User login
COVID-19’s psychological impact gets a name
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
During normal times, the U.K.-based charity No Panic offers itself as an easily accessible service to those with anxiety disorders and phobias. Visitors to the website who can receive immediate, remote support from trained volunteers. But this spring was anything but normal, as the reality of COVID-19’s worldwide spread became terrifyingly clear.
COVID-19 cases peaked in the United Kingdom in early April. Nationwide lockdown efforts contributed to a gradual but ultimately substantial decline in cases, yet, despite the favorable trend lines, No Panic has remained busier than ever.
Beyond the physical symptoms associated with COVID-19, the psychological outcomes are vast and, it seems, prolonged. Researchers have now formalized a definition of the long-term mental maladies associated with the pandemic, collectively deeming them “coronaphobia.”
The term is a catch-all phrase for the fear and the emotional and social strain experienced by the general public in response to COVID-19. Obsessive behaviors, distress, avoidance reaction, panic, anxiety, hoarding, paranoia, and depression are some of the responses associated with coronaphobia. On the surface, these appear to be normal, somewhat fitting reactions to this surreal and frightening moment in time. However, for those experiencing coronaphobia, they are distinctly maladaptive and harmful.
“We had a serious rise in the use of our services, notably the helpline and email enquiries,” explained Sarah Floyd, No Panic’s volunteer advisor and social media coordinator. “It has been up and down all along, but more of an up since lockdown is easing.”
The group’s experience offers yet more evidence that the anxieties and fears caused by this global pandemic don’t flatten alongside the curve but instead linger as chronic problems requiring ongoing care.
“Every week in my clinic, I’m seeing people who are experiencing more anxiety and hopelessness and having an emotional response that is perhaps out of proportion to what one would expect, which is directly related to what is going on in the world right now with coronavirus,” said Gregory Scott Brown, MD, founder and director of the Center for Green Psychiatry in West Lake Hills, Tex. “Simply put, I think what we are looking at is adjustment disorder. That is probably how the DSM would define it.”
Adjustment disorder is one of the most frequently diagnosed mental health conditions, although it is also relatively understudied. It is really a set of disorders that follow in the wake of a significant stressor, which can vary from serious illness or the death of a loved one to relocating or experiencing work problems. The resulting dysfunction and distress that the person experiences are considered out of proportion in duration or scale with what would normally be expected. Diagnosing an adjustment disorder is made difficult by the lack of a valid and reliable screening measure.
Recent literature suggests that coronaphobia may be likely to occur in those who feel vulnerable to disease, are predisposed to anxiety, or are intolerant of uncertainty. Preexisting mental health conditions can also be exacerbated by periods of quarantine, self-isolation, and lockdown, which can lead to panic attacks, chronophobia (fear of passing time), and suicidality.
Although imperfect comparisons, findings from earlier 21st century disease outbreaks, such as severe acute respiratory syndrome and the Ebola virus, signal that containment efforts themselves play a role in deteriorating mental health. A recent rapid review found that, in studies comparing persons who had previously undergone quarantines and those who had not, the former were significantly more likely to experience acute stress disorder, posttraumatic stress symptoms, and depression. Quarantine was found to result in long-term behavioral changes, such as avoiding crowds, among the general public and health care practitioners.
That tremendous psychological morbidity should accompany a global pandemic of this scale is not surprising, according to Amit Anand, MD, vice chair for research for the Center for Behavioral Health and director of the Mood and Emotional Disorders Across the Life Span program at the Cleveland Clinic.
“The technical definition of anxiety is an impending sense of doom, and I think all of us are living with that,” Dr. Anand said. “The basic question then becomes, what is normal and when does it become abnormal?”
He added that most classifications of psychiatric disorders are set during periods of relative stability, which the current moment is most certainly not.
“This is such an unusual situation, so I think it will depend on case-by-case basis, keeping the whole context in mind as whether the patient is thinking or behaving with an abnormal amount of anxiety,” Dr. Anand said.
Investigators are currently trying to give clinicians the tools to better make that determination. In the first scientific study of this clinical condition, Sherman Lee, MD, reported that five symptoms – dizziness, sleep disturbances, tonic immobility, appetite loss, and nausea/abdominal distress – were strong factors for distinguishing coronaphobia from otherwise normal concerns about COVID-19 that did not result in functional impairment. Dr. Lee and colleagues have since published further evidence that coronaphobia “is a unique predictor of psychological distress during the COVID-19 crisis.” They are working on validating a self-reported mental health screener for this condition.
Having the tools to identify patients struggling with coronaphobia may go some ways toward addressing another area of declining health. At the outset of the COVID-19 pandemic, there was a question as to whether doctors would be beset by a surge of the “worried well” – persons mistakenly believing themselves to be infected. Now months into the pandemic, the converse phenomenon – a fear of contracting COVID-19 that is driving patients away from practitioners – appears to be the more valid concern.
In early spring, the pandemic’s first surge was accompanied by reports of approximately 40% and 60% drops in visits to EDs and ambulatory centers, respectively. Stories of acute stroke patients avoiding treatment began to appear in the press. Major U.S. cities saw noteworthy declines in 911 calls, indicating a hesitancy to be taken to a hospital. That COVID-19 has been accompanied by mass unemployment and subsequent loss of insurance complicates the notion that fear alone is keeping people from treatment. In other countries, it has been explicitly linked. Investigators in Singapore noted that coronaphobia played a role in reducing willingness to attend in-person visits among adolescents with eating disorders. Similarly, case reports in Israel suggest that coronaphobia has contributed to delays in diagnoses of common pediatric diseases.
There is also a concern, colloquially termed “reentry anxiety,” that mental health problems caused by the pandemic, the accompanying lockdown, self-isolation, and quarantine practices will prove alarmingly durable. Even after this challenging moment in history draws to a close, many people may face substantial stress in returning to the normal activities of life – social, professional, familial – once taken for granted.
“We are in the beginning phase of that now,” said Dr. Anand. “ I think the longer it goes on for, the more difficult it will be.”
In the United States, that day may seem far away. Nonetheless, it is important to begin laying the therapeutic groundwork now, according to Dr. Brown.
“I am recommending unconventional therapies like meet-up groups, online forums,” he said. “Everything has shifted online, and so there are a lot of support groups that patients can participate to learn coping skills and really hear what other people are going through.”
Before reaching that stage, Dr. Brown recommends that clinicians first simply discuss such anxieties with their patients in order to normalize them.
“Realize that everyone essentially is going through some degree of this right now. The coronavirus pandemic is literally impacting every person on the face of the planet. Sometimes just pointing that out to people can really help,” he said.
A version of this article originally appeared on Medscape.com.
Suicide in America: The urban-rural divide
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
The gap in suicide rates between rural and urban areas has widened since 2000 for both males and females, according to a recent report from the National Center for Health Statistics.

After remaining stable from 2000 to 2007, the suicide rate for rural males rose 34% from 2007 to 2018, versus 17% among urban males over the same period. Suicide rates for females were significantly lower than those of men, but the changes were larger. For rural females, the rate increased 91% from 2000 to 2018, compared with 51% for urban females, Kristen Pettrone, MD, MPH, and Sally C. Curtin, MA, said in an NCHS Data Brief.
For 2018, the last year with available data, the age-adjusted rates look like this: 21.5 per 100,000 population for urban males, 30.7 for rural males, 5.9 per 100,000 for urban females, and 8.0 for rural females. The overall rate for the United States was 14.2 per 100,000, with combined male/female rates of 13.4 in urban areas and 19.4 in rural areas, the researchers said.
Methods of suicide also varied by sex and urban-rural status. Firearms were the leading method for males in both rural and urban areas, but females split between firearms in rural areas and suffocation (including hangings) in urban areas, said Dr. Pettrone of the Centers for Disease Control and Prevention and Ms. Curtin of the NCHS.
Suffocation, however, was the fastest-growing method from 2000 to 2018, regardless of sex or location. Suffocation-related suicide rates more than quadrupled for rural females, and more than doubled for urban females and rural males, while rates rose 85% among males in urban areas, based on data from the National Vital Statistics System.
