User login
Allergy Testing in Dermatology and Beyond
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.

Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
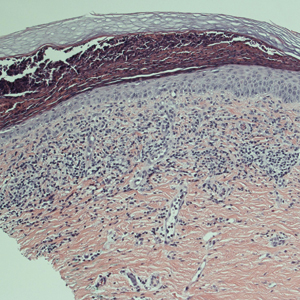
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9
Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10
There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.

Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9

Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10

There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
Allergy testing typically refers to evaluation of a patient for suspected type I or type IV hypersensitivity.1,2 The possibility of type I hypersensitivity is raised in patients presenting with food allergies, allergic rhinitis, asthma, and immediate adverse reactions to medications, whereas type IV hypersensitivity is suspected in patients with eczematous eruptions, delayed adverse cutaneous reactions to medications, and failure of metallic implants (eg, metal joint replacements, cardiac stents) in conjunction with overlying skin rashes (Table 1).1-5 Type II (eg, pemphigus vulgaris) and type III (eg, IgA vasculitis) hypersensitivities are not evaluated with screening allergy tests.

Type I Sensitization
Type I hypersensitivity is an immediate hypersensitivity mediated predominantly by IgE activation of mast cells in the skin as well as the respiratory and gastric mucosa.1 Sensitization of an individual patient occurs when antigen-presenting cells induce a helper T cell (TH2) cytokine response leading to B-cell class switching and allergen-specific IgE production. Upon repeat exposure to the allergen, circulating antibodies then bind to high-affinity receptors on mast cells and basophils and initiate an allergic inflammatory response, leading to a clinical presentation of allergic rhinitis, urticaria, or immediate drug reactions. Confirming type I sensitization may be performed via serologic (in vitro) or skin testing (in vivo).5,6
Serologic Testing (In Vitro)
Serologic testing is a blood test that detects circulating IgE levels against specific allergens.5 The first such test, the radioallergosorbent test, was introduced in the 1970s but is not quantitative and is no longer used. Although common, it is inaccurate to describe current serum IgE (s-IgE) testing as radioallergosorbent testing. There are several US Food and Drug Administration-approved s-IgE assays in common use, and these tests may be helpful in elucidating relevant allergens and for tailoring therapy appropriately, which may consist of avoidance of certain foods or environmental agents and/or allergen immunotherapy.
Skin Testing (In Vivo)
Skin testing can be performed percutaneously (eg, percutaneous skin testing) or intradermally (eg, intradermal testing).6 Percutaneous skin testing is performed by placing a drop of allergen extract on the skin, after which a lancet is used to lightly scratch the skin; intradermal testing is performed by injecting a small amount of allergen extract into the dermis. In both cases, the skin is evaluated after 15 to 20 minutes for the presence and size of a cutaneous wheal. Medications with antihistaminergic activity must be discontinued prior to testing. Both s-IgE and skin testing assess for type I hypersensitivity, and factors such as extensive rash, concern for anaphylaxis, or inability to discontinue antihistamines may favor s-IgE testing versus skin testing. False-positive results can occur with both tests, and for this reason, test results should always be interpreted in conjunction with clinical examination and patient history to determine relevant allergies.
Type IV Sensitization
Type IV hypersensitivity is a delayed hypersensitivity mediated primarily by lymphocytes.2 Sensitization occurs when haptens bind to host proteins and are presented by epidermal and dermal dendritic cells to T lymphocytes in the skin. These lymphocytes then migrate to regional lymph nodes where antigen-specific T lymphocytes are produced and home back to the skin. Upon reexposure to the allergen, these memory T lymphocytes become activated and incite a delayed allergic response. Confirming type IV hypersensitivity primarily is accomplished via patch testing, though other testing modalities exist.
Skin Biopsy
Biopsy is sometimes performed in the workup of an individual presenting with allergic contact dermatitis (ACD) and typically will show spongiosis with normal stratum corneum and epidermal thickness in the setting of acute ACD and mild to marked acanthosis and parakeratosis in chronic ACD.7 The findings, however, are nonspecific and the differential of these histopathologic findings encompasses nummular dermatitis, atopic dermatitis, irritant contact dermatitis, and dyshidrotic eczema, among others. The presence of eosinophils and Langerhans cell microabscesses may provide supportive evidence for ACD over the other spongiotic dermatitides.7,8
Patch Testing
Patch testing is the gold standard in diagnosing type IV hypersensitivities resulting in a clinical presentation of ACD. Hundreds of allergens are commercially available for patch testing, and more commonly tested allergens fall into one of several categories, such as cosmetic preservatives, rubbers, metals, textiles, fragrances, adhesives, antibiotics, plants, and even corticosteroids. Of note, a common misconception is that ACD must result from new exposures; however, patients may develop ACD secondary to an exposure or product they have been using for many years without a problem.
Three commonly used screening series are the thin-layer rapid use epicutaneous (T.R.U.E.) test (SmartPractice), North American Contact Dermatitis Group screening series, and American Contact Dermatitis Society Core 80 allergen series, which have some variation in the type and number of allergens included (Table 2). The T.R.U.E. test will miss a notable number of clinically relevant allergens in comparison to the North American Contact Dermatitis Group and American Contact Dermatitis Society Core series, and it may be of particularly low utility in identifying fragrance or preservative ACD.9

Allergens are placed on the back in chambers in a petrolatum or aqueous medium. The patches remain affixed for 48 hours, during which time the patient is asked to refrain from showering or exercising to prevent loss of patches. The patient's skin is then evaluated for reactions to allergens on 2 separate occasions: at the time of patch removal 48 hours after initial placement, then the areas of patches are marked for delayed readings at day 4 to day 7 after initial patch placement. Results are scored based on the degree of the inflammatory reaction (Table 3). Delayed readings beyond day 7 may be necessary for metals, specific preservatives (eg, dodecyl gallate, propolis), and neomycin.10

There is a wide spectrum of cutaneous disease that should prompt consideration of patch testing, including well-circumscribed eczematous dermatitis (eg, recurrent lip, hand, and foot dermatitis); patchy or diffuse eczema, especially if recently worsened and/or unresponsive to topical steroids; lichenoid eruptions, particularly of mucosal surfaces; mucous membrane eruptions (eg, stomatitis, vulvitis); and eczematous presentations that raise concern for airborne (photodistributed) or systemic contact dermatitis.11-13 Although further studies of efficacy and safety are ongoing, patch testing also may be useful in the diagnosis of nonimmediate cutaneous adverse drug reactions, especially fixed drug eruptions, acute generalized exanthematous pustulosis, systemic contact dermatitis from medications, and drug-induced hypersensitivity syndrome.3 Lastly, patients with type IV hypersensitivity to metals, adhesives, or antibiotics used in metallic orthopedic or cardiac implants may experience implant failure, regional contact dermatitis, or both, and benefit from patch testing prior to implant replacement to assess for potential allergens. Of the joints that fail, it is estimated that up to 5% are due to metal hypersensitivity.4
Throughout patch testing, patients may continue to manage their skin condition with oral antihistamines and topical steroids, though application to the site at which the patches are applied should be avoided throughout patch testing and during the week prior. According to expert consensus, immunosuppressive medications that are less likely to impact patch testing and therefore may be continued include low-dose methotrexate, oral prednisone less than 10 mg daily, biologic therapy, and low-dose cyclosporine (<2 mg/kg daily). Therapeutic interventions that are more likely to impact patch testing and should be avoided include phototherapy or extensive sun exposure within a week prior to testing, oral prednisone more than 10 mg daily, intramuscular triamcinolone within the preceding month, and high-dose cyclosporine (>2 mg/kg daily).14
An important component to successful patch testing is posttest patient counseling. Providers can create a safe list of products for patients by logging onto the American Contact Dermatitis Society website and accessing the Contact Allergen Management Program (CAMP).15 All relevant allergens found on patch testing may be selected and patient-specific identification codes generated. Once these codes are entered into the CAMP app on the patient's cellular device, a personalized, regularly updated list of safe products appears for many categories of products, including shampoos, sunscreens, moisturizers, cosmetic products, and laundry or dish detergents, among others. Of note, this app is not helpful for avoidance in patients with textile allergies. Patients should be counseled that improvement occurs with avoidance, which usually occurs within weeks but may slowly occur over time in some cases.
Lymphocyte Transformation Test (In Vitro)
The lymphocyte transformation test is an experimental in vitro test for type IV hypersensitivity. This serologic test utilizes allergens to stimulate memory T lymphocytes in vitro and measures the degree of response to the allergen. Although this test has generated excitement, particularly for the potential to safely evaluate for severe adverse cutaneous drug reactions, it currently is not the standard of care and is not utilized in the United States.16
Conclusion
Dermatologists play a vital role in the workup of suspected type IV hypersensitivities. Patch testing is an important but underutilized tool in the arsenal of allergy testing and may be indicated in a wide variety of cutaneous presentations, adverse reactions to medications, and implanted device failures. Identification and avoidance of a culprit allergen has the potential to lead to complete resolution of disease and notable improvement in quality of life for patients.
Acknowledgments
The author thanks Nina Botto, MD (San Francisco, California), for her mentorship in the arena of ACD as well as the Women's Dermatologic Society for the support they provided through the mentorship program.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
- Oettgen H, Broide DH. Introduction to the mechanisms of allergic disease. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1-32.
- Werfel T, Kapp A. Atopic dermatitis and allergic contact dermatitis. In: Holgate ST, Church MK, Broide DH, et al, eds. Allergy. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:263-286.
- Zinn A, Gayam S, Chelliah MP, et al. Patch testing for nonimmediate cutaneous adverse drug reactions. J Am Acad Dermatol. 2018;78:421-423.
- Thyssen JP, Menne T, Schalock PC, et al. Pragmatic approach to the clinical work-up of patients with putative allergic disease to metallic orthopaedic implants before and after surgery. Br J Dermatol. 2011;164:473-478.
- Cox L. Overview of serological-specific IgE antibody testing in children. Curr Allergy Asthma Rep. 2011;11:447-453.
- Dolen WK. Skin testing and immunoassays for allergen-specific IgE. Clin Rev Allergy Immunol. 2001;21:229-239.
- Keeling BH, Gavino AC, Gavino AC. Skin biopsy, the allergists' tool: how to interpret a report. Curr Allergy Asthma Rep. 2015;15:62.
- Rosa G, Fernandez AP, Vij A, et al. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J Cutan Pathol. 2016;43:498-504.
- DeKoven JG, Warshaw EM, Belsito DV. North American Contact Dermatitis Group patch test results 2013-2014. Dermatitis. 2017;28:33-46.
- Davis MD, Bhate K, Rohlinger AL, et al. Delayed patch test reading after 5 days: the Mayo Clinic experience. J Am Acad Dermatol. 2008;59:225-233.
- Rajagopalan R, Anderson RT. The profile of a patient with contact dermatitis and a suspicion of contact allergy (history, physical characteristics, and dermatology-specific quality of life). Am J Contact Dermat. 1997;8:26-31.
- Huygens S, Goossens A. An update on airborne contact dermatitis. Contact Dermatitis. 2001;44:1-6.
- Salam TN, Fowler JF. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Fowler JF, Maibach HI, Zirwas M, et al. Effects of immunomodulatory agents on patch testing: expert opinion 2012. Dermatitis. 2012;23:301-303.
- ACDS CAMP. American Contact Dermatitis Society website. https://www.contactderm.org/i4a/pages/index.cfm?pageid=3489. Accessed November 14, 2018.
- Popple A, Williams J, Maxwell G, et al. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016;13:84-91.
Lesions With a Distinct Black Pigment
The Diagnosis: Black-Spot Poison Ivy
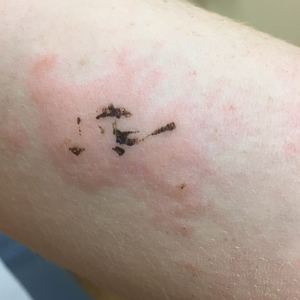
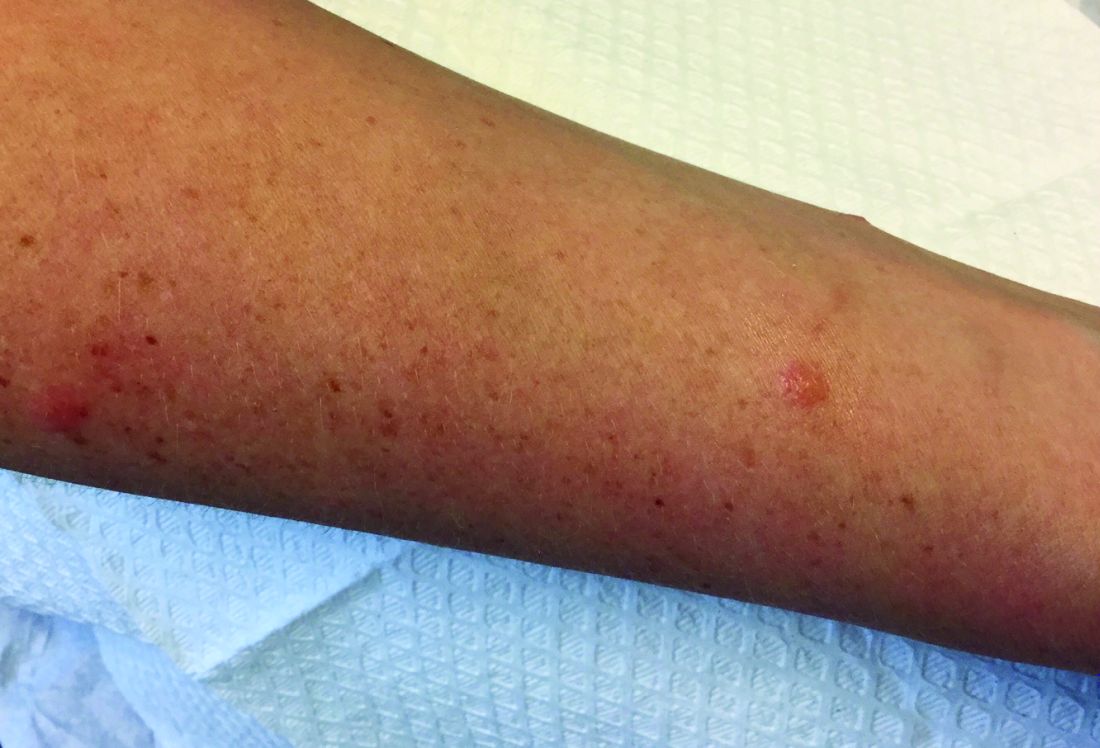
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.
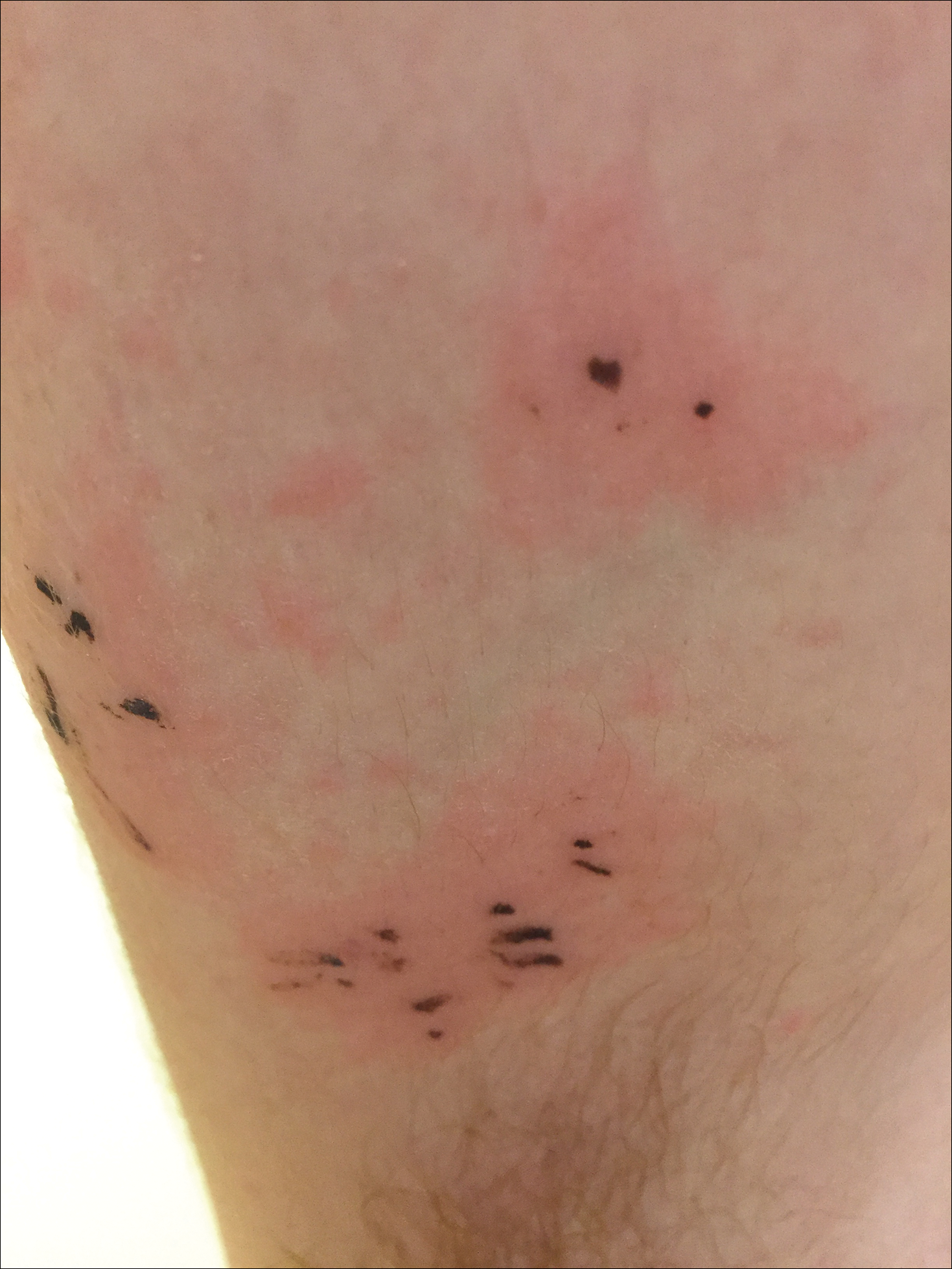
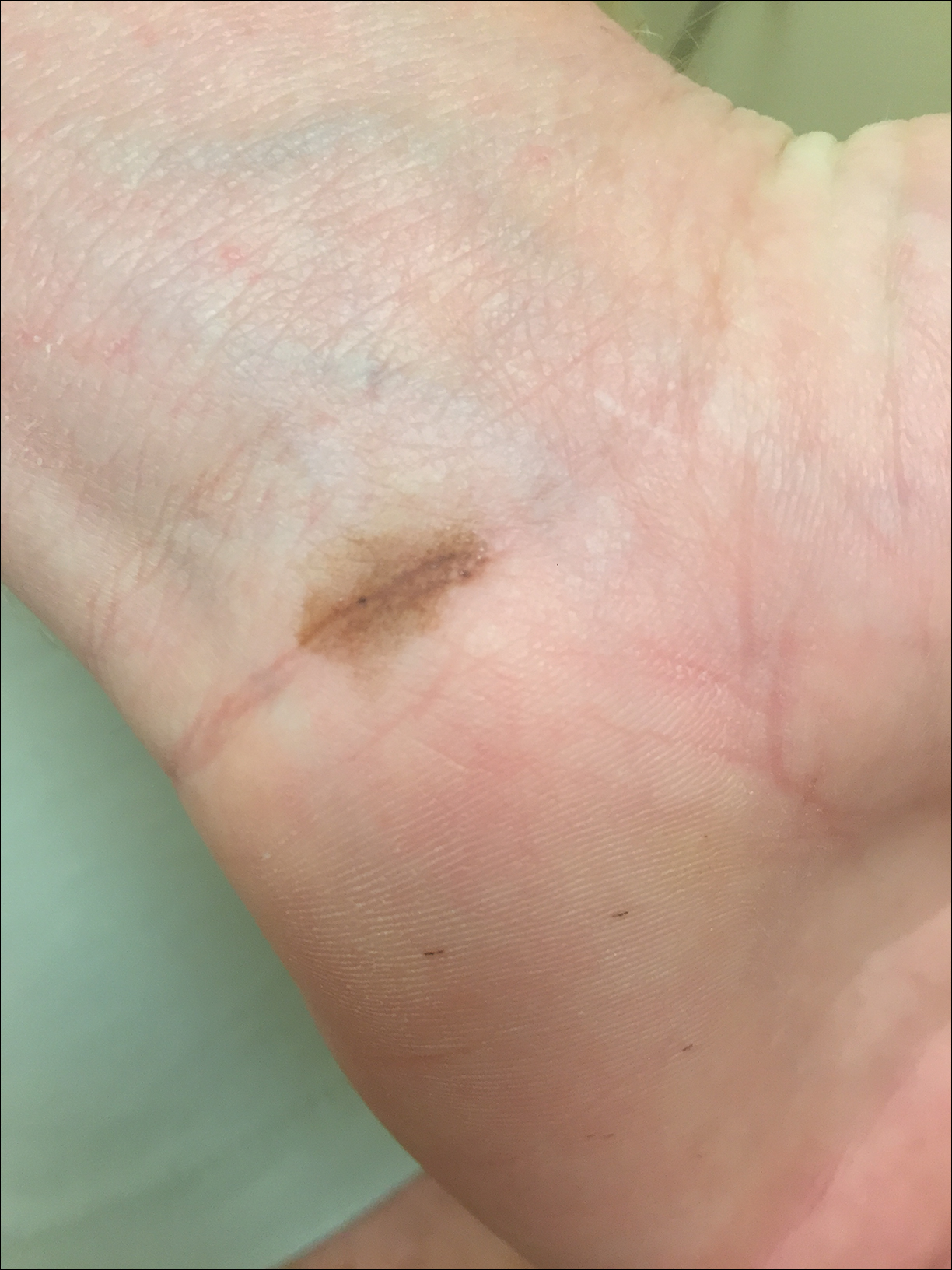
Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
The Diagnosis: Black-Spot Poison Ivy
Due to the detailed account of the patient's history including acuity of current presentation, history of recent activities, travel history, and recent exposures, as well as a thorough skin examination, a diagnosis of black-spot poison ivy was made. In this case, the linear distribution of the lesions with overlying black pigment that could not be removed (Figures 1 and 2) provided important clues to diagnosis.


