User login
Oral lichen planus prevalence estimates go global
for the general population and 0.98% among clinical patients.
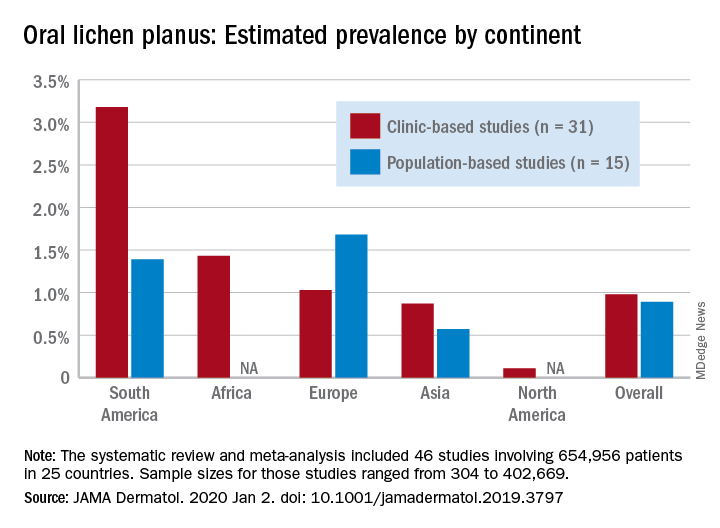
Globally, oral lichen planus (OLP) appears to be more prevalent in women than men (1.55% vs. 1.11% in population-based studies; 1.69% vs. 1.09% in clinic-based), in those aged 40 years and older (1.90% vs. 0.62% in clinic-based studies), and in non-Asian countries (see graph), Changchang Li, MD, and associates reported in JAMA Dermatology.
Of the 25 countries represented among the 46 included studies, Brazil had the highest OLP prevalence at 6.04% and India had the lowest at 0.02%. “Smokers and patients who abuse alcohol have a higher prevalence of OLP. This factor may explain why the highest prevalence … was found in Brazil, where 18.18% of residents report being smokers and 29.09% report consumption of alcoholic beverages,” wrote Dr. Li of the department of dermatology at Zhejiang University of Traditional Chinese Medicine, Wenzhou, China, and associates.
The difference in OLP prevalence by sex may be related to fluctuating female hormone levels, “especially during menstruation or menopause, and that different social roles may lead to the body being in a state of stress,” the investigators suggested.
The age-related difference in OLP could be the result of “longstanding oral habits” or changes to the oral mucosa over time, such as mucosal thinning, decreased elasticity, less saliva secretion, and greater tissue permeability. The higher prevalence among those aged 40 years and older also may be “associated with metabolic changes during aging or with decreased immunity, nutritional deficiencies, medication use, or denture wear,” they wrote.
The review and meta-analysis involved 15 studies (n = 462,993) that included general population data and 31 (n = 191,963) that used information from clinical patients. Sample sizes for those studies ranged from 308 to 402,669.
Statistically significant publication bias was seen among the clinic-based studies but not those that were population based, Dr. Li and associates wrote, adding that “our findings should be considered with caution because of the high heterogeneity of the included studies.”
The study was funded by the First-Class Discipline Construction Foundation of Guangzhou University of Chinese Medicine, the Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High-level Talents, and the Youth Research and Cultivation Project of Guangzhou University of Chinese Medicine. The investigators did not report any conflicts of interest.
SOURCE: C Li et al. JAMA Dermatol. 2020 Jan 2. doi: 10.1001/jamadermatol.2019.3797.
for the general population and 0.98% among clinical patients.

Globally, oral lichen planus (OLP) appears to be more prevalent in women than men (1.55% vs. 1.11% in population-based studies; 1.69% vs. 1.09% in clinic-based), in those aged 40 years and older (1.90% vs. 0.62% in clinic-based studies), and in non-Asian countries (see graph), Changchang Li, MD, and associates reported in JAMA Dermatology.
Of the 25 countries represented among the 46 included studies, Brazil had the highest OLP prevalence at 6.04% and India had the lowest at 0.02%. “Smokers and patients who abuse alcohol have a higher prevalence of OLP. This factor may explain why the highest prevalence … was found in Brazil, where 18.18% of residents report being smokers and 29.09% report consumption of alcoholic beverages,” wrote Dr. Li of the department of dermatology at Zhejiang University of Traditional Chinese Medicine, Wenzhou, China, and associates.
The difference in OLP prevalence by sex may be related to fluctuating female hormone levels, “especially during menstruation or menopause, and that different social roles may lead to the body being in a state of stress,” the investigators suggested.
The age-related difference in OLP could be the result of “longstanding oral habits” or changes to the oral mucosa over time, such as mucosal thinning, decreased elasticity, less saliva secretion, and greater tissue permeability. The higher prevalence among those aged 40 years and older also may be “associated with metabolic changes during aging or with decreased immunity, nutritional deficiencies, medication use, or denture wear,” they wrote.
The review and meta-analysis involved 15 studies (n = 462,993) that included general population data and 31 (n = 191,963) that used information from clinical patients. Sample sizes for those studies ranged from 308 to 402,669.
Statistically significant publication bias was seen among the clinic-based studies but not those that were population based, Dr. Li and associates wrote, adding that “our findings should be considered with caution because of the high heterogeneity of the included studies.”
The study was funded by the First-Class Discipline Construction Foundation of Guangzhou University of Chinese Medicine, the Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High-level Talents, and the Youth Research and Cultivation Project of Guangzhou University of Chinese Medicine. The investigators did not report any conflicts of interest.
SOURCE: C Li et al. JAMA Dermatol. 2020 Jan 2. doi: 10.1001/jamadermatol.2019.3797.
for the general population and 0.98% among clinical patients.

Globally, oral lichen planus (OLP) appears to be more prevalent in women than men (1.55% vs. 1.11% in population-based studies; 1.69% vs. 1.09% in clinic-based), in those aged 40 years and older (1.90% vs. 0.62% in clinic-based studies), and in non-Asian countries (see graph), Changchang Li, MD, and associates reported in JAMA Dermatology.
Of the 25 countries represented among the 46 included studies, Brazil had the highest OLP prevalence at 6.04% and India had the lowest at 0.02%. “Smokers and patients who abuse alcohol have a higher prevalence of OLP. This factor may explain why the highest prevalence … was found in Brazil, where 18.18% of residents report being smokers and 29.09% report consumption of alcoholic beverages,” wrote Dr. Li of the department of dermatology at Zhejiang University of Traditional Chinese Medicine, Wenzhou, China, and associates.
The difference in OLP prevalence by sex may be related to fluctuating female hormone levels, “especially during menstruation or menopause, and that different social roles may lead to the body being in a state of stress,” the investigators suggested.
The age-related difference in OLP could be the result of “longstanding oral habits” or changes to the oral mucosa over time, such as mucosal thinning, decreased elasticity, less saliva secretion, and greater tissue permeability. The higher prevalence among those aged 40 years and older also may be “associated with metabolic changes during aging or with decreased immunity, nutritional deficiencies, medication use, or denture wear,” they wrote.
The review and meta-analysis involved 15 studies (n = 462,993) that included general population data and 31 (n = 191,963) that used information from clinical patients. Sample sizes for those studies ranged from 308 to 402,669.
