User login
New data on IV ketamine for resistant depression in the elderly
NEW ORLEANS –
“These were patients with depression who had not responded even to intensive therapies or procedures, and we found that after a 6-week ketamine infusion regimen, there was no difference in the response to the treatment between the treatment-resistant geriatric and nongeriatric patients,” study investigator Jonathan Kim, of Emory University, Atlanta, the first author of one of two studies presented as part of the American Association for Geriatric Psychiatry annual meeting, said in an interview.
The findings are important because research on the effects of IV ketamine have not been well documented in geriatric patients, who have high rates of depression and TRD.
“There is a lack of data on IV ketamine in older adults with treatment-resistant depression, and there are some safety and tolerability concerns which may lead some older adults and their clinicians to be reluctant to pursue IV ketamine treatment,” study coinvestigator Hanadi Ajam Oughli, MD, a health sciences assistant clinical professor in the department of psychiatry and biobehavioral sciences, University of California, Los Angeles, told this news organization.
Nasal vs. IV administration
Ketamine has traditionally been used as an anesthetic that blocks N-methyl-D-aspartate (NMDA) glutamate receptors, Dr. Oughli and colleagues note.
In the treatment of TRD, an infusion of 0.5 mg/kg is typically administered over 40 minutes, producing a rapid antidepressant response. Recent research shows the drug reduces suicidality and improves mood and quality of life.
A more recent intranasal formulation of ketamine, esketamine, was approved by the U.S. Food and Drug Administration for TRD in 2019, and some experts questioned its path to approval. In addition, the drug’s high cost and poor bioavailability in comparison with IV ketamine remains an issue, said Dr. Oughli.
In the previous TRANSFORM-3 study, a placebo-controlled randomized trial, there was no difference between esketamine, used in conjunction with an antidepressant, and placebo for geriatric patients.
To better understand the effects of IV ketamine in this patient population, Mr. Kim’s team conducted a retrospective chart review of 91 older patients with TRD who received IV ketamine treatment between October 2016 and August 2022.
Patients were divided into two groups – those older than 60 years (n = 36; 44% women; mean age, 68.86) and those younger than 60 (n = 55; 49% women; mean age, 41.05). Participants in each age group received six ketamine infusions over 6 weeks.
Results showed that with regard to depression severity, as assessed using Beck Depression Inventory (BDI-II) scores, 27.8% of patients in the geriatric group had a 50% or greater improvement, vs. 25.4% of those younger than 60.
The average BDI-II scores represented a significant improvement for both groups (P < .01), and the difference in scores between the groups was not statistically significant (P = .973).
“It is important to note that our study was conducted in a real-world clinical setting with a treatment-resistant population; other clinical studies may not have such sick patients in their trials. Additional studies are therefore warranted to establish further treatment guidelines in this area,” Mr. Kim said.
Open-label trial results
In the second study, Dr. Oughli and colleagues evaluated additional key outcomes in geriatric patients treated with IV ketamine as part of a larger open-label late-life trial on TRD.
The secondary analysis of the trial focused on 23 patients (mean age, 71.5 years) who had been initially treated with twice-a-week IV ketamine for 4 weeks.
After the first 4 weeks, patients who had experienced a partial response received an additional 4 weeks of once-weekly IV ketamine.
Overall, 48% of participants achieved a response, and 24% achieved remission of depressive symptoms following the first 4 weeks of twice-weekly treatment. This effect was maintained during the continuation phase of the study.
These findings are consistent with research in younger adults and demonstrate that twice-weekly infusions yield a more sustained antidepressant response than once-weekly infusions, the authors note.
The analysis also showed important increases in psychological well-being scores on the Scale for Suicidal Ideation, improved sleep quality as measured by the Pittsburgh Sleep Quality Index, and overall psychological well-being as shown on the NIH Toolbox Positive Affect on happiness/contentment and the NIH Toolbox General Life Satisfaction scales.
In a previous analysis, published in The American Journal of Geriatric Psychiatry, the researchers also evaluated cognitive function using the NIH Cognitive Battery, which showed that geriatric patients with TRD had significant improvements in a composite of executive functioning and fluid cognition during the 4-week acute treatment period of twice-weekly IV ketamine infusions (Cohen’s d = 0.61) and that those improvements were sustained in the continuation phase of once-weekly infusions for 4 more weeks.
Those results are consistent with ketamine’s known potential procognitive effects in TRD, due to a putative antidepressant mechanism that rescues prefrontal circuit dysfunction through synaptogenesis, the researchers note.
Dr. Oughli said that in both analyses, patients tolerated ketamine well, and there were no serious adverse events.
“Adverse events, including hypertension, dissociated effects, and cravings, were rare and did not prevent the continued use of IV ketamine by older adults. We were able to use clonidine to help manage blood pressure changes seen during the infusions,” she noted.
“These findings are very promising and will need to be confirmed and extended in a larger randomized controlled trial.”
Unsettling for some older patients
George T. Grossberg, MD, director, geriatric psychiatry, Saint Louis University, noted that in his experience, IV ketamine treatment can be unsettling for some older geriatric patients, such as those in their 80s.
“Particularly with some of my older patients, the kind of psychotomimetic properties of ketamine and the out-of-body experiences [with the initial treatment] can be frightening,” he said. “They may be willing to try, but I’ve had more than one patient quit after one treatment because they became so frightened.”
However, the dire nature of TRD and failure to respond to multiple medications and combinations and other strategies may prompt patients to try ketamine as a measure with at least some potential, he noted.
“But there is a high bar for acceptance, especially on the part of older adults and their families, more than for younger people,” he said.
The investigators have disclosed no relevant financial relationships. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“These were patients with depression who had not responded even to intensive therapies or procedures, and we found that after a 6-week ketamine infusion regimen, there was no difference in the response to the treatment between the treatment-resistant geriatric and nongeriatric patients,” study investigator Jonathan Kim, of Emory University, Atlanta, the first author of one of two studies presented as part of the American Association for Geriatric Psychiatry annual meeting, said in an interview.
The findings are important because research on the effects of IV ketamine have not been well documented in geriatric patients, who have high rates of depression and TRD.
