User login
Use of Dupilumab in Severe, Multifactorial, Chronic Itch for Geriatric Patients
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
To the Editor:
Today’s geriatric population is the fastest growing in history. The National Institutes of Health predicts there will be over 1.5 billion individuals aged 65 years and older by the year 2050: 17% of the world’s population.1 Pruritus—either acute or chronic (>6 weeks)—is defined as a sensory perception that leads to an intense desire to scratch.2 Chronic pruritus is an increasing health concern that impacts quality of life within the geriatric population. Elderly patients have various risk factors for developing chronic itch, including aging skin, polypharmacy, and increased systemic comorbidities.3-7
Although the therapeutic armamentarium for chronic itch continues to grow, health care providers often are hesitant to prescribe medications for geriatric patients because of comorbidities and potential drug-drug interactions. Novel biologic therapies now provide alternatives for this complex population. Dupilumab is a fully humanized, monoclonal antibody approved for treatment-resistant atopic dermatitis. This biologic prevents helper T-cell (TH2) signaling, IL-4 and IL-13 release, and subsequent effector cell (eg, mast cell, eosinophil) activity.8-10 The combined efficacy and safety of this medication has changed the treatment landscape of resistant atopic dermatitis. We present the use of dupilumab in a geriatric patient with severe and recalcitrant itch resistant to numerous topical and oral medications.
An 81-year-old man presented to the clinic with a long history of generalized pruritic rash. His medical history was significant for insulin-dependent type 2 diabetes mellitus (T2DM), hypertension, and renal cancer following a right nephrectomy. Laboratory results approximately 14 months prior to the visit revealed a blood urea nitrogen level of 31 mg/dL (reference range, 7–20 mg/dL), creatinine level of 2.20 mg/dL (reference range, 0.7–1.3 mg/dL), and glomerular filtration rate of 29 mL/min (reference range, 90–120 mL/min). Physical examination revealed numerous pink excoriated papules on the face, neck, trunk, and extremities. Lichenified plaques were present on both arms and legs. The patient received the diagnosis of severe atopic dermatitis with greater than 10% body surface area involvement. The investigator global assessment score was 4/4, indicating severe disease burden, and biopsy results reported spongiotic dermatitis. He proceeded to trial various topical corticosteroids, including hydrocortisone ointment 2.5%, betamethasone valerate ointment 0.01%, fluocinonide ointment 0.05%, and mupirocin ointment without benefit. Three subsequent courses of oral steroids failed to provide durable relief. At this point, the peak pruritus numerical rating scale (NRS) score was 7/10, indicating severe pruritus, with a negative impact on the patient’s quality of life and sleep.
Therapy was switched to tacrolimus acetonide ointment 0.1%, betamethasone dipropionate ointment 0.05%, and triamcinolone acetonide ointment 0.1%. Eleven days later, the patient denied experiencing any response to the topical regimen and sought alternative therapy for the itch and associated poor sleep; the NRS score was 10/10, indicating very severe pruritus. Prednisone 20 mg and doxepin 10 mg were initiated for symptom management until the intended transition to dupilumab. The patient began dupilumab with a loading dose of 600 mg, then 300 mg every other week thereafter. At 2- and 4-month follow-up, the patient reported notable relief in symptoms. The rash had improved, and the NRS score decreased from 10/10 to 3/10. He endorsed improved sleep and quality of life.
Pruritus may arise from a series of age-related mechanisms such as structural and chemical changes within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease (CKD).5,6,11 Identifying the underlying etiology often is difficult and involves a complete history and physical examination as well as an appropriate contextualized laboratory workup.
Our patient’s comorbid T2DM and renal disease may have contributed to the pruritus. Type 2 diabetes mellitus can cause diabetic neuropathy, a sequela known to lead to various complications, including pruritus. One study identified a 4-fold increase in pruritus in those with diabetic polyneuropathy compared with age-matched nondiabetics.12,13 An additional study found that pruritus was present in 70% of patients with small fiber neuropathy.14 We needed to consider the role of our patient’s insulin-dependent T2DM and potential underlying neuropathy when addressing the pruritic symptoms.
Furthermore, our patient’s stage IV CKD and elevated urea level also may factor into the pruritus. The pathophysiology of CKD-associated pruritus (also referred to as uremic pruritus) remains poorly understood. Suggested mechanisms include immune-mediated neural inflammation and erroneous nociceptive-receptor activity.15,16 Although uremic pruritus is appreciated primarily in late dialysis-dependent disease, research shows that a notable portion of those with lesser disease, similar to our patient, also experience a significant itch burden.17 Diminishing pruritus is difficult and often aided by management of the underlying renal disease.18
In addition to disease management, symptomatic treatment incorporates the use of emollients, corticosteroids, and antihistamines. Unfortunately, the clinical response in the elderly population to such regimens often is poor.19 Dupilumab is an optimistic therapeutic option for chronic pruritus. By inhibiting the IL-4α receptor found on helper T cells, this biologic inhibits TH2 differentiation and subsequent inflammatory activity. One report identified an optimistic response to dupilumab in the management of uremic pruritus.20 The remarkable improvement and absence of adverse effects in our patient confirmed the utility and safety of dupilumab in complex cases such as elderly patients with multiple comorbidities. Such relief may result from inhibition of proinflammatory cytokine activity as well as decreased afferent spinal cord itch stimuli.10 The positive results from this case cast a favorable outlook on the treatment of chronic itch in the complex geriatric population.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
- World’s older population grows dramatically. News release. National Institute on Aging. Published March 28, 2016. Accessed December 23, 2022. http://www.nih.gov/news-events/news-releases/worlds-older-population-grows-dramatically
- Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23:1-11.
- Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013;310:2443-2450. doi:10.1001/jama.2013.282023
- Valdes-Rodriguez, R, Mollanazar NK, González-Muro J, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95:417-421. doi:10.2340/00015555-1968
- Li J, Tang H, Hu X, et al. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106-112.
- Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007;127:2847-2856.
- Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 pt 1):571-585.
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140:633-643. doi:10.1016/j.jaci.2017.07.006
- Kabashima K. New concept of the pathogenesis of atopic dermatitis: interplay among the barrier, allergy, and pruritus as a trinity. J Dermatol Sci. 2013;70:3-11.
- Feld M, Garcia R, Buddenkotte J, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500-508.
- Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging. 2015;32:201-215. doi:10.1007/s40266-015-0246-0
- Misery L, Brenaut E, Le Garrec R, et al. Neuropathic pruritus. Nat Rev Neurol. 2014;10:408-416.
- Yamaoka H, Sasaki H, Yamasaki H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care. 2010;33:150-155.
- Brenaut E, Marcorelles P, Genestet S, et al. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. 2015;72:328-332.
- Adigun M, Badu LA, Berner NM, et al. Uremic pruritus review. US Pharm. 2015;40:HS12-HS15.
- Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a systematic review. Am J Kidney Dis. 2017;70:638-655.
- Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. CRC Press/Taylor & Francis; 2014.
- Shirazian S, Aina O, Park Y, et al. Chronic kidney disease-associated pruritus: impact on quality of life and current management challenges. Int J Nephrol Renovasc Dis. 2017;10:11-26.
- Brummer GC, Wang LT, Sontheimer RD. A possible role for dupilumab (Dupixent) in the management of idiopathic chronic eczematous eruption of aging. Dermatol Online J. 2018;24:13030/qt55z1f6xh.
- Silverberg JI, Brieva J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019;5:339-341.
PRACTICE POINTS
- A series of age-related mechanisms within the epidermis, underlying neuropathy, medication side effects, infection, malignancy, thyroid dysregulation, liver disease, and chronic kidney disease may contribute to pruritus in elderly patients.
- Patients with mild kidney disease may still experience a recalcitrant and notable itch burden.
- Dupilumab is efficacious and safe in the management of chronic pruritus, even in complex cases such as elderly patients with multiple comorbidities.
Cutaneous Manifestations in Hereditary Alpha Tryptasemia
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
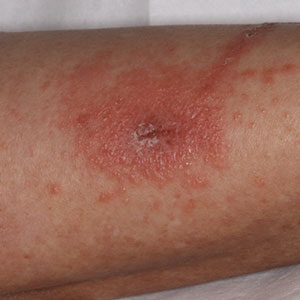
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.
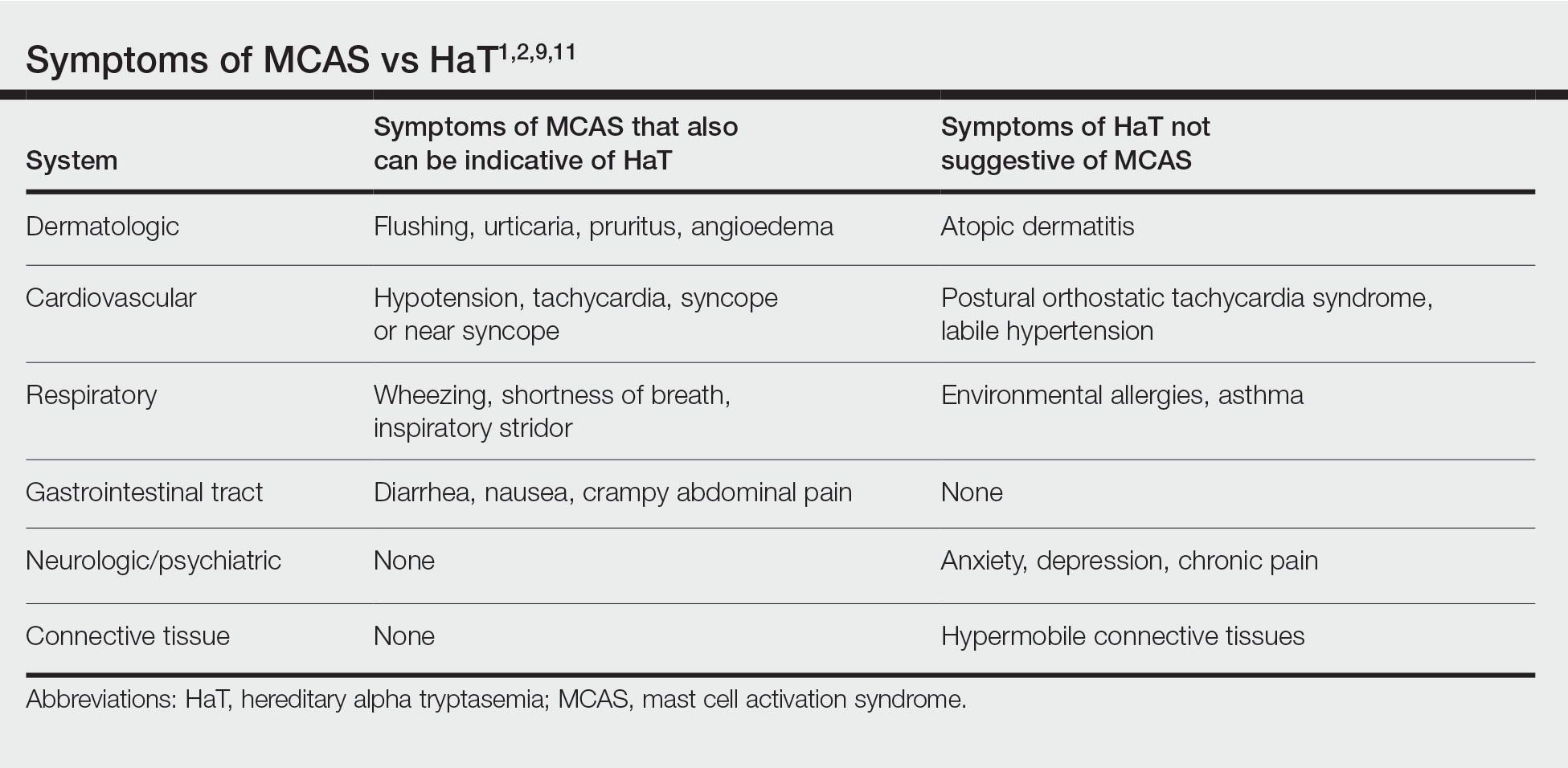
Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Practice Points
- Chronic or episodic urticaria, flushing, and pruritus are the most consistent cutaneous abnormalities associated with hereditary alpha tryptasemia (HaT), but HaT also may augment symptoms of other underlying inflammatory skin disorders, such as atopic dermatitis and psoriasis.
- Individuals with episodic dermatologic manifestations indicative of mast cell activation accompanied by symptoms affecting 1 or more organ systems should be evaluated for mast cell activation syndrome as well as HaT.