Poison ivy is an allergic contact dermatitis that affects an estimated 25 to 40 million Americans annually who are exposed to its resin. Poison ivy is a plant from the Toxicodendron genus, and an estimated 85% of the North American population report sensitivity to these plants, of which poison ivy (Toxicodendron radicans) is the most common.1 Other related plants include poison sumac and poison oak. Poison ivy and other Toxicodendron plants produce urushiol, the oleoresin responsible for one of the most common allergic contact dermatitides in the United States.2 Black-spot poison ivy is an uncommon presentation following exposure to urushiol or oleoresin,3 as sufficient concentration of urushiol on the skin rarely is achieved.3,4 This plant's resin oxidizes and turns coal black when exposed to air.5 Contact with enough of this oleoresin will produce black-spot poison ivy.6 Patients with sufficient concentrations of oleoresin on their skin to cause this black oxidation usually have similar black spots on their clothing.7 Interestingly, some Toxicodendron species, such as the Japanese lacquer tree, Toxicodendron vernicifluum, have a black lacquer sap that was historically used as ink.8 This ink was used on Chinese and Japanese jars and has caused contact dermatitis hundreds of years after they were created.7
Poison ivy is characterized by a generalized, pruritic, erythematous rash with vesicles and papules in a linear distribution.9 Black-spot poison ivy presents the same with the addition of black lacquer-like macules with surrounding erythema.10 The skin lesions usually appear on exposed areas 24 to 48 hours after contact.11 Histology of black-spot poison ivy lesions should reveal yellow material in the stratum corneum with epidermal necrosis, in addition to classic features of acute allergic contact dermatitis.3 Interestingly, because these lesions occur with the first exposure to poison ivy, a patient may not develop the typical itchy eczematous eruption characteristic of poison ivy dermatitis. Differential diagnosis includes superficial purpura; exogenous pigment such as marker, ink, or tattoo pigment; tinea nigra; purpuric allergic contact dermatitis to resins or dyes; arthropod assault; irritant contact dermatitis; and infectious and noninfectious vasculitis.11
Similar to poison ivy, treatment of black-spot poison ivy involves oral and topical steroids combined with antihistamines if the patient continues to experience pruritus.6,12 It was recommended to our patient to apply cool compresses with water or Burow solution to alleviate itching and promote drying of the lesions. Calamine lotion can provide similar outcomes.13 Once the oleoresin is oxidized and bound to skin, the black spots cannot be removed with soap, water, or alcohol. The black spots gradually desquamate 1 to 2 weeks after formation without scarring,11 and patients do not require further monitoring.1 Patients should clean or discard clothing and evaluate for possible sources of poison ivy exposure. Because this type of poison ivy dermatitis is rare, most health care workers likely have never seen black-spot poison ivy, and it is an important diagnosis to consider.13
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Usatine RP, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
- Hurwitz RM, Rivera HP, Guin JD. Black-spot poison ivy dermatitis. an acute irritant contact dermatitis superimposed upon an allergic contact dermatitis. Am J Dermatopathol. 1984;6:319-322.
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mallory SB, Hurwitz RM. Black-spot poison-ivy dermatitis. Clin Dermatol. 1986;4:149-151.
- Mallory SB, Miller OF, Tyler WB. Toxicodendron radicans dermatitis with black lacquer deposit on the skin. J Am Acad Dermatol. 1982;6:363-368.
- Rietschel R, Fowler J. Toxicodendron plants and species. Fisher's Contact Dermatitis. 4th ed. Baltimore, MD: Williams & Wilkins; 1995:469-472.
- Fisher AA. Poison ivy/oak dermatitis. part I: prevention--soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- McClanahan C, Asarch A, Swick BL. Black spot poison ivy. Int J Dermatol. 2014;53:752-753.
- Mu EW, Capell BC, Castelo-Soccio L. Black spots on a toddler's skin. Contemp Pediatr. 2013;30:31-32.
- Schram SE, Willey A, Lee PK, et al. Black-spot poison ivy. Dermatitis. 2008;19:48-51.
- Paniagua CT, Bean AS. Black-spot poison ivy: a rare phenomenon. J Am Acad Nurse Pract. 2011;23:275-277.
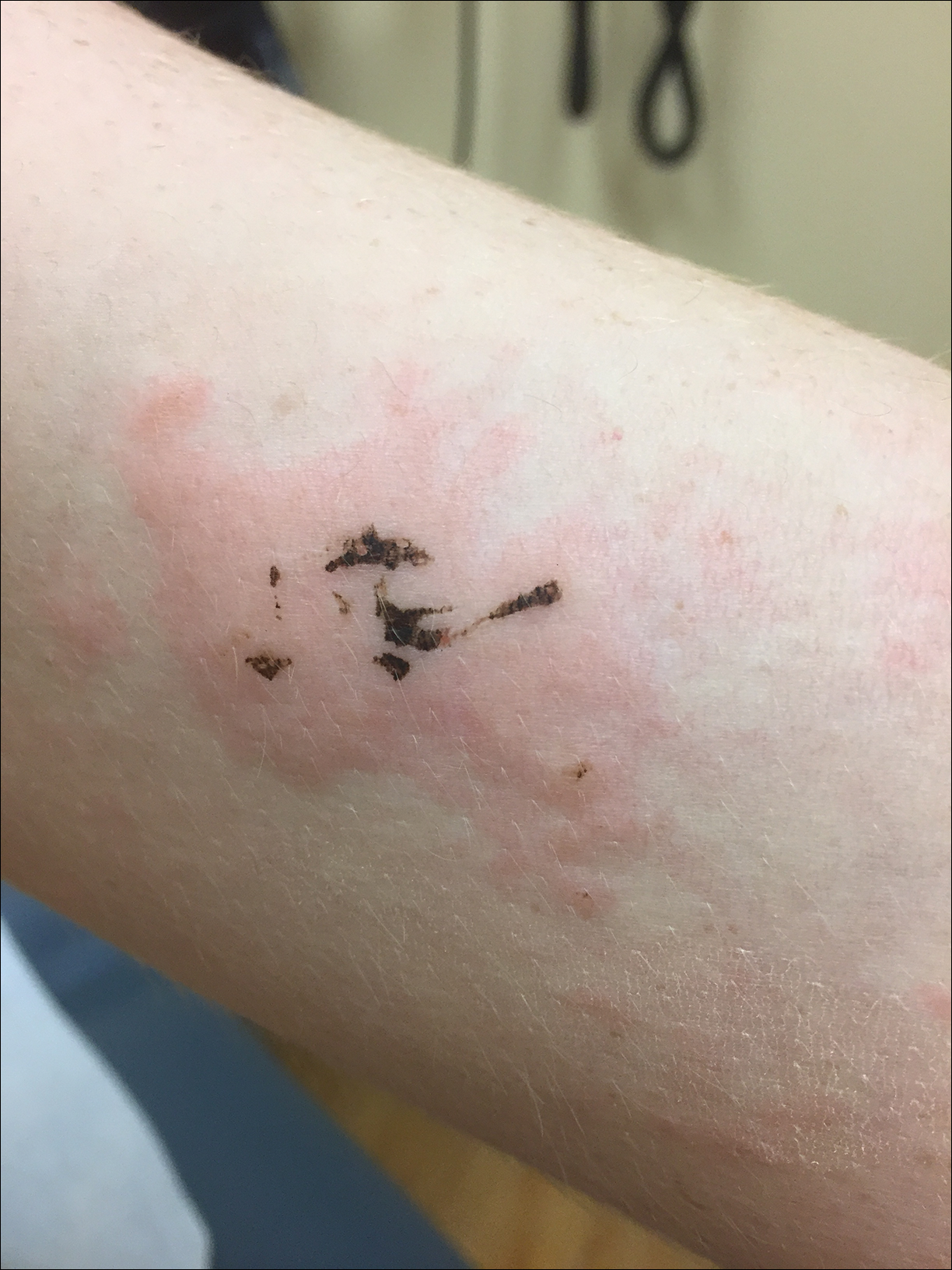
A 17-year-old adolescent boy presented to urgent care with a pruritic eruption on the bilateral arms of 1 day's duration. He was camping in the woods the night prior to presentation. On physical examination linear, erythematous, edematous plaques were observed bilaterally with overlying brown and black pigment on the arms. The pigment could not be removed with alcohol or vigorous scrubbing. The patient's condition improved with prednisone.
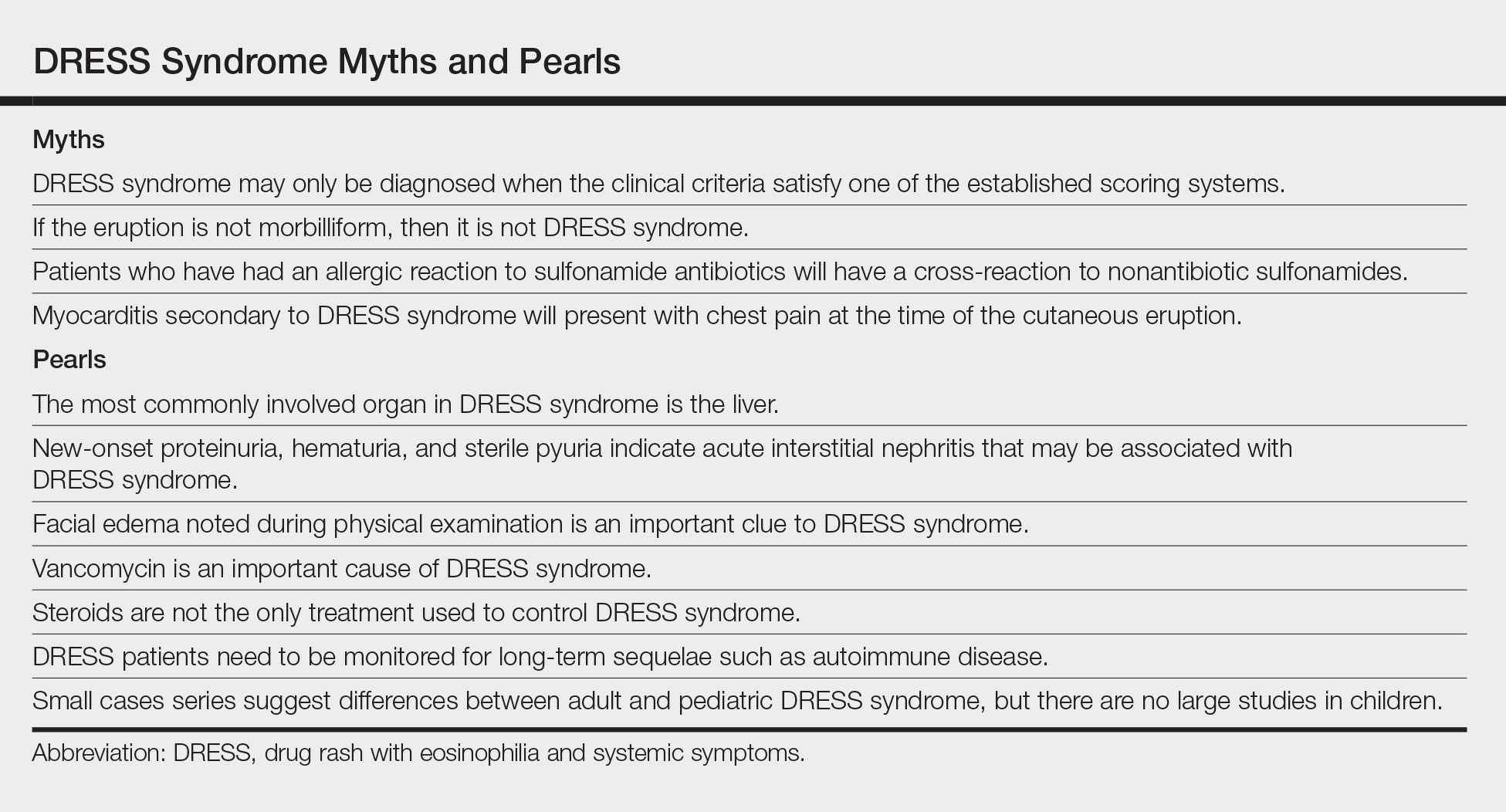
DRESS Syndrome: Clinical Myths and Pearls
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).
Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
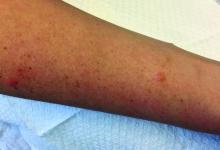
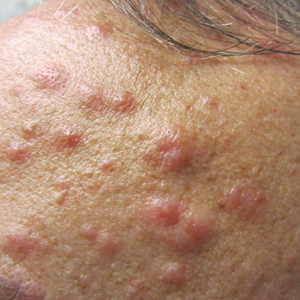
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11
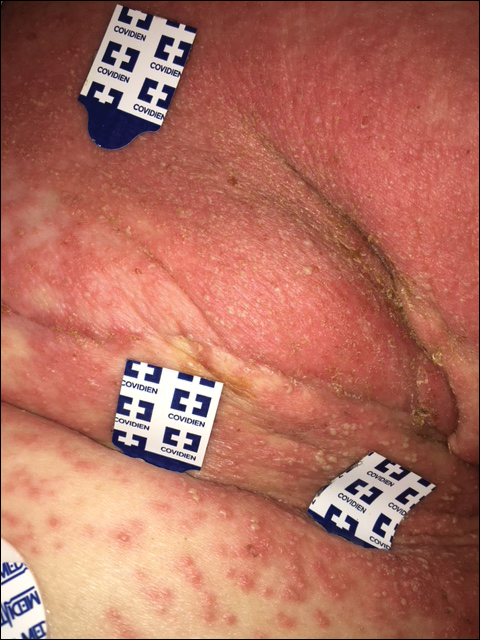
Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
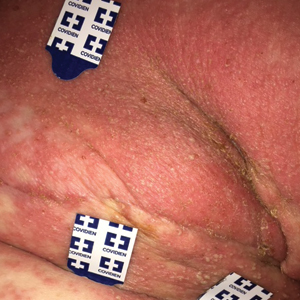
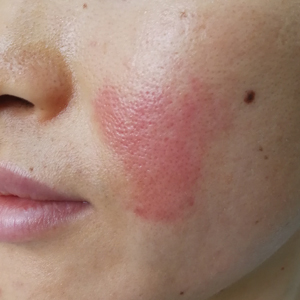
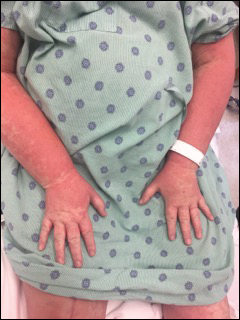
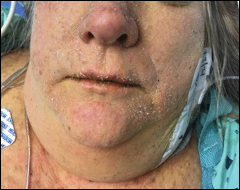
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13
Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).

Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11


Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13

Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
Drug rash with eosinophilia and systemic symptoms (DRESS syndrome), also known as drug-induced hypersensitivity syndrome, is an uncommon severe systemic hypersensitivity drug reaction. It is estimated to occur in 1 in every 1000 to 10,000 drug exposures.1 It can affect patients of all ages and typically presents 2 to 6 weeks after exposure to a culprit medication. Classically, DRESS syndrome presents with often widespread rash, facial edema, systemic symptoms such as fever, lymphadenopathy, and evidence of visceral organ involvement. Peripheral blood eosinophilia is frequently but not universally observed.1,2
Even with proper management, reported DRESS syndrome mortality rates worldwide are approximately 10%2 or higher depending on the degree and type of other organ involvement (eg, cardiac).3 Beyond the acute manifestations of DRESS syndrome, this condition is unique in that some patients develop late-onset sequelae such as myocarditis or autoimmune conditions even years after the initial cutaneous eruption.4 Therefore, longitudinal evaluation is a key component of management.
The clinical myths and pearls presented here highlight some of the commonly held assumptions regarding DRESS syndrome in an effort to illuminate subtleties of managing patients with this condition (Table).

Myth: DRESS syndrome may only be diagnosed when the clinical criteria satisfy one of the established scoring systems.
Patients with DRESS syndrome can have heterogeneous manifestations. As a result, patients may develop a drug hypersensitivity with biological behavior and a natural history compatible with DRESS syndrome that does not fulfill published diagnostic criteria.5 The syndrome also may reveal its component manifestations gradually, thus delaying the diagnosis. The terms mini-DRESS and skirt syndrome have been employed to describe drug eruptions that clearly have systemic symptoms and more complex and pernicious biologic behavior than a simple drug exanthema but do not meet DRESS syndrome criteria. Ultimately, it is important to note that in clinical practice, DRESS syndrome exists on a spectrum of severity and the diagnosis remains a clinical one.
Pearl: The most commonly involved organ in DRESS syndrome is the liver.
Liver involvement is the most common visceral organ involved in DRESS syndrome and is estimated to occur in approximately 45.0% to 86.1% of cases.6,7 If a patient develops the characteristic rash, peripheral blood eosinophilia, and evidence of liver injury, DRESS syndrome must be included in the differential diagnosis.
Hepatitis presenting in DRESS syndrome can be hepatocellular, cholestatic, or mixed.6,7 Case series are varied in whether the transaminitis of DRESS syndrome tends to be more hepatocellular8 or cholestatic.7 Liver dysfunction in DRESS syndrome often lasts longer than in other severe cutaneous adverse drug reactions, and patients may improve anywhere from a few days in milder cases to months to achieve resolution of abnormalities.6,7 Severe hepatic involvement is thought to be the most notable cause of mortality.9
Pearl: New-onset proteinuria, hematuria, and sterile pyuria indicate acute interstitial nephritis that may be associated with DRESS syndrome.
Acute interstitial nephritis (AIN) is a drug-induced form of acute kidney injury that can co-occur with DRESS syndrome. Acute interstitial nephritis can present with some combination of acute kidney injury, morbilliform eruption, eosinophilia, fever, and sometimes eosinophiluria. Although AIN can be distinct from DRESS syndrome, there are cases of DRESS syndrome associated with AIN.10 In the correct clinical context, urinalysis may help by showing new-onset proteinuria, new-onset hematuria, and sterile pyuria. More common causes of acute kidney injury such as prerenal etiologies and acute tubular necrosis have a bland urinary sediment.
Myth: If the eruption is not morbilliform, then it is not DRESS syndrome.
The most common morphology of DRESS syndrome is a morbilliform eruption (Figure 1), but urticarial and atypical targetoid (erythema multiforme–like) eruptions also have been described.9 Rarely, DRESS syndrome secondary to use of allopurinol or anticonvulsants may have a pustular morphology (Figure 2), which is distinguished from acute generalized exanthematous pustulosis by its delayed onset, more severe visceral involvement, and prolonged course.11


Another reported variant demonstrates overlapping features between Stevens-Johnson syndrome/toxic epidermal necrolysis and DRESS syndrome. It may present with mucositis, atypical targetoid lesions, and vesiculobullous lesions.12 It is unclear whether this reported variant is indeed a true subtype of DRESS syndrome, as Stevens-Johnson syndrome/toxic epidermal necrolysis may present with systemic symptoms, lymphadenopathy, hepatic, renal, and pulmonary complications, among other systemic disturbances.12
Pearl: Facial edema noted during physical examination is an important clue of DRESS syndrome.
Perhaps the most helpful findings in the diagnosis of DRESS syndrome are facial edema and anasarca (Figure 3), as facial edema is not a usual finding in sepsis. Facial edema can be severe enough that the patient’s features are dramatically altered. It may be useful to ask family members if the patient’s face appears swollen or to compare the current appearance to the patient’s driver’s license photograph. An important complication to note is laryngeal edema, which may complicate airway management and may manifest as respiratory distress, stridor, and the need for emergent intubation.13

Myth: Patients who have had an allergic reaction to sulfonamide antibiotics will have a cross-reaction to nonantibiotic sulfonamides.
A common question is, if a patient has had a prior allergy to sulfonamide antibiotics, then are nonantibiotic sulfones such as a sulfonylurea, thiazide diuretic, or furosemide likely to cause a a cross-reaction? In one study (N=969), only 9.9% of patients with a prior sulfone antibiotic allergy developed hypersensitivity when exposed to a nonantibiotic sulfone, which is thought to be due to an overall increased propensity for hypersensitivity rather than a true cross-reaction. In fact, the risk for developing a hypersensitivity reaction to penicillin (14.0% [717/5115]) was higher than the risk for developing a reaction from a nonantibiotic sulfone among these patients.14 This study bolsters the argument that if there are other potential culprit medications and the time course for a patient’s nonantibiotic sulfone is not consistent with the timeline for DRESS syndrome, it may be beneficial to look for a different causative agent.
Pearl: Vancomycin is an important cause of DRESS syndrome.
Guidelines for treating endocarditis and osteomyelitis caused by methicillin-resistant Staphylococcus aureus infection recommend intravenous vancomycin for 4 to 6 weeks.15 This duration is within the relevant time frame of exposure for the development of DRESS syndrome de novo.
One case series noted that 37.5% (12/32) of DRESS syndrome cases in a 3-year period were caused by vancomycin, which notably was the most common medication associated with DRESS syndrome.16 There were caveats to this case series in that no standardized drug causality score was used and the sample size over the 3-year period was small; however, the increased use (and misuse) of antibiotics and perhaps increased recognition of rash in outpatient parenteral antibiotic therapy clinics may play a role if vancomycin-induced DRESS syndrome is indeed becoming more common.
Myth: Myocarditis secondary to DRESS syndrome will present with chest pain at the time of the cutaneous eruption.
Few patients with DRESS syndrome–associated myocarditis actually are symptomatic during their hospitalization.4 In asymptomatic patients, the primary team and consultants should be vigilant for the potential of subclinical myocarditis or the possibility of developing cardiac involvement after discharge, as myocarditis secondary to DRESS syndrome may present any time from rash onset up to 4 months later.4 Therefore, DRESS patients should be especially attentive to any new cardiac symptoms and notify their provider if any develop.
Although no standard cardiac screening guidelines exist for DRESS syndrome, some have recommended that baseline cardiac screening tests including electrocardiogram, troponin levels, and echocardiogram be considered at the time of diagnosis.5 If any testing is abnormal, DRESS syndrome–associated myocarditis should be suspected and an endomyocardial biopsy, which is the diagnostic gold standard, may be necessary.4 If the cardiac screening tests are normal, some investigators recommend serial outpatient echocardiograms for all DRESS patients, even those who remain asymptomatic.17 An alternative is an empiric approach in which a thorough review of systems is performed and testing is done if patients develop symptoms that are concerning for myocarditis.
Pearl: Steroids are not the only treatment used to control DRESS syndrome.
A prolonged taper of systemic steroids is the first-line treatment of DRESS syndrome. Steroids at the equivalent of 1 to 2 mg/kg daily (once or divided into 2 doses) of prednisone typically are used. For severe and/or recalcitrant DRESS syndrome, 2 mg/kg daily (once or divided into 2 doses) typically is used, and less than 1 mg/kg daily may be used for mini-DRESS syndrome.
Clinical improvement of DRESS syndrome has been demonstrated in several case reports with intravenous immunoglobulin, cyclosporine, cyclophosphamide, mycophenolate mofetil, and plasmapheresis.18-21 Each of these therapies typically were initiated as second-line therapeutic agents when initial treatment with steroids failed. It is important to note that large prospective studies regarding these treatments are lacking; however, there have been case reports of acute necrotizing eosinophilic myocarditis that did not respond to the combination of steroids and cyclosporine.4,22
Although there have been successful case reports using intravenous immunoglobulin, a 2012 prospective open-label clinical trial reported notable side effects in 5 of 6 (83.3%) patients with only 1 of 6 (16.6%) achieving the primary end point of control of fever/symptoms at day 7 and clinical remission without steroids on day 30.23
Pearl: DRESS patients need to be monitored for long-term sequelae such as autoimmune disease.
Several autoimmune conditions may develop as a delayed complication of DRESS syndrome, including autoimmune thyroiditis, systemic lupus erythematosus, type 1 diabetes mellitus, and autoimmune hemolytic anemia.24-26 Incidence rates of autoimmunity following DRESS syndrome range from 3% to 5% among small case series.24,25
Autoimmune thyroiditis, which may present as Graves disease, Hashimoto thyroiditis, or painless thyroiditis, is the most common autoimmune disorder to develop in DRESS patients and appears from several weeks to up to 3 years after DRESS.24 Therefore, all DRESS patients should be monitored longitudinally for several years for signs or symptoms suggestive of an autoimmune condition.5,24,26
Because no guidelines exist regarding serial monitoring for autoimmune sequelae, it may be reasonable to check thyroid function tests at the time of diagnosis and regularly for at least 2 years after diagnosis.5 Alternatively, clinicians may consider an empiric approach to laboratory testing that is guided by the development of clinical symptoms.
Pearl: Small cases series suggest differences between adult and pediatric DRESS syndrome, but there are no large studies in children.
Small case series have suggested there may be noteworthy differences between DRESS syndrome in adults and children. Although human herpesvirus 6 (HHV-6) positivity in DRESS syndrome in adults may be as high as 80%, 13% of pediatric patients in one cohort tested positive for HHV-6, though the study size was limited at 29 total patients.27 In children, DRESS syndrome secondary to antibiotics was associated with a shorter latency time as compared to cases secondary to nonantibiotics. In contrast to the typical 2- to 6-week timeline, Sasidharanpillai et al28 reported an average onset 5.8 days after drug administration in antibiotic-associated DRESS syndrome compared to 23.9 days for anticonvulsants, though this study only included 11 total patients. Other reports have suggested a similar trend.27
The role of HHV-6 positivity in pediatric DRESS syndrome and its influence on prognosis remains unclear. One study showed a worse prognosis for pediatric patients with positive HHV-6 antibodies.27 However, with such a small sample size—only 4 HHV-6–positive patients of 29 pediatric DRESS cases—larger studies are needed to better characterize the relationship between HHV-6 positivity and prognosis.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
- Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med, 2011;124:588-597.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080.
- Intarasupht J, Kanchanomai A, Leelasattakul W, et al. Prevalence, risk factors, and mortality outcome in the drug reaction with eosinophilia and systemic symptoms patients with cardiac involvement. Int J Dermatol. 2018;57:1187-1191.
- Bourgeois GP, Cafardi JA, Groysman V, et al. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:E229-E236.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-693.e14; quiz 706-708.
- Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J Am Acad Dermatol. 2013;69:407-415.
- Lin IC, Yang HC, Strong C, et al. Liver injury in patients with DRESS: a clinical study of 72 cases. J Am Acad Dermatol. 2015;72:984-991.
- Peyrière H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422-428.
- Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? a review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391-401.
- Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82:149-162.
- Matsuda H, Saito K, Takayanagi Y, et al. Pustular-type drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms due to carbamazepine with systemic muscle involvement. J Dermatol. 2013;40:118-122.
- Wolf R, Davidovici B, Matz H, et al. Drug rash with eosinophilia and systemic symptoms versus Stevens-Johnson Syndrome—a case that indicates a stumbling block in the current classification. Int Arch Allergy Immunol. 2006;141:308-310.
- Kumar A, Goldfarb JW, Bittner EA. A case of drug rash with eosinophilia and systemic symptoms (DRESS) syndrome complicating airway management. Can J Anaesth. 2012;59:295-298.
- Strom BL, Schinnar R, Apter AJ, et al. Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics. N Engl J Med. 2003;349:1628-1635.
- Berbari EF, Kanj SS, Kowalski TJ, et al; Infectious Diseases Society of America. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:E26-E46.
- Lam BD, Miller MM, Sutton AV, et al. Vancomycin and DRESS: a retrospective chart review of 32 cases in Los Angeles, California. J Am Acad Dermatol. 2017;77:973-975.
- Eppenberger M, Hack D, Ammann P, et al. Acute eosinophilic myocarditis with dramatic response to steroid therapy: the central role of echocardiography in diagnosis and follow-up. Tex Heart Inst J. 2013;40:326-330.
- Kirchhof MG, Wong A, Dutz JP. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016;152:1254-1257.
- Singer EM, Wanat KA, Rosenbach MA. A case of recalcitrant DRESS syndrome with multiple autoimmune sequelae treated with intravenous immunoglobulins. JAMA Dermatol. 2013;149:494-495.
- Bommersbach TJ, Lapid MI, Leung JG, et al. Management of psychotropic drug-induced DRESS syndrome: a systematic review. Mayo Clin Proc. 2016;91:787-801.
- Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:E945-E949.
- Daoulah A, Alqahtani AA, Ocheltree SR, et al. Acute myocardial infarction in a 56-year-old female patient treated with sulfasalazine. Am J Emerg Med. 2012;30:638.e1-638.e3.
- Joly P, Janela B, Tetart F, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol. 2012;148:543-544.
- Kano Y, Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Matta JM, Flores SM, Cherit JD. Drug reaction with eosinophilia and systemic symptoms (DRESS) and its relation with autoimmunity in a reference center in Mexico. An Bras Dermatol. 2017;92:30-33.
- Ahluwalia J, Abuabara K, Perman MJ, et al. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090-1095.
- Sasidharanpillai S, Sabitha S, Riyaz N, et al. Drug reaction with eosinophilia and systemic symptoms in children: a prospective study. Pediatr Dermatol. 2016;33:E162-E165.
Practice Points
- Drug rash with eosinophilia and systemic symptoms (DRESS syndrome) is a clinical diagnosis, and incomplete forms may not meet formal criteria-based diagnosis.
- Although DRESS syndrome typically has a morbilliform eruption, different rash morphologies may be observed.
- The myocarditis of DRESS syndrome may not present with chest pain; a high index of suspicion is warranted.
- Autoimmune sequelae are more frequent in patients who have had an episode of DRESS syndrome.
Allergen of the year may be nearer than you think
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
MONTEREY, CALIF. – It’s only found in 2%-3% of allergy cases. So Because, a dermatologist told colleagues, it’s so common.
“If you’re allergic to it, it’s tough to stay away from it,” said Joseph F. Fowler Jr., MD, clinical professor of dermatology at the University of Louisville (Ky.) in a presentation about contact dermatitis at the annual Coastal Dermatology Symposium.
Indeed, the synthetic compound PG is found in skin care products and cosmetics, coated pills, topical medications such as corticosteroids, foods (including bread, food coloring, and such flavorings as vanilla extracts). “It’s in every topical acne product I know of,” and is even in brake fluid and so-called nontoxic antifreeze, he said. (Propylene glycol shouldn’t be confused with the poisonous toxin ethylene glycol, which also is found in antifreeze.)
Patients can be tested for allergy to PG, Dr. Fowler pointed out, but it’s important to understand that it can trigger an irritation reaction that can be mistaken for an allergic reaction.
Dr. Fowler offered the following tips related to contact dermatitis and allergens. Be aware that metals, topical antibiotics, fragrances, and preservatives are most likely to cause allergic contact dermatitis. According to 2016 figures on allergen prevalence from the North American Contact Dermatitis Group (NACDG), allergy to the metal nickel is the most common (16%); followed by neomycin (9%); fragrance mix I, a mixture of fragrances used in allergen testing (9%); bacitracin (8%); and myroxylon, also known as balsam of Peru, which is used for a variety of purposes in food, medicines, and fragrances (7%).
These are followed by the metal cobalt (6%); the preservatives quaternium 15 and formaldehyde (both 6%); para-phenylenediamine, also known as PPD, which is used in hair dye (5%); and the fragrance mix II (5%), another mix of fragrances used in allergen testing.
Dr. Fowler cautioned that nickel can trigger an intense body-wide allergic reaction in children with atopic dermatitis. “In this situation, it’s really good to be compulsive and tell parents to absolutely keep that person away from nickel as much as humanly possible,” he said.
Keep an eye out for allergens that aren’t on the NACDG list, which includes 70 items. According to Dr. Fowler, more than 20% of his patients were positive to allergens not on the NACDG list.
Contact dermatitis is as common in children as in adults and can even be more common in children. An Italian study published in 2012 found that 70% of children aged 1-15 years tested via patch test were allergic to at least one allergen, a number that’s similar in adults (Dermatitis. 2012 Nov-Dec;23[6]:275-80). There are wide disparities in reported levels of children who are allergic to nickel, cobalt, and myroxylon, Dr. Fowler said.
The T.R.U.E. Test patch test system has value, compared with standard patch tests, but beware of its limitations, he advised. T.R.U.E. is easy to use and requires no prep time, he said, but the number of allergens is limited. By contrast, his clinic mostly uses the Finn Chambers on Scanpor tape system, which can test for many more allergens and is cheaper if used at least 5-10 times a month.
He cautioned that T.R.U.E. could miss the cause of contact dermatitis as often as 39% of the time, as demonstrated in one study of children undergoing patch testing (Arch Dermatol. 2008 Oct;144[10]:1329-36). However, he said, the T.R.U.E test has value in detecting allergies to nickel, methylchloroisothiazolinone/methylisothiazolinone (MCI/MI), and neomycin (J Am Acad Dermatol. 2001 Dec;45[6]:836-9).
Consider patch testing in a child with eczema if the eczema is not in normal atopic areas, it spreads beyond normal areas, it doesn’t respond to usual treatments, or it begins later than 5 years of age.
And, Dr. Fowler added, it’s fine to perform patch testing on patients who are taking antihistamines, tumor necrosis factor–alpha inhibitors, NSAIDs, or methotrexate.
Dr. Fowler disclosed consulting for IntraDerm, serving on speakers bureaus for SmartPractice and Regeneron/Sanofi, and serving as an investigator for companies that include AbbVie, Allergan, Bayer, Dow, Galderma, Johnson & Johnson, Eli Lilly, Merck, Regeneron, SmartPractice, and Valeant (now Bausch).
The meeting was jointly presented by the University of Louisville and Global Academy for Medical Education. This publication and Global Academy for Medical Education are both owned by Frontline Medical Communications.
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Investing in the Future of Inpatient Dermatology: The Evolution and Impact of Specialized Dermatologic Consultation in Hospitalized Patients
The practice of inpatient dermatology has a rich history rooted in specialized hospital wards that housed patients with chronic dermatoses. Because systemic agents were limited, the care of these patients required skilled nursing and a distinctive knowledge of the application of numerous topical agents, including washes, baths, powders, lotions, and pastes1; however, with the evolving nature of health care in the last half a century, such dermatologic inpatient units are now rare, with only 2 units remaining in the United States, specifically at the Mayo Clinic in Minnesota and at the University of Miami.2
Although the shift away from a primary dermatologic admitting service is likely multifactorial, what is more sobering is that the majority of inpatients with dermatologic disorders are cared for by nondermatologists.2 Although the dynamics for such a diminished presence are due to various personal and professional concerns, the essential outcome for patients hospitalized with a cutaneous concern—whether directly related to their hospitalization or iatrogenic in nature—is the potential for suboptimal care.3
Fortunately, the practice of inpatient dermatology currently is undergoing a renaissance. With this renewed interest in hospital-based dermatology, there is a growing body of evidence that demonstrates how the dermatology hospitalist has become a vital member of the inpatient team, adding value to the care of patients across all specialties.
To explore the impact of consultative dermatology services, there has been a push by members of the Society for Dermatology Hospitalists to elucidate the contributions of dermatologists in the inpatient setting, which has been accomplished primarily by defining and characterizing the types of patients that dermatology hospitalists care for and, more recently, by demonstrating the improved outcomes that result from expert consultation.
Breadth of Inpatient Dermatologic Consultations
With the adaptation of dermatology consultation services, the scope of practice has shifted from the skilled management of chronic dermatoses to one with an emphasis on the identification of various acute dermatologic diseases. Although the extent of such acute disease states in the inpatient setting is vast, it is interesting to note that the majority of consultations are for common conditions, namely cutaneous infections, venous stasis dermatitis, contact dermatitis, atopic dermatitis, and cutaneous drug eruptions (Table).4,5

