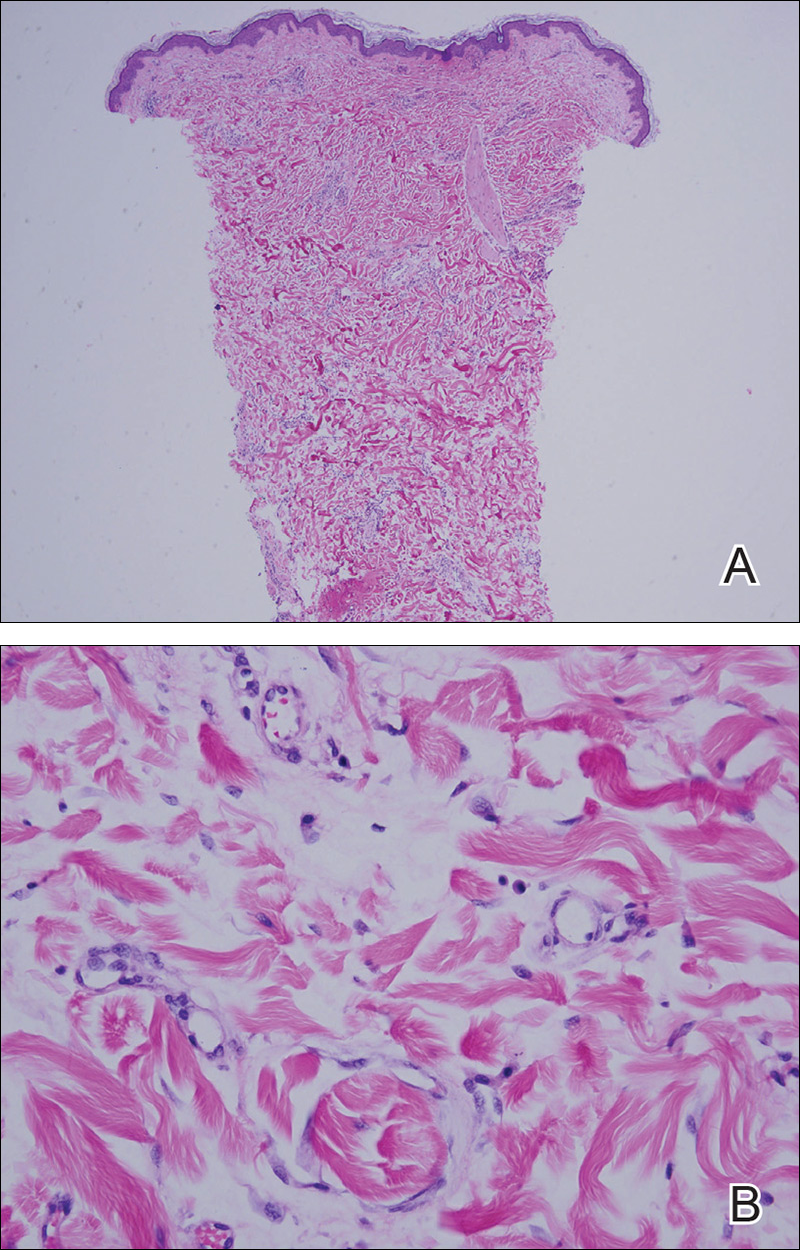
Morphea, also known as localized scleroderma, is an inflammatory skin condition characterized by sclerosis of the dermis and subcutis leading to scarlike tissue formation. Worldwide incidence ranges from 0.4 to 2.7 cases per 100,000 individuals with a predilection for white women.2 Unlike systemic scleroderma, morphea patients lack Raynaud phenomenon and visceral involvement.3,4
There are several clinical subtypes of morphea, including plaque, linear, generalized, and pansclerotic morphea. Lesions may vary in appearance based on configuration, stage of development, and depth of involvement.4 During the earliest phases, morphea lesions are asymptomatic, asymmetrically distributed, erythematous to violaceous patches or subtly indurated plaques expanding centrifugally with a lilac ring. Central sclerosis with loss of follicles and sweat glands is a later finding associated with advanced disease. Moreover, some reports of early-stage morphea have suggested a reticulated or geographic vascular morphology that may be misdiagnosed for other conditions such as a port-wine stain.5
Local skin exposures have long been hypothesized to contribute to development of morphea, including infection, especially Borrelia burgdorferi; trauma; chronic venous insufficiency; cosmetic surgery; medications; and exposure to toxic cooking oils, silicones, silica, pesticides, organic solvents, and vinyl chloride.2,6,7
Radiation therapy is an often overlooked cause of morphea. It was first described in 1905 but then rarely discussed until a 1989 case series of 9 patients, 7 of whom had received irradiation for breast cancer.8,9 Today, the increasing popularity of lumpectomy plus radiation therapy for treatment of early-stage breast cancer has led to a rise in PIM incidence.10
In contrast to other radiation-induced skin conditions, development of PIM is independent of the presence or absence of adjuvant chemotherapy, type of radiation therapy, or the total radiation dose or fractionation number, with reported doses ranging from less than 20.0 Gy to up to 59.4 Gy and dose fractions ranging from 10 to 30. In 20% to 30% of cases, PIM extends beyond the radiation field, sometimes involving distant sites never exposed to high-energy rays.1,10,11 This observation suggests a mechanism reliant on more widespread cascade rather than solely local tissue damage.
