User login
How best to approach these acute hand infections
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
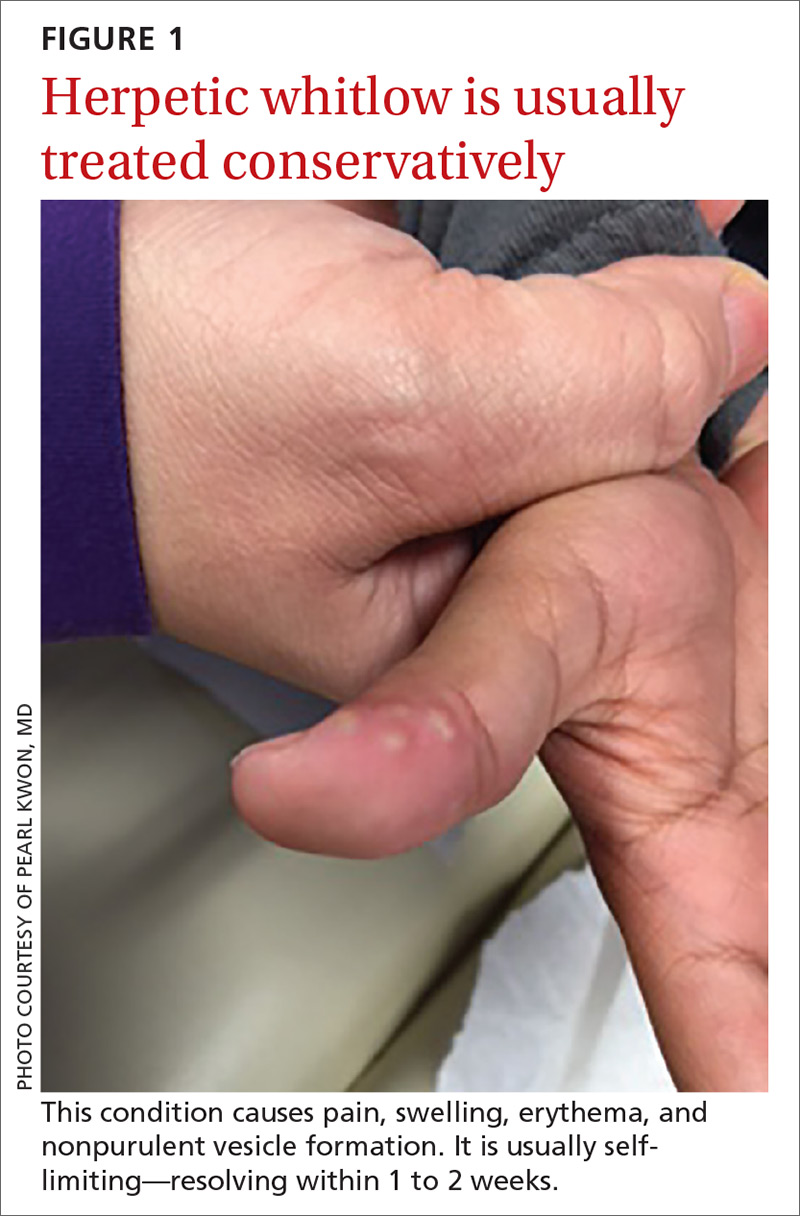
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2
Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
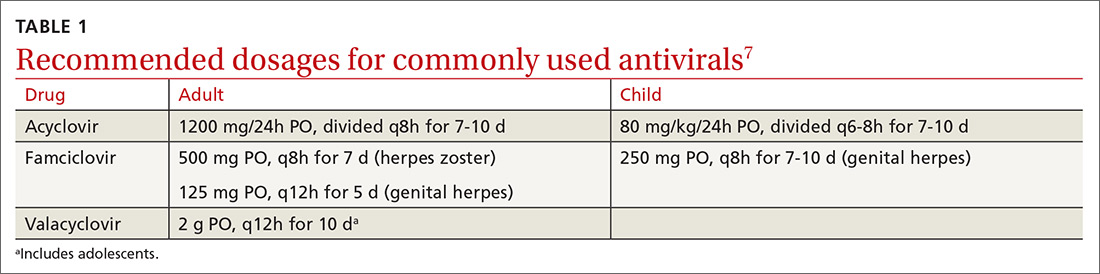
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8
Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
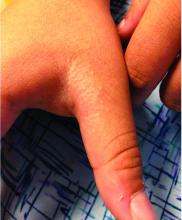
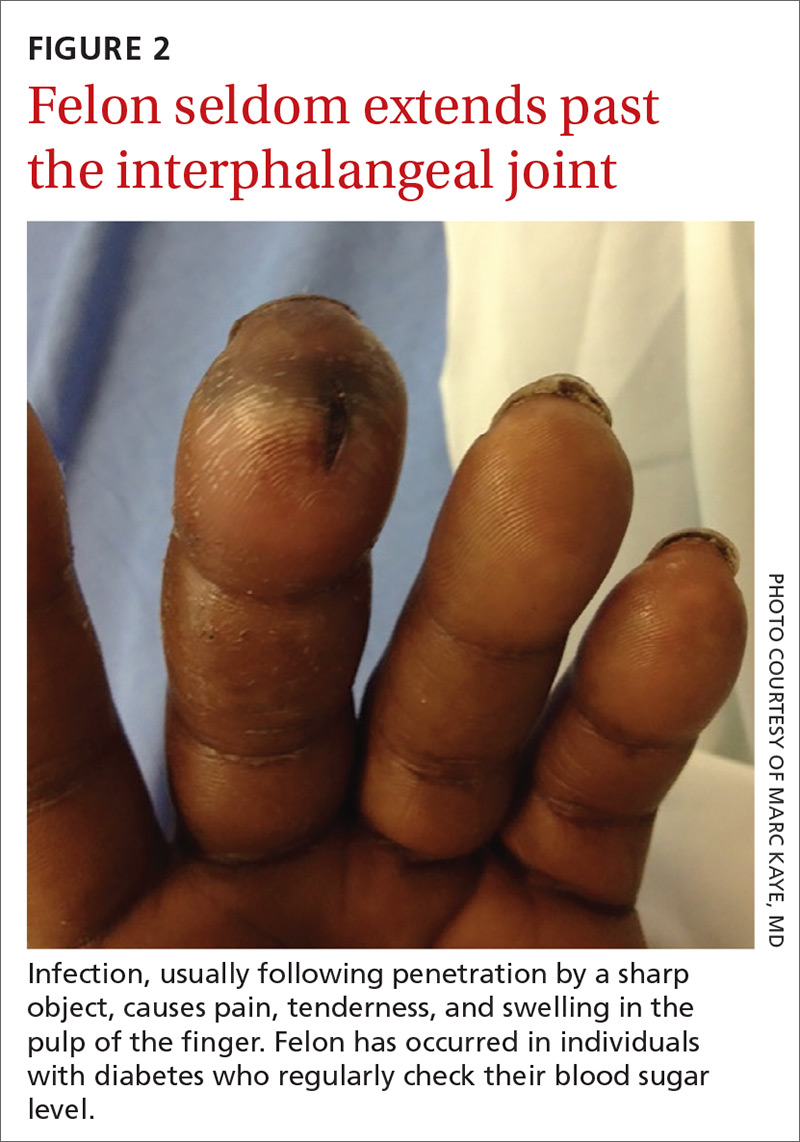
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11
How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
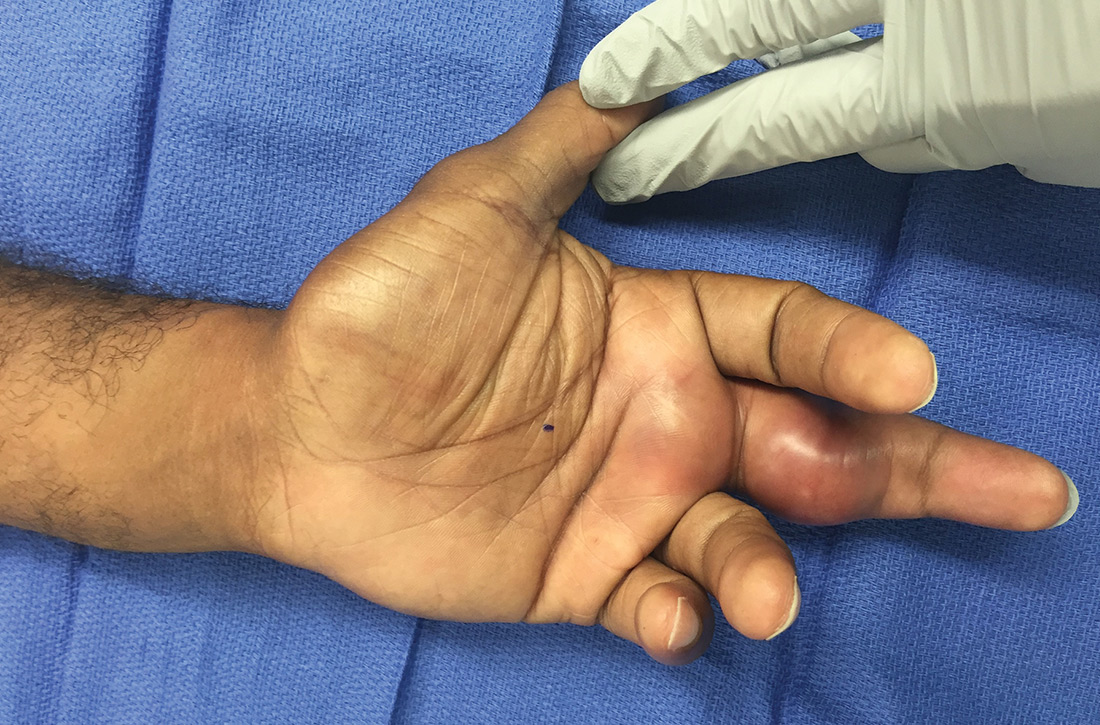
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22
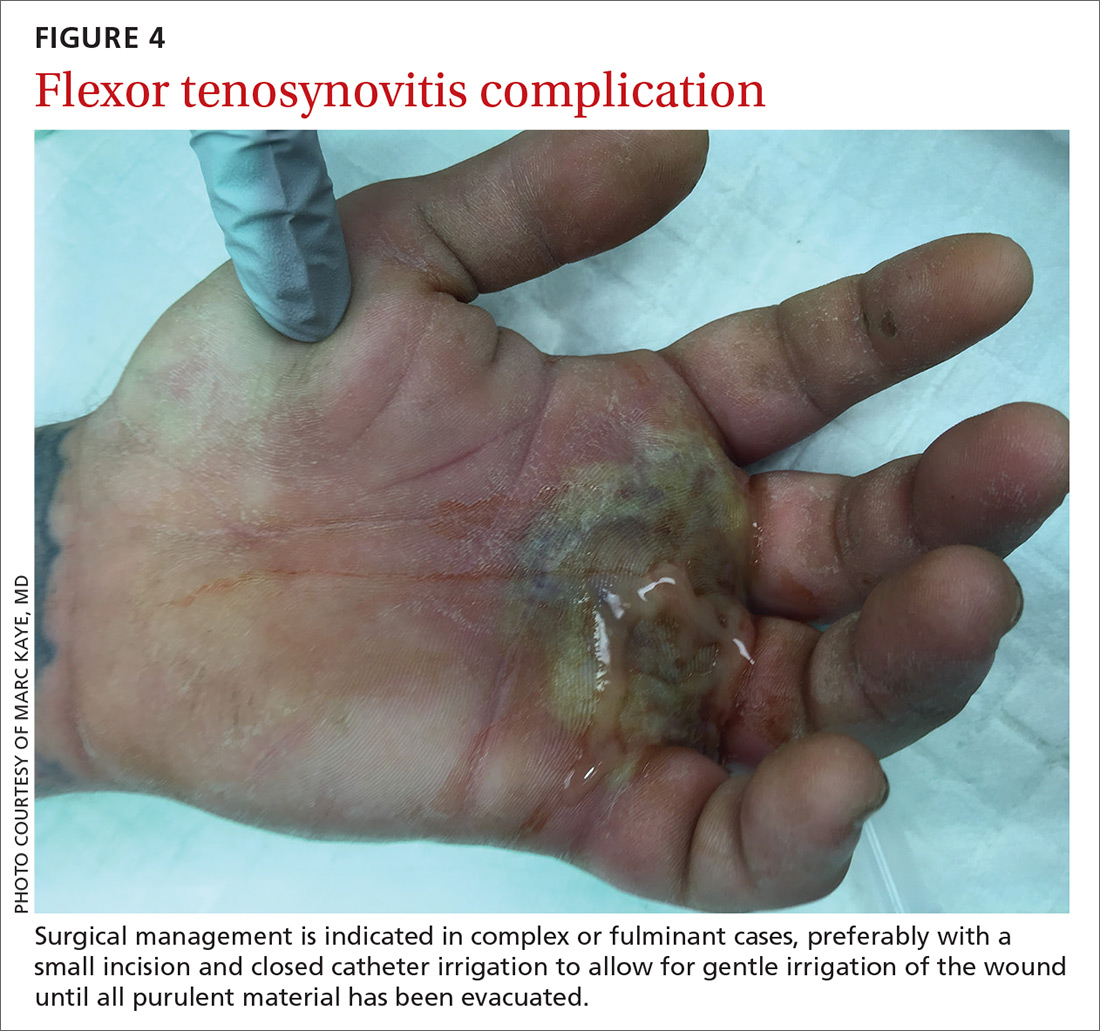
Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
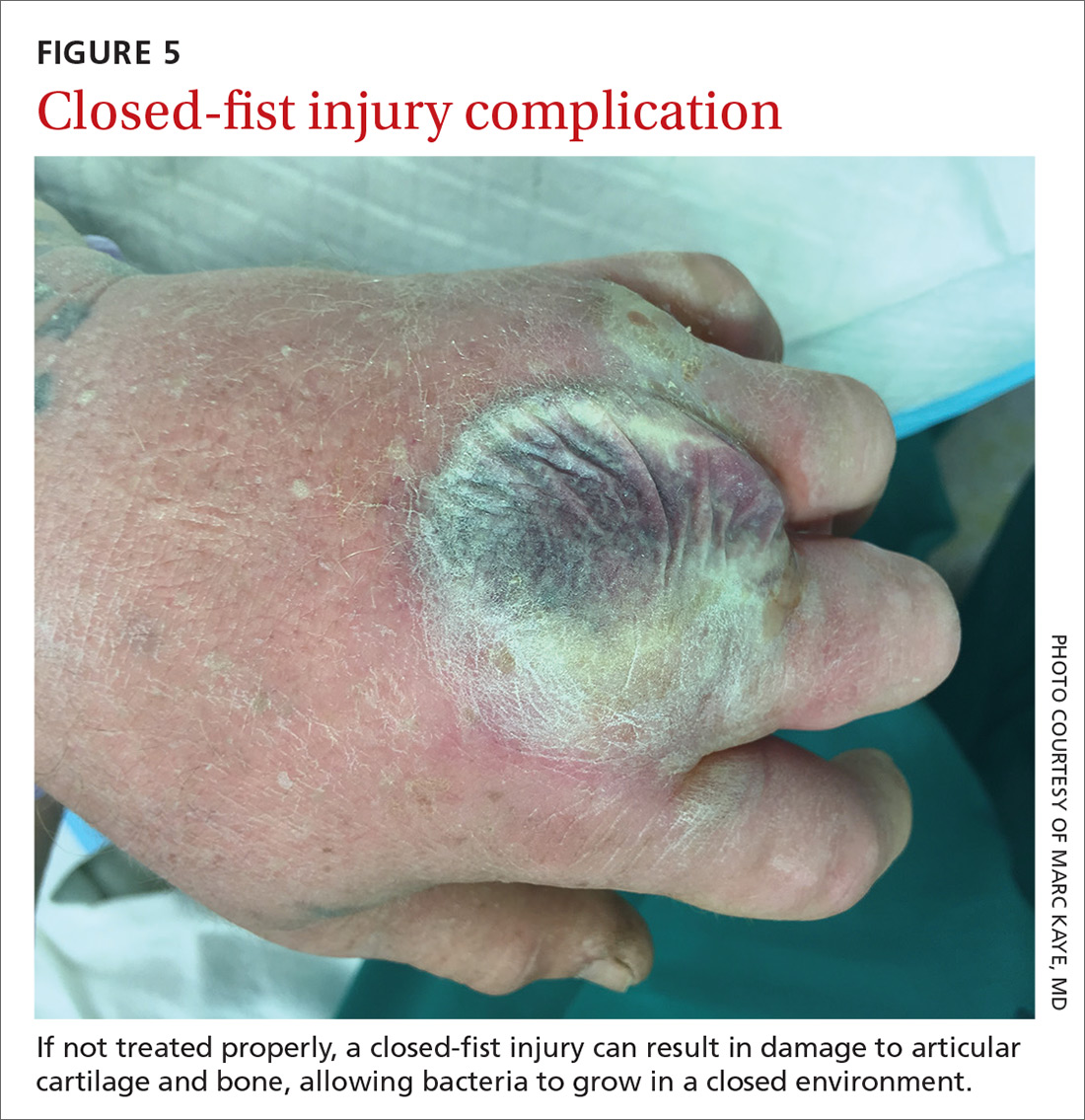
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24
Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36
CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2

Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8

Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
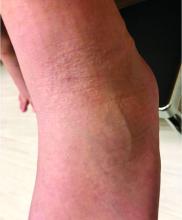
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11

How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
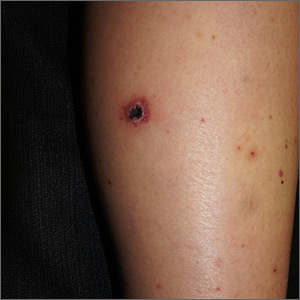
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22

Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24

Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36

CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
Hand infections, if not treated properly, can cause severe chronic morbidity. The conditions I review here range from superficial to deep seated: herpetic whitlow located in the epidermis; felon in subcutaneous tissue; pyogenic flexor tenosynovitis (FTS) in the tendon sheath; and human bite infection at any level including possibly synovium and bone.
Superficial infections usually respond to nonsurgical management. However, antimicrobial therapy is not straightforward. There is no single regimen that covers all possible pathogens. Combination therapy must usually be started and then tailored once an organism and its susceptibility are known. Subcutaneous, tendon sheath, synovial, and bone infections frequently require surgical management.
Herpetic whitlow
Herpes simplex virus type 1 (HSV-1) is common, with as much as 90% of the population exposed by 60 years of age.1 Initial infection usually occurs in the oropharynx and is known as a fever blister or cold sore. However, HSV-1 also can cause herpetic whitlow, a primary infection in the fingertip in which the virus penetrates the subcutaneous tissue, usually after a breakdown in the skin barrier either from infected saliva or the lips of an infected individual.1
What you’ll see. The lesion is characterized by pain, swelling, erythema, and nonpurulent vesicle formation (FIGURE 1). The condition is usually self-limiting, with the inflammation resolving spontaneously, leaving normal healthy skin within 1 to 2 weeks. Herpetic whitlow is an occupational hazard for medical, nursing, paramedical, and dental personnel, and standard precautions should be used when handling secretions (Strength of Recommendation [SOR]: A).2

Reactivation of HSV-1 (and HSV-2) is common, with a prodrome of a “tingling sensation” and subsequent blister formation in the same location as the previous infection. It can cause pain and discomfort and may render the individual unable to perform usual activities.1,3 This lesion is often confused with bacterial (pyogenic) infections of the pulp of the finger or thumb (felon).1 Herpetic whitlow can be distinguished from a felon by its formation of vesicles, lack of a tense pulp space, and serous, rather than purulent, drainage. Scarring is not associated with herpes infection because penetration is limited to the epidermal area. Superimposed bacterial infection can be mistaken for an abscess (felon) and lead to unnecessary incision and drainage, causing associated morbidity and potential scarring in the affected area.1
How it’s treated. Treatment of herpetic whitlow is usually conservative, and topical application of acyclovir 5% appears to be beneficial (SOR: C).4 Two studies also suggest that oral acyclovir is beneficial for herpetic whitlow and may reduce the frequency of recurrence.5,6 Controlled studies in the use of acyclovir for herpetic whitlow have not been conducted. Despite a lack of direct evidence, acyclovir, famciclovir, and valacyclovir are accepted therapies for herpetic whitlow (TABLE 17) (SOR: B).6,8

Felon
A felon is a closed-space infection affecting the pulp of the fingers or thumb. The anatomy of the finger is unique in that there are multiple septa attaching the periosteum to the skin, thereby creating several closed spaces prone to develop pockets of infection.
Continue to: What you'll see
What you’ll see. Clinical signs and symptoms include pain in the pulp of the finger with tenderness and swelling. Bacteria are usually introduced into fingertip space (fat pad) by a penetrating object. Some reported cases have involved individuals with diabetes who regularly check their blood sugar (FIGURE 2).9 A defining characteristic is that the infection usually does not extend past the interphalangeal joint. Radiologic evaluation may be necessary to detect the presence of foreign bodies or to assess bone involvement (osteomyelitis of the distal phalanx).10 The differential diagnosis includes paronychia, in which the infection starts in the nail area and pain is not as intense as in a felon infection.11

How it’s treated. Surgical treatment of felon is controversial. There is no doubt that pus should be drained; how the incision is best performed, however, has been debated.12 Before surgical debridement, obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli (AFB), and fungi (SOR: A).13,14 Several surgical techniques and their pitfalls are described in the literature.
Lateral and tip incisions may help avoid painful scars. However, multiple reports of this procedure describe injury to neurovascular bundles, leading to ischemia and anesthesia.12 The “fish-mouth” incision and the “hockey stick” or “J” incision, as well as the transverse palmar incision, are no longer recommended due to painful sequelae, sensorial alterations, and risk of cutting the digital nerves.15 The preferred surgical procedure at this time is to make a very short incision over the area of maximum tenderness, then open and drain the abscess. Avoid placing packing in the affected area. Post-surgical management includes elevation, immobilization with an appropriate splint, and application of compresses until the wound has healed.12,15,16
Since Staphylococcus aureus and Streptococcus sp are the most common bacteria causing felon, start
Acute pyogenic flexor tenosynovitis
FTS is an aggressive closed-space bacterial infection that involves the flexor tendon synovial sheath. FTS accounts for up to 10% of acute hand infections and requires prompt medical attention with wound lavage, surgical management, and antimicrobial therapy to minimize serious consequences to the digit.18
Continue to: What you'll see
What you’ll see. FTS is diagnosed using clinical criteria19,20: fusiform swelling of the finger; exquisite tenderness over the entire course of the flexor tendon sheath; pain on passive extension; and flexed posture of the digit (FIGURE 3). Patients usually recall some type of trauma or puncture wound to the affected area, but hematogenous spread of Neisseria gonorrhoeae also has been reported.21 The most common bacterial pathogens are Staphylococcus sp or Streptococcus sp. However, obtain a sample for Gram stain and culture for aerobic, anaerobic, AFB, and fungal agents before irrigating the wound with copious fluids and initiating empirical antibiotic therapy.14 Once a pathogen has been isolated, tailor antimicrobial therapy based on identified sensitivities and local antibiogram.13

How it’s treated. Early treatment of FTS is of utmost importance to avoid adverse outcomes. If FTS is diagnosed early, manage conservatively with elevation of the hand, splinting in a neutral position, and intravenous (IV) antibiotics. The use of adjunct antibiotics has improved range-of-motion outcomes compared with elevation and splinting alone (54% excellent vs 14% excellent) (SOR: A).22

Surgical management of FTS has involved either an open or closed method. The open approach consists of open incision and drainage with exposure of the flexor tendon sheath, followed by large-volume sheath irrigation and closure of incision, in some cases over a drain. The closed approach with irrigation, rather than open washout, has been associated with improved outcomes (71% excellent vs 26% excellent).22 As a result, the procedure of choice is the closed approach, which uses closed catheter irrigation. An incision and placement of an angiocatheter allows for gentle irrigation of the wound until all purulent material has been evacuated (SOR: C).22,23
Human bite injuries
A human bite injury occurs in 1 of 2 ways, and each has a distinct pattern.
What you’ll see
Closed-fist injury occurs when a clenched fist strikes the teeth of another person. The resulting lesion can easily fool a clinician by appearing to show very little damage. If not appropriately evaluated and treated, the lesion can cause considerable morbidity (FIGURE 5). Injury to the extensor mechanism and joint capsule can also damage the articular cartilage and bone, allowing bacteria to grow in a closed environment. This usually affects the metacarpophalangeal joint (MCP) due to its prominence when a hand is clenched. Initially the lesion seems to be minor, with a small laceration of 3 to 5 mm on the overlying skin, thus inoculating mouth flora deep in the hand tissue. Once the hand is relaxed, the broken skin retracts proximally, covering the wound and making it look innocuous.24