Moreover, for the services that obtain dermatologic consultation, the majority of requests originate from internal medicine and hematology/oncology.4,5 Although internal medicine often is the largest-represented specialty in the hospital and provides a proportional amount of dermatology consultations, hematology/oncology patients represent a distinct cohort who are prone to unique mucocutaneous dermatoses related to underlying malignancies, immunosuppression, and cancer-specific therapies (eg, chemotherapy, immunotherapy, stem cell transplantation). Within this subset of patients, cutaneous infections and drug eruptions constitute the majority of cases, while graft-versus-host disease and neutrophilic dermatoses account for a smaller percentage of dermatologic disease in this population. Given the complex and uncommon nature of these dermatoses, timely intervention by a dermatologist can have a considerable impact on morbidity and mortality associated with such disease states.6,7
Among pediatric patients, dermatology consultation patterns mimic those seen among adult patients, with common conditions such as atopic dermatitis and contact dermatitis representing the majority of consultations.8-11 Vascular lesions further represent a unique source of consultation among pediatric patients. Although they often are considered an outpatient concern, one group found that the majority of inpatient consultations for vascular lesions led to early identification of a syndromic association and/or complication (eg, ulceration).10 Identifying these cases in the hospital provides early opportunities for intervention and multidisciplinary care.
Adding Value to the Care of Hospitalized Patients
Following other inpatient models, hospitalist dermatology has begun to demonstrate feasibility, advances in quality improvement, and most importantly improved health care outcomes. In an effort to better characterize the enhancement of such health care delivery, recent literature around the impact of inpatient dermatology consultation has centered on improving key objective hospital-based quality measures, namely diagnosis and management as well as hospital length of stay (LOS) and readmission rates.5,12-18
When identifying cutaneous disease, recent evidence points to the increased diagnostic accuracy by way of dermatology consultation. Specifically, diagnoses were changed 30% to 70% of the time when consultations were provided.6,12-15 Interestingly, misdiagnosis regularly centered on common diagnoses, specifically cellulitis, stasis dermatitis, and hypersensitivity reactions.6,12-16 In a multi-institutional retrospective study that examined the national incidence of cellulitis misdiagnosis, the authors found that when a dermatology consultation for presumed cellulitis was called, approximately 75% (N=55) of cases represented mimickers of cellulitis, such as stasis dermatitis, contact dermatitis, and cutaneous fungal infections. Moreover, in more than 38% (N=21) of such cellulitis consultations, patients often had more than one ongoing disease process, further speaking to the diagnostic accuracy obtained from expert consultation.16 The result of such misdiagnosis is not trivial, as unnecessary hospital admission or inappropriate treatment due to misdiagnosis of cutaneous disease often leads to avoidable complications and preventable health care spending. In a cross-sectional analysis of patients diagnosed with presumed lower extremity cellulitis (N=259), approximately 30% were misdiagnosed. In these cases, more than 90% of patients received unnecessary antibiotics, with approximately 30% of them experiencing a complication or avoidable utilization of health care related to their misdiagnosis.17
Along with the profound impact on diagnostic accuracy, management and treatment are almost universally affected after dermatology consultation.5,12-14 Such findings bear importance on optimizing hospital LOS as well as readmission rates. For hospital LOS, a recent study demonstrated reductions in LOS by 2.64 days as well as 1-year cutaneous disease-specific readmissions for patients who received dermatologic consultation for their inflammatory skin disease.18 Similarly, in a recent prospective cohort study of patients diagnosed with presumed lower extremity cellulitis, hospital LOS decreased by 2 days following a diagnosis of pseudocellulitis via timely dermatologic consultation. Across the United States, such reductions in LOS associated with unnecessary hospitalization due to pseudocellulitis can result in annual health care savings of $100 to $200 million.13 As such, early dermatologic intervention plays a vital role in diagnostic accuracy, appropriate treatment implementation, expedited discharge, and the overall economics of health care delivery and utilization, thereby supporting the utility of clinical decision support through expert consultation.
Conclusion
There is a clear and distinct value that results in having specialized inpatient dermatology services. Such expert consultation enhances quality of care and reduces health care costs. Although the implementation and success of inpatient dermatology services has primarily been observed at large hospitals/tertiary care centers, there is incredible potential to further our impact through engagement in our community hospitals. With that said, all practicing dermatologists should feel empowered to employ their expert skillset in their own communities, as such access to care and specialty support is desperately needed and can remarkably impact health care outcomes. Moreover, in addition to the direct impact on health care delivery and economics, the intangible benefits of an inpatient dermatology presence are innumerable, as opportunities to promote quality research and improve trainee education also demonstrate our value. These facets together provide a positive perspective on the potential contribution that our field can have on shaping the outlook of hospital medicine. As such, in addition to enjoying the current renaissance of inpatient dermatology, it is imperative that dermatologists build on this momentum and invest in the future of consultative dermatology.
- Albert MR, Mackool BT. A dermatology ward at the beginning of the 20th century. J Am Acad Dermatol. 2000;42(1, pt 1):113-123.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Storan ER, McEvoy MT, Wetter DA, et al. Experience of a year of adult hospital dermatology consultations. Int J Dermatol. 2015;54:1150-1156.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Phillips GS, Freites-Martinez A, Hsu M, et al. Inflammatory dermatoses, infections, and drug eruptions are the most common skin conditions in hospitalized cancer patients. J Am Acad Dermatol. 2018;78:1102-1109.
- Storan ER, McEvoy MT, Wetter DA, et al. Pediatric hospital dermatology: experience with inpatient and consult services at the Mayo Clinic. Pediatr Dermatol. 2013;30:433-437.
- Afsar FS. Analysis of pediatric dermatology inpatient consultations in a pediatric teaching hospital. Arch Argent Pediatr. 2017;115:E377-E384.
- McMahon P, Goddard D, Frieden IJ. Pediatric dermatology inpatient consultations: a retrospective study of 427 cases. J Am Acad Dermatol. 2013;68:926-931.
- Peñate Y, Borrego L, Hernández N, et al. Pediatric dermatology consultations: a retrospective analysis of inpatient consultations referred to the dermatology service. Pediatr Dermatol. 2012;29:115-118.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis [published online November 2, 2016]. JAMA Dermatol. doi:10.1001/jamadermatol.2016.3816.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
The practice of inpatient dermatology has a rich history rooted in specialized hospital wards that housed patients with chronic dermatoses. Because systemic agents were limited, the care of these patients required skilled nursing and a distinctive knowledge of the application of numerous topical agents, including washes, baths, powders, lotions, and pastes1; however, with the evolving nature of health care in the last half a century, such dermatologic inpatient units are now rare, with only 2 units remaining in the United States, specifically at the Mayo Clinic in Minnesota and at the University of Miami.2
Although the shift away from a primary dermatologic admitting service is likely multifactorial, what is more sobering is that the majority of inpatients with dermatologic disorders are cared for by nondermatologists.2 Although the dynamics for such a diminished presence are due to various personal and professional concerns, the essential outcome for patients hospitalized with a cutaneous concern—whether directly related to their hospitalization or iatrogenic in nature—is the potential for suboptimal care.3
Fortunately, the practice of inpatient dermatology currently is undergoing a renaissance. With this renewed interest in hospital-based dermatology, there is a growing body of evidence that demonstrates how the dermatology hospitalist has become a vital member of the inpatient team, adding value to the care of patients across all specialties.
To explore the impact of consultative dermatology services, there has been a push by members of the Society for Dermatology Hospitalists to elucidate the contributions of dermatologists in the inpatient setting, which has been accomplished primarily by defining and characterizing the types of patients that dermatology hospitalists care for and, more recently, by demonstrating the improved outcomes that result from expert consultation.
Breadth of Inpatient Dermatologic Consultations
With the adaptation of dermatology consultation services, the scope of practice has shifted from the skilled management of chronic dermatoses to one with an emphasis on the identification of various acute dermatologic diseases. Although the extent of such acute disease states in the inpatient setting is vast, it is interesting to note that the majority of consultations are for common conditions, namely cutaneous infections, venous stasis dermatitis, contact dermatitis, atopic dermatitis, and cutaneous drug eruptions (Table).4,5