Statistically significant publication bias was seen among the clinic-based studies but not those that were population based, Dr. Li and associates wrote, adding that “our findings should be considered with caution because of the high heterogeneity of the included studies.”
The study was funded by the First-Class Discipline Construction Foundation of Guangzhou University of Chinese Medicine, the Young Top Talent Project of Scientific and Technological Innovation in Special Support Plan for Training High-level Talents, and the Youth Research and Cultivation Project of Guangzhou University of Chinese Medicine. The investigators did not report any conflicts of interest.
SOURCE: C Li et al. JAMA Dermatol. 2020 Jan 2. doi: 10.1001/jamadermatol.2019.3797.
FROM JAMA DERMATOLOGY
Experts in Europe issue guidance on atopic dermatitis in pregnancy
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Papules on hands and soles
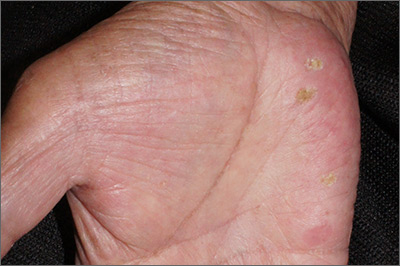
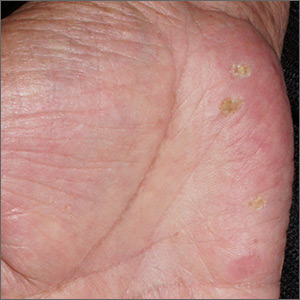
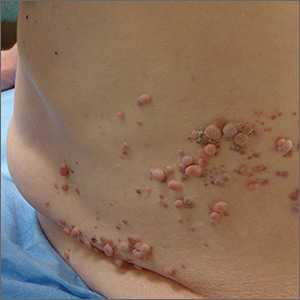
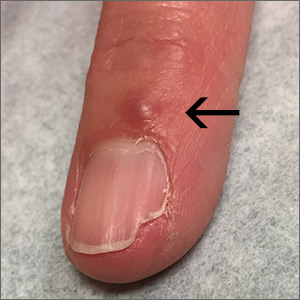
Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.

Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Although these lesions were consistent with actinic keratosis, the location on the palms and the patient’s history of growing up on a ranch in northern Mexico (where he said he was exposed to high arsenic levels in the water and soil), suggested that this was a case of arsenical keratosis. A biopsy excluded frank squamous cell carcinoma; further testing was not needed to make the diagnosis.
While both actinic and arsenical keratoses are precancerous growths with delayed presentations of 20 to 30 years, arsenical keratoses do not typically appear in sun-exposed areas. Rather, these lesions appear on a patient’s palms or soles. They also may present as plate-like hyperpigmentation of the palms and soles or corn-like keratotic papules.
Arsenic occurs naturally in soil and groundwater worldwide and acts as a toxin and carcinogen. It accumulates in the skin, hair, nails, and teeth—making these sites markers of chronic disease. Chronic exposure leads to skin and lung cancer. Cancer of other organs also is possible. Peripheral neuropathy and pancytopenia are hallmarks of chronic exposure.
If arsenical keratosis is suspected, testing for arsenic levels in water or in the work environment is recommended. That said, arsenic exposure is often remote (given the time that has elapsed) and hard to prove.
A blood count and renal and liver function tests should be considered to assess for organ damage. In this case, these tests were normal and the patient was treated with cryotherapy and monitored for recurrence with annual skin exams. Oral retinoids, such as acitretin, have had success in suppressing the frequency and severity of episodes of new lesions in small case reports.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.
US Department of Health and Human Services Agency for Toxic Substances and Disease Registry. Arsenic Toxicity. https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=0. Updated January 15, 2010. Accessed January 2, 2020.
Dupilumab-induced head and neck erythema described in atopic dermatitis patients
MADRID – A growing recognition that from their background skin disease emerged as a hot topic of discussion at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“During treatment with dupilumab, we saw something that is really different from the classic eczema that patients experienced prior to dupilumab, with no or minimal scaling, itch, or burning sensation. We do not believe this is a delayed effect of dupilumab on that specific region. We think this is a dupilumab-induced entity that we’re looking at. You should take home, in my opinion, that this is a common side effect that’s underreported in daily practice at this moment, and it’s not reported in clinical trials at all,” said Linde de Wijs, MD, of the department of dermatology, Erasmus University Medical Center in Rotterdam, the Netherlands.
She presented a detailed case series of seven affected patients which included histologic examination of lesional skin biopsies. The biopsies were characterized by a perivascular lymphohistiocytic infiltrate, an increase in ectatic capillaries in the papillary dermis, and a notable dearth of spongiosis, eosinophils, and neutrophils. Four patients had bulbous elongated rete ridges evocative of a psoriasiform dermatitis. The overall histologic picture was suggestive of a drug-induced skin reaction.
A striking finding was that, even though AD patients typically place high importance on achieving total clearing of disease on the head and neck, these seven closely studied patients nonetheless rated their treatment satisfaction as 9 out of a possible 10 points. Dr. de Wijs interpreted this as testimony to dupilumab’s potent efficacy and comparatively acceptable safety profile, especially the apparent side effect’s absence of scaling and itch.
“Remember, these are patients with really severe atopic dermatitis who’ve been treated with a lot of immunosuppressants prior to dupilumab,” she said.
Once the investigators began to suspect the existence of a novel dupilumab-induced skin reaction, they conducted a retrospective chart review of more than 150 patients treated with dupilumab (Dupixent) and determined that roughly 30% had developed this distinctive sharply demarcated patchy erythema on the head and neck characterized by absence of itch. The sequence involved clearance of the AD in response to dupilumab, followed by gradual development of the head and neck erythema 10-39 weeks after the start of treatment.
The erythema proved treatment refractory. Dr. de Wijs and her colleagues tried topical corticosteroids, including potent ones, as well as topical tacrolimus, antifungals, antibiotics, emollients, oral steroids, and antihistamines, to no avail. Patch testing to investigate allergic contact dermatitis as a possible etiology was unremarkable.
She hypothesized that, since dupilumab blocks the key signaling pathways for Th2 T-cell differentiation by targeting the interleukin-4 receptor alpha, it’s possible that the biologic promotes a shift towards activation of the Th17 pathway, which might explain the observed histologic findings.
The fact that this erythema wasn’t reported in the major randomized clinical trials of dupilumab underscores the enormous value of clinical practice registries, she said.
“We are not the only ones observing this phenomenon,” noted Dr. de Wijs, citing recently published reports by other investigators (J Am Acad Dermatol. 2020 Jan;82[1]:230-2; JAMA Dermatol. 2019 Jul 1;155[7]:850-2).
Indeed, her talk was immediately followed by a presentation by Sebastien Barbarot, MD, PhD, who reported on a French national retrospective study of head and neck dermatitis arising in patients on dupilumab that was conducted by the French Atopic Dermatitis Network using the organization’s GREAT database. Among 1,000 adult patients with AD treated with the biologic at 29 French centers, 10 developed a de novo head and neck dermatitis, and 32 others experienced more than 50% worsening of eczema signs on the head and neck from baseline beginning about 2 months after starting on dupilumab.
