User login
Let side effects guide treatment choice for refractory OCD
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
Choosing the most effective treatment for patients with obsessive-compulsive disorder requires flexibility and agility on the part of clinicians, according to Wayne K. Goodman, MD.
“There are no data at this point to suggest that one SSRI is superior to another. It’s really dealer’s choice, and it has to do with really picking medications based upon side effects,” Dr. Goodman said at the Psychopharmacology Update, presented by Current Psychiatry and Global Academy for Medical Education. Clinicians can use family history as a guide, he noted, but pharmacogenetic testing has not been helpful in his experience for selection or dosing of an SSRI.
SSRIs, such as fluvoxamine, are one of two mainstays of treatment for patients with obsessive-compulsive disorder (OCD). The other drug class is serotonin reuptake inhibitors, which include medications such clomipramine. Cognitive-behavioral therapy options, such as Exposure and Response Prevention therapy, also has some, albeit limited, efficacy.
Meanwhile, Dr. Goodman said, antidepressant classes other than SRIs and SSRIs have not been effective in treating obsessive-compulsive symptoms, and some patients do not adhere well to cognitive-behavioral therapy, said Dr. Goodman, who is the D.C. and Irene Ellwood Professor in the department of psychiatry and behavioral sciences at Baylor College of Medicine, Houston.
FROM PSYCHOPHARMACOLOGY UPDATE
How mental health care would look under a Trump vs. Biden administration
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
The COVID-19 pandemic is one of the most pressing public health challenges the United States has ever faced, and the resulting financial ruin and social isolation are creating a mental health pandemic that will continue well after COVID-19 lockdowns end. To understand which presidential candidate would best lead the mental health recovery, we identified three of the most critical issues in mental health and compared the plans of the two candidates.
Fighting the opioid epidemic
Over the last several years, the opioid epidemic has devastated American families and communities. Prior to the pandemic, drug overdoses were the leading cause of death for American adults under 50 years of age. The effects of COVID-19–enabled overdose deaths to rise even higher. Multiple elements of the pandemic – isolation, unemployment, and increased anxiety and depression – make those struggling with substance use even more vulnerable, and immediate and comprehensive action is needed to address this national tragedy.
Donald J. Trump: President Trump has been vocal and active in addressing this problem since he took office. One of the Trump administration’s successes is launching the Opioid and Drug Abuse Commission and rolling out a five-point strategy built around improving services, data, research, overdose-reversing drugs, and pain management. Last year, the Trump administration funded $10 billion over 5 years to combat both the opioid epidemic and mental health issues by building upon the 21st Century CURES Act. However, in this same budget, the administration proposed cutting funding by $600 million for SAMHSA, the Substance Abuse and Mental Health Services Administration, which is the top government agency for addressing and providing care for substance use.
President Trump also created an assistant secretary for mental health and substance use position in the Department of Health & Human Services, and appointed Elinore F. McCance-Katz, MD, PhD, a psychiatrist with a strong track record on fighting opioid abuse in Rhode Island, to the post.
Joe Biden: Former Vice President Biden emphasizes that substance use is “a disease of the brain,” refuting the long-held misconception that addiction is an issue of willpower. This stigmatization is very personal given that his own son Hunter reportedly suffered through mental health and substance use issues since his teenage years. However, Biden also had a major role in pushing forward the federal “war on drugs,” including his role in crafting the “Len Bias law.”
Mr. Biden has since released a multifaceted plan for reducing substance use, aiming to make prevention and treatment services more available through a $125 billion federal investment. There are also measures to hold pharmaceutical companies accountable for triggering the crisis, stop the flow of fentanyl to the United States, and restrict incentive payments from manufacturers to doctors so as to limit the dosing and usage of powerful opioids.
Accessing health care
One of the main dividing lines in this election has been the battle to either gut or build upon the Affordable Care Act (ACA). This will have deep ramifications on people’s access to health mental health services. Since COVID-19 started, more than 50% of Americans have reported worsening mental health. This makes it crucial that each candidate’s mental health plan is judged by how they would expand access to insurance, address unenforced parity laws, and protect those who have a mental health disorder as a preexisting condition.
Mr. Trump: Following a failed Senate vote to repeal this law, the Trump administration took a piecemeal approach to dismantling the ACA that included removing the individual mandate, enabling states to introduce Medicaid work requirements, and reducing cost-sharing subsidies to insurers.
If a re-elected Trump administration pursued a complete repeal of the ACA law, many individuals with previous access to mental health and substance abuse treatment via Medicaid expansion may lose access altogether. In addition, key mechanisms aimed at making sure that mental health services are covered by private health plans may be lost, which could undermine policies to address opioids and suicide. On the other hand, the Trump administration’s move during the pandemic to expand telemedicine services has also expanded access to mental health services.
Mr. Biden: Mr. Biden’s plan would build upon the ACA by working to achieve parity between the treatment of mental health and physical health. The ACA itself strengthened the Mental Health Parity and Addiction Equity Act (federal parity law), which Mr. Biden championed as vice president, by mandating that all private insurance cover mental health and substance abuse treatment. This act still exempts some health plans, such as larger employers; and many insurers have used loopholes in the policy to illegally deny what could be life-saving coverage.
It follows that those who can afford Mr. Biden’s proposed public option Medicare buy-in would receive more comprehensive mental health benefits. He also says he would invest in school and college mental health professionals, an important opportunity for early intervention given 75% of lifetime mental illness starts by age 24 years. While Mr. Biden has not stated a specific plan for addressing minority groups, whose mental health has been disproportionately affected by COVID-19, he has acknowledged that this unmet need should be targeted.
Addressing suicide
More than 3,000 Americans attempt suicide every day. Suicide is the second leading cause of death for America’s youth and one of the top 10 leading causes of death across the population. Numerous strategies are necessary to address suicide, but one of the most decisive is gun control. Gun violence is inextricably tied to suicide: States where gun prevalence is higher see about four times the number of suicides because of guns, whereas nonfirearm suicide rates are the same as those seen elsewhere. In 2017, of the nearly 40,000 people who died of gun violence, 60% were attributable to suicides. Since the pandemic started, there have been increases in reported suicidal thoughts and a nearly 1,000% increase in use of the national crisis hotline. This is especially concerning given the uptick during the pandemic of gun purchases; as of September, more guns have been purchased this year than any year before.
Mr. Trump: Prior to coronavirus, the Trump administration was unwilling to enact gun control legislation. In early 2017, Mr. Trump removed an Obama-era bill that would have expanded the background check database. It would have added those deemed legally unfit to handle their own funds and those who received Social Security funds for mental health reasons. During the lockdown, the administration made an advisory ruling declaring gun shops as essential businesses that states should keep open.
Mr. Biden: The former vice president has a history of supporting gun control measures in his time as a senator and vice president. In the Senate, Mr. Biden supported both the Brady handgun bill in 1993 and a ban on assault weapons in 1994. As vice president, he was tasked by President Obama to push for a renewed assault weapons ban and a background check bill (Manchin-Toomey bill).
During his 2020 presidential campaign, Mr. Biden has suggested creating universal background checks and reinstating bans on assault rifle sales. He has said that he is also open to having a federal buyback program for assault rifles from gun owners.
Why this matters
The winner of the 2020 election will lead an electorate that is reeling from the health, economic, and social consequences COVID-19. The next administration needs to act swiftly to address the mental health pandemic and have a keen awareness of what is ahead. As Americans make their voting decision, consider who has the best plans not only to contain the virus but also the mental health crises that are ravaging our nation.
Dr. Vasan is a clinical assistant professor of psychiatry at Stanford (Calif.) University, where she is founder and executive director of Brainstorm: The Stanford Lab for Mental Health Innovation. She also serves as chief medical officer of Real, and chair of the American Psychiatric Association Committee on Innovation. Dr. Vasan has no conflicts of interest. Mr. Agbafe is a fellow at Stanford Brainstorm and a first-year medical student at the University of Michigan, Ann Arbor. He has no conflicts of interest. Ms. Li is a policy intern at Stanford Brainstorm and an undergraduate student in the department of economics at the University of California, Berkeley. She has no conflicts of interest.
Is ketamine living up to the promise for depression?
After years of dormancy, psychiatric drug development is showing signs of life. There is the novel antipsychotic lumateperone, recently approved for adults with schizophrenia. Brexanolone was approved last year for postpartum depression. And perhaps generating the most attention lately among psychiatrists – and people with depression – is the use of ketamine and esketamine for depression.
Columbia psychiatrist J. John Mann, MD, a professor of translational neuroscience and a mood disorder specialist, has been involved in several notable studies of ketamine in patients with depression. He and his colleagues’ recent research efforts include a randomized study into ketamine’s ability to reduce suicidal thoughts in bipolar depression and an MRI analysis illuminating the role that dosing plays in antidepressant effects.
Dr. Sederer: Nearly 20 million people in this country alone suffer from clinical depression every year. That means they have a functional impairment in addition to their suffering and are at risk of taking their own lives. Depression is a very prevalent, painful, and disabling condition. The psychopharmacologic treatments we’ve had have been more or less the same for the past 20 or 30 years.
We’ve asked Dr. Mann to … talk to us about ketamine and its nasal preparation esketamine, which is a novel psychopharmacologic treatment. Dr. Mann, please tell us about ketamine, its utility, and the yet-to-be-answered questions.
Dr. Mann: Ketamine and esketamine, which is a component of ketamine itself, is different from traditional antidepressant medications in three fundamental ways.
The first is that it acts very quickly. Traditional antidepressants take 4, 6, 8 weeks to work. This means that the patient has to put up with a great deal of suffering while waiting for a response. And the probability of the medication working isn’t that high. About 50%-70% of individuals will respond eventually at the end of this ride. But ketamine, when it works, does so in 2 hours. That’s a totally different timescale.
The second aspect is that, when it does work, it often works very robustly, even though it’s a quick-acting antidepressant. The patient often quickly feels distinctly better.
Very often when you’re using traditional antidepressants, it takes a while for the improvement to reach the point that the patient is confident that they are clearly better, and too often that does not happen.
Dr. Sederer: As the treating doctor, you’re trying to keep that patient’s hope alive, even though we don’t have substantial evidence that they’re going to respond.
Dr. Mann: Exactly. One of the difficulties of keeping patients on track with traditional antidepressants is that, after the first dose or two, they have all the side effects and yet no benefit has emerged. In many ways patients sometimes feel that they’re going backwards. They have all of their depression plus the side effects of the medication.
But with ketamine, it’s rather different. You come in, you have the treatment, and many patients feel improved in a couple of hours. And not just a bit improved, but in many cases distinctly improved.
It’s very important for clinicians to appreciate that ketamine will work in patients who have a classically described treatment-resistant depression, meaning they’ve tried several other types of antidepressant medications that haven’t worked.
A prerequisite for treatment with ketamine is that they have had a number of treatment failures. The labeling for the intranasal esketamine states that you should try the other antidepressants first and then use this if they don’t work. The fact that ketamine can work even when the other medications have failed is a huge advantage.
Dr. Sederer: There is another feature of ketamine, in that it also has a pronounced benefit for suicidal ideation, which your research has reported on.
Dr. Mann: Yes, we’ve learned over the years that depression and suicidality are in some ways comorbid conditions. That both have to be addressed in order to keep somebody alive so that they can respond to treatment.
That’s a very important point. If the patient is suffering from depression and the antidepressant takes weeks to work, they may lose hope during that time. They may become overwhelmed by the suicidal ideation, no longer able to control or resist the impulse to take their life. A lot of the management is therefore to try to help support the patient (and family) so that these thoughts never become too compelling. Often we have to consider hospitalization to protect these patients so that they can stay alive long enough for the antidepressant to work. But ketamine not only has this very rapid effect for their depression, it also has a partly independent effect on suicidal ideation that is equally rapid and robust, which can render the patient safer.
Dr. Sederer: In other words, it’s effective and rapidly so for depression, with a bonus of reducing suicidality? This sounds almost too good to be true.
Dr. Mann: There are some limitations that we have to keep in mind. One limitation is that a single administration of ketamine will produce this robust improvement but it will only persist for most people for 5-7 days.
Dr. Sederer: Is the same duration true for scheduling the next treatment as well?
Dr. Mann: Yes, it is. The patient will gradually begin to deteriorate if you do not repeat the treatment. But as we showed in our randomized controlled clinical study, with ketamine for suicidal ideation, if you continue to deliver the medication, you can sustain the benefit.
Dr. Sederer: Can a person receive both ketamine and a conventional antidepressant at the same time?
Dr. Mann: Yes. In this study, half of the patients were actually continued on their previous medication while we added the ketamine on top of that. It worked very well.
In practice, people use two approaches. One approach used by most ketamine clinics is to give six doses of ketamine at a frequency of about two per week. Then they will reduce the frequency down to once a week for a few more doses and then once a month.
Dr. Sederer:: And this is a ketamine infusion?
Dr. Mann: Yes, this generally has been a ketamine infusion. This approach seems to work quite well. But that may not be necessary.
Another strategy is to give one, two, or three doses of ketamine. If the patient doesn’t respond robustly to two or three doses, they’re not going to respond to subsequent doses.
Dr. Sederer: So, initial responses are a predictor of future response?
Dr. Mann: Exactly. Now, if they haven’t done well with two or three doses, then you’ve got to use other treatments. But if they do well with the two or three, then you’ve got a choice: You can either complete the treatment course with ketamine and then continue them on antidepressant medications, or simply treat them with ketamine alone. What we tend to do is to treat with only antidepressant medications after a small number of ketamine treatments. We also use ketamine as a kind of “rescue medication” if they relapse into severe depression, though this is true for only a minority of patients.
Dr. Sederer: One of the things that we’ve learned is that antidepressants have a very beneficial effect for some people, but then they wear out and the person starts to relapse. Should ketamine be studied as an intervention for people who are no longer responding to the antidepressant(s) that they are on?
Dr. Mann: We do not really know the answer to that question. My experience treating very seriously ill patients is that sometimes the ketamine will work very well the first time or the second time but then in the future, if you try to use it as a rescue medication, it might not work that well. There is some clinical experience that suggests that that may be true for some people. But we have no idea about the frequency or timing with which this might happen. That’s all uncertain.
Moreover, most of our control clinical trial data come from either one dose of ketamine or from a few trials where people have received multiple doses of ketamine, followed by a bit of a taper. But there are very, very few of those types of studies. We’re still learning about the use of this medication.
Dr. Sederer: Importantly, you referenced the side effects of antidepressants. What are the side effects and risks of ketamine?
Dr. Mann: We know a lot more about the immediate short-term side effects of a single dose or a few doses of ketamine. Most people will get a kind of tripping experience. They’ll feel a bit unreal, or their circumstances or experiences of the world feel a bit distorted.
Some patients develop strange ideas. Most patients don’t enjoy those symptoms, even though I know ketamine is used as a party drug, and so on and so forth.
Dr. Sederer: It seems that the context is what matters.
Dr. Mann: Yes. And in a clinic context, most patients simply don’t enjoy these types of dissociative experiences, but they put up with them. They’re not severe, in general.
Dr. Sederer: Is part of the preparation of the patient telling them that this may happen?
Dr. Mann: Yes. We try to explain the potential for these symptoms and that most people get them. These side effects almost invariably terminate with the cessation of the administration.
Dr. Sederer: What’s the typical duration of the infusion you use?
Dr. Mann: Traditionally, infusion is 40 minutes and always in a clinic setting.
Dr. Sederer: And that’s because of the concern that a patient may have these symptoms?
Dr. Mann: Exactly. They may have dissociative effects that they’re disturbed by, and we need to monitor that. They’re probably going to remain under observation in the clinic for about the same amount of time because it takes about the same time for these effects to wear off.
