User login
Thyroid hormones predict psychotic depression in MDD patients
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
Thyroid dysfunction is common among major depressive disorder (MDD) patients, but its relationship with the psychotic depression (PD) subtype has not been well studied, wrote Pu Peng, of The Second Xiangya Hospital of Central South University, Changsha, Hunan, China, and colleagues.
Given the significant negative consequences of PD in MDD, including comorbid psychosis, suicidal attempts, and worse prognosis, more ways to identify PD risk factors in MDD are needed, they said. Previous research suggests a role for thyroid hormones in the pathophysiology of PD, but data on specific associations are limited, they noted.
In a study published in Psychiatry Research, the authors recruited 1,718 adults aged 18-60 years with MDD who were treated at a single center. The median age was 34 years, 66% were female, and 10% were identified with PD.
Clinical symptoms were identified using the positive subscale of the Positive and Negative Symptom Scale (PANSS-P), Hamilton Anxiety Rating Scale (HAMA), and Hamilton Depression Rating Scale (HAMD). The median PANSS-P score was 7. The researchers measured serum levels of thyroid stimulating hormone (TSH), anti-thyroglobulin (TgAb), and thyroid peroxidases antibody (TPOAb). Subclinical hyperthyroidism (SCH) was defined as TSH levels greater than 8.0 uIU/L and FT4 within normal values.
Overall, the prevalence of SCH, abnormal TgAb, TPOAb, FT3, and FT4 were 13%, 17%, 25%, <0.1%, and 0.3%, respectively. Serum TSH levels, TgAb levels, and TPOAb levels were significantly higher in PD patients than in non-PD patients. No differences appeared in FT3 and FT4 levels between the two groups.
In a multivariate analysis, subclinical hypothyroidism was associated with a ninefold increased risk of PD (odds ratio, 9.32) as were abnormal TPOAb (OR, 1.89) and abnormal TgAb (OR, 2.09).
The findings were limited by several factors including the cross-sectional design, and the inclusion of participants from only a single center in China, which may limit generalizability, the researchers noted.
In addition, “It should be noted that the association between thyroid hormones and PD was small to moderate and the underlying mechanism remained unexplored,” they said. Other limitations include the use of only 17 of the 20 HAMD items and the lack of data on the relationship between anxiety and depressive features and thyroid dysfunction, they wrote.
More research is needed to confirm the findings in other populations, however; the results suggest that regular thyroid function tests may help with early detection of PD in MDD patients, they concluded.
The study was funded by the CAS Pioneer Hundred Talents Program and the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose.
FROM PSYCHIATRY RESEARCH
Beyond the psychedelic effect: Ayahuasca as antidepressant
Ayahuasca is a psychoactive beverage that has long been used by indigenous people in South America in religious ceremonies and tribal rituals. In recent years, the beverage has emerged as a strong candidate for implementation into psychiatric care, particularly for patients with treatment-resistant depression.
Studies have shown that taking ayahuasca is associated with an improvement of depressive symptoms. In a study published in Frontiers in Psychiatry, a team of researchers from Brazil’s Federal University of Rio Grande do Norte (UFRN) describe an experimental ayahuasca session. They found that
Nicole Leite Galvão-Coelho, PhD, professor of physiology and behavior at UFRN, is one of the authors of that study. She is also a researcher at the NICM Health Research Institute at Western Sydney University. Dr. Galvão-Coelho spoke with this news organization about her team’s work.
A total of 72 people volunteered to participate in the study. There were 28 patients, all of whom were experiencing a moderate to severe depressive episode at screening. In addition, they had been diagnosed with treatment-resistant depression and had not achieved remission after at least two treatments with antidepressant medications of different classes. These patients had been experiencing depression for about 10.71 ± 9.72 years. The other 44 volunteers were healthy control participants. All the participants – both in the patient group and the control group – were naive to any classic serotonergic psychedelic such as ayahuasca.
In each group, half received ayahuasca, and the other half received a placebo. The dosing session was performed at UFRN’s Onofre Lopes University Hospital and lasted about 8 hours.