This 4.2% incidence is probably an underestimate, since dermatologists weren’t aware of the phenomenon and didn’t specifically ask patients about it, observed Dr. Barbarot, a dermatologist at the University of Nantes (France).
Among the key findings: No differences in clinical characteristics were found between the de novo and exacerbation groups, nearly half of affected patients had concomitant conjunctivitis, and seven patients discontinued dupilumab because of an intolerable burning sensation on the head/neck.
“I think this condition is quite different from rosacea,” Dr. Barbarot emphasized.
French dermatologists generally turned to topical corticosteroids or topical tacrolimus to treat the face and neck dermatitis, with mixed results; 22 of the 42 patients showed improvement and 8 worsened.
Marjolein de Bruin-Weller, MD, PhD, a dermatologist at Utrecht (the Netherlands) University and head of the Dutch National Eczema Expertise Center, said she and her colleagues have also encountered this dupilumab-related head and neck erythema and are convinced that a subset of affected patients have Malassezia-induced dermatitis with neutrophils present on lesional biopsies. “It responds very well to treatment. I think it’s very important to try itraconazole because sometimes it works,” she said.
Dr. de Wijs replied that she and her coworkers tried 2 weeks of itraconazole in several patients, with no effect. And none of their seven biopsied patients had an increase in neutrophils.
“It might be a very heterogenous polyform entity that we’re now observing,” she commented. Dr. de Bruin-Weller concurred.
Dr. Barbarot said he’d be interested in a formal study of antifungal therapy in patients with dupilumab-related head and neck dermatitis. Mechanistically, it seems plausible that dupilumab-induced activation of the TH17 pathway might lead to proliferation of Malassezia fungus.
Dr. de Wijs and Dr. Barbarot reported having no financial conflicts regarding their respective studies, which were conducted free of commercial sponsorship.
MADRID – A growing recognition that from their background skin disease emerged as a hot topic of discussion at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“During treatment with dupilumab, we saw something that is really different from the classic eczema that patients experienced prior to dupilumab, with no or minimal scaling, itch, or burning sensation. We do not believe this is a delayed effect of dupilumab on that specific region. We think this is a dupilumab-induced entity that we’re looking at. You should take home, in my opinion, that this is a common side effect that’s underreported in daily practice at this moment, and it’s not reported in clinical trials at all,” said Linde de Wijs, MD, of the department of dermatology, Erasmus University Medical Center in Rotterdam, the Netherlands.
She presented a detailed case series of seven affected patients which included histologic examination of lesional skin biopsies. The biopsies were characterized by a perivascular lymphohistiocytic infiltrate, an increase in ectatic capillaries in the papillary dermis, and a notable dearth of spongiosis, eosinophils, and neutrophils. Four patients had bulbous elongated rete ridges evocative of a psoriasiform dermatitis. The overall histologic picture was suggestive of a drug-induced skin reaction.
A striking finding was that, even though AD patients typically place high importance on achieving total clearing of disease on the head and neck, these seven closely studied patients nonetheless rated their treatment satisfaction as 9 out of a possible 10 points. Dr. de Wijs interpreted this as testimony to dupilumab’s potent efficacy and comparatively acceptable safety profile, especially the apparent side effect’s absence of scaling and itch.
“Remember, these are patients with really severe atopic dermatitis who’ve been treated with a lot of immunosuppressants prior to dupilumab,” she said.
Once the investigators began to suspect the existence of a novel dupilumab-induced skin reaction, they conducted a retrospective chart review of more than 150 patients treated with dupilumab (Dupixent) and determined that roughly 30% had developed this distinctive sharply demarcated patchy erythema on the head and neck characterized by absence of itch. The sequence involved clearance of the AD in response to dupilumab, followed by gradual development of the head and neck erythema 10-39 weeks after the start of treatment.
The erythema proved treatment refractory. Dr. de Wijs and her colleagues tried topical corticosteroids, including potent ones, as well as topical tacrolimus, antifungals, antibiotics, emollients, oral steroids, and antihistamines, to no avail. Patch testing to investigate allergic contact dermatitis as a possible etiology was unremarkable.
She hypothesized that, since dupilumab blocks the key signaling pathways for Th2 T-cell differentiation by targeting the interleukin-4 receptor alpha, it’s possible that the biologic promotes a shift towards activation of the Th17 pathway, which might explain the observed histologic findings.
The fact that this erythema wasn’t reported in the major randomized clinical trials of dupilumab underscores the enormous value of clinical practice registries, she said.
“We are not the only ones observing this phenomenon,” noted Dr. de Wijs, citing recently published reports by other investigators (J Am Acad Dermatol. 2020 Jan;82[1]:230-2; JAMA Dermatol. 2019 Jul 1;155[7]:850-2).
Indeed, her talk was immediately followed by a presentation by Sebastien Barbarot, MD, PhD, who reported on a French national retrospective study of head and neck dermatitis arising in patients on dupilumab that was conducted by the French Atopic Dermatitis Network using the organization’s GREAT database. Among 1,000 adult patients with AD treated with the biologic at 29 French centers, 10 developed a de novo head and neck dermatitis, and 32 others experienced more than 50% worsening of eczema signs on the head and neck from baseline beginning about 2 months after starting on dupilumab.
This 4.2% incidence is probably an underestimate, since dermatologists weren’t aware of the phenomenon and didn’t specifically ask patients about it, observed Dr. Barbarot, a dermatologist at the University of Nantes (France).
Among the key findings: No differences in clinical characteristics were found between the de novo and exacerbation groups, nearly half of affected patients had concomitant conjunctivitis, and seven patients discontinued dupilumab because of an intolerable burning sensation on the head/neck.
“I think this condition is quite different from rosacea,” Dr. Barbarot emphasized.
French dermatologists generally turned to topical corticosteroids or topical tacrolimus to treat the face and neck dermatitis, with mixed results; 22 of the 42 patients showed improvement and 8 worsened.
Marjolein de Bruin-Weller, MD, PhD, a dermatologist at Utrecht (the Netherlands) University and head of the Dutch National Eczema Expertise Center, said she and her colleagues have also encountered this dupilumab-related head and neck erythema and are convinced that a subset of affected patients have Malassezia-induced dermatitis with neutrophils present on lesional biopsies. “It responds very well to treatment. I think it’s very important to try itraconazole because sometimes it works,” she said.
Dr. de Wijs replied that she and her coworkers tried 2 weeks of itraconazole in several patients, with no effect. And none of their seven biopsied patients had an increase in neutrophils.
“It might be a very heterogenous polyform entity that we’re now observing,” she commented. Dr. de Bruin-Weller concurred.
Dr. Barbarot said he’d be interested in a formal study of antifungal therapy in patients with dupilumab-related head and neck dermatitis. Mechanistically, it seems plausible that dupilumab-induced activation of the TH17 pathway might lead to proliferation of Malassezia fungus.
Dr. de Wijs and Dr. Barbarot reported having no financial conflicts regarding their respective studies, which were conducted free of commercial sponsorship.
