User login
COVID-19 Screening and Testing Among Patients With Neurologic Dysfunction: The Neuro-COVID-19 Time-out Process and Checklist
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
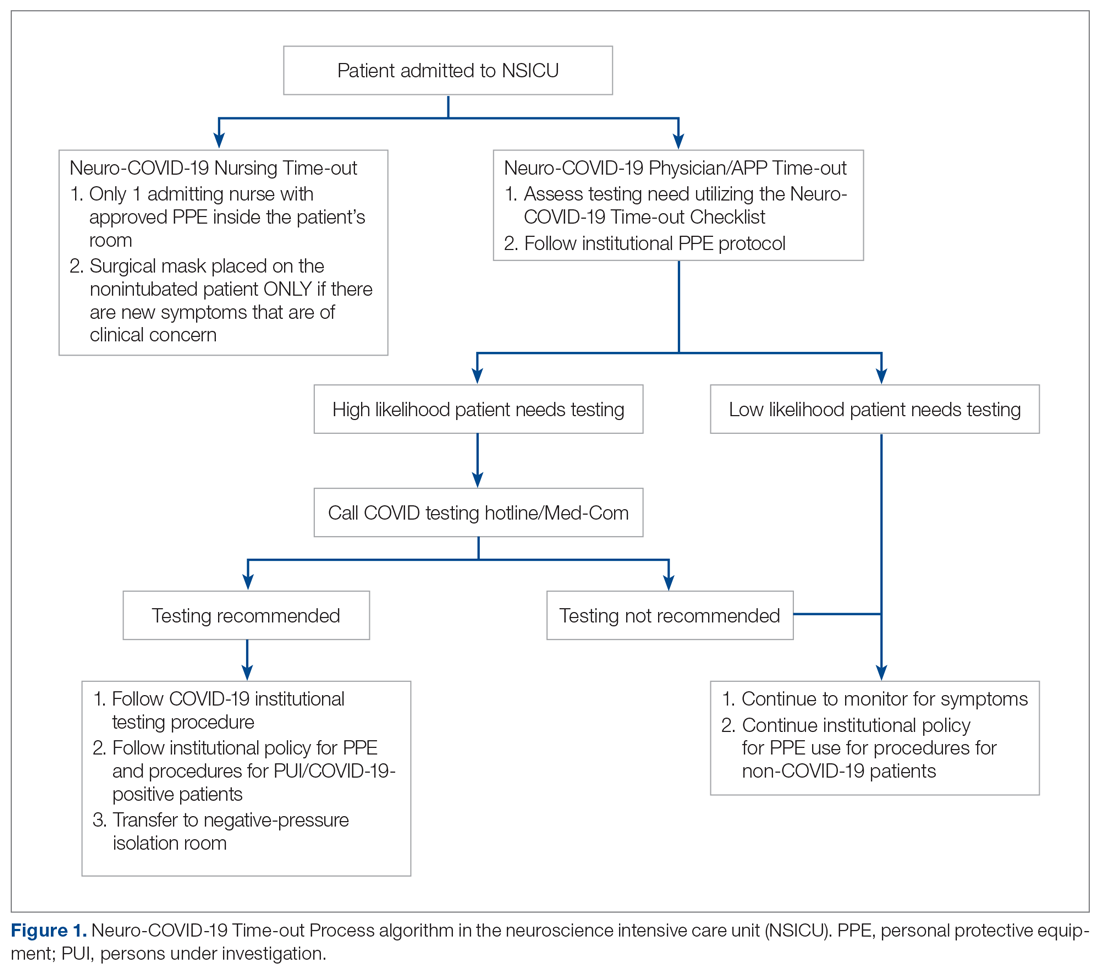
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.
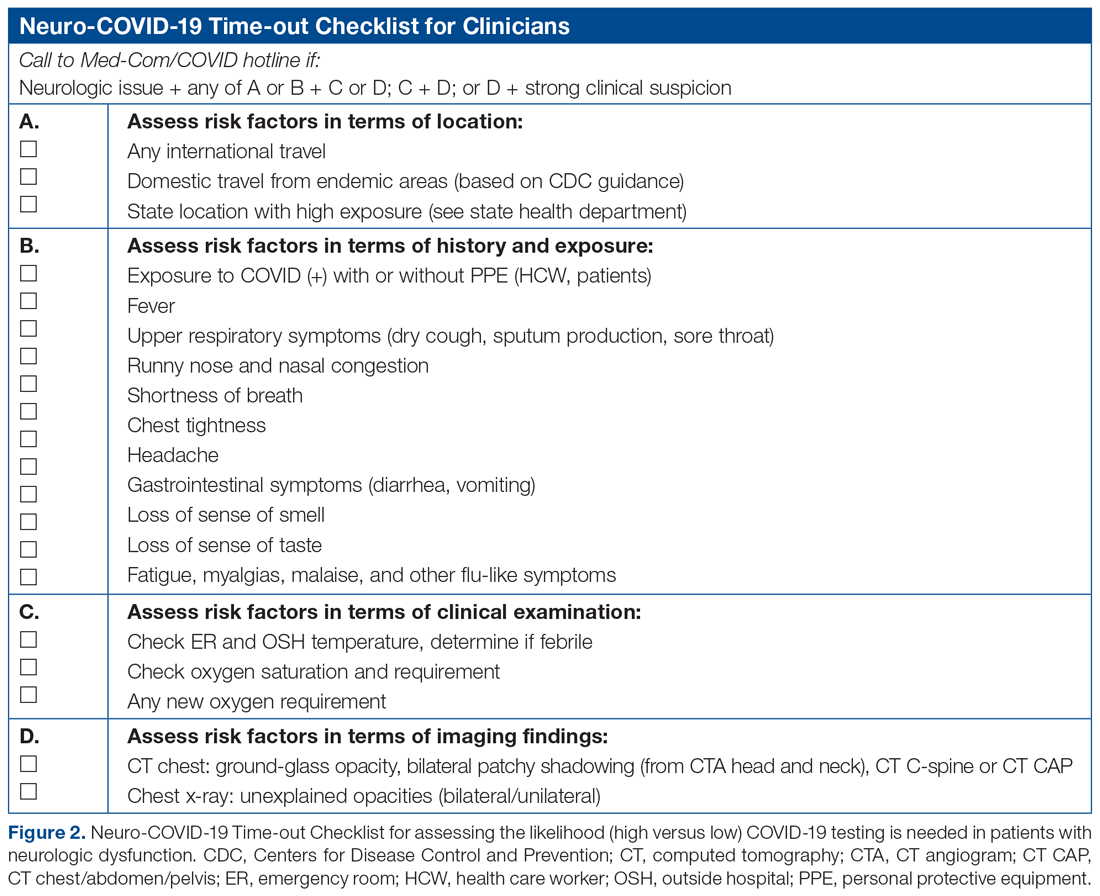
The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.
NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
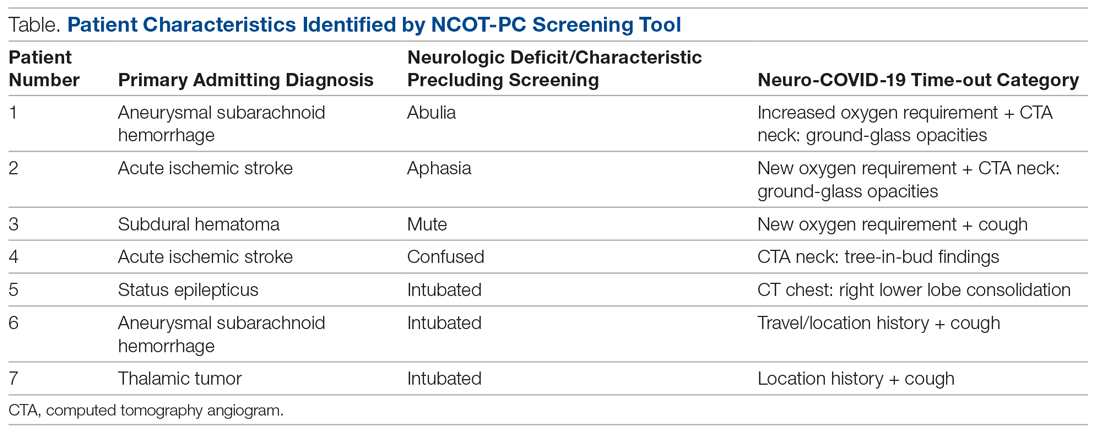
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).
Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
From the University of Mississippi Medical Center, Department of Neurology, Division of Neuroscience Intensive Care, Jackson, MS.
Abstract
Objective: To test a coronavirus disease 2019 (COVID-19) screening tool to identify patients who qualify for testing among patients with neurologic dysfunction who are unable to answer the usual screening questions, which could help to prevent unprotected exposure of patients and health care workers to COVID-19.
Methods: The Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) was implemented at our institution for 1 week as a quality improvement project to improve the pathway for COVID-19 screening and testing among patients with neurologic dysfunction.
Results: A total of 14 new patients were admitted into the neuroscience intensive care unit (NSICU) service during the pilot period. The NCOT-PC was utilized on 9 (64%) patients with neurologic dysfunction; 7 of these patients were found to have a likelihood of requiring testing based on the NCOT-PC and were subsequently screened for COVID-19 testing by contacting the institution’s COVID-19 testing hotline (Med-Com). All these patients were subsequently transitioned into person-under-investigation status based on the determination from Med-Com. The NSICU staff involved were able to utilize NCOT-PC without issues. The NCOT-PC was immediately adopted into the NSICU process.
Conclusion: Use of the NCOT-PC tool was found to be feasible and improved the screening methodology of patients with neurologic dysfunction.
Keywords: coronavirus; health care planning; quality improvement; patient safety; medical decision-making; neuroscience intensive care unit.
The coronavirus disease 2019 (COVID-19) pandemic has altered various standard emergent care pathways. Current recommendations regarding COVID-19 screening for testing involve asking patients about their symptoms, including fever, cough, chest pain, and dyspnea.1 This standard screening method poses a problem when caring for patients with neurologic dysfunction. COVID-19 patients may pre-sent with conditions that affect their ability to answer questions, such as stroke, encephalitis, neuromuscular disorders, or headache, and that may preclude the use of standard screening for testing.2 Patients with acute neurologic dysfunction who cannot undergo standard screening may leave the emergency department (ED) and transition into the neuroscience intensive care unit (NSICU) or any intensive care unit (ICU) without a reliable COVID-19 screening test.
The Protected Code Stroke pathway offers protection in the emergent setting for patients with stroke when their COVID-19 status is unknown.3 A similar process has been applied at our institution for emergent management of patients with cerebrovascular disease (stroke, intracerebral hemorrhage, and subarachnoid hemorrhage). However, the process from the ED after designating “difficult to screen” patients as persons under investigation (PUI) is unclear. The Centers for Disease Control and Prevention (CDC) has delineated the priorities for testing, with not all declared PUIs requiring testing.4 This poses a great challenge, because patients designated as PUIs require the same management as a COVID-19-positive patient, with negative-pressure isolation rooms as well as use of protective personal equipment (PPE), which may not be readily available. It was also recognized that, because the ED staff can be overwhelmed by COVID-19 patients, there may not be enough time to perform detailed screening of patients with neurologic dysfunction and that “reverse masking” may not be done consistently for nonintubated patients. This may place patients and health care workers at risk of unprotected exposure.
Recognizing these challenges, we created a Neuro-COVID-19 Time-out Process and Checklist (NCOT-PC) as a quality improvement project. The aim of this project was to improve and standardize the current process of identifying patients with neurologic dysfunction who require COVID-19 testing to decrease the risk of unprotected exposure of patients and health care workers.
Methods
Patients and Definitions
This quality improvement project was undertaken at the University of Mississippi Medical Center NSICU. Because this was a quality improvement project, an Institutional Review Board exemption was granted.
The NCOT-PC was utilized in consecutive patients with neurologic dysfunction admitted to the NSICU during a period of 1 week. “Neurologic dysfunction” encompasses any neurologic illness affecting the mental status and/or level of alertness, subsequently precluding the ability to reliably screen the patient utilizing standard COVID-19 screening. “Med-Com” at our institution is the equivalent of the national COVID-19 testing hotline, where our institution’s infectious diseases experts screen calls for testing and determine whether testing is warranted. “Unprotected exposure” means exposure to COVID-19 without adequate and appropriate PPE.
Quality Improvement Process
As more PUIs were being admitted to the institution, we used the Plan-Do-Study-Act method for process improvements in the NSICU.5 NSICU stakeholders, including attendings, the nurse manager, and nurse practitioners (NPs), developed an algorithm to facilitate the coordination of the NSICU staff in screening patients to identify those with a high likelihood of needing COVID-19 testing upon arrival in the NSICU (Figure 1). Once the NCOT-PC was finalized, NSICU stakeholders were educated regarding the use of this screening tool.

The checklist clinicians review when screening patients is shown in Figure 2. The risk factors comprising the checklist include patient history and clinical and radiographic characteristics that have been shown to be relevant for identifying patients with COVID-19.6,7 The imaging criteria utilize imaging that is part of the standard of care for NSICU patients. For example, computed tomography angiogram of the head and neck performed as part of the acute stroke protocol captures the upper part of the chest. These images are utilized for their incidental findings, such as apical ground-glass opacities and tree-in-bud formation. The risk factors applicable to the patient determine whether the clinician will call Med-Com for testing approval. Institutional COVID-19 processes were then followed accordingly.8 The decision from Med-Com was considered final, and no deviation from institutional policies was allowed.