All volunteers underwent a full clinical mental health evaluation and medical history. Blood and saliva samples were collected at baseline, approximately 4 hours before the dosing session, and 2 days after the dosing session. During the dosing session, saliva samples were collected at 1 hour 40 minutes, 2 hours 40 minutes, and 4 hours after ayahuasca intake.
The study showed that some acute measures assessed during ayahuasca dosing moderated the improvements in major depressive disorder (MDD) biomarkers 2 days after the session in patients with treatment-resistant depression. Larger acute decreases of depressive symptoms moderated higher levels of SC in those patients, while lower acute changes in SC levels were related to higher BDNF levels in patients with a larger clinical response.
The UFRN research team has been investigating the potential antidepressant effects of ayahuasca for approximately 12 years. According to Dr. Galvão-Coelho, the work reported in the most recent article – one in a series of articles that they wrote – provides a step forward as a pioneering psychedelic field study assessing the biological changes of MDD molecular biomarkers. “There have indeed been observational studies and open-label clinical studies. We were the first team, though, to conduct placebo-controlled clinical studies with ayahuasca in patients with treatment-resistant depression,” she explained. She noted that the work was carried out in partnership with Dráulio Barros de Araújo, PhD, a professor at UFRN’s Brain Institute, as well as with a multidisciplinary team of researchers in Brazil and Australia.
Dr. Galvão-Coelho said that in an earlier study, the UFRN researchers observed that a single dose of ayahuasca led to long-lasting behavioral and physiologic improvements in an animal (marmoset) model. In another study, there was improvement in depression severity for patients with treatment-resistant depression 7 days after taking ayahuasca.
As for biomarkers, Dr. Galvão-Coelho said that there is a long history of research on cortisol (the “stress hormone”) with respect to patients with depressive symptoms, given the link between chronic stress and depressive disorders. “In our patients with treatment-resistant depression, we found that before being dosed with ayahuasca, they presented hypocortisolemia,” she said. She noted that low levels of cortisol are as harmful to one’s health as high levels. According to her, the goal should be to sustain moderate levels. “In other studies, we’ve shown that patients with more recent, less chronic depression have high cortisol levels, but after a little while, the [adrenal] glands get overworked, which seems to lead to a situation where they’re not producing all those important hormones. That’s why chronic conditions of depression are marked by low levels of cortisol. But,” she pointed out, “after patients with treatment-resistant depression take ayahuasca, we no longer see hypocortisolemia.”
Another biomarker analyzed by the research team, the protein BDNF, has the capacity to induce neuroplasticity. Indeed, Dr. Galvão-Coelho mentioned a theory that antidepressant drugs work when they increase levels of this protein, which would stimulate new connections in the brain.
Because several earlier studies indicated that other psychedelic substances would promote an increase in BDNF, the UFRN researchers decided to explore the potential effects of ayahuasca on this biomarker. “We observed that there was actually an increase in serum BDNF, and the patients who showed the greatest increase [of this marker] had a more significant reduction in depressive symptoms,” Dr. Galvão-Coelho explained.
Considering all the previous findings, the team wondered whether acute parameters recorded during an ayahuasca dosing session could in some way modulate the responses of certain key MDD molecular biomarkers. They then conducted their study that was published last December.
Dr. Galvão-Coelho said that the results of that study show that acute emotional and physiologic effects of ayahuasca seem to be relevant to an improvement of key MDD molecular biomarkers (namely, SC and BDNF). She also noted that the results revealed that larger reductions of depressive symptoms during the dosing session significantly moderated higher levels of SC in patients 2 days after ayahuasca intake. In the case of BDNF, the positive correlation between clinical response and day-2 BDNF levels only occurred for patients who experienced small increases of cortisol during the experimental session. These were individuals who did not have such an intense response to stress and who felt more at ease during the session.
The findings showed which factors that arise during the psychedelic state induced by ayahuasca modulate biological response associated with the antidepressant action of these substances in patients with major depression. “We realized, for example, that to bring about a sense of comfort and trust, to get a good acute response, the dosing session had to be extremely well thought out. That seemed to be relevant to the results on the other days,” Dr. Galvão-Coelho explained.