“Suicide has remained the 10th leading cause of death in the United States since 2008,” they wrote, and
SOURCE: Pettrone K, Curtin SC. 2020 Aug. NCHS Data Brief, No 373.
Impact of the MTHFR C677T genetic variant on depression
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
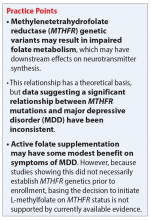
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
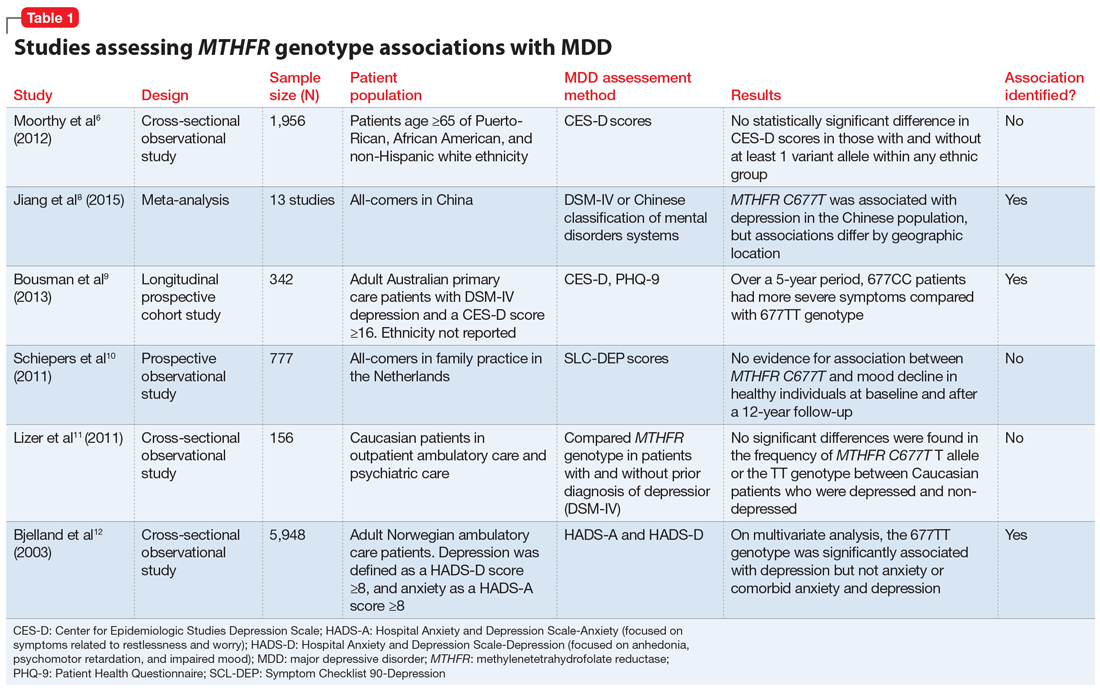
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.
Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
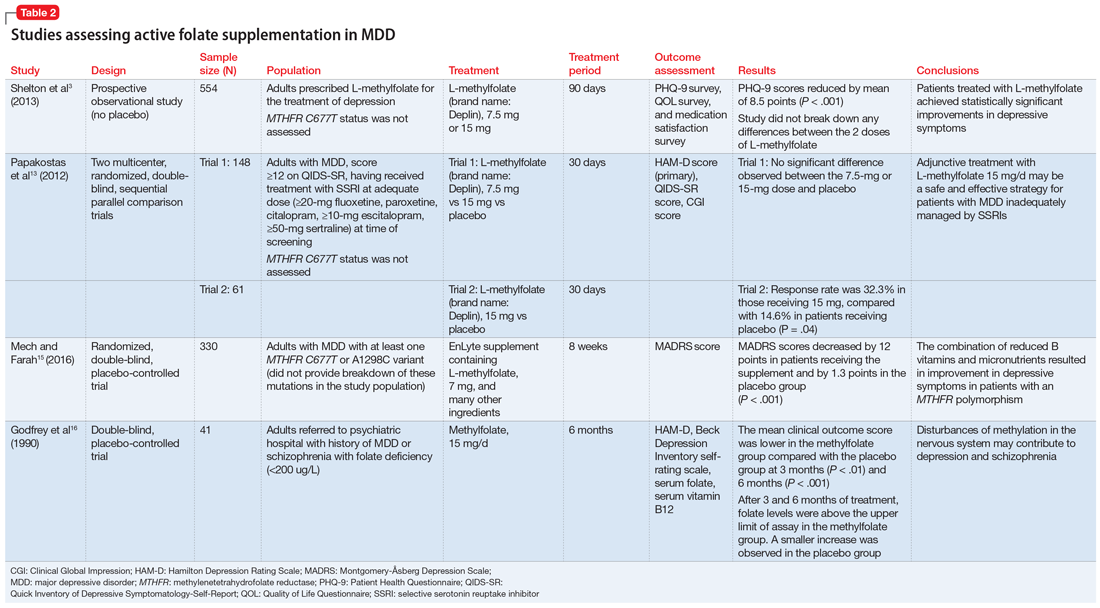
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Ms. T, age 55, presents to her psychiatrist’s clinic with a chief complaint of ongoing symptoms of anhedonia and lethargy related to her diagnosis of major depressive disorder (MDD). She also has a history of peripheral arterial disease, hypothyroidism, and generalized anxiety disorder. Her current antidepressant regimen is duloxetine, 60 mg/d, and mirtazapine, 15 mg at night. She recently elected to undergo pharmacogenetic testing, which showed that she is heterozygous for the methylenetetrahydrofolate reductase (MTHFR) C677T mutation (MTHFR C677T CT carrier). Her test report states that she may have impaired folate metabolism. Her psychiatrist adds L-methylfolate, 15 mg/d, to her current antidepressant regimen.
What is the relationship between folic acid and MTHFR?
Methylenetetrahydrofolate reductase is an intracellular enzyme responsible for one of several steps involved in converting dietary folic acid to its physiologically active form, L-methylfolate.1 Once active, L-methylfolate can be transported into the CNS, where it participates in one-carbon transfer reactions.2,3 Mutations in the MTHFR gene have been associated with decreased activity of the enzyme, which has been shown to result in accumulation of homocysteine and may lead to decreased synthesis of neurotransmitters.2,4Commercial pharmacogenetic testing panels may offer MTHFR genetic testing to assist with prescribing decisions for patients with mental illness. The most well-characterized mutation currently is C677T (rsID1801133), which is a single amino acid base pair change (cytosine [C] to thymine [T]) that leads to increased thermolability and instability of the enzyme.5 Carrying 1 or 2 T alleles can lead to a 35% or 70% reduction in enzyme activity, respectively. The T variant allele is most frequent in Hispanics (20% to 25%), Asians (up to 63%), and Caucasians (8% to 20%); however, it is relatively uncommon in African Americans (<2%).5,6 Another variant, A1289C (rs1801131), has also been associated with decreased enzyme function, particularly when analyzed in combination with C677T. However, carrying the 1289C variant allele does not appear to result in as large of a reduction of enzyme function as the 677T variant.7
What is the relationship between MTHFR C677T and depression?
Some researchers have proposed that the C677T mutation in MTHFR may be associated with depression as a result of decreased neurotransmitter synthesis, but studies have not consistently supported this hypothesis. Several studies suggest an association between MTHFR mutations and MDD8-10:
Jiang et al8 performed a meta-analysis of 13 studies including 1,295 Chinese patients and found that having at least 1 C677T variant allele was significantly associated with an increased risk of depression (for T vs C odds ratio 1.52, 95% confidence interval 1.24 to 1.85). The authors noted a stronger association identified in the Northern Chinese population compared with the Southern Chinese population.8
Bousman et al9 found that American patients with MDD and the 677CC genotype had greater Patient Health Questionnaire-9 (PHQ-9) scores at assessments at 24, 36, and 48 months post-baseline compared with those with the 677TT genotype (P = .024), which was unexpected based on previously reported associations.9
Schiepers et al10 also assessed the association between the MTHFR genotype in a Dutch ambulatory care population over 12 years. There was no association identified between scores on the depression subscale of the Symptom Checklist 90 and C677T diplotype.10
Table 16,8-12 provides summaries of these and other selected studies on MTHFR and MDD. Overall, although a pathophysiological basis for depression and decreased MTHFR function has been proposed, the current body of literature does not indicate a consistent link between MTHFR C677T genetic variants alone and depression.