NCOT-PC was utilized for consecutive days for 1 week before re-evaluation of its feasibility and adaptability.
Data Collection and Analysis
Consecutive patients with neurologic dysfunction admitted into the NSICU were assigned nonlinkable patient numbers. No identifiers were collected for the purpose of this project. The primary diagnosis for admission, the neurologic dysfunction that precluded standard screening, and checklist components that the patient fulfilled were collected.
To assess the tool’s feasibility, feedback regarding the ease of use of the NCOT-PC was gathered from the nurses, NPs, charge nurses, fellows, and other attendings. To assess the utility of the NCOT-PC in identifying patients who will be approved for COVID-19 testing, we calculated the proportion of patients who were deemed to have a high likelihood of testing and the proportion of patients who were approved for testing. Descriptive statistics were used, as applicable for the project, to summarize the utility of the NCOT-PC.
Results
We found that the NCOT-PC can be easily used by clinicians. The NSICU staff did not communicate any implementation issues, and since the NCOT-PC was implemented, no problems have been identified.
During the pilot period of the NCOT-PC, 14 new patients were admitted to the NSICU service. Nine (64%) of these had neurologic dysfunction, and the NCOT-PC was used to determine whether Med-Com should be called based on the patients’ likelihood (high vs low) of needing a COVID-19 test. Of those patients with neurologic dysfunction, 7 (78%) were deemed to have a high likelihood of needing a COVID-19 test based on the NCOT-PC. Med-Com was contacted regarding these patients, and all were deemed to require the COVID-19 test by Med-Com and were transitioned into PUI status per institutional policy (Table).

Discussion
The NCOT-PC project improved and standardized the process of identifying and screening patients with neurologic dysfunction for COVID-19 testing. The screening tool is feasible to use, and it decreased inadvertent unprotected exposure of patients and health care workers.
The NCOT-PC was easy to administer. Educating the staff regarding the new process took only a few minutes and involved a meeting with the nurse manager, NPs, fellows, residents, and attendings. We found that this process works well in tandem with the standard institutional processes in place in terms of Protected Code Stroke pathway, PUI isolation, PPE use, and Med-Com screening for COVID-19 testing. Med-Com was called only if the patient fulfilled the checklist criteria. In addition, no extra cost was attributed to implementing the NCOT-PC, since we utilized imaging that was already done as part of the standard of care for patients with neurologic dysfunction.
The standardization of the process of screening for COVID-19 testing among patients with neurologic dysfunction improved patient selection. Before the NCOT-PC, there was no consistency in terms of who should get tested and the reason for testing patients with neurologic dysfunction. Patients can pass through the ED and arrive in the NSICU with an unclear screening status, which may cause inadvertent patient and health care worker exposure to COVID-19. With the NCOT-PC, we have avoided instances of inadvertent staff or patient exposure in the NSICU.
The NCOT-PC was adopted into the NSICU process after the first week it was piloted. Beyond the NSICU, the application of the NCOT-PC can be extended to any patient presentation that precludes standard screening, such as ED and interhospital transfers for stroke codes, trauma codes, code blue, or myocardial infarction codes. In our department, as we started the process of PCS for stroke codes, we included NCOT-PC for stroke patients with neurologic dysfunction.
The results of our initiative are largely limited by the decision-making process of Med-Com when patients are called in for testing. At the time of our project, there were no specific criteria used for patients with altered mental status, except for the standard screening methods, and it was through clinician-to-clinician discussion that testing decisions were made. Another limitation is the short period of time that the NCOT-PC was applied before adoption.
In summary, the NCOT-PC tool improved the screening process for COVID-19 testing in patients with neurologic dysfunction admitted to the NSICU. It was feasible and prevented unprotected staff and patient exposure to COVID-19. The NCOT-PC functionality was compatible with institutional COVID-19 policies in place, which contributed to its overall sustainability.
The Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) were utilized in preparing this manuscript.9
Acknowledgment: The authors thank the University of Mississippi Medical Center NSICU staff for their input with implementation of the NCOT-PC.
Corresponding author: Prashant A. Natteru, MD, University of Mississippi Medical Center, Department of Neurology, 2500 North State St., Jackson, MS 39216; [email protected].
Financial disclosures: None.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
1. Coronavirus disease 2019 (COVID-19) Symptoms. www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed April 9, 2020.
2. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1-9.
3. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019. (COVID-19) pandemic. Stroke. 2020;51:1891-1895.
4. Coronavirus disease 2019 (COVID-19) evaluation and testing. www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 9, 2020.
5. Plan-Do-Study-Act Worksheet. Institute for Healthcare Improvement website. www.ihi.org/resources/Pages/Tools/PlanDoStudyActWorksheet.aspx. Accessed March 31,2020.
6. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;10.1002/jmv.25728.
7. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623.
8. UMMC’s COVID-19 Clinical Processes. www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/Clinical-Resources.html. Accessed April 9, 2020.
9. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): Revised Publication Guidelines from a Detailed Consensus Process. The EQUATOR Network. www.equator-network.org/reporting-guidelines/squire/. Accessed May 12, 2020.
Systemic Corticosteroids in Critically Ill Patients With COVID-19
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
Study Overview
Objective. To assess the association between administration of systemic corticosteroids, compared with usual care or placebo, and 28-day all-cause mortality in critically ill patients with coronavirus disease 2019 (COVID-19).
Design. Prospective meta-analysis with data from 7 randomized clinical trials conducted in 12 countries.
Setting and participants. This prospective meta-analysis included randomized clinical trials conducted between February 26, 2020, and June 9, 2020, that examined the clinical efficacy of administration of corticosteroids in hospitalized COVID-19 patients who were critically ill. Trials were systematically identified from ClinicalTrials.gov, the Chinese Clinical Trial Registry, and the EU Clinical Trials Register, using the search terms COVID-19, corticosteroids, and steroids. Additional trials were identified by experts from the WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Senior investigators of these identified trials were asked to participate in weekly calls to develop a protocol for the prospective meta-analysis.1 Subsequently, trials that had randomly assigned critically ill patients to receive corticosteroids versus usual care or placebo were invited to participate in this meta-analysis. Data were pooled from patients recruited to the participating trials through June 9, 2020, and aggregated in overall and in predefined subgroups.
Main outcome measures. The primary outcome was all-cause mortality up to 30 days after randomization. Because 5 of the included trials reported mortality at 28 days after randomization, the primary outcome was reported as 28-day all-cause mortality. The secondary outcome was serious adverse events (SAEs). The authors also gathered data on the demographic and clinical characteristics of patients, the number of patients lost to follow-up, and outcomes according to intervention group, overall, and in subgroups (ie, patients receiving invasive mechanical ventilation or vasoactive medication; age ≤ 60 years or > 60 years [the median across trials]; sex [male or female]; and the duration patients were symptomatic [≤ 7 days or > 7 days]). For each trial, the risk of bias was assessed independently by 4 investigators using the Cochrane Risk of Bias Assessment Tool for the overall effects of corticosteroids on mortality and SAEs and the effect of assignment and allocated interventions. Inconsistency between trial results was evaluated using the I2 statistic. The trials were classified according to the corticosteroids used in the intervention group and the dose administered using a priori-defined cutoffs (15 mg/day of dexamethasone, 400 mg/day of hydrocortisone, and 1 mg/kg/day of methylprednisolone). The primary analysis utilized was an inverse variance-weighted fixed-effect meta-analysis of odds ratios (ORs) for overall mortality. Random-effects meta-analyses with Paule-Mandel estimate of heterogeneity were also performed.
Main results. Seven trials (DEXA-COVID 19, CoDEX, RECOVERY, CAPE COVID, COVID STEROID, REMAP-CAP, and Steroids-SARI) were included in the final meta-analysis. The enrolled patients were from Australia, Brazil, Canada, China, Denmark, France, Ireland, the Netherlands, New Zealand, Spain, the United Kingdom, and the United States. The date of final follow-up was July 6, 2020. The corticosteroids groups included dexamethasone at low (6 mg/day orally or intravenously [IV]) and high (20 mg/day IV) doses; low-dose hydrocortisone (200 mg/day IV or 50 mg every 6 hr IV); and high-dose methylprednisolone (40 mg every 12 hr IV). In total, 1703 patients were randomized, with 678 assigned to the corticosteroids group and 1025 to the usual-care or placebo group. The median age of patients was 60 years (interquartile range, 52-68 years), and 29% were women. The larger number of patients in the usual-care/placebo group was a result of the 1:2 randomization (corticosteroids versus usual care or placebo) in the RECOVERY trial, which contributed 59.1% of patients included in this prospective meta-analysis. The majority of patients were receiving invasive mechanical ventilation at randomization (1559 patients). The administration of adjunctive treatments, such as azithromycin or antiviral agents, varied among the trials. The risk of bias was determined as low for 6 of the 7 mortality results.
A total of 222 of 678 patients in the corticosteroids group died, and 425 of 1025 patients in the usual care or placebo group died. The summary OR was 0.66 (95% confidence interval [CI], 0.53-0.82; P < 0.001) based on a fixed-effect meta-analysis, and 0.70 (95% CI, 0.48-1.01; P = 0.053) based on the random-effects meta-analysis, for 28-day all-cause mortality comparing all corticosteroids with usual care or placebo. There was little inconsistency between trial results (I2 = 15.6%; P = 0.31). The fixed-effect summary OR for the association with 28-day all-cause mortality was 0.64 (95% CI, 0.50-0.82; P < 0.001) for dexamethasone compared with usual care or placebo (3 trials, 1282 patients, and 527 deaths); the OR was 0.69 (95% CI, 0.43-1.12; P = 0.13) for hydrocortisone (3 trials, 374 patients, and 94 deaths); and the OR was 0.91 (95% CI, 0.29-2.87; P = 0.87) for methylprednisolone (1 trial, 47 patients, and 26 deaths). Moreover, in trials that administered low-dose corticosteroids, the overall fixed-effect OR for 28-day all-cause mortality was 0.61 (95% CI, 0.48-0.78; P < 0.001). In the subgroup analysis, the overall fixed-effect OR was 0.69 (95% CI, 0.55-0.86) in patients who were receiving invasive mechanical ventilation at randomization, and the OR was 0.41 (95% CI, 0.19-0.88) in patients who were not receiving invasive mechanical ventilation at randomization.
Six trials (all except the RECOVERY trial) reported SAEs, with 64 events occurring among 354 patients assigned to the corticosteroids group and 80 SAEs occurring among 342 patients assigned to the usual-care or placebo group. There was no suggestion that the risk of SAEs was higher in patients who were administered corticosteroids.
Conclusion. The administration of systemic corticosteroids was associated with a lower 28-day all-cause mortality in critically ill patients with COVID-19 compared to those who received usual care or placebo.
Commentary
Corticosteroids are anti-inflammatory and vasoconstrictive medications that have long been used in intensive care units for the treatment of acute respiratory distress syndrome and septic shock. However, the therapeutic role of corticosteroids for treating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was uncertain at the outset of the COVID-19 pandemic due to concerns that this class of medications may cause an impaired immune response in the setting of a life-threatening SARS-CoV-2 infection. Evidence supporting this notion included prior studies showing that corticosteroid therapy was associated with delayed viral clearance of Middle East respiratory syndrome or a higher viral load of SARS-CoV.2,3 The uncertainty surrounding the therapeutic use of corticosteroids in treating COVID-19 led to a simultaneous global effort to conduct randomized controlled trials to urgently examine this important clinical question. The open-label Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, conducted in the UK, was the first large-scale randomized clinical trial that reported the clinical benefit of corticosteroids in treating patients hospitalized with COVID-19. Specifically, it showed that low-dose dexamethasone (6 mg/day) administered orally or IV for up to 10 days resulted in a 2.8% absolute reduction in 28-day mortality, with the greatest benefit, an absolute risk reduction of 12.1%, conferred to patients who were receiving invasive mechanical ventilation at the time of randomization.4 In response to these findings, the National Institutes of Health COVID-19 Treatment Guidelines Panel recommended the use of dexamethasone in patients with COVID-19 who are on mechanical ventilation or who require supplemental oxygen, and recommended against the use of dexamethasone for those not requiring supplemental oxygen.5
The meta-analysis discussed in this commentary, conducted by the WHO REACT Working Group, has replicated initial findings from the RECOVERY trial. This prospective meta-analysis pooled data from 7 randomized controlled trials of corticosteroid therapy in 1703 critically ill patients hospitalized with COVID-19. Similar to findings from the RECOVERY trial, corticosteroids were associated with lower all-cause mortality at 28 days after randomization, and this benefit was observed both in critically ill patients who were receiving mechanical ventilation or supplemental oxygen without mechanical ventilation. Interestingly, while the OR estimates were imprecise, the reduction in mortality rates was similar between patients who were administered dexamethasone and hydrocortisone, which may suggest a general drug class effect. In addition, the mortality benefit of corticosteroids appeared similar for those aged ≤ 60 years and those aged > 60 years, between female and male patients, and those who were symptomatic for ≤ 7 days or > 7 days before randomization. Moreover, the administration of corticosteroids did not appear to increase the risk of SAEs. While more data are needed, results from the RECOVERY trial and this prospective meta-analysis indicate that corticosteroids should be an essential pharmacologic treatment for COVID-19, and suggest its potential role as a standard of care for critically ill patients with COVID-19.