For her, there was another takeaway from the research: New antidepressant treatments should be complemented by a more comprehensive view of the case at hand. “We have to think about the patient’s overall improvement – including, therefore, the improvement of biomarkers – and not focus solely on the clinical symptoms.”
This article was translated from the Medscape Portuguese Edition.
A version of this article first appeared on Medscape.com.
Ayahuasca is a psychoactive beverage that has long been used by indigenous people in South America in religious ceremonies and tribal rituals. In recent years, the beverage has emerged as a strong candidate for implementation into psychiatric care, particularly for patients with treatment-resistant depression.
Studies have shown that taking ayahuasca is associated with an improvement of depressive symptoms. In a study published in Frontiers in Psychiatry, a team of researchers from Brazil’s Federal University of Rio Grande do Norte (UFRN) describe an experimental ayahuasca session. They found that
Nicole Leite Galvão-Coelho, PhD, professor of physiology and behavior at UFRN, is one of the authors of that study. She is also a researcher at the NICM Health Research Institute at Western Sydney University. Dr. Galvão-Coelho spoke with this news organization about her team’s work.
A total of 72 people volunteered to participate in the study. There were 28 patients, all of whom were experiencing a moderate to severe depressive episode at screening. In addition, they had been diagnosed with treatment-resistant depression and had not achieved remission after at least two treatments with antidepressant medications of different classes. These patients had been experiencing depression for about 10.71 ± 9.72 years. The other 44 volunteers were healthy control participants. All the participants – both in the patient group and the control group – were naive to any classic serotonergic psychedelic such as ayahuasca.
In each group, half received ayahuasca, and the other half received a placebo. The dosing session was performed at UFRN’s Onofre Lopes University Hospital and lasted about 8 hours.
All volunteers underwent a full clinical mental health evaluation and medical history. Blood and saliva samples were collected at baseline, approximately 4 hours before the dosing session, and 2 days after the dosing session. During the dosing session, saliva samples were collected at 1 hour 40 minutes, 2 hours 40 minutes, and 4 hours after ayahuasca intake.
The study showed that some acute measures assessed during ayahuasca dosing moderated the improvements in major depressive disorder (MDD) biomarkers 2 days after the session in patients with treatment-resistant depression. Larger acute decreases of depressive symptoms moderated higher levels of SC in those patients, while lower acute changes in SC levels were related to higher BDNF levels in patients with a larger clinical response.
The UFRN research team has been investigating the potential antidepressant effects of ayahuasca for approximately 12 years. According to Dr. Galvão-Coelho, the work reported in the most recent article – one in a series of articles that they wrote – provides a step forward as a pioneering psychedelic field study assessing the biological changes of MDD molecular biomarkers. “There have indeed been observational studies and open-label clinical studies. We were the first team, though, to conduct placebo-controlled clinical studies with ayahuasca in patients with treatment-resistant depression,” she explained. She noted that the work was carried out in partnership with Dráulio Barros de Araújo, PhD, a professor at UFRN’s Brain Institute, as well as with a multidisciplinary team of researchers in Brazil and Australia.
Dr. Galvão-Coelho said that in an earlier study, the UFRN researchers observed that a single dose of ayahuasca led to long-lasting behavioral and physiologic improvements in an animal (marmoset) model. In another study, there was improvement in depression severity for patients with treatment-resistant depression 7 days after taking ayahuasca.
As for biomarkers, Dr. Galvão-Coelho said that there is a long history of research on cortisol (the “stress hormone”) with respect to patients with depressive symptoms, given the link between chronic stress and depressive disorders. “In our patients with treatment-resistant depression, we found that before being dosed with ayahuasca, they presented hypocortisolemia,” she said. She noted that low levels of cortisol are as harmful to one’s health as high levels. According to her, the goal should be to sustain moderate levels. “In other studies, we’ve shown that patients with more recent, less chronic depression have high cortisol levels, but after a little while, the [adrenal] glands get overworked, which seems to lead to a situation where they’re not producing all those important hormones. That’s why chronic conditions of depression are marked by low levels of cortisol. But,” she pointed out, “after patients with treatment-resistant depression take ayahuasca, we no longer see hypocortisolemia.”