Prominent culture-negative, lymphoplasmacytic inflammation is another important diagnostic clue. Radiation dermatitis and fibrosis do not have the marked erythematous to violaceous hue seen in early morphea plaques. This color seen in early morphea plaques may be intense enough and in a geographic pattern, emulating a vascular lesion.
There is no standardized treatment of PIM, but traditional therapies for morphea may provide some benefit. Several randomized controlled clinical trials have shown success with pentoxifylline and oral vitamin E supplementation to treat or prevent radiation-induced breast fibrosis.12 Extrapolating from this data, our patient was started on this combination therapy and showed marked improvement in skin color and texture.
- Morganroth PA, Dehoratius D, Curry H, et al. Postirradiation morphea: a case report with a review of the literature and summary of the clinicopathologic differential diagnosis [published online October 4, 2013]. Am J
Dermatopathol. doi:10.1097/DAD.0b013e3181cb3fdd. - Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228; quiz 229-230.
- Noh JW, Kim J, Kim JW. Localized scleroderma: a clinical study at a single center in Korea. Int J Rheum Dis. 2013;16:437-441.
- Vasquez R, Sendejo C, Jacobe H. Morphea and other localized forms of scleroderma. Curr Opin Rheumatol. 2012;24:685-693.
- Nijhawan RI, Bard S, Blyumin M, et al. Early localized morphea mimicking an acquired port-wine stain. J Am Acad Dermatol. 2011;64:779-782.
- Haustein UF, Ziegler V. Environmentally induced systemic sclerosis-like disorders. Int J Dermatol. 1985;24:147-151.
- Mora GF. Systemic sclerosis: environmental factors. J Rheumatol. 2009;36:2383-2396.
- Colver GB, Rodger A, Mortimer PS, et al. Post-irradiation morphoea. Br J Dermatol. 1989;120:831-835.
- Crocker HR. Diseases of the Skin: Their Description, Pathology, Diagnosis, and Treatment. Philadelphia, PA: P. Blakiston Son & Co; 1905.
- Laetsch B, Hofer T, Lombriser N, et al. Irradiation-induced morphea: x-rays as triggers of autoimmunity. Dermatology. 2011;223:9-12.
- Shetty G, Lewis F, Thrush S. Morphea of the breast: case reports and review of literature. Breast J. 2007;13:302-304.
- Jacobson G, Bhatia S, Smith BJ, et al. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85:604-608.