The other consideration is that some people get a little nausea. In our experience with the intravenous ketamine, there’s also a problematic side effect that their blood pressure will be slightly raised. Therefore, it’s good to know that the person’s blood pressure is under control before they begin the treatments and that you’re monitoring it during administration.
Dr. Sederer: What are the differences you’re discovering between esketamine and ketamine?
Dr. Mann: It is a bit different. We’ve just completed a very important National Institutes of Health–funded clinical trial here at Columbia showing that with esketamine or ketamine itself, the dose and the blood levels are very closely related to the robustness of the clinical response.
When you give a drug intravenously, you give a very reliable dose. When you give the drug over 40 minutes, you’re spreading the dose administration over a period of time so that it doesn’t peak very high. The side effects appear to be proportional to the peak dose.
When you give it intranasally, you give the drug over a much shorter period of time. Even if you use more than one intranasal administration to give the whole dose, it’s still a relatively shorter time, compared with the 40 minutes.
Dr. Sederer: This means that to get the equivalent dose intranasally, the patient is going to have to experience a higher peak. Can you predict that those patients who are treated intranasally are going to have more side effects?
Dr. Mann: Right. And that should be explained to the patient. You will not need an intravenous line inserted, which some people might find highly appealing and advantageous, but you will probably have more side effects.
Also, in general, intranasal absorption of drugs is more variable. The predictability of the blood level and, therefore, the degree of antidepressant effect is not as good intranasally as intravenously.
Now, all of this is anecdotal clinical experience, based on theoretical pharmacology, because nobody has actually done a head-to-head control comparison.
Dr. Sederer: What about the cost of both of these preparations?
Dr. Mann: There is a bit of a range in pricing between ketamine clinics around the country. It’s always important to find out what they charge per administration. And then it makes a difference whether you have two or three administrations versus six plus further tapered administration. Clearly, the cost can vary a great deal.
Dr. Sederer: But it’s generally not covered by insurance, so most people are paying out of pocket.
Dr. Mann: Yes. The intranasal ketamine is still in negotiation at the moment, but it should be resolved before it’s fully marketed.
Dr. Sederer: Ketamine is used for major depression. Does it have utility in bipolar depression?
Dr. Mann: We and some others have done initial studies in bipolar depression. In our view, it’s probably going to be as effective in bipolar disorder as it is in major depressive disorder, unipolar depression.
We haven’t seen any manic episodes triggered, but we don’t give repeated doses. We allow research patients to stay on anticonvulsants or mood stabilizers, so that’s helpful. Generally, people with bipolar disorder who come for ketamine treatment for their depression are coming on a mood stabilizer, because that and perhaps other conventional antidepressants have not proven to be effective. So, I think that ketamine plus mood stabilizers seems to be very promising.
Dr. Sederer: I want to return to the antisuicidal properties that you had previously mentioned. I heard from a colleague about a patient who had been admitted to a psychiatric inpatient unit. The patient was in her 20s; she did not have major depression but was persistently suicidal, constantly trying to hurt herself in any way she could. But that seemed to be more a product of borderline personality disorder, with its impulsive and self-destructive problems.
In the end, they tried intranasal ketamine. The response was, just as you described, robust. Her self-injurious behavior dropped in a very pronounced way within a day or two. But she then did require administrations a couple of times a week in order to keep that suicidality at bay.
Based on that example, I’m wondering whether there is an application here for people who are suicidal yet who may not have features of major depression or bipolar depression.
Dr. Mann: It’s a very interesting suggestion for which we have no data-based answer. However, we have a clue from the study that we published in the American Journal of Psychiatry and have since published further analyses on.
The reasons that people die by suicide, or make suicide attempts, are not entirely attributable to the fact that they suffer from a mood disorder.
Dr. Sederer: Yes, because only a minority of individuals with a mood disorder ever make suicide attempts. But there is a subgroup at risk.
Dr. Mann: Here at Columbia, we’ve promulgated the stress-diathesis model for suicidal behavior. A stressor could be external life events, but the internal stressor would be something like an acute episode of depression.
But predisposition also plays an important role, which has several elements to it. One is decision-making. These are patients with a propensity to go for a short-term, quick relief. In other words, a patient would be seeking immediate relief rather than waiting for the delayed improvement from an antidepressant. They’re more prone to act on the pain of the depression and terminate their lives – to try to end their pain – rather than wait and hope that, in time, there’s a chance that the antidepressant will work.
Dr. Sederer: What else do you want to share with our viewers about this medication and how it’s used?
Dr. Mann: My goal in treating patients is to try to use the least amount of medication possible. We do not really know yet the long-term safety of ketamine treatment.
It’s been used for many decades in anesthesia, but people don’t get repeated anesthetic doses of ketamine. And higher doses of ketamine given repeatedly have been shown in preclinical studies to produce little lesions in the brain, which is not good. But we’re using much lower doses.
As we potentially move into a time when we could be giving multiple doses of ketamine to patients, we should remember that we need to be cautious about that. If we don’t need to give them more doses, we shouldn’t. We should know that there is a potential downside that we don’t fully understand yet to giving ketamine repeatedly.
And that’s aside from its abuse potential. We know that people have employed ketamine for physical and emotional pain, and when they administer it themselves, they tend to get dependent on it. In a clinic setting it’s given in a very formal and structured fashion, a bit like the administration of opioids. In that setting, it is much safer and the risk for abuse and diversion is minimized. But we need to remember that this drug does have abuse potential and perhaps not yet fully measurable neurotoxic effects.
Dr. Sederer: If physicians, nurses, and other professional clinicians want to learn more about this medication, what are the accurate, reliable sources of information to which you suggest they turn?
Dr. Mann: The National Institute of Mental Health’s website offers good and reliable information for patients and their families. It is an unbiased, scientific, and thoughtful source of information, and better than just trolling the Internet for information.
People are much more sophisticated now than they were 20 years ago in these matters, and scientific papers are much more accessible to the public. Reading papers in recognized journals is also a useful way to gain information.
For example, one of the major papers that we published in the American Journal of Psychiatry is available to anybody on the Internet to read. So, I encourage people to make their own inquiries and talk to more than one doctor. Informed patients and families are the best partners a doctor can ever have. We encourage that in all of our patients.
Dr. Sederer: I want to thank you very much, Dr. Mann, for your work in this area and for joining us here at Medscape and Columbia Psychiatry to teach us so much about what is truly a novel psychopharmacologic agent, yet one where we still have a lot more to learn.
A version of this article originally appeared on Medscape.com.
After years of dormancy, psychiatric drug development is showing signs of life. There is the novel antipsychotic lumateperone, recently approved for adults with schizophrenia. Brexanolone was approved last year for postpartum depression. And perhaps generating the most attention lately among psychiatrists – and people with depression – is the use of ketamine and esketamine for depression.
Columbia psychiatrist J. John Mann, MD, a professor of translational neuroscience and a mood disorder specialist, has been involved in several notable studies of ketamine in patients with depression. He and his colleagues’ recent research efforts include a randomized study into ketamine’s ability to reduce suicidal thoughts in bipolar depression and an MRI analysis illuminating the role that dosing plays in antidepressant effects.
Dr. Sederer: Nearly 20 million people in this country alone suffer from clinical depression every year. That means they have a functional impairment in addition to their suffering and are at risk of taking their own lives. Depression is a very prevalent, painful, and disabling condition. The psychopharmacologic treatments we’ve had have been more or less the same for the past 20 or 30 years.
We’ve asked Dr. Mann to … talk to us about ketamine and its nasal preparation esketamine, which is a novel psychopharmacologic treatment. Dr. Mann, please tell us about ketamine, its utility, and the yet-to-be-answered questions.
Dr. Mann: Ketamine and esketamine, which is a component of ketamine itself, is different from traditional antidepressant medications in three fundamental ways.
The first is that it acts very quickly. Traditional antidepressants take 4, 6, 8 weeks to work. This means that the patient has to put up with a great deal of suffering while waiting for a response. And the probability of the medication working isn’t that high. About 50%-70% of individuals will respond eventually at the end of this ride. But ketamine, when it works, does so in 2 hours. That’s a totally different timescale.
The second aspect is that, when it does work, it often works very robustly, even though it’s a quick-acting antidepressant. The patient often quickly feels distinctly better.
Very often when you’re using traditional antidepressants, it takes a while for the improvement to reach the point that the patient is confident that they are clearly better, and too often that does not happen.
Dr. Sederer: As the treating doctor, you’re trying to keep that patient’s hope alive, even though we don’t have substantial evidence that they’re going to respond.
Dr. Mann: Exactly. One of the difficulties of keeping patients on track with traditional antidepressants is that, after the first dose or two, they have all the side effects and yet no benefit has emerged. In many ways patients sometimes feel that they’re going backwards. They have all of their depression plus the side effects of the medication.
But with ketamine, it’s rather different. You come in, you have the treatment, and many patients feel improved in a couple of hours. And not just a bit improved, but in many cases distinctly improved.
It’s very important for clinicians to appreciate that ketamine will work in patients who have a classically described treatment-resistant depression, meaning they’ve tried several other types of antidepressant medications that haven’t worked.
A prerequisite for treatment with ketamine is that they have had a number of treatment failures. The labeling for the intranasal esketamine states that you should try the other antidepressants first and then use this if they don’t work. The fact that ketamine can work even when the other medications have failed is a huge advantage.
Dr. Sederer: There is another feature of ketamine, in that it also has a pronounced benefit for suicidal ideation, which your research has reported on.
Dr. Mann: Yes, we’ve learned over the years that depression and suicidality are in some ways comorbid conditions. That both have to be addressed in order to keep somebody alive so that they can respond to treatment.
That’s a very important point. If the patient is suffering from depression and the antidepressant takes weeks to work, they may lose hope during that time. They may become overwhelmed by the suicidal ideation, no longer able to control or resist the impulse to take their life. A lot of the management is therefore to try to help support the patient (and family) so that these thoughts never become too compelling. Often we have to consider hospitalization to protect these patients so that they can stay alive long enough for the antidepressant to work. But ketamine not only has this very rapid effect for their depression, it also has a partly independent effect on suicidal ideation that is equally rapid and robust, which can render the patient safer.
Dr. Sederer: In other words, it’s effective and rapidly so for depression, with a bonus of reducing suicidality? This sounds almost too good to be true.
Dr. Mann: There are some limitations that we have to keep in mind. One limitation is that a single administration of ketamine will produce this robust improvement but it will only persist for most people for 5-7 days.
Dr. Sederer: Is the same duration true for scheduling the next treatment as well?
Dr. Mann: Yes, it is. The patient will gradually begin to deteriorate if you do not repeat the treatment. But as we showed in our randomized controlled clinical study, with ketamine for suicidal ideation, if you continue to deliver the medication, you can sustain the benefit.
Dr. Sederer: Can a person receive both ketamine and a conventional antidepressant at the same time?
Dr. Mann: Yes. In this study, half of the patients were actually continued on their previous medication while we added the ketamine on top of that. It worked very well.
In practice, people use two approaches. One approach used by most ketamine clinics is to give six doses of ketamine at a frequency of about two per week. Then they will reduce the frequency down to once a week for a few more doses and then once a month.
Dr. Sederer:: And this is a ketamine infusion?
Dr. Mann: Yes, this generally has been a ketamine infusion. This approach seems to work quite well. But that may not be necessary.
Another strategy is to give one, two, or three doses of ketamine. If the patient doesn’t respond robustly to two or three doses, they’re not going to respond to subsequent doses.
Dr. Sederer: So, initial responses are a predictor of future response?
Dr. Mann: Exactly. Now, if they haven’t done well with two or three doses, then you’ve got to use other treatments. But if they do well with the two or three, then you’ve got a choice: You can either complete the treatment course with ketamine and then continue them on antidepressant medications, or simply treat them with ketamine alone. What we tend to do is to treat with only antidepressant medications after a small number of ketamine treatments. We also use ketamine as a kind of “rescue medication” if they relapse into severe depression, though this is true for only a minority of patients.
Dr. Sederer: One of the things that we’ve learned is that antidepressants have a very beneficial effect for some people, but then they wear out and the person starts to relapse. Should ketamine be studied as an intervention for people who are no longer responding to the antidepressant(s) that they are on?
Dr. Mann: We do not really know the answer to that question. My experience treating very seriously ill patients is that sometimes the ketamine will work very well the first time or the second time but then in the future, if you try to use it as a rescue medication, it might not work that well. There is some clinical experience that suggests that that may be true for some people. But we have no idea about the frequency or timing with which this might happen. That’s all uncertain.
Moreover, most of our control clinical trial data come from either one dose of ketamine or from a few trials where people have received multiple doses of ketamine, followed by a bit of a taper. But there are very, very few of those types of studies. We’re still learning about the use of this medication.
Dr. Sederer: Importantly, you referenced the side effects of antidepressants. What are the side effects and risks of ketamine?
Dr. Mann: We know a lot more about the immediate short-term side effects of a single dose or a few doses of ketamine. Most people will get a kind of tripping experience. They’ll feel a bit unreal, or their circumstances or experiences of the world feel a bit distorted.
Some patients develop strange ideas. Most patients don’t enjoy those symptoms, even though I know ketamine is used as a party drug, and so on and so forth.
Dr. Sederer: It seems that the context is what matters.
Dr. Mann: Yes. And in a clinic context, most patients simply don’t enjoy these types of dissociative experiences, but they put up with them. They’re not severe, in general.
Dr. Sederer: Is part of the preparation of the patient telling them that this may happen?
Dr. Mann: Yes. We try to explain the potential for these symptoms and that most people get them. These side effects almost invariably terminate with the cessation of the administration.
Dr. Sederer: What’s the typical duration of the infusion you use?
Dr. Mann: Traditionally, infusion is 40 minutes and always in a clinic setting.
Dr. Sederer: And that’s because of the concern that a patient may have these symptoms?
Dr. Mann: Exactly. They may have dissociative effects that they’re disturbed by, and we need to monitor that. They’re probably going to remain under observation in the clinic for about the same amount of time because it takes about the same time for these effects to wear off.
The other consideration is that some people get a little nausea. In our experience with the intravenous ketamine, there’s also a problematic side effect that their blood pressure will be slightly raised. Therefore, it’s good to know that the person’s blood pressure is under control before they begin the treatments and that you’re monitoring it during administration.
Dr. Sederer: What are the differences you’re discovering between esketamine and ketamine?
Dr. Mann: It is a bit different. We’ve just completed a very important National Institutes of Health–funded clinical trial here at Columbia showing that with esketamine or ketamine itself, the dose and the blood levels are very closely related to the robustness of the clinical response.
When you give a drug intravenously, you give a very reliable dose. When you give the drug over 40 minutes, you’re spreading the dose administration over a period of time so that it doesn’t peak very high. The side effects appear to be proportional to the peak dose.
When you give it intranasally, you give the drug over a much shorter period of time. Even if you use more than one intranasal administration to give the whole dose, it’s still a relatively shorter time, compared with the 40 minutes.
Dr. Sederer: This means that to get the equivalent dose intranasally, the patient is going to have to experience a higher peak. Can you predict that those patients who are treated intranasally are going to have more side effects?
Dr. Mann: Right. And that should be explained to the patient. You will not need an intravenous line inserted, which some people might find highly appealing and advantageous, but you will probably have more side effects.
Also, in general, intranasal absorption of drugs is more variable. The predictability of the blood level and, therefore, the degree of antidepressant effect is not as good intranasally as intravenously.
Now, all of this is anecdotal clinical experience, based on theoretical pharmacology, because nobody has actually done a head-to-head control comparison.
Dr. Sederer: What about the cost of both of these preparations?
Dr. Mann: There is a bit of a range in pricing between ketamine clinics around the country. It’s always important to find out what they charge per administration. And then it makes a difference whether you have two or three administrations versus six plus further tapered administration. Clearly, the cost can vary a great deal.
Dr. Sederer: But it’s generally not covered by insurance, so most people are paying out of pocket.
