User login
Optimizing Narrowband UVB Phototherapy: Is It More Challenging for Your Older Patients?
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
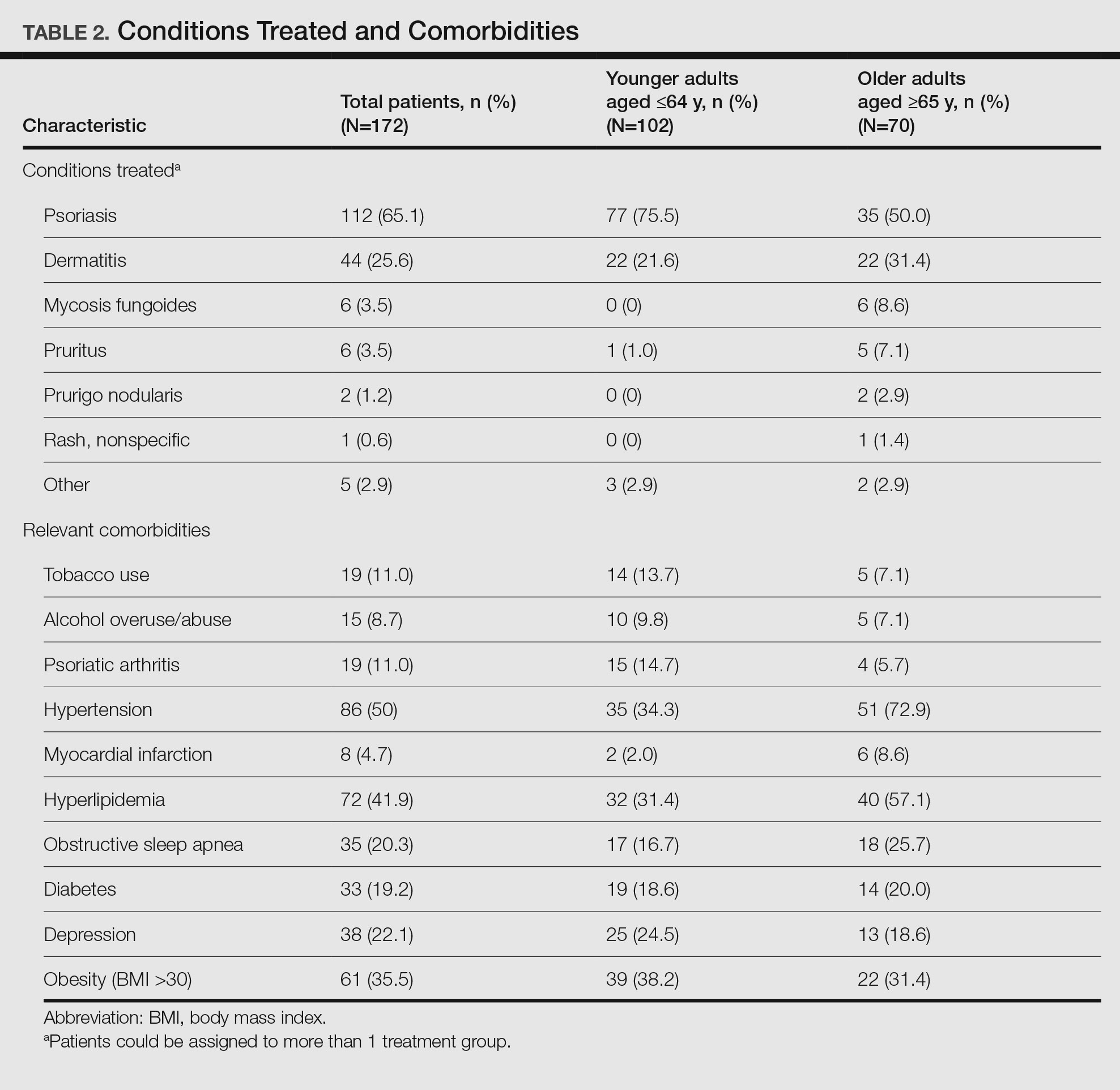
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—
Photosensitizing Medications, Clearance Levels, and Clearance Rates—
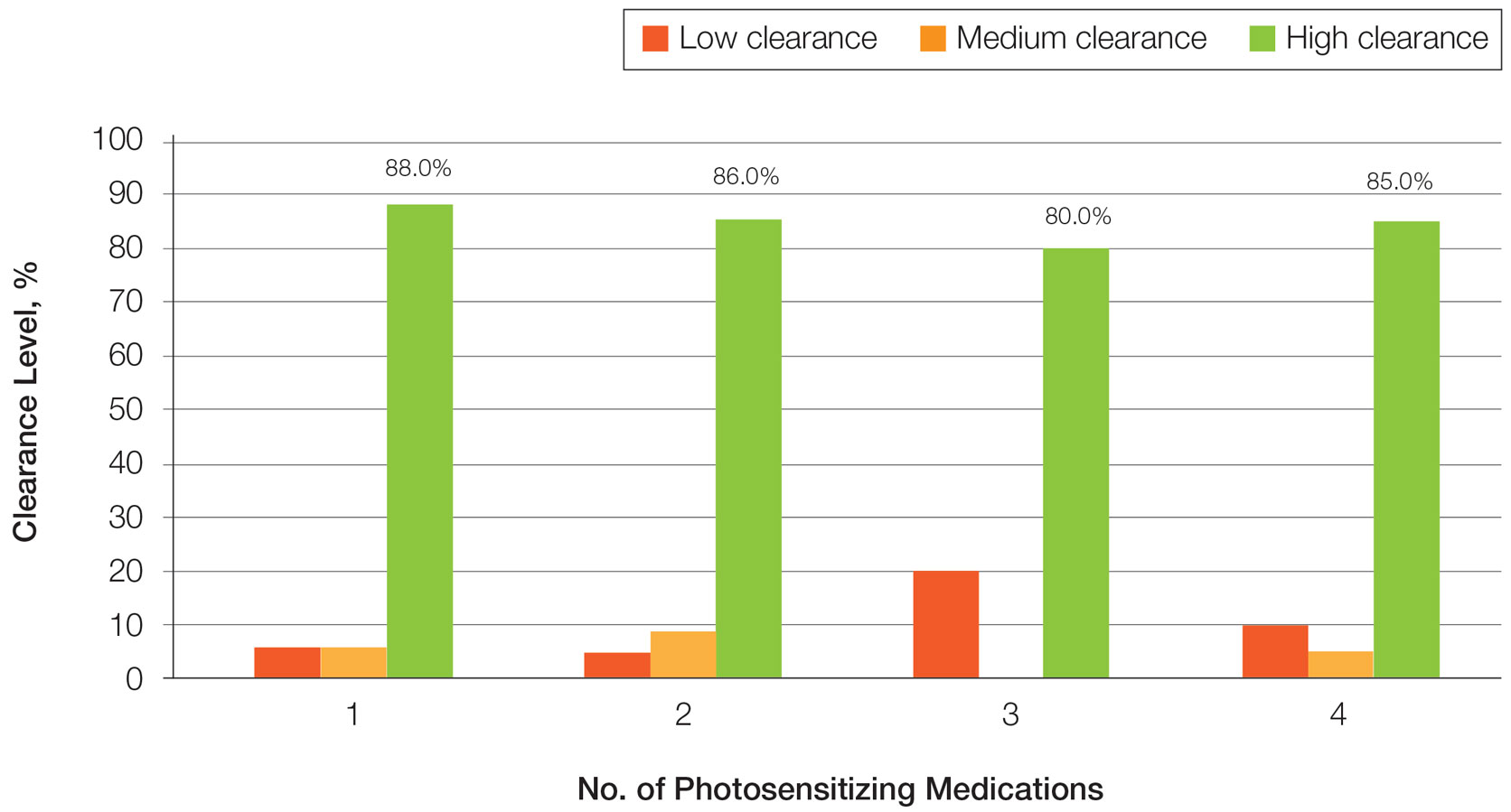
Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.
