User login
Abortion debate may affect Rx decisions for pregnant women
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Obstetrician Beverly Gray, MD, is already seeing the effects of the Roe v. Wade abortion debate in her North Carolina practice.
The state allows abortion but requires that women get counseling with a qualified health professional 72 hours before the procedure. “Aside from that, we still have patients asking for more efficacious contraceptive methods just in case,” said Dr. Gray, residency director and division director for women’s community and population health and associate professor for obstetrics and gynecology at Duke University, Durham, N.C.
Patients and staff in her clinic have also been approaching her about tubal ligation. “They’re asking about additional birth control methods because they’re concerned about what’s going to happen” with the challenge to the historic Roe v. Wade decision in the Supreme Court and subsequent actions in the states to restrict or ban abortion, she said.
This has implications not just for abortion but for medications known to affect pregnancy. “What I’m really worried about is physicians will be withholding medicine because they’re concerned about teratogenic effects,” said Dr. Gray.
With more states issuing restrictions on abortion, doctors are worried that patients needing certain drugs to maintain their lupus flares, cancer, or other diseases may decide not to take them in the event they accidentally become pregnant. If the drug is known to affect the fetus, the fear is a patient who lives in a state with abortion restrictions will no longer have the option to terminate a pregnancy.
Instead, a scenario may arise in which the patient – and their physician – may opt not to treat at all with an otherwise lifesaving medication, experts told this news organization.
The U.S. landscape on abortion restrictions
A leaked draft of a U.S. Supreme Court opinion on Mississippi’s 15-week abortion ban has sent the medical community into a tailspin. The case, Dobbs v. Jackson Women’s Health Organization, challenges the 1973 Roe v. Wade decision that affirms the constitutional right to abortion. It’s anticipated the high court will decide on the case in June.
Although the upcoming decision is subject to change, the draft indicated the high court would uphold the Mississippi ban. This would essentially overturn the 1973 ruling. An earlier Supreme Court decision allowing a Texas law banning abortion at 6 weeks suggests the court may already be heading in this direction. At the state level, legislatures have been moving on divergent paths – some taking steps to preserve abortion rights, others initiating restrictions.
More than 100 abortion restrictions in 19 states took effect in 2021, according to the Guttmacher Institute, which tracks such metrics. In 2022, “two key themes are anti-abortion policymakers’ continued pursuit of various types of abortion bans and restrictions on medication abortion,” the institute reported.
Forty-six states and the District of Columbia have introduced 2,025 restrictions or proactive measures on sexual and reproductive health and rights so far this year. The latest tally from Guttmacher, updated in late May, revealed that 11 states so far have enacted 42 abortion restrictions. A total of 6 states (Arizona, Florida, Idaho, Kentucky, Oklahoma, and Wyoming) have issued nine bans on abortion.
Comparatively, 11 states have enacted 19 protective abortion measures.
Twenty-two states have introduced 117 restrictions on medication abortions, which account for 54% of U.S. abortions. This includes seven measures that would ban medication abortion outright, according to Guttmacher. Kentucky and South Dakota collectively have enacted 14 restrictions on medication abortion, as well as provisions that ban mailing of abortion pills.
Chilling effect on prescribing
Some physicians anticipate that drugs such as the “morning-after” pill (levonorgestrel) will become less available as restrictions go into effect, since these are medications designed to prevent pregnancy.*
However, the ongoing effort to put a lid on abortion measures has prompted concerns about a trickle-down effect on other medications that are otherwise life-changing or lifesaving to patients but pose a risk to the fetus.
Several drugs are well documented to affect fetal growth and development of the fetus, ranging from mild, transitory effects to severe, permanent birth defects, said Ronald G. Grifka, MD, chief medical officer of University of Michigan Health-West and clinical professor of pediatrics at the University of Michigan Medical School, Ann Arbor. “As new medications are developed, we will need heightened attention to make sure they are safe for the fetus,” he added.
Certain teratogenic medications are associated with a high risk of abortion even though this isn’t their primary use, noted Christina Chambers, PhD, MPH, co-director of the Center for Better Beginnings and associate director with the Altman Clinical & Translational Research Institute at the University of California, San Diego.
“I don’t think anyone would intentionally take these drugs to induce spontaneous abortion. But if the drugs pose a risk for it, I can see how the laws might be stretched” to include them, said Dr. Chambers.
Methotrexate, a medication for autoimmune disorders, has a high risk of spontaneous abortion. So do acne medications such as isotretinoin.
Patients are usually told they’re not supposed to get pregnant on these drugs because there’s a high risk of pregnancy loss and risk of malformations and potential learning problems in the fetus. But many pregnancies aren’t planned, said Dr. Chambers. “Patients may forget about the side effects or think their birth control will protect them. And the next time they refill the medication, they may not hear about the warnings again.”
With a restrictive abortion law or ban in effect, a woman might think: “I won’t take this drug because if there’s any potential that I might get pregnant, I won’t have the option to abort an at-risk pregnancy.” Women and their doctors, for that matter, don’t want to put themselves in this position, said Dr. Chambers.
Rheumatologist Megan Clowse, MD, who prescribes several medications that potentially cause major birth defects and pregnancy loss, worries about the ramifications of these accumulating bans.
“Methotrexate has been a leading drug for us for decades for rheumatoid arthritis. Mycophenolate is a vital drug for lupus,” said Dr. Clowse, associate professor of medicine at Duke University’s division of rheumatology and immunology.
Both methotrexate and mycophenolate pose about a 40% risk of pregnancy loss and significantly increase the risk for birth defects. “I’m definitely concerned that there might be doctors or women who elect not to use those medications in women of reproductive age because of the potential risk for pregnancy and absence of abortion rights,” said Dr. Clowse.
These situations might force women to use contraceptives they don’t want to use, such as hormonal implants or intrauterine devices, she added. Another side effect is that women and their partners may decide to abstain from sex.
The iPLEDGE factor
Some rheumatology drugs like lenalidomide (Revlimid) require a valid negative pregnancy test in a lab every month. Similarly, the iPLEDGE Risk Evaluation and Mitigation Strategy seeks to reduce the teratogenicity of isotretinoin by requiring two types of birth control and regular pregnancy tests by users.
For isotretinoin specifically, abortion restrictions “could lead to increased adherence to pregnancy prevention measures which are already stringent in iPLEDGE. But on the other hand, it could lead to reduced willingness of physicians to prescribe or patients to take the medication,” said Dr. Chambers.
With programs like iPLEDGE in effect, the rate of pregnancies and abortions that occur in dermatology are relatively low, said Jenny Murase, MD, associate clinical professor of dermatology at the University of California, San Francisco.
Nevertheless, as a physician who regularly prescribes medications like isotretinoin in women of childbearing age, “it’s terrifying to me that a woman wouldn’t have the option to terminate the pregnancy if a teratogenic effect from the medication caused a severe birth defect,” said Dr. Murase.
Dermatologists use other teratogenic medications such as thalidomide, mycophenolate mofetil, and methotrexate for chronic dermatologic disease like psoriasis and atopic dermatitis.
The situation is especially tricky for dermatologists since most patients – about 80% – never discuss their pregnancy with their specialist prior to pregnancy initiation. Dr. Murase recalls when a patient with chronic plaque psoriasis on methotrexate in her late 40s became pregnant and had an abortion even before Dr. Murase became aware of the pregnancy.
Because dermatologists routinely prescribe long-term medications for chronic diseases like acne, psoriasis, and atopic dermatitis, it is important to have a conversation regarding the risks and benefits of long-term medication should a pregnancy occur in any woman of childbearing age, she said.
Fewer women in clinical trials?
Abortion restrictions could possibly discourage women of reproductive age to participate in a clinical trial for a new medication, said Dr. Chambers.
A female patient with a chronic disease who’s randomized to receive a new medication may be required to use certain types of birth control because of unknown potential adverse effects the drug may have on the fetus. But in some cases, accidental pregnancies happen.
The participant in the trial may say, “I don’t know enough about the safety of this drug in pregnancy, and I’ve already taken it. I want to terminate the pregnancy,” said Dr. Chambers. Thinking ahead, a woman may decide not to do the trial to avoid the risk of getting pregnant and not having the option to terminate the pregnancy.
This could apply to new drugs such as antiviral treatments, or medications for severe chronic disease that typically have no clinical trial data in pregnancy prior to initial release into the market.
Women may start taking the drug without thinking about getting pregnant, then realize there are no safety data and become concerned about its effects on a future pregnancy.
The question is: Will abortion restrictions have a chilling effect on these new drugs as well? Patients and their doctors may decide not to try it until more data are available. “I can see where abortion restrictions would change the risk or benefit calculation in thinking about what you do or don’t prescribe or take during reproductive age,” said Dr. Chambers.
The upside of restrictions?
If there’s a positive side to these developments with abortion bans, it may encourage women taking new medications or joining clinical trials to think even more carefully about adherence to effective contraception, said Dr. Chambers.
Some methods are more effective than others, she emphasized. “When you have an unplanned pregnancy, it could mean that the method you used wasn’t optimal or you weren’t using it as recommended.” A goal moving forward is to encourage more thoughtful use of highly effective contraceptives, thus reducing the number of unplanned pregnancies, she added.
If patients are taking methotrexate, “the time to think about pregnancy is before getting pregnant so you can switch to a drug that’s compatible with pregnancy,” she said.
This whole thought process regarding pregnancy planning could work toward useful health goals, said Dr. Chambers. “Nobody thinks termination is the preferred method, but planning ahead should involve a discussion of what works best for the patient.”
Patients do have other choices, said Dr. Grifka. “Fortunately, there are many commonly prescribed medications which cross the placenta and have no ill effects on the fetus.”
