User login
How to make resident mental health care stigma free
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Sarah Sofka, MD, FACP, noticed a pattern. As program director for the internal medicine (IM) residency at West Virginia University, Morgantown, she was informed when residents were sent to counseling because they were affected by burnout, depression, or anxiety. When trainees returned from these visits, many told her the same thing: They wished they had sought help sooner.
IM residents and their families had access to free counseling at WVU, but few used the resource, says Dr. Sofka. “So, we thought, let’s just schedule all of our residents for a therapy visit so they can go and see what it’s like,” she said. “This will hopefully decrease the stigma for seeking mental health care. If everybody’s going, it’s not a big deal.”
In July 2015, Dr. Sofka and her colleagues launched a universal well-being assessment program for the IM residents at WVU. The program leaders automatically scheduled first- and second-year residents for a visit to the faculty staff assistance program counselors. The visits were not mandatory, and residents could choose not to go; but if they did go, they received the entire day of their visit off from work.
Five and a half years after launching their program, Dr. Sofka and her colleagues conducted one of the first studies of the efficacy of an opt-out approach for resident mental wellness. They found that , suggesting that residents were seeking help proactively after having to at least consider it.
Opt-out counseling is a recent concept in residency programs – one that’s attracting interest from training programs across the country. Brown University, Providence, R.I.; the University of Colorado at Denver, Aurora; University of Pennsylvania, Philadelphia; and the University of California, San Francisco have at least one residency program that uses the approach.
Lisa Meeks, PhD, an assistant professor of family medicine at Michigan Medicine, in Ann Arbor, and other experts also believe opt-out counseling could decrease stigma and help normalize seeking care for mental health problems in the medical community while lowering the barriers for trainees who need help.
No time, no access, plenty of stigma
Burnout and mental health are known to be major concerns for health care workers, especially trainees. College graduates starting medical education have lower rates of burnout and depression, compared with demographically matched peers; however, once they’ve started training, medical students, residents, and fellows are more likely to be burned out and exhibit symptoms of depression. The ongoing COVID-19 pandemic is further fraying the well-being of overworked and traumatized health care professionals, and experts predict a mental health crisis will follow the viral crisis.
The Accreditation Council for Graduate Medical Education recently mandated that programs offer wellness services to trainees. Yet this doesn’t mean they are always used; well-known barriers stand between residents, medical students, and physicians and their receiving effective mental health treatment.
Two of the most obvious are access and time, given the grueling and often inflexible schedules of most trainees, says Jessica Gold, MD, a psychiatrist at Washington University, St. Louis, who specializes in treating medical professionals. Dr. Gold also points out that, to be done correctly, these programs require institutional support and investment – resources that aren’t always adequate.
“A lack of transparency and clear messaging around what is available, who provides the services, and how to access these services can be a major barrier,” says Erene Stergiopoulos, MD, a second-year psychiatry resident at the University of Toronto. In addition, there can be considerable lag between when a resident realizes they need help and when they manage to find a provider and schedule an appointment, says Dr. Meeks.
Even when these logistical barriers are overcome, trainees and physicians have to contend with the persistent stigma associated with mental health treatment in the culture of medicine, says Dr. Gold. A recent survey by the American College of Emergency Physicians found that 73% of surveyed physicians feel there is stigma in their workplace about seeking mental health treatment. Many state medical licensing boards still require physicians to disclose mental health treatment, which discourages many trainees and providers from seeking proactive care, says Mary Moffit, PhD, associate professor of psychiatry and director of the resident and faculty wellness program at Oregon Health & Science University, Portland.
How the opt-out approach works
“The idea is by making it opt-out, you really normalize it,” says Maneesh Batra, MD, MPH, associate director of the University of Washington, Seattle, Children’s Hospital residency program. Similar approaches have proven effective at shaping human behavior in other health care settings, including boosting testing rates for HIV and increasing immunization rates for childhood vaccines, Dr. Batra says.
In general, opt-out programs acknowledge that people are busy and won’t take that extra step or click that extra button if they don’t have to, says Oana Tomescu, MD, PhD, associate professor of clinical medicine and pediatrics at the University of Pennsylvania, Philadelphia.
In 2018, Dr. Sofka and her colleagues at WVU conducted a survey that showed that a majority of residents thought favorably of their opt-out program and said they would return to counseling for follow-up care. In their most recent study, published in the Journal of Graduate Medical Education in 2021, Dr. Sofka and her colleagues found that residents did just that – only 8 of 239 opted out of universally scheduled visits. Resident-initiated visits increased significantly from zero during the 2014-2015 academic year to 23 in 2018-2019. Between those periods, program-mandated visits decreased significantly from 12 to 3.
The initiative has succeeded in creating a culture of openness and caring at WVU, says 2nd-year internal medicine resident Nistha Modi, MD. “It sets the tone for the program – we talk about mental health openly,” says Dr. Modi.
Crucially, the counselors work out of a different building than the hospital where Dr. Modi and her fellow residents work and use a separate electronic medical record system to protect resident privacy. This is hugely important for medical trainees, note Dr. Tomescu, Dr. Gold, and many other experts. The therapists understand residency and medical education, and there is no limit to the number of visits a resident or fellow can make with the program counselors, says Dr. Modi.
Opt-out programs offer a counterbalance to many negative tendencies in residency, says Dr. Meeks. “We’ve normalized so many things that are not healthy and productive. ... We need to counterbalance that with normalizing help seeking. And it’s really difficult to normalize something that’s not part of a system.”
Costs, concerns, and systematic support
Providing unlimited, free counseling for trainees can be very beneficial, but it requires adequate funding and personnel resources. Offering unlimited access means that an institution has to follow through in making this degree of care available while also ensuring that the system doesn’t get overwhelmed or is unable to accommodate very sick individuals, says Dr. Gold.
Another concern that experts like Dr. Batra, Dr. Moffit, and Dr. Gold share is that residents who go to their scheduled appointments may not completely buy into the experience because it wasn’t their idea in the first place. Participation alone doesn’t necessarily indicate full acceptance. Program personnel don’t intend for these appointments to be thought of as mandatory, yet residents may still experience them that way. Several leading resident well-being programs instead emphasize outreach to trainees, institutional support, and accessible mental health resources that are – and feel – entirely voluntary.
“If I tell someone that they have to do something, it’s very different than if they arrive at that conclusion for themselves,” says Dr. Batra. “That’s how life works.”
When it comes to cost, a recent study published in Academic Medicine provides encouraging data. At the University of Colorado, an opt-out pilot program for IM and pediatrics interns during the 2017-2018 academic year cost just $940 total, equal to $11.75 per intern. As in West Virginia, the program in Colorado covered the cost of the visit, interns were provided a half day off (whether they attended their appointment or not), and the visits and surveys were entirely optional and confidential. During the 1-year pilot program, 29% of 80 interns attended the scheduled appointment, 56% opted out in advance, and 15% didn’t show up. The majority of interns who were surveyed (85%), however, thought the program should continue and that it had a positive effect on their wellness even if they didn’t attend their appointment.
In West Virginia, program costs are higher. The program has $20,000 in annual funding to cover the opt-out program and unlimited counseling visits for residents and fellows. With that funding, Dr. Sofka and her colleagues were also able to expand the program slightly last year to schedule all the critical care faculty for counseling visits. Cost is a barrier to expanding these services to the entire institution, which Dr. Sofka says she hopes to do one day.
Research in this area is still preliminary. The WVU and Colorado studies provide some of the first evidence in support of an opt-out approach. Eventually, it would be beneficial for multicenter studies and longitudinal research to track the effects of such programs over time, say Dr. Sofka and Ajay Major, MD, MBA, one of the study’s coauthors and a hematology/oncology fellow at the University of Chicago.
Whether a program goes with an opt-out approach or not, the systematic supports – protecting resident privacy, providing flexible scheduling, and more – are crucial.
As Dr. Tomescu notes, wellness shouldn’t be just something trainees have to do. “The key with really working on burnout at a huge level is for all programs and schools to recognize that it’s a shared responsibility.”
“I felt very fortunate that I was able to get some help throughout residency,” says Dr. Modi. “About how to be a better daughter. How to be content with things I have in life. How to be happy, and grateful. With the kind of job we have, I think we sometimes forget to be grateful.”
A version of this article first appeared on Medscape.com.
Fear, stigma can stymie the care of criminal justice-involved outpatients
One of the greatest challenges psychotherapists face when working with justice-involved outpatients is a lack of familiarity with the criminal legal system, according to Debra A. Pinals, MD.
“It’s certainly nothing we learned about in medical school or in our mental health training, per se,” said Dr. Pinals, director of the program in psychiatry, law, and ethics at the University of Michigan, Ann Arbor, during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Another challenge is a lack of comfort with some patient personality styles, particularly those with antisocial personality styles,” she said. “We may have countertransference issues that emerge in our work with this population. That can lead to concerns about our own safety, which may at times be reasonable but often because of stereotypes often becomes of mythical proportion. The population is a high-demand population with limited resources, usually tapping public mental health services. That becomes a challenge as well. And there can be burnout when the challenges of our patient population exceed our capacity.”
Despite such obstacles, Dr. Pinals described the outpatient treatment of individuals involved in the criminal justice system as exciting, interesting, and intellectually challenging. But she acknowledged the role that stigma and fear can play.
