User login
FDA OKs first condom for anal sex
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
specifically designed for use during anal sex has gained Food and Drug Administration approval.
Anal intercourse is considered to be much riskier than vaginal sex for the transmission of infections such as HIV and HPV, a risk factor for anal cancer, agency officials said in a statement Feb. 23 announcing the decision. And though the Centers for Disease Control and Prevention has long encouraged the use of a condom during anal intercourse, the FDA had not until now deemed this practice safe.
The latex ONE Male Condom, from prophylactic maker Global Protection Corp. of Boston, has already been available for vaginal sex. The FDA action now allows the company to market the product for anal intercourse.
“This authorization helps us accomplish our priority to advance health equity through the development of safe and effective products that meet the needs of diverse populations,” Courtney Lias, PhD, the director of the FDA’s Office of GastroRenal, ObGyn, General Hospital, and Urology Devices, said in a statement.
The FDA said it relied on an Emory University clinical study of condom safety of more than 500 men. Those who took part in the study were evenly divided between men who have sex with men and men who have sex with women. The condom failure rate, meaning that a condom either broke or slipped, was less than 1% during anal sex. The failure rate was 3 times higher during vaginal intercourse.
The Emory researchers also found that roughly 70% of men who have sex with men would be more likely to use condoms marked as safe for anal sex, according to a survey of 10,000 people.
ONE Male Condoms sell for between $3.48 for a three-pack and $14.48 for a 24-pack, according to Milla Impola, Global Protection’s director of marketing and communications. The FDA said the condom should be used with a condom-compatible lubricant when used during anal sex.
A version of this article first appeared on WebMD.com.
Federal sex education programs linked to decrease in teen pregnancy
The birth rate for U.S. teenagers dropped 3% in counties where a federally funded sex education program was introduced, a recently published paper says.
Researchers concentrated on the effects of the Teen Pregnancy Prevention program (TPP), which was introduced during the Obama administration and administered on the county level. TPP programs provide more information on sex, contraception, and reproductive health than abstinence-only programs, the paper said.
“Sex education in the United States has been hotly debated among researchers, policy makers, and the public,” Nicholas Mark, a doctoral candidate in New York University’s department of sociology and the lead author of the paper, said in a news release. “Our analysis provides evidence that funding for more comprehensive sex education led to an overall reduction in the teen birth rate at the county level of more than 3%.”
Researchers examined teen birth rates in 55 counties from 1996 to 2009, before TTP, and from 2010 to 2016, after TTP. Next, they compared teen birth rates in the 55 counties with teen birth rates in 2,800 counties that didn’t have the funding in the years before and after TPP was introduced.
In the 55 counties, teen birth rates fell 1.5% in the first year of TTP funding and fell about 7% by the fifth year of funding, for an average drop of 3%, the news release said.
“We’ve known for some time that abstinence-only programs are ineffective at reducing teen birth rates,” said Lawrence Wu, a professor in NYU’s department of sociology and the paper’s senior author. “This work shows that more wide-reaching sex education programs – those not limited to abstinence – are successful in lowering rates of teen births.”
The paper was published in the Proceedings of the National Academy of Sciences of the United States of America.
The paper said the findings probably understate the true effect of more comprehensive sex education at the individual level.
The authors said the findings are important because U.S. women are more likely to become mothers in their teens than women in other developed nations, with many teen pregnancies reported as unintended, the authors said.
As of 2020, teen birth rates and the number of births to teen mothers had dropped steadily since 1990. Teen birth rates fell by 70% over 3 decades.
A version of this article first appeared on WebMD.com.
The birth rate for U.S. teenagers dropped 3% in counties where a federally funded sex education program was introduced, a recently published paper says.
Researchers concentrated on the effects of the Teen Pregnancy Prevention program (TPP), which was introduced during the Obama administration and administered on the county level. TPP programs provide more information on sex, contraception, and reproductive health than abstinence-only programs, the paper said.
“Sex education in the United States has been hotly debated among researchers, policy makers, and the public,” Nicholas Mark, a doctoral candidate in New York University’s department of sociology and the lead author of the paper, said in a news release. “Our analysis provides evidence that funding for more comprehensive sex education led to an overall reduction in the teen birth rate at the county level of more than 3%.”
Researchers examined teen birth rates in 55 counties from 1996 to 2009, before TTP, and from 2010 to 2016, after TTP. Next, they compared teen birth rates in the 55 counties with teen birth rates in 2,800 counties that didn’t have the funding in the years before and after TPP was introduced.
In the 55 counties, teen birth rates fell 1.5% in the first year of TTP funding and fell about 7% by the fifth year of funding, for an average drop of 3%, the news release said.
“We’ve known for some time that abstinence-only programs are ineffective at reducing teen birth rates,” said Lawrence Wu, a professor in NYU’s department of sociology and the paper’s senior author. “This work shows that more wide-reaching sex education programs – those not limited to abstinence – are successful in lowering rates of teen births.”
The paper was published in the Proceedings of the National Academy of Sciences of the United States of America.
The paper said the findings probably understate the true effect of more comprehensive sex education at the individual level.
The authors said the findings are important because U.S. women are more likely to become mothers in their teens than women in other developed nations, with many teen pregnancies reported as unintended, the authors said.
As of 2020, teen birth rates and the number of births to teen mothers had dropped steadily since 1990. Teen birth rates fell by 70% over 3 decades.
A version of this article first appeared on WebMD.com.
The birth rate for U.S. teenagers dropped 3% in counties where a federally funded sex education program was introduced, a recently published paper says.
Researchers concentrated on the effects of the Teen Pregnancy Prevention program (TPP), which was introduced during the Obama administration and administered on the county level. TPP programs provide more information on sex, contraception, and reproductive health than abstinence-only programs, the paper said.
“Sex education in the United States has been hotly debated among researchers, policy makers, and the public,” Nicholas Mark, a doctoral candidate in New York University’s department of sociology and the lead author of the paper, said in a news release. “Our analysis provides evidence that funding for more comprehensive sex education led to an overall reduction in the teen birth rate at the county level of more than 3%.”
Researchers examined teen birth rates in 55 counties from 1996 to 2009, before TTP, and from 2010 to 2016, after TTP. Next, they compared teen birth rates in the 55 counties with teen birth rates in 2,800 counties that didn’t have the funding in the years before and after TPP was introduced.
In the 55 counties, teen birth rates fell 1.5% in the first year of TTP funding and fell about 7% by the fifth year of funding, for an average drop of 3%, the news release said.
“We’ve known for some time that abstinence-only programs are ineffective at reducing teen birth rates,” said Lawrence Wu, a professor in NYU’s department of sociology and the paper’s senior author. “This work shows that more wide-reaching sex education programs – those not limited to abstinence – are successful in lowering rates of teen births.”
The paper was published in the Proceedings of the National Academy of Sciences of the United States of America.
The paper said the findings probably understate the true effect of more comprehensive sex education at the individual level.
The authors said the findings are important because U.S. women are more likely to become mothers in their teens than women in other developed nations, with many teen pregnancies reported as unintended, the authors said.
As of 2020, teen birth rates and the number of births to teen mothers had dropped steadily since 1990. Teen birth rates fell by 70% over 3 decades.
A version of this article first appeared on WebMD.com.
Drospirenone vs norethindrone progestin-only pills. Is there a clear winner?
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
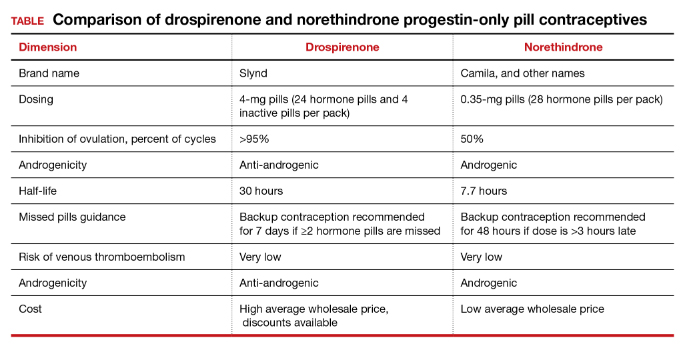
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.
Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
Contraception and family planning have improved the health of all people by reducing maternal mortality, improving maternal and child health through birth spacing, supporting full education attainment, and advancing workforce participation.1 Contraception is cost-effective and should be supported by all health insurers. One economic study reported that depending on the contraceptive method utilized, up to $7 of health care costs were saved for each dollar spent on contraceptive services and supplies.2
Progestin-only pills (POPs) are an important contraceptive option for people in the following situations who3:
- have a contraindication to estrogen-containing contraceptives
- are actively breastfeeding
- are less than 21 days since birth
- have a preference to avoid estrogen.
POPs are contraindicated for women who have breast cancer, abnormal uterine bleeding, or active liver disease and for women who are pregnant. A history of bariatric surgery with a malabsorption procedure (Roux-en-Y and biliopancreatic diversion) and the use of antiepileptic medications that are strong enzyme inducers are additional situations where the risk of POP may outweigh the benefit.3 Alternative progestin-only options include the subdermal etonogestrel implant, depot medroxyprogesterone acetate, and levonorgestrel-releasing intrauterine devices. These 3 options provide superior contraceptive efficacy to POP.
As a contraceptive, norethindrone at a dose of 0.35 mg daily has two major flaws:
- it does not reliably inhibit ovulation
- it has a short half-life.
In clinical studies, norethindrone inhibits ovulation in approximately 50% of cycles.4,5 Because norethindrone at a dose of 0.35 mg does not reliably inhibit ovulation it relies on additional mechanisms for contraceptive efficacy, including thickening of the cervical mucus to block sperm entry into the upper reproductive tract, reduced fallopian tube motility, and thinning of the endometrium.6
Norethindrone POP is formulated in packs of 28 pills containing 0.35 mg intended for daily continuous administration and no medication-free intervals. One rationale for the low dose of 0.35 mg in norethindrone POP is that it approximates the lowest dose with contraceptive efficacy for breastfeeding women, which has the benefit of minimizing exposure of the baby to the medication. Estrogen-progestin birth control pills containing norethindrone as the progestin reliably inhibit ovulation and have a minimum of 1 mg of norethindrone in each hormone pill. A POP with 1 mg of norethindrone per pill would likely have greater contraceptive efficacy. When taken daily, norethindrone acetate 5 mg (Aygestin) suppresses ovarian estrogen production, ovulation, and often causes cessation of uterine bleeding.7 The short half-life of norethindrone (7.7 hours) further exacerbates the problem of an insufficient daily dose.6 The standard guidance is that norethindrone must be taken at the same time every day, a goal that is nearly impossible to achieve. If a dose of norethindrone is taken >3 hours late, backup contraception is recommended for 48 hours.6
Drospirenone is a chemical analogue of spironolactone. Drospirenone is a progestin that suppresses LH and FSH and has anti-androgenic and partial anti-mineralocorticoid effects.8 Drospirenone POP contains 4 mg of a nonmicronized formulation that is believed to provide a pharmacologically similar area under the curve in drug metabolism studies to the 3 mg of micronized drospirenone, present in drospirenone-containing estrogen-progestin contraceptives.8 It is provided in a pack of 28 pills with 24 drospirenone pills and 4 pills without hormone. Drospirenone has a long half-life of 30 to 34 hours.8 If ≥2 drospirenone pills are missed, backup contraception is recommended for 7 days.9 The contraceptive effectiveness of drospirenone POP is thought to be similar to estrogen-progestin pills.8 Theoretically, drospirenone, acting as an anti-mineralocorticoid, can cause hyperkalemia. People with renal and adrenal insufficiency are most vulnerable to this adverse effect and should not be prescribed drospirenone. Women taking drospirenone and a medication that strongly inhibits CYP3A4, an enzyme involved in drospirenone degradation—including ketoconazole, indinavir, boceprevir, and clarithromycin—may have increased circulating levels of drospirenone and be at an increased risk of hyperkalemia. The US Food and Drug Administration (FDA) suggests that clinicians consider monitoring potassium concentration in women taking drospirenone who are also prescribed a strong CYP3A4 inhibitor.9 In people with normal renal and adrenal function, drospirenone-induced hyperkalemia is not commonly observed.9
Drospirenone 4 mg has been reported to not affect the natural balance of pro- and anti-coagulation factors in women.10 Drospirenone 4 mg daily has been reported to cause a modest decrease in systolic (-8 mm Hg) and diastolic (-5 mm Hg) blood pressure for women with a baseline blood pressure ≥130 mm Hg. Drospirenone 4 mg daily did not change blood pressure measurement in women with a baseline systolic blood pressure <130 mm Hg.11 For women using drospirenone POP, circulating estradiol concentration is usually >30 pg/mL, with a mean concentration of 51 pg/mL.12,13 Drospirenone POP does not result in a significant change in body weight.14 Preliminary studies suggest that drospirenone is an effective contraceptive in women with a BMI >30 kg/m2.14,15 Drospirenone enters breast milk and the relative infant dose is reported to be 1.5%.9 In general, breastfeeding is considered reasonably safe when the relative infant dose of a medication is <10%.16
The most common adverse effect reported with both norethindrone and drospirenone POP is unscheduled uterine bleeding. With norethindrone POP about 50% of users have a relatively preserved monthly bleeding pattern and approximately 50% have bleeding between periods, spotting and/or prolonged bleeding.17,18 A similar frequency of unscheduled uterine bleeding has been reported with drospirenone POP.14,19 Unscheduled and bothersome uterine bleeding is a common reason people discontinue POP. For drospirenone POP, the FDA reports a Pearl Index of 4.9 Other studies report a Pearl Index of 0.73 (95% confidence interval [CI], 0.31 to 1.43) for drospirenone POP.14 For norethindrone POP, the FDA reports that in typical use about 5% of people using the contraceptive method would become pregnant.6 The TABLE provides a comparison of the key features of the two available POP contraceptives. My assessment is that drospirenone has superior contraceptive properties over norethindrone POP. However, a head-to-head clinical trial would be necessary to determine the relative contraceptive effectiveness of drospirenone versus norethindrone POP.

Maintaining contraception access
Access to contraception without a copayment is an important component of a comprehensive and equitable insurance program.20 The American College of Obstetricians and Gynecologists (ACOG) advocates that all people “should have unhindered and affordable access to all U.S. Food and Drug Administration-approved contraceptives.”21 ACOG also calls for the “full implementation of the Affordable Care Act requirement that new and revised private health insurance plans cover all U.S. Food and Drug Administration approved contraceptives without cost sharing, including nonequivalent options within one method category.” The National Women’s Law Center22 provides helpful resources to ensure access to legislated contraceptive benefits, including a phone script for speaking with an insurance benefits agent23 and a toolkit for advocating for your contraceptive choice.24 We need to ensure that people have unfettered access to all FDA-approved contraceptives because access to contraception is an important component of public health. Although drospirenone is more costly than norethindrone POP, drospirenone contraception should be available to all patients seeking POP contraception. ●
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
- Kavanaugh ML, Andreson RM. Contraception and beyond: the health benefits of services provided at family planning centers, NY. Guttmacher Institute. 2013. www.gutmacher.org/pubs/helth-benefits.pdf. Accessed January 13, 2022.
- Foster DG, Rostovtseva DP, Brindis CD, et al. Cost savings from the provision of specific methods of contraception in a publicly funded program. Am J Pub Health. 2009;99:446-451.
- Curtis M, Tepper NK, Jatlaoui TC, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Rice CF, Killick SR, Dieben T, et al. A comparison of the inhibition of ovulation achieved by desogestrel 75 µg and levonorgestrel 30 µg daily. Human Reprod. 1999;14:982-985.