Continue to: Medication changes based on MTHFR: What is the evidence?
Medication changes based on MTHFR: What is the evidence?
Some evidence supports the use of active folate supplementation to improve symptoms of MDD.
Shelton et al3 conducted an observational study that assessed the effects of adding L-methylfolate (brand name: Deplin), 7.5 or 15 mg, to existing antidepressant therapy in 502 patients with MDD who had baseline PHQ-9 scores of at least 5. After an average 95 days of therapy, PHQ-9 scores were reduced by a mean of 8.5 points, with 67.9% of patients achieving at least a 50% reduction in PHQ-9 scores. The study did not take into account patients’ MTHFR genotype or differentiate results between the 2 doses of L-methylfolate.3
Papakostas et al13 performed 2 randomized, double-blind, parallel-sequential, placebo-controlled trials of L-methylfolate for patients with MDD. The first compared L-methylfolate, 7.5 and 15 mg, to placebo, without regard to MTHFR genotype.13 There was no significant difference between the 7.5-mg dose and placebo, or the 15-mg dose and placebo. However, among the group receiving the 15-mg dose, the response rate was 24%, vs 9% in the placebo group, which approached significance (P = .1). Papakostas et al13 followed up with a smaller trial comparing the 15-mg dose alone to placebo, and found the response rate was 32.3% in patients treated with L-methylfolate compared with 14.6% in the placebo group (P = .04).13
Although the Shelton et al3 and Papakostas et al13 studies showed some improvement in depressive symptom scores among patients who received L-methylfolate supplementation, an important consideration is if MTHFR genotype may predict patient response to this therapy.
Papakostas et al14 performed a post hoc analysis of their earlier study to assess potential associations amongst multiple other biomarkers of inflammation and metabolic disturbances hypothesized by the authors to be associated with MDD, as well as body mass index (BMI), with treatment outcome.14 When change in the Hamilton Depression Rating Scale-28 (HDRS-28) was analyzed by C677T and A1298C variant groups (677 CT vs TT and 1298 AC vs CC), no statistically significant improvements were identified (C677T mean change from baseline −3.8 points, P = .087; A1298C mean change from baseline −0.5 points, P = .807).14 However, statistically significant improvements in HDRS-28 scores were observed compared with baseline when the C677T genotype was pooled with other biomarkers, including methionine synthase (MTR 2756 AG/GG, −23.3 points vs baseline, P < .001) and a voltage-dependent calcium channel (CACNAIC AG/AA, −9 points vs baseline, P < .001), as well as with BMI ≥ 30 kg/m2 (−9.9 points vs baseline, P = .001).14
Continue to: Mech and Farah...
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.

CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources
Related Resources
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165(1):1-13.
- Trimmer E. Methylenetetrahydrofolate reductase: biochemical characterization and medical significance. Current Pharmaceutical Design. 2013;19(4):2574-3595.
Drug Brand Names
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
L-methylfolate • Deplin
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
1. Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480-488.
2. Jadavji N, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Molecular Genetics and Metabolism Reports. 2015;3(Issue C):1-4.
3. Shelton R, Manning J, Barrentine L, et al. Assessing effects of L-methylfolate in depression management: results of a real-world patient experience trial. Prim Care Companion CNS Disord. 2013;15(4):pii:PCC.13m01520. doi: 10.4088/PCC.13m01520.
4. Brustolin S, Giugliani R, Felix T. Genetics of homocysteine metabolism and associated disorders. Braz J Med Biol Res. 2010;43(1):1-7.
5. Blom H, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34:75-81.
6. Moorthy D, Peter I, Scott T, et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J Nutr. 2012;142:1554-1560.
7. Lievers K, Boers G, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79(9):522-528.
8. Jiang W, Xu J, Lu X, et al. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2015;21(6):675-685.
9. Bousman C, Potiriadis M, Everall I, et al. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):68-76.
10. Schiepers O, Van Boxtel M, de Groot R, et al. Genetic variation in folate metabolism is not associated with cognitive functioning or mood in healthy adults. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(7):1682-1688.
11. Lizer M, Bogdan R, Kidd R. Comparison of the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in depressed versus nondepressed patients. J Psychiatr Pract. 2011;17(6):404-409.
12. Bjelland I, Tell G, Vollset S, et al. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60(6):618-626.
13. Papakostas G, Shelton R, Zajecka J, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel sequential trials. Am J Psychiatry. 2012;169(12):1267-1274.
14. Papakostas G, Shelton R, Zajecka J, et al. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry. 2014;75(8):855-863.
15. Mech A, Farah A. Correlation of clinical response with homocysteine reduction during therapy with reduced B vitamins in patients with MDD who are positive for MTHFR C677T or A1298C polymorphism: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2016;77(5):668-671.
16. Godfrey P, Toone B, Carney M, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
Cognitive impairments in major depression cluster in three patterns
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
Objective neuropsychological tests can be used to subclassify the cognitive symptoms present in patients with major depression into three patterns having implications for treatment responsiveness, Gitte Moos Knudsen, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
“Our data highlight the importance of assessing and targeting cognitive symptoms,” said Dr. Knudsen, the ECNP president and professor of neurology at the University of Copenhagen.
She was a coauthor of the Danish NeuroPharm study, in which 92 antidepressant-free patients with moderate or severe major depressive disorder and 103 healthy controls completed a comprehensive neuropsychological test battery. The testing included a validation study of the EMOTICOM test battery, a novel neuropsychological test battery developed specifically to assess what has been called “hot” cognition, such as emotion processing, social cognition, and affective verbal memory.
Overall, the depressed patients collectively showed moderate increases in measures of guilt and shame, moderate deficits in working and verbal memory, moderately slowed reaction time, and mild to moderate negative affective bias, compared with controls. No correlation was found between performance on any of the individual cognitive domains and depression severity as measured using the Hamilton Depression Rating Scale, underscoring the concept that cognitive impairment is a distinct component of depressive pathology rather than an extension of the classic mood and somatic symptoms of major depression.
Cluster analysis revealed three distinct patterns of cognitive impairment in the study population. Unlike the individual cognitive domains, these cognitive clusters did correlate with depression severity. The implication is that as they become available.
Investigators classified 38 of the 92 patients with major depressive disorder as falling within Cluster A. That is, they exhibited marked deficits in hot cognition expressed in a greatly impaired ability to accurately identify facial emotions on photographs, with resultant high scores for emotion recognition bias and emotion misattribution bias. This impairment in hot cognition was accompanied by minimal guilt and shame and little or no deficits in the cold cognitive domains of verbal and working memory.
Cluster B, composed of 28 patients, was characterized by generally good cognitive function, with positive biases in emotion processing, near-normal guilt and shame ratings, but moderate deficits across the cold cognition domains, making for a mirror image of Cluster A.
The 26 patients in Cluster C demonstrated large deficits in both the hot and cold cognition domains, with particularly pronounced guilt and shame scores.
The three clusters didn’t differ in terms of age or sex. However, patients in Cluster C had significantly more severe core depressive symptoms as measured by Hamilton scores than in Clusters A and B.
This analysis from the NeuroPharm study was cross-sectional. Dr. Knudsen cited a recent large Chinese longitudinal study to underscore how the prevalence of patient-reported cognitive deficits in major depressive disorder is high. And while those deficits decrease over time, they nonetheless remain substantial after 6 months on antidepressant therapy.
That study included 598 Chinese outpatients with major depressive disorder. At baseline, 77% had cognitive symptoms as evidenced by a total score of 21 or more on the self-rated Perceived Deficits Questionnaire–Depression (PDQ-D). One month after going on antidepressant monotherapy, the prevalence of cognitive symptoms had dropped to 59%. At 2 months, the rate was 45%. And at month 6, a PDQ-D score of 21 or greater was still present in 32.4% of patients. High baseline PDQ-D scores were associated with worse clinical outcomes, including a lower treatment response rate at 1 month and a lower remission rate at 2 months. Moreover, high PDQ-D scores at 2 months were associated with lower remission and higher relapse rates at 6 months.