Another biomarker analyzed by the research team, the protein BDNF, has the capacity to induce neuroplasticity. Indeed, Dr. Galvão-Coelho mentioned a theory that antidepressant drugs work when they increase levels of this protein, which would stimulate new connections in the brain.
Because several earlier studies indicated that other psychedelic substances would promote an increase in BDNF, the UFRN researchers decided to explore the potential effects of ayahuasca on this biomarker. “We observed that there was actually an increase in serum BDNF, and the patients who showed the greatest increase [of this marker] had a more significant reduction in depressive symptoms,” Dr. Galvão-Coelho explained.
Considering all the previous findings, the team wondered whether acute parameters recorded during an ayahuasca dosing session could in some way modulate the responses of certain key MDD molecular biomarkers. They then conducted their study that was published last December.
Dr. Galvão-Coelho said that the results of that study show that acute emotional and physiologic effects of ayahuasca seem to be relevant to an improvement of key MDD molecular biomarkers (namely, SC and BDNF). She also noted that the results revealed that larger reductions of depressive symptoms during the dosing session significantly moderated higher levels of SC in patients 2 days after ayahuasca intake. In the case of BDNF, the positive correlation between clinical response and day-2 BDNF levels only occurred for patients who experienced small increases of cortisol during the experimental session. These were individuals who did not have such an intense response to stress and who felt more at ease during the session.
The findings showed which factors that arise during the psychedelic state induced by ayahuasca modulate biological response associated with the antidepressant action of these substances in patients with major depression. “We realized, for example, that to bring about a sense of comfort and trust, to get a good acute response, the dosing session had to be extremely well thought out. That seemed to be relevant to the results on the other days,” Dr. Galvão-Coelho explained.
For her, there was another takeaway from the research: New antidepressant treatments should be complemented by a more comprehensive view of the case at hand. “We have to think about the patient’s overall improvement – including, therefore, the improvement of biomarkers – and not focus solely on the clinical symptoms.”
This article was translated from the Medscape Portuguese Edition.
A version of this article first appeared on Medscape.com.
Ayahuasca is a psychoactive beverage that has long been used by indigenous people in South America in religious ceremonies and tribal rituals. In recent years, the beverage has emerged as a strong candidate for implementation into psychiatric care, particularly for patients with treatment-resistant depression.
Studies have shown that taking ayahuasca is associated with an improvement of depressive symptoms. In a study published in Frontiers in Psychiatry, a team of researchers from Brazil’s Federal University of Rio Grande do Norte (UFRN) describe an experimental ayahuasca session. They found that
Nicole Leite Galvão-Coelho, PhD, professor of physiology and behavior at UFRN, is one of the authors of that study. She is also a researcher at the NICM Health Research Institute at Western Sydney University. Dr. Galvão-Coelho spoke with this news organization about her team’s work.
A total of 72 people volunteered to participate in the study. There were 28 patients, all of whom were experiencing a moderate to severe depressive episode at screening. In addition, they had been diagnosed with treatment-resistant depression and had not achieved remission after at least two treatments with antidepressant medications of different classes. These patients had been experiencing depression for about 10.71 ± 9.72 years. The other 44 volunteers were healthy control participants. All the participants – both in the patient group and the control group – were naive to any classic serotonergic psychedelic such as ayahuasca.
In each group, half received ayahuasca, and the other half received a placebo. The dosing session was performed at UFRN’s Onofre Lopes University Hospital and lasted about 8 hours.
All volunteers underwent a full clinical mental health evaluation and medical history. Blood and saliva samples were collected at baseline, approximately 4 hours before the dosing session, and 2 days after the dosing session. During the dosing session, saliva samples were collected at 1 hour 40 minutes, 2 hours 40 minutes, and 4 hours after ayahuasca intake.