Botanical Briefs: Daffodils (Narcissus Species)
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Practice Points
- Narcissus species are thought to be the most common cause of irritant contact dermatitis among florists.
- Use of protective gloves and clothing to prevent Narcissus-induced contact dermatitis is recommended.
Higher metal contact allergy rates found in metalworkers
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a systematic review and meta-analysis reports.
“Metal allergy to all three metals was significantly more common in European metalworkers with dermatitis attending patch test clinics as compared to ESSCA [European Surveillance System on Contact Allergies] data, indicating a relationship to occupational exposures,” senior study author Jeanne D. Johansen, MD, professor, department of dermatology and allergy, Copenhagen University Hospital, Hellerup, Denmark, and colleagues at the University of Copenhagen wrote in Contact Dermatitis. “However, confounders could not be accounted for.”
How common is metal allergy in metalworkers?
Occupational hand eczema is known to be common in metalworkers. Touching oils, greases, metals, leather gloves, rubber materials, and metalworking fluids as they repeatedly cut, shape, and process raw metals and minerals derived from ore mining exposes metalworkers to allergens and skin irritants, the authors wrote. But the prevalence of allergy to certain metals has not been well characterized.
So they searched PubMed for full-text studies in English that reported metal allergy prevalence in metalworkers, from the database’s inception through April 2022.
They included studies with absolute numbers or proportions of metal allergy to cobalt, chromium, or nickel, in all metalworkers with suspected allergic contact dermatitis who attended outpatient clinics or who worked at metalworking plants participating in workplace studies.
The researchers performed a random-effects meta-analysis to calculate the pooled prevalence of metal allergy. Because 85%-90% of metalworkers in Denmark are male, they compared the estimates they found with ESSCA data on 13,382 consecutively patch-tested males with dermatitis between 2015 and 2018.
Of the 1,667 records they screened, they analyzed data from 29 that met their inclusion criteria: 22 patient studies and 7 workplace studies involving 5,691 patients overall from 22 studies from Europe, 5 studies from Asia, and 1 from Africa. Regarding European metalworkers, the authors found:
- Pooled proportions of allergy in European metalworkers with dermatitis referred to patch test clinics were 8.2% to cobalt (95% confidence interval, 5.3%-11.7%), 8.0% to chromium (95% CI, 5.1%-11.4%), and 11.0% to nickel (95% CI, 7.3%-15.4%).
- In workplace studies, the pooled proportions of allergy in unselected European metalworkers were 4.9% to cobalt, (95% CI, 2.4%-8.1%), 5.2% to chromium (95% CI, 1.0% - 12.6%), and 7.6% to nickel (95% CI, 3.8%-12.6%).
- By comparison, ESSCA data on metal allergy prevalence showed 3.9% allergic to cobalt (95% CI, 3.6%-4.2%), 4.4% allergic to chromium (95% CI, 4.1%-4.8%), and 6.7% allergic to nickel (95% CI, 6.3%-7.0%).
- Data on sex, age, body piercings, and atopic dermatitis were scant.