Photosensitizing Medications and Erythema Rates—
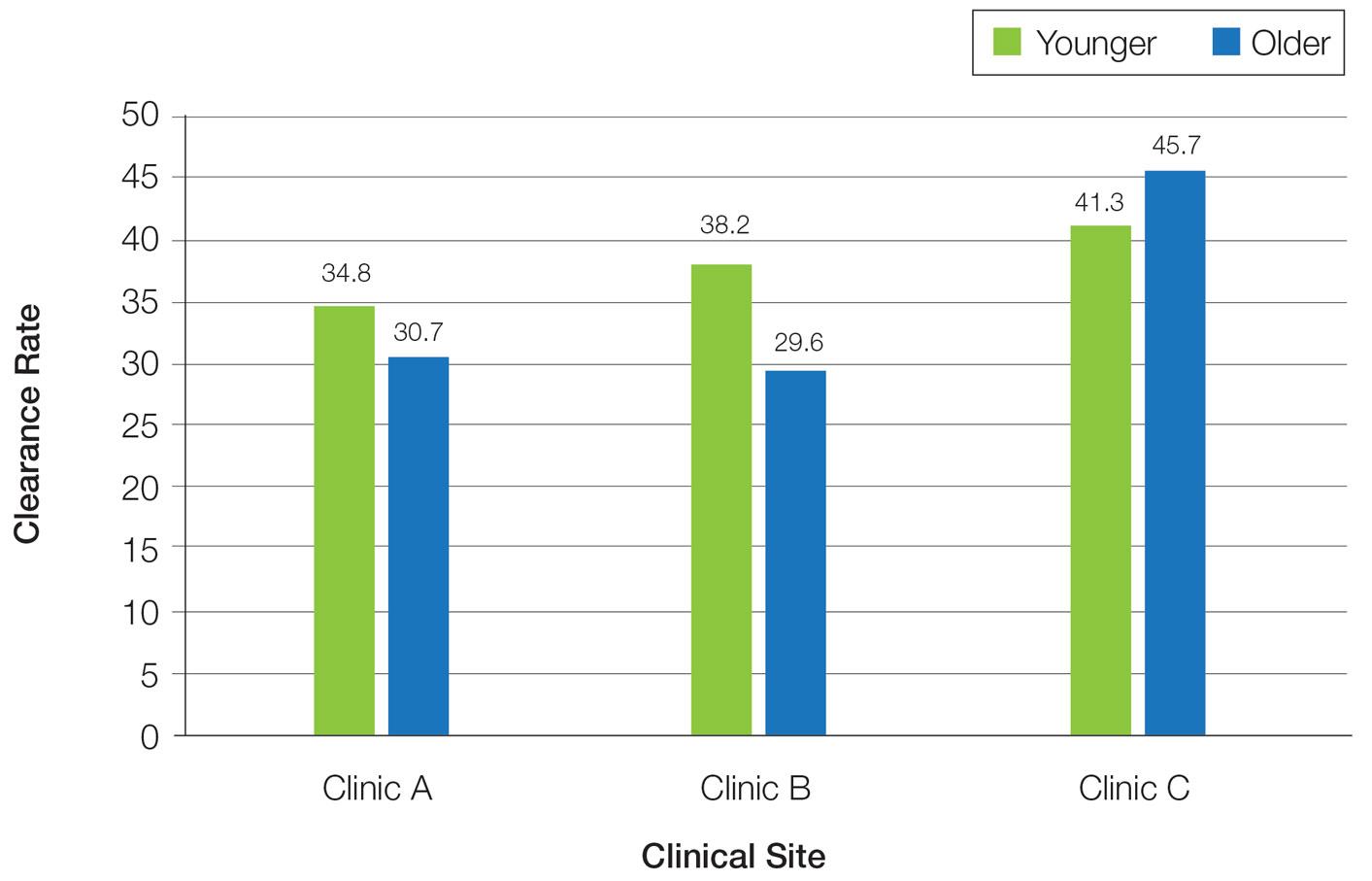
Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).
Comment
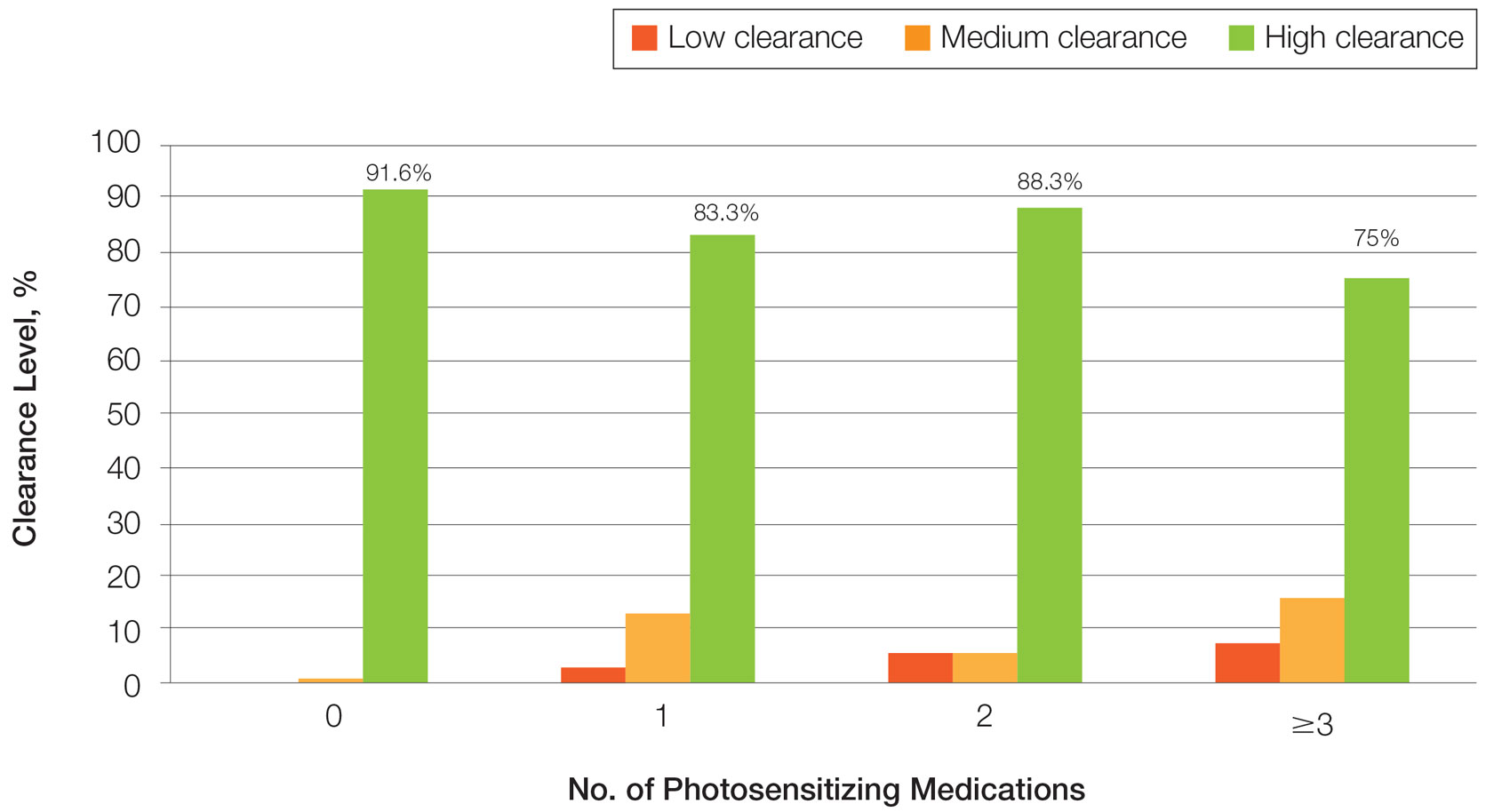
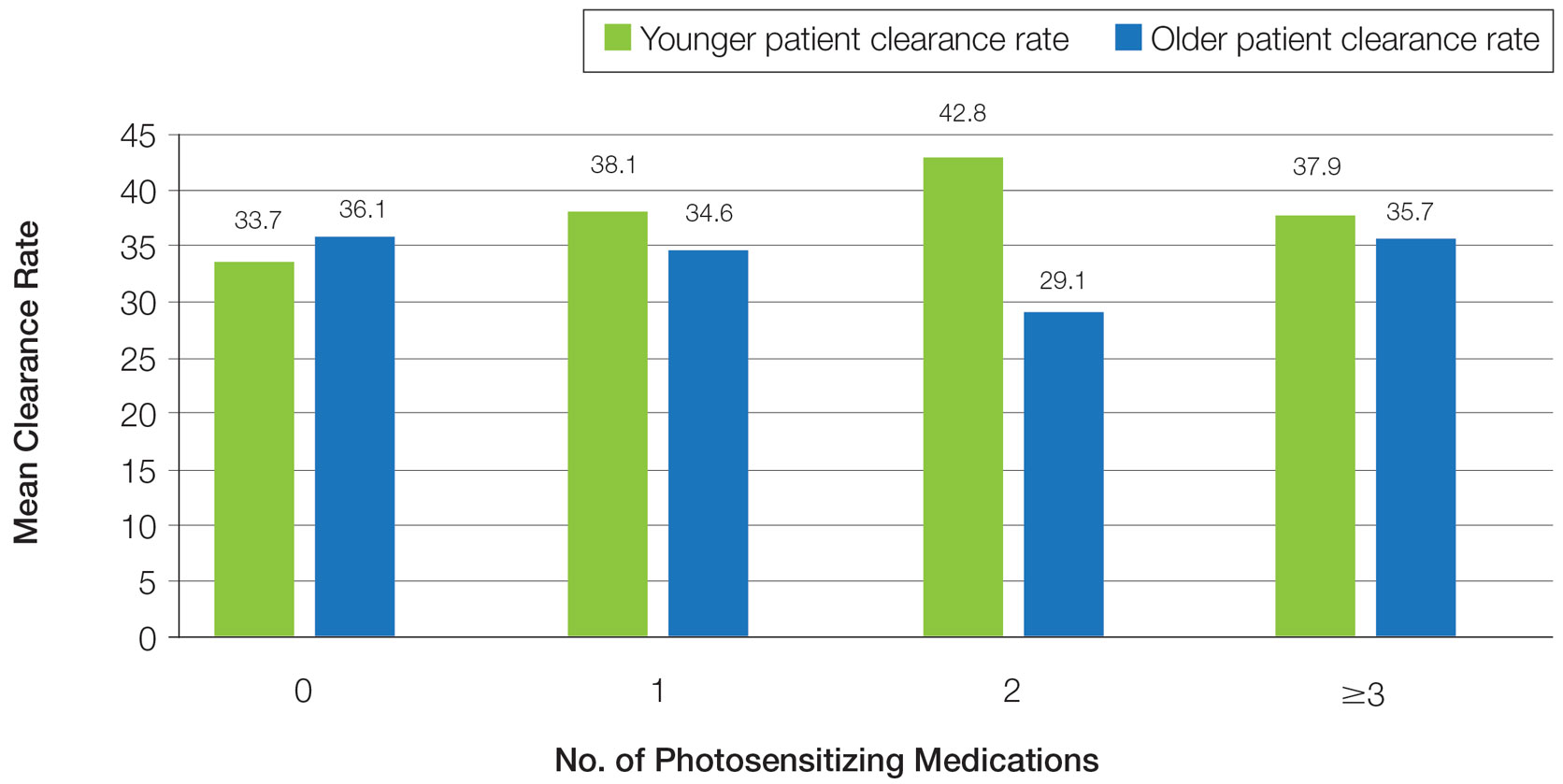
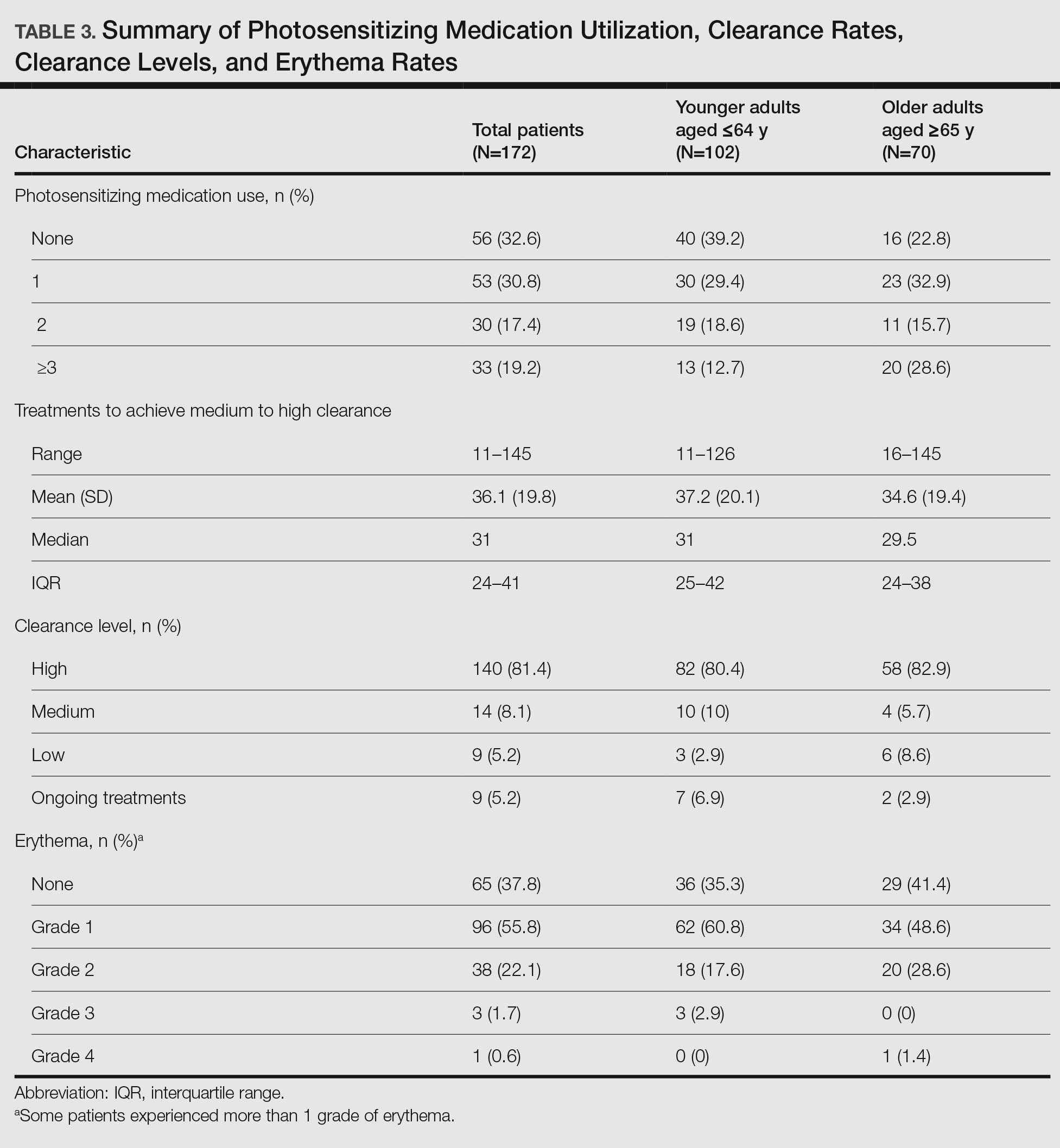
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Practice Points
- Narrowband UVB (NB-UVB) phototherapy remains a safe and efficacious nonpharmacologic treatment for dermatologic conditions in older and younger adults.
- Compared to younger adults, older adults using the same protocols need similar or even fewer treatments to achieve high levels of clearance.
- Individuals taking 3 or more photosensitizing medications, regardless of age, may be at higher risk for substantial erythema with NB-UVB phototherapy.
- Phototherapy program monitoring is important to ensure quality care and investigate opportunities for care optimization.
Dermatoses often occur in people who wear face masks
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
according to a recently published systematic review and meta-analysis.
“This report finds the most statistically significant risk factor for developing a facial dermatosis under a face mask is how long one wears the mask. Specifically, wearing a mask for more than 4 to 6 hours correlated most strongly with the development of a facial skin problem,” Jami L. Miller, MD, associate professor of dermatology, Vanderbilt University Medical Center, Nashville, Tenn., told this news organization. Dr. Miller was not involved in the study.
“The type of mask and the environment were of less significance,” she added.