MADRID – A growing recognition that from their background skin disease emerged as a hot topic of discussion at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“During treatment with dupilumab, we saw something that is really different from the classic eczema that patients experienced prior to dupilumab, with no or minimal scaling, itch, or burning sensation. We do not believe this is a delayed effect of dupilumab on that specific region. We think this is a dupilumab-induced entity that we’re looking at. You should take home, in my opinion, that this is a common side effect that’s underreported in daily practice at this moment, and it’s not reported in clinical trials at all,” said Linde de Wijs, MD, of the department of dermatology, Erasmus University Medical Center in Rotterdam, the Netherlands.
She presented a detailed case series of seven affected patients which included histologic examination of lesional skin biopsies. The biopsies were characterized by a perivascular lymphohistiocytic infiltrate, an increase in ectatic capillaries in the papillary dermis, and a notable dearth of spongiosis, eosinophils, and neutrophils. Four patients had bulbous elongated rete ridges evocative of a psoriasiform dermatitis. The overall histologic picture was suggestive of a drug-induced skin reaction.
A striking finding was that, even though AD patients typically place high importance on achieving total clearing of disease on the head and neck, these seven closely studied patients nonetheless rated their treatment satisfaction as 9 out of a possible 10 points. Dr. de Wijs interpreted this as testimony to dupilumab’s potent efficacy and comparatively acceptable safety profile, especially the apparent side effect’s absence of scaling and itch.
“Remember, these are patients with really severe atopic dermatitis who’ve been treated with a lot of immunosuppressants prior to dupilumab,” she said.
Once the investigators began to suspect the existence of a novel dupilumab-induced skin reaction, they conducted a retrospective chart review of more than 150 patients treated with dupilumab (Dupixent) and determined that roughly 30% had developed this distinctive sharply demarcated patchy erythema on the head and neck characterized by absence of itch. The sequence involved clearance of the AD in response to dupilumab, followed by gradual development of the head and neck erythema 10-39 weeks after the start of treatment.
The erythema proved treatment refractory. Dr. de Wijs and her colleagues tried topical corticosteroids, including potent ones, as well as topical tacrolimus, antifungals, antibiotics, emollients, oral steroids, and antihistamines, to no avail. Patch testing to investigate allergic contact dermatitis as a possible etiology was unremarkable.
She hypothesized that, since dupilumab blocks the key signaling pathways for Th2 T-cell differentiation by targeting the interleukin-4 receptor alpha, it’s possible that the biologic promotes a shift towards activation of the Th17 pathway, which might explain the observed histologic findings.
The fact that this erythema wasn’t reported in the major randomized clinical trials of dupilumab underscores the enormous value of clinical practice registries, she said.
“We are not the only ones observing this phenomenon,” noted Dr. de Wijs, citing recently published reports by other investigators (J Am Acad Dermatol. 2020 Jan;82[1]:230-2; JAMA Dermatol. 2019 Jul 1;155[7]:850-2).
Indeed, her talk was immediately followed by a presentation by Sebastien Barbarot, MD, PhD, who reported on a French national retrospective study of head and neck dermatitis arising in patients on dupilumab that was conducted by the French Atopic Dermatitis Network using the organization’s GREAT database. Among 1,000 adult patients with AD treated with the biologic at 29 French centers, 10 developed a de novo head and neck dermatitis, and 32 others experienced more than 50% worsening of eczema signs on the head and neck from baseline beginning about 2 months after starting on dupilumab.
This 4.2% incidence is probably an underestimate, since dermatologists weren’t aware of the phenomenon and didn’t specifically ask patients about it, observed Dr. Barbarot, a dermatologist at the University of Nantes (France).
Among the key findings: No differences in clinical characteristics were found between the de novo and exacerbation groups, nearly half of affected patients had concomitant conjunctivitis, and seven patients discontinued dupilumab because of an intolerable burning sensation on the head/neck.
“I think this condition is quite different from rosacea,” Dr. Barbarot emphasized.
French dermatologists generally turned to topical corticosteroids or topical tacrolimus to treat the face and neck dermatitis, with mixed results; 22 of the 42 patients showed improvement and 8 worsened.
Marjolein de Bruin-Weller, MD, PhD, a dermatologist at Utrecht (the Netherlands) University and head of the Dutch National Eczema Expertise Center, said she and her colleagues have also encountered this dupilumab-related head and neck erythema and are convinced that a subset of affected patients have Malassezia-induced dermatitis with neutrophils present on lesional biopsies. “It responds very well to treatment. I think it’s very important to try itraconazole because sometimes it works,” she said.
Dr. de Wijs replied that she and her coworkers tried 2 weeks of itraconazole in several patients, with no effect. And none of their seven biopsied patients had an increase in neutrophils.
“It might be a very heterogenous polyform entity that we’re now observing,” she commented. Dr. de Bruin-Weller concurred.
Dr. Barbarot said he’d be interested in a formal study of antifungal therapy in patients with dupilumab-related head and neck dermatitis. Mechanistically, it seems plausible that dupilumab-induced activation of the TH17 pathway might lead to proliferation of Malassezia fungus.
Dr. de Wijs and Dr. Barbarot reported having no financial conflicts regarding their respective studies, which were conducted free of commercial sponsorship.
REPORTING FROM THE EADV CONGRESS
Efficacy and safety of lowering dupilumab frequency for AD
Patients with moderate to severe atopic dermatitis who responded well to the approved dupilumab regimen of 300 mg every 2 weeks in pivotal phase 3 monotherapy trials were more likely to have a continued response over the longer term if they maintained this regimen rather than switching to longer dosing intervals or discontinuing the medication.
This finding comes from a 36-week, randomized, double-blind, placebo-controlled trial that enrolled 422 – SOLO 1 and SOLO 2.
The new international study – SOLO-CONTINUE – randomized these patients to continue the original regimen (weekly or every 2 weeks), to receive 300 mg of the biologic medication every 4 or 8 weeks, or to receive placebo.
Patients who continued the original regimen had the most consistent maintenance of treatment effect, while patients on longer dosage intervals or placebo had a dose-dependent reduction in response and no safety advantage. The incidence of treatment-emergent antidrug antibody was lowest with dupilumab weekly or every 2 weeks, and slightly higher with less-frequent dosing intervals, reported Margitta Worm, MD, of the Charité Universitätsmedizin Berlin, and coinvestigators.
“Because administration every 4 weeks or every 8 weeks did not provide an additional safety advantage and was numerically outperformed by administration weekly or every 2 weeks, we believe that it is prudent to adhere to the approved every 2 weeks regimen for adults and avoid less frequent treatment regimens (every 4 weeks or every 8 weeks) for long-term maintenance of efficacy,” they wrote in JAMA Dermatology.
Treatment success in the original SOLO trials was defined as having achieved an Investigator’s Global Assessment score of 0-1, or 75% improvement in Eczema Area and Severity Index Scores (EASI-75). As primary endpoints, SOLO-CONTINUE looked at the mean percentage change in EASI score over the course of the trial, and the percentage of patients who maintained EASI-75 at week 36.
Patients in the SOLO-CONTINUE trial who were randomized to receive dupilumab weekly or every 2 weeks had a mean percent change in EASI score of –0.06%. In contrast, patients assigned to the placebo group had a 21.7% decrease, and those taking the medication at 4- and 8-week intervals had mean changes of –3.84% and –6.84%, respectively. Post hoc analyses showed no apparent difference between dupilumab weekly and every 2 weeks in the maintenance of clinical response, the investigators reported.