“There is a lack of data on IV ketamine in older adults with treatment-resistant depression, and there are some safety and tolerability concerns which may lead some older adults and their clinicians to be reluctant to pursue IV ketamine treatment,” study coinvestigator Hanadi Ajam Oughli, MD, a health sciences assistant clinical professor in the department of psychiatry and biobehavioral sciences, University of California, Los Angeles, told this news organization.
Nasal vs. IV administration
Ketamine has traditionally been used as an anesthetic that blocks N-methyl-D-aspartate (NMDA) glutamate receptors, Dr. Oughli and colleagues note.
In the treatment of TRD, an infusion of 0.5 mg/kg is typically administered over 40 minutes, producing a rapid antidepressant response. Recent research shows the drug reduces suicidality and improves mood and quality of life.
A more recent intranasal formulation of ketamine, esketamine, was approved by the U.S. Food and Drug Administration for TRD in 2019, and some experts questioned its path to approval. In addition, the drug’s high cost and poor bioavailability in comparison with IV ketamine remains an issue, said Dr. Oughli.
In the previous TRANSFORM-3 study, a placebo-controlled randomized trial, there was no difference between esketamine, used in conjunction with an antidepressant, and placebo for geriatric patients.
To better understand the effects of IV ketamine in this patient population, Mr. Kim’s team conducted a retrospective chart review of 91 older patients with TRD who received IV ketamine treatment between October 2016 and August 2022.
Patients were divided into two groups – those older than 60 years (n = 36; 44% women; mean age, 68.86) and those younger than 60 (n = 55; 49% women; mean age, 41.05). Participants in each age group received six ketamine infusions over 6 weeks.
Results showed that with regard to depression severity, as assessed using Beck Depression Inventory (BDI-II) scores, 27.8% of patients in the geriatric group had a 50% or greater improvement, vs. 25.4% of those younger than 60.
The average BDI-II scores represented a significant improvement for both groups (P < .01), and the difference in scores between the groups was not statistically significant (P = .973).
“It is important to note that our study was conducted in a real-world clinical setting with a treatment-resistant population; other clinical studies may not have such sick patients in their trials. Additional studies are therefore warranted to establish further treatment guidelines in this area,” Mr. Kim said.
Open-label trial results
In the second study, Dr. Oughli and colleagues evaluated additional key outcomes in geriatric patients treated with IV ketamine as part of a larger open-label late-life trial on TRD.
The secondary analysis of the trial focused on 23 patients (mean age, 71.5 years) who had been initially treated with twice-a-week IV ketamine for 4 weeks.
After the first 4 weeks, patients who had experienced a partial response received an additional 4 weeks of once-weekly IV ketamine.
Overall, 48% of participants achieved a response, and 24% achieved remission of depressive symptoms following the first 4 weeks of twice-weekly treatment. This effect was maintained during the continuation phase of the study.
These findings are consistent with research in younger adults and demonstrate that twice-weekly infusions yield a more sustained antidepressant response than once-weekly infusions, the authors note.
The analysis also showed important increases in psychological well-being scores on the Scale for Suicidal Ideation, improved sleep quality as measured by the Pittsburgh Sleep Quality Index, and overall psychological well-being as shown on the NIH Toolbox Positive Affect on happiness/contentment and the NIH Toolbox General Life Satisfaction scales.
In a previous analysis, published in The American Journal of Geriatric Psychiatry, the researchers also evaluated cognitive function using the NIH Cognitive Battery, which showed that geriatric patients with TRD had significant improvements in a composite of executive functioning and fluid cognition during the 4-week acute treatment period of twice-weekly IV ketamine infusions (Cohen’s d = 0.61) and that those improvements were sustained in the continuation phase of once-weekly infusions for 4 more weeks.
Those results are consistent with ketamine’s known potential procognitive effects in TRD, due to a putative antidepressant mechanism that rescues prefrontal circuit dysfunction through synaptogenesis, the researchers note.
Dr. Oughli said that in both analyses, patients tolerated ketamine well, and there were no serious adverse events.
“Adverse events, including hypertension, dissociated effects, and cravings, were rare and did not prevent the continued use of IV ketamine by older adults. We were able to use clonidine to help manage blood pressure changes seen during the infusions,” she noted.
“These findings are very promising and will need to be confirmed and extended in a larger randomized controlled trial.”
Unsettling for some older patients
George T. Grossberg, MD, director, geriatric psychiatry, Saint Louis University, noted that in his experience, IV ketamine treatment can be unsettling for some older geriatric patients, such as those in their 80s.
“Particularly with some of my older patients, the kind of psychotomimetic properties of ketamine and the out-of-body experiences [with the initial treatment] can be frightening,” he said. “They may be willing to try, but I’ve had more than one patient quit after one treatment because they became so frightened.”
However, the dire nature of TRD and failure to respond to multiple medications and combinations and other strategies may prompt patients to try ketamine as a measure with at least some potential, he noted.
“But there is a high bar for acceptance, especially on the part of older adults and their families, more than for younger people,” he said.
The investigators have disclosed no relevant financial relationships. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“These were patients with depression who had not responded even to intensive therapies or procedures, and we found that after a 6-week ketamine infusion regimen, there was no difference in the response to the treatment between the treatment-resistant geriatric and nongeriatric patients,” study investigator Jonathan Kim, of Emory University, Atlanta, the first author of one of two studies presented as part of the American Association for Geriatric Psychiatry annual meeting, said in an interview.
The findings are important because research on the effects of IV ketamine have not been well documented in geriatric patients, who have high rates of depression and TRD.
“There is a lack of data on IV ketamine in older adults with treatment-resistant depression, and there are some safety and tolerability concerns which may lead some older adults and their clinicians to be reluctant to pursue IV ketamine treatment,” study coinvestigator Hanadi Ajam Oughli, MD, a health sciences assistant clinical professor in the department of psychiatry and biobehavioral sciences, University of California, Los Angeles, told this news organization.
Nasal vs. IV administration
Ketamine has traditionally been used as an anesthetic that blocks N-methyl-D-aspartate (NMDA) glutamate receptors, Dr. Oughli and colleagues note.
In the treatment of TRD, an infusion of 0.5 mg/kg is typically administered over 40 minutes, producing a rapid antidepressant response. Recent research shows the drug reduces suicidality and improves mood and quality of life.