This study has several limitations. First, not all trials systematically identified participated in the meta-analysis. Second, long-term outcomes after hospital discharge were not captured, and thus the effect of corticosteroids on long-term mortality and other adverse outcomes, such as hospital readmission, remain unknown. Third, because children were excluded from study participation, the effect of corticosteroids on pediatric COVID-19 patients is unknown. Fourth, the RECOVERY trial contributed more than 50% of patients in the current analysis, although there was little inconsistency in the effects of corticosteroids on mortality between individual trials. Last, the meta-analysis was unable to establish the optimal dose or duration of corticosteroid intervention in critically ill COVID-19 patients, or determine its efficacy in patients with mild-to-moderate COVID-19, all of which are key clinical questions that will need to be addressed with further clinical investigations.
The development of effective treatments for COVID-19 is critical to mitigating the devastating consequences of SARS-CoV-2 infection. Several recent COVID-19 clinical trials have shown promise in this endeavor. For instance, the Adaptive COVID-19 Treatment Trial (ACCT-1) found that intravenous remdesivir, as compared to placebo, significantly shortened time to recovery in adult patients hospitalized with COVID-19 who had evidence of lower respiratory tract infection.6 Moreover, there is some evidence to suggest that convalescent plasma and aerosol inhalation of IFN-κ may have beneficial effects in treating COVID-19.7,8 Thus, clinical trials designed to investigate combination therapy approaches including corticosteroids, remdesivir, convalescent plasma, and others are urgently needed to help identify interventions that most effectively treat COVID-19.
Applications for Clinical Practice
The use of corticosteroids in critically ill patients with COVID-19 reduces overall mortality. This treatment is inexpensive and available in most care settings, including low-resource regions, and provides hope for better outcomes in the COVID-19 pandemic.
Katerina Oikonomou, MD, PhD
General Hospital of Larissa, Larissa, Greece
Fred Ko, MD, MS
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
1. Sterne JAC, Diaz J, Villar J, et al. Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials. Trials. 2020;21:734.
2. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
3. Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for citically Ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
4. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report [published online ahead of print, 2020 Jul 17]. N Engl J Med. 2020;NEJMoa2021436.
5. NIH COVID-19 Treatment Guidelines. National Institutes of Health. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/immunomodulators/corticosteroids/. Accessed September 11, 2020.
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19--preliminary report [published online ahead of print, 2020 May 22]. N Engl J Med. 2020;NEJMoa2007764.
7. Casadevall A, Joyner MJ, Pirofski LA. A randomized trial of convalescent plasma for covid-19-potentially hopeful signals. JAMA. 2020;324:455-457.
8. Fu W, Liu Y, Xia L, et al. A clinical pilot study on the safety and efficacy of aerosol inhalation treatment of IFN-κ plus TFF2 in patients with moderate COVID-19. EClinicalMedicine. 2020;25:100478.
What will be the future of American medicine?
For at least the last 6 months, and what seems like much longer, the United States has been in a period of great upheaval unseen for decades. Thanks in part to a novel coronavirus that quickly spread globally, along with social and racial tensions reaching a boiling point after nationwide economic uncertainty and the deaths of George Floyd and Breonna Taylor at the hands of law enforcement. In the year of a presidential election, leaders both elected and running are looking for solutions. Medicine has also been scrambling for answers as hospitals deal with ever growing censuses and dwindling resources, which have placed a strain on budgets, employees, and communities. Through these difficult times, there appears to be a resolve to investigate how we arrived here, where do we want to go, and what will take us there. As industries look to foster more inclusive and diverse environments, health care also looks to lead this philosophical shift toward a more equitable system. In the meantime, minorities, particularly African Americans, are dying at alarming rates.
With state government shutdowns, school closures, and a transition to work from home, Americans have been increasingly cognizant of issues that are more likely to be drowned out by the routine of previously “normal” life. As the staggering coronavirus infection numbers and deaths began to be published, undeniable trends were laid bare for the country to see. While the pandemic has been a deadly scare for the entire nation, the risk of serious complications or death for others was undeniable or even likely. For many Americans of underrepresented groups, but for Black people in general, 2020 has been another checkpoint in a long straight path, as centuries of systemic injustices and racist policies enacted through legislation, health policy have left these communities far behind and incredibly unprepared for this latest challenge.
For millions of Black Americans, although there is never acceptance of it, living with inequality has become a way of life. Much is known about the eventually desegregated lunch counters and public transportation but health care also facilitated disparities that have manifested themselves in the disparate outcomes we see today. Although Brown v Board of Education eliminated the legal precedent of segregated public spaces, enforcement was not immediately unanimous. In the paper The Politics of Racial Disparities, author David Smith describes the segregation in the state hospital in the state capital of Mississippi. Accounts detailed the dismay of white patients who traveled in the same elevators as Black patients, separate floors new and expectant Black mothers were admitted to, and even policies that discouraged Black and White children from utilizing play areas at the same time. All of these policies and the resistance to change were occurring in the 1960s as the larger national appetite toward overt discrimination began to sour. Although the deep south has historically held the reputation of outdated values, this was not solely a regional problem.
Nationwide, African Americans, as well as other minorities, are very aware of the health pitfalls that await them once leaving the hospital as newborns. According to CDC data, they are more likely than White non-Hispanic White adults to be diagnosed with diabetes and hypertension. Eighty percent of African American women are overweight or obese compared with 65% of non-Hispanic White women. These comorbidities have been especially telling this year as they account for a large proportion of comorbid conditions listed on deceased COVID-19 patients’ death certificates.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and member of the White House coronavirus task force, is particularly concerned about these trends. He stated in a recent interview that the virus is, “shedding another bright light on a systemic problem that has been with us for a very long period of time.” While he does not explicitly state what the systemic problem is, you could assume it relates to racial injustice. He also goes on to say, “…social determinants of health put people of color in a position-because of employment, socioeconomic status, availability of jobs-that makes it more likely for them to be in contact with an infected person and not be able to separate themselves.”
When these statistics are quoted, discussions of personal responsibility are often discussed; however, these arguments do not stand up against the long documented, intentional exclusion of minorities, in particular Black people, from the health systems and economic opportunities the country has to offer. Lacking any significant economic power, these communities have no buffer against a pandemic, no option but to show up for work. Additionally, these jobs cannot be done in the comfort of one’s living room. Large cities, such as New York City, served as a harbinger to what could happen when masks and social distancing was ignored, as well as a tendency to blame overcrowding. More investigation unearths that the true culprit in major metropolitan areas is not the size but its effects on resident social habits. Dr. Mary Bassett explains in The New York Times, “The answer is simple: the high cost of housing.” Multigenerational households are more prevalent among minority communities, explaining the rapid spread through these epicenters.
The historical legacy of redlining and other laws that were exclusionary and hostile to racial equality have made systems much more difficult to change, even when the parties involved are willing to take a more active role in change. The question is will it be enough to have merely stopped these practices or will a more active role in reversal of policies and their intended effects be needed?
Medicine is grappling with its role in the larger context of how to provide better access and better care. The Affordable Care Act, signed into law by President Barack Obama in 2010, aimed to begin that journey. When the mandate for individual states to opt in was struck down in 2012, state legislators were able to decide whether to opt into a Medicaid agreement with the government, providing basic care to all citizens of their state. Twelve states currently have not opted into the Medicaid expansion, leaving a significant portion of their residents uninsured. Of those states, a majority have minority populations represented at levels greater than the national average.
Medicine should use this opportunity to position itself as an ally in the fight for equality. The American dream story has always been structured around innovation and discovery. The medical field shares in this delight when coincidence, discovery, and problem solving intersect. This country prides itself on its abilities to problem solve and has sold this branding to the rest of the world. America loves winning, our current President repeatedly says so. What greater win would equal care and elimination of racial disparities in chronic diseases. As our health leaders assemble solutions for a multifactorial problem, the public must become more engaged to assist in creating solutions, maintain dedication and focus on the goals, and continue to hold leaders and elected officials accountable.
Increased diversity in health-care spaces both on the ground and in leadership will help ensure less represented voices are heard. We must invest in our education system to broaden the representation of minority physicians who often do not represent their population’s share. Changes must also go beyond direct patient care and population health measures but must also address the social determinants of health, such as a livable wage, fair and affordable housing, and wealth inequality.
With federal support for biomedical research becoming more difficult, the path for the next big innovation becomes increasingly expensive and never guaranteed. We hope to create a safe and effective COVID-19 vaccine. The elimination of race as an indirect determinant of health is a worthwhile goal that, if achieved, would be near the top of the list of this country’s achievements. With 1.2 trillion spent on health care in 2019 (Brookings institute), we cannot afford not to.
Dr. Williams is Affiliate Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Mississippi; and the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Mississippi.
For at least the last 6 months, and what seems like much longer, the United States has been in a period of great upheaval unseen for decades. Thanks in part to a novel coronavirus that quickly spread globally, along with social and racial tensions reaching a boiling point after nationwide economic uncertainty and the deaths of George Floyd and Breonna Taylor at the hands of law enforcement. In the year of a presidential election, leaders both elected and running are looking for solutions. Medicine has also been scrambling for answers as hospitals deal with ever growing censuses and dwindling resources, which have placed a strain on budgets, employees, and communities. Through these difficult times, there appears to be a resolve to investigate how we arrived here, where do we want to go, and what will take us there. As industries look to foster more inclusive and diverse environments, health care also looks to lead this philosophical shift toward a more equitable system. In the meantime, minorities, particularly African Americans, are dying at alarming rates.
With state government shutdowns, school closures, and a transition to work from home, Americans have been increasingly cognizant of issues that are more likely to be drowned out by the routine of previously “normal” life. As the staggering coronavirus infection numbers and deaths began to be published, undeniable trends were laid bare for the country to see. While the pandemic has been a deadly scare for the entire nation, the risk of serious complications or death for others was undeniable or even likely. For many Americans of underrepresented groups, but for Black people in general, 2020 has been another checkpoint in a long straight path, as centuries of systemic injustices and racist policies enacted through legislation, health policy have left these communities far behind and incredibly unprepared for this latest challenge.
For millions of Black Americans, although there is never acceptance of it, living with inequality has become a way of life. Much is known about the eventually desegregated lunch counters and public transportation but health care also facilitated disparities that have manifested themselves in the disparate outcomes we see today. Although Brown v Board of Education eliminated the legal precedent of segregated public spaces, enforcement was not immediately unanimous. In the paper The Politics of Racial Disparities, author David Smith describes the segregation in the state hospital in the state capital of Mississippi. Accounts detailed the dismay of white patients who traveled in the same elevators as Black patients, separate floors new and expectant Black mothers were admitted to, and even policies that discouraged Black and White children from utilizing play areas at the same time. All of these policies and the resistance to change were occurring in the 1960s as the larger national appetite toward overt discrimination began to sour. Although the deep south has historically held the reputation of outdated values, this was not solely a regional problem.
Nationwide, African Americans, as well as other minorities, are very aware of the health pitfalls that await them once leaving the hospital as newborns. According to CDC data, they are more likely than White non-Hispanic White adults to be diagnosed with diabetes and hypertension. Eighty percent of African American women are overweight or obese compared with 65% of non-Hispanic White women. These comorbidities have been especially telling this year as they account for a large proportion of comorbid conditions listed on deceased COVID-19 patients’ death certificates.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and member of the White House coronavirus task force, is particularly concerned about these trends. He stated in a recent interview that the virus is, “shedding another bright light on a systemic problem that has been with us for a very long period of time.” While he does not explicitly state what the systemic problem is, you could assume it relates to racial injustice. He also goes on to say, “…social determinants of health put people of color in a position-because of employment, socioeconomic status, availability of jobs-that makes it more likely for them to be in contact with an infected person and not be able to separate themselves.”
When these statistics are quoted, discussions of personal responsibility are often discussed; however, these arguments do not stand up against the long documented, intentional exclusion of minorities, in particular Black people, from the health systems and economic opportunities the country has to offer. Lacking any significant economic power, these communities have no buffer against a pandemic, no option but to show up for work. Additionally, these jobs cannot be done in the comfort of one’s living room. Large cities, such as New York City, served as a harbinger to what could happen when masks and social distancing was ignored, as well as a tendency to blame overcrowding. More investigation unearths that the true culprit in major metropolitan areas is not the size but its effects on resident social habits. Dr. Mary Bassett explains in The New York Times, “The answer is simple: the high cost of housing.” Multigenerational households are more prevalent among minority communities, explaining the rapid spread through these epicenters.
The historical legacy of redlining and other laws that were exclusionary and hostile to racial equality have made systems much more difficult to change, even when the parties involved are willing to take a more active role in change. The question is will it be enough to have merely stopped these practices or will a more active role in reversal of policies and their intended effects be needed?