The study showed that some acute measures assessed during ayahuasca dosing moderated the improvements in major depressive disorder (MDD) biomarkers 2 days after the session in patients with treatment-resistant depression. Larger acute decreases of depressive symptoms moderated higher levels of SC in those patients, while lower acute changes in SC levels were related to higher BDNF levels in patients with a larger clinical response.
The UFRN research team has been investigating the potential antidepressant effects of ayahuasca for approximately 12 years. According to Dr. Galvão-Coelho, the work reported in the most recent article – one in a series of articles that they wrote – provides a step forward as a pioneering psychedelic field study assessing the biological changes of MDD molecular biomarkers. “There have indeed been observational studies and open-label clinical studies. We were the first team, though, to conduct placebo-controlled clinical studies with ayahuasca in patients with treatment-resistant depression,” she explained. She noted that the work was carried out in partnership with Dráulio Barros de Araújo, PhD, a professor at UFRN’s Brain Institute, as well as with a multidisciplinary team of researchers in Brazil and Australia.
Dr. Galvão-Coelho said that in an earlier study, the UFRN researchers observed that a single dose of ayahuasca led to long-lasting behavioral and physiologic improvements in an animal (marmoset) model. In another study, there was improvement in depression severity for patients with treatment-resistant depression 7 days after taking ayahuasca.
As for biomarkers, Dr. Galvão-Coelho said that there is a long history of research on cortisol (the “stress hormone”) with respect to patients with depressive symptoms, given the link between chronic stress and depressive disorders. “In our patients with treatment-resistant depression, we found that before being dosed with ayahuasca, they presented hypocortisolemia,” she said. She noted that low levels of cortisol are as harmful to one’s health as high levels. According to her, the goal should be to sustain moderate levels. “In other studies, we’ve shown that patients with more recent, less chronic depression have high cortisol levels, but after a little while, the [adrenal] glands get overworked, which seems to lead to a situation where they’re not producing all those important hormones. That’s why chronic conditions of depression are marked by low levels of cortisol. But,” she pointed out, “after patients with treatment-resistant depression take ayahuasca, we no longer see hypocortisolemia.”
Another biomarker analyzed by the research team, the protein BDNF, has the capacity to induce neuroplasticity. Indeed, Dr. Galvão-Coelho mentioned a theory that antidepressant drugs work when they increase levels of this protein, which would stimulate new connections in the brain.
Because several earlier studies indicated that other psychedelic substances would promote an increase in BDNF, the UFRN researchers decided to explore the potential effects of ayahuasca on this biomarker. “We observed that there was actually an increase in serum BDNF, and the patients who showed the greatest increase [of this marker] had a more significant reduction in depressive symptoms,” Dr. Galvão-Coelho explained.
Considering all the previous findings, the team wondered whether acute parameters recorded during an ayahuasca dosing session could in some way modulate the responses of certain key MDD molecular biomarkers. They then conducted their study that was published last December.
Dr. Galvão-Coelho said that the results of that study show that acute emotional and physiologic effects of ayahuasca seem to be relevant to an improvement of key MDD molecular biomarkers (namely, SC and BDNF). She also noted that the results revealed that larger reductions of depressive symptoms during the dosing session significantly moderated higher levels of SC in patients 2 days after ayahuasca intake. In the case of BDNF, the positive correlation between clinical response and day-2 BDNF levels only occurred for patients who experienced small increases of cortisol during the experimental session. These were individuals who did not have such an intense response to stress and who felt more at ease during the session.
The findings showed which factors that arise during the psychedelic state induced by ayahuasca modulate biological response associated with the antidepressant action of these substances in patients with major depression. “We realized, for example, that to bring about a sense of comfort and trust, to get a good acute response, the dosing session had to be extremely well thought out. That seemed to be relevant to the results on the other days,” Dr. Galvão-Coelho explained.
For her, there was another takeaway from the research: New antidepressant treatments should be complemented by a more comprehensive view of the case at hand. “We have to think about the patient’s overall improvement – including, therefore, the improvement of biomarkers – and not focus solely on the clinical symptoms.”