A more recent intranasal formulation of ketamine, esketamine, was approved by the U.S. Food and Drug Administration for TRD in 2019, and some experts questioned its path to approval. In addition, the drug’s high cost and poor bioavailability in comparison with IV ketamine remains an issue, said Dr. Oughli.
In the previous TRANSFORM-3 study, a placebo-controlled randomized trial, there was no difference between esketamine, used in conjunction with an antidepressant, and placebo for geriatric patients.
To better understand the effects of IV ketamine in this patient population, Mr. Kim’s team conducted a retrospective chart review of 91 older patients with TRD who received IV ketamine treatment between October 2016 and August 2022.
Patients were divided into two groups – those older than 60 years (n = 36; 44% women; mean age, 68.86) and those younger than 60 (n = 55; 49% women; mean age, 41.05). Participants in each age group received six ketamine infusions over 6 weeks.
Results showed that with regard to depression severity, as assessed using Beck Depression Inventory (BDI-II) scores, 27.8% of patients in the geriatric group had a 50% or greater improvement, vs. 25.4% of those younger than 60.
The average BDI-II scores represented a significant improvement for both groups (P < .01), and the difference in scores between the groups was not statistically significant (P = .973).
“It is important to note that our study was conducted in a real-world clinical setting with a treatment-resistant population; other clinical studies may not have such sick patients in their trials. Additional studies are therefore warranted to establish further treatment guidelines in this area,” Mr. Kim said.
Open-label trial results
In the second study, Dr. Oughli and colleagues evaluated additional key outcomes in geriatric patients treated with IV ketamine as part of a larger open-label late-life trial on TRD.
The secondary analysis of the trial focused on 23 patients (mean age, 71.5 years) who had been initially treated with twice-a-week IV ketamine for 4 weeks.
After the first 4 weeks, patients who had experienced a partial response received an additional 4 weeks of once-weekly IV ketamine.
Overall, 48% of participants achieved a response, and 24% achieved remission of depressive symptoms following the first 4 weeks of twice-weekly treatment. This effect was maintained during the continuation phase of the study.
These findings are consistent with research in younger adults and demonstrate that twice-weekly infusions yield a more sustained antidepressant response than once-weekly infusions, the authors note.
The analysis also showed important increases in psychological well-being scores on the Scale for Suicidal Ideation, improved sleep quality as measured by the Pittsburgh Sleep Quality Index, and overall psychological well-being as shown on the NIH Toolbox Positive Affect on happiness/contentment and the NIH Toolbox General Life Satisfaction scales.
In a previous analysis, published in The American Journal of Geriatric Psychiatry, the researchers also evaluated cognitive function using the NIH Cognitive Battery, which showed that geriatric patients with TRD had significant improvements in a composite of executive functioning and fluid cognition during the 4-week acute treatment period of twice-weekly IV ketamine infusions (Cohen’s d = 0.61) and that those improvements were sustained in the continuation phase of once-weekly infusions for 4 more weeks.
Those results are consistent with ketamine’s known potential procognitive effects in TRD, due to a putative antidepressant mechanism that rescues prefrontal circuit dysfunction through synaptogenesis, the researchers note.
Dr. Oughli said that in both analyses, patients tolerated ketamine well, and there were no serious adverse events.
“Adverse events, including hypertension, dissociated effects, and cravings, were rare and did not prevent the continued use of IV ketamine by older adults. We were able to use clonidine to help manage blood pressure changes seen during the infusions,” she noted.
“These findings are very promising and will need to be confirmed and extended in a larger randomized controlled trial.”
Unsettling for some older patients
George T. Grossberg, MD, director, geriatric psychiatry, Saint Louis University, noted that in his experience, IV ketamine treatment can be unsettling for some older geriatric patients, such as those in their 80s.
“Particularly with some of my older patients, the kind of psychotomimetic properties of ketamine and the out-of-body experiences [with the initial treatment] can be frightening,” he said. “They may be willing to try, but I’ve had more than one patient quit after one treatment because they became so frightened.”
However, the dire nature of TRD and failure to respond to multiple medications and combinations and other strategies may prompt patients to try ketamine as a measure with at least some potential, he noted.
“But there is a high bar for acceptance, especially on the part of older adults and their families, more than for younger people,” he said.
The investigators have disclosed no relevant financial relationships. Dr. Grossberg has received consulting fees from Acadia, Avanir, Biogen, BioXcel, Genentech, Karuna, Lundbeck, Otsuka, Roche, and Takeda.
A version of this article first appeared on Medscape.com.
AT AAGP 2023
Add-on antipsychotic beats switching meds in older adults with resistant depression
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
“We found that adding aripiprazole led to higher rates of depression remission and greater improvements in psychological well-being – which means how positive and satisfied patients felt – and this is good news,” study investigator Eric J. Lenze, MD, of the department of psychiatry, Washington University, St. Louis, said in a press statement.
“However, even that approach helped only about 30% of people in the study with treatment-resistant depression, underscoring the need to find and develop more effective treatments that can help more people,” he added.
The findings were presented here as part of the American Association for Geriatric Psychiatry annual meeting, and published concurrently in the New England Journal of Medicine.
Need for safe treatment options
Treatment-resistant depression is common in older patients, but switching medications or adding other agents can be challenging. With higher rates of comorbidity and polypharmacy, treatment decisions in this patient population are more complex compared with those involving younger patients.
To compare the benefits of augmentation vs. drug-switching strategies, the researchers conducted a multicenter, two-step trial involving 619 patients with an average baseline age of 69 who had failed to respond to two courses of selective serotonin reuptake inhibitors (SSRIs).
Patients were randomly assigned to one of three groups. These included augmentation of existing antidepressant medication with either aripiprazole (n = 211) or the dopamine and norepinephrine–reuptake inhibitor bupropion (Wellbutrin, Zyban) (n = 206), or to taper off of their current antidepressant and switch to bupropion (n = 202).
After 10 weeks, patients’ psychological well-being was assessed via the National Institutes of Health Toolbox Positive Affect and General Life Satisfaction subscales. The researchers found patients in the aripiprazole and bupropion add-on groups improved by 4.83 points and 4.33 points, respectively. The bupropion switch group had a change of 2.04 points.