- Milsom I, Korver T. Ovulation incidence with oral contraceptives: a literature review. J Fam Plann Reprod Health Care. 2008;34:237-246.
- OrthoMicronor [package insert]. OrthoMcNeil: Raritan, New Jersey. June 2008.
- Brown JB, Fotherby K, Loraine JA. The effect of norethisterone and its acetate on ovarian and pituitary function during the menstrual cycle. J Endocrinol. 1962;25:331-341.
- Romer T, Bitzer J, Egarter C, et al. Oral progestins in hormonal contraception: importance and future perspectives of a new progestin only-pill containing 4 mg drospirenone. Geburtsch Frauenheilk. 2021;81:1021-1030.
- Slynd [package insert]. Exeltis: Florham Park, New Jersey. May 2019.
- Regidor PA, Colli E, Schindlre AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24+4 cycle with desogestrel 75 µg per day. Gynecol Endocrinol. 2016;32:749-751.
- Palacios S, Colli E, Regidor PA. Efficacy and cardiovascular safety of the new estrogen-free contraceptive pill containing 4 mg drospirenone alone in a 24/4 regime. BMC Womens Health. 2020;20:218.
- Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporosis Int. 2019;30:2391-2400.
- Mitchell VE, Welling LM. Not all progestins are created equally: considering unique progestins individually in psychobehavioral research. Adapt Human Behav Physiol. 2020;6:381-412.
- Palacios S, Colli E, Regidor PA. Multicenter, phase III trials on the contraceptive efficacy, tolerability and safety of a new drospirenone-only pill. Acta Obstet Gynecol Scand. 2019;98:1549-1557.
- Archer DF, Ahrendt HJ, Drouin D. Drospirenone-only oral contraceptive: results from a multicenter noncomparative trial of efficacy, safety and tolerability. Contraception. 2015;92:439-444.
- Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100:42-52. doi: 10.1002/cpt.377.
- Belsey EM. Vaginal bleeding patterns among women using one natural and eight hormonal methods of contraception. Contraception. 1988;38:181-206.
- Broome M, Fotherby K. Clinical experience with the progestin-only pill. Contraception. 1990;42:489-495.
- Apter D, Colli E, Gemzell-Danielsson K, et al. Multicenter, open-label trial to assess the safety and tolerability of drospirenone 4.0 mg over 6 cycles in female adolescents with a 7-cycle extension phase. Contraception. 2020;101:412.
- Birth control benefits. Healthcare.gov website. https://www.healthcare.gov/coverage/birth-control-benefits/. Accessed January 13, 2022.
- American College of Obstetricians and Gynecologists. Access to contraception. Committee Opinion No. 615. Obstet Gynecol. 2015;125:250-256.
- Health care and reproductive rights. National Women’s Law Center website. https://nwlc.org/issue/health-care. Accessed January 13, 2022.
- How to find out if your health plan covers birth control at no cost to you. National Women’s Law Center website. https://nwlc.org/sites/default/files/072014-insuranceflowchart_vupdated.pdf. Accessed January 13, 2022.
- Toolkit: Getting the coverage you deserve. National Women’s Law Center website. https://nwlc.org/sites/default/files/pdfs/final_nwlclogo_preventive servicestoolkit_9-25-13.pdf. Accessed January 13, 2022.
More than a month after launch, iPLEDGE glitches persist
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
which mandates the program to prevent fetal exposure to the teratogenic effects of isotretinoin, and by the American Academy of Dermatology Association, whose members have repeatedly asked the FDA for meetings to discuss solutions. The AADA is the legislative and advocacy arm of AAD.
When the new program launched Dec. 13, 2021, the website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they tried to follow instructions to enter information. Hold times to talk to a live person stretched to hours.
The latest improvement attempt, announced Jan. 14 by the FDA, is a tool created by the Isotretinoin Products Manufacturers Group, the manufacturers responsible for the FDA-mandated REMS program. It is meant to allow prescribers and designees to send log-in links directly to patients’ email accounts through the iPLEDGE REMS portal, bypassing the troublesome call center.
And it’s not the answer, dermatologists said.
“The new tool does not solve issues such as prescribers or pharmacies not being able to access the site, unacceptably long call center wait times, inefficiencies caused by frequent attestation requirements for those who cannot become pregnant, patients becoming ‘locked out’ because they missed a window period through no fault of their own, among others,” said John Barbieri, MD, MBA, director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital and instructor in dermatology at Harvard Medical School, both in Boston.
The day after the FDA update about the new tool, Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., tweeted: “Lip service and empty words.” He noted that the situation has been “disastrous from the start” as the new platform launched.
Under the iPLEDGE program in place for the acne drug, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The aim of the new gender-neutral approach to the risk mitigation program is to make the experience more inclusive for transgender patients. The previous three risk categories (females of reproductive potential, females not of reproductive potential, and males) are now reduced to just two (those capable of getting pregnant and those not capable of getting pregnant).
The problem is the execution of the new platform. The transition from the old website to the new was done quickly. By most accounts, the Dec. 13 rollout was chaotic, a failure, and disastrous, triggering numerous expressions of frustration on Twitter and other social media, with some calling for the program to be halted until the bugs could be worked out.
“While the new gender-neutral categories are a welcome improvement to the system, the new categorization approach was not the underlying reason for the new platform and its failed rollout, which was instead due to a change in vendor,” Dr. Barbieri told this news organization.
AADA: More recent efforts to improve the system
“We have a letter to the FDA asking for a stakeholders meeting to include us, the IPMG, and pharmacists because there are ongoing problems, though there have been some improvement in terms of certain elements,” Ilona Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco, said in an interview shortly after the FDA posted the update on the new tool. “That said, there are many patients who have not gotten isotretinoin during the 1 month since the roll-out of the new platform.”
What still needs to be fixed? “We have ongoing concerns about the lack of transparency of the IPMG, about call center wait times, actual number of prescriptions on the hands of patients compared to the previous month, and those patients who can get pregnant who – despite complying with all of the REMS requirements – are being locked out because of the lack of timely attestation to their negative pregnancy status due to the website, not the patients themselves,” Dr. Frieden told this news organization.
“We are continuing to advocate to have decreased attestation requirements for individuals who cannot become pregnant – because this will improve the efficiency of the system for those patients for whom the REMS program goals are truly intended – those who can become pregnant, since the primary aim of the REMS program is to minimize fetal exposure.”
An AADA spokesperson said that the IPMG has invited the AADA to a joint stakeholders meeting on Jan. 26, along with representatives from the FDA and pharmacy industry.
Spotty progress
“The iPLEDGE situation is as frustrating as ever,” said Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, after the FDA’s Jan. 14 update was released. “It’s like they never tested the new website before deploying it.”
Among the issues he has experienced in his practice, he said, is an instance in which iPLEDGE swapped the first names of a mother and daughter, so it was impossible to fill the prescription. “It happened twice in the same day,” Dr. Goldberg said. The patient had to call iPLEDGE to fix this, but the call center wasn’t taking calls.
In today’s technology environment, he said, it’s hard to believe that “we have to put up with this.”
Some have seen success. ‘’The tool is working fine on our end,” said Mitesh Patel, PharmD, pharmacy manager at Sunshine Pharmacy in White Plains, N.Y. However, he added that some doctors and patients are still having issues. He encourages dermatologists still having issues with the system to reach out to independent pharmacies that have processed iPLEDGE prescriptions and ‘’lean on them to assist.”
This news organization contacted CVS and Walgreens about how the system is working at their locations, but has not yet received a response.
Dr. Goldberg, Dr. Frieden, Dr. Barbieri, and Dr. Peebles have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This story was updated on 1/24/22.
FDA updates status of iPLEDGE access problems
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
The, one month after a modified program was launched, the Food and Drug Administration announced on Jan. 14.
The IPMG has “created a new tool within the system to help resolve account access for some user groups without using the call center. This tool is intended to allow prescribers and designees to send login links directly to their patients’ desired email address through the Manage Patients page of the iPLEDGE REMS portal,” the FDA statement said.