Dr. Knudsen reported having no financial conflicts regarding the NeuroPharm study, which was conducted free of commercial support. She serves as an adviser to Sage Therapeutics and Sanos.
FROM ECNP 2020
Listening to Tim Ferriss
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Let me tell you about Tim Ferriss. A few years ago, I started reading his best-selling book, The 4-Hour Body. Ferris detailed how he made himself into a one-man experiment – he’d make changes to his diet, checked his weight and his labs, maybe he even had metabolic studies done.
He’d take these measures after soaking in hot baths, then ice baths, and while I admired his discipline, he did lose me during the chapter where he was using steroids. In the end, he advised a dairy-free, low-carbohydrate diet of green vegetables, beans or lentils, and protein for four meals a day, 6 days a week, with free-for-all eating on the 7th day. Then, there was a weight-lifting routine with kettle bells and ice packs to be placed on your shoulders for a set amount of time each day.
I may not remember the program’s details, but something about Ferris fascinated me. He brands himself as being a “human guinea pig,” about “lifestyle design,” and whatever that is, I like it. Perhaps I am attracted to the idea that we might control the trajectories of our generally uncontrollable lives.
Tim Ferriss graduated from Princeton, he’s written five best-selling books and has a popular podcast, he’s been a TED speaker, and he’s been on Fortune’s “40 under 40” list – and there’s so much more. Ferriss is brilliant, innovative, handsome, charismatic, prolific, extraordinarily athletic. I may have forgotten to mention that he was the National Chinese Kickboxing Champion and was a semifinalist in the World Champion Tango competition in Buenos Aires. He’s adventuresome and fearless, and if that isn’t enough, he speaks five languages. In the genetic dice roll, Mr. Ferriss did well, and he’s a driven and energetic hard worker who is open to new experiences.
I subscribe to the Tim Ferriss podcast – as of this writing, there are 466 episodes, with an incredible lineup of interviews with famous and successful guests. I also subscribe to his “5-Bullet Friday” email list where he mentions the interesting things he is reading, watching, learning about, or eating, and the products he is trying – single-ply toilet paper gets a thumbs down – then ends with a thought-provoking quote. This gentleman spends a tremendous amount of time searching and striving, working on himself and his own emotional growth and self-improvement, and yet he still has time for incredible explorations and experiences.
A search for psychic peace
Honestly, were it not for a few little details, I would like to be Tim Ferriss. Who wouldn’t? But what stops me from actually wanting to be Ferriss is that early on while listening to him, I realized that his drive has been fueled by intense psychic pain. He talks openly about being very close to suicide in college, about a tormenting mood disorder, demons to tame, and productivity as an antidote to a fear of failure, not always as a joy for life. There are moments that I have felt so sad for this remarkable stranger. Tim Ferriss is a searcher and what I believe he searches for most is his own psychic peace.
In a forum for psychiatrists, I wish I could write that Ferriss has found solace with our Food and Drug Administration–approved pharmaceuticals and with psychotherapy, but that’s not what he says. What Ferriss has found helpful, however, is psychedelics, and a wide variety of psychological and philosophical teachings ranging from meditation to Stoicism. And most notably, Ferriss has been an advocate for using hallucinogens as a legal medical intervention. Ferriss was one of four philanthropists who donated a total of $17 million to fund the Johns Hopkins Center for Psychedelic & Consciousness Research. He’s helped to move this field forward and to improve its credibility.
On Sept. 14, 2020, Tim Ferriss released a podcast he recorded with his dance partner and close friend, Debbie Millman, and when he recorded it, he was not certain he would release it. None of his usual sponsors endorsed during the podcast. He starts Episode #464 with, “For me, this is the most important podcast episode I’ve ever published. In it, I describe the most life-shaping, certainly the most difficult, and certainly the most transformative journey of my 43 years on this planet. I’ve never shared it before.”
by a babysitter’s son. He worried about how this would affect his family, if they would be left feeling guilty or devastated. He says, “Please note that I expect to be completely overwhelmed emotionally and otherwise when this is published and please understand if I’m not able to reply to any outreach.”
Ferriss and Millman had a long discussion about their sexual abuse as children. Millman was abused by her stepfather at the age of 9, and she talks about confronting him many years later. Ferriss has not confronted his perpetrator, though he has contemplated doing so.
Sexual violence and violation at any age leaves people scarred. In a recent letter to the New York Times in response to President Trump’s words of support to Ghislaine Maxwell, the woman who helped Jeffrey Epstein find his victims, Baltimore psychiatrist Robin Weiss wrote, “Thirty-eight years ago, when I was 32, I was raped. I was married, I was a doctor – you might say I was in pretty stable shape. Yet the shame and guilt I felt were overwhelming. Why didn’t I fight harder? How did I let this happen? I knew better, yet it took me years to overcome those irrational feelings.” These feelings of shame, guilt, self-doubt, and self-blame are nearly universal in survivors of sexual trauma. In children, they can be even worse, as children often don’t have an understanding that what is being done to them is wrong. They lack the language and the maturity to process the events, and ongoing abuse may be accompanied by threats to life of the child or their family members if they tell others, as was the case with Millman. She chose to process her abuse and the consequent difficulties she had by seeking psychiatric care. She took antidepressants and has been in psychoanalysis, both of which she has found to be helpful. Her treatment has tamed her demons, it is ongoing decades later and those demons have not vanished.
Abuse comes back in ‘a tidal wave’
Not surprisingly, Ferriss struggled with whether to make these events public. While so much of his story feels familiar to those of us who help patients process their trauma, it’s not completely typical. Ferriss remembered these episodes of sexual abuse “in high resolution,” while using ayahuasca, a hallucinogen, about 5 years ago. He describes suppressing and discounting these memories until he attended a 10-day silent retreat where he used psilocybin. I found it interesting that Ferriss fasted for 5 days before attending the retreat “to increase the depth of the experience.” He goes on to say, “Around day 6 of this silent retreat, all of this abuse came back to me like a tidal wave and it was replaying as if I was wearing a virtual reality headset. I was immersed, I wasn’t an observer, it was as though I was being traumatized and retraumatized 24/7.” He describes an excruciating and horrifying experience and he referred to it as a “psychotic break.”
Ferriss goes on to talk about how bringing these memories to light has affected him, how it’s explained many of his behaviors and ways of relating in a way that has helped him organize and understand his life.
“It was at the tail end of the retreat that I realized that these 17 seemingly inexplicable behaviors of mine – these vicious cycles or triggers that I had been treating like separate problems to be solved, were all downstream of this trauma. Oh, now that you click that puzzle piece into place, these really strange behaviors – this self-loathing, this rage that was seemingly so exaggerated and disproportionate – leading to the near-suicide I had in college – all these things fell into places making sense.” It gave him a sense of relief but was simultaneously overwhelming.
Both Ferriss and Millman talk about books and treatments that have been helpful to them. Their knowledge of trauma treatments and resources is impressive and can be found at: Tim Ferriss – My Healing Journey After Childhood Abuse (Includes Extensive Resource List). Their wish is to share their suffering as a way to help others, impart hope, and better connect.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatry Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Suicidality jumped in Israel during spring COVID-19 lockdown
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
Suicidality appears to have increased sharply in Israel during the initial nationwide lockdown implemented in response to the COVID-19 pandemic, Gil Zalsman, MD, MHA, reported at the virtual congress of the European College of Neuropsychopharmacology.
He presented highlights from a soon-to-be-published analysis of the content of online chat sessions fielded by a national crisis hotline (Sahar.org.il) during the first 6 months of 2020, compared with January through June 2019, in the pre-COVID-19 era.
It’s far too early to say whether actual deaths tied to suicide rose significantly during the spring lockdown, since medical examiners often take a long time before ruling suicide as cause of death. But this much is clear: The number of suicide-related chat sessions recorded at the volunteer-staffed national hotline during April 2020 was two-and-a-half times greater than in April 2019, and threefold greater in May 2020 than a year earlier, according to Dr. Zalsman, professor of psychiatry at Tel Aviv University and director of the Geha Mental Health Center in Petach Tikva, Israel, where he also directs an adolescent day unit.