Talking to patients about choices
Dr. Clowse, who spends a lot of time training rheumatologists, encourages them to have conversations with patients about pregnancy planning. It’s a lot to manage, getting the right drug to a female patient with chronic illness, especially in this current climate of abortion upheaval, she noted.
Her approach is to have an open and honest conversation with patients about their concerns and fears, what the realities are, and what the potential future options are for certain rheumatology drugs in the United States.
Some women who see what’s happening across the country may become so risk averse that they may choose to die rather than take a lifesaving drug that poses certain risks under new restrictions.
“I think that’s tragic,” said Dr. Clowse.
To help their patients, Dr. Gray believes physicians across specialties should better educate themselves about physiology in pregnancy and how to counsel patients on the impact of not taking medications in pregnancy.
In her view, it’s almost coercive to say to a patient, “You really need to have effective contraception if I’m going to give you this lifesaving or quality-of-life-improving medication.”
When confronting such scenarios, Dr. Gray doesn’t think physicians need to change how they counsel patients about contraception. “I don’t think we should be putting pressure on patients to consider other permanent methods just because there’s a lack of abortion options.”
Patients will eventually make those decisions for themselves, she said. “They’re going to want a more efficacious method because they’re worried about not having access to abortion if they get pregnant.”
Dr. Gray reports being a site principal investigator for a phase 3 trial for VeraCept IUD, funded by Sebela Pharmaceuticals. Dr. Clowse reports receiving research funding and doing consulting for GlaxoSmithKline.
*Correction, 6/2/2022: A previous version of this article misstated the intended use of drugs such as the “morning-after” pill (levonorgestrel). They are taken to prevent unintended pregnancy.
A version of this article first appeared on Medscape.com .
Contraceptive use boosted by enhanced counseling
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
Contraceptive counseling and interventions beyond usual care significantly increased the use of contraceptives with no accompanying increase in sexually transmitted infections or reduction in condom use, based on data from a new meta-analysis.
“Although effective contraception is available in the United States and guidelines support contraceptive care in clinical practice, providing contraceptive care has not been widely adopted across medical specialties as a preventive health service that is routinely offered to eligible patients, such as mammography screening,” lead author Heidi D. Nelson, MD, of Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, Calif., said in an interview.
“Access to and coverage of contraceptive care are frequently challenged by legislation and insurance policies, and influential preventive services guideline groups, such as the U.S. Preventive Services Task Force, have not issued recommendations for contraceptive care,” Dr. Nelson said.
“The evidence to determine the benefits and harms of contraceptive care as a preventive health service has not been examined using methods similar to those used for other preventive services and clinicians may lack guidance on the effectiveness of contraception services relevant to their practices,” she added.
In a study published in Annals of Internal Medicine, Dr. Nelson and colleagues reviewed data from 38 randomized, controlled trials with a total of 25,472 participants. The trials evaluated the effectiveness of various types of contraceptive counseling and provision interventions beyond usual care on subsequent contraception use, compared with nonintervention comparison groups.
Overall, higher contraceptive use was associated with counseling interventions (risk ratio, 1.39), advance provision of emergency contraception (RR, 2.12), counseling or provision of emergency contraception postpartum (RR, 1.15), or counseling or provision of emergency contraception at the time of abortion (RR, 1.19), compared with usual care or active controls across studies.
Most of the included trials were not powered to distinguish intended versus unintended pregnancy rates, but pregnancy rates were lower among intervention groups, compared with controls.
Five of the selected studies assessed the potential negative effect of contraceptive counseling with regard to increased rates of STIs and two studies examined decreased condom use. However, neither STI rates nor condom use were significantly different between study participants who received various contraceptive counseling interventions (such as advanced provision of emergency contraception, clinician training, and individual counseling) and those who did not (RR, 1.05 and RR, 1.03, respectively).
“These results indicate that additional efforts to assist patients with their contraception decisions improve its subsequent use,” and are not surprising, said Dr. Nelson.
“All clinicians providing health care to women, not only clinicians providing reproductive health care specifically, need to recognize contraceptive care as an essential preventive health service and assume responsibility for delivering contraceptive counseling and provision services appropriate for each patient,” Dr. Nelson emphasized. “Clinicians lacking contraceptive care clinical skills may require additional training or refer their patients if needed to assure high quality care.”
The study findings were limited by several factors including the variability of interventions across studies and the lack of data on unintended pregnancy outcomes, the researchers noted. However, the results suggest that various contraceptive counseling and interventions beyond usual care increased contraceptive use with no reduction in condom use or increase in STIs, they wrote.
“Additional research should further evaluate approaches to contraceptive counseling and provision to determine best practices,” Dr. Nelson said in an interview. “This is particularly important for medically high-risk populations, those with limited access to care, and additional populations and settings that have not yet been studied, including transgender and nonbinary patients. Research is needed to refine measures of pregnancy intention and planning; and create uniform definitions of contraceptive care, interventions, measures of use, and outcomes.”.
Make easy, effective contraception accessible to all
The news of a potential overturn of the 1973 Roe v. Wade Supreme Court decision that protects a pregnant person’s ability to choose abortion “shines a bright light on the importance of promoting the use of contraception,” and on the findings of the current review, Christine Laine, MD, editor-in-chief of Annals of Internal Medicine, wrote in an accompanying editorial. “Easy, effective, accessible, and affordable contraception becomes increasingly essential as ending unintended pregnancy becomes increasingly difficult, unsafe, inaccessible, and legally risky.”
The available evidence showed the benefits of enhanced counseling, providing emergency contraception in advance, and providing contraceptive interventions immediately after delivery or pregnancy termination, she wrote. The findings have strong clinical implications, especially with regard to the Healthy People 2030 goal of reducing unintended pregnancy from the current 43% to 36.5%.
Dr. Laine called on internal medicine physicians in particular to recognize the negative health consequences of unintended pregnancy, and to consider contraceptive counseling part of their responsibility to their patients.
“To expand the numbers of people who receive this essential preventive service, we must systematically incorporate contraceptive counseling into health care with the same fervor that we devote to other preventive services. The health of our patients – and their families – depends on it,” she concluded.
The study was supported by the Resources Legacy Fund. The researchers had no financial conflicts to disclose. Dr. Laine had no financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
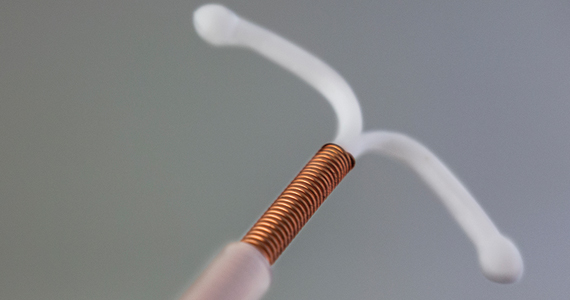
IUD cuts heavy menses in nulliparous patients with obesity
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
New phase 3 data support the use of the levonorgestrel 52-mg intrauterine device in nulliparous women with obesity and heavy menstrual bleeding. The findings, presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, showed a 97% reduction in blood loss 6 months after placement of the device, which is sold as the contraceptive Liletta by Medicines360 and AbbVie.
Experts say the results fill a gap in research because prior clinical trials of the IUD and a competitor, Mirena (Bayer), excluded significantly obese as well as nulliparous populations.
William Schlaff, MD, professor and chairman of the department of obstetrics & gynecology, Thomas Jefferson University, Philadelphia, said the absence of confirmatory evidence in these women has meant that, although use of the IUD has been “pretty widespread,” clinicians have been uncertain about the efficacy of the approach.
“Now we have objective data from a well-designed study that supports a practice that many of us have felt is probably a good one,” Dr. Schlaff, who was not involved in the new study, said in an interview.
Lead researcher Mitchell Creinin, MD, professor of obstetrics and gynecology at UC Davis Health, Sacramento, and colleagues at several centers across the country provided treatment with Liletta to 105 individuals with proven heavy menstrual bleeding. The patients’ median blood loss during two menses prior to placement of the device was 165 mL (range, 73-520 mL).
Participant demographics were: 65% White, 24% Black, 10% Hispanic, 4% Asian, and 7% who identified with other racial groups. Mean body mass index was 30.9 kg/m2, and 45% of individuals met the criteria for obesity (BMI > 30), including 13% who had a BMI of at least 40. Nearly 30% of participants in the study had never given birth and none had known medical, anatomic, infectious, or neoplastic causes of bleeding.
According to Dr. Creinin, 86 women were assessed 3 months after device placement, and their median blood loss at the time was 9.5 mL (interquartile range, 2.5-22.9 mL), representing a median 93% decrease from baseline. Median blood loss 6 months after placement of the IUD was 3.8 mL (IQR, 0-10.1 mL), a 97% reduction from baseline.
Regardless of parity or BMI, blood loss at 6 months was 97%-97.5% lower than baseline, Dr. Creinin reported.
Among the 23% of participants who did not complete the study, 4% experienced expulsions of the device, which Dr. Creinin said is a rate twice as high as that seen in women using hormone-releasing IUDs for contraception. However, he said it “is consistent with other studies among patients with quantitatively proven heavy menstrual bleeding.”
Another 6% of women who did not complete the study removed the device owing to bleeding and cramping complaints, 9% were lost to follow-up or withdrew consent, and 5% discontinued treatment for unspecified reasons, Dr. Creinin said.
“Etiologies for heavy menstrual bleeding may be different in the individuals we studied, so our findings provide assurance that these populations with heavy menstrual bleeding are equally well treated” with the IUD, Dr. Creinin said.
Dr. Creinin reported study funding from Medicines360. Dr. Schlaff reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ACOG 2022
Abortion politics lead to power struggles over family planning grants
BOZEMAN, Mont. – In a busy downtown coffee shop, a drawing of a ski lift with intrauterine devices for chairs draws the eyes of sleepy customers getting their morning underway with a caffeine jolt.