Medicine is grappling with its role in the larger context of how to provide better access and better care. The Affordable Care Act, signed into law by President Barack Obama in 2010, aimed to begin that journey. When the mandate for individual states to opt in was struck down in 2012, state legislators were able to decide whether to opt into a Medicaid agreement with the government, providing basic care to all citizens of their state. Twelve states currently have not opted into the Medicaid expansion, leaving a significant portion of their residents uninsured. Of those states, a majority have minority populations represented at levels greater than the national average.
Medicine should use this opportunity to position itself as an ally in the fight for equality. The American dream story has always been structured around innovation and discovery. The medical field shares in this delight when coincidence, discovery, and problem solving intersect. This country prides itself on its abilities to problem solve and has sold this branding to the rest of the world. America loves winning, our current President repeatedly says so. What greater win would equal care and elimination of racial disparities in chronic diseases. As our health leaders assemble solutions for a multifactorial problem, the public must become more engaged to assist in creating solutions, maintain dedication and focus on the goals, and continue to hold leaders and elected officials accountable.
Increased diversity in health-care spaces both on the ground and in leadership will help ensure less represented voices are heard. We must invest in our education system to broaden the representation of minority physicians who often do not represent their population’s share. Changes must also go beyond direct patient care and population health measures but must also address the social determinants of health, such as a livable wage, fair and affordable housing, and wealth inequality.
With federal support for biomedical research becoming more difficult, the path for the next big innovation becomes increasingly expensive and never guaranteed. We hope to create a safe and effective COVID-19 vaccine. The elimination of race as an indirect determinant of health is a worthwhile goal that, if achieved, would be near the top of the list of this country’s achievements. With 1.2 trillion spent on health care in 2019 (Brookings institute), we cannot afford not to.
Dr. Williams is Affiliate Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Mississippi; and the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Mississippi.
For at least the last 6 months, and what seems like much longer, the United States has been in a period of great upheaval unseen for decades. Thanks in part to a novel coronavirus that quickly spread globally, along with social and racial tensions reaching a boiling point after nationwide economic uncertainty and the deaths of George Floyd and Breonna Taylor at the hands of law enforcement. In the year of a presidential election, leaders both elected and running are looking for solutions. Medicine has also been scrambling for answers as hospitals deal with ever growing censuses and dwindling resources, which have placed a strain on budgets, employees, and communities. Through these difficult times, there appears to be a resolve to investigate how we arrived here, where do we want to go, and what will take us there. As industries look to foster more inclusive and diverse environments, health care also looks to lead this philosophical shift toward a more equitable system. In the meantime, minorities, particularly African Americans, are dying at alarming rates.
With state government shutdowns, school closures, and a transition to work from home, Americans have been increasingly cognizant of issues that are more likely to be drowned out by the routine of previously “normal” life. As the staggering coronavirus infection numbers and deaths began to be published, undeniable trends were laid bare for the country to see. While the pandemic has been a deadly scare for the entire nation, the risk of serious complications or death for others was undeniable or even likely. For many Americans of underrepresented groups, but for Black people in general, 2020 has been another checkpoint in a long straight path, as centuries of systemic injustices and racist policies enacted through legislation, health policy have left these communities far behind and incredibly unprepared for this latest challenge.
For millions of Black Americans, although there is never acceptance of it, living with inequality has become a way of life. Much is known about the eventually desegregated lunch counters and public transportation but health care also facilitated disparities that have manifested themselves in the disparate outcomes we see today. Although Brown v Board of Education eliminated the legal precedent of segregated public spaces, enforcement was not immediately unanimous. In the paper The Politics of Racial Disparities, author David Smith describes the segregation in the state hospital in the state capital of Mississippi. Accounts detailed the dismay of white patients who traveled in the same elevators as Black patients, separate floors new and expectant Black mothers were admitted to, and even policies that discouraged Black and White children from utilizing play areas at the same time. All of these policies and the resistance to change were occurring in the 1960s as the larger national appetite toward overt discrimination began to sour. Although the deep south has historically held the reputation of outdated values, this was not solely a regional problem.
Nationwide, African Americans, as well as other minorities, are very aware of the health pitfalls that await them once leaving the hospital as newborns. According to CDC data, they are more likely than White non-Hispanic White adults to be diagnosed with diabetes and hypertension. Eighty percent of African American women are overweight or obese compared with 65% of non-Hispanic White women. These comorbidities have been especially telling this year as they account for a large proportion of comorbid conditions listed on deceased COVID-19 patients’ death certificates.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, and member of the White House coronavirus task force, is particularly concerned about these trends. He stated in a recent interview that the virus is, “shedding another bright light on a systemic problem that has been with us for a very long period of time.” While he does not explicitly state what the systemic problem is, you could assume it relates to racial injustice. He also goes on to say, “…social determinants of health put people of color in a position-because of employment, socioeconomic status, availability of jobs-that makes it more likely for them to be in contact with an infected person and not be able to separate themselves.”
When these statistics are quoted, discussions of personal responsibility are often discussed; however, these arguments do not stand up against the long documented, intentional exclusion of minorities, in particular Black people, from the health systems and economic opportunities the country has to offer. Lacking any significant economic power, these communities have no buffer against a pandemic, no option but to show up for work. Additionally, these jobs cannot be done in the comfort of one’s living room. Large cities, such as New York City, served as a harbinger to what could happen when masks and social distancing was ignored, as well as a tendency to blame overcrowding. More investigation unearths that the true culprit in major metropolitan areas is not the size but its effects on resident social habits. Dr. Mary Bassett explains in The New York Times, “The answer is simple: the high cost of housing.” Multigenerational households are more prevalent among minority communities, explaining the rapid spread through these epicenters.
The historical legacy of redlining and other laws that were exclusionary and hostile to racial equality have made systems much more difficult to change, even when the parties involved are willing to take a more active role in change. The question is will it be enough to have merely stopped these practices or will a more active role in reversal of policies and their intended effects be needed?
Medicine is grappling with its role in the larger context of how to provide better access and better care. The Affordable Care Act, signed into law by President Barack Obama in 2010, aimed to begin that journey. When the mandate for individual states to opt in was struck down in 2012, state legislators were able to decide whether to opt into a Medicaid agreement with the government, providing basic care to all citizens of their state. Twelve states currently have not opted into the Medicaid expansion, leaving a significant portion of their residents uninsured. Of those states, a majority have minority populations represented at levels greater than the national average.
Medicine should use this opportunity to position itself as an ally in the fight for equality. The American dream story has always been structured around innovation and discovery. The medical field shares in this delight when coincidence, discovery, and problem solving intersect. This country prides itself on its abilities to problem solve and has sold this branding to the rest of the world. America loves winning, our current President repeatedly says so. What greater win would equal care and elimination of racial disparities in chronic diseases. As our health leaders assemble solutions for a multifactorial problem, the public must become more engaged to assist in creating solutions, maintain dedication and focus on the goals, and continue to hold leaders and elected officials accountable.
Increased diversity in health-care spaces both on the ground and in leadership will help ensure less represented voices are heard. We must invest in our education system to broaden the representation of minority physicians who often do not represent their population’s share. Changes must also go beyond direct patient care and population health measures but must also address the social determinants of health, such as a livable wage, fair and affordable housing, and wealth inequality.
With federal support for biomedical research becoming more difficult, the path for the next big innovation becomes increasingly expensive and never guaranteed. We hope to create a safe and effective COVID-19 vaccine. The elimination of race as an indirect determinant of health is a worthwhile goal that, if achieved, would be near the top of the list of this country’s achievements. With 1.2 trillion spent on health care in 2019 (Brookings institute), we cannot afford not to.
Dr. Williams is Affiliate Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, University of Mississippi; and the G.V. (Sonny) Montgomery VA Medical Center, Jackson, Mississippi.
Low vitamin D in COVID-19 predicts ICU admission, poor survival
Having low serum vitamin D levels was an independent risk factor for having symptomatic COVID-19 with respiratory distress requiring admission to intensive care – as opposed to having mild COVID-19 – and for not surviving, in a new study from Italy.
“Our data give strong observational support to previous suggestions that reduced vitamin D levels may favor the appearance of severe respiratory dysfunction and increase the mortality risk in patients affected with COVID-19,” the researchers report.
Luigi Gennari, MD, PhD, Department of Medicine, Surgery, and Neurosciences, University of Siena, Italy, presented these findings during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
Gennari told Medscape Medical News that this analysis suggests determining vitamin D levels (25 hydroxyvitamin D) in people testing positive for SARS-Cov-2 infection might help predict their risk of severe disease.
However, further research is needed to explore whether vitamin D supplements could prevent the risk of respiratory failure in patients with SARS-Cov-2 infection, he stressed.
In the meantime, Gennari said: “I believe that, particularly in the winter season (when the solar ultraviolet-B (UVB) radiation exposure does not allow the skin to synthesize vitamin D in most countries), the use of vitamin D supplementation and correction of vitamin D deficiency might be of major relevance for the reduction of the clinical burden of the ongoing and future outbreaks of SARS-CoV-2 infection.
Invited to comment, David Meltzer, MD, PhD, chief of hospital medicine at University of Chicago Medicine, Illinois, who was not involved with the study, agrees.
“I think this body of work suggests that people should be taking supplements if they cannot increase sun exposure on a sustained basis,” Meltzer said. “The abstract supports multiple prior findings that suggest that higher vitamin D levels are associated with improved outcomes.”
And JoAnn E. Manson, MD, DrPH, of Harvard Medical School and Brigham and Women’s Hospital, who was not involved with the research but has spoken about the topic in a video report for Medscape, said: “We know from several studies that a low vitamin D level is associated with a higher risk of having COVID-19 and severe illness, but correlation does not prove causation.”
“I think that improving vitamin D status is a promising way to reduce the risk of severe illness, but we need randomized controlled trials to prove cause and effect,” she told Medscape Medical News.
103 patients with severe COVID-19, 52 with mild COVID-19, 206 controls
Gennari said several lines of evidence suggest that vitamin D deficiency might be a risk factor for COVID-19 severity.
Countries with lower average levels of vitamin D or lower UVB radiation exposure have higher COVID-19 mortality, and “demographic groups known to be at higher risk of vitamin D deficiency (such as black individuals, the elderly, nursing home residents, and those with obesity and diabetes) are at high risk of COVID-19 hospitalization/mortality, he noted.
There is a high prevalence of vitamin D deficiency in Italy, where mortality rates from COVID-19 have been particularly high.
To examine the relationship between vitamin D levels and COVID-19 severity/mortality, the researchers studied three groups:
- 103 symptomatic patients with COVID-19 with respiratory insufficiency who were admitted to a Milan hospital from March 9 to April 30.
- 52 patients with mild COVID-19, recruited from patients and staff from a nearby nursing home who had a positive test for COVID-19.
- 206 healthy controls, matched 2:1 with symptomatic patients of the same age, weight, and gender, from 3174 patients who had vitamin D measured during a routine check-up from January to March 2020.
Patients in the hospitalized group had lower mean vitamin D levels (18.2 ng/mL) than those with mild COVID-19 (30.3 ng/mL) or those in the control group (25.4 ng/mL).
Patients with symptomatic versus mild COVID-19 were slightly older and more likely to have at least one comorbidity and less likely to be taking a vitamin D supplement at baseline (30% vs 79%).
Among symptomatic patients, mean vitamin D levels were inversely associated with interleukin (IL)-6 and C-reactive protein, “both of which are a direct expression of the inflammatory status,” Gennari noted.
About half of the hospitalized patients (49) were admitted to a ward and discharged after a mean stay of 16 days (none died).
The other 54 hospitalized patients were admitted to the intensive care unit with severe acute respiratory distress; 38 patients received continuous positive airway pressure (CPAP) and 16 patients received endotracheal intubation.
Of the 54 patients admitted to ICU, 19 patients died from respiratory distress after a mean of 19 days, “consistent with the literature,” and the other 35 patients were discharged after a mean of 21 days.
Patients with severe COVID-19 who were admitted to the ICU, as opposed to a ward, were more likely to be male, have at least one comorbidity, have higher baseline IL-6 levels and neutrophil counts, and lower lymphocyte and platelet counts.
They also had lower mean vitamin D levels (14.4 vs 22.4 ng/mL) and were more likely to have vitamin D deficiency (vitamin D <20 ng/mL; 80% vs. 45%).
Patients admitted to ICU who died had lower baseline vitamin D levels than those who survived (13.2 vs. 19.3 ng/mL).
Vitamin D levels were inversely associated with respiratory distress requiring ICU admission (odds ratio, 1.06; P = .038) and with mortality (OR, 1.18, P = 029), independent of IL-6 levels and other comorbidities.
“That vitamin D levels are associated with improved outcomes independent of IL-6 could reflect that IL-6 is an imperfect measure of the inflammatory process or that vitamin D is related to outcomes for other reasons, such as enhancement of innate or adaptive immunity,” said Meltzer.
He added that “this is not to exclude the possibility that vitamin D has important immunomodulatory effects.”
Gennari, Meltzer, and Manson have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Having low serum vitamin D levels was an independent risk factor for having symptomatic COVID-19 with respiratory distress requiring admission to intensive care – as opposed to having mild COVID-19 – and for not surviving, in a new study from Italy.
“Our data give strong observational support to previous suggestions that reduced vitamin D levels may favor the appearance of severe respiratory dysfunction and increase the mortality risk in patients affected with COVID-19,” the researchers report.