Thorough histories, protective regulations and equipment
Providers need to ask their dermatitis patients about current and past occupations and hobbies, and employers need to provide employees with equipment that protects them from exposure, Kelly Tyler, MD, associate professor of dermatology, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“Repeated exposure to an allergen is required for sensitization to develop,” said Dr. Tyler, who was not involved in the study. “Metalworkers, who are continually exposed to metals and metalworking fluids, have a higher risk of allergic contact dermatitis to cobalt, chromium, and nickel.”
“The primary treatment for allergic contact dermatitis is preventing continued exposure to the allergen,” she added. “This study highlights the importance of asking about metal or metalworking fluid in the workplace and of elucidating whether the employer is providing appropriate protective gear.”
To prevent occupational dermatitis, workplaces need to apply regulatory measures and provide their employees with protective equipment, Dr. Tyler advised.
“Body piercings are a common sensitizer in patients with metal allergy, and the prevalence of body piercings among metalworkers was not included in the study,” she noted.
The results of the study may not be generalizable to patients in the United States, she added, because regulations and requirements to provide protective gear here may differ.
“Taking a thorough patient history is crucial when investigating potential causes of dermatitis, especially in patients with suspected allergic contact dermatitis,” Dr. Tyler urged.
Funding and conflict-of-interest details were not provided. Dr. Tyler reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
Breaking the itch-scratch cycle with mindfulness
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
FROM IDS 2022
Botanical Briefs: Toxicodendron Dermatitis
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7
When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
Reactions to poison ivy, poison oak, and poison sumac, which affect 10 to 50 million Americans a year,1 are classified as Toxicodendron dermatitis; 50% to 75% of US adults are clinically sensitive to these plants.2 Furthermore, people of all ethnicities, skin types, and ages residing in most US geographical regions are at risk.3 Allergenicity is caused by urushiol, which is found in members of the Anacardiaceae family.4 Once absorbed, urushiol causes a type IV hypersensitivity reaction in those who are susceptible.5
Cutaneous Manifestations
Toxicodendron dermatitis presents with an acute eczematous eruption characterized by streaks of intensely pruritic and erythematous papules and vesicles (Figure 1). Areas of involvement are characterized by sharp margins that follow the pattern of contact made by the plant’s leaves, berries, stems, and vines.6 The fluid content of the vesicles is not antigenic and cannot cause subsequent transmission to oneself or others.3 A person with prior contact to the plant who becomes sensitized develops an eruption 24 to 48 hours after subsequent contact with the plant; peak severity manifests 1 to 14 days later.7

When left untreated, the eruption can last 3 weeks. If the plant is burned, urushiol can be aerosolized in smoke, causing respiratory tract inflammation and generalized dermatitis, which has been reported among wildland firefighters.2 Long-term complications from an outbreak are limited but can include postinflammatory hyperpigmentation and secondary bacterial infection.8 Rare reports of nephrotic syndrome also have appeared in the literature.9 Toxicodendron dermatitis can present distinctively as so-called black dot dermatitis.6
Nomenclature
Poison ivy, poison oak, and poison sumac are members of the family Anacardiaceae and genus Toxicodendron,6 derived from the Greek words toxikos (poison) and dendron (tree).10
Distribution
Toxicodendron plants characteristically are found in various regions of the United States. Poison ivy is the most common and is comprised of 2 species: Toxicodendron rydbergii and Toxicodendron radicans. Toxicodendron rydbergii is a nonclimbing dwarf shrub typically found in the northern and western United States. Toxicodendron radicans is a climbing vine found in the eastern United States. Poison oak also is comprised of 2 species—Toxicodendron toxicarium and Toxicodendron diversilobum—and is more common in the western United States. Poison sumac (also known as Toxicodendron vernix) is a small shrub that grows in moist swampy areas. It has a predilection for marshes of the eastern and southeastern United States.6,11
Identifying Features
Educating patients on how to identify poison ivy can play a key role in avoidance, which is the most important step in preventing Toxicodendron dermatitis. A challenge in identification of poison ivy is the plant’s variable appearance; it grows as a small shrub, low-lying vine, or vine that climbs other trees.