Mask wearing for infection control has been common during the COVID-19 pandemic and will likely continue for some time, study coauthors Lim Yi Shen Justin, MBBS, and Yik Weng Yew*, MBBS, MPH, PhD, Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, write in Contact Dermatitis. And cross-sectional studies have suggested a link between mask wearing and various facial dermatoses.
To evaluate this link, as well as potential risk factors for facial dermatoses, the researchers reviewed 37 studies published between 2004 and 2022 involving 29,557 adult participants self-reporting regular use of any face mask type across 17 countries in Europe and Asia. The mask types commonly studied in the papers they analyzed included surgical masks and respirators.
Facial dermatoses were self-reported in 30 studies (81.1%) and were diagnosed by trained dermatologists in seven studies (18.9%).
Dr. Justin and Dr. Yew found that:
- The overall prevalence of facial dermatoses was 55%
- Individually, facial dermatitis, itch, acne, and pressure injuries were consistently reported as facial dermatoses, with pooled prevalence rates of 24%, 30%, 31%, and 31%, respectively
- The duration of mask wearing was the most significant risk factor for facial dermatoses (P < .001)
- Respirators, including N95 masks, were not more likely than surgical masks to be linked with facial dermatoses
“Understanding risk factors of mask wearing, including situation, duration, and type of mask, may allow for targeted interventions to mitigate problems,” Dr. Yew told this news organization.
He advised taking a break from mask wearing after 4 to 6 hours to improve outcomes.
Dr. Yew acknowledged limitations, including that most of the reviewed studies relied on self-reported symptoms.
“Patient factors were not investigated in most studies; therefore, we were not able to ascertain their contributory role in the development of facial dermatoses from mask wearing,” he said. “We were also unable to prove causation between risk factors and outcome.”
Four dermatologists welcome the findings
Dr. Miller called this an “interesting, and certainly relevant” study, now that mask wearing is common and facial skin problems are fairly common complaints in medical visits.
“As the authors say, irritants or contact allergens with longer exposures can be expected to cause a more severe dermatitis than short contact,” she said. “Longer duration also can cause occlusion of pores and hair follicles, which can be expected to worsen acne and folliculitis.”
“I was surprised that the type of mask did not seem to matter significantly,” she added. “Patients wearing N95 masks may be relieved to know N95s do not cause more skin problems than lighter masks.”
Still, Dr. Miller had several questions, including if the materials and chemical finishes that vary by manufacturer may affect skin conditions.
Olga Bunimovich, MD, assistant professor, department of dermatology, University of Pittsburgh School of Medicine, Pennsylvania, called this study “an excellent step towards characterizing the role masks play in facial dermatoses.”
“The study provides a window into the prevalence of these conditions, as well as some understanding of the factors that may be contributing to it,” Dr. Bunimovich, who was not part of the study, added. But “we can also utilize this information to alter behavior in the work environment, allowing ‘mask-free’ breaks to decrease the risk of facial dermatoses.”
Elma Baron, MD, professor and director, Skin Study Center, department of dermatology, Case Western Reserve University School of Medicine, Cleveland, expected skin problems to be linked with mask wearing but didn’t expect the prevalence to be as high as 55%, which she called “very significant.”
“Mask wearing is an important means to prevent transmission of communicable infections, and the practice will most likely continue,” she said.
“Given the data, it is reasonable to advise patients who are already prone to these specific dermatoses to be proactive,” she added. “Early intervention with proper topical medications, preferably prescribed by a dermatologist or other health care provider, and changing masks frequently before they get soaked with moisture, will hopefully lessen the severity of skin rashes and minimize the negative impact on quality of life.”
Also commenting on the study, Susan Massick, MD, dermatologist and clinical associate professor of internal medicine, The Ohio State University Wexner Medical Center, Westerville, said in an interview that she urges people to wear masks, despite these risks.
“The majority of concerns are straightforward, manageable, and overall benign,” she said. “We have a multitude of treatments that can help control, address, or improve symptoms.”
“Masks are an effective and easy way to protect yourself from infection, and they remain one of the most reliable preventions we have,” Dr. Massick noted. “The findings in this article should not preclude anyone from wearing a mask, nor should facial dermatoses be a cause for people to stop wearing their masks.”
The study received no funding. The authors, as well as Dr. Baron, Dr. Miller, Dr. Bunimovich, and Dr. Massick, who were not involved in the study, reported no relevant financial relationships. All experts commented by email.
A version of this article first appeared on Medscape.com.
Correction, 9/22/22: An earlier version of this article misstated the name of Dr. Yik Weng Yew.
Can Atopic Dermatitis and Allergic Contact Dermatitis Coexist?
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
Practice Points
- Although it previously was thought that atopic dermatitis (AD) and allergic contact dermatitis (ACD) could not coexist due to their polarized immune pathways, current evidence suggests otherwise.
- When both diagnoses are suspected, patch testing should be considered as well as therapeutic strategies that can treat both AD and ACD simultaneously.
Botanical Briefs: Tulipalin A
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
Cutaneous Manifestations
Contact dermatitis is a common problem for individuals who work in the floral industry. Hand dermatitis has been reported in as many as 26% of floral employees.1Tulipa species have been identified as one of the most common causes of hand dermatitis. Tulipalin A (α-methylene-γ-butyrolactone) is the main sensitizer in tulips (Figure 1) and its precursor tuliposide A also occurs both in tulips and the Peruvian lily (Alstroemeria).

In a 1996 study, 18% (9/51) of tulip workers were found to be allergic to tulipalin A.2 In a more recent study of 164 tulip workers, 48 (29.3%) had clinical evidence of contact dermatitis and subsequently underwent patch testing; 17 (35.4%) showed a positive reaction to either tulipalin A or to tulip-bulb extract.3 Itching was the most common symptom (39 workers [81.3%]) and hand eczema at the tip of the thumb and index finger was the most common finding. In 9 (18.8%) workers, eczema had spread to other body parts including the forearm, face, legs, and abdomen.3
Peruvian lily is widely used in floral arrangements and has become a leading cause of hand dermatitis in florists (Figure 2). Large amounts of free tulipalin A are present in bulb scales of tulips, along with a small amount of tuliposide A. In young developing shoots, the situation is reversed: Both compounds are found in all parts of the plant to some degree, though tulipalin A is the major allergen, and more mature parts of the plant and bulb are most allergenic.

Cultural Considerations
In traditional Kurdish cuisine, raw herbs are part of snacking or are served as a side dish (sawza). Snacks often are consumed raw on the spot. Tulipa montana, Tulipa armena, and possibly other Tulipa species are consumed as a snack.4 Traditionally, Tulipa systola is consumed by the Kurds as an anti-inflammatory medicine and for pain relief. It also has been proposed that T systola has antioxidant properties.5 Cooked tulip also has been consumed in time of famine in Europe, though none of these dietary practices are recommended.4
Clinical Presentation
“Tulip fingers” describes the most common presentation of contact dermatitis caused by tulip bulbs. Erythematous scaling plaques are seen in the periungual skin and first and second fingertips of the dominant hand. Other manifestations include diffuse dry dermatitis of the hand; paronychia; pulpitis; and secondary spread to the face, neck, arms, and genitalia, with eczematous papules and plaques.6 Clinical signs include erythema, vesicles, hyperkeratosis, and exfoliation of the fingertips. The allergen also can cause airborne contact dermatitis and manifest as conjunctivitis, rhinitis, and asthma.2 A considerable number of tulip workers develop paresthesia and tenderness in the fingertips within several hours after working with tulip bulbs, known as “tulip fire.”7
Plant Facts
There are approximately 250 genera of bulbous plants. Tulips are members of the genus Tulipa and family Liliaceae. Tulips often are thought of as native to southwest central Asia and Turkey8; however, Tulipa sylvestris is native to Portugal, Spain, and North Africa.
Etymology and Symbolism—The word tulip is derived from the Turkish word türbent meaning a turban, possibly because the flower is compared to turbans worn by men of the Ottoman Empire in the 16th century. In Turkish culture, the tulip is a symbol of paradise on earth and can have divine status. In the Netherlands, on the other hand, the tulip represents the briefness of life.