The difference between the aripiprazole augmentation group and the switch to bupropion group was significant (difference 2.79 points; P = .014). Other between-group differences were not significantly different.
Remission rates were similar in the aripiprazole and bupropion groups at 28.9% and 28.2%, respectively. The remission rate in the bupropion switch group was 19.3%.
The study results showed patients who received adjunctive bupropion had the highest fall rate at 0.55 falls per patient, vs. 0.33 falls per patient in the aripiprazole group, suggesting that among the three treatment options, adjunctive aripiprazole may be the best choice because of its superior efficacy and lower fall risk.
A total of 248 patients enrolled in the study showed no improvement and were further randomly assigned to receive adjunctive lithium (n = 127) or switch from current therapy to nortriptyline (n = 121).
Well-being scores in the lithium group improved by 3.17 points and 2.18 points in the nortriptyline group. Remission occurred in 18.9% of patients in the lithium group and 21.5% in the nortriptyline group. Fall rates were similar among the two groups.
Overall, “this large, randomized study demonstrated that adding aripiprazole was a superior option for older adults with treatment-resistant depression,” Dr. Lenze told this news organization.
“Since neither lithium nor nortriptyline were promising against treatment-resistant depression in older adults, those medications are unlikely to be helpful in most cases,” he added.
Practice changing?
In an accompanying editorial, Gemma Lewis, PhD, and Glyn Lewis, PhD, division of psychiatry, University of College London, noted the findings “support aripiprazole augmentation as a strategy for treatment-resistant depression in older persons, largely because of the lower risk of falls than with bupropion augmentation.”
However, “in clinical practice, [it] would be important to tailor treatment in light of potential adverse effects and the preferences of the patient,” they added.
Akathisia, for instance, is a common side effect of aripiprazole, shown in one recent trial to affect 11% of the patients. In addition, weight gain, though typically lower than seen with other antipsychotics, is a consideration with aripiprazole.
With respect to fall risk, they noted that bupropion was largely used in relatively high doses of 300 mg and 450 mg, despite some recent research showing little clinical benefit from increasing antidepressant doses above minimum recommendations.
“It is possible that smaller doses of bupropion than those used in the current trial would retain effectiveness while minimizing adverse effects such as falls,” the editorialists noted.
Commenting on the study, Jennifer R. Gatchel, MD, PhD, assistant psychiatrist at Massachusetts General Hospital/McLean Hospital and assistant professor of psychiatry at Harvard Medical School, Boston, said the findings have high clinical significance in the treatment of geriatric depression.
“These results are of great impact for clinicians managing older adults with treatment-resistant depression. They provide some of the first evidence of safety and efficacy of augmentation with aripiprazole as a strategy in clinical management of older adults who fail to initially respond to treatment,” said Dr. Gatchel, who was not associated with this research.
“Of particular significance, efficacy here is based on patient-centered outcomes and psychological well-being as a primary effectiveness outcome, which could translate into strengthened physician-patient alliance.”
While adjunctive aripiprazole is not necessarily a first-line strategy when older adults fail to respond to antidepressants, there is a lack of data on the risks and benefits of any other antipsychotic medications, she noted.
“Thus, this is evidence that will impact clinical practice and hopefully contribute to reduced societal burden of depression in older adults and the morbidity and mortality associated with it,” Dr. Gatchel said.
The study received support from a Patient-Centered Outcomes Research Institute (PCORI) Award (TRD-1511-33321). Dr. Lenze received additional support from the Taylor Family Institute for Innovative Psychiatric Research at Washington University School of Medicine, as well as the Washington University Institute of Clinical and Translational Sciences grant (UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Gatchel reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAGP 2023
Depression Pathophysiology
Depression Overview
Depressive symptoms tied to higher stroke risk, worse outcomes
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Data from the international INTERSTROKE study also showed that those with depressive symptoms before a stroke had worse outcomes, including a significantly higher mortality rate in the first month after a stroke.
These findings build on prior research on the link between depression and stroke, including one study that showed an increased risk for incident stroke among those with a high number of depressive symptoms and another that found that worsening depression can precede stroke in older adults.
“Depression is an important risk factor for acute stroke and is potentially a modifiable contributor to the global burden of stroke,” lead investigator Robert Murphy, MB, a consultant in stroke and geriatric medicine and a researcher with the clinical research facility at the University of Galway, Ireland, told this news organization. “Even mild depressive symptoms were found in this study to be associated with increased risk of stroke and this adds to the literature that across the full range of depressive symptoms there is an association with increased risk of stroke.”
The findings were published online March 8 in Neurology.
Significant stroke risk
For the analysis, investigators collected data on 26,877 cases and controls across 32 countries who participated in INTERSTROKE, an international case-control study of risk factors for a first acute stroke. Participants were recruited between 2007 and 2015 and completed a series of questionnaires about stroke risk factors, including measures of depressive symptoms experienced in the past 12 months.
After adjustment for occupation, education, wealth index, diet, physical activity, alcohol consumption, and smoking history, having prestroke depressive symptoms was associated with greater odds for acute stroke (adjusted odds ratio [aOR], 1.46; 95% confidence interval [CI], 1.34-1.58), including both intracerebral hemorrhage (aOR, 1.56; 95% CI, 1.28-1.91) and ischemic stroke (aOR, 1.44; 95% CI, 1.31-1.58).
Stroke risk increased with increasing severity of depression, but even those with mild depression had a 35% increased risk (aOR, 1.35; 95% CI, 1.19-1.53).
The increased risk held even after the researchers adjusted further for diabetes, hypertension, atrial fibrillation, and body mass index, and work, home, and financial stress.
The association was consistent across geographical regions and age groups, but was stronger in men and in those without hypertension.
“This study looks at different constructs of depression and identifies that across the spectrum of mild, moderate, and severe depressive symptoms that there is an association present with acute stroke and that a biological gradient emerges with increasing burden of depressive symptoms associated with increasing risk,” Dr. Murphy said.
An antidepressant mediating effect?
While prestroke depressive symptoms were not associated with a greater odds of worse stroke severity, they were associated with worse outcomes (P < .001) and higher mortality (10% vs. 8.1%; P = .003) 1 month after a stroke.