Dr. Mann: Yes. The intranasal ketamine is still in negotiation at the moment, but it should be resolved before it’s fully marketed.
Dr. Sederer: Ketamine is used for major depression. Does it have utility in bipolar depression?
Dr. Mann: We and some others have done initial studies in bipolar depression. In our view, it’s probably going to be as effective in bipolar disorder as it is in major depressive disorder, unipolar depression.
We haven’t seen any manic episodes triggered, but we don’t give repeated doses. We allow research patients to stay on anticonvulsants or mood stabilizers, so that’s helpful. Generally, people with bipolar disorder who come for ketamine treatment for their depression are coming on a mood stabilizer, because that and perhaps other conventional antidepressants have not proven to be effective. So, I think that ketamine plus mood stabilizers seems to be very promising.
Dr. Sederer: I want to return to the antisuicidal properties that you had previously mentioned. I heard from a colleague about a patient who had been admitted to a psychiatric inpatient unit. The patient was in her 20s; she did not have major depression but was persistently suicidal, constantly trying to hurt herself in any way she could. But that seemed to be more a product of borderline personality disorder, with its impulsive and self-destructive problems.
In the end, they tried intranasal ketamine. The response was, just as you described, robust. Her self-injurious behavior dropped in a very pronounced way within a day or two. But she then did require administrations a couple of times a week in order to keep that suicidality at bay.
Based on that example, I’m wondering whether there is an application here for people who are suicidal yet who may not have features of major depression or bipolar depression.
Dr. Mann: It’s a very interesting suggestion for which we have no data-based answer. However, we have a clue from the study that we published in the American Journal of Psychiatry and have since published further analyses on.
The reasons that people die by suicide, or make suicide attempts, are not entirely attributable to the fact that they suffer from a mood disorder.
Dr. Sederer: Yes, because only a minority of individuals with a mood disorder ever make suicide attempts. But there is a subgroup at risk.
Dr. Mann: Here at Columbia, we’ve promulgated the stress-diathesis model for suicidal behavior. A stressor could be external life events, but the internal stressor would be something like an acute episode of depression.
But predisposition also plays an important role, which has several elements to it. One is decision-making. These are patients with a propensity to go for a short-term, quick relief. In other words, a patient would be seeking immediate relief rather than waiting for the delayed improvement from an antidepressant. They’re more prone to act on the pain of the depression and terminate their lives – to try to end their pain – rather than wait and hope that, in time, there’s a chance that the antidepressant will work.
Dr. Sederer: What else do you want to share with our viewers about this medication and how it’s used?
Dr. Mann: My goal in treating patients is to try to use the least amount of medication possible. We do not really know yet the long-term safety of ketamine treatment.
It’s been used for many decades in anesthesia, but people don’t get repeated anesthetic doses of ketamine. And higher doses of ketamine given repeatedly have been shown in preclinical studies to produce little lesions in the brain, which is not good. But we’re using much lower doses.
As we potentially move into a time when we could be giving multiple doses of ketamine to patients, we should remember that we need to be cautious about that. If we don’t need to give them more doses, we shouldn’t. We should know that there is a potential downside that we don’t fully understand yet to giving ketamine repeatedly.
And that’s aside from its abuse potential. We know that people have employed ketamine for physical and emotional pain, and when they administer it themselves, they tend to get dependent on it. In a clinic setting it’s given in a very formal and structured fashion, a bit like the administration of opioids. In that setting, it is much safer and the risk for abuse and diversion is minimized. But we need to remember that this drug does have abuse potential and perhaps not yet fully measurable neurotoxic effects.
Dr. Sederer: If physicians, nurses, and other professional clinicians want to learn more about this medication, what are the accurate, reliable sources of information to which you suggest they turn?
Dr. Mann: The National Institute of Mental Health’s website offers good and reliable information for patients and their families. It is an unbiased, scientific, and thoughtful source of information, and better than just trolling the Internet for information.
People are much more sophisticated now than they were 20 years ago in these matters, and scientific papers are much more accessible to the public. Reading papers in recognized journals is also a useful way to gain information.
For example, one of the major papers that we published in the American Journal of Psychiatry is available to anybody on the Internet to read. So, I encourage people to make their own inquiries and talk to more than one doctor. Informed patients and families are the best partners a doctor can ever have. We encourage that in all of our patients.
Dr. Sederer: I want to thank you very much, Dr. Mann, for your work in this area and for joining us here at Medscape and Columbia Psychiatry to teach us so much about what is truly a novel psychopharmacologic agent, yet one where we still have a lot more to learn.
A version of this article originally appeared on Medscape.com.
After years of dormancy, psychiatric drug development is showing signs of life. There is the novel antipsychotic lumateperone, recently approved for adults with schizophrenia. Brexanolone was approved last year for postpartum depression. And perhaps generating the most attention lately among psychiatrists – and people with depression – is the use of ketamine and esketamine for depression.
Columbia psychiatrist J. John Mann, MD, a professor of translational neuroscience and a mood disorder specialist, has been involved in several notable studies of ketamine in patients with depression. He and his colleagues’ recent research efforts include a randomized study into ketamine’s ability to reduce suicidal thoughts in bipolar depression and an MRI analysis illuminating the role that dosing plays in antidepressant effects.
Dr. Sederer: Nearly 20 million people in this country alone suffer from clinical depression every year. That means they have a functional impairment in addition to their suffering and are at risk of taking their own lives. Depression is a very prevalent, painful, and disabling condition. The psychopharmacologic treatments we’ve had have been more or less the same for the past 20 or 30 years.
We’ve asked Dr. Mann to … talk to us about ketamine and its nasal preparation esketamine, which is a novel psychopharmacologic treatment. Dr. Mann, please tell us about ketamine, its utility, and the yet-to-be-answered questions.
Dr. Mann: Ketamine and esketamine, which is a component of ketamine itself, is different from traditional antidepressant medications in three fundamental ways.
The first is that it acts very quickly. Traditional antidepressants take 4, 6, 8 weeks to work. This means that the patient has to put up with a great deal of suffering while waiting for a response. And the probability of the medication working isn’t that high. About 50%-70% of individuals will respond eventually at the end of this ride. But ketamine, when it works, does so in 2 hours. That’s a totally different timescale.
The second aspect is that, when it does work, it often works very robustly, even though it’s a quick-acting antidepressant. The patient often quickly feels distinctly better.
Very often when you’re using traditional antidepressants, it takes a while for the improvement to reach the point that the patient is confident that they are clearly better, and too often that does not happen.
Dr. Sederer: As the treating doctor, you’re trying to keep that patient’s hope alive, even though we don’t have substantial evidence that they’re going to respond.
Dr. Mann: Exactly. One of the difficulties of keeping patients on track with traditional antidepressants is that, after the first dose or two, they have all the side effects and yet no benefit has emerged. In many ways patients sometimes feel that they’re going backwards. They have all of their depression plus the side effects of the medication.
But with ketamine, it’s rather different. You come in, you have the treatment, and many patients feel improved in a couple of hours. And not just a bit improved, but in many cases distinctly improved.
It’s very important for clinicians to appreciate that ketamine will work in patients who have a classically described treatment-resistant depression, meaning they’ve tried several other types of antidepressant medications that haven’t worked.
A prerequisite for treatment with ketamine is that they have had a number of treatment failures. The labeling for the intranasal esketamine states that you should try the other antidepressants first and then use this if they don’t work. The fact that ketamine can work even when the other medications have failed is a huge advantage.
Dr. Sederer: There is another feature of ketamine, in that it also has a pronounced benefit for suicidal ideation, which your research has reported on.
Dr. Mann: Yes, we’ve learned over the years that depression and suicidality are in some ways comorbid conditions. That both have to be addressed in order to keep somebody alive so that they can respond to treatment.
That’s a very important point. If the patient is suffering from depression and the antidepressant takes weeks to work, they may lose hope during that time. They may become overwhelmed by the suicidal ideation, no longer able to control or resist the impulse to take their life. A lot of the management is therefore to try to help support the patient (and family) so that these thoughts never become too compelling. Often we have to consider hospitalization to protect these patients so that they can stay alive long enough for the antidepressant to work. But ketamine not only has this very rapid effect for their depression, it also has a partly independent effect on suicidal ideation that is equally rapid and robust, which can render the patient safer.
Dr. Sederer: In other words, it’s effective and rapidly so for depression, with a bonus of reducing suicidality? This sounds almost too good to be true.
Dr. Mann: There are some limitations that we have to keep in mind. One limitation is that a single administration of ketamine will produce this robust improvement but it will only persist for most people for 5-7 days.
Dr. Sederer: Is the same duration true for scheduling the next treatment as well?
Dr. Mann: Yes, it is. The patient will gradually begin to deteriorate if you do not repeat the treatment. But as we showed in our randomized controlled clinical study, with ketamine for suicidal ideation, if you continue to deliver the medication, you can sustain the benefit.
Dr. Sederer: Can a person receive both ketamine and a conventional antidepressant at the same time?
Dr. Mann: Yes. In this study, half of the patients were actually continued on their previous medication while we added the ketamine on top of that. It worked very well.
In practice, people use two approaches. One approach used by most ketamine clinics is to give six doses of ketamine at a frequency of about two per week. Then they will reduce the frequency down to once a week for a few more doses and then once a month.
Dr. Sederer:: And this is a ketamine infusion?
Dr. Mann: Yes, this generally has been a ketamine infusion. This approach seems to work quite well. But that may not be necessary.
Another strategy is to give one, two, or three doses of ketamine. If the patient doesn’t respond robustly to two or three doses, they’re not going to respond to subsequent doses.
Dr. Sederer: So, initial responses are a predictor of future response?
Dr. Mann: Exactly. Now, if they haven’t done well with two or three doses, then you’ve got to use other treatments. But if they do well with the two or three, then you’ve got a choice: You can either complete the treatment course with ketamine and then continue them on antidepressant medications, or simply treat them with ketamine alone. What we tend to do is to treat with only antidepressant medications after a small number of ketamine treatments. We also use ketamine as a kind of “rescue medication” if they relapse into severe depression, though this is true for only a minority of patients.
Dr. Sederer: One of the things that we’ve learned is that antidepressants have a very beneficial effect for some people, but then they wear out and the person starts to relapse. Should ketamine be studied as an intervention for people who are no longer responding to the antidepressant(s) that they are on?
Dr. Mann: We do not really know the answer to that question. My experience treating very seriously ill patients is that sometimes the ketamine will work very well the first time or the second time but then in the future, if you try to use it as a rescue medication, it might not work that well. There is some clinical experience that suggests that that may be true for some people. But we have no idea about the frequency or timing with which this might happen. That’s all uncertain.
Moreover, most of our control clinical trial data come from either one dose of ketamine or from a few trials where people have received multiple doses of ketamine, followed by a bit of a taper. But there are very, very few of those types of studies. We’re still learning about the use of this medication.
Dr. Sederer: Importantly, you referenced the side effects of antidepressants. What are the side effects and risks of ketamine?
Dr. Mann: We know a lot more about the immediate short-term side effects of a single dose or a few doses of ketamine. Most people will get a kind of tripping experience. They’ll feel a bit unreal, or their circumstances or experiences of the world feel a bit distorted.
Some patients develop strange ideas. Most patients don’t enjoy those symptoms, even though I know ketamine is used as a party drug, and so on and so forth.
Dr. Sederer: It seems that the context is what matters.
Dr. Mann: Yes. And in a clinic context, most patients simply don’t enjoy these types of dissociative experiences, but they put up with them. They’re not severe, in general.
Dr. Sederer: Is part of the preparation of the patient telling them that this may happen?
Dr. Mann: Yes. We try to explain the potential for these symptoms and that most people get them. These side effects almost invariably terminate with the cessation of the administration.
Dr. Sederer: What’s the typical duration of the infusion you use?
Dr. Mann: Traditionally, infusion is 40 minutes and always in a clinic setting.
Dr. Sederer: And that’s because of the concern that a patient may have these symptoms?
Dr. Mann: Exactly. They may have dissociative effects that they’re disturbed by, and we need to monitor that. They’re probably going to remain under observation in the clinic for about the same amount of time because it takes about the same time for these effects to wear off.
The other consideration is that some people get a little nausea. In our experience with the intravenous ketamine, there’s also a problematic side effect that their blood pressure will be slightly raised. Therefore, it’s good to know that the person’s blood pressure is under control before they begin the treatments and that you’re monitoring it during administration.
Dr. Sederer: What are the differences you’re discovering between esketamine and ketamine?
Dr. Mann: It is a bit different. We’ve just completed a very important National Institutes of Health–funded clinical trial here at Columbia showing that with esketamine or ketamine itself, the dose and the blood levels are very closely related to the robustness of the clinical response.
When you give a drug intravenously, you give a very reliable dose. When you give the drug over 40 minutes, you’re spreading the dose administration over a period of time so that it doesn’t peak very high. The side effects appear to be proportional to the peak dose.
When you give it intranasally, you give the drug over a much shorter period of time. Even if you use more than one intranasal administration to give the whole dose, it’s still a relatively shorter time, compared with the 40 minutes.
Dr. Sederer: This means that to get the equivalent dose intranasally, the patient is going to have to experience a higher peak. Can you predict that those patients who are treated intranasally are going to have more side effects?
Dr. Mann: Right. And that should be explained to the patient. You will not need an intravenous line inserted, which some people might find highly appealing and advantageous, but you will probably have more side effects.
Also, in general, intranasal absorption of drugs is more variable. The predictability of the blood level and, therefore, the degree of antidepressant effect is not as good intranasally as intravenously.
Now, all of this is anecdotal clinical experience, based on theoretical pharmacology, because nobody has actually done a head-to-head control comparison.
Dr. Sederer: What about the cost of both of these preparations?
Dr. Mann: There is a bit of a range in pricing between ketamine clinics around the country. It’s always important to find out what they charge per administration. And then it makes a difference whether you have two or three administrations versus six plus further tapered administration. Clearly, the cost can vary a great deal.
Dr. Sederer: But it’s generally not covered by insurance, so most people are paying out of pocket.
Dr. Mann: Yes. The intranasal ketamine is still in negotiation at the moment, but it should be resolved before it’s fully marketed.
Dr. Sederer: Ketamine is used for major depression. Does it have utility in bipolar depression?
Dr. Mann: We and some others have done initial studies in bipolar depression. In our view, it’s probably going to be as effective in bipolar disorder as it is in major depressive disorder, unipolar depression.
We haven’t seen any manic episodes triggered, but we don’t give repeated doses. We allow research patients to stay on anticonvulsants or mood stabilizers, so that’s helpful. Generally, people with bipolar disorder who come for ketamine treatment for their depression are coming on a mood stabilizer, because that and perhaps other conventional antidepressants have not proven to be effective. So, I think that ketamine plus mood stabilizers seems to be very promising.
Dr. Sederer: I want to return to the antisuicidal properties that you had previously mentioned. I heard from a colleague about a patient who had been admitted to a psychiatric inpatient unit. The patient was in her 20s; she did not have major depression but was persistently suicidal, constantly trying to hurt herself in any way she could. But that seemed to be more a product of borderline personality disorder, with its impulsive and self-destructive problems.
In the end, they tried intranasal ketamine. The response was, just as you described, robust. Her self-injurious behavior dropped in a very pronounced way within a day or two. But she then did require administrations a couple of times a week in order to keep that suicidality at bay.
Based on that example, I’m wondering whether there is an application here for people who are suicidal yet who may not have features of major depression or bipolar depression.
Dr. Mann: It’s a very interesting suggestion for which we have no data-based answer. However, we have a clue from the study that we published in the American Journal of Psychiatry and have since published further analyses on.
The reasons that people die by suicide, or make suicide attempts, are not entirely attributable to the fact that they suffer from a mood disorder.
Dr. Sederer: Yes, because only a minority of individuals with a mood disorder ever make suicide attempts. But there is a subgroup at risk.