The proportion of chats handled at the crisis hotline, many of them concerned with the standard topics – relationships, stress, fears, anxiety, and other non–suicide-related issues – was 48% greater in the first half of 2020, compared with a year earlier. Indeed, the pandemic is putting an enormous strain on crisis hotlines the world over.
“Everybody who is working hotlines knows that they’re falling apart. There are too many calls, too many chats. They need to multiply their volunteers,” Dr. Zalsman said.
The number of suicide-related online chats jumped the week of March 12, when schools closed across Israel and a partial lockdown began. The peak in suicide-related chats occurred beginning the week of April 17, when the forced total lockdown was declared.
“Everything was closed. You couldn’t go out or the police would arrest you,” Dr. Zalsman recalled.
The suicide-related chat count started to drop off in mid-May, when schools reopened, and continued to decline through the end of June.
Only a small percentage of suicide-related chats were deemed by crisis hotline volunteers and their supervisors to be truly life-threatening situations necessitating a call to the police. But the number of such exchanges was significantly greater in April and May 2020 than in January and February, or in April and May 2019.
Use of the crisis hotline is ordinarily skewed toward tech-savvy young people, or as Dr. Zalsman called them, “kids who live inside their computers.” He note that the psychological impact of the pandemic on children and adolescents is largely unexplored research territory to date.
“ You can kill your grandfather by coughing,” Dr. Zalsman said.
Older people also seek help
A finding that he and his coinvestigators didn’t anticipate was the significantly increased use of the service by individuals aged 65 and older during the pandemic. This underscores the increased vulnerability of older people, which stems in part from their heightened risk for severe infection and consequent need for prolonged physical isolation, he said.
The conventional thinking among suicidologists is that during times of crisis – wars, natural disasters – suicidality plunges, then rises quickly afterward.
“People withhold themselves. When there’s a big danger from outside they ignore the danger from inside. And once the danger from outside is gone, they’re left with emptiness, unemployment, economic crisis, and they start” taking their own lives, Dr. Zalsman explained. He expects suicidality to increase after the pandemic, or as the Israeli crisis hotline data suggest, perhaps even during it, for multiple reasons. Patients with preexisting psychiatric disorders are often going untreated. The prolonged physical isolation causes emotional difficulties for some people, especially when accompanied by social isolation and loneliness. There is grief over the loss of friends and relatives because of COVID-19. And there is an expectation of looming economic hardship, with mounting unemployment and bankruptcies.
Dr. Zalsman reported having no financial conflicts regarding his study, conducted free of commercial support.
SOURCE: Zalsman G. ECNP 2020, Session TP.06.
FROM ECNP 2020
Suicide rates up significantly among adolescents, young adults
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.
Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.

Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Suicide rates in young people aged 10-24 years increased significantly in 42 states from 2007-2009 to 2016-2018, according to a recent analysis from the National Center for Health Statistics.

Nationally, the suicide rate jumped 47%, based on the averages for the two 3-year periods, rising from 7.0 per 100,000 persons aged 10-24 years to 10.3 per 100,000. For all ages, the corresponding increase was 47%, Sally C. Curtin, MA, of the NCHS, said in a National Vital Statistics Report.
There was no state with a decrease in suicide rates for adolescents and young adults, as the other eight all had nonsignificant increases, the smallest being 14% in South Dakota. Three-year averages were used to increase statistical power for states with relatively small numbers of deaths but were still not enough to show significance for some large increases, such as the 48% rise in Delaware, Ms. Curtin noted.
In 2016-2018, Alaska’s suicide rate of 31.8 per 100,000 persons aged 10-24 years was the highest in the country, followed by South Dakota (23.6), Montana (23.2), and Wyoming (20.5). New Jersey had the lowest rate at 5.7 per 100,000, with New York and Rhode Island both slightly higher at 5.9 and Connecticut at 6.3, based on data from the National Vital Statistics System.
Even the low numbers, however, hide some large changes, as New Jersey (up by 39%) and New York (up by 44%) were among the 42 states with statistically significant increases, which ranged from 21.7% in Maryland to 110% in New Hampshire, Ms. Curtin said in the report. The increases seen in this analysis contrast with data from the preceding time period, as “the suicide rate among persons aged 10-24 was statistically stable from 2000 to 2007.”
SOURCE: Curtin SC. National Vital Statistics Reports. 2020;69(11)1-9.
Breathing enriched oxygen improves major depression
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
Maybe the hipsters patronizing trendy oxygen bars seeking elevation of mood back in the prepandemic era were actually onto something – because Israeli investigators have now shown in a pilot double-blind, placebo-controlled, randomized trial that breathing enriched oxygen on a nightly basis resulted in clinically meaningful symptomatic improvement in mild to moderate major depression.
“We saw a highly significant effect of normobaric hyperoxia therapy in lowering Hamilton Rating Scale for Depression scores,” R. Haim Belmaker, MD, reported at the virtual congress of the European College of Neuropsychopharmacology.
In addition, the patients on enriched oxygen also showed statistically significant and clinically meaningful improvements relative to sham-treated controls on the secondary endpoints of Clinical Global Impressions Scale, the World Health Organization–Five Well-Being Index, the Sheehan Disability Scale, and the Sense of Coherence Scale, added Dr. Belmaker, professor emeritus of psychiatry at the Ben Gurion University of the Negev in Beersheva, Israel.
Numerous PET imaging studies have documented diminished brain mitochondrial function in patients with depression or schizophrenia. And mitochondria need oxygen to do their work. Yet, the idea of administering enriched oxygen in an effort to boost mitochondrial energy metabolism has long been viewed with skepticism – even though it’s a simple and well-tolerated intervention – because of the fact that 90%-95% of the oxygen supply is carried bound to hemoglobin, and oxygen enrichment doesn’t further increase hemoglobin saturation in individuals with normal lung function. However, recently it has been shown that inspired enriched oxygen roughly doubles arterial oxygen tension, and while this doesn’t translate into anything close to a doubled oxygen supply to tissues, it may result in increased oxygen diffusion into brain tissue, the psychiatrist explained.
Normobaric hyperoxia therapy is not to be confused with hyperbaric oxygen therapy, which requires a special chamber to handle markedly increased atmospheric pressures and has some inherent dangers. Mobile bedside oxygen generator units for oxygen enrichment are commercially available over the counter. Those used in the Israeli study were about the size of a vacuum cleaner and weighed a little more than 40 lb. Much smaller, more convenient units are available as well, but are costlier.
Dr. Belmaker reported on 51 adults with mild or moderate symptoms of major depressive disorder and a mean 11-year disease history who were randomized double blind to breathe either 35% oxygen or normal air – that is, 21% oxygen – at 1 atm pressure delivered from an investigator-supplied oxygen generator through standard plastic nasal prongs at a flow rate of 5 L/min for 7 hours nightly for 1 month.
“Controls heard the same flow and felt the same feeling on the face but were receiving 21% oxygen,” he noted.
Oxygen generator units are capable of enriching air to more than 90% oxygen; however, the investigators wanted to be cautious in a pilot study of an untested therapy, and they found evidence from both animal and human studies that 40% oxygen is reassuringly safe. Study exclusion criteria included obesity, acute or chronic respiratory disease, psychosis, and suicidality.
The primary study endpoint was the change in Hamilton Rating Scale for Depression score at 1 month. From a mean baseline of 14.6, the score in the normobaric hyperoxia group dropped by more than 4 points while remaining unchanged in controls. In a subscale analysis, it was apparent that most of the improvement occurred in the anxiety and cognitive disturbance subscale domains, according to Dr. Belmaker.
Of note, all patients rated by blinded investigators as much improved or very much improved on the Clinical Global Impression scale came from the enriched oxygen group.
No treatment-related adverse events occurred in the study.
“We don’t know the mechanism of the benefit of oxygen on the brain. It’s complex. In stroke and acute MI we used to think oxygen was beneficial, but scientists now feel that it’s not,” the psychiatrist said. “This early data deserve replication with higher concentrations of oxygen, different time periods of application, and in different patient groups.”