In a subgroup analysis, researchers found no association between depressive symptoms and stroke risk in patients who were taking antidepressants.
While no assumptions of causality can be drawn from these findings, “this subgroup analysis does suggest that an increased risk of stroke in those with depression may be attenuated if a patient is on appropriate treatment,” Dr. Murphy said. “This is an area that warrants further exploration.”
The mechanisms that link depression to stroke are unclear, but these findings offer strong evidence that this link exists, Dr. Murphy said.
“We adjusted for potential confounders in sequential models and after adjusting for traditional cardiovascular risk factors there was a consistent association between depressive symptoms and stroke identifying that there is likely an independent association between depression and stroke,” Dr. Murphy said.
Questions remain
Commenting on the study, Daniel T. Lackland DrPH, professor, division of translational neurosciences and population studies, department of neurology, Medical University of South Carolina, Charleston, said it adds to a growing body of work on the association of stroke and depression.
“In this case, depression may be a risk factor for having a stroke,” said Dr. Lackland, who was not part of the study. In addition, the study suggests that “treating depression can have additional benefits beyond mental health, in this case, reduced stroke risks.”
However, it’s important, as with any observational study, that there may be confounding factors that may offer an alternative explanation for the findings.
“Further, it is often difficult to accurately assess depression in all individuals, and specifically in individuals who have had a stroke,” Dr. Lackland said. “While this particular study adds depression as a risk factor and suggests treatment of depression in reducing risks, it is important to emphasize that the traditional stroke risk factors including hypertension should [be] continually recognized and treat[ed] with high rigor.”
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Swedish Research Council, the Swedish Heart Lung Foundation, AFA Insurance, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), Merck Sharp & Dohme, the Swedish Heart Lung Foundation, Chest Heart & Stroke Scotland, and the Stroke Association (United Kingdom). Dr. Murphy and Dr. Lackland have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Modified ECT lowers dental, skeletal fracture risk
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“ECT is associated with a very low risk of skeletal fractures, even in high-risk patients, and is also associated with a low risk of dental fractures,” said study investigator Chittaranjan Andrade, MD, noting that preexisting bone and dental disease increase this risk.
Overall, clinicians who provide ECT “need to be aware of rare adverse effects, as well as the common ones,” Dr. Andrade, senior professor of clinical psychopharmacology and neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India, told this news organization. He added they also “need data to be able to provide reassurance.”
The findings were published online in The Journal of Clinical Psychiatry.
Avoid unmodified ECT
Dr. Andrade conducted the study because the risk of skeletal and dental fractures associated with ECT is “not commonly discussed.”
Although ECT is perhaps the most effective available treatment for major mental illness, it is associated with several adverse effects, including those associated with delivery of an electrical stimulus to the brain, which results in central and peripheral seizure, he noted.
“The central seizure is essential for the efficacy of ECT,” said Dr. Andrade. In contrast, “the motor seizure has no therapeutic value, is cosmetically displeasing, and may rarely be associated with peripheral adverse effects affecting muscles, joints, teeth, and bones,” he added.
The musculoskeletal and dental injuries are caused by stretching, twisting, compression, or direct injury. Particularly during the motor seizure, the “sudden jerk” associated with the tonic contraction of muscles as well as the repeated jerks associated with each clonic contraction can result in injuries, including skeletal and dental fractures.
To address this concern, the motor seizure is “modified” or attenuated through use of an intravenous muscle relaxant administered with other ECT premedication.
“How effectively the musculoskeletal and dental adverse effects are minimized depends on how well the motor seizure is modified,” Dr. Andrade said. He emphasized that the “use of unmodified ECT is strongly discouraged.”
Dr. Andrade reviewed prior research into the skeletal and dental risks of ECT. The infrequency of cases and ethical difficulties in conducting randomized clinical trials with such patients require reliance on anecdotal reports, he said.
Bite blocks, seizure modifiers
Population-based data showed that the fracture risk with modified ECT is two events per 100,000 ECTs. However, the risk may be as low as 0.36 events per 100,000 ECTs if calculated only with recent data, Dr. Andrade noted.
Population-based studies also suggest that the dental fracture risk with modified ECT is .02% per ECT and .17% per ECT course.
Although fractures have been reported under “unusual circumstances” among patients receiving modified ECT, many other reports point to the safety of this treatment, even in ultrahigh-risk patients.
Such patients include those with severe osteoporosis, metastatic bone disease, osteogenesis imperfecta, Ehlers-Danlos syndrome, Harrington rod implants, recent long bone fractures, multiple bone fractures, surgical repair of hip fracture, vertebroplasty, and maxillofacial repair.
Dr. Andrade noted that oral health is “poor” among patients with major mental illness for multiple reasons, including poor nutrition, self-neglect, and decreased salivation caused by the anticholinergic effects of medications.
This places these patients at increased risk for dental adverse effects during ECT because the muscles of the jaw contract forcefully during the motor seizure, causing sudden impact and, subsequently, sustained pressure on the teeth, Dr. Andrade said.
Moreover, because ECT is typically administered through repeated sessions, dental injuries may accumulate over the course of treatment.
ECT-associated skeletal risks arise from the tonic-clonic contractions of the muscles of the trunk and limbs, which need to be addressed via use of succinylcholine or other muscle relaxants included in ECT premedication.
Dr. Andrade noted that succinylcholine is effective at modifying the motor seizure at the common dose of 0.5-1.0 mg/kg. However, about 5% of patients require a higher dose (>1.5 mg/kg). If the dose is 1-2 mg/kg for patients at high risk for orthopedic complications, “muscle relaxation during ECT could be expected to be reasonably complete,” he said.
“Because of wide interpersonal variation, a neurostimulator may need to be used to identify the ideal dose for an individual patient,” he added.
In addition, use of bite blocks and effective jaw immobilization during ECT can reduce the risk. “Careful assessment of preexisting risk and good ECT practice can minimize the risk of skeletal and dental complications during ECT,” Dr. Andrade said.
Risks vs. benefits
Commenting on the study, Mark S. George, MD, distinguished professor of psychiatry, radiology, and neurology, and director of the brain stimulation division, Medical University of South Carolina, Charleston, said this was a “well-written review of how frequently patients who are undergoing modern ECT have bone fractures or dental fractures during the procedure.”