Among patients with EASI-75 response at baseline, significantly more patients maintained this response at week 36 than patients receiving placebo, and there was again an apparent dose-dependent response. The percentage with EASI-75 at week 36 was 71.6% with the weekly or every-2-weeks regimen, 58.3% with the 4-week regimen, 54.9% with the 8-week regimen, and 30.4% in the placebo group.
Continuing treatment with 300 mg weekly or every 2 weeks resulted in greater maintenance of response across multiple other clinical endpoints and patient-reported outcomes as well (such as pruritus, atopic dermatitis symptoms, sleep, pain or discomfort, quality of life, and symptoms of anxiety and depression).
The more-frequent regimens also conferred no greater risk than less-frequent administration, and there were no new safety signals over the 36-week trial. Treatment-emergent adverse events (the most common were headache, nasopharyngitis, injection-site reactions, and herpes simplex virus infection) occurred in 70.7% of patients in the weekly or every-2-weeks group, 73.6% in the 4-week group, 75% in the 8-week group, and 81.7% in the placebo group.
Unlike earlier studies, the incidence of conjunctivitis was low (less than 6%) across all treatment groups, possibly because patients in the SOLO-CONTINUE trial were all high-level responders who tend to have conjunctivitis less frequently, the authors wrote.
Patients receiving less-frequent doses of dupilumab, particularly every 8 weeks, had greater rates of skin infections, flares, and rescue medication use than patients receiving doses weekly or every 2 weeks, the investigators reported. Treatment-emergent antidrug antibody incidence was slightly higher with less-frequent doses (11.7% and 6% in the 8-week and 4-week groups, respectively, compared with 4.3% and 1.2% in the every-2-weeks and weekly groups), which indicates a “higher incidence of immunogenicity with less-frequent dosage intervals” and is “consistent with other biologics,” they wrote.
Dupilumab is a human monoclonal antibody against the interleukin-4 receptor alpha that inhibits signaling of IL-4 and IL-13. The study was conducted at 185 sites in North America, Europe, Asia, and Japan. Patients had a mean age of 38.2 years; 53.8% were male.
While the trial suggests that the approved regimen of 300 mg every 2 weeks is best for long-term treatment, “therapeutic decisions are often influenced by cost-benefit considerations, in which case practitioners and other stakeholders involved in these decisions should carefully balance potential savings against suboptimal efficacy and long-term risks associated with discontinuous treatment regimens,” the investigators wrote.
The SOLO-CONTINUE trial was funded by Sanofi and Regeneron, the companies that market dupilumab. Dr. Worm reported receiving honoraria for consulting and lecture activity from Regeneron and Sanofi during and outside the conduct of the study, among other disclosures. The other authors had multiple disclosures related to these and multiple other pharmaceutical companies, or were employees of Sanofi or Regeneron.
SOURCE: Worm M et al. JAMA Dermatol. 2019 Dec 26. doi: 10.1001/jamadermatol.2019.3617.
The desire to decrease or stop a therapy such as dupilumab may be motivated by cost, current and potential adverse effects, and individual needs. Because atopic dermatitis is a waxing-and-waning disease with a predilection for cycles of escalation, there is some thought a priori that reduced treatment schedules or discontinued use of a drug may be possible in a state of low disease activity.
The investigators of the SOLO-CONTINUE trial found, however, that continuous treatment with the dosage used in the original SOLO trials (300 mg weekly or every 2 weeks) resulted in a better maintenance of response than a less-frequent dosage and was significantly better than placebo for all endpoints. The less-frequent dosage regimens (every 4 weeks and every 8 weeks), on the other hand, produced some dose-dependent reduction in efficacy.
The development of antidrug antibodies was found in approximately 11% of individuals who received placebo or dupilumab every 8 weeks, 6% of the monthly treatment group, and only 1% in the weekly group, suggesting that less-frequent administration results in higher immunogenicity. However, most of the antidrug antibody levels were low and did not seem to have any clinical effect, making this finding of uncertain relevance to patient care.
The study is valuable because, as more patients are exposed to the drug, more will want or need to reduce the dosage or stop use over time – and although it seems optimal to continue an every-2-weeks treatment regimen, this may not always be feasible. As we integrate new therapies and learn more about atopic dermatitis, it is important that we understand the options and implications around decreasing the dosage of dupilumab. This newly concluded trial is helpful in this regard.
Peter A. Lio, MD, is with the department of dermatology at Northwestern University, Chicago, and the Chicago Integrative Eczema Center. He reported receiving grants and personal fees from Regeneron, Sanofi Genzyme, and other companies, as well as other disclosures. His comments appear in JAMA Dermatology (2019 Dec 26. doi: 10.1001/jamadermatol.2019.3331).
The desire to decrease or stop a therapy such as dupilumab may be motivated by cost, current and potential adverse effects, and individual needs. Because atopic dermatitis is a waxing-and-waning disease with a predilection for cycles of escalation, there is some thought a priori that reduced treatment schedules or discontinued use of a drug may be possible in a state of low disease activity.
The investigators of the SOLO-CONTINUE trial found, however, that continuous treatment with the dosage used in the original SOLO trials (300 mg weekly or every 2 weeks) resulted in a better maintenance of response than a less-frequent dosage and was significantly better than placebo for all endpoints. The less-frequent dosage regimens (every 4 weeks and every 8 weeks), on the other hand, produced some dose-dependent reduction in efficacy.
The development of antidrug antibodies was found in approximately 11% of individuals who received placebo or dupilumab every 8 weeks, 6% of the monthly treatment group, and only 1% in the weekly group, suggesting that less-frequent administration results in higher immunogenicity. However, most of the antidrug antibody levels were low and did not seem to have any clinical effect, making this finding of uncertain relevance to patient care.
The study is valuable because, as more patients are exposed to the drug, more will want or need to reduce the dosage or stop use over time – and although it seems optimal to continue an every-2-weeks treatment regimen, this may not always be feasible. As we integrate new therapies and learn more about atopic dermatitis, it is important that we understand the options and implications around decreasing the dosage of dupilumab. This newly concluded trial is helpful in this regard.
Peter A. Lio, MD, is with the department of dermatology at Northwestern University, Chicago, and the Chicago Integrative Eczema Center. He reported receiving grants and personal fees from Regeneron, Sanofi Genzyme, and other companies, as well as other disclosures. His comments appear in JAMA Dermatology (2019 Dec 26. doi: 10.1001/jamadermatol.2019.3331).
The desire to decrease or stop a therapy such as dupilumab may be motivated by cost, current and potential adverse effects, and individual needs. Because atopic dermatitis is a waxing-and-waning disease with a predilection for cycles of escalation, there is some thought a priori that reduced treatment schedules or discontinued use of a drug may be possible in a state of low disease activity.
The investigators of the SOLO-CONTINUE trial found, however, that continuous treatment with the dosage used in the original SOLO trials (300 mg weekly or every 2 weeks) resulted in a better maintenance of response than a less-frequent dosage and was significantly better than placebo for all endpoints. The less-frequent dosage regimens (every 4 weeks and every 8 weeks), on the other hand, produced some dose-dependent reduction in efficacy.