He emphasized that, since individuals with physical illnesses – including sleep apnea and chronic obstructive pulmonary disease – were excluded from the study, it’s not possible to say whether normobaric hyperoxia therapy would have an antidepressant effect in such patients.
“I would be especially careful with the normobaric oxygen in any patients with any cardiovascular or hypertensive disease because the increased oxygen pressure can have the side effect of contracting cardiac capillaries as a reflex action. So I certainly cannot recommend applying this study in any patients with a physical disease at this point,” Dr. Belmaker emphasized.
He reported having no financial conflicts regarding the study, funded by a grant from the Brain and Behavior Research Foundation.
SOURCE: Belmaker RH. ECNP 2020, Session S.12.
FROM ECNP 2020
ECG promising for predicting major depression, treatment response
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Individuals with major depressive disorder (MDD) have an increased heart rate – a finding that may have the potential to identify individuals at risk for the disorder and predict treatment response, early research suggests.
Using the rapid-action of the novel antidepressant ketamine and the latest wearable 24-hour electrocardiogram (ECG) technology, investigators found that heart rate could distinguish MDD patients from healthy individuals.
They also found that patients with MDD with the highest resting heart rate had a greater treatment response. In fact, on average, depressed patients had a heart rate that was roughly 10-15 beats per minute higher than healthy controls.
The innovative design of the proof-of-concept study “allowed us to see that average resting heart rate may change quite suddenly to reflect the change in mood,” lead investigator Carmen Schiweck, PhD, Goethe University, Frankfurt am Main, Germany, said in an interview.
These results could have “exciting implications for treatment selection,” and the researchers plan to assess the potential for heart rate to act as an early warning system for depression, they noted.
The findings were presented at the 33rd European College of Neuropsychopharmacology (ECNP) Congress, which was held online this year because of the COVID-19 pandemic.
Identifying trait markers
There have been recent attempts to assess heart rate or heart rate variability (HRV) in patients with MDD to identify trait markers, which are present regardless of the disease phase, or state markers, which are present only during a depressive phase.
However, heart rate and HRV are “highly variable” over a 24-hour cycle, a fact that has been ignored by recent classification efforts, the researchers noted. Moreover, most commonly used antidepressants have a long onset of action, which makes studying their impact on the heart rate challenging.
The researchers’ goal was to determine whether heart rate and HRV could be used as trait markers to distinguish MDD patients from healthy individuals and, through the use of ketamine, whether they can also act as state markers for depression.
For the study, 16 treatment-resistant patients with MDD and 16 age- and sex-matched healthy controls wore a portable ECG device for 4 consecutive days and 3 nights. Heart rate and HRV data were subsequently averaged to obtain a 24-hour ECG.
Participants then received a single infusion of intravenous ketamine for 40 minutes. After waiting for 1 hour, the patients resumed ECG recording for an additional 4 days, with changes in mood assessed using the Hamilton Rating Scale for Depression (HAM-D).
Results showed that, compared with the control group, patients with MDD had a significantly higher 24-hour heart rate (P < .001) and a significantly lower HRV, as measured by the root mean square of successive differences (P < .001).
The investigators also found a reduction in heart rate amplitude, which indicates “significant blunting of circadian rhythm variation throughout the day and less recovery at night.”
Ninety percent accuracy
Harmonic and binary regression showed that heart rate was able to identify those with MDD versus those in the control group, particularly using nighttime readings, with 90.6% accuracy. The data correctly identified 14 (87.5%) patients and 15 (93.8%) members of the control group.
Following treatment, heart rate decreased significantly among the MDD group (P < .001), but there was no significant change in HRV (P = .295).
There was a significant positive association between baseline heart rate and response to treatment on the HAM-D (r = .55; P = .046), which suggested better outcomes in patients with a higher heart rate.
Interestingly, while heart rate was positively correlated with depression severity before treatment (r = .59; P = .03), this relationship disappeared following treatment (r = –0.04; P = .90), suggesting heart rate changes were not linked to depression states.
While heart rate levels may be useful as a trait marker and, potentially, for predicting response to antidepressant treatment, they did not show potential as a state marker, the investigators noted.
They suggested that, while the results need to be confirmed in longitudinal studies, approval of a ketamine nasal spray “may open up new avenues to conceive treatment paradigms, as explored in this study.”
However, “this is a small proof-of-concept study,” the investigators acknowledged. They also point out that only six of the patients with MDD had a reduction in HAM-D scores of at least 30% in response to treatment.
Future research plans
Dr. Schiweck said in an interview that her team was able to identify differences in heart rate and HRV in MDD patients that were not observed in other studies because portable at-home devices allowed them to monitor heart rate continuously over days.
The use of ketamine may also have been advantageous because the Netherlands Study of Depression and Anxiety (NESDA), which was published in 2010, clearly showed that “traditional antidepressants,” in particular tricyclic antidepressants, have a strong influence on heart rate and HRV, Dr. Schiweck said.
because most of the recent studies “just assess patients who are remitted and patients who are currently depressed, but it’s a cross-sectional study design,” said Schiweck.
“If we can follow up the same patients over time then we might really know if it is possible to use heart rate as a state marker for depression,” she added. “That’s what we tried to do with ketamine, but our study was very, very small.”
She noted that the investigators would also like to assess individuals who are “very stressed” and may show some depressive symptoms but don’t yet have a diagnosis of depression.
Mind-body link
Commenting on the findings, Brenda W.J.H. Penninx, MD, PhD, professor of psychiatric epidemiology at the VU University Medical Center in Amsterdam, said the concept of higher heart rate and lower HRV in depression “is indicative of more sympathetic drive and less parasympathetic drive of the autonomic nervous system.”
“That fits with the overall thought that depression is a state with more continuous exposure to stress overactivation of the body, which can be reflected in the [hypothalamic-pituitary-adrenal] axis, leading to higher levels of cortisol stress hormone. But it can also be reflected in the parasympathetic and sympathetic activation of the autonomic nervous system,” she said.
Dr. Penninx, who was also the principal investigator of the NESDA study, was not involved with the current research.
She noted that the NESDA study showed that, when patients with depressive disorder are compared with a healthy controls group, they have a higher heart rate and lower HRV. “But if we then divide people into medicated and nonmedicated people ... then we see that these deviations are only seen in people using medication,” she added.
“Our findings indicate that at least the use of antidepressants is having quite a large impact on autonomic nervous system dysregulation,” Dr. Penninx said. The difference with the current study, she pointed out, is that “it examines the problem over a completely different time scale.”
Although this offers advantages, the current study did not have the large patient numbers that were included in the NESDA study and “they were not clearly able to distinguish the effect of disease from medication,” noted Dr. Penninx.
In addition, this is not an easy area to investigate because there are multiple factors that can mitigate results, including the psychiatric state of the patient, use of medications for both mental illness and cardiometabolic disease, and a patient’s age and gender.
Still, the study clearly illustrates the importance of the interplay between mental health and somatic health and that there is “a very clear indication that we don’t need to separate those,” Dr. Penninx said.
The study was funded by a TGO-IWT Grant from Belgium. The study authors and Dr. Penninx have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Reassuring findings on SSRIs and diabetes risk in children
SSRIs are associated with a much lower risk of type 2 diabetes (T2D) in children and adolescents than previously reported, new research shows.
Investigators found publicly insured patients treated with SSRIs had a 13% increased risk for T2D, compared with those not treated with these agents. In addition, those taking SSRIs continuously (defined as receiving one or more prescriptions every 3 months) had a 33% increased risk of T2D.
On the other hand, privately insured youth had a much lower increased risk – a finding that may be attributable to a lower prevalence of risk factors for T2D in this group.
“We cannot exclude that children and adolescents treated with SSRIs may be at a small increased risk of developing T2D, particularly publicly insured patients, but the magnitude of association was weaker than previous thought and much smaller than other known risk factors for T2DM, such as obesity, race, and poverty,” lead investigator Jenny Sun, PhD, said in an interview.
“When weighing the known benefits and risks of SSRI treatment in children and adolescents, our findings provide reassurance that the risk of T2DM is not as substantial as initially reported,” said Dr. Sun, a postdoctoral research fellow in the department of population medicine at Harvard Medical School’s Harvard Pilgrim Health Care Institute, Boston.