This article was translated from the Medscape Portuguese Edition.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN PSYCHIATRY
Statin disappoints for treatment-resistant depression
The randomized clinical trial findings contradict earlier, smaller studies in patients with major depressive disorder (MDD) that suggested statins may reduce symptoms.
“Given the promising results from preliminary trials of statins in MDD, I was surprised that simvastatin did not separate from placebo in our trial,” lead author M. Ishrat Husain, MBBS, MD, associate professor of psychiatry and scientific head of the Centre for Addiction and Mental Health Clinical Trials Unit at the University of Toronto, told this news organization.
“I believe that our findings suggest that statins are not effective augmentation strategies in treatment-resistant depression,” Dr. Husain said.
The findings were published online in JAMA Network Open.
Disappointing results
The double-blind, placebo-controlled randomized clinical trial was conducted in five centers in Pakistan and included 150 patients with major depressive episode whose symptoms did not improve after treatment with at least two antidepressants.
In addition to their prescribed antidepressants, participants received 20 mg/day of simvastatin (n = 77) or placebo (n = 73).
At 12 weeks, both groups reported improvements in Montgomery-Åsberg Depression Rating Scale total scores, but there was no significant difference between groups. The estimated mean difference for simvastatin vs. placebo was −0.61 (P = .7).
Researchers found similar results when they compared scores from the Generalized Anxiety Disorder Scale and Morisky Medication Adherence Scale.
“Much like several other studies in mood disorders, our study results were impacted by a large placebo response,” Dr. Husain said.
The lack of inclusion of any participants under the age of 18 and the single-country cohort were limitations of the trial. Although it is possible that could have affected the outcome, Dr. Husain said it isn’t likely.
It is also unlikely that a different statin would yield different results, he added.
“Simvastatin was selected as it is believed to be most brain penetrant of the statins given its lipophilicity,” Dr. Husain said. “Clinical trials of other statins in major depressive disorder in other settings and populations have also been congruent with our results.”
The study was funded by NIHR Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Dr. Husain reports having received grants from Compass Pathways, holds stock options in Mindset, and previously served on the Board of Trustees of the Pakistan Institute of Living and Learning. Disclosures for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
The randomized clinical trial findings contradict earlier, smaller studies in patients with major depressive disorder (MDD) that suggested statins may reduce symptoms.
“Given the promising results from preliminary trials of statins in MDD, I was surprised that simvastatin did not separate from placebo in our trial,” lead author M. Ishrat Husain, MBBS, MD, associate professor of psychiatry and scientific head of the Centre for Addiction and Mental Health Clinical Trials Unit at the University of Toronto, told this news organization.
“I believe that our findings suggest that statins are not effective augmentation strategies in treatment-resistant depression,” Dr. Husain said.
The findings were published online in JAMA Network Open.
Disappointing results
The double-blind, placebo-controlled randomized clinical trial was conducted in five centers in Pakistan and included 150 patients with major depressive episode whose symptoms did not improve after treatment with at least two antidepressants.
In addition to their prescribed antidepressants, participants received 20 mg/day of simvastatin (n = 77) or placebo (n = 73).
At 12 weeks, both groups reported improvements in Montgomery-Åsberg Depression Rating Scale total scores, but there was no significant difference between groups. The estimated mean difference for simvastatin vs. placebo was −0.61 (P = .7).
Researchers found similar results when they compared scores from the Generalized Anxiety Disorder Scale and Morisky Medication Adherence Scale.
“Much like several other studies in mood disorders, our study results were impacted by a large placebo response,” Dr. Husain said.
The lack of inclusion of any participants under the age of 18 and the single-country cohort were limitations of the trial. Although it is possible that could have affected the outcome, Dr. Husain said it isn’t likely.
It is also unlikely that a different statin would yield different results, he added.
“Simvastatin was selected as it is believed to be most brain penetrant of the statins given its lipophilicity,” Dr. Husain said. “Clinical trials of other statins in major depressive disorder in other settings and populations have also been congruent with our results.”