The development of antidrug antibodies was found in approximately 11% of individuals who received placebo or dupilumab every 8 weeks, 6% of the monthly treatment group, and only 1% in the weekly group, suggesting that less-frequent administration results in higher immunogenicity. However, most of the antidrug antibody levels were low and did not seem to have any clinical effect, making this finding of uncertain relevance to patient care.
The study is valuable because, as more patients are exposed to the drug, more will want or need to reduce the dosage or stop use over time – and although it seems optimal to continue an every-2-weeks treatment regimen, this may not always be feasible. As we integrate new therapies and learn more about atopic dermatitis, it is important that we understand the options and implications around decreasing the dosage of dupilumab. This newly concluded trial is helpful in this regard.
Peter A. Lio, MD, is with the department of dermatology at Northwestern University, Chicago, and the Chicago Integrative Eczema Center. He reported receiving grants and personal fees from Regeneron, Sanofi Genzyme, and other companies, as well as other disclosures. His comments appear in JAMA Dermatology (2019 Dec 26. doi: 10.1001/jamadermatol.2019.3331).
Patients with moderate to severe atopic dermatitis who responded well to the approved dupilumab regimen of 300 mg every 2 weeks in pivotal phase 3 monotherapy trials were more likely to have a continued response over the longer term if they maintained this regimen rather than switching to longer dosing intervals or discontinuing the medication.
This finding comes from a 36-week, randomized, double-blind, placebo-controlled trial that enrolled 422 – SOLO 1 and SOLO 2.
The new international study – SOLO-CONTINUE – randomized these patients to continue the original regimen (weekly or every 2 weeks), to receive 300 mg of the biologic medication every 4 or 8 weeks, or to receive placebo.
Patients who continued the original regimen had the most consistent maintenance of treatment effect, while patients on longer dosage intervals or placebo had a dose-dependent reduction in response and no safety advantage. The incidence of treatment-emergent antidrug antibody was lowest with dupilumab weekly or every 2 weeks, and slightly higher with less-frequent dosing intervals, reported Margitta Worm, MD, of the Charité Universitätsmedizin Berlin, and coinvestigators.
“Because administration every 4 weeks or every 8 weeks did not provide an additional safety advantage and was numerically outperformed by administration weekly or every 2 weeks, we believe that it is prudent to adhere to the approved every 2 weeks regimen for adults and avoid less frequent treatment regimens (every 4 weeks or every 8 weeks) for long-term maintenance of efficacy,” they wrote in JAMA Dermatology.
Treatment success in the original SOLO trials was defined as having achieved an Investigator’s Global Assessment score of 0-1, or 75% improvement in Eczema Area and Severity Index Scores (EASI-75). As primary endpoints, SOLO-CONTINUE looked at the mean percentage change in EASI score over the course of the trial, and the percentage of patients who maintained EASI-75 at week 36.
Patients in the SOLO-CONTINUE trial who were randomized to receive dupilumab weekly or every 2 weeks had a mean percent change in EASI score of –0.06%. In contrast, patients assigned to the placebo group had a 21.7% decrease, and those taking the medication at 4- and 8-week intervals had mean changes of –3.84% and –6.84%, respectively. Post hoc analyses showed no apparent difference between dupilumab weekly and every 2 weeks in the maintenance of clinical response, the investigators reported.
Among patients with EASI-75 response at baseline, significantly more patients maintained this response at week 36 than patients receiving placebo, and there was again an apparent dose-dependent response. The percentage with EASI-75 at week 36 was 71.6% with the weekly or every-2-weeks regimen, 58.3% with the 4-week regimen, 54.9% with the 8-week regimen, and 30.4% in the placebo group.
Continuing treatment with 300 mg weekly or every 2 weeks resulted in greater maintenance of response across multiple other clinical endpoints and patient-reported outcomes as well (such as pruritus, atopic dermatitis symptoms, sleep, pain or discomfort, quality of life, and symptoms of anxiety and depression).
The more-frequent regimens also conferred no greater risk than less-frequent administration, and there were no new safety signals over the 36-week trial. Treatment-emergent adverse events (the most common were headache, nasopharyngitis, injection-site reactions, and herpes simplex virus infection) occurred in 70.7% of patients in the weekly or every-2-weeks group, 73.6% in the 4-week group, 75% in the 8-week group, and 81.7% in the placebo group.
Unlike earlier studies, the incidence of conjunctivitis was low (less than 6%) across all treatment groups, possibly because patients in the SOLO-CONTINUE trial were all high-level responders who tend to have conjunctivitis less frequently, the authors wrote.
Patients receiving less-frequent doses of dupilumab, particularly every 8 weeks, had greater rates of skin infections, flares, and rescue medication use than patients receiving doses weekly or every 2 weeks, the investigators reported. Treatment-emergent antidrug antibody incidence was slightly higher with less-frequent doses (11.7% and 6% in the 8-week and 4-week groups, respectively, compared with 4.3% and 1.2% in the every-2-weeks and weekly groups), which indicates a “higher incidence of immunogenicity with less-frequent dosage intervals” and is “consistent with other biologics,” they wrote.
Dupilumab is a human monoclonal antibody against the interleukin-4 receptor alpha that inhibits signaling of IL-4 and IL-13. The study was conducted at 185 sites in North America, Europe, Asia, and Japan. Patients had a mean age of 38.2 years; 53.8% were male.
While the trial suggests that the approved regimen of 300 mg every 2 weeks is best for long-term treatment, “therapeutic decisions are often influenced by cost-benefit considerations, in which case practitioners and other stakeholders involved in these decisions should carefully balance potential savings against suboptimal efficacy and long-term risks associated with discontinuous treatment regimens,” the investigators wrote.
The SOLO-CONTINUE trial was funded by Sanofi and Regeneron, the companies that market dupilumab. Dr. Worm reported receiving honoraria for consulting and lecture activity from Regeneron and Sanofi during and outside the conduct of the study, among other disclosures. The other authors had multiple disclosures related to these and multiple other pharmaceutical companies, or were employees of Sanofi or Regeneron.
SOURCE: Worm M et al. JAMA Dermatol. 2019 Dec 26. doi: 10.1001/jamadermatol.2019.3617.
Patients with moderate to severe atopic dermatitis who responded well to the approved dupilumab regimen of 300 mg every 2 weeks in pivotal phase 3 monotherapy trials were more likely to have a continued response over the longer term if they maintained this regimen rather than switching to longer dosing intervals or discontinuing the medication.
This finding comes from a 36-week, randomized, double-blind, placebo-controlled trial that enrolled 422 – SOLO 1 and SOLO 2.
The new international study – SOLO-CONTINUE – randomized these patients to continue the original regimen (weekly or every 2 weeks), to receive 300 mg of the biologic medication every 4 or 8 weeks, or to receive placebo.
Patients who continued the original regimen had the most consistent maintenance of treatment effect, while patients on longer dosage intervals or placebo had a dose-dependent reduction in response and no safety advantage. The incidence of treatment-emergent antidrug antibody was lowest with dupilumab weekly or every 2 weeks, and slightly higher with less-frequent dosing intervals, reported Margitta Worm, MD, of the Charité Universitätsmedizin Berlin, and coinvestigators.