Dr. Mann: Here at Columbia, we’ve promulgated the stress-diathesis model for suicidal behavior. A stressor could be external life events, but the internal stressor would be something like an acute episode of depression.
But predisposition also plays an important role, which has several elements to it. One is decision-making. These are patients with a propensity to go for a short-term, quick relief. In other words, a patient would be seeking immediate relief rather than waiting for the delayed improvement from an antidepressant. They’re more prone to act on the pain of the depression and terminate their lives – to try to end their pain – rather than wait and hope that, in time, there’s a chance that the antidepressant will work.
Dr. Sederer: What else do you want to share with our viewers about this medication and how it’s used?
Dr. Mann: My goal in treating patients is to try to use the least amount of medication possible. We do not really know yet the long-term safety of ketamine treatment.
It’s been used for many decades in anesthesia, but people don’t get repeated anesthetic doses of ketamine. And higher doses of ketamine given repeatedly have been shown in preclinical studies to produce little lesions in the brain, which is not good. But we’re using much lower doses.
As we potentially move into a time when we could be giving multiple doses of ketamine to patients, we should remember that we need to be cautious about that. If we don’t need to give them more doses, we shouldn’t. We should know that there is a potential downside that we don’t fully understand yet to giving ketamine repeatedly.
And that’s aside from its abuse potential. We know that people have employed ketamine for physical and emotional pain, and when they administer it themselves, they tend to get dependent on it. In a clinic setting it’s given in a very formal and structured fashion, a bit like the administration of opioids. In that setting, it is much safer and the risk for abuse and diversion is minimized. But we need to remember that this drug does have abuse potential and perhaps not yet fully measurable neurotoxic effects.
Dr. Sederer: If physicians, nurses, and other professional clinicians want to learn more about this medication, what are the accurate, reliable sources of information to which you suggest they turn?
Dr. Mann: The National Institute of Mental Health’s website offers good and reliable information for patients and their families. It is an unbiased, scientific, and thoughtful source of information, and better than just trolling the Internet for information.
People are much more sophisticated now than they were 20 years ago in these matters, and scientific papers are much more accessible to the public. Reading papers in recognized journals is also a useful way to gain information.
For example, one of the major papers that we published in the American Journal of Psychiatry is available to anybody on the Internet to read. So, I encourage people to make their own inquiries and talk to more than one doctor. Informed patients and families are the best partners a doctor can ever have. We encourage that in all of our patients.
Dr. Sederer: I want to thank you very much, Dr. Mann, for your work in this area and for joining us here at Medscape and Columbia Psychiatry to teach us so much about what is truly a novel psychopharmacologic agent, yet one where we still have a lot more to learn.
A version of this article originally appeared on Medscape.com.
The psychiatric consequences of COVID-19: 8 Studies
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.
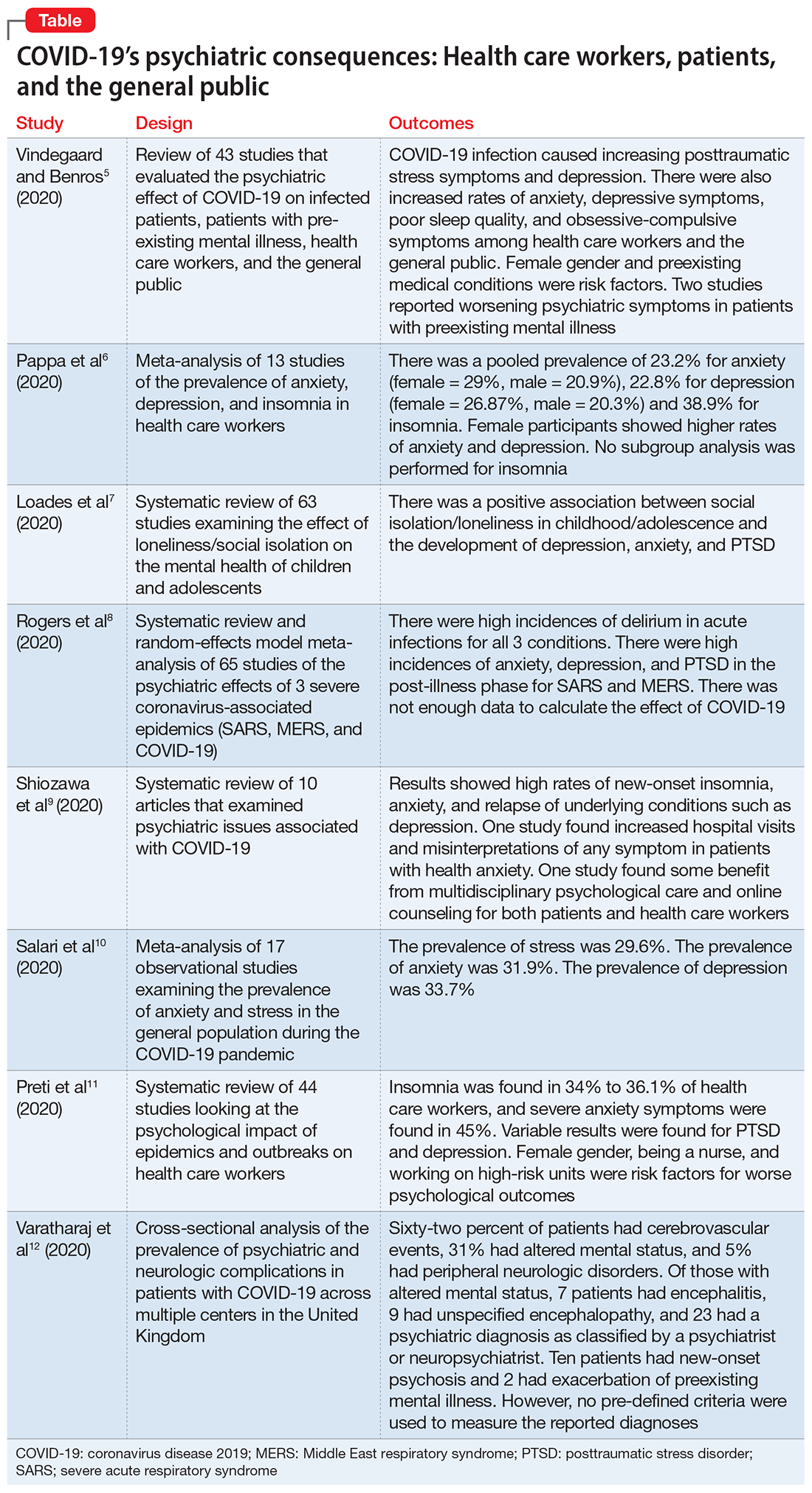
1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus that is causing the ongoing coronavirus disease 2019 (COVID-19) pandemic, was first reported in late 2019.1 As of mid-October 2020, >39 million confirmed cases of COVID-19 had been reported worldwide, and the United States was the most affected country with >8 million confirmed cases.2 Although the reported symptoms of COVID-19 are primarily respiratory with acute respiratory distress syndrome, SARS-CoV-2 has also been shown to affect other organs, including the brain, and there are emerging reports of neurologic symptoms due to COVID-19.3
Psychological endurance will be a challenge that many individuals will continue to face during and after the pandemic. Physical and social isolation, the disruption of daily routines, financial stress, food insecurity, and numerous other potential triggers for stress response have all been intensified due to this pandemic, creating a situation in which many individuals’ mental well-being and stability is likely to be threatened. The uncertain environment is likely to increase the frequency and/or severity of mental health problems worldwide. Psychiatric symptoms such as anxiety and depression have been reported among patients with SARS-CoV-1 during the previous severe acute respiratory syndrome (SARS) epidemic.4
In this article, we summarize 8 recent studies, systematic reviews, and meta-analyses to provide an overview of the psychiatric consequences of COVID-19. These studies are summarized in the Table.5-12 Clearly, the studies reviewed here are preliminary evidence, and our understanding of COVID-19’s effects on mental health, particularly its long-term sequelae, is certain to evolve with future research. However, these 8 studies describe how COVID-19 is currently affecting mental health among health care workers, patients, and the general public.

1. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
Vindegaard and Benros5 conducted a systematic review of the literature to characterize the impact of COVID-19–related psychiatric complications and COVID-19’s effect on the mental health of patients infected with COVID-19, as well as non-infected individuals.
Study design
- This systematic review included 43 studies that measured psychiatric disorders or symptoms in patients with COVID-19 and in a non-infected group.
- The non-infected group consisted of psychiatric patients, health care workers, and the general population.
- The review excluded studies with participants who were children, adolescents, or older adults, or had substance abuse or somatic disorders.
- Only 2 studies included patients with confirmed COVID-19 infection. Of the remaining 41 studies, 2 studies examined the indirect effects of the pandemic on psychiatric patients, 20 studies examined health care workers, and 19 studies examined the general population. Eighteen of the studies were case-control studies and 25 had no control group
Patients with confirmed COVID-19 infection. One case-control study showed an increased prevalence of depression in patients with COVID-19 who had recently recovered (29.2%) compared with participants who were in quarantine (9.8%). The other study showed posttraumatic stress symptoms in 96% of hospitalized patients with COVID-19 who were stable.
Continue to: Patients with preexisting psychiatric disorders
Patients with preexisting psychiatric disorders. Two studies found increased symptoms of psychiatric disorders.
Health care workers. Depression (6 studies) and anxiety symptoms (8 studies) were increased among health care workers compared with the general public or administrative staff. However, 2 studies found no difference in these symptoms among health care workers compared with the general public. Poor sleep quality and more obsessive-compulsive symptoms were reported in health care workers compared with the general public.
General public. Compared to before the COVID-19 pandemic, lower psychological well-being and increased rates of depression and anxiety were noted among the general public. Higher rates of anxiety and depression were also found in parents of children who were hospitalized during the pandemic compared with prior to the pandemic. One study found no difference between being in quarantine or not.
- Current or prior medical illness was associated with higher rates of anxiety and depression. One study found higher social media exposure was associated with increased anxiety and depression. Female health care workers had higher rates of anxiety and depression symptoms.
Conclusions/limitations
This systematic review included 39 studies from Asia and 4 from Europe, but none from other continents, which may affect the external validity of the results. Most of the studies included were not case-controlled, which limits the ability to comment on association. Because there is little research on this topic, only 2 of the studies focused on psychiatric symptoms in patients with COVID-19. In most studies, the reporting of psychiatric disorders was vague and only a few studies used assessment tools, such as the General Anxiety Disorder-7 or the Patient Health Questionnaire-9, for reporting depression and anxiety.
2. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
Pappa et al6 examined the effects of the COVID-19 pandemic on the mental health of health care workers, with specific focus on the prevalence of anxiety, depression, and insomnia.
Continue to: Study design
Study design
- Researchers searched for studies on PubMed, Medline, and Google Scholar. A random effect meta-analysis was used on the included 13 cross-sectional studies with a total of 33,062 participants. Twelve of the included studies were conducted in China and 1 in Singapore.
- Evaluation of the risk of bias of included studies was assessed using a modified form of the Newcastle-Ottawa Scale (NOS), with a score >3 considered as low risk of bias.
Outcomes
- Results were categorized by gender, rating scales, severity of depression, and professional groups for subgroup analysis.
- The primary outcomes were prevalence (p), confidence intervals (CI), and percentage prevalence (p × 100%). Studies with a low risk of bias were sub-analyzed again (n = 9).
- Anxiety was evaluated in 12 studies, depression in 10 studies, and insomnia in 5 studies (all 5 studies had a low risk of bias).
- There was a pooled prevalence of 23.2% for anxiety (29% female, 20.9% male), 22.8% for depression (26.87% female, 20.3% male), and 38.9% for insomnia. Female participants showed higher rates of anxiety and depression, while no subgroup analysis was performed for insomnia.
- The subgroup analysis of pooled data after excluding each study showed that no single study had >2% effect on the pooled analysis.
- The subgroup analysis by gender, professional group, and severity suggested that there was an increased prevalence of anxiety and depression in female health care workers, which was consistent with the increased prevalence in the general population.
Conclusions/limitations
There was a questionable effect of between-study heterogeneity. Different studies used different rating scales and different cutoff points on the same scales, which might make the results of pooled analysis unreliable, or might be assumed to increase the confidence. Despite the use of different scales and cutoff points, there was still a high prevalence of anxiety, depression, and insomnia. All studies were conducted in a single geographical region (12 in China and 1 in Singapore). None of the included studies had a control group, either from the general population or compared with pre-COVID-19 rates of depression, anxiety, and insomnia in health care workers.
3. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
The COVID-19 pandemic has led to long periods of isolation/quarantine, social distancing, and school closures, all which have resulted in significant upheaval of the lives of children and adolescents. Loades et al7 explored the impact of loneliness and disease-containment measures related to the COVID-19 pandemic on children and adolescents.
Study design
- Researchers conducted a systematic review of 63 studies examining the impact of loneliness or disease-containment measures on healthy children and adolescents. located through a search of Medline, PsycINFO, and Web of Science. Sixty-one studies were observational, and 2 were interventional.
- The search yielded studies published between 1946 and March 29, 2020.
- The quality of studies was assessed using the National Institutes of Health quality assessment tool.
Continue to: Outcomes
Outcomes
- Results by mental health symptom or disorder were categorized as follows:
Depression. Forty-five studies examined depressive symptoms and loneliness; only 6 studies included children age <10. Most reported a moderate to large correlation (0.12 ≤ r ≤ 0.81), and most of them included a measure of depressive symptoms. The association was stronger in older and female participants. Loneliness was associated with depression in 12 longitudinal studies that followed participants for 1 to 3 years. However, 3 studies (2 in children and 1 in adolescents) found no association between loneliness and depression at follow-up.
Anxiety. Twenty-three studies examined symptoms of anxiety and found a small to moderate correlation between loneliness/social isolation and anxiety (0.18 ≤ r ≤ 0.54), with duration of loneliness being more strongly associated with anxiety than intensity of loneliness. However, social anxiety or generalized anxiety were associated more with loneliness ([0.33 ≤ r ≤ 0.72] and [r = 0.37, 0.40], respectively). Three longitudinal studies found associations between loneliness and subsequent anxiety, and 1 study did not find an association between loneliness at age 5 and increased anxiety at age 12.
Mental health and well-being. Two studies found negative associations between social isolation/loneliness and well-being and mental health.
Conclusions/limitations
There is decent evidence of a strong association between loneliness/social isolation in childhood/adolescence and the development of depression, with some suggestion of increased rates in females. However, there was a small to moderate association with anxiety with increased rates in males. The length of social isolation was a strong predictor of future mental illness. Children who experienced enforced quarantine were 5 times more likely to require mental health services for posttraumatic stress symptoms.
Continue to: The compiled evidence presented in this study...
The compiled evidence presented in this study looked at previous similar scenarios of enforced social isolations; however, it cannot necessarily predict the effect of COVID-19–associated social distancing measures. Most of the studies included were cross-sectional studies and did not control for confounders. Social isolation in childhood or adolescence may be associated with developing mental health problems later in life and should be considered when implementing school closures and switching to online classes. Loades et al7 suggested that the increased rate of electronic communication and use of social media in children and adolescents may mitigate this predicted effect of social isolation.
4. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
To identify possible psychiatric and neuropsychiatric implications of the COVID-19 pandemic, Rogers et al8 examined 2 previous coronavirus epidemics, SARS and Middle East respiratory syndrome (MERS), and COVID-19.
Study design
- Researchers conducted a random-effects model meta-analysis and systematic review of 65 studies and 7 preprints from 10 countries, including approximately 3,559 case studies of psychiatric and neuropsychiatric symptoms in participants infected with the 3 major coronavirus-induced illnesses (SARS, MERS, and COVID-19).
- Pure neurologic complications and indirect effects of the epidemics were excluded.