“Though there are some unique challenges, the benefits of working with criminal justice–involved persons with serious mental illness are often not discussed,” Dr. Pinals said. “There is a tendency to overvalue the risk they may present without really looking at the specific nuances that would be involved in conducting true risk assessments and understanding that not all of these patients will be as risky as we might believe due to stigma and fear.”
Separate from how patients with criminal histories may be perceived in clinical settings. There is much to learn about the role of mental illness in crime. In a 2014 study, researchers reviewed the records of criminal arrests in 143 people with mental illness and tried to discern whether the crime itself was completely independent or completely directly connected to the symptoms of mental illness the individual was experiencing. They found that 65% were completely independent of mental illness symptoms while 8% were directly related to mental illness symptoms.
“This means that as clinicians working with outpatients, we have to understand the whole person, and what might be going on in their lives that leads them down this criminal pathway,” said Dr. Pinals, who is also a clinical professor of psychiatry at the medical school.
According to the risk-need-responsivity (RNR) paradigm, eight criminogenic risk factors are associated with recurrent involvement in the criminal legal system (Crime & Delinquency. 2006;52:7-27). The big four include history of antisocial behavior, antisocial personality pattern, antisocial cognition, and antisocial attitudes. “These are the factors that certain cognitive-behavioral therapy approaches try to address, in an effort to reduce those antisocial cognitive tendencies,” Dr. Pinals said. The other four risk factors include family or marital discord, poor school and/or work performance, few leisure or recreation activities, and substance misuse.
“You’ll notice that mental illness is not listed,” she said. “ although it might be considered a responsivity factor within this RNR paradigm. This means it’s important to address it because it may help people better respond to criminal justice supervision and thereby have an indirect effect in reducing criminal recidivism. For example, if somebody has a social anxiety disorder or agoraphobia and therefore can’t make their probation appointment, probation won’t be able to help them adhere to the terms of their probation conditions. So, we do have to treat the illnesses underlying responsivity to how the criminal justice system operates.”
To optimally serve this population, Dr. Pinals recommends that psychotherapists become familiar with the Sequential Intercept Model, which was first published in 2006. “It takes the premise that individuals move through the criminal legal system in logical steps, and if we could identify those with mental health or substance use conditions and redirect them out of the criminal legal system and into treatment, we could reduce the overall penetration of those individuals from the criminal legal system,” she said. “We know that individuals with mental illness are overrepresented in the criminal legal system.”
By understanding what happens when a patient is arrested, mental health professionals can foster communication that could facilitate treatment for their patients.
“It’s important that we remember that these are people who are going through a challenging time,” Dr. Pinals said. “Maybe we don’t like what they did. Maybe we don’t like that they were accused of committing some kind of crime. However, it is important to realize that they are patients, and we want them to achieve the best outcome, whatever setting they’re in, that continuity of care and communication across systems might be beneficial. It might reduce their chance of returning to the criminal system and having other people victimized.”
Mental health services vary across jails and prisons, she continued, but they are generally required to be commensurate with community standards.
“Of course, that’s often fraught with complexity and may not be available in particular jurisdictions” she said. Prisons, unlike local county jails, tend to have more levels of outpatient care, including inpatient, outpatient, and residential services. “Persons with mental illness can be moved in and out of these levels of care as needed,” Dr. Pinals said. “However, persons with mental illness can be at more risk for disciplinary infractions, especially if they’re not able to follow directions or if they’re psychotic or manic.”
Reentry creates certain risks to be mindful of, including social isolation, recurrent symptoms, problems acquiring medications and housing, suicide, violence, and a return to substance use. A reentry approach she recommended is the APIC model, which stands for Assess, Plan, Identify, and Coordinate. “That means individuals approaching release should be screened and assessed for their needs with a plan to meet the needs, identify critical periods and needed policies, and coordinate across systems,” Dr. Pinals said. “So, if you get a call as an outpatient provider from the reentry coordinator at a local jail trying to help you coordinate a patient’s reentry, that’s something to pay attention to.”
When first meeting with patients after a criminal justice experience, Dr. Pinals recommends asking them to discuss their arrest and criminal justice experience, and to address any emerging psychiatric or clinical issues, including trauma and adjustment associated with the arrests, incarceration, and legal processes. “The risks of rearrest are higher for those who have already touched the criminal justice system, so we want to help minimize that risk of rearrest,” she said.
Some clinics won’t allow patients with a criminal record to return, “which means you have to help potentially find alternative places for them to be seen,” she noted. “You may want to consult a specialist if you have doubts about your capacity to work with the patient. You also want to support staff who might have concerns about how to continue to treat this patient and you want to advocate for the patient’s needs and help them return to a stable treatment setting.”
Dr. Pinals concluded her presentation by underscoring the importance of delivering treatment services that are trauma informed. “There are high levels of trauma for those receiving care in psychiatric settings and among those who have spent time in jails and prisons,” she said. “We want to be sensitive to the fact that any of our patients who were involved in the criminal legal system might have a strong trauma history. Help instill a sense of safety and community, and hold hope for positive change.”
She reported consulting to jurisdictions and attorneys pertaining to behavioral health and justice, and forensic psychiatry. She reported having no relevant commercial financial disclosures.
One of the greatest challenges psychotherapists face when working with justice-involved outpatients is a lack of familiarity with the criminal legal system, according to Debra A. Pinals, MD.
“It’s certainly nothing we learned about in medical school or in our mental health training, per se,” said Dr. Pinals, director of the program in psychiatry, law, and ethics at the University of Michigan, Ann Arbor, during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Another challenge is a lack of comfort with some patient personality styles, particularly those with antisocial personality styles,” she said. “We may have countertransference issues that emerge in our work with this population. That can lead to concerns about our own safety, which may at times be reasonable but often because of stereotypes often becomes of mythical proportion. The population is a high-demand population with limited resources, usually tapping public mental health services. That becomes a challenge as well. And there can be burnout when the challenges of our patient population exceed our capacity.”
Despite such obstacles, Dr. Pinals described the outpatient treatment of individuals involved in the criminal justice system as exciting, interesting, and intellectually challenging. But she acknowledged the role that stigma and fear can play.
“Though there are some unique challenges, the benefits of working with criminal justice–involved persons with serious mental illness are often not discussed,” Dr. Pinals said. “There is a tendency to overvalue the risk they may present without really looking at the specific nuances that would be involved in conducting true risk assessments and understanding that not all of these patients will be as risky as we might believe due to stigma and fear.”
Separate from how patients with criminal histories may be perceived in clinical settings. There is much to learn about the role of mental illness in crime. In a 2014 study, researchers reviewed the records of criminal arrests in 143 people with mental illness and tried to discern whether the crime itself was completely independent or completely directly connected to the symptoms of mental illness the individual was experiencing. They found that 65% were completely independent of mental illness symptoms while 8% were directly related to mental illness symptoms.
“This means that as clinicians working with outpatients, we have to understand the whole person, and what might be going on in their lives that leads them down this criminal pathway,” said Dr. Pinals, who is also a clinical professor of psychiatry at the medical school.
According to the risk-need-responsivity (RNR) paradigm, eight criminogenic risk factors are associated with recurrent involvement in the criminal legal system (Crime & Delinquency. 2006;52:7-27). The big four include history of antisocial behavior, antisocial personality pattern, antisocial cognition, and antisocial attitudes. “These are the factors that certain cognitive-behavioral therapy approaches try to address, in an effort to reduce those antisocial cognitive tendencies,” Dr. Pinals said. The other four risk factors include family or marital discord, poor school and/or work performance, few leisure or recreation activities, and substance misuse.
“You’ll notice that mental illness is not listed,” she said. “ although it might be considered a responsivity factor within this RNR paradigm. This means it’s important to address it because it may help people better respond to criminal justice supervision and thereby have an indirect effect in reducing criminal recidivism. For example, if somebody has a social anxiety disorder or agoraphobia and therefore can’t make their probation appointment, probation won’t be able to help them adhere to the terms of their probation conditions. So, we do have to treat the illnesses underlying responsivity to how the criminal justice system operates.”
To optimally serve this population, Dr. Pinals recommends that psychotherapists become familiar with the Sequential Intercept Model, which was first published in 2006. “It takes the premise that individuals move through the criminal legal system in logical steps, and if we could identify those with mental health or substance use conditions and redirect them out of the criminal legal system and into treatment, we could reduce the overall penetration of those individuals from the criminal legal system,” she said. “We know that individuals with mental illness are overrepresented in the criminal legal system.”
By understanding what happens when a patient is arrested, mental health professionals can foster communication that could facilitate treatment for their patients.
“It’s important that we remember that these are people who are going through a challenging time,” Dr. Pinals said. “Maybe we don’t like what they did. Maybe we don’t like that they were accused of committing some kind of crime. However, it is important to realize that they are patients, and we want them to achieve the best outcome, whatever setting they’re in, that continuity of care and communication across systems might be beneficial. It might reduce their chance of returning to the criminal system and having other people victimized.”
Mental health services vary across jails and prisons, she continued, but they are generally required to be commensurate with community standards.
“Of course, that’s often fraught with complexity and may not be available in particular jurisdictions” she said. Prisons, unlike local county jails, tend to have more levels of outpatient care, including inpatient, outpatient, and residential services. “Persons with mental illness can be moved in and out of these levels of care as needed,” Dr. Pinals said. “However, persons with mental illness can be at more risk for disciplinary infractions, especially if they’re not able to follow directions or if they’re psychotic or manic.”