Continue to: Occlusive bite injury...
Occlusive bite injury occurs when one individual forcibly bites another. Such wounds tend to be less penetrating than clenched-fist injuries. However, they can vary from superficial lacerations to wounds with tissue loss, including traumatic finger amputation.24,25
One randomized prospective study compared mechanical wound care alone with combined mechanical wound care and oral or IV antibiotics and found that 47% of patients receiving wound care alone became infected vs no infection among those given oral or parenteral antibiotics (SOR: C).26 Experts in the field advise examining the wound after administering a local anesthetic, thereby allowing better visualization of possible tendon damage, joint penetration, fracture, or deep-tissue infection. The procedure should be performed by a physician experienced in treating hand wounds, whether in the emergency department (ED) or in an operating suite.
How it’s treated. There is controversy regarding whether an affected patient can be adequately treated as an outpatient. Most traumatic bite lesions occur in men, and in those abusing drugs or alcohol.25 In the latter case, individuals may be less likely to return for subsequent care or to finish the antibiotic course as prescribed. It is therefore strongly suggested that those individuals be admitted to receive IV antibiotics and physical therapy to expedite healing and avoid morbidity and sequelae of the lesions (SOR: C).25,27
Obtain cultures from the wound after giving analgesia but before starting the procedure. The sample should be sent to the Microbiology Department or outpatient reference lab for aerobic, anaerobic, AFB, and fungal cultures. Recommend that the laboratory use 10% CO2-enriched media for Eikenella corrodens isolation (SOR: A).28,29
Among oral human flora are large concentrations of anaerobic bacteria such as Bacteroides sp (including B fragilis), Prevotella sp, Peptostreptococcus sp, Fusobacterium sp, Veillonella sp, Enterobateriaceae, and Clostridum. B fragilis accounts for up to 41% of isolates in some studies.30 Most of them are beta-lactamase producers. The most common aerobic bacteria are alpha- and beta-hemolytic Streptococci, S aureus, Staphylococcus epidermis, Corynebacterium, and E corrodens. E corrodens accounts for up to 25% of bacteria isolated in clenched-fist injuries.27,29
Continue to: HSV-1 and HSV-2...
HSV-1 and HSV-2, as well as hepatitis B and C and human immunodeficiency virus (HIV) can be isolated in saliva of infected individuals and can be transmitted when contaminated blood is exposed to an open wound. Still, the presence of HIV in saliva is unlikely to result in disease transmission, due to salivary inhibitors rendering the virus non-infective in most cases.25 Obtain HIV and hepatitis B and C serology at baseline and at 3 and 6 months.25 If HIV infection is known or suspected, or if there was exposure to blood in the wound, the Centers for Disease Control and Prevention recommends postexposure prophylaxis with a 28-day course of anti-retroviral medication (SOR: A).24,31
Hepatitis B virus has an infectivity 100-fold greater than HIV.27 If possible, the 2 people involved in the altercation should be tested for hepatitis B surface antigen. If the result is positive, the individual with the skin wound should receive hepatitis B immune globulin (0.06 mL/kg/dose),32 and the vaccination schedule started if not done previously (SOR: A).33
Most experts recommend early antibiotic therapy given over 3 to 5 days for fresh, superficial wounds and specifically for wounds affecting hands, feet, joint, and genital area.11 For treatment of cellulitis or abscess, 10 to 14 days is sufficient; tenosynovitis requires 2 to 3 weeks; osteomyelitis requires 4 to 6 weeks.24 Wound care associated with daily dressing changes and antimicrobial therapy was superior to wound care alone (0% vs 47%).30 Assess tetanus status in all cases (SOR: A).17,33,34
Antimicrobial therapy for contamination with oral secretion is not straightforward. No one medication covers all possible pathogens. Use a combination therapy initially and then narrow coverage once the microorganism has been identified and susceptibilities are known. Empirical oral therapy with amoxicillin-clavulanate would be reasonable.
If IV therapy is needed, consider using ampicillin-sulbactam, cefazolin, or clindamycin. These antibiotics usually cover S aureus, Streptococcus sp, E corrodens and some anaerobes. Dicloxacillin will cover S aureus but provides poor coverage for E corrodens. First-generation cephalosporins cover S aureus, but E corrodens resistance is common. For penicillin-allergic individuals, use trimethoprim-sulfamethoxazole to cover E corrodens. Doxycycline can be used in children older than 8 years and in adults; avoid it in pregnant women.
Continue to: General principles guiding wound care, microbiology, and antibiotic management
General principles guiding wound care, microbiology, and antibiotic management
Elapsed time between injury and seeking medical attention is characterized as an early (24 hours), late (1-7 days), or delayed (> 7 days) presentation. The timing of an individual’s presentation and a determination of whether the wound is “clean” or “dirty” are both factors in the risk of infection and in associated morbidity and long-term sequelae.
General principles of surgery are important. The cornerstones of treatment are the use of topical anesthesia to provide pain control, which allows for better examination of the wound, debridement of devitalized tissue, collection of wound cultures, and irrigation of the wound with large volume of fluids to mechanically remove dirt, foreign bodies, and bacteria.
Surgical knowledge of hand anatomy increases the likelihood of favorable outcomes in morbidity and functionality. Depending on the circumstances and location, this procedure may be performed by an experienced hand surgeon or an ED physician. (Diversity in settings and available resources may explain why there is so much variability in the composition of patient populations and outcomes found in the medical literature.)
To maximize appropriate bacteriologic success, the Microbiology Department or lab needs to be informed of the type of samples that have been collected. Cultures should be sent for aerobic, anaerobic, AFB, and fungal cultures. Enrichment of aerobic culture with 10% CO2 increases the likelihood of isolating E corrodens (fastidious bacterium), which has been identified in approximately 25% of clenched-fist wounds.
Populations at heightened risk for human bite injuries include alcohol and drug abusers and those of poor socioeconomic status who may not have the resources to visit a medical facility early enough to obtain appropriate medical care. These patients are at risk for being lost to follow-up as well as medication noncompliance, so inpatient admission may diminish the possibilities of incomplete medical treatment, complications, and adverse outcomes such as loss of functionality of the affected extremity.
Continue to: Antimicrobial therapy is not easy
Antimicrobial therapy is not easy. No single regimen covers all possibilities. Start antimicrobial treatment empirically with wide-spectrum coverage, and tailor the regimen, as needed, based on microbiology results.
In clean surgical procedures, S aureus is the most common pathogen. It is acceptable to start empirical treatment with an antistaphylococcal penicillin, first-generation cephalosporin, or clindamycin. In contaminated wounds, gram-negative bacteria, anaerobes, fungal organisms, and mixed infections are more commonly seen.35-37
First-generation cephalosporin provides good coverage for gram-positive and gram-negative bacteria in clean wounds. However, in contaminated wounds with devitalized tissue, a more aggressive scheme is recommended: start with a penicillin and aminoglycoside.35-37 In some cases, monotherapy with either ampicillin/sulbactam, imipenem, meropenem, piperacillin/tazobactam, or tigecylline may be sufficient until culture results are available; at that point, antibiotic coverage can be narrowed as indicated (TABLE 27).35,36