- The systematic review followed PRISMA guidelines.
- The quality of the studies was assessed using the NOS.
Outcomes
- Outcomes measured were psychiatric signs or symptoms; symptom severity; diagnoses based on ICD-10, DSM-IV, the Chinese Classification of Mental Disorders (third edition), or psychometric scales; quality of life; and employment.
- Results were stratified as acute or post-illness:
Acute illness. Delirium was the most frequently reported symptom in all 3 coronavirus infections. Depression, anxiety, or insomnia were also reported in MERS and SARS infections. Mania was described in SARS, but it was almost entirely present in cases treated with high-dose corticosteroids, which are not used routinely for COVID-19.
Continue to: Post-illness
Post-illness. There was increased incidence of depression, anxiety, fatigue, and posttraumatic stress disorder (PTSD) in the post-illness stage of previous coronavirus epidemics (SARS and MERS), but there was no control group for comparison. There was not enough data available for COVID-19.
Conclusions/limitations
Three studies were deemed to be of high quality, 32 were low quality, and 30 were moderate quality. Despite the high incidence of psychiatric symptoms in previous coronavirus infections, it was difficult to draw conclusions due to a lack of adequate control groups and predominantly low-quality studies. The difference in treatment strategies, such as the use of high-dose corticosteroids for MERS and SARS, but not for COVID-19, made it difficult to accurately predict a response for COVID-19 based on previous epidemics.
5. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
Schiozawa et al9 conducted a systematic review of articles to identify psychiatric issues during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review of 10 articles (7 articles from China, 1 from the United States, 1 from Japan, and 1 from Korea) that described strategies for coping with the COVID-19 pandemic and/or provided a descriptive analysis of the clinical scenario, with an emphasis on psychiatric comorbidities.
- The study used PRISMA guidelines to summarize the findings of those 10 studies. There were no pre-set outcomes or inclusion criteria.
Outcomes
- The compiled results of the 10 studies showed high rates of new-onset insomnia, anxiety, and relapse of underlying conditions such as depression.
- One study found increased hospital visits and misinterpretations of any symptom in patients with health anxiety (health anxiety was not defined).
- One study found some benefit from multidisciplinary psychological care and online counseling for both patients and health care workers.
Continue to: Conclusions/limitations
Conclusions/limitations
Because each of the 10 studies examined extremely different outcomes, researchers were unable to compile data from all studies to draw a conclusion.
6. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
Salari et al10 examined the prevalence of stress, anxiety, and depression in the general population during the COVID-19 pandemic.
Study design
- Researchers conducted a systematic review and meta-analysis of 17 observational studies examining the prevalence of anxiety and stress in the general population during the COVID-19 pandemic. The STROBE checklist was used to assess the quality of studies.
- Only studies judged as medium to high quality were included in the analysis.
Outcomes
- The prevalence of stress was 29.6% (5 studies, sample size 9,074 individuals).
- The prevalence of anxiety was 31.9% (17 studies, sample size 63,439 individuals).
- The prevalence of depression was 33.7% (14 studies, sample size of 44,531 individuals).
- A sub-analysis of rates by continent revealed that Asia had highest prevalence of anxiety and depression (32.9% and 35.3%, respectively). Europe had the highest rates of stress (31.9%).
Conclusions/limitations
There is an increased prevalence of anxiety, stress, and depression in the general population amid the COVID-19 pandemic. None of the included studies compared rates to before the pandemic. Most studies used online surveys, which increased the chance of sample bias. Most studies originated from China and Iran, which had the highest rates of infection when this review was conducted.
Continue to: #7
7. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence. Curr Psychiatry Rep. 2020;22(8):43.
Preti et al11 performed a review of the literature to determine the impact of epidemic/pandemic outbreaks on health care workers’ mental health.
Study design
- Researchers conducted a rapid systematic review of 44 studies examining the psychological impact of epidemic/pandemic outbreaks on health care workers.
- Of the 44 studies, 27 (62%) referred to the SARS outbreak, 5 (11%) referred to the MERS outbreak, 5 (11%) referred to the COVID-19 outbreak, 3 (7%) referred to the influenza A virus subtype H1N1 outbreak, 3 (7%) referred to the Ebola virus disease outbreak, and 1 (2%) referred to the Asian lineage avian influenza outbreak.
Outcomes
- During these outbreaks, insomnia was found in 34% to 36.1% of health care workers, and severe anxiety symptoms in 45%.
- The prevalence of PTSD-like symptoms among health care workers during the outbreaks was 11% to 73.4%. Studies of the COVID-19 pandemic reported the highest prevalence of PTSD-like symptoms (71.5% to 73%). After 1 to 3 years following an outbreak, 10% to 40% of health care workers still had significant PTSD-like symptoms.
- Anxiety was reported in 45% of health care workers during the COVID-19 pandemic.
- A sub-analysis revealed a positive association between anxiety, PTSD, and stress symptoms and being female gender, being a nurse, and working on high-risk units.
- Perceived organizational support and confidence in protective measures were negatively associated with psychological symptoms.
Conclusions/limitations
Lessons from previous outbreaks and early data from the COVID-19 pandemic suggest that health care workers experience higher levels of psychological symptoms during outbreaks. Findings of this study suggest that organizational support and confidence in protective measures can mitigate this effect. To help preserve the well-being of health care workers, adequate training should be provided, appropriate personal protective equipment should be readily available, and support services should be well established.
8. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Varatharaj et al12 conducted a surveillance study in patients in the United Kingdom to understand the breadth of neurologic complications of COVID-19.
Continue to: Study design
Study design
- Researchers performed a cross-sectional analysis of the prevalence of psychiatric and neurologic complications in patients with COVID-19 across multiple centers in United Kingdom. Data were collected through the anonymous online reporting portals of several major neurology and psychiatric associations. Retrospective reporting was allowed.
- Evidence of SARS-CoV-2 infection was defined as:
Confirmed COVID-19 (114 cases) if polymerase chain reaction (PCR) of respiratory samples (eg, nasal or throat swab) or CSF was positive for viral RNA or if serology was positive for anti-SARS-CoV-2 immunoglobulin M (IgM) or immunoglobulin G (IgG).
Probable COVID-19 (6 cases) if a chest radiograph or chest CT was consistent with COVID-19 but PCR and serology were negative or not performed.
Possible COVID-19 (5 cases) if the disease was suspected on clinical grounds by the notifying clinician, but PCR, serology, and chest imaging were negative or not performed.
Outcomes
- Sixty-two percent of patients presented with cerebrovascular events (intracerebral hemorrhage, ischemic stroke, vasculitis, or other). Thirty-one percent of patients presented with altered mental status (AMS), and 5% had peripheral neurologic disorders.
- Of those with AMS, 18% (7 patients) had encephalitis, 23% (9 patients) had unspecified encephalopathy, and 59% (23 patients) had a psychiatric diagnosis as classified by the notifying psychiatrist or neuropsychiatrist. Ten patients (43%) of the 23 patients with neuropsychiatric disorders had new-onset psychosis, while only 2 patients had an exacerbation of a preexisting mental illness.
Continue to: Conclusions/limitations
Conclusions/limitations
This study had an over-representation of older adults. There was no control group for comparison, and the definition of confirmed COVID-19 included a positive IgM or IgG without a positive PCR or chest imaging. Although all psychiatric conditions reported were confirmed by a psychiatrist or neuropsychiatrist, there were no pre-defined criteria used for reported diagnoses.
Bottom Line
Evidence from studies of previous outbreaks and early data from the coronavirus disease 2019 (COVID-19) pandemic suggest that during outbreaks, health care workers experience higher levels of psychological symptoms than the general population. There has been an increased prevalence of anxiety, stress, poor sleep quality, obsessive-compulsive symptoms, and depression among the general population during the pandemic. COVID-19 can also impact the CNS directly and result in delirium, cerebrovascular events, encephalitis, unspecified encephalopathy, altered mental status, or peripheral neurologic disorders. Patients with preexisting psychiatric disorders are likely to have increased symptoms and should be monitored for breakthrough symptoms and acute exacerbations.
Related Resources
- Ryznar E. Evaluating patients’ decision-making capacity during COVID-19. Current Psychiatry. 2020;19(10):34-40.
- Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. 2020;19(9):24-27,33-39.
- Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future [published online August 16, 2020]. Psychiatr Q. 2020:1-13. doi: 10.1007/s11126-020-09808-4.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
1. Huang C, Wang Y, Li X, et. al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
2. John Hopkins University & Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu. Accessed October 16, 2020.
3. Montalvan V, Lee J, Bueso T, et al. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921.
4. Wu P, Fang Y, Guan Z, et al. The psychological impact of the SARS epidemic on hospital employees in China: exposure, risk perception, and altruistic acceptance of risk. Can J Psychiatry. 2009;54(5):302-311.
5. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542.
6. Pappa S, Ntella V, Giannakas T, et al. Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav Immun. 2020;88:901-907.
7. Loades ME, Chatburn E, Higson-Sweeney N, et al. Rapid systematic review: the impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19 [published online June 3, 2020]. J Am Acad Child Adolesc Psychiatry. 2020;S0890-8567(20)30337-3. doi: 10.1016/j.jaac.2020.05.009.
8. Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611-627.
9. Shiozawa P, Uchida RR. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;42(3):330-331.
10. Salari N, Hosseinian-Far A, Jalali R, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Global Health. 2020;16(1):57.
11. Preti E, Di Mattei V, Perego G, et al. The psychological impact of epidemic and pandemic outbreaks on healthcare workers: rapid review of the evidence [published online July 10, 2020]. Curr Psychiatry Rep. 2020;22(8):43.
12. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875-882.
Painful erections while being treated for OCD
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
CASE Prolonged, painful erections
Mr. G, age 27, who has a history of obsessive-compulsive disorder (OCD), presents to his internist’s office with complaints of “masturbating several times a day” and having ejaculatory delay of up to 50 minutes with intercourse. The frequent masturbation was an attempt to “cure” the ejaculatory delay. In addition, Mr. G reports that for the past 5 nights, he has awoke every 3 hours with a painful erection that lasted 1.5 to 2.5 hours, after which he would fall asleep, only to wake once again to the same phenomenon.
Mr. G’s symptoms began 3 weeks ago after his psychiatrist adjusted the dose of his medication for OCD. Mr. G had been receiving fluoxetine, 10 mg/d, for the past 3 years to manage his OCD, without improvement. During a recent consultation, his psychiatrist increased the dose to 20 mg/d, with the expectation that further dose increases might be necessary to treat his OCD.
HISTORY Concurrent GAD
Mr. G is single and in a monogamous heterosexual relationship. Three weeks earlier, when he was examined by his psychiatrist, Mr. G’s Yale-Brown Obsessive Compulsive Scale score was 28 and his Beck Anxiety Inventory score was 24. Based on these scores, the psychiatrist concluded Mr. G had concurrent generalized anxiety disorder (GAD).
EVALUATION Workup is normal
On presentation to his internist’s office, Mr. G’s laboratory values are all within normal range, including a chemistry panel, complete blood count with differential, and electrocardiogram. A human immunodeficiency virus test is negative. His internist instructs Mr. G to return to his psychiatrist.
[polldaddy:10640161]
TREATMENT Dose adjustment
Based on Mr. G’s description of painful and persistent erections in the absence of sexual stimulation or arousal, and because these episodes have occurred 5 consecutive nights, the psychiatrist makes a provisional diagnosis of stuttering priapism and reduces the fluoxetine dose from 20 to 10 mg/d.
The author’s observations
Priapism is classically defined as a persistent, unwanted penile or clitoral engorgement in the absence of sexual desire/arousal or stimulation. It can last for up to 4 to 6 hours1 orit can take a so-called “stuttering form” characterized by brief, recurrent, self-limited episodes. Priapism is a urologic emergency resulting in erectile dysfunction in 30% to 90% of patients. It is multifactorial and can be characterized as low-flow (occlusive) or high-flow (nonischemic). Most priapism is primary or idiopathic in nature; the incidence is 1.5 per 100,000 individuals (primarily men), with bimodal peaks, and it can occur in all age groups.2 Secondary priapism can occur from many causes (Table).
Mechanism is unclear
The molecular mechanism of priapism is not completely understood. Normally, nitrous oxide mediates penile erection. However, cyclic guanosine monophosphate (cGMP) acts at several levels to create smooth muscle reaction, leading to either penile tumescence or, in some cases, priapism. Stuttering or intermittent ischemic priapism is thought to be a downregulation of phosphodiesterase type 5, causing excess cGMP with subsequent smooth muscle relaxation in the penis.3
Continue to: Drug-induced priapism
Drug-induced priapism
Drug-induced priapism is commonly believed to be associated with alpha-1 adrenergic receptor blockade.4 This also results in dizziness and orthostatic hypotension.5 Trazodone is commonly associated with the development of secondary priapism; however, in the last 30 years, multiple case reports have demonstrated that a variety of psychoactive agents have been associated with low-flowpriapism.6 Most case reports have focused on new-onset priapism associated with the introduction of a new medication. Based on a recent informal search of Medline, since 1989, there have been >36 case reports of priapism associated with psychotropic use. Stuttering priapism is less frequently discussed in the literature.7
Ischemic priapism accounts for 95% of all reports. It can be associated with medication use or hematologic disorders, or it can be triggered by sexual activity. Often, patients who experience an episode will abstain from sexual contact.
The etiology of stuttering priapism is less clear. Episodes of stuttering priapism often occur during sleep and can resolve spontaneously.8 They are a form of ischemic priapism and are seen in patients with sickle cell anemia. It is not known how many patients with stuttering priapism will convert to the nonremitting form, which may require chemical or surgical intervention.9 Stuttering priapism may go unreported and perhaps may be overlooked by patients based on its frequency and intensity.
The activating selective serotonin reuptake inhibitor fluoxetine has a long half-life and is a potent inhibitor of the cytochrome P450 2D6 isoenzyme system. It inhibits serotonin transporter proteins. It is also a weak norepinephrine reuptake inhibitor, an effect that increases with increasing doses of the medication. Its 5HT2C antagonism is proposed as the mechanism of its activating properties.10 In Mr. G’s case, it is possible that fluoxetine’s weak norepinephrine reuptake inhibition resulted in an intermittent priapism effect mediated through the pathways described above.
OUTCOME Symptoms resolve
Approximately 1 week after Mr. G’s fluoxetine dose is reduced, his symptoms of priapism abated. The fluoxetine is discontinued and his ejaculatory delay resolves. Mr. G is started on fluvoxamine, 150 mg/d, which results in a significant decrease of both GAD and OCD symptoms with no notable ejaculatory delay, and no recurrence of priapism.
Continue to: The author's observations
The author’s observations
Mr. G’s case and other case reports suggest that psychiatrists should caution patients who are prescribed antidepressants or antipsychotics that stuttering priapism is a possible adverse effect.11 As seen in Mr. G’s case, fluoxetine (when used chronically) can moderate vascular responses at the pre- and post-synaptic adrenergic receptor.11 Priapism induced by a psychotropic medication will not necessarily lead to a longer-term, unremitting priapism, but it can be dramatic, frightening, and lead to noncompliance. Along with obtaining a standard history that includes asking patients about prior adverse medication events, psychiatrists also should ask their patients if they have experienced any instances of transient priapism that may require further evaluation.
Bottom Line
Any psychotropic medication that has the capacity to act on alpha adrenergic receptors can cause priapism. Ask patients if they have had any unusual erections/ clitoral engorgement while taking any psychotropic medications, because many patients will be hesitant to volunteer such information.
Related Resource
- Thippaiah SM, Nagaraja S, Birur B, et al. Successful management of psychotropics induced stuttering priapism with pseudoephedrine in a patient with schizophrenia. Psychopharmacol Bull. 2018;48(2):29-33.