The flyer touts the services of Bridgercare, a nonprofit reproductive health clinic a few miles up the road. The clinic offers wellness exams, birth control, and LGBTQ+ services – and, starting in April, it oversees the state’s multimillion-dollar share of federal family planning program funding.
In March, Bridgercare beat out the state health department to become administrator of Montana’s $2.3 million Title X program, which helps pay for family planning and preventive health services. The organization applied for the grant because its leaders were concerned about a new state law that sought to restrict which local providers are funded.
What is happening in Montana is the latest example of an ongoing power struggle between nonprofits and conservative-leaning states over who receives federal family planning money. That has intensified in recent years as the Title X program has increasingly become entangled with the politics of abortion.
This year, the federal government set aside $257 million for family planning and preventive care. The providers that get that funding often serve families with low incomes, and Title X is one of the few federal programs in which people without legal permission to be in the United States can participate.
“The program permeates into communities that otherwise would be unreached by public health efforts,” said Rebecca Kreitzer, an associate professor of public policy at the University of North Carolina at Chapel Hill.
The Montana Department of Public Health and Human Services controlled the distribution of the state’s Title X funds for decades. Bridgercare sought the administrator role to circumvent a Republican-sponsored law passed last year that required the state to prioritize the money for local health departments and federally qualified health centers. That would have put the nonprofit – which doesn’t provide abortion procedures – and similar organizations at the bottom of the list. The law also banned clinics that perform abortions from receiving Title X funds from the state health department.
Bridgercare Executive Director Stephanie McDowell said the group applied for the grant to try to protect the program from decisions coming out of the state capitol. “Because of the politicization of Title X, we’re seeing how it’s run, swinging back and forth based on partisan leadership,” Ms. McDowell said.
A U.S. Department of Health & Human Services spokesperson, Tara Broido, didn’t answer a question about whether the agency intentionally awarded grants to nonprofits to avoid state politics. Instead, she said in a statement that applicants were evaluated in a competitive process by a panel of independent reviewers based on criteria to deliver high-quality, client-centered services.
Federal law prohibits the money from being used to perform abortions. But it can cover other services provided by groups that offer abortions – the largest and best-known by far is Planned Parenthood. In recent years, conservative politicians have tried to keep such providers from receiving Title X funding.
In some cases, contraception has entered the debate around which family planning services government should help fund. Some abortion opponents have raised concerns that long-lasting forms of birth control, such as IUDs, lead to abortions. Those claims are disputed by reproductive health experts.
In 2019, the Trump administration introduced several new rules for Title X, including disqualifying from receiving the funding family planning clinics that also offered abortion services or referrals. Many clinics across the nation left the program instead of conforming to the rules. Simultaneously, the spread of COVID-19 interrupted routine care. The number of patients served by Title X plummeted.
The Biden administration reversed most of those rules, including allowing providers with abortion services back into the Title X program. States also try to influence the funding’s reach, either through legislation or budget rules.
The current Title X funding cycle is 5 years, and the amount of money available each year could shift based on the state’s network of providers or federal budget changes. Jon Ebelt, a spokesperson for the Montana Department of Public Health and Human Services, didn’t answer when asked whether the state planned to reapply to administer the funding in 2027. He said the department was disappointed with the Biden administration’s “refusal” to renew the state’s funding.
“We recognize, however, that recent proabortion federal rule changes have distorted Title X and conflict with Montana law,” he said.
Conservative states have been tangling with nonprofits and the federal government over Title X funding for more than a decade. In 2011, during the Obama administration, Texas whittled down the state’s family planning spending and prioritized sending the federal money to general primary care providers over reproductive health clinics. As a result, 25% of family planning clinics in Texas closed. In 2013, a nonprofit now called Every Body Texas joined the competition to distribute the state’s Title X dollars and won.
“Filling and rebuilding those holes have taken this last decade, essentially,” said Berna Mason, director of service delivery improvement for Every Body Texas.
In 2019, the governor of Nebraska proposed a budget that would have prohibited the money from going to any organization that provided abortions or referred patients for abortions outside of an emergency. It also would have required that funding recipients be legally and financially separate from such clinics, a restriction that would have gone further than the Trump administration’s rules. Afterward, a family planning council won the right to administer Title X money.
In 2017, the nonprofit Arizona Family Health Partnership lost its status as that state’s only Title X administrator when the state health department was given 25% of the funding to deliver to providers. That came after Arizona lawmakers ordered the department to apply for the funds and distribute them first to state- or county-owned clinics, with the remaining money going to primary care facilities. The change was backed by groups that were opposed to abortion, and reproductive health care providers saw it as an attempt to weaken clinics that offer abortion services.
However, the state left nearly all the money it received untouched, and although it’s still required by law to apply for Title X funding, it hasn’t received a portion of the grant since.
Bré Thomas, CEO of Arizona Family Health Partnership, said that, even though the nonprofit is the sole administrator of the Title X funding again, the threat remains that some or all could be taken away because of politics. “We’re at the will of who’s in charge,” Ms. Thomas said.
Nonprofits say they have an advantage over state agencies in expanding services because they have more flexibility in fundraising and fewer administrative hurdles.
In April, Mississippi nonprofit Converge took over administration of Title X funds, a role the state had held for decades. The organization’s founders said they weren’t worried that conservative politicians would restrict access to services but simply believed they could do a better job. “Service quality was very low, and it was very hard to get appointments,” said cofounder Danielle Lampton.
A Mississippi State Department of Health spokesperson, Liz Sharlot, said the agency looks forward to working with Converge.
In Montana, Bridgercare plans to restore funding to Planned Parenthood clinics that have been cut off from the program since 2019, recruit more health centers to participate, and expand the program’s reach in rural, frontier, and tribal communities using telehealth services, Ms. McDowell said.
The organization’s goal is to increase the number of patients benefiting from the federal program by at least 10% in each year of the 5-year grant cycle. The clinic also plans to apply to keep its Title X role beyond this grant.
“In 5 years, our grant application should be a clear front-runner for funding,” she said. “It’s less about ‘How do we beat someone in 5 years?’ And more about ‘How do we grow this program to serve patients?’”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
BOZEMAN, Mont. – In a busy downtown coffee shop, a drawing of a ski lift with intrauterine devices for chairs draws the eyes of sleepy customers getting their morning underway with a caffeine jolt.
The flyer touts the services of Bridgercare, a nonprofit reproductive health clinic a few miles up the road. The clinic offers wellness exams, birth control, and LGBTQ+ services – and, starting in April, it oversees the state’s multimillion-dollar share of federal family planning program funding.
In March, Bridgercare beat out the state health department to become administrator of Montana’s $2.3 million Title X program, which helps pay for family planning and preventive health services. The organization applied for the grant because its leaders were concerned about a new state law that sought to restrict which local providers are funded.
What is happening in Montana is the latest example of an ongoing power struggle between nonprofits and conservative-leaning states over who receives federal family planning money. That has intensified in recent years as the Title X program has increasingly become entangled with the politics of abortion.
This year, the federal government set aside $257 million for family planning and preventive care. The providers that get that funding often serve families with low incomes, and Title X is one of the few federal programs in which people without legal permission to be in the United States can participate.
“The program permeates into communities that otherwise would be unreached by public health efforts,” said Rebecca Kreitzer, an associate professor of public policy at the University of North Carolina at Chapel Hill.
The Montana Department of Public Health and Human Services controlled the distribution of the state’s Title X funds for decades. Bridgercare sought the administrator role to circumvent a Republican-sponsored law passed last year that required the state to prioritize the money for local health departments and federally qualified health centers. That would have put the nonprofit – which doesn’t provide abortion procedures – and similar organizations at the bottom of the list. The law also banned clinics that perform abortions from receiving Title X funds from the state health department.
Bridgercare Executive Director Stephanie McDowell said the group applied for the grant to try to protect the program from decisions coming out of the state capitol. “Because of the politicization of Title X, we’re seeing how it’s run, swinging back and forth based on partisan leadership,” Ms. McDowell said.
A U.S. Department of Health & Human Services spokesperson, Tara Broido, didn’t answer a question about whether the agency intentionally awarded grants to nonprofits to avoid state politics. Instead, she said in a statement that applicants were evaluated in a competitive process by a panel of independent reviewers based on criteria to deliver high-quality, client-centered services.
Federal law prohibits the money from being used to perform abortions. But it can cover other services provided by groups that offer abortions – the largest and best-known by far is Planned Parenthood. In recent years, conservative politicians have tried to keep such providers from receiving Title X funding.
In some cases, contraception has entered the debate around which family planning services government should help fund. Some abortion opponents have raised concerns that long-lasting forms of birth control, such as IUDs, lead to abortions. Those claims are disputed by reproductive health experts.
In 2019, the Trump administration introduced several new rules for Title X, including disqualifying from receiving the funding family planning clinics that also offered abortion services or referrals. Many clinics across the nation left the program instead of conforming to the rules. Simultaneously, the spread of COVID-19 interrupted routine care. The number of patients served by Title X plummeted.
The Biden administration reversed most of those rules, including allowing providers with abortion services back into the Title X program. States also try to influence the funding’s reach, either through legislation or budget rules.
The current Title X funding cycle is 5 years, and the amount of money available each year could shift based on the state’s network of providers or federal budget changes. Jon Ebelt, a spokesperson for the Montana Department of Public Health and Human Services, didn’t answer when asked whether the state planned to reapply to administer the funding in 2027. He said the department was disappointed with the Biden administration’s “refusal” to renew the state’s funding.
“We recognize, however, that recent proabortion federal rule changes have distorted Title X and conflict with Montana law,” he said.