Reentry creates certain risks to be mindful of, including social isolation, recurrent symptoms, problems acquiring medications and housing, suicide, violence, and a return to substance use. A reentry approach she recommended is the APIC model, which stands for Assess, Plan, Identify, and Coordinate. “That means individuals approaching release should be screened and assessed for their needs with a plan to meet the needs, identify critical periods and needed policies, and coordinate across systems,” Dr. Pinals said. “So, if you get a call as an outpatient provider from the reentry coordinator at a local jail trying to help you coordinate a patient’s reentry, that’s something to pay attention to.”
When first meeting with patients after a criminal justice experience, Dr. Pinals recommends asking them to discuss their arrest and criminal justice experience, and to address any emerging psychiatric or clinical issues, including trauma and adjustment associated with the arrests, incarceration, and legal processes. “The risks of rearrest are higher for those who have already touched the criminal justice system, so we want to help minimize that risk of rearrest,” she said.
Some clinics won’t allow patients with a criminal record to return, “which means you have to help potentially find alternative places for them to be seen,” she noted. “You may want to consult a specialist if you have doubts about your capacity to work with the patient. You also want to support staff who might have concerns about how to continue to treat this patient and you want to advocate for the patient’s needs and help them return to a stable treatment setting.”
Dr. Pinals concluded her presentation by underscoring the importance of delivering treatment services that are trauma informed. “There are high levels of trauma for those receiving care in psychiatric settings and among those who have spent time in jails and prisons,” she said. “We want to be sensitive to the fact that any of our patients who were involved in the criminal legal system might have a strong trauma history. Help instill a sense of safety and community, and hold hope for positive change.”
She reported consulting to jurisdictions and attorneys pertaining to behavioral health and justice, and forensic psychiatry. She reported having no relevant commercial financial disclosures.
One of the greatest challenges psychotherapists face when working with justice-involved outpatients is a lack of familiarity with the criminal legal system, according to Debra A. Pinals, MD.
“It’s certainly nothing we learned about in medical school or in our mental health training, per se,” said Dr. Pinals, director of the program in psychiatry, law, and ethics at the University of Michigan, Ann Arbor, during an annual psychopharmacology update held by the Nevada Psychiatric Association.
“Another challenge is a lack of comfort with some patient personality styles, particularly those with antisocial personality styles,” she said. “We may have countertransference issues that emerge in our work with this population. That can lead to concerns about our own safety, which may at times be reasonable but often because of stereotypes often becomes of mythical proportion. The population is a high-demand population with limited resources, usually tapping public mental health services. That becomes a challenge as well. And there can be burnout when the challenges of our patient population exceed our capacity.”
Despite such obstacles, Dr. Pinals described the outpatient treatment of individuals involved in the criminal justice system as exciting, interesting, and intellectually challenging. But she acknowledged the role that stigma and fear can play.
“Though there are some unique challenges, the benefits of working with criminal justice–involved persons with serious mental illness are often not discussed,” Dr. Pinals said. “There is a tendency to overvalue the risk they may present without really looking at the specific nuances that would be involved in conducting true risk assessments and understanding that not all of these patients will be as risky as we might believe due to stigma and fear.”
Separate from how patients with criminal histories may be perceived in clinical settings. There is much to learn about the role of mental illness in crime. In a 2014 study, researchers reviewed the records of criminal arrests in 143 people with mental illness and tried to discern whether the crime itself was completely independent or completely directly connected to the symptoms of mental illness the individual was experiencing. They found that 65% were completely independent of mental illness symptoms while 8% were directly related to mental illness symptoms.
“This means that as clinicians working with outpatients, we have to understand the whole person, and what might be going on in their lives that leads them down this criminal pathway,” said Dr. Pinals, who is also a clinical professor of psychiatry at the medical school.
According to the risk-need-responsivity (RNR) paradigm, eight criminogenic risk factors are associated with recurrent involvement in the criminal legal system (Crime & Delinquency. 2006;52:7-27). The big four include history of antisocial behavior, antisocial personality pattern, antisocial cognition, and antisocial attitudes. “These are the factors that certain cognitive-behavioral therapy approaches try to address, in an effort to reduce those antisocial cognitive tendencies,” Dr. Pinals said. The other four risk factors include family or marital discord, poor school and/or work performance, few leisure or recreation activities, and substance misuse.
“You’ll notice that mental illness is not listed,” she said. “ although it might be considered a responsivity factor within this RNR paradigm. This means it’s important to address it because it may help people better respond to criminal justice supervision and thereby have an indirect effect in reducing criminal recidivism. For example, if somebody has a social anxiety disorder or agoraphobia and therefore can’t make their probation appointment, probation won’t be able to help them adhere to the terms of their probation conditions. So, we do have to treat the illnesses underlying responsivity to how the criminal justice system operates.”
To optimally serve this population, Dr. Pinals recommends that psychotherapists become familiar with the Sequential Intercept Model, which was first published in 2006. “It takes the premise that individuals move through the criminal legal system in logical steps, and if we could identify those with mental health or substance use conditions and redirect them out of the criminal legal system and into treatment, we could reduce the overall penetration of those individuals from the criminal legal system,” she said. “We know that individuals with mental illness are overrepresented in the criminal legal system.”
By understanding what happens when a patient is arrested, mental health professionals can foster communication that could facilitate treatment for their patients.
“It’s important that we remember that these are people who are going through a challenging time,” Dr. Pinals said. “Maybe we don’t like what they did. Maybe we don’t like that they were accused of committing some kind of crime. However, it is important to realize that they are patients, and we want them to achieve the best outcome, whatever setting they’re in, that continuity of care and communication across systems might be beneficial. It might reduce their chance of returning to the criminal system and having other people victimized.”
Mental health services vary across jails and prisons, she continued, but they are generally required to be commensurate with community standards.
“Of course, that’s often fraught with complexity and may not be available in particular jurisdictions” she said. Prisons, unlike local county jails, tend to have more levels of outpatient care, including inpatient, outpatient, and residential services. “Persons with mental illness can be moved in and out of these levels of care as needed,” Dr. Pinals said. “However, persons with mental illness can be at more risk for disciplinary infractions, especially if they’re not able to follow directions or if they’re psychotic or manic.”
Reentry creates certain risks to be mindful of, including social isolation, recurrent symptoms, problems acquiring medications and housing, suicide, violence, and a return to substance use. A reentry approach she recommended is the APIC model, which stands for Assess, Plan, Identify, and Coordinate. “That means individuals approaching release should be screened and assessed for their needs with a plan to meet the needs, identify critical periods and needed policies, and coordinate across systems,” Dr. Pinals said. “So, if you get a call as an outpatient provider from the reentry coordinator at a local jail trying to help you coordinate a patient’s reentry, that’s something to pay attention to.”
When first meeting with patients after a criminal justice experience, Dr. Pinals recommends asking them to discuss their arrest and criminal justice experience, and to address any emerging psychiatric or clinical issues, including trauma and adjustment associated with the arrests, incarceration, and legal processes. “The risks of rearrest are higher for those who have already touched the criminal justice system, so we want to help minimize that risk of rearrest,” she said.
Some clinics won’t allow patients with a criminal record to return, “which means you have to help potentially find alternative places for them to be seen,” she noted. “You may want to consult a specialist if you have doubts about your capacity to work with the patient. You also want to support staff who might have concerns about how to continue to treat this patient and you want to advocate for the patient’s needs and help them return to a stable treatment setting.”
Dr. Pinals concluded her presentation by underscoring the importance of delivering treatment services that are trauma informed. “There are high levels of trauma for those receiving care in psychiatric settings and among those who have spent time in jails and prisons,” she said. “We want to be sensitive to the fact that any of our patients who were involved in the criminal legal system might have a strong trauma history. Help instill a sense of safety and community, and hold hope for positive change.”
She reported consulting to jurisdictions and attorneys pertaining to behavioral health and justice, and forensic psychiatry. She reported having no relevant commercial financial disclosures.
FROM NPA 2021
U.S. suicide rate in 2019 took first downturn in 14 years
In 2019, the U.S. suicide rate dropped for the first time in 14 years, driven largely by a significant decline in firearm-related deaths, according to a new analysis of National Vital Statistics System data.
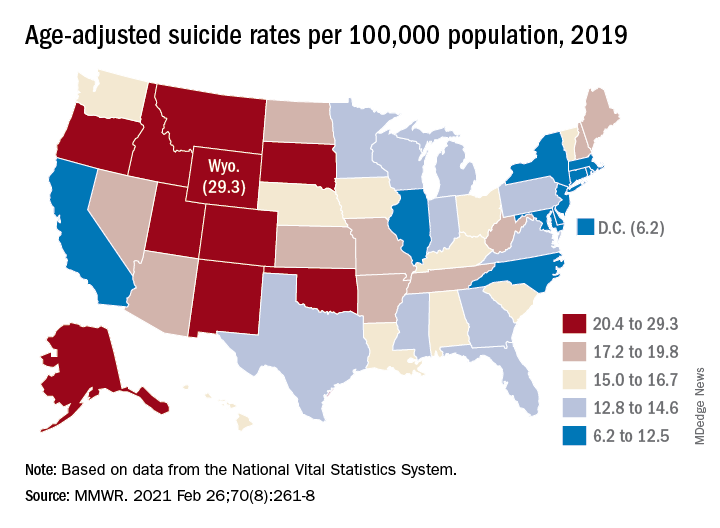
Since firearms are the “most common and most lethal” mechanism of suicide, the drop in deaths is “particularly encouraging,” Deborah M. Stone, ScD, MSW, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
The national suicide rate decreased from 14.2 per 100,000 population in 2018 to 13.9 per 100,000 in 2019, a statistically significant drop of 2.1% that reversed a 20-year trend that saw the rate increase by 33% since 1999, they said.