To the Editor:
Postirradiation morphea (PIM) is a rare but well-documented phenomenon that primarily occurs in breast cancer patients who have received radiation therapy; however, it also has been reported in patients who have received radiation therapy for lymphoma as well as endocervical, endometrial, and gastric carcinomas.1 Importantly, clinicians must be able to recognize and differentiate this condition from other causes of new-onset induration and erythema of the breast, such as cancer recurrence, a new primary malignancy, or inflammatory etiologies (eg, radiation or contact dermatitis). Typically, PIM presents months to years after radiation therapy as an erythematous patch within the irradiated area that progressively becomes indurated. We report an unusual case of PIM with a reticulated appearance occurring 3 weeks after radiotherapy, chemotherapy, and surgery for an infiltrating ductal carcinoma of the left breast.
A 62-year-old woman presented to the dermatology department with a stage IIA, lymph node–negative, estrogen and progesterone receptor–negative, human epidermal growth factor receptor 2–negative infiltrating ductal carcinoma of the left breast. She was treated with a partial mastectomy of the left breast followed by external beam radiotherapy to the entire left breast in combination with chemotherapy (doxorubicin, cyclophosphamide, paclitaxel). The patient received 15 fractions of 270 cGy (4050 cGy total) with a weekly 600-cGy boost over 21 days without any complications.
Three weeks after finishing radiation therapy, the patient developed redness and swelling of the left breast that did not encompass the entire radiation field. There was no associated pain or pruritus. She was treated by her surgical oncologist with topical calendula and 3 courses of cephalexin for suspected mastitis with only modest improvement, then was referred to dermatology 3 months later.
At the initial dermatology evaluation, the patient reported little improvement after antibiotics and topical calendula. On physical examination, there were erythematous, reticulated, dusky, indurated patches on the entire left breast. The area of most pronounced induration surrounded the surgical scar on the left superior breast. Punch biopsy for hematoxylin and eosin staining and tissue cultures was obtained at this appointment. The patient was started on doxycycline 100 mg twice daily and was instructed to apply triamcinolone ointment 0.1% twice daily to the affected area. After 1 month of therapy, she reported slight improvement in the degree of erythema with this regimen, but the involved area continued to extend outside of the radiation field to the central chest wall and medial right breast (Figure 1). Two additional biopsies—one from the central chest and another from the right breast—were then taken over the course of 4 months, given the consistently inconclusive clinicopathologic nature and failure of the eruption to respond to antibiotics plus topical corticosteroids.

Punch biopsy from the central chest revealed a sparse perivascular infiltrate comprised predominantly of lymphocytes with occasional eosinophils (Figure 2). There were foci suggestive of early dermal sclerosis, an increased number of small blood vessels in the dermis, and scattered enlarged fibroblasts. Metastatic carcinoma was not identified. Although the histologic findings were not entirely specific, the changes were most suggestive of PIM, for which the patient was started on pentoxifylline (400 mg 3 times daily) and oral vitamin E supplementation (400 IU daily). At subsequent follow-up appointments, she showed markedly decreased skin erythema and induration.