CORRESPONDENCE
Carlos A. Arango, MD, 8399 Bayberry Road, Jacksonville, FL 32256; [email protected].
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
1. Lewis MA. Herpes simplex virus: an occupational hazard in dentistry. Int Dent J. 2004;54:103-111.
2. CDC. Recommended infection-control practices for dentistry. MMWR Morb Mortal Wkly Rep. 1993;42:1-16.
3. Merchant VA, Molinari JA, Sabes WR. Herpetic whitlow: report of a case with multiple recurrences. Oral Surg. 1983;555:568-571.
4. Richards DM, Carmine AA, Brogden RN, et al. Acyclovir. A review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1983;26:378-438.
5. Laskin OL. Acyclovir and suppression of frequently recurring herpetic whitlow. Ann Int Med. 1985;102:494-495.
6. Schwandt NW, Mjos DP, Lubow RM. Acyclovir and the treatment of herpetic whitlow. Oral Surg Oral Med Oral Pathol. 1987;64:255-258.
7. Robertson J, Shilkofsk N, eds. The Harriet Lane Handbook. 17th Edition. Maryland Heights, MO; Elsevier Mosby; 2005:679-1009.
8. Usatine PR, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
9. Newfield RS, Vargas I, Huma Z. Eikenella corrodens infections. Case report in two adolescent females with IDDM. Diabetes Care. 1996;19:1011-1013.
10. Patel DB, Emmanuel NB, Stevanovic MV, et al. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014; 34:1968-1986.
11. Blumberg G, Long B, Koyfman A. Clinical mimics: an emergency medicine-focused review of cellulitis mimics. J Emerg Med. 2017;53:474-484.
12. Kilgore ES Jr, Brown LG, Newmeyer WL, et al. Treatment of felons. Am J Surgery. 1975;130:194-198.
13. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:e10-e52.
14. Baron EJ, Miller M, Weinstein MP, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2013;57:e22–e121.
15. Clark DC. Common acute hand infections. Am Fam Physician. 2003;68:2167-2176.
16. Swope BM. Panonychiae and felons. Op Tech Gen Surgery. 2002;4:270-273.
17. Yen C, Murray E, Zipprich J, et al. Missed opportunities for tetanus postexposure prophylaxis — California, January 2008-March 2014. MMWR Morb Mortal Wkly Rep. 2015;64:243-246.
18. Barry RL, Adams NS, Martin MD. Pyogenic (suppurative) flexor tenosynovitis: assessment and management. Eplasty. 2016;16:ic7.
19. The symptoms, signs, and diagnosis of tenosynovitis and major facial space abscess. In: Kanavel AB, ed. Infections of the Hand. Philadelphia, PA: Lea & Febiger; 1912:201-226.
20. Kennedy CD, Huang JI, Hanel DP. In brief: Kanavel’s signs and pyogenic flexor tenosynovitis. Clin Ortho Relat Res. 2016;474;280-284.
21. Krieger LE, Schnall SB, Holtom PD, et al. Acute gonococcal flexor tenosynovitis. Orthopedics. 1997;20:649-650.
22. Giladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. J Hand Surg Eur Vol. 2015;40:720-728.
23. Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Ann Plastic Surgery. 2002;49:350-354.
24. Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47-57.
25. Henry FP, Purcell EM, Eadie PA. The human bite injury: a clinical audit and discussion regarding the management of this alcohol fueled phenomenon. Emer Med J. 2007;24:455-458.
26. Zubowicz VN, Gravier M. Management of early human bites of the hand: a prospective randomized study. Plas Reconstr Surg. 1991;88:111-114.