The rate for firearm use, which is involved in half of all suicides, declined from 7.0 per 100,000 to 6.8, for a significant change of 2.9%, said Dr. Stone and associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control.
The only other method with a drop in suicide rate from 2018 to 2019 was suffocation – the second most common mechanism of injury – but the relative change of 2.3% was not significant, they noted.
Significant declines also occurred in several subgroups: Whites; those aged 15-24, 55-64, and 65-74 years; and those living in counties classified as large fringe metropolitan or micropolitan (urban cluster of ≥ 10,000 but less than 50,000 population), they said, based on data from the National Vital Statistics System.
the investigators wrote.
The states with significant increases were Hawaii (30.3%) and Nebraska (20.1%), while declines in the suicide rate were significant in five states – Idaho, Indiana, Massachusetts, North Carolina, and Virginia, Dr. Stone and associates reported. Altogether, the rate fell in 31 states, increased in 18, and did not change in 2.
The significance of those changes varied between males and females. Declines were significant for females in Indiana, Massachusetts, and Washington, and for males in Florida, Kentucky, Massachusetts, North Carolina, and West Virginia. Minnesota was the only state with a significant increase among females, with Hawaii and Wyoming posting increases for males, they said.
As the response to the COVID-19 pandemic continues, the investigators pointed out, “prevention is more important than ever. Past research indicates that suicide rates remain stable or decline during infrastructure disruption (e.g., natural disasters), only to rise afterwards as the longer-term sequelae unfold in persons, families, and communities.”
In 2019, the U.S. suicide rate dropped for the first time in 14 years, driven largely by a significant decline in firearm-related deaths, according to a new analysis of National Vital Statistics System data.

Since firearms are the “most common and most lethal” mechanism of suicide, the drop in deaths is “particularly encouraging,” Deborah M. Stone, ScD, MSW, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
The national suicide rate decreased from 14.2 per 100,000 population in 2018 to 13.9 per 100,000 in 2019, a statistically significant drop of 2.1% that reversed a 20-year trend that saw the rate increase by 33% since 1999, they said.
The rate for firearm use, which is involved in half of all suicides, declined from 7.0 per 100,000 to 6.8, for a significant change of 2.9%, said Dr. Stone and associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control.
The only other method with a drop in suicide rate from 2018 to 2019 was suffocation – the second most common mechanism of injury – but the relative change of 2.3% was not significant, they noted.
Significant declines also occurred in several subgroups: Whites; those aged 15-24, 55-64, and 65-74 years; and those living in counties classified as large fringe metropolitan or micropolitan (urban cluster of ≥ 10,000 but less than 50,000 population), they said, based on data from the National Vital Statistics System.
the investigators wrote.
The states with significant increases were Hawaii (30.3%) and Nebraska (20.1%), while declines in the suicide rate were significant in five states – Idaho, Indiana, Massachusetts, North Carolina, and Virginia, Dr. Stone and associates reported. Altogether, the rate fell in 31 states, increased in 18, and did not change in 2.
The significance of those changes varied between males and females. Declines were significant for females in Indiana, Massachusetts, and Washington, and for males in Florida, Kentucky, Massachusetts, North Carolina, and West Virginia. Minnesota was the only state with a significant increase among females, with Hawaii and Wyoming posting increases for males, they said.
As the response to the COVID-19 pandemic continues, the investigators pointed out, “prevention is more important than ever. Past research indicates that suicide rates remain stable or decline during infrastructure disruption (e.g., natural disasters), only to rise afterwards as the longer-term sequelae unfold in persons, families, and communities.”
In 2019, the U.S. suicide rate dropped for the first time in 14 years, driven largely by a significant decline in firearm-related deaths, according to a new analysis of National Vital Statistics System data.

Since firearms are the “most common and most lethal” mechanism of suicide, the drop in deaths is “particularly encouraging,” Deborah M. Stone, ScD, MSW, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
The national suicide rate decreased from 14.2 per 100,000 population in 2018 to 13.9 per 100,000 in 2019, a statistically significant drop of 2.1% that reversed a 20-year trend that saw the rate increase by 33% since 1999, they said.
The rate for firearm use, which is involved in half of all suicides, declined from 7.0 per 100,000 to 6.8, for a significant change of 2.9%, said Dr. Stone and associates at the Centers for Disease Control and Prevention’s National Center for Injury Prevention and Control.
The only other method with a drop in suicide rate from 2018 to 2019 was suffocation – the second most common mechanism of injury – but the relative change of 2.3% was not significant, they noted.
Significant declines also occurred in several subgroups: Whites; those aged 15-24, 55-64, and 65-74 years; and those living in counties classified as large fringe metropolitan or micropolitan (urban cluster of ≥ 10,000 but less than 50,000 population), they said, based on data from the National Vital Statistics System.
the investigators wrote.
The states with significant increases were Hawaii (30.3%) and Nebraska (20.1%), while declines in the suicide rate were significant in five states – Idaho, Indiana, Massachusetts, North Carolina, and Virginia, Dr. Stone and associates reported. Altogether, the rate fell in 31 states, increased in 18, and did not change in 2.
The significance of those changes varied between males and females. Declines were significant for females in Indiana, Massachusetts, and Washington, and for males in Florida, Kentucky, Massachusetts, North Carolina, and West Virginia. Minnesota was the only state with a significant increase among females, with Hawaii and Wyoming posting increases for males, they said.
As the response to the COVID-19 pandemic continues, the investigators pointed out, “prevention is more important than ever. Past research indicates that suicide rates remain stable or decline during infrastructure disruption (e.g., natural disasters), only to rise afterwards as the longer-term sequelae unfold in persons, families, and communities.”
FROM MMWR
Severe atopic dermatitis often puts a dent in quality of life
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
In his role as head of the division of pediatric behavioral health at National Jewish Health, Denver, Bruce G. Bender, PhD, helps children and adults navigate the adverse effects of severe atopic dermatitis (AD) on their quality of life.
“There have been many surveys of adults with AD who report impairment of their sleep, reduced activity level, increased work absence, financial burden, emotional distress, and social avoidance,” he said at the Revolutionizing Atopic Dermatitis virtual symposium. “Similarly, children with AD or their parents report emotional distress, reduced activity, and increased school absence, social avoidance, and sleep disturbance. Families report financial burdens, conflict, particularly among the adults, social avoidance, sleep disturbance in the parents, and reduction of well-being in the siblings.”
In an effort to objectively measure sleep change in this population, Dr. Bender and colleagues recruited 14 adults with AD and 14 healthy controls who wore an ActiGraph for 1 week and completed questionnaires about sleep, itch, and quality of life. Patients with AD were awake almost twice as many minutes each night as the healthy controls (a mean of 57.3 vs. 32.3 minutes, respectively; P = .0480). Consequently, their sleep efficiency was significantly reduced based on the Pittsburgh sleep quality index (a mean of 90.6 vs. 95; P = .0305).
In another study, Dr. Bender and colleagues enrolled 20 adults with AD who underwent 2 nights of polysomnography and actigraphy. The lab was set up to measure a scratching event, which was recorded when a burst of electromyographic activity of at least 3 seconds was accompanied by a visible scratching motion. “We learned that sleep efficiency as measured by both PSG and actigraphy correlated with total body surface area and scratching index,” he said. “As we might assume, the more skin involved, the more patients scratch, the less well they sleep.”
Behavioral, neurocognitive effects
In a separate study of AD, sleep, and behavior, the researchers studied 1,041 children with asthma who were enrolled in the Childhood Asthma Management Program at eight North American sites. They used baseline parent ratings on standardized sleep and behavior rating scales and found that increased awakenings were associated with increased school absence and daytime behavior problems. “So, not only do children with AD sleep less well, but this shows up to impair their functioning during the day,” said Dr. Bender, professor of psychiatry at the University of Colorado, Denver.
In a report from Australia, researchers set out to explore the association between sleep and neurocognitive function in 21 children with eczema and 20 healthy controls. Participants underwent cognitive testing and polysomnography. The authors found that the children with eczema demonstrated lower test scores. Reduced scores were correlated with parental reports of sleep problems but not polysomnography.
In a much larger study funded by the Agency for Healthcare Research and Quality, investigators analyzed data on 354,416 children and 34,613 adults from 19 U.S. population surveys including the National Health Interview Survey 1997-2013 and the National Survey of Children’s Health 2003/4 and 2007/8. They found that AD was associated with ADHD in children (adjusted odds ratio, 1.14) and adults (aOR, 1.61). Higher odds of ADHD were found in children who had significant sleep disturbance (aOR, 16.83) and other allergic disease and asthma (aOR, 1.61).
“All of these findings show that AD can impact quality of life, especially sleep, with the result of poorer daytime functioning,” Dr. Bender said. “But those studies don’t answer this question: Are patients with AD at increased risk for psychological disorders such as depression and anxiety?”