Luigi Gennari, MD, PhD, Department of Medicine, Surgery, and Neurosciences, University of Siena, Italy, presented these findings during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
Gennari told Medscape Medical News that this analysis suggests determining vitamin D levels (25 hydroxyvitamin D) in people testing positive for SARS-Cov-2 infection might help predict their risk of severe disease.
However, further research is needed to explore whether vitamin D supplements could prevent the risk of respiratory failure in patients with SARS-Cov-2 infection, he stressed.
In the meantime, Gennari said: “I believe that, particularly in the winter season (when the solar ultraviolet-B (UVB) radiation exposure does not allow the skin to synthesize vitamin D in most countries), the use of vitamin D supplementation and correction of vitamin D deficiency might be of major relevance for the reduction of the clinical burden of the ongoing and future outbreaks of SARS-CoV-2 infection.
Invited to comment, David Meltzer, MD, PhD, chief of hospital medicine at University of Chicago Medicine, Illinois, who was not involved with the study, agrees.
“I think this body of work suggests that people should be taking supplements if they cannot increase sun exposure on a sustained basis,” Meltzer said. “The abstract supports multiple prior findings that suggest that higher vitamin D levels are associated with improved outcomes.”
And JoAnn E. Manson, MD, DrPH, of Harvard Medical School and Brigham and Women’s Hospital, who was not involved with the research but has spoken about the topic in a video report for Medscape, said: “We know from several studies that a low vitamin D level is associated with a higher risk of having COVID-19 and severe illness, but correlation does not prove causation.”
“I think that improving vitamin D status is a promising way to reduce the risk of severe illness, but we need randomized controlled trials to prove cause and effect,” she told Medscape Medical News.
103 patients with severe COVID-19, 52 with mild COVID-19, 206 controls
Gennari said several lines of evidence suggest that vitamin D deficiency might be a risk factor for COVID-19 severity.
Countries with lower average levels of vitamin D or lower UVB radiation exposure have higher COVID-19 mortality, and “demographic groups known to be at higher risk of vitamin D deficiency (such as black individuals, the elderly, nursing home residents, and those with obesity and diabetes) are at high risk of COVID-19 hospitalization/mortality, he noted.
There is a high prevalence of vitamin D deficiency in Italy, where mortality rates from COVID-19 have been particularly high.
To examine the relationship between vitamin D levels and COVID-19 severity/mortality, the researchers studied three groups:
- 103 symptomatic patients with COVID-19 with respiratory insufficiency who were admitted to a Milan hospital from March 9 to April 30.
- 52 patients with mild COVID-19, recruited from patients and staff from a nearby nursing home who had a positive test for COVID-19.
- 206 healthy controls, matched 2:1 with symptomatic patients of the same age, weight, and gender, from 3174 patients who had vitamin D measured during a routine check-up from January to March 2020.
Patients in the hospitalized group had lower mean vitamin D levels (18.2 ng/mL) than those with mild COVID-19 (30.3 ng/mL) or those in the control group (25.4 ng/mL).
Patients with symptomatic versus mild COVID-19 were slightly older and more likely to have at least one comorbidity and less likely to be taking a vitamin D supplement at baseline (30% vs 79%).
Among symptomatic patients, mean vitamin D levels were inversely associated with interleukin (IL)-6 and C-reactive protein, “both of which are a direct expression of the inflammatory status,” Gennari noted.
About half of the hospitalized patients (49) were admitted to a ward and discharged after a mean stay of 16 days (none died).
The other 54 hospitalized patients were admitted to the intensive care unit with severe acute respiratory distress; 38 patients received continuous positive airway pressure (CPAP) and 16 patients received endotracheal intubation.
Of the 54 patients admitted to ICU, 19 patients died from respiratory distress after a mean of 19 days, “consistent with the literature,” and the other 35 patients were discharged after a mean of 21 days.
Patients with severe COVID-19 who were admitted to the ICU, as opposed to a ward, were more likely to be male, have at least one comorbidity, have higher baseline IL-6 levels and neutrophil counts, and lower lymphocyte and platelet counts.
They also had lower mean vitamin D levels (14.4 vs 22.4 ng/mL) and were more likely to have vitamin D deficiency (vitamin D <20 ng/mL; 80% vs. 45%).
Patients admitted to ICU who died had lower baseline vitamin D levels than those who survived (13.2 vs. 19.3 ng/mL).
Vitamin D levels were inversely associated with respiratory distress requiring ICU admission (odds ratio, 1.06; P = .038) and with mortality (OR, 1.18, P = 029), independent of IL-6 levels and other comorbidities.
“That vitamin D levels are associated with improved outcomes independent of IL-6 could reflect that IL-6 is an imperfect measure of the inflammatory process or that vitamin D is related to outcomes for other reasons, such as enhancement of innate or adaptive immunity,” said Meltzer.
He added that “this is not to exclude the possibility that vitamin D has important immunomodulatory effects.”
Gennari, Meltzer, and Manson have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Having low serum vitamin D levels was an independent risk factor for having symptomatic COVID-19 with respiratory distress requiring admission to intensive care – as opposed to having mild COVID-19 – and for not surviving, in a new study from Italy.
“Our data give strong observational support to previous suggestions that reduced vitamin D levels may favor the appearance of severe respiratory dysfunction and increase the mortality risk in patients affected with COVID-19,” the researchers report.
Luigi Gennari, MD, PhD, Department of Medicine, Surgery, and Neurosciences, University of Siena, Italy, presented these findings during the virtual American Society of Bone and Mineral Research (ASBMR) 2020 annual meeting.
Gennari told Medscape Medical News that this analysis suggests determining vitamin D levels (25 hydroxyvitamin D) in people testing positive for SARS-Cov-2 infection might help predict their risk of severe disease.
However, further research is needed to explore whether vitamin D supplements could prevent the risk of respiratory failure in patients with SARS-Cov-2 infection, he stressed.
In the meantime, Gennari said: “I believe that, particularly in the winter season (when the solar ultraviolet-B (UVB) radiation exposure does not allow the skin to synthesize vitamin D in most countries), the use of vitamin D supplementation and correction of vitamin D deficiency might be of major relevance for the reduction of the clinical burden of the ongoing and future outbreaks of SARS-CoV-2 infection.
Invited to comment, David Meltzer, MD, PhD, chief of hospital medicine at University of Chicago Medicine, Illinois, who was not involved with the study, agrees.
“I think this body of work suggests that people should be taking supplements if they cannot increase sun exposure on a sustained basis,” Meltzer said. “The abstract supports multiple prior findings that suggest that higher vitamin D levels are associated with improved outcomes.”
And JoAnn E. Manson, MD, DrPH, of Harvard Medical School and Brigham and Women’s Hospital, who was not involved with the research but has spoken about the topic in a video report for Medscape, said: “We know from several studies that a low vitamin D level is associated with a higher risk of having COVID-19 and severe illness, but correlation does not prove causation.”
“I think that improving vitamin D status is a promising way to reduce the risk of severe illness, but we need randomized controlled trials to prove cause and effect,” she told Medscape Medical News.
103 patients with severe COVID-19, 52 with mild COVID-19, 206 controls
Gennari said several lines of evidence suggest that vitamin D deficiency might be a risk factor for COVID-19 severity.
Countries with lower average levels of vitamin D or lower UVB radiation exposure have higher COVID-19 mortality, and “demographic groups known to be at higher risk of vitamin D deficiency (such as black individuals, the elderly, nursing home residents, and those with obesity and diabetes) are at high risk of COVID-19 hospitalization/mortality, he noted.
There is a high prevalence of vitamin D deficiency in Italy, where mortality rates from COVID-19 have been particularly high.
To examine the relationship between vitamin D levels and COVID-19 severity/mortality, the researchers studied three groups:
- 103 symptomatic patients with COVID-19 with respiratory insufficiency who were admitted to a Milan hospital from March 9 to April 30.
- 52 patients with mild COVID-19, recruited from patients and staff from a nearby nursing home who had a positive test for COVID-19.
- 206 healthy controls, matched 2:1 with symptomatic patients of the same age, weight, and gender, from 3174 patients who had vitamin D measured during a routine check-up from January to March 2020.
Patients in the hospitalized group had lower mean vitamin D levels (18.2 ng/mL) than those with mild COVID-19 (30.3 ng/mL) or those in the control group (25.4 ng/mL).
Patients with symptomatic versus mild COVID-19 were slightly older and more likely to have at least one comorbidity and less likely to be taking a vitamin D supplement at baseline (30% vs 79%).
Among symptomatic patients, mean vitamin D levels were inversely associated with interleukin (IL)-6 and C-reactive protein, “both of which are a direct expression of the inflammatory status,” Gennari noted.
About half of the hospitalized patients (49) were admitted to a ward and discharged after a mean stay of 16 days (none died).
The other 54 hospitalized patients were admitted to the intensive care unit with severe acute respiratory distress; 38 patients received continuous positive airway pressure (CPAP) and 16 patients received endotracheal intubation.
Of the 54 patients admitted to ICU, 19 patients died from respiratory distress after a mean of 19 days, “consistent with the literature,” and the other 35 patients were discharged after a mean of 21 days.
Patients with severe COVID-19 who were admitted to the ICU, as opposed to a ward, were more likely to be male, have at least one comorbidity, have higher baseline IL-6 levels and neutrophil counts, and lower lymphocyte and platelet counts.
They also had lower mean vitamin D levels (14.4 vs 22.4 ng/mL) and were more likely to have vitamin D deficiency (vitamin D <20 ng/mL; 80% vs. 45%).
Patients admitted to ICU who died had lower baseline vitamin D levels than those who survived (13.2 vs. 19.3 ng/mL).
Vitamin D levels were inversely associated with respiratory distress requiring ICU admission (odds ratio, 1.06; P = .038) and with mortality (OR, 1.18, P = 029), independent of IL-6 levels and other comorbidities.
“That vitamin D levels are associated with improved outcomes independent of IL-6 could reflect that IL-6 is an imperfect measure of the inflammatory process or that vitamin D is related to outcomes for other reasons, such as enhancement of innate or adaptive immunity,” said Meltzer.
He added that “this is not to exclude the possibility that vitamin D has important immunomodulatory effects.”
Gennari, Meltzer, and Manson have reported no relevant financial relationships.
This article first appeared on Medscape.com.
FROM ASBMR 2020
Noninvasive ventilation: Options and cautions for patients with COVID-19
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
Early on in the COVID-19 pandemic,
“We were concerned that, if we put them on high-flow nasal cannula or a noninvasive ventilation, that we would create aerosols that would then be a risk to clinicians,” Meghan Lane-Fall, MD, MSHP, FCCM, said at a Society for Critical Care Medicine virtual meeting called COVID-19: What’s Next. “However, we’ve gotten much more comfortable with infection control. We’ve gotten much more comfortable with controlling these aerosols, with making sure that our clinicians are protected with the appropriate protective equipment. We’ve also realized that patients who end up becoming intubated have really poor outcomes, so we’ve looked at our practice critically and tried to figure out how to support patients noninvasively when that’s possible.”
Respiratory support options
According to Dr. Lane-Fall, an associate professor of anesthesiology and critical care at the University of Pennsylvania, Philadelphia, there are two basic types of respiratory support in patients with moderate, severe, or critical COVID-19: noninvasive and invasive. Noninvasive options include CPAP or BiPAP which can be delivered through nasal pillows, masks, and helmets, as well as high-flow nasal oxygen. Invasive options include endotracheal intubation, tracheostomy, and extracorporeal membrane oxygenation (ECMO), usually the veno-venous (VV) form. “But it’s uncommon to need VV ECMO, even in patients who have critical COVID-19,” she said.
Factors that favor noninvasive ventilation include stably high oxygen requirements, normal mental status, ward location of care, and moderate to severe COVID-19. Factors that favor invasive ventilation include someone who’s deteriorating rapidly, “whose oxygen requirements aren’t stable or who is cardiopulmonary compromised,” said Dr. Lane-Fall, who is also co–medical director of the Trauma Surgery Intensive Care Unit at Penn Presbyterian Medical Center, also in Philadelphia. Other factors include the need for other invasive procedures such as surgery or if they have severe to critical COVID-19, “not just pneumonia, but [illness that’s] progressing into [acute respiratory distress syndrome],” she said.
Indications for urgent endotracheal intubation as opposed to giving a trial of noninvasive ventilation or high-flow nasal oxygen include altered mental status, inability to protect airway, copious amounts of secretions, a Glasgow Coma Scale score of less than 8, severe respiratory acidosis, hypopnea or apnea, shock, or an inability to tolerate noninvasive support. “This is a relative contraindication,” Dr. Lane-Fall said. “I’ve certainly talked people through the BiPAP mask or the helmet. If you tell a patient, ‘I don’t want to have to put in a breathing tube; I want to maintain you on this,’ often they’ll be able to work through it.”
Safety precautions
Aerosolizing procedures require attention to location, personnel, and equipment, including personal protective equipment (PPE), said Dr. Lane-Fall, who is an anesthesiologist by training. “When you are intubating someone, whether they have COVID-19 or not, you are sort of in the belly of the beast,” she said. “You are very exposed to secretions that occur at the time of endotracheal intubation. That’s why it’s important for us to have PPE and barriers to protect ourselves from potential exposure to aerosols during the care of patients with COVID-19.”