Conservative states have been tangling with nonprofits and the federal government over Title X funding for more than a decade. In 2011, during the Obama administration, Texas whittled down the state’s family planning spending and prioritized sending the federal money to general primary care providers over reproductive health clinics. As a result, 25% of family planning clinics in Texas closed. In 2013, a nonprofit now called Every Body Texas joined the competition to distribute the state’s Title X dollars and won.
“Filling and rebuilding those holes have taken this last decade, essentially,” said Berna Mason, director of service delivery improvement for Every Body Texas.
In 2019, the governor of Nebraska proposed a budget that would have prohibited the money from going to any organization that provided abortions or referred patients for abortions outside of an emergency. It also would have required that funding recipients be legally and financially separate from such clinics, a restriction that would have gone further than the Trump administration’s rules. Afterward, a family planning council won the right to administer Title X money.
In 2017, the nonprofit Arizona Family Health Partnership lost its status as that state’s only Title X administrator when the state health department was given 25% of the funding to deliver to providers. That came after Arizona lawmakers ordered the department to apply for the funds and distribute them first to state- or county-owned clinics, with the remaining money going to primary care facilities. The change was backed by groups that were opposed to abortion, and reproductive health care providers saw it as an attempt to weaken clinics that offer abortion services.
However, the state left nearly all the money it received untouched, and although it’s still required by law to apply for Title X funding, it hasn’t received a portion of the grant since.
Bré Thomas, CEO of Arizona Family Health Partnership, said that, even though the nonprofit is the sole administrator of the Title X funding again, the threat remains that some or all could be taken away because of politics. “We’re at the will of who’s in charge,” Ms. Thomas said.
Nonprofits say they have an advantage over state agencies in expanding services because they have more flexibility in fundraising and fewer administrative hurdles.
In April, Mississippi nonprofit Converge took over administration of Title X funds, a role the state had held for decades. The organization’s founders said they weren’t worried that conservative politicians would restrict access to services but simply believed they could do a better job. “Service quality was very low, and it was very hard to get appointments,” said cofounder Danielle Lampton.
A Mississippi State Department of Health spokesperson, Liz Sharlot, said the agency looks forward to working with Converge.
In Montana, Bridgercare plans to restore funding to Planned Parenthood clinics that have been cut off from the program since 2019, recruit more health centers to participate, and expand the program’s reach in rural, frontier, and tribal communities using telehealth services, Ms. McDowell said.
The organization’s goal is to increase the number of patients benefiting from the federal program by at least 10% in each year of the 5-year grant cycle. The clinic also plans to apply to keep its Title X role beyond this grant.
“In 5 years, our grant application should be a clear front-runner for funding,” she said. “It’s less about ‘How do we beat someone in 5 years?’ And more about ‘How do we grow this program to serve patients?’”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
BOZEMAN, Mont. – In a busy downtown coffee shop, a drawing of a ski lift with intrauterine devices for chairs draws the eyes of sleepy customers getting their morning underway with a caffeine jolt.
The flyer touts the services of Bridgercare, a nonprofit reproductive health clinic a few miles up the road. The clinic offers wellness exams, birth control, and LGBTQ+ services – and, starting in April, it oversees the state’s multimillion-dollar share of federal family planning program funding.
In March, Bridgercare beat out the state health department to become administrator of Montana’s $2.3 million Title X program, which helps pay for family planning and preventive health services. The organization applied for the grant because its leaders were concerned about a new state law that sought to restrict which local providers are funded.
What is happening in Montana is the latest example of an ongoing power struggle between nonprofits and conservative-leaning states over who receives federal family planning money. That has intensified in recent years as the Title X program has increasingly become entangled with the politics of abortion.
This year, the federal government set aside $257 million for family planning and preventive care. The providers that get that funding often serve families with low incomes, and Title X is one of the few federal programs in which people without legal permission to be in the United States can participate.
“The program permeates into communities that otherwise would be unreached by public health efforts,” said Rebecca Kreitzer, an associate professor of public policy at the University of North Carolina at Chapel Hill.
The Montana Department of Public Health and Human Services controlled the distribution of the state’s Title X funds for decades. Bridgercare sought the administrator role to circumvent a Republican-sponsored law passed last year that required the state to prioritize the money for local health departments and federally qualified health centers. That would have put the nonprofit – which doesn’t provide abortion procedures – and similar organizations at the bottom of the list. The law also banned clinics that perform abortions from receiving Title X funds from the state health department.
Bridgercare Executive Director Stephanie McDowell said the group applied for the grant to try to protect the program from decisions coming out of the state capitol. “Because of the politicization of Title X, we’re seeing how it’s run, swinging back and forth based on partisan leadership,” Ms. McDowell said.
A U.S. Department of Health & Human Services spokesperson, Tara Broido, didn’t answer a question about whether the agency intentionally awarded grants to nonprofits to avoid state politics. Instead, she said in a statement that applicants were evaluated in a competitive process by a panel of independent reviewers based on criteria to deliver high-quality, client-centered services.
Federal law prohibits the money from being used to perform abortions. But it can cover other services provided by groups that offer abortions – the largest and best-known by far is Planned Parenthood. In recent years, conservative politicians have tried to keep such providers from receiving Title X funding.
In some cases, contraception has entered the debate around which family planning services government should help fund. Some abortion opponents have raised concerns that long-lasting forms of birth control, such as IUDs, lead to abortions. Those claims are disputed by reproductive health experts.
In 2019, the Trump administration introduced several new rules for Title X, including disqualifying from receiving the funding family planning clinics that also offered abortion services or referrals. Many clinics across the nation left the program instead of conforming to the rules. Simultaneously, the spread of COVID-19 interrupted routine care. The number of patients served by Title X plummeted.
The Biden administration reversed most of those rules, including allowing providers with abortion services back into the Title X program. States also try to influence the funding’s reach, either through legislation or budget rules.
The current Title X funding cycle is 5 years, and the amount of money available each year could shift based on the state’s network of providers or federal budget changes. Jon Ebelt, a spokesperson for the Montana Department of Public Health and Human Services, didn’t answer when asked whether the state planned to reapply to administer the funding in 2027. He said the department was disappointed with the Biden administration’s “refusal” to renew the state’s funding.
“We recognize, however, that recent proabortion federal rule changes have distorted Title X and conflict with Montana law,” he said.
Conservative states have been tangling with nonprofits and the federal government over Title X funding for more than a decade. In 2011, during the Obama administration, Texas whittled down the state’s family planning spending and prioritized sending the federal money to general primary care providers over reproductive health clinics. As a result, 25% of family planning clinics in Texas closed. In 2013, a nonprofit now called Every Body Texas joined the competition to distribute the state’s Title X dollars and won.
“Filling and rebuilding those holes have taken this last decade, essentially,” said Berna Mason, director of service delivery improvement for Every Body Texas.
In 2019, the governor of Nebraska proposed a budget that would have prohibited the money from going to any organization that provided abortions or referred patients for abortions outside of an emergency. It also would have required that funding recipients be legally and financially separate from such clinics, a restriction that would have gone further than the Trump administration’s rules. Afterward, a family planning council won the right to administer Title X money.
In 2017, the nonprofit Arizona Family Health Partnership lost its status as that state’s only Title X administrator when the state health department was given 25% of the funding to deliver to providers. That came after Arizona lawmakers ordered the department to apply for the funds and distribute them first to state- or county-owned clinics, with the remaining money going to primary care facilities. The change was backed by groups that were opposed to abortion, and reproductive health care providers saw it as an attempt to weaken clinics that offer abortion services.
However, the state left nearly all the money it received untouched, and although it’s still required by law to apply for Title X funding, it hasn’t received a portion of the grant since.
Bré Thomas, CEO of Arizona Family Health Partnership, said that, even though the nonprofit is the sole administrator of the Title X funding again, the threat remains that some or all could be taken away because of politics. “We’re at the will of who’s in charge,” Ms. Thomas said.
Nonprofits say they have an advantage over state agencies in expanding services because they have more flexibility in fundraising and fewer administrative hurdles.
In April, Mississippi nonprofit Converge took over administration of Title X funds, a role the state had held for decades. The organization’s founders said they weren’t worried that conservative politicians would restrict access to services but simply believed they could do a better job. “Service quality was very low, and it was very hard to get appointments,” said cofounder Danielle Lampton.
A Mississippi State Department of Health spokesperson, Liz Sharlot, said the agency looks forward to working with Converge.
In Montana, Bridgercare plans to restore funding to Planned Parenthood clinics that have been cut off from the program since 2019, recruit more health centers to participate, and expand the program’s reach in rural, frontier, and tribal communities using telehealth services, Ms. McDowell said.
The organization’s goal is to increase the number of patients benefiting from the federal program by at least 10% in each year of the 5-year grant cycle. The clinic also plans to apply to keep its Title X role beyond this grant.
“In 5 years, our grant application should be a clear front-runner for funding,” she said. “It’s less about ‘How do we beat someone in 5 years?’ And more about ‘How do we grow this program to serve patients?’”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Drug combo holds promise as on-demand contraceptive: Study
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
FROM BMJ SEXUAL & REPRODUCTIVE HEALTH
How effective are sterilization procedures? Study raises questions
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
Women opt for sterilization for a variety of reasons, but the goal is the same: to avoid getting pregnant.
But a head-to-head study of two forms of female sterilization has found surprisingly high rates of failure with the procedures.
The study compared the effectiveness of hysteroscopic sterilization, a nonincisional procedure, and minimally invasive laparoscopic sterilization. Although both methods prevented pregnancy in the vast majority of women, each was associated with more than a 6% failure rate 5 years after the procedure.
That figure is “much higher than expected,” said Aileen Gariepy, MD, MPH, the director of complex family planning at Weill Cornell Medicine, New York, who led the study.