Drug Brand Names
Fluoxetine • Prozac
Fluvoxamine • Luvox
Trazodone • Desyrel, Oleptro
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
1. Kadioglu A, Sanli O, Celtik M, et al. Practical management of patients with priapism. EAU-EBU Update Series. 2006;4(4):150-160.
2. Eland IA, van der Lei J, Stricker BHC. Incidence of priapism in the general population. Urology. 2001;57(5):970-972.
3. Halls JE, Patel DV, Walkden M, et al. Priapism: pathophysiology and the role of the radiologist. Br J Radiol. 2012;85(Spec Iss 1):S79-S85.
4. Wang CS, Kao WT, Chen CD, et al. Priapism associated with typical and atypical antipsychotic medications. Int Clinical Psychopharmacology. 2006;21(4):245-248.
5. Khan Q, Tucker P, Lokhande A. Priapism: what cause: mental illness, psychotropic medications or polysubstance abuse? J Okla State Med Assoc. 2016;109(11):515-517.
6. Dent LA, Brown WC, Murney JD. Citalopram-induced priapism. Pharmacotherapy. 2002;22(4):538-541.
7. Wilkening GL, Kucherer SA, Douaihy AB. Priapism and renal colic in a patient treated with duloxetine. Mental Health Clinician. 2016;6(4):197-200.
8. Morrison BF, Burnett AL. Stuttering priapism: insight into its pathogenesis and management. Curr Urol Rep. 2012;13(4):268-276.
9. Burnett AL, Bivalacqua TJ. Priapism: current principles and practice. Urol Clin North Am. 2007;34(4):631-642.
10. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge, United Kingdom: Cambridge University Press; 2013.
11. Pereira CA, Rodrigues FL, Ruginsk SG, et al. Chronic treatment with fluoxetine modulates vascular adrenergic responses by inhibition of pre- and post-synaptic mechanisms. Eu J Pharmacol. 2017;800:70-80.
Add-on atypicals for depression carry ‘substantial’ death risk
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adding a second-generation antipsychotic to an antidepressant to treat depression carries an increased mortality risk for middle-aged adults, results of a large, observational study show.
“Our study suggests physicians should consider prescribing antipsychotics to adults with depression carefully, as the potential health risks are substantial and the benefits are quite modest and controversially debated,” lead investigator Tobias Gerhard, PhD, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, N.J., said in a news release.
The results, he added, “emphasize the importance of considering newer antipsychotics only after nonresponse to less risky, evidence-based treatment options has been established.”
The study was published online September 30 in PLOS ONE.
A last resort
Previous research has demonstrated an increased mortality risk for elderly patients with dementia who take an atypical antipsychotic, but it’s unclear whether this risk occurs among nonelderly adults who use newer antipsychotics as augmentation treatment for depression.
To investigate, Gerhard and colleagues analyzed national healthcare claims from the Medicaid program from 2001 to 2010 for 39,582 Medicaid beneficiaries (mean age, 44.5 years; 78.5% women) who had been diagnosed with depression. Patients with alternative indications for antipsychotic therapy, such as schizophrenia, psychotic depression, or bipolar disorder, were excluded.
After at least 3 months of treatment with a single antidepressant, for more than half of the patients (56.6%), treatment was augmented with an atypical antipsychotic (quetiapine, risperidone, aripiprazole or olanzapine). For the remainder (43.4%), a second antidepressant was added.
The average chlorpromazine equivalent starting dose for all atypical antipsychotics was 68 mg/d. The dose was increased to 100 mg/d during follow-up.
A total of 153 patients died during 13,328 person-years of follow-up, including 105 for whom treatment was augmented with an atypical antipsychotic and 48 for whom treatment was augmented with a second antidepressant.
(adjusted hazard ratio, 1.45; 95% CI, 1.02 – 2.06).
This equates to an absolute risk difference of 37.7 deaths per 10,000 person-years of treatment (0.38% per year) and a number needed to harm of roughly 265 per year. For higher-risk subgroups, the number needed to harm decreased substantially, the authors note. The results were robust across several sensitivity analyses.
“We don’t know the mechanisms of the increased mortality risk, but cardiac and infectious causes are leading candidates,” said Gerhard.
“Our study in nonelderly adults with depression did not identify a single predominant cause of death. However, this may be a result of both the relatively small number of deaths in our study as well as of the well-recognized concerns regarding the accuracy of cause-of-death attribution in death certificates,” Gerhard said.
“As with the potential causes of death, the pathophysiological pathways involved are not well understood but could, among others, involve adverse metabolic effects, including weight gain, diabetes, dyslipidemia, QT prolongation, sedation, and falls – all of which have been associated with at least some of the newer antipsychotics,” he added.
The researchers state that atypical antipsychotics should be considered only “after non-response to evidence-based treatment options that are less risky.”
Another red flag
Commenting for Medscape Medical News, Timothy Sullivan, MD, chair of psychiatry and behavioral sciences at Northwell Health’s Staten Island University Hospital in New York, said this is a “valid contribution” and represents the second large study that “raises the same concern.”
“We’ve been probably underestimating the risk in administering them, and that’s something people really need to know, because if you’re prescribing it for someone with mild to moderate depression, it may be helpful, but is it really worth the risk if you’re significantly increasing their risk of death?” said Sullivan, who wasn’t involved in the study.
Clearly, he said, this “raises a flag that we have to look at this a little more carefully and be a little clearer with patients about the risk. One could argue that we should not be so quick to add these drugs, even though they could be helpful, before we exhaust other less potentially risky options.”
Sullivan’s advice: “Do the three trials of antidepressants, look at antidepressant combinations, don’t be quick to jump to this particular option, because of the concerns. Certainly there are situations like psychotic depression where the risk of use is outweighed by the benefits, given the clinical syndrome, but for less severe forms, we probably should reformulate some of our algorithms.”
The study was supported by the National Institute of Mental Health (NIMH). Gerhard received grants from the NIMH and the National Institute on Aging during the conduct of the study; grants and personal fees from Bristol-Myers Squibb; and personal fees from Eisai, Merck, Pfizer, Lilly, and IntraCellular Therapies outside the submitted work. Sullivan has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Novel ‘Wingman’ program cuts suicide risk in Air Force members
A novel program that strengthens bonds, boosts morale, and encourages supportive networks among US Air Force personnel cuts suicidal ideation and depressive symptoms after 1 month, new research shows.
The so-called Wingman-Connect initiative also had a beneficial impact on work performance, and the benefits were apparent at 6-month follow-up.
“This study suggests that group training can teach skills that help with occupational functioning and reduce the likelihood of experiencing elevated depression and suicidal ideation, at least in the short term,” lead author Peter A. Wyman, PhD, professor, department of psychiatry, University of Rochester, New York, told Medscape Medical News.
The study was published online Oct. 21 in JAMA Network Open.
Significant rise in suicide rates
Suicide rates among active duty military populations have increased “significantly” in the past 15 years and have exceeded rates for the general population when comparing groups of the same age and gender, said Wyman.
The study included new personnel who were taking classes at a single training center between October 2017 and October 2019.
The Wingman-Connect intervention involved three 2-hour blocks of group classes that focused on building skills in areas such as healthy relationships and maintaining balance. Group exercises emphasized cohesion, shared purpose, and the value of a healthy unit.
Participants in the stress management group received an overview of the stress response system, information on the effect of stress on health, and cognitive and behavioral strategies to reduce stress.
Primary outcomes included the scores on the suicide scale and the depression inventory of the Computerized Adaptive Test for Mental Health.
The study included 1,485 participants (82.3% men; mean age, 20.9 years). At the 1-month follow-up, participants in Wingman-Connect classes reported less severe suicidal ideation (effect size, −0.23; 95% confidence interval, −39 to −0.09; P = .001) and depressive symptoms (ES, −0.24; 95% CI, −0.41 to −0.08; P = .002).
Unlike most suicide prevention programs, the Wingman intervention didn’t target only high-risk participants. “You’d expect smaller effect sizes” because many people were already doing well, said Wyman.
He noted that the effects at 1 month were similar to other state-of-the-art prevention programs.
Another primary endpoint was self-reported occupational impairment. A poor outcome here, said Wyman, could mean having to repeat a class or falling short of expectations behaviorally or academically.
Investigators found a 50% reduction – from approximately 10% to 5% – among the participants in the Wingman-Connect group who had occupational problems or performance concerns, said Wyman.
About 84% of participants in both study arms participated in the 6-month follow-up. At this time point, Wingman-Connect participants reported significantly lower depressive symptoms (ES, −0.16; 95% CI, −0.34 to −0.02; P = .03), but suicidal ideation severity scores were not significantly lower (ES, −0.13; 95% CI, −0.29 to 0.01; P = .06).
Universally beneficial
A beneficial effect on occupational problems was not evident after 6 months. This suggests that this type of training should be continued in later stages of military careers, said Wyman.
“This is not a one-time inoculation that will likely prevent all future problems,” he said.
Study participants experienced improvements in protective factors such as cohesion, morale, and bonds to classmates. The program was also associated with reduced anxiety and anger.
Overall, the Wingman-Connect group was about 20% less likely than the stress management group to report elevated depression at either follow-up period. In addition, on average, participants in the active intervention group were 19% less likely to have elevated suicidal ideation scores, although the difference was not significant.
The “logical interpretation” of this lack of statistical significance is that because depression was more common than suicidal ideation, “the intervention could have a slightly larger and more lasting effect on depression,” said Wyman.
There was no indication that men or women or those who started out at higher risk experienced greater benefit.
“Overall, the effects seemed to be distributed across airmen, independent of how they started,” said Wyman.
Wyman emphasized the unique nature of the Wingman-Connect program. “It’s universal prevention for all airmen – for those thriving and those struggling,” he said.
“We don’t know who necessarily will become at risk later on, or 6 months later, so it’s important to provide this kind of training for everyone.”
The “key mechanism” by which the program may prevent mental health problems is use of “units of military people working together day to day,” said Wyman.
The study did not reveal whether the intervention reduced suicidal behavior. This, say the authors, will need to be determined in future studies, as will determining which personnel are most likely to benefit.
A ‘particular challenge’
In an accompanying editorial, Roy H. Perlis, MD, department of psychiatry, Massachusetts General Hospital and Harvard Medical School, both in Boston, and Stephan D. Fihn, MD, department of medicine, University of Washington, Seattle, noted that suicide represents a “particular challenge” in the military.
This is “because soldiers are placed in extremely stressful situations, often without adequate physical or emotional support.”
The new study “adds to a literature that group-based interventions are effective in reducing depressive symptoms and may have advantages in resource-constrained environments,” they write.
Perlis and Finn note that it remains to be seen whether targeted strategies to reduce suicide “are worthwhile, rather than simply developing better treatments for depression.”
Commenting on the study for Medscape Medical News, Elspeth Cameron Ritchie, MD, former military psychiatrist and chair of the department of psychiatry, Medstar Washington Hospital Center, Washington, D.C., said the study “is based on quite a sound premise.”
Ritchie referred to the “long history” of research “repeatedly showing that units with good cohesion and morale have fewer difficulties of all kinds.”
However, the current study didn’t investigate the “converse of that,” said Ritchie. “There’s a high likelihood for suicidal ideation among those who are expelled” from the unit for various reasons.
Ritchie noted that a variety of different prevention initiatives have been launched in all military services over the years.
“Often, they have worked for a little while when there’s a champion behind them and there’s a lot of enthusiasm, and then they kind of fade out,” she said.
Ritchie agreed that such initiatives should continue throughout a person’s military career. She noted that suicide risk is elevated during periods of transition, for example, “leaving training base and going to your first duty station,” as well as when approaching retirement.
She appreciated the universal nature of the approach used in the study.
“Often, suicides are in those who have not been identified as high risk,” she said. However, she questioned whether the study’s follow-up period was long enough.
The study was supported by the Office of the Assistant Secretary of Defense for Health Affairs. Wyman, Perlis, and Cameron have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A novel program that strengthens bonds, boosts morale, and encourages supportive networks among US Air Force personnel cuts suicidal ideation and depressive symptoms after 1 month, new research shows.
The so-called Wingman-Connect initiative also had a beneficial impact on work performance, and the benefits were apparent at 6-month follow-up.
“This study suggests that group training can teach skills that help with occupational functioning and reduce the likelihood of experiencing elevated depression and suicidal ideation, at least in the short term,” lead author Peter A. Wyman, PhD, professor, department of psychiatry, University of Rochester, New York, told Medscape Medical News.
The study was published online Oct. 21 in JAMA Network Open.
Significant rise in suicide rates
Suicide rates among active duty military populations have increased “significantly” in the past 15 years and have exceeded rates for the general population when comparing groups of the same age and gender, said Wyman.
The study included new personnel who were taking classes at a single training center between October 2017 and October 2019.
The Wingman-Connect intervention involved three 2-hour blocks of group classes that focused on building skills in areas such as healthy relationships and maintaining balance. Group exercises emphasized cohesion, shared purpose, and the value of a healthy unit.
Participants in the stress management group received an overview of the stress response system, information on the effect of stress on health, and cognitive and behavioral strategies to reduce stress.
Primary outcomes included the scores on the suicide scale and the depression inventory of the Computerized Adaptive Test for Mental Health.
The study included 1,485 participants (82.3% men; mean age, 20.9 years). At the 1-month follow-up, participants in Wingman-Connect classes reported less severe suicidal ideation (effect size, −0.23; 95% confidence interval, −39 to −0.09; P = .001) and depressive symptoms (ES, −0.24; 95% CI, −0.41 to −0.08; P = .002).
Unlike most suicide prevention programs, the Wingman intervention didn’t target only high-risk participants. “You’d expect smaller effect sizes” because many people were already doing well, said Wyman.
He noted that the effects at 1 month were similar to other state-of-the-art prevention programs.
Another primary endpoint was self-reported occupational impairment. A poor outcome here, said Wyman, could mean having to repeat a class or falling short of expectations behaviorally or academically.
Investigators found a 50% reduction – from approximately 10% to 5% – among the participants in the Wingman-Connect group who had occupational problems or performance concerns, said Wyman.
About 84% of participants in both study arms participated in the 6-month follow-up. At this time point, Wingman-Connect participants reported significantly lower depressive symptoms (ES, −0.16; 95% CI, −0.34 to −0.02; P = .03), but suicidal ideation severity scores were not significantly lower (ES, −0.13; 95% CI, −0.29 to 0.01; P = .06).
Universally beneficial
A beneficial effect on occupational problems was not evident after 6 months. This suggests that this type of training should be continued in later stages of military careers, said Wyman.
“This is not a one-time inoculation that will likely prevent all future problems,” he said.
Study participants experienced improvements in protective factors such as cohesion, morale, and bonds to classmates. The program was also associated with reduced anxiety and anger.
Overall, the Wingman-Connect group was about 20% less likely than the stress management group to report elevated depression at either follow-up period. In addition, on average, participants in the active intervention group were 19% less likely to have elevated suicidal ideation scores, although the difference was not significant.
The “logical interpretation” of this lack of statistical significance is that because depression was more common than suicidal ideation, “the intervention could have a slightly larger and more lasting effect on depression,” said Wyman.
There was no indication that men or women or those who started out at higher risk experienced greater benefit.
“Overall, the effects seemed to be distributed across airmen, independent of how they started,” said Wyman.
Wyman emphasized the unique nature of the Wingman-Connect program. “It’s universal prevention for all airmen – for those thriving and those struggling,” he said.
“We don’t know who necessarily will become at risk later on, or 6 months later, so it’s important to provide this kind of training for everyone.”
The “key mechanism” by which the program may prevent mental health problems is use of “units of military people working together day to day,” said Wyman.
The study did not reveal whether the intervention reduced suicidal behavior. This, say the authors, will need to be determined in future studies, as will determining which personnel are most likely to benefit.