Impact on depression, anxiety
Two systematic reviews on the topic suggest that patients with AD are twice as likely to experience depression. One was published in 2018 and the other in 2019. The 2018 review reported a little more than a twofold increase (OR, 2.19), the 2019 review a little bit less (OR, 1.71).
“At the more severe end of the depression continuum, we sometimes see suicidal ideation and suicide attempts,” Dr. Bender said. “A number of studies have asked whether these are increased in patients with AD. Quite a few studies collectively show an increased incidence of suicidal ideation. The question of suicide attempts is reflected in fewer studies. And while the result is small, it is significant. There is a significant increase reported of suicide attempts in AD patients.”
The 2018 review also found an increased incidence of anxiety in AD patients: a little more than twofold in adults (OR, 2.19) and a little less than twofold in children (OR, 1.81).
“It’s a two-way relationship between AD and psychological factors,” Dr. Bender said. “We generally think about AD – the stress that it brings, the burden that it puts on children, adults, and families. But it can work the other way around,” he said, referring to patients who have psychological problems, experience a great deal of stress, have trouble being adherent to their treatment regimen, and find it difficult to resist scratching. “The behavioral/psychological characteristics of the patient also drive the AD. It is well established that acute and chronic stress can result in a worsening of skin conditions in AD patients.”
Behavioral health interventions that have been described in the literature include cognitive therapy, stress management, biofeedback, hypnotherapy, relaxation training, mindfulness, habit reversal, and patient education – some of which have been tested in randomized trials. “All of them report a decrease in scratching as a consequence of the behavioral intervention,” Dr. Bender said.
“Other studies have been reported that look at the impact of behavioral interventions on the severity of the skin condition. Most report an improvement in the skin condition from these behavioral interventions but it’s not a perfect literature.” Critiques of these studies include the fact that there is often not enough detail about the intervention or the framework for the intervention that would allow a clinician to test an intervention in another study or actually pull that intervention into clinical practice (Cochrane Database Syst Rev. 2014 Jan 7;2014[1]:CD004054), (Int Arch Allergy Immunol.2007;144[1]:1-9).
“Some of the studies lack rigorous designs, some have sampling bias, and some have inadequate outcome measurements,” he said. “We really need additional, high-quality studies to look at what is helpful for patients with AD.”
Dr. Bender reported having no financial disclosures.
FROM REVOLUTIONIZING AD 2020
Mepolizumab reduced exacerbations in patients with asthma and atopy, depression comorbidities
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
, according to research from the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
“Mepolizumab has clearly been shown to improve severe asthma control in many clinical trials, but atopy, obesity, and depression/anxiety affect patients with asthma at an increased rate,” Thomas B. Casale, MD, former AAAAI president and professor of medicine and pediatrics at the University of South Florida in Tampa, said in a presentation at the meeting. “Yet, few studies have examined whether asthma therapy with these comorbidities works.”
Dr. Casale and colleagues performed a retrospective analysis of patients in the United States from the MarketScan Commercial and Medicare Supplemental Database between November 2014 and December 2018 who had atopy, obesity, or depression/anxiety in addition to asthma and were receiving mepolizumab. Atopy in the study was defined as allergic rhinitis, anaphylaxis, atopic dermatitis, conjunctivitis, eosinophilic esophagitis, and food allergies. Patients were at least age 12 years, had at least one diagnosis for asthma, at least one diagnosis code for atopic disease, obesity, or depression/anxiety at baseline, and at least two administrations of mepolizumab within 180 days.
The researchers examined the number of exacerbations, oral corticosteroid (OCS) claims, and OCS bursts per year at 12-month follow-up, compared with baseline. They identified exacerbations by examining patients who had an emergency department or outpatient claim related to their asthma, and a claim for systemic corticosteroids made in the 4 days prior to or 5 days after a visit, or if their inpatient hospital admission contained a primary asthma diagnosis. Dr. Casale and colleagues measured OCS bursts as a pharmacy claim of at least 20 mg of prednisone per day for between 3 and 28 days plus a claim for an emergency department visit related to asthma in the 7 days prior or 6 days after the claim.
At baseline, patients across all groups were mean age 50.5-52.4 years with a Charleson Comorbidity Index score between 1.1 and 1.4, a majority were women (59.0%-72.0%) and nearly all were commercially insured (88.0%-90.0%). Patients who used biologics at baseline and/or used a biologic that wasn’t mepolizumab during the follow-up period were excluded.
Medication claims in the groups included inhaled corticosteroids (ICS) (36.8%-48.6%), ICS/long-acting beta-agonist (LABA) (60.2%-63.0%), LABA/ long-acting muscarinic antagonist (LAMA) (1.2%-3.5%), ICS/LABA/LAMA (21.2%-25.1%), short-acting beta-agonist (SABA) (83.2%-87.7%), LAMA alone (33.5%-42.1%), or leukotriene receptor antagonist (LTRA).
In the non–mutually exclusive group of patients with atopy (468 patients), 28.0% had comorbid obesity and 26.0% had comorbid depression/anxiety. For patients with obesity categorized in a non–mutually exclusive subgroup (171 patients), 79.0% had comorbid atopy and 32.0% had comorbid depression/anxiety. Among patients with non–mutually exclusive depression/anxiety (173 patients), 70.0% had comorbid atopy, while 32.0% had comorbid obesity.
The results showed the mean number of overall exacerbations decreased by 48% at 12 months in the atopic group (2.3 vs. 1.2; P < .001), 52% in the group with obesity (2.5 vs. 1.2; P < .001), and 38% in the depression/anxiety group (2.4 vs. 1.5; P < .001). The mean number of exacerbations leading to hospitalizations decreased by 64% in the atopic group (0.11 vs. 0.04; P < .001), 65% in the group with obesity (0.20 vs. 0.07; P < .001), and 68% in the group with depression/anxiety (0.22 vs. 0.07; P < .001).
The researchers also found the mean number of OCS claims and OCS bursts also significantly decreased over the 12-month follow-up period. Mean OCS claims decreased by 33% for patients in the atopic group (5.5 vs. 3.7; P < .001), by 38% in the group with obesity (6.1 vs. 3.8; P < .001), and by 31% in the group with depression/anxiety (6.2 vs. 4.3; P < .001).
The mean number of OCS bursts also significantly decreased by 40% in the atopic group (2.0 vs. 2.1; P < .001), 48% in the group with obesity (2.3 vs. 1.2; P < .001), and by 37% in the group with depression/anxiety (1.9 vs. 1.2; P < .001). In total, 69% of patients with comorbid atopy, 70.8% of patients with comorbid obesity, and 68.2% of patients with comorbid depression/anxiety experienced a mean decrease in their OCS dose over 12 months.
“These data demonstrate that patients with asthma and atopy, obesity, or depression and anxiety have significantly fewer exacerbations and reduced OCS use in a real-world setting with treatment of mepolizumab,” Dr. Casale said. “Thus, holistic patient care for severe asthma is critical, and mepolizumab provides tangible clinical benefit despite the complexities of medical comorbidities.”
This study was funded by GlaxoSmithKline, and the company also funded graphic design support of the poster. Dr. Casale reports he has received research funds from GlaxoSmithKline. Four authors report being current or former GlaxoSmithKline employees; three authors report holding stock and/or shares of GlaxoSmithKline. Three authors are IBM Watson Health employees, a company GlaxoSmithKline has provided research funding.
FROM AAAAI 2021
Treatment resistance is a myth!
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
For millennia, serious psychiatric brain disorders (aka mental illnesses, melancholia, madness, insanity) were written off as incurable, permanent afflictions. It’s no wonder that they were engulfed with the stigma of hopelessness.
But then came the era of serendipitous discoveries in the mid-20th century, with the felicitous arrival of antipsychotics, antidepressants, and lithium. The dogma of untreatability was shattered, but in its wake, the notion of treatment resistance emerged, and promptly became the bane of psychiatric clinicians and the practice of psychopharmacology.
Many patients with mood and psychotic disorders responded to the medications that were introduced in the 1950s and 1960s, but some either derived partial benefit or did not improve at all. These partial or poor responders were labeled “treatment-resistant,” and caring for them became a major challenge for psychiatric physicians that continues to this day. However, rapid advances in understanding the many etiologies and subtypes of the heterogeneous mood and psychotic disorders are invalidating the notion of treatment resistance, showing it is a fallacy and a misnomer. Let’s examine why.
Treatment-resistant depression (TRD)
Psychiatric clinics and hospitals are clogged with patients who do not respond to ≥2 evidence-based antidepressants and carry the disparaging label of “TRD.” But a patient manifesting what appears to be major depressive disorder (MDD) may actually have one of several types of depression that are unlikely to respond to an antidepressant, including:
- iatrogenic depression due to a prescription medication
- depression secondary to recreational drug use
- depressive symptoms secondary to a general medical condition
- bipolar depression.
Thus, a significant proportion of patients diagnosed with MDD are labeled TRD because they do not respond to standard antidepressants, when in fact they have been misdiagnosed and need a different treatment.
Even when the diagnosis of MDD is accurate, psychiatric neuroscience advances have informed us that MDD is a heterogeneous syndrome with multiple “biotypes” that share a similar phenotype.1,2 In the past, TRD has been defined as a failure to respond to ≥2 adequate trials (8 to 12 weeks at a maximum tolerated dose) of antidepressants from different classes (such as tricyclic or heterocyclic antidepressants, selective serotonin reuptake inhibitors, or serotonin-norepinephrine reuptake inhibitors). For decades, patients with TRD have been referred to electroconvulsive therapy (ECT), and have experienced an excellent response rate. So TRD is in fact an artificial concept and term, applied to a subtype of MDD that does not respond to standard antidepressants, but often responds very well to neurostimulation (ECT and transcranial magnetic stimulation [TMS]).