The American College of Obstetricians and Gynecologists reported that the chance of pregnancy after sterilization is less than 1%, Dr. Gariepy said. “Women and pregnancy-capable people considering sterilization should be informed that, after the procedure, they have at least a 6% – not 1% – chance of pregnancy in the next 5 years.”
The study was published in Fertility and Sterility.
For laparoscopic sterilization, surgeons close or sever the fallopian tubes to prevent eggs from reaching the uterus and becoming fertilized.
Hysteroscopic sterilization involves the implantation of small, flexible metal coils into each fallopian tube, a process that produces inflammation and scarring that in turn prevents pregnancy. This method, called Essure and formerly marketed by Bayer, received approval by the Food and Drug Administration in 2002. But the agency received thousands of reports of adverse events with Essure, prompting regulators in 2016 to add a boxed warning to the product label about the risk for adverse events, including perforation, migration of the coils, allergic reactions, and pain.
Bayer pulled Essure from the market in 2019, citing decreased sales of the product. Some women did not have the device removed, however, and questions remain about its effectiveness, according to the researchers.
In the new study, Dr. Gariepy and colleagues examined Medicaid claims for 5906 hysteroscopic and 23,965 laparoscopic sterilizations performed in California between 2008 and 2014. They excluded sterilizations that were performed immediately after delivery, which involve a different approach.
The average age of the women in the study was 33 years.
The study found that, 5 years after the sterilization procedure, 6% of women in either group had become pregnant.
Despite the surprising new data, Chailee Moss, MD, an assistant professor of gynecology and obstetrics at Johns Hopkins University Medical Center, Baltimore, said she did not think the study would significantly affect the way she counsels her patients.
The main reason, she said, is that the study relied on an analysis of medical claims, which “is likely inferior to careful review of individual patient records or prospective collection of clinical data.” Home pregnancy tests may easily be excluded from such data and that patients can undergo ultrasound and termination procedures that would likely not be included in the data the researchers analyzed.
Dr. Moss added that the study was limited to California and that the researchers could not determine pregnancy rates for women who moved out of the state and thus received pregnancy care elsewhere. Nor did the authors account for the use of assistive reproductive technology, which can facilitate pregnancy after sterilization despite the success of the original procedure.
Dr. Gariepy, however, said the study may in fact have undercounted pregnancies and that the failure rates might be even higher than 6%, noting that California is “one of the largest, most populous and most diverse states” in terms of race, ethnicity, and other factors, making the new findings highly generalizable.
“I agree that study results should be confirmed by new nationwide study to determine risk of pregnancy after different sterilization methods,” she said. “Nevertheless, this retrospective cohort study delivers a strong signal that doctors and patients need to know about.”
Dr. Gariepy is on the board of directors of the Society of Family Planning. Dr. Moss has received research funding from Merck.
A version of this article first appeared on Medscape.com.
FROM FERTILITY AND STERILITY
Contraception for women taking enzyme-inducing antiepileptics
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
Topiramate, introduced as an antiepileptic drug (AED), is currently most widely used for prevention of migraine headaches.
Because reproductive-aged women represent a population in which migraines are prevalent, clinicians need guidance to help women taking topiramate make sound contraceptive choices.
Several issues are relevant here. First, women who have migraines with aura should avoid estrogen-containing contraceptive pills, patches, and rings. Instead, progestin-only methods, including the contraceptive implant, may be recommended to patients with migraines.
Second, because topiramate, as with a number of other AEDs, is a teratogen, women using this medication need highly effective contraception. This consideration may also lead clinicians to recommend use of the implant in women with migraines.
Finally, topiramate, along with other AEDs (phenytoin, carbamazepine, barbiturates, primidone, and oxcarbazepine) induces hepatic enzymes, which results in reduced serum contraceptive steroid levels.
Because there is uncertainty regarding the degree to which the use of topiramate reduces serum levels of etonogestrel (the progestin released by the implant), investigators performed a prospective study to assess the pharmacokinetic impact of topiramate in women with the implant.
Ongoing users of contraceptive implants who agreed to use additional nonhormonal contraception were recruited to a 6-week study, during which they took topiramate and periodically had blood drawn.
Overall, use of topiramate was found to lower serum etonogestrel levels from baseline on a dose-related basis. At study completion, almost one-third of study participants were found to have serum progestin levels lower than the threshold associated with predictable ovulation suppression.
The results of this carefully conducted study support guidance from the Centers for Disease Control and Prevention that women seeking contraception and using topiramate or other enzyme-inducing AEDs should be encouraged to use intrauterine devices or injectable contraception. The contraceptive efficacy of these latter methods is not diminished by concomitant use of enzyme inducers.
I am Andrew Kaunitz. Please take care of yourself and each other.
Any views expressed above are the author’s own and do not necessarily reflect the views of WebMD or Medscape.
Andrew M. Kaunitz is a professor and Associate Chairman, department of obstetrics and gynecology, University of Florida, Jacksonville.
A version of this article first appeared on Medscape.com.
How common is IUD perforation, expulsion, and malposition?
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
The medicated intrauterine devices (IUDs), including the levonorgestrel-releasing IUD (LNG-IUD) (Mirena, Kyleena, Skyla, and Liletta) and the copper IUD (Cu-IUD; Paragard), are remarkably effective contraceptives. For the 52-mg LNG-IUD (Mirena, Liletta) the pregnancy rate over 6 years of use averaged less than 0.2% per year.1,2 For the Cu-IUD, the pregnancy rate over 10 years of use averaged 0.5% per year for the first 3 years of use and 0.2% per year over the following 7 years of use.3 IUD perforation of the uterus, expulsion, and malposition are recognized complications of IUD use. Our understanding of the prevalence and management of malpositioned IUDs is evolving and the main focus of this editorial.
Complete and partial uterus perforation
A complete uterine perforation occurs when the entire IUD is outside the walls of the uterus. A partial uterine perforation occurs when the IUD is outside the uterine cavity, but a portion of the IUD remains in the myometrium. When uterine perforation is suspected, ultrasound can determine if the IUD is properly sited within the uterus. If ultrasonography does not detect the IUD within the uterus, an x-ray of the pelvis and abdomen should be obtained to determine if the IUD is in the peritoneal cavity. If both an ultrasound and a pelvic-abdominal x-ray do not detect the IUD, the IUD was probably expelled from the patient.
Uterine perforation is uncommon and occurs once in every 500 to 1,000 insertions in non-breastfeeding women.4-8 The most common symptoms reported by patients with a perforated IUD are pain and/or bleeding.8 Investigators in the European Active Surveillance Study on Intrauterine Devices (EURAS) enrolled more than 60,000 patients who had an IUD insertion and followed them for 12 months with more than 39,000 followed for up to 60 months.7,8 The uterine perforation rate per 1,000 IUD insertions in non-breastfeeding women with 60 months of follow-up was 1.6 for the LNG-IUD and 0.8 for the Cu-IUD.8 The rate of uterine perforation was much higher in women who are breastfeeding or recently postpartum. In the EURAS study after 60 months of follow-up, the perforation rate per 1,000 insertions among breastfeeding women was 7.9 for the LNG-IUS and 4.7 for the Cu-IUD.8
Remarkably very few IUD perforations were detected at the time of insertion, including only 2% of the LNG-IUD insertions and 17% of the Cu-IUD insertions.8 Many perforations were not detected until more than 12 months following insertion, including 32% of the LNG-IUD insertions and 22% of the Cu-IUD insertions.8 Obviously, an IUD that has completely perforated the uterus and resides in the peritoneal cavity is not an effective contraceptive. For some patients, the IUD perforation was initially diagnosed after they became pregnant, and imaging studies to locate the IUD and assess the pregnancy were initiated. Complete perforation is usually treated with laparoscopy to remove the IUD and reduce the risk of injury to intra-abdominal organs.
Patients with an IUD partial perforation may present with pelvic pain or abnormal uterine bleeding.9 An ultrasound study to explore the cause of the presenting symptom may detect the partial perforation. It is estimated that approximately 20% of cases of IUD perforation are partial perforation.9 Over time, a partial perforation may progress to a complete perforation. In some cases of partial perforation, the IUD string may still be visible in the cervix, and the IUD may be removed by pulling on the strings.8 Hysteroscopy and/or laparoscopy may be needed to remove a partially perforated IUD. Following a partial or complete IUD perforation, if the patient desires to continue with IUD contraception, it would be wise to insert a new IUD under ultrasound guidance or assess proper placement with a postplacement ultrasound.
Continue to: Expulsion...
Expulsion
IUD expulsion occurs in approximately 3% to 11% of patients.10-13 The age of the patient influences the rate of expulsion. In a study of 2,748 patients with a Cu-IUD, the rate of expulsion by age for patients <20 years, 20–24 years, 25–29 years, 30–34 years, and ≥35 years was 8.2%, 3.2%, 3.0%, 2.3%, and 1.8%, respectively.10 In this study, age did not influence the rate of IUD removal for pelvic pain or abnormal bleeding, which was 4% to 5% across all age groups.10 In a study of 5,403 patients with an IUD, the rate of IUD expulsion by age for patients <20 years, 20–29 years, and 30–45 years was 14.6%, 7.3%, and 7.2%, respectively.12 In this study, the 3-year cumulative rate of expulsion was 10.2%.12 There was no statistically significant difference in the 3-year cumulative rate of expulsion for the 52-mg LNG-IUD (10.1%) and Cu-IUD (10.7%).12
The majority of patients who have an IUD expulsion recognize the event and seek additional contraception care. A few patients first recognize the IUD expulsion when they become pregnant, and imaging studies detect no IUD in the uterus or the peritoneal cavity. In a study of more than 17,000 patients using an LNG-IUD, 108 pregnancies were reported. Seven pregnancies occurred in patients who did not realize their IUD was expelled.14 Patients who have had an IUD expulsion and receive a new IUD are at increased risk for re-expulsion. For these patients, reinsertion of an IUD could be performed under ultrasound guidance to ensure and document optimal initial IUD position within the uterus, or ultrasound can be obtained postinsertion to document appropriate IUD position.