As the vine matures, it develops tiny, rough, “hairy” rootlets—hence the saying, “Hairy vine, no friend of mine!” Rootlets help the plant attach to trees growing near a water source. Vines can reach a diameter of 3 inches. From mature vines, solitary stems extend 1 to 2 inches with 3 characteristic leaves at the terminus (Figure 2), prompting another classic saying, “Leaves of 3, let it be!”12

Poison oak is characterized by 3 to 5 leaflets. Poison sumac has 7 to 13 pointed, smooth-edged leaves.6
Dermatitis-Inducing Plant Parts
The primary allergenic component of Toxicodendron plants is urushiol, a resinous sap found in stems, roots, leaves, and skins of the fruits. These components must be damaged or bruised to release the allergen; slight contact with an uninjured plant part might not lead to harm.2,13 Some common forms of transmission include skin contact, ingestion, inhalation of smoke from burning plants, and contact with skin through contaminated items, such as clothing, animals, and tools.14
Allergens
The catecholic ring and aliphatic chain of the urushiol molecule are allergenic.15 The degree of saturation and length of the side chains vary with different catechols. Urushiol displays cross-reactivity with poison ivy, poison oak, and poison sumac. Urushiol from these plants differs only slightly in structure; therefore, sensitization to one causes sensitization to all. There also is cross-reactivity between different members of the Anacardiaceae family, including Anacardium occidentale (tropical cashew nut), Mangifera indica (tropical mango tree), Ginkgo biloba (ginkgo tree), and Semecarpus anacardium (Indian marking nut tree).12
Poison ivy, poison oak, and poison sumac cause allergic contact dermatitis as a type IV hypersensitivity reaction. First, urushiol binds and penetrates the skin, where it is oxidized to quinone intermediates and bound to haptens. Then, the intermediates bind surface proteins on antigen-presenting cells, specifically Langerhans cells in the epidermis and dermis.5
Presentation of nonpeptide antigens, such as urushiol, to T cells requires expression of langerin (also known as CD207) and CD1a.16 Langerin is a C-type lectin that causes formation of Birbeck granules; CD1a is a major histocompatibility complex class I molecule found in Birbeck granules.5,17 After Langerhans cells internalize and process the urushiol self-hapten neoantigen, it is presented to CD4+ T cells.6 These cells then expand to form circulating activated T-effector and T-memory lymphocytes.18
The molecular link that occurs between the hapten and carrier protein determines the response. When linked by an amino nucleophile, selective induction of T-effector cells ensues, resulting in allergic contact dermatitis. When linked by a sulfhydryl bond, selective induction of suppressor cells occurs, resulting in a reduced allergic contact dermatitis response.19 In the case of activation of T-effector cells, a cell-mediated cytotoxic immune response is generated that destroys epidermal cells and dermal vasculature.2 The incidence and intensity of poison ivy sensitivity decline proportionally with age and the absence of continued exposure.20
Preventive Action—Patients should be counseled that if contact between plant and skin occurs, it is important to remove contaminated clothing or objects and wash them with soap to prevent additional exposure.14,21 Areas of the skin that made contact with the plant should be washed with water as soon as possible; after 30 minutes, urushiol has sufficiently penetrated to cause a reaction.2 Forceful unidirectional washing with a damp washcloth and liquid dishwashing soap is recommended.22
Several barrier creams are commercially available to help prevent absorption or to deactivate the urushiol antigen. These products are used widely by forestry workers and wildland firefighters.23 One such barrier cream is bentoquatam (sold as various trade names), an organoclay compound made of quaternium-18 bentonite that interferes with absorption of the allergen by acting as a physical blocker.24
Treatment
After Toxicodendron dermatitis develops, several treatments are available to help manage symptoms. Calamine lotion can be used to help dry weeping lesions.25,26 Topical steroids can be used to help control pruritus and alleviate inflammation. High-potency topical corticosteroids such as clobetasol and mid-potency steroids such as triamcinolone can be used. Topical anesthetics (eg, benzocaine, pramoxine, benzyl alcohol) might provide symptomatic relief.27,28
Oral antihistamines can allow for better sleep by providing sedation but do not target the pruritus of poison ivy dermatitis, which is not histamine mediated.29,30 Systemic corticosteroids usually are considered in more severe dermatitis—when 20% or more of the body surface area is involved; blistering and itching are severe; or the face, hands, or genitalia are involved.31,32
Clinical Uses
Therapeutic uses for poison ivy have been explored extensively. In 1892, Dakin33 reported that ingestion of leaves by Native Americans reduced the incidence and severity of skin lesions after contact with poison ivy. Consumption of poison ivy was further studied by Epstein and colleagues34 in 1974; they concluded that ingestion of a large amount of urushiol over a period of 3 months or longer may help with hyposensitization—but not complete desensitization—to contact with poison ivy. However, the risk for adverse effects is thought to outweigh benefits because ingestion can cause perianal dermatitis, mucocutaneous sequelae, and systemic contact dermatitis.2
Although the use of Toxicodendron plants in modern-day medicine is limited, development of a vaccine (immunotherapy) against Toxicodendron dermatitis offers an exciting opportunity for further research.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
- Pariser DM, Ceilley RI, Lefkovits AM, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Cruse JM, Lewis RE. Atlas of Immunology. CRC Press; 2004.
- Valladeau J, Ravel O, Dezutter-Dambuyant C, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71-81. doi:10.1016/s1074-7613(00)80160-0
- Marks JG. Poison ivy and poison oak allergic contact dermatitis. J Allergy Clin Immunol. 1989;9:497-506.
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Rytand DA. Fatal anuria, the nephrotic syndrome and glomerular nephritis as sequels of the dermatitis of poison oak. Am J Med. 1948;5:548-560. doi:10.1016/0002-9343(48)90105-3
- Gledhill D. The Names of Plants. Cambridge University Press; 2008.
- American Academy of Dermatology Association. Poison ivy, oak, and sumac: how to treat the rash. Accessed October 19, 2022. https://www.aad.org/public/everyday-care/itchy-skin/poison-ivy/treat-rash
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 suppl 1):S29-S34.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers Medical Publishers; 2016.
- Fisher AA, Mitchell JC. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 4th ed. Williams and Wilkins; 1995:461-523.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Hunger RE, Sieling PA, Ochoa MT, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701-708. doi:10.1172/JCI19655
- Hanau D, Fabre M, Schmitt DA, et al. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis “non-classical” major histocompatibility complex class Imolecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987;84:2901-2905. doi:10.1073/pnas.84.9.2901
- Gayer KD, Burnett JW. Toxicodendron dermatitis. Cutis. 1988;42:99-100.