History—By 1562, tulip bulbs had already been introduced to Holland by merchants. However, the first shipment of tulip bulbs was mistaken by the Dutch for onions and were either roasted over a fire or perished when planted in gardens with vegetables. Carolus Clusius—botanist, director of the imperial medical garden in Vienna and recipient of many plants through diplomatic channels—was particularly fond of flower bulbs and contributed to the popularity of the tulip in Europe by sending bulbs and seeds to other European countries.
In the early 17th century, the tulip craze began in France, fueled by a viral disease of tulips that produced variegated color patterns on the petals; entire properties were sold in exchange for a single tulip bulb. The tulip craze drifted from France to Holland in 1634 for 3 years before the tulip market collapsed.
More recently, in 2003 investors started a multimillion-euro tulip fund in the Netherlands to develop new varieties of tulip. Tulip bulbs were used to create money with high percentages over the selling price. With exorbitant pricing and ever-changing ownership of bulbs—bulbs were bought and sold as many as 10 times—the tulip fund collapsed 1 year later and investors lost their money. Bulb speculators then took their profit abroad. In 2006, bulb owners were charged with fraud; the tulip craze often is cited as one of the early major stock market collapses.
Tulips continue to grow in popularity. Today, nearly 6000 cultivars are registered, with 40 new cultivars registered every 5 years.9
Identifying Features
At the base of the erect tulip plant is a cluster of 2 or 3 thick bluish-green leaves. Three petals and 3 sepals make up the solitary bell-shaped flower. Many tulips can propagate only by means of their scaly bulbs. The flowers arise from the tips of stems in different solid colors, except true blue—from pure white to all shades of yellow, red, and a deep purple that is almost black. Solid-color tulips are called “self-colored.” So-called broken tulips are individual flowers with multiple colors, a condition caused by a viral disease transmitted by aphids.10
Tulip Allergen
Tuliposide A is found in many species of the genera Tulipa, Alstroemeria, and Erythronium.6 So far, 7 analogs have been identified: 1-tuliposide A and B; 6-tuliposide A and B; and tuliposides D, E, and F. 6-Tuliposide A and B are the principal tuliposides found in tulip cultivars.11 With trauma and maturation, tuliposides A and B are hydrolyzed to tulipalin A and tulipalin B, respectively.
Tulipalin A and tulipalin B have antimicrobial properties against bacteria and fungi; tulipalin A is mostly an antifungal agent, and tulipalin B has mostly bacteriostatic characteristics.12 The highest concentration of tulipalin A is found in the outer layer of the bulb, followed by (in descending order) the stem, leaves, and petals.13
The prevalence of tulipalin A allergy led the German Federal Institute for Risk Assessment to assign tuliposide A and tulipalin A to category B, which is a “solid-based indication for contact allergenic effects”; both chemicals also are considered skin sensitizers, defined by the Globally Harmonized System of Classification and Labelling of Chemicals of the United Nations as a substance that will induce an allergic response following skin contact.14 Patients who are allergic to tulips have cross-sensitivity to Alstroemeria because tuliposide A and its metabolites are found in both plants.15
Symptoms of an allergic response to tulipalin A can be immediate or delayed.14 The most common allergic contact dermatitis caused by tulip bulbs is type IV hypersensitivity, though type I reactions can occur. Symptoms of a type I reaction including contact urticaria, rhinitis, hoarseness, and dyspnea have been reported.14
The variety of tulip handled also contributes to the severity of dermatitis. Handling bulbs of Rose Copeland variety tulips and cutting the flowers of Preludium tulips have been associated with more severe allergic dermatitis presentations, whereas the Red Emperor tulip was found to have less tuliposide A and thus provoke a weaker patch-test reaction.7
A Word About Garlic—Garlic is in the subfamily Allioideae (formerly Alliaceae) taxonomically related to the tulip family (Liliaceae). Garlic also can cause hand dermatitis in cooks, with a similar clinical appearance as tulip fingers. Gas chromatography has shown that garlic contains predominantly tuliposide B, which has been found to be much less allergenic than tuliposide A.7,16
Prevention of Tulipa Dermatitis
Tuliposide A and its metabolites can be found in storehouses and trucks used to transport tulips, in clothing, and in any other place where dust containing the allergen has settled. The best prevention against contact dermatitis is to avoid the inciting plants. Gloves may prevent contact dermatitis due to tuliposide A, which penetrates vinyl but not nitrile gloves. Barrier creams have been proposed, but data are scant.1
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
- Thiboutot DM, Hamory BH, Marks JG Jr. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58. doi: 10.1016/0190-9622(90)70007-5
- Bruze M, Bjorkner B, Hellstrom AC. Occupational dermatoses in nursery workers. Am J Contact Dermat. 1996;7:100-103.
- Hassan I, Rasool F, Akhtar S, et al. Contact dermatitis caused by tulips: identification of contact sensitizers in tulip works of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64-69. doi:10.1111/cod.12870
- Pieroni A, Zahir H, Amin HI, et al. Where tulips and crocuses are popular food snacks: Kurdish traditional foraging reveals traces of mobile pastoralism in Southern Iraqi Kurdistan. J Ethnobiol Ethnomed. 2019;15:59. doi:10.1186/s13002-019-0341-0
- Amin HIM, Ibrahim MF, Hussain FHS, et al. Phytochemistry and ethnopharmacology of some medicine plants used in the Kurdistan region of Iraq. Nat Prod Commun. 2016;11:291-296.
- Crawford GH. Botanical dermatology [Plant identification – other families: Liliaceae]. Medscape. Updated June 10, 2021. Accessed August 18, 2022. https://emedicine.medscape.com/article/1090097-overview#a3
- Gette MT, Marks JE Jr. Tulip fingers. Arch Dermatol. 1990;126:203-205.
- Bruynzeel DP. Bulb dermatitis: dermatological problems in the flower bulb industries. Contact Dermatitis. 1997;37:70-77. doi:10.1111/j.1600-0536.1997.tb00042.x
- Christenhusz MJ, Govaerts RHA, David J, et al. Tiptoe through the tulips—cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot J Linn Soc. 2013;172:280-328. doi:10.1111/boj.12061
- The Editors of Encyclopaedia Britannica. Tulip. Encyclopedia Britannica. Updated July 4, 2022. Accessed August 18, 2022. https://www.britannica.com/plant/tulip
- Hausen BM. Airborne contact dermatitis caused by tulip bulbs. J Am Acad Dermatol. 1982;7:500-503. doi:10.1016/s0190-9622(82)70132-x
- Nomura T, Ogita S, Kato Y. A novel lactone-forming carboxylesterase: molecular identification of a tuliposide A-converting enzyme in tulip. Plant Physiol. 2012;159:565-578. doi:10.1104/pp.112.195388
- Khalid MM, Greenberg MI. Tulip finger. Clin Toxicol (Phila). 2018; 56:860. doi:10.1080/15563650.2018.1440588
- McCluskey J, Bourgeois M, Harbison R. Tulipalin A induced phytotoxicity. Int J Crit Illn Inj Sci. 2014;4:181-183. doi:10.4103/2229-5151.134187
- Marks JG Jr. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Sasseville D. Clinical patterns of phytodermatitis. Dermatol Clin. 2009;27:299-308. doi:10.1016/j.det.2009.05.010
Practice Points
- Tulips are a common cause of contact dermatitis among floral workers.
- Tulipalin A is the primary sensitizer in tulips causing allergic contact dermatitis.
- The best preventative for tulip contact dermatitis is avoiding the inciting plants.
Consider essential oil allergy in patient with dermatitis
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
PORTLAND, ORE. – When patients present to Brandon L. Adler, MD, with dermatitis on the eyelid, face, or neck, he routinely asks them if they apply essential oils on their skin, or if they have an essential oil diffuser or nebulizer in their home.
“The answer is frequently ‘yes,’ ” Dr. Adler, clinical assistant professor of dermatology at the University of Southern California, Los Angeles, said at the annual meeting of the Pacific Dermatologic Association. “Essential oils are widely used throughout the wellness industry. They are contained in personal care products, beauty products, natural cleaning products, and they’re being diffused by our patients into the air. More than 75 essential oils are reported to cause allergic contact dermatitis.”