“Prescribers can also send login links to their designees still having difficulty accessing their iPLEDGE account,” and users should check their emails for messages from iPLEDGE, including spam folders, the FDA advises. The iPLEDGE strategy is designed to prevent fetal exposure to isotretinoin, which is highly teratogenic.
Days after the new, gender-neutral approach to the isotretinoin risk mitigation program was launched on Dec. 13, the FDA convened an emergency meeting with representatives from the American Academy of Dermatology Association (AADA) to discuss the problematic rollout of the program, which was described as disastrous, chaotic, and a failure, with dermatologists on Twitter and elsewhere expressing anger and frustration over not being able to access the program or reach the call center.
A statement by the FDA on Dec. 23 followed, urging manufacturers to develop solutions for the website and to work with the AADA and pharmacy organizations to find solutions that would minimize treatment interruptions during the transition.
The modified REMS, launched on Dec. 13, is designed to make it more inclusive for transgender patients prescribed isotretinoin. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), patients who are prescribed isotretinoin for acne are assigned to one of two risk categories: those who can get pregnant and those who cannot get pregnant.
In the Jan. 14 statement, the FDA notes that the agency is continuing to work with the IPMG regarding the problems clinicians, pharmacists, and patients have had with accessing iPLEDGE over the last month.
“Although there has been progress, there is a significant amount of work still to be done,” the FDA acknowledged. “While we consider potential steps within the scope of FDA’s authorities, we will continue to meet with the IPMG for updates on the status of the problems with the iPLEDGE REMS and their progress towards having the system work as intended for all users.”
Medicaid expansion curbs disparities, increases immigrant access, in postpartum care
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
Expanding Medicaid coverage has proved beneficial to postpartum women and may even help reduce disparities, say two new papers.
In the first study, expansion of Medicaid coverage under the Affordable Care Act was associated with higher rates of postpartum coverage and outpatient visits, according to results published in JAMA Health Forum.
Racial and ethnic disparities were also reduced in postpartum coverage, although these disparities remained between Black and White women for outpatient visits.
In the second study, published in JAMA Network Open, researchers found that when postpartum care is covered as part of Emergency Medicaid, women who have been denied access because of their citizenship status are able to use these services, which includes contraception.
Federal law currently prohibits undocumented and documented immigrants who have been in the United States for less than 5 years from receiving full-benefit Medicaid. Coverage is limited to Emergency Medicaid, which offers benefits only for life-threatening conditions, including hospital admission for childbirth. Coverage is not available for prenatal or postpartum care, including contraception.
For the first article, lead author Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues point out that compared with other high-income countries, maternal mortality is higher in the United States and largely driven by persistent racial disparities. Compared with non-Hispanic White women, the rates of maternal death are more than twice as high among American Indian and Alaska Native women, and more than threefold greater in non-Hispanic Black women.
“To be clear, visits increased by around the same amount for Black and White individuals after Medicaid expansion, it is just that visits started off lower among Black women, and remained lower by a similar degree,” said Dr. Steenland.
One explanation is that Black women experience racial discrimination during pregnancy-related health care including childbirth hospitalizations and this may make them more reticent to seek postpartum care, she explained. “In addition, the ability to seek health care is determined by insurance as well as other social factors such as paid leave from work, childcare, and transportation, and these other factors may have remained a larger barrier for Black women after expansion.”
In this cohort study, they looked at the association of Medicaid expansion in Arkansas with continuous postpartum coverage, postpartum health care use, and change in racial disparities in the study outcomes. Using the Arkansas All-Payer Claims Database for persons with a childbirth between 2013 and 2015, the authors identified 60,990 childbirths. Of this group, 67% were White, 22% Black, and 7% Hispanic, and 72.3% were covered by Medicaid. The remaining 27.7% were paid for by a commercial payer.
Before Medicaid expansion, 50.6% of women with Medicaid had continuous coverage during the 6 months postpartum, and the share of women with Medicaid childbirth coverage who were continuously covered for 6 months postpartum increased to 69.3% in 2014 and 90.0% in 2015. Medicaid expansion was associated with a 27.8% increase in continuous coverage for 6-12 months postpartum, and 0.9 increase in visits or a relative increase of 75.0% in outpatient care compared with the visit rate of 1.2 visits within the first 6 months postpartum during the pre-expansion period.
A subgroup analysis was conducted to see if Medicaid expansion had any effect on the disparities between White and Black patients. In the 2-year period after expansion, the percentage of both Black and White women with continuous 6-month postpartum coverage increased to 87.9% and 85.9%, respectively. White individuals averaged 2 visits in the first 6 months postpartum versus 1.6 for Black individuals before expansion, and even though there was no difference in postpartum insurance coverage after expansion, racial disparities in the number of visits during the first 6 months postpartum remained after Medicaid expansion (2.5 vs. 2).
Commenting on the paper, Catherine Cansino, MD, MPH, associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, noted that she has seen the benefits of Medicaid expansion among obstetric population in California. “I’m glad to see similar expansion in other states,” she said. “But to address persistent health care inequities, I think concierge services or patient care navigation serve a role and can hopefully put a little dent in narrowing gaps.”
Dr. Cansino noted that there are many postpartum patients who need help arranging both pediatric and postpartum care, often prioritizing the newborn appointments. “They also need childcare help so they can focus on their own care as well as transportation,” she said, adding that “it would also be interesting to review racial/ethnic differences with regard to knowledge about contraceptive need immediately postpartum and also about the stigma related to postpartum mental health disorders. If patients don’t see the value of a postpartum visit, they would tend not to attend this visit especially given the many other challenges in the postpartum period.”
Access for immigrants
In the second study, the authors note that the decision to expand Emergency Medicaid options is largely up to individual states. Led by Maria I. Rodriguez, MD, MPH, of the department of obstetrics and gynecology, Oregon Health & Science University, Portland, and colleagues, they decided to compare two states – Oregon, which expanded Emergency Medicaid to include postpartum services and South Carolina, which kept only the federal minimum services – to see how it affected postpartum care among immigrant women.
Compared with South Carolina, there was a 40.6 percentage-point increase (95% confidence interval [CI] in postpartum care visits, P < .001) and postpartum contraception within 60 days grew by 33.2 percentage points (95% CI, P < .001), in Oregon after expansion went into effect.
“When postpartum care was covered for women who would have qualified for Medicaid, except for their citizenship status, their rates of attendance at a postpartum visit and use of postpartum contraception increased to levels observed in the traditional Medicaid population,” the authors wrote.
The calculations, drawn from Medicaid claims and birth certificate data from 2010 to 2019, assumed parallel trends, meaning the researchers made the assumption that use patterns would have remained the same in Oregon if the Emergency Medicaid expansion hadn’t happened and use in South Carolina would have remained consistent as well. A differential trend analysis showed significant increases in use of the services in Oregon relative to South Carolina.
“We included Oregon and South Carolina because both states have experienced similar growth in their immigrant population and have comparable immigrant populations, in terms of size and country of origin, residing in each state,” the authors noted.
Commenting on the study, Laura Mercer MD, MBA, MPH, associate professor in obstetrics and gynecology and director of the obstetrics and gynecology clerkship at the University of Arizona in Phoenix, said she was “excited and encouraged by the results” but not surprised, as it’s logical to assume that there would be more uptake of the services when they are provided free of charge or at low cost.
“Oftentimes, the mother of the family deprioritizes her own health and well-being in favor of diverting those resources to her children and her family,” said Dr. Mercer, who specializes in prenatal and postpartum care.
She added that the significant increase in contraception is a particularly representative sign of improvement as it is easier to quantify, compared to improvements in mental health or counseling.
But comprehensive postpartum care extends to physical, psychological, and social well-being. “Its components include counseling on the importance of birth spacing and providing the contraceptive method of their choice,” the authors wrote. “An absence of postpartum care has been associated with unintended pregnancy, short interpregnancy intervals, exacerbation of chronic diseases, and preterm birth.”