In February 2020, the non-for-profit Anesthesia Patient Safety Foundation published recommendations for airway management in patients with suspected COVID-19. A separate guidance was published the British Journal of Anaesthesiology based on emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China. “The idea here is that you want to intubate under controlled conditions,” said Dr. Lane-Fall, who is an author of the guidance. “You want to use the most experienced operator. You want to have full PPE, including an N95 mask, or something more protective like a powered air purifying respirator or an N95 mask with a face shield. You want the eyes, nose, and mouth of the operator covered completely.”
CPR, another aerosolizing procedure, requires vigilant safety precautions as well. “We struggled with this a little bit at our institution, because our inclination as intensivists when someone is pulseless is to run into the room and start chest compressions and to start resuscitation,” Dr. Lane-Fall said. “But the act of chest compression itself can create aerosols that can present risk to clinicians. We had to tell our clinicians that they have to put on PPE before they do CPR. The buzz phrase here is that there is no emergency in a pandemic. The idea here is that the good of that one patient is outweighed by the good of all the other patients that you could care for if you didn’t have COVID-19 as a clinician. So we have had to encourage our staff to put on PPE first before attending to patients first, even if it delays patient care. Once you have donned PPE, when you’re administering CPR, the number of staff should be minimized. You should have a compressor, and someone to relieve the compressor, and a code leader, someone tending to the airway. But in general, anyone who’s not actively involved should not be in the room.”
Risks during extubation
Extubation of COVID-19 patients is also an aerosolizing procedure not just because you’re pulling an endotracheal tube out of the airway but because coughing is a normal part of extubation. “We’ve had to be careful with how we approach extubation in COVID-19 patients,” Dr. Lane-Fall said. “Ideally you’re doing this in a negative pressure environment. We have also had to use full PPE, covering the eyes and face, and putting on a gown for precaution.”
Reintubation of COVID-19 patients is not uncommon. She and her colleagues at Penn Medicine created procedures for having intubators at the ready outside the room in case the patient were to decompensate clinically. “Another thing we learned is that it’s useful to do a leak test prior to extubation, because there may be airway edema related to prolonged intubation in these patients,” Dr. Lane-Fall said. “We found that, if a leak is absent on checking the cuff leak, the use of steroids for a day or 2 may help decrease airway edema. That improves the chances of extubation success.”
Strategies for aerosol containment
She concluded her remarks by reviewing airway control adjuncts and clinician safety. This includes physically isolating COVID-19 patients in negative pressure rooms and avoiding and minimizing aerosols, including the use of rapid intubation, “where we induce anesthesia for intubation but we don’t bag-mask the patient because that creates aerosols,” she said. The Anesthesia Patient Safety Foundation guidelines advocate for the use of video laryngoscopy so that you can visualize the glottis easily “and make sure that you successfully intubate the glottis and not the esophagus,” she said.
A smart strategy for aerosol containment is to use the most experienced laryngoscopist available. “If you are in a teaching program, ideally you’re using your most experienced resident, or you’re using fellows or attending physicians,” Dr. Lane-Fall said. “This is not the space for an inexperienced learner.”
Another way to make intubation faster and easier in COVID-19 patients is to use an intubation box, which features a plexiglass shield that enables the intubator to use their hands to get in the patient’s airway while being protected from viral droplets generated during intubation. The box can be cleaned after each use. Blueprints for an open source intubation box can be found at http://www.intubationbox.com.
Expert view on aerosol containment in COVID-19
“While there is a dearth of evidence from controlled trials, recommendations mentioned in this story are based on the best available evidence and are in agreement with guidelines from several expert groups,” said David L. Bowton, MD, FCCP, FCCM, of the department of anesthesiology at Wake Forest Baptist Health in Winston-Salem, NC. “The recommendation of Dr. Lane-Fall’s that is perhaps most controversial is the use of an intubation box. Multiple designs for these intubation/aerosol containment devices have been proposed, and the data supporting their ease of use and efficacy has been mixed [See Anaesthesia 2020;75(8):1014-21 and Anaesthesia. 2020. doi: 10.1111/anae.15188]. While bag valve mask ventilation should be avoided if possible, it may be a valuable rescue tool in the severely hypoxemic patient when used with two-person technique to achieve a tight seal and a PEEP valve and an HME over the exhalation port to minimize aerosol spread.
“It cannot be stressed enough that the most skilled individual should be tasked with intubating the patient and as few providers as possible [usually three] should be in the room and have donned full PPE. Negative pressure rooms should be used whenever feasible. Noninvasive ventilation appears safer from an infection control standpoint than initially feared and its use has become more widespread. However, noninvasive ventilation is not without its hazards, and Dr. Lane-Fall’s enumeration of the patient characteristics applicable to the selection of patients for noninvasive ventilation are extremely important. At our institution, the use of noninvasive ventilation and especially high-flow oxygen therapy has increased. Staff have become more comfortable with the donning and doffing of PPE.”
Dr. Lane-Fall reported having no financial disclosures.
FROM AN SCCM VIRTUAL MEETING
Distinguishing COVID-19 from flu in kids remains challenging
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
For children with COVID-19, rates of hospitalization, ICU admission, and ventilator use were similar to those of children with influenza, but rates differed in other respects, according to results of a study published online Sept. 11 in JAMA Network Open.
As winter approaches, distinguishing patients with COVID-19 from those with influenza will become a problem. To assist with that, Xiaoyan Song, PhD, director of the office of infection control and epidemiology at Children’s National Hospital in Washington, D.C., and colleagues investigated commonalities and differences between the clinical symptoms of COVID-19 and influenza in children.
“Distinguishing COVID-19 from flu and other respiratory viral infections remains a challenge to clinicians. Although our study showed that patients with COVID-19 were more likely than patients with flu to report fever, gastrointestinal, and other clinical symptoms at the time of diagnosis, the two groups do have many overlapping clinical symptoms,” Dr. Song said. “Until future data show us otherwise, clinicians need to prepare for managing coinfections of COVID-19 with flu and/or other respiratory viral infections in the upcoming flu season.”
The retrospective cohort study included 315 children diagnosed with laboratory-confirmed COVID-19 between March 25 and May 15, 2020, and 1,402 children diagnosed with laboratory-confirmed seasonal influenza A or influenza B between Oct. 1, 2019, and June 6, 2020, at Children’s National Hospital. The investigation excluded asymptomatic patients who tested positive for COVID-19.
Patients with COVID-19 and patients with influenza were similar with respect to rates of hospitalization (17% vs. 21%; odds ratio, 0.8; 95% confidence interval, 0.6-1.1; P = .15), admission to the ICU (6% vs. 7%; OR, 0.8; 95% CI, 0.5-1.3; P = .42), and use of mechanical ventilation (3% vs. 2%; OR, 1.5; 95% CI, 0.9-2.6; P =.17).
The difference in the duration of ventilation for the two groups was not statistically significant. None of the patients who had COVID-19 or influenza B died, but two patients with influenza A did.
No patients had coinfections, which the researchers attribute to the mid-March shutdown of many schools, which they believe limited the spread of seasonal influenza.
Patients who were hospitalized with COVID-19 were older (median age, 9.7 years; range, 0.06-23.2 years) than those hospitalized with either type of influenza (median age, 4.2 years; range, 0.04-23.1). Patients older than 15 years made up 37% of patients with COVID-19 but only 6% of those with influenza.
Among patients hospitalized with COVID-19, 65% had at least one underlying medical condition, compared with 42% of those hospitalized for either type of influenza (OR, 2.6; 95% CI, 1.4-4.7; P = .002).
The most common underlying condition was neurologic problems from global developmental delay or seizures, identified in 11 patients (20%) hospitalized with COVID-19 and in 24 patients (8%) hospitalized with influenza (OR, 2.8; 95% CI, 1.3-6.2; P = .002). There was no significant difference between the two groups with respect to a history of asthma, cardiac disease, hematologic disease, and cancer.
For both groups, fever and cough were the most frequently reported symptoms at the time of diagnosis. However, more patients hospitalized with COVID-19 reported fever (76% vs. 55%; OR, 2.6; 95% CI, 1.4-5.1; P = 01), diarrhea or vomiting (26% vs. 12%; OR, 2.5; 95% CI, 1.2-5.0; P = .01), headache (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01), myalgia (22% vs. 7%; OR, 3.9; 95% CI, 1.8-8.5; P = .001), or chest pain (11% vs. 3%; OR, 3.9; 95% CI, 1.3-11.5; P = .01).
The researchers found no statistically significant differences between the two groups in rates of cough, congestion, sore throat, or shortness of breath.
Comparison of the symptom spectrum between COVID-19 and flu differed with respect to influenza type. More patients with COVID-19 reported fever, cough, diarrhea and vomiting, and myalgia than patients hospitalized with influenza A. But rates of fever, cough, diarrhea or vomiting, headache, or chest pain didn’t differ significantly in patients with COVID-19 and those with influenza B.
Larry K. Kociolek, MD, medical director of infection prevention and control at Ann and Robert H. Lurie Children’s Hospital of Chicago, noted the lower age of patients with flu. “Differentiating the two infections, which is difficult if not impossible based on symptoms alone, may have prognostic implications, depending on the age of the child. Because this study was performed outside peak influenza season, when coinfections would be less likely to occur, we must be vigilant about the potential clinical implications of influenza and SARS-CoV-2 coinfection this fall and winter.”
Clinicians will still have to use a combination of symptoms, examinations, and testing to distinguish the two diseases, said Aimee Sznewajs, MD, medical director of the pediatric hospital medicine department at Children’s Minnesota, Minneapolis. “We will continue to test for influenza and COVID-19 prior to hospitalizations and make decisions about whether to hospitalize based on other clinical factors, such as dehydration, oxygen requirement, and vital sign changes.”
Dr. Sznewajs stressed the importance of maintaining public health strategies, including “ensuring all children get the flu vaccine, encouraging mask wearing and hand hygiene, adequate testing to determine which virus is present, and other mitigation measures if the prevalence of COVID-19 is increasing in the community.”
Dr. Song reiterated those points, noting that clinicians need to make the most of the options they have. “Clinicians already have many great tools on hand. It is extremely important to get the flu vaccine now, especially for kids with underlying medical conditions. Diagnostic tests are available for both COVID-19 and flu. Antiviral treatment for flu is available. Judicious use of these tools will protect the health of providers, kids, and well-being at large.”
The authors noted several limitations for the study, including its retrospective design, that the data came from a single center, and that different platforms were used to detect the viruses.
A version of this article originally appeared on Medscape.com.
AI can pinpoint COVID-19 from chest x-rays
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
NYC public hospitals rose to the demands of the COVID-19 crisis
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
Hospitalists at the center of the storm
Hospitalists at the center of the storm
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
New York City Health + Hospitals (NYCH+H), the country’s largest public health care system, encompasses 11 hospitals with 4,354 staffed acute beds during normal times. It serves as the safety net for 1.1 million of the 8.4 million residents of the most populous city in the United States, many of them uninsured, undocumented, covered by Medicaid, or otherwise disadvantaged.
At the very epicenter in the early days of the historic COVID-19 pandemic, NYCH+H transferred patients between its facilities, added medical and ICU beds by the hundreds, mobilized palliative care volunteers, harnessed telemedicine and a clinician hotline, and made other sweeping changes to ensure that the city’s public health system would be able to respond to demand at the peak of the surge. That peak hit in April, when an average of 9,000 new COVID-19 cases were being reported in the city every day.
Through it all, hospitalists have played critical roles in both planning for the system’s response and caring for severely ill COVID-19 patients. Their stories reflect both the unprecedented demands on the system and the dedication of frontline clinicians.
One of those, Carla Saladini-Aponte, MD, who just finished her residency in June 2019, found herself on the firing line in March 2020 as an attending physician at 457-bed NYCH+H/Jacobi Hospital in the Bronx. “I have experienced so much in my first year on the job, dealing with a disease that we’ve never seen before,” she said. “We didn’t grasp the extent of the COVID crisis in the beginning, so we were emotionally unprepared when it first hit.”
Starting on March 30, NYCH+H administration mobilized a centralized incident command structure to coordinate response systemwide to a rapidly changing situation.
Two weeks later Jacobi was a COVID-19 hospital, top to bottom, with its medical ICU beds increased from 12 to more than 100. By mid-April, Dr. Saladini-Aponte’s team, one of 11 medical teams in the hospital, had 26 patients, all of them with COVID-19. There was not a consensus in the early days on how to manage patients with severe respiratory distress. “But by the time the surge came, we had a better understanding of the scope of the situation,” she said.
Learning to be an attending
“They don’t teach you how to be an attending during residency,” Dr. Saladini-Aponte said. “At the beginning I wasn’t such a good teacher. I just wanted to prove myself and stay one step ahead of the residents. But as an academic hospitalist you have to listen to others. I learned to ask questions of the residents every morning, including how they were doing personally.”
Sometimes a visiting consultant would ask on the floor: “‘Where’s your attending?’” not recognizing Dr. Saladini-Aponte, fresh out of residency, filling that role. At times, she felt like a PGY-4 (postgraduate year 4). But she quickly grew into the attending role and was asked to be site coordinator for the mobilization of palliative medicine volunteers at Jacobi.