27. Kelly IP, Cunney RJ, Smyth EG, et al. The management of human bite injuries of the hand. Injury. 1996;27:481-484.
28. Udaka T, Hiraki N, Shiomori T, et al. Eikenella corrodens in head and neck infections. J Infect. 2007;54:343-348.
29. Decker MD. Eikenella corrodens. Infect Control. 1986;7:36-41.
30. Griego RD, Rosen T, Orengo IF, et al. Dog, cats, and human bites: a review. J Am Acad Dermatol. 1995;33:1019-1029.
31. Panlilio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54:1:17.
32. American Academy of Pediatrics. Hepatitis B. In: Kimberlin DW, Brady MT, Jackson MA, Long SS. Eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL. American Academy of Pediatrics; 2018:415.
33. Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008: recommendations of the Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2008;57:1-21.
34. Harrison M. A 4-year review of human bite injuries presenting to emergency medicine and proposed evidence-based guidelines. Injury. 2009;40:826-830.
35. Taplitz RA. Managing bite wounds. Currently recommended antibiotics for treatment and prophylaxis. Postgrad Med. 2004;116:49-52.
36. Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705-710.
37. Shapiro DB. Postoperative infection in hand surgery. Cause, prevention, and treatment. Hands Clinic. 1998;14:669-681.
PRACTICE RECOMMENDATIONS
› Obtain a sample of pus for Gram stain and for cultures of aerobic and anaerobic organisms, acid-fast bacilli, and fungi. A
› Use antibiotics as an adjunct to elevation and splinting in flexor tenosynovitis to improve range-of-motion outcomes. A
› Notify your microbiology lab to enrich cultures with 10% CO2 to isolate Eikenella corrodens. A
› Consider prescribing acyclovir, famciclovir, or valacyclovir for herpetic whitlow. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Scabies: Refine your exam, avoid these diagnostic pitfalls
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
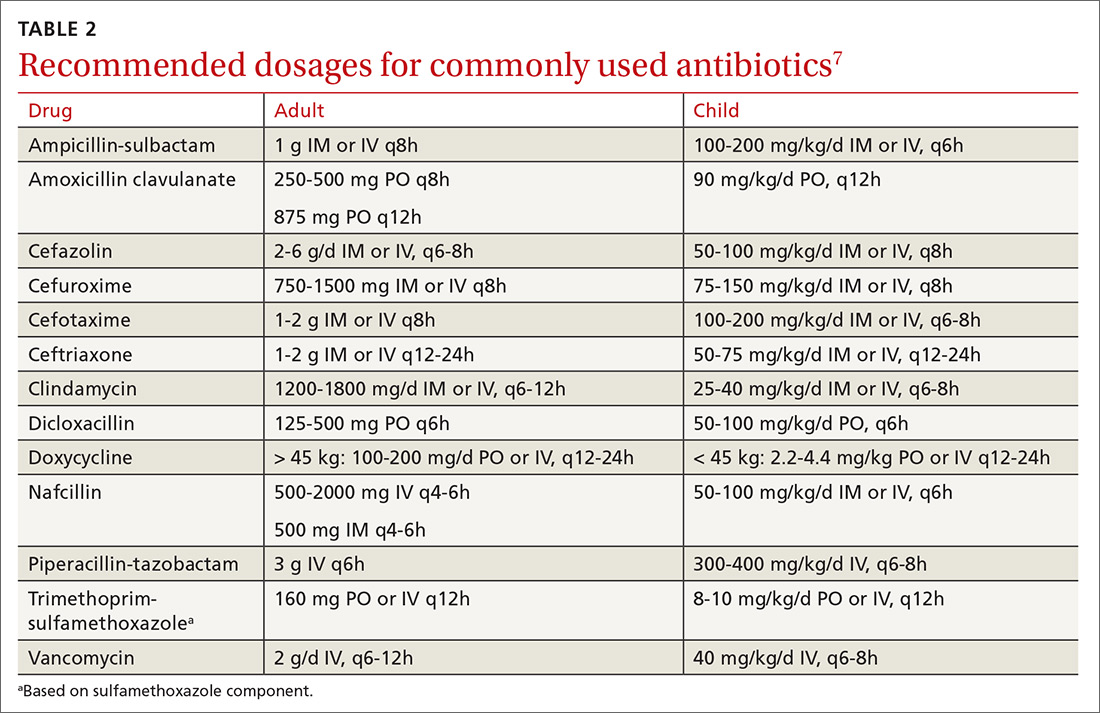
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).
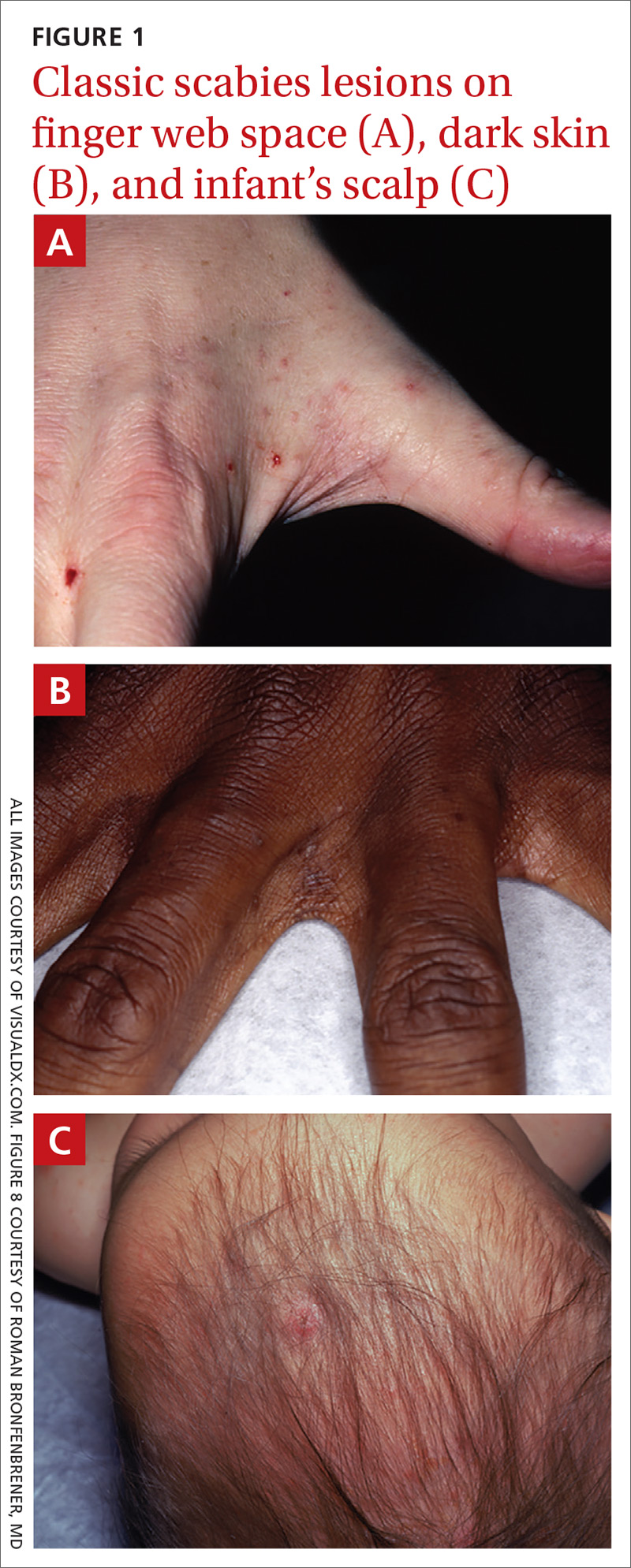
The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
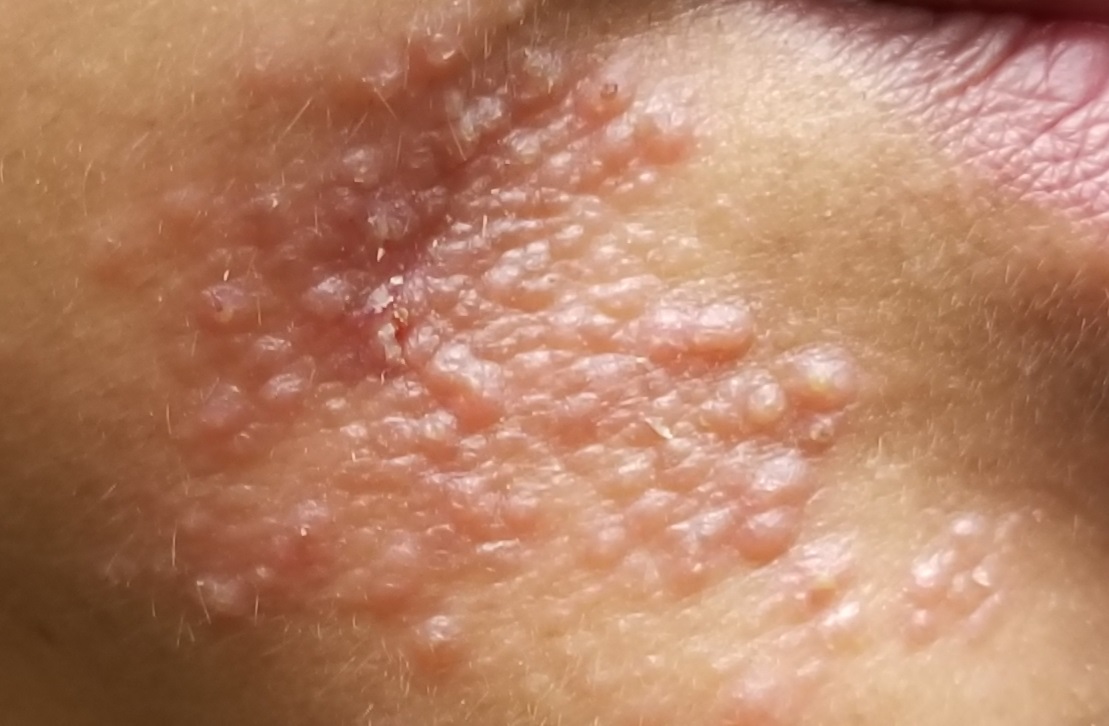
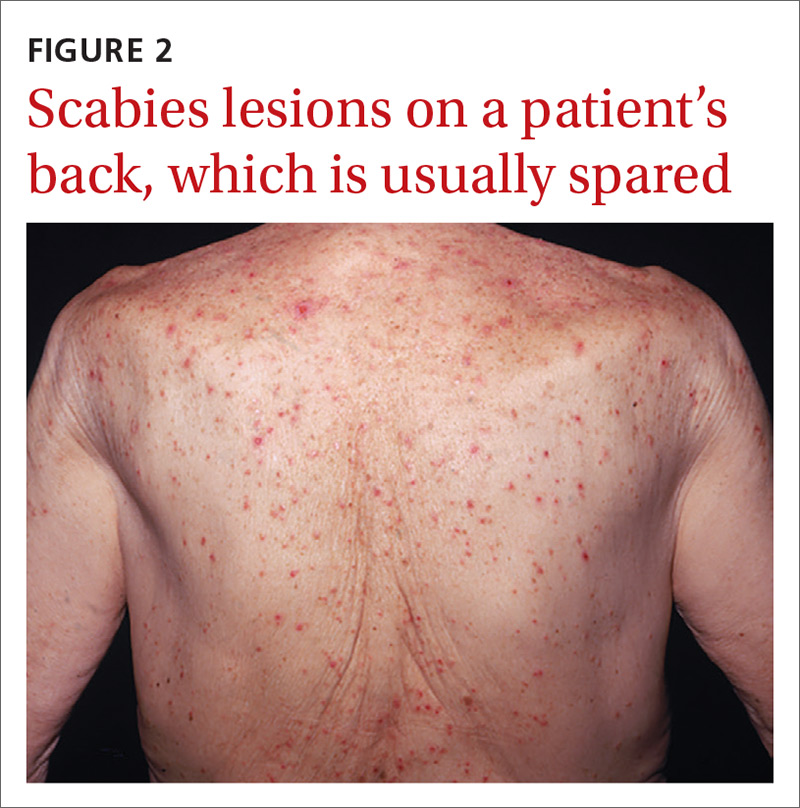
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8
Continue to: Pustular scabies...
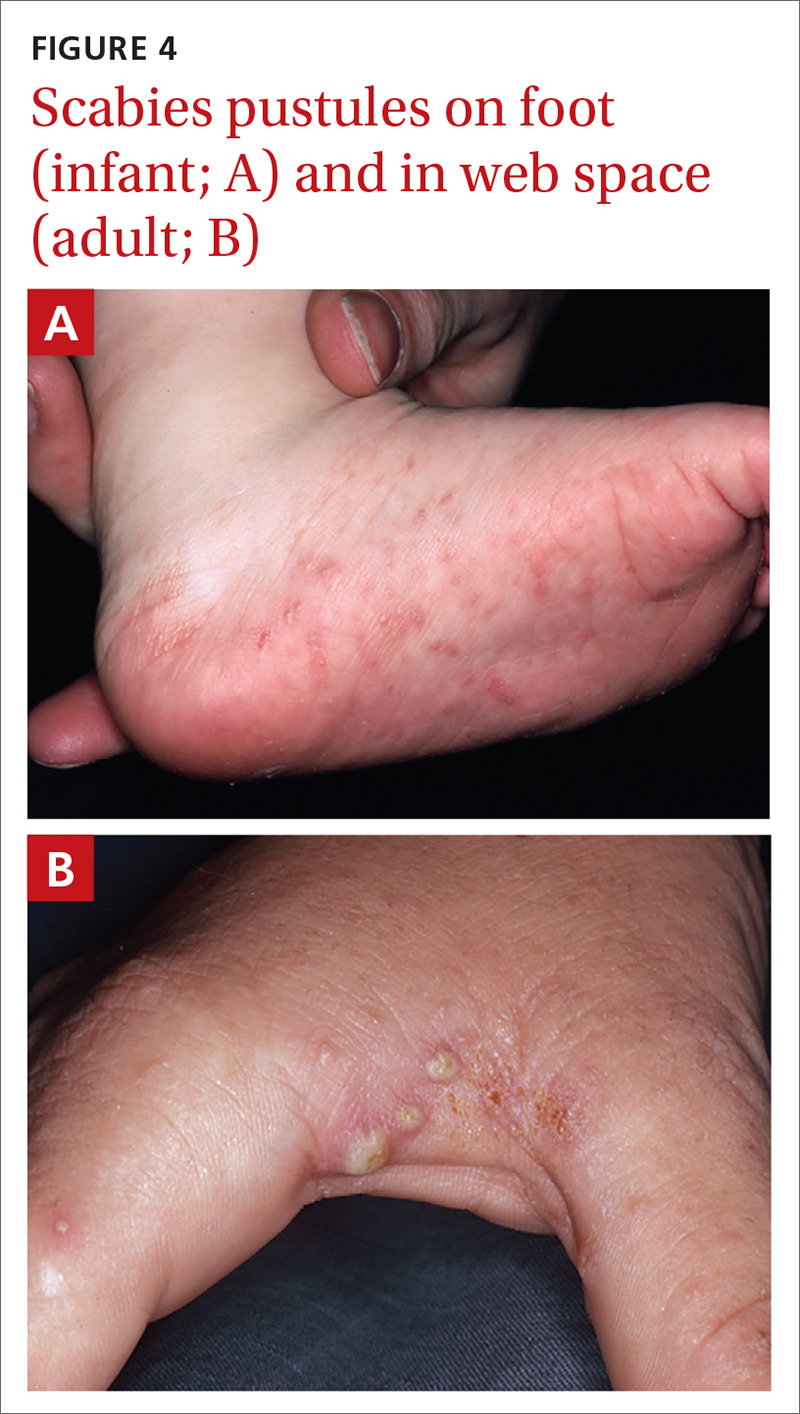
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.
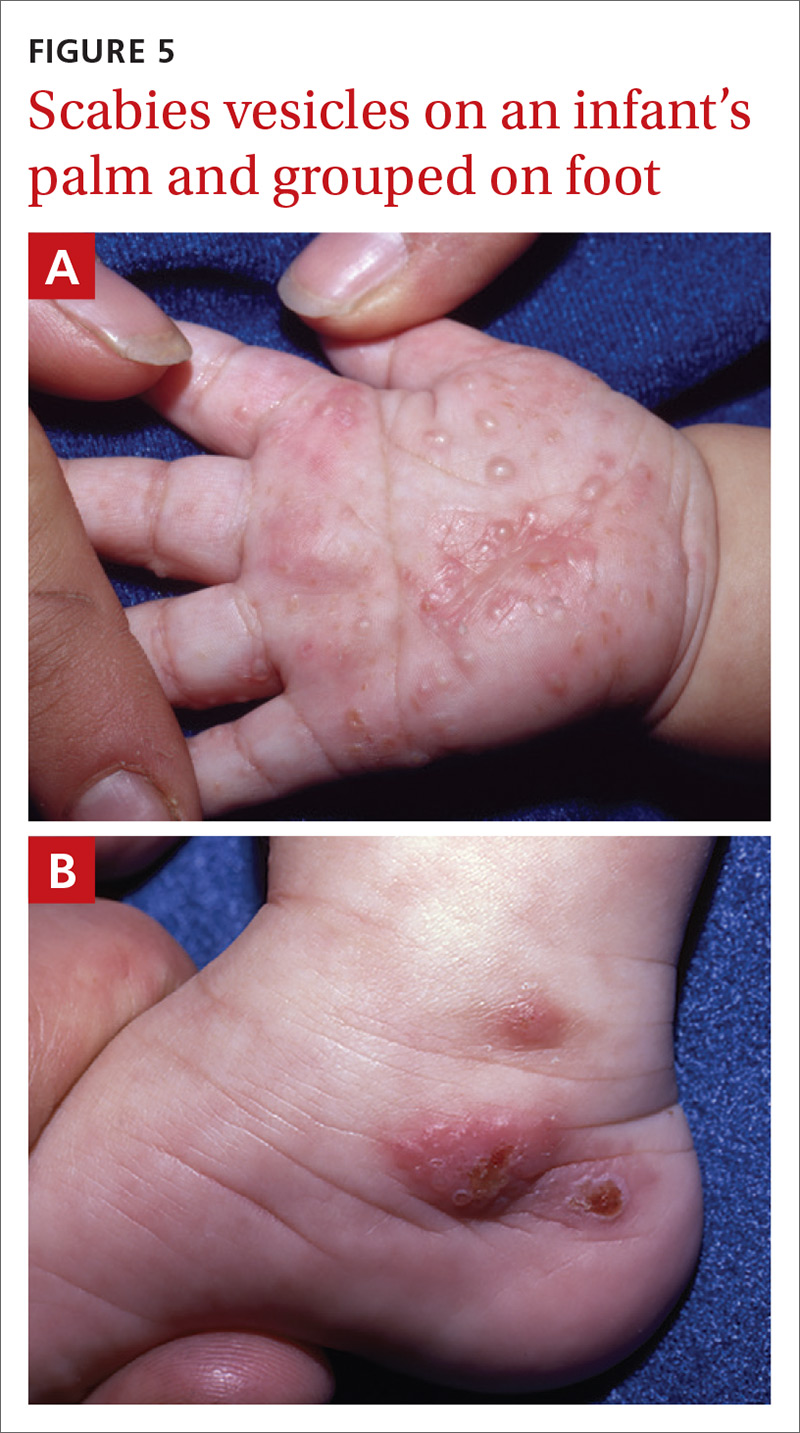
Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.
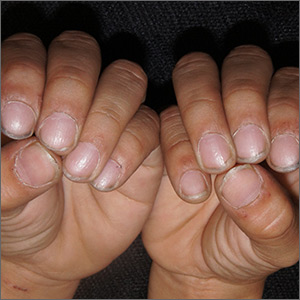
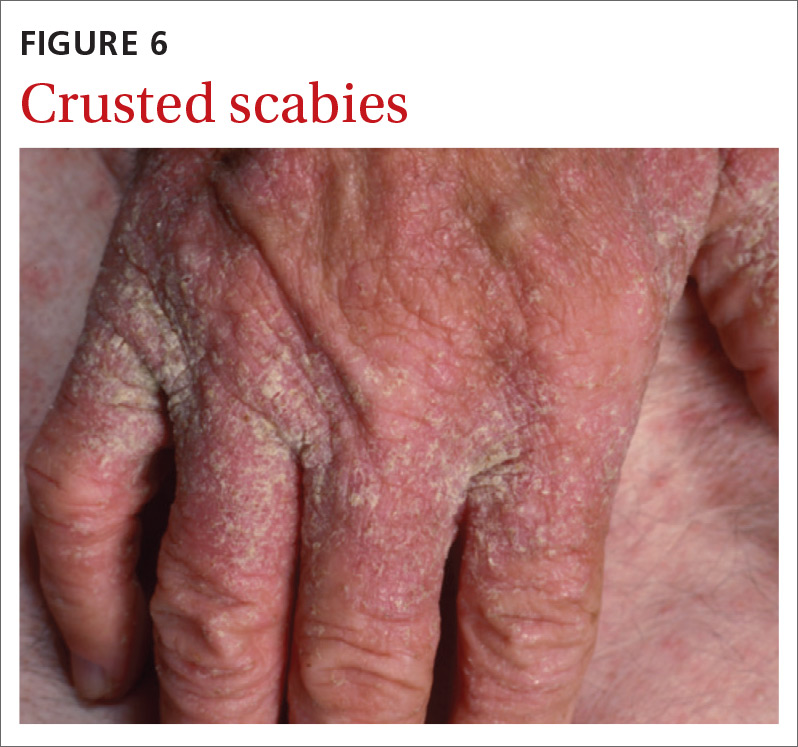
Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12
Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
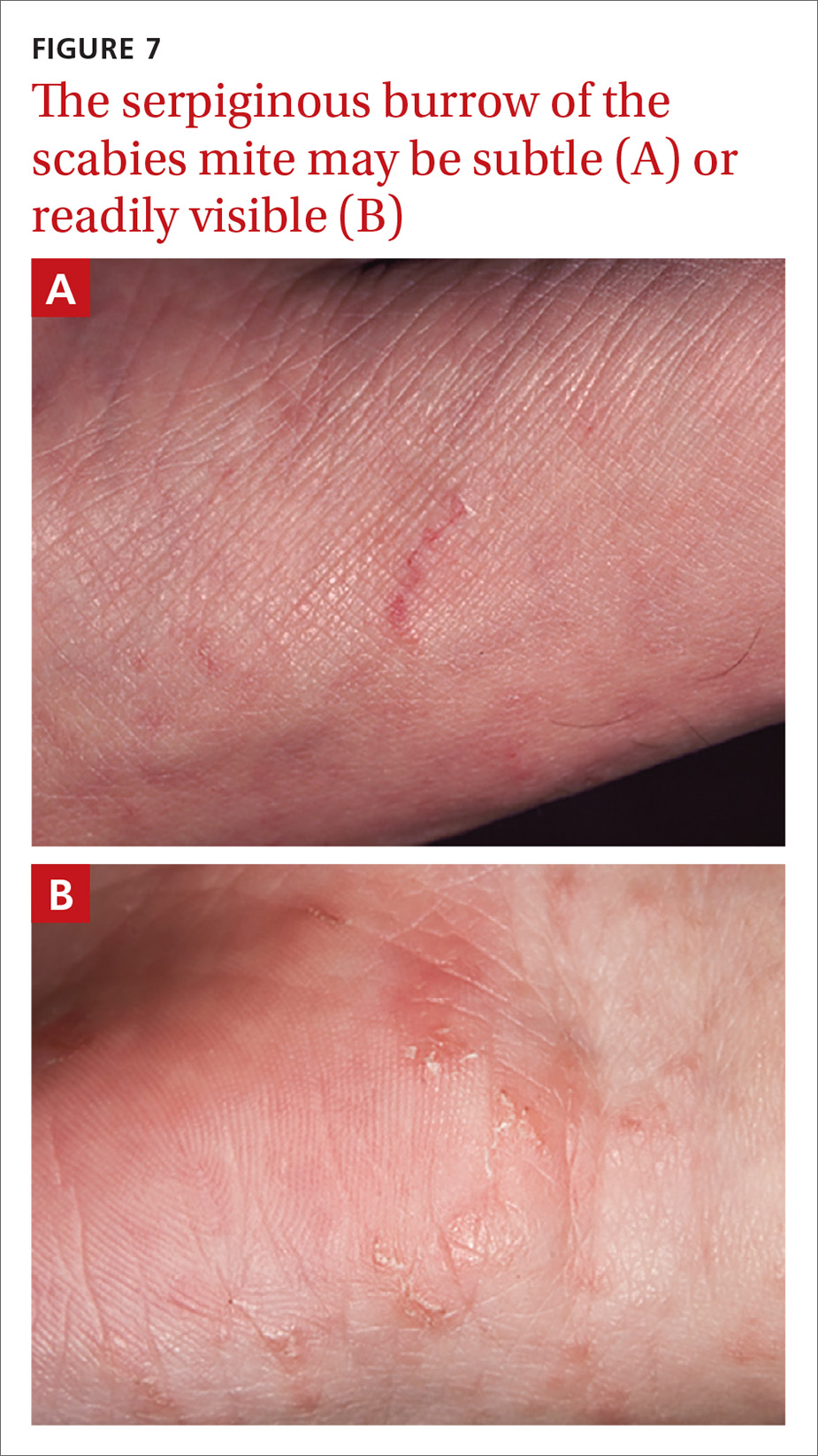
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).
Continue to: Handheld illumination with a dermatoscope...
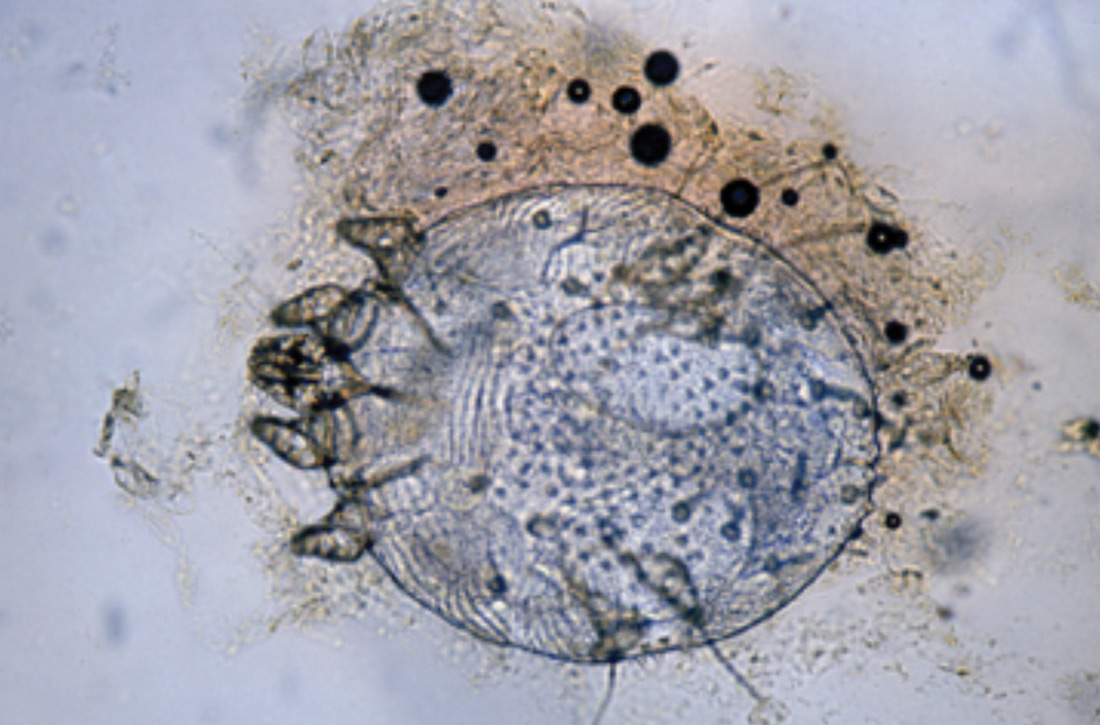
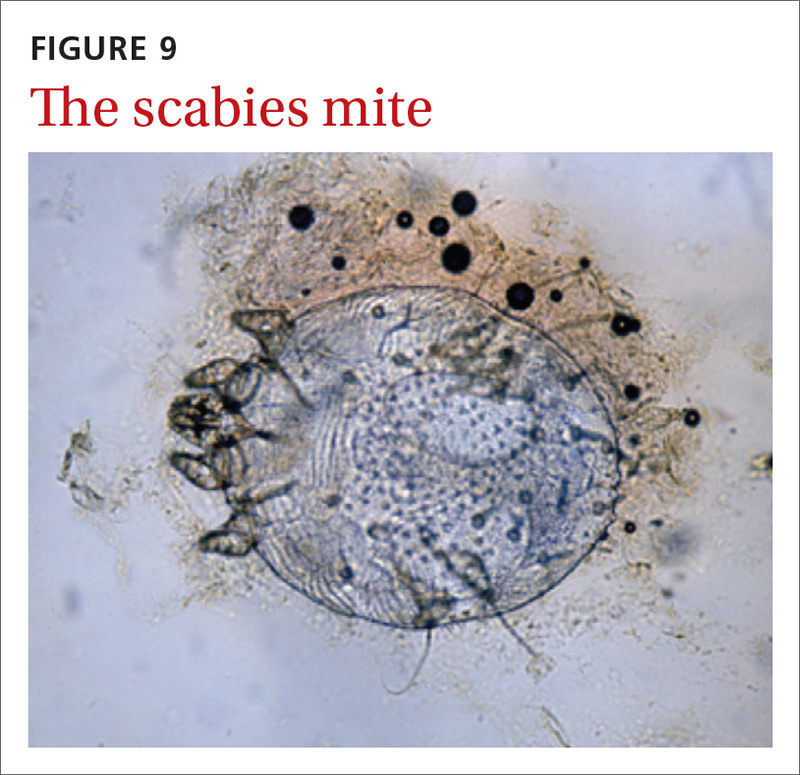
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.
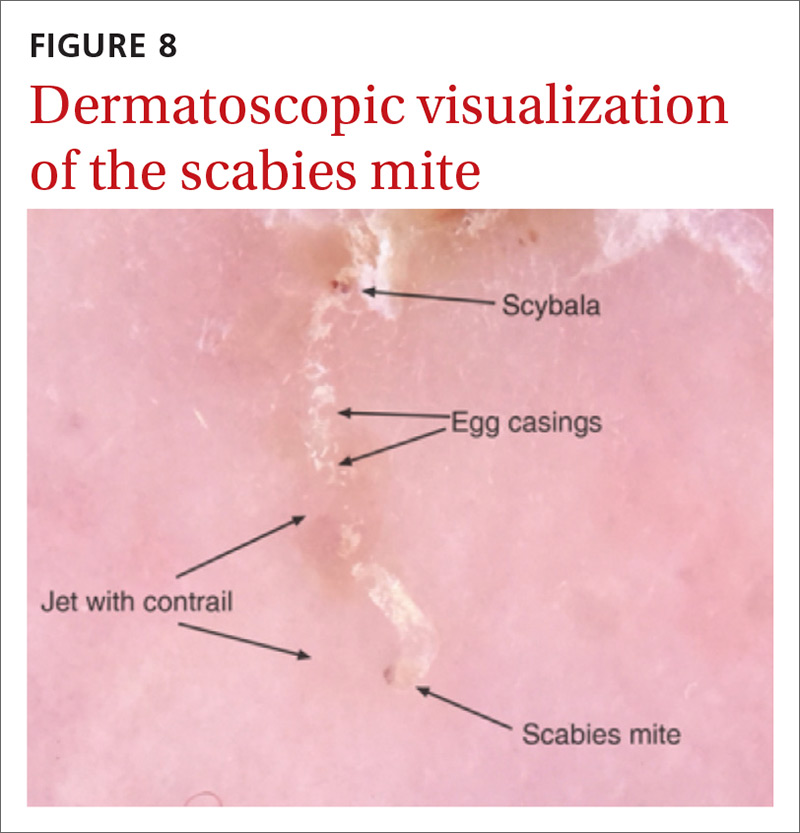
Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.
Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.
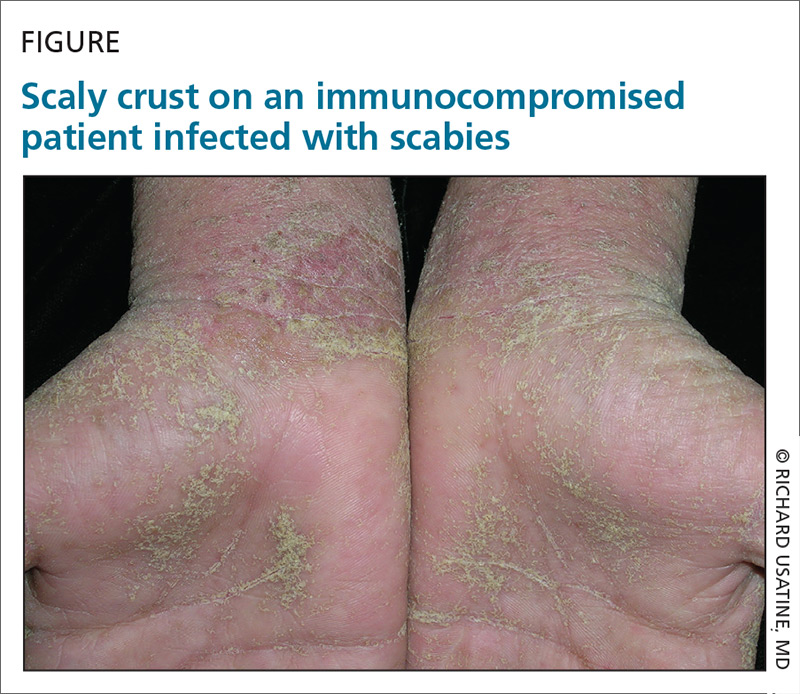
Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).