Morphea, also known as localized scleroderma, is an inflammatory skin condition characterized by sclerosis of the dermis and subcutis leading to scarlike tissue formation. Worldwide incidence ranges from 0.4 to 2.7 cases per 100,000 individuals with a predilection for white women.2 Unlike systemic scleroderma, morphea patients lack Raynaud phenomenon and visceral involvement.3,4
There are several clinical subtypes of morphea, including plaque, linear, generalized, and pansclerotic morphea. Lesions may vary in appearance based on configuration, stage of development, and depth of involvement.4 During the earliest phases, morphea lesions are asymptomatic, asymmetrically distributed, erythematous to violaceous patches or subtly indurated plaques expanding centrifugally with a lilac ring. Central sclerosis with loss of follicles and sweat glands is a later finding associated with advanced disease. Moreover, some reports of early-stage morphea have suggested a reticulated or geographic vascular morphology that may be misdiagnosed for other conditions such as a port-wine stain.5
Local skin exposures have long been hypothesized to contribute to development of morphea, including infection, especially Borrelia burgdorferi; trauma; chronic venous insufficiency; cosmetic surgery; medications; and exposure to toxic cooking oils, silicones, silica, pesticides, organic solvents, and vinyl chloride.2,6,7
Radiation therapy is an often overlooked cause of morphea. It was first described in 1905 but then rarely discussed until a 1989 case series of 9 patients, 7 of whom had received irradiation for breast cancer.8,9 Today, the increasing popularity of lumpectomy plus radiation therapy for treatment of early-stage breast cancer has led to a rise in PIM incidence.10
In contrast to other radiation-induced skin conditions, development of PIM is independent of the presence or absence of adjuvant chemotherapy, type of radiation therapy, or the total radiation dose or fractionation number, with reported doses ranging from less than 20.0 Gy to up to 59.4 Gy and dose fractions ranging from 10 to 30. In 20% to 30% of cases, PIM extends beyond the radiation field, sometimes involving distant sites never exposed to high-energy rays.1,10,11 This observation suggests a mechanism reliant on more widespread cascade rather than solely local tissue damage.
Prominent culture-negative, lymphoplasmacytic inflammation is another important diagnostic clue. Radiation dermatitis and fibrosis do not have the marked erythematous to violaceous hue seen in early morphea plaques. This color seen in early morphea plaques may be intense enough and in a geographic pattern, emulating a vascular lesion.
There is no standardized treatment of PIM, but traditional therapies for morphea may provide some benefit. Several randomized controlled clinical trials have shown success with pentoxifylline and oral vitamin E supplementation to treat or prevent radiation-induced breast fibrosis.12 Extrapolating from this data, our patient was started on this combination therapy and showed marked improvement in skin color and texture.
To the Editor:
Postirradiation morphea (PIM) is a rare but well-documented phenomenon that primarily occurs in breast cancer patients who have received radiation therapy; however, it also has been reported in patients who have received radiation therapy for lymphoma as well as endocervical, endometrial, and gastric carcinomas.1 Importantly, clinicians must be able to recognize and differentiate this condition from other causes of new-onset induration and erythema of the breast, such as cancer recurrence, a new primary malignancy, or inflammatory etiologies (eg, radiation or contact dermatitis). Typically, PIM presents months to years after radiation therapy as an erythematous patch within the irradiated area that progressively becomes indurated. We report an unusual case of PIM with a reticulated appearance occurring 3 weeks after radiotherapy, chemotherapy, and surgery for an infiltrating ductal carcinoma of the left breast.
A 62-year-old woman presented to the dermatology department with a stage IIA, lymph node–negative, estrogen and progesterone receptor–negative, human epidermal growth factor receptor 2–negative infiltrating ductal carcinoma of the left breast. She was treated with a partial mastectomy of the left breast followed by external beam radiotherapy to the entire left breast in combination with chemotherapy (doxorubicin, cyclophosphamide, paclitaxel). The patient received 15 fractions of 270 cGy (4050 cGy total) with a weekly 600-cGy boost over 21 days without any complications.
Three weeks after finishing radiation therapy, the patient developed redness and swelling of the left breast that did not encompass the entire radiation field. There was no associated pain or pruritus. She was treated by her surgical oncologist with topical calendula and 3 courses of cephalexin for suspected mastitis with only modest improvement, then was referred to dermatology 3 months later.