“Because administration every 4 weeks or every 8 weeks did not provide an additional safety advantage and was numerically outperformed by administration weekly or every 2 weeks, we believe that it is prudent to adhere to the approved every 2 weeks regimen for adults and avoid less frequent treatment regimens (every 4 weeks or every 8 weeks) for long-term maintenance of efficacy,” they wrote in JAMA Dermatology.
Treatment success in the original SOLO trials was defined as having achieved an Investigator’s Global Assessment score of 0-1, or 75% improvement in Eczema Area and Severity Index Scores (EASI-75). As primary endpoints, SOLO-CONTINUE looked at the mean percentage change in EASI score over the course of the trial, and the percentage of patients who maintained EASI-75 at week 36.
Patients in the SOLO-CONTINUE trial who were randomized to receive dupilumab weekly or every 2 weeks had a mean percent change in EASI score of –0.06%. In contrast, patients assigned to the placebo group had a 21.7% decrease, and those taking the medication at 4- and 8-week intervals had mean changes of –3.84% and –6.84%, respectively. Post hoc analyses showed no apparent difference between dupilumab weekly and every 2 weeks in the maintenance of clinical response, the investigators reported.
Among patients with EASI-75 response at baseline, significantly more patients maintained this response at week 36 than patients receiving placebo, and there was again an apparent dose-dependent response. The percentage with EASI-75 at week 36 was 71.6% with the weekly or every-2-weeks regimen, 58.3% with the 4-week regimen, 54.9% with the 8-week regimen, and 30.4% in the placebo group.
Continuing treatment with 300 mg weekly or every 2 weeks resulted in greater maintenance of response across multiple other clinical endpoints and patient-reported outcomes as well (such as pruritus, atopic dermatitis symptoms, sleep, pain or discomfort, quality of life, and symptoms of anxiety and depression).
The more-frequent regimens also conferred no greater risk than less-frequent administration, and there were no new safety signals over the 36-week trial. Treatment-emergent adverse events (the most common were headache, nasopharyngitis, injection-site reactions, and herpes simplex virus infection) occurred in 70.7% of patients in the weekly or every-2-weeks group, 73.6% in the 4-week group, 75% in the 8-week group, and 81.7% in the placebo group.
Unlike earlier studies, the incidence of conjunctivitis was low (less than 6%) across all treatment groups, possibly because patients in the SOLO-CONTINUE trial were all high-level responders who tend to have conjunctivitis less frequently, the authors wrote.
Patients receiving less-frequent doses of dupilumab, particularly every 8 weeks, had greater rates of skin infections, flares, and rescue medication use than patients receiving doses weekly or every 2 weeks, the investigators reported. Treatment-emergent antidrug antibody incidence was slightly higher with less-frequent doses (11.7% and 6% in the 8-week and 4-week groups, respectively, compared with 4.3% and 1.2% in the every-2-weeks and weekly groups), which indicates a “higher incidence of immunogenicity with less-frequent dosage intervals” and is “consistent with other biologics,” they wrote.
Dupilumab is a human monoclonal antibody against the interleukin-4 receptor alpha that inhibits signaling of IL-4 and IL-13. The study was conducted at 185 sites in North America, Europe, Asia, and Japan. Patients had a mean age of 38.2 years; 53.8% were male.
While the trial suggests that the approved regimen of 300 mg every 2 weeks is best for long-term treatment, “therapeutic decisions are often influenced by cost-benefit considerations, in which case practitioners and other stakeholders involved in these decisions should carefully balance potential savings against suboptimal efficacy and long-term risks associated with discontinuous treatment regimens,” the investigators wrote.
The SOLO-CONTINUE trial was funded by Sanofi and Regeneron, the companies that market dupilumab. Dr. Worm reported receiving honoraria for consulting and lecture activity from Regeneron and Sanofi during and outside the conduct of the study, among other disclosures. The other authors had multiple disclosures related to these and multiple other pharmaceutical companies, or were employees of Sanofi or Regeneron.
SOURCE: Worm M et al. JAMA Dermatol. 2019 Dec 26. doi: 10.1001/jamadermatol.2019.3617.
FROM JAMA DERMATOLOGY
Papules on trunk
Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.

Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.

Dermatopathologic evaluation of the tissue sample indicated that the lesion was a neurofibroma, and clinical correlation fine-tuned the diagnosis to segmental neurofibromatosis (NF). The diagnosis of segmental NF is clinical, with biopsy to confirm that the lesions are neurofibromas. Segmental NF is a mosaic form of neurofibromatosis type 1 (NF1) that results from a postzygotic mutation of the NF1 gene. While NF1 is a relatively common neurocutaneous disorder that occurs with a frequency of 1 in 3000, segmental NF is uncommon, with an estimated prevalence of 1 in 40,000.
NF1 often follows an autosomal dominant inheritance pattern, although up to 50% of patients with NF1 arise de novo from spontaneous mutations. NF1 is characterized by multiple café-au-lait macules, axillary freckling, neurofibromas, and Lisch nodules (pigmented iris hamartomas). Systemic findings associated with NF1 include malignant peripheral nerve sheath tumors, optic gliomas, and vasculopathy. While patients with segmental NF may exhibit some of these same findings, the distribution of the neurofibromas is often limited to a single dermatome. Additionally, patients with segmental NF typically do not exhibit extracutaneous lesions, systemic involvement, or a family history of NF. Segmental NF treatment typically focuses on symptomatic management or cosmetic concerns.
This patient did not have any of the systemic complications that occasionally occur with segmental NF, so no medical treatment was required. The patient was advised that cutaneous and subcutaneous neurofibromas do not require removal unless there is pain, bleeding, disfigurement, or signs of malignant transformation. The patient was not interested in removal of the nodules for cosmetic reasons, so he was encouraged to seek follow-up care, as needed.
This case was adapted from: Laurent KJ, Beachkofsky TM, Loyd A, et al. Segmental distribution of nodules on trunk. J Fam Pract. 2017;66: 765-767.
Adult atopic dermatitis brings increased osteoporosis risk
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
MADRID – – even if they’ve never taken systemic corticosteroids, according to a large observational Danish national registry study.
A key study finding was that these elevated risks were concentrated in the patients who used potent or superpotent topical corticosteroids. Adult AD patients who used mild- or moderate-potency topical steroids were not at significantly increased risk. Neither were patients on topical calcineurin inhibitors, Jacob P. Thyssen, MD, PhD, reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
“The absolute risk is low, but it’s real,” commented Dr. Thyssen, professor of dermatology at the University of Copenhagen.
His advice to colleagues: “Dermatologists should consider alternative treatments in the chronic excessive users of topical corticosteroids, or use them in combination with prophylactic treatment to preserve bone homeostasis in such patients.”
He presented the results of a retrospective case-control study of 10,636 Danish adults with AD and 87,989 matched controls. At baseline in this study, which featured a maximum of 20 years of follow-up starting in 1997, participants had no history of osteoporosis.
Dr. Thyssen expressed the absolute risk of being diagnosed with osteoporosis in the study as follows: If 10,000 adult AD patients were followed for 1 year, on average 23.5 of them would be diagnosed with osteoporosis, a rate more than double the 10.3 per 10,000 in the general population. Moreover, on average, 42.6 out of 10,000 adult AD patients would incur a major osteoporotic fracture during a year of follow-up, compared with 32.3 individuals in the general population.