- Dunn IS, Liberato DJ, Castagnoli N, et al. Contact sensitivity to urushiol: role of covalent bond formation. Cell Immunol. 1982;74:220-233. doi:10.1016/0008-8749(82)90023-5
- Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958;77:149-180. doi:10.1001/archderm.1958.01560020001001
- Derraik JGB. Heracleum mantegazzianum and Toxicodendron succedaneum: plants of human health significance in New Zealand and the National Pest Plant Accord. N Z Med J. 2007;120:U2657.
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2018;81:E25. doi:10.1016/j.jaad.2017.12.081
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? Dermatitis. 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Marks JG Jr, Fowler JF Jr, Sheretz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216. doi:10.1016/0190-9622(95)90237-6
- Baer RL. Poison ivy dermatitis. Cutis. 1990;46:34-36.
- Williford PM, Sheretz EF. Poison ivy dermatitis. nuances in treatment. Arch Fam Med. 1995;3:184.
- Amrol D, Keitel D, Hagaman D, et al. Topical pimecrolimus in the treatment of human allergic contact dermatitis. Ann Allergy Asthma Immunol. 2003;91:563-566. doi:10.1016/S1081-1206(10)61535-9
- Stephanides SL, Moore C. Toxicodendron poisoning treatment & management. Medscape. Updated June 13, 2022. Accessed October 19, 2022. https://emedicine.medscape.com/article/817671-treatment#d11
- Munday J, Bloomfield R, Goldman M, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology. 2002;205:40-45. doi:10.1159/000063138
- Yosipovitch G, Fleischer A. Itch associated with skin disease: advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617-622. doi:10.2165/00128071-200304090-00004
- Li LY, Cruz PD Jr. Allergic contact dermatitis: pathophysiology applied to future therapy. Dermatol Ther. 2004;17:219-223. doi:10.1111/j.1396-0296.2004.04023.x
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (Rhus)? J Fam Pract. 2006;55:166-167.
- Dakin R. Remarks on a cutaneous affection, produced by certain poisonous vegetables. Am J Med Sci. 1829;4:98-100.
- Epstein WL, Baer H, Dawson CR, et al. Poison oak hyposensitization. evaluation of purified urushiol. Arch Dermatol. 1974;109:356-360.
Practice Points
- Toxicodendron dermatitis is a pruritic vesicular eruption in areas of contact with the plant.
- Identification and avoidance are primary methods of preventing Toxicodendron dermatitis.
Hairdressers have ‘excess risk’ of contact allergies
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Photoallergic Contact Dermatitis: No Fun in the Sun
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.
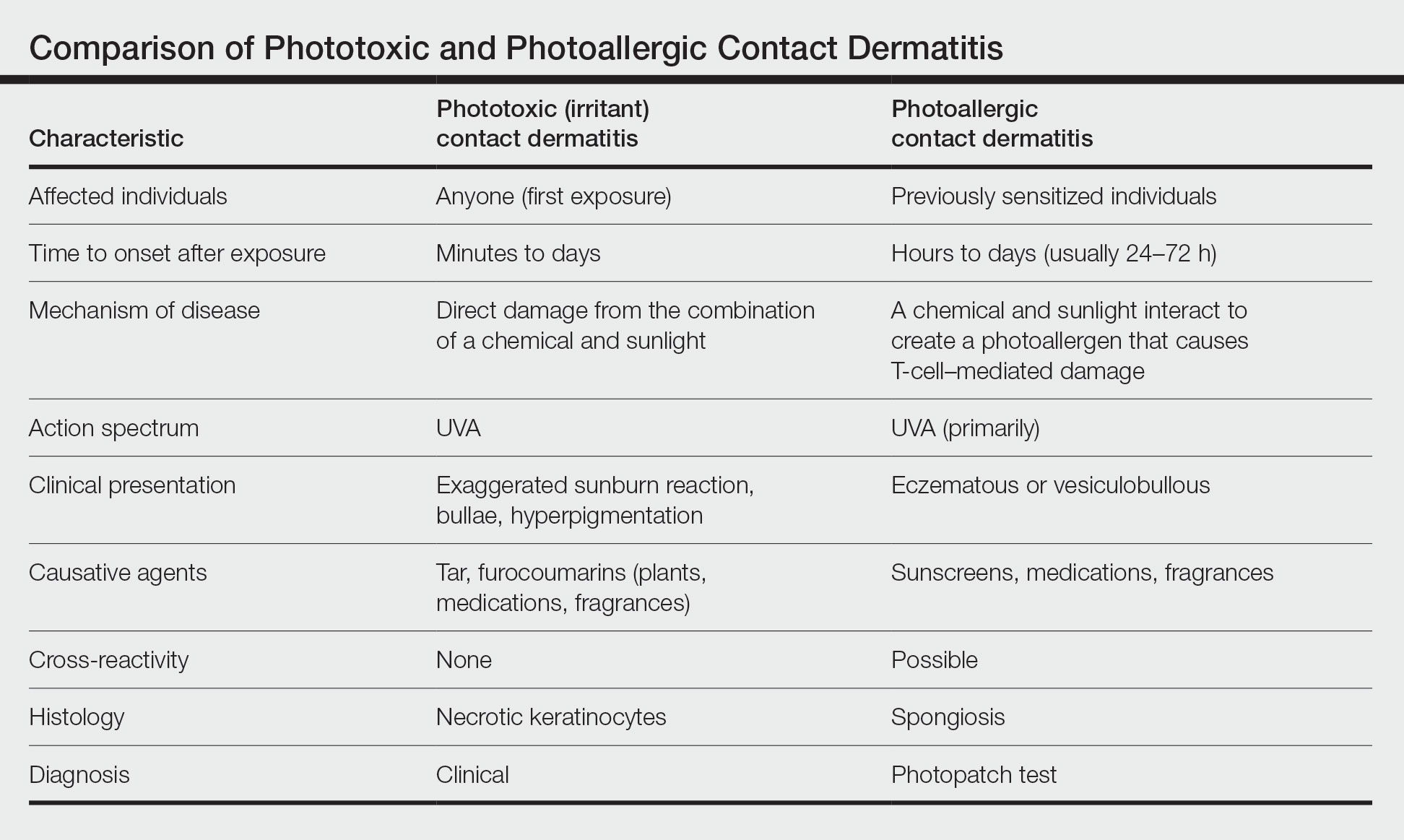
Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Practice Points
- Photoallergic contact dermatitis (PACD) presents clinically and histologically similar to allergic contact dermatitis but is concentrated in sun-exposed body sites.