When an antidepressant is approved by the FDA based on “successful” placebo-controlled double-blind trials, there is always a subset of patients who do not respond. However, the success of a controlled clinical trial is based on a decline in overall mean depression rating scale score in the antidepressant group compared with the placebo group. Not a single antidepressant has ever exerted full efficacy in 100% of patients who received it in an FDA trial because the sample is always a heterogeneous mix of patients with various depression biotypes who meet the DSM clinical diagnosis of MDD. Most often, only approximately 50% do, which is enough to be statistically significantly better than the roughly 30% response rate in the placebo group. It is impossible for a heterogeneous syndrome comprised of biologically different “diseases” to respond to any single medication! Patients who do not respond to an antidepressant medication that works in other patients represent a different subtype of depression that is not TRD. Biotypes of the depression syndrome have different neurochemical underpinnings and may respond to different mechanisms of therapeutic action, yet to be discovered.
Continue to: A very common...
A very common clinical mistake occurs when patients with bipolar depression are misdiagnosed as having MDD because most of them experience depression as their initial mood episode. These patients often end up being classified as having TRD because bipolar depression very frequently fails to respond to several of the antidepressants that are FDA-approved for MDD. When these patients are correctly diagnosed, many will respond to one of the medications specifically approved for bipolar depression that were launched over the past 15 years (quetiapine, lurasidone, and cariprazine). However, bipolar disorder is also a heterogeneous spectrum, and some patients with bipolar depression may fail to respond to any of these 3 medications and are promptly regarded as TRD. Such patients often respond to neuromodulation (TMS, ECT, or vagus nerve stimulation [VNS]), indicating that they may have a different type of bipolar depression, such as bipolar type II.
A more recent example of the falsehood of TRD as a spurious diagnosis is the dramatic and rapid response of patients who are chronically depressed (both those with MDD and those with bipolar depression) to ketamine infusions.3,4 Responders to ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, prove that nonresponders to monoamine reuptake inhibitors must not be falsely labeled as having TRD. They have a different subtype within the depression syndrome that is mediated by glutamatergic pathways, instead of monoamines such as serotonin, norepinephrine, or dopamine. In addition, unlike monoaminergic antidepressants, NMDA antagonists rapidly reverse suicidal urges, above and beyond rapidly reversing chronic, so-called TRD.
In the same vein, numerous reports have shown that buprenorphine has significant efficacy in TRD (and suicide urges, as does ketamine), which implicates opioid pathways as mediating some subtypes of TRD.5 The monoamine model of depression, which dominated the field and dragged on for half a century, has distracted psychiatric researchers from exploring and recognizing the multiple neurochemical and neuroplastic pathways of the depression syndrome, thus falsely assuming that depression is a monolithic disorder that responds to elevating the activity of brain monoamines. This major blind spot led to the ersatz concept of TRD.
Treatment-resistant schizophrenia (TRS)
Since the discovery of chlorpromazine and other antipsychotics in the 1950s, it became apparent that a subset of patients with schizophrenia do not respond to medications that block dopamine D2 receptors. Partial responders were labeled as having TRS, and complete nonresponse was called refractory schizophrenia. Many patients with severe and persistent delusions and hallucinations were permanently hospitalized, and unable to live in the community like those who responded to dopamine antagonism.
In the late 1980s, the discovery that clozapine has significant efficacy in TRS and refractory schizophrenia provided the first insight that TRS and refractory schizophrenia represent different neurobiologic subtypes of schizophrenia.6,7 The extensive heterogeneity of schizophrenia (with hundreds of genetic and nongenetic etiologies) is now widely accepted.8 Patients with schizophrenia who do not respond to dopamine receptor antagonism should not be labeled TRS, because they can respond to a different antipsychotic agent, such as clozapine, which is believed to exert its efficacy via glutamate pathways.
Continue to: But what about the 50%...
But what about the 50% of patients with TRS or refractory schizophrenia who do not respond to clozapine?9 They do not have TRS, either, but represent different schizophrenia biotypes that may respond to other medications with different mechanisms of action, such as lamotrigine,10 which is a glutamate modulator; pimavanserin,11 which is an inverse agonist of the serotonin 5HT-2A receptor; allopurinol,12,13 an adenosine modulator; or estrogen,14 a neurosteroid. Future research will continue to unravel the many biotypes of the highly heterogeneous schizophrenia syndrome that are “nondopaminergic” and do not respond to the standard class of dopamine antagonists (previously called neuroleptics and now known as antipsychotics).15 Future treatments for schizophrenia may depart from modulating various neurotransmitter receptors to targeting entirely different neurobiologic processes, such as correcting mitochondria pathology, inhibiting microglia activation, repairing white matter, reversing apoptosis pathways, inducing neuroplasticity, arresting oxidative stress and inflammation, and other neuroprotective mechanisms.
The rapid growth of biomarkers in psychiatry16 will usher in an era of precision psychiatry17 that will eliminate the term “treatment resistance.” Our psychiatric practice will then benefit from “canceling” this demoralizing and clinically unjustified term that has needlessly fostered therapeutic nihilism among psychiatric physicians.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
1. Milaneschi Y, Lamers F, Berk M, et al. Depression heterogeneity and its biological underpinnings: toward immunometabolism depression. Biol Psychiatry. 2020;88(5):369-380.
2. Akiskal HS, McKinney WT Jr. Overview of recent research in depression. Integration of ten conceptual models into a comprehensive clinical frame. Arch Gen Psychiatry. 1975;32(3):285-305.
3. Zarate CA Jr. Ketamine: a new chapter in antidepressant development. Brazilian J Psychiatry. 2020;42(6):581-582.
4. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793-802.
5. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: a systematic review. Int J Mol Sci. 2018;19(8):2410.
6. Potkin SG, Kane JM, Correll CU, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. NPJ Schizophr. 2020;6(1):1.
7. Campana M, Falkai P, Siskind D, et al. Characteristics and definitions of ultra-treatment-resistant schizophrenia - a systematic review and meta-analysis. Schizophr Res. 2021;228:218-226.
8. Kinon BJ. The group of treatment resistant schizophrenias. Heterogeneity in treatment-resistant schizophrenia (TRS). Front Psychiatry. 2019;9:757.
9. Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62(11):772-777.
10. Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109(1-3):10-14.
11. Nasrallah HA, Fedora R, Morton R. Successful treatment of clozapine-nonresponsive refractory hallucinations and delusions with pimavanserin, a serotonin 5HT-2A receptor inverse agonist. Schizophr Res. 2019;208:217-220.
12. Linden N, Onwuanibe A, Sandson N. Rapid resolution of psychotic symptoms in a patient with schizophrenia using allopurinol as an adjuvant: a case report. Clin Schizophr Relat Psychoses. 2014;7(4):231-234.
13 Lintunen J, Lähteenvuo M, Tiihonen J, et al. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophr. 2021;7(1):1.
14. Kulkarni J, Butler S, Riecher-Rössler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019;53:100743. doi: 10.1016/j.yfrne.2019.03.002
15. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present and future. Schizophr Res. 2010;122(1-3):1-23.
16. Nasrallah HA. Biomarkers in neuropsychiatric disorders: translating research to clinical applications. Biomarkers in Neuropsychiatry. 2019;1:100001. doi: 10.1016/j.bionps.2019.100001
17. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
Antidepressants: Is a higher dose always better?
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).
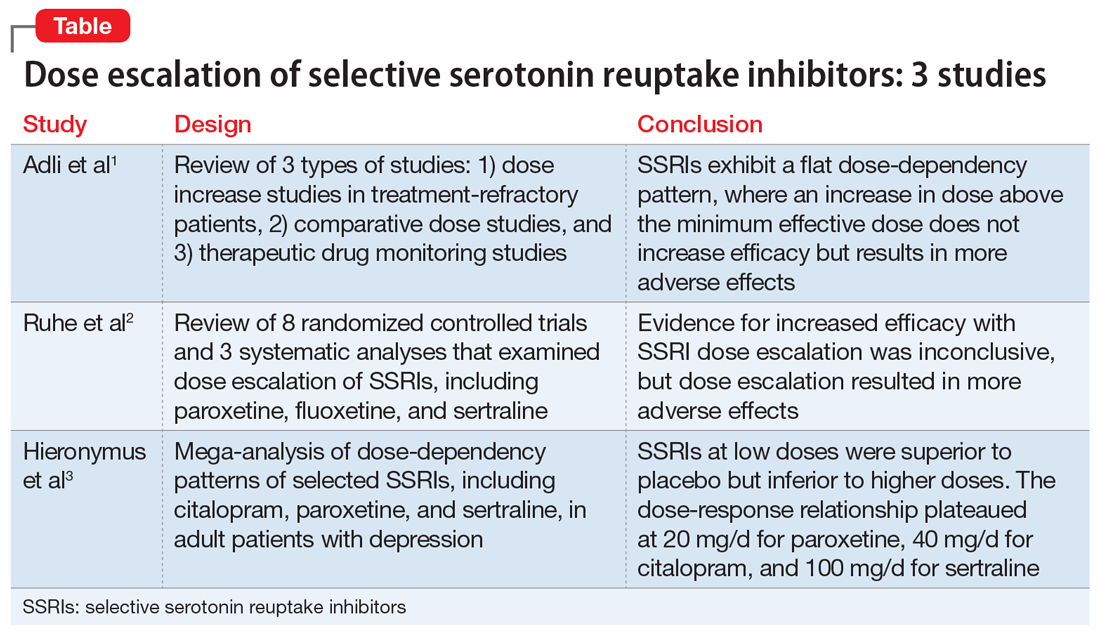
Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
Mr. E, age 39, presents to the mental health (MH) intake clinic, reporting he has had depressed mood almost every day, lack of interests, poor appetite, difficulty sleeping, inability to concentrate on daily activities, low energy and motivation, and feelings of guilt. He is diagnosed with major depressive disorder and agrees to a trial of sertraline, which is titrated up to 100 mg/d. He is also referred to the MH pharmacy clinic for interim visits.