The study was funded by NIHR Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Dr. Husain reports having received grants from Compass Pathways, holds stock options in Mindset, and previously served on the Board of Trustees of the Pakistan Institute of Living and Learning. Disclosures for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
The randomized clinical trial findings contradict earlier, smaller studies in patients with major depressive disorder (MDD) that suggested statins may reduce symptoms.
“Given the promising results from preliminary trials of statins in MDD, I was surprised that simvastatin did not separate from placebo in our trial,” lead author M. Ishrat Husain, MBBS, MD, associate professor of psychiatry and scientific head of the Centre for Addiction and Mental Health Clinical Trials Unit at the University of Toronto, told this news organization.
“I believe that our findings suggest that statins are not effective augmentation strategies in treatment-resistant depression,” Dr. Husain said.
The findings were published online in JAMA Network Open.
Disappointing results
The double-blind, placebo-controlled randomized clinical trial was conducted in five centers in Pakistan and included 150 patients with major depressive episode whose symptoms did not improve after treatment with at least two antidepressants.
In addition to their prescribed antidepressants, participants received 20 mg/day of simvastatin (n = 77) or placebo (n = 73).
At 12 weeks, both groups reported improvements in Montgomery-Åsberg Depression Rating Scale total scores, but there was no significant difference between groups. The estimated mean difference for simvastatin vs. placebo was −0.61 (P = .7).
Researchers found similar results when they compared scores from the Generalized Anxiety Disorder Scale and Morisky Medication Adherence Scale.
“Much like several other studies in mood disorders, our study results were impacted by a large placebo response,” Dr. Husain said.
The lack of inclusion of any participants under the age of 18 and the single-country cohort were limitations of the trial. Although it is possible that could have affected the outcome, Dr. Husain said it isn’t likely.
It is also unlikely that a different statin would yield different results, he added.
“Simvastatin was selected as it is believed to be most brain penetrant of the statins given its lipophilicity,” Dr. Husain said. “Clinical trials of other statins in major depressive disorder in other settings and populations have also been congruent with our results.”
The study was funded by NIHR Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King’s College London. Dr. Husain reports having received grants from Compass Pathways, holds stock options in Mindset, and previously served on the Board of Trustees of the Pakistan Institute of Living and Learning. Disclosures for the other investigators are fully listed in the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Iron deficiency in psychiatric patients
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
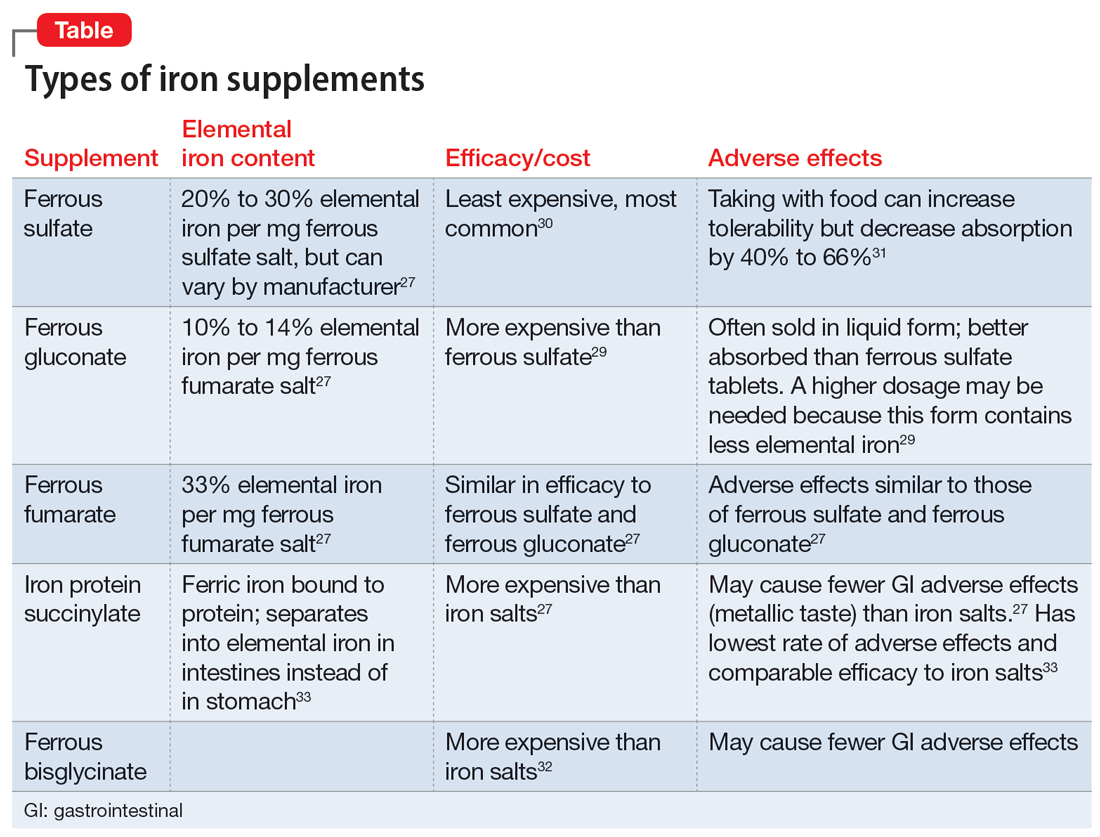
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34
Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Increased anxiety and depression after menstruation
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
‘Sighing’ tops mindfulness for reduced stress, improved mood
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
Time for a national ketamine registry, experts say