“We found ourselves having to make tough ethical decisions. Some patients, even if we provided a ventilator and maximum oxygen therapy, would still die. There were difficult discussions when we didn’t know if we had enough dialysis machines, or how to manage other limited resources. The hospital was saying: You decide, if there’s a high degree of certainty about the outcome. But we had never practiced medicine this way before,” she said.
“That’s why our hospital provided daily ethics meetings with our ethics council. There would be eight people sitting 6 feet apart in a conference room, all wearing masks. We’d talk about situations that were giving us trouble. Their role wasn’t to provide answers but to help us see the scope of the situation and the complexities,” she explained.
Dr. Saladini-Aponte said she has had many sleepless nights since the pandemic began. “Sometimes, I would come home from work and lie down on the floor and cry,” she said. “But we had so much support from volunteers helping our little hospitalist service of seven.” It was also important to keep up with the clinical information, and one of her coworkers created “cheat sheets” for the clinicians, regularly updated with the latest essential information on antibiotics, testing, and the like.
“At the peak, I was trying to read everything I could about the virus. I was just pulling myself in too many directions. I asked for help from my boyfriend to remind me not to log onto my computer when I came home from work,” she said. “One of my techniques for preventing burnout was just to avoid social media. I couldn’t deal with what was going on in the news. It just angered me. Even now, seeing people without masks makes me very uncomfortable.”
Organizing the crisis response
As chief value officer for NYCH+H, Hyung (Harry) Cho, MD, FACP, SFHM, typically focuses on issues of patient safety and overuse of medical treatments in the health system. But in the COVID-19 crisis, he found himself at the forefront of organizing its response. “We tried to provide support centrally and to standardize practice in how we test and treat,” he said.
“We were truly at the epicenter of the pandemic,” Dr. Cho said. “All of our hospitals had different experiences, and unique responses. But the system worked well.” Patients were transferred from the more overtaxed hospitals to Bellevue and other NYCH+H hospitals with spare beds. An emergency medical response structure was put in place, and every morning the system’s Tiger Team, with multidisciplinary personnel from administration, operations, logistics, and medical/technical specialists, would gather virtually to discuss needs across the system.
“It was a very open atmosphere and we asked people to report what was happening on the ground,” Dr. Cho said. “We started rapidly reviewing batches of 20 patients at a time for transfer in order to alleviate pressure in the most overtaxed ERs.”
NYCH+H also had to work through concerns about PPE, just like other U.S. hospitals. Treatment guidelines were changing by the day. Medical concerns were relayed at a rapid pace. Another priority was trying to limit unnecessary exposure for staff through a recommendation that only one clinician from a team would go into the room of an infected patient, unless another was absolutely needed.
The reality of public health
NYCH+H was created by the New York State Legislature in 1969 and rebranded in 2015. It includes a low- to no-cost health insurance plan called MetroPlus, along with outpatient centers, comprehensive case management, and social supports in the home.
“What people know about public health systems is that we typically are underresourced. That’s just the reality of public health,” Dr. Cho said. “We help the community, the underserved. The people who truly needed our help are also the ones who have been disproportionately affected by COVID-19. And that is where we really shine as a system.”
Dr. Cho lauded the performance of the health system’s frontline staff. “Watching them come together during the entire pandemic, and do their best every day, was truly inspiring,” he said. “But when they got to the peak, it really took an emotional toll on them.”
NYCH+H’s in-house staff support program, called Helping Healers Heal, was mobilized with specially trained teams at each of its 11 hospitals to provide peer-to-peer support, mental health expertise, and team-debriefing sessions to staff members following traumatic events. Support is provided both over the phone and in person on the floors, Dr. Cho said. “During the surge, everything was happening so quickly, there was no time to take a pause. Now, as we are able to catch our breath, that’s when they most need support.”
The hospitalists at NYCH+H hospitals intended to have goals-of-care conversations with all patients, but everyone was very busy – so having these conversations became harder and harder, Dr. Cho said. Recognizing limited staffing for the quadrupling of patients who needed palliative care at NYCH+H hospitals, he asked the medicine chairs about their palliative care needs and then used social media outreach to ask for help. The message went viral, attracting 413 volunteers from across the country. Sixty-seven telepalliative volunteers were put to work doing goals-of-care conversations remotely with inpatients and their families.1
Expediting transfers
For Ian Fagan, MD, a hospitalist and associate medical director for general internal medicine Inpatient Services at Bellevue Hospital in Manhattan, hospitalist shifts are a normal part of his job. But he had to give them up during the surge to focus on planning, management, and especially scheduling other doctors, with sufficient backups needed to cover last minute changes. Dr. Fagan did that by using the existing pool of hospitalist staff, physicians who were reassigned from other specialties, agency staff, military medical personnel, and volunteer doctors who flew in from around the country to help. He also worked 10- to 12-hour days for 36 consecutive days.
The impact of disparities in access to care in New York City was reflected in the greater demand for care in the hospitals in Brooklyn, Queens, and the Bronx. “With fewer patients and more hospital beds in Manhattan, we had the capacity to share our beds,” Dr. Fagan said. “It was so amazing to me how quickly we could move patients from one hospital to another. We started accepting up to 40 transfers a day. But hey, we were still really busy.”
Bellevue is the nation’s oldest public hospital. “We care for the homeless, for immigrants, and we don’t ask questions. That’s our mission. I’m so proud to work here, and so grateful,” Dr. Fagan said. “If someone is undocumented or without insurance, I will give them exactly the same care. We stepped up in a big way to care for people of New York, but we’ve always been there for them – and we were there for them during the COVID surge.”
The hospitals in the system also worked together in ways Dr. Fagan had never seen. “It helped to have a central command structure with a bird’s eye view from above the level of individual hospitals, to organize and see which hospitals could step up. It’s good to have the data to put it in perspective,” he said. The system also utilized a temporary low-acuity medical center set up by NYCH+H on Roosevelt Island, as well as field hospitals organized at the Jacob K. Javits Convention Center and the USTA Billie Jean King National Tennis Center.
“At Bellevue we tried to stay ready, with the ability to turn former hospital units that were being used as offices back to beds. We always had three units lined up that were fully ready to convert. For example, I was medical director of the preop clinic and one day they gave us 24 hours to pack everything and move out. Three days later, it was a 24-bed unit. We also built a more robust rapid response and code team,” he said.
“It was hard for me not to take hospitalist shifts, because my identity is being a doctor. I eventually came to terms with the importance of the role that I was doing every day. I felt I could protect my colleagues, and if they were having an emotional day, to give them the opportunity to talk to someone. I also did the onboarding, one-on-one, of the new doctors.”
As the crisis in New York City has ebbed, Dr. Fagan was recently able to again take a week of clinical service. “The first day back on the floor I felt that I had forgotten everything. But by the end of the day, I thought, ‘Okay, I do know how to do this, after all.’ Census is down here. It’s quiet. That’s good. We need it now,” he said.
“I think the hardest moment for me was when the head nurse on our trauma unit, Ernesto DeLeon, known to everybody here, died of COVID in our ICU in April,” Dr. Fagan said. When Mr. DeLeon died, 100 hospital personnel gathered in the halls outside the room to pay their respects. “There had been a palpable fear in our lives – and this showed us that the fear was real. Ernesto was the first person I knew well who died, who acquired COVID at work doing what we’re all doing. We haven’t lost any doctors yet, but when this nurse died, we allowed ourselves to realize that this is personal. In that moment, we needed to allow ourselves to be human.”
Joan Curcio, MD, associate director of medicine at Elmhurst Hospital, said Elmhurst was where the story started for New York City and for NYCH+H. “I trained here and have spent my entire career at this hospital. It came to feel like what a battleground must be like, with things coming at you from every direction,” she said. “It was overwhelming in ways I could not have foreseen. I had seen videos from Italy [an early COVID-19 epicenter], but until it happened here, it was just hard to process.”
Things started slowly, with a few patients with severe acute respiratory distress syndrome and a 5- to 7-day turnaround to get results of their viral infection tests. “By week 2, a greater number of patients from our clinics and testing sites were filtering through the emergency department. Then hundreds.”
The normal occupancy rate for the department of medicine at Elmhurst is 110-115%, which typically means full beds plus patients in the emergency department. “We started to grow to 160, then 180, and then a peak of 250% of occupancy. We took over a rehab surgery floor, then a 35-bed surgery and hospice floor, which went to full capacity just like that,” she said. The number of non–critical care service teams increased to 20, working with redeployed staff, volunteers, military, and agency personnel, while ICU beds increased from 20 to 105.
“We were dealing with a much higher acuity level and enduring emotional turmoil with families, trying to carve out time to call them after our shift was over,” Dr. Curcio explained. Elmhurst developed a call-in hotline and a daily call-out service for families. Technology was mobilized to provide video visits and new systems were designed for isolation and for PPE distribution and use.
“I just felt that I couldn’t get everything done. I felt continually overwhelmed, and it didn’t matter how much time I took. I never felt I was able to give enough to anybody in any area, which was hard to take,” Dr. Curcio said. “But I still felt a sense of purpose and that I was making a difference – thanks to lots of support from the central office.”
Patient volume at Elmhurst is now down, lower than Dr. Curcio has ever seen it. “One of the main issues right now, moving forward, is ‘how do we function in a post-crisis mode?’” she said. The process of transitioning back to non-COVID-19 care will be complex. “When we clear a floor and clean it to go back to being a cold [COVID-19-negative] unit, it’s a whole different level of cleaning that takes 7 days.”
One moment that was particularly jarring for Dr. Curcio occurred while she was giving a tour of the hospital to visiting military medical personnel. “We went into the emergency department and I turned around and looked into a shower room, which was full of body bags. They were all full.”
But the experience has also been inspiring. “People gave their all without complaint. We hospitalists, and all those recruited to act as hospitalists, essentially took responsibility for the COVID response,” she said. “This was, hopefully, the experience of a lifetime as a medical professional. I wouldn’t want to ever experience something as daunting as this again.”
Reference
1. Israilov S et al. National outreach of telepalliative medicine volunteers for a New York City safety net system COVID-19 pandemic response. J Pain Symptom Manag. 2020 May 29. doi: 10.1016/j.jpainsymman.2020.05.026.
COVID-19 at home: What does optimal care look like?
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Marilyn Stebbins, PharmD, fell ill at the end of February 2020. Initially diagnosed with multifocal pneumonia and treated with antibiotics, she later developed severe gastrointestinal symptoms, fatigue, and shortness of breath. She was hospitalized in early March and was diagnosed with COVID-19.
It was still early in the pandemic, and testing was not available for her husband. After she was discharged, her husband isolated himself as much as possible. But that limited the amount of care he could offer.
“When I came home after 8 days in the ICU, I felt completely alone and terrified of not being able to care for myself and not knowing how much care my husband could provide,” said Dr. Stebbins, professor of clinical pharmacy at the University of California, San Francisco.
“I can’t even imagine what it would have been like if I had been home alone without my husband in the house,” she said. “I think about the people who died at home and understand how that might happen.”
Dr. Stebbins is one of tens of thousands of people who, whether hospitalized and discharged or never admitted for inpatient care, needed to find ways to convalesce at home. Data from the Centers for Medicare & Medicaid Services show that, of 326,674 beneficiaries who tested positive for COVID-19 between May 16 and June 11, 2020, 109,607 were hospitalized, suggesting that two-thirds were outpatients.
Most attention has focused on the sickest patients, leaving less severe cases to fall through the cracks. Despite fever, cough, difficulty breathing, and a surfeit of other symptoms, there are few available resources and all too little support to help patients navigate the physical and emotional struggles of contending with COVID-19 at home.
No ‘cookie-cutter’ approach
The speed with which the pandemic progressed caught public health systems off guard, but now, “it is essential to put into place the infrastructure to care for the physical and mental health needs of patients at home because most are in the community and many, if not most, still aren’t receiving sufficient support at home,” said Dr. Stebbins.
said Gary LeRoy, MD, a family physician in Dayton, Ohio. He emphasized that there is “no cookie-cutter formula” for home care, because every patient’s situation is different.
“I begin by having a detailed conversation with each patient to ascertain whether their home environment is safe and to paint a picture of their circumstances,” Dr. LeRoy, who is the president of the American Academy of Family Physicians, said in an interview.
Dr. LeRoy suggested questions that constitute “not just a ‘medical’ checklist but a ‘whole life’ checklist.”
- Do you have access to food, water, medications, sanitation/cleaning supplies, a thermometer, and other necessities? If not, who might assist in providing those?
- Do you need help with activities of daily living and self-care?
- Who else lives in your household? Do they have signs and symptoms of the virus? Have they been tested?
- Do you have enough physical space between you and other household members?
- Do you have children? How are they being cared for?
- What type of work do you do? What are the implications for your employment if you are unable to work for an extended period?
- Do you have an emotional, social, and spiritual support system (e.g., family, friends, community, church)?
- Do you have concerns I haven’t mentioned?
Patients’ responses will inform the management plan and determine what medical and social resources are needed, he said.
Daily check-in
Dr. Stebbins said the nurse case manager from her insurance company called her daily after she came home from the hospital. She was told that a public health nurse would also call, but no one from the health department called for days – a situation she hopes has improved.
One way or another, she said, “health care providers [or their staff] should check in with patients daily, either telephonically or via video.” She noted that video is superior, because “someone who isn’t a family member needs to put eyes on a patient and might be able to detect warning signs that a family member without healthcare training might not notice.”