The study was published online Sept. 2 in JAMA Psychiatry.
Limited evidence
Previous research suggested that SSRIs increase the risk of T2D by up to 90% in children and adolescents.
However, the investigators noted, the study reporting this finding was too small to draw conclusions about the SSRI class as a whole also did not examine specific SSRIs.
In addition, although “several studies have reported that antidepressant use may be a risk factor for T2D in adults, evidence was limited in children and adolescents,” said Dr. Sun.
“Rapid changes in growth during childhood and adolescents can alter drugs’ pharmacokinetics and pharmacodynamics, so high-quality, age-specific data are needed to inform prescribing decisions,” she said.
For the current study, the researchers analyzed claims data on almost 1.6 million patients aged 10-19 years (58.3% female; mean age, 15.1 years) from two large claims databases.
The analysis focused on those with a diagnosis warranting treatment with an SSRI, including depression, generalized or social anxiety disorder, obsessive compulsive disorder, PTSD, panic disorder, or bulimia nervosa.
The Medicaid Analytic Extract database consisted of 316,178 patients insured through Medicaid or the Children’s Health Insurance Program. The IBM MarketScan database consisted of 211,460 privately insured patients. Patients were followed up for a mean of 2.3 and 2.2 years, respectively.
Patients who initiated SSRI treatment were compared with those with a similar indication but who were not taking an SSRI. Secondary analyses compared new SSRI users with patients who recently initiated treatment with bupropion, which has no metabolic side effects, or with patients who recently initiated psychotherapy.
“In observational data, it is difficult to mimic a placebo group, often used in RCTs [randomized, controlled trials], therefore several comparator groups were explored to broaden our understanding,” said Dr. Sun.
In addition, the researchers compared the individual SSRI medications, using fluoxetine as a comparator.
A wide range of more than 100 potential confounders or “proxies of confounders,” were taken into account, including demographic characteristics, psychiatric diagnoses, metabolic conditions, concomitant medications, and use of health care services.
The researchers conducted two analyses. They included an intention-to-treat (ITT) analysis that was restricted to patients with one or more additional SSRI prescriptions during the 6 months following the index exposure assessment period.
Close monitoring required
An as-treated analysis estimated the association of continuous SSRI treatment (vs. untreated, bupropion treatment, and psychotherapy), with adherence assessed at 3-month intervals.
Initiation and continuation of SSRI treatment in publicly insured patients were both associated with a considerably higher risk of T2D, compared with untreated patients, and a steeper risk, compared with their privately insured counterparts.
For newly treated publicly insured patients initiated on SSRI treatment, the ITT adjusted hazard ratio was 1.13 (95% confidence interval, 1.04-1.22).
There was an even stronger association among continuously treated publicly insured patients, with an as-treated aHR of 1.33 (95% CI, 1.21-1.47). The authors noted that this corresponds to 6.6 additional T2D cases per 10,000 patients continuously treated for at least 2 years.
The association was weaker in privately insured patients (ITT aHR, 1.01; 95% CI, 0.84-1.23; as-treated aHR, 1.10; 95% CI, 0.88-1.36).
The secondary analyses yielded similar findings: When SSRI treatment was compared with psychotherapy, the as-treated aHR for publicly insured patients was 1.44 (95% CI, 1.25-1.65), whereas the aHR for privately insured patients was lower at 1.21 (95% CI, 0.93-1.57)
The investigators found no increased risk when SSRIs were compared with bupropion, and the within-class analysis showed that none of the SSRIs carried an increased hazard of T2D, compared with fluoxetine.
“Publicly insured patients are enrolled in Medicaid and the Children’s Health Insurance Program, whereas privately insured patients are generally covered by their parent’s employer-sponsored insurance,” said Dr. Sun.
“Publicly insured patients are of lower socioeconomic status and represent a population with greater overall medical burden, more comorbidities, and a higher prevalence of risk factors for T2D, such as obesity, at the time of treatment initiation,” she said.
She added that high-risk children and youth should be closely monitored and clinicians should also consider recommending dietary modifications and increased exercise to offset T2D risk.
Useful ‘real-world data’
William Cooper, MD, MPH, professor of pediatrics and health policy at Vanderbilt University Medical Center in Nashville, Tenn., said that the study “provides a fascinating look at risks of SSRI medications in children and adolescents.”
Dr. Cooper, who was not involved with the study, said that the authors “draw from real-world data representing two different populations and carefully consider factors which might confound the associations.”
The results, he said, “provide important benefits for patients, families, and clinicians as they weigh the risks and benefits of using SSRIs for children who need treatment for depression and anxiety disorders.
The study was supported by a training grant from the program in pharmacoepidemiology at the Harvard School of Public Health. Dr. Sun disclosed no relevant financial relationships. Dr. Cooper disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SSRIs are associated with a much lower risk of type 2 diabetes (T2D) in children and adolescents than previously reported, new research shows.
Investigators found publicly insured patients treated with SSRIs had a 13% increased risk for T2D, compared with those not treated with these agents. In addition, those taking SSRIs continuously (defined as receiving one or more prescriptions every 3 months) had a 33% increased risk of T2D.
On the other hand, privately insured youth had a much lower increased risk – a finding that may be attributable to a lower prevalence of risk factors for T2D in this group.
“We cannot exclude that children and adolescents treated with SSRIs may be at a small increased risk of developing T2D, particularly publicly insured patients, but the magnitude of association was weaker than previous thought and much smaller than other known risk factors for T2DM, such as obesity, race, and poverty,” lead investigator Jenny Sun, PhD, said in an interview.
“When weighing the known benefits and risks of SSRI treatment in children and adolescents, our findings provide reassurance that the risk of T2DM is not as substantial as initially reported,” said Dr. Sun, a postdoctoral research fellow in the department of population medicine at Harvard Medical School’s Harvard Pilgrim Health Care Institute, Boston.
The study was published online Sept. 2 in JAMA Psychiatry.
Limited evidence
Previous research suggested that SSRIs increase the risk of T2D by up to 90% in children and adolescents.
However, the investigators noted, the study reporting this finding was too small to draw conclusions about the SSRI class as a whole also did not examine specific SSRIs.
In addition, although “several studies have reported that antidepressant use may be a risk factor for T2D in adults, evidence was limited in children and adolescents,” said Dr. Sun.
“Rapid changes in growth during childhood and adolescents can alter drugs’ pharmacokinetics and pharmacodynamics, so high-quality, age-specific data are needed to inform prescribing decisions,” she said.
For the current study, the researchers analyzed claims data on almost 1.6 million patients aged 10-19 years (58.3% female; mean age, 15.1 years) from two large claims databases.
The analysis focused on those with a diagnosis warranting treatment with an SSRI, including depression, generalized or social anxiety disorder, obsessive compulsive disorder, PTSD, panic disorder, or bulimia nervosa.
The Medicaid Analytic Extract database consisted of 316,178 patients insured through Medicaid or the Children’s Health Insurance Program. The IBM MarketScan database consisted of 211,460 privately insured patients. Patients were followed up for a mean of 2.3 and 2.2 years, respectively.
Patients who initiated SSRI treatment were compared with those with a similar indication but who were not taking an SSRI. Secondary analyses compared new SSRI users with patients who recently initiated treatment with bupropion, which has no metabolic side effects, or with patients who recently initiated psychotherapy.
“In observational data, it is difficult to mimic a placebo group, often used in RCTs [randomized, controlled trials], therefore several comparator groups were explored to broaden our understanding,” said Dr. Sun.
In addition, the researchers compared the individual SSRI medications, using fluoxetine as a comparator.
A wide range of more than 100 potential confounders or “proxies of confounders,” were taken into account, including demographic characteristics, psychiatric diagnoses, metabolic conditions, concomitant medications, and use of health care services.
The researchers conducted two analyses. They included an intention-to-treat (ITT) analysis that was restricted to patients with one or more additional SSRI prescriptions during the 6 months following the index exposure assessment period.
Close monitoring required
An as-treated analysis estimated the association of continuous SSRI treatment (vs. untreated, bupropion treatment, and psychotherapy), with adherence assessed at 3-month intervals.