Moreover, for the services that obtain dermatologic consultation, the majority of requests originate from internal medicine and hematology/oncology.4,5 Although internal medicine often is the largest-represented specialty in the hospital and provides a proportional amount of dermatology consultations, hematology/oncology patients represent a distinct cohort who are prone to unique mucocutaneous dermatoses related to underlying malignancies, immunosuppression, and cancer-specific therapies (eg, chemotherapy, immunotherapy, stem cell transplantation). Within this subset of patients, cutaneous infections and drug eruptions constitute the majority of cases, while graft-versus-host disease and neutrophilic dermatoses account for a smaller percentage of dermatologic disease in this population. Given the complex and uncommon nature of these dermatoses, timely intervention by a dermatologist can have a considerable impact on morbidity and mortality associated with such disease states.6,7
Among pediatric patients, dermatology consultation patterns mimic those seen among adult patients, with common conditions such as atopic dermatitis and contact dermatitis representing the majority of consultations.8-11 Vascular lesions further represent a unique source of consultation among pediatric patients. Although they often are considered an outpatient concern, one group found that the majority of inpatient consultations for vascular lesions led to early identification of a syndromic association and/or complication (eg, ulceration).10 Identifying these cases in the hospital provides early opportunities for intervention and multidisciplinary care.
Adding Value to the Care of Hospitalized Patients
Following other inpatient models, hospitalist dermatology has begun to demonstrate feasibility, advances in quality improvement, and most importantly improved health care outcomes. In an effort to better characterize the enhancement of such health care delivery, recent literature around the impact of inpatient dermatology consultation has centered on improving key objective hospital-based quality measures, namely diagnosis and management as well as hospital length of stay (LOS) and readmission rates.5,12-18
When identifying cutaneous disease, recent evidence points to the increased diagnostic accuracy by way of dermatology consultation. Specifically, diagnoses were changed 30% to 70% of the time when consultations were provided.6,12-15 Interestingly, misdiagnosis regularly centered on common diagnoses, specifically cellulitis, stasis dermatitis, and hypersensitivity reactions.6,12-16 In a multi-institutional retrospective study that examined the national incidence of cellulitis misdiagnosis, the authors found that when a dermatology consultation for presumed cellulitis was called, approximately 75% (N=55) of cases represented mimickers of cellulitis, such as stasis dermatitis, contact dermatitis, and cutaneous fungal infections. Moreover, in more than 38% (N=21) of such cellulitis consultations, patients often had more than one ongoing disease process, further speaking to the diagnostic accuracy obtained from expert consultation.16 The result of such misdiagnosis is not trivial, as unnecessary hospital admission or inappropriate treatment due to misdiagnosis of cutaneous disease often leads to avoidable complications and preventable health care spending. In a cross-sectional analysis of patients diagnosed with presumed lower extremity cellulitis (N=259), approximately 30% were misdiagnosed. In these cases, more than 90% of patients received unnecessary antibiotics, with approximately 30% of them experiencing a complication or avoidable utilization of health care related to their misdiagnosis.17
Along with the profound impact on diagnostic accuracy, management and treatment are almost universally affected after dermatology consultation.5,12-14 Such findings bear importance on optimizing hospital LOS as well as readmission rates. For hospital LOS, a recent study demonstrated reductions in LOS by 2.64 days as well as 1-year cutaneous disease-specific readmissions for patients who received dermatologic consultation for their inflammatory skin disease.18 Similarly, in a recent prospective cohort study of patients diagnosed with presumed lower extremity cellulitis, hospital LOS decreased by 2 days following a diagnosis of pseudocellulitis via timely dermatologic consultation. Across the United States, such reductions in LOS associated with unnecessary hospitalization due to pseudocellulitis can result in annual health care savings of $100 to $200 million.13 As such, early dermatologic intervention plays a vital role in diagnostic accuracy, appropriate treatment implementation, expedited discharge, and the overall economics of health care delivery and utilization, thereby supporting the utility of clinical decision support through expert consultation.
Conclusion
There is a clear and distinct value that results in having specialized inpatient dermatology services. Such expert consultation enhances quality of care and reduces health care costs. Although the implementation and success of inpatient dermatology services has primarily been observed at large hospitals/tertiary care centers, there is incredible potential to further our impact through engagement in our community hospitals. With that said, all practicing dermatologists should feel empowered to employ their expert skillset in their own communities, as such access to care and specialty support is desperately needed and can remarkably impact health care outcomes. Moreover, in addition to the direct impact on health care delivery and economics, the intangible benefits of an inpatient dermatology presence are innumerable, as opportunities to promote quality research and improve trainee education also demonstrate our value. These facets together provide a positive perspective on the potential contribution that our field can have on shaping the outlook of hospital medicine. As such, in addition to enjoying the current renaissance of inpatient dermatology, it is imperative that dermatologists build on this momentum and invest in the future of consultative dermatology.
The practice of inpatient dermatology has a rich history rooted in specialized hospital wards that housed patients with chronic dermatoses. Because systemic agents were limited, the care of these patients required skilled nursing and a distinctive knowledge of the application of numerous topical agents, including washes, baths, powders, lotions, and pastes1; however, with the evolving nature of health care in the last half a century, such dermatologic inpatient units are now rare, with only 2 units remaining in the United States, specifically at the Mayo Clinic in Minnesota and at the University of Miami.2
Although the shift away from a primary dermatologic admitting service is likely multifactorial, what is more sobering is that the majority of inpatients with dermatologic disorders are cared for by nondermatologists.2 Although the dynamics for such a diminished presence are due to various personal and professional concerns, the essential outcome for patients hospitalized with a cutaneous concern—whether directly related to their hospitalization or iatrogenic in nature—is the potential for suboptimal care.3
Fortunately, the practice of inpatient dermatology currently is undergoing a renaissance. With this renewed interest in hospital-based dermatology, there is a growing body of evidence that demonstrates how the dermatology hospitalist has become a vital member of the inpatient team, adding value to the care of patients across all specialties.
To explore the impact of consultative dermatology services, there has been a push by members of the Society for Dermatology Hospitalists to elucidate the contributions of dermatologists in the inpatient setting, which has been accomplished primarily by defining and characterizing the types of patients that dermatology hospitalists care for and, more recently, by demonstrating the improved outcomes that result from expert consultation.
Breadth of Inpatient Dermatologic Consultations
With the adaptation of dermatology consultation services, the scope of practice has shifted from the skilled management of chronic dermatoses to one with an emphasis on the identification of various acute dermatologic diseases. Although the extent of such acute disease states in the inpatient setting is vast, it is interesting to note that the majority of consultations are for common conditions, namely cutaneous infections, venous stasis dermatitis, contact dermatitis, atopic dermatitis, and cutaneous drug eruptions (Table).4,5

Moreover, for the services that obtain dermatologic consultation, the majority of requests originate from internal medicine and hematology/oncology.4,5 Although internal medicine often is the largest-represented specialty in the hospital and provides a proportional amount of dermatology consultations, hematology/oncology patients represent a distinct cohort who are prone to unique mucocutaneous dermatoses related to underlying malignancies, immunosuppression, and cancer-specific therapies (eg, chemotherapy, immunotherapy, stem cell transplantation). Within this subset of patients, cutaneous infections and drug eruptions constitute the majority of cases, while graft-versus-host disease and neutrophilic dermatoses account for a smaller percentage of dermatologic disease in this population. Given the complex and uncommon nature of these dermatoses, timely intervention by a dermatologist can have a considerable impact on morbidity and mortality associated with such disease states.6,7
Among pediatric patients, dermatology consultation patterns mimic those seen among adult patients, with common conditions such as atopic dermatitis and contact dermatitis representing the majority of consultations.8-11 Vascular lesions further represent a unique source of consultation among pediatric patients. Although they often are considered an outpatient concern, one group found that the majority of inpatient consultations for vascular lesions led to early identification of a syndromic association and/or complication (eg, ulceration).10 Identifying these cases in the hospital provides early opportunities for intervention and multidisciplinary care.
Adding Value to the Care of Hospitalized Patients
Following other inpatient models, hospitalist dermatology has begun to demonstrate feasibility, advances in quality improvement, and most importantly improved health care outcomes. In an effort to better characterize the enhancement of such health care delivery, recent literature around the impact of inpatient dermatology consultation has centered on improving key objective hospital-based quality measures, namely diagnosis and management as well as hospital length of stay (LOS) and readmission rates.5,12-18
When identifying cutaneous disease, recent evidence points to the increased diagnostic accuracy by way of dermatology consultation. Specifically, diagnoses were changed 30% to 70% of the time when consultations were provided.6,12-15 Interestingly, misdiagnosis regularly centered on common diagnoses, specifically cellulitis, stasis dermatitis, and hypersensitivity reactions.6,12-16 In a multi-institutional retrospective study that examined the national incidence of cellulitis misdiagnosis, the authors found that when a dermatology consultation for presumed cellulitis was called, approximately 75% (N=55) of cases represented mimickers of cellulitis, such as stasis dermatitis, contact dermatitis, and cutaneous fungal infections. Moreover, in more than 38% (N=21) of such cellulitis consultations, patients often had more than one ongoing disease process, further speaking to the diagnostic accuracy obtained from expert consultation.16 The result of such misdiagnosis is not trivial, as unnecessary hospital admission or inappropriate treatment due to misdiagnosis of cutaneous disease often leads to avoidable complications and preventable health care spending. In a cross-sectional analysis of patients diagnosed with presumed lower extremity cellulitis (N=259), approximately 30% were misdiagnosed. In these cases, more than 90% of patients received unnecessary antibiotics, with approximately 30% of them experiencing a complication or avoidable utilization of health care related to their misdiagnosis.17
Along with the profound impact on diagnostic accuracy, management and treatment are almost universally affected after dermatology consultation.5,12-14 Such findings bear importance on optimizing hospital LOS as well as readmission rates. For hospital LOS, a recent study demonstrated reductions in LOS by 2.64 days as well as 1-year cutaneous disease-specific readmissions for patients who received dermatologic consultation for their inflammatory skin disease.18 Similarly, in a recent prospective cohort study of patients diagnosed with presumed lower extremity cellulitis, hospital LOS decreased by 2 days following a diagnosis of pseudocellulitis via timely dermatologic consultation. Across the United States, such reductions in LOS associated with unnecessary hospitalization due to pseudocellulitis can result in annual health care savings of $100 to $200 million.13 As such, early dermatologic intervention plays a vital role in diagnostic accuracy, appropriate treatment implementation, expedited discharge, and the overall economics of health care delivery and utilization, thereby supporting the utility of clinical decision support through expert consultation.
Conclusion
There is a clear and distinct value that results in having specialized inpatient dermatology services. Such expert consultation enhances quality of care and reduces health care costs. Although the implementation and success of inpatient dermatology services has primarily been observed at large hospitals/tertiary care centers, there is incredible potential to further our impact through engagement in our community hospitals. With that said, all practicing dermatologists should feel empowered to employ their expert skillset in their own communities, as such access to care and specialty support is desperately needed and can remarkably impact health care outcomes. Moreover, in addition to the direct impact on health care delivery and economics, the intangible benefits of an inpatient dermatology presence are innumerable, as opportunities to promote quality research and improve trainee education also demonstrate our value. These facets together provide a positive perspective on the potential contribution that our field can have on shaping the outlook of hospital medicine. As such, in addition to enjoying the current renaissance of inpatient dermatology, it is imperative that dermatologists build on this momentum and invest in the future of consultative dermatology.
- Albert MR, Mackool BT. A dermatology ward at the beginning of the 20th century. J Am Acad Dermatol. 2000;42(1, pt 1):113-123.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Storan ER, McEvoy MT, Wetter DA, et al. Experience of a year of adult hospital dermatology consultations. Int J Dermatol. 2015;54:1150-1156.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Phillips GS, Freites-Martinez A, Hsu M, et al. Inflammatory dermatoses, infections, and drug eruptions are the most common skin conditions in hospitalized cancer patients. J Am Acad Dermatol. 2018;78:1102-1109.
- Storan ER, McEvoy MT, Wetter DA, et al. Pediatric hospital dermatology: experience with inpatient and consult services at the Mayo Clinic. Pediatr Dermatol. 2013;30:433-437.
- Afsar FS. Analysis of pediatric dermatology inpatient consultations in a pediatric teaching hospital. Arch Argent Pediatr. 2017;115:E377-E384.
- McMahon P, Goddard D, Frieden IJ. Pediatric dermatology inpatient consultations: a retrospective study of 427 cases. J Am Acad Dermatol. 2013;68:926-931.
- Peñate Y, Borrego L, Hernández N, et al. Pediatric dermatology consultations: a retrospective analysis of inpatient consultations referred to the dermatology service. Pediatr Dermatol. 2012;29:115-118.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis [published online November 2, 2016]. JAMA Dermatol. doi:10.1001/jamadermatol.2016.3816.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
- Albert MR, Mackool BT. A dermatology ward at the beginning of the 20th century. J Am Acad Dermatol. 2000;42(1, pt 1):113-123.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Helms AE, Helms SE, Brodell RT. Hospital consultations: time to address an unmet need? J Am Acad Dermatol. 2009;60:308-311.
- Storan ER, McEvoy MT, Wetter DA, et al. Experience of a year of adult hospital dermatology consultations. Int J Dermatol. 2015;54:1150-1156.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Phillips GS, Freites-Martinez A, Hsu M, et al. Inflammatory dermatoses, infections, and drug eruptions are the most common skin conditions in hospitalized cancer patients. J Am Acad Dermatol. 2018;78:1102-1109.
- Storan ER, McEvoy MT, Wetter DA, et al. Pediatric hospital dermatology: experience with inpatient and consult services at the Mayo Clinic. Pediatr Dermatol. 2013;30:433-437.
- Afsar FS. Analysis of pediatric dermatology inpatient consultations in a pediatric teaching hospital. Arch Argent Pediatr. 2017;115:E377-E384.
- McMahon P, Goddard D, Frieden IJ. Pediatric dermatology inpatient consultations: a retrospective study of 427 cases. J Am Acad Dermatol. 2013;68:926-931.
- Peñate Y, Borrego L, Hernández N, et al. Pediatric dermatology consultations: a retrospective analysis of inpatient consultations referred to the dermatology service. Pediatr Dermatol. 2012;29:115-118.
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis [published online November 2, 2016]. JAMA Dermatol. doi:10.1001/jamadermatol.2016.3816.
- Milani-Nejad N, Zhang M, Kaffenberger BH. Association of dermatology consultations with patient care outcomes in hospitalized patients with inflammatory skin diseases. JAMA Dermatol. 2017;153:523-528.
Practice Points
- Dermatology inpatient consultation enhances quality of care and reduces health care costs.
- Dermatology input in the inpatient setting leads to a diagnosis change in up to 70% of consultations.
- The majority of dermatologic misdiagnoses by nondermatologists involves common dermatoses such as cellulitis, stasis dermatitis, and hypersensitivity reactions.
October 2018
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
Allergic contact dermatitis (ACD) can affect individuals regardless of age, race, or sex, but ACD accounts for 20% of all contact dermatitis reactions. ACD results in an inflammatory reaction in those who have been previously sensitized to an allergen. This type of delayed hypersensitivity reaction is known as cell-mediated hypersensitivity. Generally, no reaction is elicited upon the first exposure to the allergen. In fact, it may take years of exposure to allergens for someone to develop an allergic contact dermatitis.
Once sensitized, epidermal antigen-presenting cells (APCs) called Langerhans cells process the allergen and present it in a complex on the surface of the cell to a CD4+ T cell. Subsequently, inflammatory cytokines and mediators are released, resulting in an allergic cutaneous (eczematous) reaction. Lesions may appear to be vesicular or bullous. Occasionally, a generalized eruption may occur. With repeated exposure, reactions may be acute or chronic.
Common causes of allergic contact dermatitis include toxicodendron plants (poison ivy, oak, and sumac; cashew nut tree; and mango), metals (nickel and gold), topical antibiotics (neomycin and bacitracin), fragrance and Balsam of Peru, deodorant, preservatives (formaldehyde), and rubber (elastic and gloves).
Patch testing is the standard means of detecting which allergen is causing the sensitization in an individual. The Thin-Layer Rapid Use Epicutaneous (TRUE) test or individually prepared aluminum (Finn) chambers containing the most common allergens are applied to the patient’s upper back. The patches are removed after 48 hours and read, and then reevaluated at day 4 or 5. Positive reactions appear as eczematous or vesicular papules or plaques.
Treatment includes avoidance of the allergens. Topical corticosteroid creams are helpful. For severe or generalized reactions, oral prednisone may be used. It is important to note that patient may be allergic to topical steroids. Patch testing can be performed to elucidate such allergens.
In contrast, 80% of contact dermatitis reactions are irritant, not allergic. Irritant contact dermatitis results is a local inflammatory reaction in people who have come into contact with a substance. Previous sensitization is not required. The reaction usually occurs immediately after exposure. Common causes include alkalis (detergents, soaps), acids (often found as an industrial work exposure), metals, solvents (occupational dermatitis), hydrocarbons, and chlorinated compounds.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to [email protected].
A 30-year-old female presented with 2 days of intensely pruritic erythematous papules and vesicles on her bilateral arms and hands. The lesions began appearing 1 day after a camping trip. Her neck, chest, and upper back were clear.
Latex Hypersensitivity to Injection Devices for Biologic Therapies in Psoriasis Patients
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4
Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
An allergic reaction is an exaggerated immune response that is known as a type I or immediate hypersensitivity reaction when provoked by reexposure to an allergen or antigen. Upon initial exposure to the antigen, dendritic cells bind it for presentation to helper T (TH2) lymphocytes. The TH2 cells then interact with B cells, stimulating them to become plasma cells and produce IgE antibodies to the antigen. When exposed to the same allergen a second time, IgE antibodies bind the allergen and cross-link on mast cells and basophils in the blood. Cross-linking stimulates degranulation of the cells, releasing histamine, leukotrienes, prostaglandins, and other cytokines. The major effects of the release of these mediators include vasodilation, increased vascular permeability, and bronchoconstriction. Leukotrienes also are responsible for chemotaxis of white blood cells, further propagating the immune response.1
Effects of a type I hypersensitivity reaction can be either local or systemic, resulting in symptoms ranging from mild irritation to anaphylactic shock and death. Latex allergy is a common example of a type I hypersensitivity reaction. Latex is found in many medical products, including gloves, rubber, elastics, blood pressure cuffs, bandages, dressings, and syringes. Reactions can include runny nose, tearing eyes, itching, hives, wheals, wheezing, and in rare cases anaphylaxis.2 Diagnosis can be suspected based on history and physical examination. Screening is performed with radioallergosorbent testing, which identifies specific IgE antibodies to latex; however, the reported sensitivity and specificity for the latex-specific IgE antibody varies widely in the literature, and the test cannot reliably rule in or rule out a true latex allergy.3
Allergic responses to latex in psoriasis patients receiving frequent injections with biologic agents are not commonly reported in the literature. We report the case of a patient with a long history of psoriasis who developed an allergic response after exposure to injection devices that contained latex components while undergoing treatment with biologic agents.
Case Report
A 72-year-old man presented with an extensive history of severe psoriasis with frequent flares. Treatment with topical agents and etanercept 6 months prior at an outside facility failed. At the time of presentation, the patient had more than 10% body surface area (BSA) involvement, which included the scalp, legs, chest, and back. He subsequently was started on ustekinumab injections. He initially responded well to therapy, but after 8 months of treatment, he began to have recurrent episodes of acute eruptive rashes over the trunk with associated severe pruritus that reproducibly recurred within 24 hours after each ustekinumab injection. It was decided to discontinue ustekinumab due to concern for intolerance, and the patient was switched to secukinumab.
After starting secukinumab, the patient's BSA involvement was reduced to 2% after 1 month; however, he began to develop an eruptive rash with severe pruritus again that reproducibly recurred after each secukinumab injection. On physical examination the patient had ill-defined, confluent, erythematous patches over much of the trunk and extremities. Punch biopsies of the eruptive dermatitis showed spongiform psoriasis and eosinophils with dermal hypersensitivity, consistent with a drug eruption. Upon further questioning, the patient noted that he had a long history of a strong latex allergy and he would develop a blistering dermatitis when coming into contact with latex, which caused a high suspicion for a latex allergy as the cause of the patient's acute dermatitis flares from his prior ustekinumab and secukinumab injections. Although it was confirmed with the manufacturers that both the ustekinumab syringe and secukinumab pen did not contain latex, the caps of these medications (and many other biologic injections) do have latex (Table). Other differential diagnoses included an atypical paradoxical psoriasis flare and a drug eruption to secukinumab, which previously has been reported.4