“Linalool is most classically associated with lavender, while limonene is associated with citrus, but they’re found in many different plants,” said Dr. Adler, who directs USC’s contact dermatitis clinic. “On their own, linalool and limonene are not particularly allergenic; they’re not a big deal in the patch test clinic. The problem comes when we add air to the mix, because they oxidize to hydroperoxides of linalool and limonene. These are quite potent allergens.”
According to the most recent North American Contact Dermatitis Group data, 8.9% of patients undergoing patch testing tested positive to linalool hydroperoxides and 2.6% were positive to limonene hydroperoxides.
Dr. Adler discussed the case of a female massage therapist who presented with refractory hand dermatitis and was on methotrexate and dupilumab at the time of consultation but was still symptomatic. She patch-tested positive to limonene and linalool hydroperoxides as well as multiple essential oils that she had been using with her clients, ranging from sacred frankincense oil to basil oil, and she was advised to massage using only coconut or vegetable oils.
Essential oil allergy may also be related to cannabis allergy. According to Dr. Adler, allergic contact dermatitis to cannabis has been rarely reported, but in an analysis of 103 commercial topical cannabinoid preparations that he published with Vincent DeLeo, MD, also with USC, 84% contained a NACDG allergen, frequently essential oils.
More recently, Dr. Adler and colleagues reported the case of a 40-year-old woman who was referred for patch testing for nummular dermatitis that wasn’t responding to treatment. The patient was found to be using topical cannabis and also grew cannabis at home. “She asked to be patch-tested to her homegrown cannabis and had a strong positive patch test to the cannabis, linalool and limonene hydroperoxides, and other essential oils,” Dr. Adler recalled. “We sent her cannabis sample for analysis at a commercial lab and found that it contained limonene and other allergenic terpene chemicals.
“We’re just starting to unravel what this means in terms of our patients and how to manage them, but many are using topical cannabis and topical CBD. I suspect this is a lot less rare than we realize.”
Another recent case from Europe reported allergic contact dermatitis to Cannabis sativa (hemp) seed oil following topical application, with positive patch testing.
Dr. Adler disclosed that he has received research grants from the American Contact Dermatitis Society. He is also an investigator for AbbVie and a consultant for the Skin Research Institute.
AT PDA 2022
Calcinosis Cutis Associated With Subcutaneous Glatiramer Acetate
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.
Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
Practice Points
- Glatiramer acetate is a subcutaneous injection utilized for relapsing-remitting multiple sclerosis, and common adverse effects include injection-site reactions such as calcinosis cutis.
- Development of calcinosis cutis in association with glatiramer acetate is not an indication for medication discontinuation.
- Dermatologists should be aware of this potential association, and treatment should be considered in cases of symptomatic calcinosis cutis.
Botanical Briefs: Ginkgo (Ginkgo biloba)
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.
Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
PRACTICE POINTS
- Contact with the Ginkgo biloba tree can cause allergic contact dermatitis; ingestion can cause systemic dermatitis in a previously sensitized patient.
- Ginkgo biloba can cross-react with plants of the family Anacardiaceae, such as poison ivy, poison oak, poison sumac, cashew tree, and mango.
- Ginkgo extract is widely considered safe for use; however, dermatologists should be aware that it can cause systemic dermatitis and serious adverse effects, including internal hemorrhage and convulsions.
Aluminum: The 2022 American Contact Dermatitis Society Allergen of the Year
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16
Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
Practice Points
- Aluminum is an allergen of significance relating to its use in vaccines, immunotherapies, and antiperspirants.
- There is a greater prevalence of aluminum contact allergy in children than in adults, affecting up to 5% of the pediatric patch-test population.
- The recommended patch test formulation is aluminum chloride hexahydrate 10% in petrolatum, with consideration of reducing the concentration to 2% in children younger than 8 years to avoid strong reactions.
Diabetes devices may give children contact dermatitis
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Devices that help children control their diabetes and lead fuller lives may also give them contact dermatitis, report the authors of a new study that calls for mandatory labeling of ingredients for allergy patch testing.
“A high share of patients showed positive reactions to isobornyl acrylate adhesive (IBOA) and/or their medical devices (insulin pumps or glucose devices),” the study authors write in Contact Dermatitis. “A third of patients showed positive reactions to benzoyl peroxide (BP),” used in adhesives.
“The presence of additional unidentified allergens cannot be excluded,” they add. “Overall, our experience once more highlights the importance of having access to a full description of the chemical composition of diabetes devices and related medical devices to efficiently manage patients (including children) who experience adverse skin reactions from such devices.”
Lead study author Catarina Alves da Silva, MD, of the department of dermatology and venereology of Aarhus (Denmark) University Hospital, and her colleagues conducted a retrospective study of 15 referred patients younger than 18 years who had type 1 diabetes. The children were patch tested in the university’s dermatology clinic between 2018 and 2020 in a study of skin reactions linked with diabetes devices.
Contact dermatitis from device-related allergens may be common
Many children in the study reacted to chemical compounds related to their devices.
- Of the 15 patients, seven showed positive patch test reactions to IBOA, and five showed positive reactions to BP.
- Ten children had positive patch test reactions to materials from glucose sensors and insulin pumps.
- Three showed positive reactions to adhesive remover wipes.
- Five reacted to .
Marcia Hogeling, MD, a pediatric dermatologist at UCLA Health in Santa Monica, Calif., told this news organization that she expected acrylates to cause problems but was surprised that BP caused positive patch test reactions.
BP is known to be a strong irritant but a weak allergen, the authors wrote.
“It was important to identify the allergens in these devices. Hopefully, this information will be used by manufacturers to create safer products for patients,” Dr. Hogeling, who was not involved in the study, said in an email.
Dr. Hogeling acknowledged that the small sample size is a weakness of the study, although she added that the findings may help providers select devices that do not contain their patients’ contact allergens.
Ryan J. McDonough, DO, a pediatric endocrinologist and the codirector of the Diabetes Center at Children’s Mercy Kansas City (Mo.), said in an email that, despite the small sample size, the study “highlights important device-related experiences of those living with type 1 diabetes that clinicians often encounter.
“We often spend considerable time aiding patients and their families in finding ways to mitigate the reactions,” he explained. “Having a broader understanding of these chemical compositions would help clinicians choose the right devices for their patients and prevent and treat these types of reactions.”
Dr. McDonough, who was not involved in the study, noted that the patients were in Denmark, and they were able to easily transition between insulin pumps and glucose monitoring devices.
“In the U.S., it is often more challenging to switch between devices, due to insurance-related concerns.
“The true rates of reaction in the broad type 1 diabetes population are difficult to assess,” Dr. McDonough said. “The study participants were drawn from patients referred to a dermatology clinic for evaluation of reaction. Many patients either don’t develop reactions or are treated for mild symptoms locally by their endocrinologists.
“This study should serve as a call to action for continued improvements in the transparency of the components that make up the devices and adhesives, and it can provide an opportunity to develop additional interventions to prevent these reactions,” he advised.
No information regarding funding for the study was provided. The authors, Dr. Hogeling, and Dr. McDonough reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lupus Erythematosus Tumidus Clinical Characteristics and Treatment: A Retrospective Review of 25 Patients
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.