Dr. LeRoy, who is also an associate professor of medicine at Wright State University, Dayton, Ohio, said that, given his time constraints, a nurse or medical assistant in his practice conducts the daily check-ins and notifies him if the patient has fever or other symptoms.
“Under ordinary circumstances, when a patient comes to see me for some type of medical condition, I get to meet the patient, consider what might be going on, then order a test, wait for the results, and suggest a treatment plan. But these are anything but ordinary circumstances,” said Matthew Exline, MD, a pulmonary and critical care specialist at the Ohio State University Wexner Medical Center, Columbus.
“That traditional structure broke down with COVID-19, when we may have test results without even seeing the patient. And without this interaction, it is harder to know as a physician what course of action to take,” he said in an interview.
Once a diagnosis has been made, the physician has at least some data to help guide next steps, even if there has been no prior meeting with the patient.
For example, a positive test raises a host of issues, not the least of which is the risk of spreading the infection to other household members and questions about whether to go the hospital. Moreover, for patients, positive tests can have serious ramifications.
“Severe shortness of breath at rest is not typical of the flu, nor is loss of taste or smell,” said Dr. Exline. Practitioners must educate patients and families about specific symptoms of COVID-19, including shortness of breath, loss of taste or smell, and gastrointestinal or neurologic symptoms, and when to seek emergency care.
Dr. LeRoy suggests buying a pulse oximeter to gauge blood oxygen levels and pulse rate. Together with a thermometer, a portable blood pressure monitor, and, if indicated, a blood glucose monitor, these devices provide a comprehensive and accurate assessment of vital signs.
Dr. LeRoy also educates patients and their families about when to seek medical attention.
Dr. Stebbins takes a similar approach. “Family members are part of, not apart from, the care of patients with COVID-19, and it’s our responsibility as healthcare providers to consider them in the patient’s care plan.”
Keeping family safe
Beyond care, family members need a plan to keep themselves healthy, too.
“A patient with COVID-19 at home should self-quarantine as much as possible to keep other family members safe, if they continue to live in the same house,” Dr. Exline said.
Ideally, uninfected family members should stay with relatives or friends. When that’s not possible, everyone in the household should wear a mask, be vigilant about hand washing, and wipe down all surfaces – including doorknobs, light switches, faucet handles, cellphones, and utensils – regularly with bleach or an alcohol solution.
Caregivers should also minimize the amount of time they are exposed to the patient.
“Set food, water, and medication on the night table and leave the room rather than spending hours at the bedside, since limiting exposure to viral load reduces the chances of contagion,” said Dr. Exline.
The Centers for Disease Control and Prevention offers guidance for household members caring for COVID-19 patients at home. It provides tips on how to help patients follow the doctor’s instructions and ways to ensure adequate hydration and rest, among others.
Patients with COVID-19 who live alone face more formidable challenges.
Dr. LeRoy says physicians can help patients by educating themselves about available social services in their community so they can provide appropriate referrals and connections. Such initiatives can include meal programs, friendly visit and financial assistance programs, as well as childcare and home health agencies.
He noted that Aunt Bertha, a social care network, provides a guide to social services throughout the United States. Additional resources are available on USA.gov.
Comfort and support
Patients with COVID-19 need to be as comfortable and as supported as possible, both physically and emotionally.
“While I was sick, my dogs curled up next to me and didn’t leave my side, and they were my saving grace. There’s not enough to be said about emotional support,” Dr. Stebbins said.
Although important, emotional support is not enough. For patients with respiratory disorders, such as chronic obstructive pulmonary disease, asthma, heart failure, or pneumonia, their subjective symptoms of shortness of breath, air hunger, or cough may improve with supplemental oxygen at home. Other measures include repositioning of the patient to lessen the body weight over the lungs or the use of lung percussion, Leroy said.
He added that improvement may also come from drainage of sputum from the airway passages, the use of agents to liquefy thick sputum (mucolytics), or aerosolized bronchodilator medications.
However, Dr. LeRoy cautioned, “one remedy does not work for everyone – an individual can improve gradually by using these home support interventions, or their respiratory status can deteriorate rapidly despite all these interventions.”
For this reason, he says patients should consult their personal physician to determine which, if any, of these home treatments would be best for their particular situation.
Patients who need emotional support, psychotherapy, or psychotropic medications may find teletherapy helpful. Guidance for psychiatrists, psychologists, and social workers regarding the treatment of COVID-19 patients via teletherapy can be found on the American Psychiatric Association, the American Psychological Association, and the National Association of Social Workers websites.
Pharmacists can also help ensure patient safety, Dr. Stebbins said.
If a patient has not picked up their usual medications, Dr. Stebbins said, “they may need a check-in call. Some may be ill and alone and may need encouragement to seek medical attention, and some may have no means of getting to the pharmacy and may need medications delivered.”
A home healthcare agency may also be helpful for homebound patients. David Bersson, director of operations at Synergy Home Care of Bergen County, N.J., has arranged in-home caregivers for patients with COVID-19.
The amount of care that professional caregivers provide can range from several hours per week to full-time, depending on the patient’s needs and budget, and can include companionship, Mr. Bersson said in an interview.
Because patient and caregiver safety are paramount, caregivers are thoroughly trained in protection and decontamination procedures and are regularly tested for COVID-19 prior to being sent into a client’s home.
Health insurance companies do not cover this service, Mr. Bersson noted, but the VetAssist program covers home care for veterans and their spouses who meet income requirements.
Caregiving and companionship are both vital pieces of the at-home care puzzle. “It was the virtual emotional support I got from friends, family, coworkers, and healthcare professionals that meant so much to me, and I know they played an important part in my recovery,” Dr. Stebbins said.
Dr. LeRoy agreed, noting that he calls patients, even if they only have mild symptoms and his nurse has already spoken to them. “The call doesn’t take much time – maybe just a 5-minute conversation – but it makes patients aware that I care.”
Dr. Stebbins, Dr. Exline, and Dr. LeRoy report no relevant financial relationships. Mr. Bersson is the director of operations at Synergy Home Care of Bergen County, New Jersey.
This story first appeared on Medscape.com.
Coronavirus-associated aspergillosis increased 30-day mortality risk
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
Researchers are beginning to make some headway in identifying the role of secondary infections in the course and outcomes of COVID-19.
Patients who are on ventilatory support for severe COVID-19 infections appear to be at high risk for invasive pulmonary aspergillosis, which in a small prospective study was associated with a more than threefold risk for 30-day mortality. The findings were published online in Clinical Infectious Diseases.
Among 108 patients with COVID-19 on mechanical ventilation in one of three intensive care units, 30 (27.7%) were diagnosed with coronavirus-associated pulmonary aspergillosis (CAPA) based on consensus definitions similar to those used to diagnose influenza-associated pulmonary aspergillosis (IAPA).
Of the patients with CAPA, 44% died within 30 days of ICU admission, compared with 19% of patients who did not meet the criteria for aspergillosis (P = .002). This difference translated into an odds ratio (OR) for death with CAPA of 3.55 (P = .014), reported Michele Bartoletti, MD, PhD, of the infectious diseases unit at Sant’Orsola Malpighi Hospital in Bologna, Italy, and colleagues.
When the investigators applied a proposed definition of putative invasive pulmonary aspergillosis, or “PIPA” to the same patients, the 30-day mortality rate jumped to 74% vs. 26% for patients without PIPA (P < .001), with an OR of 11.60 (P < .001). “We found a high incidence of CAPA among critically ill COVID-19 patients and that its occurrence seems to change the natural history of disease,” they wrote.
“[T]he study from Bartoletti et al. alerts the clinical audience to be aware of CAPA and take appropriate (and where needed repetitive) actions that fits their clinical setting,” Roger J. Brüggemann, PharmD, of the department of pharmacy, Radboud University Medical Center, Nijmegen, the Netherlands, and colleagues wrote in an editorial accompanying the study.
Diagnosis challenging
At the best of times, the diagnosis of pulmonary aspergillosis is difficult, subject to both false-positive and false-negative results, said a critical care specialist who was not involved in the study.
“Critically ill patients are susceptible to having aspergillus, so in reading the article, my only concerns are that I don’t know how accurate the testing is, and I don’t know if their population is truly different from a general population of patients in the ICU,” Daniel R. Ouellette, MD, FCCP, associate director of medical critical care at Henry Ford Hospital in Detroit, said in an interview.
As seen in ICU patients with severe influenza or other viral infections, patients with severe COVID-19 disease are susceptible to secondary infections, he said, making it difficult to know whether the worse outcomes seen in patients with COVID-19 and presumed aspergillosis are a reflection of their being more critically ill or whether the secondary infections themselves account for the difference in mortality.
Three ICUs
Dr. Bartoletti and colleagues conducted a study on all adult patients with microbiologically confirmed COVID-19 receiving mechanical ventilation in three ICUs in Bologna.
All patients included in the study were screened for invasive pulmonary aspergillosis with bronchoalveolar lavage and galactomannan detection and cultures. The lavage was performed on ICU admission, one day from the first day of mechanical ventilation, and if patients had evidence of clinical disease progression.
Samples that tested positive for galactomannan, a component of the aspergillus cell wall, were stored and later analyzed with a commercial quantitative real-time polymerase chain reaction assay for aspergillus; these results were not reported to clinicians on the patient floors.
The investigators defined invasive pulmonary aspergillosis according to a recently proposed definition for CAPA. This definition applies to COVID-19–positive patients admitted to an ICU with pulmonary infiltrates and at least one of the following:
- A serum galactomannan > 0.5.
- Bronchoalveolar lavage galactomannan > 1.0.
- Positive aspergillus bronchoalveolar lavage culture or cavitating infiltrate not attributed to another cause in the area of the pulmonary infiltrate.
They compared the CAPA diagnostic criteria with those of PIPA criteria as described by Stijn J. Blot, PhD, and colleagues in study published in the American Journal of Respiratory and Critical Care Medicine (2012 Jul 1;186(1):56-64).
A total of 108 patients were screened for aspergillosis, with a median age of 64. The majority of patients (78%) were male. The median age-adjusted Charlson Comorbidity Index was 2.5 (range 1-4). The median Sequential Organ Failure Assessment (SOFA) score at ICU admission was 4 (range 3-5).
As noted, probable aspergillosis by CAPA criteria was diagnosed in 30 patients (27.7%), with the diagnosis made after a median of 4 days after intubation and a median of 14 days from onset of COVID-19 symptoms.
The incidence rate of probable CAPA was 38.83 per 10,000 ICU patient days.
A comparison of clinical characteristics of patients with and without probable CAPA showed that only chronic steroid therapy at ≥ 16 mg/day prednisone for at least 15 days was significantly associated with risk for CAPA (P = .02).
At a median follow-up of 31 days, 54 patients (50%) had been discharged, 44 (41%) had died, and the remaining patients were still on follow-up.
As noted before, the mortality rate with 30 days of ICU admission was 44% for patients with probable CAPA vs. 19% for patients without. Among patients deemed to have PIPA, 74% died within 30 days of admission, compared with 26% without PIPA.
In a logistic regression model, the association of CAPA with increased risk for 30-day mortality remained even after adjustment for the need for renal replacement therapy (OR 3.02, P = .015) and SOFA score at ICU admission (OR 1.38, P = .004).
In a logistic regression using the PIPA rather than CAPA definition, the OR for 30-day mortality was 11.60 (P = .001).
Prognostic marker
The investigators noted that bronchoalveolar lavage galactomannan index appeared to be predictive of death. Each 1-point increase in the index was associated with 1.41-fold increase in the risk for 30-day mortality (P = .0070), a relationship that held up after adjustment for age, need for renal replacement therapy, and SOFA score.
Sixteen patients who met the CAPA definition received antifungal therapy, primarily voriconazole. The use of voriconazole was associated with a nonsignificant trend toward lower mortality.
They noted that the heavy use of immunomodulating agents in the patients in their study may have contributed to the high prevalence of CAPA.
Dr. Ouellette agreed that many of the therapies used to treat COVID-19 in the ICU are experimental, and that agents used to suppress the cytokine storm that is believed to contribute to disease severity may increase risk for secondary infections such as invasive aspergillosis.
“Many of our treatments may be associated with adverse consequences,” he said. “There is a trend toward treating patients with COVID-19 pneumonia with corticosteroids, and certainly that could have an immunosuppressant effect and predispose patients to secondary infections.”
He noted that the World Health Organization recommendations current in March 2020, when the pandemic began in earnest in the United States, advised against the use of corticosteroids, likely because of a lack of evidence of efficacy and concerns about risk for secondary infections.
“Regardless of the strategic choice made, all efforts should be put into improving our ability to reliably identify patients that may benefit from therapeutic interventions, which include host and risk factors, clinical factors and CAPA disease markers,” Dr. Brüggemann and colleagues wrote in their editorial.
The study was performed without external funding. The authors and Dr. Ouellette reported no conflicts of interest. Dr. Brüggemann and coauthors report grants and/or personal fees from various companies outside the submitted work.
SOURCE: Bartoletti M et al. Clin Infect Dis. 2020 Jul 28. doi: 10.1093/cid/ciaa1065.
FROM CLINICAL INFECTIOUS DISEASES