A ‘particular challenge’
In an accompanying editorial, Roy H. Perlis, MD, department of psychiatry, Massachusetts General Hospital and Harvard Medical School, both in Boston, and Stephan D. Fihn, MD, department of medicine, University of Washington, Seattle, noted that suicide represents a “particular challenge” in the military.
This is “because soldiers are placed in extremely stressful situations, often without adequate physical or emotional support.”
The new study “adds to a literature that group-based interventions are effective in reducing depressive symptoms and may have advantages in resource-constrained environments,” they write.
Perlis and Finn note that it remains to be seen whether targeted strategies to reduce suicide “are worthwhile, rather than simply developing better treatments for depression.”
Commenting on the study for Medscape Medical News, Elspeth Cameron Ritchie, MD, former military psychiatrist and chair of the department of psychiatry, Medstar Washington Hospital Center, Washington, D.C., said the study “is based on quite a sound premise.”
Ritchie referred to the “long history” of research “repeatedly showing that units with good cohesion and morale have fewer difficulties of all kinds.”
However, the current study didn’t investigate the “converse of that,” said Ritchie. “There’s a high likelihood for suicidal ideation among those who are expelled” from the unit for various reasons.
Ritchie noted that a variety of different prevention initiatives have been launched in all military services over the years.
“Often, they have worked for a little while when there’s a champion behind them and there’s a lot of enthusiasm, and then they kind of fade out,” she said.
Ritchie agreed that such initiatives should continue throughout a person’s military career. She noted that suicide risk is elevated during periods of transition, for example, “leaving training base and going to your first duty station,” as well as when approaching retirement.
She appreciated the universal nature of the approach used in the study.
“Often, suicides are in those who have not been identified as high risk,” she said. However, she questioned whether the study’s follow-up period was long enough.
The study was supported by the Office of the Assistant Secretary of Defense for Health Affairs. Wyman, Perlis, and Cameron have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A novel program that strengthens bonds, boosts morale, and encourages supportive networks among US Air Force personnel cuts suicidal ideation and depressive symptoms after 1 month, new research shows.
The so-called Wingman-Connect initiative also had a beneficial impact on work performance, and the benefits were apparent at 6-month follow-up.
“This study suggests that group training can teach skills that help with occupational functioning and reduce the likelihood of experiencing elevated depression and suicidal ideation, at least in the short term,” lead author Peter A. Wyman, PhD, professor, department of psychiatry, University of Rochester, New York, told Medscape Medical News.
The study was published online Oct. 21 in JAMA Network Open.
Significant rise in suicide rates
Suicide rates among active duty military populations have increased “significantly” in the past 15 years and have exceeded rates for the general population when comparing groups of the same age and gender, said Wyman.
The study included new personnel who were taking classes at a single training center between October 2017 and October 2019.
The Wingman-Connect intervention involved three 2-hour blocks of group classes that focused on building skills in areas such as healthy relationships and maintaining balance. Group exercises emphasized cohesion, shared purpose, and the value of a healthy unit.
Participants in the stress management group received an overview of the stress response system, information on the effect of stress on health, and cognitive and behavioral strategies to reduce stress.
Primary outcomes included the scores on the suicide scale and the depression inventory of the Computerized Adaptive Test for Mental Health.
The study included 1,485 participants (82.3% men; mean age, 20.9 years). At the 1-month follow-up, participants in Wingman-Connect classes reported less severe suicidal ideation (effect size, −0.23; 95% confidence interval, −39 to −0.09; P = .001) and depressive symptoms (ES, −0.24; 95% CI, −0.41 to −0.08; P = .002).
Unlike most suicide prevention programs, the Wingman intervention didn’t target only high-risk participants. “You’d expect smaller effect sizes” because many people were already doing well, said Wyman.
He noted that the effects at 1 month were similar to other state-of-the-art prevention programs.
Another primary endpoint was self-reported occupational impairment. A poor outcome here, said Wyman, could mean having to repeat a class or falling short of expectations behaviorally or academically.
Investigators found a 50% reduction – from approximately 10% to 5% – among the participants in the Wingman-Connect group who had occupational problems or performance concerns, said Wyman.
About 84% of participants in both study arms participated in the 6-month follow-up. At this time point, Wingman-Connect participants reported significantly lower depressive symptoms (ES, −0.16; 95% CI, −0.34 to −0.02; P = .03), but suicidal ideation severity scores were not significantly lower (ES, −0.13; 95% CI, −0.29 to 0.01; P = .06).
Universally beneficial
A beneficial effect on occupational problems was not evident after 6 months. This suggests that this type of training should be continued in later stages of military careers, said Wyman.
“This is not a one-time inoculation that will likely prevent all future problems,” he said.
Study participants experienced improvements in protective factors such as cohesion, morale, and bonds to classmates. The program was also associated with reduced anxiety and anger.
Overall, the Wingman-Connect group was about 20% less likely than the stress management group to report elevated depression at either follow-up period. In addition, on average, participants in the active intervention group were 19% less likely to have elevated suicidal ideation scores, although the difference was not significant.
The “logical interpretation” of this lack of statistical significance is that because depression was more common than suicidal ideation, “the intervention could have a slightly larger and more lasting effect on depression,” said Wyman.
There was no indication that men or women or those who started out at higher risk experienced greater benefit.
“Overall, the effects seemed to be distributed across airmen, independent of how they started,” said Wyman.
Wyman emphasized the unique nature of the Wingman-Connect program. “It’s universal prevention for all airmen – for those thriving and those struggling,” he said.
“We don’t know who necessarily will become at risk later on, or 6 months later, so it’s important to provide this kind of training for everyone.”
The “key mechanism” by which the program may prevent mental health problems is use of “units of military people working together day to day,” said Wyman.
The study did not reveal whether the intervention reduced suicidal behavior. This, say the authors, will need to be determined in future studies, as will determining which personnel are most likely to benefit.
A ‘particular challenge’
In an accompanying editorial, Roy H. Perlis, MD, department of psychiatry, Massachusetts General Hospital and Harvard Medical School, both in Boston, and Stephan D. Fihn, MD, department of medicine, University of Washington, Seattle, noted that suicide represents a “particular challenge” in the military.
This is “because soldiers are placed in extremely stressful situations, often without adequate physical or emotional support.”
The new study “adds to a literature that group-based interventions are effective in reducing depressive symptoms and may have advantages in resource-constrained environments,” they write.
Perlis and Finn note that it remains to be seen whether targeted strategies to reduce suicide “are worthwhile, rather than simply developing better treatments for depression.”
Commenting on the study for Medscape Medical News, Elspeth Cameron Ritchie, MD, former military psychiatrist and chair of the department of psychiatry, Medstar Washington Hospital Center, Washington, D.C., said the study “is based on quite a sound premise.”
Ritchie referred to the “long history” of research “repeatedly showing that units with good cohesion and morale have fewer difficulties of all kinds.”
However, the current study didn’t investigate the “converse of that,” said Ritchie. “There’s a high likelihood for suicidal ideation among those who are expelled” from the unit for various reasons.
Ritchie noted that a variety of different prevention initiatives have been launched in all military services over the years.
“Often, they have worked for a little while when there’s a champion behind them and there’s a lot of enthusiasm, and then they kind of fade out,” she said.
Ritchie agreed that such initiatives should continue throughout a person’s military career. She noted that suicide risk is elevated during periods of transition, for example, “leaving training base and going to your first duty station,” as well as when approaching retirement.
She appreciated the universal nature of the approach used in the study.
“Often, suicides are in those who have not been identified as high risk,” she said. However, she questioned whether the study’s follow-up period was long enough.
The study was supported by the Office of the Assistant Secretary of Defense for Health Affairs. Wyman, Perlis, and Cameron have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
COVID frontline physicians afraid to seek mental health care
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
A new poll of emergency physicians on the front lines of the COVID-19 pandemic shows many are fearful of seeking mental health care for fear of stigma and the potential career impact.
The results of the nationally representative poll, conducted Oct. 7-13 by the American College of Emergency Physicians, showed almost half (45%) of 862 emergency physician respondents reported being uncomfortable seeking available psychiatric care. The poll had a margin of error of plus or minus 3 percentage points.
The findings provide new insight into both the challenges of serving in emergency medicine during the pandemic and the persistent barriers to mental health care in terms of stigma and concerns about potential career setbacks.
In the poll, with another 45% report they were feeling somewhat more stressed.
When asked about causes of stress related directly to COVID-19, 83% cited concerns about family and friends contracting COVID-19. Also factoring into emergency physicians’ stress and burnout were concerns about their own safety (80%) and lack of personal protective equipment or other needed resources (60%).
In the poll, 29% of respondents reported having excellent access to mental health treatment and 42% reported having good access. Despite this, 30% of respondents still reported feeling there was a lot of stigma in their workplace about seeking mental health treatment, with another 43% reporting they felt there was some stigma.
Poll results also showed that 24% of respondents were very concerned about what might happen with their employment if they were to seek mental health treatment, with another 33% saying they were somewhat concerned.
In recent years there have been efforts to break down cultural roadblocks in medicine that deter many physicians from seeking mental health treatment, but more needs to be done, said Mark Rosenberg, DO, MBA, who was elected president of ACEP at last weekend’s annual meeting, ACEP20.
“The pandemic emphatically underscores our need to change the status quo when it comes to physicians’ mental health,” Dr. Rosenberg said.
As previously reported by Medscape Medical News, current efforts to remove such barriers include initiatives to limit inquiries into clinicians’ past or present mental health treatment.
In May, the influential Joint Commission issued a statement urging organizations to refrain from asking about any history of mental health conditions or treatment. The Joint Commission said it supports recommendations already made by the Federation of State Medical Boards and the American Medical Association to limit inquiries on licensing applications to conditions that currently impair a clinician’s ability to perform their job.
Also supporting these efforts is the Dr. Lorna Breen Heroes’ Foundation, created in honor of an emergency physician who died by suicide in April amid the pandemic.
Lorna Breen, MD, had been working intensely in the response to the pandemic. During one shift, she covered two EDs in Manhattan at locations 5 miles apart, according to a backgrounder on the foundation’s web site.
At an ACEP press conference this week, Dr. Breen’s brother-in-law, J. Corey Feist, JD, MBA, cofounder of the foundation, noted that some states’ licensing applications for physicians include questions that fall outside of the boundaries of the Americans With Disabilities Act. He cited an analysis of state medical boards’ initial licensing questions published in 2018 in the Journal of the American Academy of Psychiatry and the Law.
In many cases, states have posed questions that extend beyond an assessment of a physician’s current ability to care for patients, creating a needless hurdle to seeking care, wrote the paper’s lead author, Carol North, MD, of the University of Texas Southwestern Medical Center, Dallas.
“Over the years, many medical licensure boards have asked applicants intrusive questions about whether they have any psychiatric history. This has created a major problem for applicants, and unfortunately this has discouraged many of those who need psychiatric treatment from seeking it because of fear of the questions,” Dr. North and colleagues noted. They cited Ohio as an example of a state that had overhauled its approach to questioning to bring it in compliance with the ADA.
Ohio previously required applicants to answer lengthy questions about their mental health, including:
- Within the last 10 years, have you been diagnosed with or have you been treated for bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Have you, since attaining the age of eighteen or within the last 10 years, whichever period is shorter, been admitted to a hospital or other facility for the treatment of bipolar disorder, schizophrenia, paranoia, or any other psychotic disorder?
- Do you have, or have you been diagnosed as having, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?
In the new version, the single question reads: “In the past 5 years, have you been diagnosed as having, or been hospitalized for, a medical condition which in any way impairs or limits your ability to practice medicine with reasonable skill and safety?”
Other states such as New York pose no mental health questions on applications for licensure.
Still, even when states have nondiscriminatory laws, physicians may not be aware of them, said Mr. Feist at an ACEP press conference. In addition to his work with the foundation, Mr. Feist is the CEO of the University of Virginia Physicians Group.
He said his sister-in-law Dr. Breen may have worried without cause about potential consequences of seeking psychiatric treatment during the pandemic. In addition, physicians in need of psychiatric care may worry about encountering hitches with medical organizations and insurers.
“This stigma and this fear of professional action on your license or your credentialing or privileging is pervasive throughout the industry,” he said.
A version of this article originally appeared on Medscape.com.
Add-on psychotherapy a win in bipolar disorder
Adding psychotherapy to pharmacotherapy benefits patients with bipolar disorder (BD), particularly when delivered in family or group settings, results of a new meta-analysis confirms.
Outpatients with BD receiving drug therapy “should also be offered psychosocial treatments that emphasize illness management strategies and enhance coping skills; delivering these components in family or group format may be especially advantageous,” wrote the investigators, led by David Miklowitz, PhD, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior.
The study was published online Oct. 14 in JAMA Psychiatry.
Drugs alone not enough
It’s increasingly recognized that drug therapy alone can’t prevent recurrences of BD or fully alleviate postepisode symptoms or functional impairment, the researchers noted in their article. Several psychotherapy protocols have been shown to benefit patients with BD when used in conjunction with drug therapy, but little is known about their comparative effectiveness.
To investigate, the researchers conducted a systematic review and component network meta-analysis of 39 randomized clinical trials (36 involving adults and three involving adolescents).
The trials involved 3,863 patients with BD and compared pharmacotherapy used in conjunction with manualized psychotherapy (cognitive-behavioral therapy [CBT], family or conjoint therapy, interpersonal therapy, and/or psychoeducational therapy) with pharmacotherapy delivered in conjunction with a control intervention (supportive therapy or treatment as usual).
Across 20 two-group trials that provided usable information, manualized psychotherapies were associated with a lower probability of illness recurrence (the primary outcome), compared with control interventions (odds ratio, 0.56; 95% CI, 0.43-0.74).
Psychoeducation with guided practice of illness management skills in a family or group format was superior to these strategies delivered in an individual format (OR, 0.12; 95% CI, 0.02-0.94).
Family or conjoint therapy and brief psychoeducation were associated with lower attrition rates than standard psychoeducation.
For the secondary outcome of stabilization of depressive or manic symptoms over 12 months, CBT and, with less certainty, family or conjoint therapy and interpersonal therapy were more effective than treatment as usual.
The investigators note that the findings are in line with a network meta-analysis published earlier this year that found that combining psychotherapy with pharmacotherapy is the best option for stabilizing episodes and preventing recurrences of major depression.
“[T]here is enough evidence from this analysis and others to conclude that health care systems should offer combinations of evidence-based pharmacotherapy and psychotherapy” to outpatients with BD, the researchers note.
and active tasks to enhance coping skills (e.g., monitoring and managing prodromal symptoms) rather than being passive recipients of didactic education,” they wrote.
“When the immediate goal is recovery from moderately severe depressive or manic symptoms, cognitive restructuring, regulating daily rhythms, and communication training may be associated with stabilization,” they added.
A call to action
The coauthors of an editorial in JAMA Psychiatry noted that the findings “further reinforce extant treatment guidelines recommending medication management and adjunctive evidence-based psychosocial treatments for individuals with BD.”
The findings also “identify specific treatment components and formats most strongly associated with preventing relapse and addressing mood symptoms,” write Tina Goldstein, PhD, and Danella Hafeman, MD, PhD, from Western Psychiatric Hospital, University of Pittsburgh.
The study “may further serve as a call to action to enhance availability and uptake of these treatments in the community. Unfortunately, data suggest substantially lower rates of psychotherapy receipt (26%-50%), compared with medication management (46%-90%) among adults with BD,” they wrote.
Dr. Goldstein and Dr. Hafeman noted future steps for the field include “demonstrating effectiveness of evidence-based treatment approaches for BD in the community, maximizing accessibility, and furthering knowledge that informs individualized treatment selection with substantial promise to optimize outcomes for individuals with BD.”