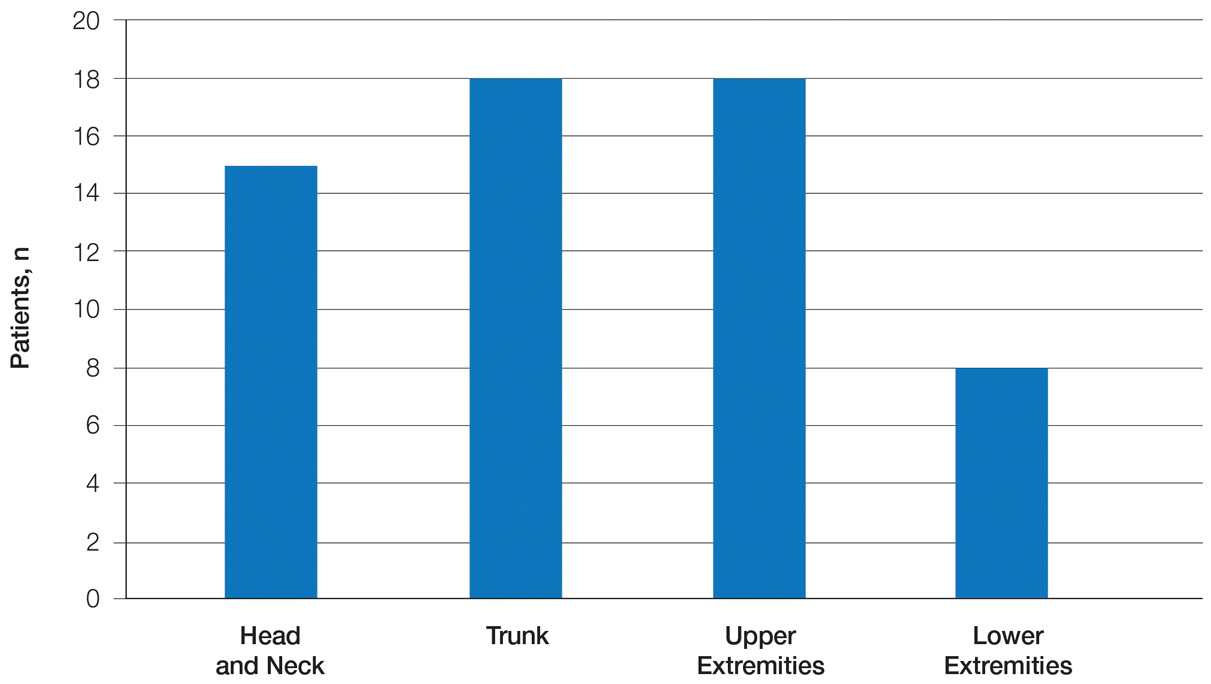
Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.
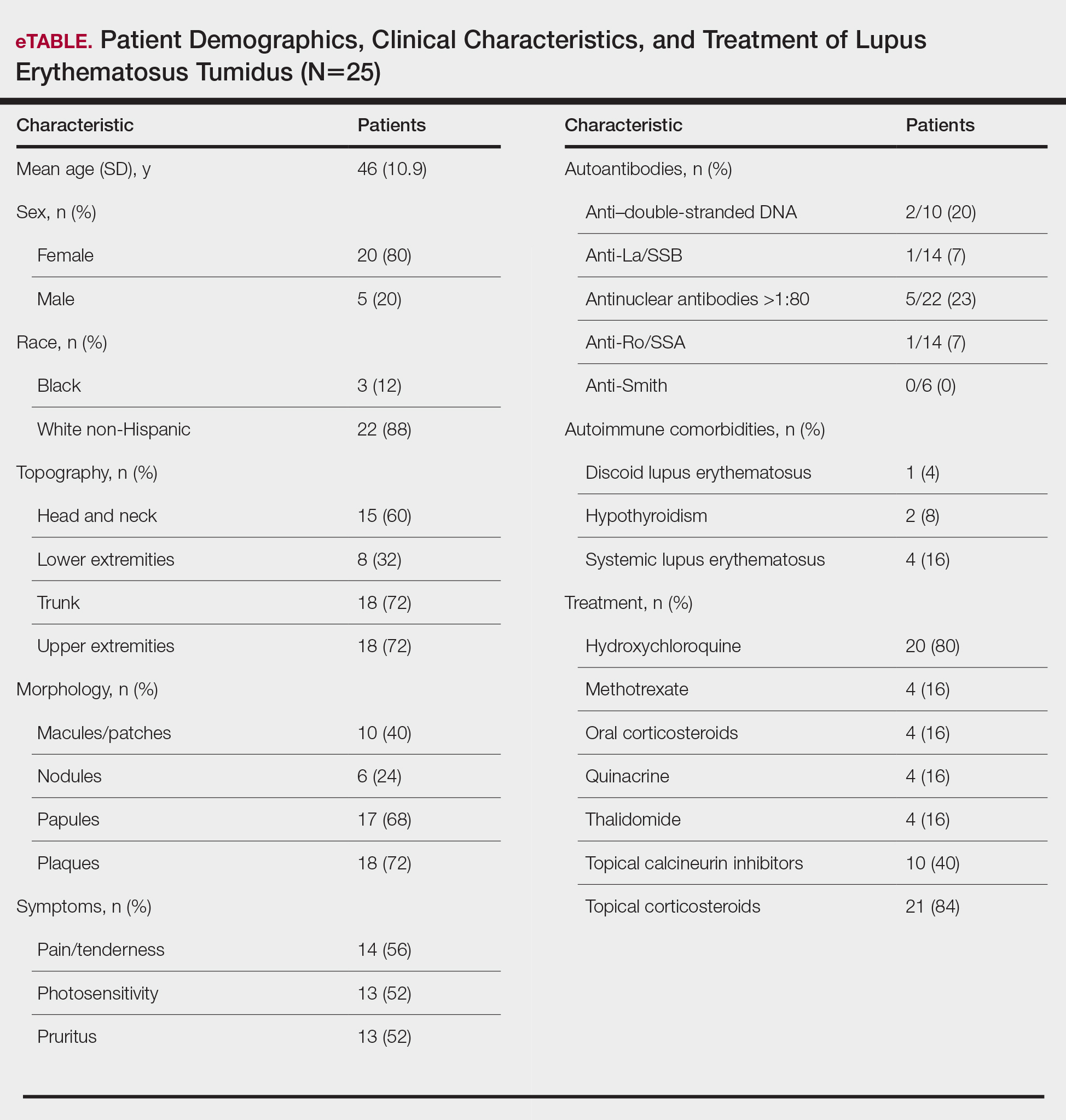
Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).
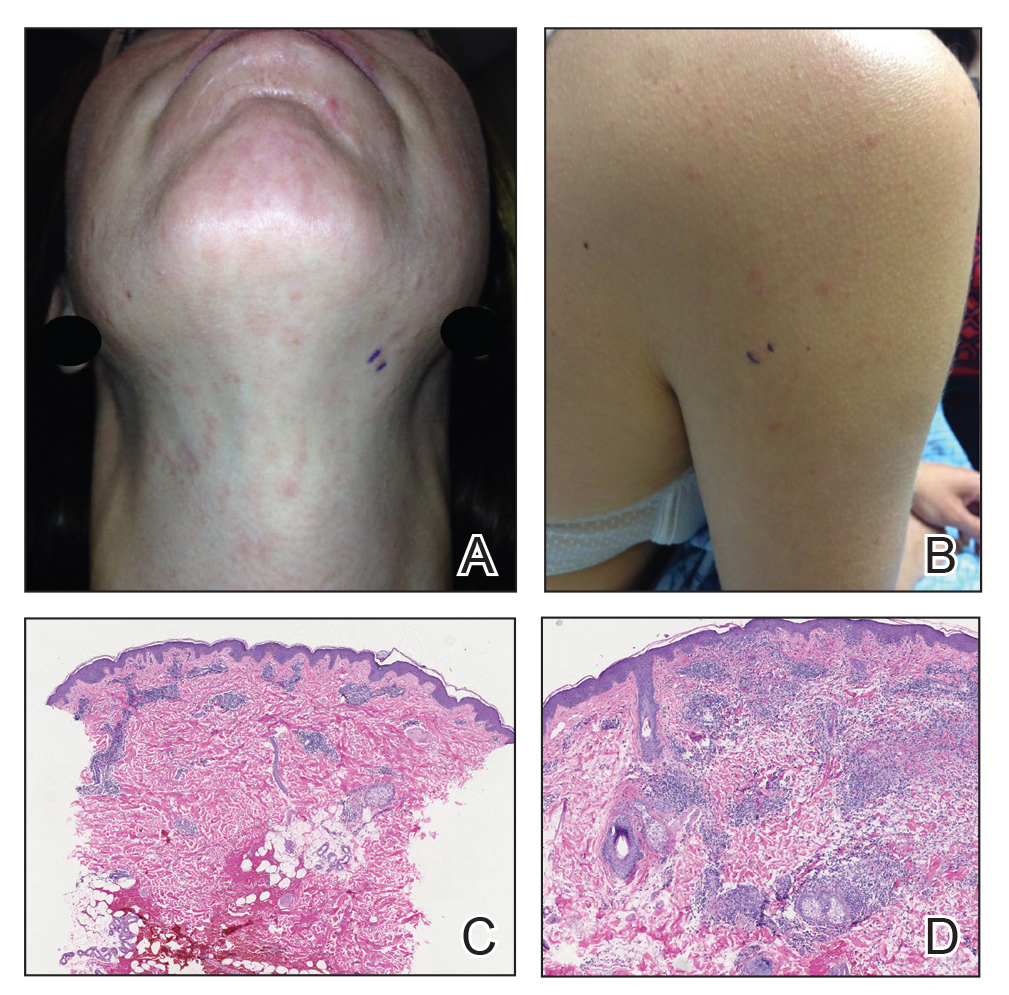
Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.

Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.

Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).

Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
Lupus erythematosus tumidus (LET) is a rare photosensitive dermatosis1 that previously was considered a subtype of chronic cutaneous lupus erythematosus; however, the clinical course and favorable prognosis of LET led to its reclassification into another category, called intermittent cutaneous lupus erythematosus.2 Although known about for more than 100 years, the association of LET with systemic lupus erythematosus (SLE), its autoantibody profile, and its prognosis are not well characterized. The purpose of this study was to describe the demographics, clinical characteristics, autoantibody profile, comorbidities, and treatment of LET based on a retrospective review of patients with LET.
Methods
A retrospective review was conducted in patients with histologically diagnosed LET who presented to the Department of Dermatology at the Wake Forest School of Medicine (Winston-Salem, North Carolina) over 6 years (July 2012 to July 2018). Inclusion criteria included males or females aged 18 to 75 years with clinical and histopathology-proven LET, which was defined as a superficial and deep lymphocytic infiltrate with abundant mucin deposition in the reticular dermis and absent or focal dermoepidermal junction alterations. Exclusion criteria included males or females younger than 18 years or older than 75 years or patients without clinical and histopathologically proven LET. Medical records were evaluated for demographics, clinical characteristics, diagnoses, autoantibodies, treatment, and recurrence. Photosensitivity was confirmed by clinical history. This study was approved by the Wake Forest School of Medicine institutional review board.

Results
Twenty-five patients were included in the study (eTable). The mean age (SD) at diagnosis was 46 (10.9) years, with a male to female ratio of 1:4. Twenty-two (88%) patients were White non-Hispanic, whereas 3 (12%) were Black. Lupus erythematosus tumidus most commonly affected the trunk (18/25 [72%]) and upper extremities (18/25 [72%]), followed by the head and neck (15/25 [60%]) and lower extremities (8/25 [32%])(Figure 1). The most common morphologies were plaques (18/25 [72%]), papules (17/25 [68%]), and nodules (6/25 [24%])(Figures 2 and 3). Most patients experienced painful (14/25 [56%]) or pruritic (13/25 [52%]) lesions as well as photosensitivity (13/25 [52%]). Of all measured autoantibodies, 5 of 22 (23%) patients had positive antinuclear antibody (ANA) titers greater than 1:80, 1 of 14 (7%) patients had positive anti-Ro (anti-SSA), 1 of 14 (7%) had positive anti-La (anti-SSB), 2 of 10 (20%) had positive anti–double-stranded DNA, and 0 of 6 (0%) patients had positive anti-Smith antibodies. Four (16%) patients with SLE had skin and joint involvement, whereas 1 had lupus nephritis. One (4%) patient had discoid lupus erythematosus (DLE). Seventeen (68%) patients reported recurrences or flares. The mean duration of symptoms (SD) was 28 (44) months.

Topical corticosteroids (21/25 [84%]) and hydroxychloroquine (20/25 [80%]) were the most commonly prescribed treatments. Hydroxychloroquine monotherapy achieved clearance or almost clearance in 12 (60%) patients. Four patients were prescribed thalidomide after hydroxychloroquine monotherapy failed; 2 achieved complete clearance with thalidomide and hydroxychloroquine, 1 achieved complete clearance with thalidomide monotherapy, and 1 improved but did not clear. Four patients were concurrently started on quinacrine (mepacrine) after hydroxychloroquine monotherapy failed; 1 patient had no clearance, 1 discontinued because of allergy, 1 improved, and 1 cleared. Four patients had short courses of prednisone lasting 1 to 4 weeks. Three of 4 patients treated with methotrexate discontinued because of adverse effects, and 1 patient improved. Other prescribed treatments included topical calcineurin inhibitors (10/25 [40%]), dapsone (1/25 [4%]), and clofazimine (1/25 [4%]).

Comment
Prevalence of LET—Although other European LET case series reported a male predominance or equal male to female ratio, our case series reported female predominance (1:4).1,3-5 Our male to female ratio resembles similar ratios in DLE and subacute lupus erythematosus, whereas relative to our study, SLE male to female ratios favored females over males.6,7

Clinical Distribution of LET—In one study enrolling 24 patients with LET, 79% (19/24) of patients had facial involvement, 50% (12/24) had V-neck involvement, 50% (12/24) had back involvement, and 46% (11/24) had arm involvement,2 whereas our study reported 72% involvement of the trunk, 72% involvement of the upper extremities, 60% involvement of the head and neck region, and 32% involvement of the lower extremities. Although our study reported more lower extremity involvement, the aforementioned study used precise topographic locations, whereas we used more generalized topographic locations. Therefore, it was difficult to compare disease distribution between both studies.2
Presence of Autoantibodies and Comorbidities—Of the 22 patients tested for ANA, 23% reported titers greater than 1:80, similar to the 20% positive ANA prevalence in an LET case series of 25 patients.5 Of 4 patients diagnosed with SLE, 3 had articular and skin involvement, and 1 had renal involvement. These findings resemble a similar LET case series.2 Nonetheless, given the numerous skin criteria in the American College of Rheumatology SLE classification criteria, patients with predominant skin disease and positive autoantibodies are diagnosed as having SLE without notable extracutaneous involvement.2 Therefore, SLE diagnosis in the setting of LET could be reassessed periodically in this population. One patient in our study was diagnosed with DLE several years later. It is uncommon for LET to be reported concomitantly with DLE.8
Treatment of LET—Evidence supporting efficacious treatment options for LET is limited to case series. Sun protection is recommended in all patients with LET. Earlier case series reported a high response rate with sun protection and topical corticosteroids, with 19% to 55% of patients requiring subsequent systemic antimalarials.3,4 However, one case series presented a need for systemic antimalarials,5 similar to our study. Hydroxychloroquine 200 to 400 mg daily is considered the first-line systemic treatment for LET. Its response rate varies among studies and may be influenced by dosage.1,3 Second-line treatments include methotrexate 7.5 to 25 mg once weekly, thalidomide 50 to 100 mg daily, and quinacrine. However, quinacrine is not currently commercially available. Thalidomide and quinacrine represented useful alternatives when hydroxychloroquine monotherapy failed. As with other immunomodulators, adverse effects should be monitored periodically.
Conclusion
Lupus erythematosus tumidus is characterized by erythematous papules and plaques that may be tender or pruritic. It follows an intermittent course and rarely is associated with SLE. Hydroxychloroquine is considered the first-line systemic treatment; however, recalcitrant disease could be managed with other immunomodulators, including methotrexate, thalidomide, or quinacrine.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Rodriguez-Caruncho C, Bielsa I, Fernandez-Figueras MT, et al. Lupus erythematosus tumidus: a clinical and histological study of 25 cases. Lupus. 2015;24:751-755.
- Patsinakidis N, Gambichler T, Lahner N, et al. Cutaneous characteristics and association with antinuclear antibodies in 402 patients with different subtypes of lupus erythematosus. J Eur Acad Dermatol Venereol. 2016;30:2097-2104.
- Petersen MP, Moller S, Bygum A, et al. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27:1424-1430.
- Dekle CL, Mannes KD, Davis LS, et al. Lupus tumidus. J Am AcadDermatol. 1999;41:250-253.
Practice Points
- Approximately 20% of patients with lupus erythematosus tumidus (LET) will have positive antinuclear antibody titers.
- Along with cutaneous manifestations, approximately 50% of patients with LET also will have pruritus, tenderness, and photosensitivity.
- If LET is resistant to hydroxychloroquine, consider using quinacrine, methotrexate, or thalidomide.