Dr. Mercer noted that closely spaced pregnancies, particularly less than 6 months but at least less than 18 months carry increased risk for mother and child. And for those who would say that immigrant women should be excluded from the Emergency Medicaid postpartum services, Dr. Mercer said she would encourage them to look at the data around the improved outcomes of comprehensive maternal care.
Being able to track health markers and intervene before a woman requires emergency care will reduce costs in the long run, she pointed out. But, regardless of the cost, policymakers have to ask themselves, “What do we value as a society? If we value families and healthy families and we want to promote the best possible outcomes, then I think this question becomes very easy to answer.”
The first study was funded by the National Institute for Health Care Management. Dr. Steenland was also supported by the Agency for Healthcare Research and Quality and the National Institutes of Health. Dr. Steenland reported grants from the Agency for Healthcare Research and Quality and from the National Institute for Child Health and Human Development during the conduct of the study. The second study was supported by the National Institute on Minority Health and Health Disparities. Dr. Rodriguez reports grants from Arnold Ventures and personal fees from the American College of Obstetricians and Gynecologists, Bayer, and Merck outside the submitted work. A coauthor reports grants from Merck/Organon and the Office of Population Affairs outside the submitted work, as well as membership on the board of directors of the Society of Family Planning and the ACOG Gynecology Clinical Practice Guideline committee. Dr. Mercer reported no relevant financial relationships.
FROM JAMA HEALTH FORUM AND JAMA NETWORK OPEN
Increased access to LARC may improve birth outcomes
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Policies increasing access to immediate postpartum long-acting reversible contraception (LARC) were associated with reductions in preterm birth and low birth weight, based on data from South Carolina’s Medicaid program.
Preterm birth and low birth weight represent the second-leading cause of infant mortality in the United States, wrote Maria W. Steenland, SD, of Brown University, Providence, R.I., and colleagues. Previous policy interventions to reduce preterm birth and low birth weight have focused on services before and during pregnancy, they said. LARC is a safe and effective postpartum intervention, but cost has been a limiting factor, they noted.
In 2012, the Medicaid program in South Carolina began reimbursing hospitals for immediate postpartum LARC independent of global maternity payments. In a previous study, the researchers found that the implementation of this policy had reduced the number of short-interval births among adolescents.
The goal of the current study, published in JAMA Pediatrics, was to analyze the association between South Carolina’s policy change and rates of preterm birth and low birth weight among individuals with Medicaid coverage during childbirth. The researchers analyzed data from 186,953 Medicaid-paid births between January 2009 and December 2015 in South Carolina. Of these, 46,414 births (24.8%) occurred in hospitals that provided immediate postpartum LARC in response to the policy change. Overall, the implementing hospitals had more annual births paid for by Medicaid compared to nonimplementing hospitals (1,105 vs. 511) and were less likely to be rural (33.3% vs. 46.8%) and had a greater share of preterm births (15.5% vs. 9.5%). Prior to the policy change, the probability of a preterm birth in the next 4 years was 4.4% for patients at implementing hospitals and 3.5% for those in nonimplementing hospitals, and the probability of a low-birth-weight birth was 3.6% and 2.9%, respectively.
The policy change was associated with a decrease of 0.4 percentage points for preterm birth and 0.3 percentage points for subsequent low-birth-weight birth.
When the results were stratified based on race and ethnicity, the policy change was associated with a decrease of 0.5 percentage points in the probability of preterm birth in both non-Hispanic Blacks and non-Hispanic Whites. No significant differences appeared in the association between the policy change and rates of preterm birth or low-birth-weight birth between non-Hispanic Black and non-Hispanic White individuals.
However, the policy was associated with a significant decrease of 0.6 percentage points in the probability of short-interval birth among non-Hispanic Blacks, and a decrease of 1.6 percentage points in the probability of another birth within 4 years overall. The policy change also was associated with a significant increase of 27 days between births among non-Hispanic Blacks, but not with any significant change among non-Hispanic Whites or the study population overall.
“In addition, although our data cannot speak to this, the policy may have affected the intendedness of subsequent pregnancies, leading to healthier behaviors before and during pregnancy, such as early initiation of prenatal care,” the researchers wrote in their discussion of the findings.
The study findings were limited by several factors including the lack of data on pregnancy intention or abortion, and the lack of data on patient-reported outcomes, notably the provision of patient-centered counseling and whether such counseling was biased, the researchers noted. Other limitations included a lack of data on infant mortality and potential confounding from risk profiles of patients in implementing vs. nonimplementing hospitals, they wrote.
Also, the study provides population-level data, which does not guide clinical decision-making about intervals between childbirth and subsequent pregnancy, the researchers emphasized.
Although the data support the value of postpartum contraception in improving birth outcomes, “it is imperative that efforts to expand access focus on assuring comprehensive access to all forms of contraception without coercion,” the researchers concluded. “Additional policy solutions are needed to improve infant health, including those that directly address structural and interpersonal racism to reduce racial disparities in infant health,” they said.
The study is important because, although immediate postpartum LARC policies were first implemented almost a decade ago in the United States, population-level evidence on the effects of these policies remains scarce, Dr. Steenland said in an interview.
Existing barriers to improving access to immediate postpartum LARC include health professional training and logistics within hospitals, as well as ensuring correct billing and timely reimbursements, Dr. Steenland said. “Simple and clear billing procedures, and advanced reimbursement so that hospitals can have devices stocked would make it easier to provide this service,” she noted.
“This service has gone from being almost completely unavailable, to available in some hospitals, mainly those that are urban, teaching, and high volume,” said Dr. Steenland. “Additional research is needed to determine how health systems can make this service available to all birthing persons,” she said. “Also, critically, additional research is needed to identify strategies to ensure that counseling for immediate postpartum LARC, and family planning more generally, is patient-centered, so that the availability of immediate postpartum LARC increases, rather than restricts, choice,” she added. “Finally, additional research is needed to determine whether postpartum people have affordable and accessible access to LARC removal services,” Dr. Steenland emphasized.
Immediate post partum is critical period
The immediate postpartum period is a critical time for access to contraception because many women do not return for postpartum visits after hospital discharge, Tracey A. Wilkinson, MD, and Jeffrey F. Peipert, MD, of Indiana University, Indianapolis, wrote in an accompanying editorial. “The focus on contraception access during the postpartum period prior to hospital discharge is important because of the potential sequelae of a subsequent unintended pregnancy or short interpregnancy intervals,” they noted. These issues may be more acute in marginalized communities, and policies to expand immediate postpartum LARC are in place in a majority of states, the editorialists said.
However, they agreed with the authors’ statements that implementation of LARC must be done in a manner that supports patient choice and avoids coercion. Given the baseline disparities of the infant outcomes studied, increased access to immediate postpartum LARC must be provided in a way that does not exacerbate these disparities, they said. “This ultimately means that plans to increase access to contraception should emphasize availability while avoiding coercion, and if a patient ultimately decides to discontinue a method, enable that to occur easily and seamlessly, including LARC device removal,” they explained.
“Future studies examining patient centeredness of these postpartum LARC implementation efforts would be an important element to augment these data and show the impact in additional spheres beyond infant outcomes,” they added.
Overcome trust barriers and offer options
“In a time of restrictive access to abortion and contraception in many states, any additional increase in access can potentially be meaningful,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview. “Additionally, given the significantly higher rates of infant and maternal morbidity and mortality among the non-Hispanic Black population, seeing an intervention that can improve outcomes for both mothers and babies is also potentially very positive,” she said.
Dr. Prager said she was not surprised by the study findings, as immediate LARC is much more common in other countries and has shown similar outcomes. “Additionally, I am reassured by the fact that the increased number of days until the next pregnancy is not higher, as this indirectly indicates that patients were able to get their LARC removed when they desired another pregnancy,” she noted.