The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8

Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.

Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.

Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12

Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).

Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.

Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.

Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.

Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
It is estimated that up to 45% of cases of scabies are misdiagnosed as another condition.1 This can occur when common clinical features are overlooked, a skin exam is rushed (and the rash is chalked up to dermatitis), or the wrong part of the pruritic lesion is scraped (the papule, rather than the burrow). There are also atypical presentations of scabies, which can confound even the most astute clinician.1 Misdiagnosis can increase health care costs due to repeat office visits or multiple referrals. In this article, we review the typical and atypical presentations of scabies and provide recommendations to aid physicians in its early recognition and correct diagnosis.
The scope of scabies infection, and its clinical stages
The prevalence of scabies, a common skin infection caused by the mite Sarcoptes scabiei, is estimated at 300 million cases worldwide annually, with the greatest incidence occurring in children and adolescents.1 In the developing world, its clinical burden is highest among the homeless, those of lower socioeconomic status, and those with poor hygiene. In the developed world, the clinical burden is highest among hospitalized patients and residents of long-term living facilities.
The S scabiei mite is an obligate parasite that elicits an adaptive immune response in susceptible hosts. The female mite lays 60 to 90 eggs that mature into adult mites after completing the mite life cycle in human hosts. In immunocompetent patients, roughly 10 to 15 surviving mites can be found at any given point in the disease process.2 In crusted or disseminated scabies, which often occur in immunocompromised patients, thousands of mites may be found at any given point in the disease process. 2
Scabies infection has 2 stages: the latent primary infection and the symptomatic secondary infection.
The primary infection starts with the initial mite invasion, typically with the transfer of impregnated females during person-to-person contact. Females deposit eggs as they burrow into the epidermis at the level of the stratum corneum with the use of proteolytic enzymes (creating the mite burrow). Surviving eggs hatch into larvae that then mature into nymphs and adult mites. After these adult mites mate, the impregnated females create new burrows and lay additional eggs.3 Patients may be asymptomatic during this initial stage and the infection may be transmitted from person to person through direct skin contact.
The second stage of infection is when patients experience severe pruritus with inflammatory papules seen on exam. The pruritus associated with scabies results from a delayed type IV hypersensitivity reaction to mite infestation. This requires host sensitization to the scabies mite. Clinically, there is a delayed onset (weeks) of numerous erythematous papules and, later, excoriated papules.
Conditions that scabies can mimic
The differential of typical scabies includes diagnoses manifesting with moderate to severe pruritus. In the immunocompetent adult, conditions to consider are atopic dermatitis, tinea corporis, papular urticaria, seborrheic dermatitis, poison ivy and other causes of contact dermatitis, drug eruptions, and irritant dermatitis. In immunocompetent infants, think of seborrheic dermatitis, atopic dermatitis, acropustulosis, and viral exanthems.
Continue to: Nodular scabies variants...
Nodular scabies variants can masquerade as pseudolymphoma, lymphoma, or leukemia cutis. In immunocompromised and elderly individuals, crusted scabies is often mistaken for psoriasis, atopic dermatitis, keratoderma, and lichen planus.2,4,5
Scabies’ classic presentation
Typically, scabies causes intensely pruritic erythematous papules. Areas commonly affected are the webs and sides of fingers (FIGURE 1A and 1B), proximal palm and wrist flexors, extensor aspects of the arms and legs, axillary folds, periumbilical areas, the peri-areolar region in women, buttocks and thigh creases, and, in males, the genitals. The head may also be affected in children (FIGURE 1C), but seldom in adults. Interestingly, the back is usually spared across all age groups, though not always (FIGURE 2).


The classic presentation also varies across age groups and populations.2 In children, vesicles, pustules, and nodular pruritic lesions may coexist with eczema and impetigo. Among homeless individuals, coinfection with impetigo and eczema is common.
Scabies subtypes with varying presentations
Clinical manifestations of scabies subtypes may make it difficult to diagnose the disease. These subtypes include nodular, pustular, vesiculobullous, and crusted scabies (Norwegian scabies). Although rare, these subtypes merit acknowledgement, as atypical cases contribute to the high rate of misdiagnosis.
Nodular scabies is a clinical variant that accounts for about 7% of scabies cases.7 It can resist traditional scabies treatment (permethrin cream, ivermectin—which we’ll discuss in a bit) and often requires topical or intralesional corticosteroid management. Nodular scabies most commonly affects male genitalia. Patients may have multiple excoriated skin-colored erythematous papules and nodules in areas involving the classic distribution of scabies (web spaces of fingers, flexural areas [FIGURE 3], scrotum, and groin).8

Continue to: Pustular scabies...
Pustular scabies is commonly seen in children and adolescents but can occur in all age groups. Patients may present with vesicular pustular lesions (FIGURE 4A and 4B).7 In some cases, topical corticosteroids have modified the classic clinical presentation into a pustular variant. However, scabies alone can cause pustular lesions.

Vesiculobullous scabies (FIGURE 5A and 5B) is a clinical subtype that may be mistaken for pemphigus vulgaris9 or bullous pemphigoid10 because of its strikingly similar clinical presentation to those disorders.

Crusted scabies (Norwegian scabies) is a severe, disseminated form of scabies that commonly affects immunocompromised patients, although cases are also seen in immunocompetent hosts. Afflicted immunocompetent patients often have a history of diabetes mellitus, liver cirrhosis, malnutrition, or alcohol abuse.11 Patients present with a thick powdery or crusted white or yellow scale involving the feet or hands (FIGURE 6) that sometimes extends onto the limbs. Severe cases can involve wide body surfaces. One unusual presentation also included a desquamating rash without pruritus.12

Highly atypical cases
Atypical presentations include lesions that appear outside of the classic distribution areas of scabies, lesions with uncharacteristic morphology, cases with coinfections, and instances in which patients are immunocompromised.13 Examples include scabies of the scalp coexisting with seborrheic dermatitis or dermatomyositis,14 scabies mimicking mastocytoma,15,16 and scabies with coinfections of impetigo or eczema.17 These coinfections and clinical variations can be particularly challenging. Other reports of atypical scabies leading to misdiagnosis include a case of crusted scabies mimicking erythrodermic psoriasis18 and a case initially attributed to suppurative folliculitis and prurigo nodularis.19
Decisive diagnostic measures
Identifying the mite’s burrow on clinical exam is essential to making the diagnosis of scabies. Microscopic examinations of burrow scrapings remain the diagnostic gold standard. Frequently, nondermatologists perform skin scrapings of the pruritic papules of scabies instead of the burrows. These papules are a hypersensitivity reaction to the mites, and no scabetic mites will be found there. Burrows appear on exam as small, linear, serpiginous lines (FIGURE 7A and 7B).

Continue to: Handheld illumination with a dermatoscope...
Handheld illumination with a dermatoscope will allow visualization of the mite (FIGURE 8). (See “How fast do scabies mites move? Dermoscopy video answers that question.”) Dermatoscope findings consistent with scabies include a “delta glider,” a dark triangular shape that is the mite’s head hidden in the burrow,20 or the classic “jet with contrail” (FIGURE 8).21 Scrape the burrow to discover the mature scabies mite (FIGURE 9) and confirm the diagnosis.

Microscopic examination of scrapings has a reported sensitivity and specificity of up to 90% and 100%, respectively, when collection of scrapings is performed accurately and contains ova, feces, or mites.14,20,22 Dermatologists increasingly use dermatoscopes to diagnose scabies. Dermoscopy's sensitivity is 91% and specificity is 85% to 86%,14 which is a reassuring frame of reference for physicians who do not routinely use dermatoscopes and instead rely on scrapings.

Continue to: How fast do scabies mites move? Dermoscopy video answers that question
SIDEBAR
How fast do scabies mites move? Dermoscopy video answers that question
Richard Usatine, MD; Ashfaq Marghoob, MD
University of Texas Health San Antonio (Dr. Usatine); Memorial Sloan Kettering Skin Cancer Center, Hauppauge, NY (Dr. Marghoob).
Dr. Marghoob reported that he has received honoraria for speaking for 3GEN. Dr. Usatine reported no potential conflict of interest relevant to this article.
A 22-year-old man infected with human immunodeficiency virus presented to a clinic with a 6-month history of intense pruritus. Physical examination revealed scale crust on the hands and wrists (FIGURE) and in the pubic region.

Dermoscopic examination of the rash on the wrist revealed scabies mites actively crawling on the surface of the skin. A superficial skin scraping revealed the Sarcoptes scabiei mite, several eggs, and scybala under the microscope. The extensive infestation in this patient with crusted scabies was related to his immunocompromised state. (The patient was successfully treated with oral ivermectin and topical permethrin.)
Unprecedented documentation of mite speed a clue to infectivity. The 36-second VIDEO captured an adult mite travelling a distance of 3 mm, translating to a speed of 5 mm/ min. Six younger adults/larvae were moving more rapidly with a maximum distance of 6 mm in 36 seconds (1 cm/min). The movement of a mite within the epidermis is slower, as the mite consumes keratin in creating burrows. The more rapid rate of movement on the skin surface may help to explain the contagious nature of scabies.

In this case, the mites and larvae were viewed on the screen of a smartphone to which a dermatoscope was attached magnetically. The mites were first visualized in the standard photo mode. Video mode was then used to capture the motion using the maximum zoom feature of the phone, to a magnification factor of 13.3×.
Literature to date has been silent on mites’ rate of motion. A Medline search yielded only 3 papers that addressed the issue of in vivo movement of scabies mites. None viewed the mites other than in their burrows and none calculated a rate of motion.
- In one study using videodermotoscopy, Micali stated that in most of the 16 cases identified, it was possible to observe the mites moving inside the burrows.1 No video images were published, and there was no mention of speed or characterization of the movement.1
- A second study used reflectance confocal microscopy (RCM) to examine a single patient with crusted scabies. The authors claim to have viewed the ectoparasite's motion within the human host but provided no details of that motion.2
- In the third study, videodermoscopy showed a slightly higher sensitivity for scabies detection than RCM (95% vs. 92%).3 The authors did not mention visualization of movement of mites in their work but did quote the Micali paper for its mention of movements of the mite.
Applying digital dermoscopy in practice. It appears that this is the first published video documenting the movement of scabies mites and larvae in vivo using dermoscopy. This should pave the way for additional observations of scabies movement on and below the skin using dermoscopy with video. We recommend using the maximum zoom capability of the device along with the dermatoscope to view this movement. What has been surmised before—that the mite must move above the skin to infect human contacts—has now been captured in vivo using the power of dermoscopy.
CORRESPONDENCE
Richard P. Usatine, MD, 903 West Martin Street, San Antonio, TX 78207; [email protected].
REFERENCES
1. Micali G, Lacarrubba F, Lo Guzzo G. Scraping versus videodermatoscopy for the diagnosis of scabies: a comparative study. Acta Derm Venereol. 1999; 79:396.
2. Gürel MS, Turgut Erdemir AV, Tekin B. A case report of real-time in vivo demonstration of Sarcoptes scabiei. Turkiye Parazitol Derg. 2017; 41:229-232.
3. Cinotti E, Labeille B, Cambazard F, et al. Videodermoscopy compared to reflectance confocal microscopy for the diagnosis of scabies. J Eur Acad Dermatol Venereol. 2016; 30:1573-1577.
Continue to: 3 diagnostic missteps to avoid
3 diagnostic missteps to avoid
Misdiagnosis is often due to an overreliance on the clinical history without performing an adequate physical exam. In such cases, the physician often diagnoses a form of dermatitis as the cause of pruritic rash. (Admittedly, diagnostic error can result in either false-positive or false-negative findings, and many patients are diagnosed with scabies when they have dermatitis.)
A second misstep? Scabies may be overlooked in a patient whose lesions are nonpruritic, such as someone with an immunocompromising condition.
And finally, crusted scabies is frequently mistaken for psoriasis or chronic dermatitis.8
Diagnostic errors are exceedingly troublesome for patients and caregivers. It is not unusual for a hospital or long-term care facility to lose significant employee work hours due to a scabies epidemic or fear of a scabies epidemic. In a 2003 outbreak of scabies in a Canadian long-term care facility, an estimated $200,000 was needed to control disease spread.23
A topical agent is a mainstay of treatment
Permethrin cream is usually the first-line treatment choice.24 Ivermectin, topical (cream) or systemic (pill), is the commonly used alternative for patients who do not respond to, or cannot tolerate, permethrin cream. A recent meta-analysis examined the effectiveness of 5% permethrin cream, 1% ivermectin cream, and oral ivermectin (200 mcg/kg single or double dose).24 Overall, findings suggested there was no difference in the efficacy or in adverse effects of permethrin cream compared with ivermectin (topical or systemic) among adults. One study reported that permethrin cream was slightly more effective than ivermectin (cream or oral) because of the more rapid treatment response (approximately 94% clearance within 2 weeks of treatment, compared with 90%).25
Continue to: Adjust treatment for special populations
Adjust treatment for special populations. Treatment of severe cases, such as crusted scabies, calls for combination therapy with oral ivermectin (200 mcg/kg) and 5% permethrin cream.26
Five percent permethrin cream is the preferred treatment for children weighing < 15 kg and pregnant women; oral ivermectin has not been studied for efficacy and safety in these populations.27
Effective response to treatment in these studies was measured by resolution of active scabies lesions and improvement in pruritus 1 to 2 weeks after treatment.
Anticipate these 3 clinical scenarios
The classic appearance of scabies usually triggers suspicion of its presence, leading to prompt identification of mite burrows and a correct diagnosis. Unfortunately, though, this is not always the case. And atypical presentations heighten the chance of diagnostic error, which overall occurs in nearly half of cases.1 Keep in mind the following common scenarios, to help improve diagnosis.
1. When a patient presents with a severe pruritic eruption, the clinician may be tempted to settle early on a form of dermatitis and not consider the possibility of scabies. When the patient is later seen by an expert, the burrows are easily identified. Solution: Whenever a patient complains of severe pruritus, use a dermatoscope to carefully examine the digits, web spaces, proximal palms, wrists, and ankles for burrows.
Continue to: A patient with distal white or yellow, thick, scaly, or crusted plaques
2. A patient with distal white or yellow, thick, scaly, or crusted plaques is often thought to have psoriasis or dermatitis. But scabies should be included in the differential diagnosis. In particular, worsening thick, scaly plaques in an immunocompromised patient should prompt consideration of scabies.
3. Smooth nodules of the genitals in males, or pruritic smooth papules and plaques in other locations, should lead to the consideration of scabies. These presentations can be mistaken for lichen planus, folliculitis, papular urticaria, insect bites, or atopic dermatitis.
Due to the limited amount of mite burrows early in the disease process of scabies, and the gross similarities to a patient with dermatitis with skin excoriations, a thorough exam is needed—one that goes beyond the traditional web spaces and includes hidden/atypical locations such as margins of the feet and hands, the scalp, and neck creases. Careful and deliberate inspection for burrows is critical before ruling out the diagnosis.
CORRESPONDENCE
Art Papier, MD, 400 Red Creek Drive, Suite 200, Rochester, NY 14623; [email protected].
ACKNOWLEDGEMENT
We thank Angela Delacenserie, MA, for reviewing the manuscript and providing editing suggestions.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
1. Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017; 30:78-84.
2. Chosidow O. Clinical practices. Scabies. N Engl J Med. 2006; 354:1718-1727.
3. Arlian LG, Morgan MS. A review of Sarcoptes scabiei: past, present and future. Parasit Vectors. 2017; 10:297.
4. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
5. Berger TG, Shive M, Harper GM. Pruritus in the older patient: a clinical review. JAMA. 2013; 310:2443-2450.
6. Hengge UR, Currie BJ, Jäger G, et al. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis. 2006; 6:769-779.
7. Reddy DR, Reddy PR. Nodular scabies: a classical case report in an adolescent boy. J Parasit Dis. 2015; 39:581-583.
8. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006; 367:1767-1774.
9. Karaca Ş, Kelekçi KH, Er O, et al. Scabies incognito presenting as a subcorneal pustular dermatosis-like eruption. Turkiye Parazitol Derg. 2015; 39:244-247.
10. Gutte RM. Bullous scabies in an adult: a case report with review of literature. Indian Dermatol Online J. 2013; 4:311-313.
11. Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005; 50:375-381.
12. Ebrahim KC, Alves JB, Tomé LA, et al. Norwegian scabies—rare case of atypical manifestation. An Bras Dermatol. 2016; 91:826-828.
13. Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007; 20:268-279.
14. Dupuy E, Dehen L, Bourrat E, et al. Accuracy of standard dermoscopy for diagnosing scabies. J Am Acad Dermatol. 2007; 56:53-62.
15. Phan A, Dalle S, Balme B, et al. Scabies with clinical features and positive Darier sign mimicking mastocytosis. Pediatr Dermatol. 2009; 26:363-364.
16. Salces IG, Alfaro J, Sáenz DE, et al. Scabies presenting as solitary mastocytoma-like eruption in an infant. Pediatr Dermatol. 2009; 26:486-488.
17. Tasani M, Tong SY, Andrews RM, et al. The importance of scabies coinfection in the treatment considerations for impetigo. Pediatr Infect Dis J. 2016; 35:374-378.
18. Fonseca V, Price HN, Jeffries M, et al. Crusted scabies misdiagnosed as erythrodermic psoriasis in a 3-year-old girl with Down syndrome. Pediatr Dermatol. 2014; 31:753-754.
19. Carr PC, Brodell RT. Images in clinical medicine: scabies. N Engl J Med. 2016; 374:e13.
20. Park JH, Kim CW, Kim SS. The diagnostic accuracy of dermoscopy for scabies. Ann Dermatol. 2012; 24:194-199.
21. Lallas A, Apalla Z, Lazaridou E, et al. Scabies escaping detection until dermoscopy was applied. Dermatol Pract Concept. 2017; 7:49-50.
22. Micali G, Lacarrubba F, Verzi AE, et al. Scabies: advances in noninvasive diagnosis. PLoS Negl Trop Dis. 2016; 10:e0004691.
23. de Beer G, Miller MA, Tremblay L, et al. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol. 2006; 27:517-518.
24. Dhana A. Yen H, Okhovat JP, et al. Ivermectin versus permethrin in the treatment of scabies: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2018; 78:194-198.
25. Sharma R, Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J Dermatol Venereol Leprol. 2011; 77:581-586.
26. Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010; 362:717-725.
27. Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017; 31:1248-1253.
VIDEO shows scabies mite in motion
PRACTICE RECOMMENDATIONS
› Consider scabies with any severe pruritic eruption. Conduct a thorough physical exam, preferably with a dermatoscope, for burrows in the webs and sides of fingers, proximal palm, and wrists. A
› Consider scabies in all patients—especially the immunocompromised—who have distal white or yellow thick, scaly, or crusted plaques. C
› Include scabies in the differential when patients present with smooth nodules of the genitals or pruritic smooth papules and plaques in other locations. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA approves first treatment for advanced epithelioid sarcoma
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
Pitting of fingernails