- Sunscreens currently are the most common photoallergens in North America, whereas topical nonsteroidal anti-inflammatory drugs are more common culprits in Europe.
- Photopatch testing is required to diagnose PACD; however, it is infrequently performed, and there currently are no North American consensus guidelines.
Training program linked to less hand eczema for hairdressers
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The study was conducted in Denmark, where about 40% of hairdressers develop occupational hand eczema (OHE), according to researchers. Hairdressers globally are exposed to wet work and myriad skin irritants and allergens, including dyes, permanent-wave solutions, persulfates, preservatives, and fragrances. The study, which was funded by the Danish hairdressers and beauticians union, was published in Contact Dermatitis.
Lead author Martin Havmose, BSc, of the National Allergy Research Center, department of dermatology and allergy, University of Copenhagen, Hellerup, Denmark, and colleagues wrote that prevention is critical, inasmuch as eczema can cut careers short and have lasting health effects.
Up to 70% of hairdressers experience some sort of work-related skin damage in their careers, as reported by this news organization.
Hand eczema also is common among hairdressers in the United States, Mark Denis Davis, MD, chair of dermatology at the Mayo Clinic in Rochester, Minn., told this news organization. It can be quite debilitating, itchy, and painful, he said.
“Often it is associated with painful fissuring, cracks in the skin, particularly involving the fingers. It may also be unsightly,” he said.
Dr. Davis said he hears anecdotally in his practice that many hairdressers are reluctant to wear gloves because of the touch and dexterity needed in their work.
The researchers evaluated the risk of OHE and compliance with skin protection measures among hairdressers who were trained before Denmark rolled out a nationwide skin protection program in hairdressing vocational schools in 2011.
Questionnaires were sent in May 2009 to all hairdressers (96.4% women; average age, 26) who had graduated from 1985 to 2007; in May 2020, questionnaires were sent to all hairdressers who had graduated from 2008 to 2018.
The average time worked in the trade was 8 years, and 28.8% no longer worked as hairdressers, data show.
The response rate was 66.6% (305/460) for the 2009 survey and 29.9% (363/1215) for the 2020 survey.
Prevalence of OHE dropped after program
The prevalence of OHE during career time dropped from 42.8% to 29% (adjusted odds ratio, 0.55; 95% confidence interval [CI], 0.40-0.77) between the two surveys.
In addition, the incidence rate of OHE decreased from 57.5 (95% CI, 48.4-68.4) to 42.0 (95% CI, 34.6-50.9) per 1,000 person-years (incidence rate ratio, 0.73; 95% CI, 0.560.95) in that period.
There was an increase in the use of gloves between the two surveys. There was more glove use when the hairdressers engaged in wet work and handled dyes, products with bleach, and permanent-wave solutions (P < .05).
The nationwide program educates hairdressing apprentices on contact allergy/urticaria, how to prevent occupational skin disease, and skin biology. Teaching materials focus on 11 recommendations, 7 of which are related to glove use.
“The lack of primary prevention of OHE in hairdressing vocational schools may be a missed opportunity in the prevention of the disease,” the authors concluded.
Dr. Davis said hairdressers with hand eczema should know that in the short term, topical corticosteroids can be used to decrease the inflammation of the skin.
He highlighted the following advice from the authors:
- Gloves should be used when washing, dyeing, bleaching, and creating perms.
- Disposable gloves should never be reused.
- Gloves should be used only as long as necessary.
- Rings should not be worn at work.
- Cotton gloves should be worn underneath protective gloves.
- For clients who are having their hair both cut and dyed, the hair should be cut before it is dyed.
- Nitrile gloves should be used without rubber accelerators.
“In the longer term,” said Dr. Davis, “the most important thing is to avoid exposure to the precipitating factors, such as wet work – working with water, which irritates the skin – and avoiding any allergens that are contributing to the eczema.”
The study was funded by an unrestricted grant from the Danish hairdressers and beauticians union. Two coauthors have links to industry, as listed in the original article. Dr. Davis reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CONTACT DERMATITIS
When is an allergic reaction to raw plant food due to tree pollen?
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.
A new guideline aims to help primary care doctors differentiate pollen food syndrome (PFS) – a cross-reactive allergic reaction to certain raw, but not cooked, plant foods – from other food allergies.