Based on the suspected cause of the eruption, the patient was instructed not to touch the cap of the secukinumab pen. Despite this recommendation, the rash was still present at the next appointment 1 month later. Repeat punch biopsy showed similar findings to the one prior with likely dermal hypersensitivity. The rash improved with steroid injections and continued to improve after holding the secukinumab for 1 month.
After resolution of the hypersensitivity reaction, the patient was started on ixekizumab, which does not contain latex in any component according to the manufacturer. After 2 months of treatment, the patient had 2% BSA involvement of psoriasis and has had no further reports of itching, rash, or other symptoms of a hypersensitivity reaction. On follow-up, the patient's psoriasis symptoms continue to be controlled without further reactions after injections of ixekizumab. Radioallergosorbent testing was not performed due to the lack of specificity and sensitivity of the test3 as well as the patient's known history of latex allergy and characteristic dermatitis that developed after exposure to latex and resolution with removal of the agent. These clinical manifestations are highly indicative of a type I hypersensitivity to injection devices that contain latex components during biologic therapy.
Comment
Allergic responses to latex are most commonly seen in those exposed to gloves or rubber, but little has been reported on reactions to injections with pens or syringes that contain latex components. Some case reports have demonstrated allergic responses in diabetic patients receiving insulin injections.5,6 MacCracken et al5 reported the case of a young boy who had an allergic response to an insulin injection with a syringe containing latex. The patient had a history of bladder exstrophy with a recent diagnosis of diabetes mellitus. It is well known that patients with spina bifida and other conditions who undergo frequent urological procedures more commonly develop latex allergies. This patient reported a history of swollen lips after a dentist visit, presumably due to contact with latex gloves. Because of the suspected allergy, his first insulin injection was given using a glass syringe and insulin was withdrawn with the top removed due to the top containing latex. He did not experience any complications. After being injected later with insulin drawn through the top using a syringe that contained latex, he developed a flare-up of a 0.5-cm erythematous wheal within minutes with associated pruritus.5
Towse et al6 described another patient with diabetes who developed a local allergic reaction at the site of insulin injections. Workup by the physician ruled out insulin allergy but showed elevated latex-specific IgE antibodies. Future insulin draws through a latex-containing top produced a wheal at the injection site. After switching to latex-free syringes, the allergic reaction resolved.6
Latex allergies are common in medical practice, as latex is found in a wide variety of medical supplies, including syringes used for injections and their caps. Physicians need to be aware of latex allergies in their patients and exercise extreme caution in the use of latex-containing products. In the treatment of psoriasis, care must be given when injecting biologic agents. Although many injection devices contain latex limited to the cap, it may be enough to invoke an allergic response. If such a response is elicited, therapy with injection devices that do not contain latex in either the cap or syringe should be considered.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
- Druce HM. Allergic and nonallergic rhinitis. In: Middleton EM Jr, Reed CE, Ellis EF, et al, eds. Allergy: Principles and Practice. 5th ed. Vol 1. St. Louis, MO: Mosby; 1998:1005-1016.
- Rochford C, Milles M. A review of the pathophysiology, diagnosis, and management of allergic reactions in the dental office. Quintessence Int. 2011;42:149-156.
- Hamilton RG, Peterson EL, Ownby DR. Clinical and laboratory-based methods in the diagnosis of natural rubber latex allergy. J Allergy Clin Immunol. 2002;110(2 suppl):S47-S56.
- Shibata M, Sawada Y, Yamaguchi T, et al. Drug eruption caused by secukinumab. Eur J Dermatol. 2017;27:67-68.
- MacCracken J, Stenger P, Jackson T. Latex allergy in diabetic patients: a call for latex-free insulin tops. Diabetes Care. 1996;19:184.
- Towse A, O'Brien M, Twarog FJ, et al. Local reaction secondary to insulin injection: a potential role for latex antigens in insulin vials and syringes. Diabetes Care. 1995;18:1195-1197.
Acute Painful Rash on the Cheek
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.
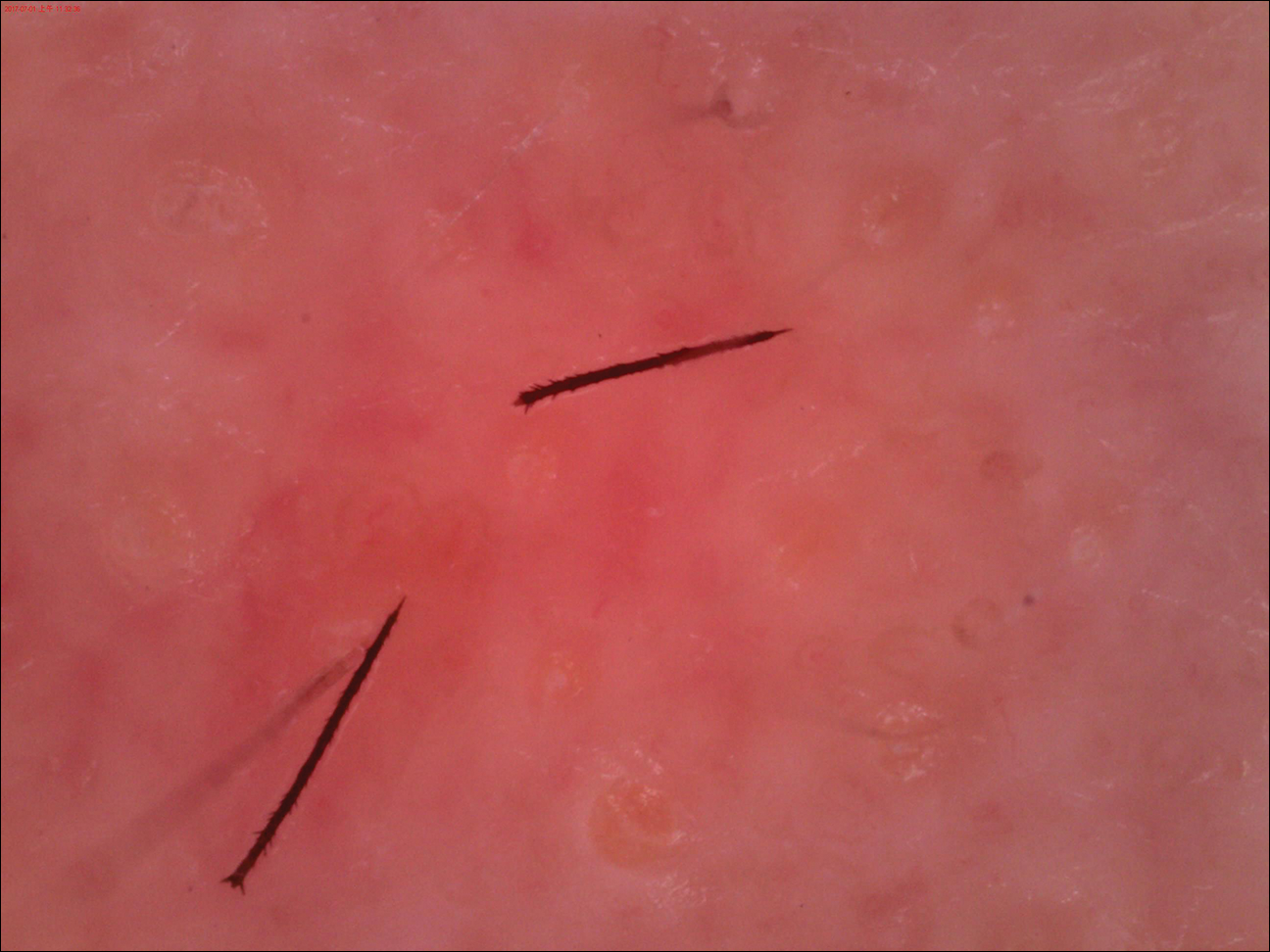
Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
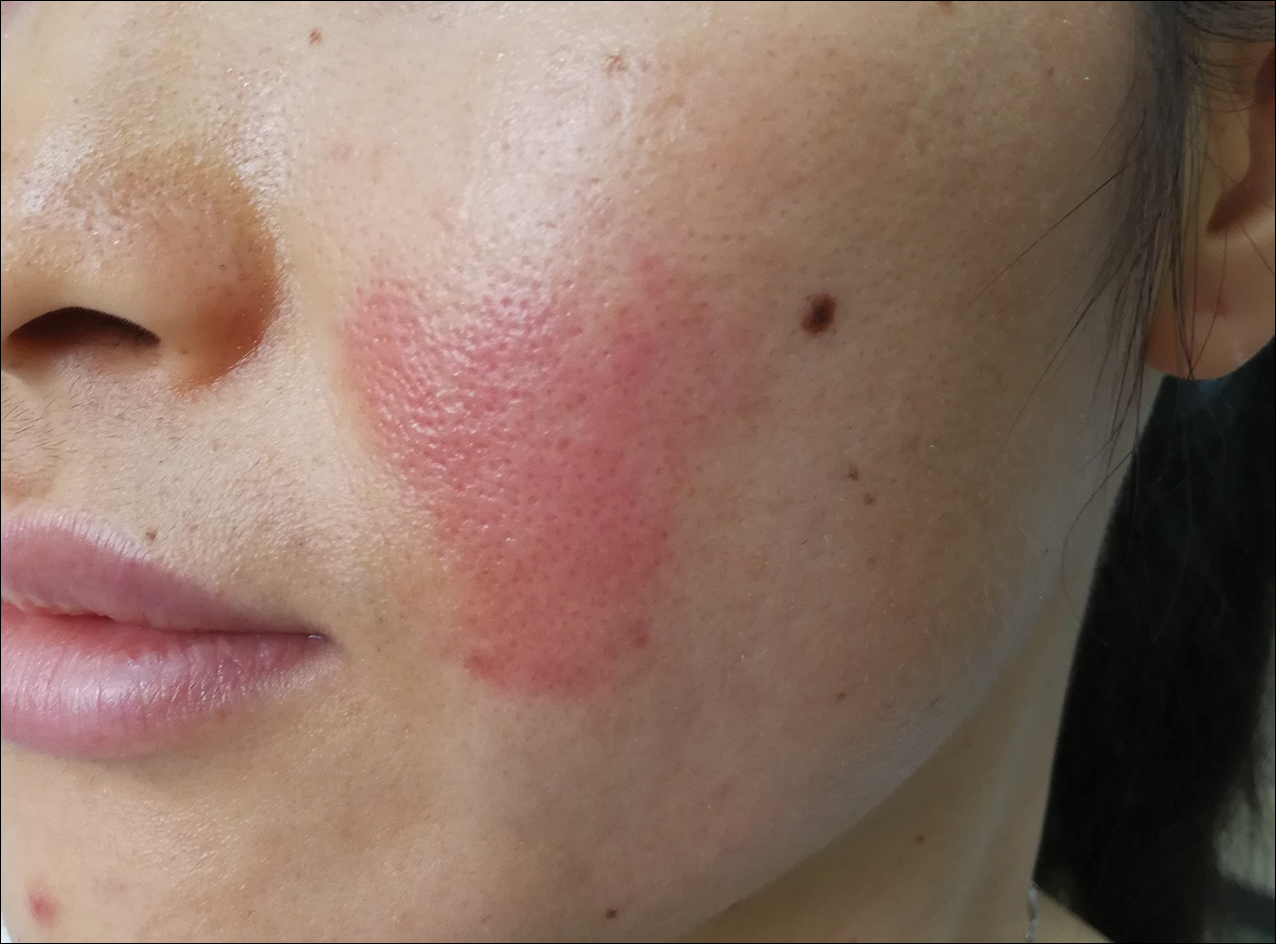
A 31-year-old woman presented to an outpatient dermatology department with acute pruritus, burning, and moderate swelling of the left cheek of 10 minutes' duration that occurred while waiting to see a hematologist in the same building. The patient was diagnosed with aplastic anemia 11 years prior and was awaiting bone marrow transplantation. Physical examination showed an edematous erythematous wheal with a relatively distinct border measuring 3 cm in diameter. No foreign material could be identified on the surface with the naked eye. Dermoscopy was performed.
Eosinophilic Pustular Folliculitis With Underlying Mantle Cell Lymphoma
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.
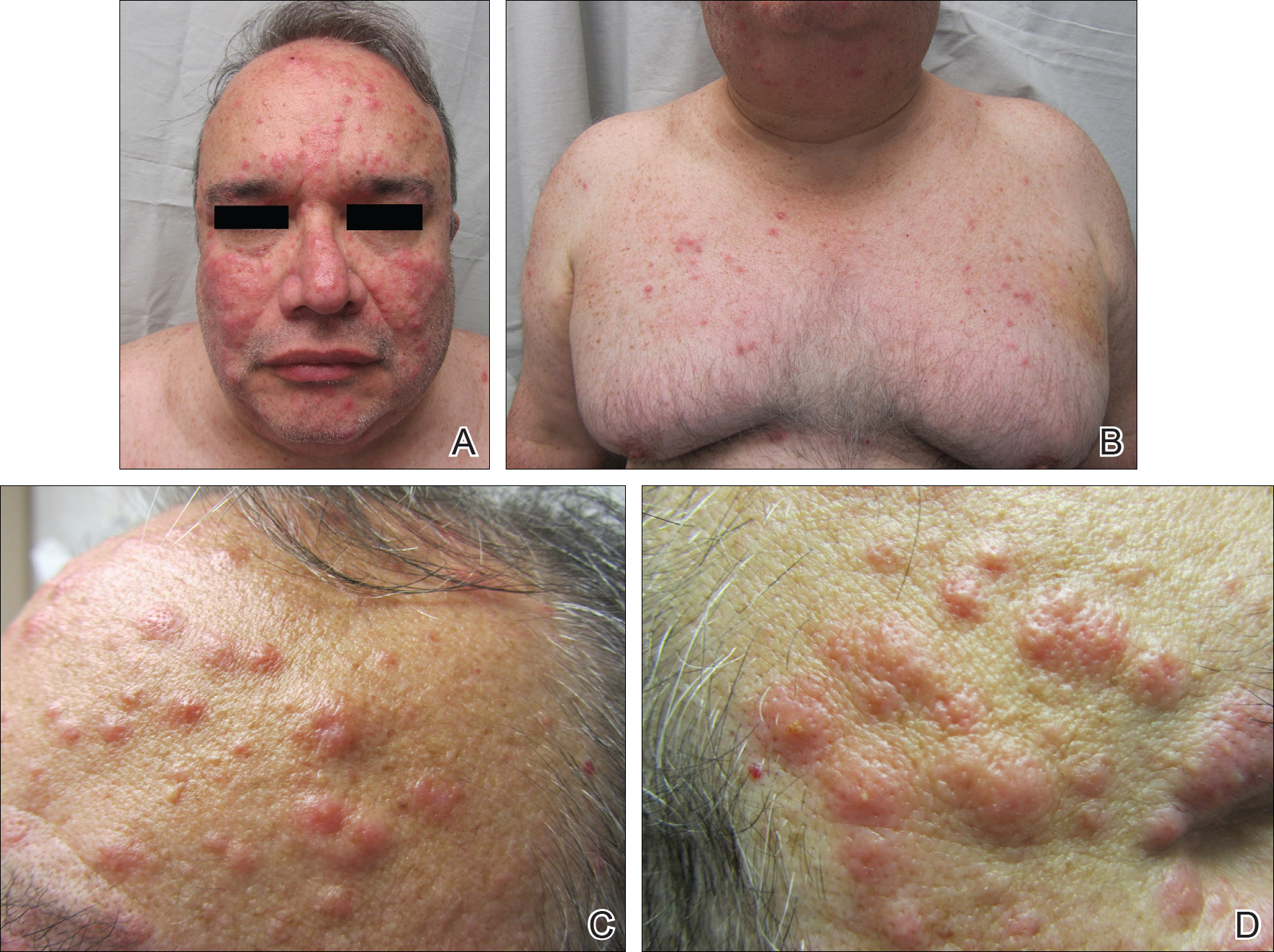
Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.
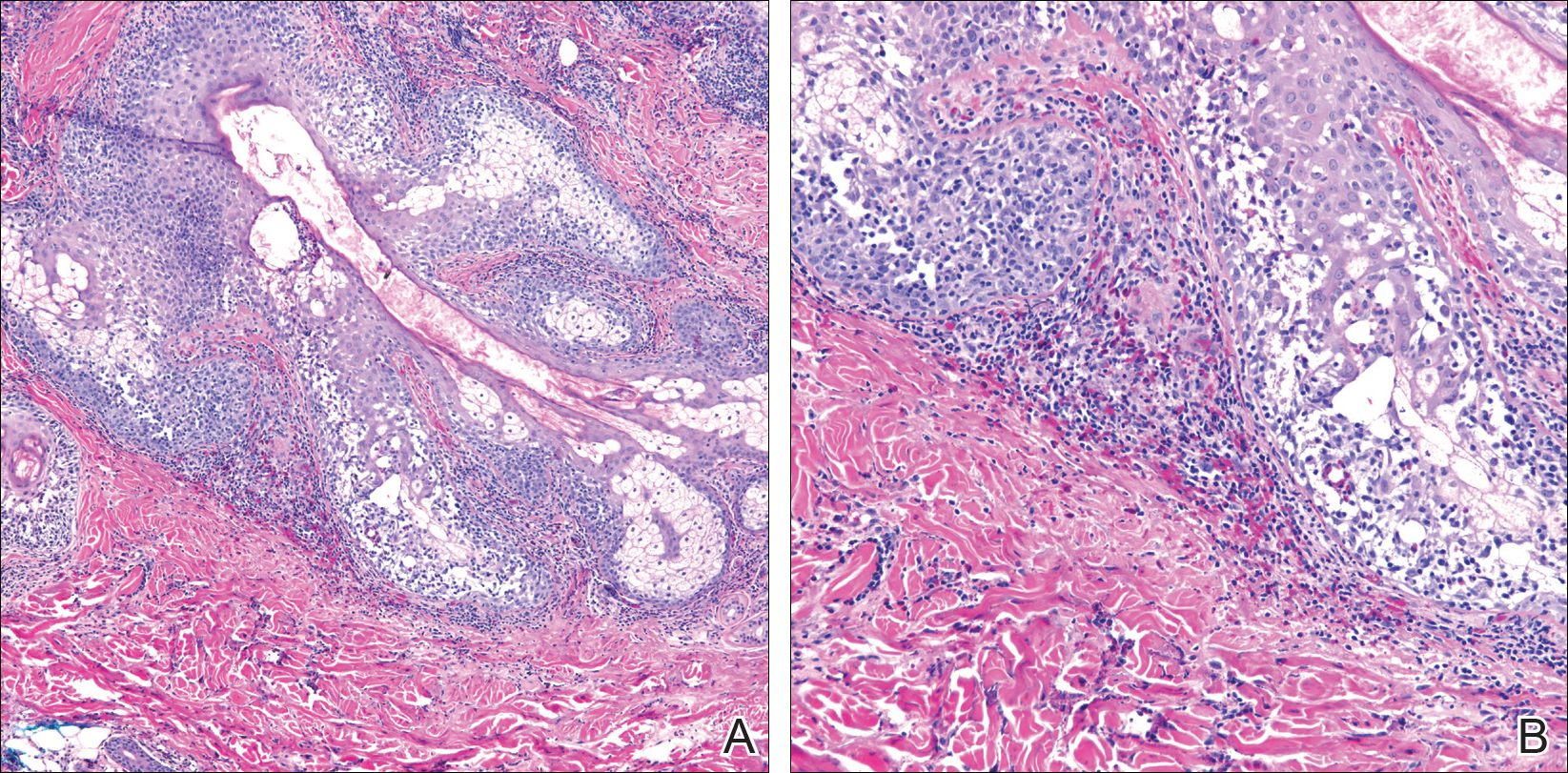
Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
Eosinophilic pustular folliculitis (EPF) was originally described in 1965 and has since evolved into 3 distinct subtypes: classic, immunosuppressed (IS), and infantile types. Immunosuppressed EPF can be further subdivided into human immunodeficiency virus (HIV) associated (IS-HIV) and non-HIV associated. Human immunodeficiency virus–seronegative cases have been associated with underlying malignancies (IS-heme) or chronic immunosuppression, such as that seen in transplant patients.
Case Report
A 52-year-old man with a medical history limited to prostate adenocarcinoma treated with a robotic prostatectomy presented with a pruritic red rash on the face, neck, shoulders, and chest of 1 month’s duration. The patient previously completed a course of azithromycin 250 mg, intramuscular triamcinolone, and oral prednisone with only minor improvement. Physical examination demonstrated multiple pink folliculocentric papules and pustules scattered on the head (Figure 1A), neck, and chest (Figure 1B), as well as edematous pink papules and plaques on the forehead (Figures 1C and 1D). The palms, soles, and oral mucosa were clear.