Malposition—prevalence and management
Our understanding of the prevalence and management of a malpositioned IUD is evolving. For the purposes of this discussion a malpositioned IUD is defined as being in the uterus, but not properly positioned within the uterine cavity. Perforation into the peritoneal cavity and complete expulsion of an IUD are considered separate entities. However, a malpositioned IUD within the uterus may eventually perforate the uterus or be expelled from the body. For example, an IUD embedded in the uterine wall may eventually work its way through the wall and become perforated, residing in the peritoneal cavity. An IUD with the stem in the cervix below the internal os may eventually be expelled from the uterus and leave the body through the vagina.
High-quality ultrasonography, including 2-dimensional (2-D) ultrasound with videoclips or 3-dimensional (3-D) ultrasound with coronal views, has greatly advanced our understanding of the prevalence and characteristics of a malpositioned IUD.15-18 Ultrasound features of an IUD correctly placed within the uterus include:
- the IUD is in the uterus
- the shaft is in the midline of the uterine cavity
- the shaft of the IUD is not in the endocervix
- the IUD arms are at a 90-degree angle from the shaft
- the top of the IUD is within 2 cm of the fundus
- the IUD is not rotated outside of the cornual plane, inverted or transverse.
Ultrasound imaging has identified multiple types of malpositioned IUDs, including:
- IUD embedded in the myometrium—a portion of the IUD is embedded in the uterine wall
- low-lying IUD—the IUD is low in the uterine cavity but not in the endocervix
- IUD in the endocervix—the stem is in the endocervical canal
- rotated—the IUD is rotated outside the cornual plane
- malpositioned arms—the arms are not at a 90-degree angle to the stem
- the IUD is inverted, transverse, or laterally displaced.
IUD malposition is highly prevalent and has been identified in 10% to 20% of convenience cohorts in which an ultrasound study was performed.15-18
Benacerraf, Shipp, and Bromley were among the first experts to use ultrasound to detect the high prevalence of malpositioned IUDs among a convenience sample of 167 patients with an IUD undergoing ultrasound for a variety of indications. Using 3-D ultrasound, including reconstructed coronal views, they identified 28 patients (17%) with a malpositioned IUD based on the detection of the IUD “poking into the substance of the uterus or cervix.” Among the patients with a malpositioned IUD, the principal indication for the ultrasound study was pelvic pain (39%) or abnormal uterine bleeding (36%). Among women with a normally sited IUD, pelvic pain (19%) or abnormal uterine bleeding (15%) were less often the principal indication for the ultrasound.15 The malpositioned IUD was removed in 21 of the 28 cases and the symptoms of pelvic pain or abnormal bleeding resolved in 20 of the 21 patients.15
Other investigators have confirmed the observation that IUD malposition is common.16-18 In a retrospective study of 1,748 pelvic ultrasounds performed for any indication where an IUD was present, after excluding 13 patients who were determined to have expelled their IUD (13) and 13 patients with a perforated IUD, 156 patients (8.9%) were diagnosed as having a malpositioned IUD.16 IUD malposition was diagnosed when the IUD was in the uterus but positioned in the lower uterine segment, cervix, rotated or embedded in the uterus. An IUD in the lower uterine segment or cervix was detected in 133 patients, representing 85% of cases. Among these cases, 29 IUDs were also embedded and/or rotated, indicating that some IUDs have multiple causes of the malposition. Twenty-one IUDs were near the fundus but embedded and/or rotated. Controls with a normally-sited IUD were selected for comparison to the case group. Among IUD users, the identification of suspected adenomyosis on the ultrasound was associated with an increased risk of IUD malposition (odds ratio [OR], 3.04; 95% confidence interval [CI], 1.08-8.52).16 In this study, removal of a malpositioned LNG-IUD, without initiating a highly reliable contraceptive was associated with an increased risk of pregnancy. It is important to initiate a highly reliable form of contraception if the plan is to remove a malpositioned IUD.16,19
In a study of 1,253 pelvic ultrasounds performed for any indication where an IUD was identified in the uterus, 263 IUDs (19%) were determined to be malpositioned.17 In this study the location of the malpositioned IUDs included17:
- the lower uterine segment not extending into the cervix (38%)
- in the lower uterine segment extending into the cervix (22%)
- in the cervix (26%)
- rotated axis of the IUD (12%)
- other (2%).
Among the 236 malpositioned IUDs, 24% appeared to be embedded in the uterine wall.17 Compared with patients with a normally-sited IUD on ultrasound, patients with a malpositioned IUD more frequently reported vaginal bleeding (30% vs 19%; P<.005) and pelvic pain (43% vs 30%; P<.002), similar to the findings in the Benacerraf et al. study.14
Connolly and Fox18 designed an innovative study to determine the rate of malpositioned IUDs using 2-D ultrasound to ensure proper IUD placement at the time of insertion with a follow-up 3-D ultrasound 8 weeks after insertion to assess IUD position within the uterus. At the 8-week 3-D ultrasound, among 763 women, 16.6% of the IUDs were malpositioned.18 In this study, IUD position was determined to be correct if all the following features were identified:
- the IUD shaft was in the midline of the uterine cavity
- the IUD arms were at 90 degrees from the stem
- the top of the IUD was within 3 to 4 mm of the fundus
- the IUD was not rotated, inverted or transverse.
IUD malpositions were categorized as:
- embedded in the uterine wall
- low in the uterine cavity
- in the endocervical canal
- misaligned
- perforated
- expulsed.
At the 8-week follow-up, 636 patients (83.4%) had an IUD that was correctly positioned.18 In 127 patients (16.6%) IUD malposition was identified, with some patients having more than one type of malposition. The types of malposition identified were:
- embedded in the myometrium (54%)
- misaligned, including rotated, laterally displaced, inverted, transverse or arms not deployed (47%)
- low in the uterine cavity (39%)
- in the endocervical canal (14%)
- perforated (3%)
- expulsion (0%).
Recall that all of these patients had a 2-D ultrasound at the time of insertion that identified the IUD as correctly placed. This suggests that during the 8 weeks following IUD placement there were changes in the location of the IUD or that 2-D ultrasound has lower sensitivity than 3-D ultrasound to detect malposition. Of note, at the 8-week follow-up, bleeding or pain was reported by 36% of the patients with a malpositioned IUD and 20% of patients with a correctly positioned IUD.17 Sixty-seven of the 127 malpositioned IUDs “required” removal, but the precise reasons for the removals were not delineated. The investigators concluded that 3-D ultrasonography is useful for the detection of IUD malposition and could be considered as part of ongoing IUD care, if symptoms of pain or bleeding occur.18
Continue to: IUD malposition following postplacental insertion...
IUD malposition following postplacental insertion
IUD malposition is common in patients who have had a postplacental insertion. Ultrasound imaging plays an important role in detecting IUD expulsion and malposition in these cases. Postplacental IUD insertion is defined as the placement of an IUD within 10 minutes following delivery of the placenta. Postplacental IUD insertion can be performed following a vaginal or cesarean birth and with a Cu-IUD or LNG-IUD. The good news is that postplacental IUD insertion reduces the risk of unplanned pregnancy in the years following birth. However, postplacental IUD insertion is associated with a high rate of IUD malposition.
In a study of 162 patients who had postplacental insertion of a Cu-IUD following a vaginal birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 8%, partial expulsion in 16%, and malposition in 15%.20 The IUD was correctly sited in 56% of patients. Seven patients (4%) had the IUD removed, and 1 patient had a perforated IUD. Among the 25 malpositioned IUDs, 14 were not within 1 cm of the fundus, and 11 were rotated outside of the axis of the cornuas. In this study partial expulsion was defined as an IUD protruding from the external cervical os on physical exam or demonstration of the distal tip of the IUD below the internal os of the cervix on ultrasound. Malposition was defined as an IUD that was >1 cm from the fundus or in an abnormal location or axis, but not partially expelled.
In a study of 69 patients who had postplacental insertion of a Cu-IUD following a cesarean birth, ultrasound and physical examination at 6 months demonstrated complete IUD expulsion in 3%, partial expulsion (stem in the cervix below the internal os) in 4% and malposition in 30%.20 The IUD was correctly positioned in 59% of the patients.21 The IUD had been electively removed in 3%. Among the 21 patients with a malpositioned IUD, 10 were rotated within the uterine cavity, 6 were inverted (upside down), 3 were low-lying, and 2 were transverse.21 Given the relatively high rate of IUD malposition following postplacental insertion, it may be useful to perform a pelvic ultrasound at a postpartum visit to assess the location of the IUD, if ultrasonography is available.
Management of the malpositioned IUD
There are no consensus guidelines on how to care for a patient with a malpositioned IUD. Clinicians need to use their best judgment and engage the patient in joint decision making when managing a malpositioned IUD. When an IUD is malpositioned and the patient has bothersome symptoms of pelvic pain or abnormal bleeding that have not responded to standard interventions, consideration may be given to a remove and replace strategy. When the stem of the IUD is below the level of the internal os on ultrasound or visible at the external os on physical examination, consideration should be given to removing and replacing the IUD. However, if the IUD is removed without replacement or the initiation of a highly reliable contraceptive, the risk of unplanned pregnancy is considerable.16,19
IUD totally or partially within the cervix or low-lying. When an IUD is in the cervix, the contraceptive efficacy of the IUD may be diminished, especially with a Cu-IUD.22 In these cases, removing and replacing the IUD is an option. In a survey of 20 expert clinicians, >80% recommended replacing an IUD that was totally or partially in the cervical canal.23 But most of the experts would not replace an IUD that was incidentally noted on ultrasound to be low-lying, being positioned more than 2 cm below the fundus, with no portion of the IUD in the cervical canal. In the same survey, for patients with a low-lying IUD and pelvic pain or bleeding, the majority of experts reported that they would explore other causes of bleeding and pelvic pain not related to the IUD itself and not replace the IUD, but 30% of the experts reported that they would remove and replace the device.23
IUD embedded in the myometrium with pelvic pain. Based on my clinical experience, when a patient has persistent pelvic pain following the insertion of an IUD and the pain does not resolve with standard measures including medication, an ultrasound study is warranted to assess the position of the IUD. If the ultrasound demonstrates that an arm of the IUD is embedded in the myometrium, removal of the IUD may be associated with resolution of the pain. Reinsertion of an IUD under ultrasound guidance may result in a correctly-sited IUD with no recurrence of pelvic pain.