In the subgroup of patients who never used systemic corticosteroids, the risk of being diagnosed with osteoporosis was 12.8 per 10,000 per year, significantly higher than the 7.4 per 10,000 rate in the general population. Similarly, the 1-year rate of major osteoporotic fractures was 33.1 per 10,000 among the AD group and 29.6 in matched controls.
In a Cox regression analysis adjusted for age, sex, socioeconomic status, body mass index, asthma, and the use of a variety of medications thought to potentially have a negative effect upon bone metabolism, the risk of osteoporosis in the entire group of 10,636 adult AD patients was 51% greater than in matched controls, and their risk of major osteoporotic fractures was 18% greater. In the subgroup of AD patients who never used systemic steroids, the risks of osteoporosis and major osteoporotic fractures were 82% and 14% greater than in controls. The medications adjusted for in the regression analysis included proton pump inhibitors, thiazide diuretics, H2 receptor blockers, statins, cyclosporine, hormone therapy, contraceptives, and psychotropic medications.
Scoring Atopic Dermatitis (SCORAD) ratings were available on roughly 4,000 of the adult AD patients. In an analysis of this large subgroup, disease severity as reflected in SCORAD scores did not explain the increased osteoporosis and fracture risks. However, the use of potent or superpotent topical corticosteroids did. Patients who used potent topical steroids had a statistically significant 16% increased risk of being diagnosed with osteoporosis than nonusers, as well as a 7% increased risk of major osteoporotic fractures. Patients who applied superpotent topical steroids had 42% and 18% increased risks of those two adverse outcomes.
In contrast, neither the use of topical calcineurin inhibitors nor mild- or mid-potency topical steroids was associated with increased risk of bone events in a Cox regression analysis adjusted for potential confounders.
A relationship between the use of high-potency topical corticosteroids and adverse bone events is biologically plausible, according to Dr. Thyssen. He and his coinvestigators have previously documented a 100%-400% increased rate of chemical penetration through atopic skin, which is notoriously barrier damaged.
“We find it very likely that, if you put topical steroids on atopic skin in high amounts and for a very long time, you may have systemic effects,” he said.
A great many adult AD patients do exactly that. When Dr. Thyssen and coworkers analyzed Danish national prescription drug registry data for their patient cohort, they found that roughly one-third of the elderly subgroup had filled prescriptions totaling greater than 2 kg of mometasone or other similar-potency steroids over the previous 10 years.
“So we know that a significant proportion of our atopic dermatitis patients are really high users of topical corticosteroids,” the dermatologist noted.
Dr. Thyssen’s national osteoporosis and fracture study was funded with a government research grant. He reported serving as an advisor to and/or recipient of research grants from AbbVie, Pfizer, Leo Pharma, Eli Lilly, Regeneron, Sanofi Genzyme, and Union Therapeutics.
REPORTING FROM EADV 2019
Calif. woman poisoned by methylmercury-containing skin cream
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
FROM MMWR
Fingernail deformity
The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The groove in the patient’s nail, and associated splitting, was due to a digital mucous cyst seen in the photo proximal to the cuticle.
In spite of the name, these cysts do not contain mucus. Instead, they contain synovial fluid and represent an outpouching of the distal interphalangeal joint of the index finger. This same process is called a ganglion cyst when it is seen at the wrist and a Baker’s cyst when it is behind the knee. These cysts typically form from an increase in synovial fluid, often due to underlying osteoarthritis, injury, or degenerative changes of the joint capsule. If the patient is asymptomatic, no treatment is required. Half of the lesions spontaneously resolve. If the patient is symptomatic, treatment options include a steroid injection; aspirating the cyst and then using a compressive cryosurgery tip to freeze the superficial and deep aspects of the cyst to scar it down; and surgical resection of the cyst.
In this case, the patient was in pain because the abnormally shaped nail was breaking. She elected to have a steroid injection into the cyst—0.25 ml of triamcinolone 20 mg/ml. The family physician (FP) advised her that this would reduce the inflammation in the distal interphalangeal joint, hopefully reducing the size of the digital mucous cyst. The FP also indicated that she might require several injections to achieve a positive outcome and that it would take 6 months for her fingernail to return to normal.
Images and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Which children are at greatest risk for atopic dermatitis?
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
MADRID – A parental history of asthma or allergic rhinitis significantly increases the risk that a child will develop atopic dermatitis, and that risk doubles if a parent has a history of atopic dermatitis rather than another atopic disease, Nina H. Ravn reported at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
She presented a comprehensive meta-analysis of 149 published studies addressing the risk of developing atopic dermatitis according to parental history of atopic disease. The studies included more than 656,000 participants. The picture that emerged from the meta-analysis was one of a stepwise increase in the risk of pediatric atopic dermatitis according to the type and number of parental atopic diseases present.
“This is something that hopefully can be useful when you talk with parents or parents-to-be with atopic diseases and they want to know how their disease might affect their child,” explained Ms. Ravn of the University of Copenhagen.
It’s also information that clinicians will find helpful in appropriately targeting primary prevention interventions if and when methods of proven efficacy become available. That’s a likely prospect, as this is now an extremely active field of research, she noted.
The meta-analysis showed that a parental history of atopic dermatitis was associated with a 3.3-fold greater risk of atopic dermatitis in the offspring than in families without a parental history of atopy. A parental history of asthma was associated with a 1.56-fold increased risk, while allergic rhinitis in a parent was linked to a 1.68-fold increased risk.
“It does matter what type of atopic disease the parents have,” she observed. “Those with a parental history of asthma or allergic rhinitis can be considered as being at more of an intermediate risk level, while those with a parental history of atopic dermatitis are a particularly high risk group.”
Of note, the risk of pediatric atopic dermatitis was the same regardless of whether the father or mother was the one with a history of atopic disease. If one parent had a history of an atopic disease, the pediatric risk was increased 1.3-fold compared to when the parental history was negative. If both parents had a history of atopic illness, the risk jumped to 2.08-fold. And if one parent had a history of more than one form of atopic disease, the pediatric risk of atopic dermatitis was increased 2.32-fold.
“An interesting result that was new to me what that fathers’ and mothers’ contribution to risk is equal,” said session cochair Andreas Wollenberg, MD, professor of dermatology at Ludwig Maximilian University of Munich. “For the past 2 decades we were always taught that the mother would have a greater impact on that risk.”
“I was also surprised by our findings,” Ms. Ravn replied. “But when we pooled all the data there really was no difference, nor in any of our subanalyses.”
She reported having no financial conflicts regarding her study.
SOURCE: Ravn NH. THE EADV CONGRESS.
REPORTING FROM The EADV CONGRESS
Key clinical point: Pediatric atopic dermatitis risk varies according to type of parental history of atopic disease.
Major finding: A parental history of atopic dermatitis is associated with a 3.3-fold increased risk of atopic dermatitis in the child, twice the risk associated with parental asthma or allergic rhinitis.
Study details: This was a systematic review and meta-analysis of 149 published studies with 656,711 participants.
Disclosures: The presenter reported having no financial conflicts regarding the study, conducted free of commercial support.
Source: Ravn NH. The EADV Congress.