Initial biopsy of the right side of the chest was nonspecific and most consistent with a reaction to an arthropod bite. The patient was started on oral doxycycline 100 mg twice daily for 2 weeks. With no improvement seen, additional biopsies were obtained from the left side of the chest and forehead. The biopsy of the chest showed ruptured folliculitis with evidence of acute and chronic inflammation. The biopsy of the forehead demonstrated eosinophilic follicular spongiosis with intrafollicular Langerhans cell microgranulomas along with abundant eosinophils adjacent to follicles, consistent with EPF (Figure 2). Serum HIV testing was negative. Serum white blood cell count was normal at 6400/µL (reference range, 4500–11,000/µL) with mild elevation of eosinophils (8%). The remaining complete blood cell count and comprehensive metabolic panel were within reference range. The patient was subsequently started on oral indomethacin 25 mg twice daily and triamcinolone cream 0.1%. Within a few days he experienced initial improvement in his symptoms of pruritus and diminution in the number of inflammatory follicular papules.

Approximately 1 month after presentation, he began to experience symptoms of dysphagia and fatigue. In addition, tonsillar hypertrophy and palpable neck and axillary lymphadenopathy were present. Computed tomography of the neck, chest, and abdomen showed diffuse lymphadenopathy. Full-body positron emission tomography–computed tomography demonstrated extensive metabolically active lymphoma in multiple nodal groups above and below the diaphragm. There also was lymphomatous involvement of the spleen. An axillary lymph node biopsy was diagnostic for mantle cell lymphoma (CD4:CD8, 1:1; CD45 negative; CD20 positive; CD5 positive). He
Comment
Subtypes of EPF
Eosinophilic pustular folliculitis was first described in a Japanese female presenting with folliculocentric pustules distributed on the face, torso, and arms.1 This noninfectious eosinophilic infiltration of hair follicles predominantly seen in the Japanese population is now regarded as the classic form. Three distinct subtypes of EPF now exist, including the originally described classic variant (Ofuji disease), an IS variant, and a rare infantile form.1
All 3 subtypes of EPF are more commonly seen in men than women. The classic form has a peak incidence between the third and fourth decades of life. It presents as chronic annular papules and sterile pustules exhibiting peripheral extension, with individual lesions lasting for approximately 7 to 10 days with frequent relapses. The face is the most common area of involvement, followed by the trunk, extremities, and more rarely the palmoplantar surfaces. Concomitant leukocytosis with eosinophilia is seen in up to 35% of patients.1 The infantile type represents the rarest EPF form. The average age of onset is 5 months, with most cases resolving by 14 months of age.1
Clinically, EPF is characterized by recurrent papules and pustules predominantly on the scalp without annular or polycyclic ring formation, as seen in the classic type. The palms and soles may be involved, which can clinically mimic infantile acropustulosis and scabies infection. Most patients exhibit a concomitant peripheral eosinophilia.1,2
In the late 1980s, the IS variant of EPF was recognized in HIV-positive (IS-HIV) and HIV-negative malignancy-associated (IS-heme) populations.1,3 This newly characterized form differs morphologically and biologically from the classic and infantile subtypes. The IS subtype has a unique presentation including intensely pruritic, discrete, erythematous, follicular papules with palmoplantar sparing and infrequent annular or circinate plaque forms.1 Frequently, with the IS-HIV form, CD4+ T-cell counts are below 300 cells/mL, and 25% to 50% of patients have lymphopenia with eosinophilia.3 Highly active antiretroviral therapy has been associated with EPF resolution in HIV-positive individuals; however, it also has been shown to induce transient EPF during the first 3 to 6 months of initiation.1,3,4
Unlike the IS-HIV form, the IS-heme form has occurred solely in males and is predominantly associated with hematologic malignancies (eg, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia, myelodysplastic syndrome) 30 to 90 days following bone marrow transplant, peripheral blood stem cell transplant, or chemotherapy treatment.5,6 Unlike the chronic and persistent IS-HIV form, prior cases of IS-heme EPF have been predominantly self-limited. Interestingly, only 2 reported cases of EPF have occurred prior to the diagnosis of malignancy including B-cell leukemia and myelodysplastic syndrome.5
Histopathology
All 3 identified forms of EPF histopathologically show acute and chronic lymphoeosinophilic infiltrate concentrated at the follicular isthmus, which can lead to follicular destruction. Scattered mononuclear cells, eosinophils, and neutrophils are found within the pilar outer root sheath, sebaceous glands, and ducts. Approximately 40% of cases demonstrate follicular mucinosis.1 Histopathology of lesional palmar skin in classic-type EPF demonstrates intraepidermal pustule formation with abundant eosinophils and neutrophils adjacent to the acrosyringium.7,8
Pathogenesis
Although the pathophysiology of EPF is largely unknown, it is thought to represent a helper T cell (TH2) response involving IL-4, IL-5, and IL-13 cytokines.9 Chemoattractant receptor homologous molecule 2, which is expressed on eosinophils and lymphocytes, is believed to play a role in the pruritus, edema, and inflammatory response seen adjacent to pilosebaceous units in EPF.10 Moreover, immunohistochemical and flow cytometry analysis has revealed a prevalence of prostaglandin D2 within the perisebocyte infiltrate in EPF.9 Prostaglandin D2 induces eotaxin-3 production within sebocytes via peroxisome proliferator-activated receptor γ, which enhances chemoattraction of eosinophils. This pathogenesis represents a prostaglandin-based mechanism and potentially explains the efficacy of indomethacin treatment of EPF through its cyclooxygenase inhibition and reduction of chemoattractant receptor homologous molecule 2 expression.9-11
Treatment
Multiple therapeutic modalities have been reported for the treatment of EPF. For all 3 subtypes, moderate- to high-potency topical corticosteroids are considered first-line therapy. UVB phototherapy 2 to 3 times weekly remains the gold standard, given its consistent efficacy.1,12 Indomethacin (50–75 mg daily) remains first-line treatment of classic EPF.4,12 Previously reported cases of classic EPF and IS-EPF have responded well to oral prednisone (1 mg/kg daily).12,13 In a retrospective review of EPF treatment data, the following treatments also have been reported to be successful: psoralen plus UVA, oral cetirizine (20–40 mg daily, particularly for IS-EPF cases), metronidazole (250 mg 3 times daily), minocycline (150 mg daily), itraconazole (200–400 mg daily, dapsone (50–200 mg daily), systemic retinoids, tacrolimus ointment 0.1%, and permethrin cream.4,12
Malignancy
Although the entity of IS-heme EPF is rare, the morphology and treatment are unique and can potentially unmask an underlying hematologic malignancy. In patients with EPF and associated malignancy, such as our patient, a differential diagnosis to consider is eosinophilic dermatosis of hematologic malignancy (EDHM). Eosinophilic dermatosis of hematologic malignancy is most commonly associated with chronic lymphocytic leukemia and can be differentiated from EPF clinically, histopathologically, and by treatment response. Eosinophilic dermatosis of hematologic malignancy clinically presents with nonspecific papules, pustules, and/or vesicles on the head, trunk, and extremities. On histopathology, EDHM shows a superficial and deep perivascular and interstitial lymphoeosinophilic infiltration. Furthermore, EDHM patients typically exhibit a poor treatment response to oral indomethacin.14
Conclusion
Eosinophilic pustular folliculitis is a noninfectious folliculocentric process comprised of 3 distinct types. The histopathology shows follicular spongiosis with increased eosinophils. The pathogenesis is most likely related to a multifactorial immune system dysregulation involving TH2 T cells, prostaglandin D2, and eotaxin-3. The treatment of EPF may involve topical corticosteroids, UVB phototherapy, or most notably oral indomethacin. In patients with EPF and malignancy, EDHM is a differential diagnosis to consider. Our case serves as a reminder that rare eosinophilic dermatoses may represent manifestations of underlying hematopoietic malignancy and, when investigated early, can lead to appropriate life-saving treatment.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
- Nervi J, Stephen. Eosinophilic pustular folliculitis: a 40 year retrospect. J Am Acad Dermatol. 2006;55:285-289.
- Hernández-Martín Á, Nuño-González A, Colmenero I, et al. Eosinophilic pustular folliculitis of infancy: a series of 15 cases and review of the literature [published online July 21, 2012]. J Am Acad Dermatol. 2013;68:150-155.
- Soepr
ono F, Schinella R. Eosinophilic pustular folliculitis in patients with acquired immunodeficiency syndrome. report of three cases. J Am Acad Dermatol. 1986;14:1020-1022. - Katoh
M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. - Keida
T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplant. J Dermatol. 2004;31:21-26. - Goiriz R, Gul-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36.
- Satoh T, Ikeda H, Yokozeki H. Acrosyringeal involvement of palmoplantar lesions of eosinophilic pustular folliculitis. Acta Derm Venereol. 2013;93:99.
- Tsuboi H, Wakita K, Fujimura T, et al. Acral variant of eosinophilic pustular folliculitis (Ofuji’s disease). Clin Exp Dermatol. 2003;28:321-324.
- Nakahig
ashi K, Doi H, Otsuka A, et al. PGD2 induces eotaxin-3 via PPARgamma from sebocytes: a possible pathogenesis of eosinophilic pustular folliculitis. J Allergy Clin Immunol. 2012;129:536-543. - Satoh
T, Shimura C, Miyagishi C, et al. Indomethacin-induced reduction in CRTH2 in eosinophilic pustular folliculitis (Ofuji’s disease): a proposed mechanism of action. Acta Derm Venereol. 2010;90:18-22. - Hagiwara A, Fujimura T, Furudate S, et al. Induction of CD163(+)M2 macrophages in the lesional skin of eosinophilic pustular folliculitis. Acta Derm Venereol. 2014;94:104-106.
- Ellis
E, Scheinfeld N. Eosinophilic pustular folliculitis: a comprehensive review of treatment options. Am J Clin Dermatol. 2004;5:189-197. - Bull R
H, Harland CA, Fallowfield ME, et al. Eosinophilic folliculitis: a self-limiting illness in patients being treated for haematological malignancy. Br J Dermatol. 1993;129:178-182. - Farber M, Forgia S, Sahu J, et al. Eosinophilic dermatosis of hematologic malignancy. J Cutan Pathol. 2012;39:690-695.
Practice Points
- Recalcitrant folliculocentric papules and pustules involving the head, trunk, arms, and legs should raise suspicion of possible eosinophilic pustular folliculitis (EPF).
- Underlying hematopoietic malignancy may be associated with cases of EPF.
Acrodermatitis Enteropathica From Zinc-Deficient Total Parenteral Nutrition
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).
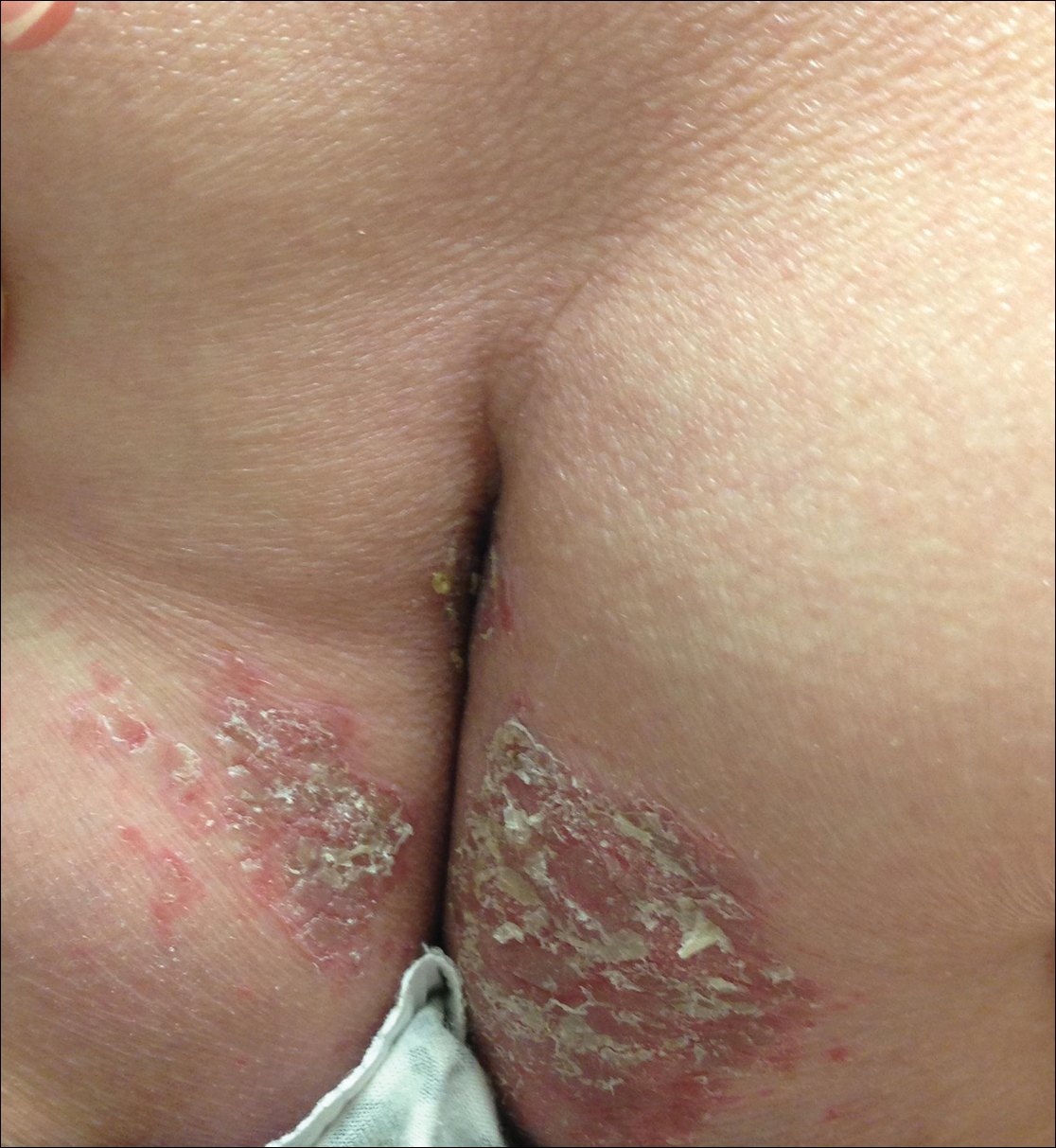
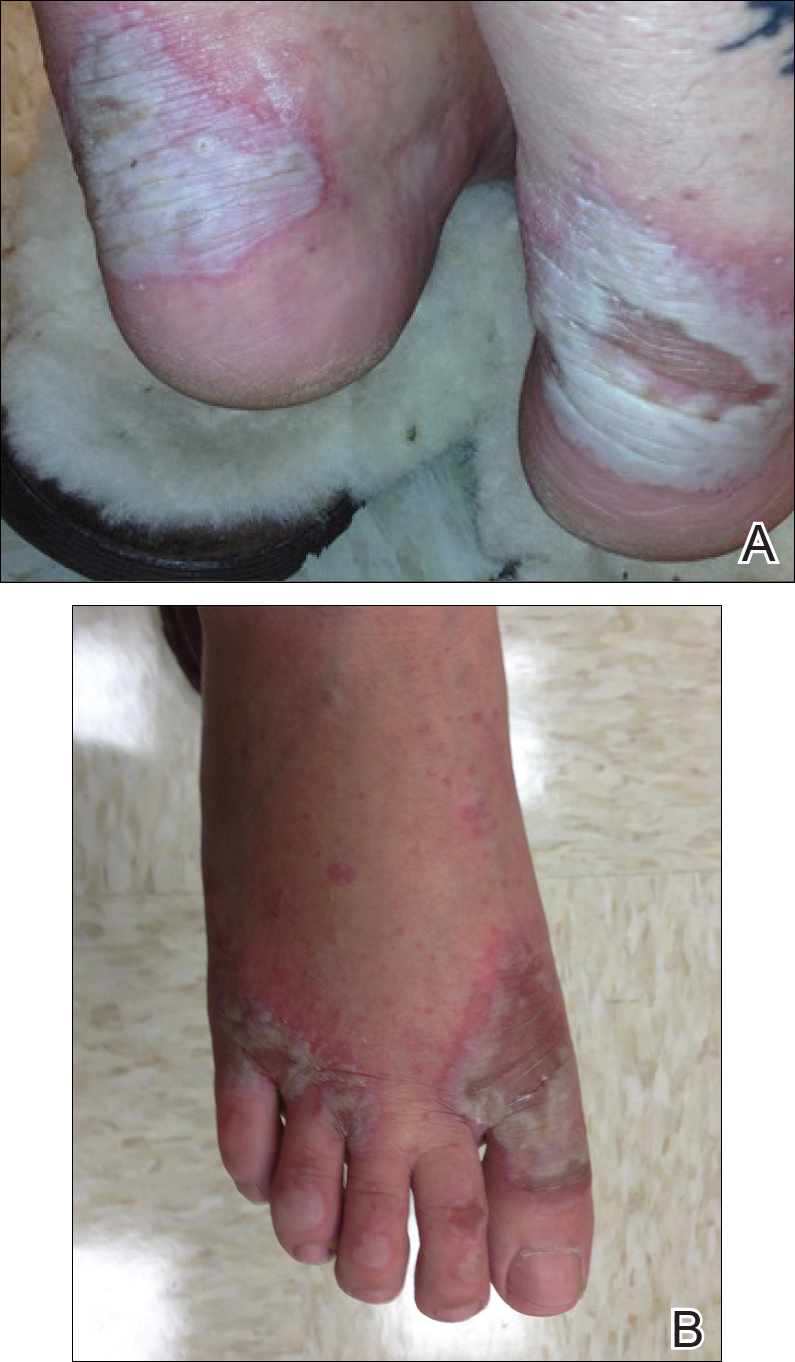
Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).
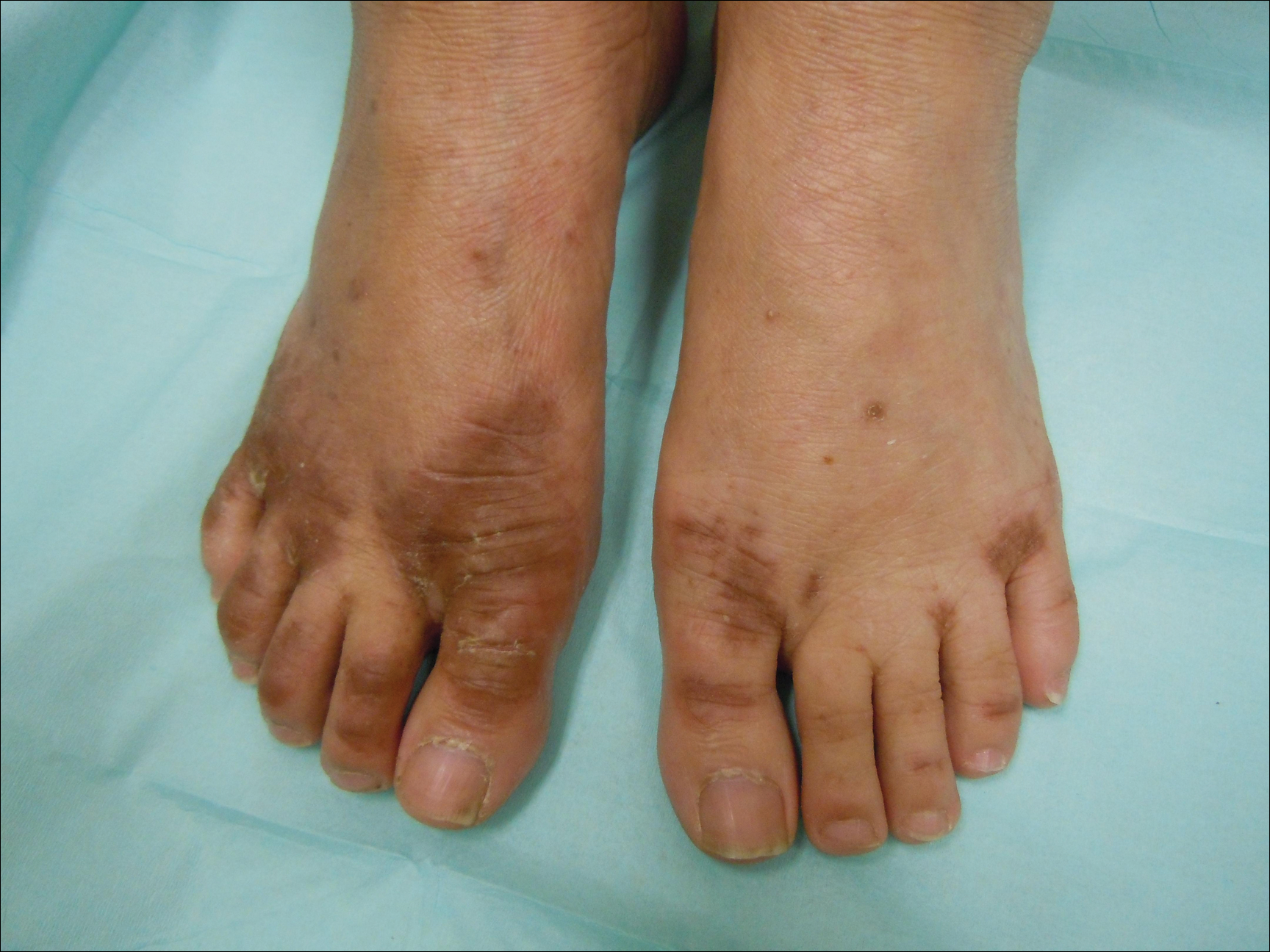
Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).


Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).

Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
Case Report
A 54-year-old woman presented with a pruritic and slightly painful skin eruption that began perinasally and progressed over 1 week to involve the labial commissures, finger webs, dorsal surfaces of the feet, heels, and bilateral gluteal folds. In addition, the eruption involved the left thigh at the donor site of a prior skin graft. She received no relief after an intramuscular steroid injection and hydrocortisone cream 1% prescribed by a primary care physician who diagnosed the rash as poison ivy contact dermatitis despite no exposure to plants. Review of systems was negative and she denied any new medication use. Her medical history was notable for extensive mesenteric injury secondary to a motor vehicle accident. She subsequently had multiple enterocutaneous fistulas that resulted in a complete small bowel enterectomy 10 months prior to presentation, which caused her to become dependent on total parenteral nutrition (TPN).
Physical examination revealed sharply demarcated, erythematous, scaly plaques perinasally, periorally, and on the bilateral gluteal folds (Figure 1). There were sharply demarcated, erythematous, scaly plaques on the right and left finger webs, dorsal surface of the right foot, and left upper thigh. Hemorrhagic bullae were appreciated on the left finger webs. Large flaccid bullae were present on the bilateral heels and dorsum of the right foot (Figure 2).


Suspecting a diagnosis of acrodermatitis enteropathica (AE), laboratory testing included a serum zinc level, which was 42 µg/dL (reference range, 70–130 µg/dL). The copper and selenium levels also were low with values of 71 µg/dL (reference range, 80–155 µg/dL) and 31 µg/dL (reference range, 79–326 µg/dL), respectively. No additional vitamin or mineral deficiencies were discovered. A complete blood cell count and comprehensive metabolic panel were performed and showed no abnormalities other than a mildly elevated sodium level of 147 mEq/L (reference range, 136–142 mEq/L).
A punch biopsy was performed. Histopathology revealed subcorneal neutrophils and neutrophilic crust, mild spongiosis, and a dense upper dermal mixed neutrophilic and lymphohistiocytic infiltrate. The specimen also exhibited mild intercellular edema and prominent capillaries (Figure 3).

After further investigation, the company providing the patient’s TPN confirmed that zinc had been removed several weeks prior to the onset of symptoms due to a critical national shortage of trace element additives. Zinc was supplemented at 15 mg daily to the TPN solution. Three days later a skin examination revealed dramatic changes with notable improvement of the finger web plaques and complete resolution of the facial lesions. The plaques and bullae on the lower extremities also had resolved (Figure 4).

Comment
Background
Acrodermatitis enteropathica is a rare autosomal-recessive disorder of zinc metabolism characterized by skin lesions predominantly distributed in acral and periorificial sites as well as alopecia and diarrhea. Acrodermatitis enteropathica was first described by Brandt1 in 1936 and later characterized by Danbolt and Closs2 in 1942 as a unique and often fatal disease of unknown etiology. More than 30 years later, the link between zinc deficiency and AE was illustrated by Moynahan3 who demonstrated clinical improvement with zinc supplementation. It was not until 2002 that the molecular pathogenesis of hypozincemia in patients with inherited AE was described. Küry et al4 identified a mutation in the SLC39A4 gene responsible for encoding the Zip4 protein, a zinc transporter found on enterocytes, particularly in the proximal small intestine.5,6 Classically, patients with inherited AE are children who present within days of birth or days to weeks after being weaned from breast milk to cow’s milk. The zinc in bovine milk is less bioavailable than breast milk, though both have similar total zinc concentrations, which results in the decreased plasma zinc levels seen in children with inherited AE.5-8 Occasionally, children present before weaning due to decreased maternal mammary zinc secretion (lactogenic AE).9,10
Clinical Presentation
Similar clinical findings are seen in patients with noninherited forms of zinc deficiency known as acquired AE. Acquired zinc deficiency may be broadly categorized as being from inadequate intake, deficient absorption, excess demand, or overexcretion.8 Such disturbances of zinc balance are most frequently seen in patients with restrictive diets, anorexia nervosa, intestinal bypass procedures, Crohn disease, pancreatic insufficiency, alcoholism, human immunodeficiency virus, and extensive cutaneous burns. Premature infants, mothers who are breastfeeding, and those dependent on TPN are at risk for developing acquired zinc deficiency.7-9,11
Differentiating Characteristics
Both acquired and inherited AE present as erythematous or pink eczematous scaly plaques with the variable presence of vesicular or bullous lesions involving periorificial, acral, and anogenital regions. Early manifestations of AE may include angular cheilitis and paronychia. Alopecia and diarrhea are characteristics of later disease. In fact, the complete triad of dermatitis, alopecia, and diarrhea is seen in only 20% of cases.7 Without treatment, patients may develop blepharitis, conjunctivitis, photophobia, irritability, anorexia, apathy, growth retardation, hypogonadism, hypogeusia, and mental slowing. Skin lesions frequently become secondarily infected with Candida albicans and/or bacteria.5,7,11
Histopathology
Histopathologic examination of skin biopsy specimens from AE lesions demonstrates nonspecific findings similar to other deficiency dermatoses, such as pellagra and glucagonoma-associated necrolytic migratory erythema. Histology typically reveals cytoplasmic pallor with vacuolization and ballooning degeneration of keratinocytes, followed by confluent keratinocyte necrosis within the stratum granulosum and stratum spinosum of the epidermis.5 Confluent parakeratosis with hypogranulosis variably associated with neutrophil crust also is seen. Scattered dyskeratotic keratinocytes may be found within all levels of the epidermis. In resolving or chronic AE lesions, psoriasiform hyperplasia is prevalent, though necrolysis may be minimal or absent.5,11
Diagnosis
Evaluation includes measurement of plasma zinc levels. Zinc levels less than 50 µg/dL are suggestive but not diagnostic of AE.5 Although plasma zinc measurement is the most useful indicator of zinc status, its utility in assessing the true total body store of zinc is limited. Plasma zinc is tightly regulated and only represents 0.1% of body stores.5,6 Additionally, zinc levels may decrease in proinflammatory states.12 Beyond zinc measurement, evaluation of alkaline phosphatase, a zinc-dependent enzyme, can provide useful diagnostic information.5,6
Zinc and TPN
Patients on TPN are at a unique risk for developing zinc and other nutritional deficiencies. Because the daily recommended dietary allowance for zinc is low (8 mg daily for adult women and 11 mg daily for adult men)5 and the element is found in a wide variety of foods, maintaining adequate zinc levels is easily achieved in healthy individuals with normal diets. Kay et al13 described 4 patients on parenteral nutrition who developed hypozincemia and an AE-like syndrome within weeks of TPN induction. The authors described rapid and drastic clinical improvement after initiating zinc supplementation, accentuating the importance of including zinc as a component of TPN.13,14 Brazin et al15 also reported a case of an AE-like syndrome from zinc-deficient hyperalimentation in a patient receiving TPN for short bowel syndrome. Chun et al16 described another case of acquired AE in a patient on TPN for acute pancreatitis. Both cases demonstrated prompt improvement of skin lesions after treatment with zinc supplementation. Other nutrient deficiencies may reveal themselves through similar dermatologic manifestations. For example, cases of scaly dermatitis secondary to the development of essential fatty acid deficiency from TPN formulations lacking adequate quantities of linoleic acid have been reported.Similar to our case, the resolution of skin lesions was seen after TPN was supplemented with the deficient nutrient.17 These cases exemplify the importance in considering deficiency dermatoses in the TPN-dependent patient population.
Conclusion
In our case, the development of skin lesions directly coincided with a recent removal of zinc from the patient’s TPN, which provided us with a unique opportunity to observe the causal relationship between decreased zinc intake and the development of clinical signs of acquired AE. This association was further elucidated by laboratory confirmation of low serum zinc levels and rapid improvement in all skin lesions after zinc supplementation was initiated.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
- Brandt T. Dermatitis in children with disturbances of general condition and absorption of food. Acta Derm Venereol. 1936;17:513-537.
- Danbolt N, Closs K. Acrodermatitis enteropathica. Acta Derm Venereol. 1942;23:127-169.
- Moynahan E. Acrodermatitis enteropathica: a lethal inherited human zinc deficiency disorder. Lancet. 1974;2:299-400.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet. 2002;31:238-240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013;68:211.e1-211.e33; quiz 244-246.
- Perafán-Riveros C, França LF, Alves AC, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Kumar P, Ranjan NR, Mondal AK. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Neldner K, Hambidge K, Walravens P. Acrodermatitis enteropathica.Int J Dermatol. 1978;17:380-387.
- Gehrig K, Dinulos J. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr. 2010;22:107-112.
- Liuzzi JP, Lichten LA, Rivera S, et al. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to hypozincemia of the acute-phase response. Proct Natl Acad Sci U S A. 2005;102:6843-6848.
- Kay RG, Tasman-Jones C, Pybus J, et al. A syndrome of acute zinc deficiency during total parenteral nutrition in man. Ann Surg. 1976;183:331-340.
- Jeejeebhoy K. Zinc: an essential trace element for parenteral nutrition. Gastroenterology. 2009;137(5 suppl):S7-S12.
- Brazin SA, Johnson WT, Abramson LJ. The acrodermatitis enteropathica-like syndrome. Arch Dermatol. 1979;115:597-599.
- Chun JH, Baek JH, Chung NG. Development of bullous acrodermatitis enteropathica during the course of chemotherapy for acute lymphocytic leukemia. Ann Dermatol. 2011;23(suppl 3):S326-S328.
- Roongpisuthipong W, Phanachet P, Roongpisuthipong C, et al. Essential fatty acid deficiency while a patient receiving fat regimen total parenteral nutrition [published June 14, 2012]. BMJ Case Rep. doi:10.1136/bcr.07.2011.4475.
Practice Points
- Acrodermatitis enteropathica (AE) may be acquired or due to a rare autosomal-recessive disorder of zinc absorption.
- Hereditary AE typically becomes symptomatic during infancy, while acquired AE may develop during hypozincemia in patients of any age.
- Both acquired and hereditary AE improve with zinc supplementation.