IUD rotated within the uterus with no pain or abnormal bleeding. For an IUD that is near the fundus and rotated on its axis within the uterus, if the patient has no symptoms of pain or abnormal bleeding, my recommendation to the patient would be to leave the device in situ.
Without available guidelines, engage in clinician-patient discussion
It is clear that IUD malposition is common, occurring in 10% to 20% of patients with an IUD. High-quality ultrasound imaging is helpful in detecting IUD malposition, including 2-D ultrasound with videoclips and/or 3-D ultrasound with coronal reconstruction. More data are needed to identify the best options for managing various types of malpositioned IUDs in patients with and without bothersome symptoms such as pain and bleeding. Until consensus guidelines are developed, clinicians need to engage the patient in a discussion of how to best manage the malpositioned IUD. Medicated IUDs and progestin subdermal implants are our two most effective reversible contraceptives. They are among the most important advances in health care over the past half-century. ●
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
- Mirena FDA approval. , 2022.
- Liletta [package insert]. Allergan USA: Irvine, California; 2019. .
- Paragard [package insert]. CooperSurgical Inc: Trumbull, Connecticut; 2019. .
- Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67:53-56.
- Van Houdenhoven K, van Kaam KJAF, van Grootheest AC, et al. Uterine perforation in women using a levonorgestrel-releasing intrauterine system. Contraception. 2006;73:257-260.
- van Grootheest K, Sachs B, Harrison-Woolrych M, et al. Uterine perforation with the levonorgestrel-releasing intrauterine device. Analysis of reports from four national pharmacovigilance centres. Drug Saf. 2011;34:83-88.
- Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
- Barnett C, Moehner S, Do Minh T, et al. Perforation risk and intra-uterine devices: results of the EURAS-IUD 5-year extension study. Eur J Contracept Reprod Health Care. 2017;22:424-428.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence and missing string. Obstet Gynecol Surv. 1981;36:335-353.
- Rivera R, Chen-Mok M, McMullen S. Analysis of client characteristics that may affect early discontinuation of the TCu-380A IUD. Contraception. 1999;60:155-160.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Madden T, McNichols, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
- Keenahan L, Bercaw-Pratt JL, Adeyemi O, et al. Rates of intrauterine device expulsion among adolescents and young women. J Pediatr Adolesc Gynecol. 2021;34:362-365.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices which are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34:110-115.
- Braaten KP, Benson CB, Maurer R, et al. Malpositioned intrauterine contraceptive devices: risk factors, outcomes and future pregnancies. Obstet Gynecol. 2011;118:1014-1020.
- Gerkowicz SA, Fiorentino DG, Kovacs AP, et al. Uterine structural abnormality and intrauterine device malposition: analysis of ultrasonographic and demographic variables of 517 patients. Am J Obstet Gynecol. 2019;220:183.e1-e8.
- Connolly CT, Fox NS. Incidence and risk factors for a malpositioned intrauterine device detected on three-dimensional ultrasound within eight weeks of placement. J Ultrasound Med. 2021 ePub Sept 27 2021.
- Golightly E, Gebbie AE. Low-lying or malpositioned intrauterine devices and systems. J Fam Plann Reprod health Care. 2014;40:108-112.
- Gurney EP, Sonalkar S, McAllister A, et al. Six-month expulsion of postplacental copper intrauterine devices placed after vaginal delivery. Am J Obstet Gynecol. 2018;219:183.e1-e9.
- Gurney EP, McAllister A, Lang B, et al. Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery. Contraception. 2020;2:100040.
- Anteby E, Revel A, Ben-Chetrit A, et al. Intrauterine device failure: relation to its location with the uterine cavity. Obstet Gynecol. 1993;81:112-114.
- Golightly E, Gebbie AE. Clinicians’ views on low-lying intrauterine devices or systems. J Fam Plann Reprod Health Care. 2014;40:113-116.
Fertility after tubal ligation – It’s a matter of ‘AGE’
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Despite the original intent of permanent contraception, tubal sterilization regret is experienced by 2%-26% of women as demonstrated by the United States Collaborative Review of Sterilization “CREST” 14-year study (Obstet Gynecol. 1999 Jun;93[6]:889-95). Regret appears to be higher in the United States than Europe and in resource-limited countries and is more common in women who are less than age 30, African-American, and unmarried. Nevertheless, requests for tubal reversal are estimated to be between 1% and 4% (Contraception. 1981 Jun;23[6]:579-89). The alternative option for fertility is in vitro fertilization (IVF) and this month’s column considers the pros and cons of both methods.
The procedure of tubal reanastomosis involves removing abnormal tissue and reapproximating the healthy tubal segments with attention to minimize adhesion formation through continued gentle irrigation. The surgery involves microsuturing using 6-0 to 10-0 sutures. Tubal patency can be confirmed during the procedure and with a subsequent hysterosalpingogram. While time from sterilization and the type of sterilization technique are factors that may influence the success rate of tubal reanastomosis, the age of the woman is the most predictive for pregnancy outcome.
In the original CREST study, the risk of ectopic pregnancy following tubal reanastomosis was contingent on the method of sterilization: Bipolar electrosurgery resulted in the highest probability of ectopic pregnancy (17.1 per 1,000 procedures at 10 years after permanent contraception), while postpartum partial salpingectomy resulted in the lowest (1.5 per 1,000 procedures at 10 years after permanent contraception) (N Engl J Med. 1997;336[11]:762). Comparatively, the ectopic pregnancy rate during an IVF cycle was 1.9% for pregnancies from transfers of fresh cleavage embryo, followed by transfers of frozen cleavage embryo (1.7%), transfers of fresh blastocyst (1.3%), and transfers of frozen blastocyst (0.8%) (Hum Reprod. 2015;30[9]:2048-54).
Reports vary regarding pregnancy rates from tubal reanastomosis. Prior use of rings and clips for sterilization appear to yield the highest outcomes as opposed to the use of electrocautery. In one large Canadian cohort study of over 300,000 women, those aged 15-30 years, 30-33 years, and 34-49 years had a conception rate of 73%, 64%, and 46%, respectively (Obstet Gynecol. 2003;101[4]:677-84). Most pregnancies were within 2 years after reversal and 48% of women achieved a delivery. Of interest, 23% of patients subsequently underwent another sterilization.
An Australian study of nearly 2,000 women found an overall cumulative live-delivery rate of 20% within the first year after reversal, 40% at 2 years, 51% at 5 years, and 52% at 10 years. As expected, the 5-year cumulative live-delivery rate was significantly lower in women who were aged 40-44 years (26%), compared with younger women. For all women below age 40 years, the live-delivery rate was approximately 50% within 5 years after tubal reanastomosis, while the rate halves after the age of 40 (Fertil Steril. 2015 Oct;104[4]:921-6).
To compare tubal reanastomosis with IVF, a retrospective cohort study of 163 patients demonstrated the cumulative delivery rate over 72 months was comparable for IVF vs. sterilization reversal (52% vs. 60%). The only significant difference was in a subset of patients aged <37 years (52% after IVF and 72% after reversal) and the lower cost of surgery. The authors advocated laparoscopic sterilization reversal in women younger than 37 years who have ≥4 cm of residual tube with IVF as the better alternative for all other women (Hum Reprod. 2007;22[10]:2660).
Indeed, tubal length is another important factor in successful reversal. The pregnancy rate after tubal anastomosis is 75% in women with tubal length of 4 cm or more, but only 19% in those with shorter tubes (Fertil Steril. 1987;48[1]:13-7). The literature does suggest equivalent pregnancy rates after laparoscopic tubal anastomosis and conventional microsurgical anastomosis. Although the laparoscopic approach may be more economical, it is more demanding technically than an open microsurgical procedure.
Tubal reanastomosis can also be performed using robot-assisted laparoscopy. In preliminary studies, robotic surgery appears to have a similar success rate and a shorter recovery time, but longer operative times and higher costs (Obstet Gynecol. 2007;109[6]:1375; Fertil Steril. 2008;90[4]:1175).
To educate women on the success of IVF based on individual characteristics, a valuable tool to approximate the cumulative outcome for a live birth following one cycle of IVF is offered by the Society for Assisted Reproductive Technology. To clarify, a cycle of IVF consists of one egg retrieval and the ultimate transfer of all embryos produced, i.e., fresh and frozen. The website also includes estimations of success following a second and third IVF cycle.