Barriers to improving access to immediate postpartum LARC in the Medicaid population may include mistrust for any long-acting contraception, “especially if they perceive that cessation of the method will be difficult to achieve,” Dr. Prager noted. “Certainly, counseling about LARC removal should be an element of counseling prior to any initiation, and lack of access to removal of an IUD or implant can be categorized as a form of reproductive coercion,” she said. Dr. Prager said that such counseling might be more effective if it occurred during prenatal visits, “so if providers are not talking about this during routine OB visits and patients only hear about immediate postpartum LARC when they are in the hospital for delivery, they may be less likely to accept the practice,” she said. “Finally, although Medicaid will cover the cost of immediate postpartum LARC, private insurers do not do so consistently in all states, so some hospitals may find this process too difficult to navigate and therefore not offer immediate postpartum LARC,” Dr. Prager emphasized.
As for additional research, Dr. Prager said she would like to see more studies in an overall United States population of pregnant people, both Medicaid patients and others, on whether the immediate postpartum timing of LARC is desired.
“I would like to couple that with patients’ impressions or experiences of their ability to access contraception outside of the immediate postpartum time period, and also their impressions or experience with ability to have LARC removed, since they are the only contraceptives not necessarily within personal control for initiation or cessation,” Dr. Prager said.
The study was supported by the National Institute for Child Health and Development, and lead author Dr. Steenland received support from other National Institutes of Health grants. The researchers had no financial conflicts to disclose. The editorial was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Peipert disclosed serving on advisory boards for Bayer and CooperSurgical, and receiving research support from Merck, Bayer, and CooperSurgical/Teva. Dr. Wilkinson had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA PEDIATRICS
IUDs may increase background enhancement on breast MRI
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Intrauterine contraceptive devices (IUDs) have been linked to increased background enhancement on breast MRI, according to research presented at the Radiological Society of North America 2021 annual meeting.
About 10.4% of women 15-49 years of age who use contraception have an IUD or contraceptive implant, according to the Centers for Disease Control and Prevention. Unlike oral or transdermal hormonal contraceptives and hormone replacement therapy, levonorgestrel-releasing IUDs release a small amount of the hormone directly into the uterus and are thought to have a much more localized effect, Luisa Huck, MD, the lead author of the study, said in an interview.
But women with IUDs have long reported adverse effects associated with other hormonal medication. “In the past, some women reported depression, headaches, sleep disorders, and panic attacks,” noted Dr. Huck, a radiology resident at RWTH Aachen University in Germany.
Christiane Kuhl, MD, chief of the department of radiology at RWTH Aachen University and senior author of the research, had also observed that women with hormonal IUDs often have increased background parenchymal enhancement (BPE) on contrast-enhanced MRI. BPE “has been established as a sensitive marker of hormonal stimulation of breast,” the study authors wrote, and previous studies have shown that women using hormonal medications have higher BPE on breast MRIs.
To better understand whether IUDs can increase BPE, Dr. Huck and colleagues used the hospital database to search for premenopausal women who had undergone breast MRIs for screening between January 2014 and July 2020. To be included, women had to have had at least two scans: one with and one without an IUD in place, with the scan conducted at least 4 weeks after IUD placement or removal. All women in the study had no history of breast cancer or hormone or antihormone intake.
The study involved 48 women with an average age of 45 years and a median of 27 months between the two scans. Forty-six of the women had the Mirena levonorgestrel-releasing IUD and two had the Jaydess IUD. To account for hormone variations between patients, the researchers used each patient as their own reference point. To control for age-related effects, 25 women had their first MRI without an IUD and their second scan with an IUD in place. The second group of 23 women underwent their first MRI with an IUD and had it removed before the second scan.
Hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging.
For 23 women in the study, background enhancement was higher on scans with the IUD than without (P < .001). For 24 women, there was no change in BPE with or without an IUD, and one woman had lower BPE with an IUD than without.
“It is very interesting and relevant to practice to consider that the presence of an intrauterine device would have potential impact on the enhancement we see in the breast on MRI imaging,” Samantha Heller, MD, PhD, associate professor of radiology at New York University, said in an interview.
However, the study used BPE as a measure for hormonal shifts, and “hormonal effects on breast enhancement are very complex, and hormonal stimulation is not always predictably correlated with changes on MRI imaging,” she noted. BPE on MRI can fluctuate, so testing actual hormone levels in patients with elevated BPE could be helpful to identify hormonal shifts, she added. It is also important to understand why half of the women in the study showed no variation in BPE, she said.
The study findings are not very surprising, considering that it is known that low levels of progesterone from IUDs circulate in the blood stream, Frances Casey, MD, MPH, associate professor in the department of obstetrics and gynecology at Virginia Commonwealth University in Richmond, said in an interview. They do not suggest that there should be any changes to IUD guidelines, she added.
However, “the study findings raise the question as to whether IUD status should be documented as a matter of course prior to performing breast MRI,” said Dr. Heller. “It is standard to document the timing of a woman’s menstrual cycle, as well as to note any hormone suppression or replacement therapy. This is in part so that the radiologist may understand the etiology of any observed variation in background enhancement,” she explained.
Although increased enhancement on MRI has sometimes been linked to higher chances of recommendations for additional imaging or biopsies, she noted, “more work would be needed to understand the impact – if any – of an IUD on breast MRI recommendations due to enhancement changes.”
Dr. Huck, Dr. Heller, and Dr. Casey disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Texas practitioners see increased interest in birth control since near-total abortion ban
In September, when Texas’ near-total abortion ban took effect, Planned Parenthood clinics in the Lone Star State started offering every patient who walked in information on Senate Bill 8, as well as emergency contraception, condoms, and two pregnancy tests. The plan is to distribute 22,000 “empowerment kits” this year.
“We felt it was very important for patients to have as many tools on hand to help them meet this really onerous law,” said Elizabeth Cardwell, lead clinician at Planned Parenthood of Greater Texas, which has 24 clinics across the northern and central regions of the state and provides care to tens of thousands of people annually.
Most of their patients – who tend to be uninsured and have annual household incomes of less than $25,000 – had not known about SB 8 the first several weeks after implementation, said Dr. Cardwell. But once they learned about it, patients seemed to rush to get on birth control, she said.
SB 8 allows private citizens, in Texas or elsewhere, to sue anyone who performs an abortion in the state or who “aided or abetted” someone getting an abortion once fetal cardiac activity is detected. This is generally around six weeks, before most people know they’re pregnant. It’s had a chilling effect in Texas, where access to abortion was already limited.
Medical staffs are doubling down on educating patients about birth control. They recognize the strategy isn’t foolproof but are desperate to prevent unintended pregnancies, nearly half of which nationwide end in abortion.
“It’s more important now than it ever has been,” said Dr. Cardwell. “I’ve been in abortion care 30-plus years, and my go-to line was ‘You’ve got plenty of time. You don’t have to feel rushed. Talk with your partner. Talk with your family,’” she said. “Now we don’t have that luxury.”
Patients, too, seem to feel a sense of urgency. During September, according to data from Planned Parenthood of Greater Texas, medical staff provided patients with some form of birth control — for example, pill packs, Depo-Provera shots or IUD implant insertions – in more than 3,750 visits, 5% more than in Sept. 2020.
Dr. Jennifer Liedtke, a family physician in West Texas, said she and her nurse practitioners explain SB 8 to every patient who comes to their private practice and saw a 20% increase in requests for long-acting reversible contraceptive methods, known as LARCs, in September.
LARCs, a category that includes intrauterine devices and hormonal implants, have become increasingly appealing because they are 99% effective at preventing pregnancy and last several years. They are also simpler than the pill, which needs to be taken daily, or the vaginal ring, which needs to be changed monthly.
Still, LARCs are not everyone’s preferred method. For example, inserting an IUD can be painful.