Four weeks later during a follow-up visit, Mr. E reports tolerating sertraline, 100 mg/d, with a slight improvement in his mood. He reports that he has started working on his previous hobbies again and tries to consistently eat 2 meals a day. He feels that his sleep remains unchanged. He would like to enroll in school again, but is concerned about his poor concentration. He asks whether a further increase in his sertraline dose would improve his symptoms. What would you advise?
Escalating antidepressant doses up to, or even above, the FDA-approved maximum dose is a strategy for clinicians to consider for patients who are nonresponders or partial responders to treatment. This practice assumes that the effectiveness of an antidepressant is dependent on the dosage. However, based on our review of available literature, this recommendation is equivocally supported for general practice.
Selective serotonin reuptake inhibitors
The Table1-3 summarizes the results of 3 studies of high-dose selective serotonin reuptake inhibitors (SSRIs).

Adli et al1 evaluated 3 types of studies—studies of patients with treatment-resistant depression receiving high-dose treatment, comparative dose studies, and studies of therapeutic drug-monitoring (TDM) of antidepressants—to assess the effectiveness of high-dose antidepressants after a treatment failure with a medium dose. They concluded that SSRIs exhibit a flat dose-dependency pattern, where increasing a dose above the minimum effective dose (MED) does not increase efficacy but results in more adverse effects. Because treatment at the MED inhibits 70% of serotonin reuptake and is only marginally less effective than medium therapeutic doses, the authors recommended reserving treatment at higher doses for patients who have failed other standard treatment options, such as augmentation.
Ruhe et al2 evaluated 8 randomized controlled trials and 3 systematic analyses that investigated dose escalation of SSRIs, including paroxetine, fluoxetine, and sertraline. The authors noted that all included studies had methodological limitations and discussed 1 study that showed potential benefit from dose escalation when dropouts due to adverse effects were excluded from analysis. They determined that the evidence for increased efficacy with dose escalation was inconclusive; however, dose escalation un-doubtedly resulted in more adverse effects.
Hieronymus et al3 found a dose-dependency pattern with selected SSRIs—citalopram, paroxetine, and sertraline—in a mega-analysis of studies of adult patients with depression. All company-funded, acute-phase, placebo-controlled fixed-dose trials of these agents were included in this analysis. It included a total of 2,859 patients: 600 patients received citalopram (10 to 60 mg/d); 1,043 patients received paroxetine (10 to 40 mg/d); 481 patients received sertraline (50 to 400 mg/d); and 735 patients received placebo. They further divided the SSRIs into “low” vs “optimal” doses based on the dose curves of these agents. For citalopram, 10 to 20 mg/d was considered low vs 40 to 60 mg/d, which was considered optimal. For paroxetine, 10 mg/d was considered low vs other doses as optimal (20, 30, and 40 mg/d). For sertraline, 50 mg was considered low vs other doses as optimal (100, 200, and 400 mg/d). The authors concluded that at low doses, these antidepressants were superior to placebo but inferior to higher doses. Interestingly, they suggested that the dose-response relationship plateaued at 20 mg/d for paroxetine, 40 mg/d for citalopram, and 100 mg/d for sertraline. One of the limitations of the study was a lack of information on the tolerability of higher vs lower doses.
Continue to: Other antidepressants
Other antidepressants
Adli et al1 found a high-dose study and several comparative studies that supported a dose-response relationship with a reasonable degree of tolerability for venlafaxine, but there were no pertinent studies that evaluated mirtazapine. The only fixed-dose study found for bupropion did not support a dose-response relationship.1
The authors also concluded that there may be evidence supporting high-dose prescribing of tricyclic and tetracyclic antidepressants (TCAs and TeCAs, respectively). Despite the lack of clinical data that directly addressed the dose-dependency of TCAs and TeCAs, the authors supported dose escalation with amitriptyline, clomipramine, imipramine, desipramine, nortriptyline, and maprotiline, based on the data from comparative dose and TDM studies.1 The authors urged caution in interpreting and applying the results of TDM studies because the pharmacodynamic of each medication—such as being linear, curvilinear, or uncorrelated— may vary, which suggests there is a targeted therapeutic dose range.1
Important considerations
Differences in the pharmacokinetic and pharmacogenetic properties of individual medications may account for the mixed outcomes found when evaluating antidepressant dose-response relationships. Genetic polymorphisms of cytochrome (CYP) P450 enzymes, mainly CYP2D6 and CYP2D19, have been shown to directly affect antidepressants’ serum levels. Depending on the patient’s phenotype expression, such as poor, intermediate, extensive (ie, normal), or ultra-metabolizers, use of a specific antidepressant at a similar dose may result in therapeutic effectiveness, ineffectiveness, or toxicity. For antidepressants such as TCAs, which have a narrow therapeutic index compared with SSRIs, the differences in pharmacokinetic and pharmacogenetic properties becomes more impactful.1,4
Escalation within approved dose ranges
Few quality studies have conclusively found a relationship between antidepressant dose escalation within the FDA-approved dose ranges and efficacy, and there are few to no recommendations for prescribing doses above FDA-approved ranges. However, in clinical practice, clinicians may consider a dose escalation within the allowable dose ranges based on anecdotal evidence from previous patient cases. Consideration of relevant pharmacokinetic parameters and the patient’s individual pharmacogenetic factors may further guide clinicians and patients in making an informed decision on dose escalation to and beyond the FDA-approved doses.
CASE CONTINUED
After reviewing the evidence of antidepressant dose escalation and Mr. E’s progress, the MH pharmacist recommends that the psychiatrist increase Mr. E’s sertraline to 150 mg/d with close monitoring.
Related Resources
- Berney P. Dose-response relationship of recent antidepressants in the short-term treatment of depression. Dialogues Clin Neurosci. 2005;7:249.
- Jakubovski E, Varigonda AL, Freemantle N, et al. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174-183.
Drug Brand Names
Amitriptyline • Elavil
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Fluoxetine • Prozac
Imipramine • Tofranil
Maprotiline • Ludiomil
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Venlafaxine • Effexor
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
1. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
2. Ruhe HG, Huyser J, Swinkels JA, et al. Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder. Bri J Psychiatry. 2006;189:309-316.
3. Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6(6):e834. doi: 10.1038/tp.2016.104
4. Nassan M, Nicholson WY, Elliott MA, et al. Pharmacokinetic pharmacogenetic prescribing guidelines for antidepressants: a template for psychiatric precision medicine. Mayo Clin Proc. 2016;91(7):897-907.
PTSD prevalent in survivors of severe COVID-19
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Posttraumatic stress disorder may occur in up to a third of patients who recover from severe COVID-19 infection, new research suggests.
A study of more than 300 patients who presented to the emergency department with the virus showed a 30.2% prevalence for PTSD 30-120 days after COVID recovery.
or having persistent medical symptoms after hospitalization.
Additional diagnoses, such as depressive and hypomanic episodes and generalized anxiety disorder (GAD), were also present in some of the survivors.
“Previous coronavirus epidemics were associated with PTSD diagnoses in postillness stages, with meta-analytic findings indicating a prevalence of 32.2%,” write the investigators, led by Delfina Janiri, MD, department of psychiatry, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome.
However, data focused specifically on COVID-19 have been “piecemeal,” they add.
The findings were published online Feb. 18 in a research letter in JAMA Psychiatry.
A traumatic event
From April to October 2020, the researchers assessed 381 consecutive patients (100% white; 56.4% men; mean age, 55.3 years) who presented to the ED and subsequently participated in a health check at the Fondazione Policlinico Universitario Agostino Gemelli.
The mean length of stay for the 309 patients hospitalized with severe COVID-19 was 18.4 days.
Results showed that 115 participants (30.2%) had PTSD, based on DSM-5 criteria, and 55.7% of the women had the disorder. Additional diagnoses found in the full patient population included:
- Depressive episodes (17.3%).
- GAD (7%).
- Hypomanic episodes (0.7%).
- Psychotic disorders (0.2%).
Patients with PTSD had higher rates than those without PTSD of a previous history of psychiatric disorders (34.8% vs. 20.7%; P = .003) and of delirium or agitation during hospitalization, as assessed with the Confusion Assessment Method (16.5% vs. 6.4%; P = .002).
In addition, 62.6% of those with PTSD had three or more persistent COVID-19 symptoms vs. 37.2% of their counterparts without PTSD (P < .001).
After logistic regression analyses, significant factors associated with a PTSD diagnosis were persistent medical symptoms (P = .002), delirium or agitation (P = .02), and being female (P = .02).