Dr. George, who was not involved with the research, added that modern medications and management “make ECT a truly safe procedure.”
“It is not without some risk, but these risks are low, especially when compared to the risks of untreated or undertreated depression or catatonia, like suicide,” he said.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made directly to registered charities, but does not benefit financially from the relationship. His travel expenses for delivering lectures and workshops have been supported by the organizers themselves or pharmaceutical companies at the behest of the organizers. He has provided advice to various pharmaceutical companies and has received “nominal compensation.” He has also received payments for developing educational materials for scientific initiatives and programs, such as for the Behavioral and Neurosciences Foundation of India, PsyBase India, Texas Tech University USA, the Nordic Association for Convulsive Therapy, and the American Society of Clinical Psychopharmacology. Dr. George reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Specialty and age may contribute to suicidal thoughts among physicians
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
A physician’s specialty can make a difference when it comes to having suicidal thoughts. Doctors who specialize in family medicine, obstetrics-gynecology, and psychiatry reported double the rates of suicidal thoughts than doctors in oncology, rheumatology, and pulmonary medicine, according to Doctors’ Burden: Medscape Physician Suicide Report 2023.
“The specialties with the highest reporting of physician suicidal thoughts are also those with the greatest physician shortages, based on the number of job openings posted by recruiting sites,” said Peter Yellowlees, MD, professor of psychiatry and chief wellness officer at UC Davis Health.
Doctors in those specialties are overworked, which can lead to burnout, he said.
There’s also a generational divide among physicians who reported suicidal thoughts. Millennials (age 27-41) and Gen-X physicians (age 42-56) were more likely to report these thoughts than were Baby Boomers (age 57-75) and the Silent Generation (age 76-95).
“Younger physicians are more burned out – they may have less control over their lives and less meaning than some older doctors who can do what they want,” said Dr. Yellowlees.
One millennial respondent commented that being on call and being required to chart detailed notes in the EHR has contributed to her burnout. “I’m more impatient and make less time and effort to see my friends and family.”
One Silent Generation respondent commented, “I am semi-retired, I take no call, I work no weekends, I provide anesthesia care in my area of special expertise, I work clinically about 46 days a year. Life is good, particularly compared to my younger colleagues who are working 60-plus hours a week with evening work, weekend work, and call. I feel really sorry for them.”
When young people enter medical school, they’re quite healthy, with low rates of depression and burnout, said Dr. Yellowlees. Yet, studies have shown that rates of burnout and suicidal thoughts increased within 2 years. “That reflects what happens when a group of idealistic young people hit a horrible system,” he said.
Who’s responsible?
Millennials were three times as likely as baby boomers to say that a medical school or health care organization should be responsible when a student or physician commits suicide.
“Young physicians may expect more of their employers than my generation did, which we see in residency programs that have unionized,” said Dr. Yellowlees, a Baby Boomer.
“As more young doctors are employed by health care organizations, they also may expect more resources to be available to them, such as wellness programs,” he added.
Younger doctors also focus more on work-life balance than older doctors, including time off and having hobbies, he said. “They are much more rational in terms of their overall beliefs and expectations than the older generation.”
Whom doctors confide in
Nearly 60% of physician-respondents with suicidal thoughts said they confided in a professional or someone they knew. Men were just as likely as women to reach out to a therapist (38%), whereas men were slightly more likely to confide in a family member and women were slightly more likely to confide in a colleague.
“It’s interesting that women are more active in seeking support at work – they often have developed a network of colleagues to support each other’s careers and whom they can confide in,” said Dr. Yellowlees.
He emphasized that 40% of physicians said they didn’t confide in anyone when they had suicidal thoughts. Of those, just over half said they could cope without professional help.
One respondent commented, “It’s just a thought; nothing I would actually do.” Another commented, “Mental health professionals can’t fix the underlying reason for the problem.”
Many doctors were concerned about risking disclosure to their medical boards (42%); that it would show up on their insurance records (33%); and that their colleagues would find out (25%), according to the report.
One respondent commented, “I don’t trust doctors to keep it to themselves.”
Another barrier doctors mentioned was a lack of time to seek help. One commented, “Time. I have none, when am I supposed to find an hour for counseling?”
A version of this article originally appeared on Medscape.com.
Even mild COVID is hard on the brain
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
Distinct suicidal thought patterns flag those at highest risk
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.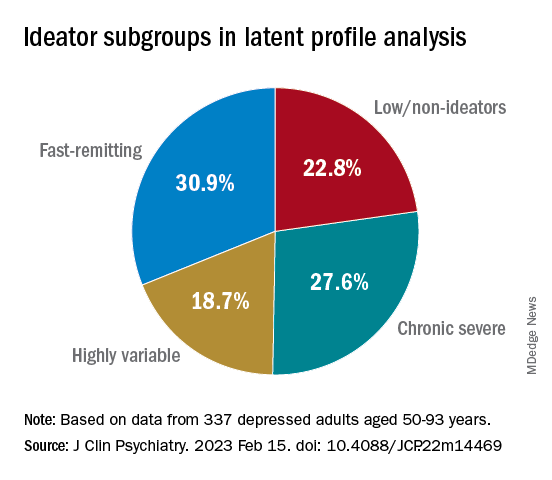
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.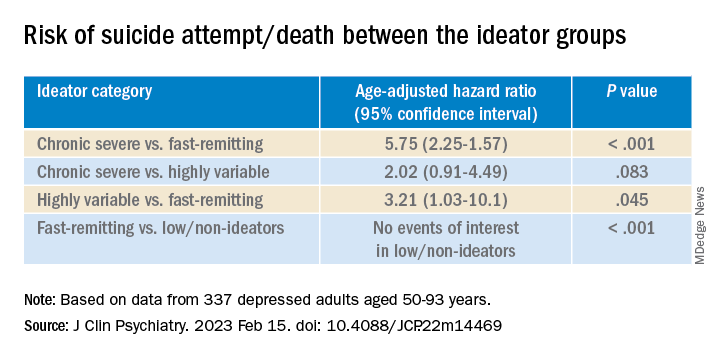
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term assessment of suicide risk and ideation in older adults may help identify distinct ideation patterns and predict potential future suicidal behavior, new research suggests.