Initiation and continuation of SSRI treatment in publicly insured patients were both associated with a considerably higher risk of T2D, compared with untreated patients, and a steeper risk, compared with their privately insured counterparts.
For newly treated publicly insured patients initiated on SSRI treatment, the ITT adjusted hazard ratio was 1.13 (95% confidence interval, 1.04-1.22).
There was an even stronger association among continuously treated publicly insured patients, with an as-treated aHR of 1.33 (95% CI, 1.21-1.47). The authors noted that this corresponds to 6.6 additional T2D cases per 10,000 patients continuously treated for at least 2 years.
The association was weaker in privately insured patients (ITT aHR, 1.01; 95% CI, 0.84-1.23; as-treated aHR, 1.10; 95% CI, 0.88-1.36).
The secondary analyses yielded similar findings: When SSRI treatment was compared with psychotherapy, the as-treated aHR for publicly insured patients was 1.44 (95% CI, 1.25-1.65), whereas the aHR for privately insured patients was lower at 1.21 (95% CI, 0.93-1.57)
The investigators found no increased risk when SSRIs were compared with bupropion, and the within-class analysis showed that none of the SSRIs carried an increased hazard of T2D, compared with fluoxetine.
“Publicly insured patients are enrolled in Medicaid and the Children’s Health Insurance Program, whereas privately insured patients are generally covered by their parent’s employer-sponsored insurance,” said Dr. Sun.
“Publicly insured patients are of lower socioeconomic status and represent a population with greater overall medical burden, more comorbidities, and a higher prevalence of risk factors for T2D, such as obesity, at the time of treatment initiation,” she said.
She added that high-risk children and youth should be closely monitored and clinicians should also consider recommending dietary modifications and increased exercise to offset T2D risk.
Useful ‘real-world data’
William Cooper, MD, MPH, professor of pediatrics and health policy at Vanderbilt University Medical Center in Nashville, Tenn., said that the study “provides a fascinating look at risks of SSRI medications in children and adolescents.”
Dr. Cooper, who was not involved with the study, said that the authors “draw from real-world data representing two different populations and carefully consider factors which might confound the associations.”
The results, he said, “provide important benefits for patients, families, and clinicians as they weigh the risks and benefits of using SSRIs for children who need treatment for depression and anxiety disorders.
The study was supported by a training grant from the program in pharmacoepidemiology at the Harvard School of Public Health. Dr. Sun disclosed no relevant financial relationships. Dr. Cooper disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SSRIs are associated with a much lower risk of type 2 diabetes (T2D) in children and adolescents than previously reported, new research shows.
Investigators found publicly insured patients treated with SSRIs had a 13% increased risk for T2D, compared with those not treated with these agents. In addition, those taking SSRIs continuously (defined as receiving one or more prescriptions every 3 months) had a 33% increased risk of T2D.
On the other hand, privately insured youth had a much lower increased risk – a finding that may be attributable to a lower prevalence of risk factors for T2D in this group.
“We cannot exclude that children and adolescents treated with SSRIs may be at a small increased risk of developing T2D, particularly publicly insured patients, but the magnitude of association was weaker than previous thought and much smaller than other known risk factors for T2DM, such as obesity, race, and poverty,” lead investigator Jenny Sun, PhD, said in an interview.
“When weighing the known benefits and risks of SSRI treatment in children and adolescents, our findings provide reassurance that the risk of T2DM is not as substantial as initially reported,” said Dr. Sun, a postdoctoral research fellow in the department of population medicine at Harvard Medical School’s Harvard Pilgrim Health Care Institute, Boston.
The study was published online Sept. 2 in JAMA Psychiatry.
Limited evidence
Previous research suggested that SSRIs increase the risk of T2D by up to 90% in children and adolescents.
However, the investigators noted, the study reporting this finding was too small to draw conclusions about the SSRI class as a whole also did not examine specific SSRIs.
In addition, although “several studies have reported that antidepressant use may be a risk factor for T2D in adults, evidence was limited in children and adolescents,” said Dr. Sun.
“Rapid changes in growth during childhood and adolescents can alter drugs’ pharmacokinetics and pharmacodynamics, so high-quality, age-specific data are needed to inform prescribing decisions,” she said.
For the current study, the researchers analyzed claims data on almost 1.6 million patients aged 10-19 years (58.3% female; mean age, 15.1 years) from two large claims databases.
The analysis focused on those with a diagnosis warranting treatment with an SSRI, including depression, generalized or social anxiety disorder, obsessive compulsive disorder, PTSD, panic disorder, or bulimia nervosa.
The Medicaid Analytic Extract database consisted of 316,178 patients insured through Medicaid or the Children’s Health Insurance Program. The IBM MarketScan database consisted of 211,460 privately insured patients. Patients were followed up for a mean of 2.3 and 2.2 years, respectively.
Patients who initiated SSRI treatment were compared with those with a similar indication but who were not taking an SSRI. Secondary analyses compared new SSRI users with patients who recently initiated treatment with bupropion, which has no metabolic side effects, or with patients who recently initiated psychotherapy.
“In observational data, it is difficult to mimic a placebo group, often used in RCTs [randomized, controlled trials], therefore several comparator groups were explored to broaden our understanding,” said Dr. Sun.
In addition, the researchers compared the individual SSRI medications, using fluoxetine as a comparator.
A wide range of more than 100 potential confounders or “proxies of confounders,” were taken into account, including demographic characteristics, psychiatric diagnoses, metabolic conditions, concomitant medications, and use of health care services.
The researchers conducted two analyses. They included an intention-to-treat (ITT) analysis that was restricted to patients with one or more additional SSRI prescriptions during the 6 months following the index exposure assessment period.
Close monitoring required
An as-treated analysis estimated the association of continuous SSRI treatment (vs. untreated, bupropion treatment, and psychotherapy), with adherence assessed at 3-month intervals.
Initiation and continuation of SSRI treatment in publicly insured patients were both associated with a considerably higher risk of T2D, compared with untreated patients, and a steeper risk, compared with their privately insured counterparts.
For newly treated publicly insured patients initiated on SSRI treatment, the ITT adjusted hazard ratio was 1.13 (95% confidence interval, 1.04-1.22).
There was an even stronger association among continuously treated publicly insured patients, with an as-treated aHR of 1.33 (95% CI, 1.21-1.47). The authors noted that this corresponds to 6.6 additional T2D cases per 10,000 patients continuously treated for at least 2 years.
The association was weaker in privately insured patients (ITT aHR, 1.01; 95% CI, 0.84-1.23; as-treated aHR, 1.10; 95% CI, 0.88-1.36).
The secondary analyses yielded similar findings: When SSRI treatment was compared with psychotherapy, the as-treated aHR for publicly insured patients was 1.44 (95% CI, 1.25-1.65), whereas the aHR for privately insured patients was lower at 1.21 (95% CI, 0.93-1.57)
The investigators found no increased risk when SSRIs were compared with bupropion, and the within-class analysis showed that none of the SSRIs carried an increased hazard of T2D, compared with fluoxetine.
“Publicly insured patients are enrolled in Medicaid and the Children’s Health Insurance Program, whereas privately insured patients are generally covered by their parent’s employer-sponsored insurance,” said Dr. Sun.
“Publicly insured patients are of lower socioeconomic status and represent a population with greater overall medical burden, more comorbidities, and a higher prevalence of risk factors for T2D, such as obesity, at the time of treatment initiation,” she said.
She added that high-risk children and youth should be closely monitored and clinicians should also consider recommending dietary modifications and increased exercise to offset T2D risk.
Useful ‘real-world data’
William Cooper, MD, MPH, professor of pediatrics and health policy at Vanderbilt University Medical Center in Nashville, Tenn., said that the study “provides a fascinating look at risks of SSRI medications in children and adolescents.”
Dr. Cooper, who was not involved with the study, said that the authors “draw from real-world data representing two different populations and carefully consider factors which might confound the associations.”
The results, he said, “provide important benefits for patients, families, and clinicians as they weigh the risks and benefits of using SSRIs for children who need treatment for depression and anxiety disorders.
The study was supported by a training grant from the program in pharmacoepidemiology at the Harvard School of Public Health. Dr. Sun disclosed no relevant financial relationships. Dr. Cooper disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.