A doctor’s office is one of the few opportunities for reliable birth control education. Texas law doesn’t require schools to teach sex education, and if they do, educators must stress abstinence as the preferred birth control method. Some doctors opt to explain abortion access in the state when naming birth control options.
Dr. Liedtke is used to having to explain new laws passed by the Texas legislature. “It happens all the time,” she said. But the controversy surrounding SB 8 confuses patients all the more as the law works its way through the court system with differing rulings, one of which briefly blocked the measure. The U.S. Supreme Court heard related arguments Nov. 1.
“People just don’t understand,” said Dr. Liedtke. “It was tied up for 48 hours, so they are like, ‘It’s not a law anymore?’ Well, no, technically it is.”
Not all providers are able to talk freely about abortion access. In 2019, the Trump administration barred providers that participate in the federally funded family planning program, Title X, from mentioning abortion care to patients, even if patients themselves raise questions. In early October, the Biden administration reversed that rule. The change will kick in this month. Planned Parenthood can discuss SB 8 in Texas because Texas affiliates do not receive Title X dollars.
Dr. Lindsey Vasquez of Legacy Community Health, the largest federally qualified health center in Texas and a recipient of Title X dollars, said she and other staff members have not discussed abortion or SB 8 because they also must juggle a variety of other priorities. Legacy’s patients are underserved, she said. A majority live at or below the federal poverty level.
Nearly two years into the Covid-19 pandemic, “we’re literally maximizing those visits,” Dr. Vasquez said. Their jobs go beyond offering reproductive care. “We’re making sure they have food resources, that they have their housing stable,” she said. “We really are trying to make sure that all of their needs are met because we know for these types of populations – patients that we serve – this may be our only moment that we get to meet them.”
Specialized family planning clinics that receive Title X dollars do have proactive conversations about contraceptive methods, according to Every Body Texas, the Title X grantee for the state.
Discussions of long-acting reversible contraception must be handled with sensitivity because these forms of birth control have a questionable history among certain populations, primarily lower-income patients. In the 1990s, lawmakers in several states, including Texas, introduced bills to offer cash assistance recipients financial incentives to get an implant or mandate insertion for people on government benefits, a move seen as reproductive coercion.
“It’s important for a client to get on the contraceptive method of their choice,” said Mimi Garcia, communications director for Every Body Texas. “Some people will just say, ‘Let’s get everyone on IUDs’ or ‘Let’s get everybody on hormonal implants’ because those are the most effective methods. ... That’s not something that’s going to work for [every] individual. ... Either they don’t agree with it philosophically, or they don’t like how it makes their body feel.”
It’s a nuanced subject for providers to broach, so some suggest starting the conversation by asking the patient about their future.
“The best question to ask is ‘When do you want to have another baby?’” said Dr. Liedtke. And then if they say, ‘Oh, gosh, I’m not even sure I want to have more kids’ or ‘Five or six years from now,’ then we start talking LARCs. ... But if it’s like, ‘Man, I really want to start trying in a year,’ then I don’t talk to them about putting one of those in.”
The Biden administration expected more demand for birth control in Texas, so Health & Human Services Secretary Xavier Becerra announced in mid-September that Every Body Texas would receive additional Title X funding, as would local providers experiencing an influx of clients as a result of SB 8.
But providers said improved access to contraception will not blunt the law’s effects. It will not protect patients who want to get pregnant but ultimately decide on abortion because they receive a diagnosis of a serious complication, their relationship status changes, or they lose financial or social support, said Dr. Elissa Serapio, an OB-GYN in the Rio Grande Valley and a fellow with Physicians for Reproductive Health.
“It’s the very best that we can do,” said Dr. Cardwell, of Planned Parenthood of Greater Texas. “There’s no 100% effective method of birth control.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In September, when Texas’ near-total abortion ban took effect, Planned Parenthood clinics in the Lone Star State started offering every patient who walked in information on Senate Bill 8, as well as emergency contraception, condoms, and two pregnancy tests. The plan is to distribute 22,000 “empowerment kits” this year.
“We felt it was very important for patients to have as many tools on hand to help them meet this really onerous law,” said Elizabeth Cardwell, lead clinician at Planned Parenthood of Greater Texas, which has 24 clinics across the northern and central regions of the state and provides care to tens of thousands of people annually.
Most of their patients – who tend to be uninsured and have annual household incomes of less than $25,000 – had not known about SB 8 the first several weeks after implementation, said Dr. Cardwell. But once they learned about it, patients seemed to rush to get on birth control, she said.
SB 8 allows private citizens, in Texas or elsewhere, to sue anyone who performs an abortion in the state or who “aided or abetted” someone getting an abortion once fetal cardiac activity is detected. This is generally around six weeks, before most people know they’re pregnant. It’s had a chilling effect in Texas, where access to abortion was already limited.
Medical staffs are doubling down on educating patients about birth control. They recognize the strategy isn’t foolproof but are desperate to prevent unintended pregnancies, nearly half of which nationwide end in abortion.
“It’s more important now than it ever has been,” said Dr. Cardwell. “I’ve been in abortion care 30-plus years, and my go-to line was ‘You’ve got plenty of time. You don’t have to feel rushed. Talk with your partner. Talk with your family,’” she said. “Now we don’t have that luxury.”
Patients, too, seem to feel a sense of urgency. During September, according to data from Planned Parenthood of Greater Texas, medical staff provided patients with some form of birth control — for example, pill packs, Depo-Provera shots or IUD implant insertions – in more than 3,750 visits, 5% more than in Sept. 2020.
Dr. Jennifer Liedtke, a family physician in West Texas, said she and her nurse practitioners explain SB 8 to every patient who comes to their private practice and saw a 20% increase in requests for long-acting reversible contraceptive methods, known as LARCs, in September.
LARCs, a category that includes intrauterine devices and hormonal implants, have become increasingly appealing because they are 99% effective at preventing pregnancy and last several years. They are also simpler than the pill, which needs to be taken daily, or the vaginal ring, which needs to be changed monthly.
Still, LARCs are not everyone’s preferred method. For example, inserting an IUD can be painful.
A doctor’s office is one of the few opportunities for reliable birth control education. Texas law doesn’t require schools to teach sex education, and if they do, educators must stress abstinence as the preferred birth control method. Some doctors opt to explain abortion access in the state when naming birth control options.
Dr. Liedtke is used to having to explain new laws passed by the Texas legislature. “It happens all the time,” she said. But the controversy surrounding SB 8 confuses patients all the more as the law works its way through the court system with differing rulings, one of which briefly blocked the measure. The U.S. Supreme Court heard related arguments Nov. 1.
“People just don’t understand,” said Dr. Liedtke. “It was tied up for 48 hours, so they are like, ‘It’s not a law anymore?’ Well, no, technically it is.”
Not all providers are able to talk freely about abortion access. In 2019, the Trump administration barred providers that participate in the federally funded family planning program, Title X, from mentioning abortion care to patients, even if patients themselves raise questions. In early October, the Biden administration reversed that rule. The change will kick in this month. Planned Parenthood can discuss SB 8 in Texas because Texas affiliates do not receive Title X dollars.
Dr. Lindsey Vasquez of Legacy Community Health, the largest federally qualified health center in Texas and a recipient of Title X dollars, said she and other staff members have not discussed abortion or SB 8 because they also must juggle a variety of other priorities. Legacy’s patients are underserved, she said. A majority live at or below the federal poverty level.
Nearly two years into the Covid-19 pandemic, “we’re literally maximizing those visits,” Dr. Vasquez said. Their jobs go beyond offering reproductive care. “We’re making sure they have food resources, that they have their housing stable,” she said. “We really are trying to make sure that all of their needs are met because we know for these types of populations – patients that we serve – this may be our only moment that we get to meet them.”
Specialized family planning clinics that receive Title X dollars do have proactive conversations about contraceptive methods, according to Every Body Texas, the Title X grantee for the state.