The woman’s age is a significant predictor of IVF success. Ovarian aging is currently best measured by combining chronologic age, antral follicle count (AFC) by transvaginal pelvic ultrasound, and serum anti-Müllerian hormone (AMH). Natural fecundity begins to decline, on average, above age 32-33 years. An AFC less than 11 reflects diminished ovarian reserve (DOR) and less than 6 is severe. AMH levels below 1.6 ng/mL have been shown to reduce the number of eggs retrieved with IVF, while levels below 0.4 ng/mL are very low. Very low AMH levels negatively affect the outcome of IVF cycles as demonstrated in the SART data study from a population of women with a mean age of 39.4 years: Cycle cancellation was 54%; of all retrieval attempts, no oocytes were obtained in 5.4%, and no embryo transfer occurred in 25.1% of cycles; the live birth rate per embryo transfer was 20.5% (9.5% per cycle start and 16.3% per retrieval) from a mean age of 36.8 years (Fertil Steril. 2016 Feb;105[2]:385-93.e3). The predictive ability of AMH on the live birth rate from IVF cycles was also shown in a study of over 85,000 women (Fertil Steril. 2018;109:258-65).
While low AMH has been shown to lessen a successful outcome from IVF, there appears to be no difference in natural pregnancy rates in women aged 30-44 years irrespective of AMH levels (JAMA. 2017;318[14]:1367-76). Of importance, the use of AMH in a population at low risk for DOR will yield a larger number of false-positive results (i.e., characterizing a woman as DOR when in fact she has normal ovarian reserve). Further, users of hormonal contraceptives have a 25.2% lower mean AMH level than nonusers.
When a patient is considering tubal reanastomosis vs. IVF, a useful acronym to remember is to check “AGE” – the A is for AMH because severely diminished ovarian reserve will reduce success with IVF as shown by the SART calculator; the G represents guy, i.e., ensuring a reasonably normal sperm analysis; and E stands for eggs representing ovulation function. In a woman who is anovulatory and who will require fertility medication, it would be reasonable to consider IVF given the need for ovarian stimulation. As in females, advanced paternal age has demonstrated a decline in fertility and sperm analysis parameters. Men above age 45 take approximately five times as long to achieve a pregnancy, compared with men less than 25 years of age. Further, there is evidence for advanced paternal age increasing risk of miscarriage, preterm birth, and birth defects. Men older than 40-45 years have twice the risk of an autistic child and five times the risk of having a child with schizophrenia (Transl Psychiatry 2017;7: e1019; Am J Psychiatry. 2002;159:1528-33).
To conclude, the data support consideration for sterilization reversal in women less than age 37 years with more than 4 cm of residual functional fallopian tube and the prior use of rings or clip sterilization. In other women, IVF may be the better option, particularly when ovulation dysfunction and/or male factor is present. IVF also offers the advantage of maintaining contraception and gender determination. However, given that AMH does not appear to reduce natural fertility, unlike during its effect during an IVF cycle, the option of tubal reversal may be more favorable in women with severe DOR.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Immediate postpartum IUD insertion increases expulsion risk
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
Expulsion of intrauterine devices was significantly more likely when the devices were inserted within the first 3 days after delivery compared with later insertions, based on data from more than 300,000 women.
Intrauterine devices are effective contraception, and current guidelines support immediate postpartum IUD insertion as a safe, effective, and convenient option, Mary Anne Armstrong, MA, of Kaiser Permanente Northern California, Oakland, and colleagues wrote. Although IUD expulsion rates are low overall, data from previous studies suggest that timing of insertion may affect expulsion rates, and that breastfeeding may play a role.
In the Association of Perforation and Expulsion of Intrauterine Devices (APEX-IUD) cohort study published in JAMA Network Open, the researchers reviewed data from the electronic health records at four sites; the study population included women aged 50 years and younger who underwent IUD insertion between 2001 and 2018.
The women were grouped by postpartum status and timing of IUD placement: 0-3 days, 4 days to 6 weeks, 6-14 weeks, 14-52 weeks, and nonpostpartum (defined as more than 52 weeks or no evidence of delivery).
The researchers also compared expulsion rates in postpartum women who were and were not breastfeeding at the time of IUD insertion based on clinical records, diagnostic codes, or questionnaires at well-baby visits.
The total study population included 326,658 women with a mean age of 32.0 years; 42% were non-Hispanic White, 17.2% were Hispanic other, 13.0% were Hispanic White, 11.9% were Asian or Pacific Islander, 8.7% were non-Hispanic Black, and 0.2% were Hispanic Black. Approximately 80% of the IUDs were levonorgestrel releasing.
A total of 8,943 expulsions were reported, for an overall expulsion rate of 13.94 per 1,000 person-years.
The adjusted hazard ratios for IUD expulsion were 5.34, 1.22, 1.06, and 1.43 for women with insertion times, respectively, of 0-3 days, 4 days to 6 or fewer weeks, 6-14 weeks, and 14-52 weeks. Women with nonpostpartum IUD insertion served as the referent.
The 5-year cumulative incidence of IUD expulsion was highest with placement between 0 and 3 days post partum and lowest with placement at 6-14 weeks postpartum (10.73% and 3.18%, respectively).
“Within the group with IUD insertions 0-3 days postpartum, the highest expulsion rates were discovered within 12 weeks of insertion, with the highest incidence rate occurring at week 6 (844 per 1,000 person-years), a time women are commonly seen post delivery,” the researchers noted.
In a subcohort of 94,817 women with known breastfeeding status, the 5-year cumulative incidence of expulsion was 3.49% for breastfeeding women and 4.57% for nonbreastfeeding women, with an adjusted HR of 0.71 for breastfeeding versus not breastfeeding.
“While women who accept immediate postpartum IUD placement report high satisfaction rates, information on women’s preferences and satisfaction associated with different timing of postpartum placement would also be helpful to understand the benefit-risk profile,” the researchers wrote in their discussion of the findings. “The fact that most expulsions in the immediate postpartum group occurred early presents an opportunity to mitigate risk of unrecognized expulsion and unintended pregnancy via counseling on signs of expulsion and follow-up examination.”
The study findings were limited by several factors including the potential misclassification of exposures and the primary outcome of expulsion, especially since some postpartum women may be lactating whether or not they are breastfeeding, the researchers noted. Other limitations included the combination of complete and partial expulsions, and the dating of IUD expulsion based on when it came to medical attention, which was not necessarily when it occurred. More data are needed on the potential association between lactational amenorrhea and lower expulsion risk among postpartum women who are breastfeeding.
However, the results were strengthened by the large and diverse study population, the use of linked mother-infant records to identify exposures, and the use electronic health records to identify outcomes, and the data can inform patient counseling for postpartum IUDs, the researchers concluded.
Study reflects findings from Europe
“The FDA mandated this study in response to a European study, EURAS-IUD1, a European prospective observational study that enrolled 61,448 participants between 2006 and 2012,” Ms. Armstrong said in an interview. In the European study “women breastfeeding at the time of device insertion or with the device inserted at 36 weeks’ postpartum or less had higher risk of uterine perforation. The FDA wanted to know if the risks were similar in the United States population”
The APEX-IUD study was designed to reflect current United States clinical practice. “The aims of APEX-IUD are to evaluate risk of IUD-related uterine perforation and device expulsion among women who are breastfeeding or within 12 months postpartum at insertion. The perforation outcome is addressed in a separate paper,” Ms. Armstrong noted.
“We were not surprised by the findings; they aligned with previous findings and confirm the overall safety of intrauterine devices,” said Ms. Armstrong. “Data from this study provides IUD expulsion risk estimates that can be used to inform clinical practice and preinsertion counseling. IUD insertions 0-3 days postpartum might decrease the risk of unintended pregnancy and provide more convenience and efficiency for new mothers. This has proven to be especially important during the pandemic. The higher risk of expulsion at 0-3 days post partum must be balanced with the low IUD-related uterine perforation risk to provide a comprehensive picture that aids in clinical decision-making.
“Potential barriers to postpartum IUD placement include lack of provision of education on the range of contraceptive options available during prenatal care and failure or inability of hospital inpatient units to stock the intrauterine devices for use when needed,” said Ms. Armstrong.
Looking ahead, “future research could evaluate risk factors for partial versus complete expulsions, the association of preinsertion counseling with recognition of potential expulsions and corresponding IUD failure rates, and whether ultrasound verification of IUD position in the uterus after insertion is associated with expulsion risk,” she said.
Identifying risk factors informs patient counseling
“The current study examines breastfeeding at time of IUD insertion as a risk factor for expulsion,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “There is biologic plausibility that breastfeeding may be a risk factor of IUD expulsion. Breastfeeding stimulates secretion of oxytocin, a hormone which plays a key role in the contraction of the uterus during labor and uterine involution postpartum. It also plays a key role in the contraction of milk ducts to allow for milk letdown. Because of its dual role some mothers may occasionally report uterine cramping with breastfeeding. Prior studies have suggested that breastfeeding may be associated with an increased risk of uterine perforation with postpartum IUD placement, but how breastfeeding may contribute to risk of IUD expulsion has not been studied extensively.”
The current data are consistent with previous studies suggesting the highest risk of IUD expulsion is with placement in the immediate postpartum period (0-3 days). “In a subcohort analysis by breastfeeding status, the risk of IUD expulsion was lower for women who were breastfeeding versus not breastfeeding;” however, “these findings may be due to amenorrhea that can also be seen with breastfeeding,” Dr. Krishna said. “Menstrual bleeding is an independent risk factor for IUD expulsion and not having menstrual bleeding while breastfeeding may lower risk of expulsion.
“Patients should be counseled on the benefits of immediate postpartum IUD placement, the risk of IUD expulsion, and alternative contraception options to be able to make an informed decision about the right contraception for them,” Dr. Krishna emphasized. “Clinicians can reassure patients that the uterine cramping they may feel while breastfeeding does not appear to increase the risk of IUD expulsion and that the amenorrhea that may result from breastfeeding also may lower the risk of IUD expulsion.”
The study was supported by Bayer through support to RTI Health Solutions, Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and the Regenstrief Institute. Ms. Armstrong and several coauthors disclosed support from Bayer during the study. Dr. Krishna had no relevant disclosures.
FROM JAMA NETWORK OPEN