Investigators studied over 300 older adults, assessing suicidal ideation and behavior for up to 14 years at least once annually. They then identified four suicidal ideation profiles.
They found that In turn, fast-remitting ideators were at higher risk in comparison to low/nonideators with no attempts or suicide.
Chronic severe ideators also showed the most severe levels of dysfunction across personality, social characteristics, and impulsivity measures, while highly variable and fast-remitting ideators displayed more specific deficits.
“We identified longitudinal ideation profiles that convey differential risk of future suicidal behavior to help clinicians recognize high suicide risk patients for preventing suicide,” said lead author Hanga Galfalvy, PhD, associate professor, department of psychiatry, Columbia University Irving Medical Center, New York.
“Clinicians should repeatedly assess suicidal ideation and ask not only about current ideation but also about the worst ideation since the last visit [because] similar levels of ideation during a single assessment can belong to very different risk profiles,” said Dr. Galfalvy, also a professor of biostatistics and a coinvestigator in the Conte Center for Suicide Prevention at Columbia University.
The study was published online in the Journal of Clinical Psychiatry.
Vulnerable population
“Older adults in most countries, including the U.S., are at the highest risk of dying of suicide out of all age groups,” said Dr. Galfalvy. “A significant number of depressed older adults experience thoughts of killing themselves, but fortunately, only a few transition from suicidal thoughts to behavior.”
Senior author Katalin Szanto, MD, professor of psychiatry, University of Pittsburgh, said in an interview that currently established clinical and psychosocial suicide risk factors have “low predictive value and provide little insight into the high suicide rate in the elderly.”
These traditional risk factors “poorly distinguish between suicide ideators and suicide attempters and do not take into consideration the heterogeneity of suicidal behavior,” said Dr. Szanto, principal investigator at the University of Pittsburgh’s Longitudinal research Program in Late-Life Suicide, where the study was conducted.
“Suicidal ideation measured at one time point – current or lifetime – may not be enough to accurately predict suicide risk,” the investigators wrote.
The current study, a collaboration between investigators from the Longitudinal Research Program in Late-Life Suicide and the Conte Center for Suicide Prevention, investigates “profiles of suicidal thoughts and behavior in patients with late-life depression over a longer period of time,” Dr. Galfalvy said.
The researchers used latent profile analysis (LPA) in a cohort of adults with nonpsychotic unipolar depression (aged 50-93 years; n = 337; mean age, 65.12 years) to “identify distinct ideation profiles and their clinical correlates” and to “test the profiles’ association with the risk of suicidal behavior before and during follow-up.”
LPA is “a data-driven method of grouping individuals into subgroups, based on quantitative characteristics,” Dr. Galfalvy explained.
The LPA yielded four profiles of ideation.
At baseline, the researchers assessed the presence or absence of suicidal behavior history and the number and lethality of attempts. They prospectively assessed suicidal ideation and attempts at least once annually thereafter over a period ranging from 3 months to 14 years (median, 3 years; IQR, 1.6-4 years).
At baseline and at follow-ups, they assessed ideation severity.
They also assessed depression severity, impulsivity, and personality measures, as well as perception of social support, social problem solving, cognitive performance, and physical comorbidities.
Personalized prevention
Of the original cohort, 92 patients died during the follow-up period, with 13 dying of suicide (or suspected suicide).
Over half (60%) of the chronic severe as well as the highly variable groups and almost half (48%) of the fast-remitting group had a history of past suicide attempt – all significantly higher than the low-nonideators (0%).
Despite comparable current ideation severity at baseline, the risk of suicide attempt/death was greater for chronic severe ideators versus fast-remitting ideators, but not greater than for highly variable ideators. On the other hand, highly variable ideators were at greater risk, compared with fast-remitting ideators.
Cognitive factors “did not significantly discriminate between the ideation profiles, although ... lower global cognitive performance predicted suicidal behavior during follow-up,” the authors wrote.
This finding “aligns with prior studies indicating that late-life suicidal behavior but not ideation may be related to cognition ... and instead, ideation and cognition may act as independent risk factors for suicidal behavior,” they added.
“Patients in the fluctuating ideator group generally had moderate or high levels of worst suicidal ideation between visits, but not when asked about current ideation levels at the time of the follow-up assessment,” Dr. Galfalvy noted. “For them, the time frame of the question made a difference as to the level of ideation reported.”
The study “identified several clinical differences among these subgroups which could lead to more personalized suicide prevention efforts and further research into the heterogeneity of suicidal behavior,” she suggested.
New insight
Commenting on the study, Ari Cuperfain, MD, of the University of Toronto said the study “adds to the nuanced understanding of how changes in suicidal ideation over time can lead to suicidal actions and behavior.”
The study “sheds light on the notion of how older adults who die by suicide can demonstrate a greater degree of premeditated intent relative to younger cohorts, with chronic severe ideators portending the highest risk for suicide in this sample,” added Dr. Cuperfain, who was not involved with the current research.
“Overall, the paper highlights the importance of both screening for current levels of suicidal ideation in addition to the evolution of suicidal ideation in developing a risk assessment and in finding interventions to reduce this risk when it is most prominent,” he stated.
The research was supported by the National Institutes of Health. The authors and Dr. Cuperfain disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Encephalitis linked to psychosis, suicidal thoughts
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators assessed 120 patients hospitalized in a neurological center and diagnosed with ANMDARE. Most had psychosis and other severe mental health disturbances. Of these, 13% also had suicidal thoughts and behaviors.
However, after medical treatment that included immunotherapy, neurologic and psychiatric pharmacotherapy, and rehabilitation and psychotherapy, almost all patients with suicidal thoughts and behaviors had sustained remission of their suicidality.
“Most patients [with ANMDARE] suffer with severe mental health problems, and it is not infrequent that suicidal thoughts and behaviors emerge in this context – mainly in patients with clinical features of psychotic depression,” senior author Jesús Ramirez-Bermúdez, MD, PhD, from the neuropsychiatry unit, National Institute of Neurology and Neurosurgery of Mexico, told this news organization.
“The good news is that, in most cases, the suicidal thoughts and behaviors as well as the features of psychotic depression improve significantly with the specific immunological therapy. However, careful psychiatric and psychotherapeutic support are helpful to restore the long-term psychological well-being,” Dr. Ramirez-Bermúdez said.