Discussions of long-acting reversible contraception must be handled with sensitivity because these forms of birth control have a questionable history among certain populations, primarily lower-income patients. In the 1990s, lawmakers in several states, including Texas, introduced bills to offer cash assistance recipients financial incentives to get an implant or mandate insertion for people on government benefits, a move seen as reproductive coercion.
“It’s important for a client to get on the contraceptive method of their choice,” said Mimi Garcia, communications director for Every Body Texas. “Some people will just say, ‘Let’s get everyone on IUDs’ or ‘Let’s get everybody on hormonal implants’ because those are the most effective methods. ... That’s not something that’s going to work for [every] individual. ... Either they don’t agree with it philosophically, or they don’t like how it makes their body feel.”
It’s a nuanced subject for providers to broach, so some suggest starting the conversation by asking the patient about their future.
“The best question to ask is ‘When do you want to have another baby?’” said Dr. Liedtke. And then if they say, ‘Oh, gosh, I’m not even sure I want to have more kids’ or ‘Five or six years from now,’ then we start talking LARCs. ... But if it’s like, ‘Man, I really want to start trying in a year,’ then I don’t talk to them about putting one of those in.”
The Biden administration expected more demand for birth control in Texas, so Health & Human Services Secretary Xavier Becerra announced in mid-September that Every Body Texas would receive additional Title X funding, as would local providers experiencing an influx of clients as a result of SB 8.
But providers said improved access to contraception will not blunt the law’s effects. It will not protect patients who want to get pregnant but ultimately decide on abortion because they receive a diagnosis of a serious complication, their relationship status changes, or they lose financial or social support, said Dr. Elissa Serapio, an OB-GYN in the Rio Grande Valley and a fellow with Physicians for Reproductive Health.
“It’s the very best that we can do,” said Dr. Cardwell, of Planned Parenthood of Greater Texas. “There’s no 100% effective method of birth control.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In September, when Texas’ near-total abortion ban took effect, Planned Parenthood clinics in the Lone Star State started offering every patient who walked in information on Senate Bill 8, as well as emergency contraception, condoms, and two pregnancy tests. The plan is to distribute 22,000 “empowerment kits” this year.
“We felt it was very important for patients to have as many tools on hand to help them meet this really onerous law,” said Elizabeth Cardwell, lead clinician at Planned Parenthood of Greater Texas, which has 24 clinics across the northern and central regions of the state and provides care to tens of thousands of people annually.
Most of their patients – who tend to be uninsured and have annual household incomes of less than $25,000 – had not known about SB 8 the first several weeks after implementation, said Dr. Cardwell. But once they learned about it, patients seemed to rush to get on birth control, she said.
SB 8 allows private citizens, in Texas or elsewhere, to sue anyone who performs an abortion in the state or who “aided or abetted” someone getting an abortion once fetal cardiac activity is detected. This is generally around six weeks, before most people know they’re pregnant. It’s had a chilling effect in Texas, where access to abortion was already limited.
Medical staffs are doubling down on educating patients about birth control. They recognize the strategy isn’t foolproof but are desperate to prevent unintended pregnancies, nearly half of which nationwide end in abortion.
“It’s more important now than it ever has been,” said Dr. Cardwell. “I’ve been in abortion care 30-plus years, and my go-to line was ‘You’ve got plenty of time. You don’t have to feel rushed. Talk with your partner. Talk with your family,’” she said. “Now we don’t have that luxury.”
Patients, too, seem to feel a sense of urgency. During September, according to data from Planned Parenthood of Greater Texas, medical staff provided patients with some form of birth control — for example, pill packs, Depo-Provera shots or IUD implant insertions – in more than 3,750 visits, 5% more than in Sept. 2020.
Dr. Jennifer Liedtke, a family physician in West Texas, said she and her nurse practitioners explain SB 8 to every patient who comes to their private practice and saw a 20% increase in requests for long-acting reversible contraceptive methods, known as LARCs, in September.
LARCs, a category that includes intrauterine devices and hormonal implants, have become increasingly appealing because they are 99% effective at preventing pregnancy and last several years. They are also simpler than the pill, which needs to be taken daily, or the vaginal ring, which needs to be changed monthly.
Still, LARCs are not everyone’s preferred method. For example, inserting an IUD can be painful.
A doctor’s office is one of the few opportunities for reliable birth control education. Texas law doesn’t require schools to teach sex education, and if they do, educators must stress abstinence as the preferred birth control method. Some doctors opt to explain abortion access in the state when naming birth control options.
Dr. Liedtke is used to having to explain new laws passed by the Texas legislature. “It happens all the time,” she said. But the controversy surrounding SB 8 confuses patients all the more as the law works its way through the court system with differing rulings, one of which briefly blocked the measure. The U.S. Supreme Court heard related arguments Nov. 1.
“People just don’t understand,” said Dr. Liedtke. “It was tied up for 48 hours, so they are like, ‘It’s not a law anymore?’ Well, no, technically it is.”
Not all providers are able to talk freely about abortion access. In 2019, the Trump administration barred providers that participate in the federally funded family planning program, Title X, from mentioning abortion care to patients, even if patients themselves raise questions. In early October, the Biden administration reversed that rule. The change will kick in this month. Planned Parenthood can discuss SB 8 in Texas because Texas affiliates do not receive Title X dollars.
Dr. Lindsey Vasquez of Legacy Community Health, the largest federally qualified health center in Texas and a recipient of Title X dollars, said she and other staff members have not discussed abortion or SB 8 because they also must juggle a variety of other priorities. Legacy’s patients are underserved, she said. A majority live at or below the federal poverty level.
Nearly two years into the Covid-19 pandemic, “we’re literally maximizing those visits,” Dr. Vasquez said. Their jobs go beyond offering reproductive care. “We’re making sure they have food resources, that they have their housing stable,” she said. “We really are trying to make sure that all of their needs are met because we know for these types of populations – patients that we serve – this may be our only moment that we get to meet them.”
Specialized family planning clinics that receive Title X dollars do have proactive conversations about contraceptive methods, according to Every Body Texas, the Title X grantee for the state.
Discussions of long-acting reversible contraception must be handled with sensitivity because these forms of birth control have a questionable history among certain populations, primarily lower-income patients. In the 1990s, lawmakers in several states, including Texas, introduced bills to offer cash assistance recipients financial incentives to get an implant or mandate insertion for people on government benefits, a move seen as reproductive coercion.
“It’s important for a client to get on the contraceptive method of their choice,” said Mimi Garcia, communications director for Every Body Texas. “Some people will just say, ‘Let’s get everyone on IUDs’ or ‘Let’s get everybody on hormonal implants’ because those are the most effective methods. ... That’s not something that’s going to work for [every] individual. ... Either they don’t agree with it philosophically, or they don’t like how it makes their body feel.”
It’s a nuanced subject for providers to broach, so some suggest starting the conversation by asking the patient about their future.
“The best question to ask is ‘When do you want to have another baby?’” said Dr. Liedtke. And then if they say, ‘Oh, gosh, I’m not even sure I want to have more kids’ or ‘Five or six years from now,’ then we start talking LARCs. ... But if it’s like, ‘Man, I really want to start trying in a year,’ then I don’t talk to them about putting one of those in.”
The Biden administration expected more demand for birth control in Texas, so Health & Human Services Secretary Xavier Becerra announced in mid-September that Every Body Texas would receive additional Title X funding, as would local providers experiencing an influx of clients as a result of SB 8.
But providers said improved access to contraception will not blunt the law’s effects. It will not protect patients who want to get pregnant but ultimately decide on abortion because they receive a diagnosis of a serious complication, their relationship status changes, or they lose financial or social support, said Dr. Elissa Serapio, an OB-GYN in the Rio Grande Valley and a fellow with Physicians for Reproductive Health.
“It’s the very best that we can do,” said Dr. Cardwell, of Planned Parenthood of Greater Texas. “There’s no 100% effective method of birth control.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
The Supreme Court 2020‒2021: What will affect ObGyns?
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