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Given the constellation of non-scarring alopecia on the patient’s posterior scalp (with no scale, papules, or plaques), the physician diagnosed alopecia areata (AA) with associated nail pitting in this patient. Although a scalp biopsy could have confirmed the diagnosis, it was not needed because the clinical picture was sufficient.
Nail pitting is commonly associated with psoriasis (although it is often less dense in presentation), but it can occur with alopecia areata. It may occur concurrently or separately from active alopecia. Pitting of the nails may occur in one or multiple fingernails and occurs in up to a third of patients with AA.
The patient’s scalp was treated with intralesional triamcinolone diluted with normal saline to a concentration of 5 mg/mL (0.5%) and injected in dermal blebs over every square centimeter of involvement. Not every 10-year-old can tolerate this modality, and many families prefer observation or topical steroids. Other topical treatments for AA of the scalp include anthralin, minoxidil, and immunotherapy with squaric acid dibutyl ester or diphencyprone. None of these therapies are approved by the Food and Drug Administration for the treatment of nail disease. There are case reports of systemic tofacitinib clearing significant AA associated nail pitting in adults.
The physician counseled the family to observe the nails and not pursue any antifungal therapies for the nails.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.
Ferreira SB, Scheinberg M2, Steiner D, et al. Remarkable improvement of nail changes in alopecia areata universalis with 10 Months of treatment with tofacitinib: a case report. Case Rep Dermatol. 2016;8:262-266.
FDA supports sunscreen safety studies
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
FROM JAMA
European marketing of Picato suspended while skin cancer risk reviewed
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
Rash on hands and feet
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
Lichenoid dermatoses are a heterogeneous group of diseases with varying clinical presentations. The term “lichenoid” refers to the popular lesions of certain skin disorders of which lichen planus (LP) is the prototype. The papules are shiny, flat topped, polygonal, of different sizes, and occur in clusters creating a pattern that resembles lichen growing on a rock. Lichenoid eruptions are quite common in children and can result from many different origins. In most instances the precise mechanism of disease is not known, although it is usually believed to be immunologic in nature. Certain disorders are common in children, whereas others more often affect the adult population.
Lichen striatus, lichen nitidus (LN), and lichen spinulosus are lichenoid lesions that are more common in children than adults.
LN – as seen in the patient described here – is an uncommon benign inflammatory skin disease, primarily of children. Individual lesions are sharply demarcated, pinpoint to pinhead sized, round or polygonal, and strikingly monomorphous in nature. The papules are usually flesh colored, however, the color varies from yellow and brown to violet hues depending on the background color of the patient’s skin. This variation in color is in contrast with LP which is characteristically violaceous. The surfaces of the papules are flat, shiny, and slightly elevated. They may have a fine scale or a hyperkeratotic plug. The lesions tend to occur in groups, primarily on the abdomen, chest, glans penis, and upper extremities. The Koebner phenomenon is observed and is a hallmark for the disorder. LN is generally asymptomatic, unlike LP, which is exceedingly pruritic.
The cause of LN is unknown; however, it has been proposed that LN, in particular generalized LN, may be associated with immune alterations in the patient. The course of LN is slowly progressive with a tendency toward remission. The lesions can remain stationary for years; however, they sometimes disappear spontaneously and completely.
The differential diagnosis of LN beyond the entities discussed above includes frictional lichenoid eruption, lichenoid drug eruption, LP, and keratosis pilaris.
LP is the classic lichenoid eruption. It is rare in children and occurs most frequently in individuals aged 30-60 years. LP usually manifests as an extremely pruritic eruption of flat-topped polygonal and violaceous papules that often have fine linear white scales known as Wickham striae. The distribution is usually bilateral and symmetric with most of the papules and plaques located on the legs, flexor wrists, neck, and genitalia. The lesions may exhibit the Koebner phenomenon, appearing in a linear pattern along the site of a scratch. Generally, in childhood cases there is reported itching, and oral and nail lesions are less common.
Frictional lichenoid eruption occurs in childhood. The lesions consist of lichenoid papules with regular borders 1-2 mm in diameter that generally are asymptomatic, although they may be mildly pruritic. The papules are found in a very characteristic distribution with almost exclusive involvement of the backs of the hands, fingers, elbows, and knees with occasional involvement of the extensor forearms and cheeks. This disorder occurs in predisposed children who have been exposed to significant frictional force during play, and typically resolves spontaneously after removal of the stimulus.
Keratosis pilaris is a rash that usually is found on the outer areas of the upper arms, upper thighs, buttocks, and cheeks. It consists of small bumps that are flesh colored to red. The bumps generally don’t hurt or itch.
The lack of symptoms and spontaneous healing have rendered treatment unnecessary in most cases. LN generally is self-limiting, thus treatment may not be necessary. However, topical treatment with mid- to high-potency corticosteroids has hastened resolution of lesions in some children, as have topical dinitrochlorobenzene and systemic treatment with psoralens, astemizole, etretinate, and psoralen-UVA.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Neither Dr. Eichenfield nor Dr. Bhatti has any relevant financial disclosures. Email them at [email protected].
References
Pickert A. Cutis. 2012 Sep;90(3):E1-3. https://mdedge-files-live.s3.us-east-2.amazonaws.com/files/s3fs-public/Document/September-2017/0900300E1.pdf Tziotzios C et al. J Am Acad Dermatol. 2018 Nov;79(5):789-804. Tilly JJ et al. J Am Acad Dermatol. 2004 Oct;51(4):606-24.
A 9-year-old healthy Kuwaiti male with no significant past medical history presents with a rash on his hands and feet that has been present for 3 years.
His mother reports that he has been seen by dermatologists in various countries and was last seen by a dermatologist in Kuwait 3 years ago. At that time, he was told that it was dryness and advised to not shower daily. Since then he has been taking showers three times weekly and using Cetaphil once weekly without improvement. He was seen by his pediatrician 6 months ago, diagnosed with xerosis, and was given hydrocortisone 2.5% to use twice daily, again without any improvement.
The rash is not itchy, and he has no oral lesions or nail involvement. Exam revealed lichenoid papules on bilateral dorsal hands and feet, bilateral upper arms, bilateral axilla, lower abdomen, and left upper chest.
Chin There, Done That—Now What?
ANSWER
The correct answer is to perform a 3-mm punch biopsy (choice “a”).
DISCUSSION
When the history and physical exam fail to point to a clear diagnosis, skin biopsy can answer a very basic question: What is the pathophysiologic process associated with the lesions? The information thus obtained doesn’t always produce a blinking neon sign of a diagnosis, but at a minimum, it can rule out a great number of things, such as cancer or infection.
More often, the types of cells seen, and the patterns in which they are arranged, tell us a great deal. In this case, accumulations of monocytes, macrophages, and activated T-lymphocytes were arranged in circular patterns, but there was no evidence of necrosis (caseation), which can be seen in tubercular granulomas. Stains performed to identify mycobacterial organisms were negative. These findings established the diagnosis of cutaneous sarcoidosis, a granulomatous disease thought to represent a reaction to an unknown antigen (eg, a microorganism) or environmental substance. It is far more common in those with darker skin types.
Although this patient’s disease was confined to the skin, sarcoidosis can affect virtually any other organ in the body—in particular, the lungs, kidneys, liver, nervous system, or even the eyes. For this reason, patients with sarcoidosis are usually referred to pulmonology for a workup intended to rule out systemic involvement and to other specialists as symptoms dictate.
Many cases of sarcoidosis remit on their own, without treatment. When treatment is initiated, the 2 most commonly used medications are systemic glucocorticoids and/or methotrexate. Both drugs have been associated with serious adverse effects and should only be prescribed by experienced providers.
This combination was prescribed for the case patient, who obtained rapid relief. Blood work (complete blood count, comprehensive metabolic panel) was within normal limits prior to and during treatment, and the pulmonologist pronounced her free of lung involvement.
ANSWER
The correct answer is to perform a 3-mm punch biopsy (choice “a”).
DISCUSSION
When the history and physical exam fail to point to a clear diagnosis, skin biopsy can answer a very basic question: What is the pathophysiologic process associated with the lesions? The information thus obtained doesn’t always produce a blinking neon sign of a diagnosis, but at a minimum, it can rule out a great number of things, such as cancer or infection.
More often, the types of cells seen, and the patterns in which they are arranged, tell us a great deal. In this case, accumulations of monocytes, macrophages, and activated T-lymphocytes were arranged in circular patterns, but there was no evidence of necrosis (caseation), which can be seen in tubercular granulomas. Stains performed to identify mycobacterial organisms were negative. These findings established the diagnosis of cutaneous sarcoidosis, a granulomatous disease thought to represent a reaction to an unknown antigen (eg, a microorganism) or environmental substance. It is far more common in those with darker skin types.
Although this patient’s disease was confined to the skin, sarcoidosis can affect virtually any other organ in the body—in particular, the lungs, kidneys, liver, nervous system, or even the eyes. For this reason, patients with sarcoidosis are usually referred to pulmonology for a workup intended to rule out systemic involvement and to other specialists as symptoms dictate.
Many cases of sarcoidosis remit on their own, without treatment. When treatment is initiated, the 2 most commonly used medications are systemic glucocorticoids and/or methotrexate. Both drugs have been associated with serious adverse effects and should only be prescribed by experienced providers.
This combination was prescribed for the case patient, who obtained rapid relief. Blood work (complete blood count, comprehensive metabolic panel) was within normal limits prior to and during treatment, and the pulmonologist pronounced her free of lung involvement.
ANSWER
The correct answer is to perform a 3-mm punch biopsy (choice “a”).
DISCUSSION
When the history and physical exam fail to point to a clear diagnosis, skin biopsy can answer a very basic question: What is the pathophysiologic process associated with the lesions? The information thus obtained doesn’t always produce a blinking neon sign of a diagnosis, but at a minimum, it can rule out a great number of things, such as cancer or infection.
More often, the types of cells seen, and the patterns in which they are arranged, tell us a great deal. In this case, accumulations of monocytes, macrophages, and activated T-lymphocytes were arranged in circular patterns, but there was no evidence of necrosis (caseation), which can be seen in tubercular granulomas. Stains performed to identify mycobacterial organisms were negative. These findings established the diagnosis of cutaneous sarcoidosis, a granulomatous disease thought to represent a reaction to an unknown antigen (eg, a microorganism) or environmental substance. It is far more common in those with darker skin types.
Although this patient’s disease was confined to the skin, sarcoidosis can affect virtually any other organ in the body—in particular, the lungs, kidneys, liver, nervous system, or even the eyes. For this reason, patients with sarcoidosis are usually referred to pulmonology for a workup intended to rule out systemic involvement and to other specialists as symptoms dictate.
Many cases of sarcoidosis remit on their own, without treatment. When treatment is initiated, the 2 most commonly used medications are systemic glucocorticoids and/or methotrexate. Both drugs have been associated with serious adverse effects and should only be prescribed by experienced providers.
This combination was prescribed for the case patient, who obtained rapid relief. Blood work (complete blood count, comprehensive metabolic panel) was within normal limits prior to and during treatment, and the pulmonologist pronounced her free of lung involvement.
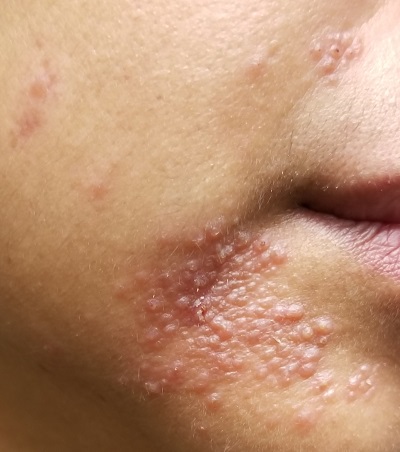
A 51-year-old woman is referred to dermatology for evaluation of a facial rash that has been present, on and off, for years. It usually affects her right chin and perioral area but occasionally manifests with smaller yet similar lesions elsewhere on the face. The lesions are slightly itchy, but the patient’s main concern is their impact on her appearance.
She has consulted several primary care providers over the years, all of whom initially diagnosed and treated for acne—to no avail. This was typically followed by a recommendation to try an OTC topical product, such as an antifungal (tolnaftate and clotrimazole), hydrocortisone 1%, or triple-antibiotic cream—again, without improvement. At no point was a biopsy suggested.
Examination reveals a dense cluster of papules covering a 5×4-cm section of the right chin and perioral area. Although the lesions appear vesicular, they are in fact solid but soft, shiny, and confluent. The papules are about the same color as her type IV skin. There are no comedones or pustules.
No adenopathy is detected in the region. There is no involvement of the adjacent oral mucosal surfaces.
The patient’s skin elsewhere is unremarkable, and in general, she appears to be in good health (certainly in no distress). She claims to be healthy otherwise, with no fever, malaise, joint pain, fatigue, or shortness of breath. No one else in her household is similarly affected.
Frequent lab testing is common, but low-yield, for isotretinoin patients
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
Abnormalities in lipids, liver enzymes, and blood counts were rare, and
In a review of 1,863 patients receiving isotretinoin, there were no cases of grade 4 abnormalities of lipids, liver enzymes, or complete blood count (CBC). Further, fewer than 1% of patients had grade 2-3 laboratory abnormalities, and no patients had cholesterol or CBC abnormalities of grade 3 or higher.
The retrospective cohort study used an electronic database to identify patients who were prescribed isotretinoin for acne from 2007 to 2017, with inclusion criteria structured to “increase the likelihood of capturing a complete course of isotretinoin therapy,” wrote John Barbieri, MD, and coauthors. The database allowed the investigators to group lab values into baseline testing, and testing by month of therapy for individual deidentified patient records.
Dr. Barbieri, a dermatologist and postdoctoral research fellow at the University of Pennsylvania, Philadelphia, and coinvestigators found that over half of all patients had baseline triglyceride, total cholesterol, AST, ALT, and platelet and white blood cell count levels.
Though the number of patients who had any of these levels checked in a given month of treatment declined over time, as did the total number of patients still on isotretinoin therapy, monthly AST and ALT monitoring occurred in 37.6%-58.5% of patients. Monthly triglyceride monitoring was conducted in between 39.6% and 61.4% of participants, and CBCs were obtained in 26.8%-37.4% of participants.
In terms of the abnormalities that were seen, grade 1 triglyceride elevations of 150-300 mg/dL were present in about 13% of patients at baseline, rising to 39% of participants who were still receiving isotretinoin at month 6. However, grade 2 elevations of up to 500 mg/dL were seen in 1.4% of patients at baseline and 2.4%-5.6% of patients during subsequent months.
Grade 1 liver enzyme abnormalities of less than three times the upper limit of normal values were seen at baseline in under 4% of patients, and in no more than 6.7% of patients through the course of treatment.
Leukopenia of between 3 x 103/mcL and the lower limit of normal occurred in 4.1% of baseline tests and in 6.6%-10.1% of tests in subsequent months. Grade 1 thrombocytopenia (values between 75 x 103/mcL and the lower limit of normal) occurred in 1.9% of baseline tests and no more than 2.9% of tests in the following months.
The results, wrote Dr. Barbieri and coauthors, affirm that most patients fare well on isotretinoin, and frequent laboratory testing is likely to be low-yield. Even using relatively low Medicare reimbursement rates for these tests yielded an estimated $134 in per-patient charges for the studied population. If baseline lipid and liver functions were followed only by repeat testing when peak isotretinoin dose was reached, charges would drop to about $87 per patient. Using the iPLEDGE database figures, this would save $17.4 million in monitoring costs annually, they wrote.
They also calculated that the monitoring regimen they observed puts the cost of detecting one single grade 3 hepatic enzyme elevation at $6,000; one grade 3 triglyceride elevation would cost $7,750.
Of the patients, 49% were female, the median age was 18.2 years, and the median duration of isotretinoin therapy was under 5 months (148 days). Nearly 90% of patients were white and non-Hispanic; 2.5% were black.
The data used for the analysis did not give the investigators access to clinician notes, but they did observe that, even when abnormal test values were seen, isotretinoin prescribing continued. This, they added, pointed toward reassuring clinical scenarios, even in cases of abnormal lab values.
“These findings are consistent with prior studies and suggest that extensive laboratory monitoring observed in this population may be of low value,” concluded Dr. Barbieri and colleagues. “In addition, changes to lipid levels observed in this study typically occurred during the first 2-3 months of therapy before stabilizing, which is consistent with findings in prior studies.”
The investigators noted that, despite mounting evidence of isotretinoin’s safety, there was no trend toward decreased CBC testing over the decade-long period of the study, and there were only “modest” decreases in hepatic enzyme and lipid monitoring. They called for an awareness campaign on the part of professional societies, and consideration for “more specific guideline recommendations” that may ease the testing burden on the adolescent and young adult population receiving isotretinoin.
The study was funded in part by the National Institutes of Health, and Dr. Barbieri receives partial salary support from Pfizer through a grant to the University of Pennsylvania. He has received support for unrelated work from Eli Lilly and Novartis. The other authors reported no conflicts of interest.
SOURCE: Barbieri J et al. J Am Acad Dermatol. 2020 Jan;82(1):72-9.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Rash on legs and abdomen
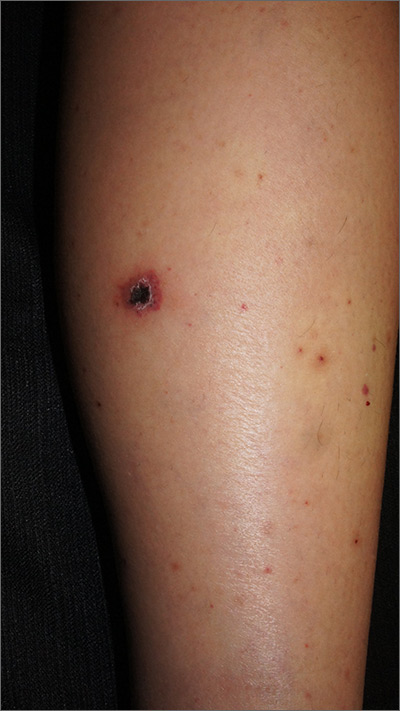
The rash was consistent with nonblanching purpura. Two punch biopsies were performed for hematoxylin and eosin stain and direct immunofluorescence, which were consistent with IgA mediated small vessel vasculitis, or Henoch-Schoenlein purpura.
IgA small vessel vasculitis commonly occurs in children after a transient viral illness or as an allergic reaction to a medication. Nonblanching purpura occurs because small vessels have been cracked open by neutrophils and lymphocytes and leaked blood cells outside of the vascular circulation. Pressure fails to move these cells downstream, and thus, the skin does not blanch.
Joint pain is common, as is a self-resolving IgA mediated nephropathy. Approximately 1% to 3% of children will progress to end stage renal disease. IgA vasculitis also occurs in adults, with a higher portion developing nephropathy. In adults, lesions that present on the abdomen are suspected to correspond with gravity dependency and the total burden of IgA, leading to a higher risk for nephropathy.
The differential diagnosis of purpura is broad, but includes leukocytoclastic vasculitis, antineutrophil cytoplasmic antibodies-associated vasculitis, capillaritis, and disseminated intravascular coagulation.
The patient in this case was given topical triamcinolone 0.1% ointment to treat the rash. She returned 3 weeks later and her blood pressure was 160/105 mm Hg and she had protein and blood in her urine. The FP recommended a renal biopsy, which confirmed severe IgA nephropathy and end stage renal failure. She was given systemic steroids and ultimately received a renal transplant. Her outcome was atypical and unfortunate. Usually IgA vasculitis is benign and self-resolves with rest. Even when nephropathy is present, it typically resolves over weeks to months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Audemard-Verger A, Terrier B, Dechartres A, et al. French Vasculitis Study Group. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis Rheumatol. 2017;69:1862-1870.