The investigators note that their results are “in line” with findings reported in research examining other traumatic events. This includes about 30% of Hurricane Katrina survivors who experienced PTSD, as did around 25% of survivors of the 2011 “Great Japan Earthquake and Tsunami.”
Study limitations cited include the “relatively small” size of the patient population, that it focused on only one participating center, and that it didn’t include a control group of non-COVID patients who reported to the ED.
“Further longitudinal studies are needed to tailor therapeutic interventions and prevention strategies,” the researchers write.
Dr. Janiri and four of the five other authors have disclosed no relevant financial relationships. The other author, Gabriele Sani, MD, reported having received personal fees from Angelini Spa, Janssen, and Lundbeck outside the submitted work.
A version of this article first appeared on Medscape.com.
Heroes: Nurses’ sacrifice in the age of COVID-19
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
This past year, the referrals to my private practice have taken a noticeable shift and caused me to pause.
More calls have come from nurses, many who work directly with COVID-19 patients, understandably seeking mental health treatment, or support. Especially in this time, nurses are facing trauma and stress that is unimaginable to many, myself included. Despite the collective efforts we have made as a society to recognize their work, I do not think we have given enough consideration to the enormous sacrifice nurses are currently undertaking to save our collective psyche.
As physicians and mental health providers, we have a glimpse into the complexities and stressors of medical treatment. In our line of work, we support patients with trauma on a regular basis. We feel deeply connected to patients, some of whom we have treated until the end of their lives. Despite that, I am not sure that I, or anyone, can truly comprehend what nurses face in today’s climate of care.
There is no denying that doctors are of value to our system, but our service has limits; nurses and doctors operate as two sides to a shared coin. As doctors, we diagnose and prescribe, while nurses explain and dispense. As doctors, we talk to patients, while nurses comfort them. Imagine spending an entire year working in a hospital diligently wiping endotracheal tubes that are responsible for maintaining someone’s life. Imagine spending an entire year laboring through the heavy task of lifting patients to prone them in a position that may save their lives. Imagine spending an entire year holding the hands of comatose patients in hopes of maintaining a sense of humanity.
And this only begins to describe the tasks bestowed upon nurses. While doctors answer pagers or complete insurance authorization forms, nurses empathize and reassure scared and isolated patients. Imagine spending an entire year updating crying family members who cannot see their loved ones. Imagine spending an entire year explaining and pleading to the outside world that wearing a mask and washing hands would reduce the suffering that takes place inside the hospital walls.
Despite the uncertainties, pressures, and demands, nurses have continued, and will continue, to show up for their patients, shift by shift. It takes a tragic number of deaths for the nurses I see in my practice to share that they have lost count. These numbers reflect people they held to feed, carried to prevent ulcers, wiped for decency, caressed for compassion, probed with IVs and tubes, monitored for signs of life, and warmed with blankets. If love were in any job description, it would fall under that of a nurse.
And we can’t ignore the fact that all the lives lost by COVID-19 had family. Family members who, without ever stepping foot in the hospital, needed a place to be heard, a place to receive explanation, and a place for reassurance. This invaluable place is cultivated by nurses. Through Zoom and phone calls, nurses share messages of hope, love, and fear between patients and family. Through Zoom and phone calls, nurses orchestrate visits and last goodbyes.
There is no denying that we have all been affected by this shared human experience. But the pause we owe our nurses feels long overdue, and of great importance. Nurses need a space to be heard, to be comforted, to be recognized. They come to our practices, trying to contain the world’s angst, while also navigating for themselves what it means to go through what they are going through. They hope that by coming to see us, they will find the strength to go back another day, another week, another month. Sometimes, they come to talk about everything but the job, in hopes that by talking about more mundane problems, they will feel “normal” and reconnected.
I hope that our empathy, congruence, and unconditional positive regard will allow them to feel heard.1 I hope that our warmth, concern, and hopefulness provide a welcoming place to voice sadness, anger, and fears.2 I hope that our processing of traumatic memory, our challenge to avoid inaccurate self-blaming beliefs, and our encouragement to create more thought-out conclusions will allow them to understand what is happening more accurately.3
Yet, I worry. I worry that society hasn’t been particularly successful with helping prior generations of heroes. From war veterans, to Sept. 11, 2001, firefighters, it seems that we have repeated mistakes. My experience with veterans in particular has taught me that for many who are suffering, it feels like society has broken its very fabric by being bystanders to the pain.
But suffering and tragedy are an inevitable part of the human experience that we share. What we can keep sight of is this: As physicians, we work with nurses. We are witnessing firsthand the impossible sacrifice they are taking and the limits of resilience. Let us not be too busy to stop and give recognition where and when it is due. Let us listen and learn from our past, and present, heroes. And let us never forget to extend our own hand to those who make a living extending theirs.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com.
References
1. Rogers CR. J Consult Psychol. 1957;21(2):95-103.
2. Mallo CJ, Mintz DL. Psychodyn Psychiatry. 2013 Mar;41(1):13-37.
3. Resick PA et al. Cognitive Processing Therapy for PTSD: A Comprehensive Manual. Guilford Publications, 2016.
Inflammatory immune findings likely in acute schizophrenia, MDD, bipolar
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
Researchers have come a long way in understanding the link between acute inflammation and treatment-resistant depression, but more work needs to be done, according to Mark Hyman Rapaport, MD.
“Inflammation has been a hot topic in the past decade, both because of its impact in medical disorders and in psychiatric disorders,” Dr. Rapaport, CEO of the Huntsman Mental Health Institute in Salt Lake City, Utah, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “We run into difficulty with chronic inflammation, which we see with rheumatic disorders, and when we think of metabolic syndrome and obesity.”
The immune system helps to control energy regulation and neuroendocrine function in acute inflammation and chronic inflammatory diseases. “We see a variety of effects on the central nervous system or liver function or on homeostasis of the body,” said Dr. Rapaport, who also chairs the department of psychiatry at the University of Utah, also in Salt Lake City. “These are all normal and necessary to channel energy to the immune system in order to fight what’s necessary in acute inflammatory response.”
A chronic state of inflammation can result in prolonged allocation of fuels to the immune system, tissue inflammation, and a chronically aberrant immune reaction, he continued. This can cause depressive symptoms/fatigue, anorexia, malnutrition, muscle wasting, cachectic obesity, insulin resistance, dyslipidemia, increased adipose tissue in the proximity of inflammatory lesion, alterations of steroid hormone axes, elevated sympathetic tone, hypertension, decreased parasympathetic tone, inflammation-related anemia, and osteopenia. “So, chronic inflammation has a lot of long-term sequelae that are detrimental,” he said.
Both physical stress and psychological stress also cause an inflammatory state. After looking at the medical literature, Dr. Rapaport and colleagues began to wonder whether inflammation and immune activation associated with psychiatric disorders are attributable to the stress of acute illness. To find out, they performed a meta-analysis of blood cytokine network alterations in psychiatric patients and evaluated comparisons between schizophrenia, bipolar disorder, and depression. A total of three meta-analyses were performed: one of acute/inpatient studies, one on the impact of acute treatment, and one of outpatient studies. The researchers hypothesized that inflammatory and immune findings in psychiatric illnesses were tied to two distinct etiologies: the acute stress of illness and intrinsic immune dysfunction.
The meta-analyses included 68 studies: 40 involving patients with schizophrenia, 18 involving those with major depressive disorder (MDD) and 10 involving those with bipolar disorder. The researchers found that levels of four cytokines were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD, compared with controls: interleukin-6, tumor necrosis factor–alpha (TNF-alpha), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA). “There has not been a consistent blood panel used across studies, be it within a disorder itself like depression, or across disorders,” Dr. Rapaport noted. “This is a challenge that we face in looking at these data.”
Following treatment of acute illness, IL-6 levels significantly decreased in schizophrenia and MDD, but no significant changes in TNF-alpha levels were observed in patients with schizophrenia or MDD. In addition, sIL-2R levels increase in schizophrenia but remained unchanged in bipolar and MDD, while IL-1RA levels in bipolar mania decreased but remained unchanged in MDD. Meanwhile, assessment of the study’s 24 outpatient studies revealed that levels of IL-6 were significantly increased in outpatients with schizophrenia, euthymic bipolar disorder, and MDD, compared with controls (P < .01 for each). In addition, levels of IL-1 beta and sIL-2R were significantly increased in outpatients with schizophrenia and bipolar disorder.
According to Dr. Rapaport, these meta-analyses suggest that there are likely inflammatory immune findings present in acutely ill patients with MDD, schizophrenia, and bipolar disorder.
“Some of this activation decreases with effective acute treatment of the disorder,” he said. “The data suggest that immune changes are present in a subset of patients with all three disorders.”
“We also need to understand the regulatory role that microglia and astroglia play within the brain,” he said. “We need to identify changes in brain circuitry and function associated with inflammation and other immune changes. We also need to carefully scrutinize publications, understand the assumptions behind the statistics, and carry out more research beyond the protein level.”
He concluded his presentation by calling for research to help clinicians differentiate acute from chronic inflammation. “The study of both is important,” he said. “We need to understand the pathophysiology of immune changes in psychiatric disorders. We need to study both the triggers and pathways to resolution.”
Dr. Rapaport disclosed that he has received research support from the National Institutes of Health, the National Institute of Mental Health, and the National Center for Complementary and Integrative Health.
FROM NPA 2021