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
Teen girls report record levels of sadness, sexual violence: CDC
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Lack of motivation to change can be deadly
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
For 15 years I rounded at Jefferson Medical College in Philadelphia as a psychiatric consultant with the chair of the department of otolaryngology, his residents, and medical students to see severely ill head and neck cancer patients.
Most of these patients were very depressed, dealing with the severe losses of disfigurement, with decreased self-esteem, and the functional losses of mastication, smell, hearing, and taste. Further exacerbating their depression were the functional limitations of social skills they experienced, with attendant alienation, decreased concentration, persistence, and pace – as well as decreased adaptive skills.
Many of these patients were interjecting a great deal of anger and were very anxious dealing with their disabling surgeries and nonideal recoveries. I witnessed patients dealing with horrific losses – of their tongues, their mandibles, and facial bones – that were chilling, even more horrific than the textbook pictures that I saw in medical school.
Many of these patients I followed with medication management and psychotherapy as outpatients after seeing them during their hospitalization. Throughout the medical literature a direct relationship has been shown between head and neck cancers and alcohol abuse, chewing tobacco, and smoking, and it became apparent that many of these patients were dealing with alcohol and tobacco issues before their cancers. I would have thought that having gone through these horrendous experiences would have been an incentive to stop abusing. To the contrary, after following these patients, I found the majority (about two-thirds) continued with their old habits, even with my interventions.
Susan A. Cohen, DMD, a dentist who has practiced for over 20 years, has also witnessed comparable outcomes, having seen and referred similar cancer patients to the appropriate medical specialists, and upon following these patients noticed that about the same percentage (two-thirds) continued their alcohol and tobacco habits. A common theme and defense mechanism of these patients was denial, and they would often say something like “I have a great doctor who can fix anything, and I don’t have to worry about my habits.” In using the primitive oral defense mechanism of denial, they had problems taking responsibility for their own actions and changing their habits.
Furthermore, Dr. Susan Cohen reveals that abusing tobacco causes severe periodontal problems, including the loss of teeth. She also notes that the same patients have exhibited decreased personal oral hygiene, which further aggravates periodontal disease, loss of dentition, and increases the likelihood of cancers of the mouth and esophagus. She discovered that the losses that occur cause patients to become more depressed and continue the vicious cycle of self-medication with alcohol and tobacco.
In conclusion, we both found that despite disfigurement and loss of function, these postsurgical patients – for the most part – continued their abusive habits.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for more than 40 years and is on the editorial advisory board for Clinical Psychiatry News. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.