The findings were published online in the Journal of Neuropsychiatry and Clinical Neurosciences.
Delayed recognition
ANMDARE is a “frequent form of autoimmune encephalitis,” the authors write. It often begins with an “abrupt onset of behavioral and cognitive symptoms, followed by seizures and movement disorders,” they add.
“The clinical care of persons with encephalitis is challenging because these patients suffer from acute and severe mental health disturbances [and] are often misdiagnosed as having a primary psychiatric disorder, for instance, schizophrenia or bipolar disorder; but, they do not improve with the use of psychiatric medication or psychotherapy,” Dr. Ramirez-Bermúdez said.
Rather, the disease requires specific treatments, such as the use of antiviral medication or immunotherapy, he added. Without these, “the mortality rate is high, and many patients have bad outcomes, including disability related to cognitive and affective disturbances,” he said.
Dr. Ramirez-Bermúdez noted that there are “many cultural problems in the conventional approach to mental health problems, including prejudices, fear, myths, stigma, and discrimination.” And these attitudes can contribute to delayed recognition of ANMDARE.
During recent years, Dr. Ramirez-Bermúdez and colleagues observed that some patients with autoimmune encephalitis and, more specifically, patients suffering from ANMDARE had suicidal behavior. A previous study conducted in China suggested that the problem of suicidal behavior is not infrequent in this population.
“We wanted to make a structured, systematic, and prospective approach to this problem to answer some questions related to ANMDARE,” Dr. Ramirez-Bermúdez said. These questions included: What is the frequency of suicidal thoughts and behaviors, what are the neurological and psychiatric features related to suicidal behavior in this population, and what is the outcome after receiving immunological treatment?
The researchers conducted an observational longitudinal study that included patients hospitalized between 2014 and 2021 who had definite ANMDARE (n = 120).
Patients were diagnosed as having encephalitis by means of clinical interviews, neuropsychological studies, brain imaging, EEG, and analysis of cerebrospinal fluid (CSF).
All participants had antibodies against the NMDA glutamate receptor in their CSF and were classified as having ANMDARE based on Graus criteria, “which are considered the best current standard for diagnosis,” Dr. Ramirez-Bermúdez noted.
Clinical measures were obtained both before and after treatment with immunotherapy, and all clinical data were registered prospectively and included a “broad scope of neurological and psychiatric variables seen in patients with ANMDARE.”
Information regarding suicidal thoughts and behaviors was gathered from patients as well as relatives, with assessments occurring at admission and at discharge.
Biological signaling
Results showed that 15 patients presented with suicidal thoughts and/or behaviors. Of this subgroup, the median age was 32 years (range, 19-48 years) and 53.3% were women.
All members of this subgroup had psychotic features, including persecutory, grandiose, nihilistic, or jealousy delusion (n = 14), delirium (n = 13), visual or auditory hallucinations (n = 11), psychotic depression (n = 10), and/or catatonia (n = 8).
Most (n = 12) had suicidal ideation with intent, three had preparatory behaviors, and seven actually engaged in suicidal self-directed violence.
Of these 15 patients, 7 had abnormal CSF findings, 8 had MRI abnormalities involving the medial temporal lobe, and all had abnormal EEG involving generalized slowing.
Fourteen suicidal patients were treated with an antipsychotic, 4 with dexmedetomidine, and 12 with lorazepam. In addition, 10 received plasmapheresis and 7 received immunoglobulin.
Of note, at discharge, self-directed violent thoughts and behaviors completely remitted in 14 of the 15 patients. Long-term follow-up showed that they remained free of suicidality.
Dr. Ramirez-Bermúdez noted that in some patients with neuropsychiatric disturbances, “there are autoantibodies against the NR1 subunit of the NMDA glutamate receptor: the main excitatory neurotransmitter in the human brain.”
The NMDA receptor is “particularly important as part of the biological signaling that is required in several cognitive and affective processes leading to complex behaviors,” he said. NMDA receptor dysfunction “may lead to states in which these cognitive and affective processes are disturbed,” frequently resulting in psychosis.
Study coauthor Ava Easton, MD, chief executive of the Encephalitis Society, told this news organization that mental health issues, self-injurious thoughts, and suicidal behaviors after encephalitis “may occur for a number of reasons and stigma around talking about mental health can be a real barrier to speaking up about symptoms; but it is an important barrier to overcome.”
Dr. Easton, an honorary fellow in the department of clinical infection, microbiology, and immunology, University of Liverpool, England, added that their study “provides a platform on which to break taboo, show tangible links which are based on data between suicide and encephalitis, and call for more awareness of the risk of mental health issues during and after encephalitis.”
‘Neglected symptom’
Commenting on the study, Carsten Finke, MD, Heisenberg Professor for Cognitive Neurology and consultant neurologist, department of neurology at Charité, Berlin, and professor at Berlin School of Mind and Brain, said that the research was on “a very important topic on a so far rather neglected symptom of encephalitis.”
Dr. Finke, a founding member of the scientific council of the German Network for Research on Autoimmune Encephalitis, was not involved in the current study.
He noted that 77% of people don’t know what encephalitis is. “This lack of awareness leads to delays in diagnoses and treatment – and poorer outcomes for patients,” Dr. Finke said.
Also commenting, Michael Eriksen Benros, MD, PhD, professor of immune-psychiatry, department of immunology and microbiology, Health and Medical Sciences, University of Copenhagen, said that the study “underlines the clinical importance of screening individuals with psychotic symptoms for suicidal ideations during acute phases,” as well as those with definite ANMDARE as a likely underlying cause of the psychotic symptoms.
This is important because patients with ANMDARE “might not necessarily be admitted at psychiatric departments where screenings for suicidal ideation are part of the clinical routine,” said Dr. Benros, who was not involved with the research.
Instead, “many patients with ANMDARE are at neurological departments during acute phases,” he added.
The study was supported by the National Council of Science and Technology of Mexico. Dr. Ramirez-Bermúdez, Dr. Easton, Dr. Benros, and Dr. Finke report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROPSYCHIATRY AND CLINICAL NEUROSCIENCES