The guideline from the British Society of Allergy and Clinical Immunology (BSACI) focuses on birch tree pollen, the major sensitizing PFS allergen in Northern Europe. Providers may be able to diagnose PFS related to birch pollen from clinical history alone, including the foods involved and the rapidity of symptom onset, write lead author Isabel J. Skypala, PhD, RD, of Imperial College London, and her colleagues.
The new BSACI guideline for diagnosis and management of PFS was published in Clinical & Experimental Allergy.
PFS is common and increasingly prevalent
PFS – also called oral allergy syndrome and pollen food allergy syndrome – is common and increasingly prevalent. PFS can begin at any age but usually starts in pollen-sensitized school-age children and adults with seasonal allergic rhinitis.
Symptoms from similar proteins in food
Mild to moderate allergic symptoms develop quickly when people sensitized to birch pollen eat raw plant foods that contain proteins similar to those in the pollen, such as pathogenesis-related protein PR-10. The allergens are broken down by cooking or processing.
Symptoms usually occur immediately or within 15 minutes of eating. Patients may have tingling; itching or soreness in the mouth, throat, or ears; mild lip and oral mucosa angioedema; itchy hands, sneezing, or eye symptoms; tongue or pharynx angioedema; perioral rash; cough; abdominal pain; nausea; and/or worsening of eczema. In children, itch and rash may predominate.
Triggers depend on pollen type
PFS triggers vary depending on a person’s pollen sensitization, which is affected by their geographic area and local dietary habits. In the United Kingdom, almost 70% of birch-allergic adults and more than 40% of birch-allergic children have PFS, the authors write.
Typical triggers include eating apples, stone fruits, kiwis, carrots, celery, hazelnuts, almonds, walnuts, soymilk, and peanuts, as well as peeling potatoes or other root vegetables. Freshly prepared vegetable or fruit smoothies or juices, celery, soymilk, raw nuts, large quantities of roasted nuts, and concentrated nut products can cause more severe reactions.
Diagnostic clinical history
If a patient answers yes to these questions, they almost certainly have PFS, the authors write:
- Are symptoms caused by raw fruits, nuts, carrots, or celery?
- Are the same trigger foods tolerated when they’re cooked well or roasted?
- Do symptoms come immediately or within a few minutes of eating?
- Do symptoms occur in the oropharynx and include tingling, itching, or swelling?
- Does the patient have seasonal allergic rhinitis or sensitization to pollen?
Testing needed for some cases
Allergy tests may be needed for people who report atypical or severe reactions or who also react to cooked or processed plant foods, such as roasted nuts, nuts in foods, fruits or vegetables in juices and smoothies, and soy products other than milk. Tests may also be needed for people who react to foods that are not linked with PFS, such as cashews, pistachios, macadamias, sesame seeds, beans, lentils, and chickpeas.
Whether PFS reactions also occur to roasted hazelnuts, almonds, walnuts, Brazil nuts, or peanuts, either alone or in composite foods such as chocolates, spreads, desserts, and snacks, is unclear.
An oral food challenge to confirm PFS is needed only if the history and diagnostic tests are inconclusive or if the patient is avoiding multiple foods.
Dietary management
PFS is managed by excluding known trigger foods. This becomes challenging for patients with preexisting food allergies and for vegetarians and vegans.
Personalized dietary advice is needed to avoid nutritional imbalance, minimize anxiety and unnecessary food restrictions, and improve quality of life. Reactions after accidental exposure often resolve without medication, and if antihistamines are needed, they rarely require self-injectable devices.
Guideline helpful beyond the United Kingdom and birch pollen
Allyson S. Larkin, MD, associate professor of pediatrics at the University of Pittsburgh School of Medicine, told this news organization in an email that the guideline summarizes in great detail the pathophysiology behind PFS and highlights how component testing may help diagnose patients and manage the condition.
“Patients worry very much about the progression and severity of allergic reactions,” said Dr. Larkin, who was not involved in the guideline development.
“As the authors note, recognizing the nutritional consequences of dietary restrictions is important, and nutrition consults and suitable alternative suggestions are very helpful for these patients, especially for those with food allergy or who are vegetarian or vegan.”
Jill A. Poole, MD, professor of medicine and chief of the Division of Allergy and Immunology at the University of Nebraska College of Medicine, Omaha, noted that PFS, although common, is underrecognized by the public and by health care providers.
“People are not allergic to the specific food, but they are allergic to a seasonal allergen, such as birch tree, that cross-reacts with the food protein, which is typically changed with cooking,” she explained in an email.
“This differs from reactions by those who have moderate to severe allergic food-specific reactions that may include systemic reactions like anaphylaxis from eating certain foods,” she said.
“Importantly, the number of cross-reacting foods with seasonal pollens continues to grow, and the extent of testing has expanded in recent years,” advised Dr. Poole, who also was not involved in the guideline development.
The authors recommend further related research into food immunotherapy and other novel PFS treatments. They also want to raise awareness of factors affecting PFS prevalence, such as increased spread and allergenicity of pollen due to climate change, pollution, the global consumption of previously local traditional foods, and the increase in vegetarian and vegan diets.
The authors, Dr. Larkin, and Dr. Poole report no relevant financial relationships involving this guideline. The guideline was not funded.
A version of this article first appeared on Medscape.com.