At the initial dermatology evaluation, the patient reported little improvement after antibiotics and topical calendula. On physical examination, there were erythematous, reticulated, dusky, indurated patches on the entire left breast. The area of most pronounced induration surrounded the surgical scar on the left superior breast. Punch biopsy for hematoxylin and eosin staining and tissue cultures was obtained at this appointment. The patient was started on doxycycline 100 mg twice daily and was instructed to apply triamcinolone ointment 0.1% twice daily to the affected area. After 1 month of therapy, she reported slight improvement in the degree of erythema with this regimen, but the involved area continued to extend outside of the radiation field to the central chest wall and medial right breast (Figure 1). Two additional biopsies—one from the central chest and another from the right breast—were then taken over the course of 4 months, given the consistently inconclusive clinicopathologic nature and failure of the eruption to respond to antibiotics plus topical corticosteroids.

Punch biopsy from the central chest revealed a sparse perivascular infiltrate comprised predominantly of lymphocytes with occasional eosinophils (Figure 2). There were foci suggestive of early dermal sclerosis, an increased number of small blood vessels in the dermis, and scattered enlarged fibroblasts. Metastatic carcinoma was not identified. Although the histologic findings were not entirely specific, the changes were most suggestive of PIM, for which the patient was started on pentoxifylline (400 mg 3 times daily) and oral vitamin E supplementation (400 IU daily). At subsequent follow-up appointments, she showed markedly decreased skin erythema and induration.

Morphea, also known as localized scleroderma, is an inflammatory skin condition characterized by sclerosis of the dermis and subcutis leading to scarlike tissue formation. Worldwide incidence ranges from 0.4 to 2.7 cases per 100,000 individuals with a predilection for white women.2 Unlike systemic scleroderma, morphea patients lack Raynaud phenomenon and visceral involvement.3,4
There are several clinical subtypes of morphea, including plaque, linear, generalized, and pansclerotic morphea. Lesions may vary in appearance based on configuration, stage of development, and depth of involvement.4 During the earliest phases, morphea lesions are asymptomatic, asymmetrically distributed, erythematous to violaceous patches or subtly indurated plaques expanding centrifugally with a lilac ring. Central sclerosis with loss of follicles and sweat glands is a later finding associated with advanced disease. Moreover, some reports of early-stage morphea have suggested a reticulated or geographic vascular morphology that may be misdiagnosed for other conditions such as a port-wine stain.5
Local skin exposures have long been hypothesized to contribute to development of morphea, including infection, especially Borrelia burgdorferi; trauma; chronic venous insufficiency; cosmetic surgery; medications; and exposure to toxic cooking oils, silicones, silica, pesticides, organic solvents, and vinyl chloride.2,6,7
Radiation therapy is an often overlooked cause of morphea. It was first described in 1905 but then rarely discussed until a 1989 case series of 9 patients, 7 of whom had received irradiation for breast cancer.8,9 Today, the increasing popularity of lumpectomy plus radiation therapy for treatment of early-stage breast cancer has led to a rise in PIM incidence.10
In contrast to other radiation-induced skin conditions, development of PIM is independent of the presence or absence of adjuvant chemotherapy, type of radiation therapy, or the total radiation dose or fractionation number, with reported doses ranging from less than 20.0 Gy to up to 59.4 Gy and dose fractions ranging from 10 to 30. In 20% to 30% of cases, PIM extends beyond the radiation field, sometimes involving distant sites never exposed to high-energy rays.1,10,11 This observation suggests a mechanism reliant on more widespread cascade rather than solely local tissue damage.
Prominent culture-negative, lymphoplasmacytic inflammation is another important diagnostic clue. Radiation dermatitis and fibrosis do not have the marked erythematous to violaceous hue seen in early morphea plaques. This color seen in early morphea plaques may be intense enough and in a geographic pattern, emulating a vascular lesion.
There is no standardized treatment of PIM, but traditional therapies for morphea may provide some benefit. Several randomized controlled clinical trials have shown success with pentoxifylline and oral vitamin E supplementation to treat or prevent radiation-induced breast fibrosis.12 Extrapolating from this data, our patient was started on this combination therapy and showed marked improvement in skin color and texture.