The study was supported in part by a grant from the National Institute for Health Research Oxford Health Biomedical Research Centre. Dr. Miklowitz has received research support from the NIHR, the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, and the Max Gray Fund; book royalties from Guilford Press and John Wiley and Sons; and served as principal investigator on four of the trials included in this meta-analysis. Dr. Goldstein has received grants from the National Institute of Mental Health, the American Foundation for Suicide Prevention, the University of Pittsburgh Clinical and Translational Science Institute, and the Brain and Behavior Research Foundation and royalties from Guilford Press outside the submitted work. Dr. Hafeman has received grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the Klingenstein Third Generation Foundation.
This article first appeared on Medscape.com.
Adding psychotherapy to pharmacotherapy benefits patients with bipolar disorder (BD), particularly when delivered in family or group settings, results of a new meta-analysis confirms.
Outpatients with BD receiving drug therapy “should also be offered psychosocial treatments that emphasize illness management strategies and enhance coping skills; delivering these components in family or group format may be especially advantageous,” wrote the investigators, led by David Miklowitz, PhD, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior.
The study was published online Oct. 14 in JAMA Psychiatry.
Drugs alone not enough
It’s increasingly recognized that drug therapy alone can’t prevent recurrences of BD or fully alleviate postepisode symptoms or functional impairment, the researchers noted in their article. Several psychotherapy protocols have been shown to benefit patients with BD when used in conjunction with drug therapy, but little is known about their comparative effectiveness.
To investigate, the researchers conducted a systematic review and component network meta-analysis of 39 randomized clinical trials (36 involving adults and three involving adolescents).
The trials involved 3,863 patients with BD and compared pharmacotherapy used in conjunction with manualized psychotherapy (cognitive-behavioral therapy [CBT], family or conjoint therapy, interpersonal therapy, and/or psychoeducational therapy) with pharmacotherapy delivered in conjunction with a control intervention (supportive therapy or treatment as usual).
Across 20 two-group trials that provided usable information, manualized psychotherapies were associated with a lower probability of illness recurrence (the primary outcome), compared with control interventions (odds ratio, 0.56; 95% CI, 0.43-0.74).
Psychoeducation with guided practice of illness management skills in a family or group format was superior to these strategies delivered in an individual format (OR, 0.12; 95% CI, 0.02-0.94).
Family or conjoint therapy and brief psychoeducation were associated with lower attrition rates than standard psychoeducation.
For the secondary outcome of stabilization of depressive or manic symptoms over 12 months, CBT and, with less certainty, family or conjoint therapy and interpersonal therapy were more effective than treatment as usual.
The investigators note that the findings are in line with a network meta-analysis published earlier this year that found that combining psychotherapy with pharmacotherapy is the best option for stabilizing episodes and preventing recurrences of major depression.
“[T]here is enough evidence from this analysis and others to conclude that health care systems should offer combinations of evidence-based pharmacotherapy and psychotherapy” to outpatients with BD, the researchers note.
and active tasks to enhance coping skills (e.g., monitoring and managing prodromal symptoms) rather than being passive recipients of didactic education,” they wrote.
“When the immediate goal is recovery from moderately severe depressive or manic symptoms, cognitive restructuring, regulating daily rhythms, and communication training may be associated with stabilization,” they added.
A call to action
The coauthors of an editorial in JAMA Psychiatry noted that the findings “further reinforce extant treatment guidelines recommending medication management and adjunctive evidence-based psychosocial treatments for individuals with BD.”
The findings also “identify specific treatment components and formats most strongly associated with preventing relapse and addressing mood symptoms,” write Tina Goldstein, PhD, and Danella Hafeman, MD, PhD, from Western Psychiatric Hospital, University of Pittsburgh.
The study “may further serve as a call to action to enhance availability and uptake of these treatments in the community. Unfortunately, data suggest substantially lower rates of psychotherapy receipt (26%-50%), compared with medication management (46%-90%) among adults with BD,” they wrote.
Dr. Goldstein and Dr. Hafeman noted future steps for the field include “demonstrating effectiveness of evidence-based treatment approaches for BD in the community, maximizing accessibility, and furthering knowledge that informs individualized treatment selection with substantial promise to optimize outcomes for individuals with BD.”
The study was supported in part by a grant from the National Institute for Health Research Oxford Health Biomedical Research Centre. Dr. Miklowitz has received research support from the NIHR, the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, and the Max Gray Fund; book royalties from Guilford Press and John Wiley and Sons; and served as principal investigator on four of the trials included in this meta-analysis. Dr. Goldstein has received grants from the National Institute of Mental Health, the American Foundation for Suicide Prevention, the University of Pittsburgh Clinical and Translational Science Institute, and the Brain and Behavior Research Foundation and royalties from Guilford Press outside the submitted work. Dr. Hafeman has received grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the Klingenstein Third Generation Foundation.
This article first appeared on Medscape.com.
Adding psychotherapy to pharmacotherapy benefits patients with bipolar disorder (BD), particularly when delivered in family or group settings, results of a new meta-analysis confirms.
Outpatients with BD receiving drug therapy “should also be offered psychosocial treatments that emphasize illness management strategies and enhance coping skills; delivering these components in family or group format may be especially advantageous,” wrote the investigators, led by David Miklowitz, PhD, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior.
The study was published online Oct. 14 in JAMA Psychiatry.
Drugs alone not enough
It’s increasingly recognized that drug therapy alone can’t prevent recurrences of BD or fully alleviate postepisode symptoms or functional impairment, the researchers noted in their article. Several psychotherapy protocols have been shown to benefit patients with BD when used in conjunction with drug therapy, but little is known about their comparative effectiveness.
To investigate, the researchers conducted a systematic review and component network meta-analysis of 39 randomized clinical trials (36 involving adults and three involving adolescents).
The trials involved 3,863 patients with BD and compared pharmacotherapy used in conjunction with manualized psychotherapy (cognitive-behavioral therapy [CBT], family or conjoint therapy, interpersonal therapy, and/or psychoeducational therapy) with pharmacotherapy delivered in conjunction with a control intervention (supportive therapy or treatment as usual).
Across 20 two-group trials that provided usable information, manualized psychotherapies were associated with a lower probability of illness recurrence (the primary outcome), compared with control interventions (odds ratio, 0.56; 95% CI, 0.43-0.74).
Psychoeducation with guided practice of illness management skills in a family or group format was superior to these strategies delivered in an individual format (OR, 0.12; 95% CI, 0.02-0.94).
Family or conjoint therapy and brief psychoeducation were associated with lower attrition rates than standard psychoeducation.
For the secondary outcome of stabilization of depressive or manic symptoms over 12 months, CBT and, with less certainty, family or conjoint therapy and interpersonal therapy were more effective than treatment as usual.
The investigators note that the findings are in line with a network meta-analysis published earlier this year that found that combining psychotherapy with pharmacotherapy is the best option for stabilizing episodes and preventing recurrences of major depression.
“[T]here is enough evidence from this analysis and others to conclude that health care systems should offer combinations of evidence-based pharmacotherapy and psychotherapy” to outpatients with BD, the researchers note.
and active tasks to enhance coping skills (e.g., monitoring and managing prodromal symptoms) rather than being passive recipients of didactic education,” they wrote.
“When the immediate goal is recovery from moderately severe depressive or manic symptoms, cognitive restructuring, regulating daily rhythms, and communication training may be associated with stabilization,” they added.
A call to action
The coauthors of an editorial in JAMA Psychiatry noted that the findings “further reinforce extant treatment guidelines recommending medication management and adjunctive evidence-based psychosocial treatments for individuals with BD.”
The findings also “identify specific treatment components and formats most strongly associated with preventing relapse and addressing mood symptoms,” write Tina Goldstein, PhD, and Danella Hafeman, MD, PhD, from Western Psychiatric Hospital, University of Pittsburgh.
The study “may further serve as a call to action to enhance availability and uptake of these treatments in the community. Unfortunately, data suggest substantially lower rates of psychotherapy receipt (26%-50%), compared with medication management (46%-90%) among adults with BD,” they wrote.
Dr. Goldstein and Dr. Hafeman noted future steps for the field include “demonstrating effectiveness of evidence-based treatment approaches for BD in the community, maximizing accessibility, and furthering knowledge that informs individualized treatment selection with substantial promise to optimize outcomes for individuals with BD.”
The study was supported in part by a grant from the National Institute for Health Research Oxford Health Biomedical Research Centre. Dr. Miklowitz has received research support from the NIHR, the Danny Alberts Foundation, the Attias Family Foundation, the Carl and Roberta Deutsch Foundation, the Kayne Family Foundation, AIM for Mental Health, and the Max Gray Fund; book royalties from Guilford Press and John Wiley and Sons; and served as principal investigator on four of the trials included in this meta-analysis. Dr. Goldstein has received grants from the National Institute of Mental Health, the American Foundation for Suicide Prevention, the University of Pittsburgh Clinical and Translational Science Institute, and the Brain and Behavior Research Foundation and royalties from Guilford Press outside the submitted work. Dr. Hafeman has received grants from the National Institute of Mental Health, the Brain and Behavior Research Foundation, and the Klingenstein Third Generation Foundation.
This article first appeared on Medscape.com.
National three-digit suicide lifeline to take effect in 2022
Beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
The number was finalized when President Donald J. Trump signed the National Suicide Hotline Designation Act on Oct. 17. It completes what has been a multiyear effort by Republican and Democratic lawmakers to make it easier for individuals to reach out during mental health emergencies.
“When your house is on fire, you can get help by calling 9-1-1,” noted Rep. Seth Moulton (D-Mass.), a key sponsor of the legislation, in a statement. The new number “is a national step forward out of the shadows of stigma that prevent too many people from getting help and into a new era when mental health care is easy to get and normal to talk about,” said Rep. Moulton, a combat veteran who has openly discussed his struggles with PTSD.
The law requires the Department of Health & Human Services to develop a strategy to provide access to specialized services for high-risk populations such as LGBTQ youth, minorities, and people who live in rural areas.
“This law is a historic victory, as this is the first explicitly LGBTQ-inclusive bill to pass unanimously in history – and 9-8-8 will undoubtedly save countless lives,” said Sam Brinton, vice president of advocacy and government affairs for the Trevor Project, in a statement, also noting that “More than half of transgender and nonbinary youth having seriously considered it.”
Robert Gebbia, CEO of the American Foundation for Suicide Prevention, said in a statement: “This easy-to-remember number will increase public access to mental health and suicide prevention crisis resources, encourage help-seeking for individuals in need, and is a crucial entry point for establishing a continuum of crisis care.”
Mr. Gabbia called for more funding for local crisis centers to “respond to what we expect will be an increased call volume and provide effective crisis services to those in need when 9-8-8 is made available in July 2022.”
In 2017, then-Senator Orrin Hatch (R-Utah) and colleague Joe Donnelly (D-Ind.) pushed for a three-digit number for people having mental health crises. Their legislation passed in the Senate that fall and passed in the House in July 2018.
The bill directed the Federal Communications Commission to submit a report to Congress that would include a recommended number, a cost-benefit analysis comparing the three-digit code with the current hotline, and an assessment of how much it might cost service providers, states, local towns, and cities.
Mr. Trump signed that bill in 2018. The FCC unanimously approved the 9-8-8 number in July 2020.
Until the new number is active in July 2022, those in crisis should continue to call the National Suicide Lifeline at 1-800-273-TALK (8255).
A version of this article originally appeared on Medscape.com.
Beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
The number was finalized when President Donald J. Trump signed the National Suicide Hotline Designation Act on Oct. 17. It completes what has been a multiyear effort by Republican and Democratic lawmakers to make it easier for individuals to reach out during mental health emergencies.
“When your house is on fire, you can get help by calling 9-1-1,” noted Rep. Seth Moulton (D-Mass.), a key sponsor of the legislation, in a statement. The new number “is a national step forward out of the shadows of stigma that prevent too many people from getting help and into a new era when mental health care is easy to get and normal to talk about,” said Rep. Moulton, a combat veteran who has openly discussed his struggles with PTSD.
The law requires the Department of Health & Human Services to develop a strategy to provide access to specialized services for high-risk populations such as LGBTQ youth, minorities, and people who live in rural areas.
“This law is a historic victory, as this is the first explicitly LGBTQ-inclusive bill to pass unanimously in history – and 9-8-8 will undoubtedly save countless lives,” said Sam Brinton, vice president of advocacy and government affairs for the Trevor Project, in a statement, also noting that “More than half of transgender and nonbinary youth having seriously considered it.”
Robert Gebbia, CEO of the American Foundation for Suicide Prevention, said in a statement: “This easy-to-remember number will increase public access to mental health and suicide prevention crisis resources, encourage help-seeking for individuals in need, and is a crucial entry point for establishing a continuum of crisis care.”
Mr. Gabbia called for more funding for local crisis centers to “respond to what we expect will be an increased call volume and provide effective crisis services to those in need when 9-8-8 is made available in July 2022.”
In 2017, then-Senator Orrin Hatch (R-Utah) and colleague Joe Donnelly (D-Ind.) pushed for a three-digit number for people having mental health crises. Their legislation passed in the Senate that fall and passed in the House in July 2018.
The bill directed the Federal Communications Commission to submit a report to Congress that would include a recommended number, a cost-benefit analysis comparing the three-digit code with the current hotline, and an assessment of how much it might cost service providers, states, local towns, and cities.
Mr. Trump signed that bill in 2018. The FCC unanimously approved the 9-8-8 number in July 2020.
Until the new number is active in July 2022, those in crisis should continue to call the National Suicide Lifeline at 1-800-273-TALK (8255).
A version of this article originally appeared on Medscape.com.
Beginning in July 2022, Americans experiencing a mental health crisis will be able to dial 9-8-8 and be connected to the services and counselors at the National Suicide Prevention Lifeline.
The number was finalized when President Donald J. Trump signed the National Suicide Hotline Designation Act on Oct. 17. It completes what has been a multiyear effort by Republican and Democratic lawmakers to make it easier for individuals to reach out during mental health emergencies.
“When your house is on fire, you can get help by calling 9-1-1,” noted Rep. Seth Moulton (D-Mass.), a key sponsor of the legislation, in a statement. The new number “is a national step forward out of the shadows of stigma that prevent too many people from getting help and into a new era when mental health care is easy to get and normal to talk about,” said Rep. Moulton, a combat veteran who has openly discussed his struggles with PTSD.
The law requires the Department of Health & Human Services to develop a strategy to provide access to specialized services for high-risk populations such as LGBTQ youth, minorities, and people who live in rural areas.
“This law is a historic victory, as this is the first explicitly LGBTQ-inclusive bill to pass unanimously in history – and 9-8-8 will undoubtedly save countless lives,” said Sam Brinton, vice president of advocacy and government affairs for the Trevor Project, in a statement, also noting that “More than half of transgender and nonbinary youth having seriously considered it.”
Robert Gebbia, CEO of the American Foundation for Suicide Prevention, said in a statement: “This easy-to-remember number will increase public access to mental health and suicide prevention crisis resources, encourage help-seeking for individuals in need, and is a crucial entry point for establishing a continuum of crisis care.”
Mr. Gabbia called for more funding for local crisis centers to “respond to what we expect will be an increased call volume and provide effective crisis services to those in need when 9-8-8 is made available in July 2022.”
In 2017, then-Senator Orrin Hatch (R-Utah) and colleague Joe Donnelly (D-Ind.) pushed for a three-digit number for people having mental health crises. Their legislation passed in the Senate that fall and passed in the House in July 2018.
The bill directed the Federal Communications Commission to submit a report to Congress that would include a recommended number, a cost-benefit analysis comparing the three-digit code with the current hotline, and an assessment of how much it might cost service providers, states, local towns, and cities.
Mr. Trump signed that bill in 2018. The FCC unanimously approved the 9-8-8 number in July 2020.
Until the new number is active in July 2022, those in crisis should continue to call the National Suicide Lifeline at 1-800-273-TALK (8255).
A version of this article originally appeared on Medscape.com.