The rash was consistent with nonblanching purpura. Two punch biopsies were performed for hematoxylin and eosin stain and direct immunofluorescence, which were consistent with IgA mediated small vessel vasculitis, or Henoch-Schoenlein purpura.
IgA small vessel vasculitis commonly occurs in children after a transient viral illness or as an allergic reaction to a medication. Nonblanching purpura occurs because small vessels have been cracked open by neutrophils and lymphocytes and leaked blood cells outside of the vascular circulation. Pressure fails to move these cells downstream, and thus, the skin does not blanch.
Joint pain is common, as is a self-resolving IgA mediated nephropathy. Approximately 1% to 3% of children will progress to end stage renal disease. IgA vasculitis also occurs in adults, with a higher portion developing nephropathy. In adults, lesions that present on the abdomen are suspected to correspond with gravity dependency and the total burden of IgA, leading to a higher risk for nephropathy.
The differential diagnosis of purpura is broad, but includes leukocytoclastic vasculitis, antineutrophil cytoplasmic antibodies-associated vasculitis, capillaritis, and disseminated intravascular coagulation.
The patient in this case was given topical triamcinolone 0.1% ointment to treat the rash. She returned 3 weeks later and her blood pressure was 160/105 mm Hg and she had protein and blood in her urine. The FP recommended a renal biopsy, which confirmed severe IgA nephropathy and end stage renal failure. She was given systemic steroids and ultimately received a renal transplant. Her outcome was atypical and unfortunate. Usually IgA vasculitis is benign and self-resolves with rest. Even when nephropathy is present, it typically resolves over weeks to months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The rash was consistent with nonblanching purpura. Two punch biopsies were performed for hematoxylin and eosin stain and direct immunofluorescence, which were consistent with IgA mediated small vessel vasculitis, or Henoch-Schoenlein purpura.
IgA small vessel vasculitis commonly occurs in children after a transient viral illness or as an allergic reaction to a medication. Nonblanching purpura occurs because small vessels have been cracked open by neutrophils and lymphocytes and leaked blood cells outside of the vascular circulation. Pressure fails to move these cells downstream, and thus, the skin does not blanch.
Joint pain is common, as is a self-resolving IgA mediated nephropathy. Approximately 1% to 3% of children will progress to end stage renal disease. IgA vasculitis also occurs in adults, with a higher portion developing nephropathy. In adults, lesions that present on the abdomen are suspected to correspond with gravity dependency and the total burden of IgA, leading to a higher risk for nephropathy.
The differential diagnosis of purpura is broad, but includes leukocytoclastic vasculitis, antineutrophil cytoplasmic antibodies-associated vasculitis, capillaritis, and disseminated intravascular coagulation.
The patient in this case was given topical triamcinolone 0.1% ointment to treat the rash. She returned 3 weeks later and her blood pressure was 160/105 mm Hg and she had protein and blood in her urine. The FP recommended a renal biopsy, which confirmed severe IgA nephropathy and end stage renal failure. She was given systemic steroids and ultimately received a renal transplant. Her outcome was atypical and unfortunate. Usually IgA vasculitis is benign and self-resolves with rest. Even when nephropathy is present, it typically resolves over weeks to months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Audemard-Verger A, Terrier B, Dechartres A, et al. French Vasculitis Study Group. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis Rheumatol. 2017;69:1862-1870.
Audemard-Verger A, Terrier B, Dechartres A, et al. French Vasculitis Study Group. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis Rheumatol. 2017;69:1862-1870.