- Morganroth PA, Dehoratius D, Curry H, et al. Postirradiation morphea: a case report with a review of the literature and summary of the clinicopathologic differential diagnosis [published online October 4, 2013]. Am J
Dermatopathol. doi:10.1097/DAD.0b013e3181cb3fdd. - Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228; quiz 229-230.
- Noh JW, Kim J, Kim JW. Localized scleroderma: a clinical study at a single center in Korea. Int J Rheum Dis. 2013;16:437-441.
- Vasquez R, Sendejo C, Jacobe H. Morphea and other localized forms of scleroderma. Curr Opin Rheumatol. 2012;24:685-693.
- Nijhawan RI, Bard S, Blyumin M, et al. Early localized morphea mimicking an acquired port-wine stain. J Am Acad Dermatol. 2011;64:779-782.
- Haustein UF, Ziegler V. Environmentally induced systemic sclerosis-like disorders. Int J Dermatol. 1985;24:147-151.
- Mora GF. Systemic sclerosis: environmental factors. J Rheumatol. 2009;36:2383-2396.
- Colver GB, Rodger A, Mortimer PS, et al. Post-irradiation morphoea. Br J Dermatol. 1989;120:831-835.
- Crocker HR. Diseases of the Skin: Their Description, Pathology, Diagnosis, and Treatment. Philadelphia, PA: P. Blakiston Son & Co; 1905.
- Laetsch B, Hofer T, Lombriser N, et al. Irradiation-induced morphea: x-rays as triggers of autoimmunity. Dermatology. 2011;223:9-12.
- Shetty G, Lewis F, Thrush S. Morphea of the breast: case reports and review of literature. Breast J. 2007;13:302-304.
- Jacobson G, Bhatia S, Smith BJ, et al. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85:604-608.
- Morganroth PA, Dehoratius D, Curry H, et al. Postirradiation morphea: a case report with a review of the literature and summary of the clinicopathologic differential diagnosis [published online October 4, 2013]. Am J
Dermatopathol. doi:10.1097/DAD.0b013e3181cb3fdd. - Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228; quiz 229-230.
- Noh JW, Kim J, Kim JW. Localized scleroderma: a clinical study at a single center in Korea. Int J Rheum Dis. 2013;16:437-441.
- Vasquez R, Sendejo C, Jacobe H. Morphea and other localized forms of scleroderma. Curr Opin Rheumatol. 2012;24:685-693.
- Nijhawan RI, Bard S, Blyumin M, et al. Early localized morphea mimicking an acquired port-wine stain. J Am Acad Dermatol. 2011;64:779-782.
- Haustein UF, Ziegler V. Environmentally induced systemic sclerosis-like disorders. Int J Dermatol. 1985;24:147-151.
- Mora GF. Systemic sclerosis: environmental factors. J Rheumatol. 2009;36:2383-2396.
- Colver GB, Rodger A, Mortimer PS, et al. Post-irradiation morphoea. Br J Dermatol. 1989;120:831-835.
- Crocker HR. Diseases of the Skin: Their Description, Pathology, Diagnosis, and Treatment. Philadelphia, PA: P. Blakiston Son & Co; 1905.
- Laetsch B, Hofer T, Lombriser N, et al. Irradiation-induced morphea: x-rays as triggers of autoimmunity. Dermatology. 2011;223:9-12.
- Shetty G, Lewis F, Thrush S. Morphea of the breast: case reports and review of literature. Breast J. 2007;13:302-304.
- Jacobson G, Bhatia S, Smith BJ, et al. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85:604-608.
Practice Points
- Radiation therapy is an often overlooked cause of morphea.
- The increasing popularity of lumpectomy plus radiation therapy for treatment of early-stage breast cancer has led to a rise in postirradiation morphea incidence.
- Tissue changes occur as early as weeks or as late as 32 years after radiation treatment.
- Postirradiation morphea may